Abstract
To investigate the dynamics of inflammation and lipid-related indicators in lung cancer patients and their impact on treatment efficacy. A retrospective analysis was conducted on 133 lung cancer patients who seek for primary treatment at Wujin Hospital Affiliated to Jiangsu University from January 2019 to August 2022. The inflammation and blood lipid-related indicators were collected 1 week before treatment and after 2 cycles of treatment. We compared the changes in these indicators among patients with different treatment methods and outcomes. The diagnostic value of the dynamic changes in each index for disease progression was calculated using the ROC curve. The risk factors influencing disease development were identified using multifactorial logistic regression analysis. After 2 cycles of treatment, the white blood cell count (WBC, P<0.001), neutrophil count (NC, P<0.001), neutrophil-to-lymphocyte ratio (NLR, P<0.001) in the disease progression (PD) group were significantly increased, triglyceride (TG, P=0.023), apolipoprotein A1 (APO-A1, P=0.009) was significantly decreased. The results showed that ∆NC had the highest sensitivity (88.24%) in predicting disease progression, and ∆WBC had the best specificity (77.78%). Multivariate regression analysis showed that ΔWBC (P<0.001), ΔTG (P=0.041), and treatment method (P=0.010) were independent risk factors for disease progression (PD). The changes of WBC and TG before and after treatment are promising indicators for predicting the progression of lung cancer and may offer a new direction for lung cancer treatment.
Keywords: NLR, lipid metabolism, lung cancer, prognosis
Introduction
Lung cancer has the highest incidence among all malignancies globally, with approximate 1.76 million fatalities annually [1]. Systemic therapy remains an important treatment for lung cancer. Despite the availability of various systemic treatment options, such as chemotherapy [2], targeted therapy [3], immunotherapy [4], the overall prognosis is still unsatisfactory. Therefore, the effective utilization of predictive biomarkers could significantly improve patient outcomes by enhancing quality of life and extending survival.
Peripheral blood circulating immune cells can reflect the general immune status and their ability to initiate or maintain an effective immune response to tumors. The presence of immune cells at the tumor site is related to their ability to migrate into the tumor, as well as the activation of T cells [5]. The dynamics of tumor and immune cells during immunotherapy might be better understood through the analysis of circulating cell-free tumor DNA (ctDNA) kinetics via liquid biopsy analyses [6,7] and by tracking the neutrophil-lymphocyte ratio (NLR) in peripheral blood [8,9]. Tumor cells recruit and activate various leukocytes, especially neutrophils (NEU) and monocytes (MONO), through T cells, specific chemokines and prostaglandins. Lymphocyte activation and intra-tumor infiltration are considered essential for the development of antitumor immune responses. Tumor-infiltrating lymphocytes (TILs) [10], presented within tumor lesions, interstitium or the adjacent tumor stroma, participate in tumor immune response and regulatory processes. The decrease in lymphocytes reflects the compromised immune response cells. Monocytes stimulate cancer cell migration and suppress anti-tumor immunity. Additionally, pro-inflammatory cytokines promote platelet activation, which is crucial for tumor growth through angiogenesis, invasion, and immune escape. And NLR, as a reflection of the body’s systemic inflammation level, is involved in tumor angiogenesis, invasion and immune escape [11]. Some studies have confirmed that NLR is associated with the prognosis of a variety of tumors [12-16] and correlates with the efficacy of immunotherapy [17] and targeted therapy [18] in lung cancer. Additionally, recent research highlights the significance of lipid metabolism in lung cancer [19,20]. Abnormal lipid metabolism, especially the abnormal synthesis of large amounts of fatty acids, supplies essential components for cell membranes and lipid signaling molecules necessary for cell proliferation, altering membrane composition and permeability, leading to tumorigenesis and progression. Lipid hydrogen peroxide, produced during lipid peroxidation, can disrupt cellular and mitochondrial membrane structure and exert cytotoxic effects. In contrast, polyunsaturated fatty acids are important regulators of inflammation and immune responses. Cholesterol is involved in the formation of cell membranes, regulates the cell membrane functions, and helps in transmembrane signaling. Low cholesterol level can impair immune defense against tumor proliferation. High-density lipoprotein cholesterol (HDL-C) is implicated in cancer development through its role in reverse cholesterol transport and its impact on cell cycle regulation, apoptosis, and inflammatory responses [21]. Therefore, inflammation and lipid metabolism are intertwined within the tumor environment.
A growing number of basic and clinical trials suggest that a persistent inflammatory response plays a crucial role in tumorigenesis. The overexpression of various pro-inflammatory mediators can damage vital cellular components, directly or indirectly leading to malignant cell transformation, invasion and metastasis. For instance, neutrophils release inflammatory factors in tumor tissue, which activate signaling pathways and induce angiogenesis, resulting in uncontrolled tumor cell proliferation. Once in the tumor tissue, blood neutrophils become tumor-associated neutrophils (TANs) [22], which kill tumor cells non-specifically by producing reactive oxygen species and secreting cytokines in the early tumor stage. Additionally, neutrophils can also inhibit tumorigenesis and progression by interacting with marginal zone (MZ) B cells to promote the production of antigenic immunoglobulins and improve the body’s immunity. TANs inhibit neutrophil-mediated tumor cell killing by upregulating CCL5 expression. However, as the tumor progresses, TANs also exhibit pro-cancer effects, synthesizing and releasing factors like neutrophil elastase (NE), cyclooxygenase 2 (COX-2), and CCL17, which promote tumor growth. Neutrophil extracellular trap networks (NETs) can promote thrombosis in lung cancer, thereby shortening the survival of tumor patients. These findings underscore the dual role of TANs in lung cancer progression. Lymphocytes play a pivotal role in immune surveillance and editing, with various types capable of inducing apoptosis in tumor cells, thus inhibiting their proliferation. Neutrophil-lymphocyte ratio (NLR), reflects systemic inflammation and is a convenient, cost-effective hematological marker. Research has demonstrated its significant correlation with the prognosis of various tumors [12-16], highlighting its potential as a predictive and prognostic tool in oncology.
