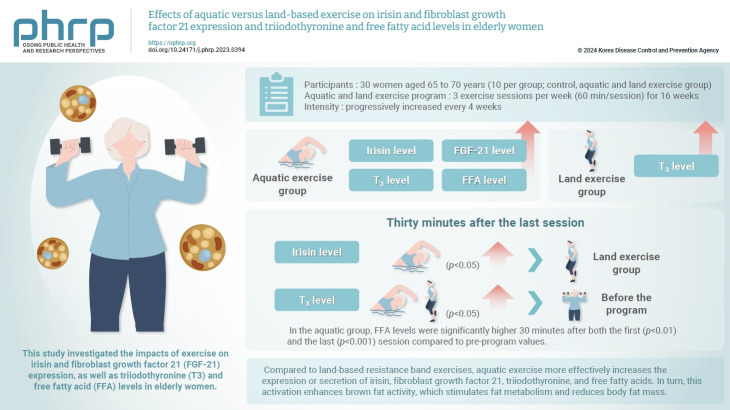
Abstract
Objectives
This study investigated the impacts of exercise on irisin and fibroblast growth factor 21 (FGF-21) expression, as well as triiodothyronine (T3) and free fatty acid (FFA) levels in elderly women.
Methods
Thirty women aged 65 to 70 years (10 per group) were randomly assigned to aquatic exercise, land exercise, and control groups. The aquatic and land groups engaged in 3 exercise sessions per week (60 min/session) for 16 weeks. The intensity was progressively increased every 4 weeks.
Results
Irisin and FGF-21 levels significantly increased in the aquatic exercise group. In the post-test, the aquatic exercise group had the highest irisin levels. Significant findings were observed for irisin and FGF-21 for the main effect between aquatic and band exercise groups (p<0.05 for both), the main effect between measurement times (p<0.01 and p<0.001, respectively), and the interaction effect (p<0.05 and p<0.001, respectively). The irisin level was significantly higher in the aquatic than in the land group 30 minutes after the last session (p<0.05). In both exercise groups, T3 levels were significantly higher 30 minutes after the final session (p<0.05) than before the program. The FFA level was significantly higher in the aquatic exercise group than the others. In the aquatic group, FFA levels were significantly higher 30 minutes after both the first (p<0.01) and the last (p<0.001) session compared to pre-program values.
Conclusion
Differences in exercise type and environment can promote fat metabolism by stimulating hormonal changes that induce brown fat activity and browning.
Keywords: Aquatic exercise, Fibroblast growth factor 21, Free fatty acid, Irisin, Triiodothyronine
Graphical abstract
Introduction
Reductions in muscle mass and strength associated with aging lead to decreased energy expenditure and fat metabolism [1]. These changes promote a gradual accumulation of body fat [2] and represent a major contributor to the onset of obesity and metabolic diseases, including type 2 diabetes. As muscle function declines and type 2 diabetes develops, conditions such as insulin resistance, hypertension, and dyslipidemia are likely to arise [3].
Human fat can be functionally categorized into 2 types: white and brown fat [4]. White adipose tissue (WAT) can store a substantial amount of energy as triglycerides, which are then released as free fatty acids (FFAs) to provide energy during periods of high demand [5]. While this fat tissue helps insulate the body and protects organs from impact injuries [6], its excessive accumulation can lead to obesity. In contrast, brown adipose tissue (BAT) plays a crucial role in regulating body temperature and expending energy through thermogenesis in response to heat, cold, and exercise [7]. This is largely due to the abundance of mitochondria expressing uncoupling protein 1 (UCP1), which has thermogenic functions. Consequently, heat is produced via the uncoupling pathway without generating adenosine triphosphate (ATP) [8]. This process enhances the rate of energy consumption by facilitating fatty acid oxidation and increasing exothermic reactions [9]. The heat produced is then circulated throughout the body [10], which is valuable for maintaining core body temperature and for the prevention and reduction of obesity [11].
Unlike conventional BAT, WAT can be converted into brown fat through various stimuli. This transformation process is known as browning, and the resulting cells are termed “brite” or “beige” adipocytes [12]. These cells facilitate the clearance of glucose and FFAs from the body through their expression of the UCP1 gene, which modulates thermogenesis in a similar process to that of conventional brown fat [9].
The hormones irisin and fibroblast growth factor 21 (FGF-21) promote BAT formation and induce the browning of WAT [13]. Irisin, a myokine, is secreted by muscles in response to shivering from cold exposure [14] or exercise [15]. It facilitates browning [16] and can serve as an indicator of muscle mass [17]. Irisin activates UCP1 in the inner mitochondrial membranes to generate thermal energy [16]. These processes enhance the body's energy expenditure through fat metabolism, leading to weight loss. Additionally, as people age, these effects are observed at lower levels in women compared to men, and in obese individuals compared to those of normal weight [16,18]. FGF-21 is secreted by the liver and adipocytes in response to physical activity or non-shivering thermogenesis [19]. This hormone contributes to body fat reduction by increasing glucose uptake [20], stimulating lipolysis [21], and triggering physiological processes such as browning, thermogenesis, and energy consumption [22]. These effects collectively increase insulin sensitivity, combat obesity, and improve metabolic health [23].
Triiodothyronine (T3) is a potential transcription factor for the UCP1 gene [14] that, in conjunction with FGF-21, may influence metabolic activity. For instance, increased T3 secretion following exercise or sustained cold exposure can raise the resting metabolic rate [24]. The expression of irisin, FGF-21, and T3 activates UCP1 in BAT and beige fat; this promotes lipolysis, leading to an increase in FFAs. These FFAs are then utilized as an energy source during thermogenesis, making them a primary substrate for thermogenesis in brown and beige fat [4].
