Abstract
Developments in the field of microfluidics have triggered technological revolutions in many disciplines, including chemical synthesis, electronics, diagnostics, single-cell analysis, micro- and nanofabrication, and pharmaceutics. In many of these areas, rapid growth is driven by the increasing synergy between fundamental materials development and new microfluidic capabilities. In this Review, we critically evaluate both how recent advances in materials fabrication have expanded the frontiers of microfluidic platforms and how the improved microfluidic capabilities are, in turn, furthering materials design. We discuss how various inorganic and organic materials enable the fabrication of systems with advanced mechanical, optical, chemical, electrical and biointerfacial properties — in particular, when these materials are combined into new hybrids and modular configurations. The increasing sophistication of microfluidic techniques has also expanded the range of resources available for the fabrication of new materials, including particles and fibres with specific functionalities, 3D (bio)printed composites and organoids. Together, these advances lead to complex, multifunctional systems, which have many interesting potential applications, especially in the biomedical and bioengineering domains. Future exploration of the interactions between materials science and microfluidics will continue to enrich the diversity of applications across engineering as well as the physical and biomedical sciences.
Microfluidic architectures (BOX 1), which usually make use of fluid flows at the micrometre scale (that is, flow rates of microlitres per minute or less and total volumes of microlitres or less), have triggered techno logical revolutions in several scientific and engineering disciplines, including diagnostics, cell-based screening technologies, single-cell research, analytical chemistry, electronics, biosensing, and micro- and nanofabrication1. For example, cell-based assays, previously conducted primarily in cell culture dishes and plates, have progressively migrated to microchip platforms over the past two decades2–4. Cells and proteins can be separated by size and type, and captured and analysed in microfluidic devices5. In diagnostics, microfluidics have made it possible to capture circulating tumour cells6,7 and to design progressively more sensitive and integrated point-of-care applications for chronic and infectious diseases using small volumes of biologically complex samples8.
Box 1 |. Microfluidics.
Microfluidics is a multidisciplinary field in which engineering, physics, chemistry, biochemistry, nanotechnology and biotechnology converge. A microfluidic system can be defined as a fluid device in which the channels have diameters that range from around 100 nanometres to several hundred micrometres. The tiny channels lead to interesting and sometimes counterintuitive properties, which enable many unconventional applications. For example, systems designed to process such small volumes of fluids can achieve multiplexing, automation and high-throughput screening. Microfluidics, which emerged at the beginning of the 1960s and was first defined by A. C. Eringen184, is expected to become a US$30 billion core industry with potential applications in a wide range of scientific disciplines, including, but not limited to, healthcare (for example, molecular biology, drug discovery, diagnostics and forensics, drug delivery and medical analysis), environmental analysis, high-value fluid production, micro- and nanoparticle fabrication, mass and energy exchange, multidimensional printing and petrochemicals.
The field of microfluidics has experienced explosive growth in the past decade, and this trend is expected to continue. The synergistic innovation of materials and microfluidic platforms is central to increasing the sophistication of microfluidics-based technologies across many scientific disciplines; new materials are expanding the applications of microfluidics, and microfluidic systems have also been shown to provide robust and flexible platforms for the fabrication of materials with well-defined physicochemical properties. A historical timeline of key events and developments at the materials–microfluidics interface is presented in FIG. 1.
Figure 1 |. Historical timeline of developments in materials and microfluidics.
Key advances in materials (red) and microfluidics (grey) have aided the interplay between the two complementary fields and their synergistic growth. PCR, polymerase chain reaction; TAS, total analysis system.
This Review provides an overview of recent developments in materials design strategies, both adapted for and derived from microfluidics, and is structured into three sections. First, we discuss how various types of materials confer new properties and capabilities in the production of microfluidic devices, ranging from inorganic and organic materials to hybrid and composite materials. Second, we discuss recent progress in the use of microfluidics as platforms for the fabrication of new materials. Last, we address challenges associated with current microfluidic platforms and the potential solutions, such as bioinspired design, that we anticipate will lead to the next generation of microfluidic systems.
Materials for microfluidic devices
The development of new materials, as well as innovative ways to combine and to configure existing materials, has led to the design of microfluidic systems with a wide range of unique functionalities. These materials can be generally categorized as inorganic, organic, and hybrid or composite. In this section, we briefly discuss the advantages and disadvantages of each type of material, and give key examples of their evolution in the fabrication of microfluidic devices with a focus on recent applications. We highlight how recent advances in synthesizing, structuring, functionalizing and combining new and conventional materials, which include glass, responsive polymers, paper and even liquid components, are creating opportunities for integrating and building on the distinctive properties of different materials.
Inorganic materials
Early microfluidic devices primarily used inorganic materials as substrates, owing to their often superior surface stability, tunable thermal conductivity and solvent compatibility9,10. Even before the introduction of the concept of microfluidics, microchannels had been used in the form of glass capillaries in gas chromatography10.
The earliest microfluidic systems originated from the microelectronics industry and were consequently constructed from silica or glass. Silicon gradually became the preferred material at the time, primarily because of the advances in and availability of microfabrication techniques. However, silicon is optically opaque, a disadvantage for applications that require optical measurements. Glass, by contrast, has excellent optical transparency, well-defined surface chemistry and superior high-pressure resistance for microfluidics applications. For example, multiplexed configurations of glass capillaries were used for the microfluidic fabrication of various functional microparticles11–14. Glass is also more compatible with electro-osmotic flow than silicon, arguably because of its lower electroconductivity9. However, a challenge lies in the difficulty of preparing high-aspect-ratio anisotropic structures using amorphous glass. Recently, liquid glass was produced in the form of a photocurable amorphous silica nanocomposite15. This advance constitutes the next step towards the prototyping and fabrication of micro fluidic microstructures based on glass at relatively low cost, with high fidelity and without the need for clean-room facilities or hazardous chemicals.
Organic materials
Organic materials are usually robust and cost-effective. They also enable faster and simpler fabrication processes than inorganic materials. Organic materials commonly used in the fabrication of microfluidic devices include polystyrene, polyvinyl chloride, polymethyl methacrylate (PMMA), cyclic olefin co-polymers, polycarbonate and polydimethylsiloxane (PDMS). In contrast to inorganic materials, these polymers possess several attractive properties, such as facile surface modification, low cost, low thermal conductivity and compatibility with biomedical applications. For example, PDMS microfluidic devices are permeable to gases and are thus able to support long-term cell culture, which cannot be achieved using silicon- or glass-based microfluidics16. Although organic materials offer many advantages, they also present challenges involving the ageing, chemical resistance, and mechanical, thermal and optical properties of the materials. For example, PDMS can restrict the detection of short-wavelength fluorescence, resulting in reduced measurement sensitivity compared with glass9. Typical organic materials used for microfluidics can be classified into five categories according to their physicochemical properties: elastomers, thermosets, plastics, hydrogels and paper10. These different classes of materials for microfluidics fabrication have been previously described in detail10,16,17; here, we focus on important recent developments.
Elastomeric materials.
Elastomers are weakly cross-linked polymers that can be easily stretched or compressed and revert to their original shapes when external forces are removed. PDMS has been the most widely used elastomer in microfluidics since Whitesides and co-workers18–20 first reported its use for this purpose in 1995. PDMS microfluidic devices can be prepared using simple mixing, casting and heating processes to replicate the numerous microchannel shapes and structures of master moulds (such as patterned silicon or SU-8)21,22. The use of PDMS imparts important properties, including optical transparency, high gas permeability, robustness, biocompatibility and low toxicity, which make it particularly well suited for microfluidic platforms designed for biomedical applications16.
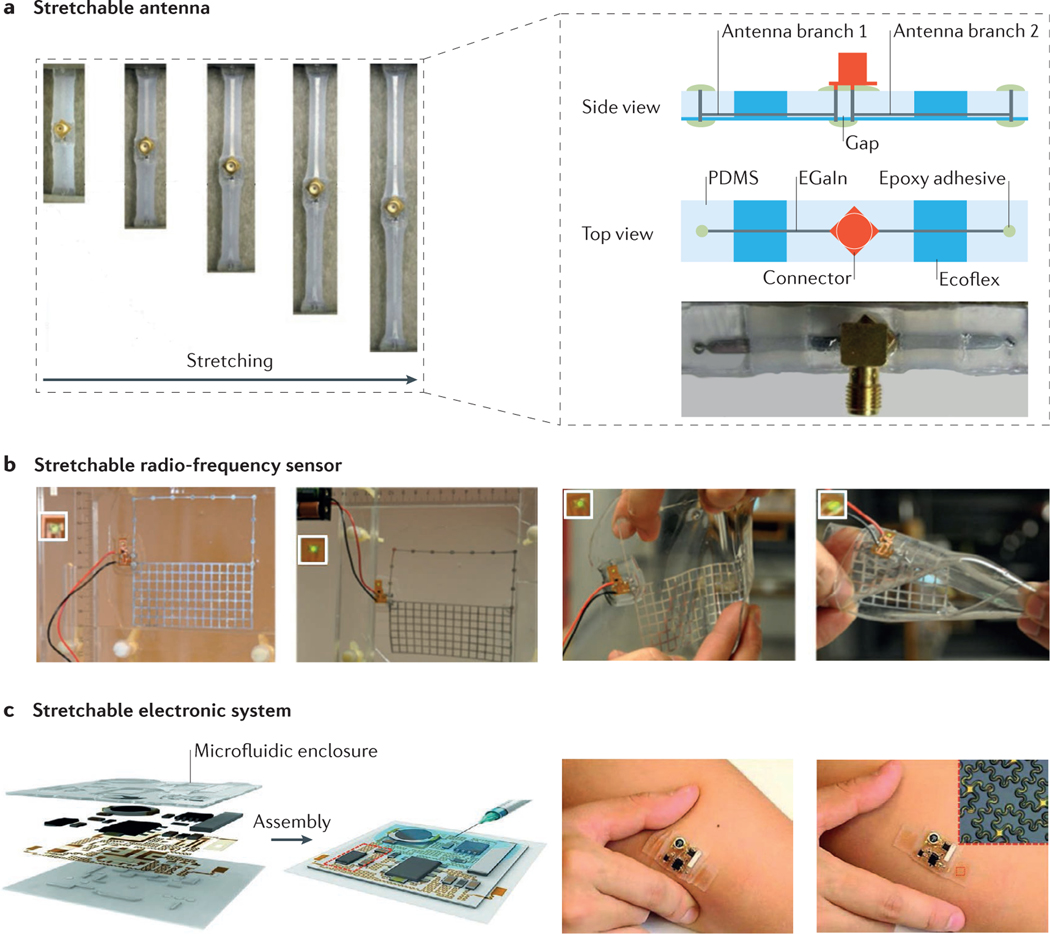
Microfluidics based on PDMS also enable the development of flexible electronics23. The evolution of the next generation of stretchable, bendable and wearable electronics has resulted in innovations such as smart bandages for health monitoring and sensors for use in monitoring the wing status of aeroplanes. However, the technology is not sufficiently developed for devices to be manufactured at large scales and low cost (see the MIT News web page). Improvements in smart, flexible microfluidics are anticipated to lead to further advances in flexible electronics. Notable examples in which this concept has already been realized include stretchable microfluidic radio-frequency electronics24,25 (FIG. 2a,b) and ultra-low-modulus, highly stretchable electronic systems26 (FIG. 2c).
