Abstract
Field studies of hantavirus infection in rodents report that a higher percentage of infected individuals are males than females. To determine whether males were more susceptible to hantavirus infection than females, adult male and female Long Evans rats (Rattus norvegicus) were inoculated with doses of Seoul virus ranging from 10−4 to 106 PFU. The 50% infective doses (ID50) were not significantly different for male and female rats (100.05 and 100.8 PFU, respectively). To determine whether sex differences in response to infection were related to circulating sex steroid hormones, sex steroid concentrations were manipulated and antibody responses and virus shedding were assessed following inoculation with the ID90. Regardless of hormone treatment, males had higher anti-Seoul virus immunoglobulin G (IgG) and IgG2a (i.e., Th1) responses than females and IgG1 (i.e., Th2) responses similar to those of females. Males also shed virus in saliva and feces longer than females. Manipulation of sex steroids in adulthood did not alter immune responses or virus shedding, suggesting that sex steroids may organize adult responses to hantavirus earlier during ontogeny.
Hantaviruses are negative-sense RNA viruses (family Bunyaviridae) encompassing over 20 different viruses that are each carried by a different host species, with rodents serving as the primary reservoirs (18). Field surveys of several rodent species, including brush mice, deer mice, harvest mice, bank voles, and cotton rats, indicate that males are more commonly infected than females (4, 8, 11, 19, 20, 27). Because these studies used serology to determine hantavirus infection, sex differences in infection could reflect either a lack of infection or the absence of sustained antibody production in females. Experimental inoculation of female rodents with hantavirus, however, illustrates that females produce long-lasting, detectable antibody (22). Alternatively, sex differences in hantavirus prevalence may reflect differences in endocrine-immune interactions (15). The extent to which sex steroids affect immune responses against hantavirus infection has not been examined.
In contrast to other rodent species, sex differences in hantavirus prevalence have not been reported consistently among natural populations of Norway rats. Among adult rats, however, males (90%) tend to be infected with Seoul virus more often than females (75%) (7, 10). Seoul virus is hypothesized to be transmitted via wounding, and adult male rats are more likely to be wounded than either females or juvenile males (10). Thus, sex differences in hantavirus prevalence may reflect complex interactions between behavior and physiology. The first goal of this study was to control for sex differences in exposure and determine whether males were more susceptible to hantavirus infection than females. At 70 to 80 days of age, 5 to 10 male and 5 to 10 female Long Evans rats (Rattus norvegicus) were inoculated with either 10−4, 10−3, 10−2, 102, 104, or 106 PFU of Seoul virus (strain SR-11) suspended in 0.2 ml of Eagle minimum essential medium (with Earle's salts; Meditach Cellgro, Va.). Seoul virus was obtained from the U.S. Army Medical Research Institute of Infectious Diseases (Ft. Detrick, Md.), where the virus was isolated from neonatal rat brains and passaged four times in Vero E6 cells. Blood samples were obtained from each animal prior to infection and then 10, 20, 30, and 40 days postinoculation under anesthesia with methoxyflurane vapors (Metofane; Schering Plough, Union, N.J.).
Plasma was used to detect anti-Seoul virus immunoglobulin G (IgG) using an enzyme-linked immunosorbent assay in which microtiter plates were coated overnight at 4°C with gamma-irradiated Vero E6 cells infected with Seoul virus or gamma-irradiated uninfected Vero E6 cells diluted 1:500 in carbonate buffer. Thawed plasma samples, as well as positive control samples (i.e., pooled plasma from rats previously determined to have anti-Seoul virus IgG) and negative control samples (i.e., pooled plasma from Seoul virus-naive rats), were diluted 1:100 in phosphate-buffered saline (PBS)–Tween (PBS-T) with 2% fetal bovine serum and added in duplicate to antigen-coated wells containing either infected or uninfected Vero E6 cells. The plates were sealed, incubated at 37°C for 1 h, and washed with PBS-T, and secondary antibody (Kirkegaard and Perry Laboratories, Gaithersburg, Md.; alkaline phosphatase-conjugated anti-rat IgG [heavy plus light chains], horseradish peroxidase-conjugated anti-rat IgG1, or horseradish peroxidase-conjugated anti-rat IgG2a diluted 1:400 in PBS with 2% fetal bovine serum) was added. The plates were resealed, incubated for 1 h at 37°C, and washed with PBS-T, and substrate buffer (0.5 mg of p-nitrophenyl phosphate per ml diluted in diethanolamine substrate buffer for alkaline phosphatase reactions or tetramethylbenzidine for horseradish peroxidase reactions) was added to each well. Plates were protected from light during the enzyme-substrate reaction, which was terminated after 30 to 45 min by adding 1.5 M NaOH to each well for alkaline phosphatase reactions or 2 N H2SO4 to each well for horseradish peroxidase reactions. The optical density (OD) was measured at 405 nm for alkaline phosphatase reactions and 450 nm for horseradish peroxidase reactions, and the average OD for each set of uninfected Vero E6 duplicates was subtracted from the average OD for each set of infected Vero E6 duplicates. Samples were considered positive if the average adjusted OD was ≥0.100. To minimize intra- and interplate variability, the average adjusted OD for each sample was expressed as a percentage of its plate-positive control OD for statistical analyses (9).