Most studies investigating irisin and FGF-21 have been focused on aerobic and resistance exercises, respectively. However, limited research has compared and analyzed these 2 types of exercise concurrently. To our knowledge, no studies have yet examined differences in irisin, FGF-21, and T3 expression between aquatic and land-based exercises while considering temperature effects. Consequently, our study was designed to investigate the differences between aquatic and land-based exercise environments. Specifically, we utilized aquatic exercises with aerobic characteristics and land exercises with resistance characteristics over a period of 16 weeks. The study objective was to compare the effects of these exercise modalities on the expression of irisin and FGF-21, as well as on the levels of T3 and FFA, in elderly women. By identifying effective exercise strategies for obesity prevention, we hope that the findings may contribute to the extension of life expectancy.
Materials and Methods
Participants
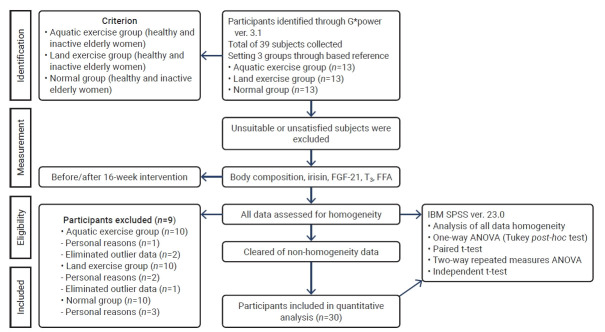
This study was conducted in women aged 65 to 70 years from Changwon city, Republic of Korea, who did not engage in regular exercise (that is, they had performed less than 1 hour of regular exercise per week over the past year). Participants were randomly selected, with G*Power 3.1 (Christian-Albrechts-Universität) utilized to calculate the appropriate sample size. The analysis indicated that 36 participants were necessary under the following conditions: an effect size of 0.40, an alpha level of 0.05, and a power of 0.80. To account for potential dropouts, 39 individuals were initially recruited. Simple random sampling was used to assign 13 participants to each of the 3 groups: control, aquatic exercise, and land exercise. Over the course of the experiment, some participants withdrew for personal reasons or were excluded due to poor attendance. Consequently, measurements and analyses were conducted among a total of 30 participants, with 10 in each of the 3 groups.
The study procedures are detailed in Figure 1.
Figure 1.
Study procedures.
FGF-21, fibroblast growth factor 21; T3, triiodothyronine; FFA, free fatty acids; ANOVA, analysis of variance.
Physique and Body Composition
Participant height, weight, body mass index, skeletal muscle mass, and body fat percentage were measured with the participants dressed in light clothing using the X-SCAN PLUS II (Jawon Medical) bioelectrical impedance analysis device. These measurements were taken in accordance with the procedures recommended by the American College of Sports Medicine [25].
Blood Tests
For control participants, blood samples were collected and analyzed under the same methods and resting conditions at 2 time points: before and after a 16-week interval. For the aquatic and land exercise groups, samples were taken at 3 points: first, at rest prior to the 16-week program; second, 30 minutes after the first exercise session; and third, 30 minutes after the final exercise session.
Blood collection was conducted between 8 AM and 9 AM on the day of the procedure, with participants instructed to fast starting at 8 PM the previous evening. After 30 minutes of rest following the completion of the respective exercise programs, a clinical pathologist drew 10 mL of blood from the antebrachial vein of individuals in the exercise groups using a vacutainer and a disposable syringe. The blood was then placed into a serum separator tube and centrifuged at 3,000 rpm for 10 minutes using a Combi-514R centrifuge (Hanil BioMed Inc.). Following centrifugation, the supernatant was decanted from the serum and transferred into a 1.5-mL microtube. These tubes were stored at −80 °C until analysis. The GC Cell Medical Foundation conducted subsequent analyses using the appropriate methods for each test.
Irisin levels were measured through an enzyme-linked immunosorbent assay (ELISA) using an irisin recombinant ELISA kit (Phoenix Pharmaceuticals). Readings were taken on a Multiskan GO spectrophotometer (Thermo Scientific) with optical density measured at 450 nm. FGF-21 levels were determined using ELISA with a human FGF-21 immunoassay kit (R&D Systems) and a microplate reader (VersaMax; Molecular Devices LLC). T3 was quantified via an electrochemiluminescence immunoassay using Elecsys T3 (Roche) and a Cobas 8000 analyzer (e801; Roche). FFA levels were assessed via calorimetry with a NEFA HR assay kit (FUJIFILM Wako Pure Chemicals) and a Cobas 8000 system (c702; Roche).
Aquatic Exercise Program
This study adapted the exercise program developed by the Korea Aquatic Exercise Association. The program was conducted in a swimming pool with an indoor air temperature of 30℃ to 32℃, relative humidity between 65% and 70%, water temperature of 27℃±1℃, and water depth of 1.2 meters. Participants engaged in the exercise program 3 times per week for 16 weeks. Each session lasted 60 minutes, comprising a 10-minute warm-up, a 40-minute main exercise routine, and a 10-minute cool-down. The intensity of the exercises was progressively increased every 4 weeks, a process conducted by measuring the participant’s heart rate and setting the rating of perceived exertion (RPE) using a heart rate monitor (Polar RS400sd; Polar Electro). The program's intensity levels were set at 40% to 50% heart rate reserve (HRR) with an RPE of 11 to 12 for the first 4 weeks, 50% to 60% HRR with an RPE of 13 to 14 for weeks 5 to 8, 60% to 65% HRR with an RPE of 14 to 15 for weeks 9 to 12, and 65% to 70% HRR with an RPE of 15 to 16 for the final 4 weeks (13–16) [25].
