Abstract
COVID-19 has affected millions worldwide, causing significant morbidity and mortality. While predominantly involving the respiratory tract, SARS-CoV-2 has also caused systemic illnesses involving other sites. Liver injury due to COVID-19 has been variably reported in observational studies. It has been postulated that liver damage may be due to direct damage by the SARS-CoV-2 virus or multifactorial secondary to hepatotoxic therapeutic options, as well as cytokine release syndrome and sepsis-induced multiorgan dysfunction. The approach to a COVID-19 patient with liver injury requires a thorough evaluation of the pattern of hepatocellular injury, along with the presence of underlying chronic liver disease and concurrent medications which may cause drug-induced liver injury. While studies have shown uneventful recovery in the majority of mildly affected patients, severe COVID-19 associated liver injury has been associated with higher mortality, prolonged hospitalization, and greater morbidity in survivors. Furthermore, its impact on long-term outcomes remains to be ascertained as recent studies report an association with metabolic-fatty liver disease. This present review provides insight into the subject by describing the postulated mechanism of liver injury, its impact in the presence of pre-existing liver disease, and its short- and long-term clinical implications.
Keywords: COVID-19 associated liver injury, cytokine release syndrome, SARS-CoV-2
Key Points
COVID-19 associated liver injury can have an unpredictable course and variable manifestations
The mechanisms of liver injury include direct damage by the virus, liver injury due to hepatotoxic drugs, cytokine release syndrome, and sepsis-induced multiorgan dysfunction.
Evaluating a COVID-19 patient with liver injury requires consideration of the pattern of hepatocellular injury, the presence of underlying chronic liver disease, and concurrent medications.
Severe COVID-19 associated liver injury is associated with higher mortality, prolonged hospitalization, and greater morbidity in survivors
Long-term clinical implications of COVID-19-associated liver injury include metabolic-associated fatty liver disease, which may have implications for overall health.
Introduction
COVID-19 has affected millions of people worldwide, causing significant morbidity and mortality. As the World Health Organization declares the end of the global health emergency due to COVID-19, the world is still grappling to understand the long-term impact of the disease.[1] SARS-CoV-2 is known to cause multi-organ damage, including injury to the lungs, kidneys, neurological, cardiovascular, and liver, as well as a myriad of hematological abnormalities. Liver injury was amongst the earliest recognized complications of COVID-19.[2,3] The incidence of liver injury has been reported to be between 16% to 29%, and a higher prevalence was observed in hospitalized patients with COVID-19.[4,5] COVID-19-associated liver injury has been defined as any identifiable liver damage that occurs as a direct result of the COVID-19 infection or during the course of the disease or treatment, irrespective of any pre-existing comorbid or liver disease.[6] The presence and extent of liver damage that occurs are primarily measured by taking quantitative measurements of liver enzymes. There are three types of liver injury described in the literature: hepatocellular, cholestatic, and mixed types. An elevated alanine aminotransferase (ALT) or aspartate aminotransferase (AST) three times greater than the upper limit of the normal value is indicative of the hepatocellular type of liver injury. Elevations in other liver enzymes, such as alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), and total bilirubin that exceed two times the upper normal value, are seen in the cholestatic type. The mixed type is a combination of both hepatocellular and cholestatic ranges.[7] Abnormalities with tests that assess liver function are common, but their overall impact is still under exploration. Furthermore, imaging modalities have also been utilized in identifying COVID-19-associated liver injury. These include ultrasound (US) (the main diagnostic tool for first evaluation), Computed Tomography Scan (CT) (most useful for overall abdominal assessment), and Magnetic Resonance Imaging (MRI) (most helpful in better clarifying liver changes in cases where changes on US and CT are indeterminate). Hepatomegaly, steatosis, acute hepatitis, drug-induced liver injury, portal vein thrombosis, and biliary involvement have all been described.[8] Liver injury has also been corroborated from liver biopsy evidence, which has shown moderate microvascular steatosis and mild lobular and portal activity.[9] It is of utmost importance for physicians and researchers to analyze and assess the mechanism of liver injury and its impact on patient outcomes and disease processes. This short review focuses on the pathophysiology of COVID-19-associated liver injury and provides an overview of the management of these patients and the consequences of COVID-19 on people living with pre-existing liver disease, primarily chronic liver disease. Moreover, it elaborates on the short and long-term implications of COVID-19-associated liver injury.
Pathophysiology of COVID-19 and Liver Injury
Liver injury was first reported by Zhang et al.[2] from Wuhan. They reported that 43.4% of the cases had mild to moderate elevation of ALT and AST. Since then, liver injury has been observed and reported in several studies, and the degree of liver damage has been associated with a greater risk of progression to severe COVID-19.[3,10] The exact pathophysiology of liver damage in SARS-CoV-2 remains unclear, but proposed mechanisms include direct damage to the liver, drug-induced injury, inflammatory immune response, and hypoperfusion (Fig. 1).[11]
Figure 1.
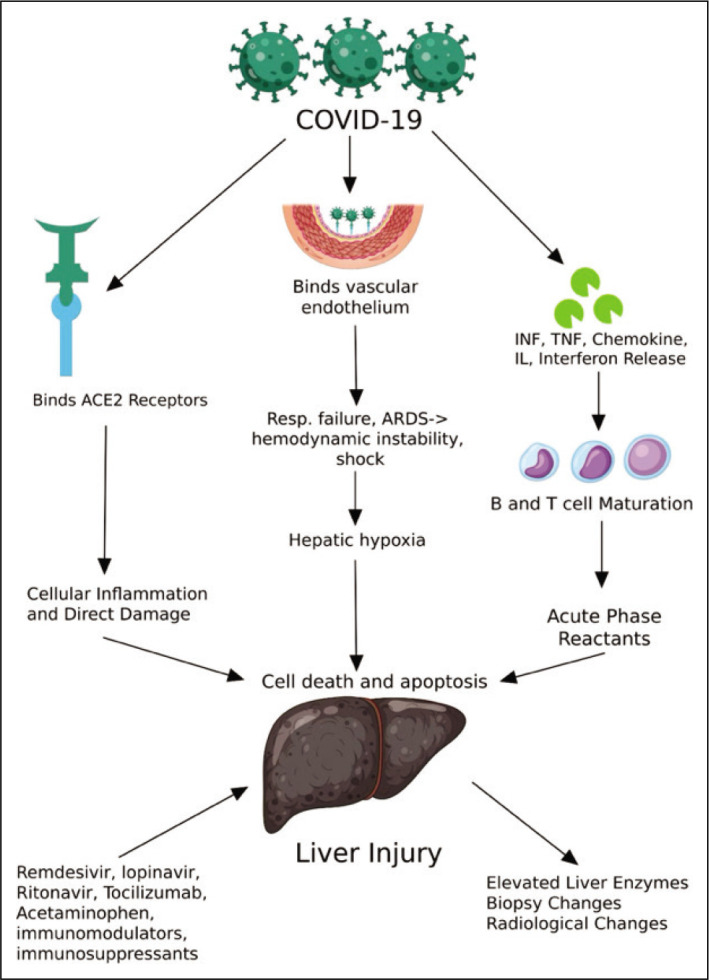
Pathophysiology of COVID-19 associated liver injury.
