Abstract
Human immunodeficiency virus (HIV) Nef downregulates the antigen recognition molecules major histocompatibility complex class I and CD4. Downregulation of surface CD4 by Nef relies on the ability of this viral protein to redirect the endocytic machinery to CD4. However, by redirecting the endocytic machinery, Nef may affect the internalization rates of other proteins. Here we show that Nef simultaneously enhances surface expression of the effector cytokines tumor necrosis factor (TNF) and LIGHT, leading to enhanced cytokine activity. A dileucine motif in Nef, which is essential for CD4 downregulation and is involved in the recruitment of adapter protein complexes by Nef, was required to increase surface levels of both cytokines. The physiological impact of the Nef-mediated interference with endocytosis was demonstrated by the fact that a TNF-responsive T-cell line chronically infected with HIV produced higher levels of p24 viral protein following expression of a Nef-green fluorescent protein (GFP) fusion protein. This enhancement was dependent on the levels of membrane-bound TNF, since it was abrogated by a recombinant soluble TNF receptor. Expression of Nef-GFP in human 293T cells reduced the endocytosis of LIGHT, whereas at the same time CD4 internalization was accelerated. Taken together, these results suggest that in infected cells Nef interferes with the internalization of these effector cytokines. By increasing TNF expression, Nef could accelerate disease progression in infected individuals. These findings may help explain the pleiotropic functions that Nef plays during infection and disease.
The nef gene of the human immunodeficiency virus (HIV) is critical for AIDS pathogenesis. Its importance is highlighted by the observation that some long-term survivors of HIV are infected with strains containing deletions or defective alleles of the nef gene (22). The role of Nef in AIDS pathogenesis has also been demonstrated by studies with animal models. Simian immunodeficiency virus (SIV) variants with Nef deletions show reduced levels of pathogenicity (21). In addition, transgenic mice expressing the Nef coding sequence of HIV type 1 (HIV-1) in CD4+ cells develop a severe AIDS-like disease which includes depletion of CD4+ cells, weight loss, wasting, and premature death (17).
Nef is a myristylated and phosphorylated 27-kDa protein abundantly expressed in cells early after infection. The nef gene is highly conserved in all primate lentivirus genomes (28, 37). Although Nef has been shown to have various functions in vitro (for a review, see reference 28), it is not yet clear how these functions contribute to the pathological properties of the virus in vivo. Nef downregulates the CD4 receptor and major histocompatibility complex (MHC) class I from the surfaces of infected cells (1, 13, 32, 34). Down-modulation of MHC class I protects infected cells from recognition by cytotoxic T lymphocytes (9), whereas the elimination of CD4 from the cell surface allows the synthesis of fully infectious HIV particles containing Env glycoproteins, a phenomenon that is blocked by CD4 (23). High levels of surface CD4 have also been shown to interfere with particle release from infected cells (31). Thus, downregulation of MHC class I and CD4 may play important roles in vivo, by allowing the virus to escape immune surveillance and by facilitating the spreading of HIV in infected individuals. The importance of the Nef-dependent CD4 downregulation is further emphasized by the fact that in infected cells the concerted, but mechanistically distinct, action of two other HIV genes, env and vpu, is required to ensure the complete elimination of CD4 from the cell surface (7). Nef also stimulates proviral DNA synthesis and enhances the infectivity of HIV particles by a CD4-independent mechanism (2, 33), and it modulates signals through the T-cell receptor (TCR) by interacting with protein kinases involved in signal transduction (3, 28).
Nef-mediated CD4 downregulation is a multistep process that has been delineated at the molecular level. Nef specifically connects the endocytic machinery of the cell with the cytoplasmic domain of CD4. The N-terminal domain of Nef is involved in binding to CD4 (15, 19), whereas a dileucine motif in the C-terminal region (LL164) is responsible for recruiting adapter protein (AP) complexes of the clathrin-coated pits (4, 11, 14). A third critical diacidic motif (EE155) is responsible for the interaction of Nef with the β-subunit of the COPI coatomer, targeting CD4 for lysosomal degradation and thus preventing its recycling to the plasma membrane (30). Nef is an abundant product in HIV-infected cells that could sequester components involved in endocytosis, thereby inhibiting the internalization of other cellular proteins. In this report, we demonstrate that expression of Nef in a CD4+ T-cell hybridoma cell line enhances the surface levels of the membrane-anchored cytokines tumor necrosis factor (TNF) and LIGHT, two members of the TNF family that activate NF-κB and c-Jun N-terminal kinase (JNK) pathways and that are involved in the regulation of immune functions (26, 39). The sustained expression of LIGHT caused by Nef is due to a reduced rate of internalization. Our findings reveal a novel role for Nef in infected cells and reinforce the need to develop strategies to target this protein for therapeutic intervention.
MATERIALS AND METHODS
Cells.
The CD4+ human T-cell hybridoma II-23.D7 has been described previously (38). The 293T cell line (human kidney embryonic fibroblast that expresses the large T antigen) was used for production of HIV vectors and HIV. ACH2 cells were obtained from the AIDS Research and Reference Reagent Program catalog. ACH2 is a CD4-negative T-cell clone chronically infected with the LAV allele of HIV-1. HIV expression is induced in these cells by treatment with phorbol myristate acetate (PMA) or TNF (12). Cell lines II-23.D7 and ACH2 were grown in RPMI medium containing penicillin, streptomycin, glutamine, HEPES, and 10% fetal bovine serum (FBS). 293T cells were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with the same reagents. Mononuclear cells from peripheral blood (PBMC) were purified from heparinized blood by centrifugation over a Ficoll-Hypaque (Pharmacia) gradient. After purification, cells were maintained in RPMI plus 10% fetal calf serum (FCS) and treated for 2 days with 5 μg of phytohemagglutinin (PHA)/ml. Cells were then maintained in the presence of 10 U of interleukin 2 (IL-2)/ml for no longer than a week.
