Abstract
Background and purpose
This study aimed to assess the diagnostic criteria, ancillary investigations and treatment response using real‐life data in multifocal motor neuropathy (MMN) patients.
Methods
Clinical and laboratory data were collected from 110 patients enrolled in the Italian MMN database through a structured questionnaire. Twenty‐six patients were excluded due to the unavailability of nerve conduction studies or the presence of clinical signs and symptoms and electrodiagnostic abnormalities inconsistent with the MMN diagnosis. Analyses were conducted on 73 patients with a confirmed MMN diagnosis and 11 patients who did not meet the diagnostic criteria.
Results
The European Federation of Neurological Societies/Peripheral Nerve Society (EFNS/PNS) diagnostic criteria were variably applied. AUTHOR:When applying the American Association of Electrodiagnostic Medicine criteria, an additional 17% of patients fulfilled the criteria for probable/definite diagnosis whilst a further 9.5% missed the diagnosis. In 17% of the patients only compound muscle action potential amplitude, but not area, was measured and subsequently recorded in the database by the treating physician. Additional investigations, including anti‐GM1 immunoglobulin M antibodies, cerebrospinal fluid analysis, nerve ultrasound and magnetic resonance imaging, supported the diagnosis in 46%–83% of the patients. Anti‐GM1 immunoglobulin M antibodies and nerve ultrasound demonstrated the highest sensitivity. Additional tests were frequently performed outside the EFNS/PNS guideline recommendations.
Conclusions
This study provides insights into the real‐world diagnostic and management strategies for MMN, highlighting the challenges in applying diagnostic criteria.
Keywords: diagnosis, diagnostic criteria, guidelines, MMN, multifocal motor neuropathy
INTRODUCTION
Multifocal motor neuropathy (MMN) is a rare, purely motor neuropathy characterized by significant upper limb involvement without sensory loss and persistent conduction block (CB) on motor nerves [1, 2, 3]. Many data point to a pathogenetic role of the immune system in this neuropathy including the frequent occurrence of high titres of immunoglobulin M (IgM) antibodies to the ganglioside GM1 [1, 2, 3] and the frequent response to therapy with high‐dose intravenous immunoglobulin (IVIg) [4]. The precise pathogenesis of this neuropathy remains unclear, however, also because, with the possible exception of cyclophosphamide, other immune therapy has not been proved to be effective in MMN [5].
A few diagnostic criteria have been used for MMN even if the criteria proposed in 2010 by the European Federation of Neurological Sciences and the Peripheral Nerve Society (EFNS/PNS) are mostly utilized [6]. These criteria are quite selective for the diagnosis of CB in MMN requiring the presence of a reduction of the area of the negative peak of the compound muscle action potential (CMAP) in relation with its duration and not of the amplitude, as allowed by the American Association of Electrodiagnostic Medicine (AAEM) consensus criteria for MMN [7, 8] and by the 2021 European Academy of Neurology/Peripheral Nerve Society (EAN/PNS) criteria for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) [9].
A number of additional diagnostic investigations have been included to support the MMN diagnosis in patients not fulfilling the electrodiagnostic criteria [6]. The relative diagnostic relevance of these investigations remains unclear, however, and they might be unnecessarily performed in these patients.
A study was conducted on patients diagnosed with MMN, under the care of specialized Italian centres for immune‐mediated neuropathies. Our aim was to analyse the criteria used for the diagnosis, the usefulness of accessory investigations for the diagnosis, and the response to therapy.
METHODS
A web‐based database on Italian MMN patients was implemented, which currently includes data from 110 patients clinically diagnosed with MMN. The treating neurologist included all the data in a web‐based electronic database expressly prepared by CINECA, Bologna, Italy. The treating neurologist initially made the diagnosis of MMN that the coordinating centre (P.E.D. and E.N.O.) reviewed and classified according to the 2010 EFNS/PNS diagnostic criteria [6]. Patients with an alternative diagnosis for the neuropathy or with symptoms and signs non‐consistent with MMN or without available nerve conduction studies were excluded from the study.
Clinical assessment
Similar to what was previously performed in patients included in the Italian CIDP database [10], all eligible patients underwent a detailed clinical history at enrolment using a structured questionnaire. This history included the time of onset and distribution and progression of symptoms, encompassing weakness, sensory symptoms, ataxia, pain, cramps, tremor, fatigue, cranial nerve impairment, dysphagia, dyspnoea and autonomic dysfunction. This information was integrated with the data reported in the medical records. The clinical evaluation at registry enrolment included assessment of muscle strength using the Medical Research Council sum score on 12 muscles (range 0–60). Neurological disability was evaluated at enrolment with the MMN Rasch‐built Overall Disability Scale (range 1–50) [11] and the Overall Neuropathy Limitation Scale (range 0–12) [12]. Response to treatment was defined as a subjective improvement objectively confirmed by an increase of at least 2 points in the Medical Research Council sum score (range 0–60) or at least 1 point in the Overall Neuropathy Limitation Scale score (range 0–12) [6, 9]. The response to treatment was prospectively evaluated by the treating neurologist and reported in the database. Whilst the evaluation of therapy response was not standardized at a predetermined time for all patients, all treated individuals had completed at least one cycle of IVIg at a dose of 2 g/kg.
