Abstract
Background
Postpartum haemorrhage (PPH) is a common and potentially life‐threatening complication of labour. Several options for preventing PPH are available, but further advances in this field are important, especially the identification of safe, easy to use and cost‐effective regimens. Tranexamic acid (TA), which is an antifibrinolytic agent that is used widely to prevent and treat haemorrhage, merits evaluation to assess whether it meets these criteria.
Objectives
To determine, from the best available evidence, whether TA is effective and safe for preventing PPH in comparison to placebo or no treatment (with or without uterotonic co‐treatment), or to uterotonic agents.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (28 January 2015) and reference lists of retrieved studies.
Selection criteria
All published, unpublished and ongoing randomised controlled trials (RCTs) evaluating the use of TA alone or in addition to uterotonics in the third stage of labour or during caesarean section (CS) to prevent PPH.
Data collection and analysis
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We entered the data into Review Manager software and checked for accuracy.
Main results
Twelve trials involving 3285 healthy women at low risk of excessive bleeding undergoing elective CS (nine trials, 2453 participants) or spontaneous birth (three trials, 832 participants) satisfied inclusion criteria and contributed data to the analysis. All participants received routine prophylactic uterotonics in accordance with the local guideline in addition to TA or placebo or no intervention. Overall, included studies had moderate risk of bias for random sequence generation, allocation concealment, blinding, selective reporting and low risk of bias for incomplete data. The quality of evidence was also as assessed using GRADE.
Blood loss greater than 400 mL or 500 mL, and more than 1000 mL was less common in women who received TA versus placebo or no intervention (risk ratio (RR) 0.52, 95% confidence interval (CI) 0.42 to 0.63, six trials, 1398 women; moderate quality evidence) and (RR 0.40, 95% CI 0.23 to 0.71, six trials, 2093 women; moderate quality evidence), respectively. TA was effective in decreasing the incidence of blood loss greater than 1000 mL in women who had undergone CS (RR 0.43, 95% CI 0.23, 0.78, four trials, 1534 women), but not vaginal birth (RR 0.28, 95% CI 0.06, 1.36, two trials 559 women). The effect of TA on blood loss greater than 500 mL or 400 mL was more pronounced in the group of women having vaginal birth than in women who had CS. Mean blood loss (from delivery until two hours postpartum) was lower in women who received TA versus placebo or no intervention (mean difference MD ‐ 77.79 mL, 95% CI ‐97.95, ‐57.64, five trials, 1186 women) and this effect was similar following vaginal birth and CS.
Additional medical interventions (moderate quality evidence) and blood transfusions were less frequent in women receiving TA versus placebo or no interventions. Mild side effects such as nausea, vomiting, dizziness were more common with the use of TA (moderate quality evidence). The effect of TA on maternal mortality, severe morbidity and thromboembolic events is uncertain (low quality evidence).
Authors' conclusions
TA (in addition to uterotonic medications) decreases postpartum blood loss and prevents PPH and blood transfusions following vaginal birth and CS in women at low risk of PPH based on studies of mixed quality. There is insufficient evidence to draw conclusions about serious side effects, but there is an increase in the incidence of minor side effects with the use of TA. Effects of TA on thromboembolic events and mortality as well as its use in high‐risk women should be investigated further.
Keywords: Female; Humans; Pregnancy; Antifibrinolytic Agents; Antifibrinolytic Agents/administration & dosage; Injections, Intravenous; Postpartum Hemorrhage; Postpartum Hemorrhage/prevention & control; Randomized Controlled Trials as Topic; Tranexamic Acid; Tranexamic Acid/administration & dosage
Plain language summary
Tranexamic acid for preventing bleeding after delivery
Postpartum haemorrhage is a common and an occasionally life‐threatening complication of labour. The majority of women receive drugs that directly stimulate the uterus (prophylactic uterotonics) during childbirth to prevent haemorrhages resulting from failure of the uterine muscle to contract normally (uterine atony).
Tranexamic acid (TA) is used to decrease blood loss in surgery and health conditions associated with increased bleeding. It works by helping to prevent the breakdown of fibrin and maintenance of blood clots. This review found that TA was also effective in reducing excessive blood loss, need for additional medical interventions to control bleeding and blood transfusions after a mother gave birth based on studies of mixed quality. Twelve trials (3285 participants) were included in the review. TA was given before caesarean section in nine randomised trials or following the vaginal birth of a baby (three randomised trials) to generally healthy women.
TA decreased blood loss greater than 400 mL or greater than 500 mL and this effect was more apparent with vaginal births. The studies had methodological shortcomings. Blood loss greater than 1000 mL decreased with the use of TA in six trials (2093 women), however, the difference was most obvious in caesarean section (two trials, 1400 women) and not in vaginal birth in which there were few such outcomes (one trial, 439 women). Mean blood loss decreased with the use of TA by 77 mL, overall (five studies, 1186 women) and with both vaginal and caesarean section births. This finding was based on studies with methodological limitations.
The studies were too small to detect the effect of TA on maternal death or blood clots. Mild side effects, which include diarrhoea, nausea and vomiting, were more common in women who received TA versus placebo or no intervention. No differences in blood loss and side effects were found when two different doses of TA were evaluated. Further larger studies are needed to investigate the effects of TA on maternal deaths and formation of clots in the blood (thromboembolism).
Summary of findings
Summary of findings for the main comparison. Tranexamic acid versus placebo/no treatment for preventing postpartum haemorrhage.
| Tranexamic acid versus placebo/no treatment for preventing postpartum haemorrhage | ||||
| Patient or population: pregnant women undergoing vaginal or caesarean birth who received TA for the prevention of postpartum haemorrhage Settings: Studies were undertaken in China, India, Iran, Pakistan, Turkey Intervention: Tranexamic acid versus placebo/no treatment1 | ||||
| Outcomes | Relative effect or Mean (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Blood loss > 400 mL or > 500 mL | RR 0.52 (0.42 to 0.63) | 1398 (6 studies) | ⊕⊕⊕⊝ moderate2 | ‐ 2 studies reported an outcome blood loss greater than 400 mL and 4 trials greater than 500 mL. ‐ 1 study investigated 2 doses of tranexamic acid, e.g. 0.5 g and 1 g and we pooled the data for both groups in this analysis. |
| Blood loss > 1000 mL | RR 0.40 (0.23 to 0.71) | 2093 (6 studies) | ⊕⊕⊕⊝ moderate3 | |
| Mean blood loss (mL) | The mean blood loss in the intervention groups was ‐ 77.79 lower (‐ 97.95 to ‐ 57.64) | 1186 (5 studies) | ⊕⊕⊕⊝ moderate 4 | ‐ 1 study investigated two doses of tranexamic acid, e.g. 0.5 g and 1 g and we pooled the data for both groups in this analysis. ‐ 1 study was opened labelled. |
| Use of additional medical interventions to control PPH | RR 0.48 (0.34 to 0.68) | 2049 (5 studies) | ⊕⊕⊕⊝ moderate5 |
|
| Maternal death or severe maternal morbidity such as seizure, thromboembolic events, need for intensive care unit admission, hysterectomy, organ failure | Not estimable | 1511 (4 studies) | ⊕⊕⊝⊝ low6 | |
| Side effects | RR 2.48 (1.36, 4.50) | 2616 (8 studies) | ⊕⊕⊕⊝ moderate7 | |
| Thromboembolic events | RR 0.98 (0.14 to 6.78) | 3012 (11 studies) | ⊕⊕⊝⊝ low6 |
|
| CI: Confidence interval; RR: Risk ratio; | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
1 Tranexamic acid was given by IV before caesarean birth or after vaginal birth in doses of 0.5 g or 10 mg/kg and 1 g or 15 mg/kg.
2 Downgraded for risk of bias (‐1). One study had high risk of selection bias; all four studies had unclear risk of performance and detection bias; one study had high risk and another study unclear risk of reporting bias.
3 Downgraded for risk of bias (‐1). One trial used computer‐generated numbers for randomisation, but the groups were unequal, e.g. women in intervention group had BMI > 30, were anaemic and had longer time of surgery and more women in control group had manual removal of placenta and more women in intervention group had placenta removed by controlled cord traction. This trial also had unclear risk of selective reporting bias. All three trials had unclear risk of performance bias and detection bias.
4 Downgraded for risk of bias (‐ 1). One trial had unclear risk and one trial had high risk of selection bias; two trials had unclear risk and two trials had high risk of performance and detection bias; one trial had high risk of attrition bias, and three trials had unclear risk of reporting bias.
5 Downgraded for risk of bias (‐1). Two trials had high risk of allocation concealment and sequence generation bias. Three trials had unclear risk of performance and detection bias.
6 Small sample size to detect this rare outcome (‐1). Also downgraded for ROB (‐1). One trial had high risk and one trial had unclear risk of selection bias; four trials had unclear risk of performance and detection bias; one trial had high risk and another one unclear risk of reporting trials.
7 Downgraded (‐1) for high statistical heterogeneity (I² = 83%).
Background
Description of the condition
Postpartum haemorrhage (PPH) remains a leading cause of maternal mortality, especially in developing countries (Ronsmans 2006). The majority (86%) of these deaths are preventable according to the committee for confidential enquiries into maternal death in South Africa (Confidential enquiries 2012). The incidence of PPH according to a recent study is 1.2% (Sheldon 2014). Up to 75% of PPH cases are caused by uterine atony (Ducloy‐Bouthors 2011), However, the cases of mortality due to obstetric haemorrhage are mainly related to other causes of PPH (Confidential enquiries 2012). Uterine atony was found only in 6.4% of cases of maternal death related to obstetric haemorrhage. Other conditions such as abruption, placenta praevia, ruptured uterus, retained placenta, morbidly adherent placenta, vaginal and cervical trauma, inverted uterus, bleeding during and after caesarean section (CS) are the cause of the vast majority of maternal deaths (Confidential enquiries 2012). Considerable efforts to assure wide‐spread access to uterotonics have been successful and the majority of women receive prophylactic uterotonics during childbirth (Sheldon 2014).
Current alternatives to tranexamic acid (TA) in preventing PPH include active management of the third stage of labour (involving administration of a prophylactic oxytocic before delivery of the placenta, and usually cord clamping and cutting, and controlled traction of the umbilical cord, over passive management: allowing the placenta to deliver spontaneously or aiding by gravity or nipple stimulation), uterotonics and prostaglandins. Widely used active management of labour is supported by a Cochrane review that showed reduced incidence of PPH greater than 1000 mL with this intervention (Begley 2015). However, assessment of individual components of active management of labour failed to identify the positive effects of early cord clamping on the amount of blood loss and the incidence of PPH in another Cochrane review (McDonald 2013). Controlled cord traction in comparison to standard placental expulsion does not decrease the incidence of PPH, but has a positive effect on the rate of retained placenta and intensity of pain and discomfort in the third stage of labour (Deneux‐Tharaux 2013). On the other hand, prophylactic oxytocin decreases the incidence of PPH, therefore, it is the uterotonic component of the active management of the third stage of labour that actually prevents PPH. There is limited evidence to support the effect of prophylactic oxytocin over ergot alkaloids on blood loss, but less side effects are reported with oxytocin use versus ergot alkaloids. There is no evidence supporting combined use of oxytocin and ergot alkaloids. Oral or sublingual misoprostol is effective in reducing the incidence of severe PPH when compared to placebo, but is less effective than uterotonic agents Tunçalp 2012. However, the issue of maternal death due to PPH remains unresolved in low‐resource settings. Attempts to address the problem need to go beyond the use of uterotonic drugs.
Description of the intervention
Tranexamic acid (TA) could be used in addition to current prophylactic uterotonic drugs in the third stage of labour, particularly in women at high risk of PPH, for example with placenta praevia, placental abruption, anaemia, multiple pregnancy or CS. It is used in the dose of 10 mg/kg given intravenously (IV) immediately after delivery of the baby (Astedt 1987) or in women undergoing CS, prior to the skin incision or after delivery of the baby. TA acts within two to three hours after oral administration and immediately after IV administration, and its half‐life is two to 10 hours (Jurema 2008). The oral route of administration is possible, but it is not ideal in the third stage of labour, when an immediate effect of the drug is required. The sublingual route may be an alternative, but has not, to our knowledge, been investigated.
Prophylactic use of TA rather than waiting for a diagnosis of PPH is supported by evidence from the CRASH‐2 study which found that TA was more effective the earlier it was administered in decreasing death due to bleeding in trauma patients (CRASH‐2 trial collaborators).
The use of TA acid for the treatment of PPH is covered by another Cochrane review (Mousa 2014).
How the intervention might work
TA potentiates the blood clotting system and is used to treat and prevent bleeding. The mechanism of action of TA is related to its antifibrinolytic effect, which makes this drug potentially very effective in the third stage of labour. TA is an inhibitor of fibrinolysis that blocks the lysine‐binding site of plasminogen to fibrin (Astedt 1987; Longstaff 1994). During placental delivery, rapid degradation of fibrinogen and fibrin occurs, as well as an increase in the activation of plasminogen activators and fibrin degradation products due to activation of the fibrinolytic system. This activation can last up to six to 10 hours postpartum, which may cause more haemorrhage. The antifibrinolytic effect of TA in the third stage of labour could make it a safe and effective alternative or adjunct to other regimens currently used in the third stage of labour for prevention of PPH.
TA could reduce blood loss associated with complications such as placenta praevia and lower genital tract trauma, as well as bleeding from the uterine body placental site. Use of TA could potentially have prevented some PPH cases if it was given to women with risk factors for PPH (Peitsidis 2011). Therefore, it may be particularly useful in preventing cases of PPH due to factors other than uterine atony, where uterotonic drugs would not be effective.
TA is an effective agent for the reduction of blood loss, which has been widely used in various areas of medicine. It has been used to decrease blood loss for many years in cases of haemorrhage, and is reported to reduce intraoperative and postoperative blood loss (Boylan 1996; Karski 1995; Katsaros 1996; Reid 1997; Vacharaksa 2002). TA is associated with a significant reduction in objective measurements of heavy menstrual bleeding when compared to placebo or other medical therapies (non‐steroidal antiinflammatory drugs (NSAIDS), oral luteal phase progestagens and ethamsylate) according to a Cochrane review (Lethaby 2000).The concerns regarding the side effects of tranexamic acid, and in particular, thromboembolic events are difficult to address because of their rare occurrence and need for large trials to be able to explore the issue adequately. The CRASH‐2 trial collaborators trial did not assess the risk of thromboembolic events associated with TA. The incidence of thromboembolic events was reported as low in a large retrospective study assessing different postoperative prophylactic anticoagulation regimens in patients with the use of TA undergoing primary total hip and knee arthroplasty (Gillette 2013). Ker and co‐authors reported that effects of TA on thromboembolic events was uncertain (deep vein thrombosis risk ratio (RR) 0.86, 95% confidence interval (CI) 0.52, 1.39 and pulmonary embolism RR 0.61, 95% CI 0.25, 1.47) based on meta‐analysis of 129 trials involving 10,488 surgical patients (Ker 2012). A Cochrane review on the use of antifibrinolytics for heavy menstrual bleeding reported no rise in side effects with TA in comparison to placebo, NSAIDS, oral luteal phase progestagens or ethamsylate (Lethaby 2000). The concerns regarding the risk of thromboembolic events related to the use of TA are especially important when this drug is used during the hypercoagulable state of pregnancy and the postpartum period. The MEGA study found that the risk of thromboembolic events was increased five‐fold during pregnancy and increased 60‐fold in the first three months after delivery compared with non‐pregnant women. Deep vein thrombosis of the leg had a 14‐fold increase and pulmonary embolism a six‐fold increase in pregnancy in this study Pomp 2008. Another large retrospective trial reported the risk of venous thromboembolism to increase by five‐fold among pregnant or postpartum women Heit 2008. Three cases of deep vein thrombosis were reported among 144 women (two in the TA group and one in the control group) in a trial on TA for treatment of PPH Ducloy‐Bouthors 2011.
