Abstract
Background and purpose
This study aimed to investigate the clinical efficacy and safety of telitacicept in patients with generalized myasthenia gravis (gMG) who tested positive for acetylcholine receptor antibodies or muscle‐specific kinase antibodies and were receiving standard‐of‐care therapy.
Methods
Patients meeting the eligibility criteria were randomly assigned to receive telitacicept subcutaneously once a week for 24 weeks in addition to standard‐of‐care treatment. The primary efficacy endpoint was the mean change in the quantitative myasthenia gravis (QMG) score from baseline to week 24. Secondary efficacy endpoints included mean change in QMG score from baseline to week 12 and gMG clinical absolute score from baseline to week 24. Additionally, safety, tolerability and pharmacodynamics were assessed.
Results
Twenty‐nine of the 41 patients screened were randomly selected and enrolled. The mean (± standard deviation [SD]) reduction in QMG score from baseline to week 24 was 7.7 (± 5.34) and 9.6 (± 4.29) in the 160 mg and 240 mg groups, respectively. At week 12, mean reductions in QMG scores for these two groups were 5.8 (± 5.85) and 9.5 (± 5.03), respectively, indicating rapid clinical improvement. Safety analysis revealed no adverse events leading to discontinuation or mortalities. All patients showed consistent reductions in serum immunoglobulin (Ig) A, IgG and IgM levels throughout the study.
Conclusion
Telitacicept demonstrated safety, good tolerability and reduced clinical severity throughout the study period. Further validation of the clinical efficacy of telitacicept in gMG will be conducted in an upcoming phase 3 clinical trial.
Keywords: autoimmune disease, B lymphocyte stimulator, clinical trial, immunotherapy, myasthenia gravis
INTRODUCTION
Generalized myasthenia gravis (gMG) is an acquired autoimmune disease characterized by muscle weakness and fatigue due to impaired neuromuscular transmission. The condition arises from autoantibodies, primarily targeting acetylcholine receptors at the neuromuscular junction [1]. It is considered rare, with an annual global incidence ranging from 4 to 18 per million individuals and a global prevalence of 2.2–36.7 cases per 100,000 people [2]. The in‐hospital mortality rate is estimated at 2.2% and rises to 4.7% during myasthenic crises (i.e., respiratory depression) [2]. Whilst current treatments such as cholinesterase inhibitors, corticosteroids and immunosuppressants have improved patient outcomes, they are often associated with adverse events, limited efficacy and high relapse rates [3]. In recent years, emerging immunotherapies, including C5 inhibitors, humanized Fc receptor inhibitors and B‐cell‐targeted therapies, have shown promise in improving outcomes for patients with gMG [4, 5, 6, 7, 8, 9, 10, 11].
B cells play an important role in the pathogenesis of several autoimmune diseases, including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) and gMG. Consequently, targeting B‐cell function presents a potential strategy to mitigate the production of pathogenic autoantibodies and improve disease outcomes. In the context of gMG, exploring B‐cell‐targeted therapies has shown promising results. Two notable treatments investigated for gMG are rituximab, a monoclonal antibody targeting CD20‐positive B cells, and belimumab, a monoclonal antibody targeting B‐cell stimulating factor (BLys) [12, 13, 14]. Whilst rituximab and belimumab are utilized in the treatment of autoimmune diseases, their effectiveness remains limited due to the crucial role of plasma cells, particularly long‐lived plasma cells, in antibody production and their involvement in both protective immunity and autoimmunity [15, 16, 17]. A randomized controlled trial evaluating rituximab's efficacy in immunoglobulin (Ig) A nephropathy, an autoimmune disease, showed no discernible clinical benefits compared to standard‐of‐care (SOC) treatment. Studies have shown that this lack of efficacy may be attributed to the absence of CD20 expression in plasma cells, which are principal contributors to antibody production [18, 19, 20]. Similarly, belimumab, which targets BLyS, does not demonstrate optimal efficacy, possibly due to the involvement of another factor, a proliferation‐inducing ligand (APRIL), in regulating B‐cell development [16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30]. Consequently, inhibiting the B‐cell activating factor of tumor necrosis factor family (BAFF)/APRIL mediated pathway has been proposed as a mechanistic targeted therapy for gMG and other autoimmune diseases.
Telitacicept, a recombinant human B lymphocyte stimulating factor receptor–IgG Fc fusion protein for injection, is a targeted therapeutic agent for B cells. Classified as a biological immunosuppressant, it comprises the BLyS receptor, the extracellular soluble part of the transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI), and the Fc part of human IgG1. It functions as a dual inhibitor targeting both BLyS and APRIL, two critical factors regulating the B‐cell population and humoral immunity. The high affinity of the TACI receptor allows it to effectively inhibit BLyS and APRIL, disrupting their interaction with cell membrane receptors (TACI, B‐cell maturation antigen [BCMA], BAFF‐R). Consequently, this blocks the biological activities of BLyS and APRIL, effectively suppressing B lymphocyte proliferation and T lymphocyte maturation and providing a treatment approach for autoimmune diseases [31, 32]. Telitacicept has received fast‐track designation from the US Food and Drugs Administration (FDA) for SLE and conditional marketing approval from the Chinese National Medical Products Administration for the treatment of active SLE [33, 34]. Furthermore, in October 2022, it obtained orphan drug designation from the US FDA for the treatment of myasthenia gravis (MG).
This study aimed to assess the clinical efficacy, safety and tolerability of telitacicept in patients diagnosed with gMG receiving SOC for their condition.
