Abstract
This study investigates whether exercise as a strategy for improving physical fitness at sea level also offers comparable benefits in the unique context of high altitudes (HA), considering the physiological challenges of hypoxic conditions. Overall, 121 lowlanders who had lived on the Tibetan Plateau for >2 years and were still living at HA during the measurements were randomly classified into four groups. Each individual of the low‐intensity (LI), moderate‐intensity (MI), and high‐intensity (HI) groups performed 20 sessions of aerobic exercise at HA (3680 m) over 4 weeks, while the control group (CG) did not undergo any intervention. Physiological responses before and after the intervention were observed. The LI and MI groups experienced significant improvement in cardiopulmonary fitness (0.27 and 0.35 L/min increases in peak oxygen uptake [O2peak], both p < 0.05) after exercise intervention, while the hematocrit (HCT) remained unchanged (p > 0.05). However, HI exercise was less efficient for cardiopulmonary fitness of lowlanders (0.02 L/min decrease in O2peak, p > 0.05), whereas both the HCT (1.74 %, p < 0.001) and glomerular filtration rate (18.41 mL/min, p < 0.001) increased with HI intervention. Therefore, LI and MI aerobic exercise, rather than HI, can help lowlanders in Tibet become more acclimated to the HA by increasing cardiopulmonary function and counteracting erythrocytosis.
Keywords: cardiopulmonary function, erythrocyte, exercise intensity, high altitude
Highlights
Low‐ and moderate‐intensity exercise at HA can improve oxygen transport of lowlanders that may help them to become more acclimated to high altitude.
High‐intensity exercise performed by lowlanders may result in erythrocytosis without improving oxygen transport.
The relationship between physiological system changes induced by exercise performed at HA is modulated by exercise intensity.
1. INTRODUCTION
It has been documented that around 400 million people live at altitudes above 1500 m, and more than 100 million lowlanders travel to high altitudes (HA) annually (Mallet et al., 2021). With increasing altitude, reductions in barometric pressure, and a fraction of inspired oxygen (FIO2), a subsequent decrease in ambient partial pressure of oxygen (PaO2), associated with a decline in arterial oxygen saturation (SaO2) will challenge the oxygen (O2) transport chain and O2 supply. Due to the limited O2 delivery, individuals who are acutely exposed to HA hypoxia environments may be at a risk for acute mountain sickness (AMS). This condition presents symptoms, including headache, anorexia, sleep difficulty, or a combination of these symptoms (Bärtsch & Swenson, 2013). However, for chronic HA exposure lowlanders (people who originally lived near sea level but moved to HA for months or years), a series of physiological acclimatization responses is required to support tissue oxygenation.
This HA acclimatization in lowlanders is a well‐coordinated set of physiological responses to ensure O2 availability through adjustment in the respiratory system, cardiovascular system, and hematologic system (Goldfarb‐Rumyantzev & Alper, 2014; Luks & Hackett, 2022). Ventilatory response is the initial major physiological response to HA exposure, which increases minute ventilation ( E) and partially attenuates reductions in PaO2 (West, 2006). A subsequent increase in cardiac output (CO), associated with an increase in heart rate (HR), alters the distribution of blood flow, and enhances O2 extraction from capillary blood to improve tissue oxygenation (Harold et al., 2012; Naeije, 2010). After chronic HA exposure, CO in lowlanders returns to normal levels with a slight increase in HR and a decrease in stroke volume (SV) (Hartley et al., 1967; Naeije, 2010). When CO remains stable and cannot contribute to an increased O2 extraction, acclimatization to HA hypoxia in most lowlanders involves an increase in hematocrit (HCT) level to maintain arterial O2 content (Gassmann et al., 2019). However, despite the increase in HCT that can contribute to acclimatization, lowlanders living at HA still have some degree of compromised adaptation to chronic hypoxic stress. This is because high HCT levels are associated with increased blood viscosity and increased risk of cardiac events, which results in a reduction in fitness and maladjustment (Vargas & Spielvogel, 2006). Meanwhile, the O2 supply in acclimated lowlanders also remains insufficient, as indicated by reduced maximal oxygen uptake (O2max) (Calbet et al., 2003). Although most of the HA residents are free of disease, they live in a state of chronic tissue hypoxia, and often have severe hypoxemia (West, 2017). How to ensure that HA lowlanders, such as the military and mountaineers, remain unaffected by unavoidable hypoxia stress is still unclear.
Exercise significantly improves aerobic capacity under normobaric normoxic conditions (Crowley et al., 2022) and enhances the release of oxygen and the deformability of red blood cells, both of which contribute to the improvement of tissue O2 supply (Mairbäurl, 2013). Given that limited O2 supply is a most prominent challenge after HA exposure, it is reasonable to predict that exercise may improve the level of HA acclimatization level by increasing tissue oxygenation. However, the relevant findings from HA hypoxia condition research remain less consistent. Previous studies have revealed that high‐intensity intermittent exercise at HA may worsen pulmonary interstitial edema (Edsell et al., 2014); however, the findings have been challenged by recent evidence. For example, a review study concluded that dual stimulation from hypoxia and exercise can induce HA acclimatization (Zhang & Chen, 2018). Another study reported that submaximal exercise performed under conditions of light or moderate hypoxia induced greater metabolic and cardiac responses (Park et al., 2022). Exercise intensity is one of the most important factors regarding the physiological responses to HA exercise, which may contribute to these contradictory findings. The intensity of exercise at HA plays a major role in the subsequent physiological acclimatization (e.g., respiratory, cardiovascular, and hematologic changes) (Mazzeo, 2008). However, most available reports attempting to classify exercise intensity are inaccurate as they do not consider the effects of HA exposure on HR and O2max (Mourot, 2018). Given that chronic HA exposure lowlanders are not fully adapted to the hypoxia environment, excessively high exercise intensity may have detrimental effects. Hence, a comprehensive investigation is needed to determine the optimal exercise intensity to promote HA physiological acclimatization.
To systematically study the effects of exercise with different intensity on the physiological acclimatization of HA lowlanders, the present study implemented exercise interventions with low‐, medium‐, and high‐intensity performed by young lowlanders who had lived at HA for more than 2 years. This was followed by measuring the cardiovascular function, O2 transport of red blood cells, renal function (Palubiski et al., 2020), and immune function (Mishra & Ganju, 2010; Pham et al., 2021), given the reports of their association with HA acclimatization. Based on accumulated evidence, hypotheses are made as follows: (H1) LI aerobic exercise does not cause changes in physiological performance; (H2) MI aerobic exercise can improve O2 transport capacity but not alter the blood viscosity; (H3) HI aerobic exercise can also improve O2 transport capacity, but increase the blood viscosity, renal, and immune response.
2. MATERIALS AND METHODS
2.1. Participants
A total of 160 male students from Tibet University were recruited. All participants were expected to meet the following inclusion criteria: Healthy Han Chinese immigrants (lowlanders) living in Lhasa, on the Tibetan Plateau (3680 m) for >2 years, and their birthplace altitude was <1500 m; had not visited HA areas before attending university; no addiction to cigarette or alcohol; no respiratory or motor system disease, and were fit for exercise according to the Physical Activity Readiness Questionnaire (Shephard, 1988). Thirty‐nine subjects were excluded from data analysis for reasons including scheduling conflicts (n = 27) and missing data (n = 12). Thus, 121 participants were included in the final data analysis. Their daily activity was assessed using the International Physical Activity Questionnaire (IPAQ). Further, all participants were asked to maintain their habitual lifestyle during the study.