Figure 2 |. Elastomer-based stretchable microfluidics.
a | Schematic illustrations and optical micrographs of a stretchable antenna. The half-wave dipole antenna was made of EGaIn (eutectic gallium–indium, a liquid metal alloy) embedded in microfluidic channels composed of polydimethylsiloxane (PDMS) and Ecoflex (an elastomeric silicone polymer that is softer than PDMS). b | Photographs of a microfluidics-based solution for the fabrication of a stretchable radio-frequency electronic radiation sensor that can operate in an ordinary office environment. The insets are the LED indicators of the sensor, which show no difference in the signal with different degrees of stretching. From left to right: non-stretched; strained with 15% elongation along the y axis; manually applied strain in both x and y directions; and severe twisting. c | Schematic illustrations and images of a soft, stretchable electronic system that integrates strain-isolated device components and a free-floating interconnecting network in a thin elastomeric microfluidic enclosure. The inset in the far right is an optical micrograph of the device, which contains a pair of epidermal electrodes in a serpentine mesh layout. LED, light-emitting diode. Panel a is adapted with permission from REF. 24, Wiley-VCH. Panel b is adapted with permission from REF. 25, Royal Society of Chemistry. Panel c is adapted with permission from REF. 26, AAAS.
Although PDMS is arguably the most popular material for microfluidic fabrication today, it has limitations for universal application27. One problem is its hydrophobic nature, which significantly increases the possibility of adsorption and absorption of hydrophobic molecules, leading to fouling in microchannels28. In addition, PDMS is incompatible with organic solvents; for example, it swells in octane (among other solvents), which alters the structural features and dimensions of the microchannels29. Strategies need to be developed to improve the chemical resistance of PDMS to a wider range of organic solvents30–32. Moreover, the elasticity of PDMS mandates that its mechanical properties be enhanced to reduce the deformation of microfluidic systems under high-pressure conditions33. Although often an advantage, the fact that PDMS is gas-permeable can lead to concentration changes during liquid handling operations in microchannels due to water evaporation. By contrast, this permeability is beneficially exploited when studying the dynamics of biomolecular crystallization34 or when promoting crystal growth for the building of complex, hierarchical microarchitectures35.
Thermoset materials.
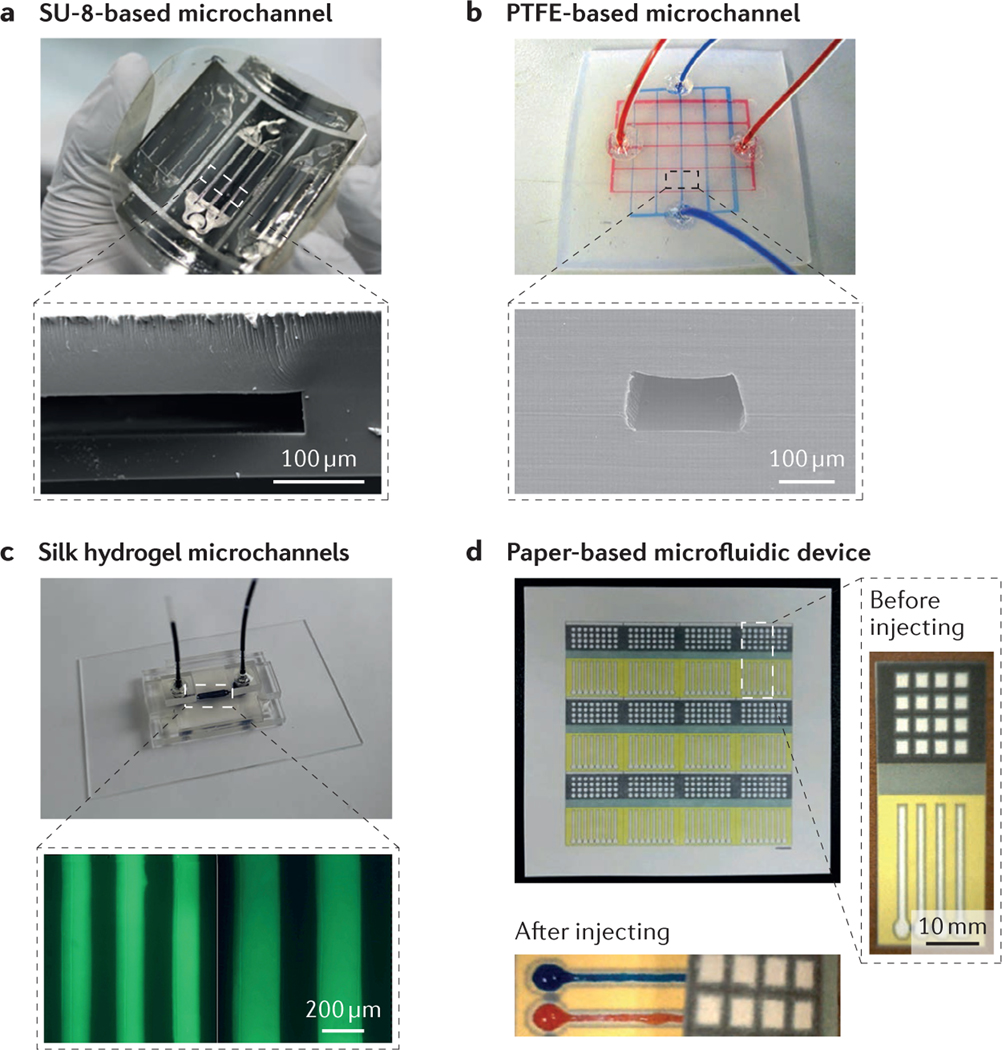
Thermosets (or thermo setting plastics) are materials that result from irreversible crosslinking of polymers in response to exposure to heat or light36. This process leads to molecules with very high molecular masses and, therefore, materials that cannot be melted and reshaped after curing, which distinguishes them from plastics. The heavily crosslinked structures of thermosets impart stability, rigidity and brittleness, even at high temperatures. Thermosets are chemically resistant to most solvents and are optically transparent. The properties of thermosets allow their use in the fabrication of microfluidic devices with free-standing structures that have high aspect ratios and are stable over a wide range of temperatures (under their decomposition thresholds) and at high pressures. Nonetheless, the current high cost of thermosets limits their application in microfluidics10. SU-8 and polyimide are the most commonly used thermosets37–40 in microfluidics, and both materials provide advantages in 3D microfabrication through photopolymerization41. For example, SU-8 is optically transparent (in the visible region but not in the UV region)17 and mechanically robust, and can be directly structured using a photolithography process to fabricate a great variety of 3D microstructures. Recently, a free-standing and flexible SU-8-based microfluidic sensor with a predictable impedance spectrum behaviour was reported42 (FIG. 3a). This design integrated electrical and microfluidic components to create a reduced-scale sensing device, revealing a new level of miniaturization.
Figure 3 |. Representative materials for microfluidic devices.
a | A free-standing SU-8-based microfluidic sensor. The inset shows a cross-sectional scanning electron microscopy (SEM) image of a microchannel. b | A polytetrafluoroethylene (PTFE)-based microfluidic device with two-layer microchannels separated by a thin membrane. The inset shows a cross-sectional SEM image of the microchannel. c | Fabrication process of a two-layer silk hydrogel microfluidic device using gelatin moulding and layer-by-layer stacking methods. The inset shows optical micrographs of the minimal microchannel diameters achievable with the silk hydrogel microfluidic device. d | Paper-based 3D microfluidic device for multiple bioassays and sequential fluidic manipulation. The inset on the right shows a magnified view of the device before liquid injection, and that on the bottom shows the device after injection. Panel a is adapted with permission from REF. 42, Copernicus Publications. Panel b is adapted with permission from REF. 47, National Academy of Sciences. Panel c is adapted with permission from REF. 60, Elsevier. Panel d is adapted with permission from REF. 70, Elsevier.
Plastic materials.
In contrast to thermosets, plastics can melt and become pliable or mouldable on application of heat; they then solidify on cooling. Acrylic (for example, PMMA), polystyrene and polytetrafluoroethylene (PTFE) are three commonly used plastics in microfluidics. These materials can be conveniently reshaped to fabricate microchannels at temperatures close to their glass transition temperatures (the temperature above which the plastic is distinctly softened)17,43–46. Because of this feature, plastics are widely used and well developed in industry. Many plastics have superior properties to PDMS, such as improved solvent compatibility47, reduced antifouling47, gas impermeability48 and greater rigidity49. Plastics were once less favoured in research laboratories for prototyping owing to the relatively sophisticated thermo-processing procedures needed for microfabrication, which are better suited for mass production16. Currently, more and more plastics are being used for prototype designs. For example, a microfluidic chip was developed with solvent-resistant photocurable perfluoropolyethers50, and microfluidics entirely based on PTFE were shown to exhibit excellent inertness to a wide range of chemicals and high resistance to many solvents47 (FIG. 3b). More recently, laser cutting technology has been used to develop PMMA and PTFE microchannels51,52. The emerging popularity of 3D printing technology has now enabled the fabrication of template moulds for building prototype microfluidic devices, which offer the advantage of producing large numbers of replicas rapidly and at low cost, while also allowing quick iterations between different designs53,54.
Hydrogel-based materials.
Hydrogels are networks of crosslinked polymers wherein water constitutes the majority of the total mass. In principle, any water-soluble polymer can be used as a matrix for a hydrogel by controlling the degree of polymerization of the polymer chains9. The highly porous structures of hydrogels along with their controllable pore sizes enable diffusion of various molecules throughout their matrices. The inherent biocompatibility, tunable degradability and high permeability of many hydrogels make them ideal for biomedical microfluidics applications10,16,55–59. Hydrogels can be functionalized to respond to external stimuli (for example, temperature, pH or chemical concentration) such that their swelling and deswelling elicit mechanical changes (expansion or contraction) inside microchannels, in which response times are determined by the rate of diffusion of the stimulus into the hydrogel matrix.
Several challenges associated with hydrogel-based microchannels are currently being addressed. For example, the production of microchannel networks with high-resolution shapes using conventional methods is difficult with hydrogel systems because of their low densities and strengths compared with other polymers. Recently, however, hydrogel-based microfluidics were developed from a silk protein elastomeric material; the resultant systems had 3D microchannel networks with a minimum feature resolution of 100 μm (REF. 60). These microfluidic platforms exhibited controllable mechanical properties, long-term stability, and tunable in vitro and in vivo degradability (FIG. 3c). Engineered hybrid or composite hydrogels with greatly improved mechanical properties may expand the use of hydrogel-based microfluidics61,62, particularly in cell and tissue culture applications.
In general, hydrogels are hydrophilic materials. However, they can be produced to include hydrophobic moieties, which can be important in the design of microfluidics for biological applications. A simple strategy was recently reported for making the surfaces of hydrogels superhydrophobic to obtain heterogeneous wettability across their surfaces and inner networks63. This strategy prevented the immediate diffusion of substances between the hydrogel and the aqueous environment, and may enable fine tuning of surface wettability in future microfluidics applications. Similarly, hydrophobicity can be imparted to hydrogel surfaces by coating with superhydrophobic microparticles64. The stimulus-responsive behaviour of hydrogels has further enabled them to be designed for switchable surface wettability.
Paper-based materials.
Paper is a highly porous, fabric matrix that is typically thin and prepared using well-developed and economical manufacturing processes by compressing cellulose fibres derived from wood, grass or rags. Some of the intrinsic characteristics of paper, such as the porosity and microstructure, increase the passive transport of liquids owing to the liquid capillary effect65 and make it particularly suitable for microfluidics applications. In addition, paper can be easily functionalized by treating it with different liquids, conferring not only the desired wettability and colours, but also other properties, such as conductivity and mechanical resistance65. Moreover, the typical white colour of paper makes it well suited for colour-based detection methods in most assays66. Paper can also be discarded or recycled easily, which is a great advantage for single-use testing devices. Paper-based microfluidics therefore offer a promising platform for diagnostic bioassays to address global health concerns in developing countries, for which simplicity, rapidity, portability and low cost are desirable10,16,17.
