Abstract
Background and purpose
Blood pressure variability, in acute stroke, may be an important modifiable determinant of functional outcome after stroke. In a large international cohort of participants with acute stroke, it was sought to determine the association of blood pressure variability (in the early period of admission) and functional outcomes, and to explore risk factors for increased blood pressure variability.
Patients and methods
INTERSTROKE is an international case–control study of risk factors for first acute stroke. Blood pressure was recorded at the time of admission, the morning after admission and the time of interview in cases (median time from admission 36.7 h). Multivariable ordinal regression analysis was employed to determine the association of blood pressure variability (standard deviation [SD] and coefficient of variance) with modified Rankin score at 1‐month follow‐up, and logistic regression was used to identify risk factors for blood pressure variability.
Results
Amongst 13,206 participants, the mean age was 62.19 ± 13.58 years. When measured by SD, both systolic blood pressure variability (odds ratio 1.13; 95% confidence interval 1.03–1.24 for SD ≥20 mmHg) and diastolic blood pressure variability (odds ratio 1.15; 95% confidence interval 1.04–1.26 for SD ≥10 mmHg) were associated with a significant increase in the odds of poor functional outcome. The highest coefficient of variance category was not associated with a significant increase in risk of higher modified Rankin score at 1 month. Increasing age, female sex, high body mass index, history of hypertension, alcohol use, and high urinary potassium and low urinary sodium excretion were associated with increased blood pressure variability.
Conclusion
Increased blood pressure variability in acute stroke, measured by SD, is associated with an increased risk of poor functional outcome at 1 month. Potentially modifiable risk factors for increased blood pressure variability include low urinary sodium excretion.
Keywords: blood pressure, blood pressure variability, functional outcomes, sodium, stroke
INTRODUCTION
Blood pressure (BP) management in acute stroke has been an intensive area of interventional research; randomized controlled trials have focused on lowering elevated BP, or increasing BP in patients where hypoperfusion is suspected [1, 2, 3, 4, 5, 6, 7]. Whilst lowering BP confers a modest benefit in patients with intracerebral haemorrhage (ICH), there is no evidence to support BP lowering in acute ischaemic stroke, based on large randomized controlled trials [1, 8, 9, 10].
Blood pressure variability, rather than absolute BP levels, has received considerably less attention in acute stroke research, and current guidelines do not consider BP variability in their recommendations [11]. Observational studies have reported an association between BP variability and functional outcome after acute stroke [12, 13], although a significant association has not been consistently reported, perhaps due to heterogeneity in the measure of BP variability and the time frame during which this was measured [14, 15]. Increased BP variability may also confound the evaluation of antihypertensive therapy in clinical trials [16].
In a non‐acute setting, BP variability is associated with an increased risk of stroke, conferring additive risk to absolute BP levels [13]. The mechanism is not well understood but is thought to relate to fluctuations in BP, impaired autoregulation and transient hypoperfusion [17, 18]. Similarly, increased BP variability in acute stroke may result in a larger burden of ischaemic damage, which may result in more unfavourable outcomes. If an association exists between acute BP variability and functional outcomes after stroke, it may present an important target for developing and evaluating future BP management interventions.
The INTERSTROKE study offers an opportunity to evaluate the association of modifiable factors, such as dietary factors, with BP variability in an international population. A previous analysis of the INTERSTROKE study reported the association between sodium and potassium excretion and risk of stroke, with lowest stroke risk in the moderate sodium excretion range and higher potassium range [19]. If an association between dietary factors and BP variability exists, this may warrant further evaluation, for example evaluating modification of fluid and nutritional management post stroke.
The aim of this analysis of the INTERSTROKE study is to explore factors (e.g., dietary exposures) that may increase the risk of BP variability and to evaluate the associations of BP variability with functional outcome 1 month following incident stroke event.
PATIENTS AND METHODS
INTERSTROKE is an international case–control study of risk factors for incident stroke. The methods have been described previously [20]. Patients (13,462 stroke patients and 13,488 matched controls) were recruited between January 2007 and August 2015. For the current analysis cases only were included (i.e., participants presenting with acute stroke). Cases were patients who presented with first acute stroke, either ischaemic or haemorrhagic (confirmation by computed tomography or magnetic resonance brain imaging), enrolled within 72 h of hospital admission or 5 days of symptom onset. For the current analyses, 13,206 cases were included, where data on BP measurements were available. The top 1% of BP variability measurements (as measured by systolic BP standard deviation [SD] and diastolic BP SD, respectively) were excluded from analyses, to reduce an effect of extreme values.
Measurement of blood pressure variability
Blood pressure was measured at three time‐points: admission, the morning after admission (from patient's chart) and the time of interview (recorded by research personnel, within 72 h of admission; median time from admission 36.7 h). Measures of BP variability were SD and coefficient of variance (CoV) of systolic and diastolic BP.
Measurement of other risk factor exposures
Standardized questionnaires were used to collect data on baseline demographics, lifestyle stroke risk factors and characteristics of acute stroke from all cases. Physical measurements (weight, height, BP) were recorded in a standardized manner. Diet quality was defined by the modified Alternative Healthy Eating Index (mAHEI); a higher score indicates a healthier cardiovascular diet than does a low score [21]. Sodium and potassium excretion was measured by non‐fasting (casual) urine samples, which were taken from cases within 72 h of recruitment, frozen at −20 to −70 °C and shipped to core laboratories (Hamilton, Canada; Beijing, China; Bangalore, India; and Istanbul, Turkey). Twenty‐four hour urine sodium and potassium excretion was estimated using the Tanaka formula [22]. This method has been validated against 24‐h urine collections and reported to be the least biased estimation for casual urine samples in an international population [23].
Measurement of functional outcome at 1 month
The modified Rankin scale (mRS) was used to measure stroke severity. A physician recorded each participant's mRS score prior to admission for acute stroke, at acute presentation and at 1‐month follow‐up.
The study was approved by the ethics committees in all participating centres. Written informed consent was obtained from participants or their proxy. This report adheres to STROBE reporting guidelines [24].
