Abstract
Assembly of infectious human immunodeficiency virus type 1 (HIV-1) virions requires incorporation of the viral envelope glycoproteins gp41 and gp120. Several lines of evidence have suggested that the cytoplasmic tail of the transmembrane glycoprotein, gp41, associates with Pr55Gag in infected cells to facilitate the incorporation of HIV-1 envelope proteins into budding virions. However, direct evidence for an interaction between gp41 and Pr55Gag in HIV-1 particles has not been reported. To determine whether gp41 is associated with Pr55Gag in HIV-1 particles, viral cores were isolated from immature HIV-1 virions by sedimentation through detergent. The cores contained a major fraction of the gp41 that was present on untreated virions. Association of gp41 with cores required the presence of the gp41 cytoplasmic tail. In HIV-1 particles containing a functional protease, a mutation that prevents cleavage of Pr55Gag at the matrix-capsid junction was sufficient for the detergent-resistant association of gp41 with the isolated cores. In addition to gp41, a major fraction of virion-associated gp120 was also detected on immature HIV-1 cores. Isolation of cores under conditions known to disrupt lipid rafts resulted in the removal of a raft-associated protein incorporated into virions but not the HIV-1 envelope proteins. These results provide biochemical evidence for a stable interaction between Pr55Gag and the cytoplasmic tail of gp41 in immature HIV-1 particles. Moreover, findings in this study suggest that the interaction of Pr55Gag with gp41 may regulate the function of the envelope proteins during HIV-1 maturation.
The replication cycle of human immunodeficiency virus type 1 (HIV-1) culminates in the release of progeny virions from an infected cell via budding from the plasma membrane. During virion assembly, incorporation of viral envelope (Env) proteins is essential for the formation of infectious particles. The HIV-1 Env complex consists of the surface glycoprotein (SU), gp120, and the transmembrane glycoprotein (TM), gp41, which are noncovalently associated. Fusion of HIV-1 particles with target cells is initiated by binding of gp120 to CD4. Secondary engagement of a chemokine receptor results in conformational changes in gp120, triggering the gp41-mediated fusion of viral and cellular membranes. HIV-1 gp41, like other lentivirus TM proteins, contains an unusually long cytoplasmic tail consisting of 150 amino acids in contrast to the cytoplasmic tails of simple retrovirus TM proteins, which are approximately 20 to 50 amino acids in length (14). Although much has been learned about mechanism of HIV-1 fusion, the role of the gp41 cytoplasmic tail in Env function remains enigmatic.
Several lines of evidence suggest that an interaction between the gp41 cytoplasmic tail and the structural protein precursor, Pr55Gag, occurs during HIV-1 assembly. This possibility was first implied by studies examining virion release from polarized epithelial cells. Coexpression of Env and Pr55Gag results in budding of HIV-1 particles exclusively from the basolateral surface of polarized epithelial cells, while expression of Pr55Gag alone results in the release of particles from both apical and basolateral sites (17, 23). Second, the matrix (MA) domain of Pr55Gag is required for incorporation of full-length Env, as evidenced by the observations that deletions or point mutations in MA inhibit the incorporation of full-length HIV-1 Env proteins into budding virions (11, 12). These mutants were rescued by truncating the cytoplasmic tail of gp41 or by pseudotyping virions with a heterologous retroviral Env containing a short cytoplasmic tail, suggesting that the MA domain of Pr55Gag is required for accommodating the long cytoplasmic domain of gp41. A third line of evidence for a gp41-Pr55Gag interaction is based on the observation that HIV-1 Env expressed in cells undergoes rapid internalization from the cell surface due to an endocytic motif present in the cytoplasmic tail of gp41 (25). Coexpression of Pr55Gag dramatically reduces Env internalization, suggesting that Pr55Gag binds the TM cytoplasmic domain and prevents its interaction with the endocytic machinery (10). Fourth, a direct interaction between the MA region of Pr55Gag and a glutathione S-transferase fusion protein containing the cytoplasmic tail of gp41 was observed in vitro using recombinant HIV-1 proteins (7). Finally, in simian immunodeficiency virus, a lentivirus closely related to HIV-1, expression of Env proteins engineered with an endoplasmic reticulum retention motif results in a dramatic decrease in the amount of viral particles released from cells (28). This finding suggested that Env and Pr55Gag interact soon after translation but before transport to the plasma membrane. Though the above data imply an interaction between gp41 and Pr55Gag in virus-infected cells, a direct interaction between Env and Pr55Gag in HIV-1 particles has yet to be demonstrated.
In this study, immature HIV-1 virions were treated with detergent to remove the viral lipid membrane, and the resulting immature cores were purified by equilibrium sedimentation centrifugation. Through the isolation of immature HIV-1 cores, we demonstrate a detergent-stable interaction of the HIV-1 Env proteins with Pr55Gag. These results provide biochemical evidence supporting an interaction between the gp41 cytoplasmic tail and MA region of Pr55Gag during HIV-1 assembly.
MATERIALS AND METHODS
Cells and viruses.
293T cells (obtained from I. Verma) were cultured at 37°C in 5% CO2 Dulbecco modified Eagle medium (DMEM; Cellgro) supplemented with fetal bovine serum (10%), penicillin (50 IU/ml), and streptomycin (50 μg/ml). Unless otherwise noted, the proviral DNA constructs used for the production of HIV-1 have been described previously (16, 31) and are as follows: R9, wild-type HIV-1; R9.PR−, protease-defective HIV-1 containing a triple alanine substitution in the protease active site; R9Tr712.PR−, protease-defective HIV-1 containing a truncation of the N-terminal 144-amino-acid gp41; NLPI.nef+ (5), HIV-1 reporter virus expressing placental alkaline phosphatase (AP); R9.MA-CA, HIV-1 containing a mutation which blocks cleavage at the matrix-capsid (MA-CA) junction of Pr55Gag (described below). R9Tr712.PR− was created by inserting the SalI-to-BamHI mutation from NLTr712 (31) into the R9.PR− provirus.