Lipids are essential for maintaining cell structure, providing energy, and facilitating cell signaling. Abnormalities in lipid metabolism can lead to cell proliferation, differentiation, and apoptosis dysregulation, contributing to tumor development and growth [23,24]. Studies have revealed a connection between abnormal lipid metabolism and the occurrence and development of various cancers, such as lung cancer and colorectal cancer [23-26]. The tumor development associated lips include fatty acids, glycerol lipids, glycerophospholipids, sphingolipids, and sterol lipids. High levels of fatty acid synthase in tumor cells not only promote tumor cell growth, but also increase the metastatic potential and resistance to cisplatin. It has been shown that targeting lung cancer stem cells with sphingosine coenzyme A desaturase (SCD) inhibitors can reverse cisplatin resistance and reduce cell proliferation [27]. Tumor cells activate SREBP-1 through PI3K/AKT/mTOR and MAPK/ERK, HIF-1α, p53, and SHH pathways, resulting in increased cholesterol production. In addition, cholesterol is involved in tumor cell signaling, growth factor secretion, mediating apoptosis and drug resistance. High-density lipoprotein (HDL) may be involved in carcinogenesis by reverse cholesterol transport, affecting cell cycle regulation, apoptosis and inflammatory response. Apolipoproteins, which transport cholesterol, triglycerides, and phospholipids, also play crucial roles. ApoA-I, an important component of plasma HDL, is involved in the pathogenesis of respiratory diseases through the ApoA-I/ABCA1-dependent pathway and plays a major protective role in respiratory diseases [28]. Low levels of APO-A and high levels of APO-B increase the risk of lung cancer. Tumor cells synthesize large amounts of signaling lipids that induce inflammatory responses, stimulate cell migration. Overall, lipids are important regulators of tumorigenesis, and lipid metabolism disorders represent a significant feature of malignancy.
Given the complexity of tumor-immune system interactions and the dynamically changing tumor immune microenvironment (TME), it is essential to identify reliable serum markers to predict patient outcomes and refine therapeutic strategies. Blood-based markers have shown promise in accurately predicting disease progression in various cancers [29]. Recent studies have explored markers such as eosinophil, NLR, platelet-lymphocyte ratio (PLR), systemic immune-inflammation index (SII), and monocyte-to-lymphocyte ratio (MLR) to assess the response of patients with non-small cell lung cancer to neoadjuvant immunotherapy [30]. However, these studies overlooked the significance of lipid-related markers and their correlation with treatment efficacy. To address this gap, our study incorporated both pre- and post-treatment inflammatory biomarkers and lipid-related indices to thoroughly investigate their relationship with treatment responses in lung cancer.
This study retrospectively analyzed the clinical data of 133 patients with primary lung cancer admitted to our hospital from January 2019 to August 2022. The changes in inflammation and blood lipid-related indicators in peripheral blood before and after treatment and their effects on treatment efficacy were studied. Inflammation-related indicators analyzed included white blood cell count (WBC), hemoglobin concentration (HGB), platelet count (PLT), absolute neutrophil count (ANC), lymphocyte count (ALC), monocyte count (AMC), albumin (ALB). Blood lipid related indicators included cholesterol (TC), TG, high density lipoprotein (HDL), low density lipoprotein (LDL), APO-A1, apolipoprotein B (APO-B). These indicators, readily obtainable from peripheral blood, offer a convenient and rapid means to assist in evaluating the therapeutic effects in lung cancer treatment.
Materials and methods
Research subjects
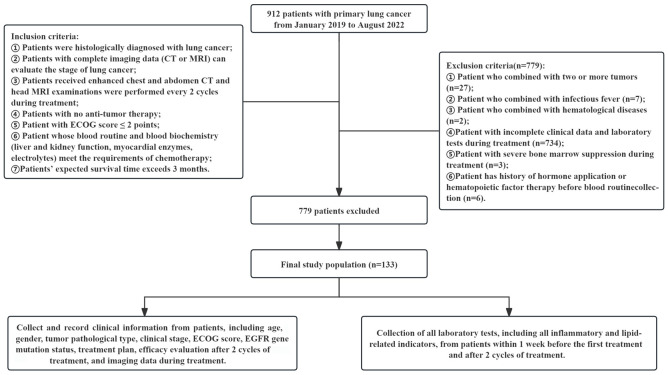
This retrospective cohort study included 133 patients with primary lung cancer treated at Wujin Hospital Affiliated to Jiangsu University from January 2019 to August 2022. Inclusion criteria: 1) Histological diagnosis of lung cancer; 2) Availability of complete imaging data (CT or MRI) for staging; 3) Enhanced chest and abdominal CT scans and head MRI every 2 cycles during treatment; 4) No prior anti-tumor therapy; 5) An ECOG performance status score of ≤2; 6) Blood routine and biochemistry (including liver and kidney function, myocardial enzymes, electrolytes) suitable for chemotherapy; 7) Expected survival time of more than 3 months. Exclusion criteria: 1) Patients with two or more concurrent tumors; 2) Presence of infectious fever; 3) Hematological diseases; 4) Incomplete clinical data or laboratory tests during treatment; 5) Severe bone marrow suppression during treatment; 6) History of hormone use or hematopoietic factor therapy before routine blood collection. This study was approved by the Ethics Committee of Wujin Hospital Affiliated to Jiangsu University. The flow chart of the study is shown in Figure 1.
Figure 1.
Flow chart of the study. CT, computed tomography; MRI, magnetic resonance imaging; ECOG, eastern cooperative oncology group; EGFR, epidermal growth factor receptor.
Clinical data collection
Clinical data were collected and recorded for each patient, including age, gender, tumor pathological type, clinical stage, ECOG score, EGFR gene mutation status, treatment plan, efficacy evaluation after 2 cycles of treatment, and imaging data during treatment. Laboratory tests encompassed all inflammatory and lipid-related indicators, collected within one week before the first treatment and after two cycles of treatment. Some calculations for inflammatory index ratios included: NLR = ANC/ALC. PLR = PLT/ALC. LMR = ALC/AMC.
Treatment methods and evaluation criteria
Tumor stage was determined according to the international lung cancer TNM stage, 8th edition. Efficacy evaluation was based on the efficacy of solid tumors (RECIST version 1.1), and the outcomes are classified into four categories: progressive disease (PD), stable disease (SD), partial remission (PR) and complete remission (CR). Patients underwent chest CT examination every 6-8 weeks to evaluate the tumor in the lungs. Owing to the retrospective nature of this study, not all patients received standard drug dosages. Chemotherapy regimens included pemetrexed (500 mg/m2), carboplatin (200-400 mg/m2), nedaplatin (80-100 mg/m2), cisplatin (50-100 mg/m2), gemcitabine (1000 mg/m2), paclitaxel (260 mg/m2), docetaxel (750 mg/m2), etoposide (1000 mg/m2). Targeted therapy regimens included gefitinib (250 mg/day), almetinib (110 mg/day), and anlotinib (12 mg/day), afatinib (40 mg/day), icotinib (125 mg/day), osimertinib (80 mg/day). Immunotherapy regimens included sintilimab injection (200 mg/time), camrelizumab injection (200 mg/time), tislelizumab injection (200 mg/time), and bevacizumab injection (400 mg/time).
