Abstract
Background and purpose
We aimed to evaluate the accuracy of plasma neurofilament light chain (NfL) in predicting Alzheimer's disease (AD) and the progression of cognitive decline in patients with subjective cognitive decline (SCD) and mild cognitive impairment (MCI).
Methods
This longitudinal cohort study involved 140 patients (45 with SCD, 73 with MCI, and 22 with AD dementia [AD‐D]) who underwent plasma NfL and AD biomarker assessments (cerebrospinal fluid, amyloid positron emission tomography [PET], and 18F‐fluorodeoxyglucose‐PET) at baseline. The patients were rated according to the amyloid/tau/neurodegeneration (A/T/N) system and followed up for a mean time of 2.72 ± 0.95 years to detect progression from SCD to MCI and from MCI to AD. Forty‐eight patients (19 SCD, 29 MCI) also underwent plasma NfL measurements 2 years after baseline.
Results
At baseline, plasma NfL detected patients with biomarker profiles consistent with AD (A+/T+/N+ or A+/T+/N−) with high accuracy (area under the curve [AUC] 0.82). We identified cut‐off values of 19.45 pg/mL for SCD and 20.45 pg/mL for MCI. During follow‐up, nine SCD patients progressed to MCI (progressive SCD [p‐SCD]), and 14 MCI patients developed AD dementia (progressive MCI [p‐MCI]). The previously identified cut‐off values provided good accuracy in identifying p‐SCD (80% [95% confidence interval 65.69: 94.31]). The rate of NfL change was higher in p‐MCI (3.52 ± 4.06 pg/mL) compared to non‐progressive SCD (0.81 ± 1.25 pg/mL) and non‐progressive MCI (−0.13 ± 3.24 pg/mL) patients. A rate of change lower than 1.64 pg/mL per year accurately excluded progression from MCI to AD (AUC 0.954).
Conclusion
Plasma NfL concentration and change over time may be a reliable, non‐invasive tool to detect AD and the progression of cognitive decline at the earliest stages of the disease.
Keywords: Alzheimer's disease, biomarker, dementia, mild cognitive impairment, neurofilament
INTRODUCTION
Subjective cognitive decline (SCD) [1] and mild cognitive impairment (MCI) [2] are considered to be first presentations of Alzheimer's disease (AD) [3] and such patients represent the main target population for clinical trials and for the administration of upcoming disease‐modifying therapies [4]. Nevertheless, MCI and SCD are very common and heterogeneous conditions with several possible trajectories and many potential underlying causes [5]. Neurofilament light chain (NfL), a component of the neuronal cytoskeleton [6], has recently emerged as a promising blood‐based biomarker for AD [7, 8]. Elevated levels of plasma NfL have been observed in individuals with SCD [9], MCI [10] and dementia due to AD (AD‐D) [10] compared to cognitively normal individuals, and longitudinal changes in NfL are related to changes in brain atrophy and cognitive outcomes in AD [10]. Previous studies demonstrated that blood‐based NfL had high accuracy in discriminating between neurodegenerative and non‐degenerative cognitive impairment [11, 12] and in forecasting the progression from SCD and MCI to AD [13, 14], but poor accuracy when attempting to differentiate between patients with positive amyloid‐beta (Aβ) pathology and those with negative Aβ pathology [12, 15, 16]. Nevertheless, to our knowledge, no prior studies have investigated the accuracy of NfL in detecting biological AD, defined as the combination of Aβ and tau pathology, in individuals with SCD or MCI. Furthermore, no specific cut‐off values have been proposed for NfL to predict AD pathology and progression to AD in individuals with SCD and MCI. Finally, the clinical meaning of NfL level change over time in non‐demented patients has been poorly explored so far [17, 18]. In this perspective, we hypothesized that plasma NfL level and its change over time may mirror the underlying AD biomarker profile and predict the progression of cognitive decline.
MATERIALS AND METHODS
Patients
We enrolled 140 consecutive patients (45 SCD, 73 MCI, 22 AD‐D) referred to the Centre for Adult Cognitive Disorders of Careggi Hospital in Florence for assessment of cognitive decline, between July 2018 and November 2022. We included patients who met the criteria for clinical diagnosis of AD‐D [19], MCI [2], or SCD [1]. Exclusion criteria were: history of head injury, current systemic and/or neurological disease other than AD, major depression or substance use disorder. At baseline, all patients underwent comprehensive clinical assessment, neurological examination, extensive neuropsychological investigation (as described in detail elsewhere [20]), and blood collection for measurement of plasma NfL concentration and apolipoprotein E (APOE) genotype analysis. We defined age at baseline as age at the time of plasma collection, disease duration as time from onset of symptoms to baseline examination, and positive family history of dementia as having one or more first‐degree relatives with documented cognitive decline. Renal function was categorized as either impaired or not impaired based on estimated glomerular filtration rate (eGFR; considered impaired if <60 mL/min/1.73 m2). eGFR was recorded only in patients with impaired renal function. A total of 110 patients (30 SCD, 60 MCI, 20 AD‐D) underwent cerebrospinal fluid (CSF) collection for Aβ42, Aβ42/Aβ40, total‐tau (t‐tau) and phosphorylated‐tau (p‐tau). Among these, 28 patients (16 SCD, 9 MCI, 3 AD‐D) also underwent cerebral amyloid positron emission tomography (PET), and 93 patients (23 SCD, 51 MCI, 19 AD‐D) also underwent 18F‐fluorodeoxyglucose‐PET brain scan (FDG‐PET). Normal values for CSF biomarkers were: Aβ42 > 670 pg/mL, Aβ42/Aβ40 ratio > 0.062, t‐tau < 400 pg/mL and p‐tau < 60 pg/mL [21]. Comparisons between patients who underwent amyloid‐PET and patients who did not are shown in Table S1.
Methods used for APOE genotyping, CSF collection and biomarker analysis, brain 18F‐FDG‐PET and amyloid‐PET acquisition and rating are described in further detail elsewhere [22, 23].
A total of 77 patients (30 with SCD and 47 with MCI) reached a follow‐up time of 2 years from blood collection and underwent a new neuropsychological examination. Blood samples were collected from 48 of these (19 SCD, 29 MCI) 2 years after baseline to repeat the NfL measure. Comparisons between patients who underwent NfL testing at follow‐up and patients who did not are reported in Table S1. Progression to MCI and to AD was defined according to the National Institute on Aging‐Alzheimer's Association (NIA‐AA) criteria [2, 19] by two trained neurologists (S.M. and V.B.) who were blinded to the plasma NfL results.
Standard protocol approvals
The study procedures and data analysis were performed in accordance with the Declaration of Helsinki and with the ethical standards of the Committee on Human Experimentation of our Institute. This study was approved by the Institutional Review Board of Careggi University Hospital (Florence, Italy, reference 15691oss). All individuals involved in this research agreed to participate and to have details and results of the research about them published.
