Abstract
Background and purpose
The association between Guillain−Barré syndrome (GBS) and severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is debated. This study reappraises, after three pandemic years, the epidemiological data and the features of GBS in SARS‐CoV‐2 patients.
Methods
A systematic review and meta‐analysis of case reports/series and cohort studies published between 1 January 2020 and 19 April 2023 was performed.
Results
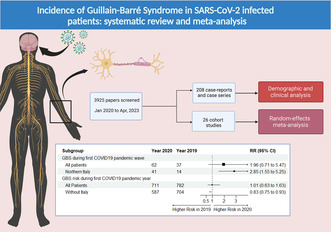
In all, 209 case reports/series (304 patients) and 26 cohort studies were included. The risk of GBS in northern Italy during the first pandemic wave was 2.85 times increased (95% confidence interval [CI] 1.54; 5.25) whereas in some countries the risk during the first pandemic year was 0.17 times reduced (risk ratio 0.83, 95% CI 0.75; 0.93). The incidence of GBS in SARS‐CoV‐2 Italian hospitalized cohorts was 8.55 per 1000 (95% CI 5.33; 12.49) with an estimated incidence of 0.13 GBS per 1000 in the SARS‐CoV‐2 infected population. In European cohorts the pooled rate of GBS with SARS‐CoV‐2 infection was 61.3% of the total. GBS patients with SARS‐CoV‐2 infection showed more frequently, but not differently from non‐infected patients, the classical clinical presentation and the demyelinating subtype. Cranial nerves were more frequently involved in SARS‐CoV‐2 infected patients.
Conclusions
An increased risk of GBS occurred in northern Italy during early COVID‐19 pandemic. The recognition of the ‘Italian factor’ reconciles contrasting results of the epidemiological studies. The slightly reduced GBS risk in other countries and the relatively high frequency of GBS associated with SARS‐CoV‐2 infection can be explained by the adopted health measures that decreased the circulation of other GBS infective antecedents.
Keywords: clinical features, epidemiology, Guillain−Barré syndrome, meta‐analysis, severe acute respiratory syndrome coronavirus, systematic review
Guillain‐Barré Syndrome during COVID‐19 pandemics: results from single cases and cohort studies.
INTRODUCTION
Guillain−Barré syndrome (GBS), a rare but potentially fatal immune‐mediated neuropathy, is thought to be an autoimmune, post‐infective disorder. Several infectious agents, including Campylobacter jejuni, Mycoplasma pneumoniae, Epstein–Barr virus, cytomegalovirus, hepatitis E virus and Zika virus have been identified as triggers [1, 2, 3, 4]. A definite pathogenic link by cross‐reactive antibodies against ganglioside epitopes has been established only for C. jejuni, although molecular mimicry is considered possible also for Haemophilus influenzae, cytomegalovirus and M. pneumoniae [5].
The GBS eponym incorporates a number of related autoimmune neuropathies including the GBS and Miller Fisher syndrome (MFS) variants and their subtypes [6]. On the basis of electrophysiological and pathological characteristics, GBS has been classified into acute inflammatory demyelinating polyradiculoneuropathy (AIDP), acute motor axonal neuropathy (AMAN) and acute motor sensory axonal neuropathy (AMSAN) [1].
Since the onset of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic, an increased number of case reports or small series of GBS occurring in SARS‐CoV‐2 infected patients have been published from all over the world suggesting a possible epidemiological association and a pathogenic link. During the first pandemic wave (March–April 2020) two studies in northern Italy reported a 2.6–7‐fold increased GBS incidence compared to the previous year [7, 8]. On the other hand, during the first five pandemic months, the incidence of GBS in the UK, as derived from the National Immunoglobulin Database, was reduced compared with the same period of the previous 5 years [9]. Similar results were recently reported in South Korean and in Swedish studies [10, 11]. These contrasting findings make the debate on GBS and SARS‐CoV‐2 infection heated.
On 5 May 2023 the World Health Organization (WHO) declared the end of the SARS‐CoV‐2 public health emergency of international concern started on 30 January 2020 with a total of 765,222,932 confirmed cases and nearly 7 million deaths (WHO's Coronavirus Dashboard). Up to now some systematic reviews on GBS and SARS‐CoV‐2 infection, but only one meta‐analysis covering the first pandemic year, have been published [12, 13, 14, 15, 16]. The aim of this work is to reappraise, after three pandemic years, the epidemiological data and the clinical features of GBS in SARS‐CoV‐2 patients compared to non‐SARS‐CoV‐2 infected contemporary or historical GBS controls.
METHODS
Study design, search strategy and inclusion criteria
An author (SC) performed a systematic search on all studies published from 1 January 2020 to 19 April 2023 via PubMed MEDLINE. All available peer‐reviewed papers published in English, French, Italian and Spanish were included. Letters, commentaries and reviews that reported original data were also included. No restriction on population sex, ethnicity, age and medical history was applied. The search terms and the flowchart of study selection are reported in the Supplementary Material, Figure S1.
The inclusion criteria were (i) definite SARS‐CoV‐2 infection confirmed by a positive nasal or throat swab polymerase chain reaction (PCR) for viral RNA or a positive serological test for anti‐SARS‐CoV‐2 antibodies; or clinically probable coronavirus disease 2019 (COVID‐19) diagnosis based on the European Centre for Disease Prevention and Control case definitions 2020 [17]; (ii) diagnosis of GBS, MFS and their subtypes according to a clinical classification [6]; (iii) occurrence of SARS‐CoV‐2 infection within 60 days from GBS onset [18]; (iv) hospitalized patients.
The certainty of GBS and MFS diagnosis was assessed by the Brighton Collaboration GBS Working Group criteria [19] or Asbury's criteria [20]. The electrodiagnosis of GBS subtypes made directly by the original authors was adopted or, when not clearly stated, an author of this paper (AU) formulated it through evaluation of the reported electrophysiological findings employing the categories of AIDP, AMAN, AMSAN, inexcitable, equivocal and normal [21].
Study quality assessment and publication bias
This study was registered in the International Prospective Register of Ongoing Systematic Reviews in 2022 (CRD42022321079) and was conducted complying with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines (Table S1) [22]. Eligible cohort studies were subjected to quality control and bias assessment through the Newcastle−Ottawa Scale [23].
Statistical analysis
All analyses were performed using R software version 4.1.0. The packages utilized and further information on statistical methodology are detailed in the Supplementary Material [24].
For case reports/series, continuous variables were expressed as median, interquartile range and range. Clinical features and laboratory findings were expressed as the number of patients in whom the variable was present in the numerator, and the total number of reported cases in the denominator: n/N (%).
The Mantel–Haenszel method was employed in a random‐effects model for calculating the pooled risk ratio (RR) of developing GBS between SARS‐CoV‐2 infected patients and non‐infected controls. For the study of GBS incidence both the Freeman−Tukey transformation (FTT) and the general linear mixed model (GLMM) were used. Average effects for the outcomes and 95% confidence intervals (CIs) were obtained using a random‐effects model. Both methods were used since conflicting opinions persist on which is the best for meta‐analysis of rare events [25]. The proportion of total variability due to between‐study heterogeneity was estimated by Cochran Q χ 2 statistics and I 2 statistics [26]. Since our analysis dealt with rare events, a cut‐off was not set for homogeneity for the Cochran Q χ 2 test p value and/or for I 2 statistics. I 2 represents what proportion of the observed variance is attributed to the variance in true effects rather than to sampling error. For a qualitative interpretation, I 2 values lower than 30% were considered to represent low variability due to between‐study heterogeneity, whilst values higher than 75% indicated considerable high variability. The Knapp−Hartung adjustment was applied to the calculation of confidence intervals when more than five studies were available, using the Paule−Mandel estimator for τ 2. Forest plots were built for each meta‐analysis end‐point and then an assessment was made for the presence of small‐study effects and possible publication bias using Egger's and Peters' method for assessment of funnel plot asymmetry when more than 10 studies were available. Wherever feasible, an influence analysis was conducted and the result was plotted using a Baujat plot. Outlier analysis was also implemented [24].
