Abstract
The introduction of genes encoding T-cell receptor (TCR) chains specific for human immunodeficiency virus into T cells of infected patients represents a means to quantitatively and qualitatively improve immunity to the virus. Our results demonstrate that the high level of TCR expression required for physiologic functioning can be reproducibly achieved with retroviral vectors encoding full-length unmodified TCR chains under the control of a strong internal constitutive phosphoglycerate kinase promoter.
The importance of the CD8+ cytotoxic-T-lymphocyte (CTL) response for containment of human immunodeficiency virus (HIV) infection has been suggested by the temporal association of the emergence of the HIV-specific CD8+ CTL response following primary infection with clearance of viremia (7, 12, 25, 27, 37), the inverse correlation between the magnitude of the HIV-specific CTL response during the chronic phase and plasma viral load (28), and the correlation between maintenance of the long-term nonprogressor state as well as the asymptomatic phase of infection with the presence of a strong CD8+ CTL response (4, 24). Direct evidence has been provided in studies with macaques infected with simian immunodeficiency virus in which in vivo depletion of CD8+ T cells led to a burst of viremia followed by disease progression (22, 38).
Our laboratory has used the adoptive transfer of CD8+ CTL clones expanded ex vivo as a means of augmenting antiviral responses in immunocompromised hosts to a magnitude not achievable by the damaged endogenous immune system (33, 34, 35, 39). Transferred CD8+ T cells specific for HIV Gag migrate to areas of viral infection in lymph nodes and mediate in vivo antiviral activity (8, 9). However, there are several obstacles to the general application of this approach. It is often difficult to isolate CTL from individuals with advanced disease with expended CTL responses (10), as well as individuals receiving prolonged therapy with highly active antiretroviral therapy, due to the fall in precursor frequency of CTL to HIV following the reduction in viral burden (2). Additionally, the T cells that can be isolated from infected individuals are often terminally differentiated and difficult to expand and may fail to recognize HIV variants with escape mutations no longer recognizable by the dominant CTL response (3, 10, 20, 21, 24, 30).
These problems have previously been addressed by genetically modifying CD8+ T cells that are not HIV specific with chimeric receptors using antibody ectodomains or CD4 receptor domains coupled to the ζ chain. Such receptors universally recognize cells infected with HIV independent of major histocompatibility complex (MHC) phenotype (36, 40), but they have some disadvantages compared with native T-cell receptor (TCR) recognition of MHC and peptide. These disadvantages include delayed T-cell dissociation from bound gp120 HIV envelope (Env) leading to apoptosis (16), binding of circulating antigen shed from the membrane leading to blockage of target recognition and/or inappropriate activation (6), inclusion of murine antibody or novel fusion sequences which may lead to an immune response targeted against the chimeric molecule, emergence of viral mutations leading to decreased affinity to CD4 (23), and the lack of complete assembly of all the component chains of the multimeric TCR complex, which may be essential for delivery of fully competent activation and survival signals (18).
Introduction of full-length TCR chains specific for a desired HIV antigen into primary CD8+ T cells represents an alternative strategy to target selected viral epitopes that takes advantage of the native affinity, MHC-peptide binding property, and intracellular signaling qualities inherent in a correctly assembled TCR. A potential obstacle to this approach has been the magnitude of TCR surface expression required to deliver an effective signal, as the introduced chains must not compete with the endogenous TCR chains for the components of the TCR complex necessary for complete assembly and export to the cell membrane.
A CD3+, CD4−, CD8+ HIV Gag-specific T-cell clone, 52H8, bearing the TCR chains Vα2.3 and Vβ3.1 was cloned from the peripheral blood mononuclear cells of an HLA A3+ HIV-seropositive volunteer by using previously described methods (8). The A3-restricted epitope recognized by 52H8 was localized to p17 Gag protein by screening targets expressing each of the individual proteins comprising full-length HIV Gag, and the peptide epitope was mapped by pulsing Epstein-Barr virus-transformed B-lymphoblastoid cell (LCL) targets with synthetic overlapping peptides corresponding to the sequence of p17 from the SF2 strain of HIV. The minimal epitope was demonstrated to be RLRPGGKKK (amino acids 20 through 28 of p17) (data not shown).