Plasma collection and NfL analysis
Blood was collected by venipuncture into standard polypropylene EDTA test tubes (Sarstedt, Nümbrecht, Germany) and centrifuged within 2 h at 1300 rcf at 4°C for 10 min. Plasma was isolated and stored at −80°C until testing. Plasma NfL analysis was performed with Simoa NF‐Light SR‐X kit (cat. No. 103400) for human samples provided by Quanterix Corporation (Lexington, Massachusetts) on the automatized Simoa SR‐X platform (GBIO, Hangzhou, China), following the manufacturer's instructions. The lower limits of quantification and detection provided by the kit were 0.316 and 0.0552 pg/mL, respectively. More details about intra‐assay precision, control samples, inter‐assay precision and assay versions are described in Appendix S1. The plasma NfL concentrations in all samples were detected in a single run. Quality controls with a low NfL concentration of 5.08 pg/mL and a high NfL concentration of 169 pg/mL were included in the array and assessed with samples. A reference calibration curve was established, and duplicate measurements of serially diluted calibrators, 4×‐diluted controls, and samples were taken. The NfL assay results are consistent with the expected values, exhibiting a coefficient of variation below 20%.
Classification of patients according to the amyloid/tau/neurodegeneration classification
Based on biomarker results, patients were classified according to the NIA‐AA Research Framework [24] (amyloid/tau/neurodegeneration [A/T/N] system). Patients were rated as A+ if at least one of the amyloid biomarkers (CSF or amyloid PET) revealed the presence of Aβ pathology, and as A− if none of the biomarkers revealed the presence of Aβ pathology. In the case of discordant CSF and amyloid PET results, we considered only the pathological result. Patients were classified as T+ or T− if CSF p‐tau concentrations were higher or lower than the cut‐off value, respectively. Patients were classified as N+ if at least one neurodegeneration biomarker was positive (CSF t‐tau higher than the cut‐off value or positive FDG‐PET). Considering our sample size, to avoid too small groups, we considered the T and N parameters together as TN+ if they were T+ and/or N+, and TN− if both T and N were negative. Finally, we defined four groups: normal AD biomarkers (A−/T−/N−), non‐AD pathological change (A−/TN+), Alzheimer's pathological change (A+, including A+/T−/N− patients and one patient with A+/T−/N+), and AD (A+/T+/N+, including A+/TN+ and A+/T+/N+).
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics software version 25 (SPSS Inc., Chicago, Illinois) and the computing environment R4.2.3 (R Foundation for Statistical Computing, Vienna, 2013). All p values were two‐tailed and the significance level for all analyses was set at p = 0.05. Distributions of all variables were assessed using the Shapiro–Wilk test. As NfL was not normally distributed, we applied log10 transformation. This transformation resulted in a more normally distributed dataset that met the assumptions of the statistical tests that we planned to use. We conducted descriptive statistics using means and standard deviation for continuous variables and frequencies or percentages and 95% confidence intervals (CIs) for categorical variables. We used the t‐test for comparison between two groups, one‐way analysis of variance (ANOVA) with Bonferroni post hoc test for comparisons among three or more groups, Pearson's correlation coefficient to evaluate correlations between groups' numeric measures, and chi‐squared tests to compare categorical data. To adjust for possible confounding factors, we used analysis of covariance (ANCOVA). We constructed receiver‐operating characteristic curves to evaluate the performance of plasma NfL in predicting ATN status and progression of cognitive decline. We used the Youden method to determine the optimal cut‐off value for NfL and calculated accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). We used Kaplan–Meier survival analyses with pairwise log‐rank to compare proportions of progression of cognitive decline among groups. We used Cox regression analysis to ascertain that the effect of NfL on progression from SCD to MCI was independent from other covariates. The consistency of NfL measures over time was computed using the intraclass correlation coefficient (ICC). We used repeated‐measures ANOVA to investigate the effect of progression of cognitive decline and ATN status on the change in NfL concentration over time. We calculated the size effect using Cohen's d for normally distributed numeric measures, η 2 for ANOVA and Cramer's V for categorical data.
RESULTS
Comparisons between groups
Neurofilament light chain levels were significantly different between the SCD, MCI and AD‐D groups (F [2136] = 14.99, p < 0.001, η 2 = 0.181; Figure 1a). Demographic features and differences among groups are summarized in Table 1. A patient with MCI and two patients with AD had impaired renal function (eGFR 47.7 mL/min/1.73 m2, 58.3 mL/min/1.73 m2, 53.0 mL/min/1.73 m2), with no differences in terms of proportion of renal impairment among the SCD, MCI and AD‐D groups. NfL concentration was correlated with age at baseline (Pearson = 0.549, p < 0.001) and Mini‐Mental State Examination (MMSE) score (Pearson = −0.291, p = 0.001). There were no differences in NfL concentrations between male and female participants (p = 0.222) or between APOEε4+ and APOEε4− subgroups (p = 0.579). The significant effect of diagnosis group (SCD, MCI and AD‐D) on NfL concentration was confirmed after controlling for age, education, MMSE score and APOE genotype (F [2119] = 30.51, p = 0.033, partial η 2 = 0.056; Table S2a). As the SCD group included an outlier patient, we conducted the comparison again after excluding this case. The difference between the groups remained significant (F [2137] = 14.23, p < 0.001, η 2 = 0.174). Post hoc analysis confirmed the differences between SCD and MCI (p = 0.005), SCD and AD (p < 0.001), as well as between MCI and AD groups (p = 0.006).
FIGURE 1.
