Abstract
Objectives:
Standard chemotherapy agents, including carboplatin, have known immunogenic properties. We sought to determine how carboplatin may influence lymphocyte trafficking to tumor sites.
Methods:
Murine models of ovarian cancer were utilized to examine lymphocyte trafficking with common clinically used agents including carboplatin, anti-PD-1 antibody, or anti-VEGFR-2 antibody. Adhesion interactions of lymphocytes with tumor vasculature were measured using intravital microscopy, lymphocyte homing with immunohistochemistry, and treatment groups followed for overall survival.
Results:
Carboplatin chemotherapy profoundly alters the tumor microenvironment to promote lymphocyte adhesive interactions with tumor vasculature and resultant improvement in lymphocyte trafficking. The measured results seen with carboplatin in the tumor microenvironment were superior to anti-PD-1 treatment or anti-VEGFR-2 which may have contributed to increased overall survival in carboplatin treated groups.
Conclusions:
These novel findings suggest a role for chemotherapeutic agents to broadly influence anti-tumor immune responses beyond the induction of immunogenic tumor cell death.
Keywords: ovarian cancer, tumor immunology, lymphocyte trafficking, tumor infiltrating lymphocytes, antiangiogenic therapy
Introduction
Epithelial ovarian cancer is responsible for the most deaths out of all the gynecologic cancers with approximately 22,240 new cases and over 14,000 related deaths annually in the United States[1]. Most patients are diagnosed at an advanced stage of disease and five-year overall survival is less than 30%[2–4]. Standard of care options include either neoadjuvant chemotherapy followed by surgical resection or primary surgery followed by adjuvant cytotoxic chemotherapy using platinum-based regimens[5]. Even with primary response rates to chemotherapy of up to 80%, the majority of patients will have a recurrence and ultimately die from disease[6, 7]. As ovarian cancers appear to be immunogenic, immunotherapy with checkpoint inhibitors, adoptive transfer of cytotoxic T-cells, cancer vaccines, and the use of oncolytic viruses have been explored with mixed success[8–10]. Similar to other cancer types, patients with ovarian cancer whose tumors contain higher quantities of tumor infiltrating lymphocytes (TIL) have a more favorable prognosis[11–14]. It is increasingly recognized that successful cancer immunotherapy requires infiltration of effector T-cells which is typically limited in the majority of patients[15–17]. Therefore, methods to influence the migration of T-cells into the tumor microenvironment may improve current protocols by rendering patients responsive to immunotherapy[18–22].
A major limiting factor to T-cell infiltration into the tumor microenvironment is the unorganized and abnormal tumor vasculature. In the tumor microenvironment there is an overexpression of proangiogenic factors such as vascular endothelial growth factor (VEGF) which lead to the creation of immature, tortuous and hyper-permeable aberrant blood vessels[23–25]. Tumor vessel diameters are inconsistent and not efficient at the delivery of nutrients or removal of waste due to impaired blood flow. These abnormal tumor vessels promote immune evasion and reduce the efficacy of immunotherapy by mechanically and functionally inhibiting the delivery of cytotoxic T lymphocytes[26–28]. Additionally, resultant hypoxia associated with these impaired vessels and oxygen delivery lead to an increase in Tregs and myeloid-derived suppressor cell populations that further promote an immunosuppressive tumor microenvironment, which nullify T-cell function and migration[29–32].
Tumor vessels are not only structurally aberrant, but also functionally deficient. T-cell trafficking is a complex process involving the interplay between T-cells and the endothelium as a well-defined lymphocyte adhesion cascade that is obligatory to accessing the tumor microenvironment[28, 33, 34]. T-cells must first tether and roll on the endothelium via selectins, followed by chemokine activation, and then firm adhesion prior to extravasation into the underlying tissue. Of the available methods to study lymphocyte trafficking in the tumor microenvironment, intravital microscopy (IVM) can visualize the adhesion cascade in real time[17]. IVM has been used successfully in preclinical models of mice to study T and B cell interactions in germinal centers, metastatic cancers of the liver, brain, lung, and melanoma[28, 35–39]. These animal models often require the use of window chambers to visualize areas of interest serially over time with IVM, but limited observations may only require exposure of the tissue of interest similar to a technique that has been recently applied in human studies[27].
In melanoma patients, our group utilized IVM for first-in-human studies evaluating the tumor vasculature by measuring vessel diameter, density, blood velocity, wall shear stress, and vessel function[27]. A major finding was that ~50% of tumor vessels did not support blood flow at the time of observation. While this study demonstrated the feasibility of performing intravital microscopy in a human patient population, the findings of lack of blood flow within tumors implied that effective treatments may never reach the tumor microenvironment. Based on these observations, we sought to determine the real-time features of tumor vasculature in a murine model of epithelial ovarian cancer and if standard clinical treatments influence lymphocyte trafficking.
Materials and Methods
Animals
Syngeneic female C57BL/6J mice, obtained from the Laboratory of Animal Resources at Roswell Park Comprehensive Cancer Center, were used to establish a murine epithelial ovarian cancer model. Transgenic OT1PL RAGKO mouse spleens were used as a source of lymphocytes for adoptive transfer.
Cell lines
The aggressive murine epithelial ovarian cancer cell line, IE9-MP1[40, 41], expressing ovalbumin was utilized. Peritoneal disease was established with 5 × 106 cells injected intraperitoneally per mouse. Prior to the intravital microscopy procedure, OT1 mice served as lymphocyte donors and were euthanized. The spleen was removed and placed into a 70 μm strainer over a conical tube and pulverized. These cells were then centrifuged at 1800 RPM for 8 minutes and the red blood cells lysed using ACK lysis buffer. Centrifugation was performed again at 1800 rpm for 8 minutes and 25 μl of Cell Tracker Green CMFDA (5-chloromethylfluorescein diacetate) (Thermo-Fisher Scientific, Waltham, MA) was used to fluorescently label the cell pellet and incubated at 37 C for 20 minutes. The cells were resuspended with 1mL of PBS yielding the final labelled lymphocyte product.