RESULTS
The flowchart of study selection is presented in Figure S1. A total of 3925 references were identified; after reviewing the title and the abstract 3640 were not pertinent and were eliminated.
Of the 285 potentially relevant papers, after reading the full text 51 papers were excluded because they did not report GBS cases or unclear diagnosis, GBS was not associated with SARS‐CoV‐2 infection, the interval between SARS‐CoV‐2 infection and GBS onset was greater than 60 days, patients were not hospitalized, insufficient or overlapping data. Overall, 234 studies were included. Since meta‐analytical results were similar using both FTT and GLMM, here FTT results only are presented, whilst GLMM results can be found in the Supplementary Material, Section 2.
Epidemiological results
A more detailed presentation of the meta‐analyses is reported in the Supplementary Material, Section 2. When missing, the inhabitant populations of selected countries were obtained from national and international databases (South Korea [https://population.un.org/wpp/], Sweden [www.scb.se], Italy [ISTAT, https://www.census.gov/popclock/]). When not clearly stated, the exact number of GBS cases for each year was obtained on consulting the authors.
Guillain−Barré syndrome risk during the first COVID‐19 pandemic wave of 2020
Three cohorts were included in the analysis of GBS risk during the first pandemic wave of COVID‐19 (March–April 2020) and the same period of 2019 [7, 8, 27].
Ninety‐nine GBS patients were identified over a total of 46,913,302 inhabitants. The random‐effects model yielded an RR of 1.96 (95% CI 0.71; 5.47) showing that the risk of developing GBS in the general population during the first COVID‐19 pandemic wave of 2020 was almost two times higher compared with the same period of the previous year (Figure 1a). The I 2 was high (74%). When limiting the analysis to the Italian cohorts, the pooled RR was 2.85 (95% CI 1.54; 5.25) (Figure 1b).
FIGURE 1.
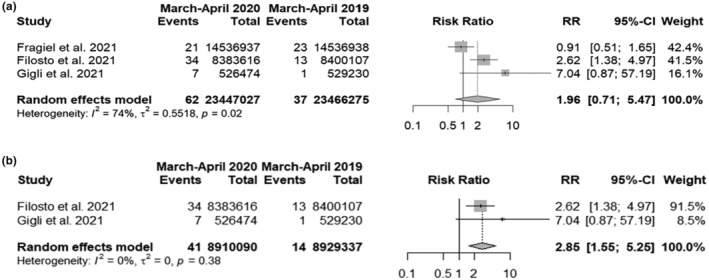
Forest plots of Guillain−Barré syndrome (GBS) risk in the general population during the first pandemic wave in 2020 compared to the same period of 2019: (a) all available studies; (b) only Italian cohorts. Events, number of GBS cases; Total, number of inhabitants.
Risk of GBS patients being positive for SARS‐CoV‐2 during the first pandemic wave
Four European cohorts were employed, three already included in the previous analysis [7, 8, 9, 27].
A total of 110 GBS cases were identified: 69 cases associated with SARS‐CoV‐2 infection and 41 without SARS‐CoV‐2 infection. The random‐effects model yielded an RR of 1.52 (95% CI 0.54; 4.30) with an I 2 of 82% (Figure 2a). The pooled rate of GBS patients with SARS‐CoV‐2 infection was 61.33% of the total GBS cases (Figure 2b).
FIGURE 2.
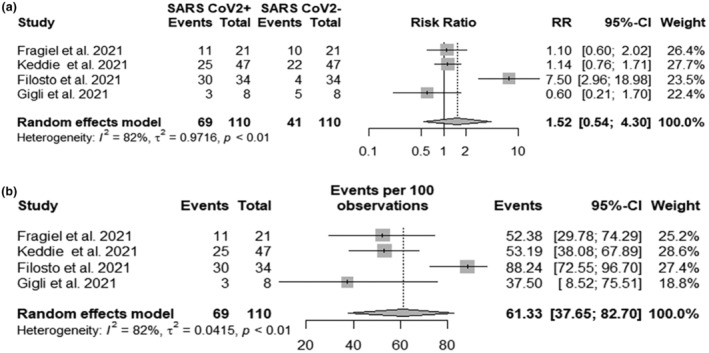
(a) Forest plot of Guillain−Barré syndrome (GBS) risk in severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infected and non‐infected patients: SARS CoV2+ events, number of GBS cases in SARS‐CoV‐2 infected patients; SARS CoV2− events, number of GBS cases in SARS‐CoV‐2 non‐infected patients; Total, total number of GBS cases. (b) Pooled incidence of GBS with SARS‐CoV‐2 infection: Events, number of GBS cases in SARS‐CoV‐2 infected patients; Total, total number of GBS cases.
Guillain−Barré syndrome risk during the first COVID‐19 pandemic year
Three cohorts were included [10, 11, 28]. The exact number of GBS cases for each year was obtained on consulting the authors. 1493 GBS were identified over a total of 123,202,390 inhabitants. The random‐effects model yielded an RR of 1.01 (95% CI 0.62; 1.63) (Figure 3a). The risk of developing GBS in the general population during the first pandemic year was nearly the same compared with the previous year. However, on re‐running the analysis excluding the Italian cohort the RR was 0.83 (95% CI 0.75; 0.93) (Figure 3b), thus slightly reduced.
FIGURE 3.
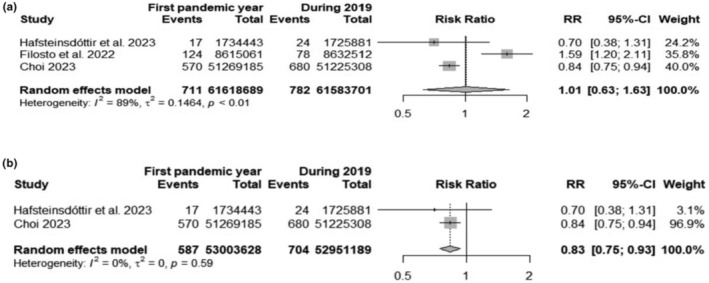
Forest plots of Guillain−Barré syndrome (GBS) risk during the first pandemic year and 2019: (a) including all available studies; (b) excluding the Italian cohorts. Events, number of GBS cases; Total, number of inhabitants.
Incidence of GBS in SARS‐CoV‐2 hospitalized patients
In 11 studies, a total of 61 GBS cases were reported over 132,132 SARS‐CoV‐2 hospitalized patients [27, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38]. The modelled proportion yielded 2.96 GBS cases per 1000 SARS‐CoV‐2 patients (95% CI 0.57; 6.75) (Figure 4a). The I 2 was very high (93.9%). Two outliers were identified [27, 33]. Removing them and re‐running the analysis yielded a proportion of 4.82 GBS cases per 1000 SARS‐CoV‐2 patients (95% CI 1.99; 8.69). The I 2 was reduced to 72.4%.
