The link between defective nutrition of the fetus and vascular disease in later life is now well established. Naveed Sattar and Ian Greer report on the intriguing probability that complications in pregnancy also predispose mothers to later vascular and metabolic disease
Plentiful evidence now links low birth weight due to intrauterine growth restriction and increased risk of vascular disease in later adult life. This is considered to be partly the result of programming through fetal nutrition.1 In contrast, much less attention has been focused on the relation between adverse pregnancy outcomes, such as pre-eclampsia, gestational diabetes, preterm delivery, and intrauterine growth restriction, and the mother's subsequent health, and interesting data are now increasingly linking the maternal vascular, metabolic, and inflammatory complications of pregnancy with an increased risk of vascular disease in later life (table). This article summarises the emerging evidence to support this fascinating concept, notes important areas for further research, and discusses potential practical implications.
Summary points
Women with a history of adverse pregnancy outcome appear to be at increased risk of metabolic and vascular diseases in later life
Pregnancy complications and coronary heart disease may have common disease mechanisms
Women with a history of gestational diabetes should be screened for type 2 diabetes and be given counselling and appropriate lifestyle advice
Women who have had a very low birthweight baby or combined complications seem to be at severalfold increased risk of mortality from cardiovascular causes and should be screened for vascular risk factors in their late 30s.
The possibility that maternal vascular risk factors, potentially ‘modifiable’ before pregnancy, correlate with increased risk of preterm delivery and low birth weight, and thus fetal programming, requires further investigation
Metabolic syndrome
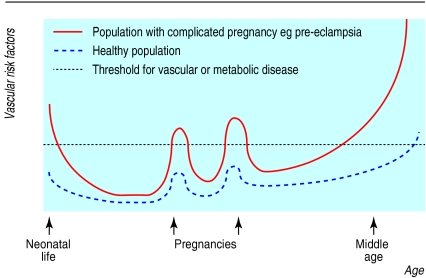
A key factor underlying cardiovascular disease and, in particular, coronary heart disease, is the metabolic syndrome. The metabolic syndrome is a spectrum of metabolic abnormalities associated with insulin resistance, which is manifest as relative hyperglycaemia, hyperlipidaemia, and disturbance of coagulation. The normal physiological response to pregnancy represents a transient excursion into a metabolic syndrome in which several components are acquired: a relative degree of insulin resistance, definite hyperlipidaemia, and an increase in coagulation factors.12,13 Normal pregnancy also involves upregulation of the inflammatory cascade and an increase in white cell count.14 Such upregulation in non-pregnant women has recently been recognised as an additional risk factor for cardiovascular disease, as markers of inflammation such as C-reactive protein, interleukin-6, and raised white cell count have been found to be independent predictors of cardiovascular events and diabetes.15 All these metabolic changes of pregnancy are likely to be the result of hormonal changes, either direct or indirect, through regulation of early fat acquisition and its rapid mobilisation in the second half of pregnancy.16 Such metabolic responses could be considered as “stress” tests of maternal carbohydrate and lipid pathways and vascular function. In this way, adverse pregnancy outcome may be an indicator of increased risk of metabolic and vascular diseases in later life (figure).
Gestational diabetes
Perhaps the best studied example of gestational diabetes is glucose metabolism in pregnancy. If the mother fails to compensate adequately for the increase in gestational insulin resistance by enhancing pancreatic insulin secretion, her regulation of glycaemia will be affected and she will have a 30% risk of developing type 2 diabetes in later life.17 In fact, pregnancy itself may accelerate the development of type 2 diabetes in susceptible women.18 Even if they remain glucose tolerant after their pregnancy, women with a history of gestational diabetes show subtle yet significant differences from controls in fasting lipid levels, blood pressure, and microvascular and large vessel function, consistent with an increased risk of diabetes.2,3 From our current knowledge of risk factors, all these observations predict an increased risk of coronary heart disease in women with previous gestational diabetes.