Transfections, virus preparations, and infections.
II-23.D7 cells were transfected with 20 μg of plasmid DNA in 2-mm gap cuvettes with a BTX electroporation system set to deliver 30-ms pulses at 100 V. Generally 10 to 30% of the cells were transfected. 293T cells were transfected by the calcium-phosphate method. For production of HIV particles we used the proviral construct NL-GI or the Nef-defective NL-GI-Nef− (8). These constructs were kindly provided by G. Cohen (AIDS Research Center, Harvard Medical School). NL-GI derives from NL4-3 HIV-1 and contains all the HIV accessory genes in addition to an enhanced version of the green fluorescent protein (GFP) gene (8). Upon infection, productively infected cells are identified by their fluorescent signal. To make HIV particles, 293T cells were transfected with these proviral constructs, and 2 days after transfection, supernatant fluids were collected, filtered through 0.45-μm-pore-size units, and stored at −80°C. Production of HIV-based vectors carrying a reporter gene has been described previously (23). Briefly, 293T cells were cotransfected with a mixture of three plasmids: the GFP reporter plasmid, a packaging construct, and a plasmid expressing vesicular stomatitis virus glycoprotein (VSVg). HIV vectors were collected and filtered as described above for HIV particles. The amount of p24 antigen in supernatant fluids was used to normalize samples containing virus. The levels of p24 antigen were estimated by an enzyme-linked immunosorbent assay (DuPont). Infections with HIV or HIV-based vectors were performed following a previously described centrifugation method using 4 μg of Polybrene/ml (9). Infections were performed in 24-well plates with 0.4 × 106 (T-cell lines) or 1.5 × 106 PBMC per well. After infection, PBMC were treated for 24 h with 5 μg of PHA/ml.
Plasmids.
To synthesize HIV-based vectors, we used plasmids pMDG (encoding VSVg) and pHR′CMV-wGFP (encoding GFP from a plasmid containing a regulatory element from woodchuck hepatitis virus), as well as the packaging construct pCMVΔR8.2 (27) (provided by Didier Trono, University of Geneva). Human LIGHT was transiently expressed from pCDNA.LIGHT (26). CD4 and the CD884 chimeric proteins were expressed from plasmids derived from pCMX (23). Nef-GFP fusion proteins were expressed from pCG-derived plasmids (15) (provided by Jacek Skowronski, Cold Spring Harbor, N.Y., and John Guatelli, University of California, San Diego). The Nef-GFP NA7 allele was PCR amplified from the pCG plasmid and subcloned into the XhoI-BamHI sites of the pHR′CMV-w vector by standard cloning procedures. The nucleotide sequence of a mutated form of GFP was added to the 3′ end of the full-length nef sequence (NA7 natural isolate). This Nef-GFP protein retains the ability to down-modulate CD4 and MHC class I, and to block signaling through the TCR-CD3 complex (15). Upon expression of the fusion protein, transfected cells can be identified by their GFP fluorescence.
Flow cytometry, antibodies, and recombinant proteins.
Labeled cells were routinely fixed with 1% paraformaldehyde after staining and were analyzed in a FACScalibur system running with the CellQuest software (Becton Dickinson). Membrane-bound TNF levels were detected with the anti-TNF (104C) monoclonal antibody (MAb) (6), followed by staining with goat anti-mouse Cy5-conjugated antibodies (CalTag). Surface expression of LIGHT was determined using an HVEM-Fc recombinant protein. In this protein the extracellular domain of the LIGHT receptor HVEM has been fused to the Fc domain of human immunoglobulin G (IgG). HVEM-Fc was expressed in insect cells infected with a baculovirus vector and purified from supernatants in a one-step protein A/G chromatography column (26). After incubation with HVEM-Fc, cells were stained with goat anti-human IgG conjugated to Cy5 (Chemicon). A TNF receptor 1 (TNFR1)-Fc recombinant protein with the extracellular domain of TNFR1 fused to the Fc domain of human IgG was expressed and purified in the same way. Recombinant TNF was purchased from R&D Systems. Antibodies specific for CD69, Fas (clone DX2), and transferrin receptor (CD71) (clone TRAP1) were all purchased from Pharmingen; a MAb against TNFRI (clone H398) was purchased from Bender MedSystems; a lymphotoxin β (LT-β) MAb (clone B9) was generously provided by J. Browning (Biogen), and the MAbs against CD4 (OKT4) and CD8 (B9) were routinely purified in our laboratory from precipitates of ascites fluids saturated with ammonium sulfate; antibodies against CD30 were obtained from Serotec. Detection of the p24 capsid antigen was performed with the Kal-1 MAb from Dako. Intracellular staining was performed with IntraStain (Dako) according to the instructions provided by the manufacturer.
Endocytosis assay.