FIGURE 4.
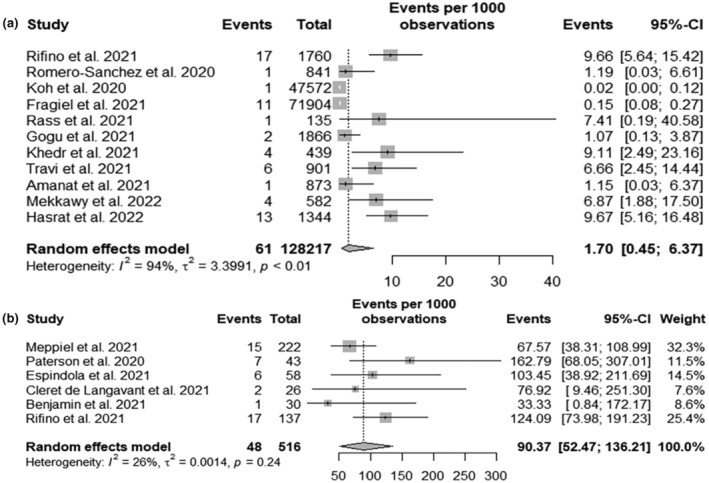
(a) Forest plot of Guillain−Barré syndrome (GBS) incidence in severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infected hospitalized patients: Events, number of GBS cases; Total, number of SARS‐CoV‐2 infected hospitalized patients. (b) Forest plot of GBS incidence in patients with neuro‐COVID: Events, number of GBS cases; Total, number of patients with neuro‐COVID.
In six studies including 516 SARS‐CoV‐2 infected patients with various neurological impairments (neuro‐COVID) [39], 48 GBS cases were reported [36, 40, 41, 42, 43, 44]. An incidence of 90.37 GBS cases per 1000 SARS‐CoV‐2 patients with neuro‐COVID was found (Figure 4b). The I 2 of this analysis was low (I 2 of 26%).
The incidence of GBS in SARS‐CoV‐2 patients was also analysed with respect to the geographical distribution. Limiting the analysis to the European cohorts and excluding the Italian studies, two cohorts from Spain and one from Romania were considered. A GBS incidence of 0.4 per 1000 SARS‐CoV‐2 patients (95% CI 0.00; 1.77) with an I 2 of 71% was found (Figure 5a). Considering only the two Italian cohorts, a GBS incidence of 8.55 per 1000 SARS‐CoV‐2 patients (95% CI 5.33; 12.49) was found (Figure 5b). Looking at African countries, two studies from Egypt were found where the incidence of GBS cases per 1000 SARS‐CoV‐2 patients was 7.77 (95% CI 3.02; 14.40) (Figure 5c).
FIGURE 5.
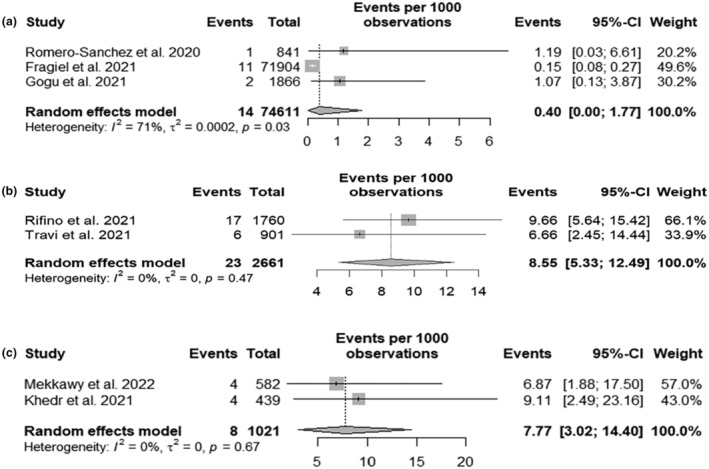
Guillain−Barré syndrome (GBS) incidence in severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infected hospitalized patients analysed by geographical region. Forest plots for (a) European cohorts without Italian studies; (b) Italian cohorts only; (c) Egyptian cohorts only. Events, number of GBS cases; Total, number of SARS‐CoV‐2 infected hospitalized patients.
Assessment of study quality and publication bias
The overall quality of included studies was high (Table S2). Assessment of funnel plot asymmetry with visual inspection and Egger's and Peters’ test showed potential asymmetry in the risk of GBS during the first pandemic wave and year (small number of included studies) and the incidence of GBS in SARS‐CoV‐2 hospitalized patients (considering both general and neuro‐COVID only populations).
Sensitivity analyses and assessment of heterogeneity
Leave‐one‐out sensitivity analysis was performed (Supplementary Material Section 2). No single study significantly affected the computed effect size for each outcome. A Baujat plot was also produced to elucidate the contribution of a single study to the overall random‐effects model heterogeneity (Supplementary Material Section 2).
Clinical results
Data were collected from 304 patients with GBS associated with SARS‐CoV‐2 infection deriving from 209 case reports/series studies: 118 cases were reported in 2020, 113 in 2021, 57 in 2022 and 16 in 2023 to 19 April. The reference list is reported in the Supplementary Material. Patients were reported from all continents except Australia, and specifically from 42 countries, mostly from Europe (39.8%). The highest percentage (15.5%) was from the USA, followed by Italy (14.8%) and India (13.5%) (Table S3). Amongst the 45 Italian cases, 93.3% were reported from Northern Italy, 2.2% from Central Italy and 4.4% from Southern Italy. In Table 1 are summarized the patients' demographic, clinical and laboratory features.
TABLE 1.
Demographic, clinical and laboratory features of 304 patients with SARS‐CoV‐2 infection and Guillain−Barré syndrome.