The results of diagnostic nerve conduction studies performed during the course of the disease were included. Motor nerve conduction studies were conducted in a non‐standardized manner, but they consistently included the clinically affected nerves. These studies encompassed measurement of distal and proximal CMAP amplitude (onset to peak), negative peak area and duration, as well as assessment of motor conduction velocities, distal motor latencies and, in most cases, F‐wave latency. All the centres were solicited to provide the data, if not done before, on CMAP area and duration. Sensory conduction studies were performed in the median, ulnar and sural nerves as well as in any nerve affected by CB or in the territory of the patients' sensory symptoms or signs. These included sensory action potential amplitude, distal latency and conduction velocity. There was no definite time point for the examination. Each centre was asked to include the most complete and diagnostic examination.
Results of cerebrospinal fluid (CSF) examination performed during the course of the disease were reported, including total protein level and cell count. As to protein level, as upper reference limit 50 mg/dL was considered for patients aged ≤50 years and 60 mg/dL for those aged >50 years [13]. The frequency of patients with CSF protein elevation ≥1 g/L was also evaluated. Results of anti‐GM1 IgM antibody testing were reported by each laboratory, including the normal value of their laboratory.
The results of brachial/lumbosacral plexus and roots magnetic resonance imaging (MRI) examination were reported and defined by the local examiner as possible supportive value for the diagnosis of MMN if they showed nerve enlargement or T2‐hyperintense signal and/or gadolinium enhancement of the brachial plexus [6, 14, 15, 16]. The results of nerve ultrasound (US) were considered of possible supportive value for the diagnosis of MMN if the local examiner reported an enlargement of the examined nerves or brachial plexus beyond their normal values [15, 16].
All the patients had been extensively investigated in each centre for the presence of possible alternative causes of neuropathy through clinical and laboratory investigation, following the EFNS/PNS guidelines [6].
The diagnosis of MMN was classified by the coordinating centre according to the EFNS/PNS diagnostic criteria into definite, probable or possible MMN [6]. Also the AAEM criteria were applied to the patients included in the study [7, 8].
The study was approved by the ethical committees of all participating centres. A written informed consent was obtained from all participants at the time of enrolment.
Statistical analysis
Descriptive statistics were reported for the entire sample of patients with MMN, and for each clinical subgroup separately. Categorical variables were described using frequencies and percentages, and continuous variables using mean, median and range. Demographic and clinical features, treatment response, strength deficit and disability level were compared between different subgroups of patients with the chi‐squared or Fisher's exact test for categorical variables and with the t test or the Wilcoxon−Mann–Whitney test for continuous variables. All tests were two‐tailed and the significance level was set to 0.05. The analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
One hundred and ten patients were included in the database from March 2019 to January 2023 from 18 Italian centres with expertise in immune‐mediated neuropathies. Ten patients were excluded since nerve conduction studies were not available and 16 patients were excluded for the presence of clinical signs or symptoms or electrodiagnostic abnormalities not consistent with the diagnosis of MMN according to the EFNS/PNS criteria (Figure 1). These included the presence of sensory symptoms and signs and abnormal sensory nerve conduction studies in nerves with motor CB in the upper limb nerves (11 patients, eventually diagnosed with multifocal CIDP), bulbar impairment (four patients, eventually diagnosed with motor neuron syndrome) and one patient who only had cramps and no evident weakness.
FIGURE 1.
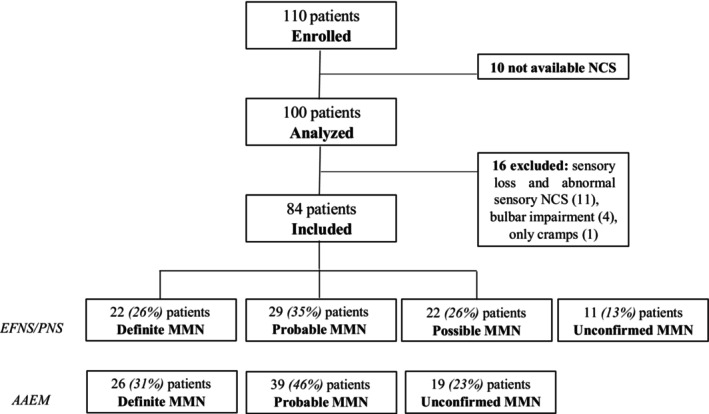
Flowchart of the Italian MMN database. AAEM, American Association of Electrodiagnostic Medicine diagnostic criteria for MMN; EFNS/PNS, European Federation of Neurological Societies/Peripheral Nerve Society diagnostic criteria for MMN; MMN, multifocal motor neuropathy; NCS, nerve conduction studies.