A mixture of LIGHT, CD4, and either wild-type Nef-GFP or the LL-to-AA mutant defective in binding to adaptins (1, 3, and 20 μg, respectively) was used to transfect 293T cells. Two days later, the cells were washed with phosphate-buffered saline (PBS)–5 mM EDTA and removed from the plate with a cell scraper. Cells were incubated either with a Cy5-conjugated CD4 MAb (Dako) or with HVEM-Fc and Cy5-conjugated goat anti-human antibodies. Antibodies were allowed to bind for 30 min at 4°C in DMEM plus 2% FCS. Unbound antibody was removed by washing with medium, and the cells were incubated at 37°C for various times to allow internalization of the bound antibody. After incubation, extracellular antibody was removed by treatment with 10 volumes of PBS-HCl (pH 2) for 2 min at 4°C. The acid was neutralized with the same volume of PBS-NaOH (pH 13), and then the cells were fixed with 1% paraformaldehyde and analyzed by flow cytometry. Total bound antibody was estimated by diluting the cells at time zero with 10 volumes of PBS (pH 7.4), and the background levels were estimated by treating the cells with acid prior to incubation at 37°C. The fraction of antibody internalized was calculated by subtracting the mean fluorescence of the initial time zero wash (background) from all values and then dividing the mean fluorescence of the acid wash by the mean fluorescence of the total bound antibody (24).
RESULTS
A Nef-GFP fusion protein enhances surface expression of TNF and LIGHT.
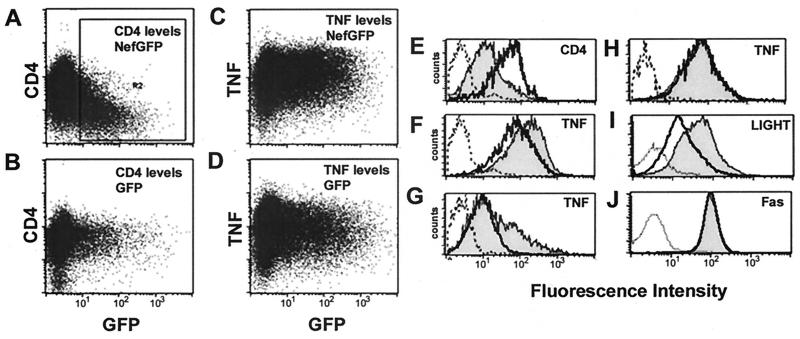
In an effort to identify new proteins modulated by Nef, we used a fluorescence-activated cell sorter (FACS)-based assay to analyze expression of cell surface molecules. Electroporation was used to introduce the Nef-GFP plasmid into II-23.D7 cells. CD4 surface expression decreased four- to fivefold in those cells actively expressing a Nef-GFP fusion protein (Fig. 1A and E). Upon activation with PMA and ionomycin, cell surface levels of TNF were increased twofold in II-23.D7 cells expressing the Nef-GFP fusion protein, compared to that in cells transfected with a GFP control protein (Fig. 1C, D, and F) or untransfected cells (GFP negative) (data not shown). These differences were statistically significant (t-test; P < 0.05). Expression of membrane-bound TNF was significantly sustained 24 h after the addition of PMA-ionomycin (51% of the Nef-GFP-transfected cells expressed TNF versus 26% of GFP-transfected cells) (Fig. 1G). The Nef-dependent modulation of TNF required a functional myristylation signal in the N terminus of the Nef protein, since a Nef-GFP version with a hemagglutinin (HA) tag in the N terminus did not increase membrane-bound TNF levels (Fig. 1H). Nef-GFP did not induce TNF surface expression in the absence of PMA-ionomycin, although similar increases in surface levels of TNF could be observed under a variety of conditions known to induce synthesis of this protein (data not shown). TNF is the prototypical member of a large superfamily of type II transmembrane proteins (39). To determine whether the Nef-mediated up-modulation of TNF is a specific phenomenon, surface levels of other proteins were analyzed in the presence of Nef-GFP or GFP. Surface levels of LIGHT, a new member of the TNF/TNFR superfamily that interacts with the herpesvirus entry mediator (HveA) and the LT-β receptor (LTβR) (26, 35), increased 290% in cells expressing Nef-GFP, compared to cells expressing GFP alone (Fig. 1I). Surface expression of TNF was increased 190% in the same experimental setting (Fig. 1F), whereas CD4 surface levels in cells expressing Nef-GFP was decreased to 23% (as estimated from their mean fluorescence). Nef-GFP did not significantly change surface levels of LT-β, CD30, Fas, TNFRI, CD69, or transferrin receptor (Fig. 1J and data not shown). These results suggest that overexpression of Nef-GFP causes a selective increase in surface levels of TNF and LIGHT.
FIG. 1.
Expression of Nef-GFP in T cells up-modulates TNF and LIGHT. (A through D) II-23.D7 cells were electroporated with 20 μg of a plasmid expressing either the wild-type NA7 nef gene with the GFP sequence fused to its 3′ region (Nef-GFP) (A and C) or GFP alone (B and D). Twenty-four hours after electroporation, cells were treated with 25 ng of PMA/ml plus 250 ng of ionomycin/ml (C and D) or were mock activated (A and B). Five hours later, the surface levels of CD4 (A and B) and membrane-bound TNF (C and D) were estimated by flow cytometry with specific antibodies. GFP fluorescence levels are shown along the x axis, whereas CD4 or TNF levels are shown along the y axis. (E through J) Histograms comparing surface levels of CD4 (E), membrane-bound TNF (F through H), LIGHT (I), and FAS (J) between cells transfected with GFP vectors (heavy solid lines) or Nef-GFP vectors (shaded curves). Transfected cells (region R2 in panel A) were revealed by their GFP-positive signal. Staining with isotype-matched control antibodies is shown by dashed lines. (E) CD4 surface levels in mock-activated cells; (F and G) membrane-bound TNF levels in cells activated for 5 or 24 h, respectively; (I and J) LIGHT and Fas surface levels, respectively, after 5 h of activation. In panel H, cells transfected with a nonmyristoylated version of Nef-GFP (HA-Nef-GFP) or GFP were analyzed after 5 h of activation.