Land Exercise Program
This study employed a modified version of the resistance exercise program originally presented by the American College of Sports Medicine [25]. The program was conducted in an indoor gym maintained at a temperature of 25℃±1℃ and a relative humidity of 50%–55%. Participants engaged in the exercise program 3 times per week for 16 weeks. Each session, performed with a TheraBand (Hygenic Corporation), lasted 60 minutes and included a 10-minute warm-up, a 40-minute main exercise routine consisting of 3 sets of 15 repetitions with rest intervals between sets, and a 10-minute cool-down period. The intensity of the exercises was progressively increased, starting with a yellow (thin) band for the first 4 weeks and transitioning to a red (medium) band for weeks 5 through 16 [26]. The length of the band was adjusted every 4 weeks to match the participants’ increasing adaptability and strength. Additionally, the RPE was used to estimate exercise intensity at the beginning of the program and was adjusted every 4 weeks. The target intensity levels were set at 40% to 50% of HRR (RPE, 11–12) for the first 4 weeks, 50% to 60% HRR (RPE, 13–14) for weeks 5 to 8, 60% to 65% HRR (RPE, 14–15) for weeks 9 to 12, and 65% to 70% HRR (RPE, 15–16) for weeks 13 to 16. The control group was instructed to continue with their usual activities without starting any new exercise programs.
Data Analysis
For this study, data processing was conducted using IBM SPSS ver. 23.0 (IBM Corp.). Descriptive statistics for each group were analyzed according to measurement items, and group homogeneity for each variable was assessed using the Levene test. To determine the average differences between groups before and after the 16-week exercise regimen, a paired t-test and 1-way analysis of variance (ANOVA) were utilized. Subsequent post hoc analysis was conducted using the Tukey test. The interaction between measurement times for the aquatic and land exercise groups was examined using 2-way repeated measures ANOVA. The average differences between these groups were assessed using an independent t-test, with the significance (alpha) level set at 0.05.
Ethics Approval
Before the experiment began, the study (derived from PNU IRB/2017_68_HR) received approval from the Institutional Human Research Committee. Participants were fully informed of the purpose of the research, and only those who willingly agreed to participate submitted their consent.
Results
Participant Characteristics and Body Composition
The demographic characteristics of the study participants are detailed in full in Table 1. For all women, we measured body composition and the levels of irisin, FGF-21, T3, and FFA before the initiation of the 16-week aquatic or land-based exercise program and again at its conclusion.
Table 1.
Changes in body composition after 16 weeks of aquatic and land exercise
| Control group (n=10) |
Aquatic exercise group (n=10) |
Land exercise group (n=10) |
||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| Age (y) | 68.59±1.83 | 68.34±1.64 | 67.71±1.35 | |||
| Height (cm) | 151.40±4.50 | 151.87±3.41 | 149.72±7.78 | |||
| Weight (kg) | 56.36±5.11 | 56.41±4.88 | 55.78±5.07 | 55.53±4.87 | 58.47±5.75 | 58.43±5.76 |
| LBM (kg) | 33.88±2.49 | 33.86±2.38 | 34.51±2.60 | 34.85±2.61 | 33.72±3.06 | 33.77±2.44 |
| BFM (kg) | 20.35±3.21 | 20.47±2.64 | 19.15±3.36 | 18.51±3.09* | 22.64±5.40 | 22.63±5.48 |
| %BF (%) | 35.94±3.20 | 36.19±2.12 | 34.17±3.54 | 33.19±3.40** | 38.37±6.29 | 38.31±6.29 |
Values are presented as mean±standard deviation.
LBM, lean body mass; BFM, body fat mass; %BF, percentage of body fat.
p<0.05,
p<0.01.
Irisin Level
Table 2 presents the variations in irisin levels within and between the groups. Within the aquatic exercise group, a significant increase was observed (p<0.05). When comparing the 3 groups, significant differences were observed in the final exercise period (p<0.01) and in the magnitude of change (p<0.01). Post hoc analysis revealed that both the irisin level after the last exercise session and the magnitude of change were higher in the aquatic exercise group compared to the control and land exercise groups.
Table 2.
Changes in irisin and FGF-21 levels throughout 16 weeks of aquatic and land exercise
| Variable | Group (n=10) | First | Second | Third | Change | Paired t-test | F | |
|---|---|---|---|---|---|---|---|---|
| Irisin (ng/mL) | CG | 166.67±15.44 | 164.37±14.85 | −2.30±13.27 | −0.548 | Group (G) | 4.480* | |
| AEG | 169.64±12.93 | 185.67±17.19 | 198.43±21.31 | 28.80±29.29 | 3.108* | Time (T) | 5.909** | |
| LEG | 172.70±12.63 | 176.86±12.32 | 175.87±15.89 | 3.17±19.44 | 0.516 | G×T | 3.717* | |
| F | 0.483 | 9.715** | 5.846** | |||||
| Post-hoc | NS | CG, LEG<AEG | CG, LEG<AEG | |||||
| FGF-21 (pg/mL) | CG | 95.29±18.19 | 93.93±21.62 | −1.36±22.54 | −0.191 | Group (G) | 4.651* | |
| AEG | 91.14±9.57 | 100.35±9.57 | 111.83±12.56 | 20.69±14.83 | 4.413* | Time (T) | 14.885*** | |
| LEG | 92.46±9.15 | 94.07±7.53 | 94.06±7.34 | 1.60±5.25 | 0.964 | G×T | 11.066*** | |
| F | 0.267 | 4.686* | 5.690** | |||||
| Post-hoc | NS | CG, LEG<AEG | CG, LEG<AEG | |||||
Values are presented as mean±standard deviation.
FGF-21, fibroblast growth factor 21; first, before the 16-week exercise program; second, 30 minutes after the first exercise session; third, 30 minutes after the last exercise session; CG, control group; AEG, aquatic exercise group; LEG, land exercise group; NS, non-significant.
p<0.05,
p<0.01,
p<0.001.
The main effects were significant both between groups (p<0.05) and across measurement times (p<0.01), as was the interaction effect (p<0.05). Thirty minutes following the last exercise session, the irisin level was significantly higher in the aquatic exercise group than in the land exercise group.
