Abstract
Background
Postpolio syndrome (PPS) may affect survivors of paralytic poliomyelitis and is characterised by a complex of neuromuscular symptoms leading to a decline in physical functioning. The effectiveness of pharmacological treatment and rehabilitation management in PPS is not yet established. This is an update of a review first published in 2011.
Objectives
To systematically review the evidence from randomised and quasi‐randomised controlled trials for the effect of any pharmacological or non‐pharmacological treatment for PPS compared to placebo, usual care or no treatment.
Search methods
We searched the following databases on 21 July 2014: Cochrane Neuromuscular Disease Group Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, PsycINFO and CINAHL Plus. We also checked reference lists of all relevant articles, searched the Database of Abstracts of Reviews of Effects (DARE), the Health Technology Assessment (HTA) Database and trial registers and contacted investigators known to be involved in research in this area.
Selection criteria
Randomised and quasi‐randomised trials of any form of pharmacological or non‐pharmacological treatment for people with PPS. The primary outcome was self perceived activity limitations and secondary outcomes were muscle strength, muscle endurance, fatigue, pain and adverse events.
Data collection and analysis
We used standard methodological procedures expected by The Cochrane Collaboration.
Main results
We included 10 pharmacological (modafinil, intravenous immunoglobulin (IVIg), pyridostigmine, lamotrigine, amantadine, prednisone) and three non‐pharmacological (muscle strengthening, rehabilitation in a warm climate (that is temperature ± 25°C, dry and sunny) and a cold climate (that is temperature ± 0°C, rainy or snowy), static magnetic fields) studies with a total of 675 participants with PPS in this review. None of the included studies were completely free from any risk of bias, the most prevalent risk of bias being lack of blinding.
There was moderate‐ and low‐quality evidence that IVIg has no beneficial effect on activity limitations in the short term and long term, respectively, and inconsistency in the evidence for effectiveness on muscle strength. IVIg caused minor adverse events in a substantial proportion of the participants. Results of one trial provided very low‐quality evidence that lamotrigine might be effective in reducing pain and fatigue, resulting in fewer activity limitations without generating adverse events. Data from two single trials suggested that muscle strengthening of thumb muscles (very low‐quality evidence) and static magnetic fields (moderate‐quality evidence) are safe and beneficial for improving muscle strength and pain, respectively, with unknown effects on activity limitations. Finally, there was evidence varying from very low quality to high quality that modafinil, pyridostigmine, amantadine, prednisone and rehabilitation in a warm or cold climate are not beneficial in PPS.
Authors' conclusions
Due to insufficient good‐quality data and lack of randomised studies, it was impossible to draw definite conclusions about the effectiveness of interventions for PPS. Results indicated that IVIg, lamotrigine, muscle strengthening exercises and static magnetic fields may be beneficial but need further investigation to clarify whether any real and meaningful effect exists.
Keywords: Humans; Cold Temperature; Exercise Therapy; Exercise Therapy/methods; Hot Temperature; Immunoglobulins, Intravenous; Immunoglobulins, Intravenous/therapeutic use; Lamotrigine; Muscle Fatigue; Muscle Strength; Postpoliomyelitis Syndrome; Postpoliomyelitis Syndrome/therapy; Randomized Controlled Trials as Topic; Triazines; Triazines/therapeutic use
Plain language summary
Treatment for postpolio syndrome
Review question
What are the effects of different treatments in people with postpolio syndrome (PPS)?
Background
PPS is a condition that can affect polio survivors years after recovery from an initial paralytic attack by the polio virus. PPS is characterised by progressive or new muscle weakness or decreased muscle endurance in muscles that were previously affected by the polio infection and in muscles that were seemingly unaffected. Other symptoms may include generalised fatigue and pain. These symptoms often lead to a decline in physical functioning, for example trouble walking. The objective of this review was to assess the benefits and harms of different drugs and rehabilitation treatments compared to placebo (a pill or procedure without any physiological effect), usual care or no treatment.
Study characteristics
We searched scientific databases to find all studies on treatments for PPS up to July 2014. We found 13 studies involving a total of 675 participants that were of sufficient quality to include in this review. Ten studies evaluated the effects of drugs (modafinil, intravenous immunoglobulin (IVIg), pyridostigmine, lamotrigine, amantadine, prednisone), and three studies evaluated other treatments (muscle strengthening, rehabilitation in a warm climate (that is temperature ± 25°C, dry and sunny) and a cold climate (that is temperature ± 0°C, rainy or snowy), static magnetic fields).
Key results and quality of the evidence
IVIg is a treatment in which antibodies that have been purified from donated blood are given as an infusion into a vein over a period of time. There was moderate‐ and low‐quality evidence that IVIg has no beneficial effect on activity limitations in the short term and long term, respectively. Evidence for effectiveness on muscle strength was inconsistent, as results differed across studies. IVIg caused minor side effects in a substantial proportion of the participants.
Lamotrigine is a drug used to help control certain kinds of epilepsy and to treat bipolar psychiatric disorder. Results of one trial provided very low‐quality evidence that lamotrigine might be effective in reducing pain and fatigue, resulting in fewer activity limitations, and in this study it was well‐tolerated. We based these conclusions on results of only one small trial with important limitations in study design.
There was very low‐quality evidence that muscle strengthening of thumb muscles is safe and beneficial for improving muscle strength. Again, we based these conclusions on results of only one small trial with important limitations in study design, and they are applicable only to thumb muscles.
Static magnetic fields is a therapy in which electrical currents are applied to the skin with the intention of reducing pain. There was moderate‐quality evidence that static magnetic fields are safe and beneficial for reducing pain directly after treatment, although functional effects on activity limitations and long‐term effects are unknown.
Finally, there was evidence varying from very low quality to high quality that modafinil, pyridostigmine, amantadine, prednisone and rehabilitation in a warm or cold climate are not beneficial in PPS.
Summary of findings
Summary of findings for the main comparison. IVIg versus placebo for postpolio syndrome.
| IVIg versus placebo for postpolio syndrome | ||||||
| Patient or population: people with postpolio syndrome Intervention: IVIg versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | IVIg | |||||
| (Change in) Activity limitations ≤ 3 months Measured with the SF‐36 PCS1 (scale from 0 to 100) | The mean activity limitations in one control group was 33.32 The mean change in activity limitations in one control group was ‐0.82 |
The mean (change in) activity limitations in the intervention groups was 2.35 higher (0.06 lower to 4.76 higher) | ‐ | 185 (2 studies) | ⊕⊕⊕⊝ moderate3 | ‐ |
| Activity limitations > 3 months Measured with the SF‐36 PCS1 (scale from 0 to 100) | The mean activity limitations in the control groups was 33.92 | Activity limitations in the intervention groups was 0.51 lower (4.63 lower to 3.60 higher) | ‐ | 91 (2 studies) |
⊕⊕⊝⊝ low4 | ‐ |
| Adverse events | See comment | See comment | Not estimable | 212 (3 studies) | See comment | See Table 11 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1SF‐36 PCS: Short Form‐36 Health Survey Physical Component Summary. Higher scores represent fewer activity limitations. 2The control group received placebo. 3Risk of bias: likely that blinding was broken in one trial due to side effects of the treatment. However, because the result was negative, it is unclear if unblinding actually did influence this result (‐1).
4Risk of bias: likely that blinding was broken in one trial due to side effects of the treatment. However, because the result was negative, it is unclear if unblinding actually did influence this result. The baseline imbalance in activity limitations in one trial reduces the quality of evidence (‐2).
1. Adverse events for pharmacological interventions.
| Study | Intervention | Serious adverse events | Minor adverse events |
| Farbu 2007 | IVIg 2 g/kg body weight, 1 infusion | Medication: flu‐like illness and chest myalgia (10%) Placebo: none reported |
Medication: chills or fever, or both (70%) Placebo: chills or fever, or both (10%) |
| Gonzalez 2006 | IVIg 90 g, 1 infusion repeated after 3 months | Medication: 1 participant (1%) developed a serious adverse event (not further specified) Placebo: 2 participants (3%) developed serious adverse events (not further specified) |
Medication: gastrointestinal disorders (22%), general disorders and administration site conditions (19%), nervous system disorders (59%), skin and subcutaneous tissue disorders (37%) Placebo: gastrointestinal disorders (3%), general disorders and administration site conditions (9%), nervous system disorders (19%), skin and subcutaneous tissue disorders (7%) |
| Bertolasi 2013 | IVIg 2 g/kg body weight, 1 infusion | None reported | Medication: transient rash (4%) Placebo: none |
| Chan 2006 | Modafinil max 2 x 200 mg/day | None reported | Medication: anxiety and dry mouth (60%) Placebo: none reported |
| Vasconcelos 2007 | Modafinil 2 x 200 mg/day | Medication: 3 participants (8%) (1. newly diagnosed endometrial cancer, 2. acute psychosis, 3. nervousness) Placebo: none reported |
Medication: insomnia (11%), nervousness (11%), dry mouth (8%), palpitation (5%), flushing (3%), abdominal discomfort (8%), urine change (11%), appetite loss (5%), upper respiratory problems (14%) Placebo: cold virus (6%), heartburn (6%), insomnia (3%), sinusitis (6%), diarrhoea (3%), dry eyes (6%), joint or back pain (6%), headache (3%) |
| Trojan 1999 | Pyridostigmine 3 x 60 mg/day | Medication: 5 participants (8%) (1. palpitations and dizziness due to benign supraventricular arrhythmia, persisted after discontinuation of treatment, 2. sepsis secondary to severe diverticulitis, 3. infiltrating ductal carcinoma of breast, 4. painful muscle and gastrointestinal cramp, 5. nausea, diarrhoea, vomiting and faintness) Placebo: 1 participant (2%) angina, shortness of breath |
Medication: 7 participants (11%) muscle cramps, abdominal pain, nausea, diarrhoea, profuse sweating, chest pain, fractured fibula, fractured rib, herpes zoster Placebo: 2 participants (3%) feeling drugged, blurred vision, nausea, diarrhoea |
| Horemans 2003 | Pyridostigmine 4 x 60 mg/day | Medication: 1 participant (3%) severe diarrhoea Placebo: none reported |
None reported |
| On 2005 | Lamotrigine 50 to 100 mg/day | None reported | None reported |
| Stein 1995 | Amantadine 2 x 100 mg/day | None reported | Medication: insomnia (73%), dry mouth (9%) Placebo: none reported |
| Dinsmore 1995 | Prednisone 80 mg/day followed by a 20‐week dose reduction schedule | Medication: 2 participants (22%) (1. severe depression, 2. transient ischaemic attack, hypertension and dyspnoea on exertion) Placebo: 1 participant (13%) increasing weakness, acne, fungal infection and insomnia |
Medication: 5 participants (56%) cataract, tinnitus, weakness, depression, acne, low back pain, irritability, hoarseness, blurred vision, urinary frequency, anxiety, fungal infection, sensitive gingiva and breasts. Placebo: 4 participants (50%) insomnia, irritability, nausea |
IVIg: intravenous immunoglobulin
Summary of findings 2. Modafinil versus placebo for postpolio syndrome.
| Modafinil versus placebo for postpolio syndrome | ||||||
| Patient or population: people with postpolio syndrome Intervention: modafinil versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Modafinil | |||||
| Activity limitations Measured with the SF‐36 PF1 (scale from 0 to 100) Follow‐up: 6 weeks | The mean activity limitations in the control group was 37.282 | The mean activity limitations in the intervention group was 1.28 higher (3.56 lower to 6.12 higher) | ‐ | 33 (1 study)3 | ⊕⊕⊕⊕ high | ‐ |
| Adverse events | See comment | See comment | Not estimable | 50 (2 studies) | See comment | See Table 11 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1SF‐36 PF: Short Form‐36 Health Survey Physical Functioning scale. Higher scores represent fewer activity limitations.
2The control group received placebo.
3In cross‐over study in which 36 participants were randomised, 33 completed required interventions. Although results were based on only one study that included relatively few participants, the confidence interval is narrow and is therefore judged as no imprecision.
Summary of findings 3. Pyridostigmine versus placebo for postpolio syndrome.
| Pyridostigmine versus placebo for postpolio syndrome | ||||||
| Patient or population: people with postpolio syndrome Intervention: pyridostigmine versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Pyridostigmine | |||||
| Change in activity limitations Measured with the SF‐36 PF1 (scale from 0 to 100) Follow‐up: 6 months | The mean change in activity limitations in the control group was 1.12 | The mean change in activity limitations in the intervention group was 2.1 higher (3.64 lower to 7.84 higher) | ‐ | 124 (1 study) | ⊕⊕⊕⊝ moderate3 | ‐ |
| Adverse events | See comment | See comment | Not estimable | 193 (2 studies) | See comment | See Table 11 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1SF‐36 PCS: Short Form‐36 Health Survey Physical Functioning scale. Higher scores represent fewer activity limitations. 2The control group received placebo. 3Risk of bias: Analysis on effectiveness of blinding provided evidence for unblinding. However, because the result was negative, it is unclear if unblinding actually did influence this result (‐1).
Summary of findings 4. Lamotrigine versus control for postpolio syndrome.
| Lamotrigine versus control for postpolio syndrome | ||||||
| Patient or population: people with postpolio syndrome Intervention: lamotrigine versus control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Lamotrigine | |||||
| Activity limitations Measured with the NHP‐PM1 (scale from 0 to 100) Follow‐up: 4 weeks | The mean activity limitations in the control group was 38.42 | The mean activity limitations in the intervention group was 23.7 lower (35.35 to 12.05 lower) | ‐ | 30 (1 study) | ⊕⊝⊝⊝ very low3,4 | ‐ |
| Adverse events | See comment | See comment | Not estimable | 30 (1 study) | See comment | See Table 11 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1NHP‐PM: Nottingham Health Profile‐Physical Mobility. Higher scores represent more activity limitations. 2The control group received usual care (advice on pacing, energy conservation, use of orthotic devices and weight loss and recommendation to start a home exercise program). 3Risk of bias: open‐label study and therefore no blinding. Randomisation procedure was unclear. Insufficient reporting on incomplete outcome data (‐2). 4Imprecision: small sample size (n = 30) and wide confidence interval (‐1).
Summary of findings 5. Amantadine versus placebo for postpolio syndrome.
| Amantadine versus placebo for postpolio syndrome | ||||||
| Patient or population: people with postpolio syndrome Intervention: amantadine versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Amantadine | |||||
| Activity limitations ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Adverse events | See comment | See comment | Not estimable | 25 (1 study) | See comment | See Table 11 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 6. Prednisone versus placebo for postpolio syndrome.
| Prednisone versus placebo for postpolio syndrome | ||||||
| Patient or population: people with postpolio syndrome Intervention: prednisone versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Prednisone | |||||
| Activity limitations ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Adverse events | See comment | See comment | Not estimable | 17 (1 study) | See comment | See Table 11 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 7. Muscle strengthening versus control for postpolio syndrome.
| Muscle strengthening versus control for postpolio syndrome | ||||||
| Patient or population: people with postpolio syndrome Intervention: muscle strengthening versus control | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Muscle strengthening | |||||
| Activity limitations ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Adverse events | See comment | See comment | Not estimable | 10 (1 study) | See comment | Deleterious effects on motor unit survival were investigated through motor unit number estimates (MUNE). Results showed that MUNE did not change after training. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 8. Rehabilitation in cold climate versus usual care for postpolio syndrome.
| Rehabilitation in cold climate versus usual care for postpolio syndrome | ||||||
| Patient or population: people with postpolio syndrome Intervention: rehabilitation in cold climate versus usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Rehabilitation in cold climate | |||||
| Activity limitations at 3 months Measured with the Sunnaas ADL‐index1 (scale from 0 to 36) | The mean activity limitations in the control group was 32.62 | The mean activity limitations in the intervention group was 2.7 lower (4.53 to 0.87 lower) | ‐ | 53 (1 study) | ⊕⊕⊝⊝ low3 | ‐ |
| Activity limitations at 6 months Measured with the Sunnaas ADL‐index1 (scale from 0 to 36) | The mean activity limitations in the control group was 32.42 | The mean activity limitations in the intervention group was 2.9 lower (4.73 to 1.07 lower) | ‐ | 53 (1 study) | ⊕⊕⊝⊝ low3 | ‐ |
| Activity limitations at 3 months Measured with the Rivermead Mobility Index4 (scale from 0 to 15) | The mean activity limitations in the control group was 13.22 | The mean activity limitations in the intervention group was 1.5 lower (2.93 to 0.07 lower) | ‐ | 53 (1 study) | ⊕⊕⊝⊝ low3 | ‐ |
| Activity limitations at 6 months Measured with the Rivermead Mobility Index4 (scale from 0 to 15) | The mean activity limitations in the control group was 13.52 | The mean activity limitations in the intervention group was 1.8 lower (3.19 to 0.41 lower) | ‐ | 53 (1 study) | ⊕⊕⊝⊝ low3 | ‐ |
| Adverse events ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Sunnaas ADL‐index: Sunnaas Index of Activities of Daily Living. Higher scores represent fewer activity limitations. 2The control group received usual care in a cold climate (rainy or snowy, temperature around 0°C). 3Risk of bias: Baseline imbalance in activity limitations scores reduced the quality of evidence. Randomisation procedure was unclear, blinding not possible (‐2). 4Rivermead Mobility Index: Higher scores represent fewer activity limitations.
Summary of findings 9. Rehabilitation in warm climate versus usual care for postpolio syndrome.
| Rehabilitation in warm climate versus usual care for postpolio syndrome | ||||||
| Patient or population: people with postpolio syndrome Intervention: rehabilitation in warm climate versus usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Rehabilitation in warm climate | |||||
| Activity limitations 3 months Measured with the Sunnaas ADL‐index1 (scale from 0 to 36) | The mean activity limitations in the control group was 32.62 | The mean activity limitations in the intervention group was 1.7 lower (3.47 lower to 0.07 higher) | ‐ | 57 (1 study) | ⊕⊕⊝⊝ low3 | ‐ |
| Activity limitations 3 months Measured with the Rivermead Mobility Index4 (scale from 0 to 15) | The mean activity limitations in the control group was 13.22 | The mean activity limitations in the intervention group was 0.9 lower (2.28 lower to 0.48 higher) | ‐ | 57 (1 study) | ⊕⊕⊝⊝ low3 | ‐ |
| Adverse events ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Sunnaas ADL‐index: Sunnaas Index of Activities of Daily Living. Higher scores represent fewer activity limitations. 2The control group received usual care in a cold climate (rainy or snowy, temperature around 0°C). 3Risk of bias: Randomisation procedure was unclear. Blinding not possible (‐2). 4Rivermead Mobility Index: Higher scores represent fewer activity limitations.
Summary of findings 10. Static magnetic fields versus placebo for postpolio syndrome.
| Static magnetic fields versus placebo for postpolio syndrome | ||||||
| Patient or population: people with postpolio syndrome Intervention: static magnetic fields versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Static magnetic fields | |||||
| Activity limitations ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Adverse events | See comment | See comment | Not estimable | 50 (1 study) | See comment | No adverse events reported directly after treatment |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Postpolio syndrome (PPS) is a complex of neuromuscular symptoms that occurs in many survivors of paralytic polio, usually 15 years or more after the acute illness. It is characterised by a gradual or, in rare cases, sudden onset of progressive and persistent new muscle weakness or decreased muscle endurance, with or without generalised fatigue, muscle atrophy or muscle and joint pain (March of Dimes Foundation 2000). Since there are no specific diagnostic tests for PPS, diagnosis is based on exclusion of other possible causes for the new symptoms.
As the large poliomyelitis epidemics occurred in Western countries in the 1940s and 1950s, many polio survivors are now experiencing the late effects of polio. The World Health Organization estimates that there are 20 million polio survivors. The prevalence of PPS has been reported to range from 15% to 80% of all people with previous paralytic polio, depending on the criteria applied and population studied (Farbu 2011). Although polio epidemics have more or less disappeared in Western countries thanks to the widespread use of polio vaccines, the continuing prevalence of polio in developing countries means that PPS will continue to be a problem for many decades to come.
PPS is considered a slowly progressive condition. Longitudinal studies with follow‐up durations of between 5 and 10 years estimate the annual rate of decline in muscle strength to vary from 1.5% to 2% (Bickerstaffe 2014; Stolwijk‐Swuste 2005; Stolwijk‐Swuste 2010). The decline in muscle mass leads to a decline in physical functioning as the reduced muscle capacity falls short to meet the demands of daily physical activities (Nollet 2003a). People with PPS also commonly report fatigue and pain, which negatively impact physical functioning (Jensen 2011; Nollet 1999).
The pathogenesis of PPS is still unclear and is probably multifactorial. The most widely accepted assumption is that the motor units, enlarged due to reinnervation in response to the denervation in acute poliomyelitis, do not remain stable throughout life (Wiechers 1981). Distal degeneration of axons occurs possibly because of persistent high metabolic stress. The initial balance between denervation and reinnervation of muscle fibres becomes disrupted, and when denervation predominates, progressive muscle weakness results. This concept is supported by the finding of single atrophic muscle fibres in muscle biopsy studies and spontaneous activity of motor unit action potentials on electromyography (Dalakas 1986; Dalakas 1988; Grimby 1989). In addition, a recent longitudinal study demonstrated that motor unit size declined in participants with PPS, that the decline was greatest in the muscles with the fewest remaining units, and that the rate of denervation was related to the rate of strength decline (Bickerstaffe 2014). Other supposed explanations for the pathogenesis of PPS include loss of whole motor units (McComas 1997), virus persistence (Jubelt 1995) or an inflammatory process with raised concentrations of pro‐inflammatory cytokines in the cerebrospinal fluid (Gonzalez 2002). Factors that may contribute to the symptoms of PPS are neuromuscular transmission defects (Trojan 1993), an impaired ability to activate muscles (Allen 1994; Beelen 2003), comorbidity (Stolwijk‐Swuste 2010), radiculopathies or entrapment neuropathies resulting from gait abnormalities and use of assistive devices, weight gain and aging effects.
Description of the intervention
We may divide the potential arsenal of treatment options for PPS into pharmacological and non‐pharmacological interventions.
Pharmacological interventions
Pharmacological treatments vary in terms of their respective points of action and targeted effects. Amantadine, bromocriptine and modafinil act on different regions of the brain and are intended to address generalised fatigue in PPS (Bruno 1996; Chan 2006; Dunn 1991; Stein 1995; Vasconcelos 2007). Insulin‐like growth factor (IGF‐I) and human growth hormone, which stimulates the secretion of IGF‐I, may be suitable agents for the treatment of PPS. It is believed that IGF‐I enhances regeneration of peripheral nerves by axonal sprouting, which in turn positively influences muscle strength (Gupta 1994; Miller 1997; Shetty 1995). Studies have examined high‐dose prednisone and intravenous immunoglobulin (IVIg) to determine whether their immunosuppressive or immunomodulating effects might have a beneficial effect on muscle strength, fatigue and pain (Dinsmore 1995; Farbu 2007; Gonzalez 2006). Pyridostigmine is a cholinesterase inhibitor, thus prolonging the survival of acetylcholine in the neuromuscular synapse. Several studies have investigated its effects on fatigue and other symptoms of PPS (Horemans 2003; Seizert 1994; Trojan 1995; Trojan 1999). Lamotrigine, a glutamate release blocker, has been studied to evaluate whether the neuroprotective effect of the drug reduces fatigue and pain in PPS (On 2005). Studies have evaluated coenzyme Q10 and selegiline for their effects on muscle metabolism and muscle strength, respectively, and effect on PPS symptoms in general (Bamford 1993; Mizuno 1997).