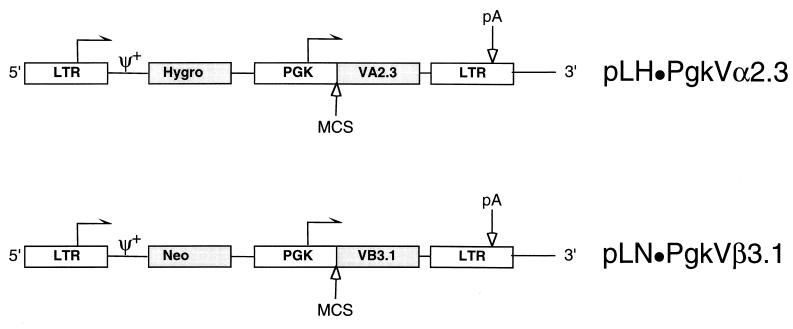
The full-length Vα2.3 and Vβ3.1 TCR genes were cloned error-free into retroviral plasmids derived from pLNSX (26) to generate pLN · PgkVβ3.1 and pLH · PgkVα2.3, respectively (Fig. 1). The internal simian virus 40 (S) promoter was replaced with a constitutive promoter derived from the murine phosphoglycerate kinase (Pgk) gene (1), based upon initial experiments demonstrating higher reporter gene expression in primary T cells compared to the long terminal repeat (LTR) or internal simian virus 40 and cytomegalovirus promoters (data not shown). The plasmids contained either the neomycin phosphotransferase (N) gene under the control of the LTR (L) or the hygromycin phosphotransferase gene (H) to permit independent selection of the two inserted genes.
FIG. 1.
Schematic of the approximately 6-kb proviral plasmids pLH · PgkVα2.3 and pLN · PgkVβ3.1 derived from the Moloney leukemia retrovirus backbone. The Vα2.3 and Vβ3.1 TCR chains under the transcriptional control of an internal Pgk promoter were cloned into a multiple cloning site (MCS) comprised of the unique AvrII, HindIII, and ClaI (not blocked by overlapping dam methylation) sites. The plasmids contain either the hygromycin B (Hygro) or neomycin (Neo) phosphotransferase genes expressed from the 5′ LTR. The arrows indicate the direction of transcription, pA indicates the polyadenylation signal, and ψ+ indicates the packaging signal.
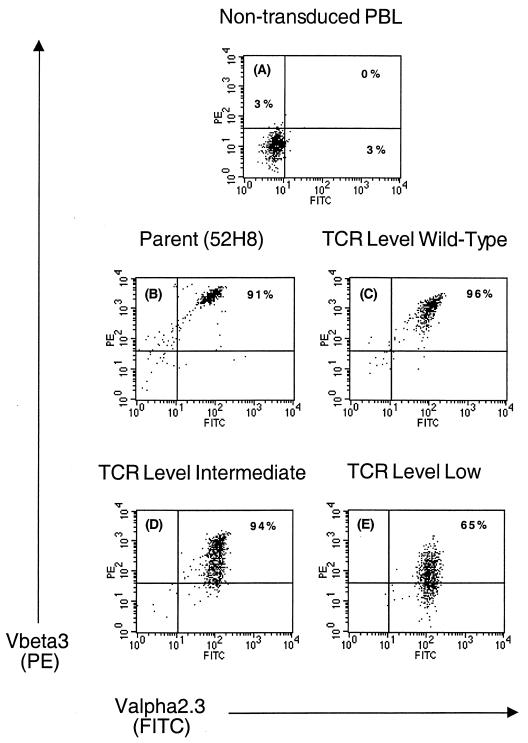
To facilitate transduction of human T cells, the proviral vectors were packaged in PG13 cells, which express the gibbon ape leukemia virus env gene, and clones of PG13 producing LN · PgkVβ3.1 and LH · PgkVα2.3 virus at approximately 105 CFU/ml were selected. Peripheral blood mononuclear cells from an HLA A3-positive HIV-seronegative volunteer were stimulated with anti-CD3 (OKT3; Ortho Biotech, Raritan, N.J.) (32) and transduced by cocultivation with PG13 cells producing LH · PgkVα2.3 virus. Hygromycin B-resistant CD8+ T cells were sorted for expression of Vα2.3, and high expressors were transduced with PG13 cells producing LN · PgkVβ3.1. T cells resistant to G418 were stimulated with autologous irradiated LCL pulsed with the peptide RLRPGGKKK to select T cells expressing a functional TCR. Wells demonstrating growth in response to antigen were cloned by limiting dilution. Twenty-four CD8+ T-cell clones were analyzed for expression of the introduced TCR chains by staining with anti-Vα2.3 and anti-Vβ3.1 monoclonal antibodies. In all clones the surface expression of Vα2.3 approximated the level observed in the parental 52H8 clone, whereas expression of Vβ3.1, which had not been used as a selection criteria by flow cytometry, was heterogeneous. Several sets of transduced T-cell clones expressing in comparison to the parental clone 52H8 either equivalent (wild type), intermediate, or low surface levels of the introduced TCR Vβ3.1 chain were selected for further study (Fig. 2). The stability of TCR surface expression in the different categories of clones in the absence of selective pressure by specific antigen stimulation or drug selection was assessed. Over a 21-week monitoring period, in which the cells were stimulated every 14 to 21 days with anti-CD3, surface levels of Vα2.3 and Vβ3.1 chains remained unchanged (data not shown).
