Abstract
Although early solid diet supplementation is a common practice to improve the growth and development in goat kids, its biological mechanism how solid diet induces rumen microbiota and epithelial development is still unknow. In this study, rumen fermentation parameters, 16S rRNA sequencing for rumen content and epithelial microbiota, transcriptomics and proteomics of epithelium were determined to classify the effects of solid diet supplementation. Here, we classified the changes of goat phenotypes (i.e., growth performance, rumen fermentation and development) and linked them to the changes of rumen microbiota, transcriptome and expressed proteins. The mechanism of solid diet improving rumen development was elucidated preliminarily. Moreover, different roles between the rumen content and epithelial microbiota were identified. Thess datasets expands our understanding of the association between the early diet intervention and rumen development, providing the useful information how nutrient strategy affects rumen function and subsequently improves the host growth. The generated data provides insights in the importance of rumen niche microbiota and microbe-host interactions, which benefits future studies.
Subject terms: Bacterial host response, Proteomics, Transcriptomics
Background & Summary
As one of the most important livestock specie in the world, the goat (Capra hircus) provides various types of products for human consumption, such as meat, milk, pelts and fiber1,2. Now, there are over 1,000 goat breeds and total goat amount is over 1 billion globally3. The rumen, the most critical digestive organ for ruminants, can degrade the high fiber-based plant components into volatile fatty acids (VFA) and microbial protein for rumen development and body nutrient requirement via microbial fermentation process4. The ability to digest fiber is the evidence of the well-developed and functional rumen in young ruminants5. However, the rumen of goat kids develops rapidly after born. Manipulation the early development of rumen becomes the most effective way to improve life-long rumen function and animal growth6,7. Early supplementation of a concentrate diet has already been widely used in ruminant production to improve its rumen and body development because of its stimulation of microbial proliferation and VFA production that initiates epithelial development8–11. However, the biological mechanism of this practise is still unclear.
Previous studies have demonstrated that early feeding starter with high grains or even alfalfa changed the rumen microbiota and improved animal growth12–14. However, most studies focused on the rumen content microbiome until now, and less researches have been conducted to determine the microbiota attached on the rumen epithelium and the rumen epithelial transcriptomics and proteomics. The rumen epithelial microbiota associated with the content microbial community might be critical for nutrient absorption as it tightly attaches on the luminal side of the rumen15–17. A previous study found differing community structure between the rumen content and the epithelial microbiome in cattle18, and the epithelial microbiota was also affected by dietary carbohydrate17. Moreover, the import roles of epithelial microbiota in maintaining host gene expression and development were also reported19. Beyond microbiota, changes of rumen fermentation and epithelial genes were reported in previous studies4,17,20,21. It is known that rumen epithelium plays key role in digestion and absorption of nutrients, such as VFAs and ammonia22. Thus, understanding the regulations of rumen epithelial gene and protein expression affected by the early diet intervention is necessary and urgent. Additionally, limit in microbe-host interactions develops a gap for understanding the connection between microbiota and rumen development as well as the goat growth. Therefore, this study was conducted to investigate the 16S rRNA gene sequences of rumen microbiota (both content and epithelium) and host transcriptomics and proteomics in goat kids consuming three diet regimes: milk replacer only (MRO), milk replacer supplemented with concentrate solid diet (MRC), and milk replacer supplemented with concentrate diet and alfalfa (MCA). This dataset, including the goat kids’ phenotypes, rumen content and epithelial microbiota, and epithelial omics (both transcriptome and proteomics), was described to illustrate the effects of early supplementation of high carbohydrates on the goat kids and explored the axis of diet-microbiota-host. As a foundation data, these omics could allow us to dig more relationship between the rumen microbiome and epithelial genes. The details of a schematic overview of the study workflow were shown in Fig. 1.
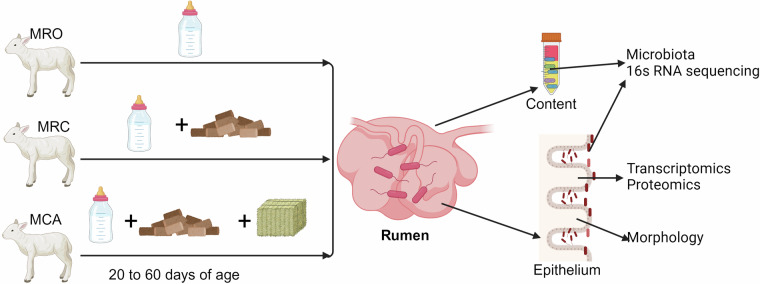
Fig. 1.
Overview of the experimental workflows. The goat kids were assigned into three treatments (milk replacer only (MRO), milk replacer supplemented concentrate (MRC) and milk replacer supplemented concentrate plus alfalfa pellets (MCA)) on 20 days of age. At the end of animal feeding trial (60 days of age), goat kids were slaughtered for rumen sample collection. After the rumen was weighted, rumen content and epithelial microbial samples were collected for 16S rRNA sequencing. The rumen epitheliums were collected for transcriptomics, proteomics, and morphology measurements.
Methods
Ethical statement
All experimental animals’ procedures in this study were approved by the Chinese Academy of Agricultural Sciences Animal Ethics Committee.