FGF-21 Level
Table 2 presents the intra- and inter-group changes in FGF-21 levels. Within the aquatic exercise group, a significant increase in FGF-21 was observed (p<0.05). When comparing the 3 groups, significant differences emerged in the final exercise session (p<0.05) and the magnitude of change (p<0.01). Post hoc analysis revealed that the aquatic exercise group displayed higher values for both the final exercise session and the magnitude of change compared to the control and land exercise groups.
The main effects were significant both between groups (p<0.05) and across measurement time point (p<0.001), as was the interaction effect (p<0.001). Thirty minutes following the last exercise session, the FGF-21 level was significantly higher in the aquatic exercise group than in the land group.
T3 Level
The changes in T3 levels over time within and between the aquatic and land exercise groups, as well as the interaction effect, are depicted in Figure 2. Significant differences were observed between measurement points, and a significant interaction effect was found (p<0.05). However, no significant difference was observed between the groups. In both aquatic and land exercise groups, T3 levels were significantly elevated 30 minutes after the final exercise session (p<0.05) compared to measurements taken before the exercise program.
Figure 2.
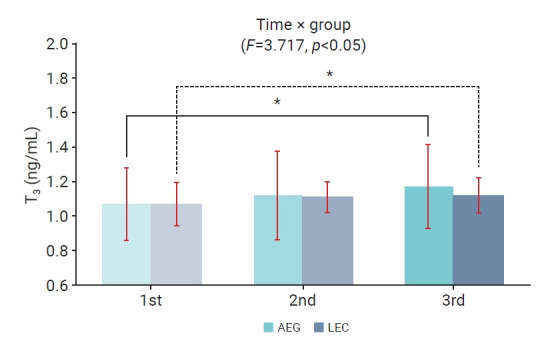
Two-way repeated measures analysis of variance of triiodothyronine (T3) levels throughout the 16-week aquatic and land exercise programs. 1st, before the 16-week exercise program; 2nd, 30 minutes after the first exercise session; 3rd, 30 minutes after the last exercise session. *p<0.05 compared with the first measurements.
AEG, aquatic exercise group; LEG, land exercise group.
FFA Level
The changes in FFA levels over time within and between the aquatic and land exercise groups, as well as the interaction effect, are depicted in Figure 3. Significant differences were observed between measurement points (p<0.05); however, no significant differences were found between the groups, nor was a significant interaction effect observed. In the aquatic exercise group, FFA levels were significantly higher 30 minutes after both the first exercise session (p<0.01) and the last session (p<0.001) relative to pre-program values. In the land exercise group, FFA levels were significantly higher 30 minutes after the initial exercise session (p<0.05) compared to the pre-program baseline.
Figure 3.
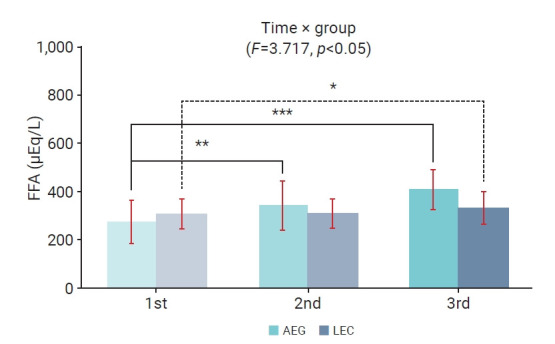
Two-way repeated measures analysis of variance of free fatty acid (FFA) levels throughout the 16-week aquatic and land exercise programs. 1st, before the 16-week exercise program; 2nd, 30 minutes after the first exercise session; 3rd, 30 minutes after the last exercise session. *p<0.05, **p<0.01, ***p<0.001 compared with the first measurements.
AEG, aquatic exercise group; LEG, land exercise group.
Discussion
This study examined the effects of a 16-week aquatic exercise and land-based exercise program on elderly women, specifically investigating the levels of irisin and FGF-21, which are known to induce brown fat activity and browning.
The present findings indicate that levels of irisin and FGF-21 varied according to the timing of blood sample collection. Previous research has indicated that irisin levels temporarily increase after exercise, then stabilize [27] at 125 minutes after moderate-intensity endurance exercise and at 15 minutes following high-intensity interval exercise [28]. Irisin typically peaks 60 minutes after engaging in aerobic and resistance exercises [29]. Meanwhile, FGF-21 levels return to a stable state 60 minutes after exercise [30], with a physiological half-life of less than or equal to 2 hours [31]. In light of these earlier findings, the present study was designed to collect blood samples 30 minutes after exercise. The results confirmed that sampling blood at this time point results in elevated levels of irisin and FGF-21 compared to their stabilized levels, aligning with the findings reported by Kim and Kim [32].
Irisin is expressed in response to various forms of exercise, including aerobic exercise [33,34] and resistance training [35,36]. Kim and Kim [32] observed that elderly women participating in a 16-week aquatic exercise program displayed increased levels of irisin. Similarly, Lee et al. [37] found that a 12-week dance sports program aimed at elderly women with obesity also led to elevated irisin levels. Additionally, Lee and So [38] reported that treadmill walking exercises resulted in higher irisin levels in elderly women. Bostrom et al. [16] noted a similar increase following a 10-week aerobic exercise program in healthy adults.
Irisin secretion is higher after high-intensity exercise than after low-intensity activity [39] and can be further increased by high-intensity sprinting [33,34]. Therefore, aerobic exercise is likely to correspond to varying levels of irisin expression based on the intensity of the exercise. In this study, the significant difference observed in the aquatic exercise program can be explained as follows. Although the intensity of the final exercise session was only moderate to high, at 65% to 70% HRR with an RPE of 15 to 16, this level of intensity was as effective as high-intensity exercise for the participants. For instance, the step exercise required participants to hop repeatedly for an hour to relatively fast-tempo music, demanding considerable effort from those with lower stamina.
