Abstract
Background
Inguinal lymph node dissection plays an important role in the management of melanoma, penile and vulval cancer. Inguinal lymph node dissection is associated with various intraoperative and postoperative complications with significant heterogeneity in classification and reporting. This lack of standardization challenges efforts to study and report inguinal lymph node dissection outcomes. The aim of this study was to devise a system to standardize the classification and reporting of inguinal lymph node dissection perioperative complications by creating a worldwide collaborative, the complications and adverse events in lymphadenectomy of the inguinal area (CALI) group.
Methods
A modified 3-round Delphi consensus approach surveyed a worldwide group of experts in inguinal lymph node dissection for melanoma, penile and vulval cancer. The group of experts included general surgeons, urologists and oncologists (gynaecological and surgical). The survey assessed expert agreement on inguinal lymph node dissection perioperative complications. Panel interrater agreement and consistency were assessed as the overall percentage agreement and Cronbach’s α.
Results
Forty-seven experienced consultants were enrolled: 26 (55.3%) urologists, 11 (23.4%) surgical oncologists, 6 (12.8%) general surgeons and 4 (8.5%) gynaecology oncologists. Based on their expertise, 31 (66%), 10 (21.3%) and 22 (46.8%) of the participants treat penile cancer, vulval cancer and melanoma using inguinal lymph node dissection respectively; 89.4% (42 of 47) agreed with the definitions and inclusion as part of the inguinal lymph node dissection intraoperative complication group, while 93.6% (44 of 47) agreed that postoperative complications should be subclassified into five macrocategories. Unanimous agreement (100%, 37 of 37) was achieved with the final standardized classification system for reporting inguinal lymph node dissection complications in melanoma, vulval cancer and penile cancer.
Conclusion
The complications and adverse events in lymphadenectomy of the inguinal area classification system has been developed as a tool to standardize the assessment and reporting of complications during inguinal lymph node dissection for the treatment of melanoma, vulval and penile cancer.
The complications and adverse events in lymphadenectomy of the inguinal area classification system has been developed as a tool to standardize the assessment and reporting of complications during inguinal lymph node dissection for the treatment of melanoma, vulvar and penile cancer.
Introduction
Inguinal lymph node dissection (ILND) plays an important role in the management of melanoma, penile and vulval cancer1–5. ILND series report a wide range of associated morbidity rates (3–97%)1,6–9. It is generally considered a procedure with a high risk of perioperative complications, with more than 50% of patients reporting at least one adverse event (AE)10,11.
ILND is associated with various types of intraoperative and postoperative complications and AEs, including skin necrosis, wound dehiscence, infection, neurovascular injury, lymphocoele, lymphorrhoea and lymphoedema1,9,10,12–16. However, there is significant heterogeneity in the surgical literature in terms of how ILND-associated complications are classified and reported. This lack of standardization challenges any effort to study and report ILND outcomes1.
In a recent systematic review, 25% of studies documented AEs after ILND with only 50% of the criteria proposed by the European Association of Urology (EAU) guidelines recommendation1,17. For some specific complications, such as lymphoedema, numerous classifications exist aiming to standardize the severity grading and management. Yet, the concordance between these classifications can be variable when evaluating lower extremities, underscoring the need for a unified approach to assessing outcomes post-ILND across all specialties. This standardization is crucial to enhance the quality of the data, more so considering the rarity of the conditions that are treated with ILND1,18.
The complications and adverse events in lymphadenectomy of the inguinal area (CALI) collaboration was established to devise a system to standardize the classification and reporting of perioperative ILND complications. Various efforts have been made in the surgical community to standardize how perioperative AEs are reported, graded and studied17,19–27, and the CALI project aspires to contribute to the field of ILND.
This paper reports the results of the CALI collaboration’s 3-round Delphi survey to establish a new perioperative AE and complication classification system for ILND. This classification system was developed with the input of global experts, and it can be widely utilized by the greater surgical community.
Methods
Study design
A modified Delphi consensus approach28 surveyed an international group of experts in ILND for melanoma, penile and vulval cancer diagnosis and treatment. The group of experts included general surgeons, gynaecological oncologists, surgical oncologists and urologists. The survey assessed expert agreement on perioperative complications and AEs clustered in macro- and microcategories that were established based on the results of our previously published systematic review on ILND complications1. The goal of this systematic review was to identify ILND complication and AE reporting to inform this newly developed classification system.
The CALI study was reviewed and approved by the institutional review board (IRB) (UP-22-00368) and is registered on clinicaltrials.gov (NCT05388786). The results of the Delphi consensus are provided according to the Checklist for Reporting Results of Internet E-Surveys (CHERRIES) and to the Accurate Consensus Reporting Document (ACCORD) guidelines (Supplementary material methods)29,30.
Study population and survey distribution
The list of experts invited to participate in the CALI Delphi survey were corresponding/senior authors of the articles identified from a previously published systematic review1. A snowball method was used, asking experts that agreed to participate in the survey to identify other experts for participation31.