Intravital microscopy
C57BL/6J mice were anesthetized with ketamine/xylazine using an intraperitoneal injection. A tail vein catheter was inserted using 30-gauge needles and flexible tubing. The mouse was secured to the Olympus BX51 WL epi-illumination intravital microscopy system heating pad and the abdomen shaved. The surgical site was cleansed with 70% ethanol followed by povidone-iodine. The abdominal skin was elevated and cut with surgical scissors and the incision was carried superiorly to the xiphoid process. The inferior incision was carried down to over the bladder. The peritoneum was then elevated and incised, and the incision length mirrored the previous skin incision. Using 5–0 silk suture the abdomen was retracted laterally bilaterally and secured to the heating pad platform (Fig 1A). The small intestines were gently retracted to the animal’s right side exposing the stomach and omentum. A glass microscope slide was laid across the abdomen and the omentum was placed on top of the slide. 50 ml conical tops were used to elevate the slide off the abdominal cavity, one on each side of the slide to reduce motion artifact (Fig 1B). The heating pad platform was then placed onto the microscopy stage (Fig 1C). The omentum was surveyed at 40X magnification for a general overview. Magnification was increased to 100X and up to 100 μl of fluorescently labeled cells (1 × 106 cells) were injected into the tail vein catheter. Observations were continued in up to 3 high powered fields for maximum of 15 minutes and digitally recorded for subsequent off-line analyses (Fig 1D).
Figure 1 –
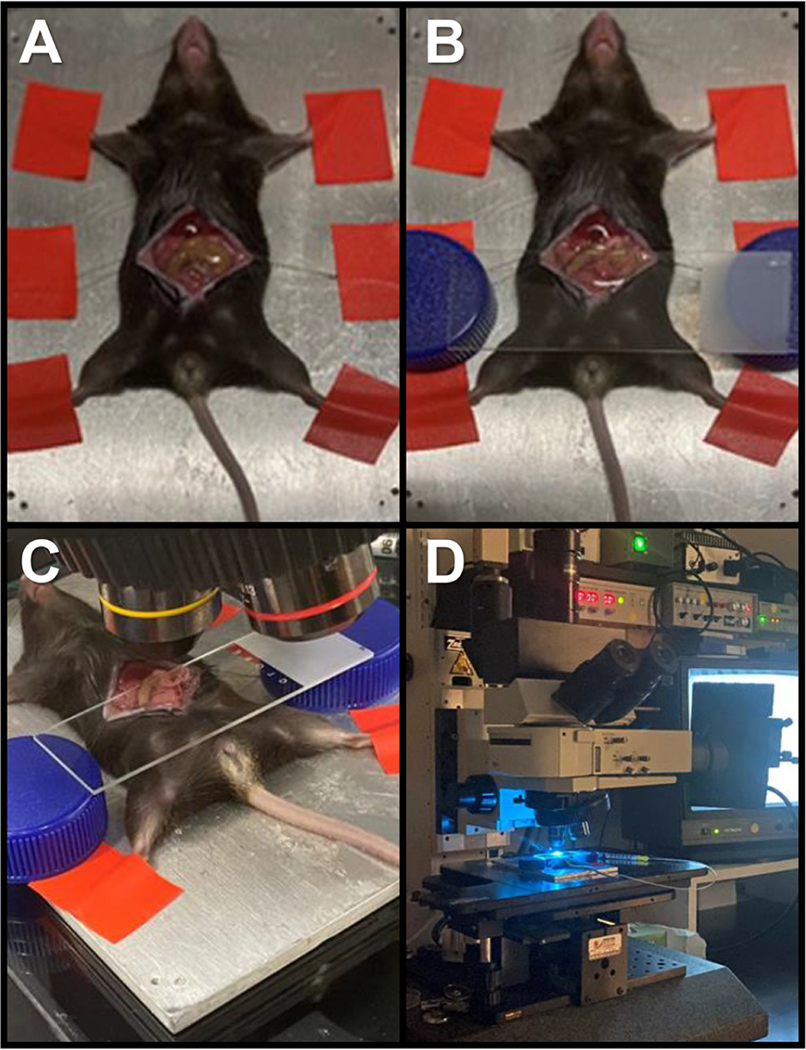
(A) Mice anesthetized with ketamine/xylazine are secured to the Olympus BX51 WL epi-illumination intravital microscopy system heating pad as abdomen and peritoneum retracted with 5–0 silk sutures. (B) Omental tissue containing tumor is exposed and placed on top of a glass microscope slide laid across the abdomen supported by 50 ml conical tops elevating the slide off the abdominal cavity to reduce motion artifact. (C) Heating pad platform placed onto the microscopy stage. (D) Omental tumor tissue identified at 40X magnification and magnification increased to 100X for recorded observation using fluorescently labeled cells (1 × 106 cells) injected via tail vein catheter.
IVM variable measurements
The rolling fraction of labeled lymphocytes was defined as the percentage of cells transiently interacting with the vasculature out of the total number of cells passing through the vessel during the observation period. Sticking efficiency was the percentage of total cells that arrested on a vessel wall for ≥30 seconds. Bleed percentage was calculated as areas of omental tissue with loss of base tissue autofluorescence that were not in defined vessels, based on the photo-absorptive nature of hemoglobin in red blood cells. Up to 50 μl of fluorescein isothiocyanate-dextran (Sigma-Aldrich, location) was injected into the tail vein catheter. The omental tissue was observed for vasculature viability and the intravital portion was concluded. The mice were euthanized per protocol. The omentum was excised and formalin-fixed and paraffin embedded or frozen using O.C.T. Compound (Thermo-Fisher Scientific, Waltham, MA) for pathologic examination.
Treatment groups
Carboplatin (Fresenius Kabi, Lake Zurich, IL) was obtained from the Department of Pharmacy, Roswell Park Comprehensive Cancer Center. Mice to receive carboplatin received the drug dosed at 20 mg/kg using a 200 μl intraperitoneal injection once per week starting 7 days post inoculation of tumor for a total of 3 doses. Mouse-specific Anti-VEGFR-2 was purchased (BioXcell, Lebanon, NH) and was dosed at 6.25 mg/kg in PBS using a 200 μl intraperitoneal injection twice per week starting at day 10 post tumor inoculation for a total of 4 doses. Mouse-specific Anti-PD-1 was obtained from BioXCell (Lebanon, New Hampshire USA) and dosed at 12.5 mg/kg in PBS using a 200 μl intraperitoneal injection three times per week starting at day 10 post tumor inoculation for 6 total doses.
