In the past few years there has been increasing concern about blood transfusion safety. Avoidable transfusion errors, mostly in patient identification, remain a serious cause of injury and death. There is also heightened awareness of the risk of transmission of viral and bacterial infections. Of particular concern in Britain is the (theoretical) possibility of transmission of variant Creutzfeldt-Jakob disease.
This review puts these risks in perspective (table) and describes the new measures that have been introduced to improve blood safety. It also describes changes in attitude and practice that will affect users of blood in all disciplines, including general practitioners advising patients of the pros and cons of transfusion. Finally it emphasises the need for careful education and training of all those involved in blood prescribing and blood component administration.
Summary points
Human error is a cause of transfusion related morbidity and mortality: these errors are entirely avoidable
The adoption of a lower “transfusion trigger” is gaining acceptance
Whether or not variant Creutzfeldt-Jakob disease is transmissible by transfusion, it may have a considerable impact on availability of blood for transfusion
Concerted efforts must now be made to reduce inappropriate blood use and to use alternatives and blood sparing agents
Pilot studies of barcode patient identification systems are assessing their feasibility in various clinical settings
Phase III clinical trials of blood substitutes (haemoglobin solutions and perfluorocarbons) are in progress
Methods
Our review is based on information from the annual reports of Serious Hazards of Transfusion (www.shot.demon.co.uk/), the guidelines of the British Committee for Standards in Haematology (www.bcshguidelines.com/), and the chief medical officer's second “Better Blood Transfusion” meeting (www.doh.gov.uk/bbt2). We also cite relevant recent publications by leading clinicians and scientists.
New measures to reduce transfusion errors
Avoidable transfusion errors remain an important if uncommon cause of death and injury. In the United States fatal misidentification errors are estimated to occur in 1 in 600 000 to 1 in 800 000 transfusions and non-fatal errors occur in 1 in 12 000 to 1 in 19 000 cases.2,3 UK data from the Serious Hazards of Transfusion (SHOT) reports suggest an error incidence of 335 per 5.5 million units of red cells transfused. The most commonly reported adverse event, “incorrect blood component transfused,” accounted for nearly 70% of reports in 1999-2000.4 Incompatibility in ABO blood groups was reported 97 times and led directly to four deaths and 29 cases of immediate major morbidity.
After the second SHOT report, updated UK national guidelines to minimise the risk of giving the wrong blood were published.5 In the past two years many hospitals have introduced hospital-wide “adverse incident reporting” schemes to identify and analyse such incidents and “near misses.” Transfusion errors feature prominently among these incidents (personal communication, F Regan). Existing adverse clinical incident reporting schemes will probably soon feed into a central UK reporting scheme managed by the National Patient Safety Agency to generate national information and recommendations. Recognition that educating staff and implementing robust hospital transfusion protocols are needed to prevent errors has resulted in these factors being incorporated in the Clinical Negligence Scheme for Trusts. However, training all staff involved in blood administration or taking samples for cross matching, including locum and agency staff, will be difficult without adequate resources.
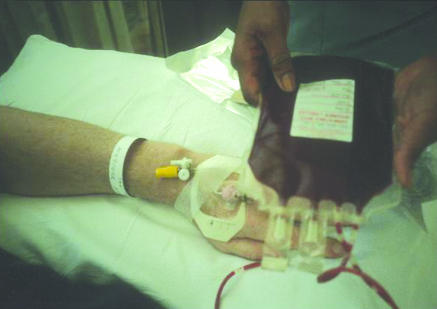
Internationally, new information technology systems are being developed to design error out of the transfusion process.6 These are based on a unique barcode on each patient's wristband, which is transferred on to the patient's cross match blood samples and transferred to each unit of blood prepared for that patient. This barcode is matched electronically with the patient's wristband before administering blood (fig 1). Pilot studies are currently assessing the feasibility of these systems in various settings including day wards, presurgical admission clinics, and inpatient wards.
Figure 1.