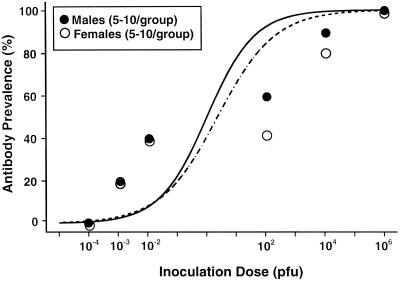
Antibody prevalence (i.e., the number of animals with detectable anti-Seoul virus IgG) by day 40 postinoculation was compared between males and females using chi-square analyses. Antibody prevalence was assessed 40 days after inoculation because previous studies illustrate that hantavirus-specific antibody is detectable 15 to 30 days postinoculation (7, 14, 22). Antibody prevalence did not differ between males and females at any of the six doses of Seoul virus (P > 0.05). Logistic regression was used to compare the infective-dose (ID) curves and estimate the 50% ID (ID50). The ID50 did not differ significantly between males (mean ± standard deviation, 1.1 ± 2.0 PFU) and females (7.6 ± 2.0 PFU) (Fig. 1).
FIG. 1.
Antibody prevalence among intact male and female rats inoculated with either 10−4, 10−3, 10−2, 102, 104, or 106 PFU of Seoul virus. Data are presented as percentages of individuals producing detectable antibody (i.e., adjusted average OD ≥ 0.100) against Seoul virus by day 40 postinoculation, with the fitted logistic regression curves for both males (solid line) and females (dashed line) included. Equal percentages of males and females seroconverted in response to each dose of Seoul virus (P > 0.05 in each case).
Although the prevalence of males and females that became infected did not differ, studies of other viral infections suggest that patterns of immune responses differ between the sexes and are mediated by sex steroid hormones (1, 15, 29). Thus, males and females may differ because testosterone suppresses and estradiol enhances several aspects of immune function (1, 15, 17, 24, 26, 29). The second aim of this study was to examine whether adult sex steroid hormone concentrations influence immune responses and virus shedding following hantavirus infection. Immunologically, patterns of helper T (Th) cell responses (i.e., Th1 or Th2) differ between males and females, with males exhibiting elevated Th1 responses (i.e., elevated gamma interferon, interleukin-2 [IL-2], and IgG2a levels) and females exhibiting increased Th2 responses (i.e., higher IL-4, IL-5, IL-6, and IL-10 levels) (5, 12, 13). Treatment of males with estradiol and females with testosterone prior to infection with pathogens, such as coxsackievirus, reverses the Th responses, suggesting that hormones can modify immune responses to virus infection (12, 13). To determine whether adult sex steroid hormone concentrations influence immune responses and virus shedding following hantavirus infection, at 70 to 80 days of age 20 male and 20 female rats were bilaterally gonadectomized under ketamine (80 mg/kg of body mass)–xylazine (6 mg/kg) anesthesia (Phoenix Pharmaceutical, St. Joseph, Mo.) and given 2 weeks to recover from surgery. After recovery, 10 castrated males were each subcutaneously implanted with a 30-mm Silastic capsule (inside diameter [i.d.] = 1.47 mm, outside diameter [o.d.] = 1.96 mm) containing 20 mm of testosterone propionate (Sigma, St. Louis, Mo.). The remaining 10 castrated males, as well as 10 intact males, were each implanted with an empty capsule of equal length. Ten ovariectomized females were each subcutaneously implanted with a 15-mm Silastic capsule (i.d. = 1.47 mm, o.d. = 1.96 mm) containing 10 mm of estradiol benzoate (Sigma). The remaining 10 ovariectomized females and 9 intact females were each implanted with an empty Silastic capsule of equal length. Silastic capsule length was based on previous reports that these hormone doses (i.e., the length of the Silastic capsule) are sufficient to maintain physiological testosterone and estradiol concentrations in male and female rats, respectively (25). At the time the Silastic capsules were implanted, all animals received an intraperitoneal inoculation of 104 PFU of Seoul virus (strain SR-11) suspended in 0.2 ml of Eagle minimum essential medium (i.e., the ID90 from the first experiment). Blood, saliva, and fecal samples were then obtained from each animal on days 0, 10, 15, 20, 30, and 40 postinoculation under anesthesia with methoxyflurane vapors. Saliva samples were collected from anesthetized rats after injecting them intraperitoneally with 2.5 mg of pilocarpine HCl (Sigma) per kg of body mass suspended in 0.9% sterile saline (6). After samples were collected on day 40 postinoculation, animals were killed and seminal vesicles were removed from the males and weighed as an index of long-term testosterone concentrations. All procedures described in this paper were approved by the Johns Hopkins Animal Care and Use Committee (protocol number RA98H536) and the Johns Hopkins Office of Health, Safety, and Environment (registration number A9902030102).