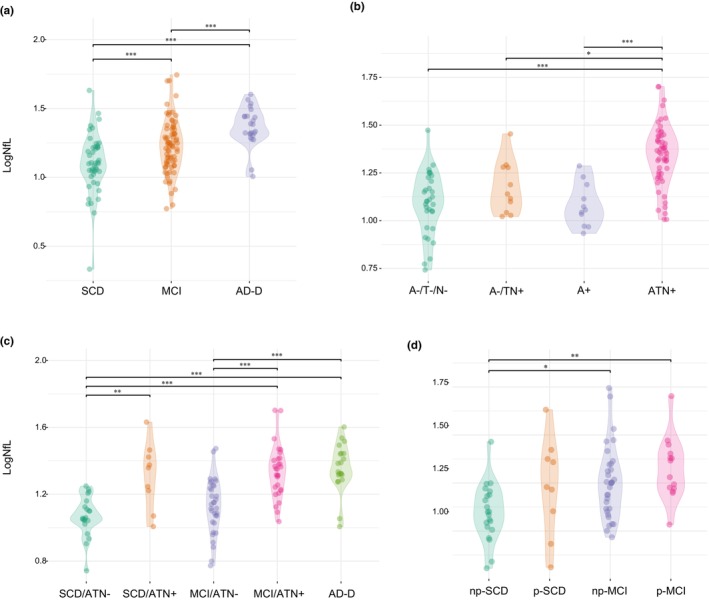
Log neurofilament light chain (logNfL) levels across groups. Values quoted in the y‐axis indicate LogNfL levels. Horizontal bars indicate significant differences between groups. (a) Comparisons between diagnosis groups: subjective cognitive decline (SCD) versus mild cognitive impairment (MCI; p = 0.002, Cohen's d = 0.671); SCD versus Alzheimer's disease (AD; p < 0.001, Cohen's d = 1.394); MCI versus AD (p = 0.010, Cohen's d = 0.723). (b) Comparisons between amyloid/tau/neurodegeneration (A/T/N) groups: A−/T−/N− versus A−/TN+ (p = 0.765, Cohen's d = 0.537); A−/T−/N− versus A+/T−/N− (p = 1.00, Cohen's d = 1.249); A−/T−/N− versus A+ (p < 0.001, Cohen's d = 1.562); A−/T−/N− versus ATN+ (p < 0.001, Cohen's d = 1.562); A−/TN+ versus A+ (p = 1.00, Cohen's d = 0.394); A−/TN+ versus ATN+ (p < 0.001, Cohen's d = 1.419); A+ versus ATN+ (p < 0.01, Cohen's d = 1.419). (c) Comparisons diagnosis/ATN groups: SCD/ATN− versus SCD/ATN+ (p = 0.003, Cohen's d = 1.571, MCI/ATN+ (p < 0.001, Cohen's d = 1.747); SCD/ATN− versus AD (p < 0.001, Cohen's d = 1.880); MCI/ATN− versus MCI/ATN+ (p < 0.001, Cohen's d = 1.343); MCI/ATN− versus AD (p < 0.001, Cohen's d = 1.476); SCD/ATN− versus MCI/ATN− (p = 1.00, Cohen's d = 0,447); SCD/ATN+ versus MCI/ATN+ (p = 1.00, Cohen's d = 0.176); MCI/ATN+ versus AD (p = 1.00, Cohen's d = 0.133). (d) Comparisons between progression groups: non‐progressive SCD (np‐SCD) versus progressive SCD (p‐SCD) (p = 0.428, Cohen's d = 0.729); np‐SCD versus non‐progressive MCI (np‐MCI) (p = 0.020, Cohen's d = 0.846); np‐SCD versus progressive MCI (p‐MCI) groups (p = 0.003, Cohen's d = 1.250); p‐SCD versus np‐MCI (p = 1.00, Cohen's d = 0.117), p‐SCD versus p‐MCI (p = 1.00, Cohen's d = 0.521); np‐MCI versus p‐MCI (p = 1.00, Cohen's d = 0.404).
TABLE 1.
Comparisons among diagnosis groups.
| SCD | MCI | AD | |
|---|---|---|---|
| N | 45 | 73 | 22 |
| Age at baseline | 66.43 (9.12) a , b | 71.26 (7.96) a | 72.64 (7.12) b |
| Disease duration | 4.61 (4.82) | 3.63 (2.63) | 3.85 (3.50) |
| Years of education | 12.37 (3.59) | 11.42 (4.33) | 9.71 (5.53) |
| MMSE score | 27.51 (2.37) c | 26.39 (2.11) d | 19.23 (4.74) c , d |
| Sex: female/male | 29/16 | 48/25 | 11/11 |
| Family history of AD | 73.81 (60.51: 7.11) | 63.64 (52.03: 75.24) | 52.94 (29.21: 76.67) |
| APOE ε4+ | 23.81 (10.93: 36.69) e | 39.73 (28.50: 50.95) | 61.90 (41.13: 82.68) e |
| Impaired renal function | 0 | 1 (1.37%) | 2 (9.09%) |
| LogNfL (pg/mL) | 1.11 (0.22) f , g | 1.21 (0.19) f ,h | 1.4 (0.12) g , h |
Note: Values quoted in the table are mean (standard deviation) for continuous variables and frequencies or percentages (95% confidence interval) for dichotomic variables. Between‐group comparisons: analysis of covariance with Bonferroni post hoc. Categorical data comparisons: χ 2 test. Size effect: Cohen's d for continuous measures, Cramer's V for categorical data. Statistical significance indicated by p < 0.05.
Abbreviations: AD, Alzheimer's disease; APOE, apolipoprotein E; MCI, mild cognitive impairment; MMSE, Mini‐Mental State Examination; NfL, neurofilament light chain; SCD, subjective cognitive decline.
p = 0.004, Cohen's d = 0.615.
p = 0.023, Cohen's d = 0.702.
p = 0.029, Cohen's d = 0.511.
p < 0.001, Cohen's d = 2.829.
χ 2 = 8.77, p = 0.003, Cramer's V = 0.47.
p = 0.002, Cohen's d = 0.671.
p < 0.001, Cohen's d = 1.394.
p = 0.010, Cohen's d = 0.723.
Biomarker profiles
Based on AD biomarker results, patients were classified as follows: 33 (30.0%) A−/T−/N− (14 SCD, 19 MCI); 11 (10.0%) A−/TN+ (3 SCD, 8 MCI); 12 (10.9%) A+ (4 SCD, 7 MCI, 1 AD); and 54 (49.1%) ATN+ patients (9 SCD, 26 MCI, 19 AD). Demographic features and differences among the groups are summarized in Table 2.
TABLE 2.
Comparisons among amyloid/tau/neurodegeneration groups.
| A−/T−/N− | A−/TN+ | A+ | ATN+ | |
|---|---|---|---|---|
| N | 33 | 11 | 12 | 53 |
| Age at baseline, years | 63.71 (8.47) a | 69.61 (7.35) | 66.73 (8.04) | 72.08 (5.98) a |
| Disease duration, years | 4.71 (4.41) | 3.68 (2.87) | 5.06 (4.69) | 3.43 (3.08) |
| Years of education | 12.33 (3.89) | 11.82 (4.58) | 11.25 (4.75) | 12.00 (4.56) |
| MMSE score | 27.10 (2.26) b | 26.54 (1.48) | 25.14 (2.98) | 23.95 (4.14) b |
| Sex: female/male | 23/10 | 6/5 | 8/4 | 29/24 |
| Family history of AD | 69.70 (54.02: 85.38) | 72.73 (46.41: 99.05) | 50.00 (21.71: 78.29) | 50.94 (37.48: 64.40) |
| APOE ε4+ | 33.33 (17.25: 49.42) f | 9.09 (0.00: 26.08) g | 33.33 (6.66: 60.01) | 61.54 (48.32: 74.76) f , g |
| Impaired renal function | 0 | 1 (11.11%) | 0 | 2 (14.29%) |
| LogNfL (pg/mL) | 1.09 (0.16) c | 1.18 (0.14) d | 1.11 (0.15) e | 1.34 (0.16) c , d , e |
Note: Values quoted in the table are mean (standard deviation) for continuous variables and frequencies or percentages (95% CI) for dichotomic variables. Between‐groups comparisons: ANOVA with Bonferroni post‐hoc. Categorical data comparisons: χ 2 test. Size effect: Cohen's d for continuous measures, Cramer's V for categorical data. Statistical significance accepted at the p < 0.05.
Abbreviations: AD, Alzheimer's disease; APOE, apolipoprotein E; A/T/N, amyloid/tau/neurodegeneration; MMSE, Mini‐Mental State Examination; NfL, neurofilament light chain.
p < 0.001, Cohen's d = 1.162.
p < 0.001, Cohen's d = 0.949.
p < 0.001, Cohen's d = 1.563.
p = 0.015, Cohen's d = 1.026.
p = 0.001, Cohen's d = 1.419.
