Abstract
Obesity is associated with hypogonadism in males, characterized by low testosterone and sperm number. Previous studies determined that these stem from dysregulation of hypothalamic circuitry that regulates reproduction, by unknown mechanisms. Herein, we used mice fed chronic high-fat diet, which mimics human obesity, to determine mechanisms of impairment at the level of the hypothalamus, in particular gonadotropin-releasing hormone (GnRH) neurons that regulate luteinizing hormone (LH), which then regulates testosterone. Consistent with obese humans, we demonstrated lower LH, and lower pulse frequency of LH secretion, but unchanged pituitary responsiveness to GnRH. LH pulse frequency is regulated by pulsatile GnRH secretion, which is controlled by kisspeptin. Peripheral and central kisspeptin injections, and DREADD-mediated activation of kisspeptin neurons, demonstrated that kisspeptin neurons were suppressed in obese mice. Thus, we investigated regulators of kisspeptin secretion. We determined that the LH response to NMDA was lower in obese mice, corresponding to fewer glutamate receptors in kisspeptin neurons, which may be critical for kisspeptin synchronization. Given that kisspeptin neurons also interact with anorexigenic POMC neurons, which are affected by obesity, we examined their cross talk, and determined that the LH response to either DREADD-mediated activation of POMC neurons or central injection of αMSH, a product of POMC, is abolished in obese mice. This was accompanied by diminished levels of αMSH receptor, MC4R, in kisspeptin neurons. Together, our studies determined that obesity leads to the downregulation of receptors that regulate kisspeptin neurons, which is associated with lower LH pulse frequency, leading to lower LH and hypogonadism.
Keywords: GnRH, high-fat diet, kisspeptin, obesity, POMC
Significance Statement
Obesity presents a significant health concern, with multiple comorbidities, including impaired reproduction. However, mechanisms are not clear, and studies are confounded by the chronic nature of this condition that leads to synaptic changes and alterations in neuron responsiveness to stimuli. Here, we demonstrate that the interaction between feeding circuitry and reproductive circuitry is altered by chronic obesity. The reason may be that chronically higher activity of POMC neurons in response to higher leptin in obesity downregulates αMSH receptors on target neurons, including kisspeptin. This may lead to the suppression of kisspeptin neurons, and their inability to regulate pulsatile secretion of GnRH, which then lowers LH pulse frequency, leading to lower LH in the circulation, lower testosterone, and lower sperm count.
Introduction
Reproduction is an energy-demanding process and proper regulation relies on the intricate interplay between the reproductive and feeding circuits in the hypothalamus. Obesity has been increasing in incidence (The Lancet Public Health, 2018) and is one of the most prevalent endocrine disorders affecting fertility in men (Sermondade et al., 2013; Santi et al., 2024). Obese men experience hypogonadism, a decrease in testosterone, sperm count, and sperm quality (Hammoud et al., 2008; Du Plessis et al., 2010). Our group and others determined that obese males experience decreased luteinizing hormone (LH) and testosterone levels, which points to a central dysregulation (Jensen et al., 2004; Wu et al., 2008; Sanchez-Garrido et al., 2014; Lainez et al., 2018). However, clear consensus in understanding of underlying mechanisms was confounded by different models: genetic or diet-induced obesity (DIO), length of diet exposure, diet composition, or whether leptin resistance was established. Here, we analyzed changes in reproductive circuitry in the hypothalamus, during chronic DIO, to mimic the human condition.
LH levels, and fertility in general, rely on the tight regulation of pulsatile gonadotropin-releasing hormone (GnRH) release from neurons that are scattered in the rostral forebrain and extend long processes to the median eminence (Goodman et al., 2022). Pulsatile GnRH secretion is regulated by afferent neurons, primarily kisspeptin neurons located in the arcuate nucleus (ARC; Pielecka-Fortuna et al., 2008; Novaira et al., 2014; Herbison, 2018). Recent findings revealed that kisspeptin neurons may synchronize their own pulse initiation via glutamate signaling (Voliotis et al., 2021; Han et al., 2023). Dysregulation of kisspeptin function can significantly impact fertility (Gottsch et al., 2011; Ronnekleiv and Kelly, 2013; Manfredi-Lozano et al., 2018). Kisspeptin neurons integrate inputs from the feeding circuitry, composed of several populations in the hypothalamus. One such population is proopiomelanocortin (POMC) that receives information on energy stores via leptin from the adipose tissue. The activity of POMC neurons increases in obesity, due to elevated leptin, which then stimulates satiety and energy expenditure (Cowley et al., 2001). POMC neurons secrete alpha melanocyte-stimulating hormone (αMSH) that acts on the melanocortin 4 receptors (MC4R) in target neurons, including kisspeptin (Fu and van den Pol, 2010; Cravo et al., 2011; Stincic and Kelly, 2022). Central injection of αMSH or an MC4R agonist stimulates LH secretion, through kisspeptin neurons (Celis, 1985; Backholer et al., 2010; Manfredi-Lozano et al., 2016). In agreement, optogenetic activation of POMC neurons activates ARC kisspeptin neurons (Stincic et al., 2018). Although our understanding of this interplay between POMC and kisspeptin neurons is beginning to emerge, whether this is altered in chronic conditions such as obesity is unknown.
Chronic obesity leads to adaptations in the POMC network, which in turn can negatively impact reproductive circuitry. Obesity leads to a reduced number of synaptic contacts to POMC neurons (Horvath et al., 2010; Paeger et al., 2017). High-fat diet increases POMC mRNA expression and increases POMC neuron excitability (Ziotopoulou et al., 2000; Benani et al., 2012). These findings suggest a complex relationship between the regulation of POMC neurons and obesity, wherein changes can impact the ability of POMC neurons to regulate energy homeostasis. Given the interconnectedness of the reproductive and feeding circuits, dysregulation of POMC neurons may have deleterious effects on reproductive health. This knowledge gap underscores the need for further research to elucidate the underlying mechanisms of obesity-mediated reproductive dysfunction.
In this study, we employed a high-fat diet (HFD) mouse model to investigate the effects of obesity on reproductive function. Our previous publications analyzed mechanisms of impaired reproductive function in obesity and determined lower LH specifically in males, but not in females (Lainez et al., 2018). We further determined changes in the pituitary transcriptomes using scRNA-sequencing (Ruggiero-Ruff et al., 2024) and changes in neuropeptide and synaptic molecule gene expression in the hypothalamus, using a custom NanoString probe panel (Lainez et al., 2018; Chen et al., 2021b). These changes were more pronounced in males than females (Lainez et al., 2018; Chen et al., 2021b). We also reported neuroinflammatory changes that were specific for males (Lainez et al., 2018; Chen et al., 2021a,b; Li et al., 2023). Here, we identified that HFD led to a remarkable increase in LH responsiveness to kisspeptin, accompanied by unchanged pituitary responsiveness, in obese male mice, suggesting dysregulation of kisspeptin neurons. Chemogenetic activation of kisspeptin and POMC neurons, along with investigation of innervation, revealed downregulation of several receptors in kisspeptin neurons that are important for kisspeptin synchronization. This change in connectivity is associated with alteration in the interaction between POMC and kisspeptin neurons in HFD. The disruption in the cross talk between the feeding and reproductive circuitry may provide a mechanistic link between HFD-induced obesity and reproductive dysfunction in obese men.
Materials and Methods
Animals
All animal procedures were performed with the approval of the University of California, Riverside, Institutional Animal Care and Use Committee under the National Institute of Health Animal Care and Use Guidelines. Three-week-old male C57BL/6J mice were obtained from Jackson Laboratory, acclimated for 1 week in our facility and randomly assigned to the high-fat diet group [HFD, catalog #D12492, 60% kcal from fat; 5.21 kcal/g; carbohydrate 20% kcal, protein 20% kcal, fat 60% kcal (lard 0.32 g/g diet, soybean oil 0.03 g/g); Research Diets] or the control diet group [CTR, D12450J, 10% kcal from fat; matching sucrose levels to HFD; 3.82 kcal/g; carbohydrate 70% kcal, protein 20% kcal, fat 10% kcal (lard 0.02 g/g diet, soybean oil 0.025 g/g); Research Diets] for 12 weeks before experimentation. Mice were maintained on a 12 h light/dark cycle and received food and water ad libitum. All mice were handled daily for 2 weeks prior to experiments by experienced lab personnel to habituate them to handling and reduce stress.
To visualize GnRH neurons, Gnrh1-GFP mice were kindly provided by Suzanne Moenter (Suter et al., 2000). To target fluorescence to kisspeptin neurons, Kiss1-Cre+/− mice were obtained from Jackson Laboratory (strain 023426) and crossed with ROSA26-EGFP mice, also from Jackson Laboratory (strain 004077; Mao et al., 2001; Cravo et al., 2011). POMC1-Cre+/− mice were obtained from Jackson Laboratory (strain 005965; Balthasar et al., 2004). All were randomly placed on CTR or HFD at 4 weeks of age for 12 weeks.
LH secretion
LH pulsatile secretion was measured by tail-tip blood sampling. Two weeks prior to sampling, mice were acclimated to handling by daily tail massage. For the experiment, a small nick was made at the tip of the tail, and 10 µl of blood was sampled every 8 min for 180 min (Czieselsky et al., 2016; McCosh et al., 2018; Kreisman et al., 2020). An ultrasensitive ELISA (Steyn et al., 2013) was used to detect LH levels, in 10 µl of blood diluted with 40 µl of 0.1 M PBS, 0.05% Tween 20, 0.2% BSA. The capture antibody was obtained from Janet Roser, University of California, Davis (anti-bovine LH beta subunit, 518B7), the detection polyclonal antibody (rabbit LH antiserum, AFP240580Rb) was obtained from the National Hormone and Peptide Program (NHPP), and HRP-conjugated polyclonal antibody (goat anti-rabbit) was purchased from DakoCytomation (D048701-2). Mouse LH reference (AFP5306A; NHPP) was used as the assay standard. Assay sensitivity was 0.016 ng/ml, the intra-assay coefficient of variation 2.2%, and the inter-assay coefficient of variation 7.3% at the low end of the curve. LH amplitude was determined by subtracting the basal LH value before the onset of the pulse from the peak level. LH pulse frequency was determined using the freeware DynPeak algorithm (Vidal et al., 2012).
Stereotaxic injections
Adeno-associated virus AAV9 driving the hM3D(Gq)-mCherry (hM3Dq) under the human synapsin promoter (AAV9-hSyn-DiO-hM3D(Gq)-mCherry, 44361-AAV9, Addgene) was used to express designer receptors activated only by designer drugs (DREADDs) specifically in kisspeptin or POMC neurons. Kiss1-Cre+/−-EGFP or POMC-Cre+/− mice (n = 6–8 per group) were fed CTR or HFD for 12 weeks as indicated above. After 12 weeks of diet exposure, mice were anesthetized with isoflurane and placed in a stereotaxic apparatus (Digital Stereotaxic Instrument, SGL M, Model: 68803, RWD) with head and nose fixed. The arcuate nucleus of the hypothalamus was targeted bilaterally using coordinates from bregma: ML: ±0.25 mm; AP: −1.32 mm; DV: −5.85 mm from the dura. In total, 400 nl of hM3Dq (5.4 × 1012 GC/ml) or control virus (AAV9-hSyn-DIO-mCherry, 50459-AAV9, Addgene) was injected at a rate of 50 nl per minute with a 1 µl 26-gauge Hamilton syringe. After injection, the syringe was left in situ for another 5 min and then, slowly over a minute, removed. Mice were placed on their respective diets for additional 2 weeks. To analyze LH secretion in response to kisspeptin and POMC activation, three baseline LH measurements were collected prior to clozapine-N-oxide injection (CNO; 34233-69-7, MedChemExpress). CNO doses of 0.3 or 1 mg/kg CNO, or saline as control, were injected intraperitoneally and tail-tip blood collected at times indicated in the figures. One week later, treatments with CNO or saline were switched while mice were fed their respective diets.
