Abstract
As numerous countries around the world have entered an aging society currently, understanding the impact of aging on human health becomes critically important. Notably, aging is associated with increased prevalence of age-related diseases, with the lungs being particularly susceptible. Aging contributes to a decline in lung function, including respiratory disorders, inflammation, and oxidative stress. Therefore, it is a very important to identify and develop active substances that can mitigate lung cell aging. In current study, we evaluated the impact of Taraxasterol on lung cell senescence, showing that Taraxasterol can alleviate lung cell senescence, as evidenced by reductions in senescence-related marker molecules, including p16 and p21. Additionally, Taraxasterol was found to ameliorate inflammation and oxidative stress in lung cells. Further mechanistic studies indicated that Taraxasterol exerts anti-aging effects through the PGC1α/NRF1 signaling pathway in lung cell models. Since aging is also closely related to lung cancer, we also explored the potential anti-tumor effect of taraxasterol. Utilizing non-small cell lung cancer cells (NSCLC) as a model, we systematically study the anti-tumor effect of Taraxasterol both in vivo and in vitro. Our findings suggest that Taraxasterol exhibited anti-cancer effect through EGFR-mediated signaling. Taken together, Taraxasterol shows dual biological activities, offering promising anti-aging and anti-lung cancer benefits.
Keywords: Taraxasterol, lung cells, lung cancer, p16
Introduction
Lung cell senescence is considered to be an irreversible arrest of growth cycle accompanied by a process of cell function impairment. This state leads to an increase in reactive oxygen species (ROS) and the expression of senescence-associated molecules, such as Sa-β-galactosidase [1]. With the increasing trend of global aging, the urgency for developing anti-aging therapies intensifies. The aging within the respiratory system is a complex physiological process that gradually declines physical function and increases the risk of chronic diseases, such as chronic obstructive pulmonary disease (COPD) and related diseases [2]. Consequently, the research on anti-lung aging drugs has garnered significant attention, aiming to provide more effective health protection and treatment methods for the elderly population.
There is a well-documented association between lung cell senescence and lung cancer, particularly prevalent in older adults. The incidence of lung cancer correlates strongly with aging [3]. Unfortunately, lung cancer is typically diagnosed at the advanced metastatic stage, severely limiting patient prognosis due to the spread of cancer to distant organs [4]. Most lung cancer patients miss the opportunity of early detection and surgical treatment, leading to enter advanced tumors [5]. Although a range of treatment options exists, chemotherapy remains foundational in managing lung cancer. Surgical resection is the main treatment for early stage NSCLC, with chemotherapy employed to improve the prognosis [4]. For advanced-stage NSCLC patients, treatment strategies often include a combination of chemotherapy, targeted therapy, and immunotherapy [6]. The challenges of managing NSCLC are compounded as many patients are diagnosed at middle to advanced stages [7]. Despite the promise shown by tyrosine kinase inhibitors (TKIs) [8], patients lacking targetable mutations face limited treatment efficacy and poor overall outcomes [8]. Improving the status of treatment and bringing survival benefits to patients is an urgent problem to be solved. In recent years, targeted programmed death 1 (PD-1) and its ligand (PD-L1) have achieved breakthroughs in the treatment of various cancer types including lung cancer [9]. The need for new anti-lung cancer drugs remains critical, with the EGF/EGFR pathway identified as a key target in ongoing research.
Moreover, the interplay between aging and lung cancer underscores the importance of identifying triggers like NSCLC. Taraxasterol, a pentacyclic-triterpene compound extracted from the traditional Chinese medicine dandelion, has many bioactivities [10]. In traditional oriental medicine, Taraxasterol is widely used in the treatment of various inflammatory or infectious diseases, such as hepatitis, upper respiratory tract infection, bronchitis, pneumonia, etc. [11]. Emerging studies have found that Taraxasterol also exhibits potential anti-tumor effects [11]. However, the specific impact of Taraxasterol on senescent cells and lung cancer is still unknown.
In our study, we systematically investigated the anti-aging effect of Taraxastero, demonstrating its capability to effectively alleviate the lung cells aging. On this basis, we also evaluated anti-tumor efficacy of Taraxasterol both in vitro and in vivo. Our findings suggest that Taraxasterol may achieve its anti-lung cancer biological function through blocking EGFR-signaling pathway and inducing cell cycle arrest. Taken together, our research presents Taraxasterol as a dual biological function that potentially prevents and treats lung cancer. Current research supports the effectiveness of Taraxasterol not only in therapeutic applications but also as a preventive measure against lung cancer.
Materials and methods
Reagents and antibodies
Antibodies Bax (ab32503, 1:200 dilution), CyclinD1 (ab16663, 1:300 dilution), CyclinE1 (ab33911, 1:400 dilution), Bcl2 (ab32124, 1:300 dilution), CDK6 (ab124821, 1:600 dilution), β-actin (ab8226, 1:300 dilution), CDK2 (ab32147, 1:200 dilution), p53 (ab26, 1:500 dilution), SIRT1 (ab110304, 1:300 dilution), HIF1α (ab179483, 1:500 dilution), 53BP1 (ab175933, 1:3000 dilution), HP1α (ab109028, 1:3000 dilution) and PCNA (ab29, 1:300 dilution) were purchased from Abcam (UK). p21 (A22460, 1:500 dilution), caspase-9 (A0281, 1:1000 dilution) and caspase-3 (A25309, 1:1000 dilution) were sourced from ABclonal (China). IL-6 (21865-1-AP, 1:500 dilution), IL-1β (16806-1-AP, 1:1000 dilution), H2A.X (29380-1-AP, 1:2000 dilution), p16 (10883-1-AP, 1:2000 dilution) were obtained from Proteintech company (China). Chemical compounds included 98% pure Taraxasterol from MCE (China). Cell culture and staining supplies such as DMEM, FBS, and MTT were purchased from Beyotime Biotechnology (Shanghai, China). Trypsin was purchased from invitrogen company (Shanghai, China). Giemsa staining solution was ordered from Solarbio company (Shanghai, China). 6-well plates were purchased from Coring Company.
Cell culture
The human nonsmall-cell lung cancer cell lines A549 and H1299, along with TC-1 and MLE-12 cells, were purchased from the cell bank of the Chinese Academy of Sciences. Cells were cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin.
Cell proliferation
The CCK-8 assays were conducted to evaluate the effect of Taraxasterol on A549 and H1299 cell proliferation. Briefly, cells were digested with 0.25% trypsin and resuspended in complete medium to prepare single-cell suspensions. The cells were then seeded into 96-well plates at a density of 6×103 cells per well. After the cells adhesion, Taraxasterol was added to culture medium for 24 h, 48 h and 72 h, alongside a control group. Absorbance (OD) at 450 nm was measured to assess the growth inhibitory effect of Taraxasterol on lung cancer cells.