Observation indicators and short-term efficacy of treatment
The changes in relevant indicators were recorded within 1 week before treatment and after 2 cycles of treatment. The short-term therapeutic effect was evaluated after 2 cycles of treatment.
Statistical analysis
SPSS 26.0 statistical software was employed for data analysis, with statistical plotting facilitated by GraphPad Prism 8.0. Qualitative data were described by rate or proportions, and quantitative data were described by mean ± standard deviation (X̅±S). Comparative analysis of quantitative indices utilized paired t-tests, independent sample t-tests, and one-way analysis of variance as appropriate. The diagnostic value of each index was comprehensively evaluated by ROC curve, area under the curve (AUC), sensitivity and specificity. The changes in indicators before and after treatment were evaluated using the formula D = pre-treatment index - post-treatment index. The factors affecting the curative effect was analyzed using multivariate logistic regression analysis (LR forward technique). rms package (R software, 4.2.0) was used to construct a Nomogram model and calibration curve for visualization. All statistical tests were two-sided, with P-values <0.05 considered statistically significant.
Results
General clinical information of the patients
Of the 133 patients, 88 patients (66.2%) were at stage IIIb-IV, while 45 patients (33.8%) were at stage I-IIIa. EGFR genetic testing was conducted on 45 patients. Majority of the patients received chemotherapy (55 patients, 41.4%), followed by patients undergoing two or more combination therapies (46 cases, 34.6%), such as surgery combined with chemotherapy, surgery combined with immunotherapy, surgery combined with targeted therapy, chemotherapy combined with immunotherapy, chemotherapy combined with targeted therapy. A smaller group received targeted therapy alone (16 patients, 12%), surgery (14 patients, 10.5%), and the fewest received only immunotherapy (2 patients, 1.5%). After 2 cycles of treatment, 32 patients (24.1%) achieved PR, 67 patients (50.4%) had SD, and 34 patients (25.5%) had PD. These details are shown in Table 1.
Table 1.
General clinical features of lung cancer patients
| Clinical features | Cases | N (%) |
|---|---|---|
| Age (year) | ||
| ≥70 | 73 | 54.9 |
| <70 | 60 | 45.1 |
| Gender | ||
| Female | 28 | 21.1 |
| Male | 105 | 78.9 |
| Histological type | ||
| Adenocarcinoma | 60 | 45.1 |
| Squamous cell carcinoma | 51 | 38.3 |
| Adenosquamous carcinoma | 1 | 0.8 |
| Sarcomatoid carcinoma | 1 | 0.8 |
| Small cell carcinoma | 20 | 15.0 |
| Stage | ||
| I-IIIa | 45 | 33.8 |
| IIIb-IV | 88 | 66.2 |
| EGFR mutation status | ||
| Wild | 23 | 17.3 |
| Mutation | 22 | 16.5 |
| Unknown | 88 | 66.2 |
| Comorbidities | ||
| COPD | 9 | 6.8 |
| Hypertension | 66 | 49.6 |
| Diabetes mellitus | 16 | 12.0 |
| Fever | 5 | 3.8 |
| Pleural effusion | 30 | 22.6 |
| Treatment modality | ||
| Surgery | 14 | 10.5 |
| Chemotherapy | 55 | 41.4 |
| Immunotherapy | 2 | 1.5 |
| Targeted therapy | 16 | 12.0 |
| Combination therapy | 46 | 34.6 |
| Metastases | ||
| Lymph nodes | 2 | 1.5 |
| Bone | 5 | 3.8 |
| Liver | 2 | 1.5 |
| Brain | 9 | 6.8 |
| Response after 2 cycles of treatment | ||
| PR | 32 | 24.1 |
| SD | 67 | 50.4 |
| PD | 34 | 25.5 |
Notes: EGFR, epidermal growth factor receptor; COPD, chronic obstructive pulmonary disease; PR, partial remission; SD, stable disease; PD, progressive disease.
Changes in each index before and after treatment
After the treatment, WBC, HGB, and ANC were significantly decreased, whereas PLT, TC, TG, and HDL were significantly increased. See Table 2.
Table 2.
Comparison of related factors before and after therapy in patients
| Index | Before therapy | After treatment | t | P |
|---|---|---|---|---|
| WBC (109/L) | 7.22±2.24 | 6.59±2.73 | 2.880 | 0.005 |
| HGB (g/L) | 130.53±18.67 | 119.87±18.67 | 7.127 | 0.000 |
| PLT (109/L) | 153.11±85.94 | 239.03±85.38 | 2.052 | 0.042 |
| ANC (109/L) | 4.93±1.96 | 4.39±2.65 | 2.494 | 0.014 |
| ALC (109/L) | 1.51±0.56 | 1.45±0.55 | 1.196 | 0.234 |
| AMC (109/L) | 0.54±0.24 | 0.56±0.25 | -0.881 | 0.380 |
| NLR (%) | 3.77±2.27 | 3.60±2.86 | 0.604 | 0.547 |
| PLR (%) | 192.55±110.73 | 183.18±89.59 | 0.908 | 0.365 |
| LMR (%) | 3.38±2.61 | 3.03±1.68 | 1.436 | 0.153 |
| ALB (g/L) | 36.56±4.53 | 36.81±5.48 | -0.500 | 0.618 |
| TC (mmol/L) | 4.23±0.86 | 4.44±1.02 | -2.676 | 0.008 |
| TG (mmol/L) | 1.29±0.71 | 1.43±0.72 | -1.996 | 0.048 |
| HDL (mmol/L) | 1.19±0.31 | 1.26±0.39 | -2.349 | 0.020 |
| LDL (mmol/L) | 2.79±0.71 | 2.87±0.84 | -1.408 | 0.162 |
| APO-A1 (g/L) | 1.17±0.27 | 1.19±0.29 | -0.628 | 0.531 |
| APO-B (g/L) | 0.85±0.22 | 0.88±0.27 | -1.889 | 0.061 |
Notes: WBC, white blood cell; HGB, hemoglobin; PLT, platelets; ANC, absolute neutrophil count; ALC, lymphocyte count; AMC, monocyte count; NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio; LMR, lymphocyte/monocyte ratio; ALB, albumin; TC, cholesterol; TG, triglyceride; HDL, high density lipoprotein; LDL, low density lipoprotein; APO-A1, apolipoprotein A1; APO-B, apolipoprotein B.