R9.MA-CA was created using PCR site-directed mutagenesis. PCR segment overlap extension, as described previously (13), was used to change a Tyr residue at amino acid 132 of the MA gene to an Ile using the forward primer of 5′-CACTATAGGAATATTTTGGCTGAC. A silent mutation encoding an SspI restriction enzyme site was engineered into the primer to aid in screening positive clones. A PCR product containing the Tyr-to-Ile mutation was transferred into the full-length proviral clone R9 using the restriction enzyme sites BssHII and SpeI. Functional verification of the mutation was determined by immunoblot analysis using capsid (CA)-specific antisera of the mutant virions produced from transfected 293T cells.
For production of viruses, 293T cells were transfected in 10-cm-diameter dishes with 20 μg of plasmid DNA as previously described (6). Virus-containing supernatants were collected 48 to 60 h after transfection, clarified by low-speed centrifugation to remove cellular debris, and passed through a 0.45-μm (pore-size) membrane filter.
Isolation of HIV-1 core structures.
Immature cores were isolated using a modification of the “spin-thru” procedure as previously described for the purification of HIV-2 cores (15). Filtered supernatants (60 ml) from transfected 293T cells were concentrated by centrifugation (120,000 × g, 3 h, 4°C) through 3 ml of 20% (wt/vol) sucrose in STE buffer (10 mM Tris-HCl [pH 7.4], 100 mM NaCl, 1 mM EDTA). The pellets were resuspended in a total volume of 0.4 ml of STE buffer by gentle pipetting, followed by incubation for 4 h at 4°C. Linear density gradients were prepared by mixing 30 and 70% sucrose in STE buffer in a gradient maker and were cooled for 2 h at 4°C. The precooled gradients were overlaid with 15% sucrose in STE buffer (0.25 ml) containing 1% Triton X-100. This layer was covered with a barrier layer of STE (0.25 ml) containing 7.5% sucrose in order to prevent premature mixing of the concentrated virions with the detergent. Concentrated HIV-1 particles were applied to the top of the barrier layer, and the tubes were centrifuged in a Beckman SW-41 rotor (100,000 × g, 16 h, 4°C). However, cores from NLPI.nef+ were isolated by treatment of the concentrated virions with 1% Triton X-100 for 1 h at either 4 or 37°C and then layering them directly onto a 30 to 70% linear sucrose gradient. Intact virions were purified in a similar manner but without exposure to detergent. Eleven 1-ml fractions were collected from the top of the gradient and assayed for CA protein and gp120 concentration by enzyme-linked immunosorbent assays (ELISAs). The density of each fraction was determined by measurement of the refractive index. Refractive index measurements were converted to density using the formula ρ = (2.6496∗η) − 2.5323, where ρ is the density in grams/milliliter and η is the index of refraction at the measured temperature.
Protein analyses.
Gradient fractions were diluted with STE buffer to reduce the density of the fraction and pelleted by ultracentrifugation (100,000 × g, 20 min, 4°C). The supernatants were removed, and the pellets were resuspended in 1× sodium dodecyl sulfate (SDS) sample buffer for subsequent immunoblot analysis. The samples were electrophoresed on a 4 to 20% Tris-HCl acrylamide gel and transferred to polyvinylidene difluoride (PVDF) membranes. Blots were probed for HIV-1 and cellular proteins using various antisera, including rabbit polyclonal anti-CA (obtained from D. Trono), human monoclonal antiserum against gp41 (C2F5) and gp120 (C2G12) (obtained from the NIH AIDS Research and Reference Program), and rabbit polyclonal anti-cyclophilin A (obtained from L. Henderson). Protein bands were revealed by chemiluminescent detection (SuperSignal; Pierce Chemical Co.) after probing the blots with appropriate peroxidase-conjugated secondary antibodies.
Concentrations of virus in the supernatants of transfected 293T cells and in the gradient fractions were determined by a CA-specific ELISA as described previously (29). Concentrations of gp120 in the gradient fractions were also determined by ELISA. For gp120 ELISA, a 96-well plate (Immulon II; Dynex) was coated with 2 μg (0.1 ml) of the mouse monoclonal gp120 capture antibody (Advanced Bioscience Laboratories) per ml in phosphate-buffered saline (PBS) and incubated overnight at 37°C. The plate was rinsed twice with PBS, followed by the addition of 0.2 ml of PBS containing 5% donor calf serum and incubation at 37°C for 1 h to block the nonspecific binding of proteins. The plate was washed four times with PBS containing 0.2% Tween 20. Samples were diluted in PBS containing 10% donor calf serum and 0.5% Triton X-100. Samples (0.1 ml) were added, and the plate was incubated for 2 h at 37°C. After four washes, rabbit polyclonal anti-gp120 primary antibody (1:5,000 dilution; Intracel Co.) was added, and the plate was incubated for 1 h at 37°C. After four washes, peroxidase-conjugated antibody to rabbit immunoglobulin (1:5,000 dilution; Pierce Chemical Co.) was added, and the mixture was incubated for 1 h at 37°C. The plate was developed using TMB peroxidase substrate (Kirkegaard & Perry Laboratories), and the absorbance at 450 nm for each well was determined. The gp120 concentration of each sample was determined by comparison to a standard curve of recombinant gp120 from HIV-1LAV (obtained from the NIH AIDS Research and Reagent Program).
Gradient fractions were assayed for the presence of human placental AP (PLAP) by both chemiluminescent and immunoblot analyses. Chemiluminescent activity assays were performed by adding 10 μl of the unpelleted gradient fraction to 50 μl of AP-Substrate (ImmTech, Inc.) and measuring the relative luminescence in a 96-well luminometer. Immunoblot analysis was performed on the pelleted gradient fractions as mentioned above using rabbit antiserum against human PLAP (obtained from Fitzgerald International Industries, Inc.).
Phospholipid analysis.
