Abstract
Background: To analyze the risk factors for benign paroxysmal positional vertigo (BPPV) and to construct a predictive nomogram model. Methods: In this retrospective study, 312 participants were enrolled, including 164 BPPV patients and 148 healthy subjects without BPPV. Risk predictors for BPPV were identified using univariate and multivariate analyses, and a clinical nomogram was constructed. The predictive accuracy was assessed by unadjusted concordance index (C-index) and calibration plot. Results: Univariate and multivariate regression analysis identified stroke (95% CI, 0.575-5.954; P=0.022), hyperlipidemia (95% CI, 0.471-4.647; P=0.003), chronic suppurative otitis media (95% CI, 1.222-45.528; P=0.005), cervical spondylosis (95% CI, 1.232-3.017; P=0.005), and osteoporosis (95% CI, 1.130-3.071; P=0.001) were the independent risk factors for BPPV. These risk factors were used to construct a clinical predictive nomogram. The regression equation was: logit (P) = -6.820 + 0.450 * stroke + hyperlipidemia * 0.312 + chronic suppurative otitis media * 0.499 + cervical spondylosis * 0.916 + osteoporosis * 0.628. The calibration curves demonstrated excellent accuracy of the predictive nomogram. Decision curve analysis showed that the predictive model is clinically applicable when the threshold probability was between 20% and 60%. Conclusions: Stroke, hyperlipidemia, chronic suppurative otitis media, cervical spondylosis and osteoporosis are independent risk predictors for BPPV. The developed nomogram is useful in predicting the risk of BPPV.
Keywords: Benign paroxysmal positional vertigo, risk factors, predictive effect, nomogram
Introduction
Benign paroxysmal positional vertigo (BPPV) is the most prevalent vertigo disease in the emergency center [1], and also the most common peripheral vestibular disease in the Department of Neurology, accounting for about 20%-30% of all vestibular complaints [2]. However, the complexity of accurately differentiating BPPV from other vertigo-inducing conditions within the “benign fatal” disease spectrum, combined with variable patient perceptions and descriptions of “dizziness” suggests that these figures are significantly underestimated. A prospective study conducted in South Korea in 2020 forecasted a 52% increase in hospital visits due to dizziness/vertigo over the next 30 years [3]. BPPV is particularly debilitating in elderly patients, severely impairing daily functions [4] and substantially increasing the risk of falls [5]. The total expenditure related to BPPV in the United States is nearly 2 billion dollars per year [6]. At present, the per capita cost of diagnosis and treatment of BPPV in China has reached 4165 yuan, figures that are projected to rise in conjunction with an aging population [7]. The primary treatment for BPPV, canalith repositioning maneuvers (CRM), has shown effectiveness in symptom amelioration. The high recurrence rate after treatment contributes to ongoing patient anxiety and diminished quality of life. Given that vertigo and dizziness can lead to falls due to impaired movement and spatial orientation. Therefore, identifying the risk factors for BPPV is imperative for better symptom and relapse prevention.
The risk factors for BPPV have attracted significant clinical research interest globally. Von Brevern et al. indicated that the incidence of BPPV increased with age. The average age for BPPV onset is 49.4 years, with individuals over 60 experiencing a prevalence rate approximately seven times higher than those aged 18-39 years [8]. Age contributes to BPPV in a multifaceted manner, enhancing the interaction of various risk factors that influence disease onset. Age-related morphologic changes in the ear, particularly the degeneration of ear cone cells, have been implicated in BPPV pathogenesis [9]. Many factors can lead to poor blood supply to the inner ear and microcirculation disorders. Elevated blood lipids, blood glucose and other vascular factors have been identified as vascular contributors to BPPV risk [10-12]. The heterogeneity and complex interplay of these factors make understanding BPPV’s etiology challenging, underscoring the need for further research.
This retrospective study aims to dissect the multifactorial risks associated with BPPV, identify independent risk factors, evaluate their predictive value, and construct a nomogram. Our goal is to furnish a theoretical framework that aids in the improved prevention of BPPV in clinical settings.
Methods and materials
Study design and ethics
This retrospective study was conducted Zibo Central Hospital between January 2020 and December 2023. A total of 312 participants were fitted into this study, including 164 BPPV patients and 148 healthy subjects without BPPV. The medical ethics committee of Zibo Central Hospital reviewed and approved the study protocol.