Statistical analysis
The following variables of BP variability were derived: systolic BP SD, systolic BP CoV (systolic BP SD/systolic BP mean), diastolic BP SD, diastolic BP CoV (diastolic BP SD/diastolic BP mean). Simple associations were assessed with frequency tables and Pearson's χ 2 tests for two independent proportions. Restricted cubic spline plots were used to explore the pattern of association between sodium and potassium excretion with BP variability (systolic BP and diastolic BP CoV) and of BP variability (continuous variables) with functional stroke outcome (mRS category at 1 month). Three level categories were derived for each of the BP variability variables, based on an assessment of univariable cubic splines. Univariable and multivariable logistic regression were used to evaluate the association of risk factors with high BP variability (systolic BP SD ≥20 mmHg, CoV ≥0.1, diastolic BP SD ≥10 mmHg, CoV ≥0.05). This multivariable model was adjusted for factors known to affect BP variability: age, sex, body mass index (BMI), diabetes mellitus, hypertension, use of antihypertensive medications, alcohol use, smoking, sodium excretion, potassium excretion, AHEI tertile and physical activity (fixed effects), and centre (random effects). Multivariable ordinal regression was used to evaluate the association between the following measures of BP: systolic BP CoV category, diastolic BP CoV category, systolic BP SD category, diastolic BP SD category with mRS category at 1 month. The following covariates were adjusted for age, sex, centre and mRS score at admission. Multicollinearity of other BP variability was assessed and covariates which were highly collinear were not included [25]. For analyses where systolic BP CoV or diastolic BP CoV were the predictors of interest, mean systolic or diastolic BP was adjusted for respectively as these variables were not above our prespecified threshold of multicollinearity. A sensitivity analysis excluding those on antihypertensives was performed. Exploratory subgroup analyses were completed by age (<70 years, >70 years), sex, stroke type, history of hypertension, admission BP above the median, in‐hospital antihypertensive use and diabetes mellitus. A likelihood ratio test was used to test for interaction between risk factor and subgroups. p for interaction was considered statistically significant when <0.05. Statistical analyses were performed using R version 4.2.2 (Innocent and Trusting).
RESULTS
The mean age of participants was 62.2 ± 13.6 years, of whom 5.9% (n = 776) of included cases received thrombolysis. 27.2% (n = 3585) of included cases had high BP variability measured as SD ≥20 mmHg and 39.9% (n = 5272) had high BP variability measured as CoV ≥0.1. Table 1 presents baseline characteristics of included participants with acute stroke, amongst systolic BP SD categories. Individuals with higher BP variability were older, more likely to be female, with more severe stroke syndromes (as measured by the mRS at admission) (Table 1). A higher proportion of those with high BP variability had a history of hypertension or were taking pre‐admission antihypertensive medications. Tables S1 and S2 present baseline characteristics of included cases by systolic BP CoV category and diastolic BP SD category, respectively.
TABLE 1.
Characteristics by systolic blood pressure variability (SD of systolic blood pressure category).
| Variable | SD (mmHg) | p value b | |||
|---|---|---|---|---|---|
| Overall | <10 | 10–20 | ≥20 | ||
| n | 13,206 a | 4817 a | 4804 a | 3585 a | |
| Age, years (SD) | 62.19 (13.56) | 61.10 (13.85) | 62.38 (13.52) | 63.40 (13.10) | <0.001 |
| Sex | |||||
| Female | 5331 (40.37%) | 1837 (38.14%) | 1964 (40.88%) | 1530 (42.68%) | <0.001 |
| Male | 7875 (59.63%) | 2980 (61.86%) | 2840 (59.12%) | 2055 (57.32%) | |
| Region | |||||
| Western Europe/Australia/North America | 1886 (14.28%) | 537 (11.15%) | 732 (15.24%) | 617 (17.21%) | <0.001 |
| Eastern/Central Europe/Middle East | 1378 (10.43%) | 447 (9.28%) | 566 (11.78%) | 365 (10.18%) | |
| Africa | 921 (6.97%) | 377 (7.83%) | 281 (5.85%) | 263 (7.34%) | |
| South Asia | 2807 (21.26%) | 1029 (21.36%) | 1070 (22.27%) | 708 (19.75%) | |
| China | 3953 (29.93%) | 1738 (36.08%) | 1339 (27.87%) | 876 (24.44%) | |
| Southeast Asia | 840 (6.36%) | 262 (5.44%) | 321 (6.68%) | 257 (7.17%) | |
| South America | 1421 (10.76%) | 427 (8.86%) | 495 (10.30%) | 499 (13.92%) | |
| Stroke subtypes | |||||
| Ischaemic | 10,230 (77.46%) | 3984 (82.71%) | 3759 (78.25%) | 2487 (69.37%) | <0.001 |
| ICH | 2976 (22.54%) | 833 (17.29%) | 1045 (21.75%) | 1098 (30.63%) | |
| Modified Rankin Scale score (admission) | |||||