A total of 47 experienced surgeons were contacted via e-mail and enrolled in the modified Delphi consensus survey. The survey was administered from July to December 2022 using Google Forms (https://docs.google.com/forms/).
The development of new definitions was rooted in a comprehensive literature review, followed by expert consensus within the CALI group1. This process is detailed in the Supplementary material. For example, some of the definitions come from the Common Terminology Criteria for Adverse Events from the US National Cancer Institute (CTCAE) or the Center for Disease Control and Prevention (CDC), Consensus Document of the International Society of Lymphology. When there were no standardized definitions, these were formulated from expert opinion (Supplementary material appendices).
Members of the CALI steering committee did not participate in the Delphi survey to avoid introducing potential bias. Multiple iterations with feedback were used to achieve consensus (greater than 80% agreement).
In the first round of the Delphi survey, survey participants were asked to report their demographics and surgical expertise, including country of practice, years of practice, surgical specialty, type of malignancy treated using ILND, type of ILND surgical approach and annual case volume. During the first round, participants were asked for their level of agreement using a 1 (strongly disagree) to 5 (strongly agree) Likert scale on a series of perioperative complications and definitions for inclusion in the new classification system. Respondents were surveyed on whether perioperative complications should be classified as intraoperative and/or postoperative and subclassified into macro- and microcategories and defined according to the existing classifications and definitions20,21,32–36. In cases where limitations in ILND perioperative complication classification systems were identified based on the previous systematic review1, new definitions were provided and experts again rated their level of agreement from 1 (strongly disagree) to 5 (strongly agree). Lastly, experts were encouraged to provide written feedback in free-text form. These responses were reviewed to improve the proposed classification system in a standardized fashion.
In the second round of the Delphi survey, the experts were asked to assess the changes implemented from the first round using a 1 to 5 Likert scale. Despite reaching a consensus on most of the classification systems and definitions following the first round, the system was refined by asking experts to provide written feedback, even in instances where consensus was reached.
Lastly, in the third round of the Delphi survey, experts were asked to evaluate the changes implemented from the second round, again using the 5-point Likert scale. After reaching consensus on each item for inclusion in the CALI classification system, a representative classification system template for assessing and reporting perioperative complications and AEs associated with ILND was created.
Statistical analysis
The interrater reliability (IRR) and consistency of the panellist responses were analysed to ensure consensus. For calculation of the agreement percentage, the 5-Likert scale responses were dichotomized, with a score of 5 (strongly agree) and 4 (agree) representing agreement, and scores of 3 (neither agree or disagree), 2 (disagree) and 1 (strongly disagree) representing disagreement. The IRR and consistency of the criteria within the expert panel were evaluated using Cronbach’s α37. Continuous and dichotomous variables were reported as median (i.q.r.), mean(s.d.), and absolute and relative frequencies as appropriate.
Results
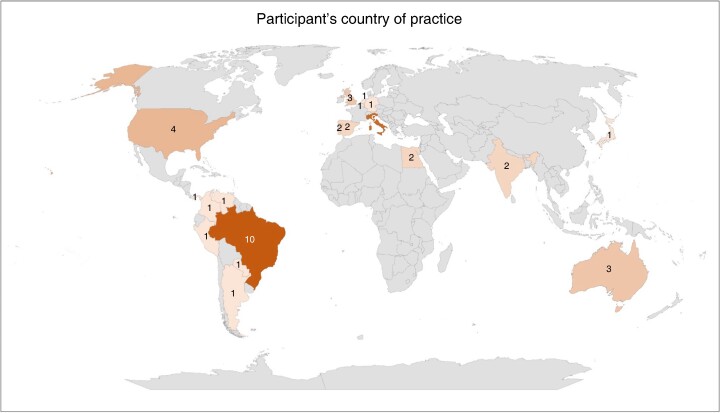
In the first round of the consensus approach, the survey was e-mailed to 218 experts and 47 responses were received (21.5%). Median responder age was 48 (i.q.r. 41–53) years, with 3 (6.4%), 13 (27.7%), 5 (10.6%), 12 (25.5%) and 13 (29.8%) of those surveyed endorsing 5–9, 10–14, 15–19, 20–24 and ≥25 years in clinical practice respectively. In terms of specialty, 26 (55.3%), 11 (23.4%), 6 (12.8%) and 4 (8.5%) were from urology, surgical oncology, general surgery and gynaecologic oncology respectively. In terms of setting, 32 (68%) were academic, followed by 7 (14.8%) community-based, 3 (6.4%) community-based university-affiliated and 5 (10.8%) private practice. The most common countries of practice among the participants were Italy (19.6%) and Brazil (19.6%). For more details regarding the participant’s country of practice, see Fig. 1.
Fig. 1.
Demographic distribution of panellists
Based on their expertise, 31 (66%), 10 (21.3%) and 22 (46.8%) of the participants treat penile cancer, vulval cancer and melanoma using ILND respectively. Median annual ILND surgical volume performed by surveyed experts was 10 (i.q.r. 5–20) cases. Various experts used multiple ILND surgical approaches as part of the treatment for penile cancer, vulval cancer or melanoma, including an open approach (91.5%), followed by laparoscopic/video endoscopic inguinal lymph node dissection (VEIL) (36.2%) and robotic (R-VEIL) (23.4%).