χ 2 = 6.42, p = 0.011, Cramer's V = 0.275;
χ 2 = 10.01, p = 0.002, Cramer's V = 0.399.
Detailed distribution of CSF biomarker concentrations and frequencies of positive amyloid‐PET and FDG‐PET as well as comparisons between groups are reported in Table S3. A detailed description of the methods used to assess Aβ pathology is reported in Table S4. Concordant and discordant results between CSF and PET are shown in Table S5.
Distribution of NfL concentration across ATN groups
Neurofilament light chain levels differed among the ATN groups, also after adjusting for age, education, MMSE and APOE (F [3, 96] = 7.21, p < 0.001, η 2 = 0.184; Table S2b). Post hoc analysis showed that ATN+ had higher NfL levels than A−/T−/N− (p < 0.001, d = 1.562), A−/TN+ (p = 0.015, d = 1.026) and A+ (p = 0.001, d = 1.419). There was no difference between A−/T−/N− and A−/TN+ (p = 0.765, d = 0.537), between A−/T−/N− and A+ (p = 1.00, d = 0.143) or between A−/TN+ and A+ (p = 1.00, d = 0.394; Figure 1b). Based on these results, for subsequent analysis, we merged A−/T−/N−, A−/TN+, and A+ into an ATN− group.
Distribution of NfL concentration across diagnosis/ATN subgroups
To explore the interaction between diagnosis and ATN group on NfL concentration, we classified patients according to diagnosis (SCD, MCI, AD‐D) and ATN classification (ATN−, ATN+). As only one AD‐D patient was ATN−, we did not split the AD‐D group. The groups consisted of 21 SCD/ATN−, nine SCD/ATN+, 33 MCI/ATN−, 27 MCI/ATN+, and 20 AD‐D patients.
The SCD/ATN− patients were younger than the SCD/ATN+ patients (61.46 ± 6.92 vs. 71.06 vs. 7.63; p = 0.002). MCI/ATN− patients were younger (67.95 ± 8.49 vs. 73.38 ± 5.56; p = 0.004) and had lower frequencies of APOEε4+ (37.50% vs. 66.67%, χ 2 = 9.31; p = 0.002) than MCI/ANT+ patients. There were no differences in neuropsychological scores between SCD/ATN− and SCD/ATN+ patients or between MCI/ATN− and MCI/ATN+ patients (Table S6). NfL levels were significantly different between the diagnosis/ATN subgroups (F [5103] = 13.50, p < 0.001, η 2 = 0.396). Differences in NfL concentration among the groups were also confirmed after controlling for age, MMSE score, MMSE score and APOE (F [4, 95] = 6.95, p < 0.001, partial η 2 = 0.226; Table S2c). Post hoc analysis showed that SCD/ATN− patients had lower NfL concentrations than SCD/ATN+ (p = 0.003, d = 1.571), MCI/ATN+ (p < 0.001, d = 1.747) and AD‐D patients (p < 0.001, d = 1.880). MCI/ATN− had lower NfL concentrations than MCI/ATN+ (p < 0.001, d = 1.343) and AD‐D patients (p < 0.001, d = 1.476). There were no differences between SCD/ATN− and MCI/ATN− (p = 1.00, d = 0.447) or between SCD/ATN+, MCI/ATN+, and AD‐D patients (p = 1.00, d = 0.133; Figure 1c).
Accuracy of plasma NfL in predicting ATN status
Neurofilament light chain showed good accuracy in distinguishing between ATN+ and ATN− patients in the SCD and MCI groups (area under the curve [AUC] 0.815 and 0.818, respectively). In the SCD group, a cut‐off of 19.45 pg/mL yielded the maximum Youden index and discriminated ATN− and ATN+ patients with excellent specificity (95.24% [95% CI 87.62: 100]), good PPV and NPV (83.33% [95% CI 70.00: 96.67]) but failed in sensitivity (55.56% [95% CI 37.77: 73.34]). Similarly, in the MCI group, a cut‐off of 20.49 pg/mL had excellent specificity (93.94% [95% CI 87.90: 99.98]), with very good PPV, fair NPV and poor sensitivity (62.96% [95% CI 50.74: 75.18]). Finally, when we merged SCD and MCI, a cut‐off of 20.03 pg/mL yielded the maximum Youden index, discriminating ATN− and ATN+ patients with excellent specificity (94.44% [95% CI 89.71: 99.18]), good PPV, fair NPV (78.46% [95% CI 69.97: 86.95]) and poor sensitivity (61.1% [95% CI 51.04: 71.18]; Figure 2).
FIGURE 2.
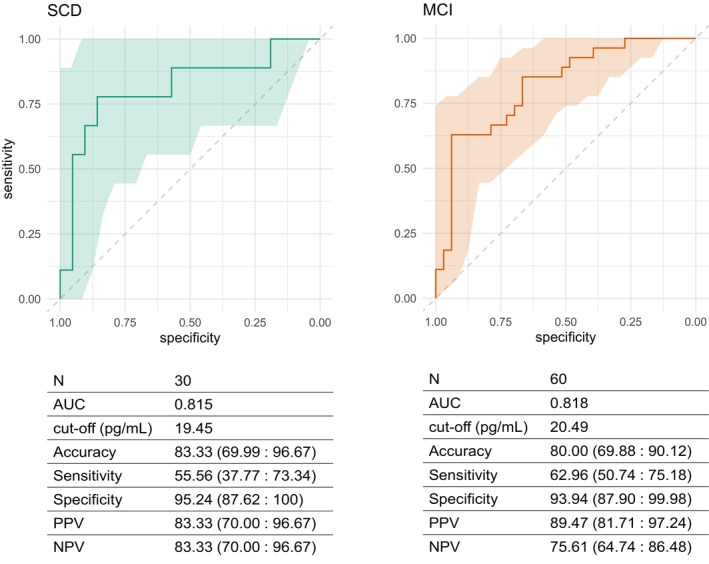
Neurofilament light chain (NfL) accuracy in predicting amyloid/tau/neurodegeneration (A/T/N) status. Receiver‐operating characteristic curves for accuracy of NfL in distinguishing ATN− and ATN+ groups in SCD and MCI. Colored shapes indicate 95% confidence interval (CI). Cut‐off values estimated by Youden's method. Accuracy, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) are expressed as percentages (95% CI). AUC, area under the curve.
Trajectories of cognitive decline over time and comparison between progression groups
During follow‐up, nine (30%) SCD patients progressed to MCI and were classified as p‐SCD. Fourteen (29.79%) MCI patients developed dementia (p‐MCI). None of the SCD patients developed dementia. Patients who did not progress were classified as np‐SCD (21, 70.00%) and np‐MCI (33, 70.21%). The p‐MCI group had a higher frequency of APOEε4+ (p = 0.037, V = 0.244) and lower MMSE scores (p = 0.002, d = 1.239; Table 3) and in two tests for verbal memory (Table S7). There were no differences between the np‐SCD and p‐SCD groups at baseline in demographic features, APOEε4+, MMSE scores, or neuropsychological scores. Baseline NfL levels were significantly different between patients who progressed and patients who did not progress from SCD to MCI or from MCI to dementia (F [5103] = 5.06, p = 0.003, η 2 = 0.172). Post hoc analysis showed that the np‐SCD group had lower NfL concentration than the np‐MCI (p = 0.020, d = 0.846) and p‐MCI groups (p = 0.003, d = 1.250; Figure 1d).
