Abstract
Background
Though acute kidney injury (AKI) is a prevalent complication in critically ill patients, knowledge on the epidemiological differences and clinical characteristics of patients with AKI admitted to medical and surgical intensive care units (ICUs) remains limited.
Methods
Electronic medical records of patients in ICUs in Pusan National University Hospital and Pusan National University Hospital Yangsan, from January 2011 to December 2020, were retrospectively analyzed. Different characteristics of AKI between patients were analyzed. The contribution of AKI to the in-hospital mortality rate was assessed using a Cox proportional hazards model.
Results
A total of 7,150 patients were included in this study. AKI was more frequent in medical (48.7%) than in surgical patients (19.7%), with the severity of AKI higher in medical patients. In surgical patients, hospital-acquired AKI was more frequent (51.0% vs. 49.0%), whereas community-acquired AKI was more common in medical patients (58.5% vs. 41.5%). 16.9% and 5.9% of medical and surgical patients died in the hospital, respectively. AKI affected patient groups to different degrees. In surgical patients, AKI patients had 4.778 folds higher risk of mortality (95% confidence interval [CI], 3.577–6.382; p < 0.001) than non-AKI patients; whereas in medical AKI patients, it was 1.239 (95% CI, 1.051–1.461; p = 0.01).
Conclusion
While the prevalence of AKI itself is higher in medical patients, the impact of AKI on mortality was stronger in surgical patients compared to medical patients. This suggests that more attention is needed for perioperative patients to prevent and manage AKI.
Keywords: Acute kidney injury, Epidemiology, Intensive care units
Introduction
Acute kidney injury (AKI) is a common condition among patients in the intensive care unit (ICU). It is associated with longer hospital stays, and a two-to three-fold increased risk of death [1–3]. The prevalence of AKI among patients in the ICU varies widely, ranging from 10% to 70% [1,4–8]. This variation has been attributed to inconsistencies in the definition of AKI. However, even after the adoption of a unified definition by the Kidney Disease: Improving Global Outcomes (KDIGO) group in 2012 [9], the prevalence of AKI remains variable across studies. Critically ill patients can be broadly categorized into medical and surgical groups, with distinct patient characteristics, comorbidities, and reasons for ICU admission. Consequently, the frequency and impact of AKI are also expected to differ between these groups.
Epidemiologic studies on AKI were conducted without distinguishing between medical and surgical patients. Limited studies have compared the prevalence or impact of AKI on patient mortality between medical and surgical ICU patients [10,11]. Previous research has shown that medical ICU patients have a higher incidence of AKI and comorbid conditions, such as sepsis and cardiovascular disease [1,10,11]. Among surgical patients, AKI was commonly complicated after cardiovascular or trauma surgery [12], and AKI patients have been shown to have a higher mortality rate than non-AKI patients.
With the differences in baseline characteristics and etiologies of AKI between medical and surgical patients, we hypothesized that the courses of AKI, clinical outcomes, and the influence of AKI may differ between both groups. If it does, differentiation and well characterization of medical and surgical AKI patients will help us to find the reasons for AKI, predict clinical courses, and plan the management of AKI at the time of diagnosis. It may also help us to plan personalized prevention of AKI in critically ill patients depending on the department.
However, there is a lack of studies directly comparing the characteristics of AKI in medical and surgical patients. This study aims to address this gap by analyzing data from the ICU-AKI cohort of two hospitals in South Korea from 2011 to 2020.
Methods
Design and setting
This is a multicenter, retrospective cohort study based on the electronic medical record (EMR)-extracted ICU cohorts in two tertiary care hospitals in South Korea: the Pusan National University Hospital (PNUH) and Pusan National University Yangsan Hospital (PNUYH).
Data of patients admitted to ICUs (medical, pulmonary, surgical, trauma, neurosurgery, and emergency) in PNUH and PNUYH between January 2011 to December 2020, were reviewed. Patients were excluded if they had end-stage kidney disease (ESKD) on maintenance dialysis, lacked prior information on baseline kidney function, and were younger than 18 years old. Patients were divided into medical and surgical groups, based on the attending physician’s department at ICU admission. Information on comorbidities, laboratory findings at ICU admission, and survival status was retrieved from the EMR. Details about cohort construction are described in our previous reports [12].