Experimental design, animal management and sampling
Based on the experimental design, 72 healthy Haimen goat kids (4.53 ± 0.52 kg body weight (BW)) were assigned into three treatment group: milk replacer only (MRO), milk replacer supplemented concentrate (MRC) and milk replacer supplemented concentrate and alfalfa pellets (MCA). Six animal replicates were included in each treatment group. Goat kids consumed these diet regimes from 20 to 60 days of age (d), respectively, and were slaughtered on d 60 to collect samples.
The animal trial was conducted at a commercial farm (the Green Sheep Valley Farm, Haimen City, China). During the trial, all goat kids had free access to water. Milk replacer, a patent product, was obtained from Beijing Precision Animal Nutrition Research Center, China. The solid diet, both concentrate and alfalfa pellets, were freely provided the MRC and MCA groups.
The feed intake was recorded daily and is shown in Table 1. At d 60, six goat kids from each treatment were weighted and slaughtered to collect rumen samples. Approximately 10 mL of rumen content was sampled from the mixed digesta and stored at −80 °C for next-generation sequencing. Rumen fluid phase approximately the 10 mL level was filtered via four layers of gauze and stored in a 15 mL tube at −20 °C for the analysis of rumen fermentation parameters. Next, rumen tissue at the bottom of the ventral sac was washed using sterilized PBS (pH = 7) to rinse the residual of rumen content or fluid filling the gap between papillae. Remaining residues attached tightly in the epithelium were abraded out to analyze for the epithelial microbiota. Concurrently, tissue sections (~4 cm2) in the ventral sac were fixed in a solution of 10% formalin for epithelial morphology detection. Samples for the remaining tissue and the epithelium-associated microbiota were snap-frozen in liquid nitrogen and stored at −80 C for host transcriptome and proteomics, respectively.
Table 1.
Effect of early supplementary solid diet on nutrient intake of goat kids.
| Items | Treatments | SEM | P value | ||
|---|---|---|---|---|---|
| MRO | MRC | MCA | |||
| Milk replacer intake /g•d−1 | 130.62 | 107.91 | 119.9 | 4.95 | 0.1696 |
| Concentrate intake /g•d−1 | — | 188.18 | 189.56 | 4.82 | 0.8956 |
| Alfalfa pellets intake /g•d−1 | — | — | 34.34 | — | — |
| Dry matter intake /g•d−1 | 122.47c | 271.50b | 309.97a | 16.12 | <0.0001 |
| Protein intake /g•d−1 | 31.33c | 58.52b | 66.37a | 4.07 | <0.0001 |
| NDF-Neutral detergent fiber intake /g•d−1 | — | 49.25b | 66.08a | 3.13 | 0.0004 |
| NFC-Non-fibrous carbohydrate intake /g•d−1 | 63.82c | 118.16b | 131.15a | 7.94 | <0.0001 |
MRO = milk replacer, MRC = milk replacer + concentrate, MCA = milk replacer + concentrate + alfalfa; SEM = Standard error of the means.
In the same row, values with different small letter superscripts mean significant difference (P < 0.05). The superscripts alphabet order represents a decreasing order of the mean among groups.
Rumen fermentation parameters measurement
Determination of the NH3-N using a phenol-sodium hypochlorite colorimetric method was performed after the rumen liquid was thawed at 4 °C. Rumen microbial proteins were analyzed according to the method described by Makkar et al.23. VFA concentration was quantified by gas chromatography (GC)24 using methyl valerate as the internal standard in an Agilent 6,890 series GC equipped with a capillary column (HP-FFAP19095F-123, 30 m, 0.53 mm diameter and 1 mm thickness). The detection results of rumen fermentation parameters are shown in Table 2.
Table 2.
Effect of early feeding on rumen fermentation parameters of goat kids.
| Items | Treatments | SEM | P value | ||
|---|---|---|---|---|---|
| MRO | MRC | MCA | |||
| pH | 7.09a | 5.88b | 6.01b | 0.15 | <0.0001 |
| NH3-N, mg/dL | 19.61a | 6.02b | 5.40b | 1.84 | <0.0001 |
| Microbial protein, mg/mL | 0.02b | 1.07a | 1.24a | 0.15 | 0.0006 |
| Total VFA, mmol/L | 34.99c | 76.53b | 91.40a | 6.28 | <0.0001 |
| Acetate, mmol/L | 22.27c | 38.75b | 45.45a | 2.64 | <0.0001 |
| Propionate, mmol/L | 9.45b | 17.54a | 22.06a | 1.64 | 0.0014 |
| Isobutyrate, mmol/L | 0.24 | 0.48 | 0.23 | 0.08 | 0.3589 |
| Butyrate, mmol/L | 1.74c | 11.39b | 17.73a | 1.84 | <0.0001 |
| Isovalerate, mmol/L | 0.56 | 0.98 | 0.91 | 0.16 | 0.547 |
| Valerate, mmol/L | 0.73b | 7.34a | 5.01a | 0.95 | 0.0045 |
| Acetate:Propionate | 2.55 | 2.23 | 2.2 | 0.13 | 0.5502 |
VFA = volatile fatty acids
In the same row, values with different small letter superscripts mean significant difference (P < 0.05). The superscripts alphabet order represents a decreasing order of the mean among groups.
