Abstract
Background and purpose
The aim was to evaluate the effect of anti‐calcitonin gene related peptide (CGRP) (ligand or receptor) antibodies on depressive symptoms in subjects with migraine and to determine whether depressive symptoms predict treatment response.
Methods
Patients with migraine treated with erenumab and fremanezumab at the Leiden Headache Centre completed daily E‐headache diaries. A control group was included. Depressive symptoms were assessed using the Hospital Anxiety and Depression Scale (HADS) and the Center for Epidemiological Studies Depression Scale (CES‐D) questionnaires at baseline (T0) and after 3 months (T1). First, the effect of treatment on the reduction in HADS‐D and CES‐D scores was assessed, with reduction in depression scores as the dependent variable and reduction in monthly migraine days (MMD) and treatment with anti‐CGRP medication as independent variables. Second, depression as a predictor of treatment response was investigated, using the absolute reduction in MMD as a dependent variable and age, gender, MMD, active depression, impact, stress and locus of control scores as independent variables.
Results
In total, n = 108 patients were treated with erenumab, n = 90 with fremanezumab and n = 68 were without active treatment. Treatment with anti‐CGRP medication was positively associated with a reduction in the HADS‐D (β = 1.65, p = 0.01) compared to control, independent of MMD reduction. However, the same effect was not found for the CES‐D (β = 2.15, p = 0.21). Active depression predicted poorer response to erenumab (p = 0.02) but not to fremanezumab (p = 0.09).
Conclusion
Anti‐CGRP (ligand or receptor) monoclonals lead to improvement of depressive symptoms in individuals with migraine, independent of migraine reduction. Depression may predict treatment response to erenumab but not to fremanezumab.
Keywords: depression, migraine, predictor, treatment response
INTRODUCTION
New preventive treatment options for migraine targeting the calcitonin gene related peptide (CGRP) pathway are available: three monoclonal antibodies targeting the CGRP ligand (eptinezumab, fremanezumab and galcanezumab) and one monoclonal antibody targeting the CGRP receptor (erenumab). As a rule of thumb for clinical practice, preventive migraine treatment in general may lead to approximately 50% reduction in monthly migraine days (MMD) in half of individuals. A large portion does not reach 50% reduction in migraine days and this often starts a long search for an effective preventive treatment. This process is often based on trial and error, as it is currently not possible to predict which patients will respond to which specific drugs.
Persons with migraine are at increased risk of depression, and shared genetic factors may underlie this association [1, 2, 3]. Comorbid depression in individuals with migraine is an important predictor for acute medication overuse and is associated with an increased risk of chronification [4, 5, 6]. In this triad, there is a role for cutaneous allodynia and the underlying mechanism central sensitization [7, 8, 9]. Depression has been associated with poorer response to acute treatment and preventive treatment with onabotulinumtoxin‐A [10, 11]. For the new anti‐CGRP (ligand or receptor) antibodies it is unknown whether depression, independently of number of migraine days, influences the treatment response. Furthermore, whether these antibodies improve symptoms of depression (in)dependently of the treatment response is also yet to be discovered.
In this prospective study the aim was (i) to assess whether treatment with erenumab or fremanezumab improves comorbid depressive symptoms, (in)dependent of reduction in MMD; (ii) to evaluate whether depressive symptoms, and other psychological factors, are predictive of response to preventive treatment with anti‐CGRP (ligand or receptor) antibodies. Increasing the understanding of treatment response and identifying determinants for response may provide an advancement in migraine treatment.
METHODS
Literature search
An extensive literature search (PubMed, Embase) up to August 2022 was performed to find all evidence regarding depression and monoclonal anti‐CGRP (ligand or receptor) antibodies. Two researchers independently evaluated the articles based on abstract and if available the whole article (SdVL and BvdA). In the case of a disagreement a discussion took place. The selection of the relevant articles and abstracts is presented in Table S1.
Participants
Participants were included who started treatment with erenumab or fremanezumab at the Leiden Headache Centre of the Leiden University Medical Centre (LUMC), and a control group was assessed in the same manner. Patients who started treatment with erenumab or fremanezumab were enrolled in a consecutive manner. They were diagnosed with migraine with or without aura by a neurologist with headache expertise according to the International Classification of Headache Disorders 3rd edition (ICHD‐3) criteria [12]. Migraine frequency had to be at least 6 migraine days per month before treatment. None of the subjects had a second primary headache disorder other than tension type headache, which is common in patients with chronic migraine [12]. None of these patients had medication overuse headache. Patients all previously failed on at least four migraine preventives (meaning being ineffective, discontinued because of side effects or being contraindicated), including at least a betablocker, candesartan, valproate and topiramate. If patients switched between different anti‐CGRP treatments, only the data of the first treatment were included.