Relationship between dynamic changes in indices and treatment efficacy
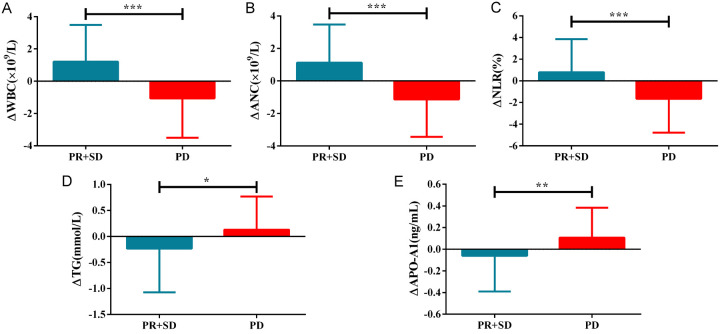
Patients were divided into a progressive disease (PD) group and a non-progressive disease (NPD) group based on their response after two treatment cycles. Patients with stable disease (SD) and partial remission (PR) comprised the NPD group. The results showed that ΔWBC, ΔANC, ΔNLR were significantly higher, while ΔTG, ΔAPO-A1 were significantly lower in the PD group, compared to NPD group (P<0.001). See Table 3 and Figure 2.
Table 3.
Comparison of dynamic changes in indicators between PD and NPD groups
| Index | NPD | PD | F | P |
|---|---|---|---|---|
| ΔWBC (109/L) | 1.21±2.28 | -1.06±2.44 | 4.930 | <0.001 |
| ΔHGB (g/L) | 11.06±18.00 | 9.47±15.01 | 0.463 | 0.644 |
| ΔPLT (109/L) | 17.15±84.18 | 5.15±62.56 | 0.762 | 0.448 |
| ΔANC (109/L) | 1.12±2.35 | -1.13±2.32 | 4.846 | <0.001 |
| ΔALC (109/L) | 0.02±0.56 | 0.18±0.67 | -1.322 | 0.189 |
| ΔAMC (109/L) | 0.00±0.26 | -0.09±0.22 | 1.809 | 0.073 |
| ΔNLR (%) | 0.80±3.06 | -1.65±3.14 | 4.001 | <0.001 |
| ΔPLR (%) | 18.45±123.57 | -17.04±102.11 | 1.507 | 0.134 |
| ΔLMR (%) | 0.32±3.15 | 0.43±1.44 | -0.198 | 0.843 |
| ΔALB (g/L) | -0.70±4.77 | 1.05±8.16 | -1.512 | 0.133 |
| ΔTC (mmol/L) | -0.28±0.92 | -0.04±0.94 | -1.300 | 0.196 |
| ΔTG (mmol/L) | -0.23±0.84 | 0.13±0.64 | -2.304 | 0.023 |
| ΔHDL (mmol/L) | -0.09±0.37 | -0.03±0.41 | -0.794 | 0.429 |
| ΔLDL (mmol/L) | -0.13±0.63 | 0.07±0.79 | -1.522 | 0.130 |
| ΔAPO-A1 (g/L) | -0.06±0.33 | 0.11±0.28 | -2.643 | 0.009 |
| ΔAPO-B (g/L) | -0.03±0.18 | -0.03±0.25 | -0.184 | 0.854 |
Notes: Δ = (pre-treatment index) - (post-treatment index); WBC, white blood cell; HGB, hemoglobin; PLT, platelets; ANC, absolute neutrophil count; ALC, lymphocyte count; AMC, monocyte count; NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio; LMR, lymphocyte/monocyte ratio; ALB, albumin; TC, cholesterol; TG, triglyceride; HDL, high density lipoprotein; LDL, low density lipoprotein; APO-A1, apolipoprotein A1; APO-B, apolipoprotein B.
Figure 2.
Changes in indicators before and after treatment in patients with different curative effects. A: Differences in ΔWBC between PD and NPD; B: Differences in ΔANC between PD and NPD; C: Differences in ΔNLR between PD and NPD; D: Differences in ΔTG between PD and NPD; E: Differences in ΔAPO-A1 between PD and NPD. *P<0.05, **P<0.01, ***P<0.001. WBC, white blood cell; ANC, absolute neutrophil count; NLR, neutrophil/lymphocyte ratio; TG, triglyceride; APO-A1, apolipoprotein A1; PR, partial remission; SD, stable disease; PD, progressive disease.
Changes in indices before and after therapy
The analysis of changes in various indicators before and after treatment was restricted to patients undergoing chemotherapy, targeted therapy, and combination therapies due to the limited number of patients receiving immunotherapy. The results indicated that different treatment modalities had distinct impacts on indicators such as ΔWBC, ΔPLT, ΔANC, ΔALC, ΔNLR, ΔPLR, ΔALB, ΔTC, ΔTG, ΔLDL, and ΔAPO-B. These variations are detailed in Table 4.
Table 4.
Comparison of dynamic changes in indicator among patients undergoing different treatments
| Index | Operation | Chemotherapy | Target treatment | Combination therapy | F | P |
|---|---|---|---|---|---|---|
| ΔWBC (×109/L) | -1.92±2.19 | 1.19±2.48 | -0.89±2.67 | 1.23±1.91 | 9.959 | 0.000 |
| ΔHGB (g/L) | 17.23±13.49 | 10.69±17.15 | 5.06±16.19 | 10.26±18.21 | 1.248 | 0.295 |
| ΔPLT (109/L) | -49.31±86.82 | 11.95±81.29 | 17.35±61.40 | 35.67±72.92 | 4.193 | 0.007 |
| ΔANC (×109/L) | -2.27±2.16 | 1.15±2.42 | -1.08±2.55 | 1.19±1.87 | 12.456 | 0.000 |
| ΔALC (×109/L) | 0.55±0.36 | 0.03±0.58 | 0.25±0.40 | -0.09±0.57 | 5.300 | 0.002 |
| ΔAMC (×109/L) | -0.13±0.19 | -0.01±0.27 | -0.03±0.21 | -0.00±0.26 | 0.938 | 0.425 |
| ΔNLR (%) | -3.16±2.79 | 0.89±2.95 | -1.96±3.24 | 1.07±2.69 | 11.536 | 0.000 |
| ΔPLR (%) | -86.74±77.10 | 9.29±74.54 | -20.10±92.23 | 49.74±157.98 | 5.445 | 0.001 |
| ΔLMR (%) | 1.79±0.95 | 0.05±2.87 | 0.66±0.99 | 0.22±3.42 | 1.447 | 0.232 |
| ΔALB (g/L) | 3.43±5.00 | -0.33±4.55 | 0.99±5.54 | -1.83±6.99 | 3.266 | 0.024 |
| ΔTC (mmol/L) | 0.47±0.84 | -0.36±0.85 | 0.01±0.63 | -0.35±1.00 | 3.836 | 0.011 |
| ΔTG (mmol/L) | 0.45±0.43 | -0.23±0.65 | -0.07±0.62 | -0.23±1.04 | 2.896 | 0.038 |
| ΔHDL (mmol/L) | 0.05±0.27 | -0.08±0.32 | -0.09±0.55 | -0.11±0.38 | 0.652 | 0.583 |
| ΔLDL (mmol/L) | 0.35±0.73 | -0.23±0.64 | 0.13±0.46 | -0.14±0.70 | 3.566 | 0.016 |
| ΔAPO-A1 (g/L) | 0.18±0.25 | -0.04±0.28 | 0.04±0.35 | -0.07±0.35 | 2.329 | 0.078 |
| ΔAPO-B (g/L) | 0.09±0.19 | -0.05±0.16 | 0.02±0.14 | -0.08±0.22 | 3.549 | 0.016 |
Notes: Δ = (pre-treatment index) - (post-treatment index); WBC, white blood cell; HGB, hemoglobin; PLT, platelets; ANC, absolute neutrophil count; ALC, lymphocyte count; AMC, monocyte count; NLR, neutrophil/lymphocyte ratio; PLR, platelet/lymphocyte ratio; LMR, lymphocyte/monocyte ratio; ALB, albumin; TC, cholesterol; TG, triglyceride; HDL, high density lipoprotein; LDL, low density lipoprotein; APO-A1, apolipoprotein A1; APO-B, apolipoprotein B.