TABLE 3.
Comparisons among progression groups.
| np‐SCD | p‐SCD | np‐MCI | p‐MCI | |
|---|---|---|---|---|
| N | 21 | 9 | 33 | 14 |
| Age at baseline, years | 66.44 (6.60) | 68.20 (10.52) | 71.04 (7.62) | 73.57 (6.73) |
| Disease duration, years | 4.76 (5.97) | 4.24 (4.95) | 3.56 (2.47) | 2.84 (1.96) |
| Years of education | 12.29 (4.23) | 12.33 (3.32) | 10.97 (4.11) | 12.00 (4.09) |
| MMSE score | 27.61 (4.42) a | 27.01 (2.42) b | 26.61 (1.86) c | 23.81 (3.05) a , b , c |
| Sex: female/male | 12/9 | 7/2 | 21/12 | 10/4 |
| Family history of AD | 61.90 (41.13: 82.68) | 77.78 (50.62: 100) | 57.58 (40.71: 74.44) | 35.71 (10.61: 60.81) |
| APOE ε4+ | 20.00 (2.47: 37.53) f | 50.00 (15.35: 84.65) | 27.27 (12.08: 42.47) g | 64.29 (39.19: 89.39) f , g |
| Impaired renal function | 0 | 0 | 2 (6.06%) | 0 |
| LogNfL (pg/mL) | 1.10 (0.15) d | 1.23 (0.26) | 1.25 (0.19) e | 1.32 (0.16) d , e |
Note: Values quoted in the table are mean (standard deviation) for continuous variables and frequencies or percentages (95% CI) for dichotomic variables. Between‐group comparisons: analysis of variance with Bonferroni post hoc. Categorical data comparisons: χ 2 test. Size effect: Cohen's d for continuous measures, Cramer's V for categorical data. Statistical significance accepted at the p < 0.05.
Abbreviations: APOE, apolipoprotein E; MMSE, Mini‐Mental State Examination; NfL, neurofilament light chain; np‐SCD, non‐progressive SCD; p‐SCD, progressive‐SCD; np‐MCI, non‐progressive MCI; p‐MCI, progressive MCI.
p = 0.003, Cohen's d = 1.14.
p = 0.020, Cohen's d = 1.16.
p = 0.006, Cohen's d = 1.11.
p = 0.020, Cohen's d = 0.846.
p = 0.003, Cohen's d = 1.250.
χ 2 = 10.040, p = 0.002, Cramer's V = 0.457.
χ 2 = 4.36, p = 0.037, Cramer's V = 0.244.
Effect of NfL group on risk of progression of cognitive decline
We classified patients according to the previously identified cut‐off values (NfL− = lower than cut‐off values; NfL+ = higher than cut‐off value): seven (15.56%) SCD patients had NfL concentrations higher than 19.45 pg/mL and 23 (31.51%) MCI patients had NfL concentrations higher than 20.49 pg/mL. A Kaplan–Meier survival analysis showed a higher proportion of progression from SCD to MCI in the SCD/NfL+ group (80.00%) as compared to SCD/NfL− (22.73%; log‐rank χ 2 = 9.79, p = 0.002). There was no difference in the distribution of progression from MCI to AD dementia (log‐rank χ 2 = 5.32, p = 0.25; Figure 3). To evaluate the effect of dichotomized NfL on the rate of conversion from SCD to MCI, adjusting for possible confounding factors, we performed Cox proportional hazards regression analysis, considering progression time as time and including age at baseline, years of education and APOE genotype as covariates (Table 4). The regression model was significant (χ 2 = 9.702, p = 0.002) and NfL group was the only significant variable (p = 0.007, hazard ratio 7.10).
FIGURE 3.

Kaplan–Meier survival analysis for comparison of distributions of progression from SCD to MCI and from MCI to AD between neurofilament light chain (NfL)− and NfL+ groups. For patients who progressed, follow‐up time indicates the time of progression. Number at risk and p values for pairwise log‐rank comparisons between groups are reported. Colored shapes indicate 95% confidence interval.
TABLE 4.
Cox's proportional hazards regression analysis.
| B | Wald | p | HR | 95% CI (min: max) | |
|---|---|---|---|---|---|
| Age at baseline | −0.032 | 0.314 | 0.575 | 0.969 | 0.867: 1.082 |
| Years of education | −0.046 | 0.086 | 0.769 | 0.955 | 0.702: 1.299 |
| MMSE score | 0.023 | 0.017 | 0.897 | 1.023 | 0.721: 1.452 |
| APOE | 0.595 | 0.421 | 0.516 | 1.814 | 0.301: 10.944 |
| NfL group | 1.992 | 6.121 | 0.013 | 7.328 | 1.513: 35.506 |
| Regression model: χ 2 = 9.702, p = 0.002 | |||||
Note: Regression coefficients (B), Wald coefficient, p value (p), hazard ratio (HR) and 95% confidence Intervals (CI) for covariates included in the model, and χ 2 and significance of the model are reported (significant differences at p < 0.05).
Abbreviations: APOE, apolipoprotein E; MMSE, Mini‐Mental State Examination; NfL, neurofilament light chain.
Accuracy of plasma NfL in predicting progression of cognitive decline
In the SCD group, the cut‐off of 19.45 pg/mL showed good accuracy (80.00% [95% CI 65.69: 94.31]) with excellent specificity (95.24% [95% CI 87.62: 100]), good PPV and NPV (80.00% [95% CI 65.69: 94.31]), but not acceptable sensitivity (44.44% [95% CI 26.66: 62.23]) in predicting progression to MCI. In the MCI group, NfL, with the cut‐off value of 20.49 pg/mL, had fair specificity (70.97% [95% CI 57.71: 84.23]) and NPV (75.86% [95% CI 63.63−:88.10]), but not acceptable sensitivity and PPV (≤50%) in predicting progression to dementia (Table 5).
TABLE 5.
Accuracy of neurofilament light chain concentration at baseline and rate of change in predicting the progression of cognitive decline.
| SCD | MCI | Rate of change (MCI) | |
|---|---|---|---|
| N | 30 | 47 | 29 |
| Cut‐off, pg/mL | 19.45 | 20.45 | 1.64 |
| Accuracy, % (95% C.I.) | 80.00 (65.69: 94.31) | 64.44 (50.46: 78.43) | 92. 86 (83.32: 100) |
| Sensitivity, % (95% C.I.) | 44.44 (26.66: 62.23) | 50.00 (35.39: 64.61) | 100.00 |
| Specificity, % (95% C.I.) | 95.24 (87.62: 100) | 70.97 (57.71: 84.23) | 91.30 (80.87: 10) |
| PPV, % (95% C.I.) | 80.00 (65.69: 94.31) | 43.75 (29.26: 58.24) | 71.43 (54.70: 88.16) |
| NPV, % (95% C.I.) | 80.00 (65.69: 94.31) | 75.86 (63.63: 88.10) | 100 |
Note: Cut‐off values estimated by Youden's method. Accuracy, sensitivity, specificity, PPV and NPV are expressed as percentages (95% confidence interval).