Why it is important to do this review
PPH remains an important cause of maternal morbidity and mortality. It is important to establish safe, inexpensive and easily available methods of PPH prevention. Administration of TA IV in the third stage of labour may be one of these methods. A particular advantage of TA is that its effect is not limited to uterine body placental site bleeding, thus, its use does not rely on accurate diagnosis of the site of the bleeding.
TA is a cost‐effective drug. A study on total hip arthroplasty reported saving blood transfusion and money (47 Euro per patient) in cases where TA was used prophylactically prior to surgery (Johansson 2005). Using TA before CS may reduce the blood loss as well.
Use of TA for preventing PPH may contribute to a reduction in blood product use, which is associated with multiple risks (transfusion reactions, transmission of blood‐borne viruses), is expensive and may be not available when it is needed. In low‐resource settings, most of the maternal deaths due to PPH occur in level one hospitals or outside the hospital without any emergency access to formal blood transfusion services. Cost savings could also be gained from avoiding the use of expensive haematological agents such as Factor VIIa, which is establishing its place in the treatment of massive PPH in modern obstetrics in well‐resourced settings despite the extreme cost and limited evidence (Welsh 2008).
The side effects described with the use of TA include gastrointestinal symptoms such as diarrhoea, nausea and vomiting that occur in about 10% of patients. Rare complications include hypotension, thrombosis, blurred vision, renal cortical necrosis and retinal artery obstruction (Astedt 1987). A study by Becassy and co‐authors reported no side effects associated with TA (Bekassy 1990).
Objectives
To determine, from the best available evidence, whether tranexamic acid (TA) is effective and safe for preventing postpartum haemorrhage (PPH) in comparison to placebo or uterotonic agents.
Methods
Criteria for considering studies for this review
Types of studies
We included all published, unpublished and ongoing randomised controlled trials (RCTs) comparing the use of tranexamic acid (TA) alone or in addition to uterotonics in the third stage of labour or prior to or during caesarean section (CS) to prevent postpartum haemorrhage (PPH). We excluded quasi‐RCTs (for example, those randomised by date of birth or hospital number or alternation) from the analysis. We included studies published in abstract if they satisfied other inclusion criteria.
Types of participants
Women undergoing vaginal or CS birth who received TA for prophylaxis of PPH. TA is given immediately after delivery of the baby following vaginal birth or in women undergoing CS, prior to the skin incision.
Types of interventions
Tranexamic acid used for the third stage of labour or at CS to decrease blood loss compared with placebo or other agents such as uterotonics; comparisons of TA dosages or routes of administration.
Comparisons
TA versus placebo/no treatment
TA versus uterotonics
Different dosages of TA
Different routes of administration of TA
Types of outcome measures
Primary outcomes
1. Blood loss 500 mL or more 2. Blood loss 1000 mL or more
Secondary outcomes
3. Mean blood loss volume (mL) 4. Use of additional medical interventions to control PPH 5. Use of additional surgical interventions to control PPH 6. Blood transfusion (not prespecified)* 7. Maternal death or severe maternal morbidity such as seizure, thromboembolic events, need for intensive care unit admission, hysterectomy, organ failure 8. Mild side effects such as nausea, vomiting, headache, skin reactions 9. Thromboembolic events
*Blood transfusion outcome was added in the current update of the review instead of haemoglobin below 6 g%. Blood transfusion was not included in the protocol and previously published version of the review. The reason behind this change is the importance of blood transfusion as an outcome when evaluating an intervention for preventing haemorrhage. Blood transfusions are used in the treatment of severe haemorrhage, they are costly, associated with significant adverse reactions and may not be available in low‐resource settings. Blood transfusion is an outcome assessed in other Cochrane reviews on PPH (Mousa 2014; Tunçalp 2012; Westhoff 2013) and TA (Ker 2013; Perel 2013).
The methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (28 January 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, see 'Novikova 2010'.
For this update, the following methods were used for assessing the 20 reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors (NN and CAC) extracted the data using the agreed form. We resolved any discrepancies through discussion. NN entered the data into Review Manager software (RevMan 2014) and JH checked them for accuracy.
When information regarding any of the above was unclear, we attempted to contact the authors of the original reports to provide further details.
In addition to the main outcomes and details on trial design, we systematically extracted the following data for each study.
Inclusion and exclusion criteria.
Mode of delivery.
Management of the third stage of labour.
Duration and technique of assessment of blood loss.
Missing data after randomisation.
Assessment of risk of bias in included studies
Two review authors independently assessed the validity of each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
We describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We describe for each included study the method used to conceal allocation to interventions prior to assignment and assess whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3) Blinding of participants, personnel and outcome assessors (checking for possible performance bias)
We describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered studies to be at low risk of bias if they were blinded, or if we judge that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel;
low, high or unclear risk of bias for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or was supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We describe for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of quality of evidence
For this update the quality of the evidence was assessed using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating to the following outcomes for the main comparison:
Blood loss 500 mL or more
Blood loss 1000 mL or more
Mean blood loss volume (mL)
Use of additional medical interventions to control PPH
Maternal death or severe maternal morbidity such as seizure, thromboembolic events, need for intensive care unit admission, hysterectomy, organ failure
Mild side effects such as nausea, vomiting, headache, skin reactions
Thromboembolic events
GRADE profiler (GRADEpro 2014) was used to import data from Review Manager 5.3 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CI).
Continuous data
For continuous data, we used the mean difference (MD) as the outcomes were measured in the same way in both trials.
In future updates of this review, we will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
If, in future updates of this review, we identify cluster‐randomised trials for inclusion, we will include them in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Handbook using an estimate of the intra‐cluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a subgroup analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials are irrelevant for this intervention, and, therefore we have not included them.
Multi‐armed trials
When analysing multi‐armed trials, we combined all relevant experimental intervention groups of the study into a single group and all relevant control intervention groups into a single control group. If the authors considered one of the arms irrelevant, we excluded it from analysis.
For dichotomous outcomes, both the sample sizes and the numbers of people with events were added from all groups. For continuous outcomes, means and standard deviations were calculated using a formula available in Table 7.7.a in Chapter 7.7.3.8 in the Handbook (Higgins 2011).
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and to analyse all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We used the Tau², I² and Chi² statistics to measure heterogeneity among the trials in each analysis. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
We planned to assess reporting biases if 10 or more studies were included in the meta‐analysis. In this update (2015), only two meta‐analyses included more than 10 studies. In future updates, if more studies are included, we will investigate reporting biases (such as publication bias) using funnel plots. We will visually assess funnel plot asymmetry.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and we discuss the clinical implications of treatment effects differing between trials. If the average treatment effect was not considered clinically meaningful, we planned not to combine trials. If we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
For analyses with substantial heterogeneity we performed random‐effects analysis and sensitivity analyses.
We assessed the following subgroup for all outcomes:
women undergoing vaginal birth versus CS.
We plan to assess primary outcomes for following subgroup in the future updates of the review if the data become available:
women who received versus women who did not receive routine uterotonics.
We compared all outcomes in subgroup "vaginal birth versus CS" because of potential differences in these two modes of birth. The secondary outcomes (for example, thromboembolic events) are included in the subgroup analysis because of its potential association with the use of TA, which is especially important in women undergoing CS because their risk of thromboembolic events is higher than in women with vaginal birth.
We planned to assess subgroup differences by interaction tests available within RevMan (RevMan 2014) and report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We performed sensitivity analyses for aspects of the review that might have affected the results, for example where there is a risk of bias associated with the quality of some of the included trials; or to explore the effects of fixed‐effect or random‐effects analyses for outcomes with statistical heterogeneity.
We planned to perform sensitivity analysis for the primary outcomes (blood loss greater than 500 mL and greater than 1000 mL).
We performed sensitivity analysis for primary outcomes by excluding the trials that did not use placebo.
Results
Description of studies
Results of the search
The search retrieved 24 new reports of 22 trials. Three trials are still ongoing (Farber 2013; Shirazi 2012; Sentilhes 2014). We have added 10 studies (Abdel‐Aleem 2013; Goswami 2013; Gungorduk 2011; Gungorduk 2013; Mirghafourvand 2013; Movafegh 2011; Senturk 2013; Shahid 2013; Xu 2013, Yehia 2014) to the two previously included trials (Gai 2004; Yang 2001). Two trials are awaiting classification published in abstract form Bhavana 2013, Ahmed 2014 and the information in the abstracts is not sufficient for appraisal. We have excluded two newly identified trials (Halder 2013; Tarabrin 2012) in addition to the previously excluded three studies (Gobbur 2011; Gohel 2007; Sekhavat 2009).
This updated review now comprises 12 included, five excluded, three ongoing and two awaiting classification trials. Data from 12 trials (3285 participants) contributed to this meta‐analysis (Figure 1).
1.
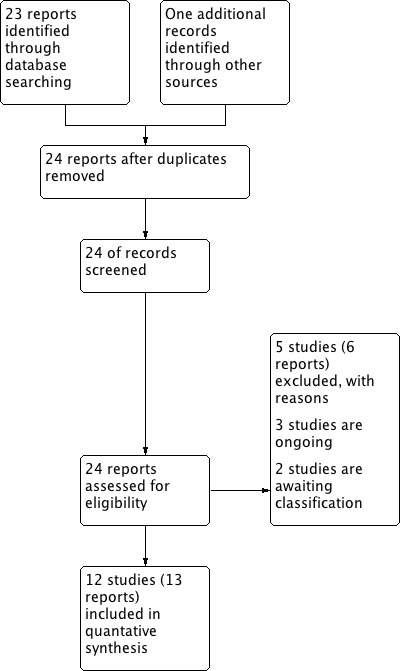
Study flow diagram
For further details see Characteristics of included studies and Characteristics of excluded studies.
Included studies
TA for vaginal birth
We included three trials (Gungorduk 2013; Mirghafourvand 2013; Yang 2001) from Turkey, Iran and China, respectively investigating the efficacy and safety of TA for preventing postpartum haemorrhage (PPH) in eight hundred thirty two women who had vaginal birth.
TA for CS
We included nine trials (2453 participants) investigating the efficacy of TA in reducing blood loss during CS undertaken in Egypt (Abdel‐Aleem 2013, Yehia 2014); China (Gai 2004, Xu 2013); India (Goswami 2013); Turkey (Gungorduk 2011; Senturk 2013); Iran (Movafegh 2011), Pakistan (Shahid 2013).
One trial included women with anaemia defined by the authors as haemoglobin between 7% and 10g% (Goswami 2013). The rest of trials' participants were low‐risk women undergoing elective CS under spinal or epidural anaesthesia who were allocated to receive either TA or placebo.
Timing of administration of intervention
TA was administered at different times in the trials:
some time (unspecified) before commencement of CS (Abdel‐Aleem 2013; Goswami 2013);
at the time of induction of anaesthesia (Yehia 2014);
10 minutes before skin incision (Gai 2004; Gungorduk 2011; Shahid 2013);
20 minutes before commencement of spinal anaesthesia (Movafegh 2011);
10 minutes before anaesthesia (Senturk 2013);
20 minutes after beginning of anaesthesia (Xu 2013).
Dose of TA
Six trials used one g of TA diluted in 20 mL of 5% glucose, given IV over five minutes (Gai 2004; Gungorduk 2011; Gungorduk 2013; Mirghafourvand 2013; Senturk 2013) and two trials the same dose given over 10 minutes (Abdel‐Aleem 2013; Shahid 2013). One trial used 1G of TA by slow intravenous injection (Yehia 2014). Two trials used 10 mg/kg TA IV in 200 mL of normal saline infused over 10 minutes (Movafegh 2011; Xu 2013). One trial used TA 10 mg/kg in 20 mL of 5% dextrose in one group and 15 mg/kg in another group diluted in 20 mL of 5% glucose given over 20 minutes (Goswami 2013). The latter trial included women with average weight of 57 kg, therefore, women received TA in an average dose of 0.6 g in one group and 0.9 g in the other group. One trial compared 0.5 g and 1 g TA given intravenously 2‐3 minutes following delivery of the baby Yang 2001. When two different doses (0.5 g or 10 mg/kg and 1 g or 15 mg/kg) (Goswami 2013; Yang 2001) were compared to each other and to placebo we have combined the treatment groups in the analysis as described above.
Comparisons
TA was compared to placebo (normal saline or dextrose solution) in all Goswami 2013; Gungorduk 2011; Gungorduk 2013; Mirghafourvand 2013; Movafegh 2011; Senturk 2013; Shahid 2013; Xu 2013, except four trials Abdel‐Aleem 2013; Gai 2004; Yang 2001; Yehia 2014 which compared TA to no intervention.
All studies used various regimes of routine prophylactic uterotonic agents administered to all participants:
Abdel‐Aleem 2013 used 5 IU (international units) oxytocin bolus intravenously and 20 IU oxytocin intravenous infusion;
Gai 2004 administered 10 IU oxytocin intravenously and 20 units into the uterine wall simultaneously after delivery of the baby;
Goswami 2013 used 20 IU oxytocin in 500 mL of normal saline intravenously over four hours;
Gungorduk 2011; Gungorduk 2013 used 5 IU oxytocin bolus and 30 IU oxytocin in 500 mL of Ringer's lactate over four hours after delivery of the baby;
Mirghafourvand 2013 used 10 IU oxytocin in 500 mL of normal saline over 20 minutes intravenously after delivery of the placenta;
Movafegh 2011 used 10 IU oxytocin in 500 mL of normal saline over 20 minutes after delivery of the baby, followed by 30 IU oxytocin over eight hours;
Senturk 2013 used 20 IU oxytocin intravenously bolus after removal of the placenta;
Shahid 2013 used 5 IU oxytocin intravenous bolus and 0.4 mg methylergometrine intravenously and 30 IU oxytocin in 500 mL Ringer's lactate over six hours;
Xu 2013 used 10 IU oxytocin in a pint of dextrose normal saline intravenously over 30 minutes;
Yang 2001; Yehia 2014 used 10 IU oxytocin intravenously immediately post the baby delivery.
Outcomes
The majority of included studies contributed data only on a few outcomes (Table 2).