As a control group people with migraine of the Leiden Headache Centre with similar distribution in gender, age and migraine diagnosis and frequency were included. To address potential selection bias, several measures were employed, including restriction (excluding patients who received active medication) and modelling (to adjust for specific variables' influence on study outcomes). In addition, 3:1 matching was used on baseline active depression in which a single untreated participant was randomly matched to three treated participants. Notably, the control group received no active medication, serving as a suitable surrogate for a placebo group. No participants received treatment aimed at reducing depressive symptoms during the duration of the study. If treatment was required patients were to be excluded.
Treatment
Participants were treated with erenumab (70 mg) or fremanezumab (225 mg), administered subcutaneously once every 4 weeks. No additional preventive treatment was used.
Headache diary
For all participants, including the control group, the clinical response was monitored using a daily headache E‐diary, validated in the Leiden Headache Centre [13]. This E‐diary contains questions on the presence of headache, headache characteristics, accompanying symptoms and the use of acutely acting migraine medication. When a headache is present, an automated algorithm based on the ICHD‐3 criteria determines whether it is a migraine day. Additionally, days in which a triptan is taken or days with the occurrence of a visual aura lasting 5–60 min (with or without headache symptoms) are also counted as migraine days. Patients started this E‐diary at least 4 weeks before starting treatment (baseline period). Diary adherence had to be ≥80%. Clinical response was based on the reduction in migraine days in the third month after initiating treatment. A month is defined as 28 days (4 weeks).
Questionnaires
At T0 and after 3 months (T1), all participants were invited to complete several questionnaires, which are described below.
Depression questionnaires
Patients filled out the Hospital Anxiety and Depression Scale (HADS) and the Centre for Epidemiological Studies Depression Scale (CES‐D). The HADS is a 14‐item questionnaire, of which seven items focus on symptoms of anxiety (HADS‐A) and seven items focus on symptoms of depression (HADS‐D) [14]. All items are answered on a 4‐point Likert scale, ranging from 0 to 3 (both total scores ranging from 0 to 21). On each of these subscales a score of ≥8 is indicative of respectively a possible anxiety or a possible depressive disorder. The CES‐D is a 20‐item questionnaire, score ranging from 0 to 60 [15]. All items are answered on a 4‐point Likert scale, from 0 (rarely or none of the time) to 3 (most or all of the time). A score of ≥16 is indicative of possible depressive disorder. The HADS‐D scale is a self‐report scale designed to measure depressive symptomatology in a medical population, whilst the CES‐D scale is likewise self‐reported but designed to measure depressive symptomatology in the general population. As such, both questionnaires were considered to be complementary to one another. Both questionnaires focus on symptoms experienced in the previous week and were completed at baseline (T0) and after 3 months of treatment (T1). Importantly, these questionnaires are not intended to provide a definitive diagnosis of major depression. Rather, they serve as indicators of the presence of depressive symptoms. For the purposes of this paper, ‘active depression’ was defined as a HADS‐D score ≥8 and/or a CES‐D score ≥16, comparable to previous studies [1, 16]. A choice was made to analyse the validated cut‐off values of the HADS‐D and CES‐D instead of the continuous scores as our interest was in clinically meaningful occurrences of depressive symptoms.
Headache Impact Test 6
The Headache Impact Test 6 (HIT‐6) is a six‐item questionnaire that assesses the impact headache has on a patient's daily life [17]. Every item is answered by a 5‐point Likert scale ranging from never (score 6) to always (score 13), comprising a total score between 36 and 78, with larger scores reflecting a higher impact. This questionnaire was completed at baseline (T0) and after 3 months of treatment (T1).
Perceived stress scale
The perceived stress scale (PSS) is a measure of the degree to which situations are appraised as stressful [18]. This questionnaire consists of 10 questions, with every item scored on a 5‐point Likert scale ranging from 0 (never) to 4 (very often). It focuses on feelings and thoughts experienced in the last month. A higher score correlates with more perceived stress. This questionnaire was completed at baseline (T0).
Headache specific locus of control
The headache specific locus of control (HSLC) assesses the individual's perceptions that headache problems and relief are determined by internal factors, healthcare professionals or chance factors [19]. It consists of 33 statements, answered on a 5‐point Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree). Every subscale (internal, healthcare professionals, chance) of the HSLC consists of 11 questions (score range 11–55). A higher score on each different subscale means higher beliefs in that subscale of the locus of control. This questionnaire was completed at baseline (T0).
Statistical analyses
Baseline characteristics
Baseline characteristics were summarized using means and standard deviations or frequencies and proportions. Failure to the preventives propranolol and metoprolol was counted as one failure (treatment class betablockers). Baseline scores of the different questionnaires (HIT‐6, PSS, HADS, CES‐D, HSLC) were summarized as means and standard deviations. For each patient, the clinical response was determined by calculating both the absolute and relative reduction in migraine days in the third month (weeks 9–12) compared to the baseline month (4 weeks before starting treatment).