Abbreviations: C.I., confidence interval; MCI, mild cognitive impairment; NPV, negative predictive value; PPV, positive predictive value; SCD, subjective cognitive decline.
Change of NfL concentration over time
Forty‐eight patients (19 SCD, 29 MCI) underwent new blood collection for NfL measurement 2 years (T2) after baseline collection (T1). NfL measures were highly consistent over time (ICC = 0.84 [95% CI 0.73: 0.91]; p < 0.001). Considering the whole sample, the mean NfL change (ΔNfL) was 1.13 ± 5.47 pg/mL in 2 years (0.71 ± 2.98 pg/mL per year), with no differences between SCD and MCI. ΔNfL was correlated with age at baseline (Pearson = 0.341, p = 0.017), while there was no effect of disease duration, education, sex, family history of AD dementia, or APOE genotype on NfL change.
Effect of ATN status and progression of cognitive decline on NfL over time
A repeated‐measures ANOVA showed a significant interaction between change in NfL and progression of cognitive decline (F [3, 44] = 5.2, p = 0.032, η 2 = 0.014), confirmed also after age‐adjustment (F [3, 41] = 4.28, p = 0.010, η 2 = 0.239; Table S2d). Post hoc analysis showed that this effect was significant between np‐SCD and p‐MCI (t = −4.32, p < 0.001) and between np‐MCI and p‐MCI (t = −2.93, p = 0.033; Figure 4a). In particular, NfL concentration showed a change of 1.63 ± 2.50 pg/mL (0.81 ± 1.25 pg/mL per year) and of −1.39 ± 3.88 pg/mL (−0.13 ± 3.24 pg/mL per year) in np‐SCD and np‐MCI respectively, and an increase of 7.05 ± 8.12 pg/mL (3.52 ± 4.06 pg/mL per year) in p‐MCI. The effect of ATN status on NfL change did not reach significance (F [1, 31] = 2.80, p = 0.056, η 2 = 0.023). Nevertheless, when performing a post hoc analysis considering the ATN groups (A−/T−/N−, A−/TN+, A+, and ATN+), we found a different effect on NfL change between A+ and ATN+ (t = −3.15, p = 0.024; Figure 4b). In particular, NfL showed a decrease of −1.94 ± 3.32 pg/mL (−0.97 ± 1.66 pg/mL per year) in the A+ group and an increase of 3.07 ± 7.21 pg/mL (1.53 ± 3.60 pg/mL per year) in the ATN+ group.
FIGURE 4.
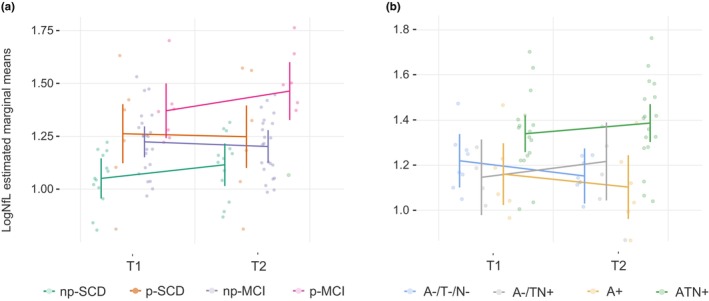
Change in log neurofilament light chain (logNfL) distribution by progression and ATN groups. T1 and T2 indicate the first and second blood collection for plasma NfL measurement. (a) Effect of cognitive decline progression on logNfL change: F [3,44] = 5.2, p = 0.032, η 2 = 0.014. Post hoc analysis: non‐progressive SCD (np‐SCD) versus progressive SCD (p‐SCD) (mean difference = −0.18, p = 0.214); np‐SCD versus non‐progressive MCI (np‐MCI) (mean difference = −0.13, p = 0.223); np‐SCD versus progressive MCI (p‐MCI) (mean difference = −0.34, p < 0.001); p‐SCD versus np‐MCI (mean difference = 0.05, p = 1.00); p‐SCD versus p‐MCI (mean difference = −0.16, p = 1.00); np‐MCI versus p‐MCI (mean difference = −0.21, p = 0.033). (b) Effect of amyloid/tau/neurodegeneration (A/T/N) on NfL change: F [1,31] = 2.80, p = 0.056, η 2 = 0.213). Post hoc analysis: A−/T−/N− versus A−/TN+ (mean difference = 0.00, p = 1.00); A−/T−/N− versus A+ (mean difference = 0.05, p = 1.00); A−/T−/N− versus ATN+ (mean difference = −0.18, p = 0.077); A−/TN+ versus A+ (mean difference = 0.05, p = 1.00); A−/TN+ versus ATN+ (mean difference = −0.18, p = 0.269); A+ versus ATN+ (mean difference = −0.23, p = 0.024).
Diagnostic accuracy of NfL change rate in predicting the progression from MCI to AD dementia
We tested the accuracy of NfL change rate (ΔNfL/per year) in distinguishing between np‐MCI and p‐MCI: a cut‐off of 1.64 pg/mL per year showed the highest Youden index, providing very good accuracy (92.86% [95% CI 83.32: 100], AUC 0.954) with very high sensitivity (100%), specificity (91.30% [95% CI 80.87: 100]) and NPV (100%) in distinguishing between np‐MCI and p‐MCI. Also in this case, PPV was fair (71.43% [95% CI 54.70: 88.16]). We did not perform the same analysis in the SCD group because the repeated‐measures ANOVA did not show a significant effect of progression on NfL change in this group.
DISCUSSION
We showed that SCD and MCI patients with biomarkers consistent with AD had higher plasma NfL concentration than patients with normal biomarkers or with isolated amyloid pathology or with suspected non‐AD pathology. We identified cut‐off values to distinguish ATN− and ATN+ with very good accuracy (19.45 and 20.49 pg/mL in SCD and MCI, respectively). These cut‐off values were very close to the cut‐off value (20 pg/mL) identified by Simrén et al. in a large cohort of healthy individuals [25]. In particular, we demonstrated excellent performance in identifying patients with SCD and MCI not associated with AD. However, the ability of plasma NfL to detect patients with AD was poor.
Additionally, we observed that differences in NfL levels among the SCD, MCI and AD groups ceased to be apparent when we stratified each diagnostic group based on ATN status. Notably, the concentration of NfL in SCD and MCI patients within the ATN+ group did not differ from patients with dementia due to AD. This suggests that these differences between SCD, MCI and AD were not driven by cognitive levels but rather by the underlying pathological substrate, as proposed by Giacomucci et al. [26]. This finding is particularly interesting for SCD patients and is in line with longitudinal studies showing that blood NfL levels increase more than a decade before the onset of clinical manifestations in carriers of amyloid precursor protein (APP), presenilin 1 (PSEN1) and presenilin 2 (PSEN2) mutations [17].