The study protocol was approved by the Institutional Review Board of Pusan National University (No. 2306-028-128), which waived the requirement for informed consent.
Data collection and definition
AKI was retrospectively defined based on the modified KDIGO serum creatinine (SCr) criteria (changes in SCr by ≥0.3 mg/dL [≥26.5 μmol/L] within 48 hours; or changes in SCr to ≥1.5 times the baseline, which is known or presumed to have occurred within the prior 7 days) [9]. AKI was categorized according to the AKI developing time, baseline kidney function status, and severity. We defined community-acquired AKI (CAAKI) as AKI diagnosed within 48 hours of hospital admission, and hospital-acquired AKI (HAAKI) as those that developed after 48 hours. AKI on chronic kidney disease (CKD) was defined as AKI diagnosed in previously diagnosed CKD patients. AKI severity was assessed based on the fold changes in peak SCr level, during the patient’s ICU stay, using the KDIGO AKI staging criteria [9]. Information on the causes of AKI was obtained by retrospective chart review. AKI causes were categorized as sepsis, volume-related, drug-related, cardiac dysfunction, hepatorenal syndrome, and obstruction of the urinary tract [4,13]. While many patients had overlapping causes of AKI, we adopted and described the single most predominant cause as the cause of AKI. Details of the criteria of each etiology were summarized in Supplementary Table 1 (available online) [14–16].
Outcomes
The primary outcome was the incidence of AKI. The secondary outcome was in-hospital mortality.
Statistical analysis
Data normality was assessed using the Kolmogorov-Smirnov test. Continuous variables are expressed as medians with interquartile ranges (IQR) or means ± standard deviations, as appropriate. Differences between the two groups were compared using the Student t test. Categorical variables are expressed as percentages, and proportions were compared using the chi-square test.
The effects of AKI on in-hospital mortality were analyzed using univariable and multivariable Cox proportional hazards models. The full model was adjusted for age, diabetes mellitus (DM), hypertension, CKD status, chronic obstructive pulmonary disease (COPD) and asthma, cancer, Sequential Organ Failure Assessment (SOFA) score, sepsis, AKI stage, and serum level of albumin. All the covariates included in the analyses are known factors associated with AKI, some of them were included in the final model. In the final model, covariates were selected by backward selection based on the Wald test, with a threshold of 0.2 for all predictors. The Cox proportional hazards model was plotted to compare the effect of AKI on mortality between medical and surgical patients who had AKI and those who did not, respectively. All analyses were restricted to the subjects with complete data on the variables involved in each analysis.
All statistical tests were two-sided, and p-values less than 0.05 were considered significant. Data analysis and plotting were performed using IBM SPSS software (ver. 29.0; IBM Corp.).
Results
Baseline characteristics
Between January 2011 and December 2020, 48,834 patients were admitted to the ICU. After excluding 100 patients (0.2%) with ESKD, 40,720 patients (97.6%) without information on their baseline kidney function status, and 861 patients (2.0%) younger than 18 years old, a total of 7,150 ICU patients were included in this study (Fig. 1). The mean age was 63.9 ± 13.4 years, and 4,289 (60.0%) were male. Among them, 3,625 (50.7%) were medical patients, and 3,525 (49.3%) were surgical patients. DM, hypertension, CKD, ischemic heart disease (IHD), lung disease, liver disease, and stroke were more prevalent among medical patients, while cancer was more common in surgical patients. At ICU admission, the mean SOFA score was 5.8 ± 3.8, which was significantly higher in medical patients (6.6 ± 3.9), who also had a higher prevalence of sepsis (43.8%) (Table 1).
Figure 1. Flowchart of the study population.