Non‐pharmacological interventions
As no curative treatment is available for PPS, rehabilitation management is considered the mainstay of treatment. The aim is to reach a functional balance by increasing capacities and reducing demands. Several different approaches can be applied. Strength training and aerobic exercise may increase functional capacities in people with PPS (Cup 2007). However, the information available in the literature is contradictory. On the one hand, people with PPS are advised to avoid muscular overuse and intensive training as this could worsen muscle weakness and fatigue and provoke a further loss of muscular strength (Farbu 2011). On the other hand, one study found that physically active people with PPS had fewer symptoms and a higher functional level than inactive people with PPS (Rekand 2004). Exercise in water may be beneficial because it minimises biomechanical stress on muscles and joints (Willen 2001). Training in a warm, dry and sunny climate may have beneficial effects on several physical, psychological and social dimensions of health in PPS (Strumse 2003). For people with PPS who have respiratory impairment, respiratory muscle training may be useful to enhance respiratory muscle endurance and improve well‐being (Klefbeck 2000). Proper orthoses and assistive devices such as crutches, wheelchairs, motorised scooters and home adaptations may facilitate daily life activities. For example, lightweight carbon orthoses may have a beneficial effect on the energy cost of walking and on walking ability (Brehm 2007; Heim 1997). Lifestyle changes including pacing of activities, taking rest intervals and reducing weight have been proposed to relieve symptoms of PPS. Many people with PPS have learned to disregard or mask their symptoms as a way to achieve an active life. Such individuals might have great difficulty adapting their lifestyle to their decreasing abilities, and psychological support may be indicated (Nollet 2003). The effectiveness of lifestyle modification in alleviating shoulder overuse symptoms has been investigated (Klein 2002), and collaborative educational sessions as a major component of a comprehensive rehabilitation program have been proposed (Davidson 2009).
Why it is important to do this review
The original version of this Cochrane review concluded that due to insufficient good‐quality data and lack of randomised studies it was impossible to draw definite conclusions on the effectiveness of interventions for PPS (Koopman 2011). Results indicated that IVIg, lamotrigine, muscle strengthening exercises and static magnetic fields may be beneficial but needed further investigation. Since September 2010, studies have been conducted that have enlarged the body of evidence for interventions included in the original review as well as assessing the effectiveness of treatment modalities not previously included in this review. This review provides guidance for daily practice in the treatment of PPS to rehabilitation physicians and neurologists. Furthermore, it provides a basis for researchers to initiate novel trials of interventions in PPS. There were no major changes in methods between the original review and this first update.
Objectives
To systematically review the evidence from randomised and quasi‐randomised controlled trials for the effect of any pharmacological or non‐pharmacological treatment for PPS compared to placebo, usual care or no treatment.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised controlled trials (RCTs) and quasi‐randomised trials of any treatment for people with PPS.
Types of participants
We included studies on participants with a diagnosis of PPS. Essential criteria to the diagnosis were:
a history of paralytic poliomyelitis;
a period of partial or complete functional recovery after acute poliomyelitis, followed by an interval of stable neurologic function;
new or increased neuromuscular symptoms.
We did not include experimental data from animal models.
Types of interventions
We included any form of pharmacological or non‐pharmacological treatment. Drugs may include cholinesterase inhibitors (pyridostigmine), steroids (prednisone or prednisolone), IVIg, dopamine‐2 receptor agonists (bromocriptine), glutamate release blockers (lamotrigine), human growth hormone, IGF‐I, amantadine, modafinil, coenzyme Q10 and selegiline. Non‐pharmacological treatment may include exercise therapy (for example aerobic exercise, muscle strengthening exercise, respiratory muscle training, warm climate training, hydro training), orthoses and other assistive devices, respiratory support, lifestyle change, weight control or surgical intervention. We also included studies that examined combinations of these treatments. We compared interventions against placebo, usual care or no treatment.
Types of outcome measures
The outcome measures listed were the outcomes of interest within whichever studies we included. We did not use outcomes as criteria for including studies.
Primary outcomes
The primary outcome measure was 'self perceived activity limitations'. We accepted any scale that measured this concept, such as the Physical Component Summary of the Short Form‐36 Health Survey (SF‐36 PCS) and the physical mobility category of the Nottingham Health Profile.
Secondary outcomes
The secondary outcome measures were:
muscle strength;
muscle endurance;
fatigue;
pain;
adverse events subdivided into minor adverse events and serious adverse events (resulting in cessation of treatment, requiring hospitalisation or being life‐threatening or fatal).
For the secondary outcome measures, we also accepted any scale that measured these concepts. We used standardised mean differences to make comparisons. Alternatively, participants may have been dichotomised into no change or improved and worse; in this case we used the numbers unchanged or improved and the numbers that were worse and calculated risk ratios. We evaluated outcomes directly post treatment. When interventions were expected to have long‐term effects, we also evaluated long‐term outcomes (greater than three months following treatment). If a study did not report change from baseline scores, but final scores were available, we used these data for the analyses. We would have considered the cost‐effectiveness of treatments in the Discussion if information had been available.
Search methods for identification of studies
We developed search strategies in consultation with the Cochrane Neuromuscular Disease Group Trials Search Co‐ordinator.
Electronic searches
We searched for relevant trials using the following databases:
Cochrane Neuromuscular Disease Group Specialized Register (21 July 2014)
Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 7 in Cochrane Library)
MEDLINE (January 1966 to July 2014)
EMBASE (January 1947 to July 2014)
PsycINFO (January 1806 to July 2014)
CINAHL Plus (January 1937 to July 2014)
We have provided the review search strategies for the different databases in: Appendix 1 (CENTRAL); Appendix 2 (MEDLINE); Appendix 3 (EMBASE); Appendix 4 (PsycINFO); Appendix 5 (CINAHL) and Appendix 6 (Cochrane Neuromuscular Disease Group Specialized Register).
Searching other resources
In an effort to identify further published, unpublished and ongoing trials, we:
checked reference lists of all relevant articles;
-
searched trial registers (Appendix 7) including:
World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/en/)
Australian New Zealand Clinical Trials Registry (www.anzctr.org.au)
U.S. National Institutes of Health (www.clinicaltrials.gov)
International Standard Randomised Controlled Trial Number Registry (www.ISRCTN.org)
UMIN Clinical Trials Registry (www.umin.ac.jp/ctr/index/htm)
Nederlands Trial Register (www.trialregister.nl);
contacted investigators known to be involved in this area of research;
searched the Database of Abstracts of Reviews of Effects (DARE) and the Health Technology Assessment (HTA) Database (2014, Issue 2 in Cochrane Library).
Data collection and analysis
Selection of studies
Two review authors (FK, AB) independently screened the search results based on titles, keywords and abstracts and read the full text of eligible studies they identified in this way. The two review authors decided on the suitability for inclusion in the review using pre‐specified inclusion criteria. Disagreements were resolved by consensus, or, if necessary, by including a third review author (NEG). Review authors were not blinded to the journals of publication, authors’ names or institutional affiliation.
Data extraction and management
Two review authors (FK, AB) extracted the data independently onto a specially designed data extraction form. They wrote to study authors for further information when necessary. Disagreements were resolved by consensus, or, if necessary, by including a third review author (NEG). One review author (FK) entered data into the Review Manager 5 software (RevMan 2014) and a second review author (AB) independently checked the data entry.
Assessment of risk of bias in included studies
The two review authors independently assessed all included studies for risk of bias according to Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008, updated Higgins 2011). We assessed randomisation sequence generation, allocation concealment, blinding (participants, administrators of the intervention and outcome assessors), incomplete outcome data (missing outcome data and intention‐to‐treat (ITT) analysis), selective outcome reporting and other sources of bias. For two domains we further specified the original criteria of the Cochrane Handbook for Systematic Reviews of Interventions. For a study to score 'low risk of bias' for the blinding domains, blinding had to be ensured for all outcome measures, including patient‐reported outcomes. For a study to score 'low risk of bias' for the ITT analysis domain, all participants had to be analysed in the groups to which they were randomised irrespective of non‐compliance and co‐interventions. This did not apply to the missing values.
Measures of treatment effect
We summarised continuous data with mean differences (MD). If studies used different outcome measurements that addressed the same clinical outcome, we used standardised mean differences (SMD). We summarised dichotomous data using risk ratios (RR). We expressed uncertainty with 95% confidence intervals (CIs).
Unit of analysis issues
We included cluster randomised trials if the study reported appropriate data to adjust for the design effect.
Assessment of heterogeneity
We explored statistical heterogeneity among results of different studies using the Chi2 test with significance set at P < 0.1. We measured the percentage of variation between trial results due to heterogeneity rather than chance using the I2 statistic, with a value greater than 50% indicating substantial heterogeneity.
Assessment of reporting biases
If there were sufficient trials, we assessed publication bias using a funnel plot. We were aware that this method is not a reliable indicator of publication bias and that any interpretations made on this basis should be made with great caution.
Data synthesis
We did not combine data from studies with different interventions. If there was more than one trial with comparable intervention and outcome measures, we calculated a pooled estimate of the treatment effect across the trials using RevMan. We used a fixed‐effect model to combine individual results if there was no significant heterogeneity among the included trials; otherwise, we used a random‐effects model.
Using the GRADEpro software we prepared a 'Summary of findings' table for each comparison in which we presented the primary outcome measure of this review, ‘self perceived activity limitations’, as well as 'adverse events'. Two review authors (FK, AB) assessed the quality of the evidence according to Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). We based evidence for downgrading studies on five factors: risk of bias, indirectness, inconsistency, imprecision and publication bias. If we found a reason for downgrading the evidence, we classified the evidence as ’serious’ (downgrading the quality rating by one level) or ’very serious’ (downgrading the quality rating by two levels). We justified decisions to downgrade the quality of studies using footnotes. We classified the quality of evidence for each outcome according to the following categories:
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Subgroup analysis and investigation of heterogeneity
If the data were available, we performed subgroup analyses to explore possible sources of clinical heterogeneity with regard to treatment. We investigated relationships between intervention effect and dose, treatment intensity or treatment duration. We were cautious about drawing conclusions if the results of the subgroup analyses were only based on between‐study differences.
Sensitivity analysis
We performed sensitivity analyses by repeating the meta‐analyses after omitting the trials in which we had identified a possible risk of bias.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
We have displayed results of the search in Figure 1. We ran the searches for the original review in October 2010. The total number of records after deduplication identified in that search was 717. Screening of the titles, keywords and abstracts of these search results resulted in a selection of 26 records, describing 23 studies, for further assessment of eligibility. Twelve studies fulfilled the selection criteria and were included in the original review. For the update we ran searches in July 2014 with updated search strategies. The numbers of records found with these updated strategies were: Cochrane Neuromuscular Disease Group Specialized Register, 28 (2 new records); the Cochrane Central Register of Controlled Trials (CENTRAL), 38 records; MEDLINE, 199 (35 new records); EMBASE, 105 (20 new records); PsycINFO, 141 (42 new records); CINAHL Plus, 165 (36 new records). The total number of records found by the search for the original review plus this update after deduplication was 812 (95 new records). We further assessed 10 new records, describing 7 new studies, for eligibility for this update. We found an additional five studies from the searches in the trial registers. The other searches did not add any further potentially eligible studies.
1.
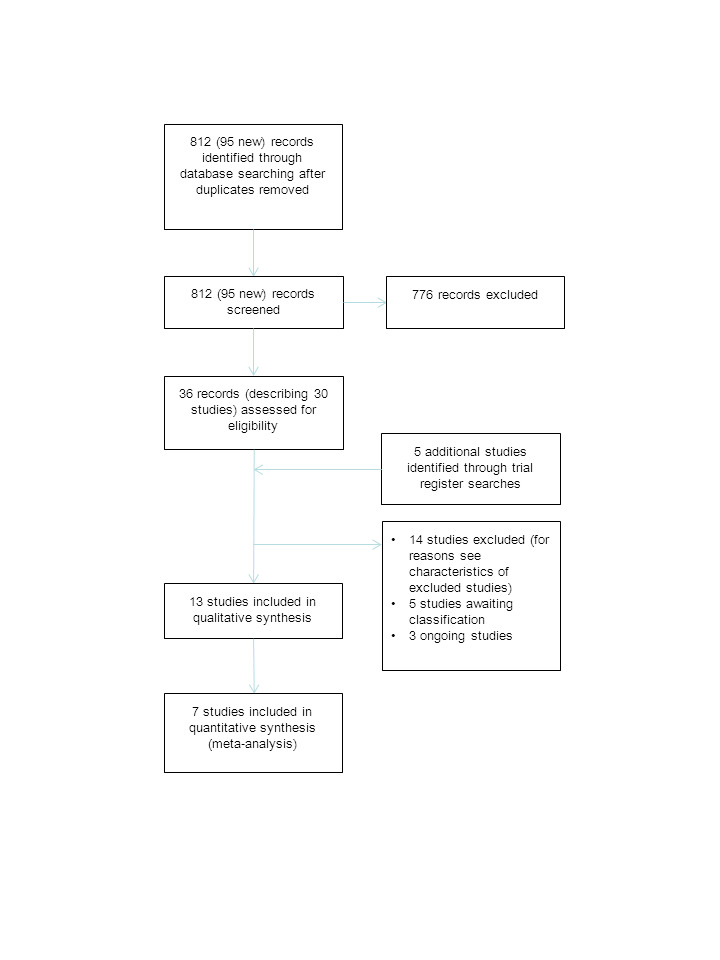
Flow diagram of the study selection process.
Included studies
One new study that evaluated the effect of IVIg fulfilled the selection criteria and was included in this review update (Bertolasi 2013). We furthermore identified a study that evaluated the long‐term effectiveness of IVIg in a subcohort of participants from the original study of Gonzalez 2006. As the authors of that study hypothesised that IVIg causes improvements over longer periods, we therefore decided by consensus to include the long‐term outcomes of IVIg in this update. As a result we have included a total of 13 studies in this update, involving a total of 675 participants. Ten studies evaluated pharmacological treatment in PPS: two studies on modafinil (Chan 2006; Vasconcelos 2007), three studies on IVIg (Bertolasi 2013; Farbu 2007; Gonzalez 2006), two studies on pyridostigmine (Horemans 2003; Trojan 1999), and three single studies that evaluated lamotrigine (On 2005), amantadine (Stein 1995), and high‐dose prednisone (Dinsmore 1995). Two non‐pharmacological studies evaluated the effect of exercise therapy: one study comparing the effect of muscle strengthening of the thumb muscles with no training (Chan 2003) and one three‐arm study comparing rehabilitation in warm climate (that is temperature ± 25°C, dry and sunny) versus rehabilitation in cold climate (that is temperature ± 0°C, rainy or snowy) versus usual care (Strumse 2003). One non‐pharmacological study evaluated the effect of static magnetic fields (Vallbona 1997).
The pharmacological treatment studies and the static magnetic fields study were placebo‐controlled studies with a parallel‐group design, except for the two modafinil studies, which used a cross‐over design, and the lamotrigine study, which was classified as an open‐label study. Because PPS is considered to be a reasonably stable chronic condition and modafinil is a drug with a temporary effect, we considered the use of a cross‐over design appropriate in the two modafinil trials. Both exercise therapy studies were classified as non‐placebo‐controlled studies with a parallel‐group design. Five studies (Bertolasi 2013; Farbu 2007; Gonzalez 2006; On 2005; Strumse 2003) included participants with PPS based on one of the definitions of Halstead (Halstead 1985; Halstead 1987; Halstead 1991); one study (Vallbona 1997) used the criteria of Dalakas (Dalakas 1995); one study (Horemans 2003) used the criteria of Borg (Borg 1996); and one study (Vasconcelos 2007) used the criteria of the March of Dimes (March of Dimes Foundation 2000). Five studies (Chan 2003; Chan 2006; Dinsmore 1995; Stein 1995; Trojan 1999) did not refer to any of these definitions but designed their own criteria. We contacted the authors of these last five studies, and they confirmed that their criteria met our pre‐specified criteria.
Excluded studies
We excluded three new studies from this update (Acler 2013; Khan 2013; Skough 2011), resulting in a total of 14 studies that were excluded from this review. One study evaluating the effect of recombinant IGF‐I against placebo was excluded because the results were only published in an abstract (Miller 1997). Three studies were excluded because they could not be classified as a RCT or quasi‐randomised trial according to the definitions described in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebre 2011). The first study evaluated the effect of bromocriptine in five people with fatigue after they had received placebo treatment for four weeks (Bruno 1996). The second study evaluated the effects of an aerobic walking program in two participants as compared to the results of a control participant who was not available for participation in the program (Dean 1988). The third study evaluated the effect of dynamic water exercise in 15 participants with PPS as compared to 13 participants who were unable to participate in the training program for practical reasons (Willen 2001). We excluded six studies that did not use a control group consisting of placebo, usual care or no treatment. The first study was a three‐arm study investigating the effects of a home‐based exercise program versus lifestyle modification versus the combination of these two interventions (Klein 2002). The second study compared the effects of a hospital‐based exercise program with a home‐based exercise program (Oncu 2009). The third study evaluated the effects of oral supplementation with coenzyme Q10 as add‐on to resistance training against the effect of a placebo and resistance training (Skough 2008). The fourth study evaluated the effects of muscular resistance training as add‐on to IVIg against the effect of usual care and IVIg (Skough 2011). The fifth study assessed whether transcranial direct current stimulation improved sleep and fatigue symptoms as compared to sham stimulation; however, all the participants underwent daily physical therapy during the intervention and were all receiving IVIg before inclusion in the study (Acler 2013). The sixth study evaluated the effect of pulsed electromagnetic field therapy in addition to stretching on hip flexor contractures against stretching only (Khan 2013). In the studies of Acler 2013, Khan 2013, Skough 2008 and Skough 2011, the intervention arm included two interventions, whereas the comparison arm consisted of one of these two interventions only. As the effectiveness of these single interventions is currently unknown, they cannot be considered placebo, usual care or no treatment; we therefore excluded these four studies from the review. Finally, we excluded four studies because they did not meet our criteria for the diagnosis of PPS. Three studies evaluated the effect of aerobic training (Dean 1991; Jones 1989; Kriz 1992).The fourth trial was a three‐arm study evaluating the effect of an online fatigue self management program versus information only versus no intervention in people with chronic neurological conditions, including PPS (Ghahari 2010).
Studies awaiting classification
We identified five completed studies for which no full‐text article was currently available. Three studies presented preliminary findings in conference abstracts (Koopman 2014; Murray 2014; Silva 2014), and we identified two studies from the trial registers (ACTRN12612000552886; ISRCTN00378146). The information these data sources provided was not sufficient to make a reliable inclusion or exclusion decision. Three studies are investigating the effectiveness of home‐based exercise therapy. The first study aims to investigate the effect of an aerobic exercise program, carried out in the home environment, using arm ergometers (Murray 2014). The second study is evaluating the effectiveness of a home‐based exercise program consisting of progressive strength‐resistance exercises (ISRCTN00378146). The third study is a three‐arm study comparing the effects of two different interventions, exercise therapy (including a home‐based aerobic training program on a cycle ergometer and a supervised group training) and cognitive behavioural therapy, versus usual care (Koopman 2014). Furthermore, Silva 2014 aims to assess whether mattress liners with far infrared bio‐ceramic components are effective in reducing pain and daytime somnolence and improving quality of life and sleep characteristics in PPS. Finally, ACTRN12612000552886 aims to determine whether taking a 100 mg capsule of coenzyme Q10 daily for a period of two months can alleviate excessive fatigue. We contacted the trial authors of these five studies, and they all confirmed that a manuscript was in preparation for publication. We will take these studies into consideration for inclusion in the next update of the review.
Ongoing studies
We identified three planned or ongoing studies from the trial registers. One planned multicentre study aims to select a dose of IVIg (1 or 2 g/kg) and confirm the efficacy of the selected IVIg dose by assessing physical performance (NCT02176863). One ongoing study is examining the efficacy of a microprocessor‐controlled knee‐ankle‐foot orthosis to improve functional mobility in individuals with lower extremity impairments, including PPS, as compared to participants' own stance control orthosis (NCT02089880). We found one study that aims to assess the efficacy of L‐carnitine and piracetam in relieving weakness, muscle fatigue and muscle pain, of which the current recruitment status is unknown (NCT01549847). When these studies are completed and results are published, we will take them into consideration for inclusion in a future update of the review.
Risk of bias in included studies
See Characteristics of included studies and Figure 2.
2.
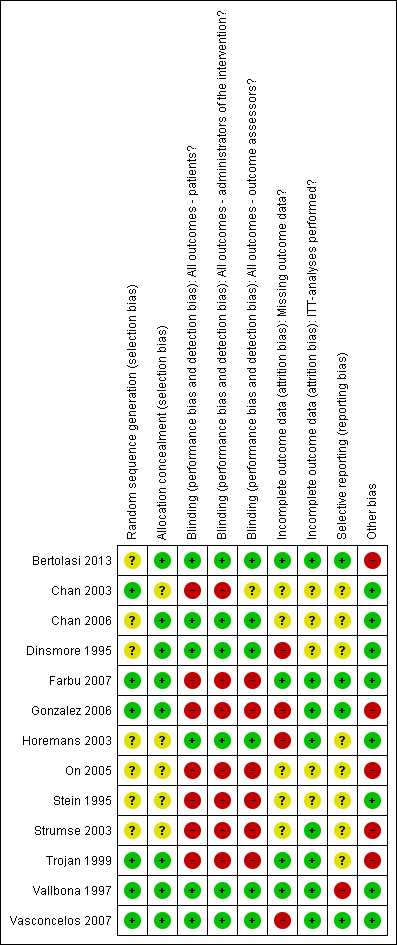
'Risk of bias' summary: review authors’ judgements about each 'Risk of bias' item for each included study.
The method of randomisation sequence generation was adequate in six studies and unclear in seven studies. Allocation concealment was adequate in eight studies and unclear in five studies. Blinding of participants, administrators of the interventions and outcome assessors was adequate in only six of the included trials (Bertolasi 2013; Chan 2006; Dinsmore 1995; Horemans 2003; Vallbona 1997; Vasconcelos 2007). In the two studies on exercise therapy (Chan 2003; Strumse 2003) and the open‐label study with lamotrigine (On 2005), participants and administrators of the interventions were aware of the treatment being given, therefore we have graded these studies as inadequate for these items. Four pharmacological‐treatment studies did blind participants and administrators of the interventions, but we graded these studies as inadequate because side effects of the treatment could have caused unblinding (Farbu 2007; Gonzalez 2006; Stein 1995; Trojan 1999). Since most of the studies included patient‐reported outcomes, grading of blinding status for outcome assessors in these studies was dependent upon the blinding status of the participant. Four studies had withdrawal of participants because of reasons considered to be related to the treatment, therefore we graded these studies as at high risk of bias for the missing outcome data domain (Dinsmore 1995; Gonzalez 2006; Horemans 2003; Vasconcelos 2007). Eight studies met our pre‐specified criteria for the ITT analysis domain (Bertolasi 2013; Farbu 2007; Gonzalez 2006; Horemans 2003; Strumse 2003; Trojan 1999; Vallbona 1997; Vasconcelos 2007). Although we rated the short‐term follow‐up assessment of Gonzalez 2006as at low risk of bias for the ITT domain, for the long‐term follow‐up assessment of Gonzalez 2006 it was unclear whether data were analysed according to the ITT principle. Protocols were available for four studies, which were published in trial registers (Bertolasi 2013; Farbu 2007; Gonzalez 2006; Vasconcelos 2007). As all pre‐specified outcomes in these study protocols had been reported in the trial articles, we rated these studies as adequate for the selective outcome reporting domain. Again, although we rated the short‐term follow‐up assessment of Gonzalez 2006 as at low risk of bias for the selective reporting domain, for the long‐term follow‐up assessment only a small selection of pre‐specified primary and secondary outcomes were reported, therefore we judged the study as at high risk of bias. We rated five studies as at high risk of bias for the other bias domain as a result of baseline imbalances between groups (Bertolasi 2013; Gonzalez 2006; On 2005; Strumse 2003; Trojan 1999). In conclusion, none of the included studies were completely free from any risk of bias, and the most prevalent risk of bias was lack of blinding.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10
Below are results for each intervention separately in relation to predefined outcome measures. We have provided adverse events for the pharmacological interventions in Table 11.