Survival studies
Experiments were repeated to conduct survival studies, consisting of the time when criteria for euthanasia reached. Each treatment group consisted of 5 mice and were followed until moribund status as stated by institutional IACUC protocol.
Immunohistochemistry
The removed omental tissues were fixed in 10% buffered formalin for 24 hours prior to processing. Tissues were processed and embedded in paraffin and then sectioned at 5 μm. Slides were de-paraffinized in several baths of xylene and then rehydrated in graded alcohols followed by ddH2O. Using an auto-stainer slides were incubated in 3% H2O2 for 15 min. To block non-specific binding, tissues were incubated with 10% normal goat (CD31, CD8) and 10% normal rabbit (CD4) serums for 30 min, followed by avidin/biotin block (Vector Labs). Primary antibody CD31 1:50 (Abcam #Ab28364), CD8 1:400 (Cell Signaling #98941S), and CD4 1:250 (Affymetrix #14–9766-82) were diluted in 1% BSA solution and incubated for 30 minutes at room temperature, followed by the biotinylated Goat anti Rabbit and Rabbit anti Goat for 15 minutes, respectively. For signal enhancement, ABC reagent (Vector labs) was applied for 30 minutes. To reveal endogenous peroxidase activity, slides were incubated with DAB substrate (Dako) for 5 minutes and then counterstained with DAKO Hematoxylin for 20 seconds. Slides were dehydrated through several baths of graded alcohols and xylenes and then cover-slipped.
Image analyses
TILs were defined as all CD4+ and CD8+ lymphocytes that invaded the tumor tissue. To measure CD4+ and CD8+ TILs, whole section slides were digitally scanned using Aperio Scanscope (Aperio Technologies, Inc., Vista, CA) with 20X bright-field microscopy. These images were then accessible using Spectrum (Aperio Technologies, Inc., Vista, CA), a web-based digital pathology information management system. Once slides are scanned, Aperio ImageScope version 12.3.3.5048 (Aperio Technologies, Inc., Vista, CA) was used to view images for image analysis. An annotation layer was created and the outlines of the tissue on the slides were circled using the free form pen tool. This annotation was used define the size of the analysis area.
The Aperio cytoplasmic algorithm was tailored to develop quantitative macros for the quantification of target cells. Briefly, these algorithms used color de-convolution to separate diaminobenzidine (DAB) from the hematoxylin counterstain thereby providing stain separation. In this case the cytoplasmic algorithm was modified to detect and quantifies the CD8+ lymphocytes. The results included the total number of cells analyzed, the number of positive cells and the size of the analysis area. Using the information produced by the analysis results, the actual number of CD4+ and CD8+ lymphocytes was then calculated. To normalize variability due to differences in tissue size, the number of CD8+ lymphocytes were reported per square millimeter.
To measure blood vessel diameter by anti CD31 IHC, histologic sections were stained with standard haematoxylin and eosin and for CD31 (Human—1:50 dilution, Clone JC70A, Dako, Carpinteria, CA, Murine—1:20 dilution, Clone SZ31, Dianova, Hamburg, Germany). Stained sections were scanned with an Aperio Scanscope XT (Leica Microsystems Inc., Buffalo Grove, IL) and evaluated using Aperio Spectrum software. Vessel density was assessed using standard ‘hot spot’ methodology. For each case, vessels in five separate hot spot fields were counted at 200X total magnification. Hot spot fields are defined as tumoral areas with large intra-tumoral vessels. Vessels with diameter ≤ 4 μm are not included. In these same fields, the maximum cross-sectional diameter was measured in all vessels with an identifiable lumen. Measurement utilized the Spectrum software toolset.
Statistical analyses
The measurements of CD4+ and CD8+ cell densities were log10 transformed. Comparisons of group means in cell densities and cell trafficking attributes were made utilizing two-sided independent sample t-tests. Trends of CD4+ and CD8+ cell densities and vessel diameters over time in non-treatment groups were investigated using linear regressions. Similarly, correlations between CD4/CD8 cell densities and vessel diameters were also examined using linear regressions. Statistical significance of coefficients was evaluated using F-tests. Difference was considered significant at p < 0.05. Probability of survival was calculated by Log Rank test.
Results
Commonly used systemic therapy agents for human ovarian cancer demonstrated the ability to alter the tumor microenvironment and tumor vessels to support lymphocyte adhesive interactions (Fig. 2). Mice treated with anti-PD-1 monotherapy were noted to have a statistically significant 2-fold increase in mean percentage of lymphocytes initiating rolling within the tumor vasculature as compared to control (untreated) mice, 17.0% versus 33.0% (p=0.008) (Fig 2A). Anti-VEGFR-2 treated mice did not show any significant differences when compared to untreated control mice for any of the trafficking variables measured during the observation period, thereby serving as a clinically relevant “treated” control when examining T cell trafficking and infiltration. In comparison to anti-VEGFR-2 treated groups, anti-PD1 treated mice and carboplatin treated groups had a 2.3 and 1.6-fold statistically significant increase in rolling fraction, respectively. Mice treated with single agent carboplatin demonstrated a statistically significant 4.2-fold increase in mean lymphocyte sticking fraction compared to untreated control (p=0.0012), and a 3.5-fold increase when compared to the anti-VEGFR-2group (p=0.0006) (Fig 2B). No significant differences were noted in sticking fraction in the anti-PD1 group when compared to both untreated and treated controls. Representative photomicrographs from individual IVM video frames from each group demonstrates tumor vasculature, fluorescently-labeled lymphocytes, and sites of vascular disruption (Fig 2C).
Figure 2 –
(A) The percent of fluorescently labeled cells with rolling or (B) sticking/firm adhesion revealed no differences between controls and anti-VEGFR-2 treated groups, but statistically significant differences between carboplatin vs. anti-VEGFR-2 for rolling (p=0.035) and sticking (p=.0006) and anti-PD-1 vs. anti-VEGFR-2 for rolling (p=0.0015 ). Two-sided independent sample t-tests, significance p≤0.05. (C) Representative photomicrographs of IVM video frames showing tumor vessels (white arrows), bleeding/vascular disruption (white arrowheads), and fluorescently labelled cells (black arrows).