Intraperitoneal injections
To determine pituitary or GnRH neuron responsiveness of CTR and HFD fed mice, 16-week-old animals were injected intraperitoneally with 1 µg/kg GnRH (L7134-5MG, Sigma-Aldrich), 1 mg/kg kisspeptin (445888-1MG, Millipore Sigma), or saline. Two blood samples were taken 10 min apart prior to injection to obtain basal levels of LH. After injection, 10 µl of tail-tip blood was sampled every 10 min at time points indicated in each figure. Samples were assayed for LH using the ultrasensitive ELISA as described above.
Intracerebroventricular injections
Fourteen-week-old C57B/J6 male mice fed CTR or HFD for 10 weeks (n = 6–7 per treatment group) were secured in a stereotaxic frame (Digital Stereotaxic Instrument, SGL M, Model: 68803, RWD), placed on 37°C heat pad, and anesthetized with isoflurane. Stereotaxic coordinates to target the lateral ventricle (ML: 1 mm; AP: −0.5 mm; DV: −2.5 mm), according to the Paxinos and George mouse brain atlas, were used for guide cannula implantation (Cannula kit#3 26G 5MM, 126GA5MMKTT, Plastics 1 Technologies). The guide cannula was secured with dental cement and then fitted with a dummy cannula. After a 2 week recovery period on respective diets, mice received a single 2 µl intracerebroventricular injection of one of the following: 3 nmol αMSH (M4135, Sigma Aldrich), 7 nmol NMDA (HY-17551, MedChemExpress), 1 nmol kisspeptin (445888-1MG, Millipore), or sterile saline (concentrations based on previous publications: D'Aniello et al., 2000; d'Anglemont deTassigny et al., 2010; Stengel et al., 2011; Manfredi-Lozano et al., 2016). As above, 10 µl of blood was taken three times, 10 min apart, before injection to measure basal levels, after which an internal cannula attached to a Hamilton syringe was used to inject the above-described compounds. The cannula was left in place for 10 min. Blood sampling continued every 10 min for times indicated in the figure legends. Mice were allowed to roam freely in their respective cages during the procedure to reduce stress. Blood was assayed for LH, as described above. Accurate cannula placement to the lateral ventricle was determined by cresyl violet dye injection and confirmed after dissection.
Immunohistochemistry
Kisspeptin neuron innervation
To investigate the effects of DIO on kisspeptin neuron innervation, Kiss1-Cre+/−-EGFP male mice were placed on either a CTR or HFD for 12 weeks, after which mice were perfused transcardially with 20 ml 0.1 M PBS and with 20 ml 4% paraformaldehyde (PFA). Brains were dissected and post-fixed in 4% PFA for 1 h on ice, then cryoprotected with 30% sucrose in 0.1 M PBS for 48 h, and the arcuate nucleus of the hypothalamus was sectioned at 55 µm. After blocking with 10% goat serum and, if necessary with M.O.M kit (mouse on mouse immunodetection kit, BMK-2202, Vector Laboratories), sections were incubated with primary antibodies for GFP (1:10,000, chicken anti-GFP, AB13970, Abcam), rabbit anti-NR2A (1:5000, 07-632, EMD Millipore), mouse anti-GluR2 (1:5000, MAB397, EMD Millipore), guinea pig anti-VGLUT2 (1:5000, 135 404, Synaptic systems), or rabbit anti-MC4R (1:5000, AMR-024, Alomone Labs) for 72 h at 4°C. NR2A, GluR2, and MC4R were amplified with a biotinylated antibody (1:5,000, anti-rabbit biotinylated, BA-1000, or 1:5000, anti-mouse biotinylated, BA-9200; both from Vector Laboratories), overnight at 4°C followed by streptavidin-Cy5 (1:2000, 434316, Invitrogen) overnight at 4°C. For anti-GFP and anti-VGLUT2, secondary antibodies anti-chicken IgG-Alexa 488 (1:2,000, A11039, Invitrogen) and anti-guinea pig IgG-Alexa 594 (1:2,000, A11076, Invitrogen) were used, respectively, and incubated overnight at 4°C. Secondary antibody-only controls were performed to determine antibody specificity. Sections were then mounted on slides and coverslipped with VECTASHIELD Vibrance containing DAPI (H-1800, Vector Laboratories). We followed our established protocol for determining puncta density and antibody specificity (Lainez et al., 2018; Ruggiero-Ruff et al., 2023; Villa et al., 2023). Puncta in close contact, where no black pixels were visible in between, were counted in the individual neurons by an investigator blinded to the group. Puncta colocalized to the soma or within 15 µm of the proximal process in 15–40 neurons were counted from four to six mice in each group and averaged for each mouse. Z-stack images were acquired by Zeiss 880 confocal laser scanning inverted microscope, and 3D reconstruction was performed using Imaris software (Bitplane).
cFOS-positive kisspeptin and POMC neurons
Kiss1-Cre+/−-EGFP and POMC neuronal activity was analyzed by determining cFOS expression. Coronal sections containing the arcuate nucleus were stained with anti-GFP (1:5,000, chicken anti-GFP, AB13970, Abcam) to visualize kisspeptin neurons, or anti-β-endorphin (1:5,000, rabbit anti-β-endorphin, H-022-33, Phoenix Pharmaceuticals) to visualize POMC neurons, and anti-cFOS (1:10,000, guinea pig anti-cFOS, 226 308, Synaptic Systems) for 72 h at 4°C. Secondary antibodies: anti-chicken IgG-Alexa 488 (1:2000, A11039, Invitrogen); anti-rabbit IgG-Alexa 488 (1:2000, A11034, Invitrogen); biotinylated anti-guinea pig (1:5,000, BA-7000 Vector Laboratories) followed by streptavidin-Cy5 (1:2,000, 434316, Invitrogen) were incubated overnight at 4°C. Secondary antibody-only controls were performed to determine antibody specificity, reported in our previous publications (Lainez et al., 2018; Ruggiero-Ruff et al., 2023; Villa et al., 2023). Ten to 15 sections, containing over 300 neurons per animal, were quantified using Fiji ImageJ version 1.45f, and data were presented as a percent of colabeled neurons.
Viral targeting of kisspeptin and POMC
To determine the percent targeting of DREADDs in kisspeptin neurons and POMC neurons, sections from Kiss1-Cre+/−-EGFP or POMC-Cre+/− mice containing the arcuate nucleus of the hypothalamus were stained using primary antibodies for GFP (1:5,000, chicken anti-GFP, AB13970, Abcam) to visualize kisspeptin neurons or β-endorphin (1:5,000, rabbit anti-β-endorphin, H-022-33, Phoenix Pharmaceuticals) to visualize POMC, and mCherry (1:5,000, rat anti-mCherry, M11217, Invitrogen) to visualize neurons with viral expression. Secondary antibodies were anti-chicken IgG-Alexa 488 (1:2,000, A11039, Invitrogen), anti-rabbit IgG-Alexa 488 (1:2,000, A11034, Invitrogen), and anti-rat IgG-Alexa 594 (1:2,000, A11007, Invitrogen). Sections were mounted on charged slides and then coverslipped with VECTASHIELD vibrance with DAPI (H-1800, Vector Laboratories). Percent targeting was determined by dividing the number of colocalized GFP and mCherry-positive kisspeptin neurons by the total number of kisspeptin neurons and dividing the number of colocalized β-endorphin and mCherry-positive POMC neurons by the total number of POMC neurons.
Statistical analyses
Power analyses were performed using JMP statistical software (SAS). Normal distribution was determined by the Shapiro–Wilk test. Statistical differences between measurements in CTR and HFD groups (p < 0.05) were determined by t test or ANOVA as appropriate, followed by Tukey's post hoc test for multiple comparisons, using Prism software (GraphPad).
Results
Male mice on a high-fat diet experience lower LH pulse frequency
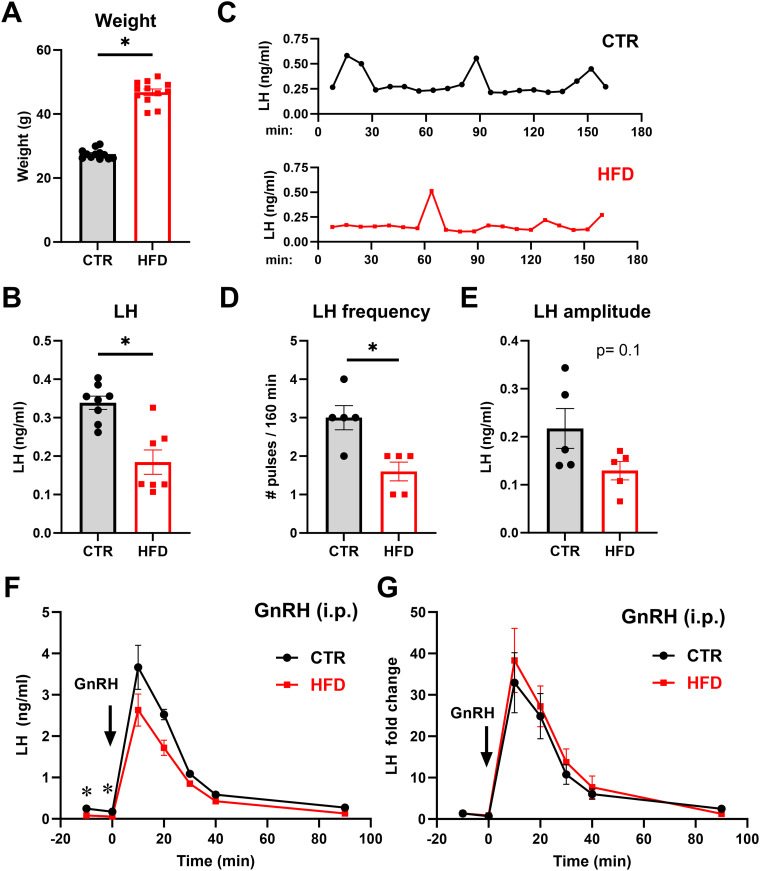
To investigate the mechanisms driving reproductive dysfunction in obese males, we utilized the DIO model. A week after weaning, mice were randomly placed on CTR or HFD for 12 weeks. We previously reported that HFD mice experience hyperleptinemia, reduced testosterone levels, and lower sperm count (Lainez et al., 2018; Chen et al., 2021a; Ruggiero-Ruff et al., 2024). Consistent with our previous reports, mice on HFD gained significantly more weight than mice on CTR (final weights after 12 weeks: CTR 27.5 g and HFD 46.88 g) and demonstrated 170% higher weight than CTR (Fig. 1A; each point represents a mouse, and bars represent group average ± SEM). HFD mice had significantly lower LH levels than CTR (Fig. 1B; CTR, 0.35 ng/ml compared with HFD, 0.18 ng/ml). To determine if lower LH levels stem from lower pulse frequency of LH secretion, we analyzed LH pulsatility by frequent blood sampling (Fig. 1C, representative pulse profiles). We determined a decrease in LH pulsatile secretion in HFD males. CTR males had three pulses in 3 h, while HFD males had 1.6 pulses during the sampling period (Fig. 1D). On the other hand, LH amplitude was not significantly affected by HFD (Fig. 1E; 0.22 ng/ml in CTR compared with 0.13 ng/ml in HFD). Given that a pulse of LH directly corresponds to a pulse of GnRH (Urbanski et al., 1988), these results suggest a dysregulation of GnRH pulsatile activity in obesity.
Figure 1.