METHODS AND MATERIALS
Study design
A randomized, multicenter, open‐label, phase 2 trial was conducted to evaluate the safety and efficacy of telitacicept in patients with gMG enrolled across 24 sites in mainland China from 2020 to 2022. This study was approved by the Institutional Review Board at Beijing Hospital Ethics Committee, with approval number 2019BJYYEC‐208‐02. All participants provided written informed consent prior to their participation in the study, detailing the purpose, procedures, potential risks and benefits involved. The study was registered at ClinicalTrials.gov (NCT04302103) and performed in accordance with the Declaration of Helsinki and Good Clinical Practice. Patients were randomly assigned in a 1:1 ratio to receive either 160 or 240 mg telitacicept subcutaneously once weekly for 24 weeks in addition to SOC for gMG. Subsequently, patients were examined every 4 weeks during the open‐label treatment period. Modifications to the telitacicept dosage were not permitted except in cases of dosing interruption or discontinuation. Patients were instructed to maintain their SOC for the treatment of gMG throughout the study and could opt out at any time and for any reason (Table S1).
Patients
Patients aged ≥18 years were considered eligible for this phase 2 study if they met the following criteria: a confirmed diagnosis of gMG, positivity for acetylcholine receptor antibodies (AChR‐Abs) or muscle‐specific kinase antibodies (MuSK‐Abs), a Myasthenia Gravis Foundation of America classification of II–IIIb, a quantitative myasthenia gravis (QMG) score ≥8 with four or more items ≥2 points at screening, and were receiving SOC treatment for gMG. A comprehensive list of accepted standard medical therapies is provided in Table S1.
Exclusion criteria encompassed patients who had received immunosuppressive agents not listed in the SOC within 1 month preceding randomization, those who had received intravenous immunoglobulin or plasma exchange within 2 months before randomization, those with other autoimmune diseases, those presenting with an active infection at screening, those with uncontrolled diabetes mellitus, and those who had undergone thymectomy or had thymoma within 6 months of screening. A detailed compilation of the inclusion and exclusion criteria is provided in Table S1.
Study procedures
Efficacy was measured using the QMG score scale [35] and the MG clinical absolute score scale [36, 37, 38]. Evaluations were conducted at baseline (week 0) and at weeks 4, 8, 12, 16, 20 and 24. The QMG and MG clinical absolute scores serve as measures of gMG severity, with higher scores indicating increased disability [35, 36, 37]. Briefly, the QMG comprises 13 items and is a clinician‐administered assessment of disease status in gMG, with each item graded for severity from 0 (none) to 3 (severe); total scores range from 0 (normal; no disease) to 39 (severe disease).
A reduction of 3 or more points in QMG score is deemed clinically meaningful [39], with an improvement of more than 5 points indicating significant therapeutic effects.
The MG clinical absolute score, widely used in China, evaluates patients across eight domains: ptosis (0–4 points for each eye), upper eyelid fatigue (0–4 points for each eye), eyeball horizontal movement (0–4 points for each eye), upper limb fatigue (0–4 points for each limb), lower limb fatigue (0–4 points for each limb), facial muscles (0–4 points), chewing and swallowing function (0–8 points) and respiratory muscle function (0–8 points). Scores range from 0 (normal; no disease) to 60 (severe disease), and a reduction of 3 in the MG clinical absolute score is considered clinically meaningful [35, 36, 37].
Endpoints
The primary efficacy endpoint was the mean change in the QMG score from baseline to week 24. Secondary efficacy endpoints included the mean change in the QMG score from baseline to week 12 and the mean change in the MG clinical absolute score from baseline to week 12 and from baseline to week 24. Additionally, the QMG responder rate was analyzed at every visit, with QMG responders defined as patients exhibiting a reduction of 3 or more points from baseline. Other endpoints included the percentage change in serum immunoglobulin levels (IgA, IgG, IgM) and the percentage change in CD19+ B‐cell counts from baseline to week 24.
Safety evaluations were conducted by monitoring the frequency and severity of adverse events (AEs), serious AEs (SAEs) and drug‐related AEs, as well as the frequency of withdrawals from the study due to AEs.
Statistical analysis
The full analysis set comprised randomly assigned patients who received at least one dose of the investigational drug and had at least one efficacy assessment after dosing. The per protocol set was a subset of the full analysis set population, excluding major protocol violators. The safety set included patients who received at least one dose of the investigational drug. The primary efficacy endpoint was analyzed using an analysis of covariance (ANCOVA) model, with treatment and center as factors and baseline values as covariates. Secondary efficacy endpoints were assessed using a t test (assuming normal distribution), chi‐squared test or Fisher's exact test, as appropriate. Descriptive statistics, such as mean, standard deviation, median and range, were provided for continuous variables, whilst counts and percentages were presented for categorical variables. Statistical analysis was performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA). Statistical significance was defined as a p value of less than 0.05.
RESULTS
Enrollment and patient characteristics
Between 2020 and 2022, a total of 41 eligible patients were screened, out of which 29 AChR‐Ab‐positive patients were randomized 1:1 to either the telitacicept 160 mg group (N = 14) or the telitacicept 240 mg group (N = 15) (Figure 1). Baseline demographics and patient characteristics were comparable between the two treatment groups (Tables 1, S2 and S3). In both the telitacicept 160 mg group and 240 mg group, the majority of enrolled patients were female (57.1% and 53.3%, respectively), of comparable ages (42.6 and 45.5 years), with similar baseline mean QMG scores (18.4 and 19.3) and similar baseline MG clinical absolute scores (23.1 and 24.8). Additionally, the rate of baseline steroid use was also similar between both groups (57.1% and 53.3%). However, the percentage of patients receiving non‐steroid immunosuppressants was higher in the telitacicept 240 mg group compared to the telitacicept 160 mg group (53.3% vs. 28.6%).
FIGURE 1.
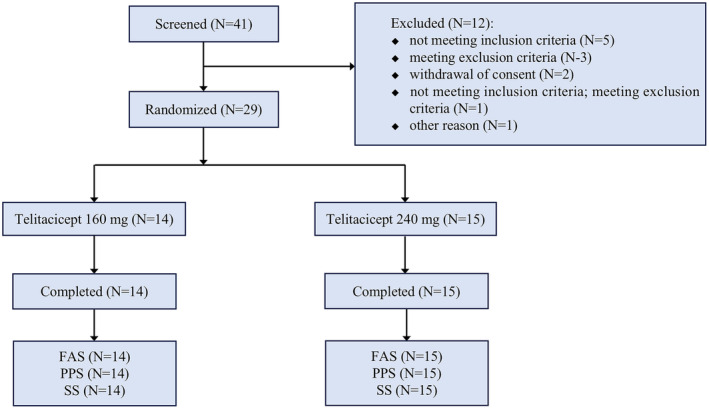
Consort flow diagram. Summary of patients screened and randomized for the two telitacicept treatment arms. FAS, full analysis set; PPS, per protocol set; SS, safety set.