Relative seminal vesicle weights (i.e., corrected for body mass) were higher among intact males (0.282 ± 0.13 g) and castrated males treated with testosterone (0.326 ± 0.12 g) than among castrated males (0.095 ± 0.06 g) [F(2, 29) = 12.75, P < 0.05]. Plasma testosterone concentrations in males and estradiol concentrations in females were assayed by radioimmunoassay using the manufacturer's protocols (ICN Biochemicals, Inc., Carson, Calif.). Testosterone concentrations were higher for intact males and castrated males treated with testosterone than for castrated male rats; castrated males treated with testosterone also had higher testosterone concentrations than intact males on days 10, 15, 20, and 30, but not on day 40, postinoculation [F(10, 179) = 19.30, P < 0.05] (Table 1). Plasma estradiol concentrations were higher for intact females and ovariectomized females treated with estradiol than for ovariectomized females 10, 15, 20, 30, and 40 days postinoculation; ovariectomized females treated with estradiol also had higher estradiol concentrations than intact females on days 10, 15, 20, 30, and 40 postinoculation [F(10, 173) = 10.29, P < 0.05] (Table 1).
TABLE 1.
Sex steroid hormone concentrationsa
| Hormone and group | Hormone concn (mean ± SE) on day postinoculationb
|
|||||
|---|---|---|---|---|---|---|
| 0 | 10 | 15 | 20 | 30 | 40 | |
| Testosterone | ||||||
| Intact males | 0.69 ± 0.17* | 0.84 ± 0.17* | 1.13 ± 0.36* | 0.92 ± 0.25* | 0.77 ± 0.19* | 0.70 ± 0.13* |
| Castrated males | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| T-treated males | 0.00 ± 0.00 | 8.24 ± 0.74*† | 6.28 ± 0.91*† | 6.62 ± 1.18*† | 2.73 ± 0.42*† | 0.71 ± 0.28* |
| Estradiol | ||||||
| Intact females | 25.8 ± 6.81* | 27.0 ± 5.57* | 20.8 ± 8.39* | 25.9 ± 7.78* | 38.2 ± 10.1* | 55.2 ± 10.2* |
| Ovx females | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| E2-treated females | 0.00 ± 0.00 | 166.6 ± 20.6*† | 123.1 ± 21.9*† | 87.5 ± 8.9*† | 162.3 ± 18.8*† | 109.7 ± 19.3*† |
Sex steroid hormone concentrations in males and females that either were intact, gonadectomized (i.e., males were castrated and females were ovariectomized [Ovx]), or gonadectomized with sex steroids replaced (i.e., gonadectomized males received testosterone [T]-filled capsules and gonadectomized females received estradiol [E2]-filled capsules).
Testosterone levels are in nanograms per milliliter, and estradiol levels are in picograms per milliliter. An asterisk indicates that intact and hormone-treated animals had higher hormone concentrations than their gonadectomized counterparts on the corresponding day, based on an analysis of variance (P < 0.05). A dagger indicates that hormone-treated animals had higher sex steroid concentrations than their intact counterparts on the corresponding day, based on an analysis of variance (P < 0.05).