Checking patient identification details on blood unit against wristband before transfusion
Measures to reduce the risk of transfusing variant Creutzfeldt-Jakob disease
Safety measures to minimise the risk of transmitting known infections through transfusion include donor selection and exclusion, testing of donor blood, and post-collection processing such as leucodepletion and viral inactivation (see below). National haemovigilance schemes to monitor adverse transfusion events have been introduced in many countries,4,7,8 and EU-wide data are being collated by the European Haemovigilance Network. Similar systems exist in the United States and Canada.
Despite these measures, the possibility of transmission of new infectious agents, including variant Creutzfeldt-Jakob disease (vCJD), remains. Although there is no evidence of vCJD transmission in humans, concern has been provoked by a study in which one of 19 asymptomatic sheep, 318 days after being given 5 g of cow brain infected with bovine spongiform encephalopathy (BSE) in their feed, seemed to transmit BSE to a second sheep via a 400 ml blood transfusion.10,11 Although no other studies have been published to validate this finding, steps have already been taken in Britain to reduce the possible risk of vCJD transmission by transfusion (box B1).12 In addition, the Department of Health's Advisory Committee on the Microbiological Safety of Blood and Tissues for Transplantation is considering excluding blood donors who themselves received transfusions between 1980 and 1996. The problem with this is it would result in a loss of about 10% of donors, and, without a corresponding reduction in blood use, blood stocks would be severely jeopardised. Furthermore, the blood supply would probably be further reduced if a blood test for vCJD becomes available.13
Box 1.
Steps taken to reduce risk of vCJD transmission via blood
- Ban on using UK plasma for manufacture of fractionated products (such as albumin, clotting factors, immunoglobulins)
- Leucodepletion of all blood, platelets, fresh frozen plasma, and cryoprecipitate (as leucocytes believed to have key role in vCJD pathogenesis)
- People who have lived in the United Kingdom for >6 months between 1980 and 1996 excluded as blood donors
Several companies are working to produce a screening test for vCJD, and one is likely to be available within two years. Once it is, the National Blood Authority will be under pressure to introduce it. (In recent litigation in relation to the transfusion of hepatitis C the National Blood Authority was found at fault for supplying a defective product, and the avoidable delay in implementing an available hepatitis C test was highlighted.14) Anonymous testing will not be an option: under EU law, donors must give consent for all tests performed on their blood and must be informed of any test results on the which the national blood authority acts (for example, discards their blood). It is likely that many donors will not agree to be tested, as the burden of knowledge will affect not only their health and happiness, but could affect availability of life insurance policies. Importing blood from BSE-free countries may seem attractive, but, as most countries face periodic blood shortages, it is unlikely that sufficient blood would be available to replace the UK blood supply of around 2.7 million units of red cells a year.
Reducing unnecessary transfusion and use of alternatives to blood
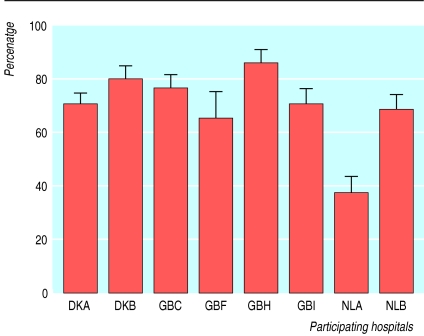
Considerable variation in transfusion practice for elective surgery is well documented (fig 2).15 Reducing unnecessary exposure to blood components by blood saving measures is particularly important in healthy patients undergoing elective surgery (box B2). A recent publication for anaesthetists summarises good transfusion practices in surgical patients.16 Implementation has been problematic, however, as until recently blood has been perceived as a safe and unlimited resource, and it has been difficult to secure funding for blood saving measures.
Figure 2.
Mean (SE) proportion of patients undergoing total hip replacement perioperatively transfused with red blood cell units in each of the participating hospitals in Europe, after adjustment for age, sex, preoperative packed cell volume, and blood loss (adapted from McClelland et al (1998)7)
Box 2.