FIG. 2.
Surface expression of TCR chains specific for HIV Gag on three representative CD8+ T-cell clones transduced with the retroviruses pLH · PgkVα2.3 and pLN · PgkVβ3.1. Staining was performed with the monoclonal antibodies Vα2.3-fluorescein isothiocyanate (x axis) and biotinylated Vβ3 followed by streptavidin-phycoerythrin (y axis), and expression was assessed 21 weeks after transduction of the PBMC by flow cytometry 10 days after anti-CD3 stimulation. There was no detectable binding of isotype- and concentration-matched nonspecific antibody (data not shown). Dead cells were excluded based on uptake of propidium iodide. (A) Nontransduced autologous peripheral blood lymphocytes (PBL), used as recipient cells for introduction of the TCR genes; (B) parental clone 52H8 from which the TCR genes were isolated; (C) representative T-cell clone expressing wild-type levels of the introduced TCR chains; (D and E) Two clones expressing less-than-physiologic levels of the pairs of the introduced TCR chains.
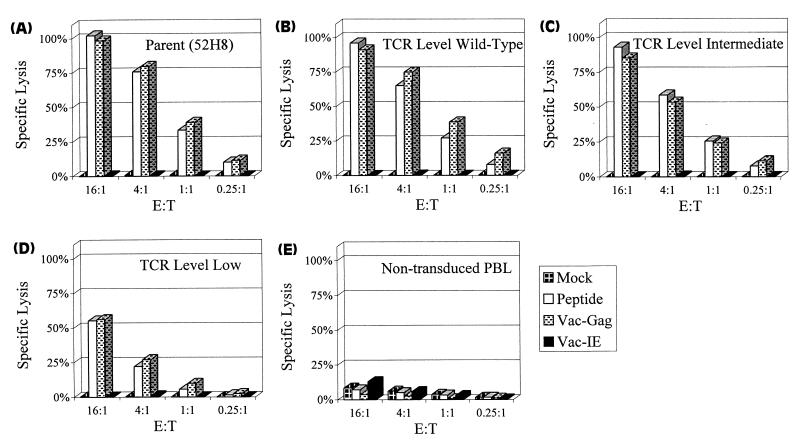
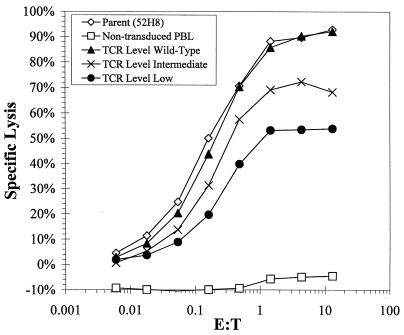
The influence of the level of TCR expression in the panel of transduced CD8+ T-cell clones on antigen recognition was assessed by lysis of HLA A3+ LCL targets expressing the HIV Gag epitope in a chromium release assay (CRA) (Fig. 3). The targets were exogenously loaded with 5 μM RLRPGGKKK peptide or infected with vaccinia virus Gag or with a control vaccinia virus immediate-early recombinant expressing the 72-kDa immediate-early protein from cytomegalovirus (19). Clones expressing levels of the introduced TCR chains similar to the parental clone 52H8 exhibited near-equivalent lysis of targets presenting the Gag epitope. T cells expressing an intermediate level of the introduced TCR chains exhibited a reduction in the ability to lyse targets expressing HIV Gag, which was further reduced in T cells expressing low levels of the introduced TCR chain pairs. Since T-cell accessory, adhesion, and signaling molecules can influence the avidity and cytolytic activity of a T cell for its target, the expression of CD28, CD11a, CD29, CD8, and TCR-ζ was determined by flow cytometry with fluorescein-conjugated antibodies. The parental clone, 52H8, and the transduced clones expressing variable levels of the introduced TCR chains did not differ in expression of these molecules (data not shown). To confirm that the specificity of the transduced T cells was dependent on the expression of the antigen-specific TCR, antibody specific for the TCR chains was used to block target recognition. The lysis of HLA A3+ LCL targets expressing the HIV Gag peptide was inhibited by antibody specific for either the Vα2.3 or Vβ3.1 chain, whereas an antibody against an irrelevant Vβ8 chain had no effect (data not shown).