As the end- product of lymphocyte-endothelial interactions, T cell infiltration into tumor tissue was measured (Fig. 3). Confirming the previously noted IVM observations, untreated control mice and anti-VEGFR-2treated mice did not demonstrate any significant differences in CD8+ T cell infiltration. However, carboplatin treated mice had a 1.4-fold increase in CD8+ TILs compared to the anti-VEGFR-2treated group (1.9 cells/mm2 vs 2.7 cells/mm2, respectively; p=0.0028) (Fig. 3A). There was no statistically significant difference in CD8+ TILs in the untreated or anti-VEGFR-2controls when compared to the anti-PD1 treated mice. CD4+ T cell infiltration was similarly measured within omental tumor specimens, and no statistically significant differences were seen between any of the treatment groups or controls (Fig. 3B). Representative photomicrographs of IHC-stained tissue sections depict co-localization of CD8+ and CD4+ TIL infiltration in carboplatin and anti-PD-1 treated groups (Fig. 3C). Combined with the quantitative measurements of TIL infiltration, this co-localization is indicative of favorable functional changes in the tumor microenvironment consistent with vascular access.
Figure 3 –
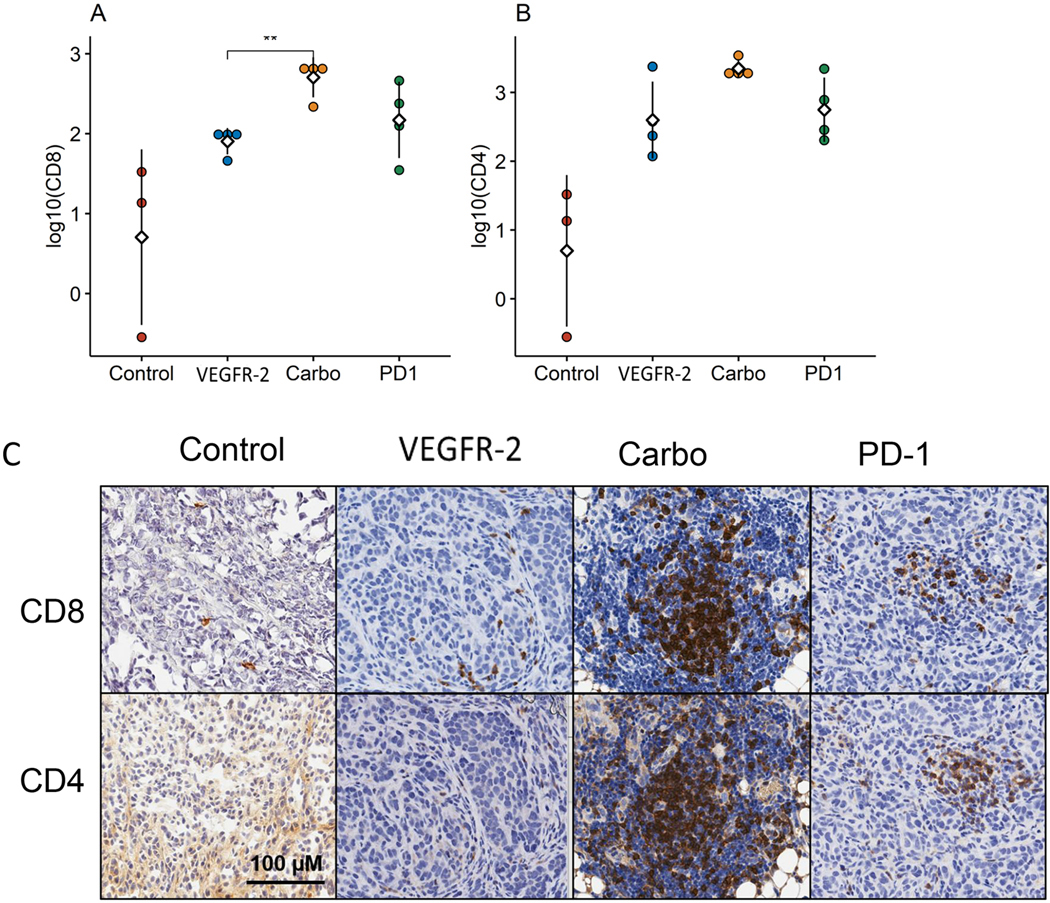
(A) CD8+ T cell infiltration is significantly increased in carboplatin treated groups compared to anti-VEGFR-2 with (B) elevated levels of CD4+ T cells noted across all treatment groups. (C) Representative photomicrographs of IHC-stained tissue sections show co-localization of CD8+ and CD4+ TIL populations in the carboplatin and anti-PD-1 treated groups and sparse detection in control or anti-VEGFR-2 groups. (n = 3–4 mice per group, in 2 experiments)
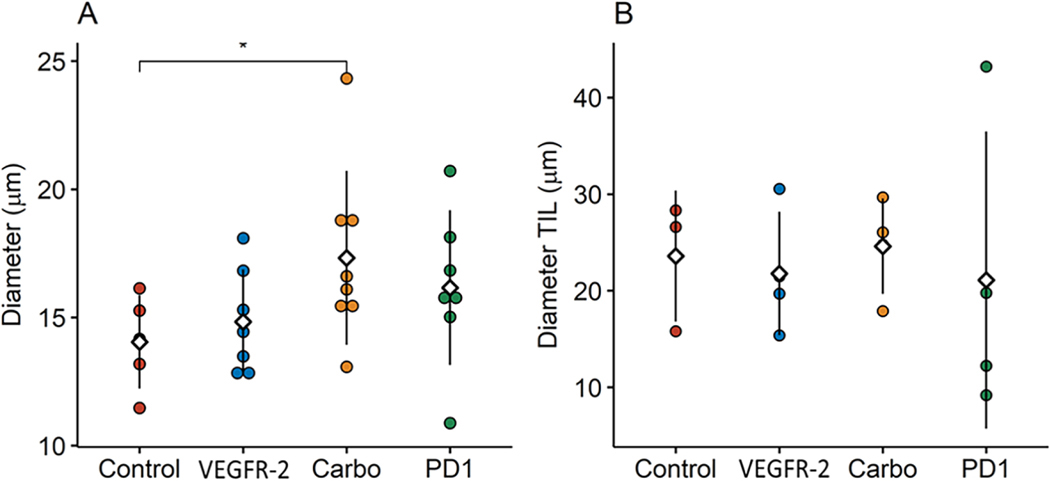
Tumor blood vessel diameter measured by IVM demonstrated an influence associated with carboplatin treatment (Fig. 4A). Blood vessel diameters measured post hoc were 23.3% larger in carboplatin treated groups as compared to untreated controls (14.0 vs. 17.3 μm, respectively; p=0.0453). No other significant differences were noted in vessel diameter in the other groups. Unlike IVM, measurement of the tumor vasculature diameters by IHC (CD31) demonstrated no significant differences between any of the groups (Fig. 4B). For comparison, the carboplatin treated mice had an average vessel diameter of 24.6 μm compared to 23.6 μm in untreated control (p=0.8371). In general, vessel diameter measurements taken via IHC were larger than those recorded by IVM with increased variability by IHC as noted by measures of standard error.