Measurement of rumen epithelial morphology
Rumen tissue sections were kept in 70% ethanol until further measurement after 24 h of fixing in formalin. All samples were stained with Yihong-hematoxylin (H.E.) at the Chinese Agriculture University (Beijing, China). The length and width of the rumen papillae and stratum corneum thickness were measured using the Axiovision software (Zeiss, Oberkochen, Germany) Image-pro express image analysis processing system. The results of rumen papilla length, papilla width, lamina propria thickness and epithelial thickness are displayed in Table 3.
Table 3.
Effects of early supplementary solid diet on growth performance and rumen fermentation parameters in goat kids.
| Items | Treatments | SEM | P value | ||
|---|---|---|---|---|---|
| MRO | MRC | MCA | |||
| Slaughter BW/kg | 7.01b | 10.47a | 10.23a | 0.41 | <0.0001 |
| Average daily gain/g | 70.28b | 123.93a | 126.25a | 5.48 | <0.0001 |
| Rumen weight/g | 47.42c | 126.70b | 175.67a | 14.94 | <0.001 |
| Papilla length/μm | 786.6b | 1191.6a | 1389.6a | 80.44 | 0.0008 |
| Papilla width/μm | 169.81b | 206.52ab | 237.13a | 9.85 | 0.0096 |
| Keratin layer thickness/μm | 33.68 | 49.98 | 36.75 | 3.38 | 0.1258 |
| Muscle layers thickness/μm | 698.6 | 628.4 | 861.3 | 47.28 | 0.1171 |
| Epithelium thickness/μm | 43.13b | 56.07a | 47.72ab | 2.19 | 0.04 |
MRO = milk replacer, MRC = milk replacer + concentrate, MCA = milk replacer + concentrate + alfalfa; SEM = Standard error of the means. BW = body weight; A:P = the ratio of acetate and propionate
In the same row, values with different small letter superscripts mean significant difference (P < 0.05). The superscripts alphabet order represents a decreasing order of the mean among groups.
Next-generation sequencing of rumen content and epithelial microbiota and analysis
Total rumen content and epithelial microbial DNA were extracted using the Magnetic Universal Genomic DNA Kit (QIAGEN Inc., Beijing, China) according to the manufacturer’s protocol, and the V3-V4 region of the bacterial 16S ribosomal RNA genes was amplified using adaptor-linked universal primers (341 F and 806 R). The concentration of DNA was determined using Qubit® DNA Assay Kit with a Qubit® 3.0 Fluorometer (Invitrogen, China). Amplicon libraries were built using all qualified products and sequenced with an Illumina HiSeq PE250 platform at the Realbio Technology Genomics Institute (Shanghai, China). More details related to the sequencing process can be found in our previous study21.
Raw sequencing files of the rumen content and epithelial microbiota were processed using the mothur program (v1.39.1)25. Forward and reverse reads were merged first, and low-quality reads were removed. The high-quality sequences were then aligned against the SILVA reference database (Full-length sequences and taxonomy references release 132, http://www.arb-silva.de/)26. Moreover, the VSEARCH algorithm was employed to remove chimeras in filtered sequences. Subsequently, high-quality sequences were clustered into operational taxonomic units (OTUs) at the 97% similarity level using the Ribosomal Database Project (RDP) database27. Alpha (Shannon Index and Observed OTUs) and beta diversities (Bray-Curtis and Jaccard distance) were calculated using mothur. The boxplots of alpha diversity and the PCoA plot of beta diversity were visualized using the ‘ggplot2’ package in R (v3.6.0). The ANalysis Of SIMilarity (ANOSIM) test was performed to test the statistical significance of beta diversity.
Transcriptomic profile of the rumen epithelial tissue
Total RNA of the rumen epithelial samples was extracted using the TRIzol reagent (Invitrogen, CA, USA). The RNA integrity was measured using an Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA). If samples had the RNA integrity equal to or over 7, it would be used for sequencing. Then, the library was prepared and sequenced at the Beijing Genomics Institution (Shenzhen, China) using the HiSeq2000 system (Illumina, CA, USA) to obtain 100-bp paired-end reads according to the manufacturer’s instructions.
Raw reads were filtered to obtain clean reads using the trimmomatic module in SOAPnuke (v1.4.0) software via the removal of adaptors and low-quality reads. Low-quality reads were defined as more than 20% of bases with a quality score smaller than 10 or having more than 5% ambiguous sequences labeled as “N”. Then, high-quality RNA reads were mapped and assembled to reference genomes (AnimalTFDB v2.0) using HISAT (v2.1.0)28. The detection of transcript expression levels was based on the number of fragments per kilobase of exon per million fragments mapped (FPKM). Differentially expressed genes (DEGs) were detected based on methods reported by Wang et al.29 and the false discovery rate (FDR) was calculated based on methods of Benjamini and Hochberg’s multiple testing correction30. The significantly DEG were confirmed at a fold change ≥ 2 and a false discovery rate (FDR) < 0.001. Using this method, the DEG were displayed through a pairwise comparison analysis (MRO-vs-MRC, MRO-vs-MCA and MRC-vs-MCA). After expression pattern clustering, the DEG from pairwise comparisons were subjected to functional annotation, including GO (Gene Ontology) functional annotation and KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway annotation. The GO terms and KEGG pathway enrichment were performed using The Database for Annotation, Visualization and Integrated Discovery (DAVID v 6.8, http://david.abcc.ncifcrf.gov)31.