TABLE 1.
Study population and baseline characteristics.
| Telitacicept 160 mg (N = 14) | Telitacicept 240 mg (N = 15) | All patients (N = 29) | |
|---|---|---|---|
| Age, years, mean ± SD | 42.6 ± 13.78 | 45.5 ± 10.60 | 44.1 ± 12.10 |
| Sex, n (%) | |||
| Male | 6 (42.9) | 7 (46.7) | 13 (44.8) |
| Female | 8 (57.1) | 8 (53.3) | 16 (55.2) |
| Duration of MG, month (m) | 50.11 (50.33) | 73.56 (90.1) | 62.24 (73.31) |
| QMG score a , mean ± SD | 18.4 ± 6.01 | 19.3 ± 4.43 | 18.9 ± 5.17 |
| MG clinical absolute score b , mean ± SD | 23.1 ± 8.47 | 24.8 ± 9.46 | 24.0 ± 8.88 |
| MGFA clinical classification | |||
| Class II, n (%) | 7 (50.0) | 9 (60.0) | 16 (55.2) |
| Class III, n (%) | 7 (50.0) | 6 (40.0) | 13 (44.8) |
| AChR‐Abs | |||
| Negative, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Positive, n (%) | 14 (100.0) | 15 (100.0) | 29 (100.0) |
| Any concomitant MG medications, n (%) | 14 (100.0) | 15 (100.0) | – |
| Cholinesterase inhibitor | |||
| Pyridostigmine bromide, n (%) | 14 (100.0) | 15 (100.0) | 29 (100.0) |
| Steroid | 8 (57.1) | 8 (53.3) | 16 (55.2) |
| Prednisone acetate, n (%) | 7 (50.0) | 6 (40.0) | 13 (44.8) |
| Methylprednisolone, n (%) | 1 (7.1) | 1 (6.7) | 2 (6.9) |
| Prednisone, n (%) | 0 (0) | 1 (6.7) | 1 (3.4) |
| Immunosuppressant, n (%) | 4 (28.6) | 8 (53.3) | 12 (41.4) |
| Tacrolimus, n (%) | 3 (21.4) | 7 (46.7) | 10 (34.5) |
| Azathioprine, n (%) | 1 (7.1) | 0 (0) | 1 (3.4) |
| Mycophenolate mofetil, n (%) | 0 (0) | 1 (6.7) | 1 (3.4) |
Abbreviations: AChR‐Abs, acetylcholine receptor antibodies; MG, myasthenia gravis; MGFA, Myasthenia Gravis Foundation of America; QMG, quantitative myasthenia gravis.
Total QMG scores range from 0 (none) to 39 (severe).
Total MG absolute clinical scores range from 0 (normal) to 60 (severe).
Efficacy outcomes
Clinical improvement was observed over the study period in both telitacicept groups. The primary efficacy endpoint, mean (± SD) change in QMG score from baseline to week 24, was −7.7 (± 5.34) in the 160 mg group and −9.6 (± 4.29) in the 240 mg group (p = 0.302) (Figure 2, Table 2). ANCOVA analysis showed a least squares change in QMG score from baseline to week 24 of −7.96 (95% confidence interval [CI] −10.07 to −5.85) in the 160 mg group and −9.37 (95% CI −11.40 to −7.33) in the 240 mg group, with a least squares difference of −1.40 (95% CI −4.34 to 1.53) between the two groups. At week 12, the mean (± SD) reduction in QMG score from baseline was 5.8 (± 5.85) in the 160 mg group and 9.5 (± 5.03) in the 240 mg group (p = 0.075), indicating rapid clinical improvement (Table 2). The mean (± SD) reduction in the MG clinical absolute score from baseline to week 24 was 13.8 (± 7.30) in the 160 mg group and 14.1 (± 9.78) in the 240 mg group (p = 0.931). From baseline to week 12, the mean (± SD) reduction in the MG clinical absolute score was 10.4 (± 4.40) in the 160 mg group and 11.2 (± 9.88) in the 240 mg group (p = 0.767). At week 12, 73.3% of patients in the 240 mg group achieved a greater improvement in the QMG score (up to an 8‐point reduction) compared to 26.6% of patients in the 160 mg group. However, this difference was not statistically significant (Figure 3). At week 24, the cumulative percentage of patients achieving a QMG response (a reduction of 3 or more points from baseline) was 92.9% in the 160 mg group and 100% in the 240 mg group (Figure 3).
FIGURE 2.
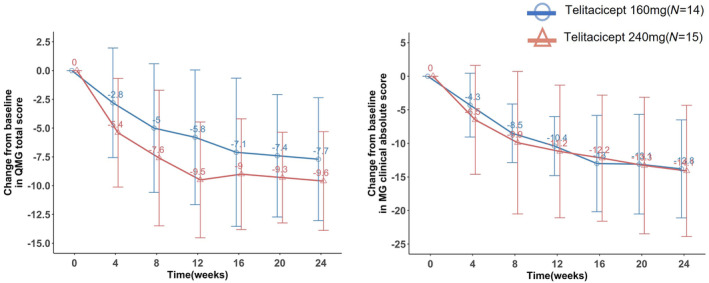
Mean change in quantitative myasthenia gravis (QMG) score and MG clinical absolute score from baseline to week 24. (a) Mean change from baseline to week 24 in QMG score (mean ± SD). (b) Mean change from baseline to week 24 in MG clinical absolute score (mean ± SD). A negative QMG score indicates a reduction in score. A QMG score reduction of 3 points or more is deemed clinically meaningful.
TABLE 2.