IVIg
Bertolasi 2013, Farbu 2007 and the long‐term follow‐up study of Gonzalez 2006 did not report change from baseline scores, therefore we have used final scores in the analyses. For the evaluation of short‐term effects, we used in the analyses the outcomes assessed at two months after infusion in Bertolasi 2013 and at three months after the (last) infusion in Farbu 2007 and Gonzalez 2006. For the evaluation of long‐term effects, we used in the analyses the outcomes assessed at four, six and nine months after the (last) infusion in Bertolasi 2013, Farbu 2007 and Gonzalez 2006, respectively.
Primary outcome measure: activity limitations
Bertolasi 2013 and Gonzalez 2006 investigated the effect of IVIg on activity limitations. Meta‐analysis showed no significant difference in activity limitations as measured with the Physical Component Summary of the Short Form‐36 Health Survey (SF‐36 PCS) between the IVIg group and the placebo group in either the short term (MD 2.35; 95% CI ‐0.06 to 4.76) (Analysis 1.1) or long term (MD ‐0.51; 95% CI ‐4.63 to 3.60) (Analysis 1.2). Removing the long‐term follow‐up data of Gonzalez 2006, where a baseline imbalance in SF‐36 PCS scores in favour of the placebo group was found, did not change the conclusion of no significant difference in activity limitations at long‐term follow‐up between IVIg and placebo (MD ‐0.70; 95% CI ‐6.33 to 4.93, 1 trial).
1.1. Analysis.
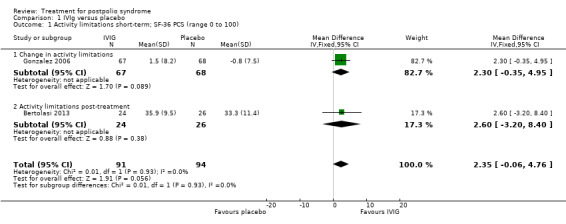
Comparison 1 IVIg versus placebo, Outcome 1 Activity limitations short‐term; SF‐36 PCS (range 0 to 100).
1.2. Analysis.
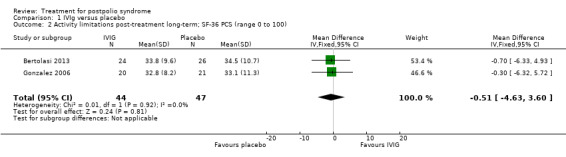
Comparison 1 IVIg versus placebo, Outcome 2 Activity limitations post‐treatment long‐term; SF‐36 PCS (range 0 to 100).
Secondary outcome measures: muscle strength, muscle endurance, fatigue and pain
All three studies measured isometric muscle strength at short‐term follow‐up. Gonzalez 2006 tested muscle strength of (1) a selected 'study muscle' in the upper leg, lower leg or hand (that is a clinically chosen polio‐affected muscle with approximately 25% to 75% of what would be the expected strength for the age and sex of the participant) and (2) the remaining muscles that were not selected as the study muscle. For this second outcome measure, different muscle groups of individual participants were recorded as multiple observations for the same outcome, therefore we could only include the study muscle in our analyses. Bertolasi 2013 and Farbu 2007 tested muscle strength of knee extensors and elbow flexors bilaterally. To reduce multiple testing, we decided by consensus to randomly choose one of the muscle groups of the lower extremities for inclusion in the analysis. The allocated outcome was muscle strength of the right knee extensor. As the outcome measures on muscle strength of Gonzalez 2006 on the one hand, and Bertolasi 2013 and Farbu 2007 on the other hand differ with respect to being symptomatic or not, we decided by consensus that pooling these measures was not justified. Gonzalez 2006 demonstrated that the IVIg group showed significant improvement in muscle strength compared to placebo in the short term (MD 8.60; 95% CI 2.81 to 14.39) (Analysis 1.3). However, the pooled data of Bertolasi 2013 and Farbu 2007 showed no significant difference in right knee extensor muscle strength between the IVIg group and the placebo group, either in the short term (MD ‐11.01; 95% CI ‐53.86 to 31.84, with I2 = 60% indicating substantial heterogeneity) (Analysis 1.4) or in the long term (MD ‐10.29; 95% CI ‐55.37 to 34.78, with I2 = 73% indicating substantial heterogeneity) (Analysis 1.5). Removing the Bertolasi 2013 data, in which a large baseline imbalance in muscle strength in favour of the placebo group was present, did not change the conclusions of no significant difference in muscle strength of the right knee extensor in either the short term (MD 12.90; 95% CI ‐29.83 to 55.63, 1 trial) or the long term (MD 13.00; 95% CI ‐20.96 to 46.96, 1 trial) between IVIg and placebo.
1.3. Analysis.
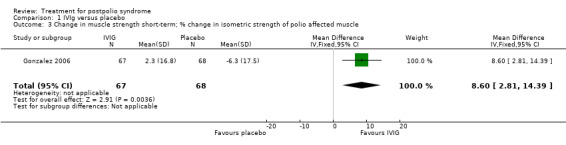
Comparison 1 IVIg versus placebo, Outcome 3 Change in muscle strength short‐term; % change in isometric strength of polio affected muscle.
1.4. Analysis.
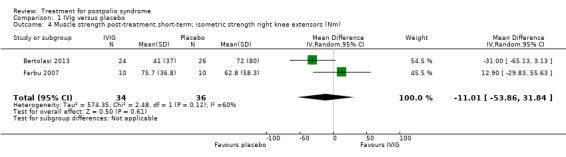
Comparison 1 IVIg versus placebo, Outcome 4 Muscle strength post‐treatment short‐term; isometric strength right knee extensors (Nm).
1.5. Analysis.
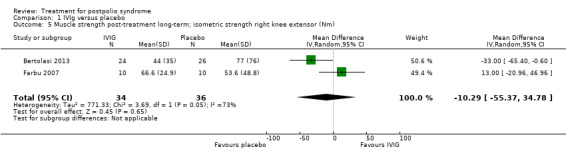
Comparison 1 IVIg versus placebo, Outcome 5 Muscle strength post‐treatment long‐term; isometric strength right knee extensor (Nm).
Fatigue was measured with the Multidimensional Fatigue Inventory (MFI) in Gonzalez 2006 and with the Fatigue Severity Scale (FSS) in Bertolasi 2013 and Farbu 2007. We could not include data obtained in Gonzalez 2006 in the meta‐analysis, as the authors of Gonzalez 2006 reported change from baseline scores, while the other two studies used final scores, which cannot be combined as SMDs (Deeks 2008). Analyses showed that there were no significant differences in change of fatigue in the short term (MFI: MD 0.00; 95% CI ‐1.05 to 1.05) (Analysis 1.6), final fatigue scores in the short term (FSS: MD 0.08; 95% CI ‐0.71 to 0.87) (Analysis 1.7) and final fatigue scores in the long term (FSS: MD ‐0.50; 95% CI ‐1.15 to 0.15) (Analysis 1.8) between the groups. Meta‐analysis showed no significant difference in pain measured with the visual analogue scale (VAS) between participants treated with IVIg and placebo in the short term (MD ‐9.27; 95% CI ‐25.11 to 6.57, with I2 = 80% indicating substantial heterogeneity) (Analysis 1.9) or in the long term (MD ‐5.61; 95% CI ‐14.95 to 3.73) (Analysis 1.12) There were also no significant differences in pain at short‐ and long‐term time points measured with the pain drawing instrument (PDI) (Farbu 2007) or 101‐point numeric rating scale for pain (101NRS) (Bertolasi 2013) (PDI short term: MD ‐6.70; 95% CI ‐23.63 to 10.23) (Analysis 1.10); (101NRS short term: MD ‐3.00; 95% CI ‐16.30 to 10.30) (Analysis 1.11); (PDI long term: MD ‐5.50; 95% CI ‐23.39 to 12.39) (Analysis 1.13); (101NRS long term: MD 0.00; 95% CI ‐13.03 to 13.03) (Analysis 1.14). Muscle endurance was not measured.
1.6. Analysis.
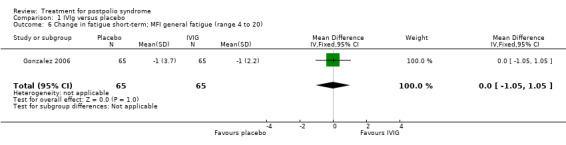
Comparison 1 IVIg versus placebo, Outcome 6 Change in fatigue short‐term; MFI general fatigue (range 4 to 20).
1.7. Analysis.
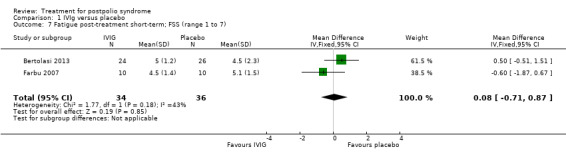
Comparison 1 IVIg versus placebo, Outcome 7 Fatigue post‐treatment short‐term; FSS (range 1 to 7).
1.8. Analysis.
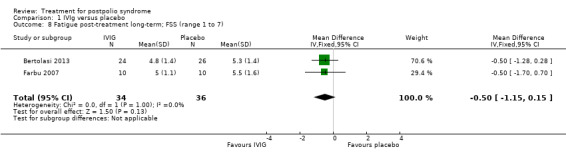
Comparison 1 IVIg versus placebo, Outcome 8 Fatigue post‐treatment long‐term; FSS (range 1 to 7).
1.9. Analysis.
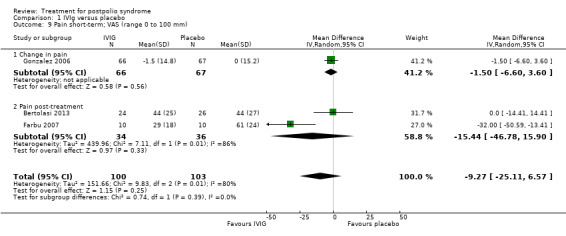
Comparison 1 IVIg versus placebo, Outcome 9 Pain short‐term; VAS (range 0 to 100 mm).
1.12. Analysis.
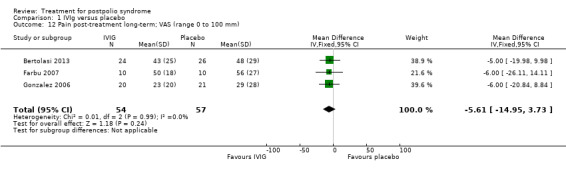
Comparison 1 IVIg versus placebo, Outcome 12 Pain post‐treatment long‐term; VAS (range 0 to 100 mm).
1.10. Analysis.
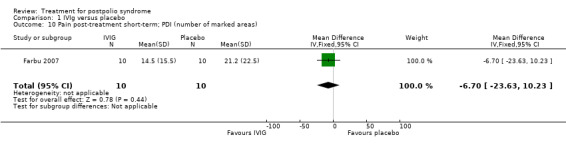
Comparison 1 IVIg versus placebo, Outcome 10 Pain post‐treatment short‐term; PDI (number of marked areas).
1.11. Analysis.
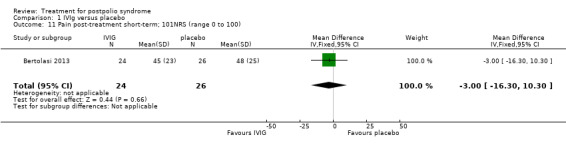
Comparison 1 IVIg versus placebo, Outcome 11 Pain post‐treatment short‐term; 101NRS (range 0 to 100).
1.13. Analysis.
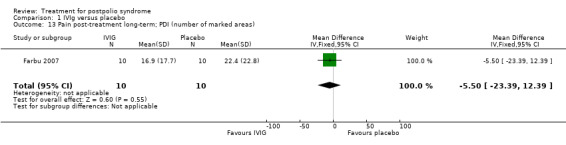
Comparison 1 IVIg versus placebo, Outcome 13 Pain post‐treatment long‐term; PDI (number of marked areas).
1.14. Analysis.
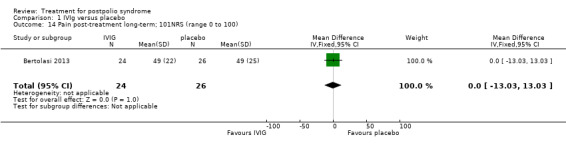
Comparison 1 IVIg versus placebo, Outcome 14 Pain post‐treatment long‐term; 101NRS (range 0 to 100).
Modafinil
Because both studies of modafinil (Chan 2006; Vasconcelos 2007) were cross‐over trials, we used the generic inverse variance method to calculate effect estimates.
Primary outcome measure: activity limitations
Vasconcelos 2007 was the only study to investigate the effect of modafinil on activity limitations. Results of this study showed that there was no significant difference in activity limitations as measured with the physical functioning scale of the SF‐36 (SF‐36 PF) between modafinil treatment and placebo (MD 1.28; 95% CI ‐3.56 to 6.12) (Analysis 2.1).
2.1. Analysis.
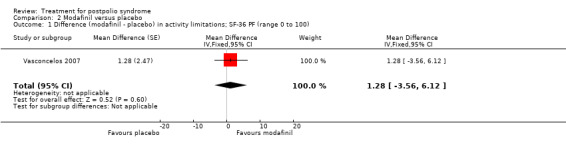
Comparison 2 Modafinil versus placebo, Outcome 1 Difference (modafinil ‐ placebo) in activity limitations; SF‐36 PF (range 0 to 100).
Secondary outcome measures: muscle strength, muscle endurance, fatigue and pain
Pooling of data on fatigue was not possible because the results of Chan 2006 were expressed as percentages of baseline values. Vasconcelos 2007 showed that there were no significant differences in fatigue between modafinil treatment and placebo treatment on any of the scales (FSS: MD 0.39; 95% CI ‐0.24 to 1.02) (Analysis 2.3); (Visual Analog Fatigue Scale: MD ‐0.01; 95% CI ‐0.93 to 0.91) (Analysis 2.4); (Fatigue Impact Scale: MD ‐3.32; 95% CI ‐15.22 to 8.58) (Analysis 2.5). Chan 2006 showed significantly less fatigue in the placebo group as compared to the modafinil group (Piper Fatigue Scale: MD 12.00; 95% CI 4.16 to 19.84) (Analysis 2.2). Also, we found no significant difference in pain between modafinil treatment and placebo treatment (MD 1.21; 95% CI ‐7.77 to 10.19) (Analysis 2.6) (Vasconcelos 2007). Muscle strength and endurance were not measured.
2.3. Analysis.
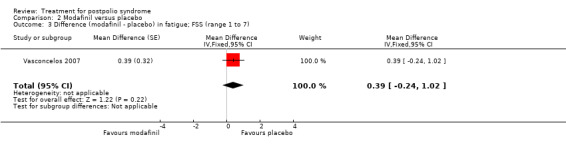
Comparison 2 Modafinil versus placebo, Outcome 3 Difference (modafinil ‐ placebo) in fatigue; FSS (range 1 to 7).
2.4. Analysis.
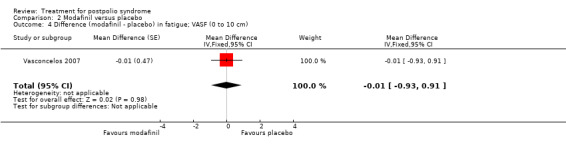
Comparison 2 Modafinil versus placebo, Outcome 4 Difference (modafinil ‐ placebo) in fatigue; VASF (0 to 10 cm).
2.5. Analysis.
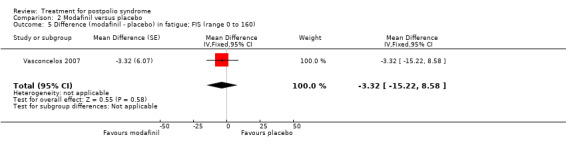
Comparison 2 Modafinil versus placebo, Outcome 5 Difference (modafinil ‐ placebo) in fatigue; FIS (range 0 to 160).
2.2. Analysis.
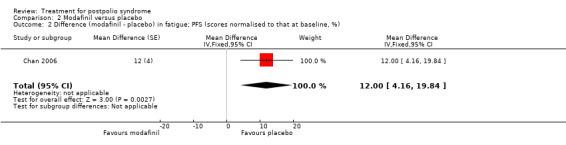
Comparison 2 Modafinil versus placebo, Outcome 2 Difference (modafinil ‐ placebo) in fatigue; PFS (scores normalised to that at baseline, %).
2.6. Analysis.
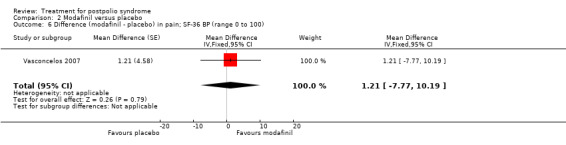
Comparison 2 Modafinil versus placebo, Outcome 6 Difference (modafinil ‐ placebo) in pain; SF‐36 BP (range 0 to 100).
Pyridostigmine
Primary outcome measure: activity limitations
Trojan 1999 was the only study to investigate the effect of pyridostigmine on activity limitations. Results showed that there was no significant difference in change in activity limitations between the pyridostigmine group and the placebo group as measured with the SF‐36 PF (MD 2.10; 95% CI ‐3.64 to 7.84) (Analysis 3.1).
3.1. Analysis.
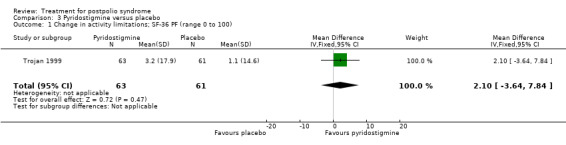
Comparison 3 Pyridostigmine versus placebo, Outcome 1 Change in activity limitations; SF‐36 PF (range 0 to 100).
Secondary outcome measures: muscle strength, muscle endurance, fatigue and pain
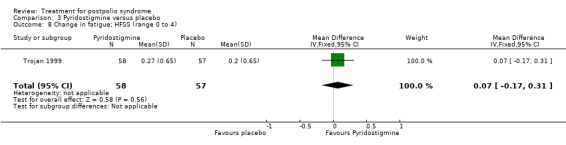
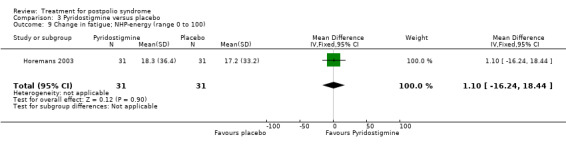
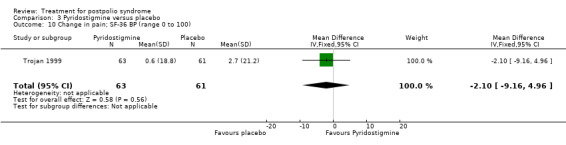
Both studies (Horemans 2003; Trojan 1999) measured isometric muscle strength. Horemans 2003 tested the symptomatic quadriceps muscle (that is quadriceps with new neuromuscular symptoms, neuromuscular transmission defects and a minimum strength of 30 Nm). Trojan 1999 tested 12 muscle groups and divided them into 3 categories of weakness. For each participant, a mean value of percent change in muscle strength for each category was calculated. Because of these substantial differences in assessment of muscle strength, we decided by consensus not to pool these data. In both studies there were no significant differences in change in muscle strength between the pyridostigmine group and the placebo group on any of the measures (very weak muscles: MD 33.90; 95% CI ‐5.49 to 73.29) (Analysis 3.2); (weak muscles: MD ‐1.80; 95% CI ‐11.75 to 8.15) (Analysis 3.3); (relatively strong muscles: MD ‐0.30; 95% CI ‐4.22 to 3.62) (Analysis 3.4); (symptomatic quadriceps muscle: MD 6.70; 95% CI ‐2.19 to 15.59) (Analysis 3.5). Only Horemans 2003 evaluated muscle endurance. Results showed that there was no significant difference in muscle endurance (that is fatigability during a 30 s sustained contraction of the quadriceps muscle) between the two groups (MD ‐0.70; 95% CI ‐2.52 to 1.12) (Analysis 3.6). Meta‐analyses of the FSS results of both trials showed no significant difference in change in fatigue between the pyridostigmine group and the placebo group (MD ‐0.06; 95% CI ‐0.34 to 0.21) (Analysis 3.7). Also, we found no significant differences in fatigue when measured with the Hare Fatigue Symptom Scale (MD 0.07; 95% CI ‐0.17 to 0.31) (Analysis 3.8) (Trojan 1999) and the energy category of the Nottingham Health Profile (NHP‐Energy) (MD 1.10; 95% CI ‐16.24 to 18.44) (Analysis 3.9) (Horemans 2003). Trojan 1999 showed that there were no significant differences between the groups' change in pain as measured with the SF‐36 Bodily Pain (MD ‐2.10; 95% CI ‐9.16 to 4.96) (Analysis 3.10).
3.2. Analysis.
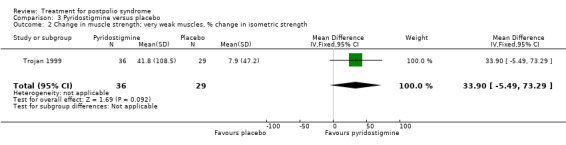
Comparison 3 Pyridostigmine versus placebo, Outcome 2 Change in muscle strength; very weak muscles, % change in isometric strength.
3.3. Analysis.
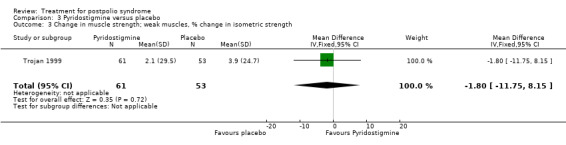
Comparison 3 Pyridostigmine versus placebo, Outcome 3 Change in muscle strength; weak muscles, % change in isometric strength.
3.4. Analysis.
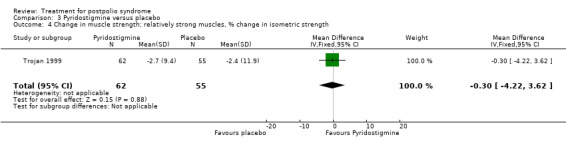
Comparison 3 Pyridostigmine versus placebo, Outcome 4 Change in muscle strength; relatively strong muscles, % change in isometric strength.
3.5. Analysis.
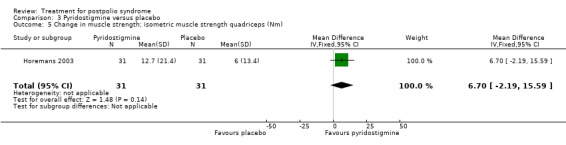
Comparison 3 Pyridostigmine versus placebo, Outcome 5 Change in muscle strength; isometric muscle strength quadriceps (Nm).
3.6. Analysis.
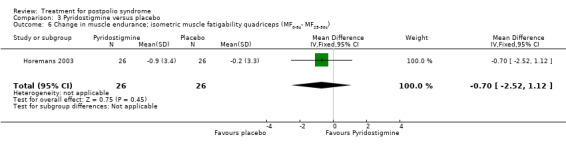
Comparison 3 Pyridostigmine versus placebo, Outcome 6 Change in muscle endurance; isometric muscle fatigability quadriceps (MF0‐5s‐ MF25‐30s).