Proteomics of the rumen epithelium
Proteins from epithelial samples were extracted using Lysis buffer 3 (8 M Urea, 40 mM Tris-HCl or TEAB with 1 mM PMSF, 2 mM EDTA and 10 mM DTT, pH 8.5) and two magnetic beads (diameter 5 mm). Then, mixtures were placed into a TissueLyser to release proteins. After centrifugating, the supernatant was transferred into a new tube, reduced with 10 mM dithiothreitol (DTT) at 56 °C for 1 hour and alkylated by 55 mM iodoacetamide (IAM) in the dark place at room temperature for 45 min. After a new round of centrifugation (25,000 g, 4 °C, 20 min), the supernatant was quantified by Bradford. Moving to next step of quality control of protein extraction, we then mixed 15–30 μg proteins with loading buffer in centrifuge tube and heated them at 95 °C for 5 minutes (min). Then, the supernatant was centrifuged at 25,000 g for 5 min and loaded to sample holes in 12% polyacrylamide gel. The SDS-PAGE in constant voltage was performed to detect proteins quality at 120 V for 120 min. Once finished, it was stained in gel with Coomassie Blue for 2 hours and added destaining solution (40% ethanol and 10% acetic acid). Ultimately, it was put on a shaker (exchange destaining solution for 3~5 times, 30 min a time). Next, the protein digestion step needed that the protein solution (100 μg) with 8 M urea was diluted 4 times with 100 mM TEAB. Then the proteins were digested at 37 °C overnight by Trypsin Gold (Promega, Madison, WI, USA) in a ratio of protein: trypsin = 40:1. After trypsin digestion, the peptides were desalted using Strata X C18 column (Phenomenex) and vacuum-dried according to the manufacturer’s protocol. Then, we did protein labeling. The peptides were dissolved in 30 μL 0.5 M TEAB with vertexing. After the iTRAQ labeling reagents were recovered to ambient temperature, they were transferred and combined with proper samples. Peptide labeling was performed by iTRAQ Reagent 8-plex Kit according to the manufacturer’s protocol. The labeled peptides with different reagents were combined, desalted with a Strata X C18 column (Phenomenex), and vacuum-dried according to the manufacturer’s protocol. Subsequently, peptide fractionation step was carried out. A Shimadzu LC-20AB HPLC Pump system coupled with a high pH RP column was employed for the separation of peptides. The peptides were reconstituted with buffer A (5% ACN, 95% H2O, adjust pH to 9.8 with ammonia) to 2 ml and loaded onto a column containing 5 μm particles (Phenomenex). The peptides were separated at a flow rate of 1 mL/min with a gradient of 5% buffer B (5% H2O, 95% ACN, adjusted pH to 9.8 with ammonia) for 10 min, 5–35% buffer B for 40 min, 35–95% buffer B for 1 min. The system was then maintained in 95% buffer B for 3 min and decreased to 5% within 1 min before equilibrating with 5% buffer B for 10 min. Elution was monitored by measuring absorbance at 214 nm, and fractions were collected per 1 min. The eluted peptides were pooled into 20 fractions and vacuum dried. Next, each fraction was resuspended in buffer A (2% ACN, 0.1% FA) and centrifuged at 20,000 g for 10 min. The supernatant was loaded onto a Thermo Scientific™ UltiMate™ 3000 UHPLC system equipped with a trap and an analytical column. The samples were loaded on a trap column at 5 μL/min for 8 min, and then eluted into the homemade nanocapillary C18 column (ID 75 μm × 25 cm, 3 μm particles) at a flow rate of 300 nl/min. The gradient of buffer B (98% ACN, 0.1% FA) was increased from 5% to 25% in 40 min, and then increased to 35% in 5 min, followed by 2 min linear gradient to 80%, then maintained at 80% B for 2 min, and finally returned to 5% in 1 min and equilibrated for 6 min. Finally, we used Mass Spectrometer to detect the proteins. The peptides separated from nanoHPLC were subjected into the tandem mass spectrometry Q EXACTIVE HF X (Thermo Fisher Scientific, San Jose, CA) for DDA (data-dependent acquisition) detection by nano-electrospray ionization. The parameters for Mass Spectrometer (MS) analysis were listed as following: electrospray voltage: 2.0 kV; precursor scan range: 350–1500 m/z at a resolution of 60,000 in Orbitrap; MS/MS fragment scan range: > 100 m/z at a resolution of 15,000 in HCD mode; normalized collision energy setting: 30%; dynamic Exclusion time: 30 s; Automatic gain control (AGC) for full MS target and MS2 target: 3e6 and 1e5, respectively. The MS/MS scan numbers followed one MS scan: 20 most abundant precursor ions above a threshold ion count of 10,000.
The raw MS/MS data was converted into Mascot Generic File (MGF) format, and the MGF files were searched by the local Mascot server against the database. Besides, quality control was performed to determine if a reanalysis step was needed. An automated software, called IQuant, was applied to analyze the labeled peptides with isobaric tags, with steps of protein identification, tag impurity correction, data normalization, missing value imputation, protein ratio calculation, statistical analysis, results presentation. All proteins with a false discovery rate (FDR) less than 1% will proceed with downstream analysis.