Amongst the 84 included patients (56 men and 28 women), 22 (26%) fulfilled the criteria for definite, 29 (35%) for probable and 22 (26%) for possible MMN according to the EFNS/PNS criteria (Figure 1). Eleven patients (13%) did not meet the diagnostic criteria for MMN, as they failed to satisfy the motor electrodiagnostic criteria. In nine of these cases, the response to IVIg therapy was unclear, thereby precluding a diagnosis of possible MMN. Furthermore, two newly diagnosed patients were included in the database before undergoing IVIg treatment. When applying the CB criteria proposed by the AAEM [7, 8], four of the patients who did not fulfil the EFNS/PNS diagnostic criteria for MMN would meet the criteria for a diagnosis of probable or definite MMN, 10 patients would shift from a diagnosis of possible to a diagnosis of probable MMN and four patients from a diagnosis of probable to a diagnosis of definite MMN, as depicted in Figures 1 and 2. This discrepancy occurred because in 14 patients only CMAP amplitude, but not area, was measured and subsequently recorded in the database by the treating physician. Of these, 10 patients improved after IVIg. In the other four patients, there was a reduction of >50% in CMAP amplitude but not in area. On the other hand, applying the AAEM criteria also resulted in 19 patients not meeting the criteria for MMN diagnosis (Figures 1 and 2). Overall, using the AAEM criteria, 14 (17%) additional patients fulfilled the criteria for probable/definite diagnosis whilst eight (9.5%) additional patients missed the diagnosis.
FIGURE 2.
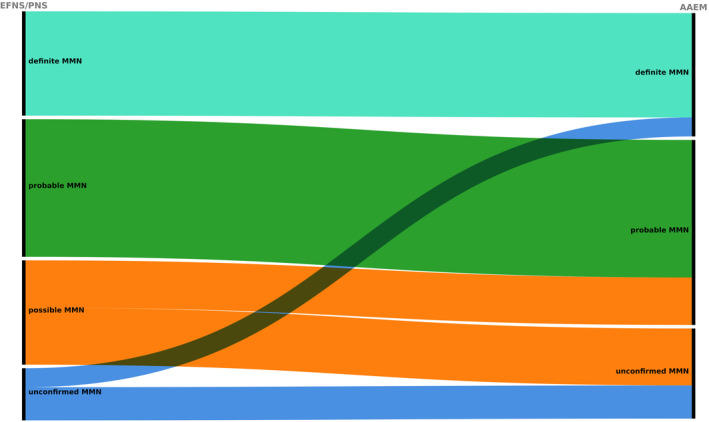
Alluvial diagram illustrating the change in diagnosis using the EFNS/PNS and the AAEM diagnostic criteria for MMN in real life. AAEM, American Association of Electrodiagnostic Medicine diagnostic criteria for MMN; EFNS/PNS, European Federation of Neurological Societies/Peripheral Nerve Society diagnostic criteria for MMN; MMN, multifocal motor neuropathy.
Table 1 summarizes the clinical and laboratory features and response to therapy of the 73 patients with a confirmed MMN diagnosis and of the 11 patients not fulfilling the EFNS/PNS criteria for MMN. Amongst MMN patients, men were more frequently affected than women with a 2:1 ratio and the mean age at disease onset was 42 years (range 21–68). At the onset of symptoms, upper limbs were exclusively affected in 63% of the patients. Upon inclusion in the study, which occurred on average 12 years later, the involvement of all four limbs increased significantly, rising from 13.6% at onset to 63%. In the upper limbs, the most frequently affected nerves, both clinically and electrophysiologically, were, in descending order, the ulnar, median, radial and musculocutaneous, whilst in the lower limbs they were the peroneal, tibial, femoral and gluteus. Throughout the disease progression, additional symptoms and signs were observed, including muscle hypotrophy primarily in the upper limbs in 53 (73%) patients, fatigue in 26 (36%) patients, cramps in 20 (27%) patients, pain in five (7%) patients and limb paraesthesia without objective sensory loss in 11 (15%) patients. The 11 patients with unconfirmed diagnosis of MMN had a shorter disease duration at inclusion (p = 0.0001). Furthermore, they exhibited a higher prevalence of selective upper limb impairment (64%) upon enrolment, in contrast to patients with MMN (33%) (p = 0.0897). Patients without a confirmed diagnosis more frequently had increased anti‐GM1 IgM antibodies and less frequently MRI/US abnormalities, but none of these differences was significant. No significant difference was observed between the two groups regarding the frequency of increased CSF proteins. Only four patients with a confirmed diagnosis had a CSF protein elevation of ≥1 g/L.
TABLE 1.
Clinical features of the included patients with MMN and unconfirmed MMN diagnosis.
| MMN | Unconfirmed MMN diagnosis | p value | |
|---|---|---|---|
| Number of patients | 73 | 11 | |
| Gender, male/female (ratio) | 49/24 (2:1) | 7/4 (1.7:1) | ns |
| Age at inclusion, years, mean (range) | 54 (31–85) | 51.5 (31–73) | ns |
| Age at onset, years, mean (range) | 42 (21–68) | 37 (21–54) | ns |
| Disease duration, years, mean (range) | 12 (0.4–35) | 2 (0.4–3) | 0.0001 |
| Definite/probable/possible MMN | 22/29/22 | ns | |
| ONLS at inclusion, mean (range) | 3.3 (0–9) | 3.0 (0–8) | ns |
| MRC at inclusion, mean (range) | 53.9 (30–60) | 55.1 (43–60) | ns |
| Limb impairment at onset, upper/lower/upper and lower | 46/17/10 | 10/1/0 | ns |
| Limb impairment at inclusion, upper/lower/upper and lower | 24/2/46 a | 7/0/3 a | ns |
| Positive anti‐GM1 IgM/tested | 24/39 (62%) | 7/8 (87%) | ns |
| Increased CSF proteins/tested | 12/26 (46%) | 2/4 (50%) | ns |
| Increased CSF proteins ≥1 g/L | 4/24 (17%) | 0/4 (0%) | ns |
| Abnormal US and/or MRI/tested | 12/17 (71%) | 2/6 (33%) | ns |
| Improved after IVIg/treated | 60/68 (88%) | 0/9 (0%) | 0.0001 |
| Improved after ScIg/treated | 7/25 (28%) | 0/2 (0%) | ns |
| Improved after cyclophosphamide/treated | 3/8 (37%) | 0/0 | na |
| Improved after steroids | 1/5 (20%) | 0/0 | na |
| Improved after other therapies b | 0/15 (0%) | 0/0 | na |
Abbreviations: CSF, cerebrospinal fluid; IgM, immunoglobulin M; IVIg, intravenous immunoglobulin; MMN, multifocal motor neuropathy; MRC, Medical Research Council; MRI, magnetic resonance imaging; na, not available; ns, not significant; ONLS, Overall Neuropathy Limitations Scale; ScIg, subcutaneous immunoglobulin; US, ultrasound.