Hypertensive complications
Pre-eclampsia, which complicates 2-4% of pregnancies, remains one of the commonest causes of maternal and fetal morbidity and mortality. However, early findings conflict with more recent data on the long term consequences for mothers. The early work by Leon Chesley and others suggested that women with pregnancy induced hypertension and eclampsia did not develop later chronic hypertension,19–21 but others have found an increase in risk of later hypertension, especially when the hypertension in pregnancy began before 30 weeks' gestation.22 There does seem to be agreement, however, that mothers who have uncomplicated pregnancies have a lower incidence of subsequent hypertension than does the general female population of similar age and race.19 Recent studies have found that women with a history of pre-eclampsia have higher circulating concentrations of fasting insulin, lipid, and coagulation factors post partum than do controls matched for body mass index.4,5 They also seem to show a specific defect of endothelial-dependent vascular function as compared with women with a history of a healthy pregnancy, independently of maternal obesity, blood pressure, and metabolic disturbances associated with insulin resistance or dyslipidaemia.6 This pattern of metabolic and vascular changes in women with a history of pre-eclampsia is nearly identical to the abnormalities seen in this condition at diagnosis—namely, exaggerated lipid and insulin levels, disturbed haemostatic factors, and endothelial dysfunction.16 It is not surprising, therefore, that the specific vascular lesion of pre-eclampsia, termed acute “atherosis,” in the placental bed, is similar to that observed in atherosclerosis, including foam cells loaded with lipid. Thus the genotypes and phenotypes underlying vascular disease may also underlie pre-eclampsia.
These changes in risk markers in women with a history of pre-eclampsia predict that they may be at an increased risk of coronary heart disease. Jonsdottir and colleagues7 examined causes of death in 374 women with a history of hypertensive complications in pregnancy and noted that their death rate from complications of coronary heart disease (standardised mortality ratio 1.47; 95% confidence interval 1.05 to 2.02) was significantly higher than expected from analysis of population data from public health and census reports during corresponding periods. Moreover, they noted that the relative risk of dying from coronary heart disease (risk ratio 2.61; 1.11 to 6.12) was significantly higher among women who had had eclampsia or pre-eclampsia (risk ratio 1.90; 1.02 to 3.52) compared with those with hypertension alone.7 A prospective cohort study using data from the Royal College of General Practitioners' oral contraceptive study also reported that a history of pre-eclampsia increased the risk of cardiovascular conditions in later life. For total ischaemic heart disease the relative risk was 1.7 (1.3 to 2.2). Furthermore, the increased risk could not be explained by underlying chronic hypertension.8 A retrospective cohort study from Scotland using hospital discharge data has also recently reported an association between pre-eclampsia and later ischaemic heart disease in the mother (risk ratio 2.0; 1.5 to 2.5).9 Prospective evaluation of women in pregnancy, with long term follow up, is now required to discover the mechanisms underlying this association. It is also important to determine whether this finding can identify risk that otherwise might not have been evident or whether the use of established risk factors such as hypertension and obesity would have identified these women as being “at risk” and offered an opportunity for primary prevention.
Low birth weight
Intriguingly, recent retrospective studies have noted that women who have delivered a baby weighing less than 2500 g have 7-11 times the risk of death from cardiovascular causes of women with babies weighing 3500 g or more.9,10 These findings seemed not to be confounded by socioeconomic status, and the association was too strong to be explained by maternal smoking. The observations suggest a link between maternal risk factors for coronary heart disease and fetal programming. The maternal genotypes and phenotypes associated with increased risk of coronary heart disease may also underlie intrauterine growth restriction and fetal programming. In turn this will lead to a perpetuation of risk factors through generations. We cannot influence genotype, but phenotype might be altered. Therefore, improving the mother's risk factor status and metabolic profiles before or early in pregnancy—for example, by stopping smoking, increasing physical activity in sedentary women, improving diet, and loss of weight by obese women—could benefit fetal development and reduce the vascular risk of future generations.
Preterm delivery
Women with a history of delivery before 37 weeks had around twice the normal risk of coronary heart disease in observational studies.9,11 Although reliable data on maternal smoking, a major potential confounder, were not available, maternal smoking seemed not to be a confounder in this relation as such women were not at increased risk of smoking related cancers. Preterm labour is recognised to be an inflammatory phenomenon with a leucocyte infiltrate in the cervical and uterine tissues, even in the absence of infection.23 The association between preterm labour and coronary heart disease might therefore be related to upregulation of chronic inflammatory pathways. Women with a “proinflammatory” phenotype may develop greater upregulation of the chronic inflammatory pathways than is seen in normal pregnancy, leading to preterm labour. This would help explain why these same women will be at increased risk of coronary heart disease in later life, as inflammation is an independent predictor of coronary heart disease in men and women.24 Again, confirmation of this important observation is needed, ideally in prospective studies, along with an exploration of the inflammatory mechanisms common to both clinical problems.