Figure 4 –
(A) Tumor vessel diameters showed a trend to increasing with treatment compared to controls with carboplatin having a statistically significant enlargement compared to controls (p=0.0453). (B) Tumor vessel diameters measured by IHC showed no significant differences with more variable measurements obtained compared to IVM. (n = 8–10 mice per group in 3 individual experiments)
Epithelial ovarian cancers are known to induce bloody ascites formation and a leaky vasculature secondary to abnormal neovascularization. Percent bleed was measured to assess the phenomenon of omental tissue with significant blood pooling and an indicator of loss of vessel integrity (Fig. 5A). In carboplatin treated mice a significant reduction in the percent bleed within omental tissue was observed from 37.2% to 14.6% as compared to anti-VEGFR-2treated groups (p=0.0138). A similar trend was noted between anti-VEGFR-2treated groups and anti-PD1 treated groups (37.2% vs 17.7%, respectively; p=0.0565). Contrary to presumed vascular normalizing effects, anti-VEGFR-2had no significant influence on percent bleed compared to untreated controls. Examining tumor vessel ability to support blood flow, carboplatin treated mice demonstrated a 2.3-fold increase in functional vasculature compared to anti-VEGFR-2treated groups (84.5% vs. 37.1%, respectively; p=0.0069) (Fig. 5B). Additionally, a 2.1-fold increase in percent functional vessels was measured in mice treated with anti-PD1 compared to anti-VEGFR-2treated groups (76.8% vs. 37.1%, respectively; p=0.0145). Anti-VEGFR-2did not appear to have any effect on the percentage of functional vessels compared to untreated controls (p=0.1838). Blood flow velocity was measured within the omental tumor vasculature and anti-VEGFR-2treatment had no demonstrable effect when compared to untreated control or any other treatment group (Fig. 5C). Wall shear stress, which is known to affect lymphocyte trafficking was also measured with none of the treatments having any demonstrable difference compared to untreated mice (Fig. 5D).
Figure 5 –
(A) Tumor vessels demonstrating disruption or bleeding were significantly higher in anti-VEGFR-2 treated mice compared to carboplatin (p=0.0138). (B) Tumor vessels capable of supporting flow at the time of the IVM observation showed statistically significant improvement in carboplatin (p=0.0069) and anti-PD-1 (p=0.0145) treatment groups compared to anti-VEGFR-2. (C) Blood flow velocity of tumor vessels and (D) wall shear stress showed no significant differences between groups. (n = 8–10 mice per group in 3 individual experiments)
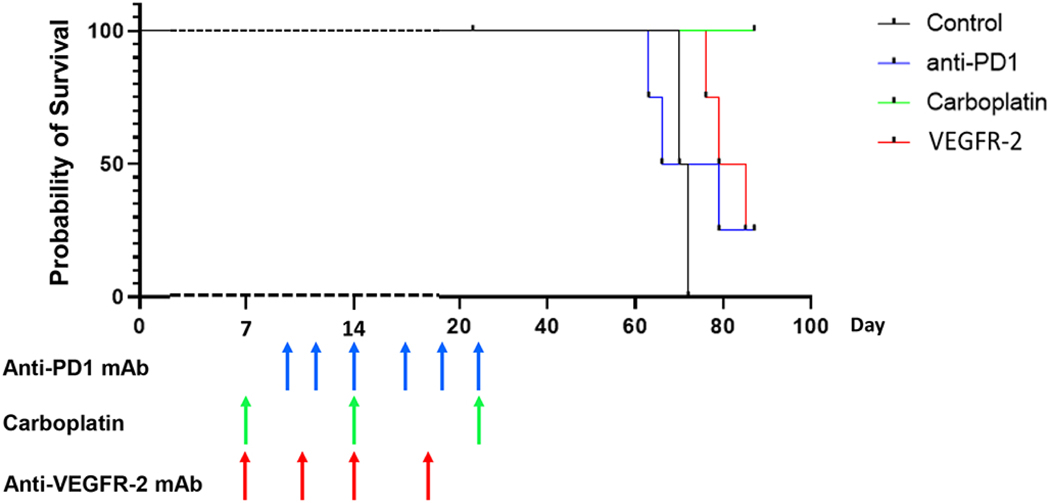
Significant survival prolongation was noted with carboplatin treatment compared to all other groups (Fig. 6) suggesting that the aforementioned changes in the tumor vasculature and TIL interactions/infiltration may partially influence clinical outcome. Interestingly, while anti-VEGFR-2 had no significant influence on lymphocyte/endothelial interactions, lymphocyte infiltration, or basic tumor vessel features, a survival benefit was noted when compared to control mice (p=0.02). A similar survival benefit was noted with PD-1 treatment, but carboplatin treatment was strikingly superior to not only anti-VEGFR-2, but PD-1 treated groups (p=0.04) which may be a reflection of each treatment’s different anti-tumor mechanisms.