Pre‐post treatment comparisons of active depression
The number of patients with (i.e., HADS‐D ≥ 8 and/or CES‐D ≥ 16) and without active depression was calculated and a McNemar test was used to determine whether there was a difference in the proportion of patients with active depression at baseline and follow‐up (T0 vs. T1).
Relation between migraine reduction and reduction in depressive symptoms
To investigate whether the benefit of treatment with anti‐CGRP medication on depressive symptoms is due to anti‐CGRP treatment or a reduction in mean MMD, two multiple linear regression models were used, one with HADS‐D reduction as the dependent variable (primary outcome questionnaire) and one with CES‐D reduction (for comparison, but less well designed for medical conditions and therefore our secondary choice), both with treatment, monthly acute medication days (MAMD) at baseline and MMD reduction as independent variables. For treatment, patients were divided into anti‐CGRP treatment (erenumab or fremanezumab) or control.
Response predictors
For erenumab and fremanezumab separately, two‐way contingency tables were made for ‘active depression’ at baseline and the outcome of <50% or ≥50% reduction in MMD in response to treatment. The chi‐squared test was used to determine whether there was an association between ‘active depression’ at baseline (T0) and the response to treatment. Furthermore, as an additional exploratory analysis this two‐way contingency table was used to calculate the sensitivity, specificity, positive predictive value and negative predictive value of ‘active depression’ at baseline (T0) for the prediction of a clinical response <50%.
Linear regression models were used to test associations, with age, gender, migraine days at baseline and the baseline responses of the above described questionnaires (‘active depression’, HIT‐6, PSS, HSLC) as predictors and the absolute migraine reduction as a dependent variable. Analyses were run as multiple regression models, adjusting for the potential confounding effects of all variables that were tested.
For all analyses, two‐tailed p values ≤0.05 were considered as statistically significant. All analyses have been performed using IBM SPSS Statistics for Windows, version 25 (IBM Corp.).
Missing data
No imputation methods were used for missing questionnaires. Missing diary days were considered headache‐free, as the average diary compliance was high (100%, interquartile range 96–100).
Standard protocol approvals, registration and patient consents
This study was approved by the Medical Ethics Committee of the Leiden University Medical Centre and patients provided written informed consent.
RESULTS
Literature search
In total eight individual articles and abstracts were identified. The results of the literature search are presented in Table S1.
Baseline characteristics
The study population consisted of 110 patients who started treatment with erenumab, 117 patients who started treatment with fremanezumab and 68 patients in the control group. All of these patients were invited to complete the questionnaires. Two patients discontinued erenumab after 2 months because of adverse events (severe daily nausea and general malaise). Of the remaining 108 erenumab patients, 101 responded to the questionnaires after 3 months (T1). In the study population of fremanezumab, no patients discontinued treatment before the 3 month period ended. In all, 27 patients previously used erenumab and thus were excluded from the analyses. In total, 78 patients responded to the questionnaires after 3 months (T1). In all groups, on average patients had failed on four migraine preventives. Baseline characteristics for the different subgroups are presented in Table 1. With the exception of patients fulfilling criteria for chronic migraine (at least 8 MMD with at least 15 monthly headache days) and MAMD at baseline, there were no differences in demographics between the groups. No patients required treatment for depressive disorder. A flowchart is presented in Figure S1.
TABLE 1.
Patient baseline characteristics.
| Erenumab (N = 108) | Fremanezumab (N = 90) | Control (N = 68) | |
|---|---|---|---|
| Female, n (%) | 92 (85) | 73 (81) | 53 (78) |
| Age (years), mean ± SD | 42.4 ± 12.5 | 44.5 ± 13.5 | 45.5 ± 9.9 |
| MMD baseline, mean ± SD | 14.0 ± 5.6 | 14.2 ± 6.3 | 14 ± 5.4 |
| MHD baseline, mean ± SD | 17.0 ± 6.2 | 17.2 ± 7.0 | 19.4 ± 5.7 |
| MAMD baseline, mean ± SD | 6.0 ± 3.6 | 5.4 ± 2.8 | 13.8 ± 5.9 |
| HADS‐D baseline, mean ± SD | 7.7 ± 4.5 | 7.9 ± 4.6 | 7.9 ± 4.3 |
| CES‐D baseline, mean ± SD | 19.9 ± 11.1 | 19.2 ± 10.5 | 18.6 ± 11.7 |
| Active depression, n (%) | 75 (70) | 55 (61) | 45 (66) |
Abbreviations: CES‐D, Center for Epidemiological Studies Depression Scale; HADS‐D, Hospital Anxiety and Depression Scale, Depression; MAMD, monthly acute medication days; MHD, monthly headache days; MMD, monthly migraine days.