Reasons to reduce blood exposure
- Immunological complications
- Red cell alloantibodies: haemolytic transfusion reaction
- HLA antibodies: refractoriness
- Transfusion related acute lung injury, post-transfusion purpura, transfusion associated graft versus host disease, etc
- Errors and “wrong blood” episodes
- Infections (bacterial, viral, and possibly prion)
- Immunomodulation (risk of infection or malignancy)
- Litigation
- Limited resource
About half of all blood transfused in the United Kingdom is to surgical patients (National Blood Service internal audit). To reduce the amount of blood used in elective surgery, detailed planning at each stage of patient care is required (box B3, fig 3). Although the cost of the blood component may be saved, other costs may be incurred and there may be no overall saving in the short term. Long term savings relating to the potential cost of transfusion transmitted infection, immunomodulation (long term mild immune suppression which occurs in recipients of blood components and can result in poorer outcome17), and litigation may be substantial but are difficult to quantify.
Box 3.
Methods of minimising transfusion
- History and examination including surgical or bleeding history
- Full blood count, “group and save,” blood chemistry, coagulation, haematinics
- Consider autologous blood deposit
- Consider erythropoietin to boost haemoglobin concentration
- Treat iron or folate deficiency
- Stop aspirin prophylaxis if possible
- Check if taking aspirin, non-steroidal anti-inflammatory drugs, anticoagulants
- Repeat full blood count and “group and save”
- Weigh patient, calculate blood volume, and estimate blood loss that would reduce packed cell volume to 0.22
- Consider acute normovolaemic haemodilution and intraoperative or postoperative cell salvage
- Consider drugs to reduce bleeding (such as aprotinin)
- Be prepared for longer duration to secure haemostasis
- Consider hypotensive surgery if appropriate
- Avoid hypothermia—give all fluids through a warmer
- Use of near patient testing
- Consider fibrin glues and sealants
- Accept lower postoperative haemoglobin concentration
- Accept transfusions of just one unit of blood, to exceed transfusion trigger
- Use continuous face mask oxygen if patient has low haemoglobin concentration
- Prescribe iron and folic acid routinely
- Consider tranexamic acid
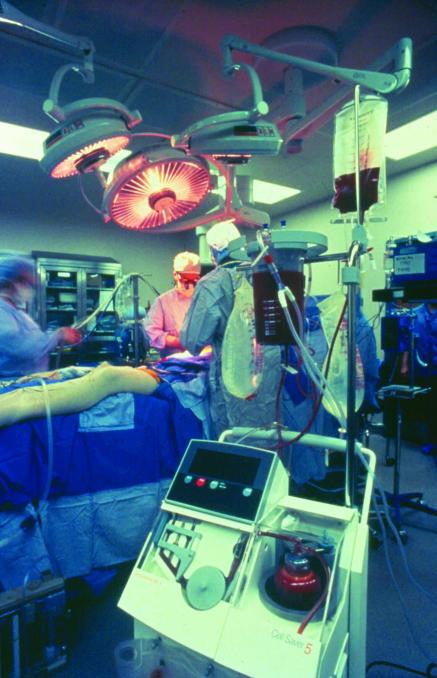
Figure 3.
Intraoperative red blood cell salvage
Implementing strategies to reduce the requirements for blood transfusion requires effective teamwork, adequate resources, and a clear understanding of the rationale for it. Blood substitutes, such as haemoglobin solutions and perfluorocarbons, are in phase III clinical trials, but their short half lives may limit usefulness.18,19 Another approach to reducing unnecessary transfusion would be to enforce, either locally or nationally, a policy of blood components being prescribed only by senior doctors.
Appointment of specialist transfusion practitioners
Over the past three years, specialist practitioners of transfusion have been appointed in over 40 UK hospitals, echoing similar developments in Europe and the United States.8,9 Most are senior nurses, but some are doctors or biomedical scientists. These posts have been created to implement recommended policies to reduce inappropriate prescribing of blood components.20 Although the cost of employing specialist transfusion practitioners has deterred some trusts, it has been found repeatedly that the savings from reducing inappropriate prescribing of blood products exceed the cost of employment.
