Abstract
Background and purpose
Susac syndrome (SuS) is a rare, autoimmune, neurological disease characterized by a clinical triad of branch retinal artery occlusion, sensorineural hearing loss and encephalopathy. Neuropsychological functioning in SuS is little researched and the prevalence, nature, and evolution over time of cognitive deficits in SuS remain unclear. This study aimed to better understand the long‐term neuropsychological outcomes of patients with SuS.
Methods
Thirteen patients with SuS (mean [SD] age 39.5 [11.1] years) were enrolled at the Ghent University Hospital by their treating neurologist. The cognitive functioning and emotional well‐being of each patient was evaluated by means of a thorough neuropsychological test battery at baseline and after 2 years. Follow‐up testing after 2 years was performed in 11 patients (mean [SD] age 42.2 [11.5] years).
Results
Patients showed normal neuropsychological test results at a group level, both at baseline and follow‐up testing. Significant improvements over time were found for information processing speed, verbal recognition, and semantic and phonological fluency. Individual test results showed interindividual variability at baseline, with most impairments being in attention, executive functioning and language, which improved after a 2‐year period. In addition, patients reported significantly lower mental and physical well‐being, both at baseline and follow‐up testing.
Conclusions
Our results suggest that neuropsychological dysfunction in SuS is limited at a group level and improves over time. Nonetheless, individual test results reveal interindividual variability, making cognitive screening essential. Furthermore, a high psycho‐emotional burden of the disease was reported, for which screening and follow‐up are necessary.
Keywords: cognitive functioning, emotional well‐being, neurology, neuropsychology, Susac syndrome
INTRODUCTION
Susac syndrome (SuS) was first described in 1979 by John O. Susac as a rare, neurological disease characterized by a clinical triad of branch retinal artery occlusion, sensorineural hearing loss and encephalopathy [1]. It is considered an autoimmune microangiopathy with small infarcts in the retina, inner ear, and brain [2] and may have a relapsing course even decades after its initial onset [3]. The fundamental immunology of the disease is not fully understood, although recent data suggest that SuS is a CD8+‐mediated endotheliopathy [4].
Up until now, approximately 500 cases have been reported in the literature [5]. However, the true prevalence and incidence of SuS remain unknown, likely because many cases are misdiagnosed [6]. Wilf‐Yarkoni et al. reported at least a 5.4‐fold increase in the annual incidence of SuS [7]. Females appear to be more vulnerable to the disease, with the male: female ratio estimated to be 1:3.5. This is in line with the finding of a female predominance in autoimmune diseases [8].
Based on common clinical and paraclinical characteristics, diagnostic criteria for SuS were proposed [9]. To enable diagnosis of SuS, retinal fluorescein angiography, auditory and vestibular testing, and magnetic resonance imaging (MRI) are required. A definite diagnosis can be made if patients show brain, retinal and vestibulocochlear involvement. However, diagnosis is often delayed as only a minority (13%) of patients show the complete triad upon presentation [9].
Indication of brain involvement includes alterations of consciousness, cognitive impairment or behavioral changes, focal neurological symptoms, and headache (migrainous or oppressive) [9]. The neuroimaging triad includes white matter lesions, deep gray matter lesions and leptomeningeal enhancement [10]. Involvement of the corpus callosum on MRI is a prerequisite for the diagnosis of SuS, with typical callosal microinfarctions with “snowball”, “icicle” and “spoke” configurations [11]. Ischemic lesions, with restriction of the apparent diffusion coefficient on diffusion‐weighted imaging sequences, are always observed [12]. In addition to damage to white matter, research has also found evidence for involvement of gray matter in at least 70% of the cases (basal ganglia and thalamus lesions that typically manifested with increased signal intensity on T2, proton density and fluid‐attenuated inversion recovery images) [10].
Although cognitive and behavioral changes are often the main complaint of patients, these have not been thoroughly described [13]. Neuropsychological functioning in SuS is little researched and only few articles have described it in detail, mostly in the form of case reports [14, 15, 16, 17, 18]. These show a variability in type and severity in cognitive deficits over individuals, ranging from psycho‐motor slowness to deficits in memory, attention and executive functioning [15].
The most detailed study on cognitive functioning in SuS including 19 patients in disease remission showed a significant slowing in processing speed and executive dysfunction [13]. The authors also found extreme atrophy in the brain and, more particularly, in the corpus callosum as measured by SIENA (Structural Image Evaluation using Normalization of Atrophy), which progressed linearly with time in all patients, independently of clinical relapses and treatment. In their sample, the annual whole brain atrophy rate was 2.1%, while that of healthy adults is 0.2%. The annual reduction of the corpus callosum was even higher, with a rate of 5.3%. However, specific normative data on the corpus callosum in healthy subjects are lacking. This raises the hypothesis that there is either a secondary fiber loss following acute lesions over several months to years, or that there is a silent progression of the disease, independently of acute flares. However, a relationship between the cognitive impairments and whole brain/corpus callosum atrophy could not be found, which raises the question of what mechanisms underlie the cognitive alterations in these patients. The authors suggested the need for cognitive evaluation over time to investigate eventual further cognitive decline.
The aim of this study, therefore, was to better understand the long‐term neuropsychological involvement in SuS. We administered a comprehensive neuropsychological test battery in a cohort of SuS patients and repeated this test battery after 2 years. Based on previous reported cases, we hypothesize that patients with SuS will show significantly lower scores on tasks of processing speed, executive functioning and visuoconstructive abilities. In addition, we hypothesize that these scores will worsen over time since previous data suggest ongoing brain atrophy in SuS.