| 0 | 441 (3.34%) | 156 (3.24%) | 172 (3.58%) | 113 (3.15%) | <0.001 |
| 1 | 2093 (15.85%) | 820 (17.02%) | 768 (15.99%) | 505 (14.09%) | |
| 2 | 2609 (19.76%) | 1012 (21.01%) | 975 (20.30%) | 622 (17.35%) | |
| 3 | 3330 (25.22%) | 1276 (26.49%) | 1176 (24.49%) | 878 (24.49%) | |
| 4 | 2966 (22.46%) | 975 (20.24%) | 1102 (22.95%) | 889 (24.80%) | |
| 5–6 | 1765 (13.37%) | 578 (12.00%) | 609 (12.68%) | 578 (16.12%) | |
| Received thrombolysis | 776 (5.88%) | 181 (4.73%) | 231 (5.62%) | 364 (6.90%) | <0.001 |
| Leisure physical activity | |||||
| Mainly inactive | 11,830 (89.63%) | 4393 (91.24%) | 4272 (88.98%) | 3165 (88.33%) | <0.001 |
| Mainly active | 1369 (10.37%) | 422 (8.76%) | 529 (11.02%) | 418 (11.67%) | |
| Alcohol | |||||
| Never | 7769 (58.93%) | 2951 (61.38%) | 2785 (58.05%) | 2033 (56.82%) | <0.001 |
| Former | 1435 (10.88%) | 479 (9.96%) | 548 (11.42%) | 408 (11.40%) | |
| Current | 3980 (30.19%) | 1378 (28.66%) | 1465 (30.53%) | 1137 (31.78%) | |
| Smoking history | |||||
| Never or former smoker | 9181 (69.55%) | 3272 (67.98%) | 3350 (69.75%) | 2559 (71.40%) | 0.003 |
| Current smoker | 4019 (30.45%) | 1541 (32.02%) | 1453 (30.25%) | 1025 (28.60%) | |
| Total AHEI score | 22.62 (6.25) | 22.26 (6.12) | 22.79 (6.22) | 22.87 (6.45) | <0.001 |
| Body mass index (kg/m2) | 25.82 (4.88) | 25.47 (4.75) | 25.98 (4.97) | 26.07 (4.89) | <0.001 |
| History of atrial fibrillation/flutter | 1314 (9.95%) | 456 (9.47%) | 492 (10.24%) | 366 (10.21%) | 0.37 |
| History of hypertension | 9571 (72.47%) | 2763 (57.36%) | 3548 (73.86%) | 3260 (90.93%) | <0.001 |
| History of diabetes or HbA1c > 6.5% | 3712 (28.12%) | 1329 (27.61%) | 1392 (28.99%) | 991 (27.65%) | 0.25 |
| Antihypertensives pre‐admission | 5254 (39.79%) | 1746 (36.25%) | 1980 (41.22%) | 1528 (42.63%) | <0.001 |
| Beta blocker use pre‐admission | 1830 (13.86%) | 561 (11.65%) | 682 (14.20%) | 587 (16.38%) | <0.001 |
| Alpha blocker use pre‐admission | 181 (1.37%) | 56 (1.16%) | 67 (1.39%) | 58 (1.62%) | 0.20 |
| Calcium channel blocker use pre‐admission | 1975 (14.96%) | 677 (14.05%) | 737 (15.34%) | 561 (15.66%) | 0.081 |
| Diuretic use pre‐admission | 1461 (11.06%) | 459 (9.53%) | 581 (12.09%) | 421 (11.75%) | <0.001 |
| ACE inhibitor use pre‐admission | 2020 (15.30%) | 628 (13.04%) | 804 (16.74%) | 588 (16.41%) | <0.001 |
| ARB use pre‐admission | 770 (5.83%) | 246 (5.11%) | 276 (5.75%) | 248 (6.92%) | 0.002 |
| Time of admission SBP | 158.09 (29.08) | 142.81 (20.68) | 156.21 (23.70) | 181.15 (30.63) | <0.001 |
| Time of admission DBP | 91.66 (16.95) | 86.12 (13.41) | 90.60 (15.45) | 100.53 (19.40) | <0.001 |
| Heart rate on admission | 80.76 (14.53) | 79.40 (13.52) | 80.89 (14.78) | 82.40 (15.30) | <0.001 |
| 24‐h urinary sodium excretion | 3.69 (1.27) | 3.73 (1.26) | 3.70 (1.22) | 3.62 (1.36) | <0.001 |
| 24‐h urinary potassium excretion | 1.57 (0.38) | 1.56 (0.38) | 1.57 (0.38) | 1.59 (0.38) | 0.025 |
Abbreviations: ACE, angiotensin‐converting enzyme; AHEI, Alternative Healthy Eating Index; ARB, angiotensin receptor blockers; DBP, diastolic blood pressure; ICH, intracerebral haemorrhage; SBP, systolic blood pressure.
Mean (SD); n (%).
Kruskal–Wallis rank sum test; Pearson's χ 2 test.
Risk factors for increased blood pressure variability
Table 2 reports the association of demographics and cardiovascular risk factors with systolic and diastolic BP variability (as measured by SD and CoV respectively). On multivariable analysis, female sex was associated with increased risk of systolic BP variability (SD ≥20 mmHg, odds ratio [OR] 1.19, 95% confidence interval [CI] 1.07–1.32; and CoV ≥0.1, OR 1.19, 95% CI 1.08–1.32) and diastolic BP variability (SD ≥10 mmHg, OR 1.20, 95% CI 1.08–1.32; and CoV ≥0.05, OR 1.25, 95% CI 1.11–1.40). Older age was associated with increased risk of systolic BP SD ≥20 mmHg (OR 1.63, 95% CI 1.24–2.15 for 70–80 years and OR 1.75, 95% CI 1.31–2.35 for >80 years; reference <40 years) and CoV >0.1 (OR 1.35, 95% CI 1.08–1.69 for 70–80 years and OR 1.39, 95% CI 1.09–1.78 for >80 years; reference <40 years). History of hypertension was associated with increased risk of systolic BP variability (SD ≥20 mmHg, OR 1.53, 95% CI 1.35–1.72; and CoV ≥0.1, OR 1.26, 95% CI 1.13–1.40) and diastolic BP variability (SD ≥10 mmHg, OR 1.45, 95% CI 1.30–1.62; and CoV ≥0.05, OR 1.17, 95% CI 1.03–1.33). Increased BMI was associated with increased risk of systolic BP variability (SD ≥20 mmHg, OR 1.18, 95% CI 1.03–1.36, and CoV ≥0.1, OR 1.38, 95% CI 1.03–1.83 for obesity (BMI ≥30); reference normal BMI) and diastolic BP variability (SD ≥10 mmHg, OR 1.16, 95% CI 1.02–1.32, and CoV ≥0.05, OR 1.12, 95% CI 0.96–1.31).
TABLE 2.