In 2008, a paper-based 3D microfluidic system was fabricated by stacking alternating layers of paper and water-impermeable tape67; this was followed by the development of pressure-driven open-channel microfluidic devices using an omniphobic paper68. In 2011, a laser treatment method was reported for the fabrication of inexpensive microfluidic platforms on paper, and the applicability of these paper-based microfluidic devices was demonstrated using a luminol-chemiluminescence assay69. Paper-based microfluidic systems have many benefits, but challenges remain — these are typically associated with the difficulties in fabricating well-controlled patterns at small scales, owing to the inability to produce microchannels with hydrophilic and/or hydrophobic barriers in paper. In 2015, wax-printed and impregnated paper-based microfluidic devices were reported; these systems featured small patterns with appropriate resolution (3.4 mm detection zones) to enable simple and robust point-of-care diagnostics70 (FIG. 3d).
Hybrid microfluidic systems
Following the first use of silicon for microfluidics applications, microchannels have subsequently been fabricated from various materials such as glass, polymers and hydrogels. However, alone, each of these materials has limitations for use in extended microfluidics applications, as discussed in the previous sections. The combination of different constituent materials or the use of composites, which is an emerging strategy, offers approaches to overcome these challenges, as well as to improve or expand functionality and to create complex, multifunctional systems suited for a variety of environments. As an illustrative example, organically modified ceramics are beneficial for many biological microfluidics applications; these materials are produced using fabrication processes similar to those used in polymer synthesis and feature glass-like surface chemistry71,72. In this section, we introduce several typical and emerging hybrid and composite material-based microfluidics approaches.
The use of PDMS in combination with other materials for the fabrication of hybrid microfluidic systems has been frequently explored. Examples include PDMS combinations with glass73–77, SU-878,79, polycarbonate80,81, PMMA82, hydrogels83,84, paper85 and biodegradable materials86. Even hybrid materials with the same components can be designed and customized for different applications. For example, one PDMS–polycarbonate hybrid microfluidic system used nanoporous polycarbonate membranes as molecular gates to control the net flow of gases80. The same hybrid device can also be used to generate perpendicular chemical and oxygen gradients suitable for cell culture by using the embedded polycarbonate film as a gas diffusion barrier81. Many other examples of hybrid microfluidics have been reported, such as silicon–polycarbonate microfluidics87. A microfluidic device was recently developed for organic synthesis based on a hybrid fluoroethylene propylene–polyimide film that offered good physical toughness at high pressure, operability at low temperature and chemical inertness88.
Multiconstituent hybrid materials for microfluidics are attracting increasing interest. The combination of the different properties of the constituent materials results in microfluidic devices of greater sophistication. The development of a hybrid microfluidic device consisting of a top PDMS substrate, a bottom quartz substrate and a patterned middle layer of SU-8 enabled isoelectric focusing of proteins with whole-channel imaging ability89. In addition, relative to a comparable device made of only PDMS, the hybrid chip exhibited increased heat dissipation owing to the high thermal conductivity of the quartz substrate, allowing larger electric fields to be used in separations, and also demonstrated a two-to-threefold improvement in sensitivity. In other examples, hybrid microfluidic devices constructed of PDMS–paper–glass and PMMA–PHEMA–glass (where PHEMA is poly(2-hydroxyethyl methacrylate)) were used for single-step multiplexed pathogen detection90 and potential cell-on-a-chip applications91. The multiconstituent designs allow each material to serve its own function in the hybrid systems, often outperforming devices based on single materials.
Coating the inner surface of existing microchannels with another material is a frequently used and simple option for the preparation of hybrid microfluidics. For example, a hybrid microfluidic device was fabricated using a UV-patternable organic–inorganic hybrid sol–gel coating92. This approach could be extended to the development of microchannel systems on silicon and glass materials. In another example, a method was established for the preparation of a solvent-resistant hybrid microfluidic device by using an inorganic–organic hybrid coating on the inner surface of PDMS microchannels to substantially increase chemical resistance to various solvents31. Metals can also be used to coat the polymer surfaces to obtain new features of relevance for microfluidics applications. For example, physical vapour deposition of silver on PDMS was used to create partially embedded, 3D clusters of silver93. This robust coating enhanced the dielectric properties of the PDMS substrate and its capacity to protect silver from oxidation. Furthermore, hydrogels may be used to coat microfluidic channels to enhance their bioactivity94.
In addition to solid-based hybrid and composite materials, liquid-based materials are providing new opportunities for microfluidics. For example, the bioinspired idea of using a fluid lining to prevent fouling inside a microchannel has resulted in anti-fouling microfluidic networks with liquid-infused porous membrane materials that show outstanding inertness to various chemicals, particles, proteins and blood95. A variant of this technology has also been used to create pressure-sensitive liquid-gated pores in the walls of microfluidic systems, enabling selective extraction of gases or liquids from multiphase flows96.
Microfluidics for materials fabrication
Advances in the application of microfluidic systems as microfabrication platforms have afforded considerable advantages and opportunities to materials scientists, biologists and bioengineers. The intrinsic properties of a material (for example, its conductivity, resistance, flexibility, hydrophobicity or hydrophilicity) can be better exploited in microfluidic devices simply because larger interfaces between materials and fluids can be achieved. In addition, currently available and emerging microfluidics-assisted platforms enable the fabrication of objects and materials with an improved level of control of key variables, such as temperature, concentration (and concentration gradients), flow rates, flow profiles and mixing parameters.
In this section, we describe important examples from a wide range of microfabrication techniques that are enabled by microfluidics. In addition, we discuss the distinctive characteristics of various microfluidic platforms that make them particularly suited for the fabrication of materials with specific shapes, architectures and/or functionalities that are otherwise difficult to achieve.
Micro- and nanoparticles
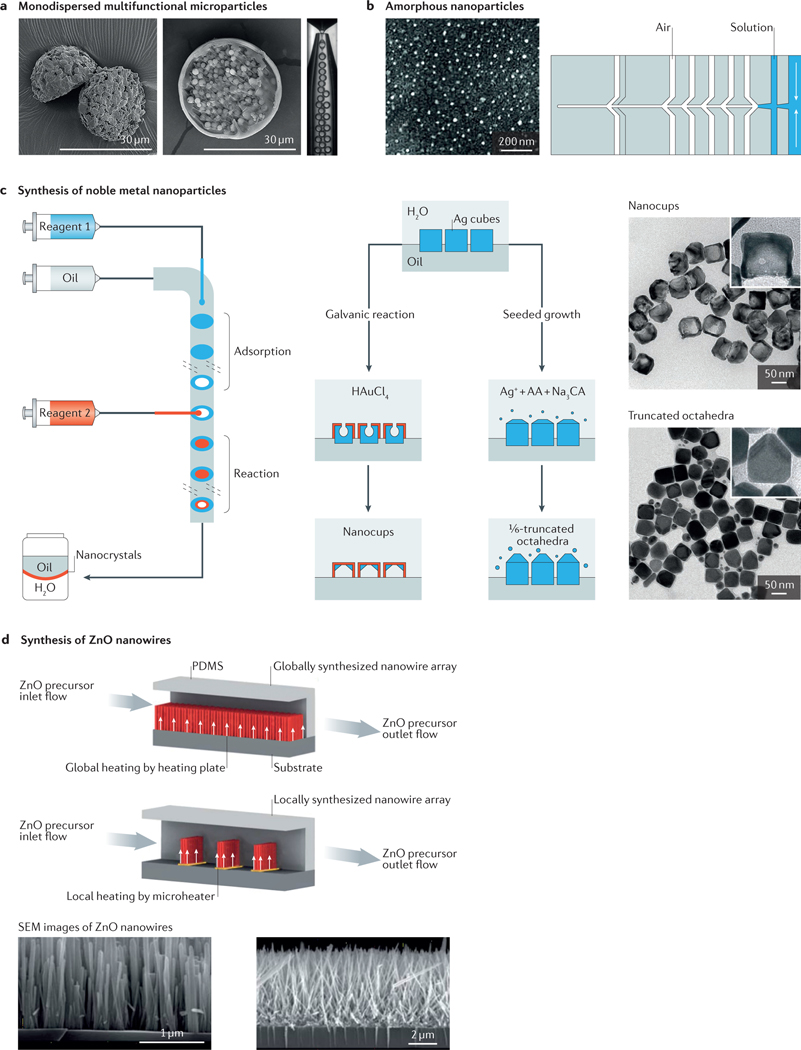
The microfabrication of monodispersed micro- and nanoparticles continues to be a technological challenge. Microfluidic devices provide an environment in which the flow properties can be finely tuned to provide suitable chemical and physical conditions to ensure particle size homogeneity from uniform emulsions14,96–99 (FIG. 4a). The state of the art in this area quickly evolved from the production of single-component particles to the fabrication of Janus (multicomponent) particles with specific architectures and characteristics, including hydrogel particles with predefined shapes11,100, magnetic and/or conductive particles101, magnetic hydrogel particles102, porous microcarriers for cell culture applications103–105, composite particles containing metal–organic frameworks106, microcapsules (microparticles containing smaller particles)13,98,107, and photo- or thermoresponsive capsules99,108,109, among other possibilities.
Figure 4 |. Fabrication of micro- and nanoparticles in microfluidic systems.
a | Microfluidic fabrication of monodispersed microparticles. The two scanning electron microscopy (SEM) images show the formation of Janus particles (left) and microcapsule particles (right). b | Amorphous nanoparticles prepared using a microfluidic nebulator (the example shown is for CaCO3 nanoparticles, although this method can be applied for a wide range of materials). c | Synthesis of noble metal nanoparticles (for example, Ag nanocubes, Ag truncated octahedra and Au–Ag nanocups) in microfluidic droplet reactors with multistep adsorption and reactions. Transmission electron microscope images of the particles are shown on the right. d | In situ synthesis of ZnO nanowires in a microfluidic chip; comparison of global synthesis in the entire fluidic channel and local synthesis by microheaters in the fluidic channel. SEM images of the nanowires are shown below. AA, ascorbic acid; CA, citric acid; PDMS, polydimethylsiloxane. Panel a is adapted with permission from REF. 98, Royal Society of Chemistry. Panel b is adapted with permission from REF. 117, AAAS. Panel c is adapted with permission from REF. 125, American Chemical Society. Panel d is adapted with permission from REF. 127, Royal Society of Chemistry.
The field of materials fabrication using microfluidics has been revolutionized by stop-flow lithography110, which conventionally could only be used to generate spherical objects, owing to the effect of surface tension generated within the emulsion system. In the initial strategy, termed continuous-flow lithography, a projection photolithographic method is combined with microfluidics: a particular pattern can then be projected to the flow within the microfluidic device. This method enables in situ photo-crosslinking of the material precursor, thus achieving continuous production of arbitrarily shaped microparticles100. The technology has been further optimized to introduce a short period in which the flow is temporarily stopped during the lithography process; the process is then repeated by resuming the flow to improve the fidelity of the microparticle synthesis111. Stop-flow lithography has also been extended to applications including the generation of cell-laden microparticles112; the production of opaque113 and shape-evolving114 microparticles; high-throughput fabrication of microparticles115; and, in combination with flow focusing, large-scale array patterning116.
Although the conventional fabrication of nanometresized particles using microfluidics has been challenging, a microfluidic nebulator was recently developed that uses supersonic spray-drying to produce monodisperse amorphous nanoparticles down to the scale of a few nanometres117 (FIG. 4b). Similarly, microfluidic approaches that promote chaotic mixing have been used to synthesize lipid nanoparticles that incorporate RNA payloads for CRISPR-based genome-editing applications118. In addition, the design of cell-laden hydrogel particles that provide a specific micro-niche for the function of particular cell types has recently received special attention105,119,120. Biomedical research will greatly benefit from these cell-associated particle fabrication techniques in the near future.