Of the experts, 91.4% (43 of 47) either agreed or strongly agreed with the importance of classifying and standardizing ILND perioperative complications into intraoperative and postoperative, and subclassifying postoperative into immediate (0–24 h), early (1–30 days) and late (31–90 days), and define them according to existing classification systems19–21. The experts were then surveyed on how to group complications. Of the presented list of intraoperative complications during ILND, 89.4% (42 of 47) agreed with the provided definitions and inclusion as part of the ILND intraoperative complication group; 93.6% (44 of 47) agreed that postoperative complications should be subclassified into five macrocategories. The survey showed agreement for the five macrocategories: 87.2% (41 of 47), 89.4% (42 of 47), 93.6% (44 of 47), 93.6% (44 of 47) and 100% (47 of 47) agreement for infectious, cutaneous, lymphatics, vascular and functional respectively. Experts were surveyed regarding their level of agreement with each complication definition and inclusion with the appropriate postoperative macrocategory. For more details regarding each complication definition and inclusion, see Supplementary material appendices.
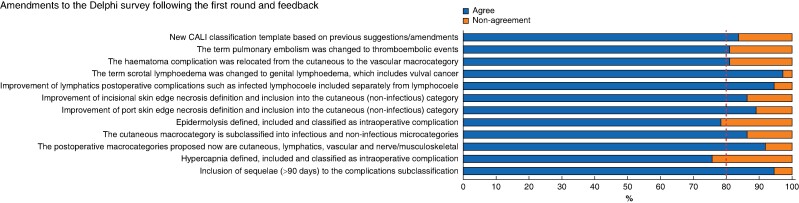
Despite reaching a consensus on all items surveyed, several amendments were made after reviewing suggestions and feedback. Supplementary material Table S1 highlights the main amendments to the Delphi survey following the first-round feedback.
The second round of the survey involved rating the agreement of the amendments made based on comments from the first round (Supplementary material Table S1). Thirty-seven of the 47 initial experts (78.7%) responded in the second round. All the amendments surpassed the minimum of greater than 80% agreement (agree or strongly agree), except for two items: whether ‘hypercapnia should be classified as an intraoperative complication, defined and included’ and whether ‘epidermolysis should be classified, defined and included within cutaneous macrocategory,’ which reached 75.7% (28 of 37) and 78.4% (29 of 37) agreement respectively. Of note, a consensus was reached for these two items in the first round. However, based on comments, they were assessed again in the second round, and here did not pass the 80% threshold (Fig. 2).
Fig. 2.
Sample round 2 results following the first round and feedback
CALI, complications and adverse events in lymphadenectomy of the inguinal area.
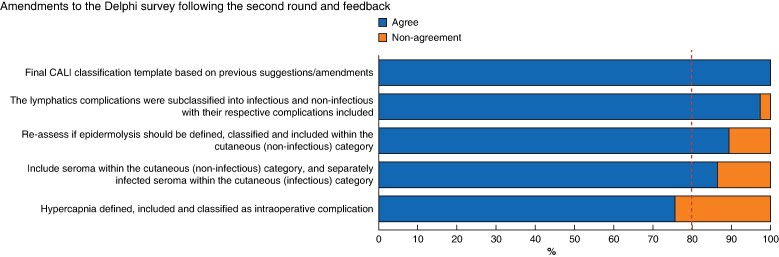
The main improvements agreed upon during the second round were the inclusion of sequelae (>90 days) to the complication subclassification system (95% agreement, 35 of 37) and restructuring macrocategories based on location/system, such as cutaneous (86.5% agreement, 32 of 37), lymphatics (95% agreement, 35 of 37), vascular (81% agreement, 30 of 37) and nerve/musculoskeletal (95% agreement, 35 of 37), rather than mixed aetiology and site of complication that was proposed before. The inclusion of infectious and non-infectious microcategories was included within the cutaneous macrocategory. Supplementary material Table S2 highlights the main amendments to the Delphi survey following the second round and feedback. For more details regarding each complication definition and inclusion, see Supplementary material appendices. Cronbach’s α for the second round of the Delphi process was 0.88 (indicating good IRR agreement37).
The third and final round consisted of assessing the rate of agreement for the two items not surpassing the threshold in round two and amendments made based on feedback from the second round (Supplementary material Table S2). All 37 experts (100%) that participated in previous rounds responded in this round. Consensus was reached on all surveyed items. This round’s main amendment was the inclusion of infectious and non-infectious microcategories with their respective complications within the lymphatics macrocategory (97.3% agreement, 36 of 37). These microcategories were re-affirmed within the cutaneous macrocategory (86.5% agreement, 32 of 37) (Fig. 3).
Fig. 3.
Sample round 3 results following the second round and feedback
CALI, complications and adverse events in lymphadenectomy of the inguinal area.