Risk factors for blood pressure variability.
| Variable | Odds of systolic BP variability a | Odds of diastolic BP variability a | ||
|---|---|---|---|---|
| SD ≥ 20 mmHg | CoV ≥0.1 | SD ≥ 10 mmHg | CoV ≥0.05 | |
| Sex | ||||
| Male | 1.0 | 1.0 | 1.0 | 1.0 |
| Female | 1.19 (1.07–1.32) | 1.19 (1.08–1.32) | 1.20 (1.08–1.32) | 1.25 (1.11–1.40) |
| Age (years) | ||||
| <40 | 1.0 | 1.0 | 1.0 | 1.0 |
| 40–50 | 1.38 (1.04–1.86) | 1.03 (0.81–1.31) | 1.11 (0.88–1.41) | 0.89 (0.67–1.18) |
| 50–60 | 1.43 (1.09–1.89) | 1.11 (0.89–1.39) | 0.92 (0.74–1.15) | 0.82 (0.62–1.06) |
| 60–70 | 1.51 (1.16–2.00) | 1.16 (0.93–1.45) | 0.86 (0.69–1.07) | 0.71 (0.54–0.92) |
| 70–80 | 1.63 (1.24–2.15) | 1.35 (1.08–1.69) | 0.80 (0.64–1.00) | 0.73 (0.56–0.95) |
| >80 | 1.75 (1.31–2.35) | 1.39 (1.09–1.78) | 0.96 (0.76–1.23) | 0.89 (0.66–1.19) |
| History of hypertension | ||||
| No | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 1.53 (1.35–1.72) | 1.26 (1.13–1.40) | 1.45 (1.30–1.62) | 1.17 (1.03–1.33) |
| Pre‐hospital use of antihypertensives | ||||
| No | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 0.66 (0.59–0.74) | 0.79 (0.70–0.88) | 0.75 (0.67–0.84) | 0.87 (0.76–0.99) |
| In‐hospital use of antihypertensives | ||||
| No | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 1.90 (1.70–2.14) | 1.60 (1.45–1.77) | 1.55 (1.40–1.72) | 1.53 (1.37–1.72) |
| Smoking | ||||
| No | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 0.96 (0.86–1.08) | 0.98 (0.88–1.09) | 0.92 (0.82–1.02) | 1.01 (0.89–1.14) |
| Alcohol | ||||
| Never | 1.0 | 1.0 | 1.0 | 1.0 |
| Former | 1.15 (0.98–1.35) | 1.19 (1.03–1.38) | 1.20 (1.04–1.39) | 1.36 (1.14–1.62) |
| Current | 1.28 (1.13–1.44) | 1.32 (1.19–1.48) | 1.36 (1.22–1.52) | 1.47 (1.29–1.67) |
| Diabetes mellitus | ||||
| No | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 0.91 (0.82–1.02) | 0.96 (0.87–1.07) | 0.89 (0.81–0.99) | 1.01 (0.90–1.14) |
| Physical activity | ||||
| Mainly active | 1.0 | 1.0 | 1.0 | 1.0 |
| Mainly inactive | 0.85 (0.73–0.98) | 0.74 (0.64–0.85) | 0.92 (0.80–1.05) | 0.88 (0.74–1.05) |
| BMI | ||||
| Normal | 1.0 | 1.0 | 1.0 | 1.0 |
| Overweight | 1.12 (1.01–1.25) | 1.15 (1.04–1.26) | 1.05 (0.95–1.16) | 1.02 (0.91–1.14) |
| Obese | 1.18 (1.03–1.36) | 1.23 (1.08–1.40) | 1.16 (1.02–1.32) | 1.12 (0.96–1.31) |
| Underweight | 1.26 (0.91–1.72) | 1.38 (1.03–1.83) | 0.94 (0.70–1.27) | 1.26 (0.89–1.83) |
| AHEI tertile | ||||
| Tertile 3 | 1.0 | 1.0 | 1.0 | 1.0 |
| Tertile 1 | 0.95 (0.84–1.07) | 0.90 (0.81–1.00) | 0.90 (0.81–1.00) | 0.82 (0.72–0.93) |
| Tertile 2 | 0.93 (0.82–1.05) | 0.94 (0.84–1.05) | 0.89 (0.80–0.99) | 0.83 (0.73–0.95) |
| Urinary Na (g/day) | ||||
| <2.8 g | 1.00 | 1.00 | 1.00 | 1.00 |
| 2.8–3.5 g | 0.81 (0.71–0.93) | 0.90 (0.80–1.02) | 0.94 (0.83–1.07) | 0.94 (0.81–1.10) |
| 3.5–4.26 g | 0.76 (0.66–0.87) | 0.85 (0.75–0.97) | 0.91 (0.80–1.03) | 0.80 (0.69–0.93) |
| >4.26 g | 0.70 (0.61–0.81) | 0.77 (0.68–0.88) | 0.86 (0.75–0.98) | 0.78 (0.67–0.90) |
| Urinary K (g/day) | ||||
| <1.34 g | 1.0 | 1.0 | 1.0 | 1.0 |
| 1.34–1.58 g | 1.08 (0.95–1.24) | 1.02 (0.91–1.16) | 1.11 (0.98–1.25) | 1.09 (0.95–1.26) |
| 1.58–1.86 g | 1.12 (0.97–1.29) | 1.04 (0.92–1.18) | 1.14 (1.00–1.29) | 1.05 (0.91–1.21) |
| >1.86 g | 1.25 (1.06–1.46) | 1.12 (0.97–1.30) | 1.18 (1.02–1.37) | 1.14 (0.96–1.35) |
Abbreviations: AHEI, Alternative Healthy Eating Index; BMI, body mass index; BP, blood pressure; CoV, coefficient of variance; K, potassium; Na, sodium; SD, standard deviation.
Multivariable model adjusts for all covariates in this table.