Inclusion criteria
(1) Age >18 years; (2) Diagnosis of BPPV aligning with the criteria set by the Otolaryngology Head and Neck Surgery Branch of the Chinese Medical Association [13]; (3) Patients without other obvious signs of nervous system and hearing impairment; (4) Presence of vertigo triggered by head position changes, confirmed by a positive Dix-Hallpike or Roll test; (5) Availability of complete clinical information.
Exclusion criteria
(1) Patients with central vertigo; (2) Patients with vertigo caused by Meniere’s disease, internal auditory artery syndrome, vestibular neuritis, migraine, vertebrobasilar ischemia, cervical-related vertigo, or epilepsy; (3) Patients with severe cervical spondylosis, severe heart disease, and postural hypotension that cannot cooperate with the examination and postural test; (4) Patients with moderate to severe cognitive or visual impairment; (5) Incomplete clinical information.
Diagnosis and grouping
The diagnosis of benign paroxysmal positional vertigo (BPPV) adhered to the criteria established by the Otolaryngology Head and Neck Surgery Branch of the Chinese Medical Association [14]: ① Typical history of transient vertigo induced by head movement to a specific position; ② Characteristic nystagmus on the affected side observed during the Dix-Hallpike test or Roll test, accompanied by a vertigo attack. Nystagmus typically presents with a 1-30 second latency period and can show signs of fatigue; ③ Brain CT or MRI examinations focused on the cochlea, along with consultations from relevant departments, to rule out inner ear or central nervous system disorders.
From January 2020 to December 2023, 164 BPPV cases were diagnosed in the Otolaryngology Head and Neck Surgery outpatient department of our hospital. Concurrently, 148 healthy subjects without BPPV were randomly selected from the physical examination center to serve as the control group.
Data collection and measurement
Data collection was guided by the criteria suggested by Worster et al. for retrospective chart reviews [15]. Two research fellows (Wenping Cao and Yang Geng) systematically reviewed medical charts to collect data. For each participant, we collected general characteristics, such as age and gender, as well as comprehensive clinical data. The clinical data encompassed onset and accompanying symptoms; detailed past medical history including stroke, hypertension, diabetes, abnormal uric acid, internal carotid artery thickening, sudden deafness, osteoporosis, chronic suppurative otitis media, vestibular neuritis, cervical spondylosis, migraine, hyperthyroidism, Meniere’s disease, and coronary atherosclerotic heart disease; lifestyle factors, such as smoking and alcohol history; and other relevant factors, including ototoxic drug exposure history, medication history, head injury history, nasal cavity and paranasal sinus surgery history, cochlear surgery history, temporal bone surgery history, and maxillofacial dental surgery history.
All patients underwent a detailed otological examination, vestibular function examination, pure tone hearing threshold examination and head MRI. Similar data on general characteristics and clinical features were also collected from the normal control group.
Statistical analysis
IBM SPSS 17.0 was used for statistical analysis. K-S (Kolmogorov Smirnov test) was used to test the normality of data. Data with normal distribution were expressed as mean ± standard deviation. Differences among multiple groups were analyzed using one-way ANOVA with post-hoc comparisons by the Least Significant Difference (LSD) test. Data with a non-normal distribution were represented by median (P25, P75) and analyzed using nonparametric test, with the Kruskal-Wallis test applied for comparisons between two groups. Categorical variables were expressed as cases (%) and analyzed using chi-square test.
The sample size was calculated by power analysis and estimated as: corrected sample size = sample size/(1 - [% attrition/100]). Finally, we settled on a sample size of around 300. The risk factors were introduced into R software (R 3.6.3) to construct a nomogram model to predict the risk of BPPV. The model incorporated identified variables and their regression coefficients, and the validity of the model was evaluated by plotting the Receiver Operating Characteristic (ROC) curve.
Result
Clinical characteristics
No significant differences were observed in age, BMI, gender, involved site, sleep disorders, coronary atherosclerotic heart disease, diabetes mellitus, Meniere disease, migraine, or head trauma between the two groups (all P>0.05). However, significant differences were observed regarding stroke, hyperlipidemia, chronic suppurative otitis media (CSOM), cervical spondylosis, osteoporosis, and hypertension between the two groups showed (all P <0.05) (Table 1).
Table 1.