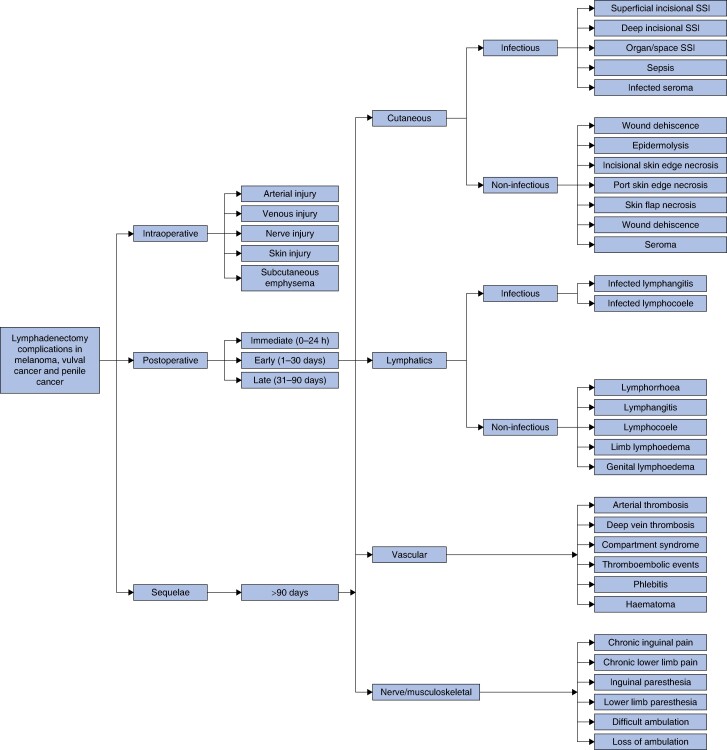
Unanimous agreement (100%, 37 of 37) was achieved with the final standardized classification system for reporting ILND complications in melanoma, vulval cancer and penile cancer (Fig. 4). IRR agreement was higher compared with the previous round (Cronbach’s α: 0.90).
Fig. 4.
Standardized classification system for reporting ILND complications in melanoma, vulval cancer and penile cancer
ILND, inguinal lymph node dissection; SSI, surgical site infection.
Discussion
In this study, a new CALI classification system to report complications associated with ILND was created. The CALI group’s end goal is to help decrease the morbidity rate associated with this procedure and to provide a standardized system to classify and report perioperative complications using common and widely accepted terminology.
A previous systematic review demonstrated that classification systems are not properly utilized in the literature1. Despite the existence of several published guidelines to standardize complication reporting, only 25% of studies report at least half of the minimum requirements for complication reporting1,17,27,38, highlighting a need for improvement.
Improving outcomes associated with ILND has several challenges. First, it is performed in the setting of rare diseases; hence, collecting a large number of patients to boost the power of the studies is difficult. Secondly, the centres of expertise are sparse, so the definitions of complications are highly variable in the literature and are mostly based on the surgeon’s personal experiences. Therefore, having a standardized system for classifying ILND complications and AEs will enable an increased body of data on this topic that can inform targets to improve patient outcomes.
This classification has several macrocategories for postoperative AEs based on location/system (cutaneous, lymphatics, vascular and nerve/musculoskeletal). Additionally, the cutaneous and lymphatics macrocategories were subclassified into infectious and non-infectious microcategories to avoid overlapping classifications. The categories were initially proposed and further refined through surveying experts. Theoretically, the macrocategories will aid in identifying common aetiologies for several complications. For instance, evidence suggests that a minimally invasive approach decreases the rate of infectious and cutaneous complications, but it is less clear if the lymphatic system morbidity rate was impacted1,11,16,39–41. The development of targeted interventions for identifying lymphatic leaks and assessing the impact of advanced energy devices is needed42–45.
An advantage of this system is that microcategory definitions were clear and standardized. For example, terms like ‘wound dehiscence’ and ‘skin flap necrosis’46 can be used interchangeably in the literature, leading to misinterpretation of true incidence.
There are limitations to this consensus study. Although this classification system was developed through surveying experts from different specialties that use different surgical approaches, it might not be representative of all surgeons. Initially, the assessment will focus on quantifying IRR across various expertise strata, followed by an external validation phase to confirm the system’s applicability and reliability in diverse clinical environments. This phase represents a pivotal progression towards the prospective application of the classification in studies related to ILNDs, with potential substantial implications for the field.
Supplementary Material
Contributor Information
René Sotelo, Catherine and Joseph Aresty Department of Urology, University of Southern California Institute of Urology, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
Aref S Sayegh, Catherine and Joseph Aresty Department of Urology, University of Southern California Institute of Urology, Keck School of Medicine, University of Southern California, Los Angeles, California, USA; Department of Surgery, MedStar Franklin Square Medical Center, Baltimore, Maryland, USA.