Pre‐post treatment comparisons of depression
First, the number of patients with an active depression at baseline (T0) and at 3 months (T1) was investigated. For erenumab, 70/101 (70%) patients were marked as having an active depression at T0, and 47/101 (47%) patients at T1. In the fremanezumab group, 46/78 (59%) patients fulfilled the criteria for active depression at T0, and 25/78 (32%) patients at T1. In the control group, 45/68 (66%) patients were marked as having active depression at T0, and 43/68 (63%) patients at T1. Exact McNemar tests showed a reduction in the proportion of patients with active depression pre‐ and post‐treatment (both p < 0.001) for erenumab and fremanezumab, but not for control (p = 0.84).
To visualize the change in HADS‐D and CES‐D, separated for erenumab and fremanezumab, our raw data are presented in Figures 1 and 2.
FIGURE 1.
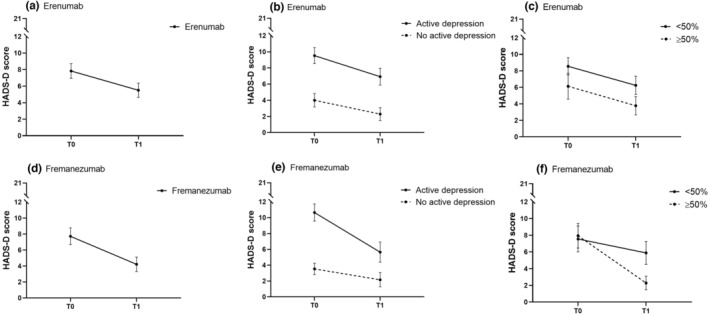
Mean HADS‐D score before (T0) and after (T1) treatment with erenumab (a)–(c) and treatment with fremanezumab (d)–(f): (a) all patients treated with erenumab; (b) patients treated with erenumab with and without active depression at baseline (T0); (c) patients with ≥50% or <50% response to erenumab after 3 months; (d) all patients treated with fremanezumab; (e) patients treated with fremanezumab with and without active depression at baseline (T0); (f) patients with ≥50% or <50% response to fremanezumab after 3 months. HADS‐D score range 0–21. Active depression is HADS‐D ≥8 and/or CES‐D ≥16. Data presented are mean ± 95% confidence interval.
FIGURE 2.
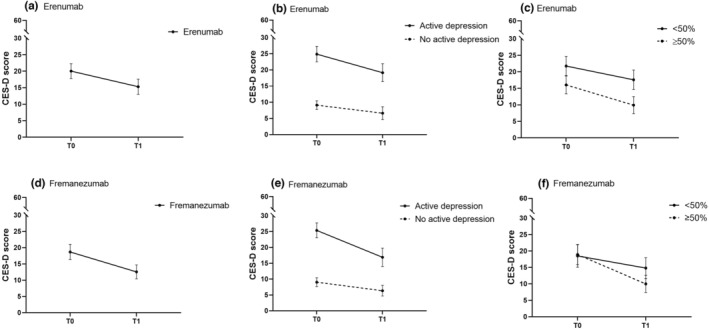
Mean CES‐D score before (T0) and after (T1) treatment with erenumab (a)–(c) and treatment with fremanezumab (d)–(f): (a) all patients treated with erenumab; (b) patients treated with erenumab with and without active depression at baseline (T0); (c) patients with ≥50% or <50% response to erenumab after 3 months; (d) all patients treated with fremanezumab; (e) patients treated with fremanezumab with and without active depression at baseline (T0); (f) patients with ≥50% or <50% response to fremanezumab after 3 months. CES‐D score range 0–60. Active depression is HADS‐D ≥8 and/or CES‐D ≥16. Data presented are mean ± 95% confidence interval.
Relation between migraine reduction and reduction in depressive symptoms
Whether the reduction in HADS‐D was dependent on anti‐CGRP treatment was analysed whilst correcting for reduction in MMD. Reduction in HADS‐D was positively associated with MMD reduction (β = 0.18, p < 0.001), but treatment with anti‐CGRP medication had an additional effect on the reduction in HADS‐D (β = 1.65, p = 0.01) (Figure 3, Table S2) compared to control.
FIGURE 3.
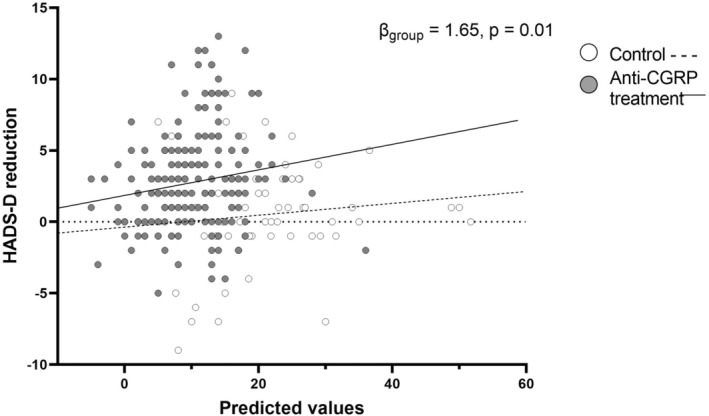
Relation predictive values (including monthly migraine days [MMD] reduction) and the reduction in depressive symptoms. Reduction in HADS‐D is positively associated with MMD reduction, but treatment with anti‐CGRP medication had an additional effect on the reduction in HADS‐D (β = 1.65, p = 0.01) compared to control.