Data Records
The raw reads files for each rumen content sample of 16S rRNA sequencing have been uploaded to the NCBI Sequence Read Archive (SRA) with accession number SRP19980432, and the raw data of the rumen epithelial samples of 16S rRNA sequencing and transcriptomics have been deposited into NCBI SRA with accession number SRP23606133. The raw proteomics data were uploaded to ProteomeXchange Consortium via the iProX partner repository with the dataset identifier PXD04784334. All these data can be used freely.
Technical Validation
Benefits of early supplementation of high carbohydrate diet was found in this dataset. As shown in Tables 1 and 2, significant increases in nutrient intake, average daily gain and body weight were observed in MRC and MCA groups. Moreover, compared to MRC, MCA had a higher intake of protein, neutral detergent fibres (NDF), and non-fibrous carbohydrates (NFC). Next, a more well-developed rumen was also found in solid diet groups as rumen weight, papilla length and width were significantly increased in MRC and MCA groups. We found that the parameters of rumen fermentation were also affected by solid diet supplementation (Table 3). Compared to MRO, lower NH3-N concentration was found in MRC and MCA, while higher concentrations of total VFA, acetate, propionate, butyrate and valerate in MRC and MCA were observed.
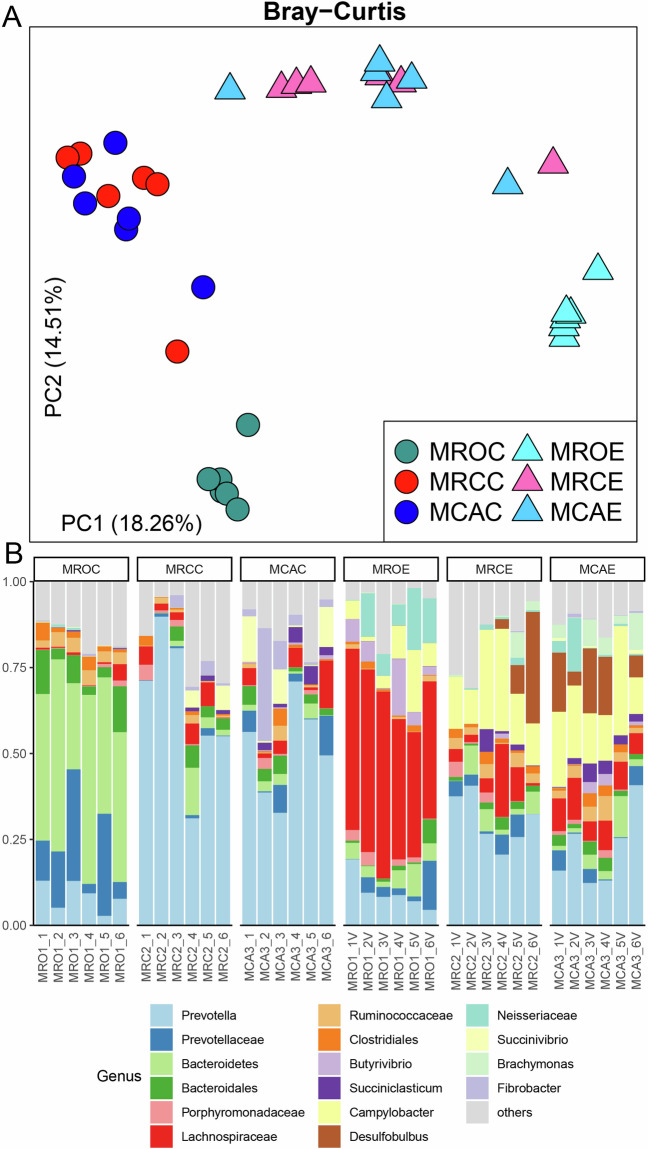
For next-generation sequencing, the DNA quality of the 16S was determined, and the DNA total amount ≥ 1 μg and concentration ≥ 30 ng/μL indicated that the DNA quality was qualified. The concentration of metagenome libraries was assessed using an Agilent 2100 Bioanalyzer instrument (Agilent DNA 1000 Reagents) and a Genomic DNA Sample Prep Kit for Illumina NovaSeq 6000 Platform, and the libraries with qualified concentration (≥10 nM) and volume (15 μL–100 μL) were subjected for sequencing. Quality control of 16S rRNA sequencing reads was performed using mothur MiSeq SOP (https://mothur.org/wiki/miseq_sop/). The quality assessment of 16S rRNA sequencing reads of both rumen content and epithelial samples is shown in Supplementary Table 1. As shown in Fig. 2A, the samples of rumen content and epithelium tended to cluster based on the organism (mainly along the first axis), the second factor of variation being the individual intra-species variability (y-axis). Thus, PCoA separated the samples according to their origin. The bacteria, including Prevotella and Bacteroidetes, dominated the rumen content communities, while epithelial samples had higher abundances of Prevotella, Lachnospiraceae unclassified, Campylobacter, and Desulfobulbus (Fig. 2B).
Fig. 2.