METHODS
Protocol approvals and patients consent
All procedures were executed in accordance with the Helsinki Declaration of 1975 as revised in 2000. The research protocol was approved by the local ethics committee of the Ghent University Hospital (ref: 2019/1443). Patients with probable or definite SuS according to the 2016 European Susac Consortium (EuSaC) criteria proposed by Kleffner et al. [9] were enrolled by their treating neurologist and written informed consent was obtained. Neurocognitive evaluation of all patients was performed by the same neuropsychologist. Demographic, clinical and paraclinical information was retrospectively collected from the electronic patient file.
Patient population
This study included patients who were recruited at the Ghent University Hospital. They had a diagnosis of SuS and were part of a larger prospective observational study of 19 patients. Neurological, audiological, vestibular and ophthalmological follow‐up was provided at the Ghent University Hospital.
Neuropsychological assessment
Since the current literature shows impairments in multiple cognitive domains are present in SuS, all patients underwent a thorough neuropsychological examination, consisting of tests evaluating five key cognitive domains: attention and executive functioning, memory, spatial cognition, language, and psychomotor functioning. In addition, we also assessed emotional well‐being. The complete neuropsychological test battery is shown in Table 1. Information on years of education and employment was retrieved before the neuropsychological testing.
TABLE 1.
Overview of neuropsychological test battery.
| Neuropsychological test | Cognitive domain |
|---|---|
| Attention and executive functioning | |
| WAIS‐III digit span | Verbal attention span and working memory |
| WAIS‐III Digit Symbol Coding Test | Information processing speed |
| WAIS‐III arithmetics | Mathematics |
| Trail Making Test | Cognitive flexibility |
| Stroop Color Word Test | Concentration effectiveness |
| Memory | |
| Rey Auditory Verbal Learning Test (version A/B) | Verbal memory |
| Rey/Taylor Complex Figure Test | Non‐verbal memory |
| Spatial cognition | |
| WAIS‐III Picture Completion Test | Visual perception |
| WAIS‐III Block Design Test | Visuoconstructive skills |
| Judgment of Line Orientation Test | Visuospatial judgement |
| Bells Test | Neglect |
| Language | |
| WAIS‐III Similarities | Verbal reasoning skills |
| Boston Naming Test | Wordfinding |
| Controlled Oral Word Association Test | Semantic and phonological word fluency |
| Psychomotor functioning | |
| Purdue Pegboard Test | Finger dexterity |
| Emotional well‐being | |
| Symptom Checklist‐90‐R | Anxiety, agoraphobia, depression, somatization, insufficiency of thoughts and behavior, distrust, hostility, sleeping problems, psychoneuroticism |
Abbreviation: WAIS‐III, Wechsler Adult Intelligence Scale 3.
To measure verbal attention span and working memory, the digit span forward and backward tests were administered. To measure information processing speed, the Digit Symbol Coding Test was used (subtests Wechsler Adult Intelligence Scale 3 [WAIS‐III]) [19]. In addition, the Trail Making Test [20] was administered to measure cognitive flexibility. We included the scaled score of the alternating condition. Another test used for executive functioning was the Stroop Color Word Test [21], which measures concentration effectiveness. The interference score was included.
Verbal and non‐verbal memory were measured respectively by means of the Rey Auditory Verbal Learning Test and the Rey Complex Figure Test [22]. For the first test, the sum of five trials, the delayed recall, correct identifiers and false positives are included. For the second test, the immediate and delayed recall are included. To avoid a possible learning effect, we used the B version of the Auditory Verbal Learning Test and the Taylor Complex Figure test at follow‐up testing.
Visuoconstructive skills were tested using the Picture Completion Test, the Block Design Test (subtests WAIS‐III) [19] and the Judgment of Line Orientation test [23]. In addition, the Bells Test [21] was administered to measure neglect. Center of cancellation is the variable included in the Bells Test.
To measure different language skills, ‘Similarities’, ‘Arithmetic’ (subtests WAIS‐III) [19], the Boston Naming Test [24] and Controlled Oral Word Association Test [21] were administered. For the first two tests, the scaled scores were included. For the third test, the number of correct responses is the variable withheld. For the last test, the number of words produced per semantic category (animals, professions) or beginning with a designated letter (N, A, K) is reported.
Finger dexterity was measured by means of the Purdue Pegboard Test [25]. The mean number of pegs placed in 30 s per condition is withheld.
Emotional well‐being was screened by the Symptom Check List‐90‐R [26]. The variables included in this test are anxiety, agoraphobia, depression, somatization, insufficiency of thoughts and behavior, distrust, hostility, sleeping problems, and psychoneuroticism.
Statistical analysis
Statistical analysis was performed using IBM SPSS statistics software (version 28.0, IBM software, UK). Group‐level mean values, standard deviations (SD) and normative values are reported. If the results deviated for more than 1.5 SD below the normative values, they were considered to be significantly lower [27]. Deficiency in a cognitive domain was defined as a score 1.5 SD below normative values on one of the domain tests. The Wilcoxon signed rank test was used to compare baseline and follow‐up test results at a group level, for which we report the p value. Because our sample size was relatively small, we also describe individual test results.
RESULTS
Patient characteristics
All patient characteristics with initial MRI findings, cumulative treatment, disease duration at time of baseline testing, individual test results and disease activity can be found in Table 2. A more comprehensive overview of our study population (information about diagnosis, MRI findings and paraclinical data) has been published previously [28]. Specific patient IDs are included in Table 2 for reference.
TABLE 2.