3.7. Analysis.
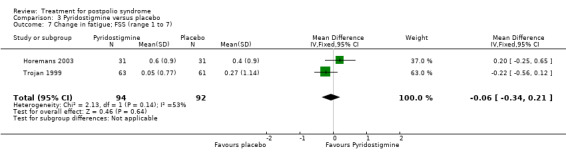
Comparison 3 Pyridostigmine versus placebo, Outcome 7 Change in fatigue; FSS (range 1 to 7).
3.8. Analysis.
Comparison 3 Pyridostigmine versus placebo, Outcome 8 Change in fatigue; HFSS (range 0 to 4).
3.9. Analysis.
Comparison 3 Pyridostigmine versus placebo, Outcome 9 Change in fatigue; NHP‐energy (range 0 to 100).
3.10. Analysis.
Comparison 3 Pyridostigmine versus placebo, Outcome 10 Change in pain; SF‐36 BP (range 0 to 100).
Lamotrigine
The study of lamotrigine (On 2005) did not report change from baseline scores, therefore we used final scores in the analyses. It should be noted that there was a baseline imbalance in all three fatigue measures, with higher levels of fatigue in the lamotrigine group.
Primary outcome measure: activity limitations
The group that received lamotrigine reported fewer problems in activity limitations after four weeks of treatment compared to the control group, as measured by the physical mobility category of the Nottingham Health Profile (MD ‐23.70; 95% CI ‐35.35 to ‐12.05) (Analysis 4.1).
4.1. Analysis.
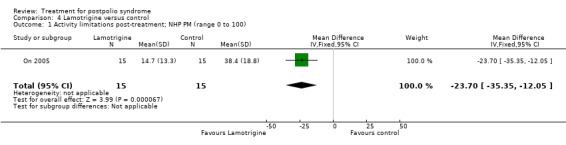
Comparison 4 Lamotrigine versus control, Outcome 1 Activity limitations post‐treatment; NHP PM (range 0 to 100).
Secondary outcome measures: muscle strength, muscle endurance, fatigue and pain
Post‐treatment fatigue (assessed with the FSS and NHP‐Energy) was lower in the group that received lamotrigine compared to the control group (FSS: MD ‐1.40; 95% CI ‐2.26 to ‐0.54) (Analysis 4.2); (NHP‐Energy: MD ‐33.30; 95% CI ‐53.13 to ‐13.47) (Analysis 4.4) despite the higher fatigue levels at baseline in the lamotrigine group. However, results of the VAS did not show a significant difference between the two groups (MD ‐1.00; 95% CI ‐3.30 to 1.30) (Analysis 4.3). Results showed less pain post‐treatment in the lamotrigine group compared to the control group (VAS: MD ‐2.80; 95% CI ‐4.36 to ‐1.24) (Analysis 4.5); (NHP‐Pain: MD ‐30.50; 95% CI ‐42.72 to ‐18.28) (Analysis 4.6). Muscle strength and endurance were not measured.
4.2. Analysis.
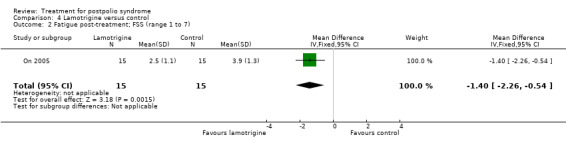
Comparison 4 Lamotrigine versus control, Outcome 2 Fatigue post‐treatment; FSS (range 1 to 7).
4.4. Analysis.
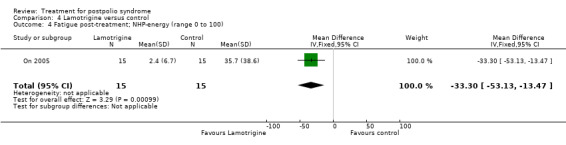
Comparison 4 Lamotrigine versus control, Outcome 4 Fatigue post‐treatment; NHP‐energy (range 0 to 100).
4.3. Analysis.
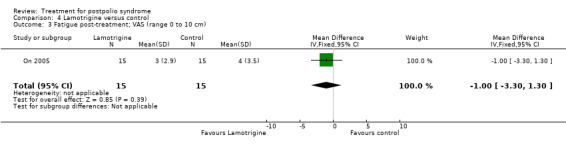
Comparison 4 Lamotrigine versus control, Outcome 3 Fatigue post‐treatment; VAS (range 0 to 10 cm).
4.5. Analysis.
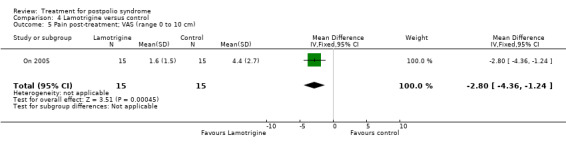
Comparison 4 Lamotrigine versus control, Outcome 5 Pain post‐treatment; VAS (range 0 to 10 cm).
4.6. Analysis.
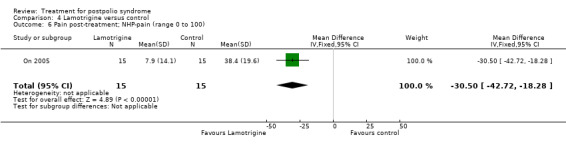
Comparison 4 Lamotrigine versus control, Outcome 6 Pain post‐treatment; NHP‐pain (range 0 to 100).
Amantadine
Primary outcome measure: activity limitations
The included trial (Stein 1995) did not measure activity limitations.
Secondary outcome measures: muscle strength, muscle endurance, fatigue and pain
Stein 1995 showed no significant differences between the amantadine group and the placebo group in number of participants improved on fatigue post‐treatment (risk ratio (RR) 2.55; 95% CI 0.81 to 7.95) (Analysis 5.1). Muscle strength, muscle endurance and pain were not measured.
5.1. Analysis.
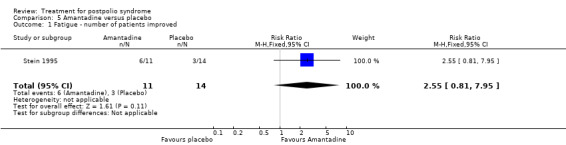
Comparison 5 Amantadine versus placebo, Outcome 1 Fatigue ‐ number of patients improved.
Prednisone
Primary outcome measure: activity limitations
The included trial (Dinsmore 1995) did not measure activity limitations.
Secondary outcome measures: muscle strength, muscle endurance, fatigue and pain
Dinsmore 1995 reported no significant difference between the prednisone group and the placebo group in number of participants improved on fatigue post‐treatment at three months of treatment (RR 1.13; 95% CI 0.75 to 1.70) (Analysis 6.1). Data on muscle strength were not adequately reported and could not be obtained from the authors because all raw data had been discarded. Muscle endurance and pain were not measured.
6.1. Analysis.
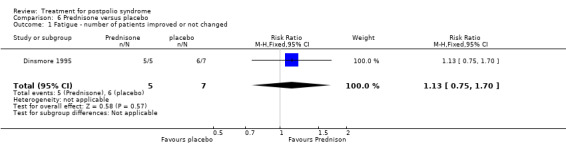
Comparison 6 Prednisone versus placebo, Outcome 1 Fatigue ‐ number of patients improved or not changed.
Muscle strengthening
Primary outcome measure: activity limitations
The included trial (Chan 2003) did not measure activity limitations.
Secondary outcome measures: muscle strength, muscle endurance, fatigue, pain and adverse events
Chan 2003 demonstrated that 12 weeks of progressive resistance training of the thenar muscles resulted in significantly more improvement in isometric muscle strength as compared to a group that received no training (MD 39.00; 95% CI 6.12 to 71.88) (Analysis 7.1). The study investigated deleterious effects of this training on motor unit survival through motor unit number estimates (MUNE). Results showed that the MUNE did not change at the end of the training. Muscle endurance, fatigue and pain were not measured.
7.1. Analysis.
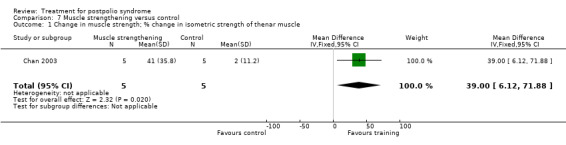
Comparison 7 Muscle strengthening versus control, Outcome 1 Change in muscle strength; % change in isometric strength of thenar muscle.
Rehabilitation in warm and cold climates
Strumse 2003 did not report change from baseline scores, therefore we used final scores in the analyses. It must be noted that there was a baseline imbalance on both measures of activity limitations between the usual care group and the group that received rehabilitation in a cold climate, with less activity limitations for the usual care group. Because outcome measurements for the usual care group were not done directly post‐treatment, we used three months' post‐treatment results in the analyses.
Primary outcome measure: activity limitations
The group that received usual care reported less activity limitations three months post‐treatment compared to the group that received rehabilitation in a cold climate (Sunnaas ADL: MD ‐2.70; 95% CI ‐4.53 to ‐0.87) (Analysis 8.1); (Rivermead Mobility Index (RMI): MD ‐1.50; 95% CI ‐2.93 to ‐0.07) (Analysis 8.2). These differences were maintained six months post‐treatment (Sunnaas ADL: MD ‐2.90; 95% CI ‐4.73 to ‐1.07) (Analysis 8.3); (RMI: MD ‐1.80; 95% CI ‐3.19 to ‐0.41) (Analysis 8.4). The baseline imbalance in favour of the usual care group probably biased these results. Rehabilitation in a warm climate did not demonstrate any significant differences in activity limitations on both scales as compared to the usual care group at three months (Sunnaas ADL: MD ‐1.70; 95% CI ‐3.47 to 0.07) (Analysis 9.1); (RMI: MD ‐0.90; 95% CI ‐2.28 to 0.48) (Analysis 9.2).
8.1. Analysis.
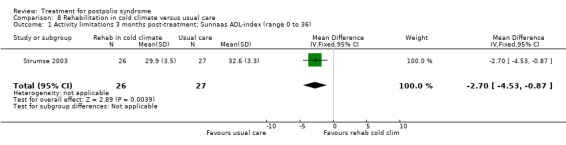
Comparison 8 Rehabilitation in cold climate versus usual care, Outcome 1 Activity limitations 3 months post‐treatment; Sunnaas ADL‐index (range 0 to 36).
8.2. Analysis.
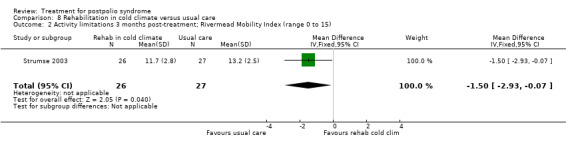
Comparison 8 Rehabilitation in cold climate versus usual care, Outcome 2 Activity limitations 3 months post‐treatment; Rivermead Mobility Index (range 0 to 15).
8.3. Analysis.
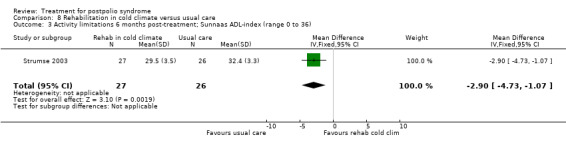
Comparison 8 Rehabilitation in cold climate versus usual care, Outcome 3 Activity limitations 6 months post‐treatment; Sunnaas ADL‐index (range 0 to 36).
8.4. Analysis.
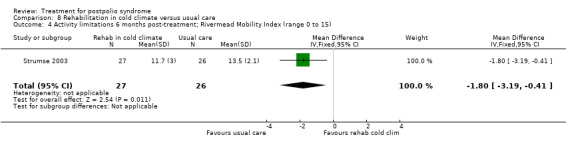
Comparison 8 Rehabilitation in cold climate versus usual care, Outcome 4 Activity limitations 6 months post‐treatment; Rivermead Mobility Index (range 0 to 15).
9.1. Analysis.
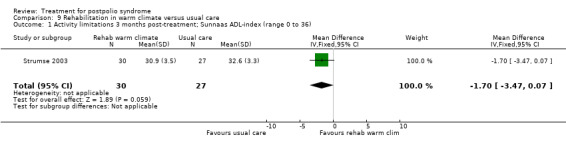
Comparison 9 Rehabilitation in warm climate versus usual care, Outcome 1 Activity limitations 3 months post‐treatment; Sunnaas ADL‐index (range 0 to 36).
9.2. Analysis.
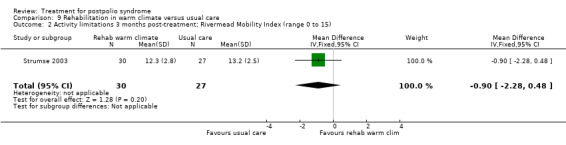
Comparison 9 Rehabilitation in warm climate versus usual care, Outcome 2 Activity limitations 3 months post‐treatment; Rivermead Mobility Index (range 0 to 15).
Secondary outcome measures: muscle strength, muscle endurance, fatigue, pain and adverse events
The study measured hand grip strength bilaterally. To reduce multiple testing we decided by consensus to randomly choose one these measures for inclusion in the analysis. The allocated outcome was hand grip strength of the right hand. Neither rehabilitation in a cold climate nor rehabilitation in a warm climate demonstrated a significant difference in grip strength of the right hand three months' post‐treatment as compared to the usual care group (MD ‐5.00; 95% CI ‐21.82 to 11.82) (Analysis 8.5); (MD 2.00; 95% CI ‐15.15 to 19.15) (Analysis 9.3). Also, both rehabilitation groups did not demonstrate any significant differences in fatigue and pain three months' post‐treatment as compared to the usual care group (FSS: MD 0.10; 95% CI ‐0.47 to 0.67) (Analysis 8.6); (VAS: MD 11.00; 95% CI ‐0.98 to 22.98) (Analysis 8.7); (FSS: MD ‐0.40; 95% CI ‐1.02 to 0.22) (Analysis 9.4); (VAS: MD ‐5.00; 95% CI ‐16.88 to 6.88) (Analysis 9.5). Muscle endurance and adverse events were not measured.
8.5. Analysis.
Comparison 8 Rehabilitation in cold climate versus usual care, Outcome 5 Muscle strength 3 months post‐treatment; Grippit Hand Grip Test, right hand (% pred).
9.3. Analysis.
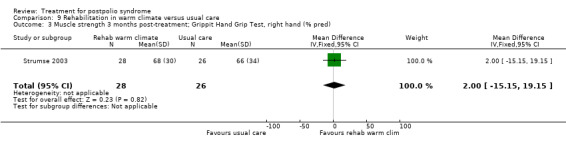
Comparison 9 Rehabilitation in warm climate versus usual care, Outcome 3 Muscle strength 3 months post‐treatment; Grippit Hand Grip Test, right hand (% pred).
8.6. Analysis.
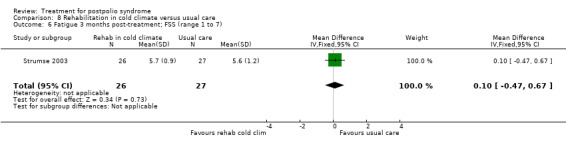
Comparison 8 Rehabilitation in cold climate versus usual care, Outcome 6 Fatigue 3 months post‐treatment; FSS (range 1 to 7).
8.7. Analysis.
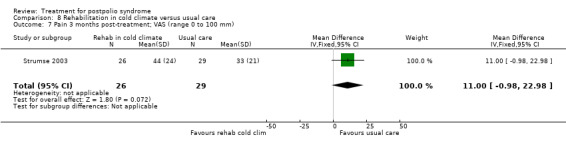
Comparison 8 Rehabilitation in cold climate versus usual care, Outcome 7 Pain 3 months post‐treatment; VAS (range 0 to 100 mm).
9.4. Analysis.
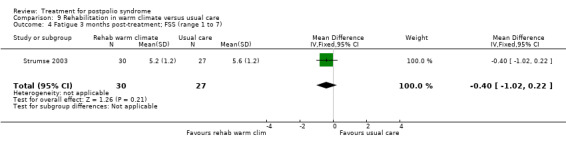
Comparison 9 Rehabilitation in warm climate versus usual care, Outcome 4 Fatigue 3 months post‐treatment; FSS (range 1 to 7).
9.5. Analysis.
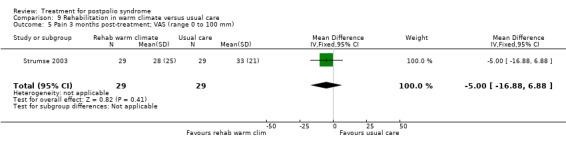
Comparison 9 Rehabilitation in warm climate versus usual care, Outcome 5 Pain 3 months post‐treatment; VAS (range 0 to 100 mm).
Static magnetic fields
Primary outcome measure: activity limitations
The included trial (Vallbona 1997) did not measure activity limitations.
Secondary outcome measures: muscle strength, muscle endurance, fatigue, pain and adverse events
Vallbona 1997 demonstrated that the application of static magnetic fields over an identified trigger point results in significantly more pain reduction immediately after application as compared to placebo (MD 4.10; 95% CI 2.75 to 5.45) (Analysis 10.1). No adverse events were reported directly after treatment. Muscle strength, muscle endurance and fatigue were not measured.
10.1. Analysis.
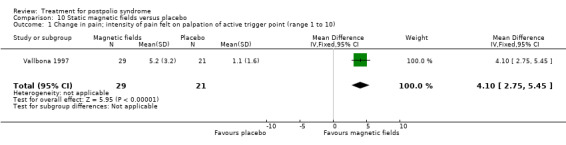
Comparison 10 Static magnetic fields versus placebo, Outcome 1 Change in pain; intensity of pain felt on palpation of active trigger point (range 1 to 10).
Discussion
Summary of main results
IVIg
Treatment with IVIg (2 infusions of 90 g or 1 infusion of 2 g/kg body weight) has no beneficial effect on activity limitations, fatigue and pain in either the short or long term (Bertolasi 2013; Farbu 2007; Gonzalez 2006). The effects on muscle strength are inconsistent; Gonzalez 2006 found a significant improvement in strength in the short term compared to placebo, in contrast to the results of the pooled data of Bertolasi 2013 and Farbu 2007, in which no difference in muscle strength between IVIg and placebo was found in the short or long term. This inconsistency might be explained by the fact that the results of Gonzalez 2006 were based on effects in symptomatic muscles, whereas the results of the other two studies were based on a pre‐selected muscle group, irrespective of being symptomatic or not. Gonzalez 2006 reported that the beneficial effect of IVIg was not demonstrable in muscles that were not selected as the (symptomatic) study muscle. As mentioned in the Results section, unfortunately we could not include these data in our analyses. Another notable finding by Gonzalez 2006 was that the degree of decline in muscle strength in the placebo group was considerably higher than in previous reports on the natural course of untreated people with PPS (Bickerstaffe 2014; Stolwijk‐Swuste 2005; Stolwijk‐Swuste 2010). This may be explained by variations in study populations or more specific variations in study muscles. The analyses of this review showed that IVIg had no effect on pain in the short term, in contrast with the conclusions of Farbu 2007. The beneficial effect on pain in Farbu 2007 was not upheld after pooling data with the non‐significant results of the larger studies of Gonzalez 2006 and Bertolasi 2013. In the original review, we had suggested that this heterogeneity in effects on pain might be explained by the finding that the participants of Farbu 2007 experienced more pain at baseline as compared to the participants of Gonzalez 2006. This explanation was supported by the positive results of IVIg on pain in a subgroup of participants from Gonzalez 2006 that reported significant pain (that is 20 mm or more out of 100 mm on the VAS). However, the results of the newly included study of Bertolasi 2013 did not corroborate these findings, as the participants of this trial reported mean baseline levels of pain comparable to those in Farbu 2007, without finding positive results of IVIg on pain. In conclusion, we found moderate‐ and low‐quality evidence that IVIg has no beneficial effect on activity limitations in the short and long term, respectively. The evidence for effectiveness of IVIg on muscle strength is inconsistent. Minor adverse events occurred at a higher rate with IVIg compared to placebo, but the number of serious adverse events was low and trial authors considered them unrelated to the IVIg intervention. More studies are needed to clarify these findings further, including the evaluation of dosing, dosing intervals and characteristics of responders. See Table 1.
Modafinil
Results of Chan 2006 and Vasconcelos 2007 showed that treatment with modafinil at a daily dose of 400 mg does not reduce activity limitations, fatigue or pain as compared to placebo and causes adverse events in a substantial proportion of those treated. From the limited but high‐quality evidence, we can conclude that there is no beneficial effect of modafinil. See Table 2.
Pyridostigmine
Pyridostigmine at a daily dose of 180 mg or 240 mg has no beneficial effects on activity limitations, muscle function, fatigue and pain and caused adverse events in a substantial proportion of the treated participants (Horemans 2003; Trojan 1999). We can conclude that there is moderate‐quality evidence of no beneficial effect for the prescription of a fixed dose of pyridostigmine of 180 mg or 240 mg. See Table 3. As it is known that daily doses up to 540 mg to 720 mg may be administered for the treatment of muscle weakness in myasthenia gravis and that plasma concentrations of this drug can vary greatly between individuals, it would be valuable to investigate the effects of individually adjusted doses of pyridostigmine for symptoms of PPS.
Lamotrigine
We found very low‐quality evidence that lamotrigine at a daily dose of 50 mg to 100 mg has a positive effect on activity limitations and pain after four weeks of treatment, without generating adverse events (On 2005). See Table 4. The beneficial effects on fatigue are inconsistent: two fatigue scales showed less fatigue in the medication group compared to the control group post‐treatment, but a third fatigue scale showed no significant difference. A major limitation of this study was the relatively short treatment period of only four weeks. Furthermore, the potential biases associated with the open‐label design of the study, which used patient‐reported outcomes, probably compromised the validity. Placebo‐controlled studies with larger sample sizes, a longer follow‐up period and adequate blinding are therefore needed to establish the efficacy of lamotrigine.
Amantadine
Six weeks of treatment with 200 mg amantadine per day does not reduce fatigue as compared to placebo and causes adverse events in a substantial proportion of the medication group (Stein 1995). The study authors stated that they found no association between serum amantadine level and clinical response. Results of this study were based on a small sample size, and there is a very serious risk of bias. We can conclude that there is very low‐quality evidence of no beneficial effect of amantadine for the treatment of fatigue in PPS. See Table 5.
Prednisone
High dose (80 mg/day for 4 weeks followed by a 20‐week tapering scheme) prednisone has no beneficial effect on fatigue (Dinsmore 1995). It is of note that both the participants treated in the prednisone group and the participants in the placebo group frequently developed (glucocorticoid‐like) adverse events, which in three cases even led to cessation of treatment. Results of this study were based on a small sample size, and there is a very serious risk of bias. We can conclude that there is very low‐quality evidence of no beneficial effect of high‐dose prednisone for the treatment of fatigue in PPS. See Table 6.
Muscle strengthening
Progressive resistance training of thumb muscles affected by polio has a beneficial effect on muscle strength (Chan 2003). To investigate whether the effects of strength training in PPS is comparable to that seen in healthy elderly, Chan 2003 also randomised and trained seven healthy elderly in a similar manner. Trial authors concluded that even though people with PPS are weaker than the healthy elderly, they are can show an improvement in their muscle strength in response to training that exceeds that of the healthy participants. Also, the study proves that training does not adversely affect motor unit survival. This study included only 10 participants, and there is a very serious risk of bias. We can therefore conclude that there is very low‐quality evidence that progressive resistance training of thumb muscles has a beneficial effect on muscle strength. See Table 7. It would be valuable to investigate whether strength training of larger muscle groups like the lower limb muscles, which are the most affected muscles in PPS, would lead to the same results. Also, effects of resistance training on activity limitations and long‐term effects need to be evaluated in further studies.