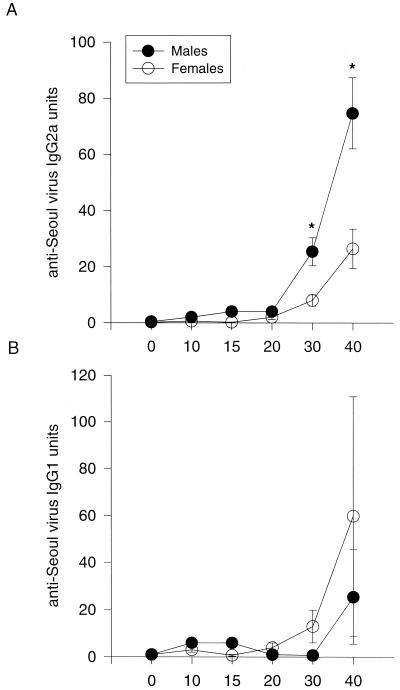
Manipulation of testosterone concentrations in males and estradiol concentrations in females did not affect production of antibody against Seoul virus (P > 0.05). Overall, males had higher anti-Seoul virus IgG responses than females on days 20, 30, and 40 postinoculation, regardless of hormone treatment [F(5, 353) = 18.72, P < 0.05] (Table 2). Male rats also had higher anti-Seoul virus IgG2a responses than females on days 30 and 40 postinoculation despite hormone manipulation [F(5, 353) = 7.81, P < 0.05] (Fig. 2A). In contrast, females tended to show higher IgG1 responses than males on days 30 and 40 postinoculation, though this did not reach statistical significance (P > 0.05) (Fig. 2B).
TABLE 2.
Plasma anti-Seoul virus IgG responsesa
| Group | Anti-Seoul virus IgG response (mean ± SE) on day postinoculationb
|
|||||
|---|---|---|---|---|---|---|
| 0 | 10 | 15 | 20 | 30 | 40 | |
| Intact males | 0.8 ± 0.6 | 4.9 ± 3.0 | 84.0 ± 22.0 | 106.0 ± 19.0* | 332.0 ± 47.0* | 342.1 ± 56.0* |
| Castrated males | 1.0 ± 0.7 | 1.0 ± 1.0 | 82.0 ± 21.0 | 106.0 ± 27.0* | 280.0 ± 71.0* | 387.3 ± 84.0* |
| T-treated males | 1.0 ± 0.7 | 2.0 ± 0.9 | 33.0 ± 10.0 | 108.0 ± 14.0* | 314.0 ± 41.0* | 426.7 ± 43.0* |
| Intact females | 3.0 ± 1.0 | 9.0 ± 4.0 | 36.0 ± 10.0 | 60.0 ± 14.0 | 189.0 ± 55.0 | 219.6 ± 63.0 |
| Ovx females | 2.0 ± 0.8 | 4.0 ± 2.0 | 7.0 ± 3.0 | 54.0 ± 16.0 | 187.0 ± 56.0 | 209.2 ± 53.0 |
| E2-treated females | 3.0 ± 1.0 | 8.0 ± 2.0 | 19.0 ± 6.0 | 39.0 ± 8.0 | 178.0 ± 42.0 | 209.1 ± 39.0 |
Plasma anti-Seoul virus IgG responses in males and females that either were intact, gonadectomized (i.e., males were castrated and females were ovariectomized [Ovx]), or gonadectomized with sex steroids replaced (i.e., gonadectomized males received testosterone [T]-filled capsules and gonadectomized females received estradiol [E2]-filled capsules).
Data are presented as IgG units, in which the mean OD of each test sample was divided by the OD of the positive control sample run on the same microtiter plate. An asterisk indicates that males had higher IgG responses than females, regardless of hormone manipulation, based on an analysis of variance (P < 0.05).
FIG. 2.
(A) Plasma anti-Seoul virus IgG2a responses (mean ± standard error) in male and female rats. (B) Plasma anti-Seoul virus IgG1 responses (mean ± standard error) in male and female rats. Blood samples were collected 0, 10, 15, 20, 30, and 40 days following inoculation with Seoul virus. For calculation of IgG2a or IgG1 units, the mean OD of each test sample was divided by the OD of the positive control sample run on the same microtiter plate. Because neither gonadectomy nor hormone replacement had an effect on antibody production, responses from the different treatments groups were collapsed and graphed together. An asterisk indicates that males had higher IgG2a responses than females (P < 0.05).
Viral RNA was identified using nested reverse transcription-PCR (RT-PCR), and the presence of virus in saliva and feces was used to determine whether virus was shed. Viral RNA was isolated using a guanidine isothiocyanate procedure (3). For RNA isolation from saliva, samples were collected from each rat and added to Trizol LS reagent (Life Technologies, Rockville, Md.) at a 3:1 ratio, with RNase-free glycogen (10 μg) added as a carrier. For RNA isolation from feces, approximately 100 mg of feces was homogenized in Tris-EDTA buffer (pH 8.0) and centrifuged at 12,000 × g for 10 min at 4°C; supernatants were collected, incubated with proteinase K (50 μg/ml; Life Technologies) and 0.5% sodium dodecyl sulfate at 50°C for 30 min to digest proteins, and then added to Trizol LS at a 3:1 ratio. To separate, precipitate, and resuspend viral RNA, the manufacturer's protocol was used (Trizol LS; Life Technologies).