The main role of the specialist transfusion practitioners is to educate staff and patients about the pros and cons of blood transfusion and to support the development and evaluation of transfusion protocols and guidelines. They also facilitate audit and implement strategies to improve blood ordering and administration.21 Where appropriate, practitioners may be directly involved in near patient testing and cell salvage techniques.
New developments in safety of plasma products
Blood components are becoming safer as more sensitive screening tests for viruses are introduced. In the United Kingdom all cellular blood components have been leucodepleted at source since November 1999 to reduce the potential transmission of vCJD, thought to be facilitated by B lymphocytes.22 Leucodepletion also reduces transmission rates of other cell associated viruses such as cytomegalovirus.23 The recent introduction of a nucleic acid test for hepatitis C in fresh frozen plasma, blood, and platelets24 has reduced the “window period” from 70 days (for antibody testing) to 13 days, and the chance of transmission by a unit of blood from 1 in 250 000 to 1 in 3 million.22
To reduce risks further, viral inactivation steps, routinely applied to pooled fractionated products such as albumin or immunoglobulin solutions, could now be applied to fresh frozen plasma and possibly cellular components.22
Pooling of plasma from over 1000 donors is required for solvent detergent treatment of fresh frozen plasma and fractionated products, for efficiency of processing and product standardisation. Pooling theoretically allows contamination of the entire pool by an infectious agent from one donor. Although the treatment kills enveloped viruses such as hepatitis B and C and HIV, not all non-enveloped viruses are affected (such as hepatitis A and parvovirus). Serological and polymerase chain reaction testing of the plasma pools is also carried out, but not all known agents are tested for, and some transmissions of parvovirus have occurred.25 Use of solvent detergent treated plasma is widespread, and in some European countries the use of untreated plasma is banned. An alternative is methylene blue treatment, which can be applied to single units of plasma. This inactivates a broader spectrum of viruses but is more costly and time consuming. Methylene blue is also potentially more toxic.26
The UK Advisory Committee on Microbiological Safety of Blood and Tissues is currently considering for which groups of patients fresh frozen plasma from UK donors should be virally inactivated. It is also looking at possible alternative sources of fresh frozen plasma. Methylene blue treatment of fresh frozen plasma, from UK donors is being introduced from May 2002 for children and infants born after 1 January 1996, the date when vCJD was officially excluded from the human food chain in Britain.
Solvent detergent and methylene blue treatments have no effect on bacteria or prions—there is no known suitable way of inactivating prions, which are resistant even to extremes of temperature. However, bacterial contamination of blood components, especially of platelets, is a more important cause of mortality and morbidity from blood transfusion than is viral transmission.4 A third method of pathogen inactivation is therefore being considered that not only inactivates all viruses but also kills bacteria, parasites, and lymphocytes. Psoralen S-59 and ultraviolet light are used together to treat individual platelet concentrates in the Helinx system, which cross links DNA and RNA.27 Another psoralen, S-303, is in development for use in red cell concentrates. Although expensive and labour intensive, this system could inactivate all potential pathogens except prions. In addition, this treatment would make it unnecessary to irradiate blood components to prevent transfusion associated graft versus host disease as the donor lymphocytes responsible would be killed. In the future it may become the pathogen inactivation system of choice, unless it is overtaken by new developments.
Additional educational resources
Serious Hazards of Transfusion (SHOT) (www.shot.demon.co.uk/)
British Committee for Standards in Haematology. BCSH guidelines (www.bcshguidelines.com/)
Department of Health. UK CMOs' better blood transfusion conference (www.doh.gov.uk/bbt2)
NHS. Building a Safer NHS for patients (www.doh.gov.uk/buildsafenhs/index.htm). Gives details of the National Patient Safety Agency
NHS Litigation Authority (www.nhsla.com/welcome_to_nhsla.htm). Gives details of the Clinical Negligence Scheme for Trusts
European Haemovigilance Network (www.ehn-org.net)
Table.