Cell surface expression of TNF and LIGHT is increased in T cells infected with HIV containing a functional nef gene.
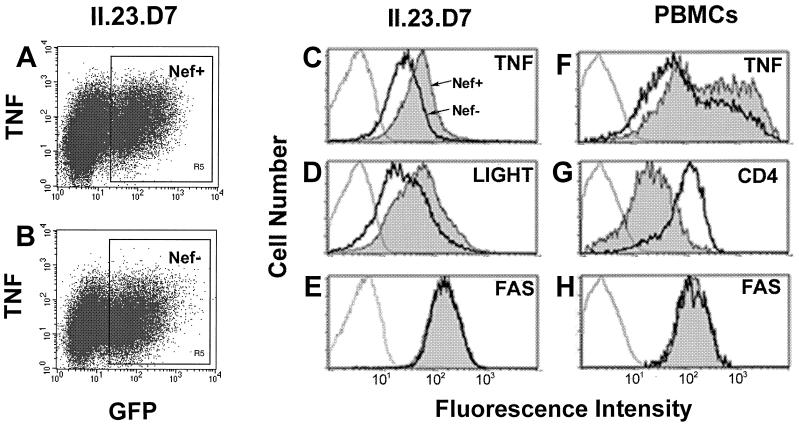
We wanted to test if the observed changes occur in HIV-infected T cells. II-23.D7 cells were infected with an HIV strain (NL4-3) carrying a GFP reporter gene (8). Cells were infected with wild-type or Nef-deficient versions of this virus, and the levels of TNF and LIGHT in GFP-positive infected cells were estimated (Fig. 2). Cells infected with either virus showed similar levels of GFP fluorescence (Fig. 2A and B). Upon activation, cells infected with virus containing a functional nef gene showed twofold increases in the surface levels of both TNF and LIGHT (Fig. 2C and D, respectively). As a control, expression of the surface protein Fas, a member of the TNFR superfamily, remained unaltered in cells infected with wild-type or Nef-deficient virus (Fig. 2E). Thus, the nef gene is responsible for the observed increase in surface levels of TNF and LIGHT in infected cells. The increase in TNF surface expression induced by Nef was also observed in infected PBMC (Fig. 2F). Surface levels of TNF were 2.5-fold higher in cells infected with wild-type virus than in cells infected with a Nef-deficient version, as determined by the mean value of the fluorescence intensity. CD4 surface levels were decreased fivefold under the same conditions, whereas Fas levels remained unchanged.
FIG. 2.
Up-modulation of TNF and LIGHT in HIV-infected T cells. II-23.D7 cells (A through E) or PBMC (F through H) were infected with an HIV strain, NL4-3, carrying an enhanced GFP gene (Nef+; filled histograms) or with a mutant version lacking the nef gene (Nef−; heavy solid lines). Twenty-four hours after infection, cells were activated with 50 ng of PMA/ml plus 50 ng of ionomycin/ml for 20 h (in the presence of 30 μM TAPI when membrane-bound TNF was analyzed). Cells were stained with a MAb specific for TNF (A through C and F), Fas (E and H), or CD4 (G), or with HVEM-Fc (D), and were then subjected to flow cytometry. Histograms C through H represent surface levels in GFP-positive infected cells (R5 region, as shown in panel A). Light lines represent staining with isotype-matched antibodies.
Nef-GFP enhances HIV expression in a chronically infected T-cell line.
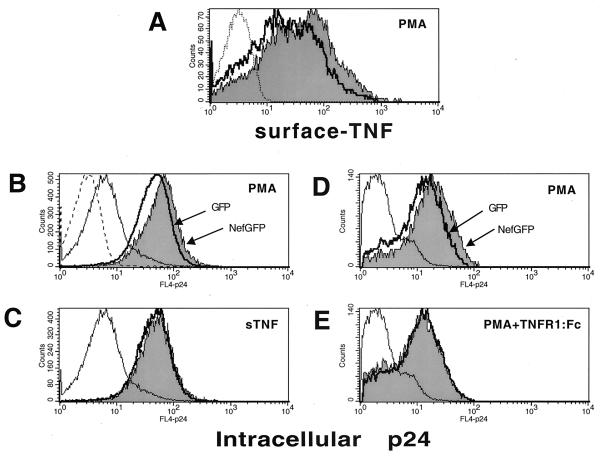
The physiological consequences of TNF modulation during HIV infection are complex. TNF is a potent activator of HIV transcription (12), and it would be expected that high levels of TNF could accelerate virus spread and disease progression. We decided to investigate whether Nef-mediated changes in TNF expression could affect the rate of HIV production. We used a T-cell clone (ACH2) chronically infected with HIV. These cells respond to TNF or PMA treatment by inducing HIV expression, which results in higher levels of viral proteins in supernatant fluids. HIV vectors encoding Nef-GFP (Fig. 3) were used to infect ACH2 cells. Expression of Nef-GFP from these vectors is sufficient to downregulate CD4 (data not shown). TNF surface levels were increased 82% in PMA-treated cells transduced with a Nef-GFP vector (Fig. 3A). The level of HIV expression in these cells was assessed by flow cytometry after intracellular staining with a p24-specific MAb. As shown in Fig. 3B, treatment of cells with PMA induced a 10-fold increase in the amount of intracellular p24 antigen. PMA-treated cells transduced with a Nef-GFP vector expressed higher levels of p24 (39% increase in fluorescence mean value) than cells expressing GFP alone. Like PMA, treatment with TNF induced a similar increase in p24 expression; however, no significant differences were observed between cells expressing Nef-GFP or GFP alone, suggesting that expression of TNF receptors and other proteins involved in the cascade signaling that induces HIV transcription is not altered. In the experiment for which results are shown in Fig. 3E, ACH2 cells were treated with PMA in the presence of TNFR1-Fc, a recombinant TNFRI soluble protein that abrogates TNF-mediated effects. Under these conditions no changes in p24 content were observed between Nef-GFP- and GFP-expressing cells (compared with Nef-GFP-expressing cells treated with PMA alone, showing a 51% increase in p24 expression [Fig. 3D]). These results suggest that the increase in p24 expression observed in PMA-treated cells expressing Nef-GFP is partially due to induction of TNF and can therefore be blocked by the addition of soluble TNF receptor.