Another area of emerging interest is the use of microfluidic droplets as microreactors for a range of chemical and nanoparticle synthesis processes, which provides more rigorous control over reaction parameters than conventional bulk methods121. The rationale behind using microdroplets as reactors is to reduce the volume to as small as possible to achieve higher homogeneity in the reaction conditions96,121,122. Indeed, micro fluidic platforms can be particularly useful for obtaining nanoparticles with narrower particle size distributions than can be achieved using conventional beaker or flask proto cols121,123. The use of microfluidic platforms to produce noble metal nanoparticles has been widely demonstrated124,125. Microfluidic platforms have recently shown sufficient flexibility to accommodate multistage reagent injections or localized heaters for the facile fabrication of nanoparticles with selected shapes, such as nanorods126, octahedra and nanocups125 (FIG. 4c), and nanowires127 (FIG. 4d), as well as nanoparticles of different sizes and/or functionalities. Microfluidic approaches can also be used to fine-tune the continuous and large-scale synthesis of nanoparticles, in which the feeding ratio of the growth solution to the seed solution, temperature and residence time determine the shape and size of the resulting nanoparticles128. This field has seen notable advances in the past few years, and exciting developments continue to be made, pushing the fabrication limits to smaller sizes and more tightly controlled conditions.
Beyond the inert materials used for micro particle fabrication, microfluidic platforms have also been increasingly adopted for the fabrication of biological materials that are anticipated to lead to the creation of miniaturized tissues. These local microenvironments or ‘niches’ are specific for each type of cell and exert their influence through a complex combination of chemical and physical stimuli129,130. Microfluidic technologies that integrate biomaterials and matrices tailored to mimic specific niches are well suited to define such microenvironments and have been increasingly demonstrated and applied. For example, the recently described microfluidics-based fabrication of hydrogel microspheres for the encapsulation of stem-cell lines may offer minimally invasive methods for effectively delivering stem-cell-based therapies to their clinical targets105. Droplet-based microfluidics approaches using hydrogels have also recently been coupled with next-generation sequencing technologies to develop analytical tools that can index large populations of individual pluripotent stem cells to map single-cell gene expression profiles, enabling the identification of heterogeneous subpopulations during early differentiation131. These microfluidic technologies, aided by advances in biomaterials design, address a vital need as the field rapidly moves closer towards the translation and deployment of clinically relevant (stem-)cell-based regenerative therapies.
Crystals and nanocrystals
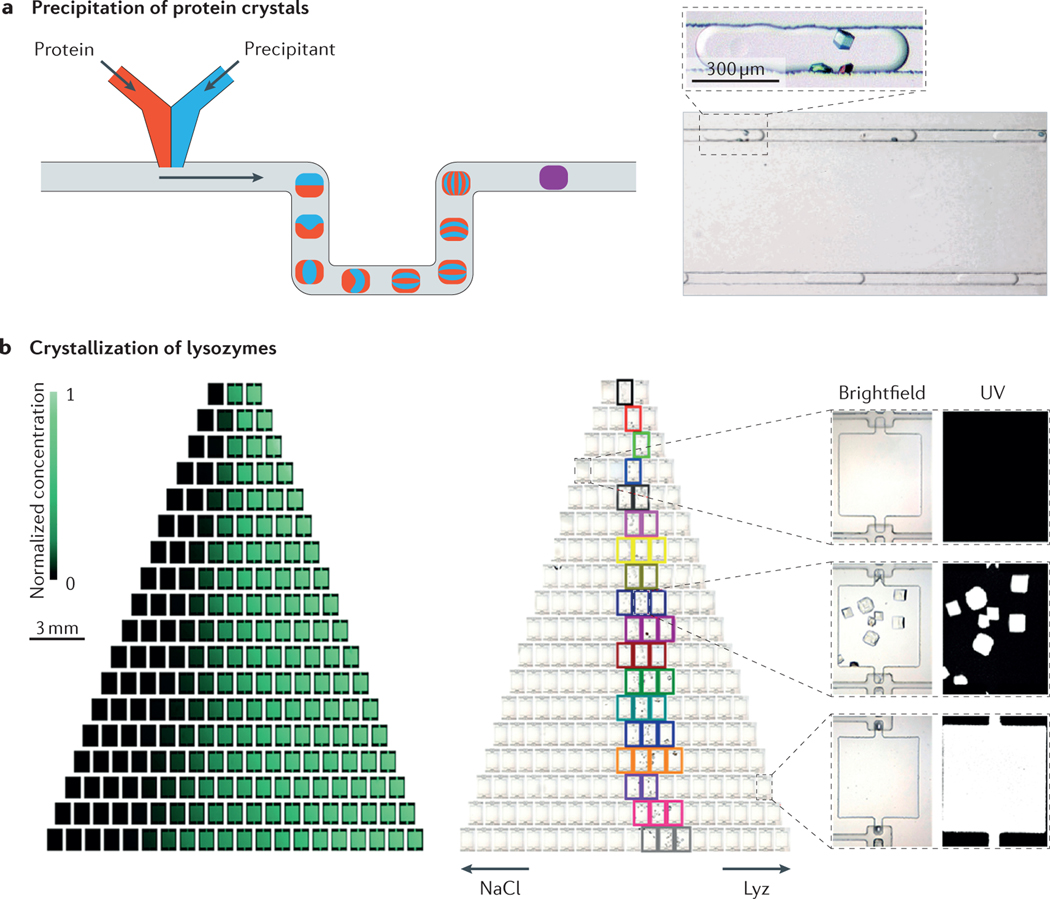
The use of microfluidic platforms addresses some of the key requirements for controlling crystallization processes. For example, crystallization is known to depend strongly on mass and heat transfer rates, as well as on local concentrations. The typical length scales of microfluidic platforms enable a high degree of control of the heat and mass transfer conditions and a reduction in diffusion distances, resulting in good control of the crystal size distributions132–135. This control is especially important in the process of synthesizing nanocrystals. This concept has evolved in complexity with the aim of developing high-throughput crystallization systems that could eventually replace classical methods for the production of nanocrystals. Microfluidic systems also enable continuity in the crystallization procedure, in which individual crystallization reaction niches136 (FIG. 5a) or multiplexed reactors operate concurrently in the same system, allowing high-throughput production or easy exploration of multiple crystallization conditions137 (FIG. 5b).
Figure 5 |. Crystallization in microfluidic systems.
a | Precipitation of protein crystals in a microfluidic reactor. By changing solvent ratios, the degree of mixing, the protein solution and other additives, different conditions can be established in each drop. b | Multichamber microfluidic system that enabled up to 144 different experimental conditions to be established, as indicated in the fluorescence images that show the gradient of fluorescein (left). In the right part, different conditions were used for investigating the optimal crystallization conditions of lysozymes (Lyz). The arrows indicate increasing concentrations of the denoted chemicals, and the coloured squares indicate the conditions under which crystals form. Panel a is adapted with permission from REFS 136, American Chemical Society. Panel b is adapted with permission from REF. 137, American Chemical Society.
Expanding the variety of materials used to fabricate microfluidic devices has resulted in a greater range of crystallization applications. The development of pharmaceuticals requires the screening of many different conditions to identify those suitable for the crystallization of a specific active pharmaceutical ingredient. These screening operations necessitate the use of different types of solvent; however, not all solvents are compatible with the materials commonly used in microfluidics. Typically, screening requires the use of 0.5 g (at least) of active ingredient — a quantity not always available in early stages of development. Recently, a microfluidic platform for crystallization was developed to be chemically resistant to many of the solvents (polar and nonpolar) most commonly used in pharmaceutical industry crystallization138. This platform is also Raman-compatible, thereby allowing the direct study of the crystallization kinetics. The use of metallic surfaces, glass and silica permits the operation of microfluidic reactors at high temperatures, resulting in a wide range of applications, such as the synthesis of semiconductor nanocrystals. For example, cadmium selenide nanocrystals were crystallized at high temperatures (180–210 °C) using a continuous microfluidic coil in which the temperature and residence times determined the average size of the resulting crystals132.
Protein crystallization continues to be one of the principal bottlenecks for protein characterization, because determination of the 3D structure of a protein by X-ray crystallography requires high-purity crystals. However, obtaining such crystals can be a cumbersome and difficult process. The integration of microfluidics with instrumentation has resulted in important advances in this area. A microfluidics-based system was recently used to study protein crystallization using very small sample volumes137. The technique is useful for building crystallization phase diagrams, which are otherwise difficult and time-intensive to construct using conventional methods. In another recent study, the concept of graphene-based microfluidics was introduced139; the adoption of a thin microfluidic chamber (less than 1 μm in thickness) with graphene walls enabled in situ X-ray diffraction studies in picolitre volumes.
Fibres with precisely controlled architecture
Many technological applications require the fabrication of fibres. For example, long conductive wires are needed for electronics applications; fibrous polymeric matrices with predetermined orientations are used to create structures with superior mechanical properties; and fibrous tissue-like structures are used to mimic the function of muscles and nerves, among other applications. Therefore, the microfabrication of fibres with precisely controlled architectures is an area of great interest and potential. The flexibility of microfluidic systems for the fabrication of a wide range of fibres of different diameters, cross-sectional shapes, lengths and materials has been demonstrated by controlling key parameters, such as flow rates and compositions of the inlet fluids140,141, the geometric features of microfluidic devices142 or the flow profiles within microfluidic channels143. Fabrication approaches can be generally divided into two classes: hydrogel fibres obtained by in situ crosslinking (for example, alginate fibres crosslinked by Ca2+ (REFS 144,145) and gelatin methacryloyl fibres crosslinked by UV light142) and polymer fibres produced using solvent extraction141.
The existence of a laminar flow regime in microfluidic devices enables the fabrication of multimaterial, multiniche or multilayer materials. This strategy has been used in different ways. For example, an inertial laminar microfluidic process was used for the fabrication of shaped microfibres143. In this system, two non-reactive streams and one central reactive stream flow through a channel containing a sequence of pillars that deflect and deform the fluids, generating a flow structure. The reactive stream is polymerized at the channel outlet using UV light. The cross-sectional shape of the resultant fibres, predicted by flow simulations, depends on the flow rate and the number and positions of the pillars within the channel.
Recent work has demonstrated the use of an interfacial microfluidic membrane-processing reactor for the fabrication of a metal–organic framework membrane on the inner surface of a hollow fibre. Two solutions — one containing Zn2+ ions and the other methylimidazole linkers — were respectively fed along the outer and inner sides of a polysulfone hollow fibre. The reaction between the two precursors resulted in the formation of a polycrystalline zeolitic imidazolate framework (ZIF-8) membrane layer on the inner wall146. The fabrication of multiniche, biologically active fibres with perfusable cylindrical hollow cores has also been demonstrated147, whereby endothelial cells were grown in the interior walls of the cylindrical channels to mimic the architecture of blood vessels. Different niches (two or three) were also created independently within these multifibre constructs, allowing the co-culture of different cell types and potentially enabling the design of multicellular fibrous tissues147.
