Supplemental Digital Content is Available in the Text.
Key Words: HIV-1, cabotegravir, rilpivirine, long-acting therapy, implementation study
Abstract
Background:
Cabotegravir + rilpivirine long-acting (CAB + RPV LA) dosed every 2 months (Q2M) is a complete regimen for the maintenance of HIV-1 virologic suppression. In this study, we report month 12 clinical outcomes in patient study participants (PSPs) in the CAB and RPV Implementation Study in European Locations (CARISEL) study.
Setting:
CARISEL is a phase 3b implementation–effectiveness study.
Methods:
CARISEL was designed as a 2-arm, unblinded study with centers randomized to either enhanced or standard implementation arms. For PSPs, this study is single arm, unblinded, and interventional; all PSPs switched from daily oral therapy to CAB + RPV LA dosed Q2M. The primary objective was to evaluate the perceived acceptability, appropriateness, and feasibility of CAB + RPV LA implementation for staff participants (presented separately). Clinical secondary endpoints assessed through month 12 included the proportion of PSPs with plasma HIV-1 RNA ≥50 and <50 copies/mL (Snapshot algorithm), incidence of confirmed virologic failure (CVF; 2 consecutive plasma HIV-1 RNA levels ≥200 copies/mL), adherence to injection visit windows, and safety and tolerability.
Results:
Four hundred thirty PSPs were enrolled and treated; the mean age was 44 years (30% ≥50 years), 25% were women (sex at birth), and 22% were persons of color. At month 12, 87% (n = 373/430) of PSPs maintained HIV-1 RNA <50 copies/mL, with 0.7% (n = 3/430) having HIV-1 RNA ≥50 copies/mL. One PSP had CVF. The safety profile was consistent with previous findings. Overall, the results were similar between implementation arms.
Conclusion:
CAB + RPV LA Q2M was well tolerated and highly effective in maintaining virologic suppression with a low rate of virologic failure.
INTRODUCTION
Current HIV treatment guidelines recommend an antiretroviral therapy (ART) that contains at least 2 active drugs from 2 or more classes, typically as a once-daily, single-tablet regimen.1,2 Despite advances in effectiveness and convenience, longstanding challenges associated with the requirement of daily oral ART remain, including fear of inadvertent disclosure, anxiety related to staying adherent, and the daily reminder of HIV status.3 These challenges have driven increased interest in long-acting (LA) HIV therapies with reduced dosing frequencies.4,5
Cabotegravir (CAB), an integrase strand transfer inhibitor (INSTI), plus rilpivirine (RPV), a non–nucleoside reverse transcriptase inhibitor, is the first and only complete LA regimen recommended for the maintenance of HIV-1 virologic suppression.1,2 CAB + RPV LA is administered monthly or every 2 months (Q2M) by intramuscular gluteal injection1,2,6–10 and therefore may be a therapeutic alternative for people living with HIV (PWH) who experience challenges related to the need for daily oral therapy. The efficacy and tolerability of CAB + RPV LA have previously been demonstrated in phase 3/3b noninferiority trials between every 4-week dosing and daily oral therapy,6,8 as well as between every 4- and 8-week dosing regimens.7
Compared with the previous standard of prescribing oral ART therapies, the novel HIV-1 treatment modality of CAB + RPV LA requires logistical, operational, and resourcing adaptations, as well as additional staff training for delivering a complete LA injectable therapy. To this effect, the CAB and RPV Implementation Study in European Locations (CARISEL) is a phase 3b implementation–effectiveness study examining strategies to support the implementation of CAB + RPV LA dosed Q2M in various clinical settings across Europe. In this study, we present the key clinical endpoints for patient study participants (PSPs) in the CARISEL study. Implementation outcomes from the CARISEL study from the perspective of staff study participants (SSPs; primary endpoint) and PSPs are the subject of separate publications.
METHODS
Study Design and Participants
CARISEL (NCT04399551) is a phase 3b, multicenter, open-label, hybrid type III implementation–effectiveness trial conducted at 18 sites across Belgium, France, Germany, the Netherlands, and Spain; clinics with no prior experience with CAB + RPV LA were preferentially selected for study participation. Both SSPs and PSPs were enrolled to participate in this study. The primary objective was to evaluate the perceived acceptability, appropriateness, and feasibility of CAB + RPV LA implementation for SSPs (presented separately). The present publication focuses on the clinical efficacy and safety outcomes of the PSPs; implementation outcomes for PSPs will be published separately.