FIG. 3.
Nef enhances HIV expression in chronically infected T cells. ACH2 cells latently infected with HIV-1 were transduced with a VSVg-pseudotyped HIV vector encoding either a Nef-GFP fusion protein or GFP alone. After infection, cells were treated for 48 h with either 10 ng of PMA/ml (A, B, and D), 1 nM TNF (C), or 10 ng of PMA/ml in the presence of 25 μg of TNFR1-Fc/ml (E) and were then subjected to flow cytometry after surface staining with a TNF-specific MAb (A) or intracellular staining with a p24-specific MAb (B through E). Histograms represent TNF or p24 levels in cells infected (GFP positive) with a Nef-GFP vector (shaded areas) or a GFP vector (heavy solid lines). p24 levels in untreated cells infected with a Nef-GFP vector are indicated by thin lines. Staining with isotype-matched antibodies for TNF and p24 is shown in panels A and B, respectively (dashed lines). Histograms in panels A through C and those in panels D through E represent data from two independent experiments which were analyzed with different flow cytometry settings.
A Nef protein lacking the dileucine motif involved in binding to adaptin fails to up-modulate TNF.
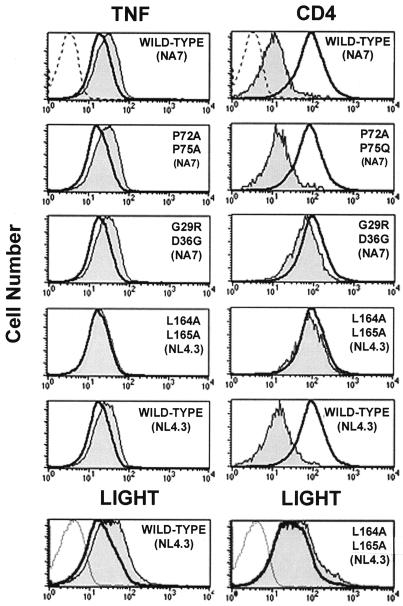
In order to understand the mechanism of action of Nef, a panel of Nef-GFP mutants was tested on II-23.D7 cells. After electroporation, cells either were mock treated and analyzed for surface CD4 or were activated with PMA-ionomycin and the membrane-bound TNF levels were estimated (Fig. 4). A double mutant (P72A P75A) defective in its ability to downregulate MHC class I but fully capable of reducing surface levels of CD4 (15) increased TNF levels. A double mutant (G29R D36G) that abolishes CD4 downregulation without impairing its effect on MHC class I (15), and which presumably disrupts the CD4 binding domain, was also active in increasing membrane-bound TNF levels. These findings suggest that interaction of Nef with CD4 is not required to enhance TNF surface levels. However, a third mutant lacking the dileucine motif implicated in binding to the clathrin AP complexes (11) neither increased TNF surface levels nor significantly downregulated CD4. These findings demonstrate that binding of Nef to the clathrin AP complex is required for Nef-dependent enhancement of TNF. The Nef-mediated enhancement of LIGHT was also dependent on the presence of a functional dileucine motif (Fig. 4, bottom panels).
FIG. 4.
Mutational analysis of Nef-induced TNF up-modulation. II-23.D7 cells were electroporated with 20 μg of pCG plasmids encoding either wild-type or mutant versions of a Nef-GFP fusion protein. After 24 h, cells were either mock treated and stained with a CD4-specific MAb (top right panels), activated with PMA-ionomycin for 5 h and analyzed with a TNF-specific MAb (top left panels), or activated with PMA-ionomycin for 20 h and analyzed with HVEM-Fc (bottom panels). Histograms represent cell surface levels of TNF, CD4, or LIGHT in transfected (GFP-positive; shaded areas) or untransfected (GFP-negative; heavy solid lines) cells. Staining controls with isotype-matched antibodies are indicated by light lines.
A Nef-GFP fusion protein inhibits endocytosis of LIGHT.