Figure 6 –
Carboplatin was associated with complete responses and prolonged overall survival compared to all other groups (p=0.0134). Anti-VEGFR-2 treated mice showed improved survival compared to control groups (p=0.0084). (n = 4 mice per group representative of 2 experiments)
Discussion
The mechanisms of action of standard chemotherapies have been well described; however, secondary effects on the tumor microenvironment have been postulated to drive subsequent immunogenic cell death that may be responsible for improved outcomes[42–44]. The specific ability of chemotherapy to influence tumor micro-vessels to support lymphocyte interactions remains relatively unexplored, but would be anticipated to be critical to mounting an ongoing anti-tumor immune response. In this study we utilized IVM in a murine intraperitoneal epithelial ovarian cancer tumor model to compare the influences of platinum chemotherapy, VEGF-targeted therapy, and immunotherapy on immune cell trafficking into the tumor microenvironment. IVM allowed the interrogation of each aspect of the lymphocyte adhesion cascade and quantitative comparisons between treatment groups. The results demonstrate that the standard chemotherapy agent carboplatin had the ability to promote lymphocyte-endothelial interactions through enhanced rolling, sticking and enhance CD8+ lymphocyte infiltration into the tumor which may have contributed toward an improved survival benefit. Also, of note, vessel diameter was increased and percentage of bleeding within omental tissue was decreased. Percentage of viable tumor vessels supporting blood flow was improved with carboplatin as well. Treatment with anti-PD-1 therapy was able to show benefit as well albeit to a lesser degree than carboplatin. Anti-PD-1 treatment demonstrated increased rolling and increase in percentages of viable blood vessels supporting blood flow, but the significantly improved survival benefit for carboplatin treatment suggests that these microvascular differences may be clinically significant.
The preclinical evidence for carboplatin to serve as an immunogenic agent is mixed[45–47], however the sole readout of immunogenic cell death may underestimate other factors including changes to tumor vascularity or improved TIL localization. Preliminary evidence from neoadjuvant clinical trials in breast cancer suggest that the addition of carboplatin is directly related to improved complete pathologic response rates and anti-tumor immune properties including increased TIL[48–51]. Additionally, dose dense chemotherapy regimens including carboplatin have been linked to CD8+ immune-mediated mechanisms[52]. Our current results demonstrate a novel finding, namely that carboplatin exerts significant influence at the level of tumor microvascular structure and function that may promote increased TIL accumulation. While anti-PD-1 treatment would also be expected to generate brisk anti-tumor immune responses, they were less pronounced compared to carboplatin. A possible explanation could be that the PD-1 axis relies on existing antigen specific anti-tumor lymphocytes and our murine model may not be temporally similar to an endogenously developing tumor. However, the presence of increased TIL in anti-PD-1 treatment groups suggests that existing anti-tumor T cells were present at the time of treatment. Furthermore, it is known that lymphocyte infiltration of tumors can be a “feed-forward” mechanism with the induction of tertiary lymphoid structures[53, 54], yet carboplatin treatment resulted in higher levels of TIL. Collectively, our data suggests that carboplatin was superior to single agent anti-PD-1 and raises the potential for synergistic applications, although this combination did not translate into clinical benefit in a recent clinical trial. The phase III JAVELIN Ovarian 100 study included 998 patients with advanced or metastatic (stage III/IV) epithelial ovarian cancer, fallopian tube cancer, or primary peritoneal cancer (ClinicalTrials.gov Identifier: NCT02718417). Patients were randomized to carboplatin/paclitaxel; carboplatin/paclitaxel with maintenance avelumab; or avelumab plus carboplatin/paclitaxel followed by maintenance avelumab. Neither of the 2 avelumab arms demonstrated a PFS benefit over the control arm of chemotherapy alone[55]. While the underlying mechanisms for the lack of clinical benefit of the combination are not yet understood, we propose that it is unlikely to be attributable to impaired lymphocyte-endothelial interactions and trafficking.
It would be anticipated that the use of antiangiogenic drugs such as anti-VEGFR-2 antibodywould shift the balance from abnormal vasculature to a more normalized structure over time; however, our results demonstrated more disrupted vessels and bleeding with decreased vessels supporting flow. There is growing evidence in glioblastoma that the effects of anti-VEGFR-2 antibody may reflect our current observations and excessive use may actually exacerbate the abnormal tumor vasculature and tissue hypoxia[56–58]. Additionally, the associated clinical toxicities of anti-VEGFR-2 treatment of ovarian cancers could have contributed to our observed survival results[59].
Limitations of this study include our use of non-sorted naïve T-cells that were used to demonstrate functional adhesive interactions during IVM observations as opposed to activated T-cells. It is likely that the adhesive interactions recorded by IVM would have been similar regardless of the reporter cell, but even if differences existed, it would be anticipated to be consistent across the treatment groups. The investigations used naïve T cell interactions to be more reflective of early time points in the elaboration of immune responses and avoid the potential for rapid tumor clearance if fully activated cells were employed. Similarly, while we investigated CD4+ and CD8+ T cell infiltration, activation and/or exhaustion markers were not analyzed, but could have influenced lymphocyte retention at these late time points. Another consideration is that the time points from initiation of chemotherapy to the time of IVM differed slightly based upon the drug utilized (Initiation - Day 21 for anti-VEGFR-2 treated groups, D18 for anti-PD-1 groups and Day 21 for carboplatin treated groups). The difference of 3 days less in the anti-PD-1 would likely have a negligible effect on these data that is 3 weeks on average. Future studies could address any true time variations between groups as defined by the current data and potentially identify time points for synergistic combinatorial or sequential treatments. Importantly, while these experiments were performed in a murine ovarian cancer model, future studies will be needed to determine whcether the currently observed properties of carboplatin are applicable across varied tumor types and locations. While the IVM platform is unable to evaluate the entire mouse omentum at high power magnification, our evaluation of 3 representative fields with the potential for hundreds of measurements per field represents a good approximation of the entire specimen regarding tumor vessel and T-cell interactions.