Immunofluorescence
After washing with PBS, cells were fixed with 4% paraformaldehyde (PFA) and permeabilized with 0.2% Triton-X100. After blocking with BSA for 1 h, the cells were incubated with primary antibodies overnight at 4°C. On the second day, fluorescently labeled secondary antibodies was added and incubated at room temperature for 60 minutes. The cells were then stained with DAPI for 5 minutes and detected by an LSM 710 confocal microscope.
MTT assay
Cells were washed with PBS and treated with 1 mL of trypsin, monitored until cell rounding was observed. After suspending in fresh culture medium, the cells were seeded in 96-well plates, divided into control and drug-treated group and cultured in an incubator under 5% CO2 at 37°C. At specific time intervals, 10 μl of MTT solution was added followed by DMSO treatment. The ELISA reader determined the optical absorbance value (OD value) of each well.
Sa-β-gal staining
The cells were treated with H2O2 at concentrations ranging from 0 to 100 μM to establish a senescent cell model. After seeding the logarithmic phase cells in a 6-well plate, cells were incubated with different concentrations of H2O2 for two hours and cultured further for 24 hours. After washing, the cells were then fixed with PFA for 20 minutes, permeabilized with 0.1% Triton X 100 for 30 minutes, and stained with SPiDER-βGal working solution for 30 minutes at 37°C. Olympus FV1200 confocal microscope was employed to conduct the final examination.
Detection of inflammation and oxidative stress levels
The levels of oxidative stress markers (SOD, Cat.no. BC5165; MDA, Cat.no. BC0025; ROS, Cat.no. CA1410; GSH, Cat.no. BC1165) were detected by corresponding commercial kits (Solarbio biological company, Beijing, China). The levels of inflammatory factors IL-1β (Cat.no. SEKM-0002) and IL-6 (Cat.no. SEKM-0007) were detected according to the instructions of the ELISA kit (Solarbio biological company, Beijing, China).
Cell cycle detection by flow cytometry
Cell density was adjusted to 1×105-106 cells. After washing, cells were digested with trypsin and resuspended as single-cell suspensions by centrifugation at 1000 g for 5 minutes. The cells were then collected and fixed with the 70% ethanol. After another PBS wash, cell samples were incubated with RNaseA solution for 30 minutes. After washing, propidium iodide was added and incubated for 30 minutes. Flow cytometry analysis was conducted using a BD system.
Western-blot analysis
Cells were lysed by RIPA buffer and the cell lysate was collected. Proteins were separated by SDS-PAGE and transferred to PVDF membrane. The membrane was blocked with nonfat milk for 60 minutes and then incubated with diluted primary antibody at 4°C overnight. On the second day, the membrane was washed with TBST, treated with secondary antibodies. After incubated with ECL chromogenic solution, the membrane was exposed with a gel imager. The target proteins were quantified using imaging software.
Detection of cell apoptosis by flow cytometry
Cells were centrifuged and resuspended before being stained with Annexin V-FITC and PI. Apoptosis levels were assessed using a BD flow cytometer.
Experimental animals
SPF Balb/c Nude nude mice, aged 5-7 weeks old and weighing 16-22 g, were purchased from Beijing Saiye Experimental Animal Co., Ltd. Mice were kept in SPF-grade animal rooms with a constant temperature of 22-23°C and a 12 h light/dark cycle with ad libitum access to food and water. All procedures in this study were approved by the Ethics Committee of the Affiliated Hospital of Guangdong Medical University.
Tumor xenograft experiment
A549 cells were prepared and injected subcutaneously into nude mice (1×107 A549 cells in 200 μl PBS per mouse). The experimental group mice received 25 μg/ml of Taraxasterol in drinking water. Tumor growth was recorded every two days. Until the tumor reached a diameter of over 1 cm, approximately 30-40 days post cells injection. After the tumor was drawn, tumors were weighed, photographed, and divided into three parts: one for total RNA extraction, one for protein extraction (both preserved in liquid nitrogen), and the third for HE sectioning and immunohistochemistry, which was fixed in 4% PFA for half an hour.
Statistical analysis
All values are presented as mean ± standard error. Statistical analyses were performed using SPASS 20.0. One-way ANOVA and Student’s methods are used to determine significant differences, with a significance level set at P<0.05.
Results
Establishment of a model of lung cell senescence
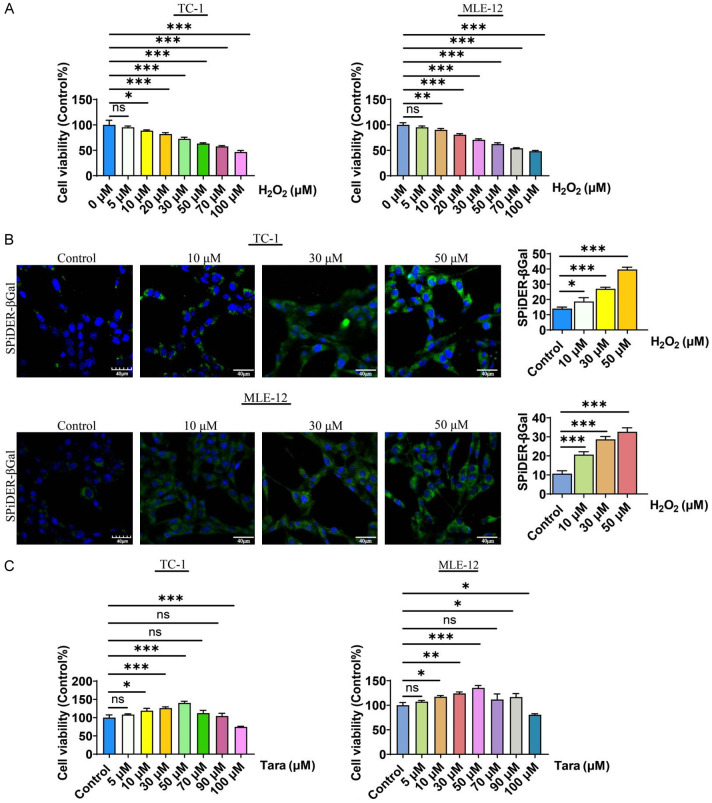
To evaluate the effects of Taraxasterol on lung cells aging, specifically in TC-1 and MLE-12 cells, our research firstly established a model of lung cell senescence by treating them with H2O2. To this end, we used different concentrations of H2O2 (0-100 μmol) to treat lung cells, and observed significant reductions in cell viability by MTT analysis (Figure 1A). In addition, there was a marked increase in the expression of the senescence marker SPiDER-βGal compared to the control group. Notably, the level of Sa-β-gal (SPiDER-βgal) increased in correlation with increasing H2O2 concentration (Figure 1B), indicating that H2O2 treatment successfully established a model of lung cell senescence. Based on these results, we selected 50 μM H2O2 to induce lung cell aging in the following experiments.