| Number of patients | 304 | |
| Gender, male–female | 202 (66.4%)–102 (33.6%) | |
| Age, years, median (IQR) (range) | 54 (39–65), (2–94) | |
| Diagnosis of SARS‐CoV‐2 infection | Nasopharyngeal swab positive | 260 (85.0%) |
| Serology positive | 29 (9.5%) | |
| High resolution chest computed tomography | 5 (1.6%) | |
| Clinical and epidemiological link | 10 (3.3%) | |
| Relationship between SARS‐CoV‐2 and GBS | ||
| First hospitalized for | Not reported | 123 (40.5%) |
| COVID‐19 | 51/181 (28.2%) | |
| GBS | 130/181 (71.8%) | |
| Nasopharyngeal swab at GBS onset | Not reported | 137 (45.1%) |
| Positive | 124/167 (74.3%) | |
| Negative | 43/167 (25.7%) | |
| Asymptomatic SARS‐CoV‐2 infection before GBS | 32 (10.5%) | |
| Time interval between SARS‐CoV‐2 symptoms onset and GBS symptoms onset (available in 261 patients), days, median (IQR), (range) | 14 (7–21), (0–60) | |
| Neurological features | Cranial nerve(s) impairment | 125 (41.1%) |
| Ophthalmoparesis | 35 (11.5%) | |
| Facial nerve palsy | 73 (24.0%) | |
| Bulbar palsy | 32 (10.5%) | |
| Four limbs weakness | 207 (68.1%) | |
| Weakness limited to LLs | 56 (18.4%) | |
| Hypo‐areflexia | 286 (94.1%) | |
| Sensory disturbances | 174 (57.2%) | |
| Ataxia | 53 (17.4%) | |
| Dysautonomia | 41 (13.5%) | |
| Blood pressure disorders and dysrhythmia | 39/41 (95.1%) | |
| Ileus paralyticus | 3/41 (7.3%) | |
| Sphincter disorders | 18/304 (5.9%) | |
| Faecal incontinence | 7/18 (38.9%) | |
| Urinary retention | 16/18 (88.9%) | |
| Urinary incontinence | 7/18 (38.9%) | |
| Hypersomnolence and consciousness disturbances | 9/304 (2.9%) | |
| Clinical classification | Classical GBS | 208/304 (68.4%) |
| Paraparetic | 56/304 (18.4%) | |
| Facial diplegia with/without paraesthesia | 7/304 (2.3%) | |
| Polyneuritis cranialis | 4/304 (1.3%) | |
| Pharyngo‐cervical‐brachial | 3/304 (1.0%) | |
| Miller Fisher syndrome | 20/304 (6.6%) | |
| Acute ataxic neuropathy | 3/304 (1.0%) | |
| Bickerstaff brainstem encephalitis | 3/304 (1.0%) | |
| Electrodiagnosis | Not done | 60/304 (19.7%) |
| AIDP | 138/244 (56.6%) | |
| AMAN | 28/244 (11.5%) | |
| AMSAN | 36/244 (14.8%) | |
| Inexcitable | 1/244 (0.4%) | |
| Equivocal | 31/244 (12.7%) | |
| Normal | 2/244 (0.8%) | |
| CSF | Not performed | 59/304 (19.4%) |
| Albumino‐cytological dissociation | 204/245 (83.6%) | |
| Normal | 41/245 (16.7%) | |
| PCR for SARS‐CoV‐2 positive | 2/65 (3.1%) | |
| Brighton criteria | Not applicable | 22 (7.2%) |
| Level 1 | 145/282 (51.4%) | |
| Level 2 | 113/282 (40.1%) | |
| Level 3 | 24/282 (8.5%) | |
| Anti‐ganglioside antibodies | ||
| Patients with reported ganglioside antibodies | 16/91 (17.6%) | |
| Ganglioside antibodies subtype | Total | |
| Anti‐GM1 | 5 | |
| Anti‐GM2 | 3 | |
| Anti‐GM3 | 1 | |
| Anti‐GD1a | 6 | |
| Anti‐GD1b | 3 | |
| Anti‐GQ1b | 5 | |
| Anti‐GT1a | 3 | |
| Anti‐GT1b | 1 | |
| MRI | Not performed | 172/304 (56.6%) |
| Normal/not contributory | 81/132 (61.4%) | |
| Enhancement of cranial nerves, roots/plexus and leptomeninges | 51/132 (38.6%) | |
| Invasive mechanical ventilation | 92/304 (30.3%) | |
| ICU admission | 95/304 (31.3%) | |
| Immunotherapy | Not reported | 16/304 (5.3%) |
| None | 12/288 (4.2%) | |
| IVIG | 214/276 (77.5%) | |
| PLEX | 23/276 (8.3%) | |
| IVIG and PLEX | 22/ 276 (8.0%) | |
| Steroids | 6/276 (2.2%) | |
| Length of hospitalization, days, median (IQR) (range) | 16.5 (11–34.25), (5–156) | |
| In‐hospital death | 22/304 (7.2%) | |
| Time interval from GBS diagnosis to death (reported in 17/21 patients): days, median (IQR) (range) | 4 (2–7.5) (1–50) | |
Abbreviations: AIDP, acute inflammatory demyelinating polyneuropathy; AMAN, acute motor axonal neuropathy; AMSAN, acute motor and sensory axonal neuropathy; COVID‐19, coronavirus disease 2019; CSF, cerebrospinal fluid; GBS, Guillain− Barré syndrome; ICU, intensive care unit; IQR, interquartile range; IVIG, intravenous immunoglobulins; LLs, lower limbs; MRI, magnetic resonance imaging; PCR, polymerase chain reaction; PLEX, plasma exchange; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
The median age was 54 years and the majority (66.4%) of patients were men. Six (2.0%) patients had a previous GBS episode, one during another SARS‐CoV‐2 infection. Five (4.9%) women developed GBS during pregnancy with foetal sufferance in two.
The diagnosis of SARS‐CoV‐2 infection was made by positive PCR of nasopharyngeal swab in 260 (85%) patients (sometimes after repeated tests), by serology in 29 (9.5%), by high‐resolution computed tomography in five (1.6%) and by clinical criteria with epidemiological link in 10 (3.3%) patients. Of the 181 patients in whom the information was available, 28.2% where first hospitalized for COVID‐19 and then developed GBS, whereas in 71.8% GBS was the reason for admission. Of 167 SARS‐CoV‐2 PCR tests done at GBS onset, 124 (74.3%) were positive.
An asymptomatic SARS‐CoV‐2 infection, before GBS onset, was reported in 32 (10.9%) patients. In these patients, the diagnosis of SARS‐CoV‐2 infection was made by nasopharyngeal swab PCR in 24 (75.0%), by serology in seven (21.8%) and by clinical features with an epidemiological link in one (3.1%).
The median interval between onset of SARS‐CoV‐2 symptoms/infection and GBS onset, reported in 261 patients, was 14 days. In only eight (3.1%) cases the interval was between 42 and 60 days.
Other infective agents known to be associated with GBS were looked for in 83 (27.3%) patients and excluded in 78 (94.0%). In one patient a cytomegalovirus reactivation (hypothesized to be due to COVID‐19 immunosuppression) was reported, in three C. jejuni seropositivity was reported, whilst in one the result was doubtful.
The classical presentation (symmetrical weakness of the limbs, sensory symptoms and reduced or absent tendon reflexes) was observed in 209 (68.8%) patients whilst the paraparetic subtype was reported in 18.4% of cases. Twenty (6.6%) MFS cases, seven (2.3%) facial diplegia, four (1.3%) polyneuritis cranialis, three (1.0%) pharyngeal‐cervico‐brachial weakness, three (1.0%) acute ataxic neuropathy and three (1.0%) cases of Bickerstaff brainstem encephalitis were reported.
Three cohorts reported the relative incidence of classical GBS and MFS in SARS‐CoV‐2 infected patients versus non‐SARS‐CoV‐2 infected controls [9, 28, 45]. The raw percentage of patients developing classical GBS was 83.7% in SARS‐CoV‐2 infected patients and 82.9% in non‐SARS‐CoV‐2 infected patients with no difference in risk ratio (RR 1.04, 95% CI 0.94; 1.14) (Figure 6a). MFS was 4.1% in SARS‐CoV‐2 infected and 9.4% in non‐SARS‐CoV‐2 infected patients, with a 0.49 times reduced risk in SARS‐CoV‐2 patients (RR 0.51, 95% CI 0.16; 1.65) (Figure 6b).
FIGURE 6.