The ethics committee of Tibet University approved this study (protocol code: XZTU2021ZRG‐06), and participants provided written informed consent. Data measurement and exercise interventions for all participants were conducted at Lhasa, Tibet.
2.2. Experimental procedure
Before the exercise intervention, blood sampling collection, self‐reported questionnaire on demographic data, basic physiological parameters (including HR, percutaneous arterial oxygen saturation [SpO2], body temperature, and blood pressure), and cardiopulmonary exercise testing (CPET) were carried out. Participants were randomly categorized into four groups: low‐intensity (LI; n = 34), moderate‐intensity (MI; n = 30), and high‐intensity (HI; n = 25) exercise groups and the control group (CG; n = 32), which did not involve any intervention. The HR range of each exercise intensity was classified according to individual heart rate reserve (HRR). HRR was calculated by subtracting resting HR (HRresting) from peak HR (HRpeak). This is as follows: LI interval = [0.3 HRR + HRresting, 0.39 HRR + HRresting]; MI interval = [0.4 HRR + HRresting, 0.59 HRR + HRresting]; HI interval = [0.6 HRR + HRresting, 0.89 HRR + HRresting] (Garber et al., 2011).
Participants in the intervention groups were instructed to perform 20 exercise sessions within 30 days. At each exercise intervention, the HR of participants was monitored using a polar heart rate meter (Polar OH1, Polar Electro Oy). We asked participants to reach their target HR within the first 5 min of exercise, and if their HR was not within the target range, they were reminded to adjust their speed. A single intervention ended when participants exercised at their target HR for 20 min, with the total duration not exceeding 30 min. Exercise was conducted on a motorized treadmill at the Exercise and Physiology Laboratory of Tibet University in August and September 2021. Participants completed the experimental posttest within 72 h after the last intervention to replicate all measures conducted before the start of the experiment.
2.3. Blood tests
Participants were asked to refrain from drinking alcohol and vigorous exercising before blood collection. Fasting blood samples were collected via standard venipuncture techniques approach in a sitting position from 7:00 a.m. to 9:00 a.m. at Fokind Medical Care hospital in Lhasa, Tibet. Venous blood samples were drawn for plasma analysis of the erythrocyte index (red blood cell count [RBC], hemoglobin [HGB], hematocrit [HCT], mean corpuscular volume [MCV], mean corpuscular hemoglobin concentration [MCHC], standard deviation of red cell distribution width [RDW‐SD], coefficient of the variation of red cell volume distribution width [RDW‐CV]), immune function (total protein [TP], globulin [GLB], albumin [ALB], and albumin/globulin [A/G]), renal function (creatinine [CREA], uric acid [UA], urea and glomerular filtration rate [GFR]). The GFR was calculated using the chronic kidney disease epidemiology collaboration equation (Levey et al., 2009). Blood samples were analyzed in a standardized environment within 12 h after collection at Fokind Medical Care.
2.4. Electrocardiography measures
Assessment of HRresting, CO, and stroke volume (SV) was done using the Biopac MP150 hardware system (MP150, BIOPAC® Systems, Inc.). Specifically, the electrocardiogram (ECG) signal was recorded using an ECG100 C module for calculating HRresting, the ECG lead numbers were 3, and the sampling frequency was 1 kHz. The impedance cardiography signal was recorded using an EBI100 C module to assess the SV and CO. The EBI100 C connected pairs of EL500 electrodes were attached to the neck and torso, and the amplifier, high‐pass filter, low‐pass filter, and frequency were set at 5 Ω/V, DC, 10 Hz, and 5 kHz, respectively. During the experiment, participants were asked to sit comfortably and quietly for 10 min. Raw ECG was used to measure the HRresting of participants, while SV and CO were exported using the noninvasive cardiac output analysis employing AcqKnowledge software (v 4.2, Biopac Systems Inc.).
2.5. Cardiopulmonary exercise testing
Cardiopulmonary function was assessed using CPET. After a 1‐min rest on the power bicycle (EC3000e, Ergoline GmbH), participants then pedaled, maintaining a speed of 55–65 revolutions/min. The resistance of the power bicycle was increased by 30 W/min, starting from 0. Exhaustion was considered to have been reached when the participant could not maintain speed and the respiratory exchange ratio was exceeding 1.1 or when neither O2 nor HR increased further with increasing speed/power (Lach et al., 2021). The O2 measured at this point was considered the true peak oxygen uptake (O2peak) of the participants in the HA environment followed by obtaining the HRpeak, E, and work rate (WR) corresponding to this point.
2.6. Statistical analysis
The Kolmogorov–Smirnov normality test was used to verify the normality of the observations. Based on the test outcome, the observations that did not satisfy the normality criterion were subjected to logarithmic transformation for subsequent analysis. One‐way analysis of variance (ANOVA) was used to compare baseline data between groups. The remaining data were analyzed using 2 (time: pretest and posttest) × 4 (group: CG, LI, MI, and HI) repeated measures ANOVA, where the group was a between‐participants factor and time was a within‐participants factor. Bonferroni post hoc test was used to detected significant differences between groups and times. The criterion for significance was 0.05, and all p‐values were two‐tailed. Effect sizes were calculated using Cohen's method; low correlation range was 0.0–0.39, moderate 0.4–0.69, and high ≥0.7 (Cohen, 1988). Data are presented as means (SD) unless otherwise indicated.
The changes in the observations were calculated by subtracting the pretest value from the posttest value for correlation and mediation analyses. Pearson's correlation was applied to estimate the relationships between the changes in physiological responses. Structural equation modeling was performed to further assess the mediation effect between physiological functions. We used the bootstrapping methods recommended by Preacher and Hayes for testing mediation hypotheses using a resampling procedure of 5000 bootstrap samples (Preacher & Hayes, 2004). The ANOVA test and correlation analysis were calculated using IBM SPSS statistics 23 (IBM Corp.) software. Further mediation analysis was performed using the Mplus statistical package (version 8, Muthe'n & Muthe'n). Data visualization was done with RStudio (Version 2022.07.2, RStudio).
3. RESULTS
Table 1 presents the fundamental demographic information of the four groups. The participants in each group were matched in age, height, weight, body mass index, SpO2, HRresting, and IPAQ (both p > 0.077). In addition, the baseline characteristics showed no significant differences across the four groups (both p > 0.05).
TABLE 1.