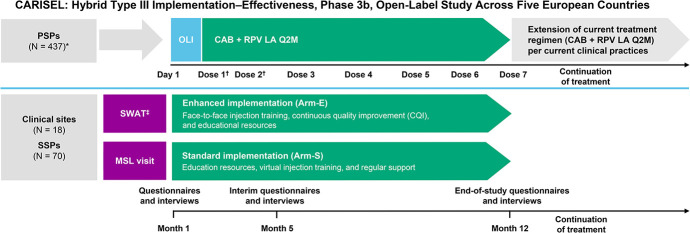
CARISEL was designed as a 2-arm, unblinded study with centers randomized to either enhanced (Arm-E) or standard (Arm-S) implementation arms (Fig. 1). Sites randomized to Arm-S were given the traditional provider support by a medical science lead, as well as product materials, including virtual injection training and provider and patient toolkits. Sites randomized to Arm-E received the same support as Arm-S, with an increased level of provider support, including face-to-face injection training, a skilled wrap-around team meeting, and 6 consecutive monthly continuous quality improvement calls (in which SSPs were asked to identify problems, plan solutions, study the results, and act accordingly to improve routine care).11,12 For PSPs, this study was single arm, unblinded, and interventional, in which all participants who fulfilled eligibility requirements were assigned to receive CAB + RPV LA Q2M and complete assessments as per the study protocol. Eligible PSPs were aged ≥18 years at the time of signing the informed consent and were virologically suppressed on guideline-recommended ART for at least 6 months before screening. Any prior switch in treatment, defined as a change of a single drug or multiple drugs simultaneously, must have occurred due to reasons of tolerability/safety, access to medications, or convenience/simplification; a switch must not have been made due to virologic failure (on treatment HIV-1 RNA ≥200 copies/mL). In addition, PSPs must have had HIV-1 RNA <50 copies/mL at screening as well as documented evidence of at least 2 plasma HIV-1 RNA measurements <50 copies/mL in the 12 months before screening: 1 within 6 months before screening and 1 within 6–12 months before screening. PSPs with any evidence of primary resistance based on the presence of any major known INSTI or non–nucleoside reverse transcriptase inhibitor resistance-associated mutations (RAMs), except for K103N, were excluded during screening. This was based on historical resistance test results, where available. Notably, CAB + RPV LA implementation began during the second wave of COVID-19 in Europe (see Figure S1, Supplemental Digital Content, http://links.lww.com/QAI/C289).
FIGURE 1.
Study design. *Four hundred thirty-seven PSPs enrolled, and 430 received CAB + RPV LA. †Dose 1 was received at month 1, dose 2 at month 2, with the remaining doses Q2M thereafter. ‡Introduce CAB + RPV LA to clinic staff and discuss what might make implementation easier and/or what might make it difficult before the first injection at the site. Meetings discussed implementation plans, how to work through challenges, and how to introduce continuous quality improvement. Arm-E, enhanced implementation arm; Arm-S, standard implementation arm; CQI, continuous quality improvement; MSL, medical scientific liaison; OLI, oral lead-in; SWAT, skilled wrap-around team.
The CARISEL study was conducted following the Declaration of Helsinki.19 All PSPs provided written informed consent. The study protocol, amendments, informed consent, and other information that required preapproval were reviewed and approved by a national, regional, or investigational center ethics committee or institutional review board.
Randomization
There was no randomization for the PSP population due to the single-arm, open-label study design (all PSPs enrolled received CAB + RPV LA Q2M). The implementation aspect of this study was a 2-arm investigation, which assumed a cluster randomization design in which investigator sites were randomized to either the Arm-E or Arm-S implementation strategy based on site location. Randomization was stratified by clinic features (such as the number of patients served).
Procedures
This study comprised a screening, intervention, and extension phase. Eligible PSPs entered the intervention phase and received oral CAB (30 mg) + RPV (25 mg) for 1 month to determine individual tolerability. PSPs then received intramuscular gluteal injections of CAB (600 mg) + RPV (900 mg) at months 1, 2, and Q2M thereafter until primary completion of the study at month 12. In the event of planned missed injection visits, CAB + RPV oral therapy was administered. If PSPs were unable to attend a visit and oral CAB + RPV could not be obtained, alternative oral ART was administered. After month 12, PSPs could remain in the extension phase of the study and continue to receive CAB + RPV LA until the study treatment was locally approved and available for their physician to prescribe. The 2 arms for the providers, Arm-E and Arm-S, contain different levels and types of support for the implementation of the HIV-1 maintenance regimen—these are summarized in Table S1, Supplemental Digital Content, http://links.lww.com/QAI/C289.
For those PSPs who met the confirmed virologic failure criterion (CVF, 2 consecutive plasma HIV-1 RNA levels ≥200 copies/mL) or withdrew from the study while meeting the suspected virologic failure criterion (SVF; a single HIV-1 RNA measurement ≥200 copies/mL), viral genotype and phenotype were analyzed from plasma samples that were collected at the SVF time point. RAMs were analyzed using Monogram Biosciences PhenoSense GT, PhenoSense Integrase, and GenoSeq Integrase testing methods for protease, RT, and integrase (IN). In addition, peripheral blood mononuclear cells were collected from all PSPs at baseline; these were only analyzed retrospectively in the case of SVF or CVF. The Monogram Biosciences GenoSure Archive assay provided HIV-1 protease, RT, and IN genotype data from integrated HIV-1 proviral DNA but did not generate phenotypic data.
Outcomes
The primary objective of this study was to evaluate the perceived acceptability, appropriateness, and feasibility of the implementation of the CAB + RPV LA regimen in the SSP population. Secondary clinical endpoints published in this study included the proportion of PSPs with plasma HIV-1 RNA ≥50 and <50 copies/mL at month 12 (US Food and Drug Administration Snapshot algorithm),14 the incidence of CVF, the incidence of genotypic and phenotypic resistance to CAB or RPV in PSPs with CVF, safety and tolerability, including the incidence and severity of adverse events (AEs), and the proportion of PSPs who discontinued treatment due to AEs. Adherence to the injection visit window was also assessed. Regarding COVID-19, assessments of suspected, probable, or confirmed cases based on the World Health Organization Case definition and European surveillance for COVID-19 were collected through a dedicated COVID-19 case report form and were reported as AEs. Examinations of efficacy outcomes and AEs across subgroups [age, sex at birth, race, baseline body mass index (BMI), and country] were also conducted.