Male mice on a high-fat diet experience lower LH pulse frequency. A, Weights of male mice after 12 week control (CTR) or high-fat diet (HFD) exposure (CTR, gray bars; HFD, red bars; each dot represents one animal, bars represent group mean ± standard error). * indicates significant difference (p < 0.0001, df = 22). B, HFD males have lower serum LH than CTR (p = 0.0007, df = 13). C, Representative LH pulse profile from CTR (top) and HFD (bottom) male mice. D, LH pulse frequency, calculated using DynPeak freeware. Significant difference between CTR and HFD was determined with t test (p = 0.0081, df = 8). E, Pulse amplitude was determined by subtracting the basal value from the LH value at the peak and averaged for each mouse. F, Intraperitoneal injection of 1 µg/kg GnRH in CTR (n = 5) and HFD (n = 5) mice was performed and LH levels in the circulation at times indicated in the graphs analyzed by ELISA. The arrow represents time point 0, when GnRH was administered; each dot represents the group mean ± standard error. G, Since basal levels of LH, prior to injection, differ between CTR and HFD, LH fold change was determined by normalizing all time points to the basal level.
Since the pituitary secretes LH in response to GnRH, and we observed lower LH in HFD males, we investigated whether HFD exposure modified pituitary responsiveness to GnRH stimulation. Two samples taken before GnRH injection determined lower basal LH in HFD males, consistent with previous results. GnRH injection resulted in rapid LH secretion with peak levels after 10 min in both groups. LH increased from 0.11 to 3.66 ng/ml in CTR and from 0.069 to 2.63 ng/ml in HFD (Fig. 1F). Since the basal LH levels differed in CTR and HFD animals, to determine pituitary responsiveness to exogenous GnRH, we normalized LH values to the basal LH level, as in previous publications analyzing gonadotropin induction in response to GnRH (Coss et al., 2004, 2007; Ely et al., 2011; Lindaman et al., 2013; Reddy et al., 2013). Fold change from basal LH was calculated and determined that in CTR, GnRH injection resulted in 33-fold increase in LH, while in HFD, GnRH injection resulted in 38-fold induction in LH levels (Fig. 1G). Fold change of LH in response to GnRH was not statistically different between CTR and HFD, implying that pituitary responsiveness was unaffected by HFD. Given that pituitary responsiveness to exogenous GnRH is not affected by HFD, the lower LH pulse frequency and lower basal LH in HFD suggests the dysregulation of the GnRH pulse generator, prompting us to investigate kisspeptin.
Peripheral and central response to kisspeptin suggests kisspeptin neurons are suppressed by HFD
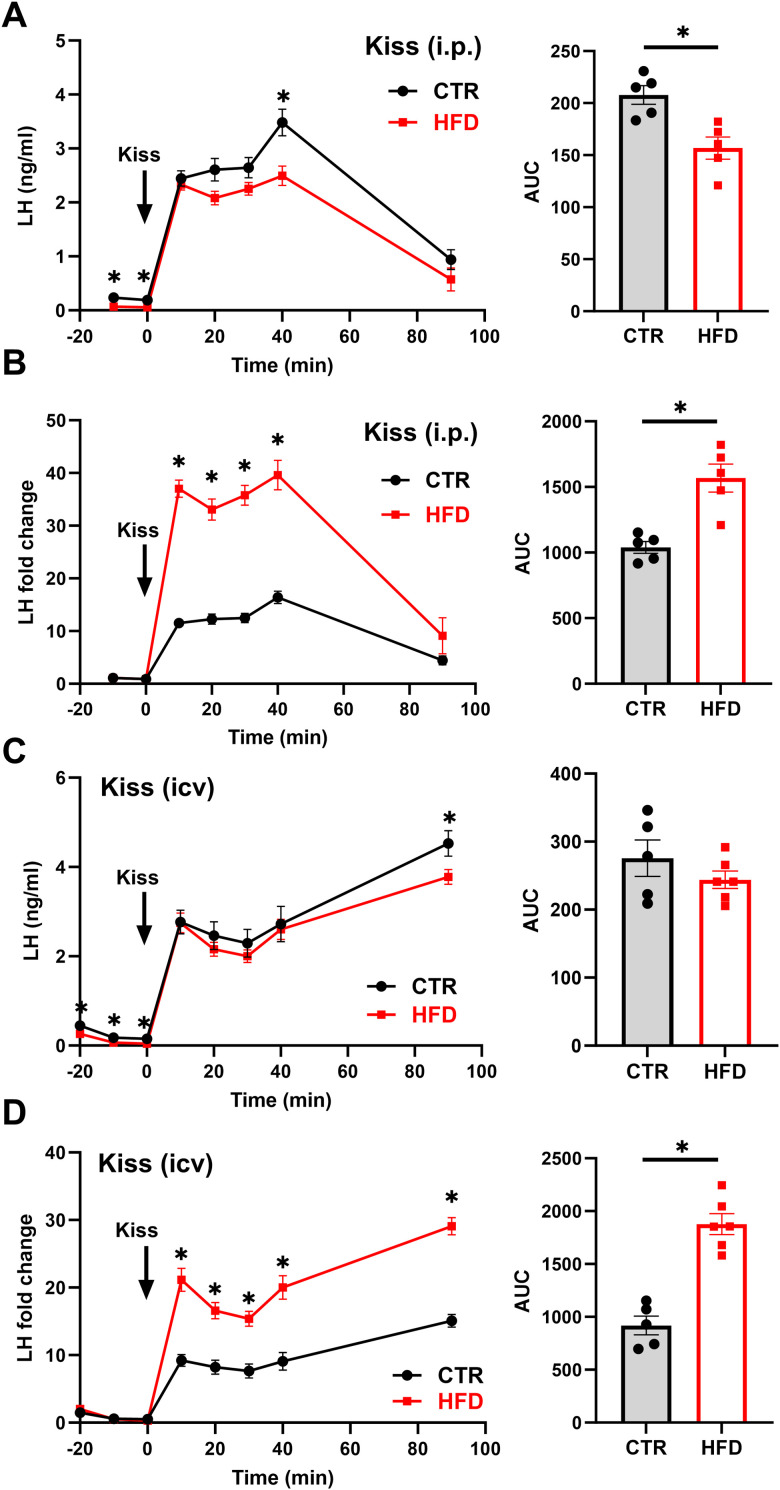
Since our data revealed decreased LH pulse frequency and kisspeptin neurons are a key population that regulates GnRH neuron pulsatility, we investigated the response to exogenous kisspeptin in HFD. CTR and HFD male mice were injected intraperitoneally with 1 mg/kg kisspeptin (Fig. 2A, kiss), and LH levels were analyzed as above. Kisspeptin injection in CTR mice caused a rapid LH secretion from 0.24 ng/ml before injection to 2.4 ng/ml after 10 min and a peak of 3.50 ng/ml after 40 min, while in HFD, LH levels increased from 0.07 to 2.3 ng/ml at 10 min and a peak of 2.50 ng/ml at 40 min (Fig. 2A, left). Area under the curve (AUC) was greater in CTR compared with that in HFD (Fig. 2A, right), which is consistent with previous studies (Sanchez-Garrido et al., 2014). Due to the differences in basal LH levels, we analyzed LH fold change as above and determined that in CTR, LH increased 10-fold at 10 min and 14.6-fold after 40 min, while in HFD, LH increased 33-fold after 10 min and 35.7-fold after 40 min (Fig. 2B, left). AUC determined that kisspeptin injection results in a remarkably higher fold induction of LH secretion in HFD compared with CTR (Fig. 2B, right).
Figure 2.
Kisspeptin treatment produces higher LH response in HFD mice than in CTR. A, C, Left, CTR (n = 5) and HFD (n = 5 in A, n = 6 in C) mice were injected intraperitoneally with 1 mg/kg kisspeptin (A) or intracerebroventricularly with 1 nmol kisspeptin (C); and LH measured at times indicated in the graph. * indicates statistical difference between CTR and HFD at the same time point. Right, AUC calculated from results presented on the left; * indicates statistically significant difference (A, p = 0.0062) between CTR and HFD. Each dot represents one animal, bars represent group average. B, D, Left, Due to the difference in basal LH levels, fold change from basal levels was calculated after intraperitoneal injection (B) or intracerebroventricular injection (D). * indicates statistical difference between CTR and HFD at the same time point. Right, AUC was calculated and statistically significant difference (* B, p = 0.0018; D, p < 0.0001) between CTR and HFD determined by t test.
Given these unexpected results, we investigated the effects of central injection of kisspeptin to circumvent peripheral influences. A cannula was placed in the lateral ventricle of CTR and HFD males, and 1 nmol kisspeptin was injected. Central injections of kisspeptin elicited a robust LH secretion, similarly to previous studies (Gottsch et al., 2004; Roseweir et al., 2009; Tonsfeldt et al., 2019). LH secretion demonstrated no differences in LH levels achieved by central kisspeptin injection at 10 and 40 min between CTR and HFD (CTR, from 0.24 to 2.77 ng/ml at 10 min; HFD from 0.13 to 2.75 ng/ml at 10 min; Fig. 2C). However, at 90 min, we observed a significantly higher LH levels in CTR of 4.2 ng/ml compared with 3.78 ng/ml in HFD (Fig. 2C). The AUC revealed no significant difference between CTR and HFD (Fig. 2C, right). As above, due to differences in basal LH levels, we calculated fold changes and determined that CTR mice had 11.5-fold increase in LH response at 10 min and 17.5-fold increase at 90 min. HFD mice again had a higher fold change of 21.1-fold at 10 min and 29.10-fold change at 90 min (Fig. 2D, left). The significant increase in LH fold change observed in HFD was consistent when AUC was calculated (Fig. 2D, right). Together, the lack of differences in LH levels after kisspeptin treatment demonstrate that GnRH neurons and the pituitary gonadotropes in HFD respond normally to exogenous kisspeptin, to either central or peripheral kisspeptin injection, implicating reduced kisspeptin signaling as a cause of lower LH in obesity. Fold change calculations result in higher LH induction in HFD males imply that endogenous kisspeptin neurons are suppressed in obesity.