Baseline characteristics for the control group and three intervention groups.
| Variables | Control group (n = 32) | Low‐intensity group (n = 34) | Moderate‐intensity group (n = 30) | High‐intensity group (n = 25) |
|---|---|---|---|---|
| Age (yrs) | 21.00 (0.98) | 21.26 (1.05) | 21.50 (1.31) | 20.88 (1.13) |
| Height (m) | 1.73 (0.07) | 1.74 (0.06) | 1.77 (0.05) | 1.75 (0.05) |
| Weight (kg) | 66.6 (15.5) | 64.0 (6.9) | 67.3 (10.3) | 63.4 (9.4) |
| BMI (kg/m2) | 22.23 (4.49) | 21.20 (2.08) | 21.55 (2.80) | 20.79 (3.06) |
| SpO2 (mmHg) | 91.3 (2.3) | 89.6 (4.0) | 90.3 (3.6) | 91.2 (2.4) |
| HRresting (bpm) | 81 (11) | 82 (12) | 80 (15) | 88 (9) |
| IPAQ | 2036 (1321) | 2602 (1752) | 3250 (3993) | 1982 (1323) |
Abbreviations: BMI, body mass index; HRresting, resting heart rate; IPAQ, international physical activity questionnaire; SaO2, arterial oxygen saturation.
3.1. Cardiopulmonary function
The changes in cardiopulmonary function before and after the exercise intervention were analyzed. The time × group interactions for O2peak [F (3, 117) = 11.561, p < 0.001, η 2 = 0.229] were significant. Figure 1A illustrates that the LI and MI exercises induced an increase in O2peak (both p < 0.001), whereas there was no significant change in HI group (p = 0.680). Although CG also exhibited a significant increase in O2peak, the improvement in O2peak was almost twofold higher in LI and MI compared with CG (Table 2). Further, CO was significantly elevated in the LI group (p = 0.001), while HRresting remained unchanged in this group (Figure 1A, Table 2). This suggested that the improvement in SV (12.64 mL/beat) could contribute to the increased CO during low intensity exercise. Furthermore, the LI and MI exercise had a significant increase in E (p = 0.002 and p < 0.001), while it was unchanged in the HI group (Table 2). The results suggested that exercise intensity is important for the improvement in cardiorespiratory fitness in HA lowlanders.
FIGURE 1.
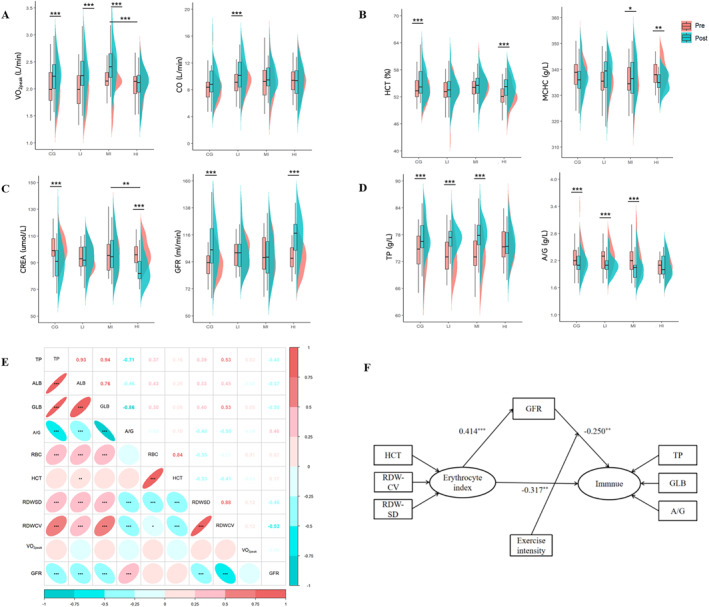
The changes and correlations among cardiopulmonary function, erythrocyte index, renal function, and immune function after exercise intervention. (A) Changes in cardiopulmonary function before and after intervention among four groups. (B) Changes in erythrocyte index before and after intervention among four groups. (C) Changes in renal function before and after intervention among four groups. (D) Changes in immune function before and after intervention among four groups. (E) The relationship between each system. The colors represent the relative direction. The numbers above the diagonal are Pearson’s correlation coefficients. (F) Two pathways by which erythrocyte function affects immune function. *p <.05, **p <.005, ***p <.001. A/G, albumin/ globulin; ALB, albumin; CO, cardiac output; CREA, creatinine; GFR, glomerular filtration rate; GLB, globulin; HCT, hematocrit; MCHC, mean corpuscular hemoglobin concentration; RBC, red blood count; RDW‐CV, coefficient of variation of red cell volume distribution width; RDW‐SD, standard deviation of red cell distribution width; TP, total protein; O2peak, peak oxygen uptake.
TABLE 2.
Cardiopulmonary function and erythrocyte index before and after the 4‐week exercise.
| Variables | Control group | Low‐intensity group | Moderate‐intensity group | High‐intensity group | Interaction effect | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Delta (Δ) | Mean (SD) | Delta (Δ) | Mean (SD) | Delta (Δ) | Mean (SD) | Delta (Δ) | p | η 2 | |
| Cardiopulmonary function | ||||||||||
| O2peak (L/min) | ||||||||||
| Pretest | 2.05 (0.38) | 0.162 a | 2.00 (0.36) | 0.271 a | 2.11 (0.32) | 0.350 a | 2.09 (0.28) | −0.020 d | <0.001 | 0.229 |
| Posttest | 2.22 (0.36) | 2.27 (0.40) | 2.46 (0.38) | 2.07 (0.29) | ||||||
| HRpeak (bpm) | ||||||||||
| Pretest | 177 (12) | −0.563 | 173 (13) | 1.529 | 174 (15) | −1.167 | 180 (8) | −8.680 a | 0.004 | 0.109 |
| Posttest | 177 (9) | 175 (12) | 173 (12) | 171 (12) | ||||||
| E (L/min) | ||||||||||
| Pretest | 119.14 (28.80) | 5.466 | 110.71 (23.55) | 12.327 a | 116.04 (26.12) | 16.020 a | 115.06 (17.29) | −4.140 d | 0.005 | 0.105 |
| Posttest | 124.61 (26.95) | 123.03 (28.38) | 132.06 (26.67) | 110.92 (20.65) | ||||||
| WR (W) | ||||||||||
| Pretest | 199 (30) | 9.375 | 204 (33) | 6.471 | 209 (33) | 6.000 | 200 (22) | 2.600 | 0.682 | 0.013 |
| Posttest | 208 (26) | 210 (29) | 215 (27) | 203 (23) | ||||||
| CO (L/min) | ||||||||||
| Pretest | 8.36 (2.29) | 0.700 | 9.09 (1.68) | 1.249 a | 9.18 (2.67) | 0.545 | 9.52 (2.14) | −0.390 | 0.041 | 0.068 |
| Posttest | 9.06 (2.30) | 10.34 (2.46) | 9.72 (2.21) | 9.13 (2.32) | ||||||
| SV (mL/beat) | ||||||||||
| Pretest | 103.42 (26.63) | 7.039 | 112.73 (24.41) | 12.640 | 116.70 (29.85) | 6.205 | 108.56 (22.72) | 7.485 | 0.603 | 0.016 |
| Posttest | 110.46 (26.40) | 125.37 (25.06) | 122.90 (32.68) | 116.05 (26.40) | ||||||
| LCWI (kg.m) | ||||||||||