ROC curve and cutoff value of ΔNLR, ΔWBC, ΔANC, ΔTG, ΔAPO-A1
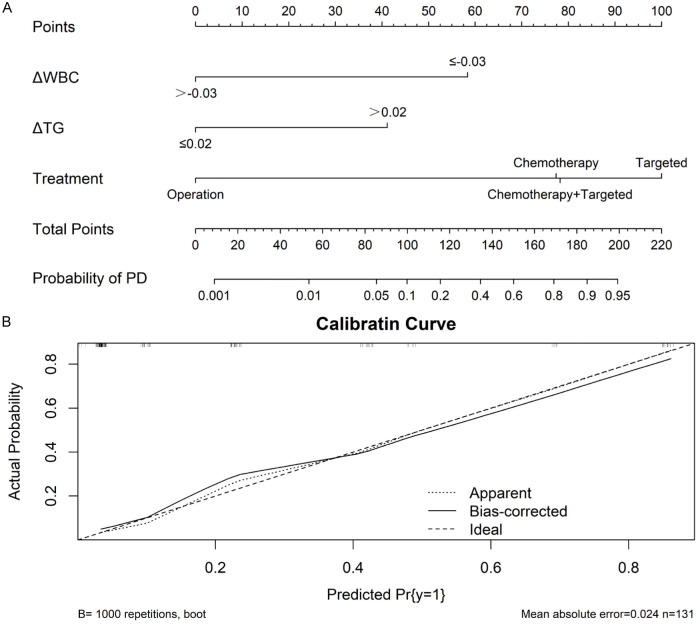
There were significant differences in ΔNLR, ΔWBC, ΔANC, ΔTG, ΔAPO-A1 between the PD and NPD groups. The diagnostic value of these indicators was assessed using ROC curves. Notably, ΔANC displayed the highest sensitivity (88.24%) and ΔWBC the greatest specificity (77.78%). See Table 5 and Figure 3. Additionally, a nomogram was constructed to visually represent these predictors, as shown in Figure 4.
Table 5.
Diagnostic value of each index for disease progression
| Index | AUC and 0.95 CI | P value | Cut point | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| ΔNLR | 0.706 (0.621, 0.782) | 0.000 | -0.443 | 58.82* | 75.76 |
| ΔWBC | 0.776 (0.695, 0.843) | 0.000 | -0.03 | 73.53* | 77.78 |
| ΔANC | 0.774 (0.694, 0.842) | 0.000 | 0.91 | 88.24 | 59.60# |
| ΔTG | 0.687 (0.601, 0.764) | 0.001 | 0.02 | 64.71* | 70.71 |
| ΔAPO-A1 | 0.652 (0.565, 0.733) | 0.006 | 0.01 | 70.59* | 60.61# |
P<0.05 vs ΔANC;
P<0.05 vs ΔWBC.
Δ = (pre-treatment index) - (post-treatment index); NLR, neutrophil/lymphocyte ratio; WBC, white blood cell; ANC, absolute neutrophil count; TG, triglyceride; APO-A1, apolipoprotein A1.
Figure 3.
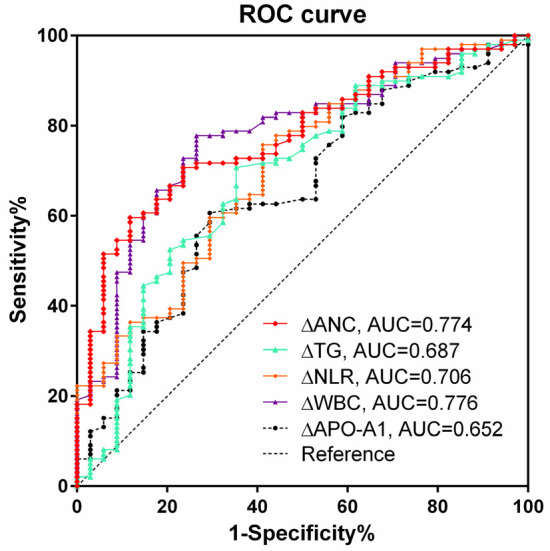
The predictive ability of alteration in each index for treatment efficacy. WBC, white blood cell; ANC, absolute neutrophil count; NLR, neutrophil/lymphocyte ratio; TG, triglyceride; APO-A1, apolipoprotein A1.
Figure 4.
Nomogram to predict patient’s treatment efficacy. A: Nomogram; B: Calibration of the nomogram. WBC, white blood cell; TG, triglyceride; PD, progressive disease; Pr, probability.
Analysis of factors affecting therapeutic efficacy of lung cancer
A logistic regression analysis was conducted to identify the factors affecting the treatment efficacy, taking disease progression after 2 cycles of treatment as the dependent variable. Factors included in the analysis were ΔNLR, ΔWBC, ΔANC, ΔTG, ΔAPO-A1, age (whether over 70), gender, treatment method, pathological type, presence of comorbidities, and staging (whether extensive). The regression results showed that ΔWBC, ΔTG, and treatment method were independent risk factors for disease progression. Patients in the chemotherapy, targeted therapy, and combination therapy groups had a higher risk of disease progression relative to the surgery alone group. See Table 6.
Table 6.