1. Outcomes reported in included studies used in the analysis.
| Study/Outcomes | PPH > 400 mL or 500 mL | PPH > 1000 mL | Mean blood loss | Additional medical interventions | Additional surgical intervention | Blood transfusion | Maternal death and other serious maternal outcomes | Side effects | Thromboembolic events |
| Abdel‐Aleem 2013 | X | X | X | X | X | X | |||
| Gai 2004 | X | X | X | ||||||
| Goswami 2013 | X | X | X | X | X | ||||
| Gungorduk 2011 | X | X | X | X | X | X | |||
| Gungorduk 2013 | X | X | X | X | X | X | X | X | X |
| Mirghafourvand 2013 | X | X | X | X | X | X | X | ||
| Movafegh 2011 | X | ||||||||
| Senturk 2013 | X | X | |||||||
| Shahid 2013 | X | X | |||||||
| Xu 2013 | X | X | X | X | |||||
| Yang 2001 | X | X | X | ||||||
| Yehia 2014 | X | X | X | X | X | X |
PPH: postpartum haemorrhage
All trials except Gungorduk 2011 used a standard method of blood loss calculation using a formula = all used materials’ weight within 2 hours postpartum plus unused materials weight minus materials weight before used)/1.05 plus directly collected blood volume. The Gungorduk 2011 trial used preoperative and postoperative haematocrit levels for estimation of blood loss and we did not include the results in the meta‐analysis.
Mean blood loss volume in vaginal birth was measured by weighing a sheet soaked from the end of the delivery till 2 hours after birth all trials (Gungorduk 2013; Mirghafourvand 2013; Yang 2001).
Mean blood loss following CS was measured in one trial from the beginning of CS until two hours postpartum inclusive of amniotic fluid (Abdel‐Aleem 2013); in another trial from incision until opening of the amniotic sac and then from placental separation until the end of surgery (Senturk 2013); in one trial from the placental delivery till the end of surgery and till six hours postpartum (Yehia 2014), one trial reported intraoperative blood loss inclusive of amniotic fluid and blood loss from the end CS until two hours postpartum (Movafegh 2011); in two trials from placental delivery until the end of surgery and from the end of surgery until two hours postpartum (Goswami 2013; Shahid 2013) and in finally in two trials from placental delivery until two hours postpartum (Gai 2004; Xu 2013).
Excluded studies
We excluded five trials for the following reasons:
two trials were published in abstract form only (Gobbur 2011; Tarabrin 2012) and one study as a preliminary report (Halder 2013) and these publications do not contain sufficient information for appraisal;
two trials were quasi‐randomised with randomisation done by the rule of odds and even (Gohel 2007; Sekhavat 2009).
Risk of bias in included studies
The risk of bias of included trials are presented in Figure 2 and Figure 3. The details of bias assessment are described in characteristics of included studies tables.
2.
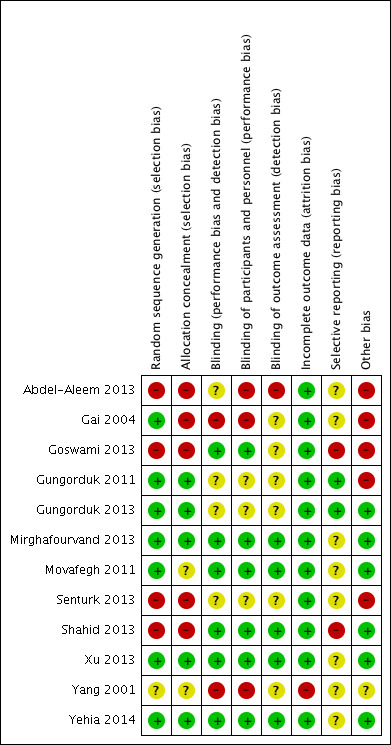
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.
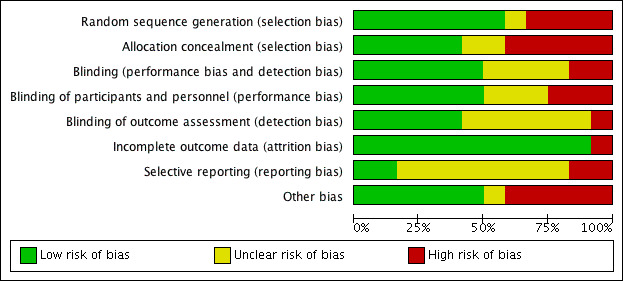
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Seven trials had low risk (Gai 2004; Gungorduk 2011; Gungorduk 2013; Mirghafourvand 2013; Movafegh 2011; Xu 2013; Yehia 2014); four trials had high risk (Abdel‐Aleem 2013; Goswami 2013; Senturk 2013Shahid 2013) and one trial had unclear risk of random sequence generation bias (Yang 2001).
Five trials had low risk of bias allocation concealment (Gungorduk 2011; Gungorduk 2013; Mirghafourvand 2013;Xu 2013; Yehia 2014). Five trials had high risk of allocation bias (Abdel‐Aleem 2013; Gai 2004; Goswami 2013; Senturk 2013; Shahid 2013) and two trials unclear risk (Movafegh 2011; Yang 2001).
Blinding
Blinding was adequate in six trials (Goswami 2013; Mirghafourvand 2013; Movafegh 2011; Shahid 2013; Xu 2013, Yehia 2014). Three trials had high risk (Abdel‐Aleem 2013; Gai 2004; Yang 2001) and three an unclear risk (Gungorduk 2011; Gungorduk 2013; Senturk 2013) of performance and detection bias.
Incomplete outcome data
All included trials had low risk of attrition bias except one (Yang 2001) with high risk.
Selective reporting
Only two trials had low risk of selective reporting bias (Gungorduk 2011; Gungorduk 2013). Two trials had a high risk of selective reporting bias Goswami 2013; Shahid 2013, and eight trials had an unclear risk of selective reporting bias (Abdel‐Aleem 2013; Gai 2004; Mirghafourvand 2013; Movafegh 2011; Senturk 2013; Xu 2013; Yang 2001; Yehia 2014).
Other potential sources of bias
We have identified other potential sources of bias in four trials (Abdel‐Aleem 2013; Gai 2004; Goswami 2013; Gungorduk 2011).
Effects of interventions
See: Table 1
All included trials used prophylactic uterotonic agents (as described above) in addition to TA or placebo or nothing. TA was used intravenously in all trials.
Comparison 1. TA versus placebo or no treatment
Primary outcomes
1. Blood loss greater than 400 mL or 500 mL
Blood loss greater than 400 mL (Gai 2004; Yang 2001) or 500 mL (Gungorduk 2013, Mirghafourvand 2013, Xu 2013, Yehia 2014) was less common in women who received TA versus placebo or no intervention (risk ratio (RR) 0.52, 95% confidence interval (CI) 0.42 to 0.63), six trials, 1398 participants) Analysis 1.1. As blood loss greater than 400 mL was reported in two included studies (Gai 2004; Yang 2001), we have taken it as a proxy for the primary outcome. The data for two different doses on TA were combined in the analysis.
1.1. Analysis.
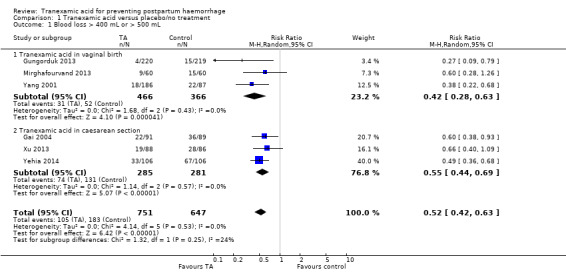
Comparison 1 Tranexamic acid versus placebo/no treatment, Outcome 1 Blood loss > 400 mL or > 500 mL.
The effect of TA on the blood loss greater than 400 mL or 500 mL was more pronounced in the group of women having vaginal birth (RR 0.42, 95% CI 0.28 to 0.63, three trials, 832 women) in comparison to women who had CS (RR 0.55, 95% CI 0.44 to 0.69, three trials, 566 participants) Analysis 1.1. see Table 1
2. Blood loss greater than 1000 mL
Blood loss greater than 1000 mL was less common in women who received TA in comparison to these who had placebo or no intervention (RR 0.40, 95% CI 0.23 to 0.71, six trials, 2093 participants) Analysis 1.2.
1.2. Analysis.
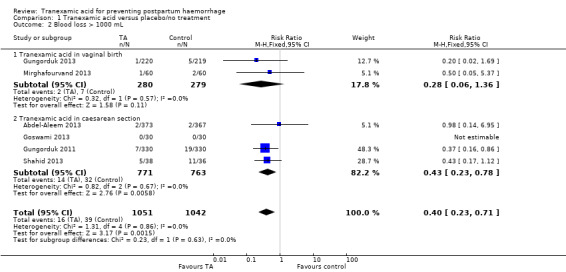
Comparison 1 Tranexamic acid versus placebo/no treatment, Outcome 2 Blood loss > 1000 mL.
TA in vaginal birth and in CS
TA was effective in decreasing the incidence of the blood loss greater than 1000 mL in women who had undergone CS (RR 0.43, 95% CI 0.23 to 0.78, four trials, 1534 participants). In women who had vaginal birth, the two trials with 559 participants had very few outcomes (two versus seven; RR 0.28, 95% CI 0.06 to 1.36) Analysis 1.2.
Secondary outcomes
3. Mean blood loss
Mean blood loss was found to be lower in women who received TA versus placebo or no intervention (mean difference (MD) ‐ 77.79 mL, 95% CI ‐97.95 to ‐57.64, five trials, 1186 women), with positive effect of TA on blood loss following both vaginal birth (MD ‐80.56 mL, 95% CI ‐ 104.80 to ‐56.33, three studies, 832 women) and CS (MD ‐ 71.57 mL, 95% CI ‐ 107.89 to ‐35.26, two studies, 354 women) Analysis 1.3.
1.3. Analysis.
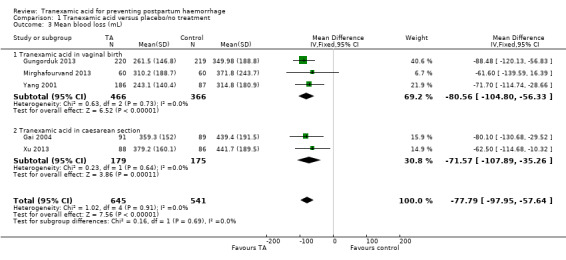
Comparison 1 Tranexamic acid versus placebo/no treatment, Outcome 3 Mean blood loss (mL).
4. Use of additional medical interventions to control PPH
Additional medical interventions such as 200 mcg intravenous methylergometrine, 20 IU oxytocin infusion in 500 mL of Ringer’s lactate and/or 800 mcg misoprostol rectally (Gungorduk 2013; Mirghafourvand 2013), intramuscular methylergometrine (Goswami 2013), additional uterotonic (unspecified) (Abdel‐Aleem 2013; Gungorduk 2013) were used less frequently in a group of women receiving TA versus placebo or no interventions (RR 0.48, 95% CI 0.34 to 0.68), five trials, 2049 participants) Analysis 1.4.
1.4. Analysis.
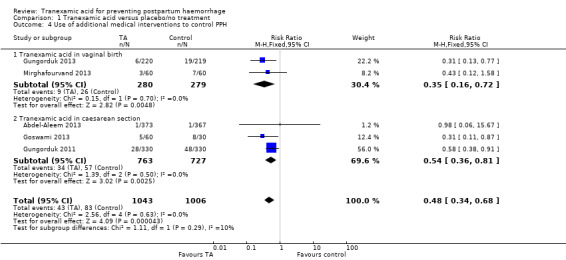
Comparison 1 Tranexamic acid versus placebo/no treatment, Outcome 4 Use of additional medical interventions to control PPH.
5. Use of additional surgical intervention to control PPH
No additional surgical interventions were used in 2051 women in four trials (Abdel‐Aleem 2013; Gungorduk 2011; Gungorduk 2013; Yehia 2014) Analysis 1.5.
1.5. Analysis.
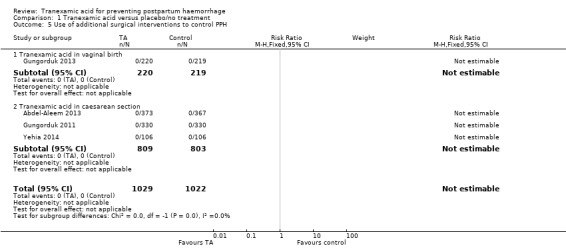
Comparison 1 Tranexamic acid versus placebo/no treatment, Outcome 5 Use of additional surgical interventions to control PPH.
6. Blood transfusion
Less blood transfusions were administered to women who received TA versus placebo or no intervention (RR 0.24, 95% CI 0.11 to 0.53, six trials, 1698 participants) Analysis 1.6, though this difference was only significant in women undergoing CS (RR 0.23, 95% CI 0.10 to 0.54) and not vaginal birth (RR 0.33, 95% CI 0.03 to 3.17).
1.6. Analysis.
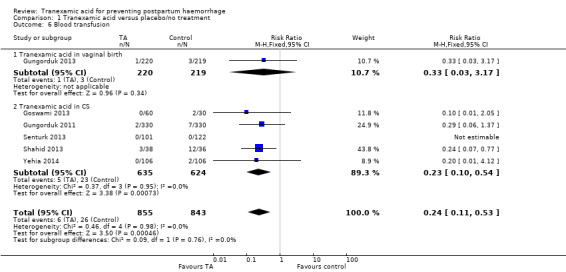
Comparison 1 Tranexamic acid versus placebo/no treatment, Outcome 6 Blood transfusion.
7. Maternal death and severe maternal morbidity
Four trials (1511 women) reported that there were no cases of maternal death or severe maternal morbidity such as seizure, thromboembolic events, need for intensive case unit admission, hysterectomy, organ failure (Goswami 2013; Gungorduk 2011; Mirghafourvand 2013, Yehia 2014) Analysis 1.7.
1.7. Analysis.
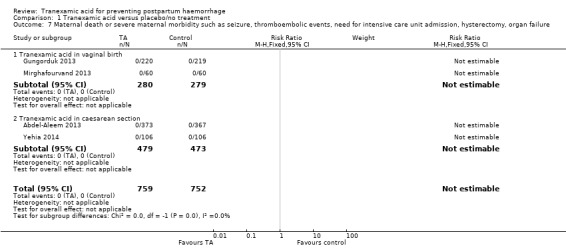
Comparison 1 Tranexamic acid versus placebo/no treatment, Outcome 7 Maternal death or severe maternal morbidity such as seizure, thromboembolic events, need for intensive care unit admission, hysterectomy, organ failure.
8. Side effects
More women who received TA versus placebo or no intervention experienced side effects ((average RR 2.48, 95% CI 1.36 to 4.50, eight trials, 2616 participants) Analysis 1.8. Considerable heterogeneity was detected in this analysis (I² = 83%; Tau² = 0.28). Analysis of side effects in women who received TA versus following vaginal birth showed that more women in the TA group in comparison to placebo or no intervention developed side effects (average RR 1.96, 95% CI 1.50 to 2.55, three trials, 740 women). There was no heterogeneity in this analysis (I² = 0%). On the other hand, a group of women who received TA before CS had a tendency to a higher frequency of side effects, which was significant (average RR 3.78, 95% CI 0.69 to 20.66, five trials, 1876 participants) with a considerable heterogeneity I² = 90%, Tau² = 2.40.