Direct Liver Damage
SARS-CoV-2 targets the angiotensin-converting enzyme 2 (ACE2) receptors, which, besides the lungs, are present in the intestinal endothelium and vascular smooth muscles.[12] ACE2 is also expressed by hepatocytes and cholangiocytes, although expression is higher in bile duct epithelium compared to hepatocytes.[13,14] SARS-CoV-2 can, therefore, cause liver injury either by binding to hepatocytes or by binding to bile duct cells, leading to upregulation of ACE2 in the liver.[14] Tian et al.,[15] however, reported that inclusion bodies of the virus were not found on autopsies of COVID-19 patients.[15] This supports bile duct damage rather than hepatocyte damage as the cause of liver injury. Zhao et al.[16] also reported that liver ductal cultures revealed bile duct injury as the possible cause of liver damage. Liver cirrhosis can lead to increased expression of ACE2 in hepatocytes. This may result in increased invasion of hepatocytes by SARS-CoV-2 and more severe liver injury in patients with chronic liver disease.[13,17]
Cytokine Release Syndrome
COVID-19 leads to multisystem dysfunction as part of the systemic inflammatory response syndrome (SIRS). Many cytokines are released, many of which are produced by the liver, including interleukins, tumor necrosis factors, interferons, and chemokines.[18] IL-6 causes activation of T-cells, B-cell differentiation, and induction of acute phase reactants in the hepatocytes.[19] The acute phase response by the liver is a protective mechanism against the pathogen.[20] The activated T-cells attack the infected cells until they are depleted. The depleted cells, when unable to control the infection, activate secondary inflammatory responses, leading to the production of more inflammatory cytokines.[21] The excessive release of inflammatory cytokines leads to shock, multiorgan dysfunction, and acute respiratory distress syndrome.[22,23]
Hypoxia Reperfusion Syndrome
SARS-CoV-2 binds to several receptors in the vascular endothelium. The subsequent vascular injury leads to respiratory failure and ARDS3. The liver is prone to the effects of hypoxemia due to its complicated vascular supply and high metabolic activity.[24] Hemodynamic instability leads to reduced flow to the liver and periphery, resulting in hypoxemia causing an acute elevation of serum transaminase levels.[25,26] Persistent hypoxia due to respiratory, cardiac failure, or shock causes lipid accumulation in hepatocytes, progressing to cell death and apoptosis.[27] This is followed by mitochondrial damage and release of reactive oxygen species causing further damage, release of proinflammatory markers, and activation of macrophages, neutrophils, and platelets.[27–29] Microcirculatory disturbance leads to coagulopathy and thrombosis. Von Willebrand factor antigen correlates with the severity of vascular endothelial damage, and higher levels are associated with increased mortality.[30]
Drug-Induced Liver Injury
Drugs may be a crucial factor leading to liver injury in COVID-19. This has been supported by autopsy reports showing microvascular steatosis and hepatic inflammation.[31] Many classes of drugs have been used to treat COVID-19 since the beginning of the pandemic; among these, many are known to be hepatotoxic. Drugs used include antipyretics (e.g., Acetaminophen), antivirals (e.g., Remdesivir, Lopinavir/Ritonavir), antibiotics (e.g., Azithromycin), immunosuppressants and immunomodulators (e.g., corticosteroids, Hydroxychloroquine), Tocilizumab, and herbal medicines. Besides this, many antimicrobial agents such as azoles, used for treating superimposed fungal infections such as COVID-19-associated pulmonary aspergillosis, are also associated with liver enzyme dysfunction. Use of multiple drugs at the same time, possible interaction between drugs for treating COVID-19, medications used to treat other comorbid conditions, and discontinuation of drugs being used by a patient for prior liver disorders may all contribute to liver injury.[24]
There is a substantial variability of transaminitis in patients with COVID-19 infection. Many patients receive multiple combinations of drugs for treatment; thus, it becomes difficult to evaluate the component of liver injury related to a particular drug. The drug-induced liver injury may either be an intrinsic liver injury or an idiosyncratic one, with an unspecified latency period. Thus, most drug-induced liver injuries may remain as a diagnosis of exclusion unless specific testing, including biopsies, are performed. Many host factors including age, gender, pregnancy, malnutrition, obesity, and comorbidities like diabetes, NAFLD have been implicated as factors in idiosyncratic drug-induced liver injury.[32] Among the common medications used in COVID-19 infections are acetaminophen, NSAIDs, antivirals like Remdesivir, Lopinavir/Ritonavir, antibiotics (e.g., Azithromycin), immunosuppressants and immunomodulators (e.g., corticosteroids, Hydroxychloroquine), antibodies (e.g., Tocilizumab), and herbal medicines. The occurrence of DILI with acetaminophen is one of the most common drug adverse events. Mortality rates have been approximated at 0.4% in overdose patients, translating to 300 deaths annually in the United States. Although the majority of patients experience mild adverse reactions, such as hepatitis, cholestasis, or asymptomatic liver enzyme elevation, APAP hepatotoxicity is generally estimated to account for approximately 48% of acute liver failure diagnoses.[33–35] DILI follows a dose-dependent toxicity ranging from 0.02% up to 30%.[36]
Remdesivir
In the initial study reporting the safety of remdesivir for COVID-19 patients, conducted by Grein et al.,[37] the effect of 5 to 10-day courses of remdesivir on the changes in the category of oxygen-support status was investigated in a small cohort of 53 patients. The most common adverse event in this study was increased hepatic enzymes, with an incidence of 23%. A similar pattern was replicated in the study on 402 patients, evaluating the optimum time course for intravenous remdesivir, conducted by Goldman et al.[38] In that study, ALT and AST elevation (7% and 6%, respectively) was reported as the most common liver adverse effects.[38] Furthermore, in the placebo-controlled double-blinded clinical trial on a total sample of 255 patients, conducted by Wang et al.,[39] grade 1–2 increased AST was detected as an adverse liver effect (12% in the placebo group, 7% in the remdesivir group), and grade 1–2 increased ALT led to drug discontinuation (1%).[39]
Lopinavir/Ritonavir
The other drug combination used for the treatment of COVID-19 infections was lopinavir/ritonavir. A significant number of studies have reported the association of lopinavir/ritonavir use in COVID-19 patients with adverse liver effects. In a study by Sun et al.[40] on a sample of 217 patients, 63% of total adverse drug reactions (ADRs) were associated with the use of lopinavir/ritonavir. Liver ADRs were the second most common ADRs, with a prevalence of 18%. However, the percentage of liver ADRs due to lopinavir/ritonavir was not reported by the same study.[40] Later, Fan et al.[41] reported that among the 148 patients, 45 patients had normal baseline liver functions, of which 48% developed an abnormality in the liver after admission to the hospital. They highlighted that among the patients with abnormal liver functions, a higher proportion had used lopinavir/ritonavir (57.8%) compared to the patients with normal liver function tests (31.3%).
Hydroxychloroquine
Hydroxychloroquine has remained in use for rheumatological disorders and is implicated in the treatment of malaria. Short-term use is associated with very limited adverse effects pertaining to liver injuries. However, it is implicated that when used in combination with other medications like ritonavir, the adverse effects may increase.
Tocilizumab
Interleukin-6 (IL-6) blockers, including tocilizumab and sarilumab, were approved in June 2021 for the treatment of patients with moderate to severe COVID-19. There has been a noticeable difference in the peak onset times of adverse reactions between the two drugs. Some studies have reported TCZ-related DILI accounted for 67.01% in the first four days after receiving TCZ therapies, and SAR-associated DILI accounted for 83.13% in the first six days after receiving SAR therapies. Furthermore, it is also associated with reactivation of Hepatitis B, as has been described in patients with rheumatological disorders being treated with tocilizumab along with DMARDs.[42,43]
Impact of COVID-19 on Patients with Pre-existing Liver Disorders
The impact of COVID-19 on liver function in patients with preexisting liver disease depends on the underlying etiology and severity of the underlying liver pathology. The disease presentation of COVID-19 is highly variable; thus, many confounding factors can be attributed to increased morbidity and mortality. Multiple studies evaluated the outcomes of COVID-19 with liver pathology and found variable and conflicting results. As compared to individuals without CLD, the patients with CLD and cirrhosis may have more severe disease and a greater COVID-19-associated mortality rate. According to a systematic review, the likelihood of COVID-19-associated severe diseases and death was 2.44 times greater in patients with CLD compared to those without underlying liver diseases.[44,45] COVID-19 can be stratified into the following groups when considering underlying liver disease.
Chronic Viral Hepatitis and COVID-19
Chronic viral hepatitis in the absence of chronic liver disease does not seem to affect morbidity and mortality among patients with COVID-19. However, studies have shown that COVID-19 patients with Hepatitis B (HBV) co-infection tend to have a poorer prognosis, with a 2.2-fold increased severity of COVID-19 and an in-hospital mortality rate of 6.0%. Similarly, chronic hepatitis C (HCV) patients with COVID-19 are more prone to hospitalization but not at a higher risk of death.[46] The results of a comprehensive meta-analysis by Hariyanto et al.[47] have also shown an association of viral hepatitis with severe COVID-19. COVID-19 can reactivate or cause a sudden increase in HBV DNA levels in the blood, hepatitis failure, and flare of acute hepatitis in patients with chronic HBV. Risk factors for HBV reactivation in patients with COVID-19 are immunosuppressive therapy, including steroids, advanced age, male sex, lymphopenia, and significant comorbidities (including chronic renal disease, hypertension, diabetes, and hypercholesterolemia).[48,49] The presence of chronic HCV infection, history of HCV infection, and acute liver injury are strong predictors of hospital mortality in patients with COVID-19. The mechanisms may be connected to baseline cytokine-mediated pro-inflammation and endothelial dysfunction, as well as extrahepatic effects of HCV that promote ACE-2/TMPRSS mechanisms of SARS-CoV-2 viral entry.[50]
Another study found that age greater than 60 years, male gender, elevated ALT and procalcitonin levels, and high HCV viral load were all individually associated with liver damage. Additionally, ALT levels in men, an elevated HCV viral load, and a higher age above 60 were all independent risk factors for all-cause mortality.[51]
It is recommended to initiate or continue anti-HBV therapy in all patients with COVID-19 with concomitant or chronic HBV infections. Based on data on reactivation of HBV with immunosuppressive therapy, studies have suggested starting antiviral prophylaxis in patients with HBV infection and receiving corticosteroids or other immunosuppressive therapy while closely monitoring HBV virological indicators and signs of liver injury.[48,52] Antiviral therapy for HCV direct-acting antiviral (DAA) should be continued to maximize the sustained virological response rate, with close monitoring for any adverse events, because the majority of COVID-19 regimens do not have significant drug-drug interactions with agents used to treat HCV.[53] Interferon therapy for both HBV and HCV should be stopped in patients with severe COVID-19, as it is one of the potent cytokines and can contribute to SARS-CoV-2-induced cytokine release syndrome.[54]
Patients with viral hepatitis encountered difficulties during the pandemic as medical resources were diverted to the COVID-19 issue. Access to standard medical care for identifying early-stage hepatitis was restricted, and follow-up visits of patients with CLD were decreased because of concerns about exposure to COVID-19 infection from HCW and healthcare facilities. Essential testing available in large centers, like ultrasonography and nucleic acid tests, was hampered by travel restrictions, and treatment interruptions were brought on by antiviral shortages due to travel restrictions and limited flight options. Infant hepatitis B vaccination rates were also similarly affected by lockdowns. Telemedicine has evolved as a valuable patient-healthcare link on a worldwide scale, but its usefulness is limited in LMIC.[55–57] Health authorities must take steps to preserve the continuity of vital programs like HCV, HBV, and TB in order to reduce the impact of future pandemic-related interruptions, especially in low-resource nations. To minimize disruptions and sustain important health services during any potential future crises, strong contingency plans, strengthened healthcare infrastructure, and flexible service delivery methods are crucial.