| Pretest | 4.83 (1.40) | 0.392 | 5.02 (1.32) | 0.712 a , b | 5.11 (1.49) | 0.271 | 5.44 (1.13) | −0.176 | 0.049 | 0.065 |
| Posttest | 5.22 (1.46) | 5.90 (1.33) | 5.38 (1.23) | 5.26 (1.24) | ||||||
| HRresting (bpm) | ||||||||||
| Pretest | 81 (11) | 3.456 | 82 (12) | 0.131 | 80 (15) | −0.159 | 88 (9) | −9.549 a | 0.001 | 0.139 |
| Posttest | 85 (12) | 82 (10) | 80 (11) | 78 (12) | ||||||
| O2/HR (mL/min/bpm) | ||||||||||
| Pretest | 14.16 (2.78) | 0.406 | 13.65 (2.55) | 1.971 a | 14.23 (2.25) | 2.500 a | 13.56 (2.20) | 1.120 a | 0.009 | 0.093 |
| Posttest | 14.56 (2.40) | 15.62 (3.14) | 16.73 (3.75) | 14.68 (2.79) | ||||||
| E/ CO2 | ||||||||||
| Pretest | 47.17 (6.53) | 1.056 | 43.32 (7.63) | 3.521 a | 42.44 (6.11) | 5.430 a | 46.44 (4.09) | 1.308 | 0.038 | 0.069 |
| Posttest | 48.23 (7.64) | 46.84 (5.85) | 47.87 (5.47) | 47.75 (5.17) | ||||||
| Erythrocyte index | ||||||||||
| RBC (10^12/L) | ||||||||||
| Pretest | 5.85 (0.41) | 0.115 | 5.87 (0.30) | 0.083 | 5.92 (0.42) | 0.042 | 5.66 (0.36) | 0.111 | 0.662 | 0.013 |
| Posttest | 5.96 (0.41) | 5.96 (0.39) | 5.96 (0.40) | 5.77 (0.34) | ||||||
| HGB (g/L) | ||||||||||
| Pretest | 182.03 (9.11) | 3.000 | 178.17 (11.93) | 2.618 | 181.47 (9.23) | 2.000 | 177.40 (8.14) | 4.080 | 0.746 | 0.010 |
| Posttest | 185.03 (9.50) | 180.79 (10.34) | 183.47 (8.99) | 181.48 (8.45) | ||||||
| HCT (%) | ||||||||||
| Pretest | 53.92 (3.01) | 1.059 a | 53.13 (3.33) | 0.521 | 54.18 (2.57) | 0.177 | 52.40 (2.56) | 1.740 a | 0.048 | 0.065 |
| Posttest | 54.98 (3.10) | 53.65 (2.99) | 54.35 (2.73) | 54.14 (2.50) | ||||||
| MCHC (g/L) | ||||||||||
| Pretest | 337.91 (6.23) | −1.281 | 335.32 (6.72) | 1.882 | 335.03 (7.47) | 2.567 a | 338.72 (4.97) | −3.560 a | 0.002 | 0.116 |
| Posttest | 336.63 (6.37) | 337.21 (7.09) | 337.60 (7.69) | 335.16 (3.68) | ||||||
| MCV (fL) | ||||||||||
| Pretest | 92.33 (3.56) | 0.034 | 90.58 (5.97) | −0.259 | 91.82 (6.10) | −0.353 | 92.67 (3.44) | 1.236 a , b | 0.005 | 0.105 |
| Posttest | 92.36 (3.40) | 90.32 (6.11) | 91.47 (6.79) | 93.91 (3.67) | ||||||
| RDW‐SD (fL) | ||||||||||
| Pretest | 43.64 (2.61) | −0.297 | 43.10 (2.44) | 0.750 a | 43.18 (3.50) | 0.647 a | 44.36 (1.76) | −0.664 | 0.009 | 0.093 |
| Posttest | 43.34 (1.93) | 43.85 (2.15) | 43.82 (3.10) | 43.69 (1.92) | 0.006 | 0.101 | ||||
| RDW‐CV (%) | ||||||||||
| Pretest | 13.26 (0.67) | −0.063 | 13.37 (0.82) | 0.274 a , b | 13.18 (0.77) | 0.217 a | 13.56 (0.72) | −0.484 a , c | <0.001 | 0.212 |
| Posttest | 13.20 (0.67) | 13.65 (0.83) | 13.40 (0.62) | 13.08 (0.55) | ||||||
Abbreviations: CO, cardiac output; Delta (Δ), the changes calculated by subtracting the pretest value from the posttest value; HCT, hematocrit; HGB, hemoglobin; HRmax, peak heart rate; HRresting, resting heart rate; LCWI, left ventricular stroke work index; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; RBC, red blood cell count; RDW‐CV, coefficient of variation of red cell volume distribution width; RDW‐SD, standard deviation of red cell distribution width; SV, stroke volume; E, ventilation; O2peak, peak oxygen uptake; , ventilatory equivalent for carbon dioxide; , maximal oxygen pulse; WR, work rate.
p < 0.05 for comparing changes in with‐in group from pretest to posttest.
p < 0.05 for the changes in posttest compared with control group.
p < 0.05 for the changes in posttest compared with low‐intensity group.
p < 0.05 for the changes in posttest compared with moderate‐intensity group.
3.2. Erythrocyte index
The exercise intervention significantly affected the blood O2 transport of lowlanders. For HCT, the time × group interaction [F (3, 117) = 2.714, p = 0.048, η 2 = 0.065] was significant. A total of 1.74% increase in HCT was observed during the 4‐week period in the HI group (p < 0.001), whereas HCT appeared to be less affected in the LI (p = 0.165) and MI (p = 0.657) groups (Figure 1B, Table 2). In contrast, MCHC, an indicator of the average oxygen‐carrying capacity of the red blood cells, significantly increased in the MI group (p = 0.038) and deceased in the HI group (p = 0.009). Interestingly, we also found an increased HCT in CG compared with baseline (p = 0.007), whereas RBC and HGB remained unchanged during the 4‐week period in both control and exercise groups (Table 2).
3.3. Renal function
The renal function outcomes are displayed in Table 3. There were no significant changes in urea and UA levels in any of the four groups. However, the significant time × group interactions for CREA [F (3, 117) = 9.405, p < 0.001, and η 2 = 0.194], and GFR [F (3, 117) = 9.672, p < 0.001, and η 2 = 0.199] were found. The CG and HI groups experienced significant decrease in CREA (both p < 0.001) and increases in GFR (both p < 0.001), whereas there were no changes in the LI and MI groups after 4 weeks of exercise (Figure 1C, Table 3). Meanwhile, the CREA was reduced to a lesser extent in the HI group than in the MI group (p = 0.005).
TABLE 3.