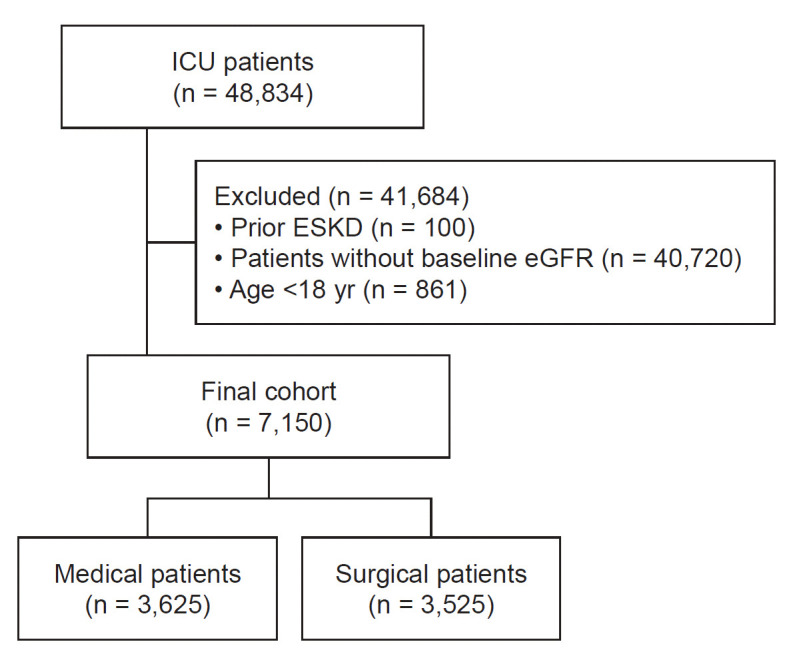
eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; ICU, intensive care unit.
Table 1.
Baseline characteristics
| Characteristic | Total | Medical group | Surgical group | p-value |
|---|---|---|---|---|
| No. of patients | 7,150 (100) | 3,625 (50.7) | 3,525 (49.3) | |
| Age (yr) | 63.88 ± 13.38 | 63.93 ± 13.91 | 63.83 ± 12.82 | 0.32 |
| Male sex | 4,289 (60.0) | 2,191 (60.4) | 2,098 (59.5) | 0.43 |
| Comorbidities | ||||
| Diabetes mellitus | 3,265 (45.7) | 1,908 (52.6) | 1,360 (38.6) | <0.001 |
| Hypertension | 4,791 (67.0) | 2,730 (75.3) | 2,062 (58.5) | <0.001 |
| Chronic kidney disease | 1,464 (20.5) | 1,004 (27.7) | 461 (13.1) | <0.001 |
| Ischemic heart disease | 1,098 (15.4) | 637 (17.6) | 462 (13.1) | <0.001 |
| Lung disease | 349 (4.9) | 235 (6.5) | 115 (3.3) | <0.001 |
| Liver disease | 696 (9.7) | 381 (10.5) | 314 (8.9) | 0.02 |
| Stroke | 729 (10.2) | 396 (10.9) | 333 (9.4) | 0.04 |
| Malignancy | 3,088 (43.2) | 1,375 (37.9) | 1,715 (48.7) | <0.001 |
| Severity of disease at ICU admission | ||||
| SOFA score | 5.77 ± 3.75 | 6.61 ± 3.93 | 4.91 ± 3.34 | <0.001 |
| Sepsis | 2,440 (34.1) | 1,586 (43.8) | 856 (24.3) | <0.001 |
| Incidence of AKI | 2,461 (34.4) | 1,765 (48.7) | 694 (19.7) | <0.001 |
Data are expressed as number (%) or mean ± standard deviation.
AKI, acute kidney injury; ICU, intensive care unit; SOFA, Sequential Organ Failure Assessment.
Differences in acute kidney injury epidemiology among medical and surgical intensive care unit patients
AKI was observed in 2,459 patients (34.4%), and it was more prevalent in medical (1,765 out of 3,625, 48.7%) than in surgical patients (694 of 3,525, 19.7%) (Fig. 2). Table 2 summarizes the differences in AKI characteristics between medical and surgical ICU patients. Stage 1 AKI was more common in surgical patients, whereas severe (stage ≥ 2) (68.4% vs. 48.7%, p < 0.001) or dialysis-requiring AKI (25.0% vs. 12.2%, p < 0.001) was more frequent in medical patients. Among patients with AKI, 1,073 (43.6%) had HAAKI, and 1,386 (56.3%) had CAAKI. HAAKI was more prevalent in surgical patients than in medical patients (49.0% vs. 41.5%, p < 0.001), while CAAKI was more common in medical patients compared to surgical patients (58.5% vs. 51.0%, p < 0.001). AKI on CKD was observed in 784 patients (31.9%) and was more frequent in medical patients than in surgical patients (36.1% vs. 21.2%, p < 0.001).