Alcohol-Associated Liver Disease and COVID-19
Due to several reasons, alcohol consumption increased during the COVID-19 pandemic, leading to higher rates of alcoholic hepatitis. Data has suggested that greater mortality and morbidity were seen among these patients, with a greater incidence of acute-on-chronic liver failure and acute-on-chronic hepatitis. One postulated mechanism of injury is related to ferritin excess in patients with alcoholic hepatitis and COVID-19 infection, leading to greater free radical damage to the liver.[58–60]
The high risk of severe COVID-19 in patients with alcohol use disorder (AUD) or alcohol-induced hepatitis is secondary to the presence of underlying comorbid illnesses, suppressed immunity, concomitant smoking in many cases with underlying respiratory diseases, social isolation leading to psychological stress and increased consumption of alcohol, and use of steroids or other immunosuppressive therapy for treatment of SARS-CoV-2 infection.[61]
The medications used for treatment of alcohol use disorder (AUD) are naltrexone, nalmefene, disulfiram, acamprosate, sodium oxybate (SO), and baclofen.[62] Patients receiving treatment for AUD or antipsychotics should be closely monitored for drug-drug interactions as well as potential adverse reactions while receiving treatment for COVID-19.
Management of AUD or alcohol-related hepatitis has faced numerous difficulties during the COVID-19 pandemic due to limited access to routine medical visits, which makes it difficult to monitor diseases and implement prompt interventions. Furthermore, forced social isolation causes psychological anguish, which could lead to increased drinking or relapse. A crucial option that enables remote patient monitoring, consultation, and action is telemedicine. The psychological effects of isolation can also be addressed through online support groups and teletherapy.[54]
Metabolic Dysfunction-associated Fatty Liver Disease (MAFLD) and Severity of COVID-19
Limited data exists regarding patients with metabolically associated liver disease, also called non-alcoholic fatty liver disease, and COVID-19. The damage to hepatocytes seems to have a similar mechanism of injury as described with alcoholic hepatitis and COVID-19. In a retrospective study by Ji D. et al.,[63] consecutive patients with COVID-19 and MAFLD were studied, and liver injury was observed in 50% on admission and 75% during hospitalization. Moreover, 33% of patients had persistent abnormal liver function from admission to the last follow-up. Patients with progressive disease were older, had higher BMI, and a higher percentage of comorbidity along with MAFLD63. Studies have reported an increased risk of severe COVID-19 in patients with a high body mass index, a combination of metabolic risk factors with increased risks related to diabetes mellitus, hypertension, and coronary heart disease, and MAFLD patients with increased noninvasive liver fibrosis scores (FIB-4 score).[64,65] MAFLD patients had a longer viral shedding time, a higher likelihood of liver damage, and a higher probability of the disease progressing to severe COVID-19.[66]
There is a high risk of respiratory complications related to COVID-19 in patients with MAFLD. There is little data on the effectiveness and safety of COVID-19 therapies for MAFLD patients. The choice of medication for these patients is based on variables such as the severity of the illness, the ALT level, and probable interactions, and medication discontinuation may be required if ALT levels are six times above average. For the treatment of MAFLD in COVID-19 cases, more research is required.[67] It is recommended that MAFLD patients receive lifestyle counseling with an emphasis on reducing risk factors (such as obesity) that indicate a poor prognosis for COVID-19.
Autoimmune Liver Disease and COVID-19
AILDs, which include overlapping syndromes, primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), autoimmune hepatitis (AIH), and primary biliary cholangitis (PBC), are a diverse collection of inflammatory disorders of the liver attributed to an autoimmune response. Patients with AILDs are not at increased risk of acquiring COVID-19 infection as compared to the general population.[68]
Autoimmune hepatitis (AIH) is a chronic inflammatory disease affecting the liver, which is treated either with steroids alone or in combination with azathioprine. Other immunosuppressive agents may be required in some cases.[69,70] Prolonged use of immunosuppression can lead to an increased risk of acquiring infections; however, results of a multicenter survey in Europe suggest that AIH is not associated with an increased risk of acquiring COVID-1968. Furthermore, according to data compiled from three large-scale international reporting registries—European Association for the Study of the Liver supported COVID-Hep registry, the European Reference Network on Hepatological Diseases (ERN RARE-LIVER), and the American Association for the Study of Liver Diseases supported SECURE-cirrhosis registry—patients with AIH did not exhibit an elevated risk of adverse outcomes, such as hospitalization, intensive care unit stay, and mortality in comparison to other causes of CLD and without any liver diseases.[71] Efe et al.[72] analyzed the factors associated with severe COVID-19 outcomes in patients with AIH. They reported 37% new-onset liver injury, and the use of antivirals was associated with liver injury. The rates of severe COVID-19 and mortality were similar in both AIH and non-AIH CLD patients. In another study, a comparison of COVID-19 patients with AIH to those without liver disease showed a higher risk of hospitalization but a similar risk of all other outcomes, including mortality.[71] In both studies, cirrhosis was an independent predictor of severe COVID-19.
Patients with AIH are on immunosuppressive therapy for prolonged periods, and several studies have shown no association between immunosuppressive therapy and the severity of COVID-19. However, a subsequent study showed an increased risk of COVID-19 severity in those patients who were on steroids and azathioprine as compared to those who were not on treatment.[71,73] Tacrolimus and mycophenolate mofetil (MMF) were associated with similar outcomes.[74] The effect of tacrolimus and MMF on COVID-19 outcomes, however, requires further validation on larger cohorts, as although there are several studies.[75–77] that support the association of these drugs with COVID-19 severity, there are others which show no impact of these drugs on COVID outcomes.[78,79]
Vaccination against SARS-CoV-2 is an important preventive measure for COVID-19. Effective vaccination has reduced the need for hospitalizations and mortality due to COVID-19 in the general population throughout the World.[80] Similar outcomes have been reported in patients with chronic liver disease (CLD).[81–83] Patients on immunosuppressive therapy and those with CLD have a low antibody response to SARS-CoV-2 vaccination, and this has also been seen in AIH. An early third booster is recommended for these patients to maintain the antibody levels in the body.[84–86] Efe et al.[87] in their study on AIH patients with COVID-19 reported a significantly reduced risk for hospitalization, supplemental oxygen, and mortality in those who had received the SARS-CoV-2 vaccination.