Future research
Most of the above findings come from observational studies with relatively small numbers of cases or end points, and so require confirmation in larger cohorts with longer periods of follow up, adequate control groups, and proper attention to confounding by smoking. These should examine whether established risk factors account for excess risk associated with pregnancy complications or if novel factors might be implicated. Simultaneously, large prospective longitudinal studies (of several thousand women) examining changes in conventional risk factor pathways (lipids, blood pressure, haemostatic factors) and novel pathways (inflammation, insulin resistance) during and after pregnancy should be undertaken. Such studies lend themselves well to long term follow up with the eventual aim of linking pregnancy outcome to maternal vascular risk factor status at the first antenatal visit in the short term, to post-pregnancy risk factor status in the medium term, and to vascular and metabolic disease end points in later life. This design could also examine whether the pattern of risk factor perturbances is unique to individual complications or similar in all. Clearly, a variety of study designs are needed to confirm associations and to work out the mechanisms and causality.
Implications
A major problem in the prevention of vascular disease has been the difficulty in identifying individuals at risk at an early enough stage for them to benefit from intervention such as modification of their lifestyle. For example, by the time type 2 diabetes is diagnosed, more than 30-50% of patients will already have evidence of vascular disease. Clearly, women with a history of gestational diabetes are candidates for screening for diabetes. This should take the form of measurement of fasting plasma glucose any time between 6 weeks and 6 months post partum, and thereafter regularly at intervals guided by initial results. A diagnosis of diabetes is now made if the plasma glucose concentration is 7 mmol/l or above on two occasions. If a result between 6.1 and 6.9 mmol/l is recorded on two occasions, then an oral glucose tolerance test is advised. All women with such a history should be counselled about their increased risk of developing type 2 diabetes and the benefits of modifying their lifestyle. This is important, as improved diet and physical activity have recently been shown to prevent the onset of type 2 diabetes in people at high risk.25,26 Even if initial plasma glucose concentrations are normal, regular checks are warranted, particularly if gestational diabetes recurs in a second pregnancy, to allow early identification and treatment of asymptomatic diabetes.
Similarly, if other adverse pregnancy outcomes—pre-eclampsia, intrauterine growth restriction, and preterm labour—are confirmed as indicators of increased vascular risk in mothers, these women may benefit from screening and primary prevention strategies. Such intervention could be focused on the perimenopausal years (a time when risk of vascular disease increases rapidly) or even earlier. This may be particularly relevant in mothers with low birthweight babies (under 2500 g), in whom relative risks for coronary heart disease seem to be increased severalfold (table). In addition, as risk ratios for complications seem to be additive, a woman with multiple pregnancy complications, such as pre-eclampsia combined with preterm delivery and a baby in the lowest fifth of birth weights, is at severalfold increased risk of coronary heart disease.9 It is notable that the absolute risk of coronary heart disease in women in their 40s is very low, thus only factors which increase risk severalfold should be targeted. Screening in these women would take the form of routine coronary heart disease assessment including measurements of blood pressure, fasting lipids (total cholesterol, triglyceride, and high density lipoprotein cholesterol), and glucose concentrations; the risk of coronary heart disease can then be ascertained from the widely available risk factor charts. To help ensure that appropriate women are screened and given relevant health education, adverse pregnancy outcomes could be used in general practitioners' computer databases for targeted health screening programmes. Indeed, such interventions could start at the routine postpartum review at six weeks, when these women could be made aware of their potentially increased risk of coronary heart disease.
The second implication of an association between maternal coronary heart disease risk and adverse pregnancy outcome, particularly low birth weight and preterm delivery, is the potential for modification of risk factors before a subsequent pregnancy or in early pregnancy. For example, increased physical activity in women who are sedentary may result in a better pregnancy outcome for both mother and child. Indeed, there are preliminary data to support this hypothesis: increasing exercise during pregnancy may increase birth weight27 and reduce the risk of gestational diabetes.28 Such data would suggest that complications are not simply genetically determined, but that lifestyle factors play a major role. At present this remains speculative, and further research is needed to examine this important question.