Similar analyses were conducted for CES‐D, in which a positive association was also found between the reduction in CES‐D and MMD reduction (β = 0.43, p < 0.001). However, our findings did not indicate an additional effect of treatment with anti‐CGRP medication on the reduction in CES‐D (β = 2.15, p = 0.21) (Table S2). This seemed to be explained by the added variable MAMD at baseline.
Predictive value of active depressive symptoms for <50% response
For erenumab, the proportion of patients with active depression differed between responder groups (<50% vs. ≥50% response) (chi‐squared test p = 0.02, Table 2). Of the 75 patients who had signs of active depression (i.e., HADS‐D ≥ 8 and/or CES‐D ≥ 16) at baseline (T0), 58 (77%) patients had <50% reduction in MMD after 3 months of treatment with erenumab. Of the patients without active depression 18/33 (55%) had <50% reduction in MMD after 3 months of treatment with erenumab. Active depression had a sensitivity of 74%, a specificity of 46%, a positive predictive value of 77% and a negative predictive value of 45% for a clinical response to erenumab of <50%.
TABLE 2.
Active depression at baseline (T0) and response to erenumab and fremanezumab after 3 months of treatment.
| Erenumab | <50% responders | ≥50% responders | |
|---|---|---|---|
| Active depression | 58 | 17 | 75 |
| No active depression | 18 | 15 | 33 |
| 76 | 32 | 108 |
| Fremanezumab | <50% responders | ≥50% responders | |
|---|---|---|---|
| Active depression | 27 | 28 | 55 |
| No active depression | 22 | 13 | 35 |
| 49 | 41 | 90 |
Note: Active depression is HADS‐D ≥8 and/or CES‐D ≥16.
Erenumab: N = 108 (all patients who filled out questionnaires at baseline and completed the 3 months follow‐up period). Chi‐squared test p = 0.02. Sensitivity 76%, specificity 47%, positive predictive value 77%, negative predictive value 45% for a <50% response.
Fremanezumab: N = 90 (all patients who filled out questionnaires at baseline and completed the 3 months follow‐up period). Chi‐squared test p = 0.28. Sensitivity 55%, specificity 32%, positive predictive value 49%, negative predictive value 37% for a <50% response.
For fremanezumab, the proportion of patients with active depression did not differ between responder groups (<50% or ≥50% response) (chi‐squared test p = 0.09, Table 2). Of the 55 patients who had active depression before starting treatment with fremanezumab (T0), 27 (49%) patients had <50% reduction in migraine days after 3 months of treatment. Of the patients without active depression 22/35 (62%) had <50% reduction in MMD after 3 months of treatment with fremanezumab. Active depression had a sensitivity of 58%, a specificity of 27%, a positive predictive value of 63% and a negative predictive value of 19% for a clinical response to fremanezumab <50%.
Response predictors
Table S3 (left column) presents the results of the multiple linear regression analysis with absolute monthly migraine reduction (baseline vs. month 3) as a response to erenumab as outcome variable. Migraine reduction in response to treatment with erenumab was negatively associated with active depression (β = −2.02, 95% confidence interval [CI] −4.04 to −0.001, p = 0.05), a higher HIT‐6 score (β = −0.29, 95% CI −0.54 to −0.04, p = 0.02) and a lower number of migraine days at baseline (β = 0.21, 95% CI 0.06–0.36, p = 0.01). Migraine reduction in response to treatment with fremanezumab was negatively associated with a lower number of migraine days at baseline (β = 0.31, 95% CI 0.14–0.49, p < 0.001, Table S3 right column).
DISCUSSION
In this study a reduction in depressive symptoms in participants with migraine after 3 months of treatment with anti‐CGRP medication was demonstrated. Importantly, this reduction in depressive symptoms was independent of the reduction in MMD. Discrepancies were observed between the primary and secondary outcome variables. The primary analysis using the HADS‐D questionnaire indicated a significant reduction in depressive symptoms following treatment with anti‐CGRP medication. However, the same effect was not found for our secondary outcome, the CES‐D. These discrepancies in outcomes can be attributed to the different purposes and designs of the questionnaires. The HADS‐D is specifically tailored for assessing depressive symptoms in patients with medical conditions, such as migraine, making it more suitable for our study population [14]. The CES‐D questionnaire is less specific and designed to measure not only depressive symptoms but also other related aspects like appetite loss and sleep problems. Thus, the CES‐D is less suitable for persons with medical conditions and more often used for epidemiological studies in a general population [15]. By adding acute medication days (MAMD) at baseline as covariate in our analyses, a specific aspect of the medical condition migraine is added leading to a non‐significant effect on the CES‐D for the treatment with anti‐CGRP medication. When leaving out MAMD as covariate, depressive symptoms as measured with CES‐D were also significantly reduced (data not shown).