The relationship between sodium and BP variability is depicted in Figure 1, demonstrating an increased risk of systolic and diastolic BP variability associated with lower sodium excretion range (Figure 1a,b). Higher sodium excretion range was associated with reduced risk of systolic BP variability (SD ≥20 mmHg, OR 0.76, 95% CI 0.66–0.87 for 3.5–4.26 g/day and OR 0.70, 95% CI 0.61–0.81 for >4.26 g/day; and CoV ≥0.1, OR 0.85, 95% CI 0.75–0.97 for 3.5–4.26 g/day and OR 0.77, 95% CI 0.68–0.88 for >4.26 g/day; reference <2.8 g/day) and diastolic BP variability (SD ≥10 mmHg, OR 0.86, 95% CI 0.75–0.98 for >4.26 g/day; and CoV ≥0.05, OR 0.80, 95% CI 0.69–0.93 for 3.5–4.26 g/day and OR 0.78, 95% CI 0.67–0.90 for >4.26 g/day; reference <2.8 g/day). The urinary potassium range >1.86 g/day was associated with increased risk of systolic BP variability (SD ≥20 mmHg, OR 1.25, 95% CI 1.06–1.46 and CoV ≥0.1, OR 1.12, 95% CI 0.97–1.29 for >1.86 g/day; reference <1.34 g/day). Table S3 reports the univariable association of these factors with BP variability metrics. A sensitivity analysis excluding samples taken ≥72 h from admission did not materially alter findings (Figure S1).
FIGURE 1.
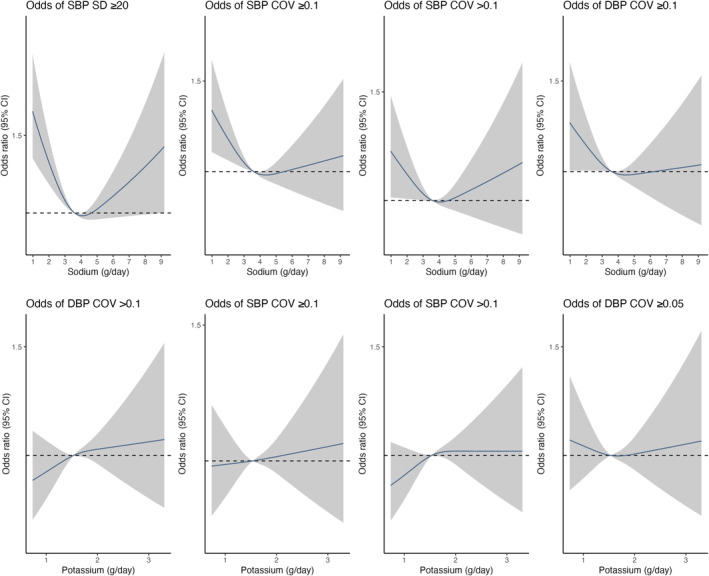
Association of estimated 24‐h sodium and potassium excretion (Tanaka) with risk of blood pressure variability. All plots were adjusted for age, sex, country. The grey ribbons indicate 95% confidence interval. The black lines represent the median value. DBP, diastolic blood pressure; SBP, systolic blood pressure.
Association of systolic blood pressure variability with modified Rankin Scale score at 1 month
Based on cubic spline analysis, increasing systolic BP measured as SD and CoV respectively was associated with a modest increased risk of higher mRS at 1 month for all stroke and ischaemic stroke (Figures 2 and 3). On multivariable ordinal regression, the highest systolic BP variability category measured as SD (≥20 mmHg) was associated with an increase in risk of higher mRS at 1 month within all stroke types (OR 1.13, 95% CI 1.03–1.24). This was consistent for ischaemic stroke cases (OR 1.09, 95% CI 0.97–1.21) and ICH stroke types (OR 1.14, 95% CI 0.95–1.37) (Figure 2 and Table S4). However, the highest CoV category (≥0.1) was not associated with a significant increase in risk of higher mRS at 1 month within all stroke types (OR 1.03, 95% CI 0.94–1.12) (Figures 2 and 3, Table S4). An exploratory subgroup analysis by age (<70 vs. >70), sex, history of hypertension, admission systolic BP (above vs. below the median) and diabetes mellitus did not alter findings (Table S5).
FIGURE 2.
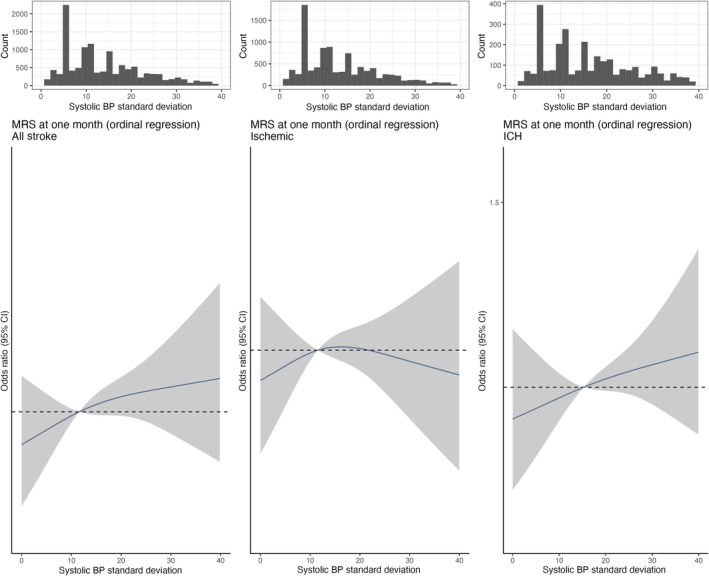
Spline of association of systolic blood pressure standard deviation (four level mRS). Association of systolic blood pressure variability (SD) with functional outcome (mRS at 1 month). (a)–(c) Restricted cubic splines of the associations between systolic blood pressure variability (SD) with functional outcome (mRS at 1 month) in all stroke types, in ischaemic stroke and in intracerebral haemorrhage (ICH) stroke subtypes. All plots were adjusted for age, sex, centre, mRS score at admission. The grey ribbons indicate 95% confidence interval. The black lines represent the median value. mRS, modified Rankin Scale.
FIGURE 3.