Figure 1.
Induction of cellular senescence in mouse lung TC-1 and MLE-12 cells. A. H2O2 treatment decreased the viability of TC-1 and MLE-12 cells in a dose-dependent manner as determined by MTT assay. B. H2O2 treatment increased the level of Sa-β-gal in TC-1 and MLE-12 cells. C. Dose response curve of Taraxasterol treatment in TC-1 and MLE-12 cells. P<0.05 indicates significant difference.
To investigate the potential of Taraxasterol in mitigating the aging process, we first evaluated its effects across a range of concentrations (1-100 μM) in the current work. The results demonstrated that Taraxasterol promoted cell proliferation at concentrations from 1 to 50 μM. However, when the concentration of Taraxasterol exceeded 50 μM, the proliferation of lung cells was partially inhibited (Figure 1C). Consequently, we selected 10, 30, and 50 μM concentrations of Taraxasterol for further investigation in our study.
Taraxasterol alleviated lung cell senescence
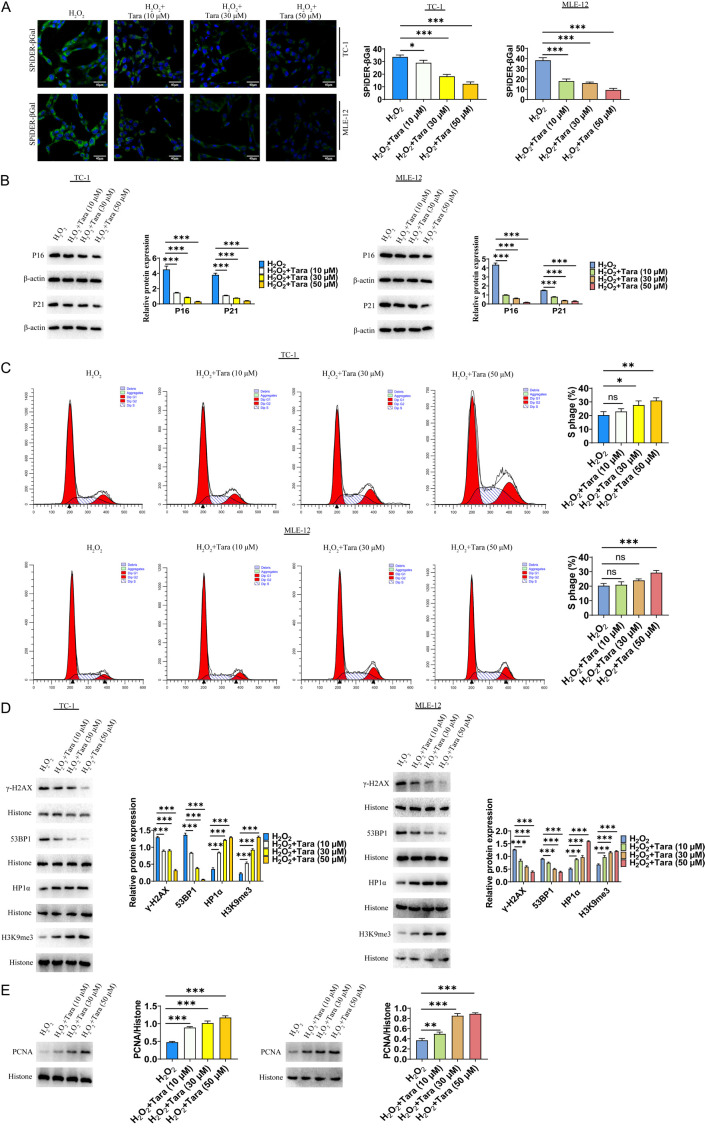
To evaluate the effect of Taraxasterol in mitigating lung cell senescence, we treated senescent lung cells with different concentrations of Taraxasterol. The results showed that the level of senescence was significantly reduced, as indicated by SpiDER-β-Gal staining (Figure 2A). Cell senescence is known to induce DNA damage and activate aging-related signaling molecules such as p16 and p21, leading to cell cycle arrest and further senescence. In this study, we found that 50 μM H2O2 markedly induced the expression of these senescence-associated proteins. In contrast, Taraxasterol treatment substantially reduced the expression of these markers by Western blot analysis (Figure 2B). Furthermore, flow cytometry analysis indicated that Taraxasterol significantly improved cell cycle (Figure 2C). We also analyzed the expression levels of DNA damage response markers (γ-H2AX), showing that Taraxasterol treatment significantly downregulated γ-H2AX expression. Further analysis of DNA damage response protein (53BP1) and heterochromatin markers (HP1α and H3K9me3) suggested Taraxasterol treatment decreased 53BP1 expression and increased HP1α and H3K9me3 expression (Figure 2D). Additionally, the PCNA expression significantly increased following Taraxasterol treatment (Figure 2E).
Figure 2.
Taraxasterol reduced the senescence of TC-1 and MLE-12 cells. A. Effect of Taraxasterol on cellular senescence determined by SpiDER-β-Gal staining. B. Effect of Taraxasterol on the expression of p16 and p21 determined by WB. C. Effect of Taraxasterol on cell cycle progression determined by Flow Cytometry. D. Effect of Taraxasterol on HP1α and H3K9me3 expression determined by WB. E. Effect of Taraxasterol on the PCNA expression determined by Western-blot. P<0.05 represents significant difference.