Luis G Medina, Catherine and Joseph Aresty Department of Urology, University of Southern California Institute of Urology, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
Laura C Perez, Catherine and Joseph Aresty Department of Urology, University of Southern California Institute of Urology, Keck School of Medicine, University of Southern California, Los Angeles, California, USA; Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Anibal La Riva, Catherine and Joseph Aresty Department of Urology, University of Southern California Institute of Urology, Keck School of Medicine, University of Southern California, Los Angeles, California, USA; Department of General Surgery, Digestive Disease & Surgery Institute, Cleveland Clinic Foundation, Cleveland, Ohio, USA.
Michael B Eppler, Catherine and Joseph Aresty Department of Urology, University of Southern California Institute of Urology, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
José Gaona, Universidad de Santander, Instituto Uromédica, Bucaramanga, Colombia.
Marcos Tobias-Machado, Department of Urology, Instituto do Câncer Arnaldo Vieira de Carvalho, São Paulo, Brazil.
Philippe E Spiess, Department of Genitourinary Oncology and Tumor Biology, Moffitt Cancer Center, Tampa, Florida, USA.
Curtis A Pettaway, The University of Texas, M.D. Anderson Cancer Center, Houston, Texas, USA.
Antonio Carlos Lima Pompeo, Department of Urology, Faculdade de Medicina do ABC, São Paulo, Brazil.
Pablo Aloisio Lima Mattos, Department of Urology, Associação Piauiense de Combate ao Câncer, Teresina, Piauí, Brazil.
Timothy G Wilson, Department of Urology, Providence St. John’s Cancer Institute, Santa Monica, California, USA.
Gustavo M Villoldo, Department of Urology, Instituto Alexander Fleming, Buenos Aires, Argentina.
Eric Chung, Department of Urology, Princess Alexandra Hospital, University of Queensland, Brisbane, Queensland, Australia.
Aldo Samaniego, Department of Urology, Servicio de Urología del Hospital Central del Instituto de Previsión Social, Asunción, Paraguay.
Antonio Augusto Ornellas, Departamento de Urologia, Instituto Nacional do Câncer do Brasil (INCA), Rio de Janeiro, Brazil.
Vladimir Pinheiro, Department of Urology, AC Camargo Cancer Center, São Paulo, Brazil.
Eder S Brazão, Jr, Department of Urology, AC Camargo Cancer Center, São Paulo, Brazil.
David Subira-Rios, Department of Urology, Gregorio Marañon Universitary Hospital, Madrid, Spain.
Leandro Koifman, Serviço de Urologia, Hospital Municipal Souza Aguiar, Rio de Janeiro, Rio de Janeiro, Brazil.
Stênio de Cassio Zequi, Department of Urology, AC Camargo Cancer Center-São Paulo, São Paulo, Brazil; Department of Urology, National Institute for Science and Technology in Oncogenomics and Therapeutic Innovation, São Paulo, Brazil; Graduate School of Urology, Escola Paulista de Medicina-Universidade Federal de São Paulo, São Paulo, Brazil.
Humberto M Pontillo Z, Department of Surgery, Sant Jaume of Calella Hospital, Barcelona, Spain.
José de Ribamar Rodrigues Calixto, Department of Medicine II, Federal University of Maranhão, São Luís, Massachusetts, Brazil.
Rafael Campos Silva, Department of Urology, Hospital Universitário Presidente Dutra—HUPD/UFMA, São Luís, Maranhão, Brazil.
B Mark Smithers, University of Queensland, Queensland Melanoma Project, Princess Alexandra Hospital, Brisbane, Queensland, Australia.
Simone Garzon, Department of Surgery, Dentistry, Pediatrics, and Gynecology, University of Verona, Verona, Italy.
Oliver Haase, Department of Surgery, University Medicine Berlin—Charité, Berlin, Germany.
Antonio Sommariva, Veneto Institute of Oncology Institute Oncology Veneto, Istituto Di Ricovero e Cura a Carattere Scientifico, Padova, Italy.
Robert Fruscio, Department of Medicine and Surgery, University of Milan Bicocca, Azienda Socio Sanitaria Territoriale Monza, Italy.
Francisco Martins, Department of Urology, Centro Hospitalar Universitário Lisboa Norte, Hospital de Santa Maria, Lisbon, Portugal.
Pedro S de Oliveira, Department of Urology, Centro Hospitalar Universitário Lisboa Norte, Hospital de Santa Maria, Lisbon, Portugal.
Giovanni Battista Levi Sandri, Department of Surgery, ASL Frosinone, Frosinone, Italy.
Marco Clementi, Department of Medicine, Health and Life, University of L'Aquila, L'Aquila, AQ, Italy.
Juan Astigueta, Department of Urology, Universidad Privada Antenor Orrego, Trujillo, Perú.
Islam H Metwally, Surgical Oncology Department, Oncology Center Mansoura University (OCMU), Mansoura, Egypt.
Rasiah Bharathan, Department of Gynaecological Oncology, Medical University of Vienna, Vienna, Austria.
Tarun Jindal, Department of Uro-oncology, Narayana Super Speciality Hospital, Howrah, India.