Statistical Analysis
The sample size was based on practical considerations in terms of the feasibility of enrolling an adequate number of sites on each implementation strategy, balanced with the desire to have interventions tested across several types of investigative sites (eg, hospital-based settings or HIV outpatient clinics), and enrolling an adequate number of PSPs. This study descriptively summarized clinical AEs, laboratory evaluations, virologic parameters, and other clinical safety and efficacy outcomes. The safety population served as the primary population for both safety and efficacy analyses and comprised all enrolled PSPs who received at least 1 dose of CAB or RPV (oral or LA).
Role of the Funding Source
This study was funded by ViiV Healthcare. The funders participated in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. All authors vouch for the accuracy and completeness of the data, data analyses and interpretation, and fidelity to the protocol.
RESULTS
Clinics, Participant Characteristics, and Disposition
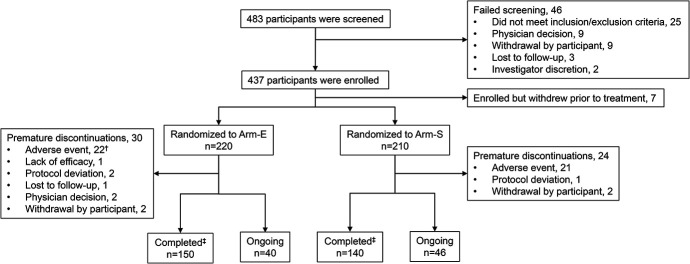
In accordance with the study design to preferentially include clinics with no prior experience with CAB + RPV LA, 72% [n = 13/18 (Arm-E, n = 6/9; Arm-S, n = 7/9)] of clinics had not previously administered the regimen at study start. In total, 483 PSPs were screened for this study, of whom 46 (10%) failed screening, with the most common reason being not meeting the inclusion or exclusion criteria (Fig. 2). A further 7 PSPs were enrolled but withdrawn before receiving study treatment, 2 of whom withdrew due to protocol deviation (eligibility criteria not met) and the remaining 5 participants withdrew consent. In total, 430 PSPs entered the intervention phase and received study treatment. As of the last participant’s last visit for the month 12 primary analysis (February 21, 2022), 54 (13%) PSPs discontinued treatment prematurely: AEs were the primary reason for premature treatment discontinuation in both arms, occurring in 10% (n = 43/430) of PSPs. Two PSPs withdrew from treatment for reasons of procedural burden and a further 2 due to frequency of visits. In addition, 3 PSPs discontinued due to protocol deviations, 2 PSPs discontinued due to physician decision (related to treatment efficacy), 1 PSP discontinued due to lack of treatment efficacy, and 1 PSP was lost to follow-up. Among those who completed the study at month 12 (n = 376), as of the last participant’s last visit, approximately 75% (n = 281/376) of PSPs transitioned to locally available CAB + RPV LA, 23% (n = 86/376) continued receiving CAB + RPV LA in the extension phase, while 2% (n = 9/376) transitioned to an alternative, oral ART.
FIGURE 2.
Randomization and treatment*. *A single PSP may have more than 1 reason for withdrawal/failure. †Includes 1 participant who was reclassified as discontinuing due to an AE before month 12, after the primary analysis was completed. ‡Completed the intervention phase (month 12). Arm-E, enhanced implementation arm; Arm-S, standard implementation arm.
Overall, baseline characteristics were similar between implementation arms (Table 1). PSPs had a mean (range) age of 44 (22–76) years, with 30% (n = 129/430) being at least 50 years old, 25% (n = 109/430) were women (sex at birth), 78% (n = 336/430) were White, and 18% (n = 76/430) were of Black or African American race.
TABLE 1.
Demographic and Clinical Characteristics at Baseline
| Baseline Demographic Characteristics | CAB + RPV LA, Arm-E (n = 220) | CAB + RPV LA, Arm-S (n = 210) | Overall (N = 430) |
| Age (yr) | |||
| Mean, range | 44.4 (22–76) | 44.1 (24–70) | 44.2 (22–76) |
| Age ≥50 yr, n (%) | 67 (30) | 62 (30) | 129 (30) |
| Sex at birth, n (%) | |||
| Female | 57 (26) | 52 (25) | 109 (25) |
| Male | 163 (74) | 158 (75) | 321 (75) |
| Self-reported sex, n (%)* | |||
| Female | 61 (28) | 54 (26) | 115 (27) |
| Male | 159 (72) | 156 (74) | 315 (73) |
| Race, n (%) | |||
| White | 169 (77) | 167 (80) | 336 (78) |
| Black or African American | 42 (19) | 34 (16) | 76 (18) |
| Asian | 3 (1) | 6 (3) | 9 (2) |
| Other races† | 6 (3) | 3 (1) | 9 (2) |
| Ethnicity, n (%) | |||
| Hispanic or Latinx | 18 (8) | 14 (7) | 32 (7) |
| Not Hispanic or Latinx | 202 (92) | 196 (93) | 398 (93) |
| BMI (kg/m2)‡ | |||
| Median, interquartile range | 25.1 (22.8–27.8) | 24.9 (22.8–27.4) | 24.9 (22.8–27.6) |
| Baseline CD4+ (cells/mm3), n (%) | |||
| <200 | 2 (<1) | 1 (<1) | 3 (<1) |
| 200 to <350 | 9 (4) | 8 (4) | 17 (4) |
| 350 to <500 | 37 (17) | 30 (14) | 67 (16) |
| ≥500 | 172 (78) | 171 (81) | 343 (80) |
A total of 6 participants had a self-reported sex that was different from the sex assigned at birth.