FIG. 3.
Specific lysis of HLA A3+ LCL expressing HIV Gag antigen by the parental clone 52H8 (A), transduced clones representing different levels of the introduced pair of TCR chains (B through D), and nontransduced CD8+ T cells (E). LCL targets were loaded with 5 μM concentrations of the peptide RLRPGGKKK derived from HIV Gag or were infected with recombinant vaccinia virus Gag. Control targets were mock-treated LCL and LCL infected with immediate-early vaccinia virus. Lysis was measured in a standard 5-h CRA. E:T, effector-to-target cell ratio.
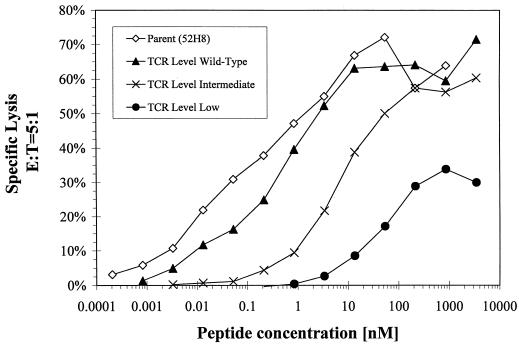
The amount of HIV antigen presented by HIV-infected cells can vary, particularly since HIV infection results in down-regulation of class I MHC molecules (15; D. A. Lewinsohn, S. R. Riddell, P. D. Greenberg, M. Emerman, and S. R. Bartz, unpublished data). To examine recognition of target cells with different antigen densities, HLA A3+ LCL were coated with a range of concentrations of the HIV Gag peptide. At all antigen densities the cytolytic activity of the transduced T-cell clones correlated with the level of TCR surface expression (Fig. 4). Transduced CD8+ T cells which expressed levels of the introduced TCR chains equivalent to the parental clone exhibited similar lytic activity even at the lowest peptide concentrations. T cells expressing diminished levels of the introduced TCR chains exhibited reduced capacity to lyse targets, which became increasingly prominent when the target antigen density was reduced.
FIG. 4.
Effect of the target epitope density on lysis by the parental and transduced CTL clones. Targets were generated by incubating A3+ LCL with serial dilutions of RLRPGGKKK peptide for 5 h and lysis by clones expressing various levels of the TCR was measured in a CRA at an effector-to-target cell (E:T) ratio of 5:1.
Vaccinia virus infection or peptide loading of targets might provide higher levels of epitope presentation than those obtained with HIV infection. Thus, recognition of HIV-infected targets by transduced CTL and clone 52H8 was directly assessed by infecting HLA A3+ CD4+ Jurkat cells with an env-deleted HIV-1 provirus (pBru3Δenv) pseudotyped with the vesicular stomatitis virus envelope glycoprotein (5) to achieve infection of a high proportion of Jurkat cells. The parental clone 52H8 and CTL clones expressing the introduced TCR genes lysed Jurkat cells infected with HIV type 1 but did not lyse uninfected Jurkat cells (Fig. 5). The level of surface expression of the introduced TCR chains again correlated with lytic activity, with clones expressing wild-type levels of the TCR chains demonstrating activity equivalent to that of the parental clone.