Furthermore, the higher levels of irisin observed during aquatic exercise compared to land exercise in this study may stem from several factors. The water temperature for aquatic exercise was 27℃±1℃ versus the indoor air temperature for land exercise (25℃±1℃). Notably, however, water is approximately 800 times denser than air, with a thermal conductivity around 25 times greater. Consequently, the increased energy expenditure required to maintain body temperature during aquatic exercise likely had a major impact. Furthermore, additional research is warranted to explore how other characteristics of water, such as buoyancy, hydrostatic pressure, and resistance, might affect irisin levels.
Previous studies investigating the effects of resistance exercise on irisin levels have reported increased levels in several contexts: following a 12-week land resistance exercise program among elderly women [40], a 12-week weight training regimen for men aged 20 to 30 years [41], and an 8-week resistance training routine in individuals with obesity [42]. However, research by Moraes et al. [43] indicated that resistance exercise in hemodialysis patients and healthy male and female adults aged 40–50 years did not lead to a significant change in irisin levels. Additionally, Norheim et al. [44] found that resistance exercise either maintained or reduced irisin levels. Collectively, these studies suggest that resistance exercise may result in an increase or no change in irisin concentration.
Resistance exercise alters irisin expression based on the method and intensity of the exercise. However, it does not influence irisin excretion, as the land exercise in this study was characterized by low intensity and high repetitions, which enhance muscle endurance rather than muscle hypertrophy associated with high-intensity, low-repetition exercise. Given that resistance exercise yields inconsistent results varying by sex, age group, and exercise intensity, future studies should establish and apply specific criteria tailored to these factors.
Previous studies have examined the impact of exercise on FGF-21 levels. Kim et al. [23] found that a single session of either moderate-intensity (50% maximal oxygen consumption [VO2 max]) or high-intensity (80% VO2 max) treadmill running significantly increased FGF-21 concentrations in men in their 20s. This increase was more pronounced following high-intensity exercise compared to low-intensity exercise. Additionally, Lee and Kim [45] observed that treadmill running at low intensity (50% VO2 max) and medium intensity (75% VO2 max) also led to a significant elevation in FGF-21 levels in men in their 20s, with the medium-intensity exercise producing a greater increase.
FGF-21 secretion increases during high-intensity exercise [46]. The intensity of the activity correlates with increased activity in the adenosine monophosphate-activated protein kinase-FGF21 signaling pathway [47], and expression levels rise with the extension of a medium-intensity exercise regimen over a relatively long term [45]. These findings indicate that exercise intensity influences FGF-21 expression. In this study, by progressively increasing exercise intensity every 4 weeks, and by engaging in a medium- or high-intensity aquatic exercise regimen for an extended duration of 16 weeks, a significant impact was observed. Notably, a significant difference in FGF-21 expression was seen in the aquatic exercise group, in contrast to the land exercise group. This discrepancy may be attributed to differences in the energy metabolism systems of the 2 exercise modalities. Aquatic exercise, which is predominantly aerobic, utilizes fat as its main energy source. In contrast, the land exercise used in this study involved resistance training, which relies primarily on glucose for energy.
Irisin and FGF-21 expression are influenced by the exercise environment and temperature [30,42,48–50]. The following summarizes previous research on the impact of exercise environment on irisin levels. Lee et al. [42] demonstrated that a single hour of cold exposure induced shivering and elevated irisin levels in 10 healthy adults. Seebacher and Glanville [49] observed no change in the expression of PGC1-α, a transcription factor for irisin, in mice subjected to 12 °C or 22 °C. However, the presence or absence of exercise at each temperature did impact expression levels. Notably, expression was higher in mice that exercised at 12 °C [49]. Kim [48] conducted a comparison between aquatic and land-based exercises in elderly women, finding that those in the aquatic exercise group exhibited higher irisin levels.
Several previous studies have investigated the impact of the exercise environment on FGF-21 levels. Lee et al. [42] demonstrated that a single 1-hour exposure to cold induced shivering and elevated irisin levels in 10 healthy adults. Hanssen et al. [50] observed that continuous cold exposure for 6 hours daily over a 10-day period led to a significant rise in irisin concentration among healthy men. Additionally, Jeon et al. [30], who studied a group of scuba divers, noted that a 1-time exposure to cold water increased irisin concentrations. These findings suggest that variations in the temperature of the exercise environment can influence the expression of irisin and FGF-21.
Heat conduction in water is approximately 25 times greater than in air, which can adversely impact temperature homeostasis during aquatic exercises [51]. Consequently, additional energy is needed to maintain body temperature [32]. Nevertheless, mild exposure to cold can stimulate energy expenditure with minimal impacts on other parts of the body [11].
In this study, levels of irisin and FGF-21 were significantly altered following aquatic exercise, while no significant changes were noted after land-based exercise. The aquatic exercise program was conducted in a swimming pool at a controlled temperature of 27 °C±1 °C (medium-temperature water). In this case, irisin and FGF-21 were likely secreted to counteract the reduction in core body temperature, which is a response to the high thermal conductivity of water that promotes non-shivering thermogenesis [50]. Perspiration increases during land exercise, which here consisted of a 1-hour session set to music with a tempo of ≤70 beats per minute, performed in an indoor fitness center with an ambient temperature of 25 °C±1 °C and 50% to 55% relative humidity. This resulted in a more pronounced decrease in plasma volume. In this study, the timing of blood collection—30 minutes after exercise completion—may have influenced the measurement of changes in irisin and FGF-21 concentrations due to the body’s fluid redistribution for homeostatic balance.
Furthermore, irisin—an exercise-induced myokine—is expressed at levels 40% higher in skeletal muscle fibers than in type II (fast-twitch) and type I (slow-twitch) fibers [52]. In this study, the predominant muscle fiber type utilized during land exercise was type II, while type I fibers were primarily engaged during aquatic exercise.