Change of the renal and immune function after the 4‐week aerobic exercise.
| Variables | Control group | Low‐intensity group | Moderate‐intensity group | High‐intensity group | Interaction effect | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Delta (Δ) | Mean (SD) | Delta (Δ) | Mean (SD) | Delta (Δ) | Mean (SD) | Delta (Δ) | p | η 2 | |
| Renal function | ||||||||||
| CREA (mg/dL) | ||||||||||
| Pretest | 100.16 (9.93) | −8.469 a | 94.09 (9.00) | −0.912 | 96.27 (13.45) | 0.367 | 97.68 (9.22) | −12.720 a , c | 0.000 | 0.194 |
| Posttest | 91.69 (14.12) | 93.18 (10.45) | 96.63 (14.37) | 84.96 (10.30) | ||||||
| GFR (mL/min) | ||||||||||
| Pretest | 93.33 (11.63) | 12.247 a | 100.31 (11.41) | 1.776 | 98.62 (15.90) | −0.013 | 96.13 (11.12) | 18.415 a , b , c | 0.000 | 0.199 |
| Posttest | 105.57 (19.24) | 102.09 (14.92) | 98.61 (17.71) | 114.54 (16.22) | ||||||
| Urea (mg/dL) | ||||||||||
| Pretest | 5.13 (1.38) | −0.103 | 4.80 (1.04) | −0.053 | 5.09 (1.03) | 0.253 b | 5.03 (1.34) | 0.468 a , b | 0.109 | 0.050 |
| Posttest | 5.02 (1.34) | 4.75 (0.93) | 5.34 (1.12) | 5.50 (1.08) | ||||||
| UA (mg/dL) | ||||||||||
| Pretest | 467.91 (93.68) | −3.594 | 474.85 (84.10) | −16.588 | 440.07 (80.88) | 4.300 | 456.80 (86.29) | 20.360 | 0.202 | 0.039 |
| Posttest | 464.31 (85.99) | 458.26 (94.26) | 444.37 (84.79) | 477.16 (68.12) | ||||||
| The immune function | ||||||||||
| TP (g/L) | ||||||||||
| Pretest | 74.27 (4.84) | 2.766 a | 73.29 (4.20) | 3.721 a | 73.24 (4.43) | 3.940 a | 75.90 (3.90) | −0.096 | 0.002 | 0.119 |
| Posttest | 77.03 (4.77) | 77.01 (2.87) | 77.18 (4.04) | 75.81 (3.47) | ||||||
| ALB (g/L) | ||||||||||
| Pretest | 51.15 (2.17) | 0.919 a | 50.65 (1.55) | 1.394 a | 50.13 (2.07) | 1.517 a | 50.97 (1.72) | 0.096 | 0.097 | 0.052 |
| Posttest | 52.07 (2.34) | 52.04 (1.43) | 51.64 (2.48) | 51.07 (1.71) | ||||||
| GLB (g/L) | ||||||||||
| Pretest | 23.12 (3.51) | 1.847 a | 22.64 (3.16) | 2.326 a | 23.11 (3.47) | 2.423 a | 24.93 (2.85) | −0.192 | 0.000 | 0.177 |
| Posttest | 24.96 (3.15) | 24.97 (2.28) | 25.54 (3.10) | 24.74 (2.71) | ||||||
| A/G | ||||||||||
| Pretest | 2.26 (0.35) | −0.147 a | 2.27 (0.26) | −0.171 a | 2.21 (0.34) | −0.157 a | 2.07 (0.22) | 0.016 | 0.000 | 0.185 |
| Posttest | 2.12 (0.24) | 2.10 (0.21) | 2.06 (0.29) | 2.08 (0.24) | ||||||
Abbreviations: A/G, albumin/globulin; ALB, albumin; CREA, creatinine; Delta (Δ), the changes calculated by subtracting the pretest value from the posttest value; GFR, glomerular filtration rate; GLB, globulin; TP, total protein; UA, uric acid.
p < 0.05 for comparing changes in with‐in group from pretest to posttest.
p < 0.05 for the changes in posttest compared with low‐intensity group.
p < 0.05 for the changes in posttest compared with moderate‐intensity group.
3.4. Immune function
As shown in Table 3, the levels of TP, ALB, and GLB increased to a similar extent in CG and LI and MI groups at posttest compared to pretest (both p < 0.001), whereas the A/G was decreased in CG and LI and MI groups (both p < 0.001). Those results suggested a slight increase in the immune responses during the 4‐week period in the above groups. In contrast, the immune function remained unaltered in the HI group after the 4‐week exercise interventions (Figure 1D, Table 3).
3.5. A moderated mediation model
The relationship between changes in physiological functions after exercise performed in an HA environment and the role of different exercise intensities were explored by calculating the changes in physiological responses by subtracting the pretest from the posttest value using a structural equation model. Before the structural equation analysis, Pearson's correlation coefficients were calculated to estimate the relationship between changes in physiological functions. The relationships between multiple datasets are shown in Figure 1E.
According to the model (Figure 1F), the kidney function mediated the relationship between erythrocyte indices and immune function (β = −0.104, 95% confidence interval [CI95] [−0.203, −0.040]). The direct effect of erythrocyte indices on immune function was significant (β = −0.317, CI95 [−0.537, −0.159]). The moderating effect of exercise intensity was also significant. When exercise intensity was low, the indirect effect of erythrocyte indices on immune function was significant (β = 0.182, CI95 [0.047, 0.357]); however, when exercise intensity was moderate (β = 0.020, CI95 [−0.066, 0.124]) or high (β = 0.009, CI95 [−0.250, 0.046]), the indirect effect was not significant. Model fit was assessed using a comparative fit index (CFI = 0.879) and the Tucker‐–Lewis index (TLI = 0.853).
4. DISCUSSION
Our study examined the effect of long‐term aerobic exercise with different intensities on the physiological responses of acclimated lowlanders. We found that LI and MI exercises are effective stimuli for the improvement in O2 transport and the prevention of erythrocytosis in acclimatized lowlanders. However, HI exercise is less effective than LI and MI exercise as results in erythrocytosis, and induces an overloaded renal adaption to HA with no improvement in O2 transport. These results have essentially validated the previously formulated hypotheses.
Given that hypobaric hypoxia is unavoidable and sustained at HA, maintaining aerobic metabolism and adjusting physiological acclimatization is important at HA (McClelland & Scott, 2019). The indigenous people living on the Tibetan plateau are a prime example of successful adaptation to HA (Wu, 2001). Studies have shown that Tibetan natives, compared to lowlanders at HA, exhibit greater exercise capacity and lower HGB concentrations (Simonson et al., 2015). Lowlanders exposed to HA obtain higher O2 uptake mainly by increasing HGB; however, excessive erythrocytosis is associated with risk for cardiovascular disease and is considered maladaptive (Julian & Moore, 2019). Our study showed that LI and MI exercises improve O2 transport without erythrocytosis. Participants in the LI and MI groups experienced an increase in O2peak after the intervention, consistent with a previous study finding that exercise training in highlanders can successfully improve aerobic capacity and exercise workload (Macarlupu et al., 2021). Additionally, we found that LI exercise increased CO, as has been reported previously (Nystoriak & Bhatnagar, 2018). Increased CO level is the initial cardiovascular response to altitude and can increase aerobic capacity; lowlanders chronically exposed to HA are known to return to the normal baseline CO (Naeije, 2010). This observation provides new insights into the simultaneous increase of CO and O2peak in an HA environment, both of which can increase the diffusional transport of O2.