Patient characteristics with initial magnetic resonance imaging findings, cumulative treatment, disease duration at time of baseline testing, individual test results and disease activity 1 year prior to and at the moment of baseline and follow‐up testing.
| Patient ID a | Sex/age at baseline testing | Years of education | Year of diagnosis | Disease duration at baseline testing, years | Initial MRI findings | Cumulative treatment | Abnormal neuropsychological test results b | Disease activity | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow‐up | 1 year prior to baseline testing | At moment of baseline testing | 1 year prior to follow‐up testing | At moment of follow‐up testing | |||||||
| 1 | F/50 | 15 | 2005, definite | 15 | CC, ST, GM, IT, DR | C, PLEX, MMF | Phonological fluency |
Cognitive flexibility, mathematics |
Inactive | Inactive | Inactive | Inactive |
| 2 | M/38 | 15 | 2016, probable | 4 | CC, ST, IT, DR, LM, CE | C, PLEX, AZA, RTX, MMF | Information processing speed, phonological fluency |
Information processing speed, cognitive flexibility, phonological fluency |
Inactive | Inactive | Inactive | Inactive |
| 4 | M/39 | 15 | 2018, definite | 2 | CC, ST, GM, IT, DR, LM, CE | CYC, NAT | Phonological fluency |
Phonological fluency |
01/2020: Disease activity with migraine |
Active |
Inactive | Inactive |
| 6 | F/43 | 17 | 2011, definite | 9 | CC, ST, IT, DR | C, PLEX, MMF, AZA |
Information processing speed, cognitive flexibility, mathematics, visual perception, semantic and phonological fluency |
Missing data | Inactive | Inactive | / | / |
| 7 | M/70 | 15 | 2018, definite | 2 | CC, ST, GM, IT, DR | C, PLEX, MMF, RTX |
Information processing speed, verbal memory, visual perception, neglect, phonological fluency, finger dexterity |
Information processing speed, cognitive flexibility, verbal memory, wordfinding, finger dexterity | Inactive | Inactive | Inactive | Inactive |
| 8 | F/32 | 14 | 2016, definite | 4 | CC, ST | RTX |
Mathematics, phonological fluency |
Phonological fluency |
10/2020: Disease activity with BRAO lesions |
Active | Inactive | Inactive |
| 10 | F/30 | 17 |
2015, definite |
5 | CC, ST, DR | C, MMF, IVIG, RTX | No cognitive impairment | No cognitive impairment | Inactive | Inactive |
01/2021: disease activity with sensory complaints |
Inactive |
| 11 | F/34 | 12 | 2019, probable | 2 | CC, ST | None |
Mathematics, semantic fluency, phonological fluency, finger dexterity |
Semantic fluency, phonological fluency, verbal reasoning |
2020: disease activity with sensory complaints, migraine and visual symptoms |
Inactive | Inactive | Inactive |
| 13 | F/35 | 15 | 2020, definite | 5 | CC, ST, DR | C, PLEX, RTX, MMF | Verbal attention, mathematics |
Cognitive flexibility, mathematics, verbal memory |
Inactive | Inactive | Inactive | Inactive |
| 14 | F/31 | 16 | 2019, definite | 2 | CC, ST | C |
Cognitive flexibility |
Missing data | Inactive | Inactive | / | / |
| 15 | M/45 | 17 |
2020, definite |
1 | CC, ST, IT, DR | C, PLEX, MMF, RTX | No cognitive impairment | No cognitive impairment | Inactive | Inactive | Inactive | Inactive |
| 16 | F/38 | 15 | 2020, probable | 1 | CC, ST | PLEX, MMF |
Phonological fluency |
No cognitive impairment | Active | Active | Inactive | Inactive |
| 17 | M/28 | 15 | 2020, probable | 1 | CC, ST, IT, DR, LM | C, PLEX, RTX, MMF | Information processing speed, visual perception, phonological fluency, wordfinding | Cognitive flexibility | 09/2020: disease activity with visual symptoms | Active | Inactive | Inactive |
Abbreviations: AZA, azathioprine; BRAO, Branch Retinal Artery Occlusion; C, corticosteroids; CC, corpus callosum; CE, contrast enhancing; CYC, cyclophosphamide; DR, diffusion restrictive; F, female; GM, grey matter; IT, infratentorial; IVIG, intravenous immunoglobulin; LM, leptomeningeal; M, male; MMF, mycophenolate mofetil; MRI, magnetic resonance imaging; NAT, natalizumab; NP, not performed; PLEX, plasma exchange; RTX, rituximab; ST, supratentorial.
The same patient ID is used as in Dekeyser et al. [28]. For a more comprehensive overview we refer to this article.
Deficits on neuropsychological tests are shown.
In total, we collected data from 13 patients with probable or definite SuS (eight female and five male patients) according to the 2016 EuSaC criteria proposed by Kleffner et al. [9]. At baseline testing, the mean (SD) patient age was 39.5 (10.7) years and patients’ educational level ranged from 12 to 17 years (mean [SD] 15.2 [1.3] years). The mean (range) time interval between SuS diagnosis and first neurocognitive testing was 3.75 (0.13–15.48) years.
At cognitive follow‐up testing 2 years after baseline testing, 11 patients were included (six female and five male patients) with a mean (SD) age of 42.2 (11.5) years. Five of the 13 patients showed disease activity 1 year prior to baseline testing. Only one patient showed disease activity within 1 year prior to follow‐up testing. Four of the 13 patients showed disease activity at the moment of baseline testing, while none of the patients showed disease activity at follow‐up testing. None of the patients had clinically significant hearing loss or deafness that would interfere with the neuropsychological evaluation during the period of this study.
Neuropsychological test results at group level
All neuropsychological test results—baseline and follow‐up—for the study group (mean and SD), their respective normative values, and results of the Wilcoxon signed ranks test can be found in Table 3.
TABLE 3.