Not reported in one.
Rituximab (7), cyclosporin (3), plasma exchange (2), azathioprine (1), interferon (1), methotrexate (1).
Across all 84 patients in the cohort, a total of seven out of 11 (64%) tested patients had a positive brachial plexus MRI. Amongst them, five patients exhibited heightened signal intensity on the T2‐weighted images of the brachial plexus. Two other patients demonstrated a more focal increase in signal intensity on the T2‐weighted images, localized in the axilla and ventral rami of the C6, C7 and C8 roots. Contrast enhancement was observed in two patients. Six patients showed plexus hypertrophy. The distribution of MRI abnormalities was asymmetrical in all patients and corresponded with the distribution of their reported symptoms. Nerve US yielded positive results in 12 out of 16 (75%) tested patients of the entire cohort. In all cases, the examination revealed the presence of at least one nerve affected by a focal increase in cross‐sectional area outside the sites of nerve entrapment. In three patients, the examination also indicated an increased size of the nerve roots. These changes were consistently unilateral or asymmetric across all patients and corresponded with the distribution of their reported symptoms. There were no significant differences in the frequency of abnormal brachial plexus MRI or nerve US between patients with MMN and those with an unconfirmed MMN diagnosis (Table 1).
The vast majority of MMN patients (60/68, 88%) improved with IVIg therapy. Conversely, none of the nine patients with unconfirmed MMN diagnosis, treated with IVIg, showed a clear response to therapy (p = 0.0001). Fifty‐three patients (78%) received ongoing IVIg maintenance treatment at the time of this study. The median duration of maintenance treatment was 8 years (range 0–35). Fifteen patients (22%) did not receive maintenance treatment due to either no beneficial effect (eight patients) or a stable disease course without treatment (seven patients). Most patients (16/25, 64%) with MMN were reported to stabilize after treatment with subcutaneous immunoglobulin, which was administered to all patients following improvement from IVIg therapy, whilst seven patients (28%) reported further improvement and two patients deteriorated. Three of the eight (37.5%) treated patients improved after therapy with intravenous or oral cyclophosphamide. This treatment was administered to all with the goal of reducing the IVIg dose. After oral steroid therapy, one out of five patients demonstrated improvement. Amongst the 15 patients who received alternative immune therapies, including rituximab (n = 7), cyclosporin (n = 3), plasma exchange (n = 2), azathioprine (n = 1), interferon (n = 1) and methotrexate (n = 1), none showed improvement (Table 1).
Within the group of patients with definite MMN (n = 22), additional diagnostic examinations were required by the treating physician in 13 (59%) patients, including anti‐GM1 IgM antibodies in 12 (of which five [42%] resulted positive), CSF examination in five (of which none had increased proteins), nerve US in three (all of which resulted diagnostic) and brachial plexus MRI in one (which resulted diagnostic) (Table 2). Within the group of patients with probable MMN (n = 29), 20 patients had two or more nerves with probable CB, whilst nine patients had one nerve with probable CB and at least two supportive criteria including response to IVIg in eight, increased anti‐GM1 IgM antibodies in four, increased CSF proteins in four, and abnormal nerve MRI in three. Additional diagnostic investigations were also required in 15 (75%) of the 20 patients with probable MMN who had two or more nerves with probable CB. Within the group of patients with possible MMN (n = 22), all met the clinical criteria in at least two nerves and exhibited a positive response to IVIg treatment. In 13 (59%) patients with possible MMN, additional ancillary tests were performed to confirm diagnosis, including anti‐GM1 IgM antibodies in 12 (of which eight [67%] resulted positive), CSF examination in seven (of which five [71%] had increased proteins), nerve US in four (of which three [75%] resulted diagnostic) and brachial plexus MRI in two (of which one [50%] resulted diagnostic) (Table 2). Overall, amongst the 73 patients with confirmed MMN, anti‐GM1 IgM antibodies were increased in 24 of the 39 (61%) patients tested, CSF proteins were increased in 12 of the 26 (46%) examined patients and MRI was found to be abnormal in four of the eight (50%) examined patients. Nerve US was performed in 12 patients and resulted diagnostic in 10 (83%).
TABLE 2.