Risks of red blood cell transfusion (adapted from British Committee for Standards in Haematology (2001)1)
| Risk factor
|
Estimated frequency per unit transfused
|
Deaths per million units
|
|---|---|---|
| Acute haemolytic reactions | 1 in 250 000 to 1 in 1 000 000 | 0.67 |
| Hepatitis B | 1 in 100 000 to 1 in 400 000* | <0.5 |
| Hepatitis C | 1 in 3 000 000† | <0.5 |
| HIV | 1 in 4 000 000 | <0.5 |
| Bacterial contamination of red cell concentrates | 1 in 500 000 | <0.25 |
Data from Kate Soldan, National Blood Service and Central Public Health Laboratory.
Data from Dr Pat Hewitt and Dr John Barbara, National Blood Service, North London.
Acknowledgments
Both authors also work at the National Blood Service, North London Centre, Colindale, London. We thank Dr Mahes de Silva and Dr Kevin Barraclough for their helpful comments on reviewing the manuscript and Ms Carmel McGinn for preparing the manuscript.
Footnotes
Competing interests: CT has a transfusion data manager funded by Ortho-Biotech, which manufactures erythropoietin, and has received fees and travel costs from the company for speaking at a symposium.
References
- 1.British Committee for Standards in Haematology; Blood Transfusion Task Force. Guidelines for the clinical use of red cell transfusions. Br J Haematol. 2001;113:24–31. [Google Scholar]
- 2.Krombach J, Kampe S, Gathof BS, Diefenbach C, Kasper S-M. Human error: the persisting risk of blood transfusion: a report of five cases. Anesth Analg. 2002;94:154–156. doi: 10.1097/00000539-200201000-00029. [DOI] [PubMed] [Google Scholar]
- 3.Linden JV, Wagner K, Voytovich AE, Sheehan J. Transfusion errors in New York State: an analysis of 10 years experience. Transfusion. 2000;40:1207–1213. doi: 10.1046/j.1537-2995.2000.40101207.x. [DOI] [PubMed] [Google Scholar]
- 4.Serious Hazards of Transfusion Steering Group. Annual report 1999-2000. Manchester: SHOT; 2001. [Google Scholar]
- 5.British Committee for Standards in Haematology; Blood Transfusion Task Force. The administration of blood and blood components and the management of the transfused patients. Transfusion Med. 1999;9:227–238. [PubMed] [Google Scholar]
- 6.Jensen NJ, Crosson JT. An automated system for bedside verification of the match between patient ID and blood unit ID. Transfusion. 1996;36:216–221. doi: 10.1046/j.1537-2995.1996.36396182138.x. [DOI] [PubMed] [Google Scholar]
- 7.McClelland B, Love E, Scott S, Williamson LM. Haemovigilance: concept, Europe and UK initiatives. Vox Sanguinis. 1998;74(suppl 2):431–439. doi: 10.1111/j.1423-0410.1998.tb05453.x. [DOI] [PubMed] [Google Scholar]
- 8.Debeir J, Noel L, Aullen JP, Frette C, Sari F, Vo Mai MP, et al. The French haemovigilance system. Vox Sanguinis. 1999;77:77–81. doi: 10.1159/000031080. [DOI] [PubMed] [Google Scholar]
- 9.Busch M, Chamberland M, Epstein J, Kleinman S, Khabbaz R, Nemo G. Oversight and monitoring of blood safety in the United States. Vox Sanguinis. 1999;77:67–76. doi: 10.1159/000031079. [DOI] [PubMed] [Google Scholar]
- 10.Houston F, Foster JD, Chong A, Hunter N, Bostock CJ. Transmission of BSE by blood transfusion in a sheep. Lancet. 2000;356:999–1000. doi: 10.1016/s0140-6736(00)02719-7. [DOI] [PubMed] [Google Scholar]
- 11.Brown P. BSE and transmission through blood. Lancet. 2000;356:955–956. doi: 10.1016/S0140-6736(00)02706-9. [DOI] [PubMed] [Google Scholar]
- 12.Brown P, Will RG, Bradley R, Asher DM, Detwiler L. Bovine spongiform encephalopathy and variant Creutzfeldt-Jakob disease: background, evolution and current concerns. Emerging Infect Dis. 2001;7:6–16. doi: 10.3201/eid0701.010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonetta L. Scientists race to develop a blood test for vCJD. Nature Med. 2001;7:261. doi: 10.1038/85359. [DOI] [PubMed] [Google Scholar]
- 14. A vs National Blood Authority [2001] 3 All ER 289.