Comparison of clinical characteristics between two groups
| BPPV group (n=164) | Control group (n=148) | t/χ2 | P | |
|---|---|---|---|---|
| Age (years) | 48.9 ± 6.3 | 49 4 ± 5.8 | 1.658 | 0.278 |
| BMI | 23.89 ± 4.01 | 22.89 ± 3.77 | 0.606 | 0.373 |
| Gender | 2.199 | 0.132 | ||
| Male | 84 (51.2%) | 86 (58.1%) | ||
| Female | 80 (48.8%) | 62 (41.9%) | ||
| Sites | 0.022 | 0.997 | ||
| Posterior semicircular canal | 72 (43.9%) | 66 (44.6%) | ||
| Horizontal semicircular canal | 32 (19.5%) | 32 (21.6%) | ||
| Anterior semicircular canal | 46 (28%) | 38 (25.7%) | ||
| Mixed type | 14 (8.5%) | 12 (8.1%) | ||
| Sleep disorders | 132 (80.5%) | 118 (79.7%) | 0.247 | 0.619 |
| Coronary atherosclerotic heart disease | 99 (60.4%) | 99 (66.9%) | 2.206 | 0.137 |
| Stroke | 129 (78.7%) | 63 (42.6%) | 8.756 | 0.009 |
| Hyperlipidemia | 80 (48.8%) | 53 (35.8%) | 12.697 | 0.002 |
| Chronic suppurative otitis media | 122 (74.4%) | 60 (40.5%) | 10.986 | 0.006 |
| Cervical spondylosis | 100 (61.0%) | 50 (33.8%) | 10.916 | 0.005 |
| Osteoporosis | 120 (73.2%) | 85 (57.4%) | 8.474 | 0.010 |
| Hypertension | 83 (50.6%) | 10 (6.8%) | 10.285 | 0.003 |
| Diabetes mellitus | 78 (47.6%) | 62 (41.9%) | 0.381 | 0.537 |
| Meniere | 83 (50.6%) | 71 (48%) | 0.628 | 0.428 |
| Migraine | 85 (51.8%) | 73 (49.3%) | 0.186 | 0.666 |
| Head trauma | 32 (19.5%) | 30 (20.3%) | 0.562 | 0.757 |
Comparison of biochemical indexes
As shown in Table 2, compared with the control group, the BPPV group exhibited significantly higher blood uric acid level (P<0.05). There were no significant differences in levels of cystatin C, total cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) between the two groups (all P>0.05).
Table 2.
Comparison of biochemical indexes
| BPPV group (n=164) | Control group (n=148) | t/χ2 | P | |
|---|---|---|---|---|
| Blood uric acid | 395.43 ± 14.98 | 277.6 ± 10.5 | 8.756 | 0.001 |
| Cystatin C | 0.998 ± 0.211 | 0.432 ± 0.315 | 0.562 | 0.757 |
| Total cholesterol | 19.6 ± 4.5 | 16.9 ± 3.5 | 2.697 | 0.102 |
| Low density lipoprotein | 3.9 ± 1.1 | 3.5 ± 1.0 | 2.984 | 0.095 |
| High density lipoprotein | 1.6 ± 0.9 | 1.9 ± 0.7 | 1.983 | 0.983 |
Univariate and multivariate analysis
Univariate and multivariate analysis identified several risk factors associated with BPPV (Tables 3 and 4): stroke (95% CI: 0.575-5.954; P=0.022), hyperlipidemia (95% CI: 0.471-4.647; P=0.003), CSOM (95% CI: 1.222-45.528; P=0.005), cervical spondylosis (95% CI: 1.232-3.017; P=0.005), and osteoporosis (95% CI: 1.130-3.071; P=0.001).
Table 3.