The effect of Taraxasterol on inflammation
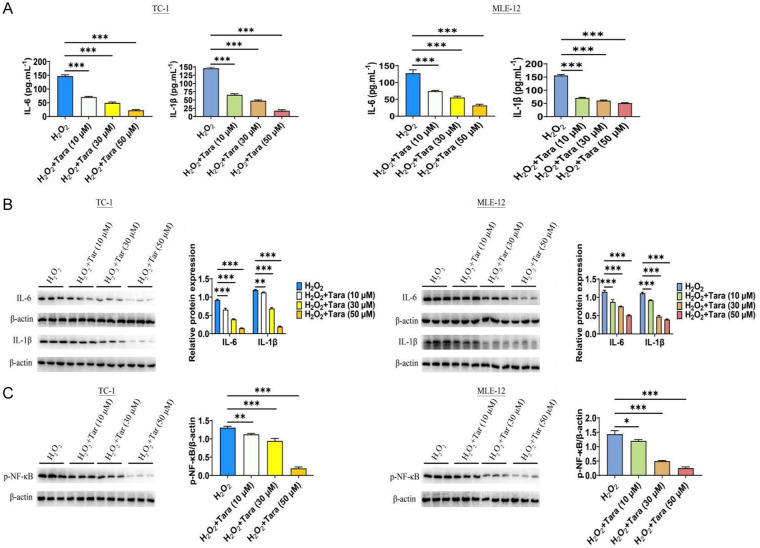
We also investigated the effect of Taraxasterol on inflammatory factors, specifically IL-6 and IL-1β. The results revealed that senescent cells exhibited significantly elevated levels of IL-6 and IL-1β. However, Taraxasterol treatment significantly decreased the secretion of IL-6 and IL-1β by ELISA analysis (Figure 3A). Western blot analysis further confirmed these results (Figure 3B). Building on these findings, we analyzed the effect of Taraxasterol on the NF-κB signaling pathway. The results showed that while the expression level of NF-κB was significantly increased in the senescent cell model, Taraxasterol significantly inhibited NF-κB activation (Figure 3C).
Figure 3.
Effect of Taraxasterol on the inflammatory pathway of TC-1 and MLE-12 cells. A. Taraxasterol decreased the secretion of IL-6/IL-1β as determined by ELISA analysis. B. Taraxasterol decreased the expression of IL-6/IL-1β as determined by WB. C. Taraxasterol inhibited p-p65 (phosphor-S536) level. P<0.05 represents significant difference.
The effect of Taraxasterol on oxidative stress
We explored the effect of Taraxasterol on cellular oxidative stress. Our results showed that Taraxasterol reduced the levels of reactive oxygen species (ROS) and malondialdehyde (MDA). Additionally, Taraxasterol increased the levels of superoxide dismutase (SOD) and glutathione (GSH) in cells. These experimental data indicated that Taraxasterol significantly alleviated oxidative stress in lung cells (Figure 4).
Figure 4.
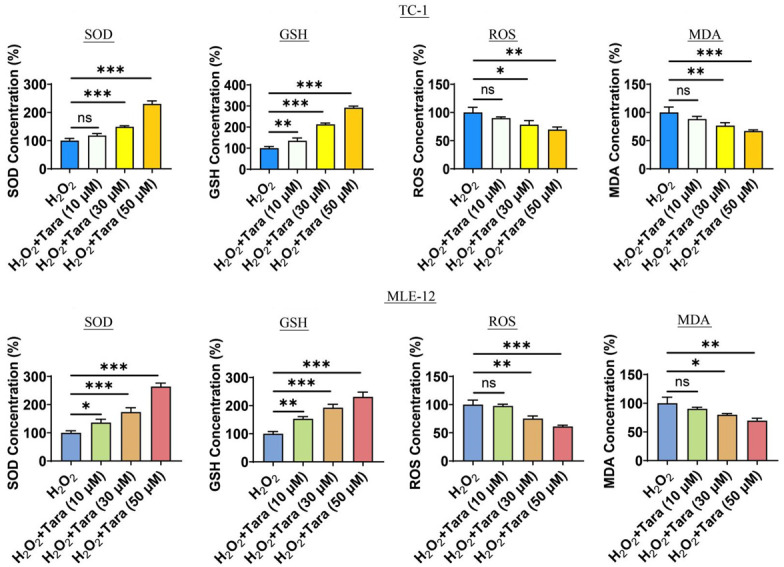
Effect of Taraxasterol on the oxidative stress of TC-1 and MLE-12 cells. The effects of taraxasterol treatment on SOD, GSH, ROS and MDA. P<0.05 represents significant difference.
Taraxasterol exerted the anti-aging effects through the PGC1α/NRF1 signaling pathway in lung cell models
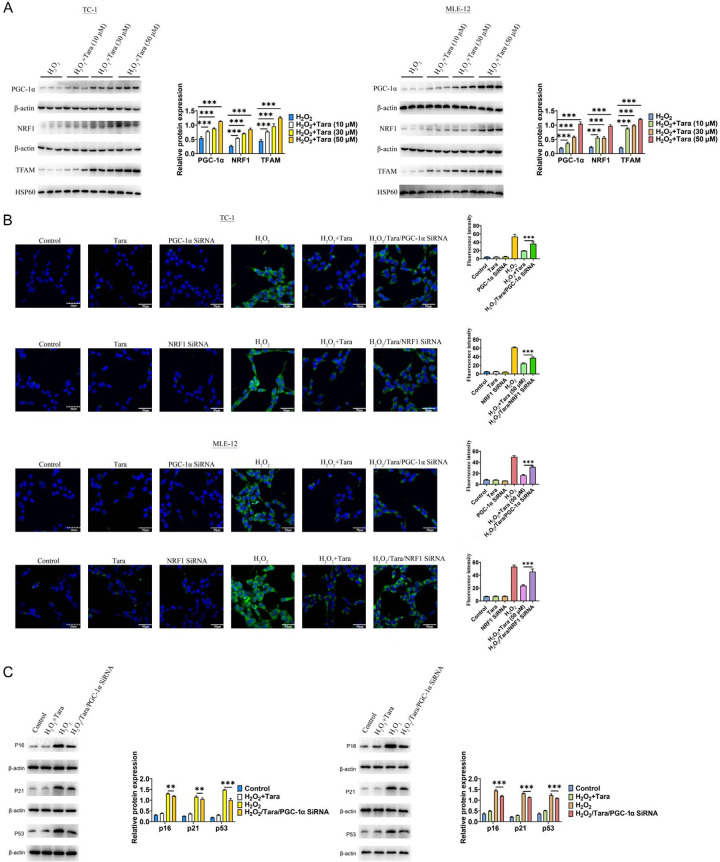
Mitochondria, critical organelles in cells, play a pivotal role in aging. One of the main functions of PGC-1α is to activate mitochondrial biogenesis and oxidative phosphorylation. Previous studies have reported that Taraxasterol exhibited the role of antioxidant stress [12,13]. In this context, our research focused on the PGC-1α/NRF1/TFAM signaling pathway, which is integral to the mitochondrial response to antioxidant stress. Initially, we observed that the expression of PGC-1α/NRF1/TFAM was significantly reduced in the senescent lung cell model. However, under Taraxasterol treatment, the expression levels of PGC-1α/NRF1/TFAM were significantly increased (Figure 5A). To further identify whether PGC-1α/NRF1 is involved in the anti-aging effect of Taraxasterol, we employed a knockdown approach and found that reducing PGC-1α or NRF1 (Supplementary Figure 1) neutralized the anti-aging effect of Taraxasterol, as evidenced by Sa-β-gal staining, confirming the critical role of this signaling axis (Figure 5B). Additionally, Western-blot analysis also corroborated these findings by detecting changes in p16, p21 and p53 (Figure 5C). These findings suggested that Taraxasterol alleviate cellular senescence, at least in part, by regulating the PGC-1α/NRF1 signaling pathway.