Autoimmune Liver Disease and COVID-19
Among other adverse effects post SARS-CoV-2 vaccine, autoimmune hepatitis-like liver injury has also been reported.[88,89] A large case series of 87 patients describes 84% hepatocellular type injury, with 57% having features of immune-mediated hepatitis. Approximately half the cases received steroids. All showed complete resolution except one patient, and no relapse was reported on follow-up.[90] Although the current recommendation is against changing immunosuppressive treatment in patients with AIH, the available data to date supports the continuation of low-dose steroid therapy to keep the disease in remission.[91]
Cirrhosis and COVID-19
Decompensated cirrhosis is among the independent predictors of mortality in patients with COVID-19. Attributable factors include worsening sepsis and immune dysregulation, along with complications of cirrhosis. Data from two international reporting registries (SECURE-cirrhosis USA and COVID-Hep.net coordinated by the University of Oxford and the European Association for the Study of the Liver) showed poorer outcomes. Hepatic decompensation during COVID-19 was strongly associated with a subsequent risk of death: 63.2% of those with new decompensation died compared to 26.2% of those without new decompensation. Notably, 24.3% of those with new hepatic decompensation had no respiratory symptoms of COVID-19 at the time of diagnosis. Hence, decompensated liver disease is a significant risk factor for mortality in patients with COVID-19.[92]
A multicenter study concluded that patients with cirrhosis and COVID-19 had similar mortality compared with patients with cirrhosis alone, albeit higher than those with only COVID-19.[93] In another study, deaths occurred in 12.2% of patients with chronic liver disease (CLD) without cirrhosis, 24% of patients with Child-Turcotte-Pugh Class A (CTP-A) cirrhosis, 43% with CTP-B cirrhosis, and 63% with CTP-C cirrhosis. The cause of death in patients with cirrhosis was reported to be due to COVID-19 lung disease in 78.7%, cardiac-related in 4.3%, and liver-related in 12.2%.[94] However, studies have been conflicting, and the pooled results have shown that CLD has played a minor role in influencing patient progression toward the severe form of the disease.[95]
Cirrhosis is common, and its course is characterized by life-limiting complications such as variceal hemorrhage, ascites, hepatic encephalopathy, and hepatocellular carcinoma (HCC) that require active surveillance. Cirrhosis has a negative impact on COVID-19; thus, management and treatment should be more rigorous in terms of medication, monitoring, and follow-up. The pandemic has severely affected the management of patients with CLD and cirrhosis, both with and without COVID-19. The delay has resulted in rapid progression of hepatic diseases, variceal bleed, increased incidence of hepatocellular carcinoma (HCC), delay in procedures including liver transplant, and overall mortality.[96]
There should be provision of holistic, personalized, and continuous care plans for patients with cirrhosis and CLD to avoid such complications in future crisis situations. There are new recommendations suggested by experts, i.e., changing HCC surveillance from 3 months to 12 months, telemedicine, prioritizing care to high-risk patients, availability of medicine, and simpler surveillance techniques in the community; the effectiveness of these is yet unknown.[97,98] More clinical data is required to determine the effect of these recommendations on patient outcomes and to ensure they are appropriate given the ongoing healthcare issues.
Approach to a COVID-19 Patient with Liver Injury
Hepatic involvement in COVID-19 can range from asymptomatic status to severe disease. Males are substantially more likely than females to get liver damage. The liver injury is predominantly found in patients with severe and critical COVID-19 and those with prior comorbid illnesses like hypertension, diabetes mellitus, cardiovascular diseases, and malignancies.[99] Studies have identified several risk factors for hepatic impairment in COVID-19 patients, including old age, obesity, severe and critical COVID-19, male gender, existing liver diseases, high C-Reactive Protein, lymphopenia, and low AST/ALT ratio.[7,100]
A significant proportion of patients with COVID-19 have abnormal liver biochemical tests at the time of presentation to a healthcare facility.[101] Furthermore, patients with normal liver functions can also develop hepatic injury during their stay in the hospital with the progression of the disease course41. Although uncommon, a few cases of fulminant hepatic failure with COVID-19 have also been reported in the literature.[102–104] The biochemical indicators of liver injury are elevated total bilirubin (TB), alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyl transferase (GGT), alkaline phosphatase (AP), increased prothrombin time (PT) including INR, and reduced levels of serum albumin in patients with COVID-19.[105] The abnormal liver function is found to be associated with critical disease, stay in high dependency units, ICU admission, need for ventilator support, prolonged hospital stay, and death.[41] Among three forms of liver impairments i.e., hepatocellular injury, cholestasis, and hepatocellular dysfunction, patients with hepatocellular dysfunction or mixed type have an increased likelihood of developing a serious illness.[106] Hence, monitoring of liver function tests is warranted for patients with severe or critical COVID-19 from the time of admission.
Management
Most COVID-19 individuals experience minor, transient liver damage which returns to normal without treatment.[24] Supportive treatment is required for the management of hepatic function impairment in patients with COVID-19. Detailed history and examination are required to identify any pre-existing liver diseases, prior medication, or hepatotoxic agent use. Careful monitoring of liver function tests is recommended, especially in the presence of risk factors for hepatotoxicity and previous liver diseases. In patients with severe COVID-19 and pre-existing liver pathology, medical management should not include more than two drugs that have the potential for drug-induced liver injury or drug-drug interactions. Close monitoring of the dosage and drug interactions, along with regular measurement of liver enzymes, is advisable, with a prompt dose adjustment or drug discontinuation as required. Anti-inflammatory liver protection and hepatoprotective drug treatment should also be initiated as required.[107] Abnormal liver functions can persist longer than clinical recovery from acute illness, which necessitates long-term monitoring of liver function tests in patients with severe COVID-19.[24]
HCV and HBV patients should continue taking antiviral medications as discontinuation in the setting of a COVID-19 infection is not recommended in the guidelines issued by the AASLD (American Association for the Study of Liver Diseases) unless there is a pattern of worsening liver enzymes, in which case drug removal can be considered. In patients not on antiviral therapy and with a history of HBV, HbsAg testing can be conducted to rule out flares.[108] In patients with liver cirrhosis, due to the high likelihood of severe complications and death, patients should continue receiving appropriate treatment.[97] Patients with comorbid conditions related to metabolic associated fatty liver disease (MAFLD) such as diabetes, dyslipidemia, and obesity should be evaluated for liver injury as a result of MAFLD in the setting of elevated liver enzymes.[24]
Changing Trends in Management
The management of COVID-19 has evolved considerably over the years with the addition of newer antivirals and use of monoclonal antibodies, as opposed to early in the pandemic when no approved drugs existed for this disease.[109] Furthermore, owing to vaccination, the disease manifests with relatively less severity, leading to lesser requirements for salvage treatment with IL-6 antagonist Tocilizumab. This has a favorable outlook for liver injury arising due to the use of Tocilizumab, as well as liver injury as a result of the disease itself. While one of the antivirals, Remdesivir, has been associated with drug-induced liver injury, the impact has rarely been significant enough to require discontinuation of treatment.
Biopsy Findings in COVID-19 Associated Liver Injury
Post-mortem analysis has shown that there are two possible common pathways that cause the histological changes typified in COVID-associated liver injury: a post-infectious immune response and immune dysregulation.[110]
Post-Infectious Immune Response
After viral infection, there may be an exaggerated immune response, characterized by acute hepatitis and prominent bile duct injury, which can lead to significant damage to the liver.[111] The histological evidence of this would be mild to severe hepatic congestion, focal confluent necrosis, hepatocyte necrosis, and lobular necroinflammation, underscoring the severity of immune-mediated liver injury.[112,113]
Immune Dysregulation and Susceptibility to Subsequent Infections
Another intriguing hypothesis is that the immune dysregulation that occurs during the primary SARS-CoV-2 infection can prime the liver to react abnormally to other pathogens, such as adenoviruses. The liver expresses ACE-2 receptors, which are active binding sites for SARS-CoV-2 in the lungs and may play a role in the binding occurring in the liver as well. This binding occurs potentially more frequently and avidly on cholangiocytes rather than hepatocytes.[114] The histological manifestation of this is present in sinusoidal dilation in zone 3, patchy hepatic necrosis, and the presence of cirrhosis.[115] The presence of cirrhosis may also suggest that there is further exacerbation or reactivation of underlying chronic liver diseases. Furthermore, a constellation of drug-induced liver injury and ischemic liver injury due to hypoxic and shock-like states may also explain the injury occurring to non-cholangiocytic cell types which have a far lower concentration of ACE-2 receptors.[12]
Common Histological Findings
Biopsy findings in patients diagnosed with COVID-19-associated liver injury frequently exhibit the following patterns:
Hepatic Congestion: Ranging from mild to severe, it may occur as a result of severe respiratory disease, which causes right ventricular failure leading to elevated venous pressure within the liver and its vascular system.[113]
Ischemic Changes: The presence of focal confluent necrosis and hepatocyte necrosis suggests the possibility of a low oxygen state within the liver.[113] This may result from shock, a shock-like state, or chronically low oxygen saturation due to a decrease in lung functionality.[115]
Steatosis: A very common finding on biopsies is the presence of increased fat and glycogen accumulation within the liver.[116] This may be present in a pan-lobular pattern and suggests an additional mechanism of liver injury.[112] This may also manifest as microvascular and macrovascular steatosis.[117]
Despite this wide range of abnormalities that may occur in the liver and cause injury that warrants further investigation, the routine usage of biopsies in cases of liver injury associated with COVID-19 infections is not recommended.[117]
Implications of COVID-19 Associated Liver Injury
Short-Term Impact on Patient Outcomes
COVID-19-associated liver injury may be mild to severe. The prevalence of mild transaminitis has been reported between 15% to 50% with ALT/AST ratio reversal, and in the majority of cases, it is self-limiting.[118] In most patients, the ALT and AST values peak after day 6 to up to 2 weeks of illness.[119] However, studies have also reported severe liver injury with incidences reported to be higher than or equal to 70% in those patients who died of the disease.[120,121] Hence, COVID-19-associated liver injury is a marker of severity of disease or a predictor for progression of disease, whether it is a consequence of direct hepatotoxicity of SARS-CoV-2 virus or a manifestation of multi-organ dysfunction. Moreover, the risk of death may be directly proportional to the degree of transaminitis.[119] In patients hospitalized with COVID-19, a moderate rise in liver function tests (LFTs) during admission was linked to a poor short-term outcome. Among patients with pre-existing liver disease, the presence of cirrhosis was reported to be an independent predictor of higher 30-day mortality.[122] Furthermore, the presence of liver injury in hospitalized COVID-19 patients results in a prolonged length of hospital stay.[123–125]
Long-Term Impact on Patient Outcomes
The majority of cases of COVID-19-associated liver injury are acute, with a transient rise in biomarkers that self-resolve over a short period of time. Even in the case of acute-on-chronic liver injury, the rise in hepatic biomarkers is non-permanent, with the patient returning to their pre-COVID baseline soon after.