FIG. 5.
Specific lysis of HIV-infected A3+ CD4+ Jurkat cells by CTL clones expressing various TCR levels. Jurkat cells were infected with a vesicular stomatitis virus envelope glycoprotein-pseudotyped HIV to improve the efficiency of target infection, and lysis was measured at multiple effector-to-target (E:T) ratios in a standard 5-h CRA. Lysis of noninfected mock-treated Jurkat cells was subtracted (<10% specific lysis) from lysis of HIV-infected targets. PBL, peripheral blood lymphocytes.
TCR chains have previously been successfully introduced into hybridomas (14, 31), and recently TCR chains specific for a melanoma antigen were transferred into human peripheral blood lymphocytes (13). However, a large fraction of these genetically altered melanoma-specific T cells recognized peptide-loaded targets but failed to recognize melanoma tumor cells. Our results suggest that this discrepancy in target recognition is likely the consequence of variable levels of TCR expression and a predictably lower epitope density on tumor targets than peptide-loaded targets. Moreover, by employing a strong constitutive promoter, such as Pgk, wild-type levels of TCR expression can be reproducibly achieved.
Expression of the introduced receptors is apparently not substantially limited by endogenous ζ chain expression required for assembly of the multimeric TCR complex and export to the cell surface. This result is supported by recent studies with mice in which two functional transgenic TCRs were expressed in mature T cells (17). However, it is possible that some of the surface expression of the introduced TCR chains reflects alternative pairing with the reciprocal endogenous TCR chains. Such receptors would not have been subjected to developmental selection and could rarely be autoreactive. Our detection of clones expressing higher levels of the introduced Vα than Vβ confirms that such alternative pairing does occur. This problem could potentially be overcome either by transducing clones that do not express the Vα or Vβ chain when inserted alone or by modifying the TCR chains to contain tails with heterodimeric leucine zipper motifs to promote pairing (11, 29).
The potential to transfer TCRs with known specificity for HIV epitopes into autologous primary T cells offers a means of providing desired effector CD8+ T-cell responses to a broad range of individuals. Improved understanding of the molecular virology of HIV and increased mapping of T-cell epitopes should make it possible to ultimately provide potent T-cell responses targeting portions of the HIV genome that are essential to the virus and cannot mutate and lead to escape variants.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (CA18029, AI43650, CA33084, AI27757, and HD28834) and fellowship support from the Leukemia and Lymphoma Society of America (L.J.N.C.), by NIH training grant CA09351 (L.J.N.C.), and by the Cancer Research Institute (M.K.).
We thank Jenny Joyce and Brian Macintosh for technical assistance.
REFERENCES
- 1.Adra C N, Boer P H, McBurney M W. Cloning and expression of the mouse Pgk-1 gene and the nucleotide sequence of its promoter. Gene. 1987;60:65–74. doi: 10.1016/0378-1119(87)90214-9. [DOI] [PubMed] [Google Scholar]
- 2.Altman J, Moss P A H, Goulder P, Barouch D, McHeyzer-Williams M, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 3.Ariyoshi K, Cham F, Berry N, Jaffar S, Sabally S, Corrah T, Whittle H. HIV-2-specific cytotoxic T-lymphocyte activity is inversely related to proviral load. AIDS. 1995;9:555–559. doi: 10.1097/00002030-199506000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Barker E, Mackewicz C E, Ryes-Terán G, Sata A, Stanford S A, Fujimura S H, Christopherson C, Chang S-Y, Levy J A. Virological and immunological features of long-term human immunodeficiency virus-infected individuals who have remained asymptomatic compared with those who have progressed to acquired immunodeficiency syndrome. Blood. 1998;92:3105–3114. [PubMed] [Google Scholar]