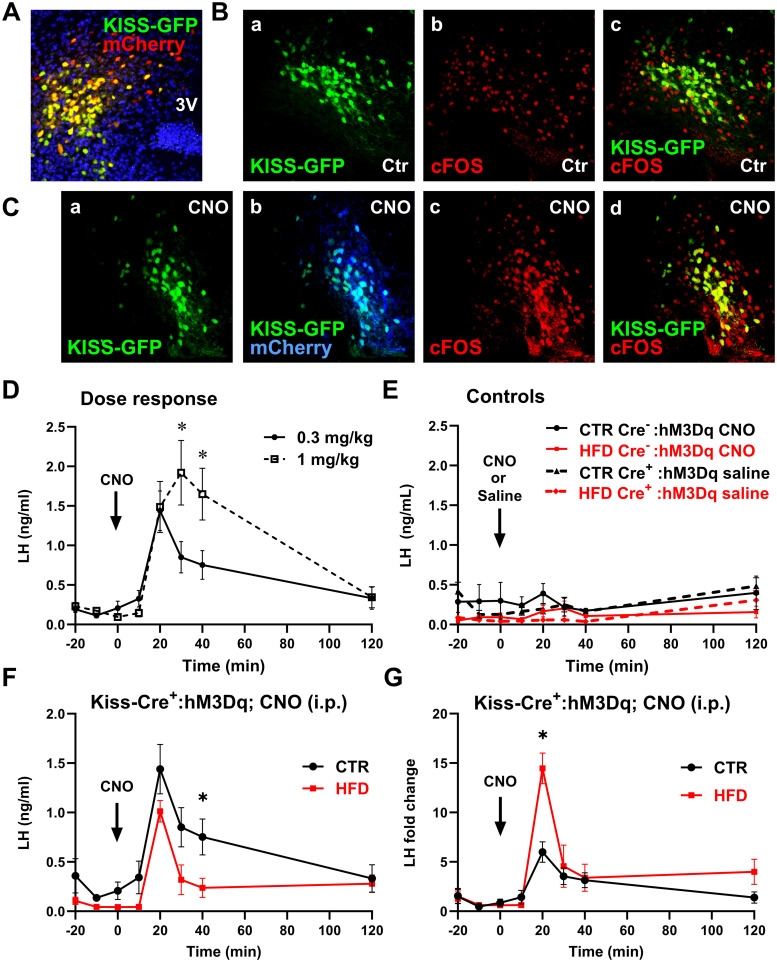
To further investigate this hypothesis, we used chemogenetics to activate endogenous kisspeptin neurons and analyze LH response. Kiss1-Cre-EGFP mice were used to express hM3Dq in kisspeptin neurons, after AAV was stereotactically injected in the ARC. Stereotaxic injection of 5.4 × 1012 GC/ml AAV9-hSyn-DiO-hM3D(Gq)-mCherry (hM3Dq) resulted in infection of 76% of kisspeptin neurons, as determined by colocalization of EGFP reporter with mCherry, after Cre-mediated recombination in kisspeptin neurons (Fig. 3A). Five percent of mCherry-immunoreactive cells did not express EGFP. To ensure that the infection with activating DREADDs resulted in neuron activation following CNO injection, we stained for cFOS as a marker of neuronal activity (Fig. 3B,C). cFOS immunoreactivity was present in 11% of kisspeptin neurons in control (Ctr) animals (Fig. 3B). Ninety minutes after CNO injection, virtually all kisspeptin neurons infected with a virus expressed cFOS; 99% of infected neurons (Fig. 3Cb, coexpression of mCherry and kisspeptin-driven GFP) express cFOS (Fig. 3Cd, coexpression of GFP and cFOS). This observation was the same in CTR and HFD mice. To confirm that DREADD activation with CNO would not maximize the response that would prevent us from observing differences in HFD compared with control, we performed a dose response analyses and injected 0.3 and 1 mg/kg CNO intraperitoneally. A robust dose-dependent secretion of LH occurred in response to CNO. A total of 0.3 mg/kg CNO resulted in 6.9-fold increase in LH at the peak after 20 min (from 0.21 ng/ml at time 0, to 1.44 ng/ml at 20 min), while 1 mg/kg CNO resulted in 10.7-fold induction at the peak after 30 min (from 0.18 to 1.92 ng/ml; Fig. 3D). To control for CNO off-target responses, Kiss-Cre− mice were also injected with hM3Dq and treated with 0.3 mg/kg CNO and a week later with 1 mg/kg CNO, which did not result in increased LH secretion (Fig. 3E, 0.3 mg/kg CNO shown with solid lines, 1 mg/kg CNO not shown since 1 mg/kg dose was not used in comparison of the response in CTR and HFD mice). In addition, Kiss-Cre+ mice, injected with hM3Dq, were treated with sterile saline, which confirmed the absence of increased LH secretion without CNO (Fig. 3E, dashed lines). These data support specific activation of Kiss1-Cre neurons expressing hM3Dq after CNO injections. Then, CTR and HFD mice with Kiss1-Cre+ neurons expressing hM3Dq were injected intraperitoneally with 0.3 mg/kg CNO, and LH was measured at times indicated in the graph (Fig. 3F). Activation of kisspeptin neurons resulted in an increase of LH from 0.23 to 1.40 ng/ml at 20 min post CNO in CTR mice, compared with an increase from 0.07 to 1.01 ng/ml in HFD males. The difference at peak levels 20 min after CNO injection between CTR and HFD was not significant (Fig. 3F). Due to the difference in basal level before CNO induction, fold change was analyzed and determined that CNO injection resulted in 6.1-fold increase in LH in CTR mice and 14.4-fold increase in LH secretion in HFD mice (Fig. 3G). These results determined that HFD male mice responded more robustly to kisspeptin than CTR male mice, in all treatments: to peripheral and central injection and DREADD-mediated neuron activation. Together, our data suggest that the ability of ARC kisspeptin neurons to regulate GnRH neurons and LH secretion is not affected by HFD, and that in fact, kisspeptin neurons are likely suppressed in obesity.
Figure 3.
Chemogenetic activation of kisspeptin neurons also resulted in higher LH induction in HFD mice. A, Kiss1-Cre+-EGFP colocalization with virally transduced hM3Dq-mCherry (red) 2 weeks after injection demonstrates DREADDs targeting to kisspeptin neurons. B, Kisspeptin neuron activity, assessed by cFOS expression, in control animals; a, GFP immunoreactivity identified kisspeptin neurons in kisspeptin-GFP mice; b, cFOS expression in arcuate nucleus; c, overlap to determine percentage of kisspeptin neurons with cFOS. C, Kisspeptin neuron activity after injection of CNO in DREADD-infected animals; a, GFP; b, colocalization of mCherry and GFP, indicating rate of infection; c, cFOS; d, colocalization of cFOS and GFP. D, LH secretion after intraperitoneal injection of 0.3 mg/kg (n = 6) or 1 mg/kg (n = 4) CNO demonstrates dose-dependent effects. * indicates statistical difference in response to different CNO concentration at the same time point. E, LH secretion in response to intraperitoneal injection of 0.3 mg/kg CNO in Kiss1-Cre− animals infected with AAV9 hM3Dq-mCherry (CTR n = 4, HFD n = 4) and in Kiss-Cre+ infected with AAV9 hM3Dq-mCherry (CTR n = 6, HFD n = 5) after injection with saline demonstrate specificity of response. F, LH levels in response to 0.3 mg/kg CNO injection (CTR, n = 6; HFD, n = 5). * indicates statistically significant difference (p = 0.043) between CTR and HFD at the same time point. G, Fold change of LH from basal levels in response to 0.3 mg/kg CNO injection. * indicates significant difference between CTR and HFD at the peak, p = 0.00119).
Glutamatergic regulation of kisspeptin neurons is suppressed in HFD
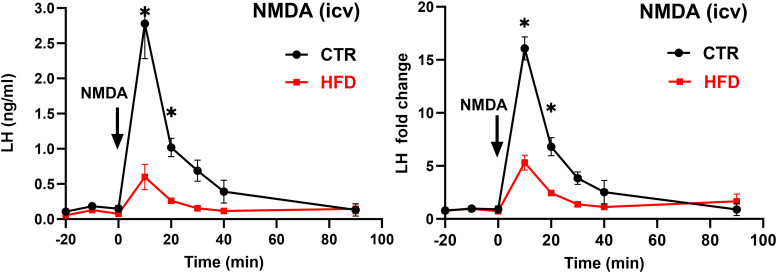
Several studies demonstrated that glutamatergic stimulation, specifically N-methyl-d-aspartate (NMDA) treatment, increases LH secretion, without increased expression of cFOS in GnRH neurons (Lee et al., 1993; d'Anglemont de Tassigny et al., 2010), while recent evidence suggested that kisspeptin neuron synchronization and pulse initiation is dependent on glutamate (Voliotis et al., 2021; Han et al., 2023). Since our result above indicated that kisspeptin neurons were impaired in obesity, we then investigated glutamatergic innervation of kisspeptin neurons and LH secretion in response to NMDA in HFD. To investigate LH response to NMDA in CTR and HFD, we implanted a cannula into the lateral ventricle and centrally injected 7 nmol NMDA. LH levels increased from 0.17 ng/ml before treatment to 2.75 ng/ml 10 min after injection in CTR, while in HFD LH levels increased from 0.11 to 0.6 ng/ml (Fig. 4A). For consistency, fold change was calculated, and CTR mice exhibited a 16.1-fold increase in LH 10 min post injection, while HFD mice had a 5.5-fold increase in LH (Fig. 4B). Our results revealed that LH secretion in response to NMDA was diminished in HFD male mice. It is possible that kisspeptin neuron synchronization and pulse initiation are dysregulated in HFD due to a decrease in glutamatergic innervation at the level of the kisspeptin neuron.
Figure 4.
LH induction by NMDA is decreased in HFD mice compared with CTR. Left, LH secretion in response to intracerebroventricular injection of 7 nmol NMDA was lower in HFD mice. Right, Fold change from basal of LH response to intracerebroventricular NMDA injection was lower in HFD mice. Each dot represents the group mean ± standard error (n = 5). Statistical significance between CTR compared with HFD at the same time point is indicated with a * (p < 0.05).
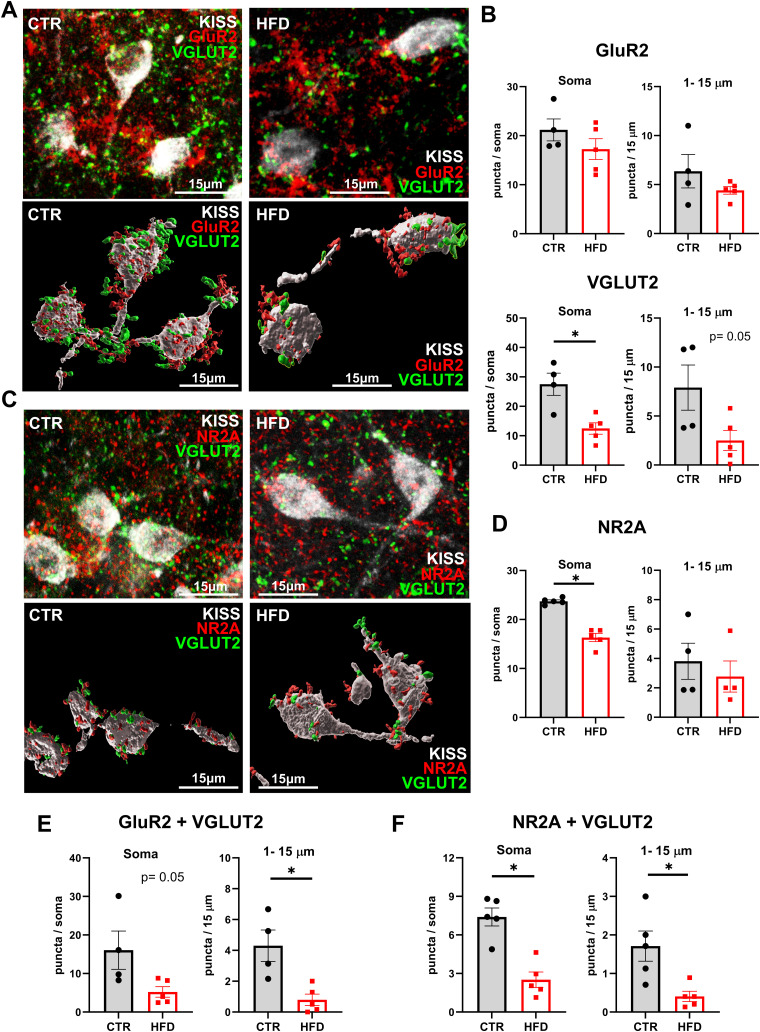
To examine glutamatergic innervation of kisspeptin neurons, CTR and HFD brains were sectioned, and sections were triple stained for GFP to visualize kisspeptin neurons and either AMPA receptors containing the GluR2 subunit and presynaptic vesicular glutamate transporter 2 (VGLUT2) or NMDA receptors containing the NR2A subunit and VGLUT2, to visualize glutamatergic innervation. GluR2 is an obligatory subunit for synaptic AMPA receptors impermeable to Ca2+ (Isaac et al., 2007), and NR2A is an obligatory subunit for synaptic NMDA receptors (Thomas et al., 2006). Representative images show relative levels of AMPA receptors (Fig. 5A, top) or NMDA receptors (Fig. 5C, top) on kisspeptin neurons in CTR and HFD, and 3D reconstruction to facilitate counting of direct contacts only with kisspeptin neurons (Fig. 5A,C, bottom). The relative numbers were quantified and revealed no difference in GluR2-containing AMPA receptor immunoreactivity (Fig. 5B, top); but a significant decrease in VGLUT2 puncta in HFD kisspeptin neurons compared with CTR. VGLUT2 levels were decreased by 55% at the soma and by 68% in 1–15 µm segment from the soma (Fig. 5B, bottom). Quantification of NMDA receptors revealed a decrease in NR2A levels in HFD animals compared with CTR; CTR had 23.7 NMDA puncta at the soma, while HFD had 16.3 puncta at the soma (Fig. 5D). More importantly, there was a significant decrease in GluR2 and VGLUT2 colocalization in kisspeptin neurons, which decreased by 67% at the soma and by 83% at the proximal process (Fig. 5E). There was also a significant decrease in NR2A and VGLUT2 colocalizations in kisspeptin neurons, from 7.4 colocalized puncta in CTR to 2.5 puncta in HFD at the soma and from 1.7 in CTR to 0.4 in HFD in the proximal process (Fig. 5F). This decrease in glutamatergic innervation can lead to diminished kisspeptin neuron pulse initiation and synchronization.