Microfluidic printing
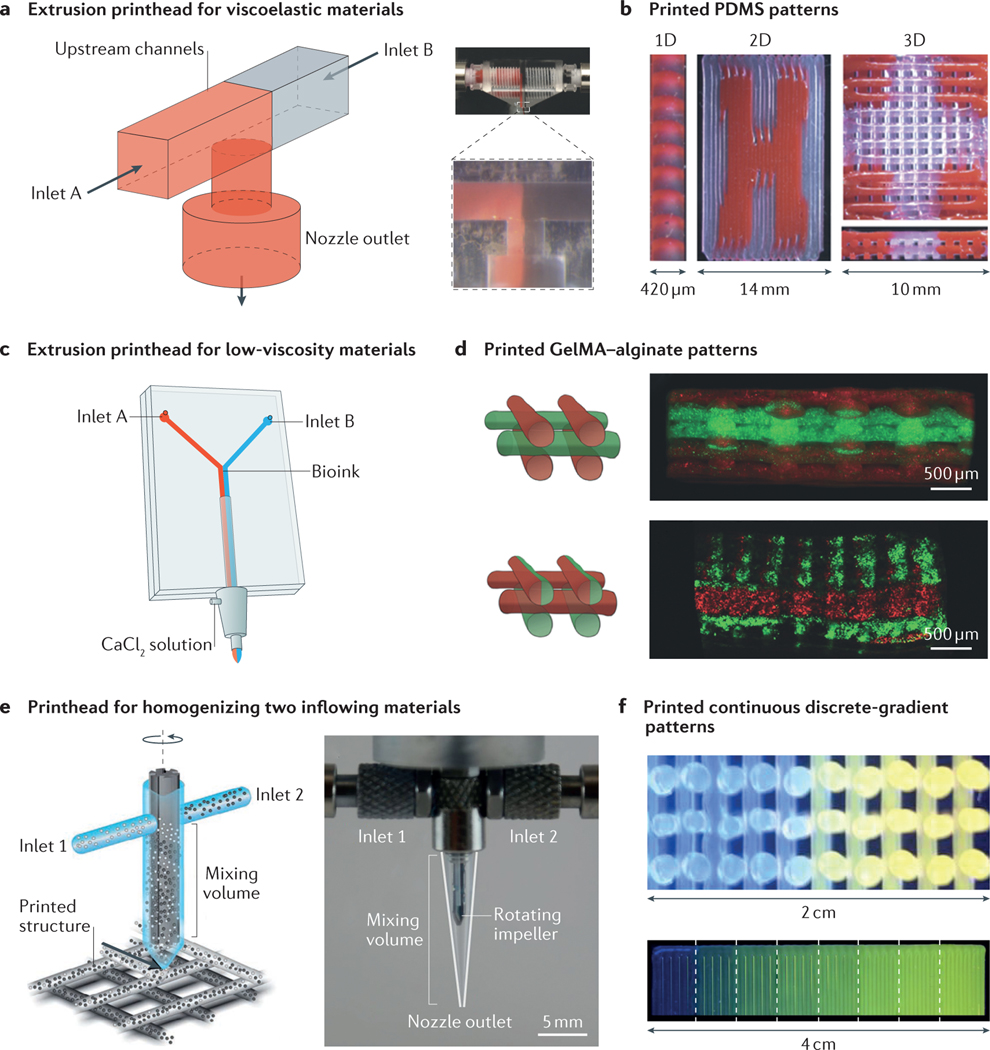
Three-dimensional printing has emerged as a versatile fabrication technology that is capable of one-step generation of well-defined volumetric architectures. Since its debut in 1986 in the form of stereolithography148, 3D printing technology has evolved into a wide variety of forms149, including inkjet printing150,151, selective sintering152, fused deposition modelling153 and laser-assisted forward transfer154. The recent extension of these fabrication techniques for the production of biological components, including cells and biomolecules (termed 3D bioprinting), is leading to unprecedented advances in the reproducible assembly of complex tissue constructs with high fidelity and spatial resolution155–158. Among the different printing techniques, those based on the extrusion of inks from nozzles are particularly versatile in terms of the choice of available inks, as well as the capability for multimaterial printing156,157. Extrusion printing integrates the concept of microfluidics to introduce precise control over the flow of bioinks, usually in the form of a hydrogel159–164 or a nanocomposite165,166, through the nozzle printhead to achieve the timed delivery of materials at desired locations.
The recent combination of extrusion printing with advanced microfluidic printheads has greatly improved our ability to deposit multiple materials with ease and speed. Although conventional multimaterial printing systems rely on the use of several physically separated nozzles160,162,167, dual-channel microfluidic extrusion printheads have recently been developed that enable programmed, sequential or simultaneous extrusion of two materials168,169. In this approach, a T- or Y-junction microfluidic chip is used as the printhead and is fitted at the tip of a printer, where the extrusion of the two materials from the side channels converges to a single outlet for printing168 (FIG. 6a). This simplified direct-extrusion microfluidic printhead enables extrusion of multiple materials of relatively high viscosities (FIG. 6b). Another form of this chip and printhead involves a more sophisticated approach with the addition of a sheath flow outside the common outlet169 (FIG. 6c). This modification enables the extrusion of materials of relatively low viscosity by adopting a fast crosslinking mechanism that is realized through delivery of the crosslinking agent from the sheath flow. For example, crosslinking of a hybrid hydrogel bioink containing gelatin methacryloyl and alginate occurs immediately on the introduction of calcium chloride carried by the sheath flow, thereby achieving layered printing of microfibrous structures (FIG. 6d).
Figure 6 |. Microfluidics-enabled 3D bioprinting.
a | Microfluidic printhead for the extrusion of two viscoelastic materials. b | Printed heterogeneous 1D, 2D and 3D polydimethylsiloxane (PDMS) patterns. c | Dual-layer microfluidic printhead for the extrusion of two materials with low viscosities. d | Printed heterogeneous gelatin methacryloyl (GelMA)–alginate patterns. e | Microfluidic printhead containing an active rotating impeller for homogenizing two inflowing materials before they are extruded from the printhead to deposit patterns. f | Printed continuous- and discrete-gradient patterns. Panels a and b are adapted with permission from REF. 168, Wiley-VCH. Panels c and d are adapted with permission from REF. 169, Wiley-VCH. Panels e and f are adapted with permission from REF. 170, National Academy of Sciences.
This printhead was further advanced to incorporate a rotating impeller within the common outlet to actively mix two materials into a homogeneous ink170 (FIG. 6e). In contrast to a passive dual-channel microfluidic chip, which only leads to the deposition of alternating materials (FIG. 6b,d) or laminated mixtures of the two (FIG. 6d), the active-mixing printhead enables the direct printing of homogenized inflowing inks. Careful tuning of the amounts of each material to be delivered into the mixer permits graduated outflowing inks and thus a continuous or discrete gradient in the material of the printed volume (FIG. 6f). This innovation marked the advance of microfluidics-enabled 3D (bio)printing, paving the way for future uses of this technology in constructing compositionally complex structures, in addition to the architectural sophistication currently possible.
Summary and outlook
The field of microfluidics is still growing rapidly, and its integration with progress in materials science, chemistry, physics, and micro- and nanotechnology will be crucial to producing smarter and more complex functional systems. Many challenges lie ahead, such as addressing fouling and stability issues, and the need to design portable, flexible, stretchable, multifunctional and controllable systems that can perform in many different environments.
Bioinspired design is an increasingly fruitful source of new directions in the development of micro fluidics and will probably lead to further advances focused on large market applications171–173. Microchannels in biological systems exhibit controlled, responsive transport of multiphase fluids, are integrated into hierarchical systems, and not only self-heal damage but also respond to clogging, pressure changes or gas build-up in the flow. One example is blood vessels, which serve as tightly regulated mass-transport networks to ensure oxygen and nutrient delivery to surrounding tissues and maintain homeostasis throughout the body. The vascular networks that regulate water pumping and balance liquid and gas pressures within trees provide a complementary set of design principles. Building systems to study and simulate these innate processes continues to represent a challenge for biomedical applications of microfluidics.
Fouling constitutes a critical challenge to the design of future microfluidic devices174. Numerous approaches have been explored to reduce fouling, including selecting low-surface-energy materials to fabricate microfluidic channels, or using bulk and surface chemical modification post-fabrication. However, these methods inevitably complicate fabrication procedures and fail to provide effective solutions to fouling issues. The bioinspired concept of using a fluid lining to prevent fouling inside microfluidic channels results in antifouling microfluidic networks that show inertness to various chemicals, particles, proteins and blood95. In addition, chemical modifications of the interior surfaces of microchannels with functional molecules that closely mimic the controllable transport properties of biological microchannels are anticipated to control mass transport through a microscale orifice in response to ambient stimuli, such as pH, applied force, light and temperature. It is anticipated that the new concept of microchannel functional molecular systems will soon be applied to build smart microfluidics with functions that can be controlled with greater precision through bioinspired design strategies175–178.
Advances in bioinspired smart materials are also leading to biofabrication technologies such as 4D bioprinting, which adds a time dimension to the conventional 3D bioprinting (that is, fabricated constructs can change their shapes on application of desired stimuli)179,180. Bioinspired design can be transformative to manufacturing, and 4D printing is the first process of its kind to offer the capability to control size, shape and structure post-manufacture.
Being inherently multifunctional and multidisciplinary, bioinspired design provides principles for integrating microfluidics across a broad range of fields. For example, the bioinspired gating concept for microfluidic applications has led to new separation techniques that provide opportunities for complex sorting in environmental, fuel, biomedical, degassing and waste treatment fields, among others, for real-world industrial application95. This is a radical shift in our understanding of microfluidics; up to this point, microfluidic technologies have remained static and rigid, but they will soon be dynamic, flexible, adaptable and tunable for on-demand performance.
Microfluidics will continue to expand the frontiers of manufacturing as it imparts extraordinary processing control. The manufacture of high-value-added products will greatly benefit from the control of key quality variables, such as temperature, pressure, and chemical or bio chemical microenvironments, which can be better achieved in microfluidic systems than in large stirred tanks. The coming years will see the use of microfluidic platforms in the fabrication of new materials and in the addition of functionality to existing materials. For example, advances are expected in the area of continuous manufacturing of pharmaceutical and biopharmaceutical products enabled by micro fluidics-based fabrication technologies181–183.
We have provided an overview of recent developments, contemporary challenges and future directions at the interface between materials science and micro fluidics. We anticipate that further progress in microfluidics will enable new real-world applications, which range from the implementation of advanced manufacturing techniques to personalized diagnostics and therapeutics for healthcare. In addition, the intersection of microfluidics and materials science is expected to grow progressively, leading to increasingly complex systems that integrate channel design and in situ materials processing. We hope that this Review will foster and inspire research towards these next-generation materials and micro fluidic technologies.
Acknowledgements
The authors acknowledge funding from the US National Institutes of Health (AR057837, DE021468, D005865, AR068258, AR066193, EB022403, EB021148), the Air Force Office of Scientific Research Award (USA, FA9550-15-1-0273), the Presidential Early Career Award for Scientists and Engineers (USA), Consejo Nacional de Ciencia y Tecnología (Mexico, scholarships 262130 and 234713), Tecnológico de Monterrey (Mexico), Massachusetts Institute of Technology (MIT) International Science and Technology Initiatives and Fundación México en Harvard. This research has been partially funded by the Tecnológico de Monterrey and MIT Nanotechnology Program. X.H. acknowledges the support of the Recruitment Program for Young Professionals (China), the National Natural Science Foundation (China, 21673197), and the Research Institute for Biomimetics and Soft Matter, Fujian Provincial Key Laboratory for Soft Functional Materials Research, Xiamen University (China), supported by the 111 Project (B16029). Y.S.Z. acknowledges the National Cancer Institute of the US National Institutes of Health Pathway to Independence Award (K99CA201603). J.R. acknowledges support from the Portuguese Foundation for Science and Technology (SFRH/BD/51679/2011). P.S.W., A.M.A. and J.A. acknowledge support from the Kavli Foundation (USA). A.M.A. acknowledges support from the Hatos Center for Neuropharmacology (USA). S.J.J. acknowledges the support of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at the University of California Los Angeles (UCLA) Training Program through its Clinical Fellowship Training Award Program, as well as the UCLA Children’s Discovery and Innovation Institute’s Fellows Research Support Award.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1. Whitesides GM The origins and the future of microfluidics. Nature 442, 368–373 (2006). A comprehensive review of the background, applications and future perspectives of microfluidics.