Figure 2. Incidence of AKI in medical and surgical ICU patients.
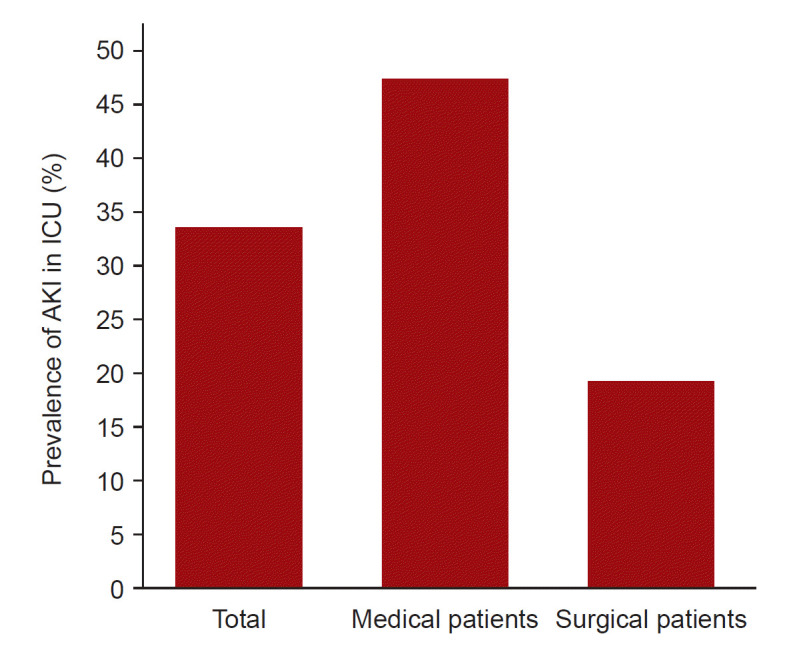
AKI, acute kidney injury; ICU, intensive care unit.
Table 2.
Differences in AKI characteristics between medical and surgical ICU patients
| Characteristic | Total | Medical group | Surgical group | p-value |
|---|---|---|---|---|
| No. of patients | 2,459 (34.4) | 1,765 (48.7) | 694 (19.7) | |
| AKI stage at peak | ||||
| AKI stage 1 | 915 (37.2) | 558 (31.6) | 356 (51.3) | <0.001 |
| AKI stage 2 | 481 (19.5) | 341 (19.3) | 140 (20.2) | 0.63 |
| AKI stage 3 | 538 (21.8) | 425 (24.1) | 113 (16.3) | <0.001 |
| AKI stage 3D | 527 (21.0) | 441 (25.0) | 85 (12.2) | <0.001 |
| AKI developing time | ||||
| Hospital-acquired | 1,073 (43.6) | 733 (41.5) | 340 (49.0) | <0.001 |
| Community-acquired | 1,386 (56.3) | 1,032 (58.5) | 354 (51.0) | <0.001 |
| AKI on CKD | 784 (31.9) | 637 (36.1) | 147 (21.2) | <0.001 |
Data are expressed as number (%).
AKI, acute kidney injury; CKD, chronic kidney disease; ICU, intensive care unit.
The causes of AKI were reviewed only in patients treated at PNUH (1,673 of 2,461, 67.9%). Among these patients, sepsis (44.8%) was the most common cause of AKI, followed by volume-related AKI (34.5%), cardiac dysfunction (9.8%), and nephrotoxin-induced AKI (3.9%). In medical patients, sepsis (616 out of 1,252, 49.2%) was the leading cause of AKI, followed by volume-related AKI (349 out of 1,252, 27.9%), while in surgical patients, volume-related AKI (228 out of 421, 54.2%) was most common. Drug-related AKI was more prevalent in medical patients (63 out of 1,252, 5.0%), whereas AKI related to cardiac dysfunction was more frequent in surgical patients (49 out of 421, 11.6%) (Table 3).