1.8. Analysis.
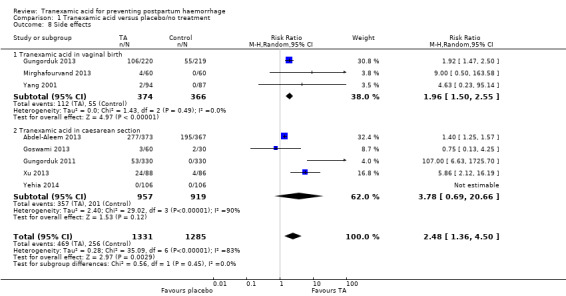
Comparison 1 Tranexamic acid versus placebo/no treatment, Outcome 8 Side effects.
One study reported on the following side effects: nausea, vomiting, headache, skin reaction (Abdel‐Aleem 2013), one study reported only nausea (Goswami 2013), one study reported on nausea, vomiting and diarrhoea (Gungorduk 2011), one study reported nausea, vomiting, diarrhoea, pyrexia, tachycardia, headaches, giddiness, shivering (Gungorduk 2013), one study reported on dizziness and nausea (Mirghafourvand 2013), one study reported nausea/vomiting, phosphenes, dizziness (Xu 2013), and one study reported nausea, vomiting, dizziness (Yang 2001).
9. Thromboembolic events
There was no difference in the number of thromboembolic events in women who received TA versus placebo or no interventions (RR 0.98, 95% CI 0.14 to 6.78, 11 trials, 3012 participants) Analysis 1.9. Two episodes of deep venous thrombosis were reported in the TA and placebo group in one study (Xu 2013).
1.9. Analysis.
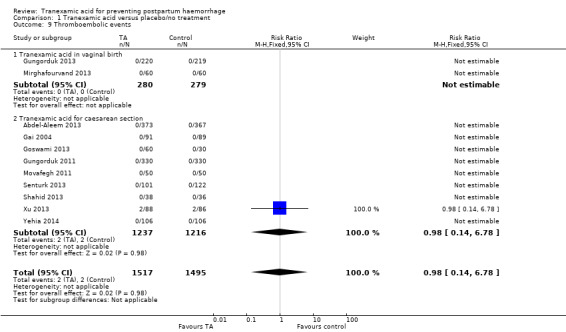
Comparison 1 Tranexamic acid versus placebo/no treatment, Outcome 9 Thromboembolic events.
Comparison 2. TA versus uterotonic
We did not find any studies comparing TA with uterotonic agents.
Comparison 3. Different doses of TA
Primary outcomes
1. Blood loss > 400 mL or > 500 mL
Only one study contributed data on the effect of different doses (0.5 g versus 1 g IV) of TA on blood loss greater than 400 mL (Yang 2001). There were no statistically significant differences in blood loss greater than 400 mL (RR 0.49, 95% CI 0.19 to 1.25, one study, 186 participants) Analysis 2.1.
2.1. Analysis.
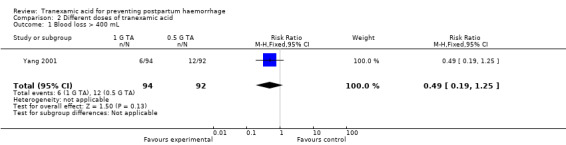
Comparison 2 Different doses of tranexamic acid, Outcome 1 Blood loss > 400 mL.
2. Blood loss > 1000 mL
Two trials (Goswami 2013; Yang 2001) that assessed different doses of TA did not study blood loss greater than 1000 mL.
Secondary outcomes
3. Mean blood loss
Two studies contributed data on the effect of different doses of TA (0.5 g or 10 mg/kg versus 1 g or 15 mg/kg) on mean blood loss and showed no difference between the groups (MD‐58.86 mL, 95% CI ‐54.82 to 172.54), two studies, 246 participants) with considerable heterogeneity (I² = 96%; Tau² = 6445.54) Analysis 2.2. Exploring heterogeneity, we found that one study (Yang 2001) investigating the effects of TA in women who had a vaginal birth had found no difference between the different doses on TA (MD ‐0.40 mL, 95% CI ‐41.06 to 40.26), one study, 186 participants). We cannot explain why the mean blood loss was slightly higher in a group of women who received 1 g of TA versus group that received 0.5 g TA. On the other hand, a study (Goswami 2013) investigating the effects of different doses of TA in women who had CS had found that women who received 15 mg/kg of TA had less blood loss than women who received 0.5 g TA (MD 115.63 mL, 95% CI 92.31 to 138.95, one trial, 60 participants).
2.2. Analysis.
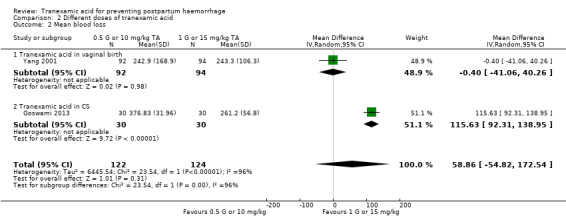
Comparison 2 Different doses of tranexamic acid, Outcome 2 Mean blood loss.
4. Use of additional medical interventions to control PPH
The same study (Goswami 2013) reported no difference in the use of additional medical interventions to control PPH (RR 1.50, 95% CI 0.27 to 8.34) Analysis 2.3)
2.3. Analysis.
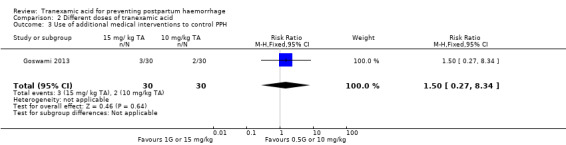
Comparison 2 Different doses of tranexamic acid, Outcome 3 Use of additional medical interventions to control PPH.
5. Use of additional surgical interventions to control PPH
No data available
6. Blood transfusion (not prespecified)
No data available
7. Maternal death or severe maternal morbidity such as seizure, thromboembolic events, need for intensive care unit admission, hysterectomy, organ failure
No data available
8. Mild side effects such as nausea, vomiting, headache, skin reactions
No difference was found in mild side effects in women who received different doses of TA (RR 2.00, 95% CI 0.19 to 20.90), one trial, 60 participants Analysis 2.4).
2.4. Analysis.
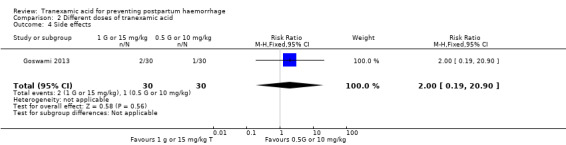
Comparison 2 Different doses of tranexamic acid, Outcome 4 Side effects.
9. Thromboembolic events
No thromboembolic events were reported in the same study (Goswami 2013) Analysis 2.5.
2.5. Analysis.
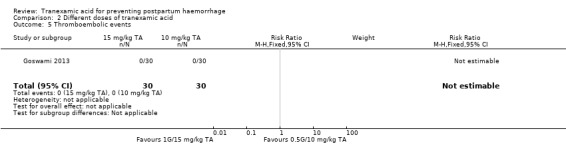
Comparison 2 Different doses of tranexamic acid, Outcome 5 Thromboembolic events.
Comparison 4. Different routes of administration of TA
TA was used intravenously in all trials, therefore, this comparison was not possible to perform.
Subgroup analysis
We planned to perform two subgroup analysis, i.e. women undergoing vaginal birth and CS; women who received and did not receive routine uterotonics. The data on the former subgroup analysis are presented within Comparison 1 above. The latter subgroup analysis was not possible as all participants in the included studies received prophylactic uterotonic agents.
Sensitivity analysis
For the purpose of sensitivity analysis, we excluded the trials that did not use placebo (Abdel‐Aleem 2013; Gai 2004; Yang 2001; Yehia 2014) from the assessment of primary outcomes. Blood loss > 400 mL or 500 mL was still less common in women undergoing vaginal birth who received TA versus placebo (RR 0.43, 95% CI 0.23 to 0.80, two trials, 559 women). Although more women who had undergone CS had blood loss > 500 mL or > 400 mL, this difference was no longer statistically significant when only trials with placebo were analysed (RR 0.66, 95% CI 0.40 to 1.09, one trial, 174 women). Overall, excluding trials without placebo did not change the outcome of blood loss > 400 mL or 500 mL (RR 0.54, 95% CI 0.37 to 0.80, three trials, 733 women).
Similar trend was observed in the outcome of blood loss >1000 mL: there was no difference in the results in women who had vaginal birth as all studies used placebo, more women had PPH >1000 mL in the TA group versus placebo, but this result was no longer statistically significant (RR 0.28, 95% CI 0.06 to 1.36, three trials, 794 women), no difference in overall result for both CS and vaginal birth studies (RR 0.37, 95%CI 0.21 to 0.67, five trials, 1353 women).
Discussion
Summary of main results
Tranexamic acid (TA) is used widely to prevent haemorrhage. Our review assessed 12 trials that fulfilled our inclusion criteria. The summary of the main results is presented in Table 1.
Our analysis showed that TA decreases blood loss greater than 400 mL or greater than 500 mL and this effect is more prominent in the vaginal birth comparison than in the CS comparison based on studies with methodological shortcomings. Blood loss greater than 1000 mL decreased with the use of TA, however, the difference was significant only in the caesarean section comparison and not in the vaginal birth comparison in which there were few outcomes.
The use of additional medical interventions to control PPH was higher in the placebo or no intervention groups versus the TA group with more prominent differences in the vaginal birth group in comparison to the CS group. No additional surgical procedures to control PPH were used in three trials that reported on this outcome. The sample size in this analysis is too small to evaluate this outcome and further trials are necessary.
All women in all studies received routine uterotonics. No comparison between TA and uterotonics was investigated, nor was the effect of TA in the absence of uterotonics, which may potentially be greater.
Maternal death or severe maternal morbidity such as seizure, need for intensive care unit admission, hysterectomy or organ failure did not occur according to the two trials that reported this outcome. A larger number of participants is required to evaluate this important outcome.
Based on pooled data from all included trials except one study (Yang 2001), we found no difference in thromboembolic episodes between the two groups (11 trials, 3012 participants). One trial (Xu 2013) evaluating TA in women undergoing CS reported two episodes of deep venous thrombosis in two participants out of 88 in the TA group and in two participants out of 86 in the control group. This is an unusual finding as the rate of this venous thromboembolism following CS reported in the literature is less than one per 1000 (James 2009). The participants in the Xu 2013 trial were low‐risk women with routine postoperative care. It is not clear why deep venous thrombosis was more common in this study population. None of the participants in the other eight trials experienced this complication.
More women who received TA versus placebo or no intervention experienced side effects. This outcome had considerable heterogeneity (I² = 83%). There was no heterogeneity in this outcome in the subgroup of women who had vaginal birth.
Women who have had a CS are expected to have more mild common side effects such as nausea, vomiting and dizziness, which can be related to surgery and/or TA. Therefore, it is difficult to explain the heterogeneity of these results and the studies did not report on the same mild side effects. The studies that have introduced heterogeneity to this analysis reported on the following side effects ‐ nausea, vomiting, headache and skin reaction in Abdel‐Aleem 2013; only nausea in Goswami 2013, Gungorduk 2011 reported on nausea, vomiting and diarrhoea Xu 2013 reported nausea/vomiting, phosphenes, dizziness.
Only two trials with unclear risk of bias and limited sample size (Goswami 2013; Yang 2001) evaluated different doses of TA and they only reported on a few outcomes. One trial compared 10 mg/kg and 15 mg/kg of TA (Goswami 2013). This trial included women with average weight of 57 kg, therefore, women received TA in the average dose of 0.6 g in one group and 0.9 g in the other group. The other trial (Yang 2001) compared 0.5 g and 1 g TA given following vaginal birth. We pooled data from both trials and combined groups 10 mL/kg with 0.5 g TA and 15 mL/kg with 1 g TA. No difference was found in blood loss greater than 400 mL and mean blood loss following vaginal birth between 0.5 g and 1 g based on this single trial. Although mean blood loss following CS was lower in women who received 15 mg/kg versus 10 mg/kg TA, no difference was seen in the use of additional medical interventions to control PPH in the same study population. The number of side effects did not differ between the groups who received 10 mg/kg versus 15 mg/kg TA based on a small number of participants. No clear conclusions can be drawn from comparison of different doses of TA for preventing PPH. One study (Yang 2001), which was of limited quality, found marginally higher blood loss with the higher dose of TA versus the lower dose.
Overall completeness and applicability of evidence
The studies included in the meta‐analysis were undertaken in low‐ and middle‐resource settings, therefore, the results have wide applicability. The study population included only low‐risk women, but there is no biological reason to expect that TA would not have similar effects in women at high risk of PPH, such as women with placenta praevia, placental abruption, multiple pregnancy, instrumental delivery, prolonged labour as well as in women for whom blood loss needs to be minimised, such as women with anaemia or those who are haemodynamically unstable.
Although we did not find any data on cost‐effectiveness of TA for preventing PPH, it is worth noting that the cost of TA is low and in bleeding trauma patients it was found to provide significant savings in low‐, middle‐ and high‐income countries (Guerriero 2011). The cost of one vile of tranexamic acid (1 g) was reported to be GBP 3.75 Sepah 2011. It can be stored at room temperature (25°C). It is easily administered by intravenous injection.
Quality of the evidence
Included studies were mixed in terms of risk of bias. Five trials had a low risk of selection bias, one trial had an unknown risk of selection bias and six had either an unknown or a high risk of selection bias. The risk of performance bias was low in six trials and unknown or high in six trials. The risk of attrition bias was low in all included trials except one. The risk of selective reporting was generally unclear. Only two trials had registered the trial protocol.
All included trials except one used the standard method of measuring blood loss. Gungorduk 2011 used preoperative and postoperative haematocrit difference to calculate the blood loss and we did not use the data on mean blood loss from this trial.
Five trials reported on blood loss from birth until two hours postpartum (Gai 2004; Gungorduk 2013; Mirghafourvand 2013; Xu 2013; Yang 2001).We included the data on mean blood loss from these trials in our analysis. Other trials included in this review had estimated blood loss at different times points, e.g. one trial included the blood loss from the beginning of CS until two hours postpartum inclusive of amniotic fluid (Abdel‐Aleem 2013); one trial reported blood loss from incision until opening of the amniotic sac and then from placental separation until the end of surgery (Senturk 2013); one trial reported intraoperative blood loss inclusive of amniotic fluid and blood loss from the end of CS until two hours postpartum (Movafegh 2011), two trials reported on blood loss from placental delivery until the end of surgery and from the end of surgery until two hours postpartum (Goswami 2013; Shahid 2013). The data from these trials were not included in this analysis.
High heterogeneity was observed in side effects outcome (I² = 83%) contributed by studies on the use of TA in CS (I² = 90%) and not vaginal birth (I² = 0%). The heterogeneity is likely to be due to different definitions of side effects in various trials. Interestingly, two trials reported no side effects in placebo (Gungorduk 2011) or no intervention (Yehia 2014) groups in women undergoing CS. Such findings are unusual as the rate of nausea and vomiting following CS is reported to be up to 35% (Noroozinia 2013). Nausea and vomiting were included as part of side effects in both trials.
Sensitivity analysis (excluding the trials that did not use placebo) did not add any further information to our results.