A negative association was found between active depression before starting treatment with erenumab and the clinical response. This association was not found for fremanezumab, which may indicate a different class‐effect between the two anti‐CGRP medications. The ability of erenumab to interact with the AMY1 receptor, which is not affected by fremanezumab, might be of influence in comorbid depression [20]. Additionally, the different modes of binding and internalization between erenumab and fremanezumab may also play a role in their respective treatment outcomes [20, 21].
Decrease in depressive symptoms after the start of preventive treatment has scarcely been described [22, 23, 24]. Although it might be presumed that depressive symptoms may improve when patients have fewer migraine attacks, our study suggests that anti‐CGRP treatment has an additional effect on reducing depressive symptoms. Interestingly, migraine and (major) depressive disorder have shared genetic factors [1, 3, 25] and both have been associated with higher levels of CGRP [1, 25, 26, 27, 28]. CGRP‐blocking medication might influence both migraine and depressive symptoms independently. However, knowledge on the effect of blockage of CGRP for depressive symptomatology is limited. Whilst the anti‐CGRP (ligand or receptor) antibodies most probably act peripherally, mood disorders have been associated with changes in several brain areas [29]. If erenumab and fremanezumab modify depressive symptoms independently from decrease in migraine days, this might suggest that central effects may be modified by a peripheral site of action. CGRP interacts with both the dopaminergic and noradrenergic systems in our brain, exerting several biochemical and behavioural effects [30]. Our study therefore demonstrates promising results for a new potential drug target for depression; however, data are still limited.
There are only limited publications on the response to anti‐CGRP treatment in subjects with a history of depression. In a brief communication on subjects with migraine treated with erenumab, researchers reported that psychological traits, such as depression, were not related to clinical outcome [31]. However, only a small sample size was investigated and treatment response was divided into three groups (non‐responders, responders and super‐responders) instead of a continuous outcome, leading to a great loss of power. Post hoc analyses of phase 3 studies demonstrated that fremanezumab effectively reduced migraine frequency in subjects with comorbid depression as measured with the Patient Health Questionnaire 9 [22, 32]. Even though these are interesting and important findings, they did not directly evaluate the effect of anti‐CGRP medication on depressive symptoms, nor how depression influences responder rate. A post hoc analysis of phase 3 studies of galcanezumab showed efficacy for reducing migraine frequency regardless of medical history of comorbid anxiety and/or depression [33]. The difference with our present study is that all anxiety and depression diagnoses, either ongoing or in the past, were included in those analyses and no separate data were presented as to what extent patients currently were affected by those disorders.
Interestingly, in the literature there is evidence that cognitive behaviour therapy for depression in people with migraine increases the response to preventive treatment [34]. Whether additional treatment of depression will lead to a more successful reduction in migraine in patients treated with CGRP‐blocking medication is yet to be determined. The patient population in the present study had a high number of MMD and was resistant to previous preventive treatment. With this, the comorbidity of depressive symptoms was high, as to be expected. As the treatment options for this patient group are very limited, it is of the utmost importance to increase the understanding of what it is that makes these patients (non‐)responders and how to improve their migraine status and quality of life, including depressive symptoms.
A clear strength of the present study is the daily E‐diary with automated algorithm. This gives an accurate assessment of the response to treatment, even more because the time‐locked aspect of the E‐diary prevents patients from changing their answers or delaying their input, which prevents reporting bias. The presence of depressive symptoms were evaluated with the HADS‐D and CES‐D. Even though these questionnaires are not diagnostic tools for a clinical depression per se, they are indicative of depressive symptoms, and they provide for an easy screening tool for comorbid depression suitable for use in a headache clinic. A limitation of our study may be the sample size. In the fremanezumab group, patients already treated with erenumab were excluded. Including these patients in the analyses (data not shown) did not influence the results. Furthermore, large commercial trials as opposed to investigator initiated studies might have more non‐adherence, more heterogeneity in patient selection, more placebo responders (particularly amongst late‐enrolling patients) and inflation of the baseline scores, and therefore might have less sensitivity [35, 36]. Another limitation might be that the control group was part of other concurring real‐world data studies which could potentially lead to selection bias. Although these patients were matched on active depression at baseline and had similar distribution in gender, age, migraine diagnosis and frequency, and failures on early preventive medication, there could be other hidden differences. However, it is believed that for these analyses the control group was comparable to the erenumab and fremanezumab groups, since possible selection bias was accounted for by restriction and modelling and patients were matched on the most critical data. Also, all patients were treated by the same healthcare providers of the Leiden Headache Centre.
CONCLUSION
Depressive symptoms in subjects with migraine improve in response to anti‐CGRP (ligand or receptor) monoclonals.