Figure 5.
The molecular mechanisms underlying the anti-aging effects of taraxasterol. A. Taraxasterol increased the expression level of PGC-1α/NRF1/TFAM. B. The anti-aging effect of Taraxasterol was mediated by PGC-1α/NRF1 signaling pathway. C. The expression level of p16/p21/p53. P<0.05 indicates significant differences.
Effect of Taraxasterol the proliferation of A549 and H1299 cells
Following our investigation into Taraxasterol’s effects on aging in lung cells, we shifted our focus to its impact on lung cancer cell proliferation. In order to study the effect of Taraxasterol on the proliferation of lung cancer, we selected two lung cancer cell lines H1299 and A549 for the following experiments. CCK-8 analysis showed that Taraxasterol concentrations ranging from 0-100 µM inhibited the proliferation of both A549 and H1299 cells in a concentration-dependent manner (Figure 6A). The IC50 value of Taraxasterol on A549 is 25.89 µM. Based on these findings, we choose 30 μM Taraxasterol for the subsequent experiments.
Figure 6.
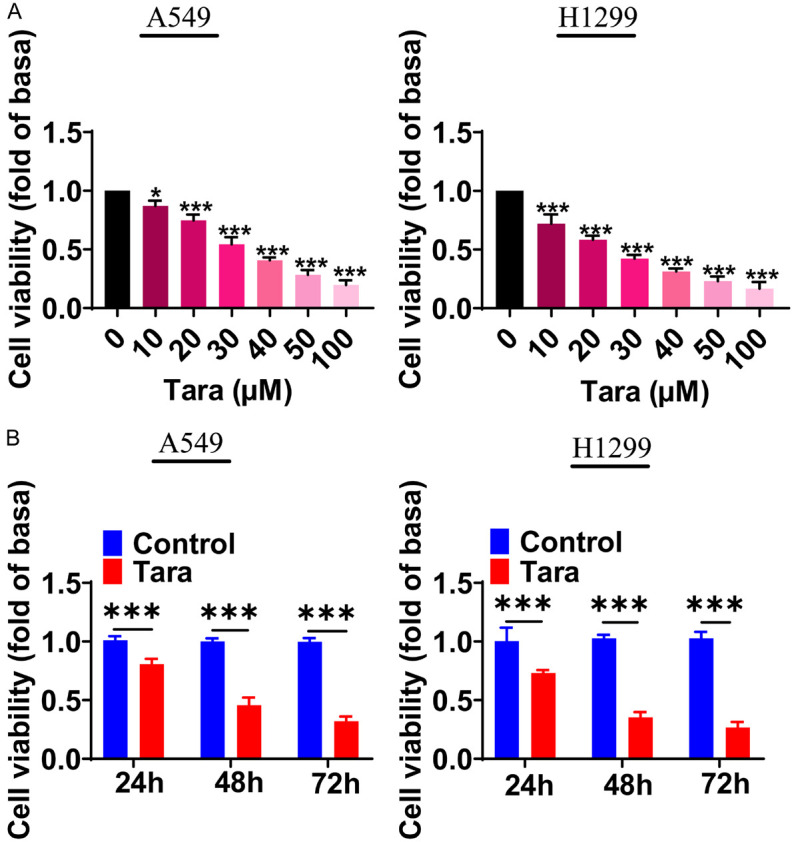
Effects of Taraxasterol on the proliferation of lung cancer A549 and H1299 cells. A. The effect of different concentrations of Taraxasterol on the proliferation of A549 and H1299 cells. B. Time course of taraxasterol-inhibited proliferation of A549 and H1299 cells. Cell proliferation was determined by CCK8 assay. *P<0.05, **P<0.01, ***P<0.001.
To further assess the effect of Taraxasterol on the proliferation of A549 and H1299 cells at different time points, the cells were treated with 30 μM of Taraxasterol for 0, 24, 48, and 72 h. CCK8 assays was used to detect the cell proliferation. The results were shown in Figure 6B, Taraxasterol treatment inhibited the proliferation of lung cancer cells at all tested each time points.
The effect of Taraxasterol on the apoptosis of lung cancer cells detected by flow cytometry
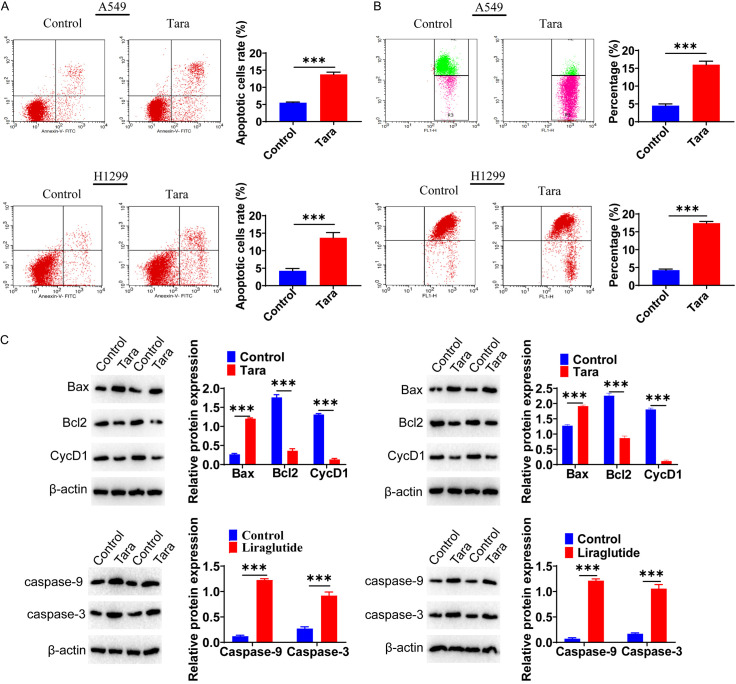
Flow cytometry was conducted to determine the effect of Taraxasterol on the apoptosis of lung cancer cells. Compared to the control group, the apoptosis rate of the Taraxasterol-treated group was significantly higher (Figure 7A). Furthermore, mitochondrial membrane potential was reduced under Taraxasterol treatment in both lung cancer cell lines (Figure 7B). We examined the expression of apoptosis-related molecules BCL2 and CyclinD1, which were down-regulated in the Taraxasterol-treated group, with a significant up-regulation of BAX. Caspase3 and Caspase9 expressions were also significantly up-regulated in the Taraxasterol-treated group (Figure 7C).