- 2.Huh D, Hamilton GA & Ingber DE From 3D cell culture to organs-on-chips. Trends Cell Biol. 21, 745–754 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatia SN & Ingber DE Microfluidic organs-on-chips. Nat. Biotechnol 32, 760–772 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Esch EW, Bahinski A. & Huh D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 14, 248–260 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes AJ & Herr AE Microfluidic western blotting. Proc. Natl Acad. Sci. USA 109, 21450–21455 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozkumur E. et al. Inertial focusing for tumor antigen–dependent and–independent sorting of rare circulating tumor cells. Sci. Transl. Med 5, 179ra47 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karabacak NM et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat. Protoc 9, 694–710 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warren AD, Kwong GA, Wood DK, Lin KY & Bhatia SN Point-of-care diagnostics for noncommunicable diseases using synthetic urinary biomarkers and paper microfluidics. Proc. Natl Acad. Sci. USA 111, 3671–3676 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian W-C & Fineout E. (eds) Microfluidics for Biological Applications (Springer, 2008). [Google Scholar]
- 10.Ren KN, Zhou JH & Wu HK Materials for microfluidic chip fabrication. Acc. Chem. Res 46, 2396–2406 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Shepherd RF et al. Microfluidic assembly of homogeneous and Janus colloid-filled hydrogel granules. Langmuir 22, 8618–8622 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Kim JW, Utada AS, Fernández-Nieves A, Hu Z. & Weitz DA Fabrication of monodisperse gel shells and functional microgels in microfluidic devices. Angew. Chem. Int. Ed 119, 1851–1854 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Chu LY, Utada AS, Shah RK, Kim JW & Weitz DA Controllable monodisperse multiple emulsions. Angew. Chem. Int. Ed 46, 8970–8974 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Shah RK et al. Designer emulsions using microfluidics. Mater. Today 11, 18–27 (2008). [Google Scholar]
- 15.Kotz F. et al. Liquid glass: a facile soft replication method for structuring glass. Adv. Mater 28, 1521–4095 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Ren KN, Chen Y. & Wu HK New materials for microfluidics in biology. Curr. Opin. Biotechnol 25, 78–85 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Nge PN, Rogers CI & Woolley AT Advances in microfluidic materials, functions, integration, and applications. Chem. Rev 113, 2550–2583 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia YN & Whitesides GM Soft lithography. Annu. Rev. Mater. Sci 28, 153–184 (1998). [Google Scholar]
- 19.Xia YN et al. Complex optical surfaces formed by replica molding against elastomeric masters. Science 273, 347–349 (1996). [DOI] [PubMed] [Google Scholar]
- 20.Kim E, Xia YN & Whitesides GM Polymer microstructures formed by molding in capillaries. Nature 376, 581–584 (1995). [Google Scholar]
- 21.McDonald JC & Whitesides GM Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res 35, 491–499 (2002). [DOI] [PubMed] [Google Scholar]
- 22.McDonald JC et al. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 21, 27–40 (2000). [DOI] [PubMed] [Google Scholar]
- 23.Rogers JA, Someya T. & Huang Y. Materials and mechanics for stretchable electronics. Science 327, 1603–1607 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Kubo M. et al. Stretchable microfluidic radiofrequency antennas. Adv. Mater 22, 2749–2752 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Cheng S. & Wu ZG Microfluidic stretchable RF electronics. Lab Chip 10, 3227–3234 (2010). [DOI] [PubMed] [Google Scholar]
- 26. Xu S. et al. Soft microfluidic assemblies of sensors, circuits, and radios for the skin. Science 344, 70–74 (2014). A seminal paper that reports the development of soft microfluidics and applications in flexible sensors.
- 27.Sollier E, Murray C, Maoddi P. & Di Carlo D. Rapid prototyping polymers for microfluidic devices and high pressure injections. Lab Chip 11, 3752–3765 (2011). [DOI] [PubMed] [Google Scholar]
- 28.Toepke MW & Beebe DJ PDMS absorption of small molecules and consequences in microfluidic applications. Lab Chip 6, 1484–1486 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Lee JN, Park C. & Whitesides GM Solvent compatibility of poly(dimethylsiloxane)-based microfluidic devices. Anal. Chem 75, 6544–6554 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Lim H. & Moon S. Stable nonpolar solvent droplet generation using a poly(dimethylsiloxane) microfluidic channel coated with poly-p-xylylene for a nanoparticle growth. Biomed. Microdevices 17, 70–77 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Kim BY, Hong LY, Chung YM, Kim DP & Lee CS Solvent-resistant PDMS microfluidic devices with hybrid inorganic/organic polymer coatings. Adv. Funct. Mater 19, 3796–3803 (2009). [Google Scholar]
- 32.Kim BY, Hong LY, Kim DP & Lee CS in Proc. 1st Shenyang Int. Colloquium Microfluidics 111–114 (Northeast Univ. Press, 2007). [Google Scholar]
- 33.Kim M, Huang Y, Choi K. & Hidrovo CH The improved resistance of PDMS to pressure-induced deformation and chemical solvent swelling for microfluidic devices. Microelectron. Eng 124, 66–75 (2014). [Google Scholar]
- 34.Shim JU et al. Control and measurement of the phase behavior of aqueous solutions using microfluidics. J. Am. Chem. Soc 129, 8825–8835 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noorduin WL, Grinthal A, Mahadevan L. & Aizenberg J. Rationally designed complex, hierarchical microarchitectures. Science 340, 832–837 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Kloxin CJ & Bowman CN Covalent adaptable networks: smart, reconfigurable and responsive network systems. Chem. Soc. Rev 42, 7161–7173 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Metz S, Jiguet S, Bertsch A. & Renaud P. Polyimide and SU-8 microfluidic devices manufactured by heatdepolymerizable sacrificial material technique. Lab Chip 4, 114–120 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Zulfiqar A, Pfreundt A, Svendsen WE & Dimaki M. Fabrication of polyimide based microfluidic channels for biosensor devices. J. Micromech. Microeng 25, 035022 (2015). [Google Scholar]
- 39.Metz S, Holzer R. & Renaud P. Polyimide-based microfluidic devices. Lab Chip 1, 29–34 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Ayuso JM et al. SU-8 based microfluidic device for oxygen/nutrients gradient three dimensional cell culture (Poster). Mol. Biol. Cell 24 (2013). [Google Scholar]
- 41.Sato H, Matsumura H, Keino S. & Shoji S. An all SU-8 microfluidic chip with built-in 3D fine microstructures. J. Micromech. Microeng 16, 2318–2322 (2006). [Google Scholar]
- 42.Schmidt M-P et al. Flexible free-standing SU-8 microfluidic impedance spectroscopy sensor for 3D molded interconnect devices application. J. Sens. Sens. Syst 5, 55–61 (2016). [Google Scholar]
- 43.Martin PM, Matson DW, Bennett WD & Hammerstrom DJ Fabrication of plastic microfluidic components. Proc. SPIE 3515, 172–176 (1998). [Google Scholar]
- 44.Man PF, Jones DK & Mastrangelo CH Microfluidic plastic capillaries on silicon substrates: a new inexpensive technology for bioanalysis chips. Proc. IEEE Micro Electro Mech. Syst 311–316 (1997). [Google Scholar]
- 45.Barker SLR et al. Fabrication, derivatization and applications of plastic microfluidic devices. Proc. SPIE 4205, 112–118 (2001). [Google Scholar]
- 46.Boone T. et al. Plastic advances microfluidic devices. Anal. Chem 74, 78a–86a (2002). [DOI] [PubMed] [Google Scholar]
- 47. Ren KN, Dai W, Zhou JH, Su J. & Wu HK Whole-Teflon microfluidic chips. Proc. Natl Acad. Sci. USA 108, 8162–8166 (2011). The first report on methods that can be used to fabricate microfluidic devices made entirely of thermoplastic materials.
- 48.Birarda G. et al. IR-live: fabrication of a low-cost plastic microfluidic device for infrared spectromicroscopy of living cells. Lab Chip 16, 1644–1651 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Lee KS & Ram RJ Plastic-PDMS bonding for high pressure hydrolytically stable active microfluidics. Lab Chip 9, 1618–1624 (2009). [DOI] [PubMed] [Google Scholar]
- 50.Rolland JP, Van Dam RM, Schorzman DA, Quake SR & DeSimone JM Solvent-resistant photocurable ‘liquid Teflon’ for microfluidic device fabrication. J. Am. Chem. Soc 126, 2322–2323 (2004). [DOI] [PubMed] [Google Scholar]
- 51.Chen X, Li T. & Shen J. CO2 laser ablation of microchannel on PMMA substrate for effective fabrication of microfluidic chips. Int. Polym. Process 31, 233–238 (2016). [Google Scholar]
- 52.Howell C. et al. Stability of surface-immobilized lubricant interfaces under flow. Chem. Mater 27, 1792–1800 (2015). [Google Scholar]
- 53. Au AK, Huynh W, Horowitz LF & Folch A. 3D-printed microfluidics. Angew. Chem. Int. Ed 55, 3862–3881 (2016). A critical review on the adoption of the 3D printing strategy for the fabrication of microfluidic devices.
- 54.Ho CMB, Ng SH, Li KHH & Yoon YJ 3D printed microfluidics for biological applications. Lab Chip 15, 3627–3637 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Peppas NA, Hilt JZ, Khademhosseini A. & Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv. Mater 18, 1345–1360 (2006). [Google Scholar]
- 56.Khademhosseini A. & Langer R. Microengineered hydrogels for tissue engineering. Biomaterials 28, 5087–5092 (2007). [DOI] [PubMed] [Google Scholar]
- 57.Nichol JW et al. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31, 5536–5544 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yue K. et al. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 73, 254–271 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loessner D. et al. Functionalization, preparation and use of cell-laden gelatin methacryloyl-based hydrogels as modular tissue culture platforms. Nat. Protoc 11, 727–746 (2016). [DOI] [PubMed] [Google Scholar]
- 60. Zhao SW et al. Bio-functionalized silk hydrogel microfluidic systems. Biomaterials 93, 60–70 (2016). Report on the development of silk hydrogel-based microfluidic systems.
- 61.Kharaziha M. et al. Tough and flexible CNT–polymeric hybrid scaffolds for engineering cardiac constructs. Biomaterials 35, 7346–7354 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y-N et al. A highly elastic and rapidly crosslinkable elastin-like polypeptide-based hydrogel for biomedical applications. Adv. Funct. Mater 25, 4814–4826 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yao X. et al. Superhydrophobic diffusion barriers for hydrogels via confined interfacial modification. Adv. Mater 28, 7383–7389 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Oliveira NM et al. Hydrophobic hydrogels: towards construction of floating (bio)microdevices. Chem. Mater 28, 3641–3648 (2016). [Google Scholar]
- 65.Glavan AC et al. Omniphobic “RF paper” produced by silanization of paper with fluoroalkyltrichlorosilanes. Adv. Funct. Mater 24, 60–70 (2014). [Google Scholar]
- 66.Songjaroen T, Dungchai W, Chailapakul O, Henry CS & Laiwattanapaisal W. Blood separation on microfluidic paper-based analytical devices. Lab Chip 12, 3392–3398 (2012). [DOI] [PubMed] [Google Scholar]
- 67. Martinez AW, Phillips ST & Whitesides GM Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc. Natl Acad. Sci. USA 105, 19606–19611 (2008). One of the first demonstrations of disposable paper-based microfluidic devices.