Comparison of the patients with definite, probable or possible MMN.
| Definite MMN | Probable MMN | Possible MMN | p value | |
|---|---|---|---|---|
| Number | 22 | 29 | 22 | |
| Gender, male/female (ratio) | 15/7 (2.1:1) | 15/14 (1.1:1) | 19/3 (6.3/1) | 0.0155 a |
| Age at inclusion, years, mean (range) | 53 (36–85) | 54 (35–85) | 55 (31–70) | ns |
| Age at onset, years, mean (range) | 40 (22–68) | 43 (25–63) | 41 (21–65) | ns |
| Disease duration, years, mean (range) | 13 (0.5–35) | 10 (1–30) | 13 (0.4–32) | ns |
| ONLS at inclusion, mean (range) | 3.0 (1–6) | 3.6 (1–9) | 3.1 (0–7) | ns |
| MRC at inclusion, mean (range) | 54.2 (46–60) | 53.2 (30–60) | 53.9 (33–60) | ns |
| Limb impairment at onset, upper/lower/upper and lower | 16/6/0 | 15/7/7 | 15/4/3 | ns |
| Limb impairment at inclusion, upper/lower/upper and lower | 5/0/16 b | 9/1/19 | 10/1/11 | ns |
| Positive anti‐GM1 IgM/tested | 5/12 (42%) | 11/15 (73%) | 8/12 (67%) | ns |
| Increased CSF proteins/tested | 0/5 (0%) | 7/14 (50%) | 5/7 (71%) | ns |
| Increased CSF proteins ≥1 g/L/tested | 0/5 (0%) | 1/14 (0.7%) | 3/7 (43%) | ns |
| Abnormal US and/or MRI/tested | 4/4 (100%) | 4/8 (50%) | 4/5 (80%) | ns |
| Improved after IVIg/treated | 14/17 (82%) | 24/29 (83%) | 22/22 (100%) | ns |
| Improved after ScIg/treated | 1/6 (17%) | 2/11 (18%) | 4/8 (50%) | ns |
| Improved after cyclophosphamide/treated | 0/2 (0%) | 3/4 (75%) | 0/2 (0%) | ns |
| Improved after other therapies/treated | 0/4 (0%) | 0/5 (0%) | 0/6 (0%) | ns |
Abbreviations: CSF, cerebrospinal fluid; IgM, immunoglobulin M; IVIg, intravenous immunoglobulin; MMN, multifocal motor neuropathy; MRC, Medical Research Council; MRI, magnetic resonance imaging; ns, not significant; ONLS, Overall Neuropathy Limitations Scale; ScIg, subcutaneous immunoglobulin; US, ultrasound.
Probable vs. possible MMN.
Not reported in one.
In Table 2, the clinical and laboratory findings and response to therapy of the patients with definite, probable or possible MMN are compared. There was no significant difference between the groups of patients besides a higher proportion of men amongst patients with possible compared to probable MMN (p = 0.0155), whilst none of the other differences was significant.
DISCUSSION
Our study showed that the current diagnostic criteria proposed by the EFNS/PNS were not strictly used in our series of patients with MMN. In a non‐negligible proportion of patients (17%), the treating physicians recorded only the CMAP amplitude data, neglecting the CMAP area data as required by the EFNS/PNS criteria. This might be explained by the fact that the criteria of the AAEM, unlike those of the EFNS/PNS, also consider CMAP amplitude reduction to define CB [6, 7, 8]. When applying the criteria for CB established by the AAEM to our cohort, an additional 17% of patients fulfilled the criteria for a probable/definite diagnosis. However, a further 9.5% of patients did not meet the diagnostic criteria. This comparison must consider the fact that, in some patients, the area data were not recorded by the treating physician, preventing a direct sensitivity comparison between the two sets of criteria. Nevertheless, the results illustrate the real‐life application of both sets of criteria.
In our cohort, patients not meeting the EFNS/PNS diagnostic criteria for MMN had a significantly shorter disease duration compared to patients with a confirmed MMN diagnosis. Moreover, two newly diagnosed patients were included in the database before undergoing IVIg treatment. It is conceivable that, over time, the diagnosis may be substantiated in some of these patients through the discovery of new CB in nerve segments that previously did not show any evidence of CB or by demonstrating a response to IVIg.
In the EFNS/PNS criteria, the choice to define CB as a decrease of CMAP area was based on studies in animal models using computer modelling of CB and temporal dispersion showing that up to 50% area reduction of the proximal to distal CMAP can be due entirely to interphase cancellation [17]. Similar studies in man have shown that distal CMAP duration and proximal CMAP duration prolongation are important factors for the definition of CB [18]. The EFNS/PNS criteria acknowledge a limited amount of evidence in relation to these aspects [6]. Furthermore, as of now, no studies have conducted a comparative analysis of the diagnostic accuracy between the EFNS/PNS and AAEM criteria for MMN.
The additional diagnostic investigations recommended by the EFNS/PNS guidelines played a valuable role in enhancing the accuracy of the diagnosis in our cohort, with sensitivities ranging from 46% to 61%. Notably, the anti‐GM1 IgM antibodies proved to be particularly effective, displaying a sensitivity of 61%. The frequent detection of these antibodies in patients not meeting the EFNS/PNS criteria in our cohort could be attributed to a potential selection bias, considering the inclusion of patients with criteria supporting the diagnosis in the absence of a diagnostic nerve conduction study, and taking into account the relatively low specificity of these antibodies [19, 20].