Figure 5.
Reduced glutamatergic innervation of kisspeptin neurons in HFD. A, AMPA receptors were quantified in kisspeptin neurons in CRL and HFD. Top, representative images of GluR2 (red) and VGLUT2 (green) colocalized to kisspeptin neurons (white, 630× magnification). Bottom, 3D reconstruction using Imaris to assure counting cell surface receptors in direct contact with kisspeptin neurons. B, Quantification of GluR2 (top) and VGLUT2 (bottom) in kisspeptin neuron soma (left) and proximal 15 μm of the process (right). Each dot represents an average for one mouse (15–40 neurons counted per animal), and bars represent the group average for CTR (gray) and HFD (red). C, Fewer NMDA receptors on kisspeptin neurons in HFD mice. Top, confocal and bottom, 3D reconstructed representative images of postsynaptic NR2A (red) and presynaptic VGLUT2 (green) on kisspeptin neurons (white). D, Quantification of NR2A numbers in CTR and HFD at the soma and 1–15 µm proximal process. E, Quantification of GluR2 and VGLUT2 colocalization at the kisspeptin neuron soma and proximal 1–15 µm process in CTR or HFD. F, Quantification of NR2A and VGLUT2 colocalization at the kisspeptin neuron soma and proximal 1–15 µm process in CTR or HFD. Each dot represents an average for one mouse (15–40 neurons counted per animal), and bars represent the group average for CTR (gray) and HFD (red). Statistical significance between CTR and HFD is indicated with a * (p < 0.05).
Lack of LH response to αMSH injection and POMC chemogenetic activation in HFD
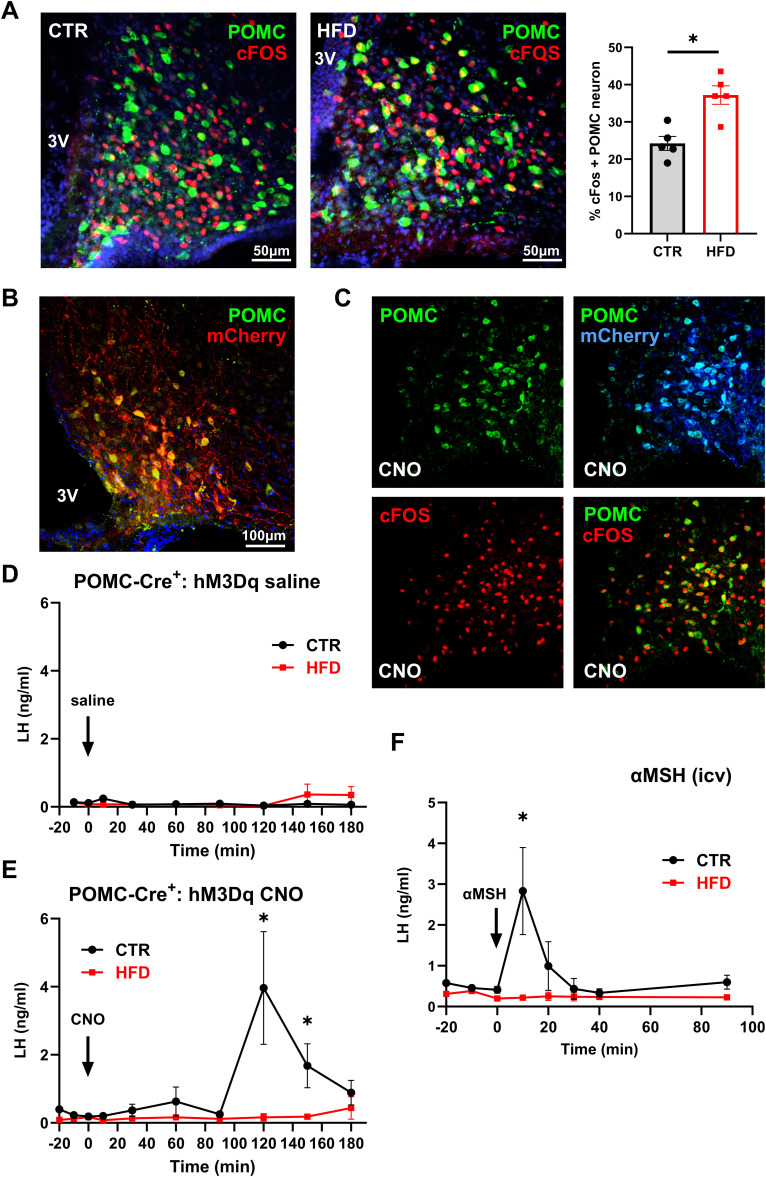
Since reproduction is a very energy-demanding process, reproductive circuitry and feeding circuitry neurons in the hypothalamus interact to integrate their functions (Manfredi-Lozano et al., 2018; Rønnekleiv et al., 2019). Specifically, kisspeptin neurons interact with POMC neurons, a population of neurons that sense energy stores through leptin secreted from fat (Nestor et al., 2016; Quarta et al., 2021; Stincic and Kelly, 2022). POMC neurons regulate satiety and energy expenditure by secreting αMSH that binds MC4R receptors (Andermann and Lowell, 2017). Given that (1) αMSH injection can stimulate LH secretion, which is dependent on kisspeptin (Manfredi-Lozano et al., 2016), (2) kisspeptin neurons express MC4R (Cravo et al., 2011), and (3) POMC neurons respond to obesity (Ziotopoulou et al., 2000), POMC neurons are a good candidate to affect kisspeptin neuron changes observed here. First, to determine if POMC neurons are affected by HFD, CTR and HFD sections were stained for β-endorphin to visualize POMC neurons, and cFOS, a marker of neuronal activity. CTR mice had 24.2% cFOS-positive POMC neurons, while HFD mice had 37.2% cFOS-positive POMC neurons, suggesting that excess adiposity leads to more active POMC neurons in HFD (Fig. 6A). There was no difference in the number of POMC neurons between diets. This increase in active POMC neurons is attributed to higher leptin levels, since leptin signaling leads to activation of POMC neurons (Cowley et al., 2001).
Figure 6.
HFD diminishes LH response to exogenous POMC activation and αMSH injection. A, Increased percentage of cFOS positive POMC neurons in HFD. Left, Images of cFOS (red) colocalization with β-endorphin in POMC (green) neuron in CTR and HFD mice. Right, Quantification of cFOS positive POMC neurons, presented as percent of all POMC neurons. Each dot represents one animal, and bars represent group average. * indicates significant difference determined by t test (p = 0.0031). B, Representative image of DREADDs (mCherry) in POMC neurons (green) to demonstrate 73% infection. C, Infected POMC neurons that express mCherry, induce cFOS 90 min after 1 mg/kg CNO injection. D, LH levels in CTR (n = 5) or HFD (n = 7) POMC-Cre+ mice expressing hM3Dq DREADDs, after saline intraperitoneal injection. E, LH levels in CTR (n = 5) or HFD (n = 7) POMC-Cre+ positive mice expressing hM3Dq DREADDs, after 1 mg/kg CNO intraperitoneal injection. F, LH levels in response to intracerebroventricular injection of 3 nmol αMSH (CTR, black line n = 5; HFD, red line n = 5). Significant difference between CTR and HFD at the peak is indicated with a * (E, p = 0.0114; F, p = 0.0147).
To further determine LH response to POMC regulation in CTR and HFD, we chemogenetically activated POMC neurons using DREADDs. POMC-Cre+ were placed on CTR or HFD for 12 weeks and then POMC-Cre+ mice were injected with 400 nl of hM3Dq and allowed to recover for 2 weeks for optimal transduction, on their respective diets. Validation of proper targeting was performed after experiments were completed and determined that 73% of POMC neurons expressed mCherry. Seven percent of mCherry immunolabeled neurons lacked β-endorphin immunoreactivity (Fig. 6B). To confirm the functionality of DREADD-mediated activation of POMC neurons following CNO injection, the percentage of POMC neurons that express cFOS was analyzed 90 min after CNO treatment (Fig. 6C). Ninety-four percent of infected POMC neurons in either CTR and HFD animals that expressed mCherry also expressed cFOS following CNO injection (Fig. 6C, compare POMC + mCherry and POMC + cFOS; then compare these with Fig. 6A, left panel CTR). Controls, saline injections in CTR and HFD POMC-Cre+ mice injected with hM3Dq, lacked the LH response (Fig. 6D). After CNO injection, LH increased from 0.3 to 3.96 ng/ml in CTR mice, while LH secretion did not increase in HFD (Fig. 6E). CNO injection of POMC-Cre+-hM3Dq mice resulted in 13.2-fold increase in LH secretion 120 min after injection in CTR males; however, there was no response in HFD males during the sampling period.
To investigate if LH response to POMC neuron product was altered in HFD, we centrally injected αMSH and analyzed LH secretion. We determined that after αMSH treatment LH increased from 0.41 to 2.83 ng/ml at 10 min post injection in CTR mice, while in the HFD mice this response was abolished (Fig. 6F). We postulate that the difference in the timing of LH secretion following CNO injection versus αMSH injection may be due to the time required to synthesize and process POMC to αMSH. αMSH stimulated a 6.9-fold increase in LH secretion 10 min after injection in CTR; however, there was no response in HFD. Lack of LH response in HFD mice to αMSH treatment and chemogenetic POMC neuron activation supports that POMC–kisspeptin interaction is dysregulated in obesity.