American Indian or Alaska Native (Arm-E, n = 4; Arm-S, n = 3); multiple races (Arm-E only, n = 2).
Per the protocol, BMI was only calculated at screening.
Arm-E, enhanced implementation arm; Arm-S, standard implementation arm.
Adherence to Dosing Window
Of the 2376 planned injection visits through month 12, 93% (n = 2211) occurred within the ±7-day dosing window, with treatment adherence similar between arms (see Figure S2, Supplemental Digital Content, http://links.lww.com/QAI/C289). Overall, 22 injection visits were missed, with 6 covered by temporary use of oral bridging with CAB + RPV or an alternative daily oral ART; the remaining 16 fully transitioned to an alternative daily oral ART regimen.
Impact of COVID-19
Overall, COVID-19 was diagnosed in 16% (n = 69/430) of PSPs and was similarly reported across each implementation arm. Important COVID-19-related protocol deviations were reported in 2 (<1%) PSPs. Four PSPs (1%) received oral CAB + RPV to cover missed injection visits due to COVID-19 infection. An additional participant received an alternative oral ART for a missed injection dose due to travel restrictions caused by COVID-19. There were no discontinuations or virologic nonresponse events due to COVID-19. Four PSPs (<1%) received home nursing visits (n = 2) or remote visits (n = 2).
Efficacy
At the month 12 Snapshot, 87% (n = 373/430) of PSPs maintained virologic suppression (HIV-1 RNA <50 copies/mL), which was consistent across implementation arms [Arm-E, 87% (n = 191/220); Arm-S, 87% (n = 182/210)]. The proportions of PSPs with HIV-1 RNA <50 copies/mL were similar by predefined baseline subgroups [age group (<50 and ≥50 years old), sex at birth, race, country, and baseline CD4+ count (<350 and ≥350 cells/mm3); Figure S3, Supplemental Digital Content, http://links.lww.com/QAI/C289]. Fewer than 1% (n = 3/430) of PSPs had HIV-1 RNA ≥50 copies/mL at month 12. All 3 PSPs with HIV-1 RNA ≥50 copies/mL were in Arm-E: 1 PSP discontinued due to lack of efficacy and 2 PSPs discontinued due to other reasons while their viral load was not below threshold (<50 copies/mL; physician decision due to SVF, n = 1; physician decision due to 2 viral load measurements between 50 and 200 copies/mL and an HIV-1 subtype A, n = 1) (Table 2). Overall, 13% (n = 54) of PSPs had no virologic data available at month 12, which was consistent across implementation arms [Arm-E, 12% (n = 26/220); Arm-S, 13% (n = 28/210)], with the reasons listed in Table 2. Of these, 7 ongoing PSPs had no data in window: 6 were reported as a protocol deviation due to a site missing the viral load assessment at month 12 followed by HIV-1 RNA <50 copies/mL at month 14. The remaining PSP was from a different site with HIV-1 RNA <50 copies/mL at month 8 and transitioned to commercial CAB + RPV LA following a missed central laboratory viral load assessment at month 12 and a local month 12 viral load of <20 copies/mL.
TABLE 2.
Snapshot Efficacy Outcomes at Month 12
| Parameter, n (%) | CAB + RPV LA, Arm-E (n = 220) | CAB + RPV LA, Arm-S (n = 210) | Overall (N = 430) |
| HIV-1 RNA <50 copies/mL | 191 (87) | 182 (87) | 373 (87) |
| HIV-1 RNA ≥50 copies/mL | 3 (1) | 0 | 3 (<1) |
| Data in window not below threshold | 0 | 0 | 0 |
| Discontinued for lack of efficacy | 1 (<1) | 0 | 1 (<1) |
| Discontinued for other reasons while not below threshold* | 2 (<1) | 0 | 2 (<1) |
| No virologic data | 26 (12) | 28 (13) | 54 (13) |
| Discontinued study due to AE or death | 20 (9) | 20 (10) | 40 (9) |
| Discontinued for other reason† | 5 (2) | 2 (<1) | 7 (2) |
| On study but missing data in window‡ | 1 (<1) | 6 (3) | 7 (2) |
Physician decision, n = 2.
Protocol deviation, n = 3; lost to follow-up, n = 1; withdrawal by participant, n = 3.
Six participants at 1 site had a missed viral load assessment at month 12 and had HIV-1 RNA <50 copies/mL at month 14. One other participant from a different site had HIV-1 RNA <50 copies/mL at month 8 and transitioned to commercial CAB + RPV LA following a missed central laboratory viral load assessment at month 12 and local month 12 viral load of <20 copies/mL.
Virologic Failure
One PSP had CVF [0.23%; female (sex at birth), BMI 29.3 kg/m2, HIV-1 subtype G] through month 12 with a viral load of 1861 copies/mL at discontinuation (month 10). At month 10, the RPV RAMs E138A + M230L were detected with no INSTI RAMs present; RPV RAM E138A was present in the baseline sample analyzed at the time of failure (see Table S2, Supplemental Digital Content, http://links.lww.com/QAI/C289). At the time of CVF (6 weeks following the prior injection), CAB and RPV plasma concentrations were 1.5 μg/mL and 78.5 ng/mL, respectively.