Figure 7.
A. Effect of Taraxasterol on the apoptosis of A549 and H1299 cells. Taraxasterol increased the apoptosis of A549 and H1299 cells as determined by Flow cytometry analysis. B. Taraxasterol caused a significant decrease in the mitochondrial membrane potential of A549 and H1299 cells. C. Effects of Taraxasterol on the expression of apoptosis-related molecules as determined by Western-blot analysis. *P<0.05, **P<0.01, ***P<0.001.
Taraxasterol treatment leaded to cycle arrest of lung cancer cells
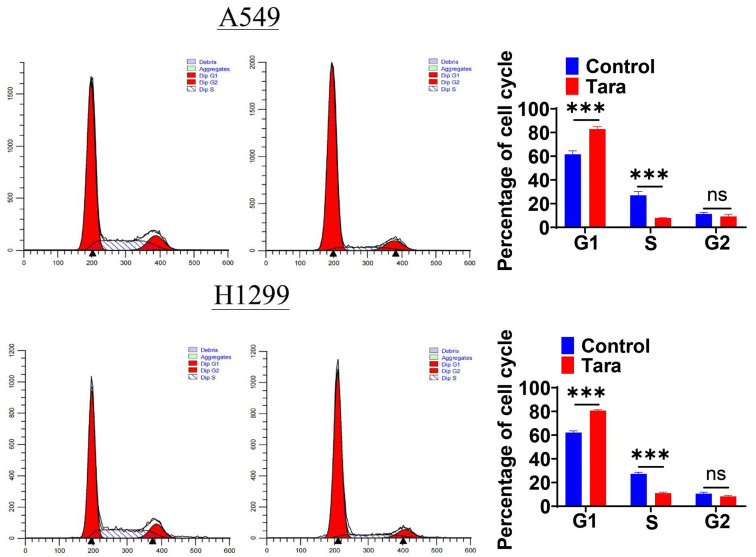
To determine whether the inhibitory effect of Taraxasterol on cancer cell growth was associated with cell cycle arrest, we performed cell cycle assays. Cells were stained with PI after Taraxasterol treatment to detected cell cycle. The results were shown in Figure 8: Post-Taraxasterol treatment, the percentage of cells in the G0/G1 phase significantly increased, with a corresponding decrease in the number of cells entering the S phase. Specifically, the proportion of A549 cells in the S phase decreased from 23.54±0.71% in the control group to 7.52±0.85% in the Taraxasterol group. For H1299 cells, the figures were 27.26±1.43% for the control and 11.05±1.26% for the taraxasterol-treated group. These findings indicates that Taraxasterol treatment could induce the cell-cycle arrest in the G0/G1 phase, preventing cells enter the S phase.
Figure 8.
Effects of Taraxasterol on the cell cycle progression of A549 and H1299 cells determined by flow cytometry. *P<0.05 indicates a significant difference.
Taraxasterol downregulated EGFR-mediated signaling
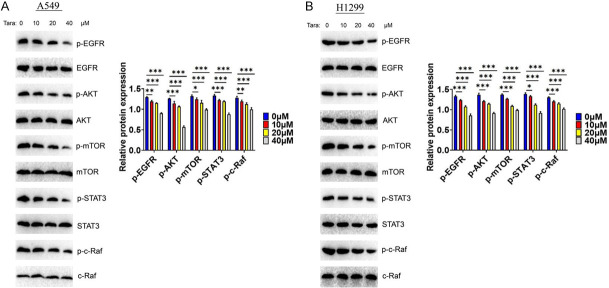
EGF/EGFR plays an important role in the development of lung cancer, providing an important anticancer drug target for lung cancer. Here, we analyzed the potential effect of Taraxasterol on the EGFR signaling. The cells were treated with Taraxasterol, showing that Taraxasterol significantly downregulated the expression of EGFR-mediated signaling, including EGFR, AKT, STAT3, m-TOR and c-RAF signaling (Figure 9A). A similar reduction was observed in H1299 (Figure 9B), suggesting that Taraxasterol inhibited the proliferation of lung cancer cells by targeting EGFR signaling pathway.
Figure 9.
Taraxasterol downregulates EGFR signaling intensity in A549 (A) and H1299 (B) cells. The expression levels of EGFR, AKT, STAT3, m-TOR and c-RAF were determined by WB.
Taraxasterol inhibited the lung cancer growth
In vitro experiments, we have proved that Taraxasterol could effectively inhibit the proliferation of lung cancer cell. To extend these findings, we studied whether Taraxasterol could also inhibit the growth of lung cancer in vivo. This experiment was divided into two groups: a control group and a Taraxasterol treatment group, where Taraxasterol was administered orally at a concentration of 30 μg/ml in drinking water for 34 days. Each group consisted of 6 nude mice, which were injected subcutaneously with 1×107 cells/mice. The tumor size was monitored every 2 days, and all nude mice were killed on the 34th day. Results indicated that the tumor growth rate of the Taraxasterol-treated group was significant slower and the tumor volume was notably smaller (P<0.05) (Figure 10A). Immunohistochemistry analysis revealed that Ki67 expression was significantly down-regulated in the Taraxasterol group (Figure 10B), while the expression of casapase-3 was significantly up-regulated (Figure 10C).
Figure 10.