Logistic multivariate analysis of the factors affecting the treatment efficacy of lung cancer (forward LR method)
| Index | β | Standard error | Wald | P | OR and 0.95 CI |
|---|---|---|---|---|---|
| ΔWBC (0 = ≤-0.03, 1 = >-0.03) | -0.525 | 0.137 | 14.763 | 0.000 | 0.591 (0.452, 0.773) |
| ΔTG (0 = ≤0.02, 1 = >0.02) | 0.660 | 0.323 | 4.180 | 0.041 | 1.935 (1.028, 3.643, 0.773) |
| Treatment (with the surgery group as the control group) | - | - | 11.450 | 0.010 | - |
| Chemotherapy | 3.541 | 1.315 | 7.244 | 0.007 | 34.486 (2.618, 454.323) |
| Targeting therapy | 4.534 | 1.368 | 10.984 | 0.001 | 93.133 (6.377, 1360.203) |
| Combination therapy | 3.379 | 1.338 | 6.378 | 0.012 | 29.350 (2.131, 404.158) |
Notes: WBC, white blood cell; TG, triglyceride; OR, odds ratio; CI, confidence interval.
Discussion
Recent advancements in immune checkpoints and driver genes, along with the ongoing development of new drugs and the strategic application of multidisciplinary comprehensive diagnosis and treatment approaches, have significantly improved the prognosis for lung cancer. Despite these advancements, lung cancer remains the malignancy with the highest mortality rate. Researchers continue to explore early biomarkers for lung cancer prognosis, aiming to enhance therapeutic outcomes through early intervention.
There are, however, few studies assess the dynamic changes in inflammation and lipid metabolism during patient therapy and their correlation with treatment efficacy and prognosis. Chen et al. [11] showed that elevated NLR in limited-stage small cell lung cancer led to a poorer prognosis. In addition, Deng et al. [31] also found a worse prognosis in the patients with high NLR and PLR among 203 adenocarcinoma patients with EGFR mutations receiving first-line targeted therapies. These findings suggest that NLR significantly impacts prognosis, regardless of gene mutation status. Studies by Cui et al. [13] identified TG level as an independent risk factor for small cell lung cancer progression. Higher TG levels are linked to more rapid disease progresses and poor patient outcomes.
In this study, after treatment, WBC, HGB, and ANC were significantly decreased, while PLT, TC, TG, and HDL were significantly increased. The dynamic changes in WBC, ANC, NLR, TG and APO-A1 were significantly correlated with the therapeutic efficacy. After 2 cycles of treatment, the NLR dramatically reduced in the NPD group, but significantly increased in the PD group, and the change in NLR (ΔNLR) was significantly lower in the NPD group compared to the PD group. This reduction in NLR post-treatment indicates a better therapeutic outcome in lung cancer patients. McLellan et al. [32] concluded that patients with metastatic pancreatic cancer who had an NLR>5 after 15 days of chemotherapy had a worse prognosis. Similarly, Lawati et al. [33] found that patients with esophageal adenocarcinoma undergoing surgery had poorer outcomes when their ΔNLR remained elevated, underscoring the link between dynamic NLR changes and survival rates. His correlation was also noted in earlier studies, such as the association of pre-treatment NLR with progression-free survival (PFS) and overall survival (OS) in patients with malignant melanoma in 2015 [34], and more recent reports connecting pretreatment NLR to OS in nivolumab-treated lung cancer patients [35,36]. After 2 cycles of treatment, the PD group exhibited significantly higher levels of WBC and ANC, and lower levels of TG and APO-A1 compared to the NPD group, which showed the opposite pattern. This suggests that decreases in WBC and ANC, along with increases in TG and APO-A1, are indicative of better therapeutic outcomes in lung cancer patients. Inflammation is associated with the development and malignant progression of most cancers. It plays a crucial role in carcinogenesis, tumor growth, metastasis, and has a substantial immunosuppressive impact on anti-tumor immune functions, increasing recurrence risks [37,38]. Neutrophils are the most abundant peripheral leukocyte type and are capable of destroying invading microorganisms through phagocytosis and intracellular degradation with the release of particles [39]. In addition, neutrophils could infiltrate various tumors and form neutrophil extracellular traps (NETs) [40]. Recent study has shown that NETs are involved in tumor progression and metastasis [41]. These findings suggest a possible mechanism by which leukocytosis can lead to adverse prognoses.
The diagnostic value of dynamic changes in WBC, ANC, NLR, TG, and APO-A1 for predicting disease progression was evaluated using ROC curves. The results demonstrated that ΔANC exhibited the highest sensitivity (88.24%), while ΔWBC provided the best specificity (77.78%). The observed changes after treatment - NLR increased by 0.443, WBC by 0.03, ANC by 0.91, while TG decreased by 0.02, and APO-A1 by 0.01 - suggest potential indicators of disease progression and suboptimal treatment efficacy. In addition, the dynamic changes in inflammation and blood lipid-related indicators are also related to treatment methods. Multivariate logistic regression analysis identified ΔWBC, ΔTG, and treatment methods as independent risk factors for disease progression. These findings indicate that changes in WBC and TG can predict the therapeutic outcome in lung cancer. Notably, compared to the surgery-only group, patients undergoing chemotherapy, targeted therapy, or combination therapies exhibited a higher likelihood of progression, suggesting that patients opting for surgery alone might have less severe disease.
This study shows that dynamic changes in WBC, ANC, NLR, TG and APO-A1 can reflect treatment efficacy. However, several critical issues remain unresolved, affecting the prognostic and therapeutic implications of these findings: 1) The thresholds for each indicator are not standardized. 2) It is unclear whether modifying inflammation and lipid metabolism markers can improve patient survival. Addressing these questions will require large-scale, prospective clinical studies to provide definitive answers and guide future therapeutic strategies.
Conclusion
In this study, we comprehensively analyzed inflammation and lipid metabolism indicators, and investigated their dynamic changes during the treatment and the subsequent impact on prognosis. ΔWBC, ΔTG, and treatment modalities were independent risk factors for disease progression. Our findings underscore that ΔWBC and ΔTG are valuable predictors for lung cancer progression. Additionally, they could serve as lung cancer therapy targets. In clinical practice, clinicians should consider the dynamic changes in serum inflammation and lipid metabolism-related indicators to make individualized treatment plan.
Further assessment of immune function in patients should extend beyond merely quantifying immune cells to include changes in subpopulations and cell function. Future study will focus on monitoring the changes in cell subpopulation and cell surface signaling molecules during lung cancer treatment, which will enhance our understanding of the immune function during the treatment and provide more theoretical support for the developing individualized immunotherapy strategies.
Disclosure of conflict of interest
None.