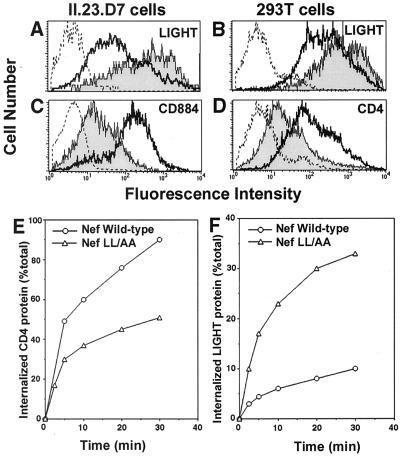
A possible explanation for the findings of this study is that Nef competes for essential endocytosis factors and thereby interferes with the internalization of other cellular proteins. Such a hypothesis predicts that modulation of TNF and LIGHT by Nef would take place at the posttranscriptional level by interfering with their rates of internalization. To rule out Nef-mediated transcriptional effects, we analyzed the surface expression of LIGHT driven from a heterologous promoter. Human LIGHT was expressed from a pCDNA plasmid introduced into II-23.D7 cells by electroporation. pCDNA.LIGHT was cotransfected with either pCG.Nef-GFP or pCG.GFP, and surface levels of LIGHT were analyzed in transfected cells. LIGHT was expressed at higher levels in cells cotransfected with Nef-GFP than in cells expressing GFP (Fig. 5A). In contrast, a chimeric molecule composed of the extracellular and transmembrane domains of CD8 and the Nef-responsive cytoplasmic domain of CD4 was downregulated as expected (Fig. 5C). Similar results were found for a human fibroblast kidney cell line (293T) (Fig. 5B and D). LIGHT surface levels were increased, whereas transiently expressed CD4 was downregulated in the same cells. To ascertain whether Nef was blocking the endocytosis of LIGHT, 293T cells were transfected with a mixture of plasmids encoding LIGHT, CD4, and either wild-type Nef or the LL-to-AA mutant version, which is unable to trigger CD4 endocytosis. Internalization rates of both CD4 and LIGHT were measured by a FACS-based method (29). As shown in Fig. 5E, wild-type Nef accelerated the rate of endocytosis of CD4, compared to that with the defective mutant lacking the dileucine motif. On the other hand, wild-type Nef reduced the endocytosis rates of LIGHT in the same cells (Fig. 5F). Similar reductions in LIGHT internalization rates were observed when the experiment was repeated in the absence of CD4 (data not shown). These findings suggest that the observed increase in surface levels of LIGHT can be explained as a reduction in its rate of endocytosis. Attempts to analyze the endocytosis rates of membrane-bound TNF using this system were uninformative, due to the fact that most of the surface TNF is cleaved and released in the supernatant fluid.
FIG. 5.
Nef blocks endocytosis of LIGHT. (A and C) II-23.D7 cells were electroporated with a mixture containing pCG.Nef-GFP or pCG.GFP (10 μg) and a plasmid encoding human LIGHT (pCDNA.LIGHT; 25 μg) (A) or CD884 (C). CD884 is a chimeric protein containing the extracellular and transmembrane domains of CD8 fused to the cytoplasmic domain of CD4. After 48 h, cells were stained with specific antibodies or recombinant Fc proteins and were subjected to flow cytometry. The histograms show the cell surface levels of these proteins in GFP-positive cells transfected with Nef-GFP (shaded curves) or GFP (heavy solid lines). (B and D) 293T cells were transfected with a mixture containing 20 μg of either Nef-GFP or GFP, together with 5 μg of pCMX.CD4 (encoding CD4) and 5 μg of pCDNA.LIGHT. After 36 h, cells were stained with either HVEM-Fc (B) or a CD4-specific MAb (D) and were analyzed by flow cytometry as explained for panels A and C. (E and F) 293T cells were cotransfected with a mixture containing pCMX.CD4 (3 μg), pCDNA.LIGHT (1 μg), and either wild-type Nef-GFP or the LL-to-AA version (NL4.3 allele; 20 μg). After 48 h the internalization rates of CD4 and LIGHT were estimated by a FACS-based assay as described in Materials and Methods.
DISCUSSION
One of the steps involved in recruiting endocytosis factors by Nef relies on the interaction of the viral protein with AP complexes, which in turn triggers the de novo formation of clathrin-coated pits specific for CD4 (29). We hypothesized that in infected cells, the recruitment of AP complexes by the abundantly expressed Nef protein might decrease the endocytosis rates of other cellular proteins internalized by a clathrin-dependent mechanism. We report here that this is indeed the case with the TNF and LIGHT cytokines. Surface levels of these proteins were increased two- to fourfold under conditions that downregulated CD4 fivefold. The modulation of LIGHT by Nef appeared to be at the posttranscriptional level, since it was observed when LIGHT was expressed from a heterologous promoter in either T cells or fibroblasts. Furthermore, Nef-GFP induced a drastic reduction in the internalization rates of LIGHT. Although the signals that make TNF and LIGHT responsive to Nef have not yet been identified, modulation of cell surface proteins by Nef does not appear to be a general phenomenon. Out of eight tested proteins expressed on the surfaces of T cells, only TNF and LIGHT were found to be significantly increased by the viral product.
The physiological consequences of TNF modulation during HIV infection are complex. TNF is an important mediator of wasting in infections (36) and may contribute significantly to the pathological manifestations of the AIDS-like disease observed in transgenic murine models and in infected patients (10, 17, 18). It is interesting that TNF levels are elevated in the sera of infected patients, and high serum TNF levels have been proposed as a marker for disease progression (5, 20). However, it remains unclear whether elevated TNF levels are the consequence of a specific viral product (e.g., Nef) or rather represent a general state of immune activation against HIV. TNF is also a potent activator of HIV transcription (12), and it would be expected that high levels of TNF in serum accelerate virus spread and disease progression. We have shown that Nef-GFP introduced into a chronically infected T-cell line which responds to TNF and PMA resulted in increased HIV expression. Although a 40 to 50% increase in TNF levels (observed upon expression of Nef in latently infected T cells) would have a minor effect on a single-replication-cycle basis, even smaller changes in the replication potential of HIV could exert a dramatic effect on the propagation of the virus in infected individuals. It should also be noted that the latent virus present in ACH2 cells encodes a functional nef gene (12), which must also contribute to the observed increase in TNF expression and partially mask the effects due to overexpression of Nef-GFP. Increased surface expression of TNF might enhance HIV transcription in a paratropic or autotropic fashion. Our experiments do not distinguish between these two possibilities. Addition of TNFR1-Fc recombinant protein would abrogate the TNF-mediated effect in both cases. Furthermore, it is likely that soluble TNF cleaved off from the cell surface by specific metalloproteinases also contributes to the enhancement of HIV expression. It is noteworthy that upregulation of TNF would make HIV-infected cells more susceptible to TNF-mediated induction of cell death. Interestingly, it has been shown that Nef negatively regulates TNF- and Fas-mediated apoptosis by inhibiting the cellular kinase ASK1 (W. C. Greene, personal communication). Therefore Nef would be exerting a dual effect, increasing HIV replication by enhancing surface expression of TNF and protecting against the deleterious effects that this protein would induce in infected cells.