Table 3.
Cause of AKI (Pusan National University Hospital)
| Cause of AKI | Total (n = 1,673) | Medical group (n = 1,252) | Surgical group (n = 421) | p-value |
|---|---|---|---|---|
| Sepsis | 749 (44.8) | 616 (49.2) | 133 (31.6) | <0.001 |
| Volume overload/depletion | 577 (34.5) | 349 (27.9) | 228 (54.2) | <0.001 |
| Cardiac dysfunction | 164 (9.8) | 115 (9.2) | 49 (11.6) | <0.001 |
| Drug-related | 65 (3.9) | 63 (5.0) | 2 (0.5) | <0.001 |
| Hepatorenal syndrome | 44 (2.6) | 38 (3.0) | 6 (1.4) | <0.001 |
| Obstruction of urinary tract | 10 (0.6) | 7 (0.6) | 3 (0.7) | 0.22 |
| Others | 19 (1.1) | 19 (1.5) | 0 (0) | <0.001 |
Data are expressed as number (%).
AKI, acute kidney injury.
Differences in patient outcomes by acute kidney injury status between medical and surgical intensive care unit patients
The average length of stay (LOS) at the hospital was 16 days (IQR, 9–30 days). Medical ICU patients had a longer LOS of 18 days, while surgical ICU patients had a LOS of 15 days. Both medical and surgical patients with AKI stayed longer in the hospital than those without, even after being transferred from the ICU (Supplementary Table 2, available online).
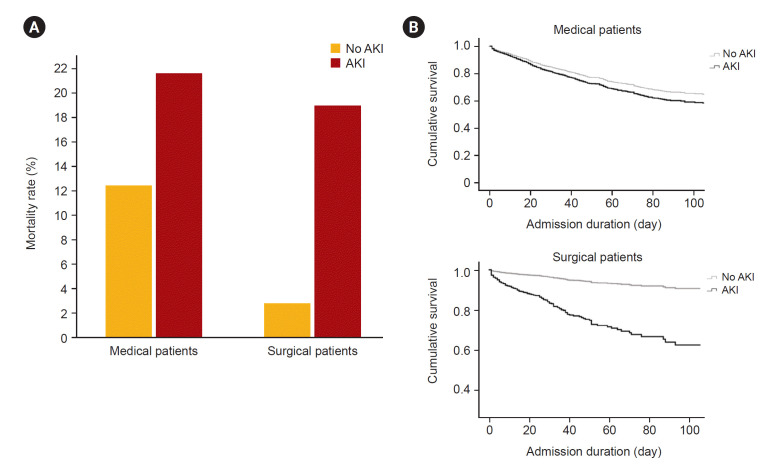
Out of the total patient population, 819 (11.5%) died in the hospital. The in-hospital mortality rate was higher in medical patients (612 out of 3,620, 16.9%) compared to surgical patients (207 in 3,525, 5.9%; p < 0.001). In medical patients with and without AKI, in-hospital mortality rate was 21.6% and 12.6% and in surgical patients, it was 18.9% and 2.7%, respectively (Fig. 3A; Supplementary Table 2, available online). The influence of AKI on mortality differed between medical and surgical patients. In medical patients, the risk of in-hospital mortality was 1.239 (95% confidence interval [CI], 1.051–1.461; p = 0.01) fold higher in AKI patients compared to no AKI, whereas it was 4.778 (95% CI, 3.577–6.382; p < 0.001) in surgical patients (Fig. 3B).
Figure 3. In-hospital mortality.
(A) It shows differences in in-hospital mortality rates between medical and surgical patients by the presence of acute kidney injury (AKI). (B) Cox proportional hazards model of patients by department and presence of AKI.
The proportion of comorbidities and mortality rate increased with increasing AKI severity in both medical and surgical patient groups (Supplementary Fig. 1, available online).