- 68.Glavan AC et al. Rapid fabrication of pressure-driven open-channel microfluidic devices in omniphobic RF paper. Lab Chip 13, 2922–2930 (2013).23719764 [Google Scholar]
- 69.Chitnis G, Ding ZW, Chang CL, Savran CA & Ziaie B. Laser-treated hydrophobic paper: an inexpensive microfluidic platform. Lab Chip 11, 1161–1165 (2011). [DOI] [PubMed] [Google Scholar]
- 70.Choi S, Kim SK, Lee GJ & Park HK Paper-based 3D microfluidic device for multiple bioassays. Sens. Actuators B 219, 245–250 (2015). [Google Scholar]
- 71.Singh A, Scotti G, Sikanen T, Jokinen V. & Franssila S. Laser direct writing of thick hybrid polymers for microfluidic chips. Micromachines 5, 472–485 (2014). [Google Scholar]
- 72.Aura S, Sikanen T, Kotiaho T. & Franssila S. Novel hybrid material for microfluidic devices. Sens. Actuators B 132, 397–403 (2008). [Google Scholar]
- 73.Shameli SM, Glawdel T, Liu Z. & Ren CL Bilinear temperature gradient focusing in a hybrid PDMS/glass microfluidic chip integrated with planar heaters for generating temperature gradients. Anal. Chem 84, 2968–2973 (2012). [DOI] [PubMed] [Google Scholar]
- 74.Moraes FC et al. Glass/PDMS hybrid microfluidic device integrating vertically aligned SWCNTs to ultrasensitive electrochemical determinations. Lab Chip 12, 1959–1962 (2012). [DOI] [PubMed] [Google Scholar]
- 75.Crabtree HJ et al. Inhibition of on-chip PCR using PDMS–glass hybrid microfluidic chips. Microfluid. Nanofluid 13, 383–398 (2012). [Google Scholar]
- 76.Li OA et al. A glass/PDMS hybrid microfluidic chip embedded with integrated electrodes for contactless conductivity detection. Chromatographia 68, 1039–1044 (2008). [Google Scholar]
- 77.Matsui T, Franzke J, Manz A. & Janasek D. Temperature gradient focusing in a PDMS/glass hybrid microfluidic chip. Electrophoresis 28, 4606–4611 (2007). [DOI] [PubMed] [Google Scholar]
- 78.Guo HL, Zhao P, Xiao GZ, Zhang ZY & Yao JP Optical manipulation of microparticles in an SU-8/PDMS hybrid microfluidic chip incorporating a monolithically integrated on-chip lens set. IEEE J. Select. Top. Quantum Electron. 16, 919–926 (2010). [Google Scholar]
- 79.Wu MH et al. A SU-8/PDMS hybrid microfluidic device with integrated optical fibers for online monitoring of lactate. Biomed. Microdevices 7, 323–329 (2005). [DOI] [PubMed] [Google Scholar]
- 80.Kuo TC, Cannon DM, Shannon MA, Bohn PW & Sweedler JV Hybrid three-dimensional nanofluidic/microfluidic devices using molecular gates. Sens. Actuators A 102, 223–233 (2003). [Google Scholar]
- 81.Chang CW et al. A polydimethylsiloxane–polycarbonate hybrid microfluidic device capable of generating perpendicular chemical and oxygen gradients for cell culture studies. Lab Chip 14, 3762–3772 (2014). [DOI] [PubMed] [Google Scholar]
- 82.Suzuki Y, Yamada M. & Seki M. Sol-gel based fabrication of hybrid microfluidic devices composed of PDMS and thermoplastic substrates. Sens. Actuators B 148, 323–329 (2010). [Google Scholar]
- 83.Leung JCK, Hilliker AJ & Rezai P. An integrated hybrid microfluidic device for oviposition-based chemical screening of adult Drosophila melanogaster. Lab Chip 16, 709–719 (2016). [DOI] [PubMed] [Google Scholar]
- 84.Wong I. et al. An agar gel membrane–PDMS hybrid microfluidic device for long term single cell dynamic study. Lab Chip 10, 2710–2719 (2010). [DOI] [PubMed] [Google Scholar]
- 85.Dou MW, Dominguez DC, Li XJ, Sanchez J. & Scott GA Versatile PDMS/paper hybrid microfluidic platform for sensitive infectious disease diagnosis. Anal. Chem 86, 7978–7986 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hesari Z. et al. A hybrid microfluidic system for regulation of neural differentiation in induced pluripotent stem cells. J. Biomed. Mater. Res. A 104, 1534–1543 (2016). [DOI] [PubMed] [Google Scholar]
- 87.Chartier I. et al. Fabrication of a hybrid plastic–silicon microfluidic device for high-throughput genotyping. Proc. SPIE 4982, 208–219 (2003). [Google Scholar]
- 88.Kim H. et al. Submillisecond organic synthesis: outpacing Fries rearrangement through microfluidic rapid mixing. Science 352, 691–694 (2016). [DOI] [PubMed] [Google Scholar]
- 89.Ou JJ, Glawdel T, Ren CL & Pawliszyn J. Fabrication of a hybrid PDMS/SU-8/quartz microfluidic chip for enhancing UV absorption whole-channel imaging detection sensitivity and application for isoelectric focusing of proteins. Lab Chip 9, 1926–1932 (2009). [DOI] [PubMed] [Google Scholar]
- 90.Zuo P, Li XJ, Dominguez DC & Ye B-C A PDMS/paper/glass hybrid microfluidic biochip integrated with aptamer-functionalized graphene oxide nano-biosensors for one-step multiplexed pathogen detection. Lab Chip 13, 3921–3928 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Santaniello T. et al. A room-temperature bonding technique for the packaging of hydrogel-based hybrid microfluidic devices. Microfluid. Nanofluid 19, 31–41 (2015). [Google Scholar]
- 92.Zhang X, Lu H, Qian M. & Zeng X. Fabrication of microfluidic devices using photopatternable hybrid sol-gel coatings. J. Sol-Gel Sci. Technol 48, 143–147 (2008). [Google Scholar]
- 93.Connatser RM, Riddle LA & Sepaniak MJ Metalpolymer nanocomposites for integrated microfluidic separations and surface enhanced Raman spectroscopic detection. J. Sep. Sci 27, 1545–1550 (2004). [DOI] [PubMed] [Google Scholar]
- 94.Annabi N. et al. Hydrogel-coated microfluidic channels for cardiomyocyte culture. Lab Chip 13, 3569–3577 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hou X, Hu YH, Grinthal A, Khan M. & Aizenberg J. Liquid-based gating mechanism with tunable multiphase selectivity and antifouling behaviour. Nature 519, 70–73 (2015). Microfluidic gating mechanism for separating liquids with tunable multiphase selectivity.
- 96.Shestopalov I, Tice JD & Ismagilov RF Multi-step synthesis of nanoparticles performed on millisecond time scale in a microfluidic droplet-based system. Lab Chip 4, 316–321 (2004). [DOI] [PubMed] [Google Scholar]
- 97.Xu S. et al. Generation of monodisperse particles by using microfluidics: control over size, shape, and composition. Angew. Chem. Int. Ed 44, 724–728 (2005). [DOI] [PubMed] [Google Scholar]
- 98.Li W, Dong H, Tang G, Ma T. & Cao X. Controllable microfluidic fabrication of Janus and microcapsule particles for drug delivery applications. RSC Adv. 5, 23181–23188 (2015). [Google Scholar]
- 99.Kim B, Lee HS, Kim J. & Kim S-H Microfluidic fabrication of photo-responsive hydrogel capsules. Chem. Commun 49, 1865–1867 (2013). [DOI] [PubMed] [Google Scholar]
- 100.Dendukuri D, Pregibon DC, Collins J, Hatton TA & Doyle PS Continuous-flow lithography for high-throughput microparticle synthesis. Nat. Mater 5, 365–369 (2006). [DOI] [PubMed] [Google Scholar]
- 101.Lee SW, Choi JS, Cho KY & Yim J-H Facile fabrication of uniform-sized, magnetic, and electroconductive hybrid microspheres using a microfluidic droplet generator. Eur. Polymer J. 80, 40–47 (2016). [Google Scholar]
- 102.Hwang DK, Dendukuri D. & Doyle PS Microfluidic-based synthesis of non-spherical magnetic hydrogel microparticles. Lab Chip 8, 1640–1647 (2008). [DOI] [PubMed] [Google Scholar]
- 103.Wang J. et al. Microfluidic generation of porous microcarriers for three-dimensional cell culture. ACS Appl. Mater. Interfaces 7, 27035–27039 (2015). [DOI] [PubMed] [Google Scholar]
- 104.Velasco D, Tumarkin E. & Kumacheva E. Microfluidic encapsulation of cells in polymer microgels. Small 8, 1633–1642 (2012). [DOI] [PubMed] [Google Scholar]
- 105.Zhao X. et al. Injectable stem cell-laden photocrosslinkable microspheres fabricated using microfluidics for rapid generation of osteogenic tissue constructs. Adv. Funct. Mater 26, 2809–2819 (2016). [Google Scholar]
- 106. Faustini M. et al. Microfluidic approach toward continuous and ultrafast synthesis of metal–organic framework crystals and hetero structures in confined microdroplets. J. Am. Chem. Soc 135, 14619–14626 (2013). Microfluidic synthesis of metal–organic framework crystals in a continuous manner.
- 107.Priest C. et al. Microfluidic polymer multilayer adsorption on liquid crystal droplets for microcapsule synthesis. Lab Chip 8, 2182–2187 (2008). [DOI] [PubMed] [Google Scholar]
- 108.Kim B, Soo Lee H, Kim J. & Kim SH Microfluidic fabrication of photo-responsive hydrogel capsules. Chem. Commun 49, 1865–1867 (2013). [DOI] [PubMed] [Google Scholar]
- 109.Amstad E, Kim SH & Weitz DA Photo-and thermoresponsive polymersomes for triggered release. Angew. Chem. Int. Ed 124, 12667–12671 (2012). [DOI] [PubMed] [Google Scholar]
- 110.Dendukuri D. & Doyle PS The synthesis and assembly of polymeric microparticles using microfluidics. Adv. Mater 21, 4071–4086 (2009). [Google Scholar]
- 111.Dendukuri D, Gu SS, Pregibon DC, Hatton TA & Doyle PS Stop-flow lithography in a microfluidic device. Lab Chip 7, 818–828 (2007). [DOI] [PubMed] [Google Scholar]
- 112.Panda P. et al. Stop-flow lithography to generate cell-laden microgel particles. Lab Chip 8, 1056–1061 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Suh SK, Bong KW, Hatton TA & Doyle PS Using stop-flow lithography to produce opaque microparticles: synthesis and modeling. Langmuir 27, 13813–13819 (2011). [DOI] [PubMed] [Google Scholar]
- 114.Hwang DK et al. Stop-flow lithography for the production of shape-evolving degradable microgel particles. J. Am. Chem. Soc 131, 4499–4504 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Le Goff GC, Lee J, Gupta A, Hill WA & Doyle PS High-throughput contact flow lithography. Adv. Sci 2, 1500149 (2015). Extension of contact flow lithography to high-throughput lithography.
- 116.Kim JJ, Bong KW, Reategui E, Irimia D. & Doyle PS Porous microwells for geometry-selective, large-scale microparticle arrays. Nat. Mater 16, 139–146 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Amstad E. et al. Production of amorphous nanoparticles by supersonic spray-drying with a microfluidic nebulator. Science 349, 956–960 (2015). The fabrication of nanoparticles using a microfluidic nebulator featuring a multistage supersonic spray-drying process.