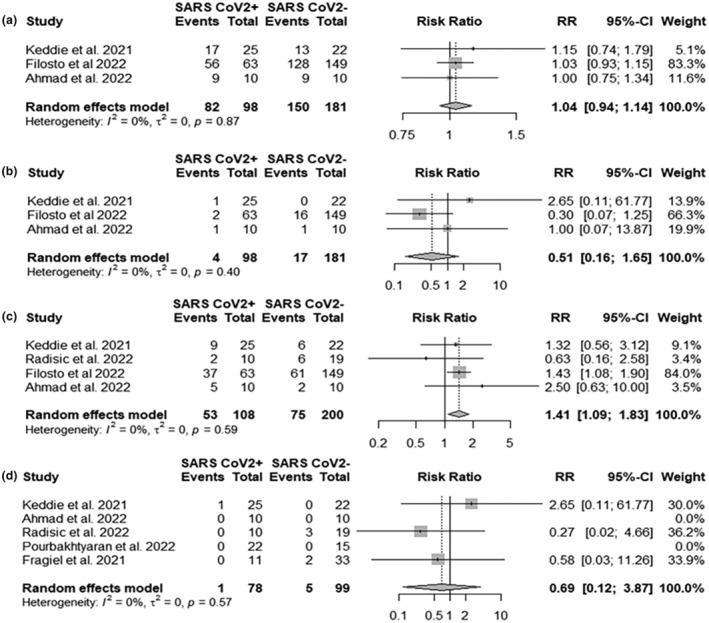
Forest plots for (a) classical Guillain−Barré syndrome (GBS) risk ratio; (b) Miller Fisher syndrome (MFS) risk ratio; (c) cranial nerve risk ratio; (d) mortality risk ratio. SARSCoV2+ events, number of classical GBS cases, MFS cases, number of patients with cranial nerve involvement, number of deaths in severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infected patients; SARSCoV2− events, number of classical GBS cases, MFS cases, number of patients with cranial nerve involvement, number of deaths in SARS‐CoV‐2 non‐infected patients; Total, total number of GBS cases.
In four cohorts the raw percentage of cranial nerve involvement was 37.5% in non‐SARS‐CoV‐2 infected patients and 49.1% in SARS‐CoV‐2 infected patients with 1.41 RR of cranial nerve involvement (RR 1.41, 95% CI 1.09; 1.83) (Figure 6c) [9, 28, 45, 46].
Electrophysiology was performed in case reports/series studies in 244 (80.2%) patients. AIDP was the most common GBS subtype (56.6%) followed by AMSAN (14.8%) and AMAN (11.5%). In 31 (12.7%) patients electrophysiological findings were equivocal, being abnormal but not fulfilling the criteria for a primary demyelinating or axonal neuropathy. Two patients (0.8%) showed normal electrophysiology. In six cohorts the summed percentage of patients with AIDP was 60.8% in SARS‐CoV‐2 infected patients and 51.8% in non‐infected patients with a non‐significant difference (RR 1.13, 95% CI 0.87; 1.47) (Figure 7a) [9, 28, 45, 46, 47, 48]. Axonal GBS (AMAN and AMSAN) was 21.7% in SARS‐CoV‐2 infected and 25.7% in non‐infected patients with a 0.24 times lower risk of axonal GBS in SARS‐CoV‐2 infected patients (RR 0.76, 95% CI 0.56; 1.12) (Figure 7b).
FIGURE 7.
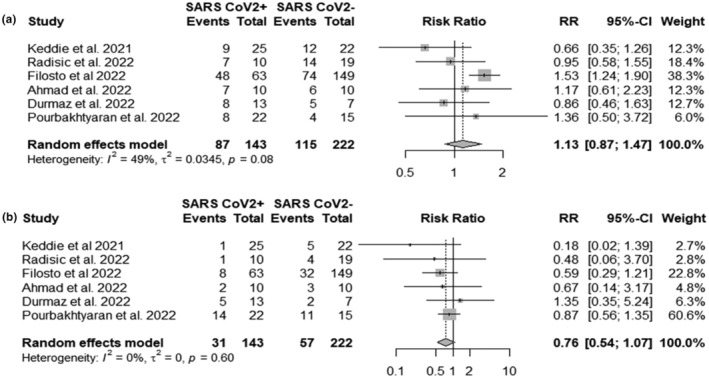
Forest plots of (a) severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection and risk of acute inflammatory demyelinating polyradiculoneuropathy (AIDP), (b) SARS‐CoV‐2 infection and risk of axonal Guillain−Barré syndrome (GBS) (acute motor axonal neuropathy and acute motor sensory axonal neuropathy): SARSCoV2+ events, number of AIDP or axonal GBS cases in SARS‐CoV‐2 infected patients; SARSCoV2− events, number of AIDP or axonal GBS cases in SARS‐CoV‐2 non‐infected patients; Total, total number of GBS cases.
In case reports/series studies cerebrospinal fluid (CSF) examination was performed in 245 patients. Albumino‐cytological dissociation was present in 204 (83.6%). Only two out of 62 patients (3.2%) showed a positive SARS‐CoV‐2 PCR in CSF.
The Brighton criteria for diagnostic certainty of GBS and MFS were not applicable in four patients because of missing data and in 18 patients diagnosed as facial diplegia, polyneuritis cranialis, acute ataxic neuropathy and Bickerstaff brainstem encephalitis. In the remaining 282 patients 51.4% reached level 1 of certainty, 40.1% level 2 and 8.5% level 3, making the diagnosis quite certain in the great majority of cases.
Anti‐ganglioside antibodies were searched in 91 patients and immunoglobulin M (IgM) and/or IgG against different gangliosides were found in 16 (17.6%) patients, with anti‐GD1a, anti‐GM1 and anti‐GQ1b being the most frequent antibodies.
In five cohort studies the summed percentage of deceased patients was 1.28% in SARS‐CoV‐2 infected patients and 5.05% in non‐infected patients (Figure 6d) [9, 27, 45, 46, 48]. A 0.31 times reduced risk of death was found in the SARS‐CoV‐2 infected GBS population (RR 0.69, 95% CI 0.12; 3.87).
DISCUSSION
The risk of developing GBS in northern Italy during the first COVID‐19 pandemic wave was 2.85 times increased compared with the same period of the previous year, whereas, when considering the first pandemic year, the overall GBS risk was in some countries 0.17 times reduced [7, 8, 10, 11]. In northern Italy the GBS risk decreased from 2.6 in March–April 2020 to 1.41 considering the whole first pandemic year [7, 28].
The overall incidence of GBS in SARS‐CoV‐2 hospitalized patients was 2.69 per 1000 but an analysis by geographical distribution showed that in northern Italy the incidence was 8.5 per 1000, whereas in other European cohorts it was only 0.4 per 1000 [27, 30, 36, 37, 38]. About 90 per 1000 hospitalized patients with neuro‐COVID had GBS similarly to what was reported in another meta‐analysis [15].
The overall picture seems blurred, but it is considered that the contrasting results of the epidemiological studies and the high proportion of the variance in some meta‐analyses can be reconciled by the recognition of an ‘Italian factor’ on the basis of the following remarks: (i) the number of GBS cases reported from northern Italy, although it could be ascribed to a publication bias, was similar to the number of cases reported from the USA but with less than one‐twelfth of the population; (ii) the almost three times increased risk of GBS in northern Italy during the first pandemic wave; (iii) the 21‐fold increased incidence of GBS in SARS‐CoV‐2 hospitalized patients in northern Italy compared to other European countries [36, 38].