Further analysis showed that LI and MI exercises did not produce a significant increase in the erythrocyte index after the intervention, which differs from the results of the “Living Low‐Training High” (LLTH) model. The success of this model is attributable to the erythropoietic effects of hypoxia (Wilber et al., 2007). Some studies have confirmed that the erythrocytes of lowlanders during prolonged exposure to HA tend to stabilize as the duration of altitude exposure extends (Luks & Hackett, 2022). Therefore, the LI exercise does not disrupt homeostasis and does not lead to erythrocytosis. It is sufficient to change the body's respiratory function (Crowley et al., 2022) and improve O2 uptake. In particular, we found an increase in immune response after LI and MI exercise. The results are consistent with previous studies reporting an increase in oxidative stress and the subsequent inflammatory response in LI exercise at chronic HA exposure (Quindry et al., 2016). This may serve a protective role by setting an immune response in motion (Pham et al., 2021). Thus, the upregulated immune response in our study could be considered as an adaptive regulation of inflammation to promote O2 supply (Harris et al., 2014).
However, in the present study, lowlanders experienced some adverse effects when performing HI exercise at HA, such as erythrocytosis without any improvement in aerobic capacity. Unlike the LLTH study that was performed at moderate altitudes, our study was performed at 3680 m, and showed that the increase in RBC induced by HA exercise did not lead to an increase in O2 transport capacity. This may be because exercise is a stressor, and the effect of stress on organisms is dose‐dependent (Salminen & Kaarniranta, 2010), suggesting that a higher intensity of stress overwhelms the organism and can evoke detrimental effects (Gems & Partridge, 2008) and disrupt physiological homeostasis. Moreover, an elevated RBC level increases HCT level and blood viscosity; therefore, the O2 uptake of the participants did not improve by exercise (Crawford et al., 2017). In addition, we found that different from LI and MI of exercises, GFR increased as HCT increased after the HI exercise. However, previous studies showed that the increase in HCT was accompanied by an increase in blood viscosity at chronic exposure to HA, which may lead to a decreased GFR (Palubiski et al., 2020). In our study, given the physiological challenge of both hypoxia and HI exercise, renal adaption to HA could be overloaded, potentially resulting in an elevated GFR.
Notably, during the current experiment, some changes occurred in the CG, including slight increases in O2peak, HCT, GFR, and TP, which may have been caused by a decrease in FIO2 and oxygen concentration in atmosphere. Shi et al. (2021) found that oxygen concentration on the Tibetan plateau is positively related to air temperature. Our study was conducted over a month from August to September, when the air temperature gradually decreased from 18°C to 13°C and the oxygen concentration decreased from 20.282% to 20.176%. Thus, the increase of O2peak in CG is associated with increases in blood viscosity and renal function responses, which may be a potential compensation mechanism to resist the adverse impacts of decrease in O2 supply (Luks, 2015; Palubiski et al., 2020; Simonson et al., 2015). This mechanism is commonly employed by the lowlanders who experience chronic exposure to HA to ensure enough O2 supply (West, 2017). However, for LI and MI groups, the increase in O2peak did not lead to physiological compensation, even when accounting for the challenge of a decline in oxygen concentration.
Finally, our study demonstrated that GFR mediates the effect of erythrocytes on the immune function, which was strengthened by lower intensity activity. Considering that human erythrocytes have an important function in the innate immune system (Hotz et al., 2018), the direct relationship between the erythrocyte index and immunity may be expected. Additionally, as mentioned above, the blood viscosity of lowlanders was increased at chronic HA exposure due to the increase in HCT, which was accompanied by an adaptative response in renal plasma flow and caused the change in GFR (Palubiski et al., 2020). The kidneys further regulate fluid and acid–base homeostasis to ensure physiological adaptation to HA, which may be related to individual response to inflammation (Goldfarb‐Rumyantzev & Alper, 2014), since resistance to hypoxia stress is associated with anti‐inflammation responses. By this means, renal function mediates the relationship between erythrocytes and immunity. The intensity and duration of exercise stress have a dose‐dependent effect on the induction of in vivo immunity in humans (Diment et al., 2015). The change in renal function during LI exercise positively affects the change in immune function, which is a normal physiological regulation of the body. However, intense physical activity at HA exacerbates the extent of the oxidative challenge (Dosek et al., 2007). Therefore, this regulatory response gradually diminishes with an increase in exercise intensity. This finding provides further evidence that an intensity threshold exists for exercise performed at HA and that excessively high intensities of exercise can have negative effects.
While this study fills the gap in understanding the physiological response after HA exercise with different intensities, it also unavoidably possesses certain limitations. First, the participants were homogeneous and the experiment was conducted at a fixed altitude (3680 m), challenging the generalizability of the results. Second, the measurements used to evaluate the individual systems and mechanistic explanation were not comprehensive; for example, only immune proteins in the blood were used to assess immune function. Future studies combining enzyme‐linked immunosorbent assay (ELISA) need to be conducted to explore the molecular mechanism of exercise effect on HA acclimatization. Third, although previous studies found similar HRpeak values (Basset & Boulay, 2003; Zhou et al., 1997) or slightly higher values (Price et al., 2022) in treadmill testing compared with cycle ergometers, conducting an HRpeak testing through cycle ergometers, but using treadmill exercise for intervention limits the accuracy of the exercise prescription of this study. The uniformity in measurement and intervention is expected to be improved in the future.
In conclusion, for lowlanders at HA, the benefits of exercise cannot be generalized. This study demonstrates that LI and MI exercises improve O2 uptake capacity while avoiding an excessive increase in red blood cells and inflammation (Figure 2); thus, further improving HA acclimatization among lowlanders on the Tibetan plateau. However, HI exercise does not improve O2 uptake capacity and increases blood viscosity. Therefore, subsequent studies should consider exercise mode, duration, and altitude to identify more effective exercise approaches to promote HA acclimatization.
FIGURE 2.
Changes in physiological acclimatization of lowlanders after low‐moderate intensity exercise. O2max and CO increased after the exercise intervention, and the erythrocyte index positively affected renal function, as indicated by the red arrow. The erythrocyte index and renal function negatively affected immune function, indicated by the gray arrows. ALB, albumin; CO, cardiac output; GFR, glomerular filtration rate; GLB, globulin; TP, total protein; O2max, maximal oxygen uptake.
AUTHOR CONTRIBUTIONS
Rui Su: Conceptualization, methodology, writing—reviewing and Editing. Chenxiao Han: Methodology, visualization, formal analysis. Guiquan Chen: Intervention, resources. Wanying Liu and Chengzhi Wang: Data curation. Yuming Zhang and Hao Li: Investigation, validation. Delong Zhang: Reviewing and Editing. Hailin Ma: Supervision, project administration, funding acquisition. All the authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors report no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation Regional innovation development joint fund project of China (U23A20476); Science and Technology Major Project of Tibetan Autonomous Region of China (XZ202201ZD0001G); National Natural Science Foundation of China (32260212); and Scientific Development funds for Local Region from the Chinese Government in 2023 (XZ202301YD0032C).