We then tested whether the identified cut‐off values were also able to detect progression from SCD to MCI and from MCI to AD. In this case, NfL exhibited a high prognostic performance in excluding progression from SCD to MCI over a span of 2 years. However, it demonstrated a lack of sensitivity in identifying patients who would progress to either MCI or AD.
Finally, we explored the change in NfL concentrations over time. Overall, NfL concentration increased by 0.71 pg/mL per year, in line with previous reports [27]. We showed that the rate of increase was higher in patients who progressed from MCI to AD dementia compared to patients who did not progress. In particular, the rate of increase in MCI patients who progressed to dementia was approximately 3.5 times higher than in MCI patients who remained stable, consistent with a previous report by de Wolf et al. [18] Moreover, we showed that an increase lower than 1.64 pg/mL per year can exclude progression from MCI to AD with high accuracy, in line with a previous study that showed that the rate of change of serum NfL was able to discriminate carriers of a mutation in APP, PSEN1 or PSEN2 genes from non‐carriers [17].
Although the ANOVA comparing NfL changes among ATN groups revealed only a suggestive trend towards significance (likely due to the limited sample size), post hoc analysis demonstrated that the rate of increase was more pronounced in patients with ATN+ status compared to those with isolated A+. A similar trend towards significance was also observed when comparing NfL changes between patients with normal AD biomarkers and those with ATN+ status. Specifically, NfL levels increased in both the ATN+ and A−/TN+ groups, while they decreased in the A−/T−/N− and A+ groups. In future studies, an expansion of our sample size will enable us to elucidate the interplay between pathological substrates and changes in NfL levels, thus providing valuable insights for the interpretation of repeated NfL measurements.
The high specificity of NfL in distinguishing between ATN+ and ATN− and between SCD patients who progressed and those who did not progress to MCI might appear to be in contrast to the general assumption that NfL is a highly sensitive but poorly specific biomarker [28]. Indeed, previous studies showed low accuracy of NfL when distinguishing AD from other neurological diseases [29]. By contrast, when compared to cognitively healthy individuals, NfL was shown to be the most accurate blood‐based biomarker [29]. Therefore, our results may suggest that, if applied to unimpaired patients complaining of memory decline (in which other possible non‐degenerative causes have been ruled out by first‐line assessments), plasma NfL may be highly suggestive of underlying AD.
It should be noted that patients with isolated Aβ biomarker positivity had the same NfL levels at baseline and showed the same change of NfL over years as patients with normal AD biomarkers, in line with previous reports [15, 16, 26] and with the hypothesis raised by Benedet et al. [30] that NfL concentration increases when Aβ pathology and tauopathy are associated. This evidence may have a relevant clinical implication in terms of the risk of AD and progression to dementia. Indeed, although part of the AD continuum, isolated Aβ pathology is not sufficient to define AD [3] and is associated with the lowest risk of AD dementia [31]. In this regard, it would also be interesting to conduct a more in‐depth investigation into whether the distribution of NfL in this particular group is correlated with cognitive status. Unfortunately, because our current sample size was limited, we were unable to perform a comprehensive analysis in this regard. Nonetheless, we have aspirations to delve into this aspect more extensively in our future studies.
Our study has several limitations. Firstly, the follow‐up time was relatively short, and only a subgroup of patients reached the 2‐year follow‐up mark for a new neuropsychological examination. Consequently, the group of patients who progressed from SCD to MCI was notably smaller than the group of patients who remained stable, in accordance with rates of progression reported in previous works [32, 33], and potentially introducing a bias that could impact our results. We intend to prolong the follow‐up duration to confirm the pattern we have currently identified. Secondly, we did not include a sample of healthy controls. Thirdly, our study protocol did not encompass factors that are recognized to influence NfL; specifically, we did not collect data on body mass index, and glomerular filtration rate was reported only for patients with impaired renal function. Furthermore, since there were only three patients with renal impairment, we refrained from conducting a comparison of NfL levels between individuals with impaired renal function and those without. Fourthly, tau pathology was assessed only through CSF p‐tau, potentially leading to an underestimation of positive T frequencies. Lastly, not all the patients underwent amyloid‐PET at baseline and NfL measurement at follow‐up. Specifically, these final two points could potentially introduce significant selection biases. To mitigate this potential bias, we conducted an analysis that demonstrated no notable differences between patients who underwent amyloid‐PET and those who did not, as well as between patients who received NfL measurements during follow‐up and those who did not. However, we recognize that this does present a notable limitation of our study. In future research, we intend to address this limitation by conducting the necessary assessments that were initially missing.
Strengths of the study include the assessment of Aβ by both CSF Aβ42 and Aβ42/40 and amyloid‐PET, as well as the assessment of neurodegeneration by CSF total‐tau and FDG‐PET. In addition, this is one of the few studies [26, 31] that tested plasma NfL in an SCD cohort. Notably, while most studies assessed the accuracy of NfL in detecting Aβ pathology [15, 16], we classified patients according to ATN status, also considering tau pathology and neurodegeneration biomarkers. Moreover, follow‐up data were used to validate the performance of the identified cut‐off value also in predicting progression to MCI and dementia. Finally, our study explores one of the main advantages of blood‐based biomarkers: as they are non‐invasive, their measurement can be repeated many times. In this sense, our study adds useful information, showing that the NfL trajectory may accurately distinguish between patients who will progress to dementia and patients who will not.
In conclusion, our results have potential implications for the clinical management of patients with SCD and MCI. Individuals with negative NfL levels had a lower risk of being carriers of AD and not progressing to MCI or dementia. Therefore, they may require monitoring for cognitive decline and investigation of other causes of cognitive decline. In contrast, patients with NfL levels exceeding the cut‐off value had a higher risk of AD and cognitive decline progression. In the era of disease‐modifying therapies, this may enable earlier identification of patients suitable for treatment in the earliest stages of the disease.
AUTHOR CONTRIBUTIONS
Salvatore Mazzeo: Conceptualization; investigation; writing – original draft; methodology; software; formal analysis; project administration; data curation. Assunta Ingannato: Investigation; validation; data curation; visualization. Giulia Giacomucci: Data curation; visualization; investigation. Alberto Manganelli: Data curation; visualization. Valentina Moschini: Data curation; investigation; visualization. Juri Balestrini: Data curation; investigation; visualization. Arianna Cavaliere: Investigation; visualization; data curation. Carmen Morinelli: Data curation; visualization; investigation. Giulia Galdo: Investigation; visualization; data curation. Filippo Emiliani: Data curation; visualization; investigation. Diletta Piazzesi: Visualization; data curation. Chiara Crucitti: Data curation; visualization; investigation. Daniele Frigerio: Investigation; visualization; data curation. Cristina Polito: Visualization; investigation. Valentina Berti: Visualization; investigation; resources. Silvia Bagnoli: Conceptualization; investigation; methodology; validation; visualization; resources. Sonia Padiglioni: Conceptualization; investigation; methodology; visualization; data curation. Sandro Sorbi: Resources; visualization; supervision. Benedetta Nacmias: Supervision; resources; funding acquisition; validation. Valentina Bessi: Conceptualization; investigation; funding acquisition; methodology; visualization; project administration; supervision; resources.