Rehabilitation in warm and cold climates
Rehabilitation treatment in a warm climate (temperature ± 25°C, dry and sunny) does not reduce activity limitations or improve muscle strength, fatigue and pain as compared to usual care (Strumse 2003). The beneficial effect of usual care on activity limitations as compared to rehabilitation treatment in a cold climate (temperature ± 0°C, rainy or snowy) is probably the result of a baseline imbalance. This assumption is supported by the finding that usual care did not have a beneficial effect on muscle strength, fatigue and pain compared to treatment in a cold climate. A more detailed description of the different components of the program and an outcome assessment for the usual care group directly post‐treatment would have provided more insight into the short‐term individual effects of both rehabilitation groups and possibly a better understanding of the results of this study. In conclusion, there is low‐quality evidence of no beneficial effect of rehabilitation treatment in warm and cold climates three months after treatment for PPS. See Table 8 and Table 9.
Static magnetic fields
We found moderate‐quality evidence that application of static magnetic fields over a pain trigger point has a beneficial effect in reducing pain directly after treatment, without generating adverse events (Vallbona 1997). See Table 10. The clinical relevancy of the immediate effect on pain is unclear since the study did not investigate sustained effects. Further studies evaluating long‐term effects on pain and effects on activity limitations are needed.
Overall completeness and applicability of evidence
We included in this review studies on 10 different interventions, both pharmacological as well as non‐pharmacological. However, we excluded a considerable number of intervention studies, mainly because these studies' designs did not meet our pre‐specified criteria. Although there appears to be a positive trend of interventions being investigated in randomised designs, we could not include a substantial number of randomised studies in this review because they did not include a control group consisting of placebo, usual care or no treatment as the comparator. Also, more than half of the included studies reported the effects of an intervention on various PPS‐related symptoms, but did not include disability (or activities) as an outcome. Some of the pharmacological studies we excluded examined the effects of bromocriptine, IGF‐I, human growth hormone, coenzyme Q10 and selegiline. Preliminary evidence from these studies indicates that these interventions are not effective or may cause serious adverse events (Bamford 1993; Bruno 1996; Miller 1997; Skough 2008), which may explain why these pharmacological interventions were never investigated in larger, properly controlled studies. Some of the non‐pharmacological studies we excluded examined the effectiveness of aerobic exercise, hydrotraining, respiratory muscle training, respiratory support, orthoses, lifestyle changes and weight control. The European Federation of Neurological Societies (EFNS) task force recommends all of these interventions to a certain degree (Farbu 2011); these recommendations are based on consensus within the task force group or on studies that could not be included in this review. Preliminary evidence from more recently investigated interventions that were excluded from this review claim positive effects of transcranial direct current stimulation on fatigue and sleep problems (Acler 2013), positive effects of stretching combined with pulsed electromagnetic field therapy on hip flexion contractures and pain (Khan 2013), a protective effect of oral bisphosphonates on fracture risk (Alvarez 2010), and positive effects of multidisciplinary or individualised goal‐oriented comprehensive interdisciplinary rehabilitation on physical, psychological and functional outcomes (Davidson 2009; Larsson Lund 2010). There is preliminary evidence that whole‐body vibration training has no effects on muscle strength and gait performance (Brogårdh 2010). Interventions that are planned or that are currently being investigated in randomised studies are different doses of IVIg (NCT02176863), home‐based exercise therapy (ISRCTN00378146; Koopman 2014; Murray 2014), cognitive behavioural therapy (Koopman 2014), far infrared bio‐ceramic components incorporated in mattress liners (Silva 2014), coenzyme Q10 (ACTRN12612000552886), microprocessor‐controlled knee‐ankle‐foot orthosis (NCT02089880), and L‐carnitine and piracetam (NCT01549847).
Quality of the evidence
Both the amount of evidence as well as the evidence quality in this review are limited. Although we included 13 trials (675 participants), for each of the 10 different interventions we evaluated, we based evidence on a maximum of 3 included studies with the number of participants varying from 10 to 203 per comparison. There are several reasons why the quality of the evidence in this review is rather low. Blinding of participants and administrators of the intervention was a prevalent risk of bias. Admittedly, blinding is cumbersome in trials on exercise therapy and on medication with substantial side effects. In addition, many of these trials used patient‐reported outcomes, which make blinded outcome assessment unfeasible.
We also noted the occurrence of a large number of negative (that is nonsignificant) results. The most reasonable explanation for this finding is that the investigated interventions actually have no effects. This might be partially caused by the fact that targeting interventions is very difficult when the exact pathogenesis of a disorder is still unclear as is the case with PPS.
However, other possible factors have been put forth, explaining the large amount of negative results in intervention studies in PPS (Dalakas 1999; Nollet 2000; Nollet 2010). Firstly, people with PPS constitute a highly heterogeneous group, which may hinder balanced randomisation in a trial, and it may be that certain interventions are only effective in subgroups of those with the condition. Secondly, the slow progression of PPS warrants long‐term follow‐up for interventions aimed at preventing deterioration in signs and symptoms. Finally, relevant outcome measures are lacking. For example, most of the questionnaires used in PPS research are generic, non‐disease‐specific measures, which may not be responsive enough to detect relevant changes.
Potential biases in the review process
As there are few experts in this field and we supplemented our search strategy with checking references, searching trial registers and contacting experts, we likely identified all relevant studies in this review.
Given that there was nearly complete consensus between the two review authors responsible for study selection, the risk of selection bias in this part of the review process is probably low.
In a considerable number of studies it was unclear whether participants met our inclusion criteria for the diagnosis of PPS. Also, many studies did not report outcomes in such a way that they could be used in our analyses. All but one trial author responded to our requests for further information about these issues, and the trial authors who responded provided most of the requested information.
Agreements and disagreements with other studies or reviews
In 2006, an expert task force appointed by the scientific committee of the EFNS evaluated the existing evidence for the effectiveness of therapeutic interventions and provided a clinical guideline for management of PPS (Farbu 2006). This guideline was updated in 2011 (Farbu 2011). There were some major differences between the methodology used in this review and the EFNS report. Firstly, the EFNS report had no restrictions on study design, including evidence obtained from RCTs, uncontrolled studies, case series, case reports and expert opinion. Secondly, in the EFNS report both within‐group and between‐group differences were taken into account depending on which differences were reported by the authors of the included studies. Thirdly, the EFNS report used a different method of grading the quality of the evidence. These differences in methodology hampered a comparison between results and conclusions of the EFNS report and results and conclusions of this review. With respect to the pharmacological studies, conclusions on the direction of the effects were the same in both the EFNS report and this review. However, for most interventions, the EFNS report had more confidence in the effect when compared to this review, as illustrated by the higher quality evidence rating. As a result of having no restrictions on study design, the EFNS report included considerably more non‐pharmacological interventions compared to this review. With respect to the effectiveness of muscle strengthening exercises, the EFNS report gave a more detailed recommendation, and again had more confidence in the effect compared to this review. Although based on evidence obtained from the same RCT, the EFNS report and this review do not agree on the direction of the effect of rehabilitation in warm climate: the EFNS report concluded that there was a positive effect of this intervention on several symptoms of PPS, while this review found no effects. This difference in conclusions can be explained by the fact that the EFNS report based their conclusions on within‐group differences, while this review considered between‐group differences.
Authors' conclusions
Implications for practice.
We found moderate‐ and low‐quality evidence that IVIg has no beneficial effect on activity limitations in the short and long term, respectively, and inconsistent evidence of the effectiveness of IVIg on muscle strength. IVIg caused minor adverse events in a substantial proportion of those who received it. Results of one trial provided very low‐quality evidence that lamotrigine might be effective in reducing pain, and fatigue and activity limitations without generating adverse events. Data from two single trials suggested that muscle strengthening of thumb muscles (very low‐quality evidence) and static magnetic fields (moderate‐quality evidence) are safe and beneficial for improving muscle strength and pain, respectively, with unknown effects on activity limitations. Finally, we found evidence varying from very low quality to high quality that modafinil, pyridostigmine, amantadine, prednisone and rehabilitation in a warm or cold climate are not beneficial in PPS. However, due to a lack of good‐quality data and randomised studies, it was impossible to draw definitive conclusions about the effectiveness of interventions in people with PPS.
Implications for research.
More studies are needed to further clarify the effects of IVIg, including the evaluation of dosing, dosing intervals and characteristics of responders. For lamotrigine, placebo‐controlled studies with larger sample sizes, a longer follow‐up period and adequate blinding are needed to establish the effect in PPS. Muscle strengthening of varying intensity and muscle groups and long‐term effects on activity limitations should be evaluated in the future. Although this review was unable to demonstrate a positive effect of rehabilitation in a warm or cold climate in PPS, future studies should evaluate the effects of individualised goal‐oriented comprehensive rehabilitation. We also recommend that future studies on the effects of climate, differences between simply being treated in and actually living in a particular climate are taken into account. It might be valuable to investigate the effect of individually adjusted doses of pyridostigmine and the long‐term effects of static magnetic fields on pain and activity limitations. Finally, other possible treatments not evaluated in this review such as orthoses, cognitive behavioural interventions and aerobic exercise should be tested in RCTs, and monitoring and reporting of adverse effects of both pharmacological and non‐pharmacological interventions should be systematically addressed.
What's new
| Date | Event | Description |
|---|---|---|
| 7 October 2014 | New citation required and conclusions have changed | One new trial included in this update and conclusions have slightly changed. Kimi Uegaki withdrew from authorship |
| 31 July 2014 | New search has been performed | Searches updated to July 2014. |
History
Protocol first published: Issue 2, 2009 Review first published: Issue 2, 2011
| Date | Event | Description |
|---|---|---|
| 10 March 2011 | Amended | Adjustment to forest plot scales |
| 8 February 2011 | Amended | Correction to reference |
Acknowledgements
The review authors gratefully acknowledge the assistance of the Cochrane Neuromuscular Disease Group. We also thank Dr Kimi Uegaki (KU) who co‐authored the original review.
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to the Cochrane Neuromuscular Disease Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health. The Cochrane Neuromuscular Disease Group also receives support from the Motor Neurone Disease Association and the MRC Centre for Neuromuscular Disease.
Appendices
Appendix 1. CENTRAL search strategy
1 Postpoliomyelitis Syndrome (MeSH) 2 post next polio* 3 (late NEAR/3 polio*) OR (late next effect* NEAR/3 polio*) OR (late next onset NEAR/3 polio*) OR (lateonset NEAR/3 polio*) 4 polio* NEAR/3 survivor* 5 prior next polio*
6 (#1 OR #2 OR #3 OR #4 OR #5)
Appendix 2. MEDLINE (OvidSP) search strategy
Database: Ovid MEDLINE(R) <1946 to July Week 2 2014> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial.pt. (378583) 2 controlled clinical trial.pt. (88802) 3 randomized.ab. (276556) 4 placebo.ab. (147643) 5 drug therapy.fs. (1717215) 6 randomly.ab. (195852) 7 trial.ab. (286811) 8 groups.ab. (1257501) 9 or/1‐8 (3225927) 10 exp animals/ not humans.sh. (3968663) 11 9 not 10 (2745937) 12 Postpoliomyelitis Syndrome/ (731) 13 (post?polio* or post polio$).mp. (1075) 14 ((late adj3 polio$) or (late effect$ adj3 polio$) or (late?onset adj3 polio$) or (late onset adj3 polio$)).mp. (179) 15 (polio$ adj3 survivor$).mp. (188) 16 (prior?polio$ or prior polio$).mp. (55) 17 or/12‐16 (1233) 18 11 and 17 (210) 19 remove duplicates from 18 (199)
Appendix 3. EMBASE (OvidSP) search strategy
Database: Embase <1980 to 2014 Week 29> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 crossover‐procedure.sh. (39531) 2 double‐blind procedure.sh. (114388) 3 single‐blind procedure.sh. (18551) 4 randomized controlled trial.sh. (345939) 5 (random$ or crossover$ or cross over$ or placebo$ or (doubl$ adj blind$) or allocat$).tw,ot. (1043737) 6 trial.ti. (159871) 7 clinical trial/ (832647) 8 or/1‐7 (1631583) 9 (animal/ or nonhuman/ or animal experiment/) and human/ (1273903) 10 animal/ or nonanimal/ or animal experiment/ (3231185) 11 10 not 9 (2707772) 12 8 not 11 (1532844) 13 limit 12 to embase (1265402) 14 Postpoliomyelitis Syndrome/ (865) 15 (post?polio* or post polio$).mp. (1350) 16 ((late adj3 polio$) or (late effect$ adj3 polio$) or (late?onset adj3 polio$) or (late onset adj3 polio$)).mp. (197) 17 (polio$ adj3 survivor$).mp. (230) 18 (prior?polio$ or prior polio$).mp. (60) 19 or/14‐18 (1531) 20 13 and 19 (105) 21 remove duplicates from 20 (105)
Appendix 4. PsycINFO (OvidSP) search strategy
Database: PsycINFO <1806 to July Week 3 2014> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 (post?polio* or post polio$).mp. (119) 2 ((late adj3 polio$) or (late effect$ adj3 polio$) or (late?onset adj3 polio$) or (late onset adj3 polio$)).mp. (18) 3 (polio$ adj3 survivor$).mp. (31) 4 (prior?polio$ or prior polio$).mp. (4) 5 poliomyelitis/ and syndromes/ (30) 6 1 or 2 or 3 or 4 or 5 (141) 7 remove duplicates from 6 (141)
Appendix 5. CINAHL (EBSCOhost) search strategy
Monday, July 21, 2014 10:04:29 AM S27 S25 AND S26 14 S26 EM 20120914‐ 634,258 S25 S18 and S24 165 S24 S19 or S20 or S21 or S22 or S23 738 S23 (prior polio*) or (prior?polio*) 23 S22 (polio* W3 survivor*) 202 S21 (late W3 polio*) or (late effect* W3 polio*) or (late onset W3 polio*) 77 S20 (post polio*) or (post?polio*) or (postpolio*) 642 S19 (MH "Postpoliomyelitis Syndrome") or (MH "Polio Survivors") 570 S18 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 688,041 S17 ABAB design* 85 S16 TI random* or AB random* 138,577 S15 ( TI (cross?over or placebo* or control* or factorial or sham? or dummy) ) or ( AB (cross?over or placebo* or control* or factorial or sham? or dummy) ) 278,861 S14 ( TI (clin* or intervention* or compar* or experiment* or preventive or therapeutic) or AB (clin* or intervention* or compar* or experiment* or preventive or therapeutic) ) and ( TI (trial*) or AB (trial*) ) 97,006 S13 ( TI (meta?analys* or systematic review*) ) or ( AB (meta?analys* or systematic review*) ) 31,731 S12 ( TI (single* or doubl* or tripl* or trebl*) or AB (single* or doubl* or tripl* or trebl*) ) and ( TI (blind* or mask*) or AB (blind* or mask*) ) 21,830 S11 PT ("clinical trial" or "systematic review") 119,831 S10 (MH "Factorial Design") 920 S9 (MH "Concurrent Prospective Studies") or (MH "Prospective Studies") 238,160 S8 (MH "Meta Analysis") 19,817 S7 (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") 41 S6 (MH "Quasi‐Experimental Studies") 6,799 S5 (MH "Placebos") 8,823 S4 (MH "Double‐Blind Studies") or (MH "Triple‐Blind Studies") 29,591 S3 (MH "Clinical Trials+") 177,179 S2 (MH "Crossover Design") 11,921 S1 (MH "Random Assignment") or (MH "Random Sample") or (MH "Simple Random Sample") or (MH "Stratified Random Sample") or (MH "Systematic Random Sample") 66,325
Appendix 6. Cochrane Neuromuscular Disease Group Specialized Register (CRS) search strategy
#1 post?polio* or "post polio" or "post poliomyelitis" or "post‐polio" or "post‐poliomyelitis" [REFERENCE] [STANDARD] #2 late near5 polio* [REFERENCE] [STANDARD] #3 late near2 effect* near5 polio* [REFERENCE] [STANDARD] #4 late?onset NEAR3 polio* [REFERENCE] [STANDARD] #5 "late onset" NEAR3 polio* [REFERENCE] [STANDARD] #6 polio* NEAR3 survivor* [REFERENCE] [STANDARD] #7 prior?polio* [REFERENCE] [STANDARD] #8 "prior polio*" [REFERENCE] [STANDARD] #9 prior NEXT polio* [REFERENCE] [STANDARD] #10 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 [REFERENCE] [STANDARD] #11 (#10) AND (INREGISTER) [REFERENCE] [STANDARD]
Appendix 7. Trials registers searches
poliomyelitis
postpolio syndrome
Data and analyses
Comparison 1. IVIg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activity limitations short‐term; SF‐36 PCS (range 0 to 100) | 2 | 185 | Mean Difference (IV, Fixed, 95% CI) | 2.35 [‐0.06, 4.76] |
| 1.1 Change in activity limitations | 1 | 135 | Mean Difference (IV, Fixed, 95% CI) | 2.3 [‐0.35, 4.95] |
| 1.2 Activity limitations post‐treatment | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [‐3.20, 8.40] |
| 2 Activity limitations post‐treatment long‐term; SF‐36 PCS (range 0 to 100) | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐4.63, 3.60] |
| 3 Change in muscle strength short‐term; % change in isometric strength of polio affected muscle | 1 | 135 | Mean Difference (IV, Fixed, 95% CI) | 8.6 [2.81, 14.39] |
| 4 Muscle strength post‐treatment short‐term; isometric strength right knee extensors (Nm) | 2 | 70 | Mean Difference (IV, Random, 95% CI) | ‐11.01 [‐53.86, 31.84] |
| 5 Muscle strength post‐treatment long‐term; isometric strength right knee extensor (Nm) | 2 | 70 | Mean Difference (IV, Random, 95% CI) | ‐10.29 [‐55.37, 34.78] |
| 6 Change in fatigue short‐term; MFI general fatigue (range 4 to 20) | 1 | 130 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.05, 1.05] |
| 7 Fatigue post‐treatment short‐term; FSS (range 1 to 7) | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.71, 0.87] |
| 8 Fatigue post‐treatment long‐term; FSS (range 1 to 7) | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.15, 0.15] |
| 9 Pain short‐term; VAS (range 0 to 100 mm) | 3 | 203 | Mean Difference (IV, Random, 95% CI) | ‐9.27 [‐25.11, 6.57] |
| 9.1 Change in pain | 1 | 133 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐6.60, 3.60] |
| 9.2 Pain post‐treatment | 2 | 70 | Mean Difference (IV, Random, 95% CI) | ‐15.44 [‐46.78, 15.90] |
| 10 Pain post‐treatment short‐term; PDI (number of marked areas) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐6.70 [‐23.63, 10.23] |
| 11 Pain post‐treatment short‐term; 101NRS (range 0 to 100) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐3.00 [‐16.30, 10.30] |
| 12 Pain post‐treatment long‐term; VAS (range 0 to 100 mm) | 3 | 111 | Mean Difference (IV, Fixed, 95% CI) | ‐5.61 [‐14.95, 3.73] |
| 13 Pain post‐treatment long‐term; PDI (number of marked areas) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐5.5 [‐23.39, 12.39] |
| 14 Pain post‐treatment long‐term; 101NRS (range 0 to 100) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐13.03, 13.03] |
Comparison 2. Modafinil versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Difference (modafinil ‐ placebo) in activity limitations; SF‐36 PF (range 0 to 100) | 1 | Mean Difference (Fixed, 95% CI) | 1.28 [‐3.56, 6.12] | |
| 2 Difference (modafinil ‐ placebo) in fatigue; PFS (scores normalised to that at baseline, %) | 1 | Mean Difference (Fixed, 95% CI) | 12.0 [4.16, 19.84] | |
| 3 Difference (modafinil ‐ placebo) in fatigue; FSS (range 1 to 7) | 1 | Mean Difference (Fixed, 95% CI) | 0.39 [‐0.24, 1.02] | |
| 4 Difference (modafinil ‐ placebo) in fatigue; VASF (0 to 10 cm) | 1 | Mean Difference (Fixed, 95% CI) | ‐0.01 [‐0.93, 0.91] | |
| 5 Difference (modafinil ‐ placebo) in fatigue; FIS (range 0 to 160) | 1 | Mean Difference (Fixed, 95% CI) | ‐3.32 [‐15.22, 8.58] | |
| 6 Difference (modafinil ‐ placebo) in pain; SF‐36 BP (range 0 to 100) | 1 | Mean Difference (Fixed, 95% CI) | 1.21 [‐7.77, 10.19] |
Comparison 3. Pyridostigmine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in activity limitations; SF‐36 PF (range 0 to 100) | 1 | 124 | Mean Difference (IV, Fixed, 95% CI) | 2.1 [‐3.64, 7.84] |
| 2 Change in muscle strength; very weak muscles, % change in isometric strength | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | 33.9 [‐5.49, 73.29] |
| 3 Change in muscle strength; weak muscles, % change in isometric strength | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐11.75, 8.15] |
| 4 Change in muscle strength; relatively strong muscles, % change in isometric strength | 1 | 117 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐4.22, 3.62] |
| 5 Change in muscle strength; isometric muscle strength quadriceps (Nm) | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 6.70 [‐2.19, 15.59] |
| 6 Change in muscle endurance; isometric muscle fatigability quadriceps (MF0‐5s‐ MF25‐30s) | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | ‐0.7 [‐2.52, 1.12] |
| 7 Change in fatigue; FSS (range 1 to 7) | 2 | 186 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.34, 0.21] |
| 8 Change in fatigue; HFSS (range 0 to 4) | 1 | 115 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.17, 0.31] |
| 9 Change in fatigue; NHP‐energy (range 0 to 100) | 1 | 62 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐16.24, 18.44] |
| 10 Change in pain; SF‐36 BP (range 0 to 100) | 1 | 124 | Mean Difference (IV, Fixed, 95% CI) | ‐2.1 [‐9.16, 4.96] |
Comparison 4. Lamotrigine versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activity limitations post‐treatment; NHP PM (range 0 to 100) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐23.7 [‐35.35, ‐12.05] |
| 2 Fatigue post‐treatment; FSS (range 1 to 7) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.4 [‐2.26, ‐0.54] |
| 3 Fatigue post‐treatment; VAS (range 0 to 10 cm) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐3.30, 1.30] |
| 4 Fatigue post‐treatment; NHP‐energy (range 0 to 100) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐33.30 [‐53.13, ‐13.47] |
| 5 Pain post‐treatment; VAS (range 0 to 10 cm) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐2.80 [‐4.36, ‐1.24] |
| 6 Pain post‐treatment; NHP‐pain (range 0 to 100) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐30.50 [‐42.72, ‐18.28] |
Comparison 5. Amantadine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fatigue ‐ number of patients improved | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [0.81, 7.95] |
Comparison 6. Prednisone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fatigue ‐ number of patients improved or not changed | 1 | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.75, 1.70] |
Comparison 7. Muscle strengthening versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in muscle strength; % change in isometric strength of thenar muscle | 1 | 10 | Mean Difference (IV, Fixed, 95% CI) | 39.0 [6.12, 71.88] |
Comparison 8. Rehabilitation in cold climate versus usual care.