Univariate analysis
| BPPV group (n=164) | Control group (n=148) | t/χ2 | P | |
|---|---|---|---|---|
| Age | 3.316 | 0.069 | ||
| <35 years | 114 (69.5%) | 124 (83.8%) | ||
| ≥35 years | 50 (30.5%) | 24 (16.2%) | ||
| BMI | 2.695 | 0.058 | ||
| ≥22 | 129 (78.7%) | 124 (83.8%) | ||
| <22 | 35 (21.3%) | 24 (16.2%) | ||
| Sites | 0.022 | 0.997 | ||
| Posterior semicircular canal | 72 (43.9%) | 66 (44.6%) | ||
| Horizontal semicircular canal | 32 (19.5%) | 32 (21.6%) | ||
| Anterior semicircular canal | 46 (28%) | 38 (25.7%) | ||
| Mixed type | 14 (8.5%) | 12 (8.1%) | ||
| Stroke | 0.342 | 0.036 | ||
| Yes | 129 (78.7%) | 63 (42.6%) | ||
| No | 35 (21.3%) | 85 (57.4%) | ||
| Sleep disorders | 0.247 | 0.619 | ||
| Yes | 132 (80.5%) | 118 (79.7%) | ||
| No | 32 (19.5%) | 30 (20.3%) | ||
| Hyperlipidemia | 11.286 | 0.007 | ||
| Yes | 80 (48.8%) | 53 (35.8%) | ||
| No | 84 (51.2%) | 95 (64.2%) | ||
| Chronic suppurative otitis media | 10.607 | 0.016 | ||
| Yes | 122 (74.4%) | 60 (40.5%) | ||
| No | 44 (26.8%) | 48 (32.4%) | ||
| Coronary atherosclerotic heart disease | 2.206 | 0.137 | ||
| Yes | 99 (60.4%) | 99 (66.9%) | ||
| No | 65 (39.6%) | 49 (33.1%) | ||
| Cervical spondylosis | 6.697 | 0.011 | ||
| Yes | 100 (61.0%) | 50 (33.8%) | ||
| No | 64 (39.0%) | 98 (66.2%) | ||
| Osteoporosis | 8.474 | 0.010 | ||
| Yes | 120 (73.2%) | 85 (57.4%) | ||
| No | 44 (25.6%) | 63 (42.6%) | ||
| Hypertension | 10.285 | 0.003 | ||
| Yes | 83 (50.6%) | 10 (6.8%) | ||
| No | 81 (49.4%) | 138 (93.2%) | ||
| Anemia | 0.381 | 0.537 | ||
| Yes | 78 (47.6%) | 62 (41.9%) | ||
| No | 86 (52.4%) | 86 (58.1%) | ||
| Hypoproteinemia | 0.628 | 0.428 | ||
| Yes | 83 (50.6%) | 71 (48%) | ||
| No | 81 (49.4%) | 77 (52%) | ||
| Diabetes mellitus | 0.381 | 0.537 | ||
| Yes | 78 (47.6%) | 66 (44.6%) | ||
| No | 86 (52.4%) | 82 (55.4%) | ||
| Meniere | 0.628 | 0.428 | ||
| Yes | 83 (50.6%) | 71 (48%) | ||
| No | 81 (49.4%) | 77 (52%) | ||
| Migraine | 0.186 | 0.666 | ||
| Yes | 85 (51.8%) | 73 (49.3%) | ||
| No | 79 (51.2%) | 75 (54.1%) | ||
| Head trauma | 0.562 | 0.757 | ||
| Yes | 32 (19.5%) | 30 (20.3%) | ||
| No | 132 (80.5%) | 118 (79.7%) | ||
| Blood uric acid | 395.43 ± 14.98 | 277.6 ± 10.5 | 8.756 | 0.001 |
| Cystatin C | 0.998 ± 0.211 | 0.432 ± 0.315 | 0.562 | 0.757 |
| Total cholesterol | 19.6 ± 4.5 | 16.9 ± 3.5 | 2.697 | 0.102 |
| Low density lipoprotein | 3.9 ± 1.1 | 3.5 ± 1.0 | 2.984 | 0.095 |
| High density lipoprotein | 1.6 ± 0.9 | 1.9 ± 0.7 | 1.983 | 0.983 |
BMI: body mass index.
Table 4.
Multivariate regression analysis
| Factor | β | SE | Wals | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Stroke | ||||||
| No | - | - | 3.546 | 0.059 | 1 | |
| Yes | 0.515 | 0.296 | 15.064 | 0.022 | 2.450 | 0.575-5.954 |
| Hyperlipidemia | ||||||
| No | - | - | 1.981 | 0.104 | 1 | |
| Yes | 0.891 | 0.484 | 10.149 | 0.003 | 2.499 | 0.471-4.647 |
| Chronic suppurative otitis media | ||||||
| No | - | - | 3.981 | 0.304 | 1 | |
| Yes | 2.037 | 0.909 | 15.024 | 0.005 | 1.916 | 1.222-45.528 |
| Cervical spondylosis | ||||||
| No | - | - | 4.981 | 0.354 | 1 | |
| Yes | 0.661 | 0.226 | 13.514 | 0.005 | 0.628 | 1.232-3.017 |
| Osteoporosis | ||||||
| No | - | - | 14.379 | 0.0001 | 1 | |
| Yes | -0.459 | 0.801 | 0.324 | 0.001 | 0.632 | 1.130-3.071 |
Development of a nomogram model
The nomogram prediction model (Figure 1) for BPPV was established by R software 3.6.3. The model calculates the probability of BPPV by summing the integrals of each risk factor, which are then converted into a risk score. The regression equation for the model is: logit (P) = -6.820 + 0.450 * stroke + hyperlipidemia * 0.312 + CSOM * 0.499 + cervical spondylosis * 0.916 + osteoporosis * 0.628.