Radiolabeled immature virions were produced by transfecting two 10-cm dishes of 293T cells with R9.PR−. Following the removal of the calcium phosphate precipitate, 1 mCi of [32P]orthophosphate was added in phosphate-free DMEM (3 ml) supplemented with fetal bovine serum (10%, dialyzed against 150 mM NaCl), penicillin (50 IU/ml), and streptomycin (50 μg/ml). Labeled virions were filtered, mixed with unlabeled virions, and concentrated by ultracentrifugation. Concentrated virions were resuspended in PBS, and half of the sample was treated with 1% Triton X-100 at 37°C for 1 h to isolate cores. The remaining half of the virion sample was incubated in parallel in the absence of detergent at 4°C for 1 h. To separate free lipids from HIV-1 cores, the samples were pelleted through a 20% sucrose cushion by ultracentrifugation (100,000 × g, 20 min, 4°C).
Pellets were resuspended in 100 μl of PBS and assayed for Pr55Gag content by p24 ELISA, and lipids were extracted according to the method of Bligh and Dyer (3). The samples were subsequently analyzed by thin-layer chromatography on silica plates with chloroform-methanol-water (60:35:8 [vol/vol/vol]) as a solvent. Radiolabeled 32P-containing lipids were visualized by autoradiography.
RESULTS
Isolation of immature HIV-1 cores.
We and others have reported the isolation and characterization of mature HIV-1 cores (16, 30). These particles contained normal quantities of CA, reverse transcriptase, and integrase but lacked significant quantities of the viral Env proteins. To further examine the interaction of the HIV-1 Env proteins with HIV-1 particles, we have isolated immature cores by a similar procedure. Immature HIV-1 particles were harvested from 293T cells transfected with an HIV-1 molecular clone encoding an inactive protease and were concentrated by ultracentrifugation through a 20% sucrose cushion. Concentrated virions were then layered onto a linear 30 to 70% sucrose gradient containing a layer of 1% Triton X-100 at the top of the gradient. Upon centrifugation, the immature virions pass through the layer of detergent and sediment to their equilibrium density in the sucrose gradient. As a control, intact immature virions were isolated on a similar 30 to 70% sucrose gradient lacking a detergent layer. One-milliliter fractions were collected from the top of gradient, and the Pr55Gag concentration for each fraction was determined by HIV-1 p24 ELISA. The particles in each fraction were pelleted in a microultracentrifuge and assayed for protein composition by immunoblotting.
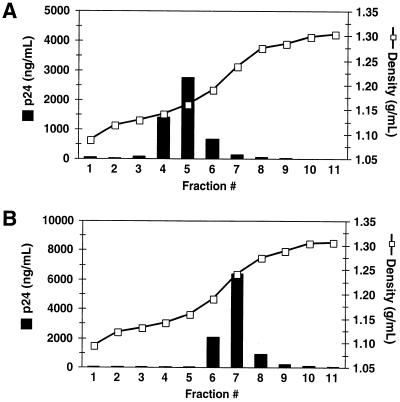
Analysis of the gradient fractions revealed that the peak of Pr55Gag for intact virions (Fig. 1A) was located in fraction 5 of the gradient, corresponding to a density of 1.16 g/ml, which is typical for retroviral particles. In contrast, the peak of Pr55Gag for the immature cores (Fig. 1B) was detected in fraction 7 of the gradient, corresponding to a density of 1.24 g/ml, which is similar to that observed for mature HIV-1 cores (16). The profiles demonstrate that a 30 to 70% sucrose allowed virions and cores to be distinguished using physical criteria. Furthermore, the sharp increase in density of the isolated cores indicated that most, if not all, of the lipid was removed from the viral particles during exposure to detergent. Unlike mature cores, which are recovered at approximately 15% of the input virion associated CA (reference 16 and data not shown), the immature cores proved to be remarkably stable, allowing recovery of approximately 80% of the input virus as determined by an ELISA for CA protein.
FIG. 1.
Isolation of immature HIV-1 core structures. Immature HIV-1 particles were harvested from 293T cells transiently transfected with the R9.PR− proviral plasmid and were filtered to remove cellular debris. Concentrated virions were sedimented through a layer of 1% Triton X-100 into a linear 30 to 70% sucrose gradient. Fractions (1 ml) were collected from the top of the gradient and assayed for CA concentration (p24) by ELISA and for density by refractometry. (A) Virions subjected to ultracentrifugation on a control gradient lacking detergent. (B) Detergent-treated virions.
Detergent-stable association of gp41 with the immature core.
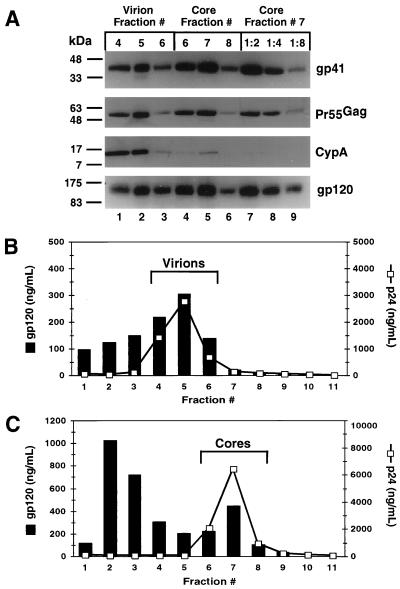
To test for the association of the HIV-1 Env proteins with immature HIV-1 cores, Pr55Gag-containing fractions from the gradients in Fig. 1 were analyzed by pelleting the particles and immunoblotting using gp41- and CA-specific antisera. A majority of the gp41 molecules remained associated with the immature cores following exposure to 1% Triton X-100 compared to intact virions isolated in a similar manner (Fig. 2A, compare lanes 2 and 7). To determine whether brief exposure to detergent was sufficient to disrupt the lipid bilayer of the virus, immature virions and cores were tested for the presence of the cellular protein cyclophilin A (CypA). CypA is specifically incorporated into HIV-1 virions but is removed upon isolation of mature HIV-1 cores (30). Reprobing the blot with antiserum specific for CypA revealed a significant depletion of CypA in immature HIV-1 cores relative to intact virions (Fig. 2A, compare lanes 2 and 7). These results, together with the increase in density of the immature HIV-1 cores, demonstrate that exposure to 1% Triton X-100 effectively disrupts the viral lipid envelope and that gp41 remains associated with the immature HIV-1 core.