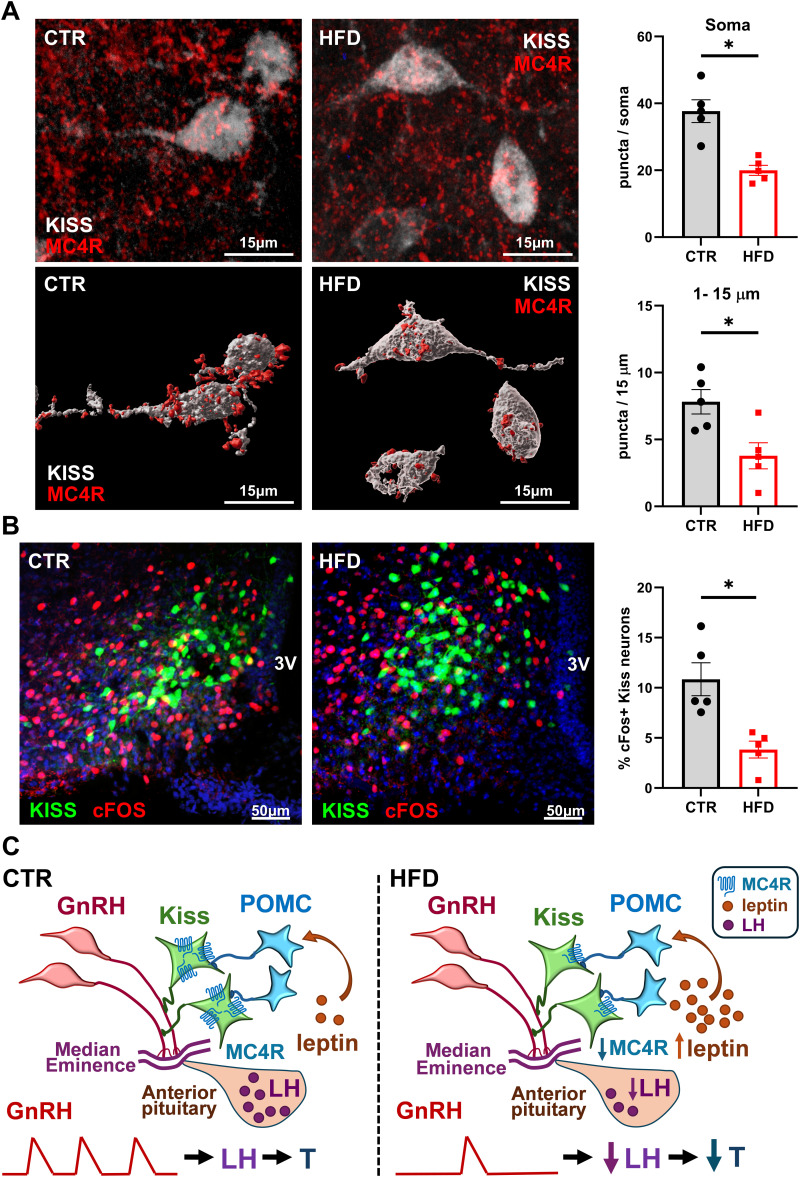
To investigate POMC–kisspeptin interaction in CTR and HFD, we analyzed levels of MC4R in kisspeptin neurons by immunohistochemistry. Kiss1-Cre+-EGFP mice on CTR and HFD were stained for GFP and MC4R (Fig. 7A, top, confocal images; Fig. 7A, bottom, 3D reconstruction). Kisspeptin neurons in HFD males had significantly fewer MC4R. HFD resulted in a 47% decrease in the immunoreactivity of receptors at the soma and 52% decrease in the proximal process (Fig. 7A, right).
Figure 7.
Fewer active kisspeptin neurons in HFD mice compared with CTR. A, Kiss-EGFP (n = 5) mice were fed CTR or HFD for 12 weeks, after which immunohistochemistry was performed to analyze MC4R and cFOS levels. Top, MC4R receptor (red) colocalized to kisspeptin neurons, identified by GFP (white); Bottom, 3D reconstruction; Right, MC4R puncta in 15–25 neurons per mouse were counted and averaged. Each dot represents one animal, bars represent group average, * indicates statistical difference between CTR and HFD by t test (top, p = 0.0014; bottom, p = 0.0162). B, Left, Images of Kiss-GFP and cFOS after CTR and HFD; Right, percent of cFOS positive kisspeptin neurons determined through colocalization of cFOS (red) and kisspeptin neurons (green). Each dot represents one animal, bars represent group average, * indicates statistical difference between CTR and HFD by t test (p = 0.0052). C, Model of HFD-mediated effects on hypothalamic circuitry, based on results presented here. Left, GnRH secretion into the median eminence capillaries is regulated by kisspeptin neurons that integrate input from POMC neurons. Pulsatile secretion of GnRH stimulates LH synthesis and secretion from the anterior pituitary, which in turn regulates testosterone synthesis in the gonads. Right, HFD-induced obesity leads to increased leptin that causes higher activity of POMC neurons and downregulation of MC4R, a receptor for POMC product αMSH, in kisspeptin neurons. HFD exposure results in lower activity of kisspeptin neurons, which leads to lower pulse frequency of GnRH secretion, and diminished levels of LH and T in the circulation.
We postulated that kisspeptin neurons were suppressed in obesity. To assess the activity of kisspeptin neurons in CTR and HFD mice, we quantified the percentage of cFOS-positive kisspeptin neurons, as an indication of their activity. We determined CTR mice had 10.9% cFOS-positive kisspeptin neurons, compared with 3.8% in HFD (Fig. 7B). Therefore, HFD resulted in a 65% decrease in active kisspeptin neurons, consistent with our previous hypothesis that kisspeptin neurons are suppressed in HFD. Together, our results revealed that obesity results in a decrease in kisspeptin neuron activity, resulting in a lower LH (and GnRH) pulse frequency, and reduced LH in the circulation. LH secretion was impaired in response to NMDA, αMSH, and POMC neuron activation in HFD animals. Lack of LH response to αMSH and chemogenetic POMC activation in HFD likely stems from overactive POMC neurons due to higher leptin, and subsequent downregulation of MC4R in kisspeptin neurons, which dysregulates the communication between the feeding and reproductive circuit (Fig. 7C).
Discussion
Mechanisms that underlie the negative effects of obesity on reproductive function are not clear, despite clinical findings that obesity causes decreased testosterone, lowered sperm numbers, and impaired sperm quality (Hammoud et al., 2008; Du Plessis et al., 2010). Studies in animal models by us and others confirm clinical findings in humans (Bakos et al., 2011; McGee et al., 2014; Sanchez-Garrido et al., 2014; Vendramini et al., 2014; DiVall et al., 2015; Fernandez et al., 2017; Lainez et al., 2018). Males are more prone to obesity-mediated pathologies, while females may be protected (Parks et al., 2015; Gerdts and Regitz-Zagrosek, 2019) and our previous studies focused on delineating mechanisms of sex differences in response to obesity (Lainez et al., 2018; Chen et al., 2021a,b; Li et al., 2023). Herein, we focus on understanding the mechanisms of reproductive impairment in males. Several studies, including ours, determined that the primary effects of DIO are central, at the level of the hypothalamus, resulting in lower basal LH (Hill et al., 2008; Evans and Anderson, 2017; Lainez et al., 2018; Manfredi-Lozano et al., 2018). Obesity-associated reduction in testosterone is accompanied by lower LH, compared with the age-related lower testosterone, which is correlated with higher LH, due to a lack of negative feedback (Wu et al., 2008), again indicating central effects in obesity. However, mechanisms whereby obesity affects reproductive circuitry in the hypothalamus are not clear. Herein, we report several novel findings that contribute to our understanding of obesity-mediated hypogonadism: (1) we determined lower LH pulse frequency in obese animals, implying dysregulation of GnRH pulse generator; (2) we determined that kisspeptin neurons are suppressed in obesity and exogenous kisspeptin results in higher LH response in obese animals; (3) consistent with previous studies, we identified synaptic changes; in particular, we showed fewer glutamate receptors in kisspeptin neurons that may be important for the initiation of the pulse; and (4) the cross talk of POMC neurons and kisspeptin neurons is altered due to diminished receptor levels. We postulate that changes in receptor levels may stem from chronically higher activity of POMC neurons in obesity that is a result of increased leptin and in turn causes an increase in αMSH section, which leads to downregulation of MC4R on target neurons (Fig. 7C). New results presented here expand our understanding of obesity-mediated hypothalamic impairment.
Our results here indicate that pituitary responsiveness, more specifically, gonadotrope response to GnRH injection, is unaltered in obese animals. Our recent study analyzed the pituitary changes in obesity and demonstrated that somatotrope and lactotrope populations exhibit plasticity; however changes in the gonadotrope may result from either hypothalamic effects, since GnRH regulates gonadotropin transcription, or from direct impact on the gonadotrope population (Ruggiero-Ruff et al., 2024). One study postulated direct effects on the pituitary, since LH pulses showed lower amplitude but unaltered frequency, during overnight sampling (Jain et al., 2007). A more recent study by the same group determined that pituitary responsiveness to GnRH is unchanged and that in both obese and lean individuals GnRH injection caused the same fold increase of LH (Jones et al., 2020), which agrees with our results presented here. Lower basal LH in our experiments here in mice, the abovementioned study in humans (Jones et al., 2020), and rats (Sanchez-Garrido et al., 2014) may obscure LH induction in response to treatment, a reason we analyzed LH secretion as the fold change from basal. Normalizing to basal level allows us to determine the level of response. This permits us to uncover that pituitary responsiveness to GnRH is unchanged, while kisspeptin treatment caused higher LH secretion in HFD. Results without normalizing were also presented and illustrate the lack of differences in LH levels after kisspeptin. They demonstrate the normal response of GnRH neurons to kisspeptin in HFD, suggesting that reduced LH in obesity stems from impaired kisspeptin signal.
We demonstrated that in obesity kisspeptin neurons are suppressed and that exogenous kisspeptin, administered via peripheral or central injection, or by chemogenetic kisspeptin neuron activation, results in higher LH fold response in obese animals than in controls. We also showed that kisspeptin neurons are less active since in obese mice, a lower percentage of kisspeptin neurons express cFOS, a marker of neuronal activation. They may be suppressed because they exhibit fewer glutamate receptors, and glutamate may play a role in kisspeptin pulse generation (Han et al., 2023). Kisspeptin neurons in obesity also have lower levels of MC4R, an αMSH receptor that allows stimulation by POMC neurons that produce αMSH. Bidirectional communication between POMC and kisspeptin neurons has been demonstrated, indicating close integration of feeding and reproductive circuits (Stincic and Kelly, 2022). Kisspeptin excites POMC neurons, via kisspeptin neuropeptide and glutamate release (Fu and van den Pol, 2010; Nestor et al., 2016; Qiu et al., 2018), while POMC neurons project to ARC kisspeptin neurons (Cravo et al., 2011) and POMC product, αMSH stimulates LH secretion via kisspeptin (Manfredi-Lozano et al., 2016). It is not clear how this interaction is altered during chronic obesity, which we examined here, since long-lasting obesity may lead to receptor downregulation, leptin resistance (Myers et al., 2010), biphasic changes in αMSH levels (Wu et al., 2023), and endoplasmic reticulum stress that modulates neuropeptide processing (Çakir et al., 2013), all of which may exert different effects on POMC–kisspeptin cross talk. Due to its chronic nature, it is also difficult to distinguish cause and effect or pinpoint the timing of each change. Nonetheless, we determined changes in kisspeptin neuron activity and postulate that these changes are caused by alterations in neuropeptide receptors and/or synaptic, neurotransmitter receptor levels.
Our studies show that interaction between feeding circuitry, in particular POMC neurons, and reproductive circuitry, specifically kisspeptin neurons, is impeded by obesity. Several studies determined that αMSH treatment, a product of POMC neurons, causes LH secretion in humans (Reid et al., 1984; Limone et al., 1997) and rodents, in the estrous cycle stage-dependent manner (Alde and Celis, 1980; Evans and Anderson, 2017). MC4R receptor agonist also stimulates LH in sheep (Backholer et al., 2009, 2010) and rodents (Manfredi-Lozano et al., 2016). In this study, we also show that αMSH intracerebroventricular treatment causes rapid LH secretion. Although αMSH elicited ∼7-fold change in LH levels, response was more variable than response to kisspeptin or NMDA, and we postulate that variability may stem from the timing of food intake prior to injection. Consistent with a response to αMSH, here we show for the first time that chemogenetic activation of POMC neurons stimulates LH release. LH secretion following POMC neuron activation with CNO occurred after 2 h, compared with 20 min after kisspeptin neuron activation. This may imply indirect effects of POMC neuron activation, likely via kisspeptin neurons. Additionally, this timing may reflect the need to synthesize and process POMC to αMSH. Previous studies using DREADD-mediated approaches to regulate POMC neuron activity are in agreement with our studies that demonstrated cFOS induction in POMC neurons 90 min after CNO injection, a time frame close to LH section (Zhan et al., 2013; Jiang et al., 2020). The physiological effects on food intake, which reflect the function of POMC neurons, required 3 d of CNO treatment (Zhan et al., 2013). To our knowledge, the shortest time frame to observe the effects of POMC neuron activation via DREADDs was 8 h (Koch et al., 2015). Considering these, the time frame in our studies may not be unexpected. Surprisingly, HFD-mediated obesity completely abolished LH response to αMSH injection and to POMC neuron activation. It is possible that LH secretion is delayed past the sampling period. However, we postulate the lack of response is due to the MC4R receptor downregulation, since MC4R levels in kisspeptin neurons are 50% lower in HFD mice. We hypothesize that changes in MC4R levels may stem from increased leptin-mediated chronic activation of POMC neurons (Pinto et al., 2004). This implies the regulation of POMC–kisspeptin interaction by tightly controlled receptor levels.