Whilst the utilization of MRI in our cohort was limited, probably due to availability constraints, it still contributed to refining diagnoses in a subset of patients. Our results do not confirm the observations made by Beecher and coauthors who noted the absence of hypertrophy in the brachial plexus amongst MMN patients, with its occurrence solely in patients with Lewis−Sumner syndrome [21]. Notably, the average disease duration in the seven patients with a positive MRI (mean 5 years, range 1–12) still leaves the possibility of progression to Lewis−Sumner syndrome, based on previous observations by other authors [20].
Although not recommended by the EFNS/PNS guidelines, nerve US demonstrated remarkably high sensitivity (83%). This underlines the potential of nerve US as a supplementary diagnostic tool for the diagnosis of MMN [15, 16].
The EFNS/PNS criteria for MMN recommend ancillary tests to support the diagnosis solely in patients with probable CB [6]. Our study shows that a significant proportion of patients (41 out of 64, 64%) underwent additional testing beyond the EFNS/PNS guidelines [6]. Given the absence of studies assessing the diagnostic accuracy of the EFNS/PNS electrophysiological criteria for MMN, the appropriateness of these supplementary tests for patients already meeting the electrophysiological criteria remains uncertain.
It is perhaps worth making a distinction between non‐invasive examinations, such as anti‐GM1 antibody testing or MRI, and more invasive procedures like CSF analysis. Moreover, a distinction should be drawn between performing additional investigations in patients with a definite MMN diagnosis based on the electrophysiological criteria and those meeting only the criteria for a possible diagnosis. For the latter group, the EFNS/PNS guidelines recommend IVIg treatment responsivity as the sole diagnostic supportive criterion [6]. Given the considerable cost of IVIg therapy, advocating for additional diagnostic tests before treatment initiation seems reasonable. Furthermore, the administration of IVIg treatment, in the absence of a confirmed diagnosis, could potentially lead to reimbursement issues.
Our study confirms the limited treatment options for MMN patients [5, 6]. Amongst the seven patients subjected to rituximab treatment, none exhibited a favourable response. Responses to cyclosporin, plasma exchange, azathioprine, interferon and methotrexate were not observed, although administered to a limited subset of patients. Notably, in our cohort, 37% of patients (three out of eight) receiving cyclophosphamide treatment showed improvement. Cyclophosphamide was not recommended by one group of experts because concern exists about its short‐ and long‐term toxicity and lack of evidence of efficacy in MMN [22]. This study also confirms that IVIg therapy is effective in most patients with MMN [6, 23] and that subcutaneous immunoglobulin is mainly effective in maintaining the improvement achieved with IVIg [6, 24, 25], although some patients may develop increasing weakness [25]. It remains a possibility that the response rate to IVIg could have been higher if assessed in all patients after cumulative doses of IVIg or with higher IVIg doses.
The main limitation of this study is its retrospective nature with information collected from medical charts and by clinical history using a structured questionnaire. Also the presence of selection bias cannot be excluded as, compared with the general population, patients seen in our centres might be more complex cases. It is also possible that this study is only representative of the Italian population and might not be extended to other populations. The study provides, however, real‐life data on the current diagnostic strategy and management of patients with MMN in Italian centres with expertise in immune‐mediated neuropathies. It also allows for the assessment of the usefulness and challenges of the currently employed diagnostic criteria within a real‐world clinical practice setting.
AUTHOR CONTRIBUTIONS
Eduardo Nobile‐Orazio: Conceptualization; investigation; funding acquisition; validation; methodology; visualization; writing – review and editing; formal analysis; data curation; supervision; resources. Pietro Emiliano Doneddu: Conceptualization; investigation; writing – original draft; methodology; writing – review and editing; formal analysis; data curation. Luca Gentile: Investigation; data curation; writing – review and editing; visualization; validation. Dario Cocito: Investigation; validation; visualization; writing – review and editing; data curation. Raffaella Fazio: Investigation; validation; visualization; writing – review and editing; data curation. Marco Luigetti: Investigation; validation; visualization; writing – review and editing; data curation. Chiara Briani: Investigation; validation; visualization; writing – review and editing; data curation. Massimiliano Filosto: Investigation; validation; visualization; writing – review and editing; data curation. Gabriele Siciliano: Investigation; validation; visualization; writing – review and editing; data curation. Luana Benedetti: Investigation; validation; visualization; writing – review and editing; data curation. Giovanni Antonini: Investigation; validation; visualization; writing – review and editing; data curation. Sabrina Matà: Investigation; validation; visualization; writing – review and editing; data curation. Girolama Alessandra Marfia: Investigation; validation; visualization; writing – review and editing; data curation. Maurizio Inghilleri: Investigation; validation; visualization; writing – review and editing; data curation. Fiore Manganelli: Investigation; validation; visualization; writing – review and editing; data curation. giuseppe cosentino: Investigation; validation; visualization; writing – review and editing; data curation. Filippo Brighina: Investigation; validation; visualization; writing – review and editing; data curation. Marinella Carpo: Investigation; validation; visualization; writing – review and editing; data curation. Francesca Carta: Investigation; validation; visualization; writing – review and editing; data curation. Anna Mazzeo: Investigation; validation; visualization; writing – review and editing; data curation. Erdita Peci: Investigation; validation; visualization; writing – review and editing; data curation. Camilla Strano: Investigation; validation; visualization; writing – review and editing; data curation. Angela Romano: Investigation; validation; visualization; writing – review and editing; data curation. Marta Campagnolo: Investigation; validation; visualization; writing – review and editing; data curation. Stefano Cotti‐Piccinelli: Investigation; validation; visualization; writing – review and editing; data curation. Divina Valeria Viola: Investigation; validation; visualization; writing – review and editing; data curation. Francesco Germano: Investigation; validation; visualization; writing – review and editing; data curation. Luca Leonardi: Investigation; validation; visualization; writing – review and editing; data curation. Martina Sperti: Investigation; validation; visualization; writing – review and editing; data curation. Giorgia Mataluni: Investigation; validation; visualization; writing – review and editing; data curation. Marco Ceccanti: Investigation; validation; visualization; writing – review and editing; data curation. Emanuele Spina: Investigation; validation; visualization; writing – review and editing; data curation. Elisa Vegezzi: Investigation; validation; visualization; writing – review and editing; data curation. Vincenzo Di Stefano: Investigation; validation; visualization; writing – review and editing; data curation.