Given the increased trafficking improvement seen with single agent carboplatin and single agent anti-PD-1, future studies will investigate combination therapy of platinum agents and checkpoint blockade. Combination therapy may have synergy with adoptive cell therapies and could be examined with the use of activated T-cells in this model system. This study demonstrates how lymphocyte trafficking to the tumor as a marker of prognosis and response, is influenced by standard clinical therapies that have demonstrated efficacy. These results raise important questions of actual mechanisms of standard clinical treatments and whether they can be leveraged to improve outcomes.
Highlights.
Carboplatin promotes lymphocyte adhesive interactions with tumor vasculature and increases lymphocyte trafficking
Carboplatin was associated with increased vessel diameters and percentage of viable tumor vessels supporting blood flow
The influence of carboplatin on the tumor microenvironment suggests synergy with existing immunotherapy approaches
Funding
This work was supported by Roswell Park Comprehensive Cancer Center with the National Cancer Institute grant P30CA016056 and the Roswell Park Alliance Foundation and National Cancer Institute training grant T32CA108456 in surgical oncology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Torre LA, Trabert B, DeSantis CE et al. Ovarian cancer statistics, 2018. CA Cancer J Clin 2018; 68: 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora T, Mullangi S, Lekkala MR. Ovarian Cancer. In StatPearls. Treasure Island (FL): 2021. [Google Scholar]
- 3.Huang Z, Yan H, Chavan D et al. Effective treatment of a patient with stage IV ovarian cancer: A case report. Oncol Lett 2018; 15: 588–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raja FA, Chopra N, Ledermann JA. Optimal first-line treatment in ovarian cancer. Ann Oncol 2012; 23 Suppl 10: x118–127. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong DK, Alvarez RD, Bakkum-Gamez JN et al. Ovarian Cancer, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021; 19: 191–226. [DOI] [PubMed] [Google Scholar]
- 6.Chandra A, Pius C, Nabeel M et al. Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med 2019; 8: 7018–7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGuire WP, Hoskins WJ, Brady MF et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 1996; 334: 1–6. [DOI] [PubMed] [Google Scholar]
- 8.Yang C, Xia BR, Zhang ZC et al. Immunotherapy for Ovarian Cancer: Adjuvant, Combination, and Neoadjuvant. Front Immunol 2020; 11: 577869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Odunsi K. Immunotherapy in ovarian cancer. Ann Oncol 2017; 28: viii1-viii7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gil M, Komorowski MP, Seshadri M et al. CXCL12/CXCR4 blockade by oncolytic virotherapy inhibits ovarian cancer growth by decreasing immunosuppression and targeting cancer-initiating cells. J Immunol 2014; 193: 5327–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuji T, Eng KH, Matsuzaki J et al. Clonality and antigen-specific responses shape the prognostic effects of tumor-infiltrating T cells in ovarian cancer. Oncotarget 2020; 11: 2669–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.James FR, Jiminez-Linan M, Alsop J et al. Association between tumour infiltrating lymphocytes, histotype and clinical outcome in epithelial ovarian cancer. BMC Cancer 2017; 17: 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kroeger DR, Milne K, Nelson BH. Tumor-Infiltrating Plasma Cells Are Associated with Tertiary Lymphoid Structures, Cytolytic T-Cell Responses, and Superior Prognosis in Ovarian Cancer. Clin Cancer Res 2016; 22: 3005–3015. [DOI] [PubMed] [Google Scholar]
- 14.Sato E, Olson SH, Ahn J et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005; 102: 18538–18543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salas-Benito D, Conde E, Tamayo-Uria I et al. The mutational load and a T-cell inflamed tumour phenotype identify ovarian cancer patients rendering tumour-reactive T cells from PD-1(+) tumour-infiltrating lymphocytes. Br J Cancer 2021; 124: 1138–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hao J, Yu H, Zhang T et al. Prognostic impact of tumor-infiltrating lymphocytes in high grade serous ovarian cancer: a systematic review and meta-analysis. Ther Adv Med Oncol 2020; 12: 1758835920967241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher DT, Chen Q, Skitzki JJ et al. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J Clin Invest 2011; 121: 3846–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGray AJR, Eppolito C, Miliotto A et al. A prime/boost vaccine platform efficiently identifies CD27 agonism and depletion of myeloid-derived suppressor cells as therapies that rationally combine with checkpoint blockade in ovarian cancer. Cancer Immunol Immunother 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Majidpoor J, Mortezaee K. The efficacy of PD-1/PD-L1 blockade in cold cancers and future perspectives. Clin Immunol 2021; 226: 108707. [DOI] [PubMed] [Google Scholar]
- 20.Chen M, Brackett CM, Burdelya LG et al. Stimulation of an anti-tumor immune response with “chromatin-damaging” therapy. Cancer Immunol Immunother 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zsiros E, Lynam S, Attwood KM et al. Efficacy and Safety of Pembrolizumab in Combination With Bevacizumab and Oral Metronomic Cyclophosphamide in the Treatment of Recurrent Ovarian Cancer: A Phase 2 Nonrandomized Clinical Trial. JAMA Oncol 2021; 7: 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pitt JM, Marabelle A, Eggermont A et al. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol 2016; 27: 1482–1492. [DOI] [PubMed] [Google Scholar]
- 23.Majidpoor J, Mortezaee K. Angiogenesis as a hallmark of solid tumors - clinical perspectives. Cell Oncol (Dordr) 2021. [DOI] [PubMed] [Google Scholar]
- 24.Garrido MP, Torres I, Vega M, Romero C. Angiogenesis in Gynecological Cancers: Role of Neurotrophins. Front Oncol 2019; 9: 913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhani NC, Oza AM. Targeting Angiogenesis: Taming the Medusa of Ovarian Cancer. Hematol Oncol Clin North Am 2018; 32: 1041–1055. [DOI] [PubMed] [Google Scholar]