For RT-PCR, a 280-bp nucleotide sequence of the SR-11 small (S) genome was amplified using two 20-bp primers, HTN-S4 (5′ GATAGGTGTCCACCAACATG 3′) and HTN-S6 (5′ AGCTCTGGATCCATGTCATC 3′), that amplified positions 979 through 1259 (3). The DNA fragment obtained from the RT-PCR was further amplified using primers HTN-S3 (5′ GCCTTCTTTTCTATACTTCAGG 3′) and HTN-S5 (5′ CCAGGCAACCATAAACATAAC 3′), designed to amplify a 176-bp nucleotide sequence (positions 1031 through 1207). First-strand cDNA was prepared using the GeneAmp RNA PCR kit protocol (Perkin-Elmer, Branchburg, N.J.), incubated in a DNA thermocycler (Techne Genius) at 42°C for 15 min, 99°C for 5 min, and 5°C for 5 min, and then held at 4°C. The reaction mixture contained 5 mM MgCl2, 1 mM deoxynucleoside triphosphates, 1 U of RNase inhibitor, and 2.5 U of murine leukemia virus reverse transcriptase. The positive control was SR-11 RNA isolated from virus stock, and the negative control was diethyl pyrocarbonate water that was included in the cDNA syntheses and primary and secondary amplifications.
The 280-bp sequence was amplified in a 100-μl reaction mixture containing 20 μl of the cDNA, 0.3 μM HTN-S6 primer, and 2.5 U of polymerase (AmpliTaq; Perkin-Elmer). Reactions were amplified for one cycle at 94°C for 3 min and 40 cycles of 94°C for 30 s, 55°C for 45 s, and 72°C for 60 s, followed by 10 min at 72°C. The nested 176-bp sequence was amplified in a 100-μl reaction mixture containing 2 μl of the product of the first DNA amplification, 20 μM HTN-S3 primer, 20 μM HTN-S5 primer, 10 mM MgCl2, 1 mM deoxynucleoside triphosphates, and 2.5 U of polymerase. Nested-PCR products were amplified using the same cycle series as was used for the primary amplification. The PCR products were electrophoresed on a 4% gel (3% NuSieve plus 1% SeaKem; FMC Bioproducts, Rockland, Maine), stained with ethidium bromide, and examined for bands of the appropriate size. Randomly selected positive PCR products from saliva and fecal samples from males and females, as well as positive and negative control products, were purified using QIAquick (Qiagen, Valencia, Calif.) and sequenced.
Virus shedding in saliva and feces was not altered by hormone manipulation (P > 0.05) (Table 3). Overall, more males shed virus in saliva than females 10 days (χ2 = 3.82, df = 1, P = 0.051) and 30 days (χ2 = 8.19, df = 1, P < 0.05) after inoculation with Seoul virus (Table 3). The prevalence of Seoul virus in feces also differed between males and females on day 30 postinoculation; more males shed virus in feces than females (χ2 = 6.88, df = 1, P < 0.05) (Table 3). In general, males shed virus in saliva and feces more consistently than females, regardless of hormone manipulation (Table 3). The PCR product obtained from saliva and feces of males and females was sequenced and verified as Seoul virus DNA.
TABLE 3.
Virus sheddinga
| Sample and group | No. of virus-shedding rats/total on day postinoculationb
|
||||
|---|---|---|---|---|---|
| 10 | 15 | 20 | 30 | 40 | |
| Saliva samples | |||||
| Intact males | 6/11 | 7/10 | 6/11 | 6/11 | 6/11 |
| Castrated males | 4/9 | 4/9 | 6/9 | 5/9 | 8/9 |
| T-treated males | 9/10 | 7/10 | 4/10 | 6/10 | 7/10 |
| Total males | 19/30* | 18/29 | 16/30 | 17/30* | 21/30 |
| Intact females | 3/9 | 6/9 | 5/9 | 2/9 | 2/9 |
| Ovx females | 4/10 | 7/10 | 2/10 | 2/10 | 6/10 |
| E2-treated females | 3/10 | 10/10 | 3/10 | 1/10 | 6/10 |
| Total females | 10/29 | 23/29 | 11/29 | 5/29 | 14/29 |
| Fecal samples | |||||
| Intact males | 5/11 | 4/11 | 4/11 | 5/11 | 1/11 |
| Castrated males | 6/9 | 5/9 | 7/9 | 4/8 | 1/9 |
| T-treated males | 4/10 | 6/10 | 7/10 | 7/9 | 1/10 |
| Total males | 15/30 | 15/30 | 18/30 | 16/29* | 3/30 |
| Intact females | 7/9 | 4/9 | 4/9 | 1/8 | 0/9 |
| Ovx females | 9/10 | 4/10 | 6/10 | 2/10 | 2/10 |
| E2-treated females | 6/9 | 5/10 | 8/10 | 2/10 | 1/10 |
| Total females | 22/28 | 13/29 | 18/29 | 5/28 | 3/29 |
Virus shedding in saliva and feces from males and females that either were intact, gonadectomized (i.e., males were castrated and females were ovariectomized [Ovx]), or gonadectomized with sex steroids replaced (i.e., gonadectomized males received testosterone [T]-filled capsules and gonadectomized females received estradiol [E2]-filled capsules).