The quality of evidence was assessed using the GRADE approach for each of seven outcomes. The evidence was downgraded to moderate quality due to concerns regarding study design for blood loss 500 mL or more; blood loss 1000 mL or more; mean blood loss volume (mL); and use of additional medical interventions to control PPH. The evidence was downgraded to low quality for maternal death or severe maternal morbidity and thromboembolic events due to study design limitations and imprecision (sample size too small to detect this rare outcome). The evidence was downgraded to moderate quality for mild side effects due to inconsistency in the evidence (high heterogeneity I² = 83%).
Potential biases in the review process
We pooled results for PPH with blood loss greater than 400 mL with the prespecified outcome blood loss greater than 500 mL together. Only blood loss greater than 400 mL, which differed to the primary outcome selected for this review (greater then 500 mL) was reported in two trials (Gai 2004; Yang 2001).The reasons behind a choice of an outcome such as blood loss greater than 400 mL are not clear from these papers. The possibility exists that this end‐point may have been chosen retrospectively.
We have updated the outcomes being assessed in this review, e.g. we added blood transfusion to secondary outcomes instead of haemoglobin below 6 g%. The reasons behind this change is the importance of blood transfusion as an outcome when evaluating an intervention for preventing haemorrhage. Blood transfusions are used in the treatment of severe haemorrhage, they are costly, associated with significant adverse reactions and may not be available in low‐resource settings. Blood transfusion is an outcome assessed in other Cochrane reviews on PPH (Mousa 2014) and TA (Ker 2013; Perel 2013).
Mean blood loss following vaginal birth was measured at different time points (from birth till placental delivery, from placental delivery till two hours postpartum and from birth till two hours postpartum). Although initially our prespecified outcome was mean blood loss from placental delivery until two hours postpartum, we changed it to the mean blood loss from birth until two hours postpartum to be able to pool data from all three trials on TA for preventing PPH in vaginal birth.
All the above‐mentioned measures increased the numbers in the meta‐analysis. The heterogeneity remained low.
One study (Yang 2001) used aminomethylbenzoic acid in one group, which was considered a placebo by the trial authors, though there was also a group that did not received any intervention. We included only the latter group.
Agreements and disagreements with other studies or reviews
A recent meta‐analysis of observational trials and randomised controlled trials (RCTs) on usage of TA for obstetric haemorrhage found that TA reduces the amount of blood loss following CS and vaginal birth, and reduces the requirement for blood transfusion (Peitsidis 2011), which is in agreement with our results. Peitsidis and co‐authors published a meta‐analysis, which included studies with considerable heterogeneity (Peitsidis 2011). Abdel‐Aleem and co‐authors presented a meta‐analysis on TA effects on blood loss following CS and also found positive effects of TA with considerable heterogeneity (Abdel‐Aleem 2013). Our results did not have heterogeneity because we included only RCTs that evaluated the prespecified outcomes in a similar manner.
One trial included in the Cochrane review on treatment of primary PPH (Mousa 2014) showed a trend to decrease the incidence of severe PPH (greater than 800 mL) in a group of women who received TA versus placebo (Ducloy‐Bouthors 2011). TA decreases the probability of blood transfusion following emergency surgery (Perel 2013).
The effect of TA on mortality following emergency surgery was reported to be uncertain in a recent Cochrane review that included three trials (260 participants) (Perel 2013). A large high‐quality RCT showed a decrease in all‐causes mortality and death due to bleeding in trauma patients who received a short course of TA versus placebo (CRASH‐2 trial collaborators). Mousa and co‐authors did not show any benefits of TA for treatment of primary PPH in relation to maternal mortality, admission to intensive care unit and hysterectomy following vaginal birth based on the same trial (Ducloy‐Bouthors 2011; Mousa 2014). This trial was underpowered to evaluate these rare outcomes. Findings of our review are consistent with previous reports on the need for further well‐designed studies with a larger number of participants to evaluate the effect of TA on maternal mortality, severe morbidity and additional surgical interventions to control PPH.
Prophylactic oxytocin in comparison to placebo is effective in preventing PPH greater than 500 mL according to a recent Cochrane review (RR 0.53, 95% CI 0.38 to 0.74, 6 trials 4203 women) (Tunçalp 2012) and the relative risk is comparable to the effects of TA on PPH greater than 400 or 500 mL in our meta‐analysis (0.53, 95% CI 0.41, 0.69, five trials, 1188 women). Prophylactic ergometrine‐oxytocin is associated with a small reduction of PPH greater than 500 mL (OR 0.82, 95% CI 0.71 to 0.95) according to another Cochrane review (McDonald 2004). Interestingly, the effect of TA in addition to prophylactic oxytocin in our meta‐analysis is more prominent than the effect of ergometrine.
A recent well‐designed trial on the use of TA following vaginal birth for treatment of PPH (144 participants) reported three cases of deep vein thrombosis at the site of intravenous catheter (two in the tranexamic acid group and one in the control group) (Ducloy‐Bouthors 2011). Although the association of TA with thromboembolic events has been hypothesised, but has not been proven to date (CRASH‐2 trial collaborators; Gandhi 2013; Ker 2012; Ker 2013; Poeran 2014), this review is in agreement with other publications suggesting that larger studies are required to assess this outcome and establish the safety of TA.
Mild side effects are more common in TA versus placebo or no intervention (Ducloy‐Bouthors 2011; Peitsidis 2011).
Authors' conclusions
Implications for practice.
The evidence presented in this review suggests that TA is an effective drug for preventing PPH based on data from trials in low‐risk women. The increase in mild side effects with the use of TA versus placebo or no intervention should be considered.
Implications for research.
Further research is needed to examine the effects of TA on maternal mortality, severe morbidity and thromboembolic events. Studies assessing TA for preventing PPH in high‐risk women with placenta praevia, placental abruptio, uterine rupture and other conditions causing PPH are important. Comparison of different doses of TA as well as prophylactic use of TA without prophylactic uterotonics is necessary, using large, well‐designed trials.
What's new
| Date | Event | Description |
|---|---|---|
| 10 February 2015 | New citation required and conclusions have changed | Ten new trials incorporated. The review now includes a total of 12 trials. The review's conclusions have changed: the previous review concluded that TA decreases postpartum blood loss after vaginal birth and CS based on two RCTs of unclear quality which reported only a few outcomes. Following inclusion of ten studies the new conclusion has changed to "TA (in addition to uterotonic medications) decreases postpartum blood loss and prevents PPH following vaginal birth and CS in low‐risk women. TA is not associated with severe side effects. The evidence suggests that TA should be considered as part of routine management for prevention of PPH". We have added information in the methods section on how the trials with multiple groups are handled. We have updated the secondary outcomes (added blood transfusion, removed haemoglobin < 6 G%). We have added a description of blood loss measurement in methods / outcomes section. A 'Summary of findings' table has been incorporated for this update. |
| 10 February 2015 | New search has been performed | Search updated. Methods updated. We identified 20 reports of 19 trials. We have added ten studies: Abdel‐Aleem 2013; Goswami 2013; Gungorduk 2011; Gungorduk 2013; Mirghafourvand 2013; Movafegh 2011; Senturk 2013; Shahid 2013; Xu 2013; Yehia 2014 to the previously included two trials. Two trials are still ongoing Farber 2013; Shirazi 2012. One trial is awaiting classification Bhavana 2013. Sentilhes 2014 is a protocol of ongoing trial, Ahmed 2014 was published in abstract form only and is lacking information for adequate assessment at this stage. We have excluded four newly identified trials (Gobbur 2011; Gohel 2007; Sekhavat 2009; Tarabrin 2012). |
History
Protocol first published: Issue 3, 2009 Review first published: Issue 7, 2010
| Date | Event | Description |
|---|---|---|
| 15 February 2011 | New search has been performed | Search updated. We identified and excluded three new trials Gobbur 2011; Gohel 2007; Sekhavat 2009 |
Acknowledgements
Cochrane Pregnancy and Childbirth Group team for technical support.
Andrew Weeks and Bukola Fawole for their comprehensive reviewers comments.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team) and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Tranexamic acid versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss > 400 mL or > 500 mL | 6 | 1398 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.42, 0.63] |
| 1.1 Tranexamic acid in vaginal birth | 3 | 832 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.28, 0.63] |
| 1.2 Tranexamic acid in caesarean section | 3 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.44, 0.69] |
| 2 Blood loss > 1000 mL | 6 | 2093 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.23, 0.71] |
| 2.1 Tranexamic acid in vaginal birth | 2 | 559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.06, 1.36] |
| 2.2 Tranexamic acid in caesarean section | 4 | 1534 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.23, 0.78] |
| 3 Mean blood loss (mL) | 5 | 1186 | Mean Difference (IV, Fixed, 95% CI) | ‐77.79 [‐97.95, ‐57.64] |
| 3.1 Tranexamic acid in vaginal birth | 3 | 832 | Mean Difference (IV, Fixed, 95% CI) | ‐80.56 [‐104.80, ‐56.33] |
| 3.2 Tranexamic acid in caesarean section | 2 | 354 | Mean Difference (IV, Fixed, 95% CI) | ‐71.57 [‐107.89, ‐35.26] |
| 4 Use of additional medical interventions to control PPH | 5 | 2049 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.34, 0.68] |
| 4.1 Tranexamic acid in vaginal birth | 2 | 559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.16, 0.72] |
| 4.2 Tranexamic acid in caesarean section | 3 | 1490 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.36, 0.81] |
| 5 Use of additional surgical interventions to control PPH | 4 | 2051 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Tranexamic acid in vaginal birth | 1 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Tranexamic acid in caesarean section | 3 | 1612 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Blood transfusion | 6 | 1698 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.11, 0.53] |
| 6.1 Tranexamic acid in vaginal birth | 1 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.17] |
| 6.2 Tranexamic acid in CS | 5 | 1259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.10, 0.54] |
| 7 Maternal death or severe maternal morbidity such as seizure, thromboembolic events, need for intensive care unit admission, hysterectomy, organ failure | 4 | 1511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 Tranexamic acid in vaginal birth | 2 | 559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Tranexamic acid in caesarean section | 2 | 952 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Side effects | 8 | 2616 | Risk Ratio (M‐H, Random, 95% CI) | 2.48 [1.36, 4.50] |
| 8.1 Tranexamic acid in vaginal birth | 3 | 740 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [1.50, 2.55] |
| 8.2 Tranexamic acid in caesarean section | 5 | 1876 | Risk Ratio (M‐H, Random, 95% CI) | 3.78 [0.69, 20.66] |
| 9 Thromboembolic events | 11 | 3012 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.14, 6.78] |
| 9.1 Tranexamic acid in vaginal birth | 2 | 559 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Tranexamic acid for caesarean section | 9 | 2453 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.14, 6.78] |
Comparison 2. Different doses of tranexamic acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss > 400 mL | 1 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.19, 1.25] |
| 2 Mean blood loss | 2 | 246 | Mean Difference (IV, Random, 95% CI) | 58.86 [‐54.82, 172.54] |
| 2.1 Tranexamic acid in vaginal birth | 1 | 186 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐41.06, 40.26] |
| 2.2 Tranexamic acid in CS | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 115.63 [92.31, 138.95] |
| 3 Use of additional medical interventions to control PPH | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.27, 8.34] |
| 4 Side effects | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 20.90] |
| 5 Thromboembolic events | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdel‐Aleem 2013.
| Methods | Randomised controlled trial undertaken in Egypt. | |
| Participants | 740 pregnant women with singleton fetus at > 37 weeks' gestation undergoing elective CS were considered potentially eligible for the study. Women were considered ineligible if they had any of the following: history of medical disorders, pre‐eclampsia, antepartum haemorrhage, history of thromboembolic disorders, polyhydramnios, macrosomia, history of sensitivity to TA and taking anticoagulant therapy. | |
| Interventions | Intervention group (N = 373) received TA 1 g in 20 mL of 5% glucose, given slowly IV over 10 minutes before the operation commenced. The control group (N = 367) received routine care. All received oxytocin (5 IU IV bolus and 20 IU IV infusion) according to hospital policy or judgement of the surgeon. |
|
| Outcomes | Primary outcome ‐ mean blood loss (mL) (during operation and for 2 hours after operation). Secondary outcomes ‐ incidence of PPH, cases with postpartum blood loss > 500 mL and > 1000 mL, use of additional uterotonics, use of additional surgical intervention to control PPH, the incidence of mild side effects such as (nausea, vomiting, headache, skin reaction), mean change in haematocrit value, mean change in the haemoglobin value, number of hospital admission days, serious adverse events as thromboembolic event, admission to ICU and state of the patient at discharge. |
|
| Notes | More women in intervention group had BMI > 30, were anaemic and had longer time of surgery. More women in control group had manual removal of placenta and more women in intervention group had placenta removed by controlled cord traction. Blood loss measurement ‐ blood loss included blood loss during CS and 2 hours following operation determined using calibrated drapes. Amniotic fluid was also included. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Computer‐generated random numbers, however, groups were unequal ‐ women in intervention group had BMI > 30, were anaemic and had longer time of surgery and more women in control group had manual removal of placenta and more women in intervention group had placenta removed by controlled cord traction. |
| Allocation concealment (selection bias) | High risk | Women were enrolled in the operating room before induction of anaesthesia by opening the opaque sealed envelopes that were consecutively numbered. See above. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The injection was given by the anaesthetist as requested by the researcher without knowing the nature of the trial. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The control group did not receive any intervention, there was no placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The researcher was aware of allocation to the groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all outcomes complete. |
| Selective reporting (reporting bias) | Unclear risk | No protocol was registered prior to the trial. |
| Other bias | High risk | 1. Imbalances despite randomisation: Despite high‐quality randomisation and allocation concealment more women in intervention group had BMI > 30, were anaemic and had longer time of surgery and more women in control group had manual removal of placenta and more women in intervention group had placenta removed by controlled cord traction. |
Gai 2004.
| Methods | Randomised controlled trial undertaken in 3 centres in China. | |
| Participants | 180 primiparas healthy women at term with singleton who underwent CS. | |
| Interventions | Intervention group (N = 91) ‐ 1 g (10 mL) of TA diluted in 20 mL 5% glucose given IV slow infusion over 5 minutes 10 minutes before incision. Control (N = 89) ‐ received routine care. |
|
| Outcomes | Blood loss from placental delivery until 2 hours postpartum, incidence of PPH (blood loss > 400 mL), vital signs, uterine contractility, placental separation, neonatal manifestations, side effects. | |
| Notes | No placebo was used. Women in both control and study groups received 10 units of oxytocin IV and 20 units of oxytocin into the intrauterine wall. The same surgical team performed CS in each hospital. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised consecutive numbered chart. |
| Allocation concealment (selection bias) | High risk | Control group did not receive any intervention, therefore, allocation was not concealed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No intervention was administered to control group, therefore, blinding was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The data seem complete. |
| Selective reporting (reporting bias) | Unclear risk | No prior public registration of protocol. |
| Other bias | High risk | PPH > 400 mL and not conventional > 500 mL. No placebo used. No sample size calculation. |
Goswami 2013.