Regarding the last point, the pooled incidence of 8.55 GBS cases per 1000 SARS‐CoV‐2 infected in northern Italy during the first pandemic wave surely overestimated the incidence in the general population because only hospitalized patients were considered. The study by Rifino and coworkers was made in the Bergamo province, which carried until 30 April 2020 the sad record of the highest number (n = 11,313) of confirmed SARS‐CoV‐2 cases in Italy [36]. Taking this number as denominator, the incidence of GBS decreases from 9.66 to 1.5 per 1000 confirmed SARS‐CoV‐2 infections in the general population. Data on seroprevalence in northern Italy during the first pandemic wave are scarce. However, extrapolating the 12% infection rate found in the healthcare workers at the end of the first pandemic wave in the Lombardy region [49] to the Bergamo province population (1,108,126 inhabitants), the estimated incidence of GBS decreases further to 0.13 per 1000 SARS‐CoV‐2 infections and it is similar to the 0.15 per 1000 incidence estimated in a previous meta‐analysis [15]. This value is lower than the estimated incidence reported for C. jejuni infection (0.25–0.65 per 1000) or during the Zika virus outbreak in French Polynesia (0.24 per 1000) [50]. Northern Italy was the first European region to face a sudden, exceptional emergency that almost overwhelmed the health system. The high number of SARS‐CoV‐2 infected patients may contribute to explain the increased risk of GBS in northern Italy during the first pandemic wave. Other hypothetical explanations for the ‘Italian factor’ are the occurrence of a variant of SARS‐CoV‐2 strain with an increased capability to trigger GBS or an increased susceptibility in the population to develop GBS after SARS‐CoV‐2 infection, as suggested for ACE2 gene variants [51].
With regard to the slightly reduced GBS risk in some countries it should be underlined that the SARS‐CoV‐2 pandemic was characterized by unique and drastic public health measures that by reducing the circulation of infective agents known to trigger GBS decreased the overall risk. Indeed, in two South Korean studies, most respiratory and common gastrointestinal infections, including C. jejuni, decreased significantly during the pandemic [52, 53].
The pooled incidence of GBS associated with SARS‐CoV‐2 infection was 61% of the total, and in the Spanish Emergency Departments study patients with SARS‐CoV‐2 infection were six times more likely to develop GBS compared to patients without SARS‐CoV‐2 infection [27]. This estimated relative frequency of GBS associated with SAR‐CoV‐2 infection is considerably higher compared to the frequency of other known infective antecedents (30% for C. jejuni, 10% for M. pneumoniae, 4% for cytomegalovirus, 3% for hepatitis E virus and 1% for Epstein–Barr virus [3]) and supports the association between GBS and SARS‐CoV‐2 infection.
Considering the clinical features of GBS associated with SARS‐CoV‐2 infection it was found that the median interval between the onset of COVID‐19 and GBS was 14 days. Although the distinction between parainfective and post‐infective disorders based solely on the time interval seems simplistic in SARS‐CoV‐2 infection and GBS [16], this time interval in addition to the very low rate of positive PCR for SARS‐CoV‐2 in CSF suggest a post‐infective mechanism.
Most SARS‐CoV‐2 infected patients showed the classical GBS presentation: 68.4% in case reports/series studies and up to 88.8% in the largest cohort study [28]. However, all clinical variants and subtypes were reported. The meta‐analysis showed that the risk of developing classical GBS was the same in SARS‐CoV‐2 infected and non‐infected populations whereas the risk of developing MFS was found to be about half of the non‐infected population. However, the confidence intervals for MFS analysis were wide, so a clear conclusion on this point is still to be reached. Cranial nerves, excluding the olfactory nerve, appeared to be more involved in SARS‐CoV‐2 infected GBS.
In the case reports/series studies respiratory failure requiring invasive ventilation and intensive care unit (ICU) admission occurred in about 30% of patients. In the largest cohort study 49.2% of GBS cases associated with SARS‐CoV‐2 infection were admitted to the ICU compared with 14.7% of SARS‐Co‐2 negative patients [28]. It is likely, at least for the patients in whom GBS occurred during hospitalization for COVID‐19, that COVID‐19‐related respiratory and systemic impairment contributed to the higher frequency of admissions to the ICU.
In the case reports/series studies death occurred in 7.5% of patients with a median interval from GBS diagnosis to exitus of 4 days. This quite high percentage and the ‘fulminating’ course was in most cases due to a coexisting severe COVID‐19. On the other hand, the meta‐analysis of cohorts showed a 0.21 times reduced risk of death in SARS‐CoV‐2 infected patients. This apparent discrepancy can be explained by a selection bias in case report analysis with respect to cohorts, in which control groups were present.
In case reports/series and cohort studies, the great majority of GBS cases associated with SARS‐CoV‐2 were treated with intravenous immunoglobulins and plasma exchange and no significant difference in the response rate to therapy with SARS‐CoV‐2 non‐associated GBS was found in a cohort study [28].
In case reports/series studies the majority of SARS‐CoV‐2 infected patients showed the AIDP electrophysiological subtype; however, the actual criteria adopted for electrodiagnosis were only occasionally reported. In the largest published cohort, employing a criterion set that demonstrated in previous studies a high diagnostic accuracy, the AIDP subtype was 76.2% [21, 28]. A meta‐analysis showed that the risk of developing AIDP amongst SARS‐CoV‐2 infected patients is the same compared to non‐SARS‐CoV‐2 infected patients whereas the risk of developing axonal GBS (AMAN and AMSAN) is 0.24 reduced in SARS‐CoV‐2 infected patients. These data support the association between SARS‐CoV‐2 infection and prevalently demyelinating nerve damage.
In case reports/series CSF examination showed albumino‐cytological dissociation in 82.6% and in the largest cohort in 61.2% of GBS patients with SARS‐CoV‐2 infection with no significant difference from SARS‐CoV‐2 non‐infected cases [28].
Anti‐ganglioside antibodies were found in 17.6% of patients. The surface of the SARS‐CoV‐2 spike protein is heavily glycosylated and in 40 sera of COVID‐19 patients high levels of antibodies to numerous self‐glycans, even non‐human, were described [54]. Specifically, antibodies to gangliosides reported in GBS were found in 15% of sera but it is not stated if these patients actually had GBS. Overall, molecular mimicry between SARS‐CoV‐2 and GBS and whether anti‐ganglioside antibodies are capable of primarily inducing nerve damage or are only an epiphenomenon are to be demonstrated.
This study presents some limitations. The reduced number of published cohorts, the small sample size in some of them and the high number of SARS‐CoV‐2 infected individuals, even asymptomatic, make it hard to draw an accurate statistical statement about the overall true risk and incidence of GBS with respect to SARS‐CoV‐2 infection. Moreover, since a minimum of 10 studies for each of our analyses was hardly reached, a precise estimation of the variability of the effect size across studies would be biased [55]. Because of the health emergency, overwhelming during the first pandemic period in some countries, it is likely that mild GBS cases, either associated or not with SARS‐CoV‐2 infection, were not admitted or not recognized. Additionally, in critically ill COVID‐19 patients GBS could have been misdiagnosed as critical illness polyneuropathy when electrophysiological and CSF testing were not performed.
CONCLUSIONS
The increased overall risk of GBS in the general population and the high GBS incidence in SARS‐CoV‐2 hospitalized patients indicate that something happened in northern Italy during the first pandemic wave. The slightly decreased risk of GBS in some countries and the considerably higher frequency of GBS associated with SARS‐CoV‐2 infection during the pandemic were probably due to the adopted public health measures that decreased the circulation of other GBS infective antecedents. The above observations support, in our opinion, an association between SARS‐CoV‐2 infection and GBS and the recognition of the ‘Italian factor’, although not easily explainable, sheds some light on an apparently confused picture.