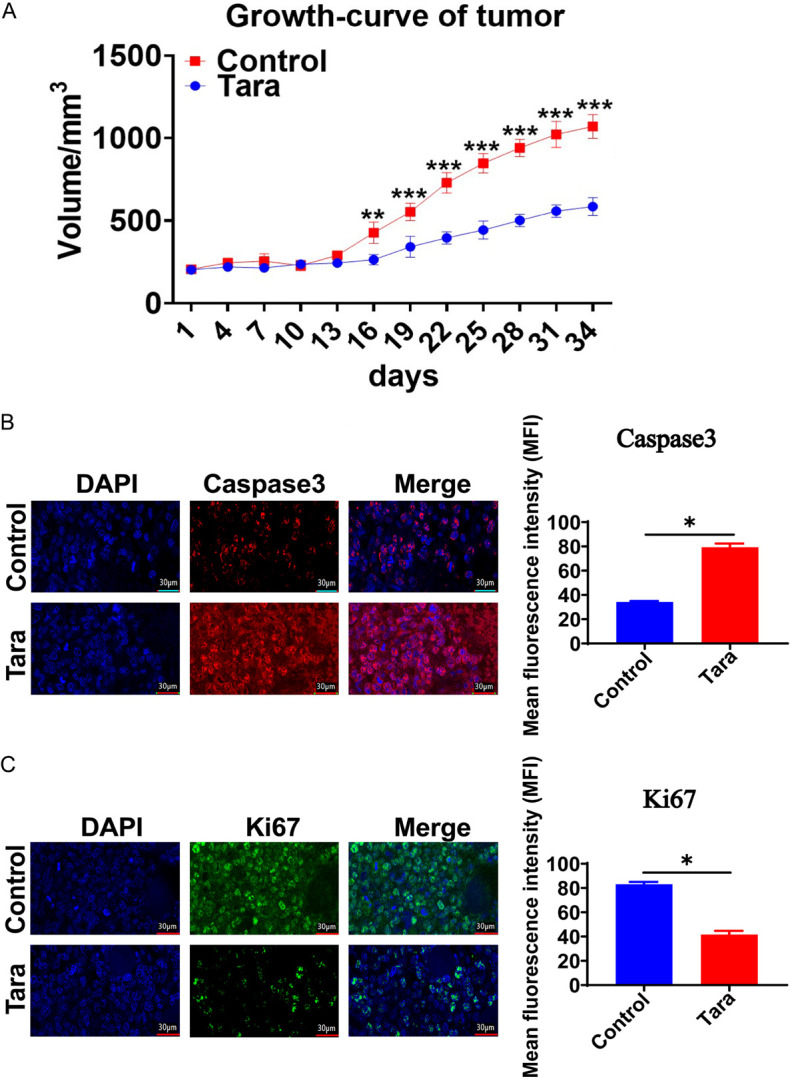
Taraxasterol inhibited the growth of lung cancer cells in vivo in a tumor xenograft model (A). (B) Taraxasterol treatment increased Caspase3 expression in tumor samples. (C) Taraxasterol treatment reduced Ki67 expression in tumor samples. *P<0.05 indicates a significant difference.
Discussion
Global aging is an irreversible trend, characterized by extended life expectancy and declining fertility rates [14]. This demographic shift is leading to an increase in the proportion of the elderly population, which in turn impacts global health and healthcare systems significantly. As the elderly population grows, the incidence of chronic diseases is also on the rise. Chronic diseases are long-term illnesses that usually require long-term treatment and management, such as chronic respiratory diseases. These diseases are more common in the elderly population and impose a huge burden on healthcare resources. Consequently, exploring anti-lung aging drugs is an important scientific issue.
Anti-aging molecules could potentially delay the aging process and improve the quality of life of the elderly. As people age, many elderly individuals face respiratory system decline. In our research, we conducted the relevant studies using lung cells as a model and found that Taraxasterol can effectively ameliorate lung cell aging by enhancing cellular activity and reducing the expression of aging marker. The overall experimental data suggest that Taraxasterol is able to alleviate senescence of lung cells.
Aging is closely related to oxidative stress [15], a condition where the balance between oxidants and antioxidants in the body is disrupted, leading to cellular damage. Lung cell aging is characterized by an irreversible growth cycle arrest accompanied by impaired cell function, leading to increase in reactive oxygen species (ROS) and the expression of aging-related molecules. When an animal’s body is exposed by harmful substances, it produces excessive ROS, exceeding the body’s clearance rate. As the respiratory system ages, the production of free radicals increases, while the body’s scavenging ability decreases. This leads to an accumulation of excessive free radicals, which exceeded the body’s repair and compensatory capabilities, exacerbateing aging-related DNA damage accelerating the deterioration of tissue and organ function. Here, we found that Taraxasterol can significantly alleviate oxidative stress in aging lung cells, as evidenced by the levels of ROS, MDA, and GSH. In addition, we also found that Taraxasterol can mitigate inflammatory responses in lung cells.
Aging is closely related to the occurrence and progression of lung cancer. Currently, chemotherapy remains as one of the main methods of lung cancer treatment [16], though surgery is the optimal treatment for early-stage lung cancer. Unfortunately, many patients with non-small cell lung cancer (NSCLC) are diagnosed too late for surgery to be an option [16]. In addition to traditional surgery, radiotherapy, and chemotherapy, biological therapy is emerging as a significant alternative due to the severe side effects and drug resistance of chemoradiotherapy, resulting in unsatisfactory treatment effects. In the current study, we found that Taraxasterol shows a good potential in the treatment of lung cancer both in vitro and in vivo, implying a potential application against lung cancer.
To demonstrate the anti-lung cancer effect of Taraxasterol, we utilized two cell lines, H1299 and A549. The results of CCK-8 and MTT showed that various concentrations of Taraxasterol effectively inhibited lung cancer cell proliferation. Furthermore, flow cytometry analysis revealed that Taraxasterol can induce lung tumor cell apoptosis. Previous research has also highlighted Taraxasterol has anti-tumor properties. For example, Bao et al reported that Taraxasterol could suppress liver cancer by blocking Hint1 expression [17]. Similarly, Taraxasterol also showed the antitumor effects on gastric cancer [18], further substantiating its potential as a broad-spectrum anticancer agent.
We also discovered that Taraxasterol can downregulate the EGFR signaling pathway [19], an important anticancer drug target [20,21]. Studies have shown that elevated EGFR signaling levels are associated with tumor metastasis and angiogenesis [22], promoting cell proliferation and tumor progression [23]. In our current study, Taraxerol treatment effectively downregulated EGFR-mediated signaling pathways, suggesting a potential molecular mechanisms for its anti-tumor activity. However, the specific molecular details of this interaction warrant further investigation. Recently, Hu et al reported that Taraxasterol suppresses lung cancer proliferation and alters the tumor microenvironment, marking an important advancement. Building on this, our research has identified novel aspects of Taraxasterol’s anti-cancer effects in lung cancer, including its potential to prevent lung cancer associated with aging. We have demonstrated that Taraxasterol possesses dual biological functions, enhancing its utility as a therapeutic agent [24].
Conclusion
This study systematically investigates the anti-aging and anti-tumor effect of Taraxasterol, revealing its dual biological function. The findings suggest that Taraxasterol has broad potential applications in treating and preventing lung cancer.
Acknowledgements
This study was supported by Zhanjiang Science and Technology Plan Project (2022A01192), and Young Scientific Research and Cultivation Fund of Guangdong Medical University (GDMUQ2021004).
Disclosure of conflict of interest
None.