Mean change in quantitative myasthenia gravis (QMG) score and MG clinical absolute score from baseline to week 12 and from baseline to week 24.
| Telitacicept 160 mg (N = 14) | Telitacicept 240 mg (N = 15) | p value | |
|---|---|---|---|
| Change in QMG score from baseline to week 24, mean ± SD | −7.7 ± 5.34 | −9.6 ± 4.29 | 0.302 |
| Change in QMG score from baseline to week 12, mean ± SD | −5.8 ± 5.85 | −9.5 ± 5.03 | 0.075 |
| Change in MG clinical absolute score from baseline to week 24, mean ± SD | −13.8 ± 7.30 | −14.1 ± 9.78 | 0.931 |
| Change in MG clinical absolute score from baseline to week 12, mean ± SD | −10.4 ± 4.40 | −11.2 ± 9.88 | 0.767 |
Note: Mean change from baseline to week 24 in QMG score (mean ± SD). Mean change from baseline to week 24 in MG clinical absolute score (mean ± SD).
Abbreviations: MG, myasthenia gravis; QMG, quantitative myasthenia gravis.
FIGURE 3.
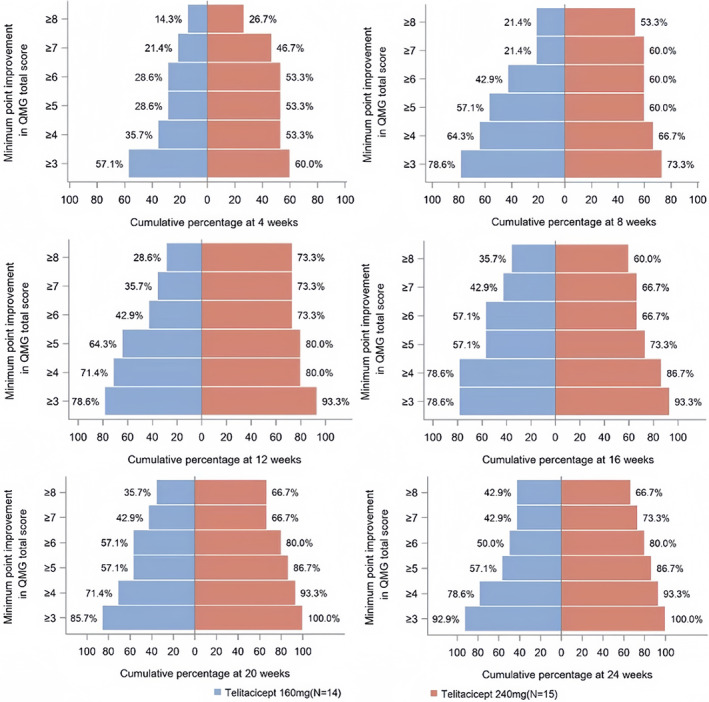
Proportion of patients with a QMG improvement ≥3 points over the 24‐week study period. QMG responders were defined as patients with a reduction of 3 or more points from baseline. QMG responder rate at 4, 8, 12, 16, 20 and 24 weeks. The cumulative percentage of patients in each group with a clinical improvement (i.e., QMG score reduction of 3 or more points from baseline) is indicated next to the corresponding bar.
Whilst there were no statistically significant differences between the two telitacicept groups in the primary and secondary efficacy endpoints, both groups exhibited a reduction in their QMG score and MG clinical absolute score at week 4 compared to baseline, indicating early clinical improvement in gMG. These reductions were sustained throughout the study period (Figures 2 and 3), demonstrating that patients in both telitacicept groups achieved clinical improvement in gMG severity over the 24‐week study period.
Serum immunoglobulin levels and CD19 + B‐cell counts
Both telitacicept groups achieved substantial reductions in serum immunoglobulin levels (IgG, IgA and IgM) over the 24‐week study period (Figure 4). From baseline to week 24, the telitacicept 160 mg group showed reductions in IgG, IgA and IgM of 29.5%, 53.8% and 63.2%, respectively. Similarly, the telitacicept 240 mg group demonstrated reductions in IgG, IgA and IgM of 28.2%, 56.7% and 70%, respectively. From baseline to week 4, CD19+ B‐cell counts increased by 7.8% in the telitacicept 160 mg group and by 27% in the telitacicept 240 mg group (Figure 4). Subsequently, from week 4 to week 12, the CD19+ B‐cell counts decreased by 3.6% in the 160 mg group and by 1.7% in the 240 mg group. Between week 12 and week 16, CD19+ B‐cell counts increased in both groups. However, from week 20 to week 24, CD19+ B‐cell counts increased in the telitacicept 160 mg group but decreased in the telitacicept 240 mg group (Figure 4). No significant differences were observed between the two groups regarding immunoglobulin levels or CD19+ B‐cell counts.
FIGURE 4.
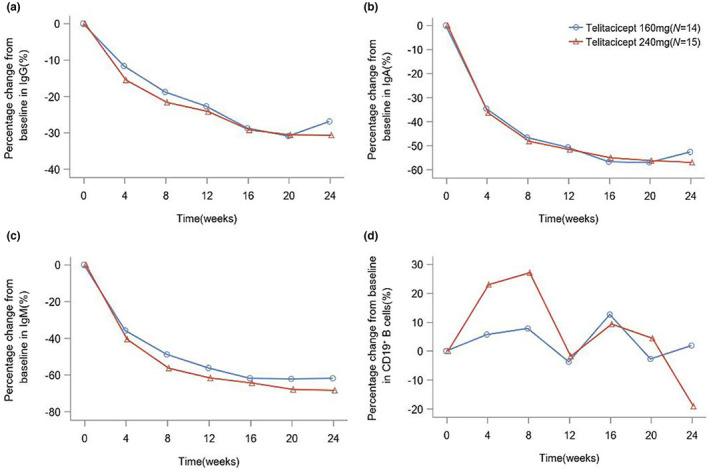
Mean percentage change in immunoglobulin levels and CD19+ B‐cell counts over the 24‐week study period. (a) Changes in IgG levels over the study period. (b) Changes in IgA levels over the study period. (c) Changes in IgM levels over the study period. (d) Changes in CD19+ B‐cell counts over the study period. Values are expressed as the percentage change from baseline to week 24 in immunoglobulin levels and CD19+ B‐cell counts.