Following discontinuation, the PSP switched to darunavir/cobicistat/emtricitabine/tenofovir alafenamide and resuppressed at the long-term follow-up month 16 visit. An additional PSP met the SVF criterion twice [0.23%; male (sex at birth), BMI 30.4 kg/m2, HIV-1 subtype B]: at month 4 (585 copies/mL), which was not confirmed, and again at withdrawal (month 6; 386 copies/mL). The PSP resuppressed on darunavir/cobicistat/emtricitabine/tenofovir alafenamide at the month 6 visit. RPV RAM E138K and INSTI RAM N155N/S were detected in the SVF sample at month 4; no INSTI or RPV RAMs were present in baseline peripheral blood mononuclear cells. Hence, SVF or CVF with detected resistance was observed in 2 participants (0.5%), 1 of whom also had baseline RAMs. No pharmacokinetic data were available for the participants with SVF.
Safety
The overall safety profiles were similar between PSPs in Arm-E and Arm-S (Table 3).
TABLE 3.
AEs Through Last Participant’s Last Visit
| Parameter, N (%) | CAB + RPV LA, Arm-E (n = 220) | CAB + RPV LA, Arm-S (n = 210) | Overall (N = 430) |
| Any AE | 213 (97) | 206 (98) | 419 (97) |
| Excluding ISRs | 190 (86) | 173 (82) | 363 (84) |
| Any grade ≥3 AE | 21 (10) | 28 (13) | 49 (11) |
| Excluding ISRs | 17 (8) | 20 (10) | 37 (9) |
| Any drug-related AEs | 197 (90) | 192 (91) | 389 (90) |
| Excluding ISRs | 81 (37) | 75 (36) | 156 (36) |
| Any grade ≥3 drug-related AEs | 9 (4) | 16 (8) | 25 (6) |
| Excluding ISRs | 4 (2) | 6 (3) | 10 (2) |
| Any AE leading to withdrawal | 22 (10) | 21 (10) | 43 (10)* |
| Excluding ISRs | 9 (4) | 16 (8) | 25 (6) |
| Drug-related excluding ISRs | 8 (4) | 13 (6) | 21 (5)† |
| SAEs | 11 (5) | 4 (2) | 15 (3) |
| Drug-related excluding ISRs | 1 (<1) | 0 | 1 (<1)‡ |
| Fatal SAEs | 0 | 0 | 0 |
| Common (≥5% of participants) AEs (excluding ISRs) | |||
| COVID-19 | 33 (15) | 36 (17) | 69 (16) |
| Pyrexia | 18 (8) | 19 (9) | 37 (9) |
| Headache | 16 (7) | 20 (10) | 36 (8) |
| Back pain | 21 (10) | 12 (6) | 33 (8) |
| Diarrhea | 10 (5) | 17 (8) | 27 (6) |
| Asthenia | 14 (6) | 11 (5) | 25 (6) |
| Nasopharyngitis | 14 (6) | 11 (5) | 25 (6) |
| Common (≥3% in any arm) drug-related AEs (excluding ISRs) | |||
| Headache | 10 (5) | 14 (7) | 24 (6) |
| Pyrexia | 10 (5) | 12 (6) | 22 (5) |
| Asthenia | 11 (5) | 7 (3) | 18 (4) |
| Diarrhea | 3 (1) | 11 (5) | 14 (3) |
| Fatigue | 2 (<1) | 9 (4) | 11 (3) |
Includes 1 participant who was reclassified as discontinuing due to an ISR AE before month 12, after the primary analysis was completed.
Weight gain, n = 2; dizziness, n = 1; myalgia, n = 1; nausea, fever, and vomiting, n = 1; noncardiac chest pain, n = 1; suicidal ideation, n = 1; upper abdominal pain, abdominal distension, and diarrhea, n = 1; abdominal distension and diarrhea, n = 1; asthenia, insomnia, and irritability, n = 1; decreased appetite, depressed mood, postural dizziness, headaches, malaise, and pain, n = 1; depression, n = 1; diarrhea, n = 1; sciatica, n = 1; asthenia and leg pain, n = 1; anxiety, n = 1; acute hepatitis, n = 1; chills, fever, and night sweats, n = 1; weight gain and lipodystrophy, n = 1; insomnia, n = 1; pain in right leg, chills, and fever, n = 1.
Suicidal ideation, n = 1.
SAE, serious adverse event.
Excluding injection site reactions (ISRs), AEs occurred in 84% (n = 363/430) of PSPs, with 36% (n = 156/430) of PSPs experiencing drug-related AEs; 10 (2%) PSPs had drug-related AEs of Grade ≥3. Excluding ISRs, AEs leading to withdrawal occurred in 6% (n = 25/430) of PSPs (6 of whom also had an ISR AE at the time of withdrawal). Overall, 5% (n = 21/430) of PSPs had drug-related AEs (excluding ISRs) leading to withdrawal. In total, 3% (n = 15) of PSPs experienced a serious adverse event; 1 serious adverse event was considered by the investigator to be related to study treatment (suicidal ideation). This participant previously reported suicidal ideation at the time of HIV diagnosis, approximately 5 years before initiating CAB + RPV, and recovered in 18 days. There were no fatal AEs. The most frequent non-ISR AEs, excluding COVID-19, were pyrexia (9%, n = 37/430), headache (8%, n = 36/430), and back pain (8%, n = 33/430). Headache (6%, n = 24), pyrexia (5%, n = 22), and asthenia (4%, n = 18) were the most common non-ISR drug-related AEs. The safety profiles were generally similar by age, sex at birth, and race (see Table S3, Supplemental Digital Content, http://links.lww.com/QAI/C289), as well as by BMI and country (see Table S4, Supplemental Digital Content, http://links.lww.com/QAI/C289).