- 5.Bartz S R, Vodicka M A. Production of high-titer human immunodeficiency virus type 1 pseudotyped with the vesicular stomatitis virus glycoprotein. Methods Enzymol. 1997;12:337–342. doi: 10.1006/meth.1997.0487. [DOI] [PubMed] [Google Scholar]
- 6.Bitton N, Gorochov G, Debre P, Eshhar Z. Gene therapy approaches to HIV-infection: immunological strategies: use of T bodies and universal receptors to redirect cytolytic T-cells. Front Biosci. 1999;4:D386–D393. doi: 10.2741/bitton. [DOI] [PubMed] [Google Scholar]
- 7.Borrow P, Lewicki H, Hahn B H, Shaw G M, Oldstone M B A. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodie S J, Lewinsohn D A, Patterson B K, Jiyamara D, Krieger J, Corey L, Greenberg P D, Riddell S R. In vivo migration and function of transferred HIV-1-specific cytotoxic T cells. Nat Med. 1999;5:34–41. doi: 10.1038/4716. [DOI] [PubMed] [Google Scholar]
- 9.Brodie S J, Patterson B K, Lewinsohn D A, Diem K, Spach D, Greenberg P D, Riddell S R, Corey L. HIV-specific cytotoxic T lymphocytes traffic to lymph nodes and localize at sites of HIV replication and cell death. J Clin Investig. 2000;105:1407–1417. doi: 10.1172/JCI8707. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J Exp Med. 1993;177:249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang H-C, Bao Z-Z, Yao Y, Tse A G D, Goyarts E C, Madsen M, Kawasaki E, Brauer P P, Sacchettini J C, Nathenson S G, Reinherz E L. A general method for facilitating heterodimeric pairing between two proteins: application to expression of α and β T-cell receptor extracellular elements. Proc Natl Acad Sci USA. 1994;91:11408–11412. doi: 10.1073/pnas.91.24.11408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheynier R, Langlade-Demoyen P, Marescot M R, Blanche S, Blondin G, Wain-Hobson S, Griscelli C, Vilmer E, Plata F. Cytotoxic T lymphocyte responses in the peripheral blood of children born to human immunodeficiency virus-1-infected mothers. Eur J Immunol. 1992;22:2211–2217. doi: 10.1002/eji.1830220905. [DOI] [PubMed] [Google Scholar]
- 13.Clay T, Custer M C, Sachs J, Hwu P, Rosenberg S A, Nishimura M I. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol. 1999;163:507–513. [PubMed] [Google Scholar]
- 14.Cole D J, Weil D P, Shilyansky J, Custer M, Kawakami Y, Rosenberg S A, Nishimura M I. Characterization of the functional specificity of a cloned T-cell receptor heterodimer recognizing MART-1 melanoma antigen. Cancer Res. 1995;55:748–752. [PubMed] [Google Scholar]
- 15.Collins K L, Chen B K, Kalams S A, Walker B D, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 16.Davis M M, Boniface J J, Reich Z, Lyons D, Hampl J, Arden B, Chien Y-H. Ligand recognition by αβ T cell receptors. Annu Rev Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 17.Fossati G, Cooke A, Papafio R Q, Haskins K, Stockinger B. Triggering a second T cell receptor on diabetogenic T cells can prevent induction of diabetes. J Exp Med. 1999;190:577–583. doi: 10.1084/jem.190.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Germain R N, Stefanová I. The dynamics of T cell receptor signaling: complex orchestration and the key roles of tempo and cooperation. Annu Rev Immunol. 1999;17:467–522. doi: 10.1146/annurev.immunol.17.1.467. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert M J, Riddell S R, Plachter B, Greenberg P D. Cytomegalovirus selectively blocks antigen processing and presentation of its immediate-early gene product. Nature. 1996;383:720–722. doi: 10.1038/383720a0. [DOI] [PubMed] [Google Scholar]
- 20.Gotch F M, Koup R A, Safrit J T. New observations on cellular immune responses to HIV and T-cell epitopes. AIDS. 1997;11(Suppl. A):S99–S107. [PubMed] [Google Scholar]