Exercise impacts levels of both T3 and FFAs. Miller et al. [53] observed that exercise prompts an increase in thyroid-stimulating hormone release, which in turn stimulates the secretion of T3. Similarly, Hackney et al. [54] found that both high-intensity training and endurance exercise elevated T3 levels in participants, suggesting that exercise can influence thyroid hormone activity. Additionally, Goto et al. [55] reported that 60 minutes of continuous exercise at 60% VO2 max intensity led to an increase in FFAs. Complementing these findings, Kim et al. [56] demonstrated that both intermittent and continuous fast walking exercises increased FFA levels 30 minutes after exercise in female college students, a result that aligns with the findings of the present study.
Coggan et al. [57] reported that the rise in serum T3 concentration observed immediately after exercise is influenced by norepinephrine and the activation of adrenaline. In this study, the difference in the elevated T3 levels during the recovery period compared to the levels stabilized 30 minutes post-exercise may result from excess post-exercise oxygen consumption. Additionally, the high FFA levels following both types of exercise indicate that fat metabolism as an energy source during the latter portion of the exercise session can mobilize more energy. This suggests a potential for body fat reduction during the post-exercise recovery period.
The stress of cold exposure and exercise activates the sympathetic nervous system, and the activity of T3 induces lipolysis in both BAT and WAT. This process of lipid metabolism elevates the FFA concentration [22]. Additionally, Cuevas-Ramos et al. [21] found that PPAR-α, a transcription factor critical for FGF-21 expression, is stimulated by FFAs and that lipolysis leads to the expression of FGF-21. These findings imply that sympathetic nerve stimulation results in increased FFA levels and a subsequent rise in FGF-21 secretion due to lipolysis. The present study confirmed that levels of T3 (which activates the sympathetic nervous system), as well as FFAs and FGF-21, were highest following the final exercise session. Thus, an increase in FFA levels is closely related to the increased expression of FGF-21.
Irisin, FGF-21, and T3, which are secreted during shivering, non-shivering thermogenesis, and exercise, activate BAT and induce browning [16,22]. This process enhances triglyceride consumption through lipid metabolism. Consequently, the increase in energy expenditure through thermogenesis may help prevent weight gain [9,58] and combat obesity. However, further research is needed to investigate the expression levels of irisin, FGF-21, T3, and FFA under various exercise conditions, including ambient and water temperatures, program content, and the specific goals of aquatic and land-based exercise regimens.
Our study has certain limitations. First, due to the relatively small sample size (n=10 per group), it may be challenging to generalize the results to broader populations. Second, participants necessarily engaged in additional, unrecorded activities of daily living, which could have confounded the results. Third, further research is required to confirm our findings; additionally, future studies should compare the effects of aquatic and land exercise programs on various subgroups (obese and non-obese individuals, men and women, etc.) categorized by age or type of exercise, and develop effective aerobic and land exercise programs for different age groups. Additionally, safety concerns for the elderly female participants prevented full exposure to cold temperatures during aquatic exercises. Future studies should consider the exercise environment from multiple perspectives.
Conclusion
This study demonstrates that levels of irisin, FGF-21, T3, and FFAs were highest 30 minutes following the final exercise session. The data suggest that variations in the expression of irisin and FGF-21, as well as fluctuations in T3 and FFA levels, could be attributed not only to the heightened energy expenditure from exercise but also to differences in the exercise environment. Specifically, aquatic exercise may be more effective than land-based activity in enhancing the secretion of irisin and FGF-21.
Different exercise types and environments can enhance fat metabolism by triggering hormonal changes that activate brown fat and encourage the conversion of white to brown fat. This process can ultimately aid in preventing and lessening obesity through body fat reduction.
Further research is needed to ascertain how the expressions of irisin and FGF-21, as well as the levels of T3 and FFAs, can be elevated by modifying exercise environment conditions—including ambiance, water temperature, and program content—to prevent and ameliorate obesity.
HIGHLIGHTS
• Compared to land-based resistance band exercises, aquatic exercise more effectively increases the expression or secretion of irisin, fibroblast growth factor 21, triiodothyronine, and free fatty acids. In turn, this activation enhances brown fat activity, which stimulates fat metabolism and reduces body fat mass.
• Variations in exercise type and environment can trigger hormonal changes that induce brown fat activation and fat browning. Ultimately, this process promotes fat metabolism, contributing to a cumulative effect that helps prevent and lessen obesity through fat reduction.
Footnotes
Ethics Approval
Before the experiment began, the study (derived from PNU IRB/2017_68_HR) received approval from the Institutional Human Research Committee. Participants were fully informed of the purpose of the research, and only those who willingly agreed to participate submitted their consent.
Conflicts of Interest
The authors have no conflicts of interest to declare.
Funding
This work was supported by a 2-year research grant from Pusan National University.
Availability of Data
The datasets are not publicly available but can be obtained from the corresponding author upon reasonable request.
Authors’ Contributions
Conceptualization: DWK, DYK; Data curation: KSH, MKK; Formal analysis: DWK, SHK, MKK; Investigation: DWK, SHK, MKK; Methodology: DWK, SHK, DYK; Project administration: DYK; Resources: SHK, MKK; Software: SHK, MKK; Supervision: DWK; Validation: DWK, DYK; Visualization: SHK, MKK; Writing–original draft: DWK, DYK; Writing–review & editing: all authors. All authors read and approved the final manuscript.