- 15.Sanguis Study Group. Use of blood products for elective surgery in 43 European Hospitals. Transfusion Med. 1994;4:251–268. [PubMed] [Google Scholar]
- 16.Association of Anaesthetists of Great Britain and Ireland. Blood transfusion and the anaesthetist: red cell transfusion. London: Association of Anaesthetists of Great Britain and Ireland; 2001. [Google Scholar]
- 17.Blajchman MA, Dzik S, Vamvakas EC, Sweeney J, Snyder EL. Clinical and molecular basis of transfusion induced immunomodulation: summary of the proceedings of a state-of-the-art conference. Transfusion Med Rev. 2001;15:108–135. doi: 10.1053/tmrv.2001.22614. [DOI] [PubMed] [Google Scholar]
- 18.Prowse CV. Alternatives to standard blood transfusion: availability and promise. Transfusion Med. 1999;9:287–299. doi: 10.1046/j.1365-3148.1999.00212.x. [DOI] [PubMed] [Google Scholar]
- 19.Watson N, Taylor C. Allogeneic blood transfusion—the alternatives. Hosp Pharmacist. 2000;7:124–129. [Google Scholar]
- 20.NHS Executive. Better blood transfusion. London: Department of Health; 1998. . (Health Service Circular (HSC) 1998/224.) [Google Scholar]
- 21.Clark P, Rennie I, Rawlinson S. Effect of a formal education programme on safety of transfusions. BMJ. 2001;323:1118–1120. doi: 10.1136/bmj.323.7321.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Council of Europe expert committee in blood transfusion study group on pathogen inactivation of labile blood components. Pathogen inactivation of labile blood products. Transfusion Med. 2001;11:149–175. doi: 10.1046/j.1365-3148.2001.00310.x. [DOI] [PubMed] [Google Scholar]
- 23.Dumont LJ, Luka J, VandenBroeke T, Whitley P, Ambruso DR, Elfath MD. The effect of leukocyte-reduction method on the amount of cytomegalovirus in blood products: a comparison of apheresis and filtration methods. Blood. 2001;97:3640–3647. doi: 10.1182/blood.v97.11.3640. [DOI] [PubMed] [Google Scholar]
- 24.Roth WK, Weber M, Seifried E. Feasibility and efficacy of routine PCR screening of blood donations for hepatitis C virus, hepatitis B virus and HIV-1 in a blood bank setting. Lancet. 1999;353:359–363. doi: 10.1016/S0140-6736(98)06318-1. [DOI] [PubMed] [Google Scholar]
- 25.Koenigbauer UF, Eastlund T, Day JW. Clinical illness due to parvovirus B19 after infusion of solvent/detergent-treated pooled plasma. Transfusion. 2000;40:1203–1206. doi: 10.1046/j.1537-2995.2000.40101203.x. [DOI] [PubMed] [Google Scholar]
- 26.Pamphilon D. Viral inactivation of fresh frozen plasma. Br J Haematol. 2000;109:680–693. doi: 10.1046/j.1365-2141.2000.02019.x. [DOI] [PubMed] [Google Scholar]
- 27. Barbara J. Pathogen inactivation treatment of platelet components: advancing from theory to clinical practice. Semin Hematol 2001;38(4 suppl 11). [DOI] [PubMed]