Yasuhiro Nakamura, Department of Skin Oncology/Dermatology, Saitama Medical University International Medical Center, Saitama, Japan.
Hisham Abdel Mageed, Surgical Oncology Department, National Cancer Institute Cairo University, Cairo, Egypt.
Sakthiushadevi Jeevarajan, Department of Surgical Oncology, Regional Cancer Centre, Kanchipuram, Tamil Nadu, India.
Ramón Rodriguez Lay, Clinic Urology Service, Complejo Hospitalario Metropolitano, Madrid, Panama.
Herney Andrés García-Perdomo, Division of Urology/Urooncology, Department of Surgery, School of Medicine, Universidad del Valle, Cali, Colombia.
Omaira Rodríguez González, Chief of Surgical Department, Clínicas Caracas Hospital, Faculty of Medicine, Central University of Venezuela, Caracas, Venezuela.
Saum Ghodoussipour, Sections of Urologic Oncology, Rutgers Cancer Institute of New Jersey and Rutgers Robert Wood Johnson Medical School, New Brunswick, New Jersey, USA.
Inderbir Gill, Catherine and Joseph Aresty Department of Urology, University of Southern California Institute of Urology, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
Giovanni E Cacciamani, Catherine and Joseph Aresty Department of Urology, University of Southern California Institute of Urology, Keck School of Medicine, University of Southern California, Los Angeles, California, USA.
Funding
The authors have no funding to declare.
Disclosure
Inderbir S. Gill has equity interest in OneLine Health and Karkinos, USA. The authors declare no other conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Author contributions
René Sotelo (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Visualization, Writing—original draft, Writing—review & editing), Aref S. Sayegh (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing—original draft, Writing—review & editing), Luis G. Medina (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing—original draft, Writing—review & editing), Laura C. Perez (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing—original draft, Writing—review & editing), Anibal La Riva (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing—original draft, Writing—review & editing), Michael B. Eppler (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing—original draft, Writing—review & editing), Jose Gaona (Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing—review & editing), Marcos Tobias-Machado (Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing—review & editing), Philippe E. Spiess (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Curtis A. Pettaway (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Antonio Carlos Lima Pompeo (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Pablo Aloisio Lima Mattos (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Timothy G. Wilson (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Gustavo Villoldo (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Eric Chung (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Aldo Samaniego (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Antonio Augusto Ornellas (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Vladimir Pinheiro (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Eder S. Brazao Jr (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), David Subira-Rios (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Leandro Koifman (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Stênio de Cassio Zequi (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Humberto M. Pontillo. Z (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), José de Ribamar Rodrigues Calixto (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Rafael Campos Silva (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), B. Mark Smithers (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Simone Garzon (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Oliver Haase (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Antonio Sommariva (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Robert Fruscio (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Francisco Martins (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Pedro S. de Oliveira (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Giovanni Battista Levi Sandri (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Marco Clementi (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Juan Astigueta (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Islam H. Metwally (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Rasiah Bharathan (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Tarun Jindal (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Yasuhiro Nakamura (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Hisham Abdel Mageed (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Sakthiushadevi Jeevarajan (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Ramon Rodriguez Lay (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Herney García-Perdomo (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Omaira Rodríguez González (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Saum Ghodoussipour (Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing), Inderbir Gill (Conceptualization, Data curation, Investigation, Methodology, Supervision, Writing—review & editing) and Giovanni E. Cacciamani (Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Visualization, Writing—original draft, Writing—review & editing)
References