Next-generation sequencing of the rumen content and epithelial microbiota in goat kids. (A) Beta diversity of the rumen content and epithelial microbiota based on Bray–Curtis. One point represents one sample. (B) Rumen microbial composition at the genus level. Each column represents a sample, and each bar represents one bacterium. MROC, MRCC and MCAC represent content samples in animals that received MRO, MRC and MCA diets, while MROE, MRCE and MCAE represent the epithelial microbiota from the three diets, respectively. The MRO treatment was fed only milk replacer, the MRC treatment was fed milk replacer with concentrate and the MCA treatment was fed milk replacer with concentrate plus alfalfa.
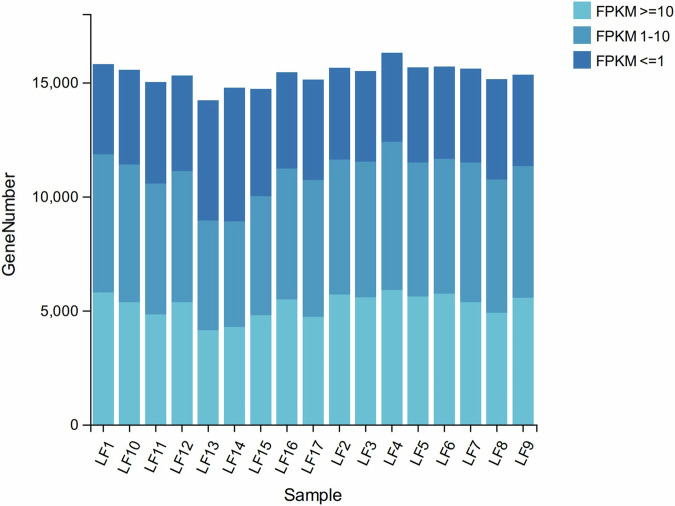
To ensure the quality of the transcriptomic sequencing data, a state-of-the-art equipment for molecular biology was employed to determine the purity, concentration, and integrity of RNA. Subsequently, the library’s quality was assessed through testing. Once the requirements are met, computer sequencing can be conducted. A total of 109 Gb of clean data were generated, with an average of 6.44 Gb per subject. After filtration using trimmomatic, the proportion of clean reads with quality score over 30 was 96.79% (Supplementary Table 2). When mapping the high-quality reads to reference genome, we found the average of the mapping rations of all samples was 76.75% (Supplementary Table 2). The gene expressions of each epithelial sample were shown in Fig. 3.
Fig. 3.
Gene expression stacked bar plot of each epithelial sample. LF1-LF6, LF7-LF12 and LF13-LF17 belong to MRO, MRC and MCA treatments, respectively. MRO = milk replacer, MRC = milk replacer + concentrate, MCA = milk replacer + concentrate + alfalfa.
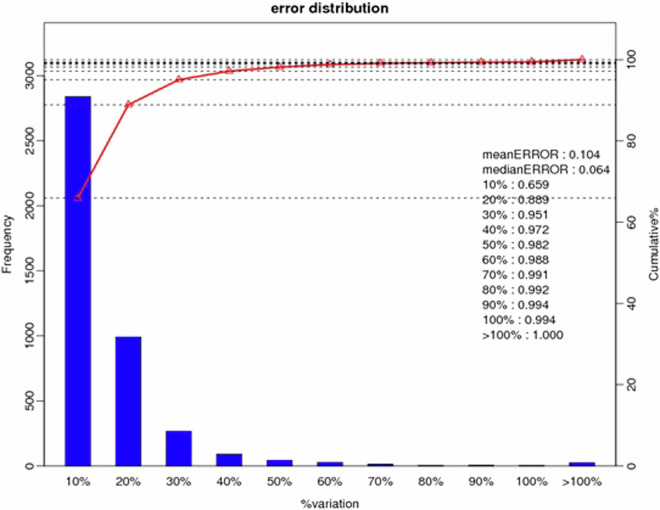
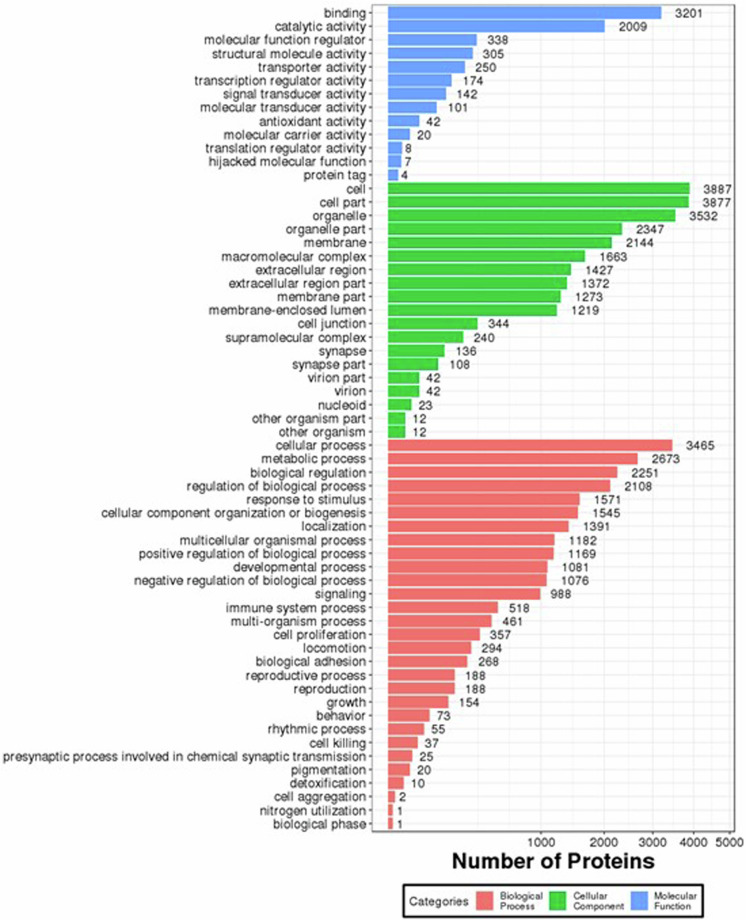
The iTRAQ (Isobaric tags for relative and absolute quantitation, iTRAQ) technology was confirmed to have its high precision in protein quantitative method. Three technical duplicate experiments were conducted for each sample. Totally 1,443,120 spectrums were generated, 26,793 peptides and 6,003 proteins were identified with 1% FDR. Coefficient of Variation (CV) defined as the ratio of the standard deviation (SD) to the mean was used to evaluate the reproducibility (Fig. 4). The lower the CV, the better the reproducibility. The Gene Ontology (GO) annotation for all identified proteins were displayed (Fig. 5).