REFERENCES
- Bärtsch, Peter , and Swenson Erik R.. 2013. “Clinical Practice: Acute High‐Altitude Illnesses.” New England Journal of Medicine 368(24): 2294–2302. 10.1056/NEJMcp1214870. [DOI] [PubMed] [Google Scholar]
- Basset, F. A. , and Boulay M. R.. 2003. “Treadmill and Cycle Ergometer Tests Are Interchangeable to Monitor Triathletes Annual Training.” Journal of Sports Science and Medicine 2(3): 110–116. https://www.jssm.org/jssm‐02‐110.xml%3EFulltext. [PMC free article] [PubMed] [Google Scholar]
- Calbet, J. A. L. , Boushel R., Rådegran G., Søndergaard H., Wagner P. D., and Saltin B.. 2003. “Why Is VO2max after Altitude Acclimatization Still Reduced Despite Normalization of Arterial O2 Content?” American Journal of Physiology ‐ Regulatory, Integrative and Comparative Physiology 284(2): R304–R316. 10.1152/ajpregu.00156.2002. [DOI] [PubMed] [Google Scholar]
- Cohen, J . 1988. Statistical Power Analysis for the Behavioral Sciences. (2nd ed.). Hillsdale, NJ: Lawence Erlbaum. 10.4324/9780203771587. [DOI] [Google Scholar]
- Crawford, Jacob E. , Amaru Ricardo, Song Jihyun, Julian Colleen G., Racimo Fernando, Cheng Jade Yu, Guo Xiuqing, et al. 2017. “Natural Selection on Genes Related to Cardiovascular Health in High‐Altitude Adapted Andeans.” The American Journal of Human Genetics 101(5): 752–767. 10.1016/j.ajhg.2017.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley, Emmet , Powell Cormac, Carson Brian P., and W Davies Robert. 2022. “The Effect of Exercise Training Intensity on VO2max in Healthy Adults: an Overview of Systematic Reviews and Meta‐Analyses.” Translational Sports Medicine 2022: 1–10. 10.1155/2022/9310710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diment, Bethany C. , Fortes Matthew B., Edwards Jason P., Hanstock Helen G., Ward Mark D., Dunstall Huw M., Friedmann Peter S., and Walsh Neil P.. 2015. “Exercise Intensity and Duration Effects on In Vivo Immunity.” Medicine & Science in Sports & Exercise 47(7): 1390–1398. 10.1249/MSS.0000000000000562. [DOI] [PubMed] [Google Scholar]
- Dosek, Agoston , Ohno Hideko, Acs Zoltan, Taylor Albert W., and Radak Zsolt. 2007. “High Altitude and Oxidative Stress.” Respiratory Physiology & Neurobiology 158(2–3): 128–131. 10.1016/j.resp.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Edsell, Mark E. , Wimalasena Yashvi H., Malein William L., Ashdown Kimberly M., Gallagher Carla A., Imray Chris H., Wright Alex D., Myers Stephen D., and Society B. M. R. E.. 2014. “High‐intensity Intermittent Exercise Increases Pulmonary Interstitial Edema at Altitude but Not at Simulated Altitude.” Wilderness and Environmental Medicine 25(4): 409–415. 10.1016/j.wem.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Garber, Carol Ewing , Blissmer Bryan, Deschenes Michael R., Franklin Barry A., Lamonte Michael J., Lee I.‐Min, Nieman David C., Swain David P.. and Amer Coll Sports Med . 2011. “Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise.” Medicine & Science in Sports & Exercise 43(7): 1334–1359. 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- Gassmann, Max , Mairbäurl Heimo, Livshits Leonid, Seide Svenja, Hackbusch Matthes, Malczyk Monika, Kraut Simone, et al. 2019. “The Increase in Hemoglobin Concentration with Altitude Varies Among Human Populations.” Annals of the New York Academy of Sciences 1450(1): 204–220. 10.1111/nyas.14136. [DOI] [PubMed] [Google Scholar]
- Gems, David , and Partridge Linda. 2008. “Stress–response Hormesis and Aging: “That Which Does Not Kill Us Makes Us Stronger”.” Cell Metabolism 7(3): 200–203. 10.1016/j.cmet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Goldfarb‐Rumyantzev, A. S. , and Alper S. L.. 2014. “Short‐term Responses of the Kidney to High Altitude in Mountain Climbers.” Nephrology Dialysis Transplantation 29(3): 497–506. 10.1093/ndt/gft051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley, L. H. , Alexander J. K., Modelski M., and Grover R. F.. 1967. “Subnormal Cardiac Output at Rest and during Exercise in Residents at 3,100 M Altitude.” Journal of Applied Physiology 23(6): 839–848. 10.1152/jappl.1967.23.6.839. [DOI] [PubMed] [Google Scholar]
- Hotz, Meghan J. , Qing Danielle, Shashaty Michael G. S., Zhang Peggy, Faust Hilary, Sondheimer Neal, Rivella Stefano, Worthen G. Scott, and Mangalmurti Nilam S.. 2018. “Red Blood Cells Homeostatically Bind Mitochondrial DNA through TLR9 to Maintain Quiescence and to Prevent Lung Injury.” American Journal of Respiratory and Critical Care Medicine 197(4): 470–480. 10.1164/rccm.201706-1161OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian, Colleen G. , and Moore Lorna G.. 2019. “Human Genetic Adaptation to High Altitude: Evidence from the Andes.” Genes 10(2): 150. 10.3390/genes10020150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lach, Jacek , Wiecha Szczepan, Śliż Daniel, Price Szymon, Zaborski Mateusz, Cieśliński Igor, Postuła Marek, Knechtle Beat, and Mamcarz Artur. 2021. “HR Max Prediction Based on Age, Body Composition, Fitness Level, Testing Modality and Sex in Physically Active Population.” Frontiers in Physiology 12: 695950. 10.3389/fphys.2021.695950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin, M. Harold , Davis Michael J., Secher Niels H., van Lieshout Johannes J., Arce‐Esquivel Arturo A., Simmons Grant H., Bender Shawn B., et al. 2012. “Peripheral Circulation.” Comprehensive Physiology 2(1): 321–447. 10.1002/cphy.c100048. [DOI] [PubMed] [Google Scholar]
- Levey, Andrew S. , Stevens Lesley A., Schmid Christopher H., Zhang Yaping Lucy, Castro Alejandro F., Feldman Harold I., Kusek John W., et al. 2009. “A New Equation to Estimate Glomerular Filtration Rate.” Annals of Internal Medicine 150(9): 604–612. 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luks, Andrew M . 2015. “Physiology in Medicine: A Physiologic Approach to Prevention and Treatment of Acute High‐Altitude Illnesses.” Journal of Applied Physiology 118(5): 509–519. 10.1152/japplphysiol.00955.2014. [DOI] [PubMed] [Google Scholar]
- Luks, Andrew M. , and Hackett Peter H.. 2022. “Medical Conditions and High‐Altitude Travel.” New England Journal of Medicine 386(4): 364–373. 10.1056/NEJMra2104829. [DOI] [PubMed] [Google Scholar]