Comparison 9. Rehabilitation in warm climate versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Activity limitations 3 months post‐treatment; Sunnaas ADL‐index (range 0 to 36) | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐3.47, 0.07] |
| 2 Activity limitations 3 months post‐treatment; Rivermead Mobility Index (range 0 to 15) | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐2.28, 0.48] |
| 3 Muscle strength 3 months post‐treatment; Grippit Hand Grip Test, right hand (% pred) | 1 | 54 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐15.15, 19.15] |
| 4 Fatigue 3 months post‐treatment; FSS (range 1 to 7) | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.02, 0.22] |
| 5 Pain 3 months post‐treatment; VAS (range 0 to 100 mm) | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐16.88, 6.88] |
Comparison 10. Static magnetic fields versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in pain; intensity of pain felt on palpation of active trigger point (range 1 to 10) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 4.1 [2.75, 5.45] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bertolasi 2013.
| Methods | Double‐blind, placebo‐controlled RCT | |
| Participants | N = 50 (IVIg 24, placebo 26) Mean age: 54.9 years (IVIg), 58.3 years (placebo) Gender distribution, male: 50% (IVIg), 50% (placebo) Inclusion: diagnosis of PPS according to the Halstead criteria (Halstead 1991) including clinical electrophysiological evaluation, age between 18 and 70 years. Exclusion: systemic or malignant disease, previous allergic reaction to IVIg, immune‐modulating treatments other than IVIg within the last 6 months and conditions associated with prolonged coagulation time, hypothyroidism, diabetes (not fully controlled), or medical or orthopedic disorders that could give rise to symptoms mimicking PPS, obesity‐related comorbidities, a BMI greater than 30 or unstable weight, serum IgA deficiency, and increased central conduction times on somatosensory or motor evoked potentials |
|
| Interventions | Treatment intervention: 1 infusion of IVIg with a dose of 0.4 g/kg body weight/day infused over 5 consecutive days Control intervention: placebo |
|
| Outcomes | Measurements at baseline, 2 and 4 months Primary: HRQoL (SF‐36 PCS) Secondary: HRQoL (SF‐36 MCS, SF‐36 individual domain scores), isometric muscle strength of elbow flexors and knee extensors, 6‐MWT, pain (VAS and 101NRS), fatigue (FSS) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Low risk | "The hospital pharmacy ensured and kept the blinding scheme" and "patients and the study personnel, including outcome assessors, were blinded throughout the study" |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | "Patients and the study personnel, including outcome assessors, were blinded throughout the study" Comment: 1 participant treated with IVIg had a transient rash 3 days after infusion and the code was kept closed, therefore it was unlikely that this led to unblinding |
| Blinding (performance bias and detection bias) All outcomes ‐ administrators of the intervention? | Low risk | "Patients and the study personnel, including outcome assessors, were blinded throughout the study" Comment: 1 participant treated with IVIg had a transient rash 3 days after infusion and the code was kept closed, therefore it was unlikely that this led to unblinding |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | "Patients and the study personnel, including outcome assessors, were blinded throughout the study" Comment: Self reported outcomes were used, and there was a low risk that blinding of participants was broken |
| Incomplete outcome data (attrition bias) Missing outcome data? | Low risk | No missing outcome data |
| Incomplete outcome data (attrition bias) ITT‐analyses performed? | Low risk | "Data for all randomised patients were included to calculate the primary end‐point according to an ITT‐analysis" |
| Selective reporting (reporting bias) | Low risk | Study protocol available in trial register (NCT01537575); pre‐specified primary outcome has been reported |
| Other bias | High risk | Large baseline imbalance in peak isometric muscle strength of right knee extensors |
Chan 2003.
| Methods | RCT | |
| Participants | N = 10 (strength training 5, no training 5) Mean age: 65 years (strength training), 65 years (no training) Gender distribution, male: 20% (strength training), 0% (no training) Inclusion: unequivocal history of prior poliomyelitis in an otherwise healthy subject, 1 or both upper limbs affected by polio, further strength decline after stable period, moderate motor neuronal loss in the median‐innervated thenar muscles (MUNE between 10 and 90) |
|
| Interventions | Treatment intervention: supervised progressive resistance training consisting of 3 sets of 8 isometric contractions of the thumb muscles, 3 times weekly for 12 weeks. Training load 50% to 70% MVC. Control intervention: no training |
|
| Outcomes | Measurements at baseline, 4, 8 and 12 weeks Outcomes: muscle function of thumb muscles: isometric strength, voluntary activation, MUNE, tetanic tension |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was done using the random number generation function in a commercially available software program." |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Not possible |
| Blinding (performance bias and detection bias) All outcomes ‐ administrators of the intervention? | High risk | Not possible |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Unclear risk | No information |
| Incomplete outcome data (attrition bias) Missing outcome data? | Unclear risk | Insufficient reporting |
| Incomplete outcome data (attrition bias) ITT‐analyses performed? | Unclear risk | Insufficient reporting |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available |
| Other bias | Low risk | |
Chan 2006.
| Methods | Double‐blind, placebo‐controlled, cross‐over RCT | |
| Participants | N = 14 (phase 1: modafinil 7, placebo 7; phase 2: modafinil 7, placebo 7) Mean age: 57.7 years Gender distribution, male: 36% Inclusion: unequivocal history of polio, new neuromuscular symptoms after stable period, moderate to severe fatigue Exclusion: presence of any medical condition or medication that could influence level of fatigue |
|
| Interventions | Treatment intervention: a 5‐week course of modafinil of maximal 200 mg 2 times per day. From day 14, participants were given the option of adjusting their daily dosage between 200 mg and 400 mg based on how they felt Control intervention: placebo Wash‐out interval: 1 week |
|
| Outcomes | Measurements at baseline, and at weekly intervals throughout the study Primary: fatigue (Piper Fatigue Scale) Secondary: daytime sleepiness (Epworth Sleepiness Scale), short‐term memory (forward and backward aural digit span test), reaction time |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient reporting |
| Allocation concealment (selection bias) | Low risk | "The randomisation code was generated by Draxis Pharmaceuticals, which was not otherwise directly involved in the study. Neither the subjects nor the investigators had access to the sealed codes." |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | "Subjects were randomised in a double blind manner" Comment: Although there were more side effects experienced during modafinil treatment, analysis on effectiveness of blinding provided evidence for successful blinding (57% correct guessing) |
| Blinding (performance bias and detection bias) All outcomes ‐ administrators of the intervention? | Low risk | "Subjects were randomised in a double blind manner" and "neither the subjects nor the investigators had access to the sealed codes" |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | "Subjects were randomised in a double blind manner" and "neither the subjects nor the investigators had access to the sealed codes" |
| Incomplete outcome data (attrition bias) Missing outcome data? | Unclear risk | Insufficient reporting; Although all 14 participants completed the trial, it was unclear whether they all completed the outcome measurements |
| Incomplete outcome data (attrition bias) ITT‐analyses performed? | Unclear risk | Insufficient reporting |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available |
| Other bias | Low risk | Because PPS is considered a reasonably stable chronic condition and modafinil is a medicament with a temporary effect, we considered the use of a cross‐over design appropriate |
Dinsmore 1995.
| Methods | Double‐blind, placebo‐controlled RCT | |
| Participants | N = 17 (high‐dose prednisone 9, placebo 8) Mean age: 50.2 years (high‐dose prednisone), 47.8 years (placebo) Gender distribution, male: 56% (high‐dose prednisone), 38% (placebo) Inclusion: history of acute paralytic poliomyelitis, followed by 10 to 20 years of stable neuromuscular function, followed by new muscle weakness unrelated to other cause Exclusion: contraindications to receive steroids, medical diseases causing fatigue, major depression, older than 60 years |
|
| Interventions | Treatment intervention: 4 weeks of prednisone 80 mg once daily followed by a 20‐week dose reduction schedule. From week 25 discontinuation Control intervention: placebo |
|
| Outcomes | Measurements at baseline, 3 months (primary) and 6 months Primary: muscle strength (Tufts Quantitative Neuromuscular Exam) Secondary: muscle strength (MMT), fatigue (4‐point scale) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Low risk | "NIH Pharmacy performed the randomisation and maintained blinding to treatment assignment" |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | "The patients were blinded to treatment assignment" Comment: Side effects were experienced in both groups, therefore it was unlikely that this led to unblinding |
| Blinding (performance bias and detection bias) All outcomes ‐ administrators of the intervention? | Low risk | "Treating physicians were blinded to treatment assignment" Comment: Side effects were experienced in both groups, therefore it was unlikely that this led to unblinding |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | "Staff performing muscle strength evaluations was blinded to treatment assignment" |
| Incomplete outcome data (attrition bias) Missing outcome data? | High risk | Missing outcomes: high‐dose prednisone 2/9, placebo 1/8 Comment: Reasons for missing outcome data were likely related to true outcome. |
| Incomplete outcome data (attrition bias) ITT‐analyses performed? | Unclear risk | Insufficient reporting |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available |
| Other bias | Low risk | |
Farbu 2007.
| Methods | Double‐blind, placebo‐controlled RCT | |
| Participants | N = 20 (IVIg 10, placebo 10) Mean age: 59.9 years (IVIg), 58.7 years (placebo) Gender distribution, male: 40% (IVIg), 30% (placebo) Inclusion: diagnosis of PPS according to the criteria of Halstead of 1991 (Halstead 1991) Exclusion: wheelchair dependence, cardiac disease, diabetes mellitus, renal insufficiency, warfarin treatment, previous thromboembolic episode, increased thrombotic risk, previous IVIg treatment, IgA deficit, other ongoing autoimmune disease |
|
| Interventions | Treatment intervention: 1 infusion of IVIg with a dose of 2 g/kg body weight Control intervention: placebo |
|
| Outcomes | Measurements at baseline, 1 month, 3 months (primary) and 6 months Primary: pain (VAS, pain drawing instrument), fatigue (FSS), isometric muscle strength of elbow flexors and knee extensors Secondary: cerebrospinal fluid cytokine levels |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The hospital pharmacy prepared a randomisation scheme with 20 notes marked with either IVIg or placebo. As the patients were enrolled prospectively, one note was drawn for each patient." |
| Allocation concealment (selection bias) | Low risk | "The blinding scheme was kept by the pharmacy and was not broken during the trial." |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | "Patients were blinded throughout the study." Comment: It was likely that blinding was broken due to side effects of the treatment |
| Blinding (performance bias and detection bias) All outcomes ‐ administrators of the intervention? | High risk | "Study personnel was blinded throughout the study." Comment: It was likely that blinding was broken due to side effects of the treatment |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | "Study personnel was blinded throughout the study." Comment: Self reported outcomes were used and blinding of participants could have been broken |
| Incomplete outcome data (attrition bias) Missing outcome data? | Low risk | No missing outcome data |
| Incomplete outcome data (attrition bias) ITT‐analyses performed? | Low risk | ITT analyses were probably done since all participants received the intervention to which they were randomised |
| Selective reporting (reporting bias) | Low risk | Study protocol available in trial register (NCT00231439); pre‐specified outcomes have been reported |
| Other bias | Low risk | |
Gonzalez 2006.
| Methods | Double‐blind, placebo‐controlled RCT | |
| Participants | N = 142 (IVIg 73, placebo 69) Mean age: 61.5 years (IVIg), 59.0 years (placebo) Gender distribution, male: 29% (IVIg), 42% (placebo) Inclusion: diagnosis of PPS according to the criteria of Halstead of 1987 (Halstead 1987) with increased muscle weakness, muscle fatigue and pain in muscle groups previously affected by the poliomyelitis, age between 18 and 75 years Exclusion: obesity or unstable weight, other disorders explaining PPS symptoms, S‐IgA deficiency 1‐year follow‐up study: N = 41 (IVIg 20, placebo 21) Mean age: 61.7 years (IVIg), 61.9 years (placebo) Gender distribution, male: 30% (IVIg), 43% (placebo) |
|
| Interventions | Treatment intervention: infusion of 90 g in total of IVIg during 3 consecutive days, repeated after 3 months Control intervention: placebo |
|
| Outcomes | Measurements at baseline and 3 months after the second infusion Primary: muscle strength in a selected study muscle, HRQoL (SF‐36 PCS) Secondary: vitality (SF‐36 vitality), 6‐MWT, TUG, muscle strength in muscles not chosen as the study muscle, physical activity (PASE), pain (VAS), fatigue (MFI‐20), balance, sleep quality 1‐year follow‐up study: Measurements at baseline and at 1 year (i.e. 9 months after the second infusion) HRQoL (SF‐36), 6‐MWT, pain (VAS) |
|
| Notes | The follow‐up study is an extension of the Gonzalez 2006 study. This follow‐up study consisted of a cohort of 41 participants from 1 of the 4 participating centres of the original study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer generated list with permuted blocks of randomly varying size (2,4,6) allocated consecutive patient numbers to treatment group" |
| Allocation concealment (selection bias) | Low risk | "Randomisation was done by an independent contract research organisation" |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | "Patients were unaware of treatment allocation throughout the study." Comment: It was likely that blinding was broken due to side effects of the treatment |
| Blinding (performance bias and detection bias) All outcomes ‐ administrators of the intervention? | High risk | "Physicians and nurses were unaware of treatment allocation throughout the study." Comment: It was likely that blinding was broken due to side effects of the treatment |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | "Physiotherapists were unaware of treatment allocation throughout the study." Comment: Self reported outcomes were used and blinding of participants could have been broken |
| Incomplete outcome data (attrition bias) Missing outcome data? | High risk | 1/143 received no medication; reason unclear Missing outcomes: IVIg 6/73, placebo 1/69 Comment: Reason for missing outcome data was likely related to true outcome |
| Incomplete outcome data (attrition bias) ITT‐analyses performed? | Low risk | ITT analyses with the last results carried forward did not differ from the per‐protocol analysis |
| Selective reporting (reporting bias) | Low risk | Study protocol available in trial register (NCT00160082); pre‐specified outcomes have been reported |
| Other bias | High risk | Baseline imbalance in gender and for the follow‐up study a baseline imbalance in SF‐36 scores |
Horemans 2003.
| Methods | Double‐blind, placebo‐controlled RCT | |
| Participants | N = 67 (pyridostigmine 34, placebo 33) Mean age: 51 years (pyridostigmine), 52 years (placebo) Gender distribution, male: 30% (pyridostigmine), 39% (placebo) Inclusion: symptoms of PPS muscle dysfunction in at least 1 quadriceps according to the criteria of Borg (Borg 1996), neuromuscular transmission defects and minimum strength of 30 Nm in the symptomatic quadriceps, fatigue, age between 18 and 70 years Exclusion: significant neurological, orthopaedic, cardiovascular, pulmonary or endocrine disorders |
|
| Interventions | Treatment intervention: a 14‐week course of pyridostigmine 60 mg 4 times per day Control intervention: placebo |
|
| Outcomes | Measurements at baseline, 5 and 14 weeks (primary) and 3 weeks after cessation of treatment Primary: fatigue (NHP‐Energy) Secondary: fatigue (FSS), 2‐MWT, 75‐meters walk test, daily physical activity (activity monitor), muscle function of quadriceps: isometric strength, voluntary activation, fatigability, transmission defects |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | "Treatment allocations were concealed for the patients" Comment: Extra effort was taken to improve blinding (e.g. placebo atropine), and analysis on effectiveness of blinding provided evidence for successful blinding |
| Blinding (performance bias and detection bias) All outcomes ‐ administrators of the intervention? | Low risk | "Treatment allocations were concealed for the researchers" |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | "Treatment allocations were concealed for the researchers" and "The data analyst remained blinded until after the primary outcome analyses." |
| Incomplete outcome data (attrition bias) Missing outcome data? | High risk | At 14 weeks: Missing outcomes: pyridostigmine 3/34, placebo 2/33 Comment: Reason for missing outcome data was likely related to true outcome |
| Incomplete outcome data (attrition bias) ITT‐analyses performed? | Low risk | "Analyses were based on an ITT approach" |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available |
| Other bias | Low risk | |
On 2005.
| Methods | RCT | |
| Participants | N = 30 (lamotrigine + usual care 15, usual care 15) Mean age: 36.6 years (lamotrigine + usual care), 35.9 years (usual care) Inclusion: diagnosis of PPS according to the criteria of Halstead of 1985 (Halstead 1985), lower extremity involvement Exclusion: non‐ambulatory or wheelchair‐dependent patients, medical illnesses that could be contributing to any secondary deterioration in muscle performance |
|
| Interventions | Treatment intervention: a 4‐week course of lamotrigine of 50 to 100 mg per day + usual care (i.e. advice on pacing, energy conservation, use of orthotic devices and weight loss and recommendation to start a home exercise program) Control intervention: usual care (as described under treatment intervention) |
|
| Outcomes | Measurements at baseline, 2 and 4 weeks Outcomes: pain (VAS), fatigue (VAS, FSS), muscle cramps (VAS), HRQoL (NHP‐6 dimensions) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | No blinding |
| Blinding (performance bias and detection bias) All outcomes ‐ administrators of the intervention? | High risk | No blinding |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Comment: Self reported outcomes were used and participants were not blinded |
| Incomplete outcome data (attrition bias) Missing outcome data? | Unclear risk | Insufficient reporting |
| Incomplete outcome data (attrition bias) ITT‐analyses performed? | Unclear risk | Insufficient reporting |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available |
| Other bias | High risk | Baseline imbalance in fatigue severity |
Stein 1995.
| Methods | Double‐blind, placebo‐controlled RCT | |
| Participants | N = 25 (amantadine 11, placebo 14) Mean age: range total sample 34 to 59 years Gender distribution, male: total sample 76% Inclusion: diagnosis of PPS according to the criteria of Dalakas (Dalakas 1995), prominent fatigue (FSS score > 3) Exclusion: medical conditions or medication that may cause fatigue |
|
| Interventions | Treatment intervention: a 6‐week course of amantadine of 100 mg 2 times per day Control intervention: placebo |
|
| Outcomes | Measurements at baseline, post‐treatment Outcomes: fatigue (VAS, FSS), overall effectiveness, neuropsychological tests |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | "double‐blind study" Comment: It was not explicitly stated who was blinded, and it is likely that blinding was broken due to side effects of the treatment |
| Blinding (performance bias and detection bias) All outcomes ‐ administrators of the intervention? | High risk | "double‐blind study" Comment: It was not explicitly stated who was blinded, and it is likely that blinding was broken due to side effects of the treatment |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | "double‐blind study" Comment: It was not explicitly stated who was blinded and blinding of patients could have been broken. Self reported outcomes are used |
| Incomplete outcome data (attrition bias) Missing outcome data? | Unclear risk | Insufficient reporting |
| Incomplete outcome data (attrition bias) ITT‐analyses performed? | Unclear risk | Insufficient reporting |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available |
| Other bias | Low risk | |
Strumse 2003.
| Methods | RCT | |
| Participants | N = 88 (warm‐climate rehabilitation 30, cold‐climate rehabilitation 29, usual care 29) Mean age: 57.3 years (warm‐climate rehabilitation), 57.4 years (cold‐climate rehabilitation), 58.6 years (usual care) Gender distribution, male: 27% (warm‐climate rehabilitation), 31% (cold‐climate rehabilitation), 34% (usual care) Inclusion: diagnosis of PPS according to the criteria of Halstead of 1987 (Halstead 1987) Exclusion: other medical conditions that could influence the rehabilitation programme |
|
| Interventions | Treatment intervention 1 (warm‐climate rehabilitation): outdoor treatment in a rehabilitation centre in Tenerife (dry, sunny, temperature around 25°C) consisting of a combination of individual and group therapy with daily treatment in a swimming pool (45 min), physiotherapy, individually adapted training program for 4 weeks Treatment intervention 2 (cold‐climate rehabilitation): indoor treatment as described above in a rehabilitation centre in Norway (rainy or snowy, temperature around 0°C) Control intervention: usual care in a cold climate as described under treatment intervention 2 |
|
| Outcomes | Measurements at baseline, post‐treatment (only interventions 1 and 2), 3 and 6 months following intervention Outcomes: pain (VAS), fatigue (FSS), health‐related problems (Ursin Holger Inventorium), depression (BDI), life satisfaction (Life Satisfaction Scale), ADL (Sunnaas ADL‐index), mobility (RMI), lung function (spirometry), handgrip strength, endurance (6‐MWT), walking (20 min fast walking), movement (TUG) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Not possible |
| Blinding (performance bias and detection bias) All outcomes ‐ administrators of the intervention? | High risk | Not possible |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient‐reported outcomes were included, and participants were not blinded. Insufficient reporting of blinding status for objective outcome measures |
| Incomplete outcome data (attrition bias) Missing outcome data? | Unclear risk | No information |
| Incomplete outcome data (attrition bias) ITT‐analyses performed? | Low risk | Participants were analysed in the groups to which they were randomised |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available |
| Other bias | High risk | Baseline imbalance in activity limitations outcomes and no direct post‐treatment outcome assessment for the usual care group |
Trojan 1999.
| Methods | Double‐blind, placebo‐controlled RCT | |
| Participants | N = 126 (pyridostigmine 64, placebo 62) Mean age: 56.8 years (pyridostigmine), 55.7 years (placebo) Gender distribution, male: 34% (pyridostigmine), 45% (placebo) Inclusion: ambulatory, history and physical examination consistent with past paralytic polio followed by at least 10 years of functional stability, new symptoms of general fatigue or muscular fatigue and new weakness of at least 1 year's duration Exclusion: medical conditions that could produce similar symptoms to PPS, contraindications to usage of pyridostigmine |
|
| Interventions | Treatment intervention: a 6‐month course of pyridostigmine 60 mg 3 times per day Control intervention: placebo |
|
| Outcomes | Measurements at baseline, 6 and 10 weeks and 6 months (primary) Primary: physical functioning (SF‐36 PF) Secondary: HRQoL (SF‐36), isometric muscle strength (modified Tufts Quantitative Neuromuscular Exam), fatigue (Hare Fatigue Symptom Scale, FSS), IGF‐I serum levels |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation scheme was computer generated" |
| Allocation concealment (selection bias) | Low risk | "The randomisation scheme was kept at the coordinating centre with a copy at the pharmaceutical and packaging company." |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | "Study patients were blinded to patient treatment assignment during the course of the study." Comment: It was likely that blinding was broken due to side effects of the treatment "Analysis on effectiveness of blinding provided evidence for unblinding" Comment: Authors stated that unblinding probably did not influence the results since the study was negative. However, unblinding remains a risk of bias. |
| Blinding (performance bias and detection bias) All outcomes ‐ administrators of the intervention? | High risk | "Physicians were blinded to patient treatment assignment during the course of the study." Comment: It was likely that blinding was broken due to side effects of the treatment "Analysis on effectiveness of blinding provided evidence for unblinding" Comment: Authors stated that unblinding probably did not influence the results since the study was negative. However, unblinding remains a risk of bias |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | "Study personnel were blinded to patient treatment assignment during the course of the study." Comment: Self reported outcomes were used and blinding of participants was probably broken |
| Incomplete outcome data (attrition bias) Missing outcome data? | Low risk | At 6 months: no dropouts, some missing data for the main outcome measure per group, no imputation. Reason for missing outcome data unlikely related to true outcome |
| Incomplete outcome data (attrition bias) ITT‐analyses performed? | Low risk | "The primary analysis used an ITT approach" |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available |
| Other bias | High risk | Baseline imbalance for growth hormone |
Vallbona 1997.