Figure 1.
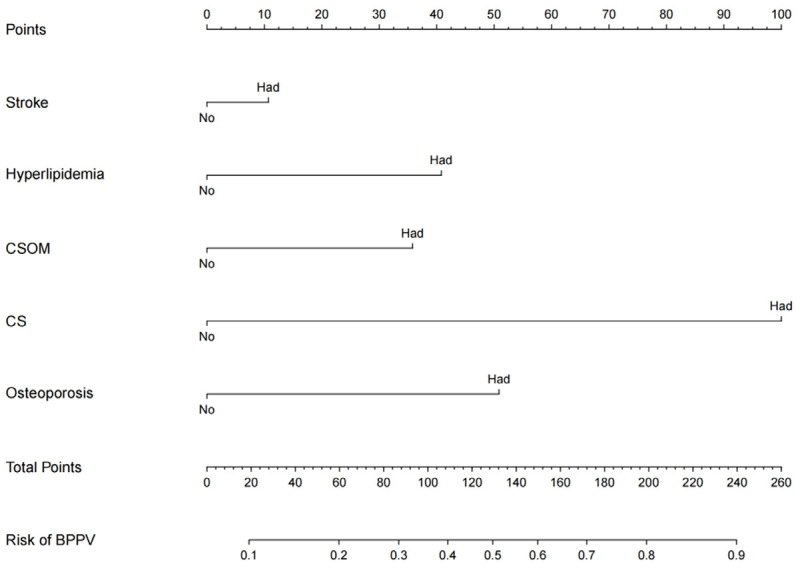
Nomogram for predicting the risk of BPPV. CSOM: chronic suppurative otitis media; CS: cervical spondylosis.
Validation of a nomogram model
The nomogram’s accuracy was assessed using the unadjusted concordance index (C-index), which was 0.892 [95% CI, 0.715-0.984], indicating high predictive accuracy. The calibration plot of the nomogram is shown in Figure 2. The Area Under the Curve (AUC) for the nomogram was 0.8355882 (Figure 3), indicating good discrimination and consistency in predicting BPPV risk.
Figure 2.

Calibration curves for nomogram in predicting BPPV risk. BPPV: Benign paroxysmal positional vertigo.
Figure 3.
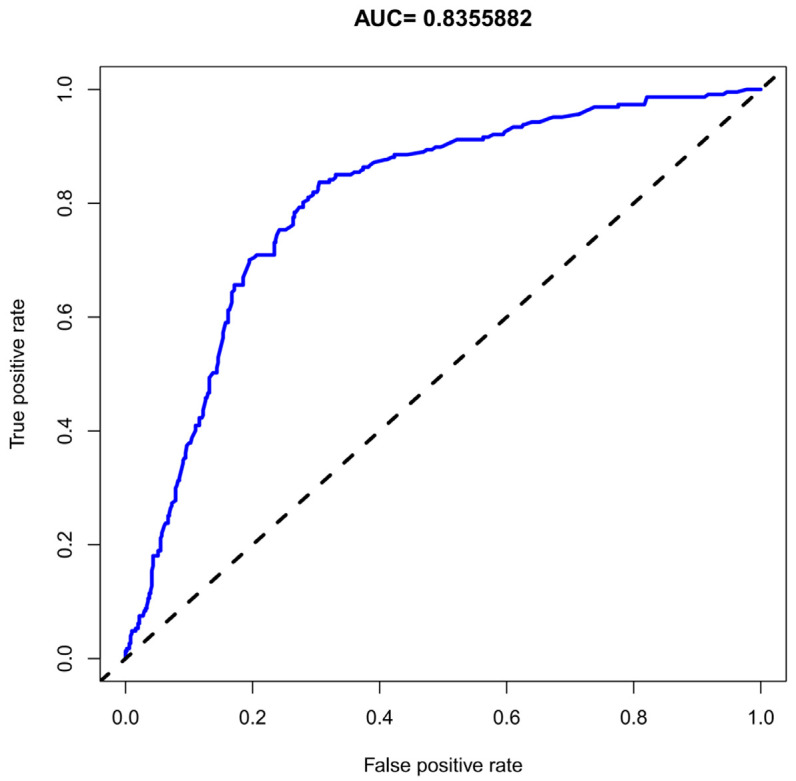
ROC curves of the monogram in predicting the BPPV risk. BPPV: Benign paroxysmal positional vertigo.