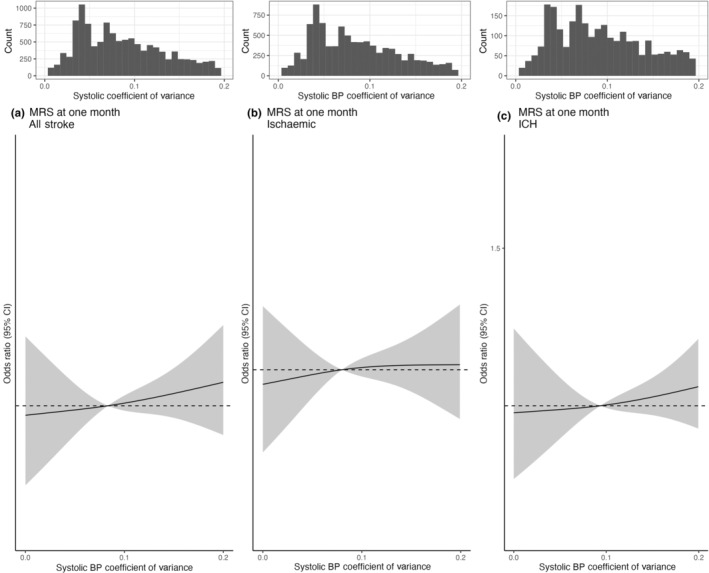
Spline of association of systolic blood pressure coefficient of variance with function outcomes (four level mRS at 1 month). Association of systolic blood pressure variability (CoV) with functional outcome (mRS at 1 month). (a)–(c) Restricted cubic splines of the associations between systolic blood pressure variability (CoV) with functional outcome (mRS at 1 month) in all stroke types, in ischaemic stroke and in intracerebral haemorrhage (ICH) stroke subtypes. All plots were adjusted for age, sex, centre, mRS score at admission. The grey ribbons indicate 95% confidence interval. The black lines represent the median value. mRS, modified Rankin Scale.
Diastolic blood pressure variability and functional outcome
Based on cubic spline analysis, increasing diastolic BP CoV was associated with a modest increased risk of higher mRS at 1 month for all stroke (Figure S2). However, on multivariable ordinal regression, the highest diastolic BP CoV category (≥0.1) was not associated with a significant increase in risk of higher mRS at 1 month within all stroke types (OR 1.10, 95% CI 0.97–1.23), ischaemic stroke (OR 1.07, 95% CI 0.94–1.23) or ICH (OR 1.16, 95% CI 0.90–1.49) (Figure S2 and Table S4). An exploratory subgroup analysis by age (<70 vs. >70), sex, history of hypertension, admission systolic BP (above vs. below the median) and diabetes mellitus did not alter findings (Table S6). The highest diastolic BP SD category (≥10 mmHg) was associated with a significant increase in risk of higher mRS at 1 month within all stroke types (OR 1.15, 95% CI 1.04–1.26); this was consistent for ischaemic stroke (OR 1.12, 95% CI 1.01–1.26) and ICH stroke types (OR 1.16, 95% CI 0.95–1.42) (Figure S3 and Table S4).
DISCUSSION
In this international study of incident stroke, high BP variability was observed in a considerable proportion of cases. Female sex, prior history of hypertension, use of antihypertensive medications, increased physical activity, high BMI and alcohol intake were associated with increased BP variability at the time of acute stroke. Moderate sodium excretion range (3.5–4.26 g/day) was associated with a reduced risk of increased BP variability. A modest association of BP variability was observed with worse functional outcome (mRS) at 1 month, but the association was not consistent amongst all measures of BP variability and was strongest for systolic BP SD and diastolic BP SD.
A reduced risk of both systolic BP and diastolic BP variability is reported, measured as CoV, with urinary sodium excretion >3.5 g (Figure 1). Our findings suggest that very low urinary sodium excretion, a surrogate for intake, may be associated with increased BP variability in acute stroke, which may suggest a role of pre‐admission sodium intake in diet. Animal studies have reported increased BP variability with low salt diets [26], and increased salt intake is a recommended treatment for orthostatic hypotension [27] as such low sodium intake may increase the risk of BP variability, but this requires additional research. If BP variability is an important determinant of functional outcome after stroke, which may be of particular relevance following endovascular thrombectomy [28], this may have implications for fluid and nutrition management. Current guidelines, for example the American Heart Association/American Stroke Association guidelines, advise a reduction of 1 g sodium/day for secondary stroke prevention whilst acknowledging the knowledge gap regarding optimal range in this population [29]. Previous studies evaluating the effect of reducing sodium intake on BP variability, in a general population, have not demonstrated a significant reduction in BP variability with reduced salt intake [30]. There are no clinical studies evaluating different regimens of intravenous fluid (e.g., normal saline, Hartmann's solution) which have different amounts of sodium, which may be an approach to intervening in future studies. Other risk factors of increased BP variability include non‐modifiable risk factors of increasing age, which has been consistently reported, and female sex, which has not been consistently reported [31, 32, 33], and increased BMI [34]. Modifiable risk factors are also reported including alcohol intake and hypertension.
Previous studies have reported the association of BP variability with increased risk of stroke [12, 13]; however, studies report conflicting results, perhaps due to differences in study population, time frame of BP measurement, measures of variability and sample size [14, 15]. Given the lack of standardization of measurement and classification of BP variability, the European Society of Hypertension recently issued a position paper regarding methodological aspects of BP variability measurement and its clinical relevance [35]. They outline BP variability classification by measurement interval: very short‐term (beat‐to‐beat), short‐term (within 24 h), mid‐term (day‐to‐day) and long‐term BP variability [35]. In this study mid‐term variability is considered, measured within days.
Strengths and limitations of our study
The INTERSTROKE study is a large international study which provides evidence of the association of BP variability from an acute stroke population, the majority of whom did not receive thrombolysis or thrombectomy (due to the enrolment time frame: 2007–2015), recruited from high‐, middle‐ and low‐income countries. mRS scores were collected by a physician at admission and at 1‐month follow‐up. Stroke types were classified by neuroimaging. Our study has several potential limitations. First, BP measurements from admission and the morning after admission were recorded from the patient's chart, rather than contemporaneously recorded. The number of measurements used to calculate variability was small; however, large numbers of measurements were not practical in this large international observational study. Whilst a larger number of BP measurements would improve accuracy, our large sample size may mitigate random error incurred by a limited number of BP measurements. To limit the impact of potentially inaccurate readings the top 1% of BP variability measurements were excluded. Secondly, sodium and potassium excretion was measured with a casual urine sample and formula derived (Tanaka), rather than with a 24‐h urine sample which would have been impractical. Thirdly, a change in oral intake or intravenous fluids due to acute stroke may impact sodium and potassium excretion. A sensitivity analysis excluding samples taken ≥72 h after admission was performed which did not materially alter findings.