FUNDING INFORMATION
This project was funded by Tuscany Region (PRedicting the EVolution of SubjectIvE Cognitive Decline to Alzheimer's Disease With machine learning – PREVIEW – CUP. D18D20001300002) and by Ministry of Health (Alzheimer's and Dementia Fund – 22 ALDE).
CONFLICT OF INTEREST STATEMENT
All the authors declared that they have no conflicts of interest relevant to this work.
Supporting information
Appendix S1
Table S1–S7
Mazzeo S, Ingannato A, Giacomucci G, et al. Plasma neurofilament light chain predicts Alzheimer's disease in patients with subjective cognitive decline and mild cognitive impairment: A cross‐sectional and longitudinal study. Eur J Neurol. 2024;31:e16089. doi: 10.1111/ene.16089
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement. 2014;10:844‐852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jack CR, Bennett DA, Blennow K, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cummings J, Zhou Y, Lee G, Zhong K, Fonseca J, Cheng F. Alzheimer's disease drug development pipeline: 2023. Alzheimer's Dement. 2023;9:e12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Margolis SA, Kelly DA, Daiello LA, et al. Anticholinergic/sedative drug burden and subjective cognitive decline in older adults at risk of Alzheimer's disease. J Gerontol A Biol Sci Med Sci. 2021;76:1037‐1043. [DOI] [PubMed] [Google Scholar]
- 6. Gafson AR, Barthélemy NR, Bomont P, et al. Neurofilaments: neurobiological foundations for biomarker applications. Brain. 2020;143:1975‐1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019;90:870‐881. [DOI] [PubMed] [Google Scholar]
- 8. Rissin DM, Kan CW, Campbell TG, et al. Single‐molecule enzyme‐linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat Biotechnol. 2010;28:595‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bangen KJ, Thomas KR, Weigand AJ, et al. Elevated plasma neurofilament light predicts a faster rate of cognitive decline over 5 years in participants with objectively‐defined subtle cognitive decline and MCI. Alzheimers Dement. 2021;17:1756‐1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mattsson N, Andreasson U, Zetterberg H, Blennow K. For the Alzheimer's disease neuroimaging initiative. Association of Plasma Neurofilament Light with Neurodegeneration in patients with Alzheimer disease. JAMA Neurology. 2017;74:557‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Götze K, Vrillon A, Bouaziz‐Amar E, et al. Plasma neurofilament light chain in memory clinic practice: evidence from a real‐life study. Neurobiol Dis. 2023;176:105937. [DOI] [PubMed] [Google Scholar]
- 12. Sarto J, Ruiz‐García R, Guillén N, et al. Diagnostic performance and clinical applicability of blood‐based biomarkers in a prospective memory clinic cohort. Neurology. 2023;100:e860‐e873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Planche V, Bouteloup V, Pellegrin I, et al. Validity and performance of blood biomarkers for Alzheimer disease to predict dementia risk in a large clinic‐based cohort. Neurology. 2023;100:e473‐e484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Silva‐Spínola A, Lima M, Leitão MJ, et al. Blood biomarkers in mild cognitive impairment patients: relationship between analytes and progression to Alzheimer disease dementia. Eur J Neurol. 2023;30:1565‐1573. [DOI] [PubMed] [Google Scholar]
- 15. Tosun D, Veitch D, Aisen P, et al. Detection of β‐amyloid positivity in Alzheimer's disease neuroimaging initiative participants with demographics, cognition, MRI and plasma biomarkers. Brain Commun. 2021;3:fcab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chatterjee P, Pedrini S, Ashton NJ, et al. Diagnostic and prognostic plasma biomarkers for preclinical Alzheimer's disease. Alzheimers Dement. 2022;18:1141‐1154. [DOI] [PubMed] [Google Scholar]
- 17. Preische O, Schultz SA, Apel A, et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer's disease. Nat Med. 2019;25:277‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Wolf F, Ghanbari M, Licher S, et al. Plasma tau, neurofilament light chain and amyloid‐β levels and risk of dementia; a population‐based cohort study. Brain. 2020;143:1220‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mazzeo S, Lassi M, Padiglioni S, et al. PRedicting the EVolution of SubjectIvE cognitive decline to Alzheimer's disease with machine learning: the PREVIEW study protocol. BMC Neurol. 2023;23:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alcolea D, Pegueroles J, Muñoz L, et al. Agreement of amyloid PET and CSF biomarkers for Alzheimer's disease on Lumipulse. Ann Clin Transl Neurol. 2019;6:1815‐1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bessi V, Balestrini J, Bagnoli S, et al. Influence of ApoE genotype and clock T3111C interaction with cardiovascular risk factors on the progression to Alzheimer's disease in subjective cognitive decline and mild cognitive impairment patients. J Pers Med. 2020;10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mazzeo S, Polito C, Padiglioni S, et al. Linguistic profiles, brain metabolic patterns and rates of amyloid‐β biomarker positivity in patients with mixed primary progressive aphasia. Neurobiol Aging. 2020;96:155‐164. [DOI] [PubMed] [Google Scholar]
- 24. Jack CR, Bennett DA, Blennow K, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Simrén J, Andreasson U, Gobom J, et al. Establishment of reference values for plasma neurofilament light based on healthy individuals aged 5–90 years. Brain Commun. 2022;4:fcac174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Giacomucci G, Mazzeo S, Bagnoli S, et al. Plasma neurofilament light chain as a biomarker of Alzheimer's disease in subjective cognitive decline and mild cognitive impairment. J Neurol. 2022;269:4270‐4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khalil M, Pirpamer L, Hofer E, et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat Commun. 2020;11:812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mattsson N, Andreasson U, Zetterberg H, Blennow K, Alzheimer's Disease Neuroimaging Initiative . Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74:557‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Benussi A, Cantoni V, Rivolta J, et al. Classification accuracy of blood‐based and neurophysiological markers in the differential diagnosis of Alzheimer's disease and frontotemporal lobar degeneration. Alzheimers Res Ther. 2022;14:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Benedet AL, Leuzy A, Pascoal TA, et al. Stage‐specific links between plasma neurofilament light and imaging biomarkers of Alzheimer's disease. Brain. 2020;143:3793‐3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ebenau JL, Timmers T, Wesselman LMP, et al. ATN classification and clinical progression in subjective cognitive decline. Neurology. 2020;95:e46‐e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bessi V, Mazzeo S, Padiglioni S, et al. From subjective cognitive decline to Alzheimer's disease: the predictive role of neuropsychological assessment, personality traits, and cognitive reserve. A 7‐year follow‐up study. J Alzheimers Dis. 2018;63:1523‐1535. [DOI] [PubMed] [Google Scholar]
- 33. Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta‐analysis. Acta Psychiatr Scand. 2014;130:439‐451. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Table S1–S7
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