References
- 1.Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS. Lung cancer. Lancet. 2021;398:535–554. doi: 10.1016/S0140-6736(21)00312-3. [DOI] [PubMed] [Google Scholar]
- 2.El-Hussein A, Manoto SL, Ombinda-Lemboumba S, Alrowaili ZA, Mthunzi-Kufa P. A review of chemotherapy and photodynamic therapy for lung cancer treatment. Anticancer Agents Med Chem. 2021;21:149–161. doi: 10.2174/1871520620666200403144945. [DOI] [PubMed] [Google Scholar]
- 3.Majeed U, Manochakian R, Zhao Y, Lou Y. Targeted therapy in advanced non-small cell lung cancer: current advances and future trends. J Hematol Oncol. 2021;14:108. doi: 10.1186/s13045-021-01121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lahiri A, Maji A, Potdar PD, Singh N, Parikh P, Bisht B, Mukherjee A, Paul MK. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. 2023;22:40. doi: 10.1186/s12943-023-01740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pernot S, Terme M, Radosevic-Robin N, Castan F, Badoual C, Marcheteau E, Penault-Llorca F, Bouche O, Bennouna J, Francois E, Ghiringhelli F, De La Fouchardiere C, Samalin E, Baptiste Bachet J, Borg C, Boige V, Voron T, Stanbury T, Tartour E, Gourgou S, Malka D, Taieb J. Infiltrating and peripheral immune cell analysis in advanced gastric cancer according to the Lauren classification and its prognostic significance. Gastric Cancer. 2020;23:73–81. doi: 10.1007/s10120-019-00983-3. [DOI] [PubMed] [Google Scholar]
- 6.Anagnostou V, Forde PM, White JR, Niknafs N, Hruban C, Naidoo J, Marrone K, Sivakumar IKA, Bruhm DC, Rosner S, Phallen J, Leal A, Adleff V, Smith KN, Cottrell TR, Rhymee L, Palsgrove DN, Hann CL, Levy B, Feliciano J, Georgiades C, Verde F, Illei P, Li QK, Gabrielson E, Brock MV, Isbell JM, Sauter JL, Taube J, Scharpf RB, Karchin R, Pardoll DM, Chaft JE, Hellmann MD, Brahmer JR, Velculescu VE. Dynamics of tumor and immune responses during immune checkpoint blockade in non-small cell lung cancer. Cancer Res. 2019;79:1214–1225. doi: 10.1158/0008-5472.CAN-18-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Zhou F, Qiao M, Li X, Zhao C, Cheng L, Chen X, Zhou C. The role of circulating tumor DNA in advanced non-small cell lung cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Oncol. 2021;11:671874. doi: 10.3389/fonc.2021.671874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moschetta M, Uccello M, Kasenda B, Mak G, McClelland A, Boussios S, Forster M, Arkenau HT. Dynamics of neutrophils-to-lymphocyte ratio predict outcomes of PD-1/PD-L1 blockade. Biomed Res Int. 2017;2017:1506824. doi: 10.1155/2017/1506824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Passiglia F, Galvano A, Castiglia M, Incorvaia L, Calò V, Listì A, Mazzarisi S, Perez A, Gallina G, Rizzo S, Soto Parra H, Bazan V, Russo A. Monitoring blood biomarkers to predict nivolumab effectiveness in NSCLC patients. Ther Adv Med Oncol. 2019;11:1758835919839928. doi: 10.1177/1758835919839928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bremnes RM, Busund LT, Kilvær TL, Andersen S, Richardsen E, Paulsen EE, Hald S, Khanehkenari MR, Cooper WA, Kao SC, Dønnem T. The role of tumor-infiltrating lymphocytes in development, progression, and prognosis of non-small cell lung cancer. J Thorac Oncol. 2016;11:789–800. doi: 10.1016/j.jtho.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Yang H, Cai D, Xiang L, Fang W, Wang R. Preoperative peripheral blood neutrophil-to-lymphocyte ratios (NLR) and platelet-to-lymphocyte ratio (PLR) related nomograms predict the survival of patients with limited-stage small-cell lung cancer. Transl Lung Cancer Res. 2021;10:866–877. doi: 10.21037/tlcr-20-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grassadonia A, Graziano V, Iezzi L, Vici P, Barba M, Pizzuti L, Cicero G, Krasniqi E, Mazzotta M, Marinelli D, Amodio A, Natoli C, Tinari N. Prognostic relevance of neutrophil to lymphocyte ratio (NLR) in luminal breast cancer: a retrospective analysis in the neoadjuvant setting. Cells. 2021;10:1685. doi: 10.3390/cells10071685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Divsalar B, Heydari P, Habibollah G, Tamaddon G. Hematological parameters changes in patients with breast cancer. Clin Lab. 2021;67 doi: 10.7754/Clin.Lab.2020.201103. [DOI] [PubMed] [Google Scholar]
- 14.Mansour R, Abu-Shawer O, Lattouf A, Sultan H, Al-Hussaini M. Hematological indices of distant metastases and prognostic nomogram in gastro-pancreatic and biliary tract cancers. Cancer Manag Res. 2020;12:9775–9786. doi: 10.2147/CMAR.S259197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao W, Yao X, Cen D, Zhi Y, Zhu N, Xu L. The prognostic role of platelet-to-lymphocyte ratio on overall survival in gastric cancer: a systematic review and meta-analysis. BMC Gastroenterol. 2020;20:16. doi: 10.1186/s12876-020-1167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abu-Shawer M, Abu-Shawer O, Souleiman M, Akkawi M, Alshakhatreh O, Altamimi T, Al-Omari A, Al-Hussaini M. Hematologic markers of lung metastasis in stage IV colorectal cancer. J Gastrointest Cancer. 2019;50:428–433. doi: 10.1007/s12029-018-0089-0. [DOI] [PubMed] [Google Scholar]
- 17.Russo A, Russano M, Franchina T, Migliorino MR, Aprile G, Mansueto G, Berruti A, Falcone A, Aieta M, Gelibter A, Russo A, Barni S, Maio M, Martelli O, Pantano F, Iacono D, Calvetti L, Quadrini S, Roca E, Vasile E, Imperatori M, Occhipinti M, Galvano A, Petrelli F, Calabrò L, Pasquini G, Intagliata S, Ricciardi GRR, Tonini G, Santini D, Adamo V. Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and outcomes with nivolumab in pretreated non-small cell lung cancer (NSCLC): a large retrospective multicenter study. Adv Ther. 2020;37:1145–1155. doi: 10.1007/s12325-020-01229-w. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Xu H, Yang G, Yang L, Li J, Wang Y. The value of blood biomarkers of progression and prognosis in ALK-positive patients with non-small cell lung cancer treated with crizotinib. Asia Pac J Clin Oncol. 2020;16:63–69. doi: 10.1111/ajco.13284. [DOI] [PubMed] [Google Scholar]
- 19.Dong Y, Wang H, Shan D, Yu Z. Research progress on the relationship between blood lipids and lung cancer risk and prognosis. Zhongguo Fei Ai Za Zhi. 2020;23:824–829. doi: 10.3779/j.issn.1009-3419.2020.102.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui YN, Gao Q, Zhu SS, Jin HF. Effect of serum lipid level on prognosis of patients with small cell lung cancer at the initial treatment. Zhonghua Zhong Liu Za Zhi. 2021;43:318–323. doi: 10.3760/cma.j.cn112152-20190813-00517. [DOI] [PubMed] [Google Scholar]