Fig. 4.
Quantification repeat analysis of the rumen epithelial proteomics. X-axis is the deviation between the protein ratio of the repetitive samples. Y-axis is the quantified protein amount at the corresponding range.
Fig. 5.
Bar plot of the Gene Ontology Analysis using proteomics. The bar chart shows the distribution of corresponding GO terms. Different colors represent different GO categories.
Usage Notes
Our comprehensive dataset of rumen microbiota and epithelial omics resulting from solid diet provides insights into association between the critical microbiota and host gene expression. Although our preliminary findings reveal how solid diet and its nutrients drive rumen microbiome, epithelial gene and proteins, more interesting biological pathways can be dug via re-analysing our omics dataset, which allow us to deeply understand the interactions between microbiome and host. Moreover, common bioinformatic software and pipeline used in this study is great for reuse of the data.
Supplementary information
Acknowledgements
This study was funded in part by grants from the National Key Research and Development Program (2022YFA1304201), the National Natural Science Foundation of China (31872385, 32222081), and the Inner Mongolia Science and Technology Key Project (2021SZD0014).
Author contributions
J.C. X.L. and Y.Z. contributed to animal trials, and sample collection. J.C. contributed to data analysis, visualization, primary drafting, and proof. Q.D., and K.C. helped with sample collection and investigation. F.D. and Y.L. contributed to proof and revision. N.Z. contributed to the experimental design, visualization, project management, and draft proof. All authors have read and agreed to the published version of the manuscript.
Code availability
Serum enzyme activity, antioxidant capacity, and immune parameter were statistically processed and analyzed using R version 3.6.0, default parameters or parameters recommended by the developer were used. A reproducible version of partial microbiome analysis and computer code is available at https://github.com/chaichai9521/goat-rumen-mcirobiome-analysis.git.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41597-024-03584-7.
References
- 1.Cao Y, et al. The multi-kingdom microbiome of the goat gastrointestinal tract. Microbiome. 2023;11:219. doi: 10.1186/s40168-023-01651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizrahi I, Wallace RJ, Morais S. The rumen microbiome: balancing food security and environmental impacts. Nat Rev Microbiol. 2021;19:553–566. doi: 10.1038/s41579-021-00543-6. [DOI] [PubMed] [Google Scholar]
- 3.Dong Y, et al. Sequencing and automated whole-genome optical mapping of the genome of a domestic goat (Capra hircus) Nat Biotechnol. 2013;31:135–141. doi: 10.1038/nbt.2478. [DOI] [PubMed] [Google Scholar]
- 4.Chai J, et al. Solid diet manipulates rumen epithelial microbiota and its interactions with host transcriptomic in young ruminants. Environ Microbiol. 2021;23:6557–6568. doi: 10.1111/1462-2920.15757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chai J, Zhuang Y, Cui K, Bi Y, Zhang N. Metagenomics reveals the temporal dynamics of the rumen resistome and microbiome in goat kids. Microbiome. 2024;12:14. doi: 10.1186/s40168-023-01733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisler MC, et al. Agriculture: Steps to sustainable livestock. Nature. 2014;507:32–34. doi: 10.1038/507032a. [DOI] [PubMed] [Google Scholar]
- 7.Chai J, et al. Diet and monensin influence the temporal dynamics of the rumen microbiome in stocker and finishing cattle. J Anim Sci Biotechnol. 2024;15:12. doi: 10.1186/s40104-023-00967-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun DM, Mao SY, Zhu WY, Liu JH. Effect of starter diet supplementation on rumen epithelial morphology and expression of genes involved in cell proliferation and metabolism in pre-weaned lambs. Animal. 2018;12:2274–2283. doi: 10.1017/S1751731118000290. [DOI] [PubMed] [Google Scholar]
- 9.Vi RLB, McLeod KR, Klotz JL, Heitmann RN. Rumen development, intestinal growth and hepatic metabolism in the pre-and postweaning ruminant. J Dairy Sci. 2004;87:E55–E65. doi: 10.3168/jds.S0022-0302(04)70061-2. [DOI] [Google Scholar]
- 10.Heinrichs, A. J. Rumen development in the dairy calf. Calf and heifer rearing, 53-65 (2005).