| Methods | Randomised controlled trial undertaken in India. | |
| Participants | 90 women ASA grade I and II patients, age more than 18 years and anaemic patients with haemoglobin 7‐10 g%, undergoing CS. Exclusion criteria: ASA physical status III and IV; history of coagulopathy or thromboembolism, or both; patients who had received Acenocoumerol or platelet antiaggregant such as aspirin in the week before surgery or non‐steroidal anti inflammatory drugs 2 days before surgery; preoperative plasma creatinine greater than 130 μmol/L; myocardial infarction or chronic arteriopathy or unstable angina in the previous 12 months; mental status preventing them from understanding the study prospectus; renal or hepatic impairment or any hypersensitivity to TA. |
|
| Interventions | Control group (N = 30) – 5 mL of distilled water in 20 mL of 5% glucose. Group T1 (N = 30) – TA 10 mg/kg in 20 mL of 5% glucose. Group T2 (N = 30) – TA 15 mg/kg in 20 mL of 5% glucose. The drug in all the groups was given IV over 20 minutes before skin incision. |
|
| Outcomes | Blood loss was measured intraoperatively and postoperatively up to 24 hours, uterine contractility, placental separation, neonatal condition and any side effects; methylergometrine intramuscular ‐ a rescue uterotonic treatment when required; postoperative haemoglobin, haematocrit, serum creatinine, prothrombin time, and INR values were recorded at 24 hours. | |
| Notes | This trial included only anaemic women with haemoglobin of 7‐10 g%. We used blood loss during surgery for mean blood loss outcome. We combined 2 intervention groups that received different doses of TA. Definition of PPH is not mentioned in the report. Reported that there was no incidence of PPH in any of the patients. Blood loss ‐ was measured during the surgery. Volume in suction bottle before placental delivery was not included. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "All participants were recruited as a consecutive series to one of the three study groups of 30 patients each." |
| Allocation concealment (selection bias) | High risk | Neither the patient nor the investigator was aware of the group assignment. An anaesthetist not related to the study prepared the drug for every patient. It is unclear how the allocated intervention was concealed, as the IV infusion would be visible to all. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Neither the patient nor the investigator was aware of the group assignment. An anaesthetist not related to the study prepared the drug for every patient. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Neither the investigator nor the patient was aware of group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data are complete. |
| Selective reporting (reporting bias) | High risk | No pre‐trial public registration of protocol. Although the authors stated that the blood loss was measured during surgery and every 2 hours for 6 hours postoperatively and then 6 hourly for 24 hours the data were presented only on intraoperative blood loss. The authors stated that postoperative blood loss was minimal. |
| Other bias | High risk | Definition of PPH not mentioned in the report. Women enrolled were anaemic with haemoglobin 7‐10 g%. It is unclear how the sample size was calculated. The groups were not equal ‐ the duration of CS was longest in group 2. We combined groups that were administered different dosages of TA using above‐described methods. |
Gungorduk 2011.
| Methods | Randomised controlled trial undertaken in Turkey. | |
| Participants | Women more than 38 gestational weeks requiring elective CS without risk factors for PPH (anaemia with haemoglobin < 7 g%, multiple gestation, antepartum haemorrhage (placenta praevia or placental abruption), abnormal placentation, uterine fibroids, polyhydramniosis, emergency CS, history of uterine atony or PPH, history of significant disease. | |
| Interventions | Intervention group (N = 330) ‐ 1 g/10 mL TA diluted in 5% 20 mL glucose given IV over 5 minutes 10 minutes prior to skin incision. Control group (N = 330) ‐ 30 mL 5% dextrose given in the same manner as above. Both groups received 5 IU of IV oxytocin as a bolus following delivery of the baby followed by 30 IU of oxytocin in 500 mL lactate Ringer's solution infused at 125 mL/hr. |
|
| Outcomes | Primary outcome ‐ estimated blood loss (calculated based on a formula using preoperative and postoperative haematocrit at 48 hours and women's weight). Secondary outcomes ‐ PPH, use of additional uterotonics, need for blood transfusion, TA side effects, duration of mother's hospital stay, neonatal outcome. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by a pharmacist who prepared infusion bags. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Infusion bags with TA and placebo were prepared by a pharmacist and marked A or B. Neither study staff nor participants were aware of the content of the infusion bags, however, having bags marked differently might introduced bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Neither study staff nor participants were aware of the content of the infusion bags with the study medication or placebo, however, having bags marked differently might introduced bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Low risk | The trial protocol was registered at http://www.controlled‐trials.com ‐ ISRCTN42314355. All prespecified outcomes were reported. |
| Other bias | High risk | Blood loss calculation was done differently to all other trials based on patients weight and difference in haematocrit preoperatively and 48 hours after CS. This method of estimating blood loss differs from clinical practice. |
Gungorduk 2013.
| Methods | Randomised controlled trial undertaken in Turkey. | |
| Participants | Gestational age 34 to 42 weeks, a live fetus, cephalic presentation, expected vaginal birth. Exclusion criteria: multiple gestation, polyhydramnios, estimated fetal weight > 4500 g, grand multiparity (5 or more), pre‐eclampsia, or previous PPH, placenta praevia, placental abruption, CS, or any uterine scarring, abnormal placentation (accreta, increta, percreta), history of thromboembolic disease, and a current or previous history of significant disease, including heart disease and liver and renal disorders. |
|
| Interventions | Intervention group (N = 220) ‐ 1 g/10 mL TA diluted in 5% 20 mL glucose given IV over 5 minutes at the time of delivery of the anterior shoulder of the baby. Control group (N = 219) ‐ 30 mL 5% glucose given in the same manner as above. Routine management including uterotonics of third and 4th stages of labour in both groups. |
|
| Outcomes | Primary outcome ‐ the volume of blood loss from placental delivery until 2 hours postpartum Other outcomes ‐ PPH (> 500 mL), severe PPH (> 1000 mL), need for blood transfusion, need for additional uterotonic drugs (200 mcg IV methylergometrine, 20 IU oxytocin infusion in 500 mL of Ringer’s lactate and/or 800 mcg misoprostol rectally), and side effects of TA injection (such as thrombotic events and gastrointestinal side effects including nausea, vomiting, or diarrhoea). |
|
| Notes | Blood loss measured from the end of delivery until 2 hours postpartum. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by a pharmacist who prepared infusion bags. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Infusion bags with TA and placebo were prepared by a pharmacist and marked A or B. Neither study staff nor participants were aware of the content of the infusion bags, however, having bags marked differently might have introduced bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Neither study staff nor participants were aware of the content of the infusion bags, however, having bags marked differently might have introduced bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After randomisation 7 (3%) participants excluded from control group (5 had CS, 1 chorioamnionitis, 1 delivery in bed) and 8 (3%) participants excluded from intervention group (6 had CS, 2 delivery in bed) though they have received the study intervention. It is not clear why participants delivered in bed were excluded from the study. There might have been difficulties measuring blood loss in such cases. |
| Selective reporting (reporting bias) | Low risk | Trial was registered at clinicaltrial.gov ‐ NCT01338454. |
| Other bias | Low risk | No other bias identified. |
Mirghafourvand 2013.
| Methods | Randomised controlled trial undertaken in Iran. | |
| Participants | 120 pregnant women 18‐35 years with a singleton pregnancy, cephalic presentation and normal blood pressure who had indication for vaginal delivery. Exclusion criteria were grand multiparity, long‐term induction in first stage of labour (oxytocin administration for at least 12 hours), previous history of caesarean delivery or uterine surgery, uterine myoma, history of heart, liver, renal and brain diseases, history of coagulation disorders and thromboembolic disease, blood disorders, history of diabetes and pre‐eclampsia, PPH and haemorrhage during current pregnancy, placenta praevia, abnormal placenta, multiple pregnancies, macrosomia, polyhydramnios, instrumental delivery or continuous pressure in the fundus of the uterus before the fetal expulsion |
|
| Interventions | Intervention group (N = 60) ‐ 1 g TA IV in 200 mL of normal saline over 10 minutes after delivery of the anterior shoulder. Control group (N = 60) ‐ 200 mL normal saline administered in the same manner as above. Both groups got 10 units oxytocin after placenta delivery. |
|
| Outcomes | Haemoglobin and haematocrit levels were measured 1‐12 hours before and 12‐24 hours after delivery. Blood loss was measured from fetus delivery until placental delivery and from placental delivery until 2 hours postpartum, blood loss > 500 mL, use of additional uterotonics (20 IU oxytocin in 500 mL of Ringer's lactate, 200 mcg methylergometrine intramuscular and 800 mcg misoprostol rectally, side effects (nausea and dizziness). | |
| Notes | Publsihed in abstract form only in English. The full paper is currently in press in ANZJOG. The data included in meta‐analysis provided by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation, stratified according to number of births (first and second and more childbirths) with block sizes of 4 and 6 and allocation ratio of 1:1. |
| Allocation concealment (selection bias) | Low risk | Opaque sequentially numbered sealed packages. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Data assessor, participants and analysts had no knowledge of type of intervention was administered. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Data assessor, participants and analysts had no knowledge of type of intervention was administered. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data assessor, participants and analysts had no knowledge of type of intervention was administered. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol was registered. |
| Other bias | Low risk | No other bias identified. |
Movafegh 2011.
| Methods | Randomised controlled trial undertaken in Iran. | |
| Participants | 100 women aged 20–40 years with a singleton pregnancy at between 38 weeks + 5 days and 40 weeks’ gestation, who were categorised as class 1 ASA and were scheduled to undergo CS by Pfannenstiel incision under spinal anaesthesia for malpresentation, contracted pelvis, or patient request. Exclusion criteria were previous history of CS or intra‐abdominal surgery; polyhydramniosis, macrosomia, pre‐eclampsia or abnormal placenta; thrombophilia, anaemia, or coagulopathy; cardiovascular, renal, or liver disorders; or contraindication to any drug used in the study protocol. |
|
| Interventions | Intervention group (N = 50) ‐ 10 mg/kg TA IV in 200 mL of normal saline infused over 10 minutes, 20 minutes before beginning spinal anaesthesia. Control group (N = 50) ‐ 200 mL of normal saline. Following delivery of the placenta both groups received 10 units of oxytocin in 500 mL of normal saline IV over20 minutes. All patients then received 30 units of oxytocin during the first 8 hours postoperatively. A further 10 units of oxytocin was infused in the case of uterine atony. |
|
| Outcomes | Blood loss intraoperative and 2 hours after surgery (reported separately), the amount of oxytocin administered in units, postoperative haemoglobin, platelets, PT, PTT and their decline. | |
| Notes | No side effects in TA group. Blood loss was reported separately during CS and 2 hours after CS. Blood loss included amniotic fluid volume. We have used intraoperative blood loss. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All of the solutions were prepared by an anaesthetist who was not involved in patient management or assessment; both the patients and investigators were blinded to the group assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both the patients and investigators were blinded to the group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigators were blinded to the group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete data. |
| Selective reporting (reporting bias) | Unclear risk | No prior registration of the protocol. |
| Other bias | Low risk | None identified. |
Senturk 2013.
| Methods | Randomised controlled trial undertaken in Turkey. | |
| Participants | Women undergoing elective or urgent CS. Exclusion criteria ‐ high BMI, venous thromboembolism, uterine myoma, active liver or kidney diseases, polyhydramnios and overweight fetus, allergies to TA or other drugs, especially NSAID, or patients receiving antithrombotic treatment. |
|
| Interventions | Intervention group (N = 122) ‐ 1 g TA in 20 mL 5% glucose IV over 5 minutes 10 minutes before anaesthesia. Control group (N = 101) ‐ 20 mL 5 % glucose solution administered in the same manner as in intervention group. All patients received 20 IU oxytocin IV in bolus form after removal of placenta. |
|
| Outcomes | Blood loss (from incision until opening of the amniotic sac and then from placental separation until the end of surgery). Other outcomes ‐ haemoglobin difference, haematocrit difference, red blood cell count difference, pre‐ and postoperative haemoglobin. |
|
| Notes | All patients were operated under spinal anaesthesia by 2 surgeons. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random number table. |
| Allocation concealment (selection bias) | High risk | Not described.Discrepancy in numbers (122 vs 101). |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind in title, but no description in methods. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind in title, but no description in methods. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind in title, but no description in methods. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete data. |
| Selective reporting (reporting bias) | Unclear risk | No prior registration of protocol. |
| Other bias | High risk | No description of blinding. |
Shahid 2013.
| Methods | Randomised controlled trial undertaken in Pakistan. | |
| Participants | Women at term primipara/multiparas (parity not more than 2) with a singleton pregnancy being delivered by CS. Exclusion criteria ‐ medical problems involving the heart, liver, kidney, brain, having blood disorders, allergy to TA, history of thromboembolic disorders, abnormal placentation, severe pre‐eclampsia, multiple pregnancy, macrosomia, polyhydramnios and those requiring blood transfusion due to anaemia. |
|
| Interventions | Intervention group (N = 38) ‐ 1 g/10 mL TA in 20 mL of 5% glucose administered 10 minutes prior to skin incision. Control group (N = 36) ‐ distilled water was diluted with 20 mL of 5% glucose administered in the same manner as above. Both groups ‐ 5 IU IV bolus of oxytocin and 0.4 mg methylergometrine after delivery of the neonate and 30 IU oxytocin in 500 mL of lactated Ringer's solution IV infusion at a rate of 125 mL/hour over 6 hours. |
|
| Outcomes | Heart rate, respiratory rate and blood pressure checked and noted before the surgery, immediately after placental delivery and 1 and 2 hours after birth. The blood loss was measured following placental delivery to the end of the surgery, and from the end of the operation to 2 hours after birth. Blood collected from the suction container (the volume was measured in mL as marked on the container) was noted and soaked mops, pads, and operation table sheet were weighed by electronic scale before and after the surgery. Uterine contractility, placental separation, neonatal manifestations, and side effects were noted. Haemoglobin, urine analysis, liver and renal function were noted before and on the 3rd day after the operation. |
|
| Notes | Blood loss measured from placental delivery until the end of surgery and from the end of surgery until 2 hours postpartum separately. PPH definition was not mentioned, but reported decrease in PPH from 30% in placebo group to 13% in TA group. We were unable to use any data on blood loss outcomes from this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "The investigator has packed 50 pieces of TA (1 G) and 50 pieces of distilled water in identical opaque paper bags. Randomisation was simple, by picking‐up the opaque paper bags containing either 1 G TA, or equivalent numbers of distilled water (placebo) ampoules at random." It is not clear how sequence generation was done. |
| Allocation concealment (selection bias) | High risk | The study medications packed by investigator were in opaque paper bags. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Anaesthetist administered study medication. Neither investigator nor patient were aware of what medication was administered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Neither investigator nor patient were aware of what medication was administered. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Neither investigator nor patient were aware of what medication was administered. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data complete. |
| Selective reporting (reporting bias) | High risk | No prior registration of protocol. Some data are not presented (e.g. side effects) and the report states that there was no difference between the groups. |
| Other bias | Low risk | |
Xu 2013.