Overall, 5844 injections (Arm-E, 2962; Arm-S, 2882) were administered with 1858 ISR events reported (Arm-E, 941; Arm-S, 917); the ISR profile was similar between implementation arms. Injection pain was the most frequent ISR event, occurring with 26% (n = 1533/5844) of injections (see Table S5, Supplemental Digital Content, http://links.lww.com/QAI/C289). Of all ISR events, 98% (n = 1828/1858) were grade 1 or 2 in severity; no grade 4 or 5 events were reported. The median duration of ISR events was 3 days (interquartile range, 2–5). The proportion of PSPs reporting ISRs at each visit decreased over time in both implementation arms (see Figure S4, Supplemental Digital Content, http://links.lww.com/QAI/C289). In total, 6% (n = 24/430) of PSPs withdrew due to injection-related reasons [Arm-E, 7% (n = 15); Arm-S, 4% (n = 9)]. Of these participants, 6 also had a non-ISR AE at the time of withdrawal and were therefore included in both categories.
DISCUSSION
The clinical parameters of CAB + RPV LA have been extensively studied in the context of 4 large randomized clinical trials, Antiretroviral Therapy as Long Acting Suppression (ATLAS), First Long-Acting Injectable Regimen (FLAIR), ATLAS-2M, and Switch Onto Long-Acting Regimen (SOLAR)6–8,12 ; however, studies evaluating the LA regimen in the context of real-world use are required for improving the implementation and helping realize the maximum impact of this novel intervention for PWH. To this end, and by design, CARISEL enrolled a diverse population of PSPs (in terms of sex, race, and age), representing a broad spectrum of PWH across Europe. Overall, the clinical findings are consistent with those in previous phase 3/3b randomized controlled trials for CAB + RPV LA, supporting its effectiveness as a maintenance regimen.
Treatment adherence and study visit compliance were high during the study, with the majority (93%) of injections occurring within the ±7-day treatment window, suggesting that the regimen was well tolerated, and PSPs were comfortable with the treatment regimen.
At the month 12 Snapshot, 87% of PSPs maintained HIV-1 virologic suppression and 0.7% of PSPs had HIV-1 RNA ≥50 copies/mL across both arms, with similar efficacy results for both levels of implementation. This is consistent with the primary outcome in CARISEL (reported separately), in which the mean scores of acceptability, appropriateness, and feasibility of the implementation of CAB + RPV LA reported by SSPs demonstrated no significant difference between implementation arms.15 The virologic outcomes in CARISEL are similar to those observed at week 48 in the 3 large phase 3/3b CAB + RPV LA trials.6–8 When examined across predefined subgroups [age group (<50 and ≥50 years old), sex at birth, race, country, and baseline CD4+ count (<350 and ≥350 cells/mm3)], rates of virologic suppression were similar.
In this large and diverse population, the rate of CVF was low with 1 PSP (0.23%) meeting the CVF criterion through month 12, supporting the effectiveness of CAB + RPV LA. This rate is slightly lower than that observed in the CAB + RPV LA arms during the phase 3/3b FLAIR, ATLAS, and ATLAS-2M studies through 48 weeks (∼1%) 6–8; however, it is consistent with the CARLOS and CUSTOMIZE implementation studies, in which the incidence of CVF ranged from 0% to 0.5%.13,14 Variation in the rates of CVF between studies may be driven by differences in the study populations, including the respective prevalence of risk factors for CVF. The presence of 2 or more baseline factors (of the following 3: preexisting RPV RAMs, HIV-1 subtype A6/A1, and/or BMI ≥30 kg/m2) has been previously associated with an increased risk of CVF during CAB + RPV LA treatment in multivariable analyses.15,16 In CARISEL, the PSP with CVF had RPV RAMs present at baseline (E138A) and failure (E138A + M230L) with no INSTI RAMs detected. CAB and RPV plasma concentrations were consistent with previous observations for Q2M dosing.7,17 However, these results should be interpreted with caution as the samples were obtained 6 weeks following the prior injection (at the time of CVF) and, therefore, do not represent the Q2M trough concentrations. An additional PSP met the SVF criterion with no RAMs observed at baseline; however, RPV (E138K) and INSTI (N155N/S) RAMs were detected at failure. Both PSPs had one of the aforementioned factors present: baseline RPV RAMs for the PSP with CVF; BMI ≥30 kg/m2 for the PSP with SVF. Following discontinuation, both PSPs resuppressed on darunavir/cobicistat/emtricitabine/tenofovir alafenamide.
Overall, the CAB + RPV LA regimen was well tolerated with no notable differences between implementation arms or subgroups (age, sex at birth, and race). ISRs were mostly mild to moderate (98% grade 1 or 2) and short-lived (median 3 days), with 6% of PSPs withdrawing due to injection-related reasons. The overall safety profile of CAB + RPV LA in this implementation study is consistent with those observed in phase 3/3b clinical studies,6–8,12 with no new safety signals identified.