| Methods | Double‐blind, placebo‐controlled RCT | |
| Participants | N = 50 (magnetic treatment 29, placebo 21) Mean age: 51.5 years (magnetic treatment), 55.9 years (placebo) Gender distribution, male: 17% (magnetic treatment), 29% (placebo) Inclusion: diagnosis of PPS according to the criteria of Dalakas (Dalakas 1995), significant muscular or arthritic pain for at least 4 weeks, a trigger point or a circumscribed painful region by palpation, body weight less than 140% of predicted for age and height |
|
| Interventions | Treatment intervention: application of an active 300 to 500 Gauss magnetic device directly to a pain trigger point for 45 minutes Control intervention: application of placebo device |
|
| Outcomes | Measurements pre‐treatment and directly post‐treatment Outcome: intensity of pain felt on palpation of the active trigger point |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "an envelope....was randomly selected from a box" |
| Allocation concealment (selection bias) | Low risk | "The manufacturer supplied us with an equal number of active and placebo devices, placed in number coded envelopes. The code numbers were not broken until all patients completed the study" |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | "Double‐blind"; "active and placebo devices were of identical size and shape"; "the code numbers were not broken until all patients completed the study" |
| Blinding (performance bias and detection bias) All outcomes ‐ administrators of the intervention? | Low risk | "Double‐blind"; "active and placebo devices were of identical size and shape"; "the code numbers were not broken until all patients completed the study" |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | "Double‐blind"; "active and placebo devices were of identical size and shape"; "the code numbers were not broken until all patients completed the study" |
| Incomplete outcome data (attrition bias) Missing outcome data? | Low risk | No missing outcomes |
| Incomplete outcome data (attrition bias) ITT‐analyses performed? | Low risk | ITT analysis was probably done since all participants received the intervention to which they were randomised |
| Selective reporting (reporting bias) | High risk | Study protocol was not available. Pre‐specified outcome measure (McGill Pain Questionnaire) was not reported |
| Other bias | Low risk | |
Vasconcelos 2007.
| Methods | Double‐blind, placebo‐controlled, cross‐over RCT | |
| Participants | N = 36 (phase 1: modafinil 18, placebo 18; phase 2: modafinil 18, placebo 15) Mean age: 63.1 years (modafinil first), 59.3 years (placebo first) Gender distribution, male: 33% (modafinil first), 39% (placebo first) Inclusion: diagnosis of PPS according to a modified version (interval ≥ 10 years of stable function) of the criteria of the March of Dimes (March of Dimes Foundation 2000), ≥ 18 years old Exclusion: no or minimal fatigue, presence of confounding medical conditions, allergic to modafinil, pregnant and breastfeeding women, patients who report pain as their dominant symptom |
|
| Interventions | Treatment intervention: a 6‐week period of modafinil of 200 mg 2 times per day Control intervention: placebo Wash‐out interval: 14 days |
|
| Outcomes | Measurements at baseline and post‐treatment Primary: fatigue (FSS) Secondary: fatigue (VAS, FIS), HRQoL (SF‐36) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were allocated to treatment using computerized block randomisation" |
| Allocation concealment (selection bias) | Low risk | "The pharmacist formulated matching modafinil and placebo capsules, and concealed allocations from investigators by securing treatment codes." |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Low risk | "double‐blind study" Comment: Although there were more side effects experienced during modafinil treatment, analysis on effectiveness of blinding provided evidence for successful blinding |
| Blinding (performance bias and detection bias) All outcomes ‐ administrators of the intervention? | Low risk | "...concealed allocations from investigators by securing treatment codes." |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Low risk | "...concealed allocations from investigators by securing treatment codes." |
| Incomplete outcome data (attrition bias) Missing outcome data? | High risk | Missing outcomes: modafinil first 3/18, placebo first 0/18 Comment: Reason for missing outcome data was likely related to true outcome |
| Incomplete outcome data (attrition bias) ITT‐analyses performed? | Low risk | Results in the ITT sample did not differ from the per‐protocol sample |
| Selective reporting (reporting bias) | Low risk | Study protocol available in trial register (NCT00067496); pre‐specified outcomes have been reported |
| Other bias | Low risk | Because PPS is considered a reasonably stable chronic condition and modafinil is a medicament with a temporary effect, we considered the use of a cross‐over design appropriate |
101NRS: 101‐point numeric rating scale 2‐MWT: 2 Minute Walking Test 6‐MWT: 6 Minute Walking Test ADL: activities of daily living BDI: Beck Depression Inventory FIS: Fatigue Impact Scale FSS: Fatigue Severity Scale HRQoL: health‐related quality of life IGF‐1: insulin‐like growth factor 1 ITT: intention‐to‐treat IVIg: intravenous immunoglobulin MFI: Multidimensional Fatigue Inventory MMT: manual muscle testing MUNE: motor unit number estimates MVC: maximal voluntary contraction NHP: Nottingham Health Profile PASE: Physical Activity Scale for the Elderly PPS: postpolio syndrome RCT: randomised controlled trial RMI: Rivermead Mobility Index SF‐36: Short Form‐36 Health Survey SF‐36 MCS: Mental Component Summary of the Short Form‐36 Health Survey SF‐36 PCS: Physical Component Summary of the Short Form‐36 Health Survey S‐IgA: secretory immunoglobulin A TUG: Timed Up and Go Test VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acler 2013 | Did not include a control group consisting of placebo, usual care or no treatment; all participants underwent daily physical therapy during the intervention period and were all receiving IVIg before inclusion in the study |
| Bruno 1996 | No randomisation |
| Dean 1988 | No randomisation |
| Dean 1991 | Did not meet our pre‐specified criteria for PPS |
| Ghahari 2010 | Did not meet our pre‐specified criteria for PPS |
| Jones 1989 | Did not meet our pre‐specified criteria for PPS |
| Khan 2013 | Did not include a control group consisting of placebo, usual care or no treatment |
| Klein 2002 | Did not include a control group consisting of placebo, usual care or no treatment |
| Kriz 1992 | Did not meet our pre‐specified criteria for PPS |
| Miller 1997 | No full text available |
| Oncu 2009 | Did not include a control group consisting of placebo, usual care or no treatment |
| Skough 2008 | Did not include a control group consisting of placebo, usual care or no treatment |
| Skough 2011 | Did not include a control group consisting of placebo, usual care or no treatment |
| Willen 2001 | No randomisation |
IVIg: intravenous immunoglobulin PPS: postpolio syndrome
Characteristics of studies awaiting assessment [ordered by study ID]
ACTRN12612000552886.
| Methods | Double‐blind, placebo‐controlled RCT |
| Participants | Target sample size: 110 Inclusion: polio survivors with PPS or the late effects of polio with excessive fatigue, minimum age 50 years Exclusion: diagnosis with another medical condition that may account for the excessive fatigue, e.g. diabetes, anaemia, thyroid deficiency or fibromyalgia, treatment with warfarin, already taking coenzyme Q10 on a regular basis |
| Interventions | Treatment intervention: oral supplementation by 100 mg capsule of coenzyme Q10 daily for a period of 2 months Control intervention: placebo |
| Outcomes | Primary outcome: fatigue (FSS and Multidimensional Assessment of Fatigue Scale) |
| Notes | Study completed; no full‐text publication available as of 14 July 2014; manuscript in preparation |
ISRCTN00378146.
| Methods | RCT |
| Participants | N = 40 Inclusion: paralytic poliomyelitis survivors with 1 (or 2) lower limb(s) affected more than 20 years ago Exclusion: medical contraindication for physical exercise, doing regular physical exercise within 6 months before trial |
| Interventions | Treatment intervention: 3 months of home‐based physical exercises including 2 1‐hour sessions per week with progressive strength‐resistance exercises Control intervention: usual care |
| Outcomes | Measurements at baseline, 3 months and 6 months Outcomes: HRQoL (EQ‐5D, SF‐36), back disorders, fatigue, neuromuscular function, fitness, cost‐effectiveness |
| Notes | Study completed; no full‐text publication available as of 14 July 2014; manuscript in preparation |
Koopman 2014.
| Methods | Multicentre, single‐blind RCT |
| Participants | N = 68 Inclusion: diagnosis of PPS according to the criteria of the March of Dimes (March of Dimes Foundation 2000), severe perceived fatigue, age between 18 and 75 years, life expectancy longer than 1 year, walking ability at least indoors with or without a walking aid, ability to cycle on a cycle ergometer against a load of at least 25 watt Exclusion: use of psychotropic drugs or other psychiatric treatment, clinical depression, disabling comorbidity, respiratory insufficiency or assisted ventilation, cognitive impairment, insufficient mastery of the Dutch language, pregnancy |
| Interventions | (1) exercise therapy and usual care versus (2) cognitive behavioural therapy and usual care versus (3) usual care only |
| Outcomes | Measurements at baseline, at discharge from the program, and at 3 months and 6 months follow‐up Primary outcomes: fatigue (Checklist Individual Strength; domain fatigue), HRQoL (SF‐36), daily activity performance (Sickness Impact Profile; domains mobility range, mobility control, social behavior) Secondary outcomes: pain, emotional states, sleep disturbances, cardiorespiratory fitness, neuromuscular capacity, physical activity level in daily life, perceived participation, functional capacity, illness cognitions, coping, general self efficacy, cost‐effectiveness |
| Notes | Study completed; no full‐text publication available as of 14 July 2014; manuscript in preparation |
Murray 2014.
| Methods | Single‐blind RCT |
| Participants | N = 55 Inclusion: a history of poliomyelitis affecting at least 1 lower limb confirmed by the neurologist, capable of walking for 6 minutes with or without an aid/appliance, good upper limb strength confirmed objectively using maximum voluntary isometric contraction, 7 out of 10 tested upper limb movements must lie above the 5th percentile of the normal range, completion of the Physical Activity Readiness Medical Examination assessment and cleared by a medical practitioner as safe for exercise, age 18 to 75 years Exclusion: unstable cardiac or respiratory conditions, including oxygen dependence, uncontrolled hypertension, significant upper limb pain greater than 4/10 on a VAS or more than 3 specific sites of pain in the upper limbs, neck or upper back, severe fatigue (> 5 on the FSS), recent onset of upper limb weakness or severe upper limb weakness, steroid use in last 3 months, pregnancy |
| Interventions | Treatment intervention: an 8‐week, home‐based arm ergometry aerobic exercise programme Control intervention: usual care |
| Outcomes | Measurements at baseline and at 8 weeks Primary outcomes: physical fitness measured using the Six‐Minute Arm Test Secondary outcomes: self reported physical activity, body composition, energy cost of walking, fatigue, HRQoL, pain, upper limb strength, handgrip motor fatigue |
| Notes | Study completed; no full‐text publication available as of 14 July 2014; manuscript in preparation |
Silva 2014.
| Methods | Double‐blind RCT |
| Participants | N = 52 Inclusion: adults diagnosed with PPS according to the criteria of the March of Dimes (March of Dimes Foundation 2000) and documented periodic limb movement disorder during sleep Exclusion: untreated sleep‐disordered breathing |
| Interventions | Treatment intervention: mattress liners with far infrared bio‐ceramic components for 4 weeks Control intervention: mattress liners without far infrared bio‐ceramic components for 4 weeks |
| Outcomes | Measurements at baseline and post‐treatment Outcomes: pain, daytime somnolence, HRQoL, sleep data by nocturnal polysomnography |
| Notes | Study completed; no full‐text publication available as of 14 July 2014; manuscript in preparation |
EQ‐5D: EuroQol 5 Dimensions questionnaire FSS: Fatigue Severity Scale HRQoL: health‐related quality of life PPS: postpolio syndrome RCT: randomised controlled trial SF‐36: Short Form‐36 Health Survey VAS: visual analogue scale
Characteristics of ongoing studies [ordered by study ID]
NCT01549847.
| Trial name or title | A phase III, randomised, double‐blind, placebo‐controlled trial to evaluate the therapeutic effect of the association of L‐carnitine and piracetam as an adjuvant therapy in the treatment of weakness, muscle fatigue and muscle pain in PPS |
| Methods | Double‐blind, placebo‐controlled RCT |
| Participants | Target sample size: 120 Inclusion: people with PPS with diagnosis confirmed over a year, electromyography test compatible with poliomyelitis, preserved ability to swallow medication, oral communication ability preserved, preserved ability to perform pedaling test in at least 1 lower limb affected by PPS, ability to understand information about the study and to document the decision to participate in the trial by signing the informed consent form, age 18 to 60 years Exclusion: complete list of exclusion criteria is provided in the trial registration (clinicaltrials.gov/show/NCT01549847) |
| Interventions | Treatment intervention: L‐carnitine and piracetam Control intervention: placebo |
| Outcomes | Measurements at baseline and at 26 weeks Primary outcome: fatigue, muscle weakness Secondary outcomes: daily function, daytime sleepiness, depressive mood, muscle pain, oxidative capacity in skeletal muscle, HRQoL, adverse events |
| Starting date | Unknown |
| Contact information | AS Bulle Oliveira, Federal University of Sao Paulo |
| Notes |
NCT02089880.
| Trial name or title | Microprocessor‐controlled knee‐ankle‐foot orthosis (C‐Brace) versus stance‐control knee‐ankle‐foot orthosis (SCO): functional outcomes in individuals with lower extremity impairment |
| Methods | Cross‐over RCT |
| Participants | Target sample size: 24 Inclusion: lower extremity functional impairment due to neurologic or neuromuscular disease, orthopaedic disease or trauma (including PPS), prior active and compliant use of unilateral SCO, age 18 to 80 years, demonstrate a reciprocal gait pattern using current SCO, cognitive ability to understand and the willingness to sign a written informed consent, ability to turn the global positioning sensor and actigraph units on and off and sufficient memory ability to wear the devices each day during use of the orthoses Exclusion: passive ankle range of motion < 2°, body weight > 275 pounds, unstable neurological or cardiovascular/pulmonary disease or cancer, knee flexion contracture resulting in the inability to actively use C‐Brace, participating in physical therapy specific to orthotic use and gait training currently or within 1 month of starting protocol |
| Interventions | Treatment intervention: C‐Brace Control intervention: SCO |
| Outcomes | Measurements at baseline, at 8 weeks (i.e. after using device 1) and at 16 weeks (i.e. after using device 2) Primary outcome: 6‐MWT Secondary outcomes: 10 metre walk test, 5 times sit‐to‐stand test, Berg Balance Scale, Cross Walk Blinking Signal Test, Functional Gait Assessment, GAITRite data capture, Hill Assessment Index, muscle strength, passive and active range of motion, Stair Assessment Index |
| Starting date | February 2014 |
| Contact information | A. Jayaraman, email: ajayaraman@ricres.org |
| Notes |
NCT02176863.
| Trial name or title | Study of the efficacy and safety of immune globulin intravenous (human) Flebogamma® 5% DIF in people with PPS |
| Methods | Double‐blind, placebo‐controlled RCT |
| Participants | Target sample size: 210 Inclusion: March of Dimes clinical criteria for diagnosis of PPS (March of Dimes Foundation 2000), age 18 to 75 years, body mass index < 30 kg/m2, ambulatory or able to walk with a cane or other aids, at least 2 newly weakened muscle groups, and 1 of them in a lower extremity as defined by medical history and having a modified Medical Research Council scale score of ≥ 3, female of childbearing potential must have a negative test for pregnancy, female of childbearing potential and their sexual partners have agreed to practice contraception using a method of proven reliability, able to walk a 2‐MWT of at least 50 m Exclusion: complete list of exclusion criteria is provided in the trial registration (clinicaltrials.gov/ct2/show/NCT02176863) |
| Interventions | Treatment intervention: IVIg 2 g/kg, or IVIg 1 g/kg Control intervention: placebo |
| Outcomes | Measurements at baseline and at 52 weeks Primary outcome: 2‐MWT Secondary outcomes: HRQoL (SF‐36), 6‐MWT, pain (VAS) |
| Starting date | September 2014 |
| Contact information | K. Rucker, email: karen.rucker@grifols.com |
| Notes |
2‐MWT: 2 Minute Walking Test 6‐MWT: 6 Minute Walking Test FSS: Fatigue Severity Scale HRQoL: health‐related quality of life IVIg: intravenous immunoglobulin PPS: postpolio syndrome SF‐36: Short Form‐36 Health Survey VAS: visual analogue scale
Differences between protocol and review
Kimi Uegaki withdrew from review authorship prior to the start of this update.
In the update of this review we:
used a new RCT filter for EMBASE and removed the MEDLINE records that have been added to EMBASE (Appendix 3);
added a search strategy for the Cochrane Neuromuscular Disease Group Specialized Register (Appendix 6);
included the World Health Organization International Clinical Trials Registry Platform in the searches of the trial registers;
searched the Database of Abstracts of Reviews of Effects (DARE) and the Health Technology Assessment (HTA) Database;
removed 'sensitivity analyses were performed by repeating the meta‐analyses after omitting the trials that did not use the recent criteria for PPS defined by the March of Dimes' from the Methods section;
added a study flow diagram to the Results section;
included data on long‐term effectiveness of IVIg (i.e. long‐term follow‐up data from two studies included in the original review (Farbu 2007; Gonzalez 2006) and one new included study (Bertolasi 2013));
included the data of only one randomly chosen muscle group from studies that reported results of multiple muscle groups (Bertolasi 2013; Farbu 2007; Strumse 2003).
Contributions of authors
Contributions of review authors to the original review:
Writing of protocol and review: FK, KU, NEG, AB, MdV, FN
Screening of titles and abstracts: FK, KU
Assessment for inclusion: FK, KU
'Risk of bias' assessment: FK, KU
Disagreement resolution: NEG
Data extraction: FK, KU
Data entry into RevMan: FK
Data analysis: FK, KU
Assessment of quality of evidence: FK, AB
Interpretation of results: FK, KU, NEG, AB, MdV, FN
For this update of the review the tasks of KU were taken over by AB.
Sources of support
Internal sources
No sources of support supplied
External sources
Prinses Beatrix Spierfonds (The Dutch Public Fund for Neuromuscular Disorders)/ ZonMw (The Netherlands Organisation for Health Research and Development), Netherlands.
Declarations of interest
FK is involved in a RCT on the effectiveness of exercise therapy and cognitive behavioural therapy in PPS (Koopman 2014); its results are not yet published. Her work on the review was supported by a grant from Prinses Beatrix Spierfonds (The Dutch Public Fund for Neuromuscular Disorders)/ ZonMw (The Netherlands Organisation for Health Research and Development), Netherlands.
AB carried out a RCT on the effect of pyridostigmine in PPS (Horemans 2003). She is involved in Koopman 2014. Her work on the review was supported by a grant from Prinses Beatrix Spierfonds (The Dutch Public Fund for Neuromuscular Disorders)/ ZonMw (The Netherlands Organisation for Health Research and Development), Netherlands.
NEG was involved in a RCT on the effect of IVIg in PPS (Farbu 2007). He has received payment for scientific lectures and travel support to scientific meetings form the pharmaceutical companies Baxter, Octapharma and Merck Serono.
FN was an investigator on Horemans 2003. He is involved in Koopman 2014. He is also involved in a planned RCT on the effectiveness of IVIg (NCT02176863). He received study grants from the Netherlands Organisation for Health Research and Development and the Prinses Beatrix Spierfonds, Otto Bock, Fior & Gentz, OIM Orthopedie and for consultancy from Grifols Pharmaceuticals. All of these grants were paid to his institution.
MdV was an investigator on Horemans 2003. She is involved in Koopman 2014.