FUNDING INFORMATION
The study was supported by Baxalta, now part of Takeda, with investigator initiated grant no. IIR‐ITA‐BXLT‐001955/IISR‐2017‐104226, Italy. The study was also supported by IRCCS, Humanitas Research Hospital (Rozzano, Milan, Italy). The funders and supporters had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICT OF INTEREST STATEMENT
Pietro Emiliano Doneddu has received travel grants to attend scientific meetings from CSL Behring and Kedrion. Fiore Manganelli reports personal fees for scientific events from CSL Behring and has received travel grants to attend scientific meetings from CSL Behring and Kedrion. Dario Cocito has received honoraria for lecturing from Shire, CSL Behring and Kedrion and travel grants to attend scientific meetings from Shire, Kedrion and CSL Behring. Raffaella Fazio has served on scientific advisory boards for CSL Behring and has received travel grants from Kedrion and CSL Behring to attend scientific meetings. Marco Luigetti received financial grants from Ackea, Alnylam, Sobi and Pfizer, and travel grants from Ackea, Alnylam, Sobi, Pfizer, Kedrion and Grifols. Chiara Briani has served on scientific advisory boards for Pfizer, Alnylam and Akcea, and has received travel grants from Kedrion and CSL Behring to attend scientific meetings. Giovanni Antonini served on advisory boards and received travel grants from Alnylam, Alexion, UCB, Kedrion, and honoraria for lecturing from Kedrion. Anna Mazzeo has received travel grants from Kedrion and CSL Behring to attend scientific meetings. Massimiliano Filosto has served on scientific advisory boards for CSL Behring, Sanofi and Amicus and has received travel grants from Sanofi, Biogen, Kedrion and CSL Behring to attend scientific meetings. Giuseppe Cosentino has received travel grants to attend scientific meetings from CSL Behring and Kedrion. Girolama Alessandra Marfia has received travel grants to attend scientific meetings from CSL Behring and Kedrion. Maurizio Inghilleri has received travel grants to attend scientific meetings from CSL Behring and Alexion. Erdita Peci has received travel grants to attend scientific meetings from CSL Behring. Eduardo Nobile‐Orazio reports personal fees for advisory or scientific board from ArgenX (Belgium), Takeda (Italy and USA), CSL Behring (Italy and USA), Janssen (USA), Kedrion (Italy), LFB (France), Roche (Switzerland), Sanofi (USA). The other authors declare no conflict of interest.
ETHICS STATEMENT
The study was approved by the Ethical Committee of IRCCS Humanitas Clinical and Research Centre (no. 413/17, 19/09/2017) and of each participating centre.
PATIENT CONSENT FOR PUBLICATION
Obtained.
Doneddu PE, Gentile L, Cocito D, et al. Assessment of diagnostic criteria for multifocal motor neuropathy in patients included in the Italian database. Eur J Neurol. 2024;31:e16248. doi: 10.1111/ene.16248
DATA AVAILABILITY STATEMENT
Anonymised data used for this study are available upon reasonable request from the corresponding author.