An asterisk indicates that more males shed virus than females on the respective day postinoculation, based on chi-square analyses (P < 0.05).
Sex differences in the prevalence of hantavirus infection have been observed in several natural rodent populations, including deer mice, brush mice, harvest mice, bank voles, and cotton rats (4, 8, 11, 19, 20, 27). In each case, males are infected more often than females. Field studies of Norway rats suggest that sex differences in hantavirus prevalence reflect sex differences in behaviors, like aggression, that increase the likelihood of males being infected (10). High circulating testosterone concentrations increase the probability of engaging in aggressive encounters in several vertebrate species (21). In addition to modulating aggression, sex steroid hormones can affect immune responses against infection. Studies of viral infections, such as coxsackievirus, suggest that sex differences in both the prevalence and intensity of infection are due to differences in endocrine-immune interactions (12, 13).
Despite the known effects of sex steroids on infection, in the present study, manipulation of adult sex steroids had no effect on immune responses or virus shedding following exposure to Seoul virus. Specifically, males had higher antibody responses and shed virus longer than females, regardless of adult hormone manipulation. Sex steroid hormones affect physiology and behavior at two distinct times during ontogeny (2, 16, 23). During perinatal development, sex steroids cause sex differences in the differentiation or organization of central and peripheral structures. In adulthood, exposure to sex steroids serves to activate preexisting hormonal circuits. The data from the present study may suggest that sex steroid hormones are not involved in hantavirus infection. Alternatively, these data may illustrate that manipulation of activational sex steroids does not alter responses to infection because the hormonal circuitry was organized earlier during development. If sex steroids organize adult responses to infection, then manipulation of neonatal sex steroids should alter adult responses to hantavirus infection.
Regardless of hormone manipulation, males had higher anti-Seoul virus IgG2a responses than females. Recent data from our laboratory indicate that following Seoul virus inoculation, males have elevated IL-2 and gamma interferon concentrations and females have elevated IL-4 responses (S. L. Klein and G. E. Glass, unpublished data). Taken together, these data suggest that males may have higher Th1 responses to hantavirus infection than females. Studies of other viral infections in rodents suggest that females typically have higher Th2 responses than males and that this is due, in part, to the effects of estrogens on cytokine production (12). In the present study, females tended to produce higher IgG1 responses than males. In contrast to estrogens, androgens promote differentiation of CD4+ T cells to a Th1 phenotype (12). In the present study, however, castrated and intact males had similar IgG2a responses, suggesting that increased Th1 responses are not contingent on the direct effects of androgens.
High antibody responses in males may indicate that males have more efficient immune responses against infection than females. This outcome seems unlikely given the rapid increase and long duration of virus shedding in males compared to females. Alternatively, males may have higher antibody responses than females because virus replication is increased in males. Higher Th1 responses are associated with increased susceptibility to infections caused by coxsackievirus and Sindbis virus in mice (12, 28). Although quantitative analyses were not conducted, males shed Seoul virus longer than females, suggesting that higher Th1 responses among males may be a consequence of increased virus replication.
In summary, although males and females are equally susceptible to infection with Seoul virus, males shed virus longer and produce higher Th1 responses against Seoul virus than females. Increased virus shedding among males may explain why males are more likely to acquire Seoul virus infection following aggressive encounters among natural populations of Norway rats (10). In the present study, manipulation of adult sex steroid hormones did not alter immune responses or virus shedding following inoculation with Seoul virus. Although sex steroid hormones may not mediate sex differences in response to hantavirus infection, sex differences in infection among adults may be altered by sex steroids earlier during development. Alternatively, sex differences in infection may reflect other neuroendocrine changes, such as differences in glucocorticoids, that may affect responses to Seoul virus infection.