Neuropsychological test results at baseline and follow‐up of study participants (mean and standard deviation), their respective normative values and the p value of the Wilcoxon signed rank test.
| Baseline mean (SD) n = 13 | Normative value mean (SD) | Follow‐up mean (SD) n = 11 | Normative value mean (SD) | Wilcoxon signed rank test p value | |
|---|---|---|---|---|---|
| WAIS Digit Span | 10.1 (2.6) | 10 (3) | 10.8 (3.4) | 10 (3) | 0.511 |
| WAIS Digit Symbol Coding Test | 9.6 (3.9) | 10 (3) | 10.9 (4.06) | 10 (3) | 0.047 |
| WAIS Arithmetics | 8.0 (3.2) | 10 (3) | 8.7 (2.7) | 10 (3) | 0.393 |
| TMT alternating | 9.2 (3.9) | 10 (3) | 7.0 (5.1) | 10 (3) | 0.112 |
| SCWT interference | 36.2 (22.2) | 31 b | 35.8 (19.3) | 34 b | 0.624 |
| AVLT sum of 5 trials | 59.2 (9.8) | 59.1 (7.5) | 56.3 (13.9) | 55.3 (8.5) | 0.507 |
| AVLT delayed recall | 12.6 (2.7) | 12.0 (2.1) | 12.7 (2.8) | 11.3 (2.7) | 0.750 |
| AVLT correct identifiers | 14.5 (0.5) | 40–50 b | 14.9 (0.3) | 30–40 b | 0.046 |
| AVLT false positives | 0.3 (0.9) | 20–30 b | 0.6 (1.5) | 30–40 b | 0.180 |
| CFT immediate recall | 25.4 (4.2) | 24.5 (6.3) | 28.7 (5.6) | 26.5 (4.7) | 0.028 c |
| CFT delayed recall | 25.8 (4.8) | 24.2 (5.9) | 29.3 (5.3) | 25.4 (5.5) | 0.007 c |
| WAIS Picture Completion Test | 9.4 (4.2) | 10 (3) | 11.4 (2.2) | 10 (3) | 0.150 |
| WAIS Block Design Test | 9.3 (2.2) | 10 (3) | 10.2 (1.5) | 10 (3) | 0.120 |
| JOLOT | 26.5 (2.7) | 21 a | 26.2 (2.09) | 21 a | 0.787 |
| Bells Test – Center of Cancelation | 0.03 (0.1) | 0.08 a | 0.0 (0.03) | 0.08 a | 0.889 |
| WAIS Similarities | 10.1 (2.4) | 10 (3) | 10.0 (2.8) | 10 (3) | 0.719 |
| BNT | 55.0 (3.4) | 50 a | 56.0 (4.6) | 50 a | 0.159 |
| COWA Animals | 25.1 (4.4) | 28.6 (5.4) | 26.5 (8.6) | 28.6 (5.4) | 0.504 |
| COWA Professions | 16.5 (4.2) | 19.1 (5.4) | 18.0 (5.4) | 19.1 (5.4) | 0.036 |
| COWA – N | 9.0 (3.6) | 14.0 (4.5) | 11.1 (3.5) | 14.0 (4.5) | 0.036 |
| COWA – A | 8.3 (3.9) | 14.3 (5.2) | 11.4 (5.6) | 14.3 (5.2) | 0.010 |
| COWA – K | 11.8 (3.7) | 18.6 (5.7) | 13.3 (4.9) | 18.6 (5.7) | 0.168 |
| PPT – preferred hand | 14.8 (2.3) | 15.9 (1.5) | 15.0 (2.9) | 15.9 (1.5) | 0.624 |
| PPT – non‐preferred hand | 14.3 (1.9) | 15.2 (1.5) | 13.8 (2.8) | 15.2 (1.5) | 0.284 |
| PPT – both hands | 11.7 (2.2) | 13.1 (1.6) | 11.6 (2.2) | 13.1 (1.6) | 0.406 |
| SCL‐90‐R – Anxiety | 16.7 (4.5) | 14 a | 13.2 (1.8) | 14 a | 0.043 |
| SCL‐90‐R – Agoraphobia | 7.5 (0.5) | 7 a | 7.9 (1.5) | 7 a | 0.317 |
| SCL‐90‐R – Depression | 25.8 (7.5) | 22 a | 23.2 (6.2) | 22 a | 0.202 |
| SCL‐90‐R – Somatization | 20.5 (5.3) | 18 a | 19.8 (5.5) | 18 a | 0.645 |
| SCL‐90‐R – Insufficiency of thoughts and behavior | 18.5 (4.0) | 15 a | 16.0 (3.7) | 15 a | 0.086 |
| SCL‐90‐R – Distrust | 26.8 (5.2) | 27 a | 24.5 (6.4) | 27 a | 0.265 |
| SCL‐90‐R – Hostility | 7.9 (1.8) | 7 a | 7.6 (1.8) | 7 a | 0.248 |
| SCL‐90‐R – Sleeping problems | 8.3 (4.0) | 5 a | 6.5 (2.9) | 5 a | 0.153 |
| SCL‐90‐R – Psychoneuroticism | 145.5 (20.2) | 130 a | 130.5 (20.6) | 130 a | 0.037 |
Note: Significant p‐values (all improved) are in bold.
Abbreviations: AVLT, Auditory Verbal Learning Test; BNT, Boston Naming Test; CFT, Complex Figure Rey; COWA, Controlled Oral Word Association test; JOLOT, Judgment of Line Orientation Test; PPT, Purdue Pegboard Test; SCL‐90‐R, Symptom Checklist‐90‐Revised; SCWT, Stroop Color Word Test; TMT, Trail Making Test; WAIS, Wechsler Adult Intelligence Scale.
Cut‐off values.
Percentile scores.