None of the review authors have financial conflicts of interest in the findings of this review.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Bertolasi 2013 {published and unpublished data}
- Bertolasi L, Frasson E, Turri M, Gajofatto A, Bordignon M, Zanolin E, et al. A randomized controlled trial of IV immunoglobulin in patients with postpolio syndrome. Journal of the Neurological Sciences 2013;330(1‐2):94‐9. [PUBMED: 23683859] [DOI] [PubMed] [Google Scholar]
Chan 2003 {published and unpublished data}
- Chan KM, Amirjani N, Sumrain M, Clarke A, Strohschein FJ. Randomized controlled trial of strength training in post‐polio patients. Muscle & Nerve 2003;27(3):332‐8. [PUBMED: 12635120] [DOI] [PubMed] [Google Scholar]
- Chan KM, Sumrain M, Clarke A, Strohschein F. A randomized controlled trial of strength training in post‐polio patients. Muscle & Nerve 2002;26(4):574. [DOI: 10.1002/mus.1243] [DOI] [PubMed] [Google Scholar]
Chan 2006 {published and unpublished data}
- Chan KM, Strohschein FJ, Rydz D, Allidina A, Shuaib A, Westbury CF. Randomized controlled trial of modafinil for the treatment of fatigue in postpolio patients. Muscle & Nerve 2006;33(1):138‐41. [PUBMED: 16175627] [DOI] [PubMed] [Google Scholar]
Dinsmore 1995 {published and unpublished data}
- Dinsmore S, Dambrosia J, Dalakas MC. A double‐blind, placebo‐controlled trial of high‐dose prednisone for the treatment of post‐poliomyelitis syndrome. Annals of the New York Academy of Sciences 1995;753:303‐13. [PUBMED: 7611639] [DOI] [PubMed] [Google Scholar]
Farbu 2007 {published data only}
- Farbu E, Rekand T, Vik‐Mo E, Lygren H, Gilhus NE, Aarli JA. Post‐polio syndrome patients treated with intravenous immunoglobulin: a double‐blinded randomized controlled pilot study. European Journal of Neurology 2007;14(1):60‐5. [PUBMED: 16403993] [DOI] [PubMed] [Google Scholar]
Gonzalez 2006 {published and unpublished data}
- Gonzalez H, Khademi M, Borg K, Olsson T. Intravenous immunoglobulin treatment of the post‐polio syndrome: sustained effects on quality of life variables and cytokine expression after one year follow up. Journal of Neuroinflammation 2012;9:167. [PUBMED: 22776106] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez H, Sunnerhagen KS, Sjöberg I, Kaponides G, Olsson T, Borg K. Intravenous immunoglobulin for post‐polio syndrome: a randomised controlled trial. The Lancet Neurology 2006;5(6):493‐500. [PUBMED: 16713921] [DOI] [PubMed] [Google Scholar]
Horemans 2003 {published data only}
- Horemans HL, Nollet F, Beelen A, Drost G, Stegeman DF, Zwarts MJ, et al. Pyridostigmine in postpolio syndrome: no decline in fatigue and limited functional improvement. Journal of Neurology, Neurosurgery, & Psychiatry 2003;74(12):1655‐61. [PUBMED: 14638885] [DOI] [PMC free article] [PubMed] [Google Scholar]
On 2005 {published data only}
- On AY, Oncu J, Uludag B, Ertekin C. Effects of lamotrigine on the symptoms and life qualities of patients with post polio syndrome: a randomized, controlled study. NeuroRehabilitation 2005;20(4):245‐51. [PUBMED: 16403993] [PubMed] [Google Scholar]
Stein 1995 {published and unpublished data}
- Stein DP, Dambrosia JM, Dalakas MC. A double‐blind, placebo‐controlled trial of amantadine for the treatment of fatigue in patients with the post‐polio syndrome. Annals of the New York Academy of Sciences 1995;753:296‐302. [PUBMED: 7611638] [DOI] [PubMed] [Google Scholar]
Strumse 2003 {published and unpublished data}
- Strumse YA, Stanghelle JK, Utne L, Ahlvin P, Svendsby EK. Treatment of patients with postpolio syndrome in a warm climate. Disability and Rehabilitation 2003;25(2):77‐84. [PUBMED: 12554382] [PubMed] [Google Scholar]
- Strumse YA, Stanghelle JK, Utne L, Ahlvin P, Svendsby EK. Treatment of patients with postpolio syndrome in warm climate. Tidsskrift for Den Norske Laegeforening 2001;121(17):2003‐7. [PUBMED: 11875895] [PubMed] [Google Scholar]
Trojan 1999 {published data only}
- Trojan DA, Collet JP, Shapiro S, Jubelt B, Miller RG, Agre JC, et al. A multicenter, randomized, double‐blinded trial of pyridostigmine in postpolio syndrome. Neurology 1999;53(6):1225‐33. [PUBMED: 10522877] [DOI] [PubMed] [Google Scholar]
Vallbona 1997 {published data only}
- Vallbona C, Hazlewood CF, Jurida G. Response of pain to static magnetic fields in postpolio patients: a double‐blind pilot study. Archives of Physical Medicine & Rehabilitation 1997;78(11):1200‐3. [PUBMED: 9365349] [DOI] [PubMed] [Google Scholar]
Vasconcelos 2007 {published data only}
- Vasconcelos OM, Prokhorenko OA, Salajegheh MK, Kelley KF, Livornese K, Olsen CH, et al. Modafinil for treatment of fatigue in post‐polio syndrome: a randomized controlled trial. Neurology 2007;68(20):1680‐6. [PUBMED: 17502549] [DOI] [PubMed] [Google Scholar]
- Vasconcelos OM, Prokhorenko OA, Vo AH, Kelly KF, Dalakas MC, Lauro HS, et al. Controlled trial of modafinil for the treatment of fatigue in post‐polio syndrome. Neurology 2006;66(Suppl 2):A248. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Acler 2013 {published data only}
- Acler M, Bocci T, Valenti D, Turri M, Priori A, Bertolasi L. Transcranial direct current stimulation (tDCS) for sleep disturbances and fatigue in patients with post‐polio syndrome. Restorative Neurology and Neuroscience 2013;31(5):661‐8. [DOI] [PubMed] [Google Scholar]
Bruno 1996 {published data only}
- Bruno RL, Zimmerman JR, Creange SJ, Lewis T, Molzen T, Frick NM. Bromocriptine in the treatment of post‐polio fatigue: a pilot study with implications for the pathophysiology of fatigue. American Journal of Physical Medicine and Rehabilitation 1996;75(5):340‐7. [PUBMED: 8873700] [DOI] [PubMed] [Google Scholar]
Dean 1988 {published data only}
- Dean E, Ross J. Modified aerobic walking program: effect on patients with postpolio syndrome symptoms. Archives of Physical Medicine and Rehabilitation 1988;69(12):1033‐8. [PUBMED: 3214262] [PubMed] [Google Scholar]
Dean 1991 {published data only}
- Dean E, Ross J. Effect of modified aerobic training on movement energetics in polio survivors. Orthopedics 1991;14(11):1243‐6. [PUBMED: 1758791] [DOI] [PubMed] [Google Scholar]
Ghahari 2010 {published and unpublished data}
- Ghahari S, Leigh Packer T, Passmore AE. Effectiveness of an online fatigue self‐management programme for people with chronic neurological conditions: a randomized controlled trial. Clinical Rehabilitation 2010;24(8):727‐44. [PUBMED: 20543022] [DOI] [PubMed] [Google Scholar]
- Ghahari S, Packer TL, Passmore AE. Development, standardisation and pilot testing of an online fatigue self‐management program. Disability and Rehabilitation 2009;31(21):1762‐72. [PUBMED: 19479510] [DOI] [PubMed] [Google Scholar]
Jones 1989 {published and unpublished data}
- Jones DR, Speier J, Canine K, Owen R, Stull GA. Cardiorespiratory responses to aerobic training by patients with postpoliomyelitis sequelae. JAMA 1989;261(22):3255‐8. [PUBMED: 2654435] [PubMed] [Google Scholar]
Khan 2013 {published data only}
- Khan S, Gajapathi M, Pandit M. Additive effect of pulsed electromagnetic field therapy (PEMF) on hip flexor contracture in post polio syndrome (PPS). Indian Journal of Physiotherapy & Occupational Therapy 2013;7(2):196‐201. [Google Scholar]
Klein 2002 {published and unpublished data}
- Klein MG, Whyte J, Esquenazi A, Keenan MA, Costello R. A comparison of the effects of exercise and lifestyle modification on the resolution of overuse symptoms of the shoulder in polio survivors: a preliminary study. Archives of Physical Medicine and Rehabilitation 2002;83(5):708‐13. [PUBMED: 11994812] [DOI] [PubMed] [Google Scholar]
Kriz 1992 {published and unpublished data}
- Kriz JL, Jones DR, Speier JL, Canine JK, Owen RR, Serfass RC. Cardiorespiratory responses to upper extremity aerobic training by postpolio subjects. Archives of Physical Medicine and Rehabilitation 1992;73(1):49‐54. [PUBMED: 1729974] [PubMed] [Google Scholar]
Miller 1997 {published and unpublished data}
- Miller RG, Gelinas DF, Kent‐Braun J, Dobbins T, Dao H, Dalakas M. The effect of recombinant insulin‐like growth factor 1 (rhIGF‐1), upon exercise induced fatigue and recovery in patients with post‐polio syndrome. Neurology 1997;48:A217. Abstract. [Google Scholar]
Oncu 2009 {published data only}
- Oncu J, Durmaz B, Karapolat H. Short‐term effects of aerobic exercise on functional capacity, fatigue, and quality of life in patients with post‐polio syndrome. Clinical Rehabilitation 2009;23(2):155‐63. [PUBMED: 19164403] [DOI] [PubMed] [Google Scholar]
Skough 2008 {published and unpublished data}
- Skough K, Krossén C, Heiwe S, Theorell H, Borg K. Effects of resistance training in combination with coenzyme Q10 supplementation in patients with post‐polio: a pilot study. Journal of Rehabilitation Medicine 2008;40(9):773‐5. [PUBMED: 18843432] [DOI] [PubMed] [Google Scholar]
Skough 2011 {published data only}
- Skough K. Effects of resistance training in combination with IVIG treatment in patients with post‐polio. Journal of Rehabilitation Medicine. 1st European Polio Conference: ‘Post polio syndrome – a challenge of today'; 2011 August 31 – September 2; Copenhagen. 2011; Vol. Suppl 49:33.
- Skough K, Borg K, Henriksson M. Effects of resistance training in combination with IVIG treatment in patients with post‐polio. European Journal of Neurology. 15th Congress of the EFNS Budapest. 2011.
Willen 2001 {published and unpublished data}
- Willén C, Sunnerhagen KS, Grimby G. Dynamic water exercise in individuals with late poliomyelitis. Archives of Physical Medicine and Rehabilitation 2001;82(1):66‐72. [PUBMED: 11239288] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
ACTRN12612000552886 {published and unpublished data}
- ACTRN12612000552886. Oral supplementation of polio survivors with post polio syndrome or late effects of polio by coenzyme Q10 for relief of their excessive fatigue, as measured by fatigue questionnaires. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612000552886 (accessed 14 July 2014).
ISRCTN00378146 {published and unpublished data}
- ISRCTN00378146. Cost‐utility and efficacy of a home‐based exercise program in poliomyelitis survivors: paralytic poliomyelitis survivors with one (or two) lower limb affected more than twenty years ago, recruited through local association. http://www.controlled‐trials.com/ISRCTN00378146 (accessed 14 July 2014).
Koopman 2014 {published and unpublished data}
- Koopman FS, Beelen A, Gerrits KH, Bleijenberg G, Abma TA, Visser M, et al. Exercise therapy and cognitive behavioural therapy to improve fatigue, daily activity performance and quality of life in postpoliomyelitis syndrome: the protocol of the FACTS‐2‐PPS trial. BMC Neurology 2010;10:8. [PUBMED: 20082714] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman FS, Voorn E, Brehm M, Beelen A, Nollet F. Exercise therapy and cognitive behavioural therapy to improve fatigue in post‐polio syndrome: preliminary results of a randomized controlled trial. Journal of Rehabilitation Medicine. 2nd European Polio Conference: 'Post‐Polio Syndrome Conference: a condition without boundaries’. 2014 June 25–27; Amsterdam. 2014; Vol. 46:589.
Murray 2014 {published and unpublished data}
- Murray D, Horgan F, Campion A, Vance R, Meldrum D, Hardiman O. The effects of a home‐based arm ergometry exercise programme on physical fitness, fatigue and activity in polio survivors; a randomised controlled trial. Journal of Rehabilitation Medicine. 2nd European Polio Conference: ‘Post‐Polio Syndrome Conference: a condition without boundaries’. 2014 June 25–27; Amsterdam. 2014; Vol. 46:589.
- Murray D, Meldrum D, Moloney R, Campion A, Horgan F, Hardiman O. The effects of a home‐based arm ergometry exercise programme on physical fitness, fatigue and activity in polio survivors: protocol for a randomised controlled trial. BMC Neurology 2012;12:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Silva 2014 {published and unpublished data}
- Moreira G, Silva T, Pradella‐Hallinan M, Quadros A, Oliveira A, Tufik S. Impact of BioceramicTM mattress on sleep of patients with postpolio syndrome and periodic leg movement. A randomized controlled study. Journal of Sleep Research. 20th Congress of the European Sleep Research Society. 2010 September 14‐18; Lisbon. 2010:335.
- Silva TM. Analyses of sleep characteristics in Post‐Polio Syndrome Patients. Journal of Rehabilitation Medicine. 1st European Polio Conference: ‘Post Polio Syndrome – a Challenge of Today'. 2011 August 31 – September 2; Copenhagen. 2011; Vol. Suppl 49:47.
References to ongoing studies
NCT01549847 {published data only}
- NCT01549847. A phase III, randomized, double blind, placebo controlled trial to evaluate the therapeutic effect of the association of L‐carnitine and piracetam as an adjuvant therapy in the treatment of weakness, muscle fatigue and muscle pain in the postpoliomyelitis syndrome. http://www.clinicaltrials.gov/ct2/results?term=NCT01549847 (accessed 14 July 2014).
NCT02089880 {published data only}
- NCT02089880. Micro‐processor controlled knee‐ankle‐foot orthosis (C‐brace) versus stance‐control knee‐ankle‐foot orthosis (SCO): functional outcomes in individuals with lower extremity impairment. http://www.clinicaltrials.gov/ct2/results?term=NCT02089880 (accessed 14 July 2014).
NCT02176863 {published data only}
- NCT02176863. Study of the efficacy and safety of immune globulin intravenous (human) Flebogamma® 5% DIF in patients with post‐polio syndrome. http://www.clinicaltrials.gov/ct2/show/record/NCT02176863?term=NCT02176863 (accessed 14 July 2014).
Additional references
Allen 1994
- Allen GM, Gandevia SC, Neering IR, Hickie I, Jones R, Middleton J. Muscle performance, voluntary activation and perceived effort in normal subjects and patients with prior poliomyelitis. Brain 1994;117:661‐70. [PUBMED: 7922455] [DOI] [PubMed] [Google Scholar]
Alvarez 2010
- Alvarez A, Kremer R, Weiss DR, Benedetti A, Haziza M, Trojan DA. Response of postpoliomyelitis patients to bisphosphonate treatment. Physical Medicine and Rehabilitation 2010;2(12):1094‐103. [DOI] [PubMed] [Google Scholar]
Bamford 1993
- Bamford CR, Montgomery EB Jr, Munoz JE, Stumpf C, Pry S, Namerow NS. Postpolio syndrome‐‐response to deprenyl (selegiline). The International Journal of Neuroscience 1993;71(1‐4):183‐8. [PUBMED: 8407144] [DOI] [PubMed] [Google Scholar]
Beelen 2003
- Beelen A, Nollet F, Visser M, Jong BA, Lankhorst GJ, Sargeant AJ. Quadriceps muscle strength and voluntary activation after polio. Muscle & Nerve 2003;28(2):218‐26. [PUBMED: 12872327] [DOI] [PubMed] [Google Scholar]
Bickerstaffe 2014
- Bickerstaffe A, Dijk JP, Beelen A, Zwarts MJ, Nollet F. Loss of motor unit size and quadriceps strength over 10 years in post‐polio syndrome. Clinical Neurophysiology 2014;125(6):1255‐60. [DOI] [PubMed] [Google Scholar]
Borg 1996
- Borg K. Post‐polio muscle dysfunction 29th ENMC workshop 14‐16 October 1994, Naarden, the Netherlands. Neuromuscular Disorders 1996;6(1):75‐80. [PUBMED: 8845722] [DOI] [PubMed] [Google Scholar]
Brehm 2007
- Brehm MA, Beelen A, Doorenbosch CA, Harlaar J, Nollet F. Effect of carbon‐composite knee‐ankle‐foot orthoses on walking efficiency and gait in former polio patients. Journal of Rehabilitation Medicine 2007;39(8):651‐7. [PUBMED: 17896058] [DOI] [PubMed] [Google Scholar]
Brogårdh 2010
- Brogårdh C, Flansbjer UB, Lexell J. No effects of whole‐body vibration training on muscle strength and gait performance in persons with late effects of polio: a pilot study. Archives of Physical Medicine and Rehabilitation 2010;91(9):1474‐7. [DOI] [PubMed] [Google Scholar]
Cup 2007
- Cup EH, Pieterse AJ, Broek‐Pastoor JM, Munneke M, Engelen BG, Hendricks HT, et al. Exercise therapy and other types of physical therapy for patients with neuromuscular diseases: a systematic review. Archives of Physical Medicine & Rehabilitation 2007;88(11):1452‐64. [DOI] [PubMed] [Google Scholar]
Dalakas 1986
- Dalakas MC, Elder G, Hallett M, Ravits J, Baker M, Papadopoulos N, et al. A long‐term follow‐up study of patients with post‐poliomyelitis neuromuscular symptoms. The New England Journal of Medicine 1986;314(15):959‐63. [DOI] [PubMed] [Google Scholar]
Dalakas 1988
- Dalakas MC. Morphologic changes in the muscles of patients with postpoliomyelitis neuromuscular symptoms. Neurology 1988;38(1):99‐104. [PUBMED: 3336469] [DOI] [PubMed] [Google Scholar]
Dalakas 1995
- Dalakas MC. The post‐polio syndrome as an evolved clinical entity. Definition and clinical description. Annals of the New York Academy of Sciences 1995;753:68‐80. [PUBMED: 7611661] [DOI] [PubMed] [Google Scholar]
Dalakas 1999
- Dalakas MC. Why drugs fail in postpolio syndrome: lessons from another clinical trial. Neurology 1999;53(6):1166‐7. [PUBMED: 10522866] [DOI] [PubMed] [Google Scholar]
Davidson 2009
- Davidson AC, Auyeung V, Luff R, Holland M, Hodgkiss A, Weinman J. Prolonged benefit in post‐polio syndrome from comprehensive rehabilitation: a pilot study. Disability and Rehabilitation 2009;31(4):309‐17. [PUBMED: 18608421] [DOI] [PubMed] [Google Scholar]
Deeks 2008
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008:243‐96. [Google Scholar]
Dunn 1991
- Dunn MG. Post‐polio fatigue treated with amantadine. Archives of Neurology 1991;48(6):570. [PUBMED: 2039376] [DOI] [PubMed] [Google Scholar]
Farbu 2006
- Farbu E, Gilhus NE, Barnes MP, Borg K, Visser M, Driessen A, et al. EFNS guideline on diagnosis and management of post‐polio syndrome. Report of an EFNS task force. European Journal of Neurology 2006;13(8):795‐801. [PUBMED: 16879288] [DOI] [PubMed] [Google Scholar]
Farbu 2011
- Farbu E, Gilhus NE, Barnes MP, Borg K, Visser M, Howard R, et al. Post‐polio syndrome. In: Gilhus NE, Barnes MP, Brainin M editor(s). European Handbook of Neurological Management. 2nd Edition. Vol. 1, Blackwell Publishing Ltd, 2011:311‐9. [Google Scholar]
Gonzalez 2002
- Gonzalez H, Khademi M, Andersson M, Wallström E, Borg K, Olsson T. Prior poliomyelitis‐evidence of cytokine production in the central nervous system. Journal of the Neurological Sciences 2002;205(1):9‐13. [DOI] [PubMed] [Google Scholar]
Grimby 1989
- Grimby G, Einarsson G, Hedberg M, Aniansson A. Muscle adaptive changes in post‐polio subjects. Scandinavian Journal of Rehabilitation Medicine 1989;21(1):19‐26. [PUBMED: 2711135] [PubMed] [Google Scholar]
Gupta 1994
- Gupta KL, Shetty KR, Agre JC, Cuisinier MC, Rudman IW, Rudman D. Human growth hormone effect on serum IGF‐I and muscle function in poliomyelitis survivors. Archives of Physical Medicine and Rehabilitation 1994;75(8):889‐94. [PUBMED: 8053796] [DOI] [PubMed] [Google Scholar]
Halstead 1985
- Halstead LS, Rossi CD. New problems in old polio patients: Results of a survey of 539 polio survivors. Orthopedics. 1985;8(7):845‐50. [PUBMED: 3867865] [DOI] [PubMed] [Google Scholar]
Halstead 1987
- Halstead LS, Rossi CD. Post‐polio syndrome: clinical experience with 132 consecutive outpatients. Birth Defects Original Article Series 1987;23(4):13‐26. [PUBMED: 3620612] [PubMed] [Google Scholar]
Halstead 1991
- Halstead LS. Assessment and differential diagnosis for post‐polio syndrome. Orthopedics 1991;14(11):1209‐17. [PUBMED: 1758788] [DOI] [PubMed] [Google Scholar]
Heim 1997
- Heim M, Yaacobi E, Azaria M. A pilot study to determine the efficiency of lightweight carbon fibre orthoses in the management of patients suffering from post‐poliomyelitis syndrome. Clinical Rehabilitation 1997;11(4):302‐5. [PUBMED: 9408670] [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008:187‐241. [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jensen 2011
- Jensen MP, Alschuler KN, Smith AE, Verrall AM, Goetz MC, Molton IR. Pain and fatigue in persons with postpolio syndrome: independent effects on functioning. Archives of Physical Medicine and Rehabilitation 2011;92(11):1796‐801. [DOI] [PubMed] [Google Scholar]
Jubelt 1995
- Jubelt B, Salazar‐Grueso EF, Roos RP, Cashman NR. Antibody titer to the poliovirus in blood and cerebrospinal fluid of patients with post‐polio syndrome. Annals of the New York Academy of Sciences 1995;753:201‐7. [PUBMED: 7611629] [DOI] [PubMed] [Google Scholar]
Klefbeck 2000
- Klefbeck B, Lagerstrand L, Mattsson E. Inspiratory muscle training in patients with prior polio who use part‐time assisted ventilation. Archives of Physical Medicine and Rehabilitation 2000;81(8):1065‐71. [PUBMED: 10943756] [DOI] [PubMed] [Google Scholar]
Larsson Lund 2010
- Larsson Lund M, Lexell J. A positive turning point in life‐‐how persons with late effects of polio experience the influence of an interdisciplinary rehabilitation programme. Journal of Rehabilitation Medicine 2010;42(6):559‐65. [DOI] [PubMed] [Google Scholar]
Lefebre 2011
- Lefebre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011.. Available from www.cochrane‐handbook.org. Chichester: John Wiley & Sons.
March of Dimes Foundation 2000
- March of Dimes Birth Defects Foundation. Identifying best practices in diagnosis & care. Proceedings of the International Conference on Post Polio Syndrome; 2000 May 19; Warm Springs, GA. New York. http://www.polioplace.org/sites/default/files/files/MOD‐%20Identifying.pdf. (accessed 14 August 2014).
McComas 1997
- McComas AJ, Quartly C, Griggs RC. Early and late losses of motor units after poliomyelitis. Brain 1997;120 Pt 8:1415‐21. [DOI] [PubMed] [Google Scholar]
Mizuno 1997
- Mizuno M, Quistorff B, Theorell H, Theorell M, Chance B. Effects of oral supplementation of coenzyme Q10 on 31P‐NMR detected skeletal muscle energy metabolism in middle‐aged post‐polio subjects and normal volunteers. Molecular Aspects of Medicine 1997;18 Suppl:S291‐8. [PUBMED: 9266539] [DOI] [PubMed] [Google Scholar]
Nollet 1999
- Nollet F, Beelen A, Prins MH, Visser M, Sargeant AJ, Lankhorst GJ, et al. Disability and functional assessment in former polio patients with and without postpolio syndrome. Archives of Physical Medicine and Rehabilitation 1999;80(2):136‐43. [PUBMED: 10025486] [DOI] [PubMed] [Google Scholar]
Nollet 2000
- Nollet F, Horemans H, Beelen A. A multicenter, randomized, double‐blinded trial of pyridostigmine in postpolio syndrome. Neurology 2000;55(6):899‐901. [PUBMED: 10994028] [DOI] [PubMed] [Google Scholar]
Nollet 2003
- Nollet F. Post‐polio syndrome. https://www.orpha.net/data/patho/GB/uk‐PP.pdf (accessed 14 August 2014).
Nollet 2003a
- Nollet F, Beelen A, Twisk JW, Lankhorst GJ, Visser M. Perceived health and physical functioning in postpoliomyelitis syndrome: a 6‐year prospective follow‐up study. Archives of Physical Medicine and Rehabilitation 2003;84(7):1048‐56. [PUBMED: 12881833] [DOI] [PubMed] [Google Scholar]
Nollet 2010
- Nollet F. Postpolio syndrome: unanswered questions regarding cause, course, risk factors, and therapies. The Lancet Neurology 2010;9(6):561‐3. [PUBMED: 20494317] [DOI] [PubMed] [Google Scholar]
Rekand 2004
- Rekand T, Kõrv J, Farbu E, Roose M, Gilhus NE, Langeland N, et al. Lifestyle and late effects after poliomyelitis. A risk factor study of two populations. Acta Neurologica Scandinavica 2004;109(2):120‐5. [PUBMED: 14705974] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Seizert 1994
- Seizert BP, Speier JL, Canine K. Pyridostigmine effect on strength, endurance and fatigue in postpolio patients. Archives of Physical Medicine and Rehabilitation 1994;75:1049. Abstract. [Google Scholar]
Shetty 1995
- Shetty KR, Gupta KL, Agre JC, Rudman IW, Rudman D. Effect of human growth hormone on muscle function in post‐polio syndrome. Annals of the New York Academy of Sciences 1995;753:386‐9. [PUBMED: 7611652] [DOI] [PubMed] [Google Scholar]
Stolwijk‐Swuste 2005
- Stolwijk‐Swüste JM, Beelen A, Lankhorst GJ, Nollet F, CARPA Study Group. The course of functional status and muscle strength in patients with late‐onset sequelae of poliomyelitis: a systematic review. Archives of Physical Medicine and Rehabilitation 2005;86(8):1693‐701. [PUBMED: 16084828] [DOI] [PubMed] [Google Scholar]
Stolwijk‐Swuste 2010
- Stolwijk‐Swüste JM, Tersteeg I, Beelen A, Lankhorst GJ, Nollet F, CARPA Study Group. The impact of age and comorbidity on the progression of disability in late‐onset sequelae of poliomyelitis. Archives of Physical Medicine and Rehabilitation 2010;91(4):523‐8. [DOI] [PubMed] [Google Scholar]
Trojan 1993
- Trojan DA, Gendron D, Cashman NR. Stimulation frequency‐dependent neuromuscular junction transmission defects in patients with prior poliomyelitis. Journal of the Neurological Sciences 1993;118(2):150‐7. [PUBMED: 8229063] [DOI] [PubMed] [Google Scholar]
Trojan 1995
- Trojan DA, Cashman NR. An open trial of pyridostigmine in post‐poliomyelitis syndrome. Canadian Journal of Neurological Sciences 1995;22(3):223‐7. [PUBMED: 8529175] [DOI] [PubMed] [Google Scholar]
Wiechers 1981
- Wiechers DO, Hubbell SL. Late changes in the motor unit after acute poliomyelitis. Muscle & Nerve 1981;4(6):524‐8. [PUBMED: 6273721] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Koopman 2011
- Koopman FS, Uegaki K, Gilhus NE, Beelen A, Visser M, Nollet F. Treatment for postpolio syndrome. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD007818] [DOI] [PubMed] [Google Scholar]