Safety outcomes
During the study period, no AEs leading to discontinuation were reported, and no patients died in either treatment group (Table 3). Adverse drug reactions were observed in 64.3% of patients in the telitacicept 160 mg group and 80.0% of patients in the 240 mg group. Furthermore, AEs occurred in 78.6% of patients in the telitacicept 160 mg group and 100.0% of patients in the 240 mg group. Notably, most reported AEs were of mild to moderate severity (Common Terminology Criteria for Adverse Events Grade 1 and Grade 2). One patient (7.1%) in the telitacicept 160 mg group experienced a Grade 3 AE due to a decrease in lymphocyte count, which was considered an adverse reaction. Another patient (7.1%) in the telitacicept 160 mg group experienced an SAE (pneumonia); however, telitacicept was not discontinued as the condition resolved after treatment. In the telitacicept 160 mg group, one patient (7.1%) experienced two AEs necessitating dosing interruption associated with a decrease in lymphocyte count. Meanwhile, in the telitacicept 240 mg group, one patient (6.7%) experienced an AE leading to a dosing interruption, attributed to a decrease in IgM.
TABLE 3.
Summary of adverse events in all patients.
| Telitacicept 160 mg (N = 14) | Telitacicept 240 mg (N = 15) | All patients (N = 29) | |
|---|---|---|---|
| AE, n (%) | 11 (78.6) | 15 (100.0) | 26 (89.7) |
| ADR, n (%) | 9 (64.3) | 12 (80.0) | 21 (72.4) |
| SAE, n (%) | 1 (7.1) | 0 (0) | 1 (3.4) |
| SADR, n (%) | 0 (0) | 0 (0) | 0 (0) |
| AE leading to dosing interruption, n (%) | 1 (7.1) | 1 (6.7) | 2 (6.9) |
| ADR leading to dosing interruption, n (%) | 1 (7.1) | 1 (6.7) | 2 (6.9) |
| AE leading to discontinuation n (%) | 0 (0) | 0 (0) | 0 (0) |
| Withdrawal owing to AE, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Severe AE, n (%) | 1 (7.1) | 0 (0) | 1 (3.4) |
| Severe ADR, n (%) | 1 (7.1) | 0 (0) | 1 (3.4) |
| Death, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Most common TEAEs (occurring in ≥2 patients in any group), n (%) | |||
| Upper respiratory tract infection | 4 (28.6) | 3 (20.0) | 7 (24.1) |
| Blood immunoglobulin M decreased | 3 (21.4) | 4 (26.7) | 7 (24.1) |
| Blood immunoglobulin A decreased | 2 (14.3) | 4 (26.7) | 6 (20.7) |
| Diarrhea | 2 (14.3) | 3 (20.0) | 5 (17.2) |
| Blood immunoglobulin G decreased | 3 (21.4) | 2 (13.3) | 5 (17.2) |
| Injection site reactions | 1 (7.1) | 4 (26.7) | 5 (17.2) |
| Immunoglobulin decreased | 1 (7.1) | 2 (13.3) | 3 (10.3) |
| Urinary tract infection | 1 (7.1) | 2 (13.3) | 3 (10.3) |
| Dizziness | 0 (0) | 2 (13.3) | 2 (6.9) |
| Blood glucose increased | 0 (0) | 2 (13.3) | 2 (6.9) |
| Hemorrhoids | 2 (14.3) | 0 (0) | 2 (6.9) |
| Natural killer cell count decreased | 0 (0) | 2 (13.3) | 2 (6.9) |
Abbreviations: ADR, adverse drug reaction; AE, adverse event; SADR, serious adverse drug reaction; SAE, serious adverse event; TEAE, treatment emergent adverse event.
DISCUSSION
The results of this open‐label phase 2 study demonstrated that telitacicept was well tolerated and effectively reduced the severity of gMG over the 24‐week study period. This study showed a mean reduction in QMG score of 7.7 points in the telitacicept 160 mg group and 9.6 points in the telitacicept 240 mg group at week 24, suggesting an improvement in the clinical condition of patients with gMG. Similarly, the MG clinical absolute score showed a reduction from baseline to 4 weeks in both groups, which continued throughout the study. Notably, no patients withdrew from the study due to AEs, and most AEs were classified as mild or moderate.
The broader mechanism of telitacicept's efficacy differs from that of anti‐CD20 antibodies or BAFF inhibitors. BAFF and APRIL bind to different receptors on B cells and plasma cells. BAFF binds to three receptors on B cells: BAFF‐R (also known as BR3), TACI and BCMA. APRIL binds to TACI and BCMA receptors on B cells but not to BAFF‐R. Studies suggest that BAFF is crucial for the survival, differentiation and maturation of B cells, whereas APRIL is more influential in modulating the function and survival of long‐lived plasma cells, thereby impacting antibody production. Consequently, dual BAFF/APRIL inhibitors may hold therapeutic potential for autoimmune diseases and may be advantageous compared to other B‐cell‐targeted therapies.
Telitacicept, a fusion protein that combines the extracellular domain of TACI (the receptor for both BAFF and APRIL) with the Fc region of a human antibody, has shown promising results in several clinical trials for the treatment of SLE, RA, IgA nephropathy and Sjögren's syndrome. A first‐in‐human single‐blind, placebo‐controlled clinical study exploring the short‐term efficacy and safety of telitacicept in patients with RA found that telitacicept was safe and well tolerated in Chinese patients with RA [39]. Furthermore, in a randomized, double‐blind, multicenter, placebo‐controlled phase 2 clinical trial of telitacicept versus placebo in combination with SOC in patients with SLE, telitacicept substantially reduced B‐cell counts and immunoglobulin levels, increased complement levels, and substantially decreased the recurrence rate of severe SLE [40, 41].