Decision curve analysis
Decision curve analysis (DCA), illustrated in Figure 4, suggests that using the nomogram for predicting BPPV risk is clinically advantageous when the predicted probability of occurrence ranges from 20% to 80%.
Figure 4.

Decision curve analysis for the nomogram. BPPV: Benign paroxysmal positional vertigo.
Discussion
In our study, stroke (95% CI, 0.575-5.954; P=0.022), hyperlipidemia (95% CI, 0.471-4.647; P=0.003), chronic suppurative otitis media (95% CI, 1.222-45.528; P=0.005), cervical spondylosis (95% CI, 1.232-3.017; P=0.005), and osteoporosis (95% CI, 1.130-3.071; P=0.001) were identified as independent risk factors for BPPV. Furthermore, we constructed a predictive nomogram model to predict the risk of developing BPPV. This nomogram model demonstrated good accuracy and clinical applicability, with a high C-index and AUC for the nomogram. DCA highlighted the model’s practical value in clinical settings, particularly in aiding the early identification of individuals at heightened risk for BPPV.
This study identified stroke as an independent risk factor of BPPV. A stroke occurs due to an acute neurological deficit caused by a disturbance in cerebral blood circulation. The blood supply to the brain comes from the internal carotid artery system and the vertebral basilar artery system. The labyrinthine artery, a crucial branch of the basilar artery - predominantly originating from the anterior inferior cerebellar artery and the posterior inferior cerebellar artery - plays a significant role in this context. This artery, with limited collateral circulation, is particularly sensitive to changes in blood flow [16]. During a stroke, especially one impacting the vertebral-basilar system, the labyrinthine artery may undergo spasms due to disrupted cerebral circulation. This can damage the vestibular system’s oval cysts, leading to physiologic and pathologic changes that may cause otoliths to dislodge, subsequently triggering BPPV.
Multivariate logistic regression analysis showed that hypertension was also an independent risk factor for BPPV. For patients with long-term hypertension, the high aortic pressure and the heart’s impaired ability to pump blood lead to diminished blood flow to various body organs, including the vestibular organs. The vestibule, particularly sensitive to ischemia, may suffer from an insufficient blood supply, triggering vasospasm and other pathologic changes that affect the oval sac and balloon, potentially leading to otolith shedding [17,18].
This study found that the prevalence of BPPV in patients with hyperlipidemia was significantly higher than those without. Lipid plaques forming on the vascular walls may cause narrowing, which over time can result in vasospasm and ischemic conditions conducive to otolith shedding [19]. De Stefano et al. reported that the occurrence of BPPV was significantly correlated with hypertension and hyperlipidemia and may be associated with stroke [20].
Chronic suppurative otitis media (CSOM) has been identified as another independent risk factor for BPPV. The association likely stems from the alterations in the inner ear’s lymphatic environment resulting from otological infections. Such infections can disrupt the microcirculation perfusion within the inner ear, leading to metabolic imbalances and epithelial degeneration. These physiologic changes can impact the oval sac, potentially causing otoliths to dislodge and precipitate episodes of BPPV [21-24].
This study found that the prevalence of BPPV in patients with previous cervical spondylosis was higher than those without. The potential pathogenesis linking cervical spondylosis to BPPV may involve several degenerative changes in the cervical spine, such as vertebral instability, loosening, nucleus pulposus prolapse, bone spur formation, ligament hypertrophy and secondary spinal canal stenosis. Such pathologic changes may irritate or compress the vertebral artery and cervical sympathetic nerve. Chronic recurrence of these conditions can lead to ischemia and spasm in the vertebral-basilar system, subsequently reducing blood flow to the vestibular system. This reduction in blood supply may cause otoliths to dislodge, triggering BPPV [25-28]. However, further research is required to substantiate this hypothesis and clarify the underlying mechanisms.
This study found that the prevalence of BPPV in patients with osteoporosis was significantly higher than those without. Vibert et al. [29] suggested that the occurrence of BPPV in elderly female patients may be re-lated to osteoporosis and bone loss. Osteoporosis leads to disturbances in calcium metabolism, a critical factor since the primary component of otoliths is calcium. Disruptions in calcium metabolism could alter the composition of otoliths, making them more likely to detach from the oval sac [30-32].
This study has some limitations. Its retrospective nature and single-center study might limit the generalizability of the findings. Whether the prediction model can be applied to other populations is still uncertain. Therefore, a multi-center study with a large sample size is needed for further verification. Besides, the potential mechanism underlying the prognostic effect of nomogram still needs more detailed investigation to better understand and verify these initial findings.