Although COVID-19 is a self-limited viral infection, its effects can be felt for weeks and even months after resolution. The major manifestation of this is in the form of long-COVID, a constellation of signs and symptoms that affects multiple organ systems and presents after the resolution of the initial acute infection, presenting from 14 to 110 days post-infection with an estimated incidence rate of 80%.[126] The major symptoms are fatigue, breathlessness, arthralgia, and chest pain; however, there is multi-system involvement, including but not limited to respiratory, cardiovascular, neurologic, renal, mental health, and hepatic.[127,128]
A study examining long-term outcomes of liver function derangements in COVID-19 found that approximately 28% of patients have persistent abnormalities at one year of follow-up.[129] In contrast, another study from China reports persistent abnormalities in transaminase levels in 13%, with the majority recovering uneventfully at 12-month follow-up.[130]
After the end of the acute phase, there is still an underlying risk of an increased level of hepatic biomarkers that do not return to their pre-infection baseline.[131] In a study conducted on low-risk individuals with post-COVID-19 syndrome, it was found that 28% had mild injury and increased fat accumulation in the liver, which was associated with a need for hospitalization.[132] Another study looking at patients who had persisting symptoms found that there was increased liver stiffness and steatosis, suggesting that there is an increased buildup of fats and fibrotic/necrotic tissue within the liver.[133]
Furthermore, there is also evidence of damage to the bile ducts, leading to post-COVID cholangiopathy. This results from ischemic changes, microthrombosis, direct liver injury, drug-induced and autoimmune factors. Management with ursodeoxycholic acid and cholestyramine offered no significant clinical benefit.[134]
Moreover, a study exploring liver fibrosis after COVID-19 using a liver fibrosis index found a 5% incidence of liver fibrosis in the post-COVID-19 group when they followed the cohort for 3–6 months.[135] Post-acute COVID syndrome (PACS) is now a well-recognized clinical entity and is associated with several systemic manifestations including metabolic-associated fatty liver disease (MAFLD). A recent study has described an increased prevalence of this condition amongst post-COVID-19 patients. The long-term consequences of MAFLD as a post-COVID-19 condition remain to be ascertained and may potentially have an impact on cardiovascular health outcomes.[136]
Based on the understanding that long-term complications of COVID-19 on the liver are widely reported, the most potent method to ensure that further damage via fibrosis does not take place is to ensure that patients who have suffered liver injury in the acute setting, or are at risk of developing long-COVID related liver injury, be screened to ensure that there is a decrease in hepatic biomarkers.
Conclusion
In conclusion, COVID-19-associated liver injury can occur through multiple mechanisms, and recognition and prompt management are crucial due to its significant impact on mortality and long-term morbidity. Further research and understanding of COVID-19-associated liver injury are needed to improve patient outcomes and develop effective management strategies.
Footnotes
How to cite this article: Nasir N, Khanum I, Habib K, Wagley A, Arshad A, Majeed A. Insight into COVID-19 associated liver injury: Mechanisms, evaluation, and clinical implications. Hepatology Forum 2024; 5(3):139–149.
Author Contributions
Concept – NN, KH, IK, AM, AW, AA; Design – NN, KH, IK, AM, AW, AA; Supervision – NN, KH, IK, AM, AW, AA; Analysis and/or Interpretation – NN, KH, IK, AM, AW, AA; Literature Search – NN, KH, IK, AM, AW, AA; Writing – NN, KH, IK, AM, AW, AA; Critical Reviews – NN, KH, IK, AM, AW, AA.
Conflict of Interest
The authors have no conflict of interest to declare.
Use of AI for Writing Assistance
Not declared.
Financial Disclosure
The authors declared that this study has received no financial support.
Peer-review
Externally peer-reviewed.
References
- 1.Wise J. Covid-19: WHO declares end of global health emergency. BMJ. 2023;381:1041. doi: 10.1136/bmj.p1041. [DOI] [PubMed] [Google Scholar]
- 2.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du M, Yang S, Liu M, Liu J. COVID-19 and liver dysfunction: Epidemiology, association and potential mechanisms. Clin Res Hepatol Gastroenterol. 2022;46(2):101793. doi: 10.1016/j.clinre.2021.101793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cichoż-Lach H, Michalak A. Liver injury in the era of COVID-19. World J Gastroenterol. 2021;27(5):377–390. doi: 10.3748/wjg.v27.i5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41(1):20–32. doi: 10.1111/liv.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu D, Du Q, Yan S, Guo XG, He Y, Zhu G, et al. Liver injury in COVID-19: Clinical features and treatment management. Virol J. 2021;18(1):121. doi: 10.1186/s12985-021-01593-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ippolito D, Maino C, Vernuccio F, Cannella R, Inchingolo R, Dezio M, et al. Liver involvement in patients with COVID-19 infection: A comprehensive overview of diagnostic imaging features. World J Gastroenterol. 2023;29(5):834–850. doi: 10.3748/wjg.v29.i5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu WS, Jiang FY, Shu W, Zhao R, Cao JM, Wang DP. Liver injury in COVID-19: A minireview. World J Gastroenterol. 2022;28(47):6716–6731. doi: 10.3748/wjg.v28.i47.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. Covid-19: Abnormal liver function tests. J Hepatol. 2020;73(3):566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrido I, Liberal R, Macedo G. Review article: Covid-19 and liver disease-What we know on 1st May 2020. Aliment Pharmacol Ther. 2020;52(2):267–275. doi: 10.1111/apt.15813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herath CB, Warner FJ, Lubel JS, Dean RG, Jia Z, Lew RA, et al. Upregulation of hepatic angiotensin-converting enzyme 2 (ACE2) and angiotensin-(1-7) levels in experimental biliary fibrosis. J Hepatol. 2007;47(3):387–395. doi: 10.1016/j.jhep.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pirola CJ, Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: Putative mechanisms of liver involvement in Covid-19. Liver Int. 2020;40(8):2038–2040. doi: 10.1111/liv.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33(6):1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11(10):771–775. doi: 10.1007/s13238-020-00718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi X, Wang J, Li X, Wang Z, Liu Y, Yang H, et al. Clinical course of COVID-19 in patients with pre-existing decompensated cirrhosis: Initial report from China. Hepatol Int. 2020;14(4):478–482. doi: 10.1007/s12072-020-10051-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, et al. Clinical best practice advice for hepatology and liver transplant providers during the Covid-19 pandemic: AASLD expert panel consensus statement. Hepatology. 2020;72(1):287–304. doi: 10.1002/hep.31281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mojtabavi H, Saghazadeh A, Rezaei N. Interleukin-6 and severe Covid-19: A systematic review and meta-analysis. Eur Cytokine Netw. 2020;31(2):44–49. doi: 10.1684/ecn.2020.0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, et al. COVID-19 infection: The perspectives on immune responses. Cell Death Differ. 2020;27(5):1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, et al. COVID-19 and liver dysfunction: Current insights and emergent therapeutic strategies. J Clin Transl Hepatol. 2020;8(1):18–24. doi: 10.14218/JCTH.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized With Covid-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am J Emerg Med. 2008;26(6):711–715. doi: 10.1016/j.ajem.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 24.Li P, Liu Y, Cheng Z, Yu X, Li Y. COVID-19-associated liver injury: Clinical characteristics, pathophysiological mechanisms and treatment management. Biomed Pharmacother. 2022;154:113568. doi: 10.1016/j.biopha.2022.113568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henrion J, Schapira M, Luwaert R, Colin L, Delannoy A, Heller FR. Hypoxic hepatitis: Clinical and hemodynamic study in 142 consecutive cases. Medicine (Baltimore) 2003;82(6):392–406. doi: 10.1097/01.md.0000101573.54295.bd. [DOI] [PubMed] [Google Scholar]
- 26.Seeto RK, Fenn B, Rockey DC. Ischemic hepatitis: Clinical presentation and pathogenesis. Am J Med. 2000;109(2):109–113. doi: 10.1016/s0002-9343(00)00461-7. [DOI] [PubMed] [Google Scholar]