- 21.Sotiropoulos GP, Dalamaga M, Antonakos G, Marinou I, Vogiatzakis E, Kotopouli M, Karampela I, Christodoulatos GS, Lekka A, Papavassiliou AG. Chemerin as a biomarker at the intersection of inflammation, chemotaxis, coagulation, fibrinolysis and metabolism in resectable non-small cell lung cancer. Lung Cancer. 2018;125:291–299. doi: 10.1016/j.lungcan.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Zuo Y, Leng G, Leng P. Identification and validation of molecular subtype and prognostic signature for lung adenocarcinoma based on neutrophil extracellular traps. Pathol Oncol Res. 2023;29:1610899. doi: 10.3389/pore.2023.1610899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pope ED 3rd, Kimbrough EO, Vemireddy LP, Surapaneni PK, Copland JA 3rd, Mody K. Aberrant lipid metabolism as a therapeutic target in liver cancer. Expert Opin Ther Targets. 2019;23:473–483. doi: 10.1080/14728222.2019.1615883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kouba S, Ouldamer L, Garcia C, Fontaine D, Chantome A, Vandier C, Goupille C, Potier-Cartereau M. Lipid metabolism and Calcium signaling in epithelial ovarian cancer. Cell Calcium. 2019;81:38–50. doi: 10.1016/j.ceca.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Akinci Ozyurek B, Sahin Ozdemirel T, Buyukyaylaci Ozden S, Erdogan Y, Kaplan B, Kaplan T. Prognostic value of the neutrophil to lymphocyte ratio (NLR) in lung cancer cases. Asian Pac J Cancer Prev. 2017;18:1417–1421. doi: 10.22034/APJCP.2017.18.5.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pakiet A, Kobiela J, Stepnowski P, Sledzinski T, Mika A. Changes in lipids composition and metabolism in colorectal cancer: a review. Lipids Health Dis. 2019;18:29. doi: 10.1186/s12944-019-0977-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tyler A, Johansson A, Karlsson T, Gudey SK, Brännström T, Grankvist K, Behnam-Motlagh P. Targeting glucosylceramide synthase induction of cell surface globotriaosylceramide (Gb3) in acquired cisplatin-resistance of lung cancer and malignant pleural mesothelioma cells. Exp Cell Res. 2015;336:23–32. doi: 10.1016/j.yexcr.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Yao X, Gordon EM, Figueroa DM, Barochia AV, Levine SJ. Emerging roles of apolipoprotein E and apolipoprotein A-I in the pathogenesis and treatment of lung disease. Am J Respir Cell Mol Biol. 2016;55:159–169. doi: 10.1165/rcmb.2016-0060TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huai Q, Luo C, Song P, Bie F, Bai G, Li Y, Liu Y, Chen X, Zhou B, Sun X, Guo W, Gao S. Peripheral blood inflammatory biomarkers dynamics reflect treatment response and predict prognosis in non-small cell lung cancer patients with neoadjuvant immunotherapy. Cancer Sci. 2023;114:4484–4498. doi: 10.1111/cas.15964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li C, Wu J, Jiang L, Zhang L, Huang J, Tian Y, Zhao Y, Liu X, Xia L, E H, Gao P, Hou L, Yang M, Ma M, Su C, Zhang H, Chen H, She Y, Xie D, Luo Q, Chen C. The predictive value of inflammatory biomarkers for major pathological response in non-small cell lung cancer patients receiving neoadjuvant chemoimmunotherapy and its association with the immune-related tumor microenvironment: a multi-center study. Cancer Immunol Immunother. 2023;72:783–794. doi: 10.1007/s00262-022-03262-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng C, Zhang N, Wang Y, Jiang S, Lu M, Huang Y, Ma J, Hu C, Hou T. High systemic immune-inflammation index predicts poor prognosis in advanced lung adenocarcinoma patients treated with EGFR-TKIs. Medicine (Baltimore) 2019;98:e16875. doi: 10.1097/MD.0000000000016875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLellan P, Henriques J, Ksontini F, Doat S, Hammel P, Desrame J, Trouilloud I, Louvet C, Pietrasz D, Vernerey D, Bachet JB. Prognostic value of the early change in neutrophil-to-lymphocyte ratio in metastatic pancreatic adenocarcinoma. Clin Res Hepatol Gastroenterol. 2021;45:101541. doi: 10.1016/j.clinre.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Al Lawati Y, Cools-Lartigue J, Ramirez-GarciaLuna JL, Molina-Franjola JC, Pham D, Skothos E, Mueller C, Spicer J, Ferri L. Dynamic alteration of neutrophil-to-lymphocyte ratio over treatment trajectory is associated with survival in esophageal adenocarcinoma. Ann Surg Oncol. 2020;27:4413–4419. doi: 10.1245/s10434-020-08521-7. [DOI] [PubMed] [Google Scholar]
- 34.Ferrucci PF, Gandini S, Battaglia A, Alfieri S, Di Giacomo AM, Giannarelli D, Cappellini GC, De Galitiis F, Marchetti P, Amato G, Lazzeri A, Pala L, Cocorocchio E, Martinoli C. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer. 2015;112:1904–1910. doi: 10.1038/bjc.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, Kosteva JA, Ciunci CA, Gabriel PE, Thompson JC, Stonehouse-Lee S, Sherry VE, Gilbert E, Eaby-Sandy B, Mutale F, DiLullo G, Cohen RB, Vachani A, Langer CJ. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1–7. doi: 10.1016/j.lungcan.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 36.Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, Templeton AJ, Früh M. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–181. doi: 10.1016/j.lungcan.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32:1267–1284. doi: 10.1101/gad.314617.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51:27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosales C. Neutrophil: a cell with many roles in inflammation or several cell types? Front Physiol. 2018;9:113. doi: 10.3389/fphys.2018.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu L, Saxena S, Awaji M, Singh RK. Tumor-associated neutrophils in cancer: going pro. Cancers (Basel) 2019;11:564. doi: 10.3390/cancers11040564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, Upadhyay P, Uyeminami DL, Pommier A, Küttner V, Bružas E, Maiorino L, Bautista C, Carmona EM, Gimotty PA, Fearon DT, Chang K, Lyons SK, Pinkerton KE, Trotman LC, Goldberg MS, Yeh JT, Egeblad M. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science. 2018;361:eaao4227. doi: 10.1126/science.aao4227. [DOI] [PMC free article] [PubMed] [Google Scholar]