CONCLUSION
Increased BP variability (measured as SD), which is more common in older women and those with low urinary sodium excretion, is associated with an increased risk of poor functional outcome 1 month after stroke. Further research is required to evaluate the optimal time frame and measurement of BP variability.
FUNDING INFORMATION
The INTERSTROKE study was funded by the Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, Canadian Stroke Network, Swedish Research Council, Swedish Heart and Lung Foundation, the Health and Medical Care Committee of the Regional Executive Board, Regiona Västra Götaland (Sweden), and through unrestricted grants from several pharmaceutical companies with major contributions from AstraZeneca, Boehringer Ingelheim (Canada), Pfizer (Canada), MSD, Chest, Heart and Stroke Scotland, and the Stroke Association, with support from the UK Stroke Research Network. The Department of Neurology at the University Duisburg‐Essen received research grants from the German Research Council (DFG), German Ministry of Education and Research (BMBF), European Union, National Institutes of Health, Bertelsmann Foundation and Heinz‐Nixdorf Foundation. CR was supported by the Irish Clinical Academic Training (ICAT) Programme, the Wellcome Trust and the Health Research Board (grant number 203930/B/16/Z), the Health Service Executive, National Doctors Training and Planning, and the Health and Social Care, Research and Development Division, Northern Ireland. The sponsors had no role in data collection, analyses or the decision to submit for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare no financial or other conflicts of interest.
ETHICAL APPROVAL
The study was approved by the ethics committees in all participating centres. Guarantors CR and MOD had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
INFORMED CONSENT
Written informed consent was obtained from participants or their proxy.
Supporting information
Appendix S1.
ACKNOWLEDGEMENT
Open access funding provided by IReL.
Reddin C, Murphy R, Hankey GJ, et al. Blood pressure variability in acute stroke: Risk factors and association with functional outcomes at 1 month. Eur J Neurol. 2024;31:e16314. doi: 10.1111/ene.16314
DATA AVAILABILITY STATEMENT
No additional data are available.
REFERENCES
- 1. Lee M, Ovbiagele B, Hong KS, et al. Effect of blood pressure lowering in early ischemic stroke: meta‐analysis. Stroke. 2015;46(7):1883‐1889. doi: 10.1161/STROKEAHA.115.009552 [DOI] [PubMed] [Google Scholar]
- 2. Yang P, Song L, Zhang Y, et al. Intensive blood pressure control after endovascular thrombectomy for acute ischaemic stroke (ENCHANTED2/MT): a multicentre, open‐label, blinded‐endpoint, randomised controlled trial. Lancet. 2022;400(10363):1585‐1596. doi: 10.1016/S0140-6736(22)01882-7 [DOI] [PubMed] [Google Scholar]
- 3. Mazighi M, Richard S, Lapergue B, et al. Safety and efficacy of intensive blood pressure lowering after successful endovascular therapy in acute ischaemic stroke (BP‐TARGET): a multicentre, open‐label, randomised controlled trial. Lancet Neurol. 2021;20(4):265‐274. doi: 10.1016/S1474-4422(20)30483-X [DOI] [PubMed] [Google Scholar]
- 4. Nam HS, Kim YD, Heo J, et al. Intensive vs conventional blood pressure lowering after endovascular thrombectomy in acute ischemic stroke: the OPTIMAL‐BP randomized clinical trial. JAMA. 2023;330(9):832‐842. doi: 10.1001/jama.2023.14590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma L, Hu X, Song L, et al. The third intensive care bundle with blood pressure reduction in acute cerebral haemorrhage trial (INTERACT3): an international, stepped wedge cluster randomised controlled trial. Lancet. 2023;402(10395):27‐40. doi: 10.1016/S0140-6736(23)00806-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bang OY, Chung JW, Kim SK, et al. Therapeutic‐induced hypertension in patients with noncardioembolic acute stroke. Neurology. 2019;93(21):e1955‐e1963. doi: 10.1212/WNL.0000000000008520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nasi LA, Martins SCO, Gus M, et al. Early manipulation of arterial blood pressure in acute ischemic stroke (MAPAS): results of a randomized controlled trial. Neurocrit Care. 2019;30(2):372‐379. doi: 10.1007/s12028-018-0642-5 [DOI] [PubMed] [Google Scholar]
- 8. Boulouis G, Morotti A, Goldstein JN, Charidimou A. Intensive blood pressure lowering in patients with acute intracerebral haemorrhage: clinical outcomes and haemorrhage expansion. Systematic review and meta‐analysis of randomised trials. J Neurol Neurosurg Psychiatry. 2017;88(4):339‐345. doi: 10.1136/jnnp-2016-315346 [DOI] [PubMed] [Google Scholar]
- 9. Moullaali TJ, Wang X, Sandset EC, et al. Early lowering of blood pressure after acute intracerebral haemorrhage: a systematic review and meta‐analysis of individual patient data. J Neurol Neurosurg Psychiatry. 2022;93(1):6‐13. doi: 10.1136/jnnp-2021-327195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu L, Xie X, Pan Y, et al. Early versus delayed antihypertensive treatment in patients with acute ischaemic stroke: multicentre, open label, randomised, controlled trial. BMJ. 2023;383:e076448. doi: 10.1136/bmj-2023-076448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sandset EC, Anderson CS, Bath PM, et al. European Stroke Organisation (ESO) guidelines on blood pressure management in acute ischaemic stroke and intracerebral haemorrhage. Eur Stroke J. 2021;6(2):XLVIII‐LXXXIX. doi: 10.1177/23969873211012133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang H, Li M, Xie S, et al. Visit‐to‐visit systolic blood pressure variability and stroke risk: a systematic review and meta‐analysis. CURR Med SCI. 2019;39(5):741‐747. doi: 10.1007/s11596-019-2100-9 [DOI] [PubMed] [Google Scholar]