AUTHOR CONTRIBUTIONS
Simone de Vries Lentsch: Conceptualization; writing—original draft; formal analysis; methodology; writing—review and editing. Britt W. H. van der Arend: Conceptualization; methodology; writing—review and editing; formal analysis. Irene de Boer: Methodology; writing—review and editing. Erik W. van Zwet: Formal analysis; methodology. Antoinette MaassenVanDenBrink: Writing—review and editing. Gisela M. Terwindt: Supervision; conceptualization; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
G.M. Terwindt reports consultancy support from Abbvie/Allergan, Lilly, Lundbeck, Novartis and Teva, and independent support from the Dutch Organization for Scientific Research, the Dutch Heart and Brain Foundations, International Retinal Research Foundation (IRRF) and Dioraphte. A. MaassenVanDenBrink reports consultancy or industry grant support from Novartis, Lilly and Teva, and independent support from the Dutch Research Council and the Dutch Heart and Brain Foundations. Irene de Boer reports independent support from the Dutch Heart Foundation and IRRF. S. de Vries Lentsch, B.W.H. van der Arend and E.W. van Zwet report no disclosures.
Supporting information
Figure S1
Tables S1–S3
de Vries Lentsch S, van der Arend BWH, de Boer I, van Zwet EW, MaassenVanDenBrink A, Terwindt GM. Depression and treatment with anti‐calcitonin gene related peptide (CGRP) (ligand or receptor) antibodies for migraine. Eur J Neurol. 2024;31:e16106. doi: 10.1111/ene.16106
Simone de Vries Lentsch and Britt W. H. van der Arend shared first author status.
Antoinette MaassenVanDenBrink and Gisela M. Terwindt shared last author status.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Stam AH, de Vries B, Janssens ACJW, Vanmolkot KRJ, Aulchenko YS, Henneman P. Shared genetic factors in migraine and depression. Neurology. 2010;74:288‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang Y, Zhao H, Boomsma DI, et al. Molecular genetic overlap between migraine and major depressive disorder. Eur J Hum Genet. 2018;26:1202‐1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brainstorm Consortium . Analysis of shared heritability in common disorders of the brain. Science. 2018;360:eaap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ashina S, Serrano D, Lipton RB, et al. Depression and risk of transformation of episodic to chronic migraine. J Headache Pain. 2012;13:615‐624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lipton RB, Fanning KM, Buse DC, et al. Migraine progression in subgroups of migraine based on comorbidities. Neurology. 2019;93:e2224‐e2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bigal ME, Lipton RB. Modifiable risk factors for migraine progression (or for chronic daily headaches)—clinical lessons. Headache. 2006;46(Suppl 3):S144‐S146. [DOI] [PubMed] [Google Scholar]
- 7. Louter MA, Bosker JE, van Oosterhout WP, et al. Cutaneous allodynia as a predictor of migraine chronification. Brain. 2013;136:3489‐3496. [DOI] [PubMed] [Google Scholar]
- 8. Louter MA, Wardenaar KJ, Veen G, et al. Allodynia is associated with a higher prevalence of depression in migraine patients. Cephalalgia. 2014;34:1187‐1192. [DOI] [PubMed] [Google Scholar]
- 9. Bigal ME, Ashina S, Burstein R, et al. Prevalence and characteristics of allodynia in headache sufferers: a population study. Neurology. 2008;70:1525‐1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lanteri‐Minet M, Radat F, Chautard MH, Lucas C. Anxiety and depression associated with migraine: influence on migraine subjects' disability and quality of life, and acute migraine management. Pain. 2005;118:319‐326. [DOI] [PubMed] [Google Scholar]
- 11. Schiano di Cola F, Caratozzolo S, Liberini P, Rao R, Padovani A. Response predictors in chronic migraine: medication overuse and depressive symptoms negatively impact onabotulinumtoxin—a treatment. Front Neurol. 2019;10:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Headache Classification Committee of the International Headache Society . The international classification of headache disorders, 3rd edition. Cephalalgia. 2018;38:1‐211. [DOI] [PubMed] [Google Scholar]
- 13. van Casteren DS, Verhagen IE, de Boer I, et al. E‐diary use in clinical headache practice: a prospective observational study. Cephalalgia. 2021;41:1161‐1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bjellanda I, Dahlb AA, Haugc TT, Neckelmannd D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69‐77. [DOI] [PubMed] [Google Scholar]
- 15. Radloff LS. The CES‐D scale: a self‐report depression scale for research in the general population. Appl Psychol Measur. 1977;1:385‐401. [Google Scholar]
- 16. Louter MA, Pelzer N, de Boer I, et al. Prevalence of lifetime depression in a large hemiplegic migraine cohort. Neurology. 2016;87:2370‐2374. [DOI] [PubMed] [Google Scholar]