- 21.Goulder P J, Phillips R E, Colbert R A, McAdam S, Ogg G, Nowak M A, Giangrande P, Luzzi G, Morgan B, Edwards A, McMichael A J, Rowland-Jones S. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997;3:212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- 22.Jin X, Bauer D E, Tuttleton S E, Lewin S, Gettie A, Blanchard J, Irwin C E, Safrit J T, Mittler J, Weinberger L, Kostrikis L G, Perelson A S, Ho D D. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klasse P J, McKeating J A. Soluble CD4 and CD4 immunoglobulin-selected HIV-1 variants: a phenotypic characterization. AIDS Res Hum Retrovir. 1993;9:595–604. doi: 10.1089/aid.1993.9.595. [DOI] [PubMed] [Google Scholar]
- 24.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P, Eeftinck-Schattenkerk J K, Osterhaus A D, Schuitemaker H, Miedema F. Kinetics of gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1371. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller A D, Miller D G, Garcia J V, Lynch C M. Use of retroviral vectors for gene transfer and expression. Methods Enzymol. 1993;217:581–599. doi: 10.1016/0076-6879(93)17090-r. [DOI] [PubMed] [Google Scholar]
- 27.Musey L, Hughes J, Schacker T, Shea T, Corey L, McElrath M J. Cytotoxic T cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N Engl J Med. 1997;337:1267–1274. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 28.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowack M A, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantification of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 29.O'Shea E K, Lumb K J, Kim P S. Peptide “velcro”: design of a heterodimeric coiled coil. Curr Biol. 1993;3:658–667. doi: 10.1016/0960-9822(93)90063-t. [DOI] [PubMed] [Google Scholar]
- 30.Pantaleo G, Koenig S, Baseler M, Lane H C, Fauci A S. Defective clonogenic potential of CD8+ T lymphocytes in patients with AIDS. J Immunol. 1990;144:1696–1704. [PubMed] [Google Scholar]
- 31.Pogulis R J, Pease L R. A retroviral vector that directs simultaneous expression of alpha and beta T cell receptor genes. Hum Gene Ther. 1998;9:2299–2304. doi: 10.1089/hum.1998.9.15-2299. [DOI] [PubMed] [Google Scholar]
- 32.Riddell S R, Greenberg P D. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128:189–201. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- 33.Riddell S R, Greenberg P D. Principles for adoptive T cell therapy of human viral diseases. Annu Rev Immunol. 1995;13:545–586. doi: 10.1146/annurev.iy.13.040195.002553. [DOI] [PubMed] [Google Scholar]
- 34.Riddell S R, Watanabe K S, Goodrich J M, Li C-R, Agha M E, Greenberg P D. Restoration of viral immunity in immunocompromised humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 35.Riddell S R, Gilbert M J, Greenberg P D. CD8+ cytotoxic T cell therapy of cytomegalovirus and HIV infection. Curr Opin Immunol. 1993;5:484–491. doi: 10.1016/0952-7915(93)90027-p. [DOI] [PubMed] [Google Scholar]
- 36.Roberts M R, Qin L, Zhang D, Smith D H, Tran A-C, Dull T J, Groopman J E, Capon D J, Byrn R A, Finer M H. Targeting of human immunodeficiency virus-infected cells by CD8+ T lymphocytes armed with universal T-cell receptors. Blood. 1994;84:2878–2889. [PubMed] [Google Scholar]
- 37.Rowland-Jones S, McMichael A. Role of cellular immunity in protection against HIV infection. Adv Immunol. 1997;65:277–346. [PubMed] [Google Scholar]
- 38.Schmitz J E, Kuroda M J, Santra S, Sasseville V G, Simon M A, Lifton M A, Racz P, Tenner-Racz K, Dalesandro M, Scallon B J, Ghrayeb J, Forman M A, Montefiori D C, Rieber E P, Letvin N L, Reimann K A. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 39.Walter E A, Greenberg P D, Gilbert M J, Finch R J, Watanabe K S, Thomas E D, Riddell S R. Reconstitution of cellular immunity against CMV in recipients of allogeneic bone marrow by adoptive transfer of T cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 40.Yang O O, Tran A C, Kalams S A, Johnson R P, Roberts M R, Walker B D. Lysis of HIV-1-infected cells and inhibition of viral replication by universal receptor T cells. Proc Natl Acad Sci USA. 1997;94:11478–11483. doi: 10.1073/pnas.94.21.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]