- 27.Zhang XJ, Cheng X, Yan ZZ, Fang J, Wang X, Wang W, et al. An ALOX12-12-HETE-GPR31 signaling axis is a key mediator of hepatic ischemia-reperfusion injury. Nat Med. 2018;24(1):73–83. doi: 10.1038/nm.4451. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Lei J, Li Z, Yan L. Potential effects of coronaviruses on the liver: An update. Front Med (Lausanne) 2021;8:651658. doi: 10.3389/fmed.2021.651658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caraceni P, Domenicali M, Vendemiale G, Grattagliano I, Pertosa A, Nardo B, et al. The reduced tolerance of rat fatty liver to ischemia reperfusion is associated with mitochondrial oxidative injury. J Surg Res. 2005;124(2):160–168. doi: 10.1016/j.jss.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Philippe A, Chocron R, Gendron N, Bory O, Beauvais A, Peron N, et al. Circulating Von Willebrand factor and high molecular weight multimers as markers of endothelial injury predict Covid-19 in-hospital mortality. Angiogenesis. 2021;24(3):505–517. doi: 10.1007/s10456-020-09762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chalasani NP, Hayashi PH, Bonkovsky HL, Navarro VJ, Lee WM, Fontana RJ. ACG Clinical Guideline: The diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950–967. doi: 10.1038/ajg.2014.131. [DOI] [PubMed] [Google Scholar]
- 33.Clark R, Fisher JE, Sketris IS, Johnston GM. Population prevalence of high dose paracetamol in dispensed paracetamol/opioid prescription combinations: An observational study. BMC Clin Pharmacol. 2012;12:11. doi: 10.1186/1472-6904-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herndon CM, Dankenbring DM. Patient perception and knowledge of acetaminophen in a large family medicine service. J Pain Palliat Care Pharmacother. 2014;28(2):109–116. doi: 10.3109/15360288.2014.908993. [DOI] [PubMed] [Google Scholar]
- 35.Bunchorntavakul C, Reddy KR. Acetaminophen-related hepatotoxicity. Clin Liver Dis. 2013;17(4):587–607. doi: 10.1016/j.cld.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 36.Yoon E, Babar A, Choudhary M, Kutner M, Pyrsopoulos N. Acetaminophen-induced hepatotoxicity: A comprehensive update. J Clin Transl Hepatol. 2016;4(2):131–142. doi: 10.14218/JCTH.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382(24):2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020;383(19):1827–1837. doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun J, Deng X, Chen X, Huang J, Huang S, Li Y, et al. Incidence of adverse drug reactions in covid-19 patients in China: An active monitoring study by hospital pharmacovigilance system. Clin Pharmacol Ther. 2020;108(4):791–797. doi: 10.1002/cpt.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, et al. Clinical features of COVID-19-related liver functional abnormality. Clin Gastroenterol Hepatol. 2020;18(7):1561–1566. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ridruejo E, Soza A. The liver in times of COVID-19: What hepatologists should know. Ann Hepatol. 2020;19(4):353–358. doi: 10.1016/j.aohep.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perreault G, Ching C, Nobel YR. COVID-19 in patients with liver disease and liver transplant: Clinical implications, prevention, and management. Therap Adv Gastroenterol. 2023;16:17562848231188586. doi: 10.1177/17562848231188586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagarajan R, Krishnamoorthy Y, Rajaa S, Hariharan VS. COVID-19 severity and mortality among chronic liver disease patients: A systematic review and meta-analysis. Prev Chronic Dis. 2022;19:E53. doi: 10.5888/pcd19.210228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liatsos GD. SARS-CoV-2 induced liver injury: Incidence, risk factors, impact on COVID-19 severity and prognosis in different population groups. World J Gastroenterol. 2023;29(16):2397–2432. doi: 10.3748/wjg.v29.i16.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hariyanto TI, Jodhinata C, Halim DA, Kurniawan A. Association between viral hepatitis and increased risk of severe coronavirus disease 2019 (COVID-19) outcome: A systematic review and meta-analysis. Gastroenterol Hepatol Bed Bench. 2022;15(1):9–14. [PMC free article] [PubMed] [Google Scholar]
- 48.Sagnelli C, Montella L, Grimaldi P, Pisaturo M, Alessio L, De Pascalis S, et al. COVID-19 as another trigger for HBV reactivation: Clinical Case and review of literature. Pathogens. 2022;11(7):816. doi: 10.3390/pathogens11070816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodríguez-Tajes S, Miralpeix A, Costa J, López-Suñé E, Laguno M, Pocurull A, et al. Low risk of hepatitis B reactivation in patients with severe COVID-19 who receive immunosuppressive therapy. J Viral Hepat. 2021;28(1):89–94. doi: 10.1111/jvh.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ronderos D, Omar AMS, Abbas H, Makker J, Baiomi A, Sun H, et al. Chronic hepatitis-C infection in COVID-19 patients is associated with in-hospital mortality. World J Clin Cases. 2021;9(29):8749–8762. doi: 10.12998/wjcc.v9.i29.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cerbu B, Pantea S, Bratosin F, Vidican I, Turaiche M, Frent S, et al. Liver impairment and hematological changes in patients with chronic hepatitis C and COVID-19: A retrospective study after one year of pandemic. Medicina (Kaunas) 2021;57(6):597. doi: 10.3390/medicina57060597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yip TC, Gill M, Wong GL, Liu K. Management of hepatitis B virus reactivation due to treatment of COVID-19. Hepatol Int. 2022;16(2):257–268. doi: 10.1007/s12072-022-10306-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong GL, Wong VW, Thompson A, Jia J, Hou J, Lesmana CRA, et al. Management of patients with liver derangement during the COVID-19 pandemic: An Asia-Pacific position statement. Lancet Gastroenterol Hepatol. 2020;5(8):776–787. doi: 10.1016/S2468-1253(20)30190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamid S, Alvares Da Silva MR, Burak KW, Chen T, Drenth JPH, Esmat G, et al. WGO guidance for the care of patients with COVID-19 and liver disease. Available at https://ses.library.usyd.edu.au/handle/2123/25300 Accessed on May 22, 2024. [DOI] [PMC free article] [PubMed]
- 55.Pley CM, McNaughton AL, Matthews PC, Lourenço J. The global impact of the COVID-19 pandemic on the prevention, diagnosis and treatment of hepatitis B virus (HBV) infection. BMJ Glob Health. 2021;6(1):e004275. doi: 10.1136/bmjgh-2020-004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rehman ST, Rehman H, Abid S. Impact of coronavirus disease 2019 on prevention and elimination strategies for hepatitis B and hepatitis C. World J Hepatol. 2021;13(7):781–789. doi: 10.4254/wjh.v13.i7.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ismail Z, Aborode AT, Oyeyemi AA, Khan H, Hasan MM, Saha A, et al. Impact of COVID-19 pandemic on viral hepatitis in Africa: Challenges and way forward. Int J Health Plann Manage. 2022;37(1):547–552. doi: 10.1002/hpm.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Julien J, Ayer T, Tapper EB, Barbosa C, Dowd WN, Chhatwal J. Effect of increased alcohol consumption during COVID-19 pandemic on alcohol-associated liver disease: A modeling study. Hepatology. 2022;75(6):1480–1490. doi: 10.1002/hep.32272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cholankeril G, Goli K, Rana A, Hernaez R, Podboy A, Jalal P, et al. Impact of COVID-19 pandemic on liver transplantation and alcohol-associated liver disease in the USA. Hepatology. 2021;74(6):3316–3329. doi: 10.1002/hep.32067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Testino G, DI Biagio A, Fagoonee S, Pellicano R. SARS-CoV-2, alcohol consumption and liver injury. Minerva Med. 2022;113(2):333–342. doi: 10.23736/S0026-4806.21.07809-5. [DOI] [PubMed] [Google Scholar]
- 61.Da BL, Im GY, Schiano TD. Coronavirus disease 2019 hangover: A rising tide of alcohol use disorder and alcohol-associated liver disease. Hepatology. 2020;72(3):1102–1108. doi: 10.1002/hep.31307. [DOI] [PubMed] [Google Scholar]
- 62.Antonelli M, Ferrulli A, Sestito L, Vassallo GA, Tarli C, Mosoni C, et al. Alcohol addiction–The safety of available approved treatment options. Expert Opin Drug Saf. 2018;17(2):169–177. doi: 10.1080/14740338.2018.1404025. [DOI] [PubMed] [Google Scholar]
- 63.Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, et al. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73(2):451–453. doi: 10.1016/j.jhep.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, et al. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69(8):1545–1547. doi: 10.1136/gutjnl-2020-321611. [DOI] [PubMed] [Google Scholar]