FIG. 2.
Immunoblot and ELISA analysis of immature HIV-1 virions and cores. (A) The peak Pr55Gag-containing fractions of virions (lanes 1 to 3) and cores (lanes 4 to 6) were diluted with STE buffer, and particles were pelleted by ultracentrifugation. Pellets were dissolved in 1× SDS loading dye, subjected to electrophoresis on a 4 to 20% acrylamide gel, transferred to PVDF membrane, and probed with the indicated antisera. Protein bands were revealed by chemiluminescent detection after probing with the appropriate HRP-conjugated secondary antiserum. For comparative purposes, twofold serial dilutions of the peak cores fraction (lanes 7 to 9) were analyzed on the same gel. Molecular mass markers are shown in kilodaltons on the left of each panel. (B and C) Quantitation of gp120 associated with immature HIV-1 virions and cores by ELISA. Gradient fractions of HIV-1 immature virions and cores were analyzed for the presence of gp120 and Pr55Gag by ELISA. The gp120 concentration for each sample was determined using a standard curve of recombinant HIV-1LAV gp120. (B) Virions subjected to ultracentrifugation on a control gradient lacking detergent. (C) Detergent-treated virions.
Detergent-stable association of gp120 with the HIV-1 immature core.
Assays of spontaneous and CD4-induced gp120 shedding from mature HIV-1 particles and Env-expressing cells have shown that the association of gp41 with gp120 is relatively unstable on laboratory-adapted strains of HIV-1 (18, 19). To determine whether gp120 remains associated with gp41 on immature cores, these particles were analyzed for gp120 content by immunoblot using a gp120-specific antiserum (Fig. 2A). Remarkably, a large fraction of the gp120 present on untreated virions remained associated with the immature cores. Quantitation of the gp120 associated with the immature cores was performed by ELISA of gradient fractions from the immature virions and cores (Fig. 2B and C). Immature HIV-1 cores retained approximately 60% of the gp120 present on intact virions (compare fraction 5 in Fig. 2B and fraction 7 in Fig. 2C) after normalizing for Pr55Gag content. These results demonstrate that the interaction of gp41 and gp120 on immature virions is highly resistant to exposure to 1% Triton X-100.
Association of HIV-1 Env proteins with immature cores is dependent on the presence of the gp41 cytoplasmic tail.
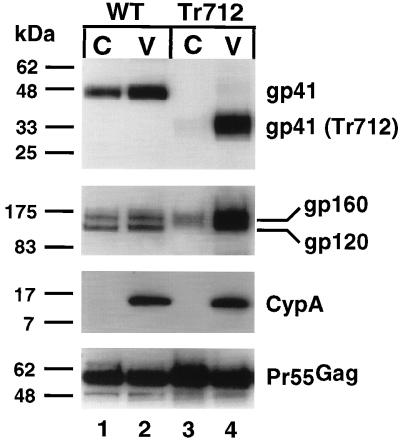
Previous studies have provided strong indirect evidence for an interaction between the gp41 cytoplasmic tail and Pr55Gag in HIV-1-infected cells. However, this interaction has not been observed in HIV-1 particles. To determine whether the gp41 cytoplasmic tail is required for the association of Env with immature HIV-1 cores, we isolated cores from immature virions containing a truncated form of gp41 lacking all but 6 of the 150 amino acids of the gp41 cytoplasmic tail. This mutant has been previously shown to incorporate high levels of Env into virions, probably due to the high level of expression of the Env proteins on the cell surface (31). Virions were produced by transfection of the PR-defective HIV-1 clone R9Tr712.PR− encoding the truncated Env protein. As previously observed for immature HIV-1 particles expressing wild-type Env, the peak of Pr55Gag for the intact virions was found in fraction 5 of the gradient, corresponding to a density of approximately 1.16 g/ml (data not shown). The peak of Pr55Gag for the Env mutant immature cores was found in fraction 7 of the gradient, corresponding to a density of approximately 1.24 g/ml (data not shown). The yield of immature cores lacking the cytoplasmic tail of gp41 was identical to that of the immature cores containing the full-length form of gp41, indicating that the cytoplasmic tail of gp41 does not influence the stability of the HIV-1 particles (data not shown). The peak Pr55Gag-containing fractions from the virions and cores were concentrated, normalized for Pr55Gag content by CA-specific ELISA, and assayed for gp41 and gp120 by immunoblotting (Fig. 3). In contrast to immature HIV-1 particles containing full-length Env, detergent treatment of immature R9Tr712.PR− virions removed essentially all of the gp41 and gp120 from immature virions lacking the gp41 cytoplasmic tail (Fig. 3, lane 3). A small amount of unprocessed Env protein (gp160) remained associated with the cores, a result that was occasionally observed in repeated experiments. Reprobing the blot with anti-CA antiserum confirmed that similar quantities of Pr55Gag were analyzed, and probing with anti-CypA antiserum verified that the viral lipid envelope was disrupted by exposure to 1% Triton X-100 (Fig. 3A). These results demonstrate that the detergent-resistant association of the HIV-1 Env proteins with immature HIV-1 particles requires the gp41 cytoplasmic tail.
FIG. 3.
Immunoblot analysis of immature HIV-1 cores and virions lacking the cytoplasmic tail of gp41. The peak Pr55Gag-containing fraction of cores (C, lanes 1 and 3) and virions (V, lanes 2 and 4), containing either a full-length form of gp41 (WT) or a truncated form of gp41 lacking the cytoplasmic tail (Tr712), were diluted with STE buffer and pelleted by ultracentrifugation. Pellets were dissolved in 1× SDS loading buffer, subjected to electrophoresis on a 4 to 20% acrylamide gel, and transferred to PVDF membrane, and the blot was probed with the indicated antisera. Protein bands were revealed by chemiluminescent detection after incubation of the blot with appropriate HRP-conjugated secondary antisera. Molecular mass markers are shown in kilodaltons on the left of each panel.