Because the recall of the Rey Figure is harder than the recall of the Taylor Figure, the comparison between baseline and follow‐up testing was recalculated by means of z‐scores. This is reported in the section of the group results.
According to age‐, gender‐ and education‐matched normative data, we found normal neuropsychological test results at a group level for every cognitive test. We found significant differences in information processing speed, verbal recognition, and semantic and phonological fluency between baseline and follow‐up testing.
There was a significant difference between baseline and follow‐up testing using the Digit Symbol Coding Test (Z = −1.983, p = 0.047). Relative to the baseline testing, six patients achieved a higher test result, two patients achieved the same result and one patient achieved a lower result. This effect can be considered “large”, r = 0.6. For verbal recognition (Z = −2.000, p = 0.046), four patients achieved a higher test result, seven patients achieved the same result and no patient achieved a lower result compared to baseline testing. This effect can be considered “large”, r = 0.61. In addition, the Wilcoxon signed rank test indicated that there was a significant difference between baseline and follow‐up testing of verbal semantic fluency (“profession”; Z = −2.094, p = 0.036) and verbal phonological fluency (letter “N”; Z = −2.094, p = 0.036 and letter “A”; Z = −2.586, p = 0.010). Compared to the baseline testing of verbal semantic fluency, seven patients achieved a higher test result, two patients achieved the same result and two patients achieved a lower result. This effect can be considered “large”, r = 0.63. For verbal phonological fluency (letter “N”), eight patients achieved a higher result, two patients achieved a lower result and one patient achieved the same result. This effect can be considered “large”, r = 0.63. For phonological fluency (letter “A”), nine patients achieved a better result at follow‐up testing, two patients achieved a worse result and no patient achieved the same result. This effect can be considered “large”, r = 0.78.
Although the Wilcoxon signed rank test showed a significant difference between baseline and follow‐up testing of the immediate and delayed recall of a complex figure, these results should be interpreted with caution. Although the copy administration of the Rey and Taylor Complex Figures are of equivalent difficulty, the recall of the Rey Complex Figure is harder than the recall of the Taylor Complex Figure [29]. Therefore, the comparison between baseline and follow‐up testing was recalculated by means of z‐scores. For the immediate recall of the complex figure, the patients' scores were higher than the population average, both for baseline (z = 0.14) and follow‐up (z = 0.5) testing. For the delayed recall, the patients' scores were also higher, both for baseline (z = 0.27) and follow‐up (z = 0.7) testing. Based on the calculation of these z‐scores, the difference between baseline and follow‐up was not clinically significant.
Individual neuropsychological test results
Individual test results can be found in Table 2. Graphs were created to provide a visual overview of differences at baseline and follow‐up. Individual results for each cognitive test are described in Figure 1a. We compared the results of the 11 patients for whom we had follow‐up data. For attention and executive functioning, one patient (Patient 13) had a significantly lower result for verbal attention and working memory at baseline, but not at follow‐up testing. Three patients (Patients 2, 7 and 17) had a significantly lower score on information processing speed at baseline. At follow‐up, Patient 17 had a normal score on this test. At baseline, all patients had a normal score on cognitive flexibility. At follow‐up, however, five patients (Patients 13 and 17) had a significantly lower score. For arithmetics, three patients (Patients 8, 11 and 13) had a significantly lower score at baseline. At follow‐up, two patients had this (Patients 1 and 13). For memory, the individual test results show that one patient (Patient 7) had a significantly lower score on a verbal memory test at baseline. At follow‐up, there was one more patient with a significantly lower score (Patient 13). No patient had a significantly lower score on the visual memory test. For spatial cognition, one patient (Patient 7) showed signs of neglect at baseline. This score normalized at follow‐up. In addition, two patients (Patients 7 and 17) had a significantly lower score on visual perception. These scores also normalized at follow‐up. Looking at individual test results for language, eight of the 11 patients had significantly more difficulty with phonological fluency at baseline. At follow‐up four patients had a significantly lower score. One patient (Patient 11) had a significantly lower score on semantic fluency, at baseline and follow‐up testing. At baseline, one patient had a significantly lower score on wordfinding (Patient 17) and another at follow‐up (Patient 7). For psychomotor functioning, individual test results show that two patients (Patients 7 and 11) had a significantly lower score on finger dexterity at baseline. For Patient 11, this score normalized at follow‐up.
FIGURE 1.