Table.
Association of adverse pregnancy outcomes with risk of diabetes or risk factors for coronary heart disease and vascular disease
|
Pregnancy outcome
|
Incidence in pregnancy (%)
|
Risk factors shown to be perturbed after pregnancy
|
Association or risk ratio (95% CI)
|
|---|---|---|---|
| Gestational diabetes | 1.9-5.0* | Lipids2 | Increased risk of type 2 diabetes, especially if recurrence of gestational diabetes in a subsequent pregnancy. No data on coronary heart disease risk |
| Blood pressure2 | |||
| Large vessel function3 | |||
| Small vessel function3 | |||
| Pre-eclampsia | 2-4 | Lipids4 Clotting4 |
1.9 (1.0 to 3.5) v pregnancy induced hypertension alone7 |
| Fasting insulin5 | 1.7 (1.3 to 2.2) v no pre-eclampsia8 | ||
| Large vessel function6 | 2.0 (1.5 to 2.5) v no pre-eclampsia9 | ||
| Low birth weight (<2500 g) | 5 | Not studied | 11.3 (3.5 to 36.1) v ⩾3500 g9 |
| 7.1 (2.6 to 18.7) v ⩾3500 g10 | |||
| Preterm delivery (<37 weeks) | 5-6 | Not studied | 1.8 (1.3 to 2.5) v term delivery9 |
| 2.1 (1.2 to 3.5) v term delivery11 |
Dependent on population studied, ethnic group, and diagnostic criteria.
Figure.
Risk factors for vascular disease are identifiable during excursions into the metabolic syndrome of pregnancy
Footnotes
Funding: None.
Competing interests: None declared.
References
- 1.Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr. 2000;71:1344–152S. doi: 10.1093/ajcn/71.5.1344s. [DOI] [PubMed] [Google Scholar]
- 2.Meyers-Seifer CH, Vohr BR. Lipid levels in former gestational diabetic women. Diabetologia. 1996;19:1351–1356. doi: 10.2337/diacare.19.12.1351. [DOI] [PubMed] [Google Scholar]
- 3.Hu J, Norman M, Wallensteen M, Gennser G. Increased larger arterial stiffness and impaired acetylcholine induced skin vasodilatation in women with previous gestational diabetes. Br J Obstet Gynaecol. 1998;105:1279–1287. doi: 10.1111/j.1471-0528.1998.tb10006.x. [DOI] [PubMed] [Google Scholar]
- 4.He S, Silveira A, Hamsten A, Blomback M, Bremme K. Haemostatic, endothelial and lipoprotein parameters and blood pressure levels in women with a history of pre-eclampsia. Thromb Haemost. 1999;81:538–542. [PubMed] [Google Scholar]
- 5.Laivuori H, Tikkanen MJ, Ylikorkala O. Hyperinsulinaemia 17 years after pre-eclamptic first pregnancy. J Clin Endocrinol Metab. 1996;81:2908–2911. doi: 10.1210/jcem.81.8.8768850. [DOI] [PubMed] [Google Scholar]
- 6.Chambers JC, Fusi L, Malik IS, Haskard DO, De Swiet M, Kooner JS. Association of maternal endothelial dysfunction with preeclampsia. JAMA. 2001;285:1607–1612. doi: 10.1001/jama.285.12.1607. [DOI] [PubMed] [Google Scholar]
- 7.Jonsdottir LS, Arngrimsson R, Geirsson RT, Sigvaldason H, Sigfusson N. Death rates from ischaemic heart disease in women with a history of hypertension in pregnancy. Acta Obstet Gynecol Scand. 1995;74:772–776. doi: 10.3109/00016349509021195. [DOI] [PubMed] [Google Scholar]
- 8.Hannaford P, Ferry S, Hirsch S. Cardiovascular sequelae of toxaemia of pregnancy. Heart. 1997;77:154–158. doi: 10.1136/hrt.77.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith GCS, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study of 129 290 births. Lancet. 2001;357:2002–2006. doi: 10.1016/S0140-6736(00)05112-6. [DOI] [PubMed] [Google Scholar]