Inhibition of cleavage at the MA-CA junction allows retention of the HIV-1 Env proteins on a PR-competent core.
Mutations in the MA region of Pr55Gag have been reported to block the incorporation of Env into nascent virions, suggesting that the MA region of Pr55Gag associates with gp41 during virion assembly (12). Structural studies of MA suggest that upon cleavage from Pr55Gag, the free MA protein assumes a different conformation than when part of the full-length Pr55Gag (27). Based on these observations, we hypothesized that inhibiting cleavage at the MA-CA junction, in the context of an active PR, would be sufficient for retention of gp41 by the isolated cores. To test this hypothesis, we created an MA-CA cleavage site mutant by mutating the P1 position of the MA-CA junction, converting the tyrosine (Tyr) to an isoleucine (Ile) codon. This strategy was based on reports that substitution of residues containing β-branched side chains in the P1 position of a cleavage site has been shown to block cleavage by HIV-1 PR (24).
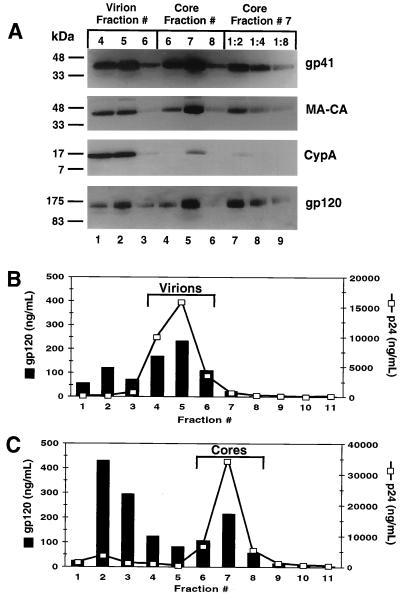
Intact virions and cores from the MA-CA mutant were isolated, and the peak Pr55Gag-containing fractions (virion fractions 4-6 and core fractions 6-8) were analyzed for the presence of Env proteins (Fig. 4A) by immunoblotting. As observed with the immature cores, a large fraction of the gp41 and gp120 present on virions remained associated with the MA-CA cores (Fig. 4A, compare lanes 2 and 7, which contain similar amounts of Gag protein). Reprobing the blot with a CA-specific antiserum verified that cleavage at the MA-CA junction of Pr55Gag was blocked in the mutant virus. The low levels of CypA detected in the cores fractions confirmed that the viral lipid envelope was disrupted by treatment with 1% Triton X-100. The amount of gp120 in the gradient fractions of MA-CA virions and cores was measured by a gp120-specific ELISA (Fig. 4B and C). The peak Pr55Gag-containing fraction of the MA-CA cores (Fig. 4C, fraction 7) retains approximately 45% of the gp120 protein that is present on the intact MA-CA virions (Fig. 4B, fraction 5). These results demonstrate that blocking cleavage at the MA-CA junction is sufficient for the retention of both HIV-1 Env proteins on the isolated core structures.
FIG. 4.
Immunoblot and ELISA analysis of HIV-1 virions and cores inhibited for cleavage at the MA-CA junction of Pr55Gag. (A) The peak MA-CA-containing fractions of virions (lanes 1 to 3) and cores (lanes 4 to 6) were analyzed by immunoblotting as described in the legend to Fig. 2. (B and C) The gp120 concentrations of the gradient fractions were determined as described in the legend to Fig. 2. (B) Analysis of virions. (C) Analysis of cores.
The detergent-stable association of gp41 with Pr55Gag is independent of lipid rafts.
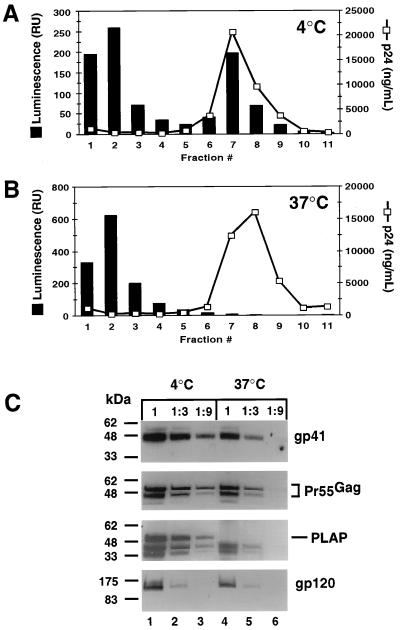
Recently, it was reported that HIV-1 assembly occurs in cholesterol-rich membrane microdomains known as lipid rafts (22). The lipid composition of HIV-1 particles is consistent with the presence of lipid rafts (1, 2), suggesting that HIV-1 contains raft microdomains within the lipid envelope on the virion. A characteristic property of lipid rafts is their insolubility in 1% Triton X-100 at 4°C (26), conditions similar to those used in the isolation of cores. We therefore tested the possibility that the HIV-1 Env proteins were present on immature cores due to the presence of detergent-insoluble lipid rafts. For this purpose, we utilized a reporter virus (NLPI.nef+) that encodes PLAP, a raft-associated protein made in the presence of an HIV-1 PR inhibitor to prevent Pr55Gag cleavage and thus maintain the immature virion phenotype. PLAP is a glycophosphatidylinositol-anchored protein that localizes to lipid rafts on cells and is used as a marker to identify lipid rafts (4). Immature HIV-1 particles were produced transfecting 293T cells with plasmid DNA and culturing in the presence of an HIV-1 PR inhibitor. The PLAP protein is incorporated into budding virions (data not shown) and was therefore used as a marker for the presence of rafts on the immature cores. Immature cores were isolated by incubating concentrated virions with 1% Triton X-100 for 1 h at either 4°C, conditions known to preserve lipid rafts, or at 37°C, which disrupts lipid rafts (4). The particles were then purified by sedimentation centrifugation. Gradient fractions from virions and cores were tested for the presence of PLAP using a quantitative chemiluminescent activity assay for AP (Fig. 5). Virions treated with 1% Triton X-100 at 4°C exhibited a peak of PLAP activity that cosedimented with a peak of Pr55Gag in the immature cores (Fig. 5A, fraction 7). In contrast, cores isolated by treatment of virions with 1% Triton X-100 at 37°C lacked detectable levels of AP activity in the cores (Fig. 5B, fraction 8). It is noteworthy that virions treated at 37°C sedimented at a slightly higher density, 1.26 g/ml (data not shown), suggesting that removal of the lipid rafts from the virions altered the density of the isolated cores. To determine whether the HIV-1 Env proteins were associated with immature cores independently of the presence of lipid rafts, the peak Pr55Gag-containing gradient fraction from each gradient was pelleted and assayed for the presence of Env proteins by immunoblotting (Fig. 5C). Similar levels of both gp41 and gp120 were present on virions following detergent treatment at either 4 or 37°C. Reprobing the blot with a PLAP-specific antiserum verified that treatment with detergent at 37°C was sufficient to remove PLAP from the cores. These results indicate that the stable association of the HIV-1 Env proteins with immature cores is not due to the presence of lipid rafts. Furthermore, these data indicate that the interactions between gp41 and Pr55Gag and between gp41 and gp120 are remarkably stable, persisting after treatment with 1% Triton X-100 for 1 h at 37°C.