Abbreviation
- Tara
taraxasterol
Supporting Information
References
- 1.Barnes PJ, Baker J, Donnelly LE. Cellular senescence as a mechanism and target in chronic lung diseases. Am J Respir Crit Care Med. 2019;200:556–564. doi: 10.1164/rccm.201810-1975TR. [DOI] [PubMed] [Google Scholar]
- 2.Parikh P, Wicher S, Khandalavala K, Pabelick CM, Britt RD Jr, Prakash YS. Cellular senescence in the lung across the age spectrum. Am J Physiol Lung Cell Mol Physiol. 2019;316:L826–L842. doi: 10.1152/ajplung.00424.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacco PC, Casaluce F, Sgambato A, Rossi A, Maione P, Palazzolo G, Napolitano A, Gridelli C. Current challenges of lung cancer care in an aging population. Expert Rev Anticancer Ther. 2015;15:1419–1429. doi: 10.1586/14737140.2015.1096201. [DOI] [PubMed] [Google Scholar]
- 4.Norouzi M, Hardy P. Clinical applications of nanomedicines in lung cancer treatment. Acta Biomater. 2021;121:134–142. doi: 10.1016/j.actbio.2020.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Pettit N, Al-Hader A, Thompson CA. Emergency department associated lung cancer diagnosis: case series demonstrating poor outcomes and opportunities to improve cancer care. Curr Probl Cancer Case Rep. 2021;3:100059. [Google Scholar]
- 6.Wang M, Herbst RS, Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med. 2021;27:1345–1356. doi: 10.1038/s41591-021-01450-2. [DOI] [PubMed] [Google Scholar]
- 7.Grant MJ, Herbst RS, Goldberg SB. Selecting the optimal immunotherapy regimen in driver-negative metastatic NSCLC. Nat Rev Clin Oncol. 2021;18:625–644. doi: 10.1038/s41571-021-00520-1. [DOI] [PubMed] [Google Scholar]
- 8.He J, Huang Z, Han L, Gong Y, Xie C. Mechanisms and management of 3rd-generation EGFR-TKI resistance in advanced non-small cell lung cancer. Int J Oncol. 2021;59:90. doi: 10.3892/ijo.2021.5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu J, Mei Q, Liu L, Cheng T, Wang P, Chen L, Zhou J. The progress and challenge of anti-PD-1/PD-L1 immunotherapy in treating non-small cell lung cancer. Ther Adv Med Oncol. 2021;13:1758835921992968. doi: 10.1177/1758835921992968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu W, Yu Q, Wang F, Li Y, Zhang G, Tao S. Taraxasterol attenuates melanoma progression via inactivation of reactive oxygen species-mediated PI3K/Akt signaling pathway. Hum Exp Toxicol. 2022;41:9603271211069034. doi: 10.1177/09603271211069034. [DOI] [PubMed] [Google Scholar]
- 11.Sharma K, Zafar R. Occurrence of taraxerol and taraxasterol in medicinal plants. Pharmacogn Rev. 2015;9:19–23. doi: 10.4103/0973-7847.156317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li C, Zheng Z, Xie Y, Zhu N, Bao J, Yu Q, Zhou Z, Liu J. Protective effect of taraxasterol on ischemia/reperfusion-induced acute kidney injury via inhibition of oxidative stress, inflammation, and apoptosis. Int Immunopharmacol. 2020;89:107169. doi: 10.1016/j.intimp.2020.107169. [DOI] [PubMed] [Google Scholar]
- 13.Ge B, Sang R, Wang W, Yan K, Yu Y, Kong L, Yu M, Liu X, Zhang X. Protection of taraxasterol against acetaminophen-induced liver injury elucidated through network pharmacology and in vitro and in vivo experiments. Phytomedicine. 2023;116:154872. doi: 10.1016/j.phymed.2023.154872. [DOI] [PubMed] [Google Scholar]
- 14.Jakovljevic M, Westerman R, Sharma T, Lamnisos D. Aging and global health. Handbook of Global Health. 2021:73–102. [Google Scholar]
- 15.Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mech Ageing Dev. 2004;125:811–826. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Ettinger DS. Ten years of progress in non-small cell lung cancer. J Natl Compr Canc Netw. 2012;10:292–295. doi: 10.6004/jnccn.2012.0029. [DOI] [PubMed] [Google Scholar]
- 17.Bao T, Ke Y, Wang Y, Wang W, Li Y, Wang Y, Kui X, Zhou Q, Zhou H, Zhang C, Zhou D, Wang L, Xiao C. Taraxasterol suppresses the growth of human liver cancer by upregulating Hint1 expression. J Mol Med (Berl) 2018;96:661–672. doi: 10.1007/s00109-018-1652-7. [DOI] [PubMed] [Google Scholar]
- 18.Chen W, Li J, Li C, Fan HN, Zhang J, Zhu JS. Network pharmacology-based identification of the antitumor effects of taraxasterol in gastric cancer. Int J Immunopathol Pharmacol. 2020;34:2058738420933107. doi: 10.1177/2058738420933107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gazdar AF. Personalized medicine and inhibition of EGFR signaling in lung cancer. N Engl J Med. 2009;361:1018–1020. doi: 10.1056/NEJMe0905763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seshacharyulu P, Ponnusamy MP, Haridas D, Jain M, Ganti AK, Batra SK. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:15–31. doi: 10.1517/14728222.2011.648617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lurje G, Lenz HJ. EGFR signaling and drug discovery. Oncology. 2009;77:400–410. doi: 10.1159/000279388. [DOI] [PubMed] [Google Scholar]
- 22.Quesnelle KM, Boehm AL, Grandis JR. STAT-mediated EGFR signaling in cancer. J Cell Biochem. 2007;102:311–319. doi: 10.1002/jcb.21475. [DOI] [PubMed] [Google Scholar]
- 23.Morello V, Cabodi S, Sigismund S, Camacho-Leal MP, Repetto D, Volante M, Papotti M, Turco E, Defilippi P. β1 integrin controls EGFR signaling and tumorigenic properties of lung cancer cells. Oncogene. 2011;30:4087–4096. doi: 10.1038/onc.2011.107. [DOI] [PubMed] [Google Scholar]
- 24.Lu J, Shuai B, Shou Z, Guo W, Zhou C, Ouyang X, Zhou H, Li J, Cui J, Jiang F, Jin KY, Sarapultsev A, Li F, Zhang G, Luo S, Hu D. Taraxasterol inhibits tumor growth by inducing apoptosis and modulating the tumor microenvironment in non-small cell lung cancer. Cancers (Basel) 2022;14:4645. doi: 10.3390/cancers14194645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.