In conclusion, stroke, hyperlipidemia, chronic suppurative otitis media, cervical spondylosis and osteoporosis are independent risk predictors for BPPV. Furthermore, the developed predictive nomogram model demonstrates good accuracy and clinical applicability, providing valuable insights for clinical decision-making and patient management.
Disclosure of conflict of interest
None.
References
- 1.Kim JS, Zee DS. Clinical practice. Benign paroxysmal positional vertigo. N Engl J Med. 2014;370:1138–1147. doi: 10.1056/NEJMcp1309481. [DOI] [PubMed] [Google Scholar]
- 2.Imai T, Inohara H. Benign paroxysmal positional vertigo. Auris Nasus Larynx. 2022;49:737–747. doi: 10.1016/j.anl.2022.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Habs M, Strobl R, Grill E, Dieterich M, Becker-Bense S. Primary or secondary chronic functional dizziness: does it make a difference? A DizzyReg study in 356 patients. J Neurol. 2020;267(Suppl 1):212–222. doi: 10.1007/s00415-020-10150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alla S, Rao MY, Aslam SM. Clinical profiles of elderly patients presenting with persistent dizziness. J Natl Med Assoc. 2020;112:362–373. doi: 10.1016/j.jnma.2020.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Lim YH, Kang K, Lee HW, Kim JS, Kim SH. Gait in benign paroxysmal positional vertigo. Front Neurol. 2021;12:633393. doi: 10.3389/fneur.2021.633393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole SR, Honaker JA. Benign paroxysmal positional vertigo: effective diagnosis and treatment. Cleve Clin J Med. 2022;89:653–662. doi: 10.3949/ccjm.89a.21057. [DOI] [PubMed] [Google Scholar]
- 7.Laurent G, Vereeck L, Verbecque E, Herssens N, Casters L, Spildooren J. Effect of age on treatment outcomes in benign paroxysmal positional vertigo: a systematic review. J Am Geriatr Soc. 2022;70:281–293. doi: 10.1111/jgs.17485. [DOI] [PubMed] [Google Scholar]
- 8.Aron M, Bance M. Insights into horizontal canal benign paroxysmal positional vertigo from a human case report. Laryngoscope. 2013;123:3197–3200. doi: 10.1002/lary.24260. [DOI] [PubMed] [Google Scholar]
- 9.Zang J, Jiang X, Feng S, Zhang H. The influence of cerebral small vessel diseases on the efficacy of repositioning therapy and prognosis of benign paroxysmal positional vertigo. Int J Med Sci. 2022;19:1227–1234. doi: 10.7150/ijms.73080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sreenivas V, Sima NH, Philip S. The role of comorbidities in benign paroxysmal positional vertigo. Ear Nose Throat J. 2021;100:NP225–NP230. doi: 10.1177/0145561319878546. [DOI] [PubMed] [Google Scholar]
- 11.Fu CY, Zhang ZZ, Chen J, Jaiswal SK, Yan FL. Unhealthy lifestyle is an important risk factor of idiopathic BPPV. Front Neurol. 2020;11:950. doi: 10.3389/fneur.2020.00950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarsitthithum K, Wisupagan T, Kiatthanabumrung S, Jariengprasert C. The association between serum vitamin D levels and benign paroxysmal positional vertigo. Ear Nose Throat J. 2023;102:473–477. doi: 10.1177/01455613211008561. [DOI] [PubMed] [Google Scholar]
- 13.Li S, Wang Z, Liu Y, Cao J, Zheng H, Jing Y, Han L, Ma X, Xia R, Yu L. Risk factors for the recurrence of benign paroxysmal positional vertigo: a systematic review and meta-analysis. Ear Nose Throat J. 2022;101:NP112–NP134. doi: 10.1177/0145561320943362. [DOI] [PubMed] [Google Scholar]
- 14.Del Risco A, Cherches A, Smith SL, Riska KM. Guideline adherence to benign paroxysmal positional vertigo treatment and management in primary care. Otolaryngol Head Neck Surg. 2023;169:865–874. doi: 10.1002/ohn.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Worster A, Bledsoe RD, Cleve P, Fernandes CM, Upadhye S, Eva K. Reassessing the methods of medical record review studies in emergency medicine research. Ann Emerg Med. 2005;45:448–451. doi: 10.1016/j.annemergmed.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Hanna J, Malhotra A, Brauer PR, Luryi A, Michaelides E. A comparison of benign positional vertigo and stroke patients presenting to the emergency department with vertigo or dizziness. Am J Otolaryngol. 2019;40:102263. doi: 10.1016/j.amjoto.