FIG. 5.
Association of HIV-1 Env proteins on immature HIV-1 cores independently of the presence of lipid rafts. Immature HIV-1 cores were isolated from a PLAP-encoding virus by incubation for 1 h in detergent at either 4 or 37°C, followed by sedimentation centrifugation. Fractions were examined for the presence of PLAP and HIV-1 Env proteins. (A and B) Levels of PLAP were determined by incubation of gradient fractions with an AP substrate and quantitated on a luminometer as relative units (RU) of luminescence. A CA-specific ELISA was used to determine the amount of virus in each gradient fraction. (C) Immunoblot analysis of the peak Pr55Gag-containing gradient fractions from immature cores treated with detergent at either 4 or 37°C (lanes 1 and 4, respectively) was performed as described in the legend to Fig. 2. Threefold serial dilutions of the peak Pr55Gag-containing fractions were also analyzed (lanes 2 and 3 and lanes 5 and 6).
Phospholipid analysis of immature HIV-1 virions and cores.
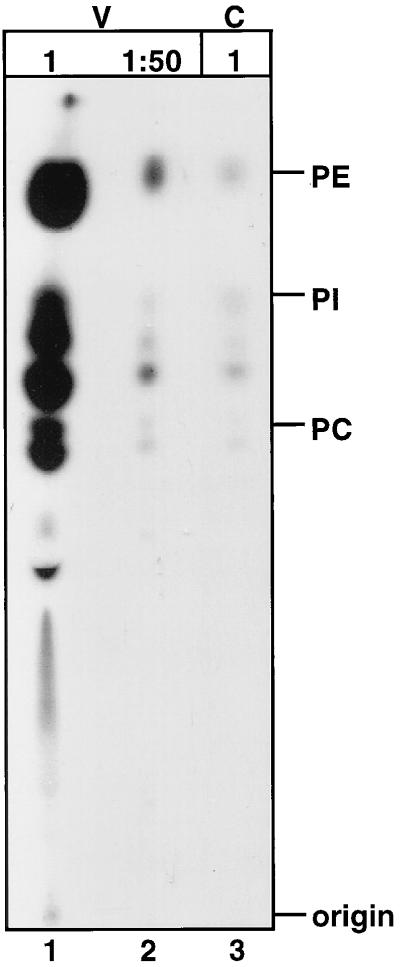
Our results suggested that a direct interaction between the cytoplasmic tail of gp41 and Pr55Gag was responsible for the stable association of Env with immature cores. However, this conclusion was based on the assumption that the core isolation procedure efficiently removed the viral lipid envelope. To determine the efficiency of lipid removal during Triton X-100 treatment of immature HIV-1 particles, we labeled the phospholipids of virions by culturing producer cells with [32P]orthophosphate. The 32P-labeled immature virions were treated with 1% Triton X-100 for 1 h at 37°C, and the resulting cores were pelleted by ultracentrifugation. Lipids were extracted from 32P-labeled cores and control virions and were analyzed by thin-layer chromatography and visualized by autoradiography (Fig. 6). Analysis of lipids extracted from similar quantities of intact virions (Fig. 6, lane 1, 30 μg of p24) and cores (Fig. 6, lane 3, 26 μg of p24) demonstrated that treatment with 1% Triton X-100 at 37°C for 1 h efficiently removed a majority of the phospholipids from the virions. Comparison of the cores sample to a 1:50 dilution of lipids extracted from intact virions (Fig. 6, lane 2) revealed that <2% of the analyzed phospholipids remained on the isolated cores. We conclude that the procedure used to isolate immature HIV-1 cores resulted in efficient removal of phospholipids.
FIG. 6.
Analysis of phospholipids present immature HIV-1 virions and cores. Immature HIV-1 particles were harvested from 32P-labeled 293T cells transiently transfected with the R9.PR− proviral plasmid. The virions were concentrated by ultracentrifugation and then treated with 1% Triton X-100 for 1 h at 37°C. The remaining core structures were separated from free proteins and lipids by pelleting them through a 20% sucrose cushion. Lipids from virions (V) and cores (C) were extracted by chloroform-methanol (2:1) extraction, analyzed by thin-layer chromatography, and visualized by autoradiography. Lipids were extracted from similar quantities of immature virions (lane 1, 30 μg of p24) and immature cores (lane 3, 26 μg of p24) and analyzed. A 1:50 dilution of the intact virions (lane 2) was analyzed for quantitative comparison. Phosphatidylethanolamine (PE), phosphatidylinositol (PI), and phosphatidylcholine (PC) standards were analyzed in parallel.
DISCUSSION
In this study, we isolated immature cores from PR-defective virions with the goal of dissecting the putative interaction of the HIV-1 TM protein with Pr55Gag. The inherent stability of these immature particles allowed us to recover greater than 80% of the initial virus. Based on this result, we conclude that biochemical observations made using these particles are likely to be representative of the whole virion population and not a consequence of the presence of a minor hyperstable subset of virions.