- 118.Yin H. et al. Therapeutic genome editing by combined viral and non-viral delivery of CRISPR system components in vivo. Nat. Biotechnol 34, 328–333 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Choi CH et al. One-step generation of cell-laden microgels using double emulsion drops with a sacrificial ultra-thin oil shell. Lab Chip 16, 1549–1555 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Allazetta S, Kolb L, Zerbib S, Bardy J. & Lutolf MP Cell-instructive microgels with tailor-made physicochemical properties. Small 11, 5647–5656 (2015). [DOI] [PubMed] [Google Scholar]
- 121.Lignos I. et al. Synthesis of cesium lead halide perovskite nanocrystals in a droplet-based microfluidic platform: fast parametric space mapping. Nano Lett. 16, 1869–1877 (2016). [DOI] [PubMed] [Google Scholar]
- 122.Hung L-H & Lee AP Microfluidic devices for the synthesis of nanoparticles and biomaterials. J. Med. Biol. Eng 27, 1–6 (2007). [Google Scholar]
- 123.Yashina A, Lignos I, Stavrakis S, Choo J. & deMello AJ Scalable production of CuInS2/ZnS quantum dots in a two-step droplet-based microfluidic platform. J. Mater. Chem C 4, 6401–6408 (2016). [Google Scholar]
- 124.Hafermann L. & Köhler JM Photochemical micro continuous-flow synthesis of noble metal nanoparticles of the platinum group. Chem. Eng. Technol 38, 1138–1143 (2015). [Google Scholar]
- 125.Zhang L, Wang Y, Tong L. & Xia Y. Synthesis of colloidal metal nanocrystals in droplet reactors: the pros and cons of interfacial adsorption. Nano Lett. 14, 4189–4194 (2014). [DOI] [PubMed] [Google Scholar]
- 126.Watt J, Hance BG, Anderson RS & Huber DL Effect of seed age on gold nanorod formation: a microfluidic, real-time investigation. Chem. Mater 27, 6442–6449 (2015). [Google Scholar]
- 127.Kim J, Li Z. & Park I. Direct synthesis and integration of functional nanostructures in microfluidic devices. Lab Chip 11, 1946–1951 (2011). [DOI] [PubMed] [Google Scholar]
- 128.Boleininger J, Kurz A, Reuss V. & Sonnichsen C. Microfluidic continuous flow synthesis of rod-shaped gold and silver nanocrystals. Phys. Chem. Chem. Phys 8, 3824–3827 (2006). [DOI] [PubMed] [Google Scholar]
- 129.Lane SW, Williams DA & Watt FM Modulating the stem cell niche for tissue regeneration. Nat. Biotechnol 32, 795–803 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wagers AJ The stem cell niche in regenerative medicine. Cell Stem Cell 10, 362–369 (2012). [DOI] [PubMed] [Google Scholar]
- 131. Klein AM et al. Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell 161, 1187–1201 (2015). Microfluidic production of droplets that encapsulate single cells with uniquely barcoded primers for transcriptome analysis.
- 132.Chan EM, Mathies RA & Alivisatos AP Size-controlled growth of CdSe nanocrystals in microfluidic reactors. Nano Lett. 3, 199–201 (2003). [Google Scholar]
- 133.Hansen CL, Skordalakes E, Berger JM & Quake SR A robust and scalable microfluidic metering method that allows protein crystal growth by free interface diffusion. Proc. Natl Acad. Sci. USA 99, 16531–16536 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hansen CL, Classen S, Berger JM & Quake SR A microfluidic device for kinetic optimization of protein crystallization and in situ structure determination. J. Am. Chem. Soc 128, 3142–3143 (2006). [DOI] [PubMed] [Google Scholar]
- 135.Hansen CL, Sommer MO & Quake SR Systematic investigation of protein phase behavior with a microfluidic formulator. Proc. Natl Acad. Sci. USA 101, 14431–14436 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zheng B, Roach LS & Ismagilov RF Screening of protein crystallization conditions on a microfluidic chip using nanoliter-size droplets. J. Am. Chem. Soc 125, 11170–11171 (2003). [DOI] [PubMed] [Google Scholar]
- 137.Abdallah BG, Roy-Chowdhury S, Fromme R, Fromme P. & Ros A. Protein crystallization in an actuated microfluidic nanowell device. Cryst. Growth Des. 16, 2074–2082 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Goyal S. et al. Solvent compatible microfluidic platforms for pharmaceutical solid form screening. RSC Adv. 6, 13286–13296 (2016). [Google Scholar]
- 139.Sui S. et al. Graphene-based microfluidics for serial crystallography. Lab Chip 16, 3082–3096 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chae SK, Kang E, Khademhosseini A. & Lee SH Micro/nanometer-scale fiber with highly ordered structures by mimicking the spinning process of silkworm. Adv. Mater 25, 3071–3078 (2013). [DOI] [PubMed] [Google Scholar]
- 141.Wang G. et al. Microfluidic crystal engineering of π-conjugated polymers. ACS Nano 9, 8220–8230 (2015). [DOI] [PubMed] [Google Scholar]
- 142.Shi X. et al. Microfluidic spinning of cell-responsive grooved microfibers. Adv. Funct. Mater 25, 2250–2259 (2015). [Google Scholar]
- 143.Nunes JK et al. Fabricating shaped microfibers with inertial microfluidics. Adv. Mater 26, 3712–3717 (2014). [DOI] [PubMed] [Google Scholar]
- 144.Onoe H. et al. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater 12, 584–590 (2013). [DOI] [PubMed] [Google Scholar]
- 145. Kang E. et al. Digitally tunable physicochemical coding of material composition and topography in continuous microfibres. Nat. Mater 10, 877–883 (2011). Microfluidic fabrication of digitally coded multimaterial microfibres.
- 146.Cacho-Bailo F. et al. Metal–organic framework membranes on the inner-side of a polymeric hollow fiber by microfluidic synthesis. J. Membr. Sci 476, 277–285 (2015). [Google Scholar]
- 147.Cheng Y. et al. Controlled fabrication of bioactive microfibers for creating tissue constructs using microfluidic techniques. ACS Appl. Mater. Interfaces 8, 1080–1086 (2016). [DOI] [PubMed] [Google Scholar]
- 148.Hull CW Apparatus for production of three-dimensional objects by stereolithography. US Patent 4,575,330 (1986).
- 149.Sheth R. et al. Three-dimensional printing: an enabling technology for IR. J. Vasc. Interv. Radiol 27, 859–865 (2016). [DOI] [PubMed] [Google Scholar]
- 150.Minemawari H. et al. Inkjet printing of single-crystal films. Nature 475, 364–367 (2011). [DOI] [PubMed] [Google Scholar]
- 151.Chou D-T et al. Novel processing of iron–manganese alloy-based biomaterials by inkjet 3D printing. Acta Biomater. 9, 8593–8603 (2013). [DOI] [PubMed] [Google Scholar]
- 152.Agarwala M, Bourell D, Beaman J, Marcus H. & Barlow J. Direct selective laser sintering of metals. Rapid Prototyp. J 1, 26–36 (1995). [Google Scholar]
- 153.Zein I, Hutmacher DW, Tan KC & Teoh SH Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 23, 1169–1185 (2002). [DOI] [PubMed] [Google Scholar]
- 154.Fardel R, Nagel M, Nüesch F, Lippert T. & Wokaun A. Fabrication of organic light-emitting diode pixels by laser-assisted forward transfer. Appl. Phys. Lett 91, 061103 (2007). [Google Scholar]
- 155.Malda J. et al. 25th anniversary article: engineering hydrogels for biofabrication. Adv. Mater 25, 5011–5028 (2013). [DOI] [PubMed] [Google Scholar]
- 156.Murphy SV & Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol 32, 773–785 (2014). [DOI] [PubMed] [Google Scholar]
- 157.Zhang YS et al. 3D bioprinting for tissue and organ fabrication. Ann. Biomed. Eng 45, 148–163 (2017). [Au:OK?] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang YS et al. Bioprinting the cancer microenvironment. ACS Biomater. 2, 1710–1721 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bertassoni LE et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab Chip 14, 2202–2211 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kolesky DB et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater 26, 3124–3130 (2014). [DOI] [PubMed] [Google Scholar]
- 161.Lee VK et al. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials 35, 8092–8102 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kang H-W et al. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol 34, 312–319 (2016). [DOI] [PubMed] [Google Scholar]
- 163.Kolesky DB, Homan KA, Skylar-Scott MA & Lewis JA Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl Acad. Sci. USA 113, 3179–3184 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hong S. et al. 3D printing of highly stretchable and tough hydrogels into complex, cellularized structures. Adv. Mater 27, 4035–4040 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shin SR et al. A bioactive carbon nanotube-based ink for printing 2D and 3D flexible electronics. Adv. Mater 28, 3280–3289 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xavier JR et al. Bioactive nanoengineered hydrogels for bone tissue engineering: a growth-factor-free approach. ACS Nano 9, 3109–3118 (2015). [DOI] [PubMed] [Google Scholar]
- 167.Chang R, Nam J. & Sun W. Effects of dispensing pressure and nozzle diameter on cell survival from solid freeform fabrication–based direct cell writing. Tissue Eng. Part A 14, 41–48 (2008). [DOI] [PubMed] [Google Scholar]
- 168.Hardin JO, Ober TJ, Valentine AD & Lewis JA Microfluidic printheads for multimaterial 3D printing of viscoelastic inks. Adv. Mater 27, 3279–3284 (2015). [DOI] [PubMed] [Google Scholar]
- 169.Colosi C. et al. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low viscosity bioink. Adv. Mater 28, 677–684 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Ober TJ, Foresti D. & Lewis JA Active mixing of complex fluids at the microscale. Proc. Natl Acad. Sci. USA 112, 12293–12298 (2015). Design of a microfluidic printhead containing an embedded impeller that actively mixes multiple fluids for printing gradient structures.
- 171.Barber RW & Emerson DR Optimal design of microfluidic networks using biologically inspired principles. Microfluid. Nanofluid 4, 179–191 (2008). [Google Scholar]
- 172.Domachuk P, Tsioris K, Omenetto FG & Kaplan DL Bio-microfluidics: biomaterials and biomimetic designs. Adv. Mater 22, 249–260 (2010). [DOI] [PubMed] [Google Scholar]
- 173.Buchberger G. et al. Bio-inspired microfluidic devices for passive, directional liquid transport: model-based adaption for different materials. Procedia Eng. 120, 106–111 (2015). [Google Scholar]
- 174.Mukhopadhyay R. When microfluidic devices go bad. Anal. Chem 77, 429a–432a (2005). [PubMed] [Google Scholar]
- 175.Hou X. (ed.) Design, Fabrication, Properties and Applications of Smart and Advanced Materials (CRC Press, 2016). [Google Scholar]
- 176.Hou X. & Jiang L. Learning from nature: building bio-inspired smart nanochannels. ACS Nano 3, 3339–3342 (2009). [DOI] [PubMed] [Google Scholar]
- 177.Liu K. & Jiang L. Multifunctional integration: from biological to bio-inspired materials. ACS Nano 5, 6786–6790 (2011). [DOI] [PubMed] [Google Scholar]
- 178.Liao W-S et al. Small-molecule arrays for sorting G-protein-coupled receptors. J. Phys. Chem C 117, 22362–22368 (2013). [Google Scholar]
- 179.Tibbits S. 4D printing: multi-material shape change. Archit. Design 84, 116–121 (2014). [Google Scholar]
- 180.Sydney Gladman A, Matsumoto EA, Nuzzo RG, Mahadevan L. & Lewis JA Biomimetic 4D printing. Nat. Mater 15, 413–418 (2016). [DOI] [PubMed] [Google Scholar]
- 181.Adamo A. et al. On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system. Science 352, 61–67 (2016). [DOI] [PubMed] [Google Scholar]
- 182.Garza-García LD et al. Continuous flow microbioreactors for the production of biopharmaceuticals: the effect of geometry, surface texture, and flow rate. Lab Chip 14, 1320–1329 (2014). [DOI] [PubMed] [Google Scholar]
- 183.Timm AC, Shankles PG, Foster CM, Doktycz MJ & Retterer ST Toward microfluidic reactors for cell-free protein synthesis at the point-of-care. Small 12, 810–817 (2016). [DOI] [PubMed] [Google Scholar]
- 184.Eringen AC Simple microfluids. Int. J. Eng. Sci 2, 205–217 (1964). [Google Scholar]