In addition to neuropeptide receptor downregulation, it is possible that change in kisspeptin neuron activity and GnRH neuron pulsatility stem from synaptic changes and alterations in the neurotransmitter receptor levels. We observed changes in the neurotransmitter receptor levels here and in previous studies (Lainez et al., 2018). Synaptic changes in obesity have been an area of intense investigation and may occur due to alterations in neuron activity after prolonged stimulation or neuroinflammation-mediated synaptic remodeling by activated astrocyte and microglia. We postulated in our previous studies that neuroinflammation leads to synaptic remodeling (Lainez et al., 2018; Lainez and Coss, 2019). A decrease in the number of synapses following HFD was reported previously in the arcuate nucleus, specifically fewer inhibitory synapses on POMC neurons, which was postulated to cause increased POMC expression and higher POMC neuron activity (Horvath et al., 2010). Reduced levels of synaptic proteins, synapses, and fewer dendritic spines in HFD were observed in hippocampal neurons (Hao et al., 2016), and in the prefrontal cortex (Bocarsly et al., 2015), in addition to our studies in the hypothalamus (Lainez et al., 2018; Chen et al., 2021b). We reported decreased synapses in GnRH neurons in our previous study (Lainez et al., 2018) and in kisspeptin neurons in the current. Future studies will determine mechanisms of synaptic changes that alter POMC–kisspeptin cross talk in obesity.
In summary, studies presented here demonstrate impaired POMC–kisspeptin cross talk in obesity, resulting from lower neuropeptide receptor levels and fewer innervations, that lead to suppression of kisspeptin neurons and lower pulse frequency of LH, which results in lower basal LH and ultimately hypogonadisms.
References
- Alde S, Celis ME (1980) Influence of alpha-melanotropin on LH release in the rat. Neuroendocrinology 31:116–120. 10.1159/000123061 [DOI] [PubMed] [Google Scholar]
- Andermann ML, Lowell BB (2017) Toward a wiring diagram understanding of appetite control. Neuron 95:757–778. 10.1016/j.neuron.2017.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backholer K, Bowden M, Gamber K, Bjørbaek C, Iqbal J, Clarke IJ (2010) Melanocortins mimic the effects of leptin to restore reproductive function in lean hypogonadotropic ewes. Neuroendocrinology 91:27–40. 10.1159/000260060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backholer K, Smith J, Clarke IJ (2009) Melanocortins may stimulate reproduction by activating orexin neurons in the dorsomedial hypothalamus and kisspeptin neurons in the preoptic area of the ewe. Endocrinology 150:5488–5497. 10.1210/en.2009-0604 [DOI] [PubMed] [Google Scholar]
- Bakos HW, Mitchell M, Setchell BP, Lane M (2011) The effect of paternal diet-induced obesity on sperm function and fertilization in a mouse model. Int J Androl 34:402–410. 10.1111/j.1365-2605.2010.01092.x [DOI] [PubMed] [Google Scholar]
- Balthasar N, et al. (2004) Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42:983–991. 10.1016/j.neuron.2004.06.004 [DOI] [PubMed] [Google Scholar]
- Benani A, et al. (2012) Food intake adaptation to dietary fat involves PSA-dependent rewiring of the arcuate melanocortin system in mice. J Neurosci 32:11970–11979. 10.1523/JNEUROSCI.0624-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocarsly ME, Fasolino M, Kane GA, LaMarca EA, Kirschen GW, Karatsoreos IN, McEwen BS, Gould E (2015) Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. Proc Natl Acad Sci U S A 112:15731–15736. 10.1073/pnas.1511593112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çakir I, Cyr NE, Perello M, Litvinov BP, Romero A, Stuart RC, Nillni EA (2013) Obesity induces hypothalamic endoplasmic reticulum stress and impairs proopiomelanocortin (POMC) post-translational processing*. J Biol Chem 288:17675–17688. 10.1074/jbc.M113.475343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis ME (1985) Release of LH in response to alpha-MSH administration. Acta Physiol Pharmacol Latinoam 35:281–290. [PubMed] [Google Scholar]
- Chen KE, Lainez NM, Coss D (2021a) Sex differences in macrophage responses to obesity-mediated changes determine migratory and inflammatory traits. J Immunol 206:141–153. 10.4049/jimmunol.2000490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KE, Lainez NM, Nair MG, Coss D (2021b) Visceral adipose tissue imparts peripheral macrophage influx into the hypothalamus. J Neuroinflammation 18:140. 10.1186/s12974-021-02183-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coss D, Hand CM, Yaphockun KK, Ely HA, Mellon PL (2007) P38 mitogen-activated kinase is critical for synergistic induction of the FSH beta gene by gonadotropin-releasing hormone and activin through augmentation of c-Fos induction and Smad phosphorylation. Mol Endocrinol 21:3071–3086. 10.1210/me.2007-0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coss D, Jacobs SB, Bender CE, Mellon PL (2004) A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone beta gene by gonadotropin-releasing hormone. J Biol Chem 279:152–162. 10.1074/jbc.M304697200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ (2001) Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411:480–484. 10.1038/35078085 [DOI] [PubMed] [Google Scholar]
- Cravo RM, et al. (2011) Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 173:37–56. 10.1016/j.neuroscience.2010.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czieselsky K, Prescott M, Porteous R, Campos P, Clarkson J, Steyn FJ, Campbell RE, Herbison AE (2016) Pulse and surge profiles of luteinizing hormone secretion in the mouse. Endocrinology 157:4794–4802. 10.1210/en.2016-1351 [DOI] [PubMed] [Google Scholar]
- D'Aniello A, Di Fiore MM, Fisher GH, Milone A, Seleni A, D'Aniello S, Perna AF, Ingrosso D (2000) Occurrence of D-aspartic acid and N-methyl-D-aspartic acid in rat neuroendocrine tissues and their role in the modulation of luteinizing hormone and growth hormone release. FASEB J 14:699–714. 10.1096/fasebj.14.5.699 [DOI] [PubMed] [Google Scholar]
- de Tassigny XDA, Ackroyd KJ, Chatzidaki EE, Colledge WH (2010) Kisspeptin signaling is required for peripheral but not central stimulation of gonadotropin-releasing hormone neurons by NMDA. J Neurosci 30:8581–8590. 10.1523/JNEUROSCI.5486-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiVall SA, Herrera D, Sklar B, Wu S, Wondisford F, Radovick S, Wolfe A (2015) Insulin receptor signaling in the GnRH neuron plays a role in the abnormal GnRH pulsatility of obese female mice. PLoS One 10:e0119995. 10.1371/journal.pone.0119995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Plessis SS, Cabler S, McAlister DA, Sabanegh E, Agarwal A (2010) The effect of obesity on sperm disorders and male infertility. Nat Rev Urol 7:153–161. 10.1038/nrurol.2010.6 [DOI] [PubMed] [Google Scholar]
- Ely HA, Mellon PL, Coss D (2011) GnRH induces the c-Fos gene via phosphorylation of SRF by the calcium/calmodulin kinase II pathway. Mol Endocrinol 25:669–680. 10.1210/me.2010-0437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MC, Anderson GM (2017) Neuroendocrine integration of nutritional signals on reproduction. J Mol Endocrinol 58:R107–R128. 10.1530/JME-16-0212 [DOI] [PubMed] [Google Scholar]
- Fernandez MO, Sharma S, Kim S, Rickert E, Hsueh K, Hwang V, Olefsky JM, Webster NJ (2017) Obese neuronal PPARgamma knockout mice are leptin sensitive but show impaired glucose tolerance and fertility. Endocrinology 158:121–133. 10.1210/en.2016-1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu LY, van den Pol AN (2010) Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci 30:10205–10219. 10.1523/JNEUROSCI.2098-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdts E, Regitz-Zagrosek V (2019) Sex differences in cardiometabolic disorders. Nat Med 25:1657–1666. 10.1038/s41591-019-0643-8 [DOI] [PubMed] [Google Scholar]
- Goodman RL, Herbison AE, Lehman MN, Navarro VM (2022) Neuroendocrine control of gonadotropin-releasing hormone: pulsatile and surge modes of secretion. J Neuroendocrinol 34:e13094. 10.1111/jne.13094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch ML, et al. (2011) Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology 152:4298–4309. 10.1210/en.2011-1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA (2004) A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077. 10.1210/en.2004-0431 [DOI] [PubMed] [Google Scholar]
- Hammoud AO, Wilde N, Gibson M, Parks A, Carrell DT, Meikle AW (2008) Male obesity and alteration in sperm parameters. Fertil Steril 90:2222–2225. 10.1016/j.fertnstert.2007.10.011 [DOI] [PubMed] [Google Scholar]
- Han SY, Morris PG, Kim JC, Guru S, Pardo-Navarro M, Yeo SH, McQuillan HJ, Herbison AE (2023) Mechanism of kisspeptin neuron synchronization for pulsatile hormone secretion in male mice. Cell Rep 42:111914. 10.1016/j.celrep.2022.111914 [DOI] [PubMed] [Google Scholar]
- Hao S, Dey A, Yu X, Stranahan AM (2016) Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav Immun 51:230–239. 10.1016/j.bbi.2015.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE (2018) The gonadotropin-releasing hormone pulse generator. Endocrinology 159:3723–3736. 10.1210/en.2018-00653 [DOI] [PubMed] [Google Scholar]
- Hill JW, Elmquist JK, Elias CF (2008) Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab 294:E827–E832. 10.1152/ajpendo.00670.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, et al. (2010) Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci U S A 107:14875–14880. 10.1073/pnas.1004282107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Lancet Public Health (2018) Tackling obesity seriously: the time has come. Lancet Public Health 3:e153. 10.1016/S2468-2667(18)30053-7 [DOI] [PubMed] [Google Scholar]
- Isaac JT, Ashby MC, McBain CJ (2007) The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54:859–871. 10.1016/j.neuron.2007.06.001 [DOI] [PubMed] [Google Scholar]
- Jain A, et al. (2007) Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab 92:2468–2473. 10.1210/jc.2006-2274 [DOI] [PubMed] [Google Scholar]
- Jensen TK, Andersson AM, Jorgensen N, Andersen AG, Carlsen E, Petersen JH, Skakkebaek NE (2004) Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril 82:863–870. 10.1016/j.fertnstert.2004.03.056 [DOI] [PubMed] [Google Scholar]
- Jiang J, Morgan DA, Cui H, Rahmouni K (2020) Activation of hypothalamic AgRP and POMC neurons evokes disparate sympathetic and cardiovascular responses. Am J Physiol Heart Circ Physiol 319:H1069–H1077. 10.1152/ajpheart.00411.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K, Ryan S, Carlson NE, Chosich J, Bradford AP, Santoro N, Polotsky AJ (2020) Aromatase inhibition ameliorates decreased LH output found in obese women. Reprod Sci 27:1018–1023. 10.1007/s43032-019-00105-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, et al. (2015) Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature 519:45–50. 10.1038/nature14260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisman MJ, McCosh RB, Tian K, Song CI, Breen KM (2020) Estradiol enables chronic corticosterone to inhibit pulsatile luteinizing hormone secretion and suppress Kiss1 neuronal activation in female mice. Neuroendocrinology 110:501–516. 10.1159/000502978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainez NM, Coss D (2019) Obesity, neuroinflammation, and reproductive function. Endocrinology 160:2719–2736. 10.1210/en.2019-00487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainez NM, Jonak CR, Nair MG, Ethell IM, Wilson EH, Carson MJ, Coss D (2018) Diet-induced obesity elicits macrophage infiltration and reduction in spine density in the hypothalami of male but not female mice. Front Immunol 9:1992. 10.3389/fimmu.2018.01992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WS, Abbud R, Hoffman GE, Smith MS (1993) Effects of N-methyl-D-aspartate receptor activation on cFos expression in luteinizing hormone-releasing hormone neurons in female rats. Endocrinology 133:2248–2254. 10.1210/endo.133.5.8404677 [DOI] [PubMed] [Google Scholar]