Guillain–Barré syndrome patients with SARS‐CoV‐2 infection showed more frequently, and not differently from non‐infected patients, the classical clinical presentation and the demyelinating electrophysiological subtype. Cranial nerves were more frequently affected in SARS‐CoV‐2 infected patients. Respiratory insufficiency, requiring invasive ventilation, and ICU admission were more frequent in SARS‐CoV‐2 infected GBS patients probably because of concomitant severe COVID‐19.
How SARS‐CoV‐2 may trigger GBS is currently unknown. Nonetheless, the time interval between GBS onset and SARS‐CoV‐2 infection, as well as the very low rate of SARS‐CoV‐2 RNA found in CSF, indicate a post‐infective disorder.
AUTHOR CONTRIBUTIONS
Conceptualization and design of the study: Stefano Censi, Giandomenico Bisaccia, Antonino Uncini. Data search and analysis: Stefano Censi, Giandomenico Bisaccia. Methodology: Stefano Censi, Giandomenico Bisaccia. Supervision: Antonino Uncini, Valentina Tomassini, Sabina Gallina. Writing—original draft: Stefano Censi, Antonino Uncini. Writing, review and editing: all.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Supporting information
Data S1.
Censi S, Bisaccia G, Gallina S, Tomassini V, Uncini A. Guillain−Barré syndrome and SARS‐CoV‐2 infection: a systematic review and meta‐analysis on a debated issue and evidence for the ‘Italian factor’. Eur J Neurol. 2024;31:e16094. doi: 10.1111/ene.16094
DATA AVAILABILITY STATEMENT
Data extracted from included studies, data used for all analyses, analytic code are available upon reasonable request.
REFERENCES
- 1. Yuki N, Hartung H‐P. Guillain–Barré syndrome. N Engl J Med. 2012;366:2294‐2304. doi: 10.1056/NEJMra1114525 [DOI] [PubMed] [Google Scholar]
- 2. Leonhard SE, Bresani‐Salvi CC, Lyra Batista JD, et al. Guillain−Barré syndrome related to Zika virus infection: a systematic review and meta‐analysis of the clinical and electrophysiological phenotype. PLoS Negl Trop Dis. 2020;14:e0008264. doi: 10.1371/journal.pntd.0008264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leonhard SE, Van Der Eijk AA, Andersen H, et al. An international perspective on preceding infections in Guillain−Barré syndrome: the IGOS‐1000 cohort. Neurology. 2022;99:e1299‐e1313. doi: 10.1212/WNL.0000000000200885 [DOI] [PubMed] [Google Scholar]
- 4. Wakerley BR, Yuki N. Infectious and noninfectious triggers in Guillain–Barré syndrome. Expert Rev Clin Immunol. 2013;9:627‐639. [DOI] [PubMed] [Google Scholar]
- 5. Yuki N. Ganglioside mimicry and peripheral nerve disease. Muscle Nerve. 2007;35:691‐711. doi: 10.1002/mus.20762 [DOI] [PubMed] [Google Scholar]
- 6. GBS Classification Group , Wakerley BR, Uncini A, Yuki N. Guillain–Barré and Miller Fisher syndromes—new diagnostic classification. Nat Rev Neurol. 2014;10:537‐544. doi: 10.1038/nrneurol.2014.138 [DOI] [PubMed] [Google Scholar]
- 7. Filosto M, Piccinelli SC, Gazzina S, et al. Guillain−Barré syndrome and COVID‐19: an observational multicentre study from two Italian hotspot regions. J Neurol Neurosurg Psychiatry. 2021;92:751‐756. [DOI] [PubMed] [Google Scholar]
- 8. Gigli GL, Bax F, Marini A, et al. Guillain−Barré syndrome in the COVID‐19 era: just an occasional cluster? J Neurol. 2021;268:1195‐1197. doi: 10.1007/s00415-020-09911-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keddie S, Pakpoor J, Mousele C, et al. Epidemiological and cohort study finds no association between COVID‐19 and Guillain−Barré syndrome. Brain. 2021;144:682‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hafsteinsdóttir B, Dalemo E, Elíasdóttir Ó, Ólafsson E, Axelsson M. Decreased incidence of Guillain−Barré syndrome during the COVID‐19 pandemic: a retrospective population‐based study. Neuroepidemiology. 2023;57:1‐6. doi: 10.1159/000527726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi SA, Hwang J, Lim BC, Chae SA. Incidence of Guillain−Barré syndrome in South Korea during the early COVID‐19 pandemic. Front Neurol. 2023;14:1125455. doi: 10.3389/fneur.2023.1125455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abu‐Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M. Guillain−Barré syndrome spectrum associated with COVID‐19: an up‐to‐date systematic review of 73 cases. J Neurol. 2021;268:1133‐1170. doi: 10.1007/s00415-020-10124-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caress JB, Castoro RJ, Simmons Z, et al. COVID‐19‐associated Guillain−Barré syndrome: the early pandemic experience. Muscle Nerve. 2020;62:485‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Finsterer J, Scorza FA. Guillain−Barré syndrome in 220 patients with COVID‐19. Egypt J Neurol Psychiatr Neurosurg. 2021;57:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Palaiodimou L, Stefanou M‐I, Katsanos AH, et al. Prevalence, clinical characteristics and outcomes of Guillain−Barré syndrome spectrum associated with COVID‐19: a systematic review and meta‐analysis. Eur J Neurol. 2021;28:3517‐3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Uncini A, Vallat J‐M, Jacobs BC. Guillain−Barré syndrome in SARS‐CoV‐2 infection: an instant systematic review of the first six months of pandemic. J Neurol Neurosurg Psychiatry. 2020;91:1105‐1110. [DOI] [PubMed] [Google Scholar]
- 17. ECDC . EDCD guidelines . ECDC COVID‐19 diagnosis guidelines. Consulted online on 16/05/2023.
- 18. Leung J, Sejvar JJ, Soares J, Lanzieri TM. Guillain−Barré syndrome and antecedent cytomegalovirus infection, USA 2009–2015. Neurol Sci. 2020;41:885‐891. doi: 10.1007/s10072-019-04156-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sejvar JJ, Kohl KS, Gidudu J, et al. Guillain−Barré syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29:599‐612. [DOI] [PubMed] [Google Scholar]
- 20. Asbury AK. Diagnostic considerations in Guillain−Barré syndrome. Ann Neurol. 1981;9:1‐5. doi: 10.1002/ana.410090703 [DOI] [PubMed] [Google Scholar]
- 21. Uncini A, Kuwabara S. The electrodiagnosis of Guillain−Barré syndrome subtypes: where do we stand? Clin Neurophysiol. 2018;129:2586‐2593. doi: 10.1016/j.clinph.2018.09.025 [DOI] [PubMed] [Google Scholar]
- 22. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006‐1012. doi: 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 23. Wells G, Shea B, O'Connell D, et al. The Newcastle−Ottawa scale (NOS) for assessing the quality if nonrandomized studies in meta‐analyses (available online).