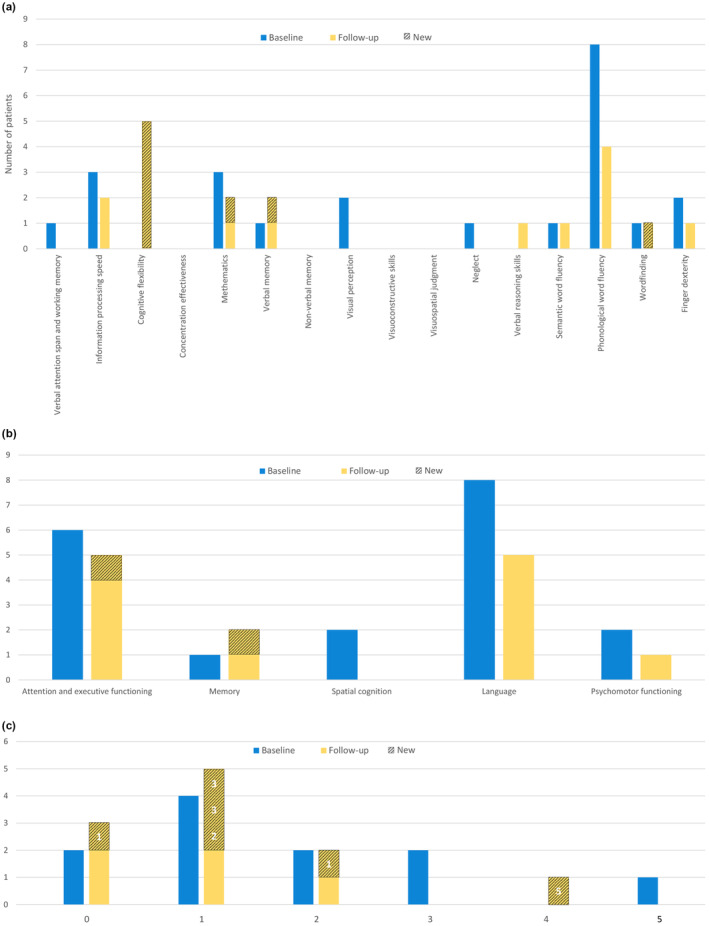
(a) Number of patients with a significant lower score on each cognitive test at baseline and follow‐up testing. (b) Number of patients with cognitive impairment in each cognitive domain at baseline and follow‐up testing. Patients who are new compared to baseline are shown in strikethrough hatching. For example: at baseline none of the patients showed an impairment in cognitive flexibility. At follow‐up, 5 patients had an impairment on this cognitive test. These were all new compared to baseline. (c) Number of patients with a cognitive impairment in one or more cognitive domains at baseline and follow‐up testing. Patients who are new compared to baseline are shown in strikethrough hatching. The number of cognitive domains they had an impairment in at baseline is shown in white. For example: two patients had an impairment in none of the cognitive domains. At follow‐up the same two patients showed no cognitive impairment. Another patient, who had an impairment in one cognitive domain at baseline, joined this group.
Looking at the different cognitive domains (Figure 1b), attention, executive functioning and language were most affected. Six of 11 patients had significant difficulty with attention and executive functioning at baseline, and five at follow‐up. Eight of 11 patients had significant difficulty with language at baseline, of whom three had normalized scores at follow‐up. These scores are mostly caused by the frequent impairments in phonological fluency. One patient showed impairment in the memory domain at baseline. At follow‐up, one extra patient had a deficit in this domain. In addition, two patients showed impairments in the domain of spatial cognition at baseline, which normalized at follow‐up. For psychomotor functioning, two patients had significant difficulty at baseline, of whom one had normalized results at follow‐up.
Overall, patients showed deficits in zero to five cognitive domains at baseline (Figure 1c). At follow‐up, all patients, except for one, showed an improvement in their cognitive functioning. At baseline, only two of 11 patients had normal cognitive functioning. At follow‐up, there was one more patient, who previously had an impairment in one cognitive domain. Additionally, four patients had an impairment in one cognitive domain at baseline. At follow‐up, this number was five, of whom three patients had a cognitive impairment in two or three domains at baseline. The patient who had deficits in five cognitive domains at baseline also improved and had deficits in four cognitive domains at follow‐up. Only one patient showed a decline in cognitive functioning; this patient went from deficits in one cognitive domain to two cognitive domains at follow‐up.
Emotional well‐being
Compared to normative data, patients scored significantly higher in all subcategories at a group level, except for “distrust”, at baseline. This implies that they experienced low physical and mental well‐being at baseline. At follow‐up, they scored higher in the subcategories “depression”, “somatization”, “insufficiency of thoughts and behavior”, “hostility” and “sleeping problems”.
A Wilcoxon signed rank test indicated that there was a significant difference between baseline and follow‐up testing of anxiety (Z = −2.023, p = 0.043) and psychoneuroticism (Z = −2.090, p = 0.037). Compared to the baseline testing of anxiety, four patients reported higher scores, six reported lower scores and one reported the same score. This effect can be considered “large”, r = 0.61. Compared to the baseline testing of psychoneuroticism, three patients reported higher scores, eight patients reported lower scores and no patient reported the same score. This effect can be considered “large”, r = 0.63.
DISCUSSION
At baseline, cognitive tests fell into the normal range at a group level, with significant improvement in information processing speed, verbal recognition, semantic and phonological fluency at 2‐year follow‐up. Looking at individual test results, patients seemed to show an interindividual variability, with most impairments being in attention, executive functioning and language. More specifically, cognitive flexibility and phonological word fluency were most impaired. Overall, patients showed deficits in zero to five cognitive domains at baseline and improved after a 2‐year period. However, only three out of 11 SuS patients had scores in the normal range for all domains at 2‐year follow‐up.
A previous prospective study of Machado et al. showed a significant slowing in processing speed, executive dysfunction and a reduced phonological word fluency in a cohort of 19 patients with SuS [13]. Although this cannot be confirmed at a group level, our individual test results are in line with these findings.
Factors that need to be considered are the total years of education, the thorough treatment our cohort received and their disease (in)activity. Regarding the first, our patients had an advantage as the mean total years of education in our cohort was 15.2. According to the active cognitive reserve model, the brain has the capacity to cope with damage through compensatory mechanisms, or through flexible and adaptive networks. Individuals with a higher cognitive reserve could have more efficient networks, allowing them to achieve better performances on cognitive tasks. Years of education is one of the proxies used to determine cognitive reserve [30].
Additionally, in our cohort, SuS was aggressively treated with corticosteroids, azathioprine, rituximab, mycophenolate mofetil, chronic plasma exchange, cyclophosphamide, natalizumab and intravenous immunoglobulin in various combinations. For specific details on this treatment, we refer to Dekeyser et al. [28]. Machado et al. did not discuss the treatment in their study in detail [13]. Because of this we could not compare the possible effect it had on preserving cognition. This needs further investigation in the future, as the necessity for aggressive immunotherapy, and the risks that come with this, in SuS remains a matter of debate. As demonstrated in other conditions such as multiple sclerosis, aggressive immunotherapy may prevent brain atrophy due to chronic active disease. Knowledge of the impact of treatment regimens on brain atrophy in SuS may help shed light on these questions.