- 1. Cacciamani GE, Medina LG, Sayegh AS, La Riva A, Perez LC, Eppler MB et al. Assessment and reporting of perioperative adverse events and complications in patients undergoing inguinal lymphadenectomy for melanoma, vulval cancer, and penile cancer: a systematic review and meta-analysis. World J Surg 2023;47:962–974 [DOI] [PubMed] [Google Scholar]
- 2. Greer BE, Koh W-J. New NCCN guidelines for vulval cancer. J Natl Compr Canc Netw 2016;14:656–658 [DOI] [PubMed] [Google Scholar]
- 3. Swetter SM, Thompson JA, Albertini MR, Barker CA, Baumgartner J, Boland G et al. NCCN guidelines® insights: melanoma: cutaneous, version 2.2021: featured updates to the NCCN guidelines. J Natl Compr Canc Network 2021;19:364–376 [DOI] [PubMed] [Google Scholar]
- 4. Clark PE, Spiess PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE et al. Penile cancer. J Natl Compr Canc Network 2013;11:594–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hakenberg OW, Compérat EM, Minhas S, Necchi A, Protzel C, Watkin N. EAU guidelines on penile cancer: 2014 update. Eur Urol 2015;67:142–150 [DOI] [PubMed] [Google Scholar]
- 6. Matin SF, Cormier JN, Ward JF, Pisters LL, Wood CG, Dinney CP et al. Phase 1 prospective evaluation of the oncological adequacy of robotic assisted video-endoscopic inguinal lymphadenectomy in patients with penile carcinoma. BJU Int 2013;111:1068–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wills A, Obermair A. A review of complications associated with the surgical treatment of vulval cancer. Gynecol Oncol 2013;131:467–479 [DOI] [PubMed] [Google Scholar]
- 8. Chang SB, Askew RL, Xing Y, Weaver S, Gershenwald JE, Lee JE et al. Prospective assessment of postoperative complications and associated costs following inguinal lymph node dissection (ILND) in melanoma patients. Ann Surg Oncol 2010;17:2764–2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sood A, Rudzinski JK, Spiess PE, Pettaway CA. The acute complications after surgery for penile carcinoma and strategies for their management: a systematic review of the literature. Semin Oncol Nurs 2022;38:151285. [DOI] [PubMed] [Google Scholar]
- 10. Spiess PE, Hernandez MS, Pettaway CA. Contemporary inguinal lymph node dissection: minimizing complications. World J Urol 2009;27:205–212 [DOI] [PubMed] [Google Scholar]
- 11. Chua KJ, Balraj V, Patel HV, Srivastava A, Doppalapudi SK, Elsamra SE et al. Wound complication rates after inguinal lymph node dissection: contemporary analysis of the NSQIP database. J Am Coll Surg 2023;236:18–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel KN, Salunke A, Bakshi G, Jayaprakash D, Pandya SJ. Robotic-assisted video-endoscopic inguinal lymphadenectomy (RAVEIL) and video-endoscopic inguinal lymphadenectomy (VEIL) versus open inguinal lymph-node dissection (OILND) in carcinoma of penis: comparison of perioperative outcomes, complications and oncological outcomes. A systematic review and meta-analysis. Urol Oncol 2022;40:112.e11–112.e22 [DOI] [PubMed] [Google Scholar]
- 13. Muñoz Guillermo V, Rosino Sánchez A, Rivero Guerra Á, Barceló Bayonas I, Pardo Martínez A, Jiménez Peralta D et al. Video endoscopic inguinal lymphadenectomy in penile cancer: systematic review. Arch Esp Urol 2019;72:992–999 [PubMed] [Google Scholar]
- 14. Gkegkes ID, Minis EE, Iavazzo C. Robotic-assisted inguinal lymphadenectomy: a systematic review. J Robot Surg 2019;13:1–8 [DOI] [PubMed] [Google Scholar]
- 15. Azizi M, Chipollini J, Peyton CC, Cheriyan SK, Spiess PE. Current controversies and developments on the role of lymphadenectomy for penile cancer. Urol Oncol 2019;37:201–208 [DOI] [PubMed] [Google Scholar]
- 16. Shao Y, Hu X, Ren S, Liao D, Yang Z, Liu Y et al. Comparison of different surgical methods and strategies for inguinal lymph node dissection in patients with penile cancer. Sci Rep 2022;12:2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mitropoulos D, Artibani W, Graefen M, Remzi M, Rouprêt M, Truss M. Reporting and grading of complications after urologic surgical procedures: an ad hoc EAU guidelines panel assessment and recommendations. Eur Urol 2012;61:341–349 [DOI] [PubMed] [Google Scholar]
- 18. Şahinoğlu E, Ergin G, Karadibak D. The agreement between three classification systems used to grade the severity of lymphedema in patients with upper and lower extremity lymphedema: a retrospective study. Physiother Theory Pract 2022;40:874–879 [DOI] [PubMed] [Google Scholar]
- 19. Cacciamani GE, Sholklapper T, Dell'Oglio P, Rocco B, Annino F, Antonelli A et al. The intraoperative complications assessment and reporting with universal standards (ICARUS) global surgical collaboration project: development of criteria for reporting adverse events during surgical procedures and evaluating their impact on the postoperative course. Eur Urol Focus 2022;8:1847–1858 [DOI] [PubMed] [Google Scholar]
- 20. Cacciamani G, Sholklapper T, Sotelo R, Desai M, Gill I. A protocol for the development of the intraoperative complications assessment and reporting with universal standards criteria: the ICARUS project. Int J Surg Protocols 2021;25:160–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clavien PA, Barkun J, De Oliveira ML, Vauthey JN, Dindo D, Schulick RD et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–196 [DOI] [PubMed] [Google Scholar]