We and others have shown that the HIV-1 Env proteins are efficiently removed from mature HIV-1 particles when cores are isolated by exposure of virions to 1% Triton X-100 (16, 30). In contrast, immature cores isolated by an identical procedure contained high levels of both gp41 and gp120. HIV-1 assembly was recently reported to take place in lipid rafts (22), which are membrane microdomains enriched in cholesterol, glycolipids, and some proteins (26). The isolation conditions we used to isolate HIV-1 cores are known to preserve lipid rafts, and immature HIV-1 cores also contained a known raft-associated protein, PLAP. However, detergent treatment of virions under conditions known to disrupt lipid rafts resulted in the efficient removal of PLAP but not of the HIV-1 Env proteins. We conclude that immature HIV-1 virions incorporate lipid rafts during assembly. Our results further demonstrate that the stable association of HIV-1 Env proteins with immature cores does not require the presence of lipid rafts. The interactions mediating this association are remarkably stable, persisting during a 1-h exposure to 1% Triton X-100 at 37°C. The observation that gp41 exhibits a detergent-stable association with immature cores extends previously reported genetic data and represents the first biochemical evidence for an interaction between gp41 and Pr55Gag in HIV-1 particles.
The precise function of the 150-residue gp41 cytoplasmic tail in HIV-1 replication is not known. In some cell lines, such as MT-4, HIV-1 lacking the gp41 cytoplasmic domain replicates efficiently, while in others, including peripheral blood mononuclear cells, the TM cytoplasmic tail is required for Env incorporation (21). In our experiments, HIV-1 particles were produced in 293T cells, a cell type that is permissive for virion incorporation of tail-less HIV-1 Env proteins. Our finding, that the detergent-stable interaction of gp41 with immature HIV-1 particles requires the gp41 cytoplasmic domain, suggests that the gp41 tail mediates the strong association of Env proteins with Pr55Gag in immature HIV-1 particles. We conclude that this interaction is probably essential for incorporation of Env in cell types in which the gp41 tail is required for HIV-1 replication.
Although removal of the TM cytoplasmic tail demonstrated that this domain is necessary for the interaction of gp41 with immature cores, the region of Pr55Gag required for gp41 binding remains to be dissected. Although other retroviruses cannot be pseudotyped by full-length HIV-1 proteins, the MA domain of HIV-1 Gag is sufficient for the incorporation of full-length HIV-1 Env in particles produced from chimeric HIV-1–visna virus Gag proteins (8). Our demonstration that cores isolated from PR-competent HIV-1 particles containing uncleaved MA-CA precursor retained large quantities of gp41 supports the hypothesis that the MA domain of Pr55Gag, when part of a larger Gag precursor, binds the gp41 cytoplasmic domain. It remains to be determined whether other regions of Gag also participate in this interaction.
Although our findings do not formally exclude the possibility of a third protein mediating the interaction between gp41 and Pr55Gag, published genetic data argue against this (20). Such a bridging protein must be present at high concentrations within the virion, must lose the ability to bridge gp41 and Pr55Gag when the gp41 tail is mutated, and must regain this ability when compensatory mutations are introduced in MA. Likewise, our data do not completely exclude the possibility of a requirement for a lipid moiety as being required for the gp41-Pr55Gag association. However, our lipid analysis demonstrated that more than 98% of the phospholipid was removed from immature cores, while approximately half of the Env molecules remained associated with immature cores. With these caveats, the most straightforward interpretation of our data is that the gp41 cytoplasmic tail binds directly to the MA region of Pr55Gag in immature HIV-1 particles.
A surprising outcome of this study was the finding that, in addition to gp41, gp120 was stably associated with the immature cores. Previous studies employing measurements of gp120 shedding from mature HIV-1 particles concluded that the gp120-gp41 interaction is unstable in laboratory-adapted strains of HIV-1 (18, 19). We observed that approximately 50% of the gp120 remained associated with immature HIV-1 cores. This result suggests that the binding of the gp41 cytoplasmic tail to Pr55Gag stabilizes the gp120-gp41 interaction. We hypothesize that the interaction of Pr55Gag with the cytoplasmic tail of gp41 regulates the conformation of the Env protein complex on the immature virion surface, thereby preventing entry until the virion has matured. Further studies will be required to determine whether gp41 binding to Pr55Gag regulates fusion of HIV-1 particles.
ACKNOWLEDGMENTS
We thank B. Chen for the plasmid pNLPI, V. Bosch for pNLTr712, and D. Trono and L. Henderson for antibodies. We also thank A. D. De Silva and S. Joyce for expert assistance with the phospholipid analysis. The following reagents were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 monoclonal antibodies against gp41 (C2F5) and gp120 (C2G12) from Hermann Katinger.
This study was supported by NIH grant AI47056 and a Discovery Grant from Vanderbilt University. D.J.W. was supported by NIH training grant T32 CA09385.