Acknowledgments
This research was supported by NASA grant NCC5-305 (G.E.G.) and NIH NRSA AI 10324 (S.L.K.).
We thank Connie Schmaljohn and Cindy Rossi for providing hantavirus reagents, Alan Scott and Aimee Marson for assistance with PCR development, and Randy Nelson and Deborah Drazen for assistance with radioimmunoassays. We also thank Diane Griffin and Alan Scott for helpful comments on early drafts of the manuscript.
REFERENCES
- 1.Alexander J, Stimson W H. Sex hormones and the course of parasitic infection. Parasitol Today. 1988;4:189–193. [Google Scholar]
- 2.Arnold A P, Breedlove S M. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19:469–498. doi: 10.1016/0018-506x(85)90042-x. [DOI] [PubMed] [Google Scholar]
- 3.Arthur R R, Lofts R S, Gomez J, Glass G E, LeDuc J W, Childs J E. Grouping of hantaviruses by small (S) genome segment polymerase chain reaction and amplification of viral RNA from wild-caught rats. Am J Trop Med Hyg. 1992;47:210–224. doi: 10.4269/ajtmh.1992.47.210. [DOI] [PubMed] [Google Scholar]
- 4.Bernshtein A D, Apekina N S, Mikhailova T V, Myasnikov Y A, Khlyap L A, Korotkov Y S, Gavrilovskaya I N. Dynamics of Puumala hantavirus infection in naturally infected bank voles (Clethrionomys glareolus) Arch Virol. 1999;144:2415–2428. doi: 10.1007/s007050050654. [DOI] [PubMed] [Google Scholar]
- 5.Bijlsma J W J, Cutolo M, Masi A T, Chikanza I C. The neuroendocrine immune basis of rheumatic diseases. Trends Immunol. 1999;20:298–301. doi: 10.1016/s0167-5699(98)01422-4. [DOI] [PubMed] [Google Scholar]
- 6.Bodner L, Baum B J. Characteristics of stimulated parotid gland secretion in the aging rat. Mech Ageing Dev. 1985;31:337–342. doi: 10.1016/0047-6374(85)90099-5. [DOI] [PubMed] [Google Scholar]
- 7.Childs J E, Korch G W, Glass G E, LeDuc J W, Shah K V. Epizootiology of hantavirus infections in Baltimore: isolation of a virus from Norway rats, and characteristics of infected rat populations. Am J Epidemiol. 1987;126:55–68. doi: 10.1093/oxfordjournals.aje.a114662. [DOI] [PubMed] [Google Scholar]
- 8.Childs J E, Ksiazek T G, Spiropoulou C F, Krebs J W, Morzunov S, Maupin G O, Gage K L, Rollin P E, Sarisky J, Enscore R E, Frey J K, Peters C J, Nichol S T. Serologic and genetic identification of Peromyscus maniculatus as the primary reservoir for a new hantavirus in the southwest United States. J Infect Dis. 1994;169:1271–1280. doi: 10.1093/infdis/169.6.1271. [DOI] [PubMed] [Google Scholar]
- 9.de Savigny D, Voller A. The communication of ELISA data from laboratory to clinician. J Immunoass. 1980;1:105–128. doi: 10.1080/01971528008055779. [DOI] [PubMed] [Google Scholar]
- 10.Glass G E, Childs J E, Korch G W, LeDuc J W. Association of intraspecific wounding with hantaviral infection in wild rats (Rattus norvegicus) Epidemiol Infect. 1988;101:459–472. doi: 10.1017/s0950268800054418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glass G E, Livingstone W, Mills J N, Hlady W G, Fine J B, Biggler W, Coke T, Frazier D, Atherley S, Rollin P E, Ksiazek T G, Peters C J, Childs J E. Black Creek Canal virus infection in Sigmodon hispidus in southern Florida. Am J Trop Med Hyg. 1998;59:699–703. doi: 10.4269/ajtmh.1998.59.699. [DOI] [PubMed] [Google Scholar]
- 12.Huber S A, Kupperman J, Newell M K. Hormonal regulation of CD4+ T-cell responses in coxsackievirus B3-induced myocarditis in mice. J Virol. 1999;73:4689–4695. doi: 10.1128/jvi.73.6.4689-4695.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huber S A, Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J Virol. 1994;68:5126–5132. doi: 10.1128/jvi.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutchinson K L, Rollin P E, Shieh W-J, Zaki S, Greer P W, Peters C J. Transmission of Black Creek Canal virus between cotton rats. J Med Virol. 2000;60:70–76. doi: 10.1002/(sici)1096-9071(200001)60:1<70::aid-jmv12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 15.Klein, S. L. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci. Biobehav. Rev., in press. [DOI] [PubMed]