In the multivariable Cox proportional hazard model, using surgical patients without AKI as the reference group, medical patients without AKI had a 2.5-fold higher risk of mortality, surgical patients with AKI had a 2.7-fold higher risk, and medical patients with AKI had a 2.9-fold higher risk (Table 4). Other factors associated with in-hospital mortality included old age, DM, hypertension, COPD, malignancy, sepsis, and low serum albumin levels at ICU admission (Table 4). Higher SOFA score and higher AKI stage were AKI-specific risk factors for in-hospital mortality in patients with AKI admitted to the ICU (Supplementary Table 3, available online). Patients with CKD showed better survival, regardless of AKI, during ICU stay (Table 4; Supplementary Table 3, available online).
Table 4.
Multivariate Cox regression analysis for in-hospital mortality
| Factor | Unadjusted model |
Adjusted model |
||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age | 1.01 (1.01–1.02) | <0.001 | 1.01 (1.01–1.02) | <0.001 |
| Diabetes mellitus | 1.85 (1.61–2.14) | <0.001 | 1.37 (1.18–1.59) | <0.001 |
| Hypertension | 3.05 (2.47–3.76) | <0.001 | 2.26 (1.81–2.82) | <0.001 |
| Chronic kidney disease | 1.22 (1.04–1.43) | 0.02 | 0.83 (0.70–0.99) | 0.03 |
| COPD, asthma | 1.40 (1.33–2.17) | <0.001 | 1.32 (1.03–1.70) | <0.001 |
| Malignancy | 1.59 (1.39–1.83) | <0.001 | 1.46 (1.26–1.68) | <0.001 |
| Sepsis | 2.25 (1.96–2.59) | <0.001 | 1.68 (1.45–1.94) | <0.001 |
| Albumin level | 0.48 (0.42–0.55) | <0.001 | 0.73 (0.64–0.84) | <0.001 |
| Total SOFA score | 1.14 (1.12–1.16) | <0.001 | 1.09 (1.07–1.11) | <0.001 |
| Acute kidney injury | ||||
| No | ||||
| Surgical department | Reference | Reference | ||
| Medical department | 4.04 (3.11–5.24) | <0.001 | 2.47 (1.89–3.23) | <0.001 |
| Yes | ||||
| Surgical department | 4.53 (3.41–6.02) | <0.001 | 2.69 (2.06–3.51) | <0.001 |
| Medical department | 5.05 (3.94–6.48) | <0.001 | 2.94 (2.20–3.94) | <0.001 |
CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; SOFA, Sequential Organ Failure Assessment.
Discussion
While previous epidemiologic studies have established the association between AKI and high nosocomial mortality [17–21] and substantial healthcare costs, particularly in dialysis-requiring cases [22–24], studies on the epidemiology of AKI in ICU patients in South Korea remain limited. Even less is known about the differences in characteristics between heterogeneous patient groups.
There were a few studies that categorized ICU patients into medical and surgical groups, but these were at the level of baseline characteristics. Patients were stratified by AKI status to analyze final outcomes such as incidence or mortality of AKI [10,11].
We aimed to conduct simple and intuitive phenotype of medical and surgical AKI patients in ICU, including clinically important factors such as reasons, courses, severity, and mortality outcomes. We created this ICU-AKI cohort using EMR-extracted data from 2011 to 2020, to allow this analysis. This study investigated how patient characteristics vary across departments, and whether and how these characteristics influence the incidence of AKI. Furthermore, the mortality outcomes of patients, considering the presence of AKI and other risk factors including AKI severity, were also evaluated.
Medical patients exhibited a higher prevalence of comorbidities, such as DM, hypertension, CKD, IHD, lung disease, and liver disease, along with a more severe illness at ICU admission. In contrast, surgical patients had a higher incidence of underlying malignancy. AKI was more prevalent in medical patients (48.1%) compared to surgical patients (19.7%). The severity of AKI was higher in medical patients, with sepsis being a major contributing factor. In surgical patients, volume status played a more significant role in the development of AKI. In-hospital mortality was substantially higher in AKI patients (20.8%) compared to non-AKI patients (6.5%). The mortality gap between surgical patients with and without AKI was more pronounced (18.9% vs. 2.7%) compared to that observed in medical patients. The incidence of AKI in our cohort aligns with previous reports. Hoste et al. [4] reported an AKI incidence of 56.7% among ICU-admitted patients, 18.4% in stage 1, 8.9% in stage 2, 30.0% in stage 3. Bagshaw et al. [25] reported an AKI incidence of 36.1% in ICU patients, while this study found an overall AKI incidence of 34.4% in ICU patients.