Notably, the implementation of CAB + RPV LA began during the second wave of COVID-19 in Europe, which posed several challenges in terms of local lockdowns, travel restrictions, limited site staff resource capacity, and potential difficulties in getting access to medicines. Despite this, there were no discontinuations or virologic nonresponse events due to COVID-19, highlighting the ability to successfully incorporate CAB + RPV LA therapy even during a time when clinic resources may be limited. Furthermore, the impact of COVID-19 on the study overall was minimal and the interpretation of the safety and efficacy results was not affected. Although CARISEL was designed to emulate real-world use of CAB + RPV LA where possible, it should be noted that its design is still a more controlled environment than a true real-world evaluation (drug was also centrally supplied to sites). Encouraging real-world data on CAB + RPV LA have been generated from the ongoing OPERA cohort and the noninterventional CARLOS study.18,20 When results were compared by demographic characteristics, the small sample size of some subgroups limited the ability to draw robust conclusions. Nevertheless, this study has several strengths. First, 72% of sites had no previous experience administering CAB + RPV LA, representing real-world implementation for clinics introducing this LA therapy into routine practice. Second, the protocol-defined goal to enroll at least 20% female (sex at birth) PSPs was achieved with 25% of PSPs being women. Last, a diverse population was enrolled, with 22% of PSPs being persons of color (18% of PSPs identifying as Black) and 30% aged at least 50 years, providing necessary data on previously underrepresented groups.
CONCLUSIONS
Across diverse European clinical settings and PSPs in the CARISEL study, CAB + RPV LA dosed Q2M was well tolerated and highly effective in maintaining virologic HIV-1 suppression with a low rate of virologic failure.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank everyone who has contributed to the success of this study: all study participants and their families, and the other lead clinical investigators [Leïla Belkhir, Cliniques universitaires Saint-Luc (UCLouvain), Brussels, Belgium; Linos Vandekerckhove, Universitair Ziekenhuis Gent, Belgium; Berend J. van Welzen, UMC Utrecht, Utrecht, the Netherlands; Thomas Lutz, Infektio Research GmbH & Co, Frankfurt, Germany; Julián Olalla Sierra, Hospital Costa del Sol, Marbella, Spain; Joaquin Portilla, Hospital General Universitario de Alicante, Alicante, Spain; María Crusells-Canales, Hospital Clinico Lozano Blesa, Zaragoza, Spain; Laurence Slama, Hôpital Hôtel Dieu, Paris, France; Eliette Jeanmaire, CHRU NANCY, France] and their staff. The authors thank Janssen for the provision of the drug RPV LA and oral RPV for this study. CARISEL was funded by ViiV Healthcare. Professional medical writing and editorial assistance was provided by Daniel Williams and Poppie Cooper of Nucleus Global, funded by ViiV Healthcare.
Footnotes
Presented at: ID Week; October 19–23, 2022; Virtual and Washington, DC. 24th International AIDS Conference, July 29–August 02, 2022; Virtual and Montreal, Canada.
C.J.-O. has received fees for advisory board meetings from Gilead, Janssen-Cilag, Merck Sharp & Dohme, and ViiV Healthcare; received speaking fees from Janssen-Cilag, ViiV Healthcare, and Clinical Care Options; and served as a consultant to ViiV Healthcare. J.G. has received fees for advisory board meetings from AstraZeneca, Gilead, MSD, Roche, Theratechnologies, and ViiV Healthcare and consulting fees from ViiV Healthcare. M.v.d.V. has received fees for advisory board meetings and research grants all paid to his institution from ViiV Healthcare, Gilead, and Merck Sharp & Dohme. E.F. has received research grants paid to their institution from ViiV Healthcare and Gilead, and fees paid to their institution for attending meetings and/or travel. F.V. has received speaking fees from ViiV Healthcare, Gilead, Merck Sharpe & Dohme, Janssen, and AbbVie. S.D.W. has received financial support or grants paid to his institution from ViiV Healthcare, Gilead, Merck Sharpe & Dohme, and Janssen; and has participated in data safety monitoring or advisory boards for ViiV Healthcare and CureVac. A.R. has received fees for advisory board meetings from ViiV Healthcare and Gilead. F.B. has received fees for advisory board meetings and speaking fees from Gilead and ViiV Healthcare and grants paid to his institution from Gilead and ViiV Healthcare. L.H. has received personal fees and nonfinancial support from Gilead, Janssen, Merck Sharp & Dohme, and ViiV Healthcare. K.H., M.A.-.K., G.B., C.L.L., C.A.G., M.C., R.D.A., and J.v.W. are employees of ViiV Healthcare and stockholders of GSK. R.D., S.I., and M.G. are employees and stockholders of GSK.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.jaids.com).
Contributor Information
Celia Jonsson-Oldenbüttel, Email: cjo@mvz-mag.de.
Jade Ghosn, Email: jade.ghosn@aphp.fr.
Marc van der Valk, Email: m.vandervalk@amsterdamumc.nl.
Eric Florence, Email: eflorence@itg.be.
Francisco Vera, Email: franciscovera72@gmail.com.
Stéphane De Wit, Email: stephane.dewit@stpierre-bru.be.