- 24. Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing Meta‐Analysis with R: A Hands‐on Guide. Chapman and Hall/CRC; 2021. [Google Scholar]
- 25. Chen Y, Chen D, Wang Y, Han Y. Using Freeman−Tukey double arcsine transformation in meta‐analysis of single proportions. Aesthetic Plast Surg. 2022;47:83‐84. [DOI] [PubMed] [Google Scholar]
- 26. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539‐1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 27. Fragiel M, Miró Ò, Llorens P, et al. Incidence, clinical, risk factors and outcomes of Guillain−Barré in COVID‐19. Ann Neurol. 2021;89:598‐603. [DOI] [PubMed] [Google Scholar]
- 28. Filosto M, Cotti Piccinelli S, Gazzina S, et al. Guillain−Barré syndrome and covid‐19: a 1‐year observational multicenter study. Eur J Neurol. 2022;29:3358‐3367. doi: 10.1111/ene.15497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Amanat M, Rezaei N, Roozbeh M, et al. Neurological manifestations as the predictors of severity and mortality in hospitalized individuals with COVID‐19: a multicenter prospective clinical study. BMC Neurol. 2021;21:116. doi: 10.1186/s12883-021-02152-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gogu AE, Motoc AG, Stroe AZ, et al. Clinical spectrum and neuroimagistic features in hospitalized patients with neurological disorders and concomitant coronavirus‐19 infection. Brain Sci. 2021;11:1138. doi: 10.3390/brainsci11091138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hasrat NH, Kadhum HJ, Hashim AR, Yakob ZA, Kadhim LA, Farid HA. Neurographic evidence of inflammatory polyneuropathies in peri‐COVID‐19 circumstances and their relationship with acute disease severity and inflammatory storm. Cureus. 2022;14(3):e23517. doi: 10.7759/cureus.23517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Khedr EM, Abo‐Elfetoh N, Deaf E, et al. Surveillance study of acute neurological manifestations among 439 Egyptian patients with COVID‐19 in Assiut and Aswan university hospitals. Neuroepidemiology. 2021;55:109‐118. doi: 10.1159/000513647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koh JS, De Silva DA, Quek AML, et al. Neurology of COVID‐19 in Singapore. J Neurol Sci. 2020;418:117118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mekkawy DA, Hamdy S, Abdel‐Naseer M, et al. Neurological manifestations in a cohort of Egyptian patients with COVID‐19: a prospective, multicenter, observational study. Brain Sci. 2022;12:74. doi: 10.3390/brainsci12010074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rass V, Beer R, Schiefecker AJ, et al. Neurological outcome and quality of life 3 months after COVID‐19: a prospective observational cohort study. Eur J Neurol. 2021;28:3348‐3359. doi: 10.1111/ene.14803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rifino N, Censori B, Agazzi E, et al. Neurologic manifestations in 1760 COVID‐19 patients admitted to Papa Giovanni XXIII Hospital, Bergamo, Italy. J Neurol. 2021;268:2331‐2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Romero‐Sánchez CM, Díaz‐Maroto I, Fernández‐Díaz E, et al. Neurologic manifestations in hospitalized patients with COVID‐19: the ALBACOVID registry. Neurology. 2020;95:e1060‐e1070. doi: 10.1212/WNL.0000000000009937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Travi G, Rossotti R, Merli M, et al. Neurological manifestations in patients hospitalized with COVID‐19: a retrospective analysis from a large cohort in northern Italy. Eur J Neurosci. 2021;53:2912‐2922. doi: 10.1111/ejn.15159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chou SH‐Y, Beghi E, Helbok R, et al. Global incidence of neurological manifestations among patients hospitalized with COVID‐19—a report for the GCS‐NeuroCOVID consortium and the ENERGY consortium. JAMA Netw Open. 2021;4:e2112131. doi: 10.1001/jamanetworkopen.2021.12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Benjamin LA, Paterson RW, Moll R, et al. Antiphospholipid antibodies and neurological manifestations in acute COVID‐19: a single‐centre cross‐sectional study. EClinicalMedicine. 2021;39:101070. doi: 10.1016/j.eclinm.2021.101070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID‐19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104‐3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cleret de Langavant L, Petit A, Nguyen QTR, et al. Clinical description of the broad range of neurological presentations of COVID‐19: a retrospective case series. Rev Neurol. 2021;177:275‐282. doi: 10.1016/j.neurol.2021.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Espíndola OM, Brandão CO, Gomes YCP, et al. Cerebrospinal fluid findings in neurological diseases associated with COVID‐19 and insights into mechanisms of disease development. Int J Infect Dis. 2021;102:155‐162. doi: 10.1016/j.ijid.2020.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meppiel E, Peiffer‐Smadja N, Maury A, et al. Neurologic manifestations associated with COVID‐19: a multicentre registry. Clin Microbiol Infect. 2021;27:458‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ahmad L, Businaro P, Regalbuto S, et al. COVID‐19 and Guillain−Barré syndrome: a single‐center prospective case series with a 1‐year follow‐up. Medicine. 2022;101:e29704. doi: 10.1097/MD.0000000000029704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Radišić V, Ždraljević M, Perić S, et al. Is there a difference between GBS triggered by COVID‐19 and those of other origins? Egypt J Neurol Psychiatr Neurosurg. 2022;58:54. doi: 10.1186/s41983-022-00486-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eroğlu Durmaz Ş, Uluca Kaya B, Gümüşyayla Ş. Association between Guillain−Barré syndrome and COVID‐19 infection: experience of a Turkish neurophysiology laboratory. Noro Psikiyatr Ars. 2022;59:255‐259. doi: 10.29399/npa.27855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pourbakhtyaran E, Heidari M, Akbari MG, et al. Childhood Guillain−Barré syndrome in the SARS‐CoV‐2 era: is there any causative relation? Clin Case Rep. 2022;10:e6772. doi: 10.1002/ccr3.6772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Poletti P, Tirani M, Cereda D, et al. Seroprevalence of and risk factors associated with SARS‐CoV‐2 infection in health care workers during the early COVID‐19 pandemic in Italy. JAMA Netw Open. 2021;4:e2115699. doi: 10.1001/jamanetworkopen.2021.15699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cao‐Lormeau V‐M, Blake A, Mons S, et al. Guillain−Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case−control study. Lancet. 2016;387:1531‐1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Benetti E, Tita R, Spiga O, et al. ACE2 gene variants may underlie interindividual variability and susceptibility to COVID‐19 in the Italian population. Eur J Hum Genet. 2020;28:1602‐1614. doi: 10.1038/s41431-020-0691-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huh K, Jung J, Hong J, et al. Impact of nonpharmaceutical interventions on the incidence of respiratory infections during the coronavirus disease 2019 (COVID‐19) outbreak in Korea: a nationwide surveillance study. Clin Infect Dis. 2021;72:e184‐e191. doi: 10.1093/cid/ciaa1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee H, Heo N, Kwon D, Ha J. Deciphering changes in the incidence of the Guillain−Barré syndrome during the COVID‐19 pandemic: a nationwide time‐series correlation study. BMJ Neurol Open. 2022;4:e000378. doi: 10.1136/bmjno-2022-000378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Butler DL, Imberti L, Quaresima V, et al. Abnormal antibodies to self‐carbohydrates in SARS‐CoV‐2‐infected patients. PNAS Nexus. 2022;1:pgac062. doi: 10.1093/pnasnexus/pgac062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Borenstein M, Higgins JPT, Hedges LV, Rothstein HR. Basics of meta‐analysis: I 2 is not an absolute measure of heterogeneity. Res Syn Meth. 2017;8:5‐18. doi: 10.1002/jrsm.1230 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
Data extracted from included studies, data used for all analyses, analytic code are available upon reasonable request.