- 26.Gabriel EM, Kim M, Fisher DT et al. Dynamic control of tumor vasculature improves antitumor responses in a regional model of melanoma. Sci Rep 2020; 10: 13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher DT, Muhitch JB, Kim M et al. Intraoperative intravital microscopy permits the study of human tumour vessels. Nat Commun 2016; 7: 10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikucki ME, Fisher DT, Matsuzaki J et al. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat Commun 2015; 6: 7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasidharan Nair V, Saleh R, Toor SM et al. Metabolic reprogramming of T regulatory cells in the hypoxic tumor microenvironment. Cancer Immunol Immunother 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandula JK, Rodriguez PC. Tumor-related stress regulates functional plasticity of MDSCs. Cell Immunol 2021; 363: 104312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semenza GL. Intratumoral Hypoxia and Mechanisms of Immune Evasion Mediated by Hypoxia-Inducible Factors. Physiology (Bethesda) 2021; 36: 73–83. [DOI] [PubMed] [Google Scholar]
- 32.Augustin RC, Delgoffe GM, Najjar YG. Characteristics of the Tumor Microenvironment That Influence Immune Cell Functions: Hypoxia, Oxidative Stress, Metabolic Alterations. Cancers (Basel) 2020; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sackstein R, Schatton T, Barthel SR. T-lymphocyte homing: an underappreciated yet critical hurdle for successful cancer immunotherapy. Lab Invest 2017; 97: 669–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skitzki J, Craig RA, Okuyama R et al. Donor cell cycling, trafficking, and accumulation during adoptive immunotherapy for murine lung metastases. Cancer Res 2004; 64: 2183–2191. [DOI] [PubMed] [Google Scholar]
- 35.Matuszak J, Tabuchi A, Kuebler WM. Ventilation and Perfusion at the Alveolar Level: Insights From Lung Intravital Microscopy. Front Physiol 2020; 11: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seano G, Jain RK. Vessel co-option in glioblastoma: emerging insights and opportunities. Angiogenesis 2020; 23: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Zeng Z. Live Imaging of Innate and Adaptive Immune Responses in the Liver. Front Immunol 2020; 11: 564768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boulch M, Grandjean CL, Cazaux M, Bousso P. Tumor Immunosurveillance and Immunotherapies: A Fresh Look from Intravital Imaging. Trends Immunol 2019; 40: 1022–1034. [DOI] [PubMed] [Google Scholar]
- 39.Weigert R, Sramkova M, Parente L et al. Intravital microscopy: a novel tool to study cell biology in living animals. Histochem Cell Biol 2010; 133: 481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang RY, Eppolito C, Lele S et al. LAG3 and PD1 co-inhibitory molecules collaborate to limit CD8+ T cell signaling and dampen antitumor immunity in a murine ovarian cancer model. Oncotarget 2015; 6: 27359–27377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang RY, Francois A, McGray AR et al. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. Oncoimmunology 2017; 6: e1249561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma Y, Yang H, Pitt JM et al. Therapy-induced microenvironmental changes in cancer. J Mol Med (Berl) 2016; 94: 497–508. [DOI] [PubMed] [Google Scholar]
- 43.Vacchelli E, Aranda F, Eggermont A et al. Trial Watch: Chemotherapy with immunogenic cell death inducers. Oncoimmunology 2014; 3: e27878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Apetoh L, Obeid M, Tesniere A et al. Immunogenic chemotherapy: discovery of a critical protein through proteomic analyses of tumor cells. Cancer Genomics Proteomics 2007; 4: 65–70. [PubMed] [Google Scholar]
- 45.Galluzzi L, Vitale I, Warren S et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer 2020; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flieswasser T, Van Loenhout J, Freire Boullosa L et al. Clinically Relevant Chemotherapeutics Have the Ability to Induce Immunogenic Cell Death in Non-Small Cell Lung Cancer. Cells 2020; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamazaki T, Buque A, Ames TD, Galluzzi L. PT-112 induces immunogenic cell death and synergizes with immune checkpoint blockers in mouse tumor models. Oncoimmunology 2020; 9: 1721810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lynce F, Nunes R. Role of Platinums in Triple-Negative Breast Cancer. Curr Oncol Rep 2021; 23: 50. [DOI] [PubMed] [Google Scholar]
- 49.Loibl S, O’Shaughnessy J, Untch M et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol 2018; 19: 497–509. [DOI] [PubMed] [Google Scholar]
- 50.Sikov WM, Berry DA, Perou CM et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 2015; 33: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von Minckwitz G, Schneeweiss A, Loibl S et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 2014; 15: 747–756. [DOI] [PubMed] [Google Scholar]
- 52.Chang CL, Hsu YT, Wu CC et al. Dose-dense chemotherapy improves mechanisms of antitumor immune response. Cancer Res 2013; 73: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colbeck EJ, Jones E, Hindley JP et al. Treg Depletion Licenses T Cell-Driven HEV Neogenesis and Promotes Tumor Destruction. Cancer Immunol Res 2017; 5: 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peske JD, Thompson ED, Gemta L et al. Effector lymphocyte-induced lymph node-like vasculature enables naive T-cell entry into tumours and enhanced anti-tumour immunity. Nat Commun 2015; 6: 7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ledermann JA, Oza AM, Birrer MJ et al. Avelumab in combination with and/or following chemotherapy vs chemotherapy alone in patients with previously untreated epithelial ovarian cancer: Results from the phase 3 javelin ovarian 100 trial. In SGO 20/20. Toronto, ON: 2020. [Google Scholar]
- 56.Huang S, Michalek JE, Reardon DA et al. Assessment of tumor hypoxia and perfusion in recurrent glioblastoma following bevacizumab failure using MRI and (18)F-FMISO PET. Sci Rep 2021; 11: 7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Michaelsen SR, Staberg M, Pedersen H et al. VEGF-C sustains VEGFR2 activation under bevacizumab therapy and promotes glioblastoma maintenance. Neuro Oncol 2018; 20: 1462–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu-Emerson C, Duda DG, Emblem KE et al. Lessons from anti-vascular endothelial growth factor and anti-vascular endothelial growth factor receptor trials in patients with glioblastoma. J Clin Oncol 2015; 33: 1197–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee SP, Hsu HC, Tai YJ et al. Bevacizumab Dose Affects the Severity of Adverse Events in Gynecologic Malignancies. Front Pharmacol 2019; 10: 426. [DOI] [PMC free article] [PubMed] [Google Scholar]