In SLE patients, a decrease in serum C3 and C4 levels is often indicative of disease activity. In the context of telitacicept treatment for SLE clinical trials, it serves as a targeted therapy against BLyS and APRIL. By inhibiting these factors, telitacicept reduces the production of pathogenic antibodies in SLE patients, consequently diminishing the deposition of immune complexes in organs and ultimately leading to an elevation in serum complement levels. In individuals afflicted with MG, the pathological cascade involves the binding of autoantibodies to the AChR, culminating in the activation of the complement system. This activation instigates an inflammatory response and subsequent degradation of the neuromuscular junction, thereby exacerbating the symptoms of muscle weakness characteristic of MG. Telitacicept, functioning as a B‐cell‐targeted therapeutic agent without a direct impact on complement, modulates the immune response by impeding B‐cell differentiation and hindering plasma cell maturation. By attenuating this process, telitacicept aims to curtail the production of autoantibodies, thereby mitigating the autoimmune assault on the neuromuscular junction. Consequently, in the context of this study, an independent analysis of the complement system was not pursued.
In the present study, both telitacicept groups achieved substantial reductions in serum immunoglobulin levels (IgG, IgA and IgM) over the 24‐week study period. Whilst the precise relationship between immunoglobulin levels and drug efficacy in gMG remains unclear, these results suggest a potential association between decreased immunoglobulin levels and improved gMG symptoms. Additionally, fluctuations in CD19+ B‐cell count were more pronounced in the 240 mg group than in the 160 mg group (Figure 4). Previous studies on telitacicept have shown similar fluctuations in CD19+ B‐cell count, where an initial increase followed by a decrease was noted after treatment initiation [40]. Whilst the precise mechanism underlying these changes and their interaction remains unclear, it is hypothesized that, at the initiation of treatment, telitacicept might initially increase the number of CD19+ B cells by binding to receptors and inducing B‐cell proliferation. However, over time, the drug's impact on B‐cell survival and maturation probably becomes more pronounced, leading to a decline in CD19+ B‐cell counts. As a biomarker expressed on the surface of early B cells [42], further investigation is warranted to ascertain whether fluctuations in CD19+ B‐cell counts reflect an effect of the drug intervention on early B‐cell maturation and what implications this may hold for evaluating drug efficacy.
Despite these promising findings, this study had a few limitations. First, the study was open‐label and the lack of blinding introduces the possibility of bias, potentially impacting the resulting interpretation. Secondly, the absence of a control or placebo group limits our ability to compare the outcomes observed in the treatment group and draw definitive conclusions about the efficacy of telitacicept. Thirdly, the study's small sample size reduces its generalizability and statistical power, thereby increasing the risk of chance findings. Finally, most of the immunosuppressants employed in SOC treatment may require several months to exert their full effects, potentially overlapping with the study period. For example, whilst prednisone may lead to clinical improvement within 1–2 weeks, its efficacy may persist for several additional months. Similarly, cyclosporine or tacrolimus might initiate action early, but both also have delayed therapeutic efficacy.
In conclusion, this phase 2 study demonstrated that telitacicept improved gMG symptoms over a 24‐week study period whilst maintaining a favorable safety profile. To further validate these preliminary findings, a multicenter, placebo‐controlled phase 3 study based on the data from this phase 2 trial is currently under way.
AUTHOR CONTRIBUTIONS
Jian Yin: Conceptualization; methodology; data curation; validation; supervision; funding acquisition; project administration; writing – original draft; writing – review and editing; investigation; formal analysis; visualization; resources. Mingming Zhao: Conceptualization; methodology; data curation; validation; resources; project administration; writing – original draft; writing – review and editing; investigation; visualization; software. Xianhao Xu: Conceptualization; methodology; validation; writing – review and editing. Meini Zhang: Conceptualization; methodology; project administration; validation; formal analysis; writing – review and editing. Zucai Xu: Conceptualization; methodology; validation; formal analysis; project administration; writing – review and editing. Zunbo Li: Conceptualization; methodology; validation; formal analysis; project administration; writing – review and editing. Xinyue Qin: Software; data curation; writing – review and editing; validation. Zhuyi Li: Software; data curation; writing – review and editing; validation. Chongbo Zhao: Software; data curation; validation; writing – review and editing. Hongyu Zhou: Software; data curation; writing – review and editing. Ying Ma: Software; data curation; writing – review and editing. Wenfeng Cao: Software; data curation; writing – review and editing. Guoping Wang: Software; data curation; writing – review and editing. Yongzhong Lin: Software; data curation; writing – review and editing. Jizhong Zhang: Software; data curation; writing – review and editing. Xu Zhang: Software; data curation; writing – review and editing. Hongbin Cai: Software; data curation; writing – review and editing. Weidong Qian: Software; data curation; writing – review and editing. Yiqi Wang: Software; data curation; writing – review and editing. Xinghu Zhang: Software; data curation; writing – review and editing. Guangzhi Liu: Software; data curation; writing – review and editing. Jiawei Wang: Software; data curation; writing – review and editing. Wei Qiu: Software; data curation; writing – review and editing. Lianqiu Min: Software; data curation; writing – review and editing. Jing Li: Software; data curation; writing – review and editing. Hui Deng: Software; data curation; writing – review and editing. Lan Chu: Software; data curation; writing – review and editing. Yifan Zhang: Software; data curation; writing – review and editing. Jianmin Fang: Conceptualization; writing – review and editing; writing – original draft; methodology; funding acquisition; formal analysis; project administration; supervision; data curation; investigation; visualization.
FUNDING INFORMATION
Funding for this research was provided by National High Level Hospital Clinical Research Funding (BJ‐2023‐111) and Yantai RemeGen Co. Ltd.
CONFLICT OF INTEREST STATEMENT
The authors received compensation from Yantai RemeGen Co. Ltd and declare no other conflicts of interest.
Supporting information
Table S1.
Table S2.
Table S3.
ACKNOWLEDGEMENTS
The authors thank all the patients who participated in our study. All the authors approved the submitted version for publication.