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Waissbluth S, Becker J, Sepúlveda V, Iribarren J, García-Huidobro F. Benign paroxysmal positional vertigo secondary to acute unilateral peripheral vestibulopathy: evaluation of cardiovascular risk factors. J Int Adv Otol. 2023;19:28–32. doi: 10.5152/iao.2023.22703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu CT, Zhao XQ, Ju Y, Wang Y, Chen MM, Cui Y. Clinical characteristics and risk factors for the recurrence of benign paroxysmal positional vertigo. Front Neurol. 2019;10:1190. doi: 10.3389/fneur.2019.01190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim CH, Jeong H, Shin JE. Incidence of idiopathic benign paroxysmal positional vertigo subtype by hospital visit type: experience of a single tertiary referral centre. J Laryngol Otol. 2023;137:57–60. doi: 10.1017/S0022215121003923. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Zhang S, Cui K, Liu C. Risk factors for benign paroxysmal positional vertigo recurrence: a systematic review and meta-analysis. J Neurol. 2021;268:4117–4127. doi: 10.1007/s00415-020-10175-0. [DOI] [PubMed] [Google Scholar]
- 21.Cao L, Jiang B, Liao Y. Analysis of clinical characteristics and risk factors in patients with benign paroxysmal positional vertigo. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2021;35:905–909. doi: 10.13201/j.issn.2096-7993.2021.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramos BF, Cal R, Mangabeira Albernaz PL, Zuma E Maia F. Practical approach for lateral canal benign paroxysmal positional vertigo. J Neurol Sci. 2022;434:120180. doi: 10.1016/j.jns.2022.120180. [DOI] [PubMed] [Google Scholar]
- 23.Zuma E Maia F, Ramos BF, Cal R, Brock CM, Mangabeira Albernaz PL, Strupp M. Management of lateral semicircular canal benign paroxysmal positional vertigo. Front Neurol. 2020;11:1040. doi: 10.3389/fneur.2020.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alzuphar SJ, Maire R. Anterior semicircular canal benign paroxysmal positional vertigo. Rev Med Suisse. 2016;12:1665–1669. [PubMed] [Google Scholar]
- 25.Saruhan G, Gökçay A, Gökçay F, Çelebisoy N. Cervical vestibular evoked myogenic potentials in patients with the first episode of posterior canal benign paroxysmal positional vertigo before and after repositioning. Acta Otolaryngol. 2021;141:147–151. doi: 10.1080/00016489.2020.1837393. [DOI] [PubMed] [Google Scholar]
- 26.Kadanka Z Jr, Kadanka Z Sr, Jura R, Bednarik J. Vertigo in patients with degenerative cervical myelopathy. J Clin Med. 2021;10:2496. doi: 10.3390/jcm10112496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rashad UM. Patients with benign paroxysmal positional vertigo and cervical spine problems: is Epley’s manoeuvre contraindicated, and is a proposed new manoeuvre effective and safer? J Laryngol Otol. 2010;124:1167–1171. doi: 10.1017/S0022215110000927. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Wang W, Gong SS. Related factors of benign paroxysmal positional vertigo: investigation of 310 patients. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;30:748–750. doi: 10.13201/j.issn.1001-1781.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 29.Miśkiewicz-Orczyk K, Pluskiewicz W, Kos-Kudła B, Misiołek M. Assessment of osteoporosis and vitamin D3 deficiency in patients with idiopathic benign paroxysmal positional vertigo (BPPV) Medicina (Kaunas) 2023;59:862. doi: 10.3390/medicina59050862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.González-Aguado R, Domènech-Vadillo E, Álvarez-Morujo de Sande MG, Guerra-Jiménez G, Domínguez-Durán E. Subjective benign paroxysmal positional vertigo in patients with osteoporosis or migraine. Braz J Otorhinolaryngol. 2020;86:83–90. doi: 10.1016/j.bjorl.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruintjes TD, van der Zaag-Loonen HJ, Eggelmeijer F, van Leeuwen RB. The prevalence of benign paroxysmal positional vertigo in patients with osteoporosis. Eur Arch Otorhinolaryngol. 2018;275:3083–3086. doi: 10.1007/s00405-018-5164-4. [DOI] [PubMed] [Google Scholar]
- 32.Si L, Ling X, Li Z, Li K, Shen B, Yang X. Clinical characteristics of patients with multi-canal benign paroxysmal positional vertigo. Braz J Otorhinolaryngol. 2022;88:89–100. doi: 10.1016/j.bjorl.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