References
- 1.Chau D, Cho LM, Jani P, et al. Individualizing recommendations for weight management in the elderly. Curr Opin Clin Nutr Metab Care. 2008;11:27–31. doi: 10.1097/MCO.0b013e3282f31744. [DOI] [PubMed] [Google Scholar]
- 2.Sakuma K, Yamaguchi A. Sarcopenic obesity and endocrinal adaptation with age. Int J Endocrinol. 2013;2013:204164. doi: 10.1155/2013/204164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lafontan M. Adipose tissue and adipocyte dysregulation. Diabetes Metab. 2014;40:16–28. doi: 10.1016/j.diabet.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Saito M. Human brown adipose tissue: regulation and anti-obesity potential. Endocr J. 2014;61:409–16. doi: 10.1507/endocrj.ej13-0527. [DOI] [PubMed] [Google Scholar]
- 5.Attie AD, Scherer PE. Adipocyte metabolism and obesity. J Lipid Res. 2009;50(Suppl):S395–9. doi: 10.1194/jlr.R800057-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trayhurn P. Adipocyte biology. Obes Rev. 2007;8 Suppl 1:41–4. doi: 10.1111/j.1467-789X.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- 7.Fenzl A, Kiefer FW. Brown adipose tissue and thermogenesis. Horm Mol Biol Clin Investig. 2014;19:25–37. doi: 10.1515/hmbci-2014-0022. [DOI] [PubMed] [Google Scholar]
- 8.Cannon B, Nedergaard J. Metabolic consequences of the presence or absence of the thermogenic capacity of brown adipose tissue in mice (and probably in humans) Int J Obes (Lond) 2010;34 Suppl 1:S7–16. doi: 10.1038/ijo.2010.177. [DOI] [PubMed] [Google Scholar]
- 9.Bartelt A, Bruns OT, Reimer R, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–5. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 10.Ricquier D. Uncoupling protein 1 of brown adipocytes, the only uncoupler: a historical perspective. Front Endocrinol (Lausanne) 2011;2:85. doi: 10.3389/fendo.2011.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cypess AM, Chen YC, Sze C, et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci U S A. 2012;109:10001–5. doi: 10.1073/pnas.1207911109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klingenspor M, Herzig S, Pfeifer A. Brown fat develops a brite future. Obes Facts. 2012;5:890–6. doi: 10.1159/000346337. [DOI] [PubMed] [Google Scholar]
- 13.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–63. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 14.Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–25. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 15.Catoire M, Mensink M, Kalkhoven E, et al. Identification of human exercise-induced myokines using secretome analysis. Physiol Genomics. 2014;46:256–67. doi: 10.1152/physiolgenomics.00174.2013. [DOI] [PubMed] [Google Scholar]
- 16.Bostrom P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huh JY, Mougios V, Kabasakalis A, et al. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J Clin Endocrinol Metab. 2014;99:E2154–61. doi: 10.1210/jc.2014-1437. [DOI] [PubMed] [Google Scholar]
- 18.Loffler D, Muller U, Scheuermann K, et al. Serum irisin levels are regulated by acute strenuous exercise. J Clin Endocrinol Metab. 2015;100:1289–99. doi: 10.1210/jc.2014-2932. [DOI] [PubMed] [Google Scholar]
- 19.Salminen A, Kaarniranta K, Kauppinen A. Integrated stress response stimulates FGF21 expression: systemic enhancer of longevity. Cell Signal. 2017;40:10–21. doi: 10.1016/j.cellsig.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Kharitonenkov A, Shiyanova TL, Koester A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–35. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuevas-Ramos D, Almeda-Valdes P, Meza-Arana CE, et al. Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS One. 2012;7:e38022. doi: 10.1371/journal.pone.0038022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Virtanen KA. BAT thermogenesis: linking shivering to exercise. Cell Metab. 2014;19:352–4. doi: 10.1016/j.cmet.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Kim KH, Kim SH, Min YK, et al. Acute exercise induces FGF21 expression in mice and in healthy humans. PLoS One. 2013;8:e63517. doi: 10.1371/journal.pone.0063517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva JE. Physiological importance and control of non-shivering facultative thermogenesis. Front Biosci (Schol Ed) 2011;3:352–71. doi: 10.2741/s156. [DOI] [PubMed] [Google Scholar]
- 25.American College of Sports Medicine (US) ACSM’s guidelines for exercise testing and prescription. Wolters Kluwer Health; 2016. [DOI] [PubMed] [Google Scholar]
- 26.Phil P, Todd E. Strength band training. 2nd ed. Human Kinetics Publisher; 2011. [Google Scholar]
- 27.Huh JY, Dincer F, Mesfum E, et al. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int J Obes (Lond) 2014;38:1538–44. doi: 10.1038/ijo.2014.42. [DOI] [PubMed] [Google Scholar]
- 28.Winn NC, Grunewald ZI, Liu Y, et al. Plasma irisin modestly increases during moderate and high-intensity afternoon exercise in obese females. PLoS One. 2017;12:e0170690. doi: 10.1371/journal.pone.0170690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuchiya Y, Ando D, Takamatsu K, et al. Resistance exercise induces a greater irisin response than endurance exercise. Metabolism. 2015;64:1042–50. doi: 10.1016/j.metabol.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Jeon YN, Ra SG, Kim CK. Effect of a bout of acute cold water immersion on circulating FGF21, irisin and T3 hormones in SCUBA divers. Exerc Sci. 2015;24:283–9. Korean. [Google Scholar]