- 11.Suarez BJ, et al. Effects of supplementing concentrates differing in carbohydrate composition in veal calf diets: I. Animal performance and rumen fermentation characteristics. J Dairy Sci. 2006;89:4365–4375. doi: 10.3168/jds.S0022-0302(06)72483-3. [DOI] [PubMed] [Google Scholar]
- 12.Yang B, He B, Wang SS, Liu JX, Wang JK. Early supplementation of starter pellets with alfalfa improves the performance of pre- and postweaning Hu lambs. J Anim Sci. 2015;93:4984–4994. doi: 10.2527/jas.2015-9266. [DOI] [PubMed] [Google Scholar]
- 13.Yang B, et al. Alfalfa Intervention Alters Rumen Microbial Community Development in Hu Lambs During Early Life. Front Microbiol. 2018;9:574. doi: 10.3389/fmicb.2018.00574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiao JZ, Huang JY, Zhou CS, Tan ZL. Taxonomic Identification of Ruminal Epithelial Bacterial Diversity during Rumen Development in Goats. Appl Environ Microbiol. 2015;81:3502–3509. doi: 10.1128/AEM.00203-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seddik, H., Xu, L., Wang, Y. & Mao, S. Y. A rapid shift to high-grain diet results in dynamic changes in rumen epimural microbiome in sheep. Animal, 1-9 (2018). [DOI] [PubMed]
- 16.Malmuthuge N, Liang GX, Guan LL. Regulation of rumen development in neonatal ruminants through microbial metagenomes and host transcriptomes. Genome Biol. 2019;20:172. doi: 10.1186/s13059-019-1786-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petri RM, Kleefisch MT, Metzler-Zebeli BU, Zebeli Q, Klevenhusen F. Changes in the rumen epithelial microbiota of cattle and host gene expression in response to alterations in dietary carbohydrate composition. Appl Environ Microbiol. 2018;84:e00384–18. doi: 10.1128/AEM.00384-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scharen M, et al. Alterations in the Rumen Liquid-, Particle- and Epithelium-Associated Microbiota of Dairy Cows during the Transition from a Silage- and Concentrate-Based Ration to Pasture in Spring. Front Microbiol. 2017;8:744. doi: 10.3389/fmicb.2017.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiao JZ, et al. Linkages between Epithelial Microbiota and Host Transcriptome in the Ileum during High-Grain Challenges: Implications for Gut Homeostasis in Goats. J Agr Food Chem. 2019;67:551–561. doi: 10.1021/acs.jafc.8b05591. [DOI] [PubMed] [Google Scholar]
- 20.Jing XP, et al. Dietary supplements during the cold season increase rumen microbial abundance and improve rumen epithelium development in Tibetan sheep. J Anim Sci. 2018;96:293–305. doi: 10.1093/jas/skx032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lv X, et al. The Signature Microbiota Drive Rumen Function Shifts in Goat Kids Introduced to Solid Diet Regimes. Microorganisms. 2019;7:516. doi: 10.3390/microorganisms7110516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCann JC, Wickersham TA, Loor JJ. High-throughput Methods Redefine the Rumen Microbiome and Its Relationship with Nutrition and Metabolism. Bioinform Biol Insights. 2014;8:109–125. doi: 10.4137/BBI.S15389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makkar HPS, Sharma OP, Dawra RK, Negi SS. Simple Determination of Microbial Protein in Rumen Liquor. J Dairy Sci. 1982;65:2170–2173. doi: 10.3168/jds.S0022-0302(82)82477-6. [DOI] [PubMed] [Google Scholar]
- 24.Jiao JZ, et al. In vitro evaluation on neutral detergent fiber and cellulose digestion by post-ruminal microorganisms in goats. J Sci Food Agr. 2014;94:1745–1752. doi: 10.1002/jsfa.6485. [DOI] [PubMed] [Google Scholar]
- 25.Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. 2013;79:5112–5120. doi: 10.1128/AEM.01043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pruesse E, et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007;35:7188–7196. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cole JR, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141–145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang LK, Feng ZX, Wang X, Wang XW, Zhang XG. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics. 2010;26:136–138. doi: 10.1093/bioinformatics/btp612. [DOI] [PubMed] [Google Scholar]
- 30.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J R Stat Soc B. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 31.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 32.2019. NCBI Sequence Read Archive. SRP199804
- 33.2019. NCBI Sequence Read Archive. SRP236061
- 34.ProteomeXchange Consortiumhttps://www.iprox.cn/page/project.html?id=IPX0007706000 (2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- 2019. NCBI Sequence Read Archive. SRP199804
- 2019. NCBI Sequence Read Archive. SRP236061
Supplementary Materials
Data Availability Statement
Serum enzyme activity, antioxidant capacity, and immune parameter were statistically processed and analyzed using R version 3.6.0, default parameters or parameters recommended by the developer were used. A reproducible version of partial microbiome analysis and computer code is available at https://github.com/chaichai9521/goat-rumen-mcirobiome-analysis.git.