The role of LIGHT during HIV infection remains unknown. LIGHT is a new member of the TNF superfamily that was identified as the ligand for HveA, an entry factor for α-herpesvirus in T lymphocytes and dendritic cells (26). LIGHT also binds the LTβR, and signaling through this receptor has been shown to stimulate HIV replication by itself or in cooperation with TNF (25). Whether LIGHT induces HIV expression through engagement of the LTβR or HveA is an interesting possibility that is currently being tested in our laboratory.
Future research should focus on studying how Nef affects the endocytosis of cellular proteins in peripheral blood lymphocytes and macrophages, which are natural targets for HIV infection, and on identifying the signals which make the internalization of these proteins responsive to Nef. It is also evident that the growing list of in vitro functions of Nef and their possible implications for AIDS pathogenesis make this protein an attractive target for therapeutic intervention.
ACKNOWLEDGMENTS
We thank Jacek Skowroski, Didier Trono, John Guatelli, George Cohen, and Jeffrey Browning, who kindly provided various reagents used in this work. We are indebted to Theresa Banks and Chris Benedict for critical reading of the manuscript. Thanks to Cheryl McLaughlin for graphics assistance.
This work was supported by grants to J. L. from the Center for AIDS Research at UCSD (NIH-funded program P30 AI36214-905) and the University of California Universitywide AIDS Research Program (R99-LJIAI-058) and by NIH grants to C.F.W. (AI03368 and P01CA69381) and J.L. (DA13866).
Footnotes
Publication 315 of the La Jolla Institute for Allergy and Immunology.
REFERENCES
- 1.Aiken C, Konner J, Landau N R, Lenburg M E, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 2.Aiken C, Trono D. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J Virol. 1995;69:5048–5056. doi: 10.1128/jvi.69.8.5048-5056.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baur A S, Sawai E T, Dazin P, Fantl W J, Cheng Mayer C, Peterlin B M. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity. 1994;1:373–384. doi: 10.1016/1074-7613(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 4.Bresnahan P A, Yonemoto W, Ferrell S, Williams-Herman D, Geleziunas R, Greene W C. A dileucine motif in HIV-1 Nef acts as an internalization signal for CD4 downregulation and binds the AP-1 clathrin adaptor. Curr Biol. 1998;8:1235–1238. doi: 10.1016/s0960-9822(07)00517-9. [DOI] [PubMed] [Google Scholar]
- 5.Brown C C, Poli G, Lubaki N, St Louis M, Davachi F, Musey L, Manzila T, Kovacs A, Quinn T C, Fauci A S. Elevated levels of tumor necrosis factor-alpha in Zairian neonate plasmas: implications for perinatal infection with the human immunodeficiency virus. J Infect Dis. 1994;169:975–980. doi: 10.1093/infdis/169.5.975. [DOI] [PubMed] [Google Scholar]
- 6.Browning J, Ribolini A. Studies on the differing effects of tumor necrosis factor and lymphotoxin on the growth of several human tumor lines. J Immunol. 1989;143:1859–1867. [PubMed] [Google Scholar]
- 7.Chen B K, Gandhi R T, Baltimore D. CD4 down-modulation during infection of human T cells with human immunodeficiency virus type 1 involves independent activities of Vpu, Env, and Nef. J Virol. 1996;70:6044–6053. doi: 10.1128/jvi.70.9.6044-6053.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen G, Gandhi R, Davis D M, Mandelboim O, Chen B, Strominger J, Baltimore D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 9.Collins K L, Chen B K, Kalams S A, Walker B D, Baltimore D. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature. 1998;391:397–401. doi: 10.1038/34929. [DOI] [PubMed] [Google Scholar]
- 10.Coodley G O, Loveless M O, Merryll T M. The HIV wasting syndrome. J Acquir Immune Defic Syndr. 1994;7:681–694. [PubMed] [Google Scholar]
- 11.Craig H M, Pandori M W, Guatelli J C. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc Natl Acad Sci USA. 1998;95:1229–11234. doi: 10.1073/pnas.95.19.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folks T M, Clouse K A, Justement J, Rabson A, Duh E, Kehrl J H, Fauci A S. Tumor necrosis factor α induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci USA. 1989;86:2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia J V, Miller A D. Serine phosphorylation-independent downregulation of cell-surface CD4 by Nef. Nature. 1991;350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg M, DeTulleo L, Rapoport I, Skowronski J, Kirchhausen T. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr Biol. 1998;8:1239–1242. doi: 10.1016/s0960-9822(07)00518-0. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg M, Lafrate A, Skowronski J. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate tracking of class I MHC complexes. EMBO J. 1998;17:2777–2789. doi: 10.1093/emboj/17.10.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenberg M E, Bronson S, Lock M, Neumann M, Pavlakis G N, Skowronski J. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 1997;16:6964–6976. doi: 10.1093/emboj/16.23.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanna Z, Kay D, Rebai N, Guimond A, Jothy S, Jolicoeur P. Nef harbors a major determinant of pathogenicity for an AIDS-like disease induced by HIV-1 in transgenic mice. Cell. 1998;95:163–175. doi: 10.1016/s0092-8674(00)81748-1. [DOI] [PubMed] [Google Scholar]