- Macarlupú, José Luis , Vizcardo‐Galindo Gustavo, Figueroa‐Mujíca Rómulo, Voituron Nicolas, Richalet J.‐Paul, and Villafuerte Francisco C.. 2021. “Sub‐maximal Aerobic Exercise Training Reduces Haematocrit and Ameliorates Symptoms in Andean Highlanders with Chronic Mountain Sickness.” Experimental Physiology 106(11): 2198–2209. 10.1113/EP089975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mairbäurl, Heimo . 2013. “Red Blood Cells in Sports: Effects of Exercise and Training on Oxygen Supply by Red Blood Cells.” Frontiers in Physiology 4: 332. 10.3389/fphys.2013.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet, Robert T. , Burtscher Johannes, Richalet J.‐Paul, Millet Gregoire P., and Burtscher Martin. 2021. “Impact of High Altitude on Cardiovascular Health: Current Perspectives.” Vascular Health and Risk Management 17: 317–335. 10.2147/VHRM.S294121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzeo, Robert S . 2008. “Physiological Responses to Exercise at Altitude: an Update. Sports Medicine.” Sports Medicine 38(1): 1–8. 10.2165/00007256-200838010-00001. [DOI] [PubMed] [Google Scholar]
- McClelland, Grant B. , and Scott Graham R.. 2019. “Evolved Mechanisms of Aerobic Performance and Hypoxia Resistance in High‐Altitude Natives.” Annual Review of Physiology 81(1): 561–583. 10.1146/annurev-physiol-021317-121527. [DOI] [PubMed] [Google Scholar]
- Mishra, K. P. , and Ganju Lilly. 2010. “Influence of High Altitude Exposure on the Immune System: A Review.” Immunological Investigations 39(3): 219–234. 10.3109/08820131003681144. [DOI] [PubMed] [Google Scholar]
- Mourot, Laurent . 2018. “Limitation of Maximal Heart Rate in Hypoxia: Mechanisms and Clinical Importance.” Frontiers in Physiology 9: 972. 10.3389/fphys.2018.00972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeije, Robert . 2010. “Physiological Adaptation of the Cardiovascular System to High Altitude.” Progress in Cardiovascular Diseases 52(6): 456–466. 10.1016/j.pcad.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Nystoriak, Matthew A. , and Bhatnagar Aruni. 2018. “Cardiovascular Effects and Benefits of Exercise.” Frontiers in Cardiovascular Medicine 5: 135. 10.3389/fcvm.2018.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palubiski, Lisa M. , O'Halloran Ken D., and O'Neill Julie. 2020. “Renal Physiological Adaptation to High Altitude: A Systematic Review.” Frontiers in Physiology 11: 756. 10.3389/fphys.2020.00756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, H.‐Young , Kim J.‐Weon, and Nam S.‐Seok. 2022. “Metabolic, Cardiac, and Hemorheological Responses to Submaximal Exercise under Light and Moderate Hypobaric Hypoxia in Healthy Men.” Biology 11(1): 144. 10.3390/biology11010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham, Kathy , Parikh Keval, and Heinrich Erica C.. 2021. “Hypoxia and Inflammation: Insights from High‐Altitude Physiology.” Frontiers in Physiology 12: 676782. 10.3389/fphys.2021.676782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher, Kristopher J. , and Hayes Andrew F.. 2004. “SPSS and SAS Procedures for Estimating Indirect Effects in Simple Mediation Models.” Behavior Research Methods 36(4): 717–731. 10.3758/BF03206553. [DOI] [PubMed] [Google Scholar]
- Price, Szymon , Wiecha Szczepan, Cieśliński Igor, Śliż Daniel, Kasiak Przemysław Seweryn, Lach Jacek, Gruba Grzegorz, Kowalski Tomasz, and Mamcarz Artur. 2022. “Differences between Treadmill and Cycle Ergometer Cardiopulmonary Exercise Testing Results in Triathletes and Their Association with Body Composition and Body Mass Index.” International Journal of Environmental Research and Public Health 19(6): 3557. 10.3390/ijerph19063557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quindry, John , Dumke Charles, Slivka Dustin, and Ruby Brent. 2016. “Impact of Extreme Exercise at High Altitude on Oxidative Stress in Humans.” The Journal of Physiology 594(18): 5093–5104. 10.1113/JP270651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen, Antero , and Kaarniranta Kai. 2010. “ER Stress and Hormetic Regulation of the Aging Process.” Ageing Research Reviews 9(3): 211–217. 10.1016/j.arr.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Shephard, Roy J . 1988. “PAR‐Q, Canadian Home Fitness Test and Exercise Screening Alternatives.” Sports Medicine 5(3): 185–195. 10.2165/00007256-198805030-00005. [DOI] [PubMed] [Google Scholar]
- Shi, Peijun , Chen Yanqiang, Zhang Gangfeng, Tang Haiping, Chen Zhi, Yu Deyong, Yang Jing, et al. 2021. “Factors Contributing to Spatial‐Temporal Variations of Observed Oxygen Concentration over the Qinghai‐Tibetan Plateau.” Scientific Reports 11(1): 17338. 10.1038/s41598-021-96741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson, T. S. , Wei G., Wagner H. E., Wuren T., Qin G., Yan M., Wagner P. D., and Ge R. L.. 2015. “Low Haemoglobin Concentration in Tibetan Males Is Associated with Greater High‐Altitude Exercise Capacity.” The Journal of Physiology 593(14): 3207–3218. 10.1113/JP270518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas P, Enrique , and Spielvogel Hilde. 2006. “Chronic Mountain Sickness, Optimal Hemoglobin, and Heart Disease.” High Altitude Medicine & Biology 7(2): 138–149. 10.1089/ham.2006.7.138. [DOI] [PubMed] [Google Scholar]
- Walmsley, Sarah , Harris Alison, Thompson A. A. Roger, and Whyte MoiraK. B.. 2014. “HIF‐Mediated Innate Immune Responses: Cell Signaling and Therapeutic Implications.” Hypoxia 2: 47–58. 10.2147/HP.S50269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, J. B . 2006. “Human Responses to Extreme Altitudes.” Integrative and Comparative Biology 46(1): 25–34. 10.1093/icb/icj005. [DOI] [PubMed] [Google Scholar]
- West, John B . 2017. “Physiological Effects of Chronic Hypoxia.” New England Journal of Medicine 376(20): 1965–1971. 10.1056/NEJMra1612008. [DOI] [PubMed] [Google Scholar]
- Wilber, Randall L. , Stray‐Gundersen James, and Levine Benjamin D.. 2007. “Effect of Hypoxic “Dose” on Physiological Responses and Sea‐Level Performance.” Medicine & Science in Sports & Exercise 39(9): 1590–1599. 10.1249/mss.0b013e3180de49bd. [DOI] [PubMed] [Google Scholar]
- Wu, Tianyi . 2001. “The Qinghai‐Tibetan Plateau: How High Do Tibetans Live?” High Altitude Medicine & Biology 2(4): 489–499. 10.1089/152702901753397054. [DOI] [PubMed] [Google Scholar]
- Zhang, Ying , and Chen Ning. 2018. “Autophagy Is a Promoter for Aerobic Exercise Performance during High Altitude Training.” Oxidative Medicine and Cellular Longevity 2018: 3617508–3617511. 10.1155/2018/3617508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, S. , Robson S. J., King M. J., and Davie A. J.. 1997. “Correlations between Short‐Course Triathlon Performance and Physiological Variables Determined in Laboratory Cycle and Treadmill Tests.” The Journal of Sports Medicine and Physical Fitness 37(2): 122. 10.1016/S1050-6411(97)88884-0. [DOI] [PubMed] [Google Scholar]