- 22. Cacciamani GE, Eppler M, Sayegh AS, Sholklapper T, Mohideen M, Miranda G et al. Recommendations for intraoperative adverse events data collection in clinical studies and study protocols. An ICARUS global surgical collaboration study. Int J Surg: Protoc 2023;27:23–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sayegh AS, Eppler M, Ballon J, Hemal S, Goldenberg M, Sotelo R et al. Strategies for improving the standardization of perioperative adverse events in surgery and anesthesiology: “the long road from assessment to collection, grading and reporting”. J Clin Med 2022;11:5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eppler M, Sayegh AS, Goldenberg M, Sholklapper T, Hemal S, Cacciamani GE. If you know them, you avoid them: the imperative need to improve the narrative regarding perioperative adverse events. J Clin Med 2022;11:4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cacciamani GE, Sholklapper T, Dell-Kuster S, Biyani SC, Francis N, Kaafarani HM et al. Standardizing the intraoperative adverse events assessment to create a positive culture of reporting errors in surgery and anesthesiology. Ann Surg 2022;276:e75–e76 [DOI] [PubMed] [Google Scholar]
- 26. Soliman C, Mulholland CJ, Santaguida P, Sathianathen NJ, Lawrentschuk N, Giannarini G et al. Protocol for CAMUS Delphi study: a consensus on comprehensive reporting and grading of complications after urological surgery. Eur Urol Focus 2022;8:1493–1511 [DOI] [PubMed] [Google Scholar]
- 27. Sayegh AS, Eppler M, Sholklapper T, Goldenberg MG, Perez LC, La Riva A et al. Severity grading systems for intraoperative adverse events. A systematic review of the literature and citation analysis. Ann Surg 2023;278:e973–e980 [DOI] [PubMed] [Google Scholar]
- 28. Clayton MJ. Delphi: a technique to harness expert opinion for critical decision-making tasks in education. Educ Psychol 1997;17:373–386 [Google Scholar]
- 29. Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of internet E-surveys (CHERRIES). J Med Int Res 2004;6:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gattrell WT, Logullo P, van Zuuren EJ, Price A, Hughes EL, Blazey P et al. ACCORD (ACcurate COnsensus reporting document): a reporting guideline for consensus methods in biomedicine developed via a modified Delphi. PLoS Med 2024;21:e1004326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Naderifar M, Goli H, Ghaljaie F. Snowball sampling: a purposeful method of sampling in qualitative research. Strides Dev Med Educ 2017;14. doi: 10.5812/sdme.67670 [DOI] [Google Scholar]
- 32. U.S. Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf
- 33. Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Healthcare Quality Promotion (DHQP) . https://www.cdc.gov/nhsn/pdfs/opc/opc-ssi-protocol-current-508.pdf
- 34. Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR et al. Centers for Disease Control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg 2017;152:784–791 [DOI] [PubMed] [Google Scholar]
- 35. The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020;53:3–19 [PubMed] [Google Scholar]
- 36. Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315:762–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Graham B, Regehr G, Wright JG. Delphi as a method to establish consensus for diagnostic criteria. J Clin Epidemiol 2003;56:1150–1156 [DOI] [PubMed] [Google Scholar]
- 38. Sholklapper TN, Ballon J, Sayegh AS, La Riva A, Perez LC, Huang S et al. Bibliometric analysis of academic journal recommendations and requirements for surgical and anesthesiologic adverse events reporting. Int J Surg 2023;109:1489–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tobias-Machado M, Tavares A, Molina WR Jr, Forseto PH Jr, Juliano RV, Wroclawski ER. Video endoscopic inguinal lymphadenectomy (VEIL): minimally invasive resection of inguinal lymph nodes. Int Braz J Urol 2006;32:316–321 [DOI] [PubMed] [Google Scholar]
- 40. Tobias-Machado M, Tavares A, Silva MNR, Molina J, Rica W, Forseto PH et al. Can video endoscopic inguinal lymphadenectomy achieve a lower morbidity than open lymph node dissection in penile cancer patients? J Endourol 2008;22:1687–1692 [DOI] [PubMed] [Google Scholar]
- 41. Tobias-Machado M, Corona-Montes VE, Moschovas MC, Sotelo RJ. Robotic-assisted video endoscopic inguinal lymphadenectomy (R-VEIL) technique and outcomes for penile cancer. In: Wiklund P, Mottrie A, Gundeti MS, Patel V (eds.), Robotic Urologic Surgery. Cham: Springer International Publishing, 2022, 843–855 [Google Scholar]
- 42. Yamamoto T, Yamamoto N, Doi K, Oshima A, Yoshimatsu H, Todokoro T et al. Indocyanine green-enhanced lymphography for upper extremity lymphedema: a novel severity staging system using dermal backflow patterns. Plast Reconstr Surg 2011;128:941–947 [DOI] [PubMed] [Google Scholar]
- 43. Yamamoto T, Narushima M, Doi K, Oshima A, Ogata F, Mihara M et al. Characteristic indocyanine green lymphography findings in lower extremity lymphedema: the generation of a novel lymphedema severity staging system using dermal backflow patterns. Plast Reconstr Surg 2011;127:1979–1986 [DOI] [PubMed] [Google Scholar]
- 44. van Heumen S, Riksen JJ, Bramer WM, van Soest G, Vasilic D. Imaging of the lymphatic vessels for surgical planning: a systematic review. Ann Surg Oncol 2023;30:462–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gerken ALH, Herrle F, Jakob J, Weiß C, Rahbari NN, Nowak K et al. Definition and severity grading of postoperative lymphatic leakage following inguinal lymph node dissection. Langenbeck's Arch Surg 2020;405:697–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. West C, Saleh D, Peach H. Combined clearance of pelvic and superficial nodes for clinical groin melanoma. J Plas Reconstr Aesthet Surg 2014;67:1711–1718 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.