Yin J, Zhao M, Xu X, et al. A multicenter, randomized, open‐label, phase 2 clinical study of telitacicept in adult patients with generalized myasthenia gravis. Eur J Neurol. 2024;31:e16322. doi: 10.1111/ene.16322
Jian Yin and Mingming Zhao contributed equally to this work and should be considered co‐first author.
Contributor Information
Jian Yin, Email: m13611177824@163.com.
Jianmin Fang, Email: jfang@tongji.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Conti‐Fine BM, Milani M, Kaminski HJ. Myasthenia gravis: past, present, and future. J Clin Invest. 2006;116(11):2843‐2854. doi: 10.1172/JCI29894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bubuioc AM, Kudebayeva A, Turuspekova S, Lisnic V, Leone MA. The epidemiology of myasthenia gravis. J Med Life. 2021;14(1):7‐16. doi: 10.25122/jml-2020-0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Menon D, Urra Pincheira A, Bril V. Emerging drugs for the treatment of myasthenia gravis. Expert Opin Emerg Drugs. 2021;26(3):259‐270. doi: 10.1080/14728214.2021.1952982 [DOI] [PubMed] [Google Scholar]
- 4. Wijnsma KL, Ter Heine R, Moes DJAR, et al. Pharmacology, pharmacokinetics and pharmacodynamics of eculizumab, and possibilities for an individualized approach to eculizumab. Clin Pharmacokinet. 2019;58(7):859‐874. doi: 10.1007/s40262-019-00742-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Howard JF Jr, Vissing J, Gilhus NE, et al. Zilucoplan: an investigational complement C5 inhibitor for the treatment of acetylcholine receptor autoantibody‐positive generalized myasthenia gravis. Expert Opin Investig Drugs. 2021;30(5):483‐493. doi: 10.1080/13543784.2021.1897567 [DOI] [PubMed] [Google Scholar]
- 6. Meisel A, Annane D, Vu T, et al. Long‐term efficacy and safety of ravulizumab in adults with anti‐acetylcholine receptor antibody‐positive generalized myasthenia gravis: results from the phase 3 CHAMPION MG open‐label extension. J Neurol. 2023;270(8):3862‐3875. doi: 10.1007/s00415-023-11699-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Howard JF Jr, Bril V, Vu T, et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo‐controlled, phase 3 trial [published correction appears in Lancet Neurol. 2021 Aug;20(8):e5]. Lancet Neurol. 2021;20(7):526‐536. doi: 10.1016/S1474-4422(21)00159-9 [DOI] [PubMed] [Google Scholar]
- 8. Menon D, Barnett C, Bril V. Novel treatments in myasthenia gravis. Front Neurol. 2020;11:538. Published 2020 Jun 30. doi: 10.3389/fneur.2020.00538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yi JS, Guptill JT, Stathopoulos P, Nowak RJ, O'Connor KC. B cells in the pathophysiology of myasthenia gravis. Muscle Nerve. 2018;57(2):172‐184. doi: 10.1002/mus.25973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Howard JF Jr, Bril V, Burns TM, et al. Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology. 2019;92(23):e2661‐e2673. doi: 10.1212/WNL.0000000000007600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saccà F, Barnett C, Vu T, et al. Efgartigimod improved health‐related quality of life in generalized myasthenia gravis: results from a randomized, double‐blind, placebo‐controlled, phase 3 study (ADAPT). J Neurol. 2023;270(4):2096‐2105. doi: 10.1007/s00415-022-11517-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Piehl F, Eriksson‐Dufva A, Budzianowska A, et al. Efficacy and safety of rituximab for new‐onset generalized myasthenia gravis: the RINOMAX randomized clinical trial. JAMA Neurol. 2022;79(11):1105‐1112. doi: 10.1001/jamaneurol.2022.2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hewett K, Sanders DB, Grove RA, et al. Randomized study of adjunctive belimumab in participants with generalized myasthenia gravis. Neurology. 2018;90(16):e1425‐e1434. doi: 10.1212/WNL.0000000000005323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Samy E, Wax S, Huard B, Hess H, Schneider P. Targeting BAFF and APRIL in systemic lupus erythematosus and other antibody‐associated diseases. Int Rev Immunol. 2017;36(1):3‐19. doi: 10.1080/08830185.2016.1276903 [DOI] [PubMed] [Google Scholar]
- 15. Khodadadi L, Cheng Q, Radbruch A, Hiepe F. The maintenance of memory plasma cells. Front Immunol. 2019;10:721. Published 2019 Apr 5. doi: 10.3389/fimmu.2019.00721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schrezenmeier E, Jayne D, Dörner T. Targeting B cells and plasma cells in glomerular diseases: translational perspectives. J Am Soc Nephrol. 2018;29(3):741‐758. doi: 10.1681/ASN.2017040367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang YM, Zhang H. Insights into the role of mucosal immunity in IgA nephropathy. Clin J Am Soc Nephrol. 2018;13(10):1584‐1586. doi: 10.2215/CJN.04370418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maixnerova D, Tesar V. Emerging modes of treatment of IgA nephropathy. Int J Mol Sci. 2020;21(23):9064. doi: 10.3390/ijms21239064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Selvaskandan H, Cheung CK, Muto M, Barratt J. New strategies and perspectives on managing IgA nephropathy. Clin Exp Nephrol. 2019;23(5):577‐588. doi: 10.1007/s10157-019-01700-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lafayette RA, Canetta PA, Rovin BH, et al. A randomized, controlled trial of rituximab in IgA nephropathy with proteinuria and renal dysfunction. J Am Soc Nephrol. 2017;28(4):1306‐1313. doi: 10.1681/ASN.2016060640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baert L, Manfroi B, Casez O, Sturm N, Huard B. The role of APRIL—a proliferation inducing ligand—in autoimmune diseases and expectations from its targeting. J Autoimmun. 2018;95:179‐190. doi: 10.1016/j.jaut.2018.10.016 [DOI] [PubMed] [Google Scholar]