REFERENCES
- 1. Nobile‐Orazio E, Cappellari A, Priori A. Multifocal motor neuropathy: current concepts and controversies. Muscle Nerve. 2005;31:663‐680. [DOI] [PubMed] [Google Scholar]
- 2. Vlam L, van der Pol W‐L, Cats EA, et al. Multifocal motor neuropathy: diagnosis, pathogenesis and treatment strategies. Nat Rev Neurol. 2011;8:48‐58. [DOI] [PubMed] [Google Scholar]
- 3. Yeh WZ, Dyck PJ, van den Berg LH, Kiernan MC, Taylor BV. Multifocal motor neuropathy: controversies and priorities. J Neurol Neurosurg Psychiatry. 2020;91:140‐148. [DOI] [PubMed] [Google Scholar]
- 4. van Schaik IN, van den Berg LH, de Haan R, Vermeulen M. Intravenous immunoglobulin for multifocal motor neuropathy. Cochrane Database Syst Rev. 2005;2:CD004429. [DOI] [PubMed] [Google Scholar]
- 5. Umapathi T, Hughes R, Nobile‐Orazio E, Leger J. Immunosuppressant and immunomodulatory treatments for multifocal motor neuropathy. Cochrane Database Syst Rev. 2015;3:CD003217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Joint Task Force of the EFNS and the PNS . European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of multifocal motor neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. First revision. J Peripher Nerv Syst. 2010;15:295‐301. [DOI] [PubMed] [Google Scholar]
- 7. Olney R, Lewis RA, Putnam TD, Campellone JV Jr. American Association of Electrodiagnostic Medicine Consensus criteria for the diagnosis of multifocal motor neuropathy. Muscle Nerve. 2003;27:117‐121. [DOI] [PubMed] [Google Scholar]
- 8. Olney R. Consensus criteria for the diagnosis of partial conduction block. Muscle Nerve. 1999;22:S225‐S229. [PubMed] [Google Scholar]
- 9. Van den Bergh PYK, Van Doorn PA, Hadden RD, et al. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force—second revision. J Peripher Nerv Syst. 2021;26:242‐268. [DOI] [PubMed] [Google Scholar]
- 10. Doneddu PE, Cocito D, Manganelli F, et al. Atypical CIDP: diagnostic criteria, progression and treatment response. Data from the Italian CIDP database. J Neurol Neurosurg Psychiatry. 2019;90:125‐132. [DOI] [PubMed] [Google Scholar]
- 11. Vanhoutte EK, Faber CG, van Nes SI, et al. Rasch‐built overall disability scale for multifocal motor neuropathy (MMN‐RODS(©)). J Peripher Nerv Syst. 2015;20:296‐305. [DOI] [PubMed] [Google Scholar]
- 12. Graham RC, Hughes RA. A modified peripheral neuropathy scale: the overall neuropathy limitations scale. J Neurol Neurosurg Psychiatry. 2006;77:973‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Breiner A, Moher D, Brooks J, et al. Adult CSF total protein upper reference limits should be age partitioned and significantly higher than 0.45 g/L: a systematic review. J Neurol. 2019;266:616‐624. [DOI] [PubMed] [Google Scholar]
- 14. Kronlage M, Knop KC, Schwarz D, et al. Amyotrophic lateral sclerosis versus multifocal motor neuropathy: utility of MR neurography. Radiology. 2019;292:149‐156. [DOI] [PubMed] [Google Scholar]
- 15. Goedee HS, Jongbloed BA, van Asseldonk J‐TH, et al. A comparative study of brachial plexus sonography and magnetic resonance imaging in chronic inflammatory demyelinating neuropathy and multifocal motor neuropathy. Eur J Neurol. 2017;24:1307‐1313. [DOI] [PubMed] [Google Scholar]
- 16. Oudeman J, Eftimov F, Strijkers GJ, et al. Diagnostic accuracy of MRI and ultrasound in chronic immune‐mediated neuropathies. Neurology. 2020;94:e62‐e74. [DOI] [PubMed] [Google Scholar]
- 17. Rhee EK, England JD, Sumner AJ. A computer simulation of conduction block: effects produced by actual block versus interphase cancellation. Ann Neurol. 1990;28:146‐156. [DOI] [PubMed] [Google Scholar]
- 18. Van Asseldonk JT, Van den Berg LH, Wieneke GH, Wokke JH, Franssen H. Criteria for conduction block based on computer simulation studies of nerve conduction with human data obtained in the forearm segment of the median nerve. Brain. 2006;129:2447‐2460. [DOI] [PubMed] [Google Scholar]
- 19. Shelly S, Mills JR, Martinez‐Thompson JM, et al. IgM‐gammopathy strongly favours immune treatable MMN and MADSAM over ALS. J Neurol Neurosurg Psychiatry. 2020;91:324‐326. [DOI] [PubMed] [Google Scholar]
- 20. Beecher G, Shelly S, Dyck PJB, et al. Pure motor onset and IgM‐gammopathy occurrence in multifocal acquired demyelinating sensory and motor neuropathy. Neurology. 2021;97:e1392‐e1403. [DOI] [PubMed] [Google Scholar]
- 21. Beecher G, Howe BM, Shelly S, et al. Plexus MRI helps distinguish the immune‐mediated neuropathies MADSAM and MMN. J Neuroimmunol. 2022;371:577953. [DOI] [PubMed] [Google Scholar]
- 22. Hughes PR, 79(th) ENMC International Workshop: multifocal motor neuropathy . Hilversum, The Netherlands. Neuromuscul Disord. 2000;2001(11):309‐314. [DOI] [PubMed] [Google Scholar]
- 23. Keddie S, Eftimov F, van den Berg LH, Brassington R, de Haan RJ, van Schaik IN. Immunoglobulin for multifocal motor neuropathy. Cochrane Database Syst Rev. 2022;1:CD004429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gentile L, Russo M, Rodolico C, et al. Long‐term treatment with subcutaneous immunoglobulin in multifocal motor neuropathy. Sci Rep. 2021;11:9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Katzberg HD, Rasutis V, Bril V. Subcutaneous immunoglobulin for treatment of multifocal motor neuropathy. Muscle Nerve. 2016;54:856‐863. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymised data used for this study are available upon reasonable request from the corresponding author.