The majority of the patients in our study had inactive disease (only four patients showed disease activity at baseline and none at follow‐up testing). Those who were symptomatic, showed no encephalopathy. Although not correlating with cognitive outcome, Machado et al. showed a pronounced ongoing global and callosal atrophy in clinically stable SuS patients [13]. This finding raised the question of potential further cognitive decline over time. However, our results instead demonstrated cognitive improvement over time. As already demonstrated in multiple sclerosis, cognitive functioning worsens during a relapse, after which it recovers but never to its initial level [31]. Our results suggest that SuS patients might follow the same pattern, which might explain why individual test results improve after a 2‐year period but are not completely normal compared to healthy controls (only three patients showed no cognitive impairment at follow‐up). It is possible that they already had attained a “new normal baseline” after a previous SuS relapse, and that because of the inactivity of the disease, we could not measure much difference after a 2‐year period.
Finally, patients included in this study seemed to experience low physical and mental well‐being. This implies a high burden of the disease and early screening and treatment of these problems at the beginning of the disease course is necessary. This burden seemed to decrease over time, although scores remained high, despite SuS treatment. Follow‐up of mental well‐being throughout the disease is therefore recommended in SuS.
Mood disorders, such as major depressive disorder, are known to impact cognitive functioning, including executive functioning, attention, memory, learning, psychomotor speed and verbal processing. However, recent evidence suggests that these cognitive deficits persist even when there is remission of the mood symptoms. This emphasizes the need to screen for and treat cognition separately from mood symptoms [32].
Strengths and limitations
The major strength of this study is that all studies up until now only cross‐sectionally investigated cognition in SuS but did not monitor cognition over time. Furthermore, this study is only the second prospective study to examine neuropsychological functioning in a larger group of patients with SuS, whereas previous articles reported individual case studies. Another strength is the extensiveness of the neuropsychological test battery used in our study.
Our study also has limitations. First, patients with a high educational level and therefore a larger cognitive reserve were overrepresented. Second, the majority of our patients had inactive disease, both at baseline and follow‐up testing. This, in combination with a relatively short time between the two assessments, might explain why the cognitive impact and change over time was relatively small. Thirdly, we did not control for a possible effect of mood disorders on cognition.
Scientific and clinical implications
Future studies should include a larger sample size with different types of educational level and should monitor cognition over a period longer than 2 years, including cognitive evaluation during and in‐between relapses. In addition, the association with the intensity and type of treatment should be further investigated to explore whether this has a potential effect on cognition. It is also important to look at the association with the location and severity of structural brain lesions and atrophy and investigate whether there is an association with the presence of mood disorders.
Due to the rarity of SuS, collecting larger cognitive datasets is only possible by standardization of a cognitive battery to be applied in several centers. Consensus on a minimal and feasible cognitive battery in SuS is needed. Systematic and standardized cognitive and neuropsychiatric testing may be necessary for adequate intervention (e.g., cognitive rehabilitation and psychological guidance). Based on our current and previous research, emphasis on processing speed, executive functioning and language are key in this standard battery.
CONCLUSION
This is the first prospective study to evaluate cognition in SuS over time and our data suggest normal cognitive functioning at a group level. Individual data showed interindividual variability, with most impairments being in attention, executive functioning and language. These improved after a 2‐year period, but patients only rarely performed normally in all cognitive domains over time. The high educational level of our patients, the treatment modalities and the activity of the disease, however, are factors that need to be taken into account. Patients reported significantly lower physical and mental well‐being, at the beginning of and throughout the disease course, emphasizing the importance of screening and treatment of these issues. Standardized multicentric testing is crucial to gain further insight into the impact of SuS on cognition.
AUTHOR CONTRIBUTIONS
Tineke Van Vrekhem: Conceptualization; investigation; writing – original draft; methodology; visualization; writing – review and editing; formal analysis; data curation. Marijke Miatton: Writing – review and editing; supervision; methodology; conceptualization. Dimitri Hemelsoet: Writing – review and editing; supervision; methodology; conceptualization. Liesbeth Van Hijfte: Writing – review and editing. Cathérine Dekeyser: Writing – review and editing. Julie De Zaeytijd: Writing – review and editing. Veroniek Van Driessche: Writing – review and editing. Helen Van Hoecke: Writing – review and editing. Leen Maes: Writing – review and editing. Guy Laureys: Writing – review and editing; supervision; methodology; formal analysis; conceptualization; investigation.
FUNDING INFORMATION
Published with support of the Universitaire Stichting van België. [Correction added on 15 February 2024 after first online publication: The funding information has been revised in this version.].
CONFLICT OF INTEREST STATEMENT
None.