REFERENCES
- 1.Aloia R C, Jensen F C, Curtain C C, Mobley P W, Gordon L M. Lipid composition and fluidity of the human immunodeficiency virus. Proc Natl Acad Sci USA. 1988;85:900–904. doi: 10.1073/pnas.85.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aloia R C, Tian H, Jensen F C. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc Natl Acad Sci USA. 1993;90:5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 4.Brown D A, Rose J K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 5.Chen B K, Feinberg M B, Baltimore D. The κB sites in the human immunodeficiency virus type 1 long terminal repeat enhance virus replication yet are not absolutely required for viral growth. J Virol. 1997;71:5495–504. doi: 10.1128/jvi.71.7.5495-5504.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosson P. Direct interaction between the envelope and matrix proteins of HIV-1. EMBO J. 1996;15:5783–5788. [PMC free article] [PubMed] [Google Scholar]
- 8.Dorfman T, Mammano F, Haseltine W A, Gottlinger H G. Role of the matrix protein in the virion association of the human immunodeficiency virus type 1 envelope glycoprotein. J Virol. 1994;68:1689–1696. doi: 10.1128/jvi.68.3.1689-1696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubay J W, Roberts S J, Hahn B H, Hunter E. Truncation of the human immunodeficiency virus type 1 glycoprotein cytoplasmic domain blocks virus infectivity. J Virol. 1992;66:6616–6625. doi: 10.1128/jvi.66.11.6616-6625.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan M A, Carruth L M, Rowell J F, Yu X, Siliciano R F. Human immunodeficiency virus type 1 envelope protein endocytosis mediated by a highly conserved intrinsic internalization signal in the cytoplasmic domain of gp41 is suppressed in the presence of the Pr55gag precursor protein. J Virol. 1996;70:6547–56. doi: 10.1128/jvi.70.10.6547-6556.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freed E, Martin M A. Virion incorporation of envelope glycoproteins with long but not short cytoplasmic tails is blocked by specific, single amino acid substitutions in the human immunodeficiency virus type 1 matrix. J Virol. 1995;69:1984–1989. doi: 10.1128/jvi.69.3.1984-1989.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freed E O, Martin M A. Domains of the human immunodeficiency virus type 1 matrix and gp41 cytoplasmic tail required for envelope incorporation. J Virol. 1996;70:341–351. doi: 10.1128/jvi.70.1.341-351.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horton R M, Cai Z L, Ho S N, Pease L R. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. BioTechniques. 1990;8:528–35. [PubMed] [Google Scholar]
- 14.Hunter E, Swanstrom R. Retrovirus envelope glycoproteins. Curr Top Microbiol Immunol. 1990;157:187–253. doi: 10.1007/978-3-642-75218-6_7. [DOI] [PubMed] [Google Scholar]
- 15.Kewalramani V N, Emerman M. Vpx association with mature core structures of HIV-2. Virology. 1996;218:159–168. doi: 10.1006/viro.1996.0176. [DOI] [PubMed] [Google Scholar]
- 16.Kotov A, Zhou J, Flicker P, Aiken C. Association of Nef with the human immunodeficiency virus type 1 core. J Virol. 1999;73:8824–8830. doi: 10.1128/jvi.73.10.8824-8830.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lodge R, Lalonde J-P, Lemay G, Cohen E A. The membrane-proximal intracytoplasmic tyrosine residue of HIV-1 envelope glycoprotein is critical for basolateral targeting of viral budding in MDCK cells. EMBO J. 1997;16:695–705. doi: 10.1093/emboj/16.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKeating J A, McKnight A, Moore J P. Differential loss of envelope glycoprotein gp120 from virions of human immunodeficiency virus type 1 isolates: effects on infectivity and neutralization. J Virol. 1991;65:852–860. doi: 10.1128/jvi.65.2.852-860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore J P, McKeating J A, Weiss R A, Sattentau Q J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990;250:1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- 20.Murakami T, Freed E O. Genetic evidence for an interaction between human immunodeficiency virus type 1 matrix and alpha-helix 2 of the gp41 cytoplasmic tail. J Virol. 2000;74:3548–3554. doi: 10.1128/jvi.74.8.3548-3554.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami T, Freed E O. The long cytoplasmic tail of gp41 is required in a cell type-dependent manner for HIV-1 envelope glycoprotein incorporation into virions. Proc Natl Acad Sci USA. 2000;97:343–348. doi: 10.1073/pnas.97.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen D H, Hildreth J E. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J Virol. 2000;74:3264–3272. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owens R J, Dubay J W, Hunter E, Compans R W. Human immunodeficiency virus envelope protein determines the site of virus release in polarized epithelial cells. Proc Natl Acad Sci USA. 1991;88:3987–3991. doi: 10.1073/pnas.88.9.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pettit S C, Simsic J, Loeb D D, Everitt C A, Hutchison III C A, Swanstrom R. Analysis of retroviral protease cleavage sites reveals two types of cleavage sites and the structural requirements of the P1 amino acid. J Biol Chem. 1991;266:14539–14547. [PubMed] [Google Scholar]
- 25.Rowell J F, Stanhope P E, Siliciano R F. Endocytosis of endogenously synthesized HIV-1 envelope protein. Mechanism and role in processing for association with class II MHC. J Immunol. 1995;155:473–488. [PubMed] [Google Scholar]
- 26.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 27.Spearman P, Horton R, Ratner L, Kuli-Zade I. Membrane binding of human immunodeficiency virus type 1 matrix protein in vivo supports a conformational myristyl switch mechanism. J Virol. 1997;71:6582–6592. doi: 10.1128/jvi.71.9.6582-6592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vincent M J, Melsen L R, Martin A S, Compans R W. Intracellular interaction of simian immunodeficiency virus Gag and Env proteins. J Virol. 1999;73:8138–8144. doi: 10.1128/jvi.73.10.8138-8144.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wehrly K, Chesebro B. p24 antigen capture assay for quantification of human immunodeficiency virus using readily available inexpensive reagents. Methods. 1997;12:288–293. doi: 10.1006/meth.1997.0481. [DOI] [PubMed] [Google Scholar]
- 30.Welker R, Hohenberg H, Tessmer U, Huckhagel C, Krausslich H G. Biochemical and structural analysis of isolated mature cores of human immunodeficiency virus type 1. J Virol. 2000;74:1168–1177. doi: 10.1128/jvi.74.3.1168-1177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilk T, Pfeiffer T, Bosch V. Retained in vitro infectivity and cytopathogenicity of HIV-1 despite truncation of the C-terminal tail of the env gene product. Virology. 1992;189:167–177. doi: 10.1016/0042-6822(92)90692-i. [DOI] [PubMed] [Google Scholar]
- 32.Yu X, Yuan X, Matsuda Z, Lee T-H, Essex M. The matrix protein of human immunodeficiency virus type 1 is required for incorporation of viral envelope protein into mature virions. J Virol. 1992;66:4966–4971. doi: 10.1128/jvi.66.8.4966-4971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]