- 10.Davey-Smith G, Harding S, Rosato M. Relation between infants' birth weight and mothers' mortality: prospective observational study. BMJ. 2000;320:839–840. doi: 10.1136/bmj.320.7238.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davey-Smith G, Whitley E, Gissler M, Hemminki E. Birth dimensions of offspring, premature birth, and the mortality of mother. Lancet. 2000;356:2066–2067. doi: 10.1016/S0140-6736(00)03406-1. [DOI] [PubMed] [Google Scholar]
- 12.Greer IA. Thrombosis in pregnancy: maternal and fetal issues. Lancet. 1999;10:1258–1265. doi: 10.1016/S0140-6736(98)10265-9. [DOI] [PubMed] [Google Scholar]
- 13.Martin U, Davies C, Hayavi S, Hartland A, Dunne F. Is normal pregnancy atherogenic? Clinical Science. 1999;96:421–425. doi: 10.1042/cs0960421. [DOI] [PubMed] [Google Scholar]
- 14.Sacks GP, Studena K, Sargent IL, Redman CWG. Normal pregnancy and pre-eclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;170:80–86. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 15.Haffner S. Do interventions to reduce coronary heart disease reduce the incidence of type 2 diabetes? A possible role for inflammatory factors. Circulation. 2001;103:346–347. doi: 10.1161/01.cir.103.3.346. [DOI] [PubMed] [Google Scholar]
- 16.Sattar N, Gaw A, Packard CJ, Greer IA. Potential pathogenic roles of aberrant lipoprotein and fatty acid metabolism in pre-eclampsia. Br J Obstet Gynaecol. 1996;103:614–620. doi: 10.1111/j.1471-0528.1996.tb09827.x. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann RC, Schleyhahn FT, Huffman DG, Amankwah KS. Gestational diabetes diagnostic criteria: long-term maternal follow-up. Am J Obstet Gynecol. 1995;172:621–625. doi: 10.1016/0002-9378(95)90582-0. [DOI] [PubMed] [Google Scholar]
- 18.Peters RK, Kjos SL, Xiang A, Buchanan TA. Long-term diabetogenic effect of single pregnancy in women with previous gestational diabetes mellitus. Lancet. 1996;347:227–230. doi: 10.1016/s0140-6736(96)90405-5. [DOI] [PubMed] [Google Scholar]
- 19.Chesley LC, Annitto JE, Cosgrove RA. The remote prognosis of eclamptic women: sixth periodic report. Am J Obstet Gynecol. 1976;124:446–459. doi: 10.1016/0002-9378(76)90168-x. [DOI] [PubMed] [Google Scholar]
- 20.Bryans CI. The remote prognosis in toxaemia of pregnancy. Clin Obstet Gynecol. 1966;9:973–980. doi: 10.1097/00003081-196612000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Fisher KA, Luger A, Spargo BH, Lindheimer MD. Hypertension in pregnancy: clinical-pathological correlations and remote prognosis. Medicine. 1981;60:267. [PubMed] [Google Scholar]
- 22.Sibai B, el-Nazer A, Gonzalez-Ruiz A. Severe preeclampsia-eclampsia in young primigravid women: subsequent pregnancy outcome and remote prognosis. Am J Obstet Gynecol. 1986;155:1011–1016. doi: 10.1016/0002-9378(86)90336-4. [DOI] [PubMed] [Google Scholar]
- 23.Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14:229–236. [PubMed] [Google Scholar]
- 24.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 25.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Finnish diabetes prevention study group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 26.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 27.Clapp JF, 3rd, Kim H, Burciu B, Lopez B. Beginning regular exercise in early pregnancy: effect on fetoplacental growth. Am J Obstet Gynecol. 2000;183:1484–1488. doi: 10.1067/mob.2000.107096. [DOI] [PubMed] [Google Scholar]
- 28.Dornhorst A, Michela R. Risk and prevention of type 2 diabetes in women with gestational diabetes. Diabetes Care. 1998;21(suppl 2):43–49. B. [PubMed] [Google Scholar]