- 18.Hanna Z, Kay D G, Cool M, Jothy S, Rebai N, Jolicoeur P. Transgenic mice expressing human immunodeficiency virus type 1 in immune cells develop a severe AIDS-like disease. J Virol. 1998;72:121–132. doi: 10.1128/jvi.72.1.121-132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iafrate A J, Bronson S, Skowronski J. Separable functions of Nef disrupt two aspects of T cell receptor machinery: CD4 expression and CD3 signaling. EMBO J. 1997;16:673–684. doi: 10.1093/emboj/16.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalinkovich A, Engelmann H, Harpaz N, Burstein R, Barak V, Kalickman I, Wallach D, Bentwich Z. Elevated serum levels of soluble tumour necrosis factor receptors (sTNF-R) in patients with HIV infection. Clin Exp Immunol. 1992;89:351–355. doi: 10.1111/j.1365-2249.1992.tb06961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kestler H W, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus load and development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 22.Kirchhoff F, Greenough T C, Brettler D B, Sullivan J L, Desrosiers R C. Absence of intact nef sequences in a long-term survivor with nonprogressive HIV infection. N Engl J Med. 1995;332:228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- 23.Lama J, Mangasarian A, Trono D. Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr Biol. 1999;9:622–631. doi: 10.1016/s0960-9822(99)80284-x. [DOI] [PubMed] [Google Scholar]
- 24.Mangasarian A, Foti M, Aiken C, Chin D, Carpentier J L, Trono D. The HIV-1 Nef protein acts as a connector with sorting pathways in the Golgi and the plasma membrane. Immunity. 1997;6:67–77. doi: 10.1016/s1074-7613(00)80243-5. [DOI] [PubMed] [Google Scholar]
- 25.Marshall W L, Brinkman B M N, Ambrose C M, Pesavento P A, Uglialoro A M, Teng E, Finberg R W, Browning J L, Goldfeld A E. Signaling through the lymphotoxin-beta receptor stimulates HIV-1 replication alone and in cooperation with soluble or membrane-bound TNF-alpha. J Immunol. 1999;162:6016–6023. [PubMed] [Google Scholar]
- 26.Mauri D, Ebner R, Montgomery R, Kochel K, Cheung T, Yu G, Ruben S, Murphy M, Eisenberg R, Cohen G, Spear P, Ware C. LIGHT, a new member of the TNF superfamily, and lymphotoxin A are ligands for herpesvirus entry mediator. Immunity. 1998;8:21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 27.Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 28.Peter F. HIV Nef: the mother of all evil? Immunity. 1998;9:433–437. doi: 10.1016/s1074-7613(00)80626-3. [DOI] [PubMed] [Google Scholar]
- 29.Piguet V, Chen Y-L, Mangasarian F, Carpentier J-L, Trono D. Mechanisms of Nef-induced CD4 endocytosis: Nef connects CD4 with the μ chain of adaptor complexes. EMBO J. 1998;17:2472–2481. doi: 10.1093/emboj/17.9.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piguet V, Gu F, Foti M, Demaurex N, Gruenberg J, Carpentier J-L, Trono D. Nef-induced CD4 degradation: a diacidic-based motif in Nef functions as a lysosomal targeting signal through the binding of beta-COP in endosomes. Cell. 1999;97:63–72. doi: 10.1016/s0092-8674(00)80715-1. [DOI] [PubMed] [Google Scholar]
- 31.Ross T M, Oran A, Cullen B R. Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral Nef protein. Curr Biol. 1999;9:613–621. doi: 10.1016/s0960-9822(99)80283-8. [DOI] [PubMed] [Google Scholar]
- 32.Salmon P, Olivier R, Riviere Y, Brisson E, Gluckman J C, Kieny M P. Loss of CD4 membrane expression and CD4 mRNA during acute human immunodeficiency virus replication. J Exp Med. 1988;168:1953–1969. doi: 10.1084/jem.168.6.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz O, Marechal V, Danos O, Heard J M. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J Virol. 1995;69:4053–4059. doi: 10.1128/jvi.69.7.4053-4059.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard J M. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 35.Tamada K, Shimozaki K, Chapoval A, Zhu G, Sica G, Flies D, Boone D, Hsu H, Fu Y, Naghata S, Ni J, Chen L. Modulation of T-cell mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway. Nat Med. 2000;6:283–289. doi: 10.1038/73136. [DOI] [PubMed] [Google Scholar]
- 36.Tracey K J. The acute and chronic pathophysiologic effects of TNF: mediation of septic shock and wasting (cachexia) In: Beutler B, editor. Tumor necrosis factors. New York, N.Y: Raven Press; 1992. pp. 255–273. [Google Scholar]
- 37.Trono D. HIV accessory proteins: leading roles for the supporting cast. Cell. 1995;82:189–192. doi: 10.1016/0092-8674(95)90306-2. [DOI] [PubMed] [Google Scholar]
- 38.Ware C F, Crowe P D, Grayson M H, Androlewicz M J, Browning J L. Expression of surface lymphotoxin and tumor necrosis factor on activated T, B, and natural killer cells. J Immunol. 1992;149:3881–3888. [PubMed] [Google Scholar]
- 39.Ware C F, Santee S, Glass A. Tumor necrosis factor-related ligands and receptors. In: Thomson A, editor. The Cytokine Handbook. 3rd ed. London, United Kingdom: Academic Press Ltd.; 1998. pp. 549–592. [Google Scholar]