- 22. La Cava A. Targeting the BLyS‐APRIL signaling pathway in SLE. Clin Immunol. 2013;148(3):322‐327. doi: 10.1016/j.clim.2012.11.010 [DOI] [PubMed] [Google Scholar]
- 23. Moore PA, Belvedere O, Orr A, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285(5425):260‐263. doi: 10.1126/science.285.5425.260 [DOI] [PubMed] [Google Scholar]
- 24. Smulski CR, Eibel H. BAFF and BAFF‐receptor in B cell selection and survival. Front Immunol. 2018;9:2285. doi: 10.3389/fimmu.2018.02285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Castigli E, Wilson SA, Scott S, et al. TACI and BAFF‐R mediate isotype switching in B cells. J Exp Med. 2005;201(1):35‐39. doi: 10.1084/jem.20032000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shabgah AG, Shariati‐Sarabi Z, Tavakkol‐Afshari J, Mohammadi M. The role of BAFF and APRIL in rheumatoid arthritis. J Cell Physiol. 2019;234(10):17050‐17063. doi: 10.1002/jcp.28445 [DOI] [PubMed] [Google Scholar]
- 27. Bossen C, Schneider P. BAFF, APRIL and their receptors: structure, function and signaling. Semin Immunol. 2006;18(5):263‐275. doi: 10.1016/j.smim.2006.04.006 [DOI] [PubMed] [Google Scholar]
- 28. Tsuji S, Cortesão C, Bram RJ, Platt JL, Cascalho M. TACI deficiency impairs sustained Blimp‐1 expression in B cells decreasing long‐lived plasma cells in the bone marrow. Blood. 2011;118(22):5832‐5839. doi: 10.1182/blood-2011-05-353961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Salazar‐Camarena DC, Ortiz‐Lazareno PC, Cruz A, et al. Association of BAFF, APRIL serum levels, BAFF‐R, TACI and BCMA expression on peripheral B‐cell subsets with clinical manifestations in systemic lupus erythematosus. Lupus. 2016;25(6):582‐592. doi: 10.1177/0961203315608254 [DOI] [PubMed] [Google Scholar]
- 30. Shi F, Xue R, Zhou X, Shen P, Wang S, Yang Y. Telitacicept as a BLyS/APRIL dual inhibitor for autoimmune disease. Immunopharmacol Immunotoxicol. 2021;43(6):666‐673. doi: 10.1080/08923973.2021.1973493 [DOI] [PubMed] [Google Scholar]
- 31. Qin Q, Chang Y, Wang D, Wu Y, Zhang LL, Wei W. TACI‐Ig induces immune balance of Th cells in MLN via BLyS/APRIL‐receptors signaling in rats with adjuvant‐induced arthritis. Int Immunopharmacol. 2011;11(12):2167‐2175. doi: 10.1016/j.intimp.2011.09.014 [DOI] [PubMed] [Google Scholar]
- 32. Liu Y, Zhang L, Wu Y, et al. Therapeutic effects of TACI‐Ig on collagen‐induced arthritis by regulating T and B lymphocytes function in DBA/1 mice. Eur J Pharmacol. 2011;654(3):304‐314. doi: 10.1016/j.ejphar.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 33. Fan Y, Gao D, Zhang Z. Telitacicept, a novel humanized, recombinant TACI‐Fc fusion protein, for the treatment of systemic lupus erythematosus. Drugs Today (Barc). 2022;58(1):23‐32. doi: 10.1358/dot.2022.58.1.3352743 [DOI] [PubMed] [Google Scholar]
- 34. Dhillon S. Telitacicept: first approval. Drugs. 2021;81(14):1671‐1675. doi: 10.1007/s40265-021-01591-1 [DOI] [PubMed] [Google Scholar]
- 35. Jaretzki A 3rd, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology. 2000;55(1):16‐23. doi: 10.1212/wnl.55.1.16 [DOI] [PubMed] [Google Scholar]
- 36. Liu GC, Gao BL, Yang HQ, Qi GY, Liu P. The clinical absolute and relative scoring system—a quantitative scale measuring myasthenia gravis severity and outcome used in the traditional Chinese medicine. Complement Ther Med. 2014;22(5):877‐886. doi: 10.1016/j.ctim.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 37. Hai‐feng LI, Huang Y, Chen XJ. Establishment of common data element for myasthenia gravis and its significance in the researches of phenotypes. Chin J Contemp Neurol Neurosurg. 2020;20(1):34‐47. doi: [DOI] [Google Scholar]
- 38. Barnett C, Katzberg H, Nabavi M, Bril V. The quantitative myasthenia gravis score: comparison with clinical, electrophysiological, and laboratory markers. J Clin Neuromuscul Dis. 2012;13(4):201‐205. doi: 10.1097/CND.0b013e31824619d5 [DOI] [PubMed] [Google Scholar]
- 39. Chen X, Zhao Q, Hou Y, et al. Pharmacokinetics, pharmacodynamics, short term efficacy and safety of RCT‐18, a novel BLyS/APRIL fusion protein, in patients with rheumatoid arthritis. Br J Clin Pharmacol. 2016;82(1):41‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhao Q, Chen X, Hou Y, et al. Pharmacokinetics, pharmacodynamics, safety, and clinical activity of multiple doses of RCT‐18 in Chinese patients with systemic lupus erythematosus. J Clin Pharmacol. 2016;56(8):948‐959. doi: 10.1002/jcph.686 [DOI] [PubMed] [Google Scholar]
- 41. Jesus D, Matos A, Henriques C, et al. Derivation and validation of the SLE disease activity score (SLE‐DAS): a new SLE continuous measure with high sensitivity for changes in disease activity. Ann Rheum Dis. 2019;78(3):365‐371. doi: 10.1136/annrheumdis-2018-214502 [DOI] [PubMed] [Google Scholar]
- 42. Hammer O. CD19 as an attractive target for antibody‐based therapy. MAbs. 2012;4(5):571‐577. doi: 10.4161/mabs.21338 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.
Table S3.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