- 13. Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit‐to‐visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375(9718):895‐905. doi: 10.1016/S0140-6736(10)60308-X [DOI] [PubMed] [Google Scholar]
- 14. Manning LS, Mistri AK, Potter J, Rothwell PM, Robinson TG. Short‐term blood pressure variability in acute stroke: post hoc analysis of the controlling hypertension and hypotension immediately post stroke and continue or stop post‐stroke antihypertensives collaborative study trials. Stroke. 2015;46(6):1518‐1524. doi: 10.1161/STROKEAHA.115.009078 [DOI] [PubMed] [Google Scholar]
- 15. Yang C, Liu K, Song Y, et al. Day‐by‐day blood pressure variability is associated with neurological functional outcome after acute ischemic stroke. Front Neurol. 2020;11:566825. doi: 10.3389/fneur.2020.566825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kollias A, Stergiou GS, Kyriakoulis KG, Bilo G, Parati G. Treating visit‐to‐visit blood pressure variability to improve prognosis: is amlodipine the drug of choice? Hypertension. 2017;70(5):862‐866. doi: 10.1161/HYPERTENSIONAHA.117.10087 [DOI] [PubMed] [Google Scholar]
- 17. Havlik RJ, Foley DJ, Sayer B, Masaki K, White L, Launer LJ. Variability in midlife systolic blood pressure is related to late‐life brain white matter lesions: the Honolulu–Asia aging study. Stroke. 2002;33(1):26‐30. doi: 10.1161/hs0102.101890 [DOI] [PubMed] [Google Scholar]
- 18. Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta‐analysis. BMJ. 2016;354:i4098. doi: 10.1136/bmj.i4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Judge C, O'Donnell MJ, Hankey GJ, et al. Urinary sodium and potassium, and risk of ischemic and hemorrhagic stroke (INTERSTROKE): a case–control study. Am J Hypertens. 2021;34(4):414‐425. doi: 10.1093/ajh/hpaa176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Donnell MJ, Chin SL, Rangarajan S, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case–control study. Lancet. 2016;388(10046):761‐775. doi: 10.1016/S0140-6736(16)30506-2 [DOI] [PubMed] [Google Scholar]
- 21. Dehghan M, Mente A, Teo KK, et al. Relationship between healthy diet and risk of cardiovascular disease among patients on drug therapies for secondary prevention: a prospective cohort study of 31 546 high‐risk individuals from 40 countries. Circulation. 2012;126(23):2705‐2712. doi: 10.1161/CIRCULATIONAHA.112.103234 [DOI] [PubMed] [Google Scholar]
- 22. Tanaka T, Okamura T, Miura K, et al. A simple method to estimate populational 24‐h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens. 2002;16(2):97‐103. doi: 10.1038/sj.jhh.1001307 [DOI] [PubMed] [Google Scholar]
- 23. Mente A, O'Donnell MJ, Dagenais G, et al. Validation and comparison of three formulae to estimate sodium and potassium excretion from a single morning fasting urine compared to 24‐h measures in 11 countries. J Hypertens. 2014;32(5):1005‐1015. doi: 10.1097/HJH.0000000000000122 [DOI] [PubMed] [Google Scholar]
- 24.Elm E von, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shrestha N. Detecting multicollinearity in regression analysis. AJAMS. 2020;8(2):39‐42. doi: 10.12691/ajams-8-2-1 [DOI] [Google Scholar]
- 26. Sun F, Zhao LP, Zhang JY, Jin C, Jin Q. The effects of dietary salt on blood pressure variability, cerebral perfusion and cognitive function in young‐adult male rats. Phys Ther. 2023;38(S1):5732586. doi: 10.1152/physiol.2023.38.S1.5732586 [DOI] [Google Scholar]
- 27. Brignole M, Moya A, de Lange FJ, et al. 2018 ESC Guidelines for the diagnosis and management of syncope. Eur Heart J. 2018;39(21):1883‐1948. doi: 10.1093/eurheartj/ehy037 [DOI] [PubMed] [Google Scholar]
- 28. Palaiodimou L, Joundi RA, Katsanos AH, et al. Association between blood pressure variability and outcomes after endovascular thrombectomy for acute ischemic stroke: an individual patient data meta‐analysis. Eur Stroke J. 2024;9(1):88‐96. doi: 10.1177/23969873231211157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American Heart Association/American Stroke Association. Stroke. 2021;52(7):e364‐e467. doi: 10.1161/STR.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 30. Zhou TL, Schütten MTJ, Kroon AA, et al. Urinary sodium excretion and salt intake are not associated with blood pressure variability in a white general population. JAHA. 2023;12(1):e026578. doi: 10.1161/JAHA.122.026578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schutte R, Thijs L, Liu YP, et al. Within‐subject blood pressure level—not variability—predicts fatal and nonfatal outcomes in a general population. Hypertension. 2012;60(5):1138‐1147. doi: 10.1161/HYPERTENSIONAHA.112.202143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. De La Sierra A, Redon J, Banegas JR, et al. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension. 2009;53(3):466‐472. doi: 10.1161/HYPERTENSIONAHA.108.124008 [DOI] [PubMed] [Google Scholar]
- 33. Gosmanova EO, Mikkelsen MK, Molnar MZ, et al. Association of systolic blood pressure variability with mortality, coronary heart disease, stroke, and renal disease. J Am Coll Cardiol. 2016;68(13):1375‐1386. doi: 10.1016/j.jacc.2016.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Webb AJ, Lawson A, Mazzucco S, Li L, Rothwell PM. Age and sex distribution of beat‐to‐beat blood pressure variability after transient ischemic attack and minor stroke: a population‐based study. Int J Stroke. 2021;16(6):683‐691. doi: 10.1177/1747493020971905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Parati G, Bilo G, Kollias A, et al. Blood pressure variability: methodological aspects, clinical relevance and practical indications for management—a European Society of Hypertension position paper*. J Hypertens. 2023;41(4):527‐544. doi: 10.1097/HJH.0000000000003363 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.
Data Availability Statement
No additional data are available.