- Li J, Ruggiero-Ruff RE, He Y, Qiu X, Lainez N, Villa P, Godzik A, Coss D, Nair MG (2023) Sexual dimorphism in obesity is governed by RELMα regulation of adipose macrophages and eosinophils. Elife 12:e86001. 10.7554/eLife.86001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limone P, Calvelli P, Altare F, Ajmone-Catt P, Lima T, Molinatti GM (1997) Evidence for an interaction between alpha-MSH and opioids in the regulation of gonadotropin secretion in man. J Endocrinol Invest 20:207–210. 10.1007/BF03346904 [DOI] [PubMed] [Google Scholar]
- Lindaman LL, Yeh DM, Xie C, Breen KM, Coss D (2013) Phosphorylation of ATF2 and interaction with NFY induces c-Jun in the gonadotrope. Mol Cell Endocrinol 365:316–326. 10.1016/j.mce.2012.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredi-Lozano M, et al. (2016) Defining a novel leptin-melanocortin-kisspeptin pathway involved in the metabolic control of puberty. Mol Metab 5:844–857. 10.1016/j.molmet.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredi-Lozano M, Roa J, Tena-Sempere M (2018) Connecting metabolism and gonadal function: novel central neuropeptide pathways involved in the metabolic control of puberty and fertility. Front Neuroendocrinol 48:37–49. 10.1016/j.yfrne.2017.07.008 [DOI] [PubMed] [Google Scholar]
- Mao X, Fujiwara Y, Chapdelaine A, Yang H, Orkin SH (2001) Activation of EGFP expression by Cre-mediated excision in a new ROSA26 reporter mouse strain. Blood 97:324–326. 10.1182/blood.V97.1.324 [DOI] [PubMed] [Google Scholar]
- McCosh RB, Kreisman MJ, Breen KM (2018) Frequent tail-tip blood sampling in mice for the assessment of pulsatile luteinizing hormone secretion. J Vis Exp 137:57894. 10.3791/57894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee WK, Bishop CV, Pohl CR, Chang RJ, Marshall JC, Pau FK, Stouffer RL, Cameron JL (2014) Effects of hyperandrogenemia and increased adiposity on reproductive and metabolic parameters in young adult female monkeys. Am J Physiol Endocrinol Metab 306:E1292–E1304. 10.1152/ajpendo.00310.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG Jr, Leibel RL, Seeley RJ, Schwartz MW (2010) Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab 21:643–651. 10.1016/j.tem.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor CC, Qiu J, Padilla SL, Zhang C, Bosch MA, Fan W, Aicher SA, Palmiter RD, Ronnekleiv OK, Kelly MJ (2016) Optogenetic stimulation of arcuate nucleus Kiss1 neurons reveals a steroid-dependent glutamatergic input to POMC and AgRP neurons in male mice. Mol Endocrinol 30:630–644. 10.1210/me.2016-1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novaira HJ, Sonko ML, Hoffman G, Koo Y, Ko C, Wolfe A, Radovick S (2014) Disrupted kisspeptin signaling in GnRH neurons leads to hypogonadotrophic hypogonadism. Mol Endocrinol 28:225–238. 10.1210/me.2013-1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paeger L, Pippow A, Hess S, Paehler M, Klein AC, Husch A, Pouzat C, Brüning JC, Kloppenburg P (2017) Energy imbalance alters Ca(2+) handling and excitability of POMC neurons. Elife 6:e25641. 10.7554/eLife.25641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks BW, et al. (2015) Genetic architecture of insulin resistance in the mouse. Cell Metab 21:334–347. 10.1016/j.cmet.2015.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM (2008) Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 149:1979–1986. 10.1210/en.2007-1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL (2004) Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 304:110–115. 10.1126/science.1089459 [DOI] [PubMed] [Google Scholar]
- Qiu J, Rivera HM, Bosch MA, Padilla SL, Stincic TL, Palmiter RD, Kelly MJ, Ronnekleiv OK (2018) Estrogenic-dependent glutamatergic neurotransmission from kisspeptin neurons governs feeding circuits in females. Elife 7:e35656. 10.7554/eLife.35656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarta C, Claret M, Zeltser LM, Williams KW, Yeo GSH, Tschöp MH, Diano S, Brüning JC, Cota D (2021) POMC neuronal heterogeneity in energy balance and beyond: an integrated view. Nat Metab 3:299–308. 10.1038/s42255-021-00345-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy GR, Xie C, Lindaman LL, Coss D (2013) GnRH increases c-Fos half-life contributing to higher FSHbeta induction. Mol Endocrinol 27:253–265. 10.1210/me.2012-1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RL, Ling N, Yen SSC (1984) Gonadotropin-releasing activity of of α-melanocyte-stimulating hormone in normal subjects and in subjects with hypothalamic-pituitary dysfunction*. J Clin Endocrinol Metab 58:773–777. 10.1210/jcem-58-5-773 [DOI] [PubMed] [Google Scholar]
- Ronnekleiv OK, Kelly MJ (2013) Kisspeptin excitation of GnRH neurons. Adv Exp Med Biol 784:113–131. 10.1007/978-1-4614-6199-9_6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rønnekleiv OK, Qiu J, Kelly MJ (2019) Arcuate kisspeptin neurons coordinate reproductive activities with metabolism. Semin Reprod Med 37:131–140. 10.1055/s-0039-3400251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseweir AK, et al. (2009) Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci 29:3920–3929. 10.1523/JNEUROSCI.5740-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero-Ruff RE, Le BH, Villa PA, Lainez NM, Athul SW, Das P, Ellsworth BS, Coss D (2024) Single-cell transcriptomics identifies pituitary gland changes in diet-induced obesity in male mice. Endocrinology 165:1. 10.1210/endocr/bqad196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero-Ruff RE, Villa PA, Hijleh SA, Avalos B, DiPatrizio NV, Haga-Yamanaka S, Coss D (2023) Increased body weight in mice with fragile X messenger ribonucleoprotein 1 (Fmr1) gene mutation is associated with hypothalamic dysfunction. Sci Rep 13:12666. 10.1038/s41598-023-39643-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Garrido MA, et al. (2014) Obesity-induced hypogonadism in the male: premature reproductive neuroendocrine senescence and contribution of Kiss1-mediated mechanisms. Endocrinology 155:1067–1079. 10.1210/en.2013-1584 [DOI] [PubMed] [Google Scholar]
- Santi D, et al. (2024) Does an increase in adipose tissue ‘weight’ affect male fertility? A systematic review and meta-analysis based on semen analysis performed using the WHO 2010 criteria. Andrology 12:123–136. 10.1111/andr.13460 [DOI] [PubMed] [Google Scholar]
- Sermondade N, et al. (2013) BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update 19:221–231. 10.1093/humupd/dms050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Wang L, Goebel-Stengel M, Taché Y (2011) Centrally injected kisspeptin reduces food intake by increasing meal intervals in mice. Neuroreport 22:253–257. 10.1097/WNR.0b013e32834558df [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C (2013) Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology 154:4939–4945. 10.1210/en.2013-1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stincic TL, Grachev P, Bosch MA, Rønnekleiv OK, Kelly MJ (2018) Estradiol drives the anorexigenic activity of proopiomelanocortin neurons in female mice. eNeuro 5:1. 10.1523/eneuro.0103-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stincic TL, Kelly MJ (2022) Estrogenic regulation of reproduction and energy homeostasis by a triumvirate of hypothalamic arcuate neurons. J Neuroendocrinol 34:e13145. 10.1111/jne.13145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM (2000) Genetic targeting of green fluorescent protein to gonadotropin- releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology 141:412–419. 10.1210/endo.141.1.7279 [DOI] [PubMed] [Google Scholar]
- Thomas CG, Miller AJ, Westbrook GL (2006) Synaptic and extrasynaptic NMDA receptor NR2 subunits in cultured hippocampal neurons. J Neurophysiol 95:1727–1734. 10.1152/jn.00771.2005 [DOI] [PubMed] [Google Scholar]
- Tonsfeldt KJ, Schoeller EL, Brusman LE, Cui LJ, Lee J, Mellon PL (2019) The contribution of the circadian gene Bmal1 to female fertility and the generation of the preovulatory luteinizing hormone surge. J Endocr Soc 3:716–733. 10.1210/js.2018-00228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanski HF, Pickle RL, Ramirez VD (1988) Simultaneous measurement of gonadotropin-releasing hormone, luteinizing hormone, and follicle-stimulating hormone in the orchidectomized rat. Endocrinology 123:413–419. 10.1210/endo-123-1-413 [DOI] [PubMed] [Google Scholar]
- Vendramini V, Cedenho AP, Miraglia SM, Spaine DM (2014) Reproductive function of the male obese Zucker rats: alteration in sperm production and sperm DNA damage. Reprod Sci 21:221–229. 10.1177/1933719113493511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal A, Zhang Q, Médigue C, Fabre S, Clément F (2012) DynPeak: an algorithm for pulse detection and frequency analysis in hormonal time series. PLoS One 7:e39001. 10.1371/journal.pone.0039001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa PA, Lainez NM, Jonak CR, Berlin SC, Ethell IM, Coss D (2023) Altered GnRH neuron and ovarian innervation characterize reproductive dysfunction linked to the fragile X messenger ribonucleoprotein (Fmr1) gene mutation. Front Endocrinol 14:1129534. 10.3389/fendo.2023.1129534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voliotis M, Li XF, De Burgh RA, Lass G, Ivanova D, McIntyre C, O'Byrne K, Tsaneva-Atanasova K (2021) Modulation of pulsatile GnRH dynamics across the ovarian cycle via changes in the network excitability and basal activity of the arcuate kisspeptin network. Elife 10:e71252. 10.7554/eLife.71252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu FC, et al. (2008) Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab 93:2737–2745. 10.1210/jc.2007-1972 [DOI] [PubMed] [Google Scholar]
- Wu Q, Chen J, Hua T, Cai J (2023) Alpha-melanocyte-stimulating hormone-mediated appetite regulation in the central nervous system. Neuroendocrinology 113:885–904. 10.1159/000530804 [DOI] [PubMed] [Google Scholar]
- Zhan C, Zhou J, Feng Q, Zhang J-E, Lin S, Bao J, Wu P, Luo M (2013) Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci 33:3624–3632. 10.1523/JNEUROSCI.2742-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziotopoulou M, Mantzoros CS, Hileman SM, Flier JS (2000) Differential expression of hypothalamic neuropeptides in the early phase of diet-induced obesity in mice. Am J Physiol Endocrinol Metab 279:E838–E845. 10.1152/ajpendo.2000.279.4.E838 [DOI] [PubMed] [Google Scholar]