| Methods | Randomised controlled trial undertaken in China. | |
| Participants | 176 primipara with a singleton pregnancy undergoing CS by Pfannenstiel incision under spinal anaesthesia. Exclusion criteria: < 18 years, severe medical and surgical complications involving the heart, liver or kidney, brain disease and blood disorders were present, allergy to TA, multiple pregnancies, macrosomia or polyhydramnios, known haemostatic abnormalities before pregnancy. |
|
| Interventions | Intervention group (N = 88) ‐ 10 mg/kg TA in 200 mL of normal saline IV infused over 10 minutes, 20 minutes after beginning of anaesthesia. Control group (N = 86) ‐ 200 mL of normal saline administered in the same manner as above Both groups ‐ 10 units of oxytocin in a pint of dextrose normal saline IV over 30 minutes and 0.4 mg of methylergometrine IV after delivery of the neonate. |
|
| Outcomes | Blood loss ‐ from placental delivery to 2 hours postpartum, PPH > 500 mL. Severe side effects ‐ deep vein thrombosis, renal failure, seizures, maternal death; non‐severe side effects ‐ nausea/vomiting, phosphenes, dizziness. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Anaesthetist not involved in the study prepared the solutions. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Anaesthetist not involved in the study prepared the solutions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Anaesthetist not involved in the study prepared the solutions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 patients out of 176 excluded due protocol violation, therefore, the data presented for 174 patients. |
| Selective reporting (reporting bias) | Unclear risk | No prior publication of protocol. |
| Other bias | Low risk | No other bias. |
Yang 2001.
| Methods | Randomised controlled trial undertaken in China. | |
| Participants | 400 primiparous women with term singleton pregnancy, vertex presentation, spontaneous delivery, normal antenatal care, no antepartum haemorrhage, no above moderate PIH, no polyhydramnios, no abnormal birth process or other complications were enrolled in the 2nd stage of labour. Had 10 units of oxytocin injected immediately post delivery. | |
| Interventions | 4 groups: I) ‐ TA 1 g IV (N = 94), 2) ‐ TA 0.5 g IV (N = 92), 3) ‐ aminomethylbenzoic acid 0.5 g IV (N = 92), 4) ‐ no treatment (N = 87). TA was given IV 2 to 3 minutes after the delivery. |
|
| Outcomes | Incidence of PPH, mean blood loss (from placental delivery until 2 hours postpartum), side effects. | |
| Notes | Aminomethylbenzoic acid is an antifibrinolytic. There was no placebo used in group 4. Not blinded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned to 4 groups. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open labelled. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 35 cases of fetal macrosomia (> 4000 g) were excluded from the study. |
| Selective reporting (reporting bias) | Unclear risk | No prior publication/registration of protocol. |
| Other bias | Unclear risk | We have combined 2 groups which received 2 different doses of TA using the above‐described methods. We did not include a group that received aminomethylbenzoic acid in this analysis. |
Yehia 2014.
| Methods | Randomised controlled double‐blinded trial. | |
| Participants | 223 women undergoing elective CS. Exclusion criteria ‐ medical disorders in pregnancy, bleeding tendency, risk of thromboembolism, known allergy to TA, antepartum haemorrhage, abnormal site of placenta, macrosomic baby, twin pregnancy, polyhydramnios. |
|
| Interventions | Intervention group ‐ TA 1 g with induction of anaesthesia by slow IV injection over 2 minutes in addition to 10 IU of oxytocin injection after delivery of the baby. Control group ‐ 10 IU of oxytocin injection after delivery of the baby. |
|
| Outcomes | Primary ‐ blood loss during CS after delivery of placenta. Secondary outcomes ‐ vital data during first 2 hours postpartum, vaginal bleeding during 6 hours postpartum, 24 hours postoperative haemoglobin and haematocrit values, need for other surgical measures to stop bleeding (B‐Lynch, uterine artery ligation, internal iliac artery ligation, hysterectomy, transfusion of blood or blood products), maternal and neonatal side effects of medications given. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation coding tables were used. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | See below. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Surgeons and investigators were not aware whether patient received TA or not. No placebo was used, therefore, participants were aware of allocation group. However, participants knowledge of the group allocation is unlikely to have influenced the blood loss or other objective outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Randomisation coding tables were concealed from investigators till the end of the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 women in group 1 and five women in group 2 were excluded from this study (either due to uterine contractions before elective CS or no available suction set in the operative room). |
| Selective reporting (reporting bias) | Unclear risk | No prior registration/publication of the study protocol. |
| Other bias | Low risk | None identified. |
BMI: body mass index CS: caesarean section ICU: intensive care unit INR: international normalised ratio IU: international units IV: intravenously NSAID: non‐steroidal anti‐inflammatory drug PIH: pregnancy‐induced hypertension PPH: postpartum haemorrhage PT: prothrombin time PTT: partial thromboplastin time TA: tranexamic acid
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gobbur 2011 | Randomised controlled trial, but sequence generation and allocation concealment were not described. Intervention group (N = 50) ‐ 1 g of TA IV 20 minutes prior to CS. Control group (N = 50) ‐ received no intervention. Outcomes ‐ blood loss from placental delivery until the end of CS and until 2 hours postpartum. This study was published only in abstract form in 2011. The information in the abstract is not sufficient for adequate appraisal. |
| Gohel 2007 | This was a quasi‐randomised trial (randomisation by the rule of odds and even). This study reported decreased blood loss in 50 women who received 1 g of TA IV 20 minutes prior to CS, in comparison to 50 women who did not receive the drug. No side effects were reported in either group. |
| Halder 2013 | Although the title of this report says "randomised case‐controlled study" it is not clear from the report whether the study was indeed randomised. It states that participants were divided in 2 groups, but there is not any description of sequence generation or allocation concealment. The blood loss is measured from placental delivery to 2 days postpartum and no difference between the groups was found. |
| Sekhavat 2009 | This was a quasi‐randomised trial (randomisation by the rule of odds and even). This study reported decreased blood loss from the end of CS until 2 hours postpartum in 45 women who received 1 g of TA IV 10 minutes prior to CS, in comparison to 45 women who did not receive the drug. Haemoglobin level was significantly greater in TA group. No side effects were reported in either group. |
| Tarabrin 2012 | This trial in published only in 2 abstracts. Information provided in abstracts is not sufficient for adequate appraisal. |
CS: caesarean section IV: intravenously PPH: postpartum haemorrhage TA: tranexamic acid
Characteristics of studies awaiting assessment [ordered by study ID]
Ahmed 2014.
| Methods | Prospective randomised trial, patients equally divided into 2 groups. |
| Participants | 124 women undergoing CS. |
| Interventions | Intervention group ‐ 10 mg/kg TA 5 minutes before skin incision. Both groups received 10 unit of oxytocin and 1 mL of ergometrine after delivery of the baby. |
| Outcomes | Blood loss from placental delivery till the end of surgery and from the end of surgery until 2 hours postpartum, haemoglobin and haematocrit levels on day 3 postpartum, maternal and neonatal adverse effects. |
| Notes | Blood loss 391 mL in intervention groups versus 597 mL in control group. Published in abstract form only. Unable to contact the authors to get more information. The abstract does not contain sufficient information on study design (randomisation, allocation concealment) for appraisal. Awaiting full publication of the study at this stage. |
Bhavana 2013.
| Methods | Randomised controlled trial. |
| Participants | 200 term pregnant women. |
| Interventions | Intervention group (N = 100) received IV 1 g TA in 20 mL 5% dextrose and before CS. Control group (N = 100) received 20 mL normal saline. All participants received 10 U oxytocin IM after delivery of neonate. |
| Outcomes | Blood loss occurred during and 6 hours after surgery; requirement of additional oxytocics; blood transfusion; side effects and adverse effects. |
| Notes | The blood loss estimated during surgery was less in the TA group (511.3 +/‐164.45 mL) than the control group (637.9+/‐429.77 mL) P < 0.0065. The blood loss in the postoperative period for 6 hours was less in the TA group (P < 0.0015).The need for additional oxytocics and blood transfusion was less in the TA group. Similarly the drop of haemoglobin and haematocrit after surgery was less in the TA group. Side effects observed were mild nausea, vomiting, diarrhoea. No difference in Apgar score and birthweight of neonates was observed in both of the groups. The abstract does not contain sufficient information on study design (randomisation, allocation concealment) for appraisal. |
CS: caesarean section IM: intramuscularly IV: intravenously TA: tranexamic acid
Characteristics of ongoing studies [ordered by study ID]
Farber 2013.
| Trial name or title | Tranexamic acid and thromboelastography during cesarean delivery. |
| Methods | Randomised, double‐blind, controlled trial with 4 study arms. Assignment parallel. |
| Participants | Control ‐ low risk for PPH (50 patients) and high risk for PPH (50 patients). Intervention ‐ low risk for PPH (50 patients) and high risk for PPH (50 patients). Women ASA class I or II aged 18‐50 years with singleton vertex pregnancy scheduled for elective CS (with or without prior labour) with a planned Pfannensteil incision. Exclusion criteria: allergy to tranexamic acid; history of inherited or acquired thrombophilia; history of deep vein thrombosis or pulmonary embolism, or use of anticoagulant medication; pre‐eclampsia, haemolysis, elevated liver enzymes, low platelet syndrome, seizure disorder. |
| Interventions | Intervention group ‐ 1 g tranexamic acid in 100 mL normal saline given over 10 minutes. Control group ‐ 100 mL 0.9% normal saline. |
| Outcomes | Thromboelastography values will be compared in patients who receive prophylactic tranexamic acid or placebo before surgery, during elective caesarean delivery, and 2 hours postpartum. Intraoperative blood loss ‐ during surgery in the operating room. Blood loss will be measured using the following methods: visual estimate of blood in the suction canister; weight of surgical sponges; postoperative haemoglobin values. At 6 weeks postpartum ‐ delayed bleeding complications, defined as obstetric bleeding requiring surgical intervention, blood transfusion, or both; thrombotic complications including: venous thrombus seen on ultrasound, thromboembolic event. |
| Starting date | JAN 2014. |
| Contact information | Dr Michaela Kristina Faber, Brigham and Women's Hospital, United States, mkfarber@partners.org |
| Notes |
Sentilhes 2014.
| Trial name or title | Tranexamic acid for preventing postpartum haemorrhage following a vaginal delivery (TRAAP). |
| Methods | Randomised controlled double blind trial. |
| Participants | Estimated 4000 women undergoing planned vaginal delivery, older than 18 years, with singleton pregnancy, more than 35 weeks' gestation. |
| Interventions | 1 g TA IV within 2 minutes of birth and prophylactic oxytocin administration versus placebo. |
| Outcomes | Primary ‐ incidence of PPH (> 500 mL) , Secondary ‐ mean blood loss at 15 minutes after birth, mean total blood loss up to 24 hours after birth, incidence of severe PPH (> 1000 mL) 24 hours after birth, need for supplementary uterotonic treatment, proportion of women requiring supplementary uterotonic treatment including sulprostone, postpartum transfusion, proportion of women transfused in postpartum, need for invasive second‐line procedures for PPH (any of the following: arterial embolization, pelvic arterial ligation, uterine compression suture, hysterectomy), haemoglobin peripartum delta (2 days postpartum), mean difference between the haemoglobin values before delivery and on the 2nd day postpartum in the absence of a transfusion of packed red blood cells, haematocrit peripartum delta (2 days postpartum), mean difference between the haematocrit values before delivery and on the 2nd day postpartum in the absence of a transfusion of packed red blood cells, haemodynamic tolerance (time frame: 15, 30, 45, 60 and 120 minutes after delivery ‐ heart rate, blood pressure), mild adverse effects, stay in labour ward, nausea, vomiting, phosphenes, dizziness, tolerance lab tests (urea, creatinaemia, prothrombin time, active prothrombin time, fibrinogenaemia, aspartate and alanine transaminase, total bilirubin). Severe adverse effects (up to 12 weeks after delivery ‐ deep venous thrombosis, pulmonary embolism, myocardial infarction, renal failure needing dialysis), other outcome measures: women's satisfaction, psychological status. |
| Starting date | January 2015. |
| Contact information | Mathilde Moreau Angers Univeristy Hospital, Angers, France 49933 Ph:+33241356329, E‐mail: mathilde.moreau@chu‐angers.fr |
| Notes |
Shirazi 2012.
| Trial name or title | A placebo‐controlled clinical trial to assess efficacy of tranexamic acid in reducing haemorrhage after vaginal delivery. |
| Methods | Randomised, not blinded, assignment ‐ parallel. |
| Participants | Women with term, singleton pregnancy candidate for the first vaginal delivery. Exclusion criteria ‐ severe medical complications involving the heart, brain, kidney; allergy to tranexamic acid; visual problems; severe pregnancy complications such as pre‐eclampsia; thromboembolic events; placenta abruption. |
| Interventions | Intervention group ‐ 1 g tranexamic acid in 20 mL 5% dextrose IV soon after neonatal delivery. Control group ‐ 20 mL 5% dextrose with 10 mL normal saline IV soon after neonatal delivery. |
| Outcomes | Primary outcomes ‐ haemoglobin before and after delivery, need for transfusion, surgery, hysterectomy, death, thrombotic events. |
| Starting date | 10 OCT 2012. |
| Contact information | Dr Fereshteh Heidari Shirazi. Shahid Beheshti Hospital 5th km of Ghotberavandi blvd. Kashan Iran Kashan Isfahan ISLAMIC REPUBLIC OF IRAN 87159/88141 hamiidi@yahoo.com |
| Notes |
CS: caesarean section IV: intravenously PPH: postpartum haemorrhage
Differences between protocol and review
The methods text has been updated to reflect the latest Cochrane Handbook (Higgins 2011) and the current standard methods text used by the Cochrane Pregnancy and Childbirth Group.
We have updated the outcomes being assessed in this review, e.g. we added blood transfusion to secondary outcomes instead of haemoglobin below 6 g%.
Contributions of authors
N Novikova participated in designing the review, and writing the protocol and review. She undertook the initial data analysis. GJ Hofmeyr conceived the review, and provided guidance in designing the review. He also provided a clinical perspective, and performed duplicate data extraction.
For the current update N Novikova assessed the new studies, extracted the data, prepared the review. GJ Hofmeyr reviewed the drafts of the update. CAC performed the duplicate data extraction and reviewed the draft of the update.