- 17. Rendas‐Baum R, Yang M, Varon SF, Bloudek LM, DeGryse RE, Kosinski M. Validation of the Headache Impact Test (HIT‐6) in patients with chronic migraine. Health Qual Life Outcomes. 2014;12:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385‐396. [PubMed] [Google Scholar]
- 19. Willekens MC, Postel D, Keesenberg MDM, Lindeboom R. Dutch translation and validation of the headache‐specific locus of control scale (HSLC‐DV). Pain Res Manag. 2018;2018:3046235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhakta M, Vuong T, Taura T, Wilson DS, Stratton JR, Mackenzie KD. Migraine therapeutics differentially modulate the CGRP pathway. Cephalalgia. 2021;41:499‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gingell JJ, Rees TA, Hendrikse ER, et al. Distinct patterns of internalization of different calcitonin gene‐related peptide receptors. ACS Pharmacol Transl Sci. 2020;3:296‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cohen JM, Yang R, Galic M, et al. Efficacy of fremanezumab in patients with chronic migraine and comorbid moderate to moderately severe depression. Neurol Sci. 2019;40(Supplement 2):S226. [Google Scholar]
- 23. Maizels M, Buse D, Jedynak JP, Hand A, Ford JH, Detke H. Assessment of anxiety and depression in a randomized, double‐blind, placebo‐controlled study of galcanezumab in adults with treatment‐resistant migraine: results from the Conquer study. J Neurol Sci. 2019;405(Supplement):129‐130. [Google Scholar]
- 24. Russo A, Silvestro M, Scotto Di Clemente F, et al. Multidimensional assessment of the effects of erenumab in chronic migraine patients with previous unsuccessful preventive treatments: a comprehensive real‐world experience. Journal of Headache and Pain. 2020;21(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schur EA, Noonan C, Buchwald D, Goldberg J, Afari N. A twin study of depression and migraine: evidence for a shared genetic vulnerability. Headache. 2009;49:1493‐1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ashina M, Bendtsen L, Jensen R, Schifter S, Olesen J. Evidence for increased plasma levels of calcitonin gene‐related peptide in migraine outside of attacks. Pain. 2000;86:133‐138. [DOI] [PubMed] [Google Scholar]
- 27. Mathé AA, Agren H, Lindström L, Theodorsson E. Increased concentration of calcitonin gene‐related peptide in cerebrospinal fluid of depressed patients. A possible trait marker of major depressive disorder. Neuroscience. 1994;182:138‐142. [DOI] [PubMed] [Google Scholar]
- 28. van Dongen RM, Zielman R, Noga M, et al. Migraine biomarkers in cerebrospinal fluid: a systematic review and meta‐analysis. Cephalalgia. 2017;37:49‐63. [DOI] [PubMed] [Google Scholar]
- 29. Zhang FF, Peng W, Sweeney JA, Jia ZY, Gong QY. Brain structure alterations in depression: psychoradiological evidence. CNS Neurosci Ther. 2018;24:994‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mathé AA, Ågren H, Wallin A, Blennow K. Calcitonin gene‐related peptide and calcitonin in the CSF of patients with dementia and depression: possible disease markers. Progress in Neuro‐Psychopharmacology and Biological Psychiatry. 2002;26:41‐48. [DOI] [PubMed] [Google Scholar]
- 31. Altamura C, Costa C, Fofi L, et al. Migraineurs' psychological traits do not influence response to erenumab. Neurological Sciences. 2020;41:467‐468. [DOI] [PubMed] [Google Scholar]
- 32. Lipton RB, Cohen JM, Ramirez‐Campos V, et al. Efficacy with fremanezumab in migraine patients with comorbid moderate to severe depression and documented inadequate response to 2‐4 classes of migraine preventive treatments: subgroup analysis of the randomised, placebo‐controlled focus study. Cephalalgia. 2019;39(1 Supplement):210‐211. [Google Scholar]
- 33. Smitherman TA, Tietjen GE, Schuh K, et al. Efficacy of galcanezumab for migraine prevention in patients with a medical history of anxiety and/or depression: a post hoc analysis of the phase 3, randomized, double‐blind, placebo‐controlled REGAIN, and pooled EVOLVE‐1 and EVOLVE‐2 studies. Headache. 2020;60:2202‐2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martin PR, Aiello R, Gilson K, Meadows G, Milgrom J, Reece J. Cognitive behavior therapy for comorbid migraine and/or tension‐type headache and major depressive disorder: an exploratory randomized controlled trial. Behav Res Ther. 2015;73:8‐18. [DOI] [PubMed] [Google Scholar]
- 35. Liu KS, Snavely DB, Ball WA, Lines CR, Reines SA, Potter WZ. Is bigger better for depression trials? J Psychiatr Res. 2008;42:622‐630. [DOI] [PubMed] [Google Scholar]
- 36. Smith SM, Fava M, Jensen MP, et al. Loeser award lecture: size does matter, but it isn't everything: the challenge of modest treatment effects in chronic pain clinical trials. Pain. 2020;161(Suppl 1):S3‐S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Tables S1–S3
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