Van Vrekhem T, Miatton M, Hemelsoet D, et al. Cognitive outcomes in Susac syndrome: A 2‐year neuropsychological follow‐up study. Eur J Neurol. 2024;31:e16186. doi: 10.1111/ene.16186
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Susac J, Hardman J, Selhorst J. Microangiopathy of the brain and retina. Neurology. 1979;29(3):313. [DOI] [PubMed] [Google Scholar]
- 2. García‐Carrasco M, Mendoza‐Pinto C, Cervera R. Diagnosis and classification of Susac syndrome. Autoimmun Rev. 2014;13(4–5):347‐350. [DOI] [PubMed] [Google Scholar]
- 3. Vodopivec I, Prasad S. Treatment of Susac syndrome. Curr Treat Options Neurol. 2016;18(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 4. Gross CC, Meyer C, Bhatia U, et al. CD8+ T cell‐mediated endotheliopathy is a targetable mechanism of neuro‐inflammation in Susac syndrome. Nat Commun. 2019;10(1):5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Willekens B, Kleffner I. Susac syndrome and pregnancy: a review of published cases and considerations for patient management. Ther Adv Neurol Diso. 2021;14:1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Seifert‐Held T, Langner‐Wegscheider BJ, Komposch M, et al. Susac's syndrome: clinical course and epidemiology in a central European population. Int J Neurol. 2017;127(9):776‐780. [DOI] [PubMed] [Google Scholar]
- 7. Wilf‐Yarkoni A, Elkayam O, Aizenstein O, et al. Increased incidence of Susac syndrome: a case series study. BMC Neurol. 2020;20:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dörr J, Krautwald S, Wildemann B, Jarius S. Characteristics of Susac syndrome: a review of all reported cases. Nat Rev Neurol. 2013;9(6):307‐316. [DOI] [PubMed] [Google Scholar]
- 9. Kleffner I, Dörr J, Ringelstein M, Gross C. Diagnostic criteria for Susac syndrome. J Neurol Neurosurg Psychiatry. 2016;87(12):1287‐1295. [DOI] [PubMed] [Google Scholar]
- 10. Susac JO, Murtagh F, Egan R, et al. MRI findings in Susac's syndrome. Neurology. 2003;61(12):1783‐1788. [DOI] [PubMed] [Google Scholar]
- 11. Rennebohm R, Susac JO, Egan RA, Daroff RB. Susac's syndrome – update. J Neurol Sci. 2010;299(1–2):86‐91. [DOI] [PubMed] [Google Scholar]
- 12. David C, Sacré K, Henri‐Feugeas MC, et al. Susac syndrome: a scoping review. Autoimmun Rev. 2022;21(6):103097. [DOI] [PubMed] [Google Scholar]
- 13. Machado S, Jouvent E, Klein I, De Guio F. Cognitive dysfunction and brain atrophy in Susac syndrome. J Neurol. 2020;267(4):994‐1003. [DOI] [PubMed] [Google Scholar]
- 14. Lemonda B, Peck C, Giles K, Bowers D. Neurocognitive profile of a woman with Susacs syndrome: further evidence of cognitive variability. Clin Neuropsychol. 2015;29(5):689‐706. [DOI] [PubMed] [Google Scholar]
- 15. Roessler‐Górecka M, Mendel T, Wiśniowska J, Seniów J. Neuropsychological characteristics of encephalopathy in Susac's syndrome – case report. Neurol Neurochir Pol. 2017;51(2):174‐179. [DOI] [PubMed] [Google Scholar]
- 16. Barritt A, Wickremaratchi M, Anderson S. Neuropsychological outcome of a case of Susac syndrome: a two‐year follow‐up study. Appl Neuropsychol. 2019;26(1):89‐95. [DOI] [PubMed] [Google Scholar]
- 17. Bolton C, Lacy M. Long‐term neuropsychological and psychiatric outcomes in Susac's syndrome. J Neuropsych Clin N. 2019;31(2):181‐182. [DOI] [PubMed] [Google Scholar]
- 18. Say MJ, Spring PJ, Hardy TA. Longitudinal improvement in neuropsychological profile following treatment of severe encephalopathy in Susac syndrome. Neuroimmun Rep. 2021;1:100017. [Google Scholar]
- 19. Wechsler D. WAIS‐III Administration and Scoring Manual. Psychol Corp; 1997. [Google Scholar]
- 20. Delis D, Kaplan E. Delis‐Kaplan Executive Function System® (D‐KEFS®): Flexibility of Thinking, Concept Formation, Problem Solving, Planning, Creativity, Impluse Control, Inhibition. Pearson; 2001. [Google Scholar]
- 21. Lezak MD. Neuropsychological Assessment. 5th ed. Oxford University Press; 2012. [Google Scholar]
- 22. Rey A. L' Examen Clinique En Psychologie. Presses universitaires de France; 1970. [Google Scholar]
- 23. Benton A, Hamsher K, Varney NR, Spreen O. Judgment of Line Orientation. Oxford University Press; 1983. [Google Scholar]
- 24. Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Lea & Febiger; 1983. [Google Scholar]
- 25. Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford University Press; 1991. [Google Scholar]
- 26. Arrindell WA, Ettema JHM. Symptom Checklist 90. Pearson; 2003. [Google Scholar]
- 27. Hendriks M, Mol B, Kessels R. Opinie: uniformiteit in de kwalitatieve beschrijving van scores op prestatietaken. Tijdschr voor Neuropsychol. 2020;3:166‐176. [Google Scholar]
- 28. Dekeyser C, Vanhoorne A, Hemelsoet D, et al. Atypical clinical and novel radiological findings in Susac syndrome: experience from a large monocentric cohort. J Neuroimmunol. 2023;376:578032. [DOI] [PubMed] [Google Scholar]
- 29. Lannoo E, Vingerhoets G. Flemish normative data on common neuropsychological tests: influence of age, education, and gender. Psychologica Belgica. 1997;37(3):141‐155. [Google Scholar]
- 30. Leoń I, Garciá‐García J, Roldań‐Tapia L. Estimating cognitive reserve in healthy adults using the cognitive reserve scale. PLoS One. 2014;9(7):39‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Benedict RH, Pol J, Yasin F, et al. Recovery of cognitive function after relapse in multiple sclerosis. Mult Scler J. 2021;27(1):71‐78. [DOI] [PubMed] [Google Scholar]
- 32. Knight MJ, Baune BT. Cognitive dysfunction in major depressive disorder. Curr Opin Psychiatry. 2018;31(1):26‐31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