Sources of support
Internal sources
Effective Care Research Unit, University of Witwatersrand, University of Fort Hare, South Africa, South Africa.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abdel‐Aleem 2013 {published data only}
- Abdel‐Aleem H, Alhusaini TK, Abdel‐Aleem MA, Menoufy M, Gulmezoglu AM. Effectiveness of tranexamic acid on blood loss in patients undergoing elective cesarean section: randomized clinical trial. Journal of Maternal‐Fetal & Neonatal Medicine 2013;26(17):1705‐9. [DOI] [PubMed] [Google Scholar]
Gai 2004 {published data only}
- Gai MY, Wu LF, Su QF, Tatsumoto K. Clinical observation of blood loss reduced by tranexamic acid during and after caesarian section: a multi‐center, randomized trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 2004;112:154‐7. [DOI] [PubMed] [Google Scholar]
Goswami 2013 {published data only}
- Goswami U, Sarangi S, Gupta S, Babbar S. Comparative evaluation of two doses of tranexamic acid used prophylactically in anemic parturients for lower segment cesarean section: a double‐blind randomized case control prospective trial. Saudi Journal of Anaesthesia 2013;7(4):427‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gungorduk 2011 {published data only}
- Gungorduk K, Yildirim G, Asicioglu O, Gungorduk OC, Sudolmus S, Ark C. Efficacy of intravenous tranexamic acid in reducing blood loss after elective cesarean section: a prospective, randomized, double‐blind, placebo‐controlled study. American Journal of Perinatology 2011;28(3):233‐40. [DOI] [PubMed] [Google Scholar]
Gungorduk 2013 {published data only}
- Gungorduk K, Asicioglu O, Yildirim G, Ark C, Tekirdag AI, Besimoglu B. Can intravenous injection of tranexamic acid be used in routine practice with active management of the third stage of labor in vaginal delivery? A randomized controlled study. American Journal of Perinatology 2013;30(5):407‐13. [DOI] [PubMed] [Google Scholar]
Mirghafourvand 2013 {published data only}
- Mirghafourvand M, Alizadeh SM, Abasalizadeh F, Shirdel M. The effect of intravenous tranexamic acid on hemoglobin and hematocrit levels after vaginal delivery: a randomized controlled trial. Iranian Journal of Obstetrics, Gynecology and Infertility 2013;16(60):1‐8. [Google Scholar]
- Mirghafourvand M, Mohammad‐Alizadeh S, Abasalizadeh F, Shirdel M. The effect of prophylactic intravenous tranexamic acid on blood loss after vaginal delivery in low risk of postpartum haemorrhage women: a double‐blind randomized controlled trial. Australian and New Zealand Journal of Obstetrics and Gynaecology in press. [DOI] [PubMed]
Movafegh 2011 {published data only}
- Movafegh A, Eslamian L, Dorabadi A. Effect of intravenous tranexamic acid administration on blood loss during and after cesarean delivery. International Journal of Gynecology and Obstetrics 2011;115(3):224‐6. [DOI] [PubMed] [Google Scholar]
Senturk 2013 {published data only}
- Senturk MB, Cakmak Y, Yildiz G, Yildiz P. Tranexamic acid for cesarean section: a double‐blind, placebo‐controlled, randomized clinical trial. Archives of Gynecology and Obstetrics 2013;287(4):641‐5. [DOI] [PubMed] [Google Scholar]
Shahid 2013 {published data only}
- Shahid A, Khan A. Tranexamic acid in decreasing blood loss during and after caesarean section. Journal of the College of Physicians and Surgeons‐‐Pakistan: JCPSP 2013;23(7):459‐62. [PubMed] [Google Scholar]
Xu 2013 {published data only}
- Xu J, Gao W, Ju Y. Tranexamic acid for the prevention of postpartum hemorrhage after cesarean section: a double‐blind randomization trial. Archives of Gynecology and Obstetrics 2013;287(3):463‐8. [DOI] [PubMed] [Google Scholar]
Yang 2001 {published and unpublished data}
- Yang H, Zheng S, Shi C. [Clinical study on the efficacy of tranexamic acid in reducing postpartum blood lose: a randomized comparative, multicenter trial] [Chinese]. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics and Gynaecology] 2001;36(10):590‐2. [PubMed] [Google Scholar]
Yehia 2014 {published data only}
- Yehia AH, Koleib MH, Abdelazim IA, Atik A. Tranexamic acid reduces blood loss during and after cesarean section: A double blinded, randomized, controlled trial. Asian Pacific Journal of Reproduction 2014;3(1):53‐6. [Google Scholar]
References to studies excluded from this review
Gobbur 2011 {published data only}
- Gobbur VR, Reddy SV, Bijapur UJ. Efficacy of tranexamic acid in reducing blood loss during lower segment caesarean section. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:92.
Gohel 2007 {published data only}
- Gohel M, Patel P, Gupta A, Desai P. Efficacy of tranexamic acid in decreasing blood loss during and after cesarean section: a randomized case controlled prospective study. Journal of Obstetrics and Gynaecology of India 2007;57(3):228‐30. [Google Scholar]
Halder 2013 {published data only}
- Halder S, Samanta B, Sardar R, Chattopadhyay S. Tranexamic acid used before caesarean section reduces blood loss based on pre‐ and postoperative hemoglobin level: a case‐control study. Journal of the Indian Medical Association 2013;111(3):184‐6. [PubMed] [Google Scholar]
Sekhavat 2009 {published data only}
- Sekhavat L, Tabatabaii A, Dalili M, Farajkhoda T, Tafti AD. Efficacy of tranexamic acid in reducing blood loss after cesarean section. Journal of Maternal‐Fetal & Neonatal Medicine 2009;22(1):72‐5. [DOI] [PubMed] [Google Scholar]
Tarabrin 2012 {published data only}
- Tarabrin O, Kaminskiy V, Galich S, Tkachenko R, Gulyaev A, Shcherbakov S, et al. Efficacy of tranexamic acid in decreasing blood loss during cesarean section. Critical Care 2012;16 Suppl 1:S157. [Google Scholar]
- Zaporozhan V, Tarabrin O, Gavrychenko D, Mazurenko G, Saleh O, Lyoshenko I. Effcacy of tranexamic acid in decreasing blood loss during cesarean section. Critical Care 2013;17 (Suppl 2):S135‐S136. [Google Scholar]
References to studies awaiting assessment
Ahmed 2014 {published data only}
Bhavana 2013 {published data only}
- Bhavana G, Mittal S. Evaluation of efficacy of prophylactic injection tranexamic acid in decreasing blood loss before and after caesarean section. BJOG: an international journal of obstetrics and gynaecology 2013;120(Suppl s1):32. [Google Scholar]
References to ongoing studies
Farber 2013 {published data only}
- Farber MK. Tranexamic acid and thromboelastography during cesarean delivery (TA TEG). ClinicalTrials.gov (http://clinicaltrials.gov) (accessed 21 January 2014) 2013.
Sentilhes 2014 {published data only}
- Sentilhes L. Tranexamic acid for preventing postpartum haemorrhage following a vaginal delivery (TRAAP). ClinicalTrials.gov (http://clinicaltrials.gov) [accessed 10 January 2015] 2014.
Shirazi 2012 {published data only}
- Shirazi FH. A placebo‐controlled clinical trial to assess efficacy of tranexamic acid in reducing hemorrhage after vaginal delivery. IRCT Iranian Registry of Clinical Trials (accessed 3 December 2013) 2013.
Additional references
Astedt 1987
- Astedt B. Clinical pharmacology of tranexamic acid. Scandinavian Journal of Gastroenterology 1987;137:22‐5. [PubMed] [Google Scholar]
Begley 2015
- Begley CM, Gyte GML, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD007412.pub4] [DOI] [PubMed] [Google Scholar]
Bekassy 1990
- Bekassy Z, Astedt B. Treatment with the fibrinolytic inhibitor tranexamic acid‐risk for thrombosis?. Acta Obstetricia et Gynecologica Scandinavica 1990;69(4):353‐4. [DOI] [PubMed] [Google Scholar]
Boylan 1996
- Boylan JF, Klinck JR, Sandler AN, Arellano R, Greig PD, Nierenberg H, et al. Tranexamic acid reduces blood loss, transfusion requirements, and coagulation factor use in primary orthotopic liver transplantation. Anesthesiology 1996;85(5):1043‐8. [DOI] [PubMed] [Google Scholar]
Confidential enquiries 2012
- Pattinson RC. Saving mothers. Fifth Report on Confidential Enquiries into Maternal Deaths in South Africa 2008‐2010. Pretoria, South Africa: Department of Health, 2012. [Google Scholar]
CRASH‐2 trial collaborators
- CRASH‐2 trial collaborators1, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH‐2): a randomised, placebo‐controlled trial. Lancet 2010;376(9734):23‐32. [DOI: 10.1016/S0140-6736(10)60835-5] [DOI] [PubMed] [Google Scholar]
Deneux‐Tharaux 2013
- Deneux‐Tharaux C, Sentilhes L, Maillard F, Closset E, Vardon D, Lepercq J, et al. Effect of routine controlled cord traction as part of the active management of the third stage of labour on postpartum haemorrhage: multicentre randomised controlled trial (TRACOR). BMJ 2013;346:1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ducloy‐Bouthors 2011
- Ducloy‐Bouthors AS, Jude B, Duhamel A, Broisin F, Huissoud C, Keita‐Meyer, et al. High‐dose tranexamic acid reduces blood loss in postpartum haemorrhage. Critical Care 2011;15:R177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gandhi 2013
- Gandhi R, Evans HMK, Mahomed SR, Mahomed NN. Tranexamic acid and reduction of blood loss in total knee and hip arthroplasty: a meta‐analysis. BMC Research Notes 2013;6:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillette 2013
- Gillette BP, DeSimone LJ, Trousdale RT, Pagnano MW, Sierra RJ. Low risk of thromboembolic complications with tranexamic acid after primary total hip and knee arthroplasty. Clinical Orthopaedics and Related Research 2013;471(1):150‐4. [DOI: 10.1007/s11999-012-2488-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.6. The Cochrane Collaboration, 2008.
Guerriero 2011
- Guerriero C, Cairns J, Perel P, Shakur H, Roberts I. Cost‐effectiveness analysis of administering tranexamic acid to bleeding trauma patients using evidence from the CRASH‐2 trial. PLoS ONE 2011;6(5):e18987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heit 2008
- Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30‐year population‐based study. Annals of Internal Medicine 2005;143(10):697‐706. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
James 2009
- James AH. Venous thromboembolism: mechanisms, treatment, and public awareness. Arteriosclerosis, Thrombosis, and Vascular Biology 2009;29:326‐31. [DOI] [PubMed] [Google Scholar]
Johansson 2005
- Johansson T, Pettersson L, Lisander B. Tranexamic acid in total hip arthroplasty saves blood and money. Acta Orthopaedica 2005;76(3):314‐9. [PubMed] [Google Scholar]
Jurema 2008
- Jurema M, Zacur HA. Patient information: Heavy or prolonged periods (menorrhagia). http://www.uptodate.com/patients/content/topic.do?topicKey=wom_issu/4769&title=Menstrual+cycle (accessed 2008).
Karski 1995
- Karski JM, Teasdale SJ, Norman P, Carroll J, VanKessel K, Wong P, et al. Prevention of bleeding after cardiopulmonary bypass with high‐dose tranexamic acid. Double‐blind, randomized clinical trial. Journal of Thoracic and Cardiovascular Surgery 1995;110(3):835‐42. [DOI] [PubMed] [Google Scholar]
Katsaros 1996
- Katsaros D, Petricevic M, Snow NJ, Woodhall DD, Bergen R. Tranexamic acid reduces postbypass blood use: a double‐blinded, prospective, randomized study of 210 patients. Annals of Thoracic Surgery 1996;61(4):1131‐5. [DOI] [PubMed] [Google Scholar]
Ker 2012
- Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta‐analysis. BMJ 2012;344:e3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ker 2013
- Ker K, Beecher D, Roberts I. Topical application of tranexamic acid for the reduction of bleeding. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD010562.pub2] [DOI] [PubMed] [Google Scholar]
Lethaby 2000
- Lethaby A, Farquhar C, Cooke I. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD000249] [DOI] [PubMed] [Google Scholar]
Longstaff 1994
- Longstaff C. Studies on the mechanisms of action of aprotinin and tranexamic acid as plasmin inhibitors and antifibrinolytic agents. Blood Coagulation & Fibrinolysis 1994;5(4):537‐42. [PubMed] [Google Scholar]
McDonald 2004
- McDonald SJ, Abbott JM, Higgins SP. Prophylactic ergometrine‐oxytocin versus oxytocin for the third stage of labour. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD000201.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2013
- McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD004074.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mousa 2014
- Mousa HA, Blum J, Abou El Senoun G, Shakur H, Alfirevic Z. Treatment for primary postpartum haemorrhage. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD003249.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noroozinia 2013
- Noroozinia H, Mahoori A, Hasani E, Gerami‐Fahim M, Sepehrvand N. The effect of acupressure on nausea and vomiting afer cesarean section under spinal anesthesia. Acta Medica Iranica 2013;51(3):163‐7. [PubMed] [Google Scholar]
Peitsidis 2011
- Peitsidis P, Kadir RA. Antifibrinolytic therapy with tranexamic acid in pregnancy and postpartum. Expert Opinion on Pharmacotherapy 2011;12(4):503‐16. [DOI] [PubMed] [Google Scholar]
Perel 2013
- Perel P, Ker K, Morales Uribe CH, Roberts I. Tranexamic acid for reducing mortality in emergency and urgent surgery. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD010245.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poeran 2014
- Poeran J, Rasul R, Suzuki S, Danninger T, Opperer, Boettner F, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ 2014;349:4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pomp 2008
- Pomp ER, Lenselink AM, Rosendaal FR, Doggen CJ. Pregnancy, the postpartum period and prothrombotic defects: risk of venous thrombosis in the MEGA study. Journal of Thrombosis and Haemostasis 2008;6(4):632‐7. [DOI] [PubMed] [Google Scholar]
Reid 1997
- Reid RW, Zimmerman AA, Laussen PC, Mayer JE, Gorlin JB, Burrows FA. The efficacy of tranexamic acid versus placebo in decreasing blood loss in pediatric patients undergoing repeat cardiac surgery. Anesthesia & Analgesia 1997;84(5):990‐6. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ronsmans 2006
- Ronsmans C, Graham WJ. Maternal mortality: who, when, where, and why. Lancet 2006;368(9542):1189‐200. [DOI] [PubMed] [Google Scholar]
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [PUBMED: 19839216] [DOI] [PubMed] [Google Scholar]
Sepah 2011
- Sepah YJ, Umer M, Ahmad T, Nasim F, Chaudhry UM, Umar M. Use of tranexamic acid is a cost effective method in preventing blood loss during and after total knee replacement. Journal of Orthopaedic Surgery 2011;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheldon 2014
- Sheldon WR, Blum J, Vogel JP, Souza JP, Gulmezoglu AM, Winikoff B, et al. Postpartum haemorrhage management, risks, and maternal outcomes: findings from the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG: an international journal of obstetrics and gynaecology 2014;121(Suppl 1):5‐13. [DOI] [PubMed] [Google Scholar]
Tunçalp 2012
- Tunçalp Ö, Hofmeyr GJ, Gülmezoglu AM. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD000494.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vacharaksa 2002
- Vacharaksa K, Prakanrattana U, Suksompong S, Chumpathong S. Tranexamic acid as a means of reducing the need for blood and blood component therapy in children undergoing open heart surgery for congenital cyanotic heart disease. Journal of the Medical Association of Thailand 2002;85(Suppl 3):904‐9. [PubMed] [Google Scholar]
Welsh 2008
- Welsh A, McLintock C, Gatt S, Somerset D, Popham P, Ogle R. Guidelines for the use of recombinant activated factor VII in massive obstetric haemorrhage. Australian and New Zealand Journal of Obstetrics and Gynaecology 2008;48(1):12‐6. [DOI] [PubMed] [Google Scholar]
Westhoff 2013
- Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD001808.pub2] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Novikova 2010
- Novikova N, Hofmeyr GJ. Tranexamic acid for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD007872.pub2] [DOI] [PubMed] [Google Scholar]