- 16.Konstadoulakis M M, Syrigos K N, Baxevanis C N, Syrigou E I, Papamichail M, Peveretos P, Anapliotou M, Golematis B C. Effect of testosterone administration, pre- and postnatally, on the immune system of rats. Horm Metab Res. 1995;27:275–278. doi: 10.1055/s-2007-979958. [DOI] [PubMed] [Google Scholar]
- 17.Mankau S K, Hamilton R. The effect of sex and sex hormones on the infection of rats by Trichinella spiralis. Can J Zool. 1972;50:597–602. doi: 10.1139/z72-082. [DOI] [PubMed] [Google Scholar]
- 18.Mertz G J, Hjelle B L, Bryan R T. Hantavirus infection. Adv Intern Med. 1997;42:369–421. [PubMed] [Google Scholar]
- 19.Mills J N, Johnson J M, Ksiazek T G, Ellis B A, Rollin P E, Yates T L, Mann M O, Johnson M R, Campbell M L, Miyashiro J, Patrick M, Zyzak M, Lavender D, Novak M G, Schmidt K, Peters C J, Childs J E. A survey of hantavirus antibody in small-mammal populations in selected United States national parks. Am J Trop Med Hyg. 1998;58:525–532. doi: 10.4269/ajtmh.1998.58.525. [DOI] [PubMed] [Google Scholar]
- 20.Mills J N, Ksiazek T G, Ellis B A, Rollin P E, Nichol S T, Yates T L, Gannon W L, Levy C E, Engelthaler D M, Davis T, Tanda D T, Frampton J W, Nichols C R, Peters C J, Childs J E. Patterns of association with host and habitat: antibody reactive with Sin Nombre virus in small mammals in the major biotic communities of the southwestern United States. Am J Trop Med Hyg. 1997;56:273–284. doi: 10.4269/ajtmh.1997.56.273. [DOI] [PubMed] [Google Scholar]
- 21.Nelson R J. An introduction to behavioral endocrinology. 2nd ed. Sunderland, Mass: Sinauer Associates, Inc.; 2000. [Google Scholar]
- 22.Nuzum E O, Rossi C A, Stephenson E H, LeDuc J W. Aerosol transmission of Hantaan and related viruses to laboratory rats. Am J Trop Med Hyg. 1988;38:636–640. doi: 10.4269/ajtmh.1988.38.636. [DOI] [PubMed] [Google Scholar]
- 23.Pheonix C H, Goy R W, Gerall A A, Young W C. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 24.Rajan T V, Nelson F K, Shultz L D, Shultz K L, Beamer W G, Yates J, Greiner D L. Influence of gonadal steroids on susceptibility to Brugia malayi in scid mice. Acta Trop. 1994;56:307–314. doi: 10.1016/0001-706x(94)90102-3. [DOI] [PubMed] [Google Scholar]
- 25.Smith E R, Damassa D A, Davidson J M. Hormone administration: peripheral and intracranial implants. In: Myers R D, editor. Methods in psychobiology. New York, N.Y: Academic Press; 1977. pp. 259–279. [Google Scholar]
- 26.Tiuria R, Horii Y, Tateyama S, Tsuchiya K, Nawa Y. The Indian soft-furred rat, Millardia meltada, a new host for Nippostrongylus brasiliensis, showing androgen-dependent sex difference in intestinal mucosal defence. Int J Parasitol. 1994;24:1055–1057. doi: 10.1016/0020-7519(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 27.Weigler B J, Ksiazek T G, Vandenbergh J G, Levin M, Sullivan W T. Serological evidence for zoonotic hantaviruses in North Carolina rodents. J Wildl Dis. 1996;32:354–357. doi: 10.7589/0090-3558-32.2.354. [DOI] [PubMed] [Google Scholar]
- 28.Wesselingh S L, Levine B, Fox R J, Choi S, Griffin D E. Intracerebral cytokine mRNA expression during fatal and nonfatal alphavirus encephalitis suggests a predominant type 2 T cell response. J Immunol. 1994;152:1289–1297. [PubMed] [Google Scholar]
- 29.Zuk M, McKean K A. Sex differences in parasite infections: patterns and processes. Int J Parasitol. 1996;26:1009–1024. [PubMed] [Google Scholar]