- 65.Xu L, Mao Y, Chen G. Risk factors for 2019 novel coronavirus disease (COVID-19) patients progressing to critical illness: A systematic review and meta-analysis. Aging (Albany NY) 2020;12(12):12410–12421. doi: 10.18632/aging.103383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang R, Zhu L, Wang J, Xue L, Liu L, Yan X, et al. Clinical features of patients with COVID-19 with nonalcoholic fatty liver disease. Hepatol Commun. 2020;4(12):1758–1768. doi: 10.1002/hep4.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jeeyavudeen MS, Chaudhari R, Pappachan JM, Fouda S. Clinical implications of COVID-19 in patients with metabolic-associated fatty liver disease. World J Gastroenterol. 2023;29(3):487–502. doi: 10.3748/wjg.v29.i3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zecher BF, Buescher G, Willemse J, Walmsley M, Taylor A, Leburgue A, et al. Prevalence of COVID-19 in patients with autoimmune liver disease in Europe: A patient-oriented online survey. United European Gastroenterol J. 2021;9(7):797–808. doi: 10.1002/ueg2.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nur Dagli S, Efe C. Coronavirus disease 2019 (COVID-19) in autoimmune hepatitis. Hepatol Forum. 2022;3(2):68–70. doi: 10.14744/hf.2022.2022.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 Practice guidance and guidelines from the American Association for the study of liver diseases. Hepatology. 2020;72(2):671–722. doi: 10.1002/hep.31065. [DOI] [PubMed] [Google Scholar]
- 71.Marjot T, Buescher G, Sebode M, Barnes E, Barritt AS 4th, Armstrong MJ, et al. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol. 2021;74(6):1335–1343. doi: 10.1016/j.jhep.2021.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Efe C, Dhanasekaran R, Lammert C, Ebik B, Higuera-de la Tijera F, Aloman C, et al. Outcome of COVID-19 in patients with autoimmune hepatitis: An international multicenter study. Hepatology. 2021;73(6):2099–2109. doi: 10.1002/hep.31797. Erratum 2022 Hepatology 75 3 774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gerussi A, Rigamonti C, Elia C, Cazzagon N, Floreani A, Pozzi R, et al. Coronavirus disease 2019 in Autoimmune hepatitis: A lesson from ımmunosuppressed patients. Hepatol Commun. 2020;4(9):1257–1262. doi: 10.1002/hep4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Efe C, Lammert C, Taşçılar K, Dhanasekaran R, Ebik B, Higuera-de la Tijera F, et al. Effects of immunosuppressive drugs on COVID-19 severity in patients with autoimmune hepatitis. Liver Int. 2022;42(3):607–614. doi: 10.1111/liv.15121. [DOI] [PubMed] [Google Scholar]
- 75.Rabiee A, Sadowski B, Adeniji N, Perumalswami PV, Nguyen V, Moghe A, et al. Liver injury in liver transplant recipients with coronavirus disease 2019 (COVID-19): US multicenter experience. Hepatology. 2020;72(6):1900–1911. doi: 10.1002/hep.31574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Webb GJ, Marjot T, Cook JA, Aloman C, Armstrong MJ, Brenner EJ, et al. Outcomes following SARS-CoV-2 infection in liver transplant recipients: An international registry study. Lancet Gastroenterol Hepatol. 2020;5(11):1008–1016. doi: 10.1016/S2468-1253(20)30271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Belli LS, Fondevila C, Cortesi PA, Conti S, Karam V, Adam R, et al. Protective role of tacrolimus, deleterious role of age and comorbidities in liver transplant recipients with Covid-19: Results from the ELITA/ELTR multi-center European Study. Gastroenterology. 2021;160(4):1151–1163. doi: 10.1053/j.gastro.2020.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74(1):148–155. doi: 10.1016/j.jhep.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nørgård BM, Nielsen J, Knudsen T, Nielsen RG, Larsen MD, Jølving LR, et al. Hospitalization for COVID-19 in patients treated with selected immunosuppressant and immunomodulating agents, compared to the general population: A Danish cohort study. Br J Clin Pharmacol. 2021;87(4):2111–2120. doi: 10.1111/bcp.14622. [DOI] [PubMed] [Google Scholar]
- 80.Khandker SS, Godman B, Jawad MI, Meghla BA, Tisha TA, Khondoker MU, et al. A systematic review on COVID-19 vaccine strategies, their effectiveness, and ıssues. Vaccines (Basel) 2021;9(12):1387. doi: 10.3390/vaccines9121387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.John BV, Deng Y, Scheinberg A, Mahmud N, Taddei TH, Kaplan D, et al. Association of BNT162b2 mRNA and mRNA-1273 vaccines with COVID-19 infection and hospitalization among patients with cirrhosis. JAMA Intern Med. 2021;181(10):1306–1314. doi: 10.1001/jamainternmed.2021.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moon AM, Webb GJ, García-Juárez I, Kulkarni AV, Adali G, Wong DK, et al. SARS-CoV-2 infections among patients with liver disease and liver transplantation who received COVID-19 vaccination. Hepatol Commun. 2022;6(4):889–897. doi: 10.1002/hep4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.John BV, Deng Y, Schwartz KB, Taddei TH, Kaplan DE, Martin P, et al. Postvaccination COVID-19 infection is associated with reduced mortality in patients with cirrhosis. Hepatology. 2022;76(1):126–138. doi: 10.1002/hep.32337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duengelhoef P, Hartl J, Rüther D, Steinmann S, Brehm TT, Weltzsch JP, et al. SARS-CoV-2 vaccination response in patients with autoimmune hepatitis and autoimmune cholestatic liver disease. United European Gastroenterol J. 2022;10(3):319–329. doi: 10.1002/ueg2.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Galmiche S, Luong Nguyen LB, Tartour E, de Lamballerie X, Wittkop L, Loubet P, et al. Immunological and clinical efficacy of COVID-19 vaccines in immunocompromised populations: A systematic review. Clin Microbiol Infect. 2022;28(2):163–177. doi: 10.1016/j.cmi.2021.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chauhan M, Nzeako I, Li F, Thuluvath PJ. Antibody response after a booster dose of SARS-CoV-2 vaccine in liver transplant recipients and those with chronic liver diseases. Ann Hepatol. 2022;27(4):100702. doi: 10.1016/j.aohep.2022.100702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Efe C, Taşçılar K, Gerussi A, Bolis F, Lammert C, Ebik B, et al. SARS-CoV-2 vaccination and risk of severe COVID-19 outcomes in patients with autoimmune hepatitis. J Autoimmun. 2022;132:102906. doi: 10.1016/j.jaut.2022.102906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shroff H, Satapathy SK, Crawford JM, Todd NJ, VanWagner LB. Liver injury following SARS-CoV-2 vaccination: A multicenter case series. J Hepatol. 2022;76(1):211–214. doi: 10.1016/j.jhep.2021.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bril F, Al Diffalha S, Dean M, Fettig DM. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: Causality or casualty? J Hepatol. 2021;75(1):222–224. doi: 10.1016/j.jhep.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Efe C, Kulkarni AV, Terziroli Beretta-Piccoli B, Magro B, Stättermayer A, Cengiz M, et al. Liver injury after SARS-CoV-2 vaccination: Features of immune-mediated hepatitis, role of corticosteroid therapy and outcome. Hepatology. 2022;76(6):1576–1586. doi: 10.1002/hep.32572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Floreani A, De Martin S. COVID-19 and autoimmune liver diseases. J Clin Med. 2022;11(10):2681. doi: 10.3390/jcm11102681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74(3):567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bajaj JS, Garcia-Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, et al. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: Multicentre matched cohort. Gut. 2021;70(3):531–536. doi: 10.1136/gutjnl-2020-322118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moon AM, Webb GJ, Aloman C, Armstrong MJ, Cargill T, Dhanasekaran R, et al. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: Preliminary results from an international registry. J Hepatol. 2020;73(3):705–708. doi: 10.1016/j.jhep.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lippi G, de Oliveira MHS, Henry BM. Chronic liver disease is not associated with severity or mortality in Coronavirus disease 2019 (COVID-19): A pooled analysis. Eur J Gastroenterol Hepatol. 2021;33(1):114–115. doi: 10.1097/MEG.0000000000001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tapper EB, Asrani SK. The COVID-19 pandemic will have a long-lasting impact on the quality of cirrhosis care. J Hepatol. 2020;73(2):441–445. doi: 10.1016/j.jhep.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boettler T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Cornberg M, et al. Care of patients with liver disease during the COVID-19 pandemic: EASL-ESCMID position paper. JHEP Rep. 2020;2(3):100113. doi: 10.1016/j.jhepr.2020.100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang JH, Chang KC, Kee KM, Chen PF, Yen YH, Tseng PL, et al. Hepatocellular carcinoma surveillance at 4-vs. 12-month intervals for patients with chronic viral hepatitis: A randomized study in community. Am J Gastroenterol. 2013;108(3):416–424. doi: 10.1038/ajg.2012.445. [DOI] [PubMed] [Google Scholar]