Agathe Rami, Email: agathe.rami@aphp.fr.
Fabrice Bonnet, Email: fabrice.bonnet@chu-bordeaux.fr.
Laurent Hocqueloux, Email: laurent.hocqueloux@chr-orleans.fr.
Mounir Ait-Khaled, Email: mounir.c.ait-khaled@viivhealthcare.com.
Rebecca DeMoor, Email: rebecca.demoor@gmail.com.
Gilda Bontempo, Email: gilda.x.bontempo@viivhealthcare.com.
Christine L. Latham, Email: christine.l.talarico@viivhealthcare.com.
Cassidy A. Gutner, Email: cassidy.x.gutner@viivhealthcare.com.
Supriya Iyer, Email: supriya.x.ramnathiyer@gsk.com.
Martin Gill, Email: martin.9.gill@gsk.com.
Maggie Czarnogorski, Email: maggie.x.czarnogorski@viivhealthcare.com.
Ronald D'Amico, Email: ronald.d.damico@viivhealthcare.com.
Jean van Wyk, Email: jean.x.andre-van-wyk@viivhealthcare.com.
REFERENCES
- 1.U.S. Department of Health and Human Services. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV; 2021. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/whats-new-guidelines. Accessed August, 2023. [Google Scholar]
- 2.Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA Panel. JAMA. 2020;324:1651–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Los Rios P, Young B, Marcotullio S, et al. 1329. Experiences and emotional challenges of antiretroviral treatment (ART)—findings from the positive perspectives study. Open Forum Infect Dis. 2019;6(suppl_2):S481. [Google Scholar]
- 4.Akinwunmi B, Buchenberger D, Scherzer J, et al. Factors associated with interest in a long-acting HIV regimen: perspectives of people living with HIV and healthcare providers in four European countries. Sex Transm Infect. 2021;97:566–573. [DOI] [PubMed] [Google Scholar]
- 5.Dandachi D, Dang BN, Lucari B, et al. Acceptability and preferences for long-acting antiretroviral formulations among people with HIV infection. AIDS Care. 2021;33:801–809. [DOI] [PubMed] [Google Scholar]
- 6.Orkin C, Arasteh K, Górgolas Hernández-Mora M, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med. 2020;382:1124–1135. [DOI] [PubMed] [Google Scholar]
- 7.Overton ET, Richmond G, Rizzardini G, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 48-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet. 2021;396:1994–2005. [DOI] [PubMed] [Google Scholar]
- 8.Swindells S, Andrade-Villanueva JF, Richmond GJ, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med. 2020;382:1112–1123. [DOI] [PubMed] [Google Scholar]
- 9.ViiV Healthcare. Cabenuva PI; 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/212888s005s006lbl.pdf. Accessed October, 2022. [Google Scholar]
- 10.European Medicines Agency. Vocabria; 2021. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/vocabria. Accessed September, 2022. [Google Scholar]
- 11.Colton D. Quality improvement in health care. Conceptual and historical foundations. Eval Health Prof. 2000;23:7–42. [DOI] [PubMed] [Google Scholar]
- 12.Leis JA, Shojania KG. A primer on PDSA: executing plan–do–study–act cycles in practice, not just in name. BMJ Qual Saf. 2017;26:572–577. [DOI] [PubMed] [Google Scholar]
- 13.Sinclair G, Benson P, Mena L, et al. Clinical outcomes during customize: a hybrid III implementation-effectiveness study focused on implementation of cabotegravir plus rilpivirine (CAB + RPV) LA in US healthcare settings. Poster Presented at: 11th IAS Conference on HIV Science; July 18–21, 2021; Virtual. Poster PED416.
- 14.Borch J, Scherzer J, Jonsson-Oldenbüttel C, et al. 6-month outcomes of every 2 months long-acting cabotegravir and rilpivirine in a real-world setting – effectiveness, adherence to injections, and patient-reported outcomes of people living with HIV in the German CARLOS Cohort. Presented at: HIV Drug Therapy Glasgow 2022; Virtual and Glasgow, Scotland. Presentation 043.
- 15.van Welzen BJ Vandekerckhove L Jonsson-Oldenbüttel C, et al. Implementation of cabotegravir and rilpivirine long-acting (CAB+RPV LA): primary results from the CAB+RPV Implementation Study in European Locations (CARISEL). Poster presented at HIV Glasgow 2022. 2022;P069. [Google Scholar]
- 16.Cutrell AG, Schapiro JM, Perno CF, et al. Exploring predictors of HIV-1 virologic failure to long-acting cabotegravir and rilpivirine: a multivariable analysis. AIDS. 2021;35:1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390:1499–1510. [DOI] [PubMed] [Google Scholar]
- 18.Borch J Scherzer J Jonsson-Oldenbüttel C, et al. 6-month outcomes of every 2 months long-acting cabotegravir and rilpivirine in a real-world setting – effectiveness, adherence to injections, and patient-reported outcomes of people living with HIV in the German CARLOS cohort. Presented at: HIV Drug Therapy Glasgow 2022; Virtual and Glasgow, Scotland. Presentation 043. [Google Scholar]
- 19.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 20.Sension MG Brunet L Hsu RK, et al. Cabotegravir + rilpivirine long-acting injections for HIV treatment in the US: real world data from the OPERA cohort. Infect Dis Ther. 2023;12:2807–2817. [DOI] [PMC free article] [PubMed] [Google Scholar]