- 31.Lee P, Brychta RJ, Linderman J, et al. Mild cold exposure modulates fibroblast growth factor 21 (FGF21) diurnal rhythm in humans: relationship between FGF21 levels, lipolysis, and cold-induced thermogenesis. J Clin Endocrinol Metab. 2013;98:E98–102. doi: 10.1210/jc.2012-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JH, Kim DY. Aquarobic exercises improve the serum blood irisin and brain-derived neurotrophic factor levels in elderly women. Exp Gerontol. 2018;104:60–5. doi: 10.1016/j.exger.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 33.Timmons JA, Baar K, Davidsen PK, et al. Is irisin a human exercise gene? Nature. 2012;488:E9–11. doi: 10.1038/nature11364. [DOI] [PubMed] [Google Scholar]
- 34.Kraemer RR, Shockett P, Webb ND, et al. A transient elevated irisin blood concentration in response to prolonged, moderate aerobic exercise in young men and women. Horm Metab Res. 2014;46:150–4. doi: 10.1055/s-0033-1355381. [DOI] [PubMed] [Google Scholar]
- 35.Pekkala S, Wiklund PK, Hulmi JJ, et al. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J Physiol. 2013;591:5393–400. doi: 10.1113/jphysiol.2013.263707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J, Lee Y, Kye S, et al. Association of serum vitamin D with osteosarcopenic obesity: Korea National Health and Nutrition Examination Survey 2008-2010. J Cachexia Sarcopenia Muscle. 2017;8:259–66. doi: 10.1002/jcsm.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JW, Zhang SA, Kim CY. Effects of 12-weeks dance sports exercise on body composition, irisin, adiponectin and blood lipids in obesity elderly women. Korean J Phys Educ. 2019;58:271–84. Korean. [Google Scholar]
- 38.Lee SS, So YS. The effects of endurance exercise on and cognitive function, irisin and BDNF in elderly women. Korean Soc Sports Sci. 2014;23:1149–59. Korean. [Google Scholar]
- 39.Tsuchiya Y, Ando D, Goto K, et al. High-intensity exercise causes greater irisin response compared with low-intensity exercise under similar energy consumption. Tohoku J Exp Med. 2014;233:135–40. doi: 10.1620/tjem.233.135. [DOI] [PubMed] [Google Scholar]
- 40.Kim HJ, So B, Choi M, et al. Resistance exercise training increases the expression of irisin concomitant with improvement of muscle function in aging mice and humans. Exp Gerontol. 2015;70:11–7. doi: 10.1016/j.exger.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Yoon JK, Kim CK. Effects of 12-week high-intensity resistance training on blood irisin level and muscular function in healthy males. Exerc Sci. 2014;23:357–64. Korean. [Google Scholar]
- 42.Lee P, Linderman JD, Smith S, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–9. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moraes C, Leal VO, Marinho SM, et al. Resistance exercise training does not affect plasma irisin levels of hemodialysis patients. Horm Metab Res. 2013;45:900–4. doi: 10.1055/s-0033-1354402. [DOI] [PubMed] [Google Scholar]
- 44.Norheim F, Langleite TM, Hjorth M, et al. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014;281:739–49. doi: 10.1111/febs.12619. [DOI] [PubMed] [Google Scholar]
- 45.Lee CJ, Kim TW. Effects of cardiopulmonary exercise intensity on FGF-21 expression and FFA levels. Korean Soc Sports Sci. 2017;26:1073–80. Korean. [Google Scholar]
- 46.Martin-Rincon M, Perez-Lopez A, Azzinnari M, et al. FGF21 is produced by active skeletal muscle during intense exercise in humans. Med Sci Sports Exerc. 2017;49:88–9. [Google Scholar]
- 47.Lee JS. The effect of exercise intensity and type on metabolic variables via FGF21/Akt pathway after fasting [dissertation] Korea University; 2015. [Google Scholar]
- 48.Kim JH. Effects of aquarobic and LBE programs on irisin, BDNF, HSP27, and β-amyloid in elderly women [dissertation] Pusan National University; 2018. [Google Scholar]
- 49.Seebacher F, Glanville EJ. Low levels of physical activity increase metabolic responsiveness to cold in a rat (Rattus fuscipes) PLoS One. 2010;5:e13022. doi: 10.1371/journal.pone.0013022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanssen MJ, Broeders E, Samms RJ, et al. Serum FGF21 levels are associated with brown adipose tissue activity in humans. Sci Rep. 2015;5:10275. doi: 10.1038/srep10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nadel ER, Holmer I, Bergh U, et al. Energy exchanges of swimming man. J Appl Physiol. 1974;36:465–71. doi: 10.1152/jappl.1974.36.4.465. [DOI] [PubMed] [Google Scholar]
- 52.Roca-Rivada A, Castelao C, Senin LL, et al. FNDC5/irisin is not only a myokine but also an adipokine. PLoS One. 2013;8:e60563. doi: 10.1371/journal.pone.0060563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller PB, Forstein DA, Styles S. Effect of short-term diet and exercise on hormone levels and menses in obese, infertile women. J Reprod Med. 2008;53:315–9. [PubMed] [Google Scholar]
- 54.Hackney AC, Kallman A, Hosick KP, et al. Thyroid hormonal responses to intensive interval versus steady-state endurance exercise sessions. Hormones (Athens) 2012;11:54–60. doi: 10.1007/BF03401537. [DOI] [PubMed] [Google Scholar]
- 55.Goto K, Ishii N, Mizuno A, et al. Enhancement of fat metabolism by repeated bouts of moderate endurance exercise. J Appl Physiol (1985) 2007;102:2158–64. doi: 10.1152/japplphysiol.01302.2006. [DOI] [PubMed] [Google Scholar]
- 56.Kim MJ, Park HC, Jung AR, et al. The effects of intermittent brisk or continuant brisk walking on fat metabolism. Korean J Phys Educ. 2009;48:545–53. Korean. [Google Scholar]
- 57.Coggan AR, Raguso CA, Gastaldelli A, et al. Fat metabolism during high-intensity exercise in endurance-trained and untrained men. Metabolism. 2000;49:122–8. doi: 10.1016/s0026-0495(00)90963-6. [DOI] [PubMed] [Google Scholar]
- 58.Taheri M, Farzian S, Esmaeili A, et al. The effect of water therapy and jogging exercises on the health-related factors of physical fitness of elderly women. Int J Sport Stud Health. 2020;3:e114813 [Google Scholar]