- 99.Shen JX, Zhuang ZH, Zhang QX, Huang JF, Chen GP, Fang YY, et al. Risk factors and prognosis in patients with COVID-19 and liver injury: A retrospective analysis. J Multidiscip Healthc. 2021;14:629–637. doi: 10.2147/JMDH.S293378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen F, Chen W, Chen J, Xu D, Xie W, Wang X, et al. Clinical features and risk factors of COVID-19-associated liver injury and function: A retrospective analysis of 830 cases. Ann Hepatol. 2021;21:100267. doi: 10.1016/j.aohep.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xing QQ, Dong X, Ren YD, Chen WM, Zeng DY, Cai YY, et al. Liver chemistries in patients with COVID-19 who were discharged alive or died: A meta-analysis. Hepatol Commun. 2020;5(1):12–23. doi: 10.1002/hep4.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ding ZY, Li GX, Chen L, Shu C, Song J, Wang W, et al. Association of liver abnormalities with in-hospital mortality in patients with COVID-19. J Hepatol. 2021;74(6):1295–1302. doi: 10.1016/j.jhep.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aldossary B, Hassan A, Moussa M, Alsaif HS, Alfaraj D. Fulminant hepatic failure in a patient testing re-positive for SARS-CoV-2: A case report. Int J Emerg Med. 2021;14(1):24. doi: 10.1186/s12245-021-00349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Melquist S, Estepp K, Aleksandrovich Y, Lee A, Beiseker A, Hamedani FS, et al. COVID-19 presenting as fulminant hepatic failure: A case report. Medicine (Baltimore) 2020;99(43):e22818. doi: 10.1097/MD.0000000000022818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kovalic AJ, Huang G, Thuluvath PJ, Satapathy SK. Elevated liver biochemistries in hospitalized chinese patients with severe COVID-19: Systematic review and meta-analysis. Hepatology. 2021;73(4):1521–1530. doi: 10.1002/hep.31472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mohammed SA, Eid KM, Anyiam FE, Wadaaallah H, Muhamed MAM, Morsi MH, et al. Liver injury with COVID-19: Laboratory and histopathological outcome-systematic review and meta-analysis. Egypt Liver J. 2022;12(1):9. doi: 10.1186/s43066-022-00171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sodeifian F, Seyedalhosseini ZS, Kian N, Eftekhari M, Najari S, Mirsaeidi M, et al. Drug-induced liver injury in COVID-19 patients: A systematic review. Front Med (Lausanne) 2021;8:731436. doi: 10.3389/fmed.2021.731436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.AASLD AASLD expert panel consensus statement: Covid-19 clinical best practice advice for hepatology and liver transplant providers. Available at https://www.aasld.org/sites/default/files/2022-10/AASLD%20COVID-19%20Guidance%20Document%2010.06.2022F.pdf Accessed on May 22, 2024. [DOI] [PMC free article] [PubMed]
- 109.Yapali S. What hepatologists need to know about COVID-19? Hepatol Forum. 2020;1(2):41–43. doi: 10.14744/hf.2020.2020.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alexander EC, Deep A. Characterization of a hepatitis outbreak in children, 2021 to 2022. JAMA Netw Open. 2022;5(10):e2237091. doi: 10.1001/jamanetworkopen.2022.37091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kleiner DE. Liver biopsy shines a light on COVID-19-related liver injury. Cell Mol Gastroenterol Hepatol. 2021;11(3):881–882. doi: 10.1016/j.jcmgh.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lagana SM, Kudose S, Iuga AC, Lee MJ, Fazlollahi L, Remotti HE, et al. Hepatic pathology in patients dying of COVID-19: A series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33(11):2147–2155. doi: 10.1038/s41379-020-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Beigmohammadi MT, Jahanbin B, Safaei M, Amoozadeh L, Khoshavi M, Mehrtash V, et al. Pathological findings of postmortem biopsies from lung, heart, and liver of 7 deceased COVID-19 patients. Int J Surg Pathol. 2021;29(2):135–145. doi: 10.1177/1066896920935195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 expression in cholangiocytes may cause liver damage After 2019-nCoV infection. Available at: https://www.biorxiv.org/content/10.1101/2020.02.03.931766v1 Accessed on May 22, 2024.
- 115.Deshmukh V, Motwani R, Kumar A, Kumari C, Raza K. Histopathological observations in COVID-19: A systematic review. J Clin Pathol. 2021;74(2):76–83. doi: 10.1136/jclinpath-2020-206995. [DOI] [PubMed] [Google Scholar]
- 116.Li Y, Xiao SY. Hepatic involvement in COVID-19 patients: Pathology, pathogenesis, and clinical implications. J Med Virol. 2020;92(9):1491–1494. doi: 10.1002/jmv.25973. [DOI] [PubMed] [Google Scholar]
- 117.Hamid S, Alvares da Silva MR, Burak KW, Chen T, Drenth JPH, Esmat G, et al. WGO guidance for the care of patients with COVID-19 and liver disease. J Clin Gastroenterol. 2021;55(1):1–11. doi: 10.1097/MCG.0000000000001459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.D’Antiga L. Coronaviruses and immunosuppressed patients: The facts during the third epidemic. Liver Transpl. 2020;26(6):832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 119.Shafran N, Issachar A, Shochat T, Shafran IH, Bursztyn M, Shlomai A. Abnormal liver tests in patients with SARS-CoV-2 or influenza-prognostic similarities and temporal disparities. JHEP Rep. 2021;3(3):100258. doi: 10.1016/j.jhepr.2021.100258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Amin M. COVID-19 and the liver: Overview. Eur J Gastroenterol Hepatol. 2021;33(3):309–311. doi: 10.1097/MEG.0000000000001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Grgurevic I, Lucijanić M, Pastrovic F, Barisic Jaman M, Tjesic Drinkovic I, Zelenika M, et al. Short-term outcomes of patients with chronic liver disease hospitalised with COVID-19. Intern Med J. 2022;52(11):1891–1899. doi: 10.1111/imj.15817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou F, Xia J, Yuan HX, Sun Y, Zhang Y. Liver injury in COVID-19: Known and unknown. World J Clin Cases. 2021;9(19):4980–4989. doi: 10.12998/wjcc.v9.i19.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int. 2020;40(6):1321–1326. doi: 10.1111/liv.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Youssef M, H Hussein M, Attia AS, M Elshazli R, Omar M, Zora G, et al. COVID-19 and liver dysfunction: A systematic review and meta-analysis of retrospective studies. J Med Virol. 2020;92(10):1825–1833. doi: 10.1002/jmv.26055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci Rep. 2021;11(1):16144. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Michelen M, Manoharan L, Elkheir N, Cheng V, Dagens A, Hastie C, et al. Characterising long COVID: A living systematic review. BMJ Glob Health. 2021;6(9):e005427. doi: 10.1136/bmjgh-2021-005427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Akbarialiabad H, Taghrir MH, Abdollahi A, Ghahramani N, Kumar M, Paydar S, et al. Long COVID, a comprehensive systematic scoping review. Infection. 2021;49(6):1163–1186. doi: 10.1007/s15010-021-01666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhu X, Wang J, Du J, Chen S, Chen S, Li J, et al. Changes in serum liver function for patients with COVID-19: A 1-year follow-up study. Infect Drug Resist. 2022;15:1857–1870. doi: 10.2147/IDR.S356181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liao X, Li D, Ma Z, Zhang L, Zheng B, Li Z, et al. 12-month post-discharge liver function test abnormalities among patients with COVID-19: A single-center prospective cohort study. Front Cell Infect Microbiol. 2022;12:864933. doi: 10.3389/fcimb.2022.864933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Daugherty SE, Guo Y, Heath K, Dasmariñas MC, Jubilo KG, Samranvedhya J, et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: Retrospective cohort study. BMJ. 2021;373:n1098. doi: 10.1136/bmj.n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dennis A, Wamil M, Alberts J, Oben J, Cuthbertson DJ, Wootton D, et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: A prospective, community-based study. BMJ Open. 2021;11(3):e048391. doi: 10.1136/bmjopen-2020-048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bende F, Tudoran C, Sporea I, Fofiu R, Bâldea V, Cotrău R, et al. A multidisciplinary approach to evaluate the presence of hepatic and cardiac abnormalities in patients with post-acute COVID-19 syndrome-A pilot study. J Clin Med. 2021;10(11):2507. doi: 10.3390/jcm10112507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Veerankutty FH, Sengupta K, Vij M, Rammohan A, Jothimani D, Murali A, et al. Post-COVID-19 cholangiopathy: Current understanding and management options. World J Gastrointest Surg. 2023;15(5):788–798. doi: 10.4240/wjgs.v15.i5.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kolesova O, Vanaga I, Laivacuma S, Derovs A, Kolesovs A, Radzina M, et al. Intriguing findings of liver fibrosis following COVID-19. BMC Gastroenterol. 2021;21(1):370. doi: 10.1186/s12876-021-01939-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Milic J, Barbieri S, Gozzi L, Brigo A, Beghé B, Verduri A, et al. Metabolic-associated fatty liver disease is highly prevalent in the postacute COVID syndrome. Open Forum Infect Dis. 2022;9(3):ofac003. doi: 10.1093/ofid/ofac003. [DOI] [PMC free article] [PubMed] [Google Scholar]