The incidence and mortality of AKI in medical patients are higher than in surgical patients due to the high frequency of comorbidities such as chronic hypertension, DM, or CKD which can lead to tissue hypoperfusion and inflammation.
Hoste et al. [26] demonstrated that AKI contributes to a higher mortality rate in ICUs due to complications arising from AKI and renal replacement therapy, which can lead to the deterioration of other vital organs. Other studies have shown an increase in ICU mortality associated with increasing disease severity and the presence of AKI [27,28]. AKI systemically affects the body through inflammatory pathways, increasing the risk of infection and potentially causing sepsis or worsening preexisting sepsis [3,29]. Inflammation, rather than an ischemic component, results in renal endothelial dysfunction, leading to microvascular disturbances [30,31]. Consistent with this pathogenesis, this study highlights that sepsis was the primary cause of AKI in both medical and surgical groups, contributing more significantly to mortality and the need for renal replacement therapy, as reported in several studies [32,33].
In surgical patients, volume overload/depletion was the main etiology of AKI (54.2%). Shock related to bleeding plays a major role, with some other mechanisms affecting the development of AKI. Even if we did not categorize the anatomical type of surgery in this study, it has been suggested that direct injury to kidney and/or urinary tract leads to reduced kidney function. Furthermore, intra-abdominal packing, which is usually used for bleeding control, may also deteriorate kidney function [34]. However, with the development of trauma centers, adequate volume management, and perioperative management such as prophylactic treatment of complications such as rhabdomyolysis in high-risk patients might be a factor in lowering the incidence and mortality of AKI in surgical patients compared to medical patients.
The LOS in the hospital was longer, and the mortality rate was higher in patients with AKI compared to those without AKI. Some previous studies have reported a positive correlation between prolonged LOS and mortality [35,36], due to an increased risk of various AKI-associated complications. Among the AKI patients, in-hospital mortality was higher in medical patients compared to surgical patients. Nevertheless, the contribution of AKI to mortality was greater in surgical patients. The prevalence of AKI had a greater impact on mortality (18.9% in the surgical AKI group vs. 2.7% in the surgical non-AKI group) in surgical patients than in medical patients. This can be explained by several factors.
First, AKI-related fluid imbalance is a challenging issue, especially if it coexists with perioperative fasting or a bleeding condition, which is inevitable with major surgery [34]. Second, even though we did not differentiate between types of surgery in this present study, the higher rate of underlying cancer in surgical patients implies that it is more difficult for them to recover from AKI. Third, AKI itself played a critical role in in-hospital mortality. Surgical patients had fewer comorbidities and a lower SOFA score compared to medical patients, and surgical patients without AKI showed the best survival rate. Meanwhile, medical patients without AKI had a 2.5-fold higher risk of mortality, possibly associated with their comorbidities and higher disease burden. Once surgical patients developed AKI, their probability of mortality was higher than that of medical patients without AKI. We speculate that AKI has a more significant contribution to mortality than other comorbidities in critically ill patients. Therefore, preventing AKI is essential even in surgical patients with fewer comorbidities.
Consistent with previous studies [5,13,25,28,32,37], this multivariate analysis identified old age, multiple comorbidities, a higher SOFA score, and the presence of sepsis as risk factors for in-hospital mortality. Mortality is lower in patients with pre-diagnosed CKD (hazard ratio, 0.831 referenced by surgical ICU patients without AKI). Oppert et al. [38] previously reported that patients with septic AKI and preexisting non-dialysis-dependent CKD had a lower mortality rate than those without preexisting CKD. This finding led to the suggestion by Parmar et al. [37] that preexisting CKD might modify the response to stressful conditions, including sepsis. Additionally, we speculate that awareness of CKD by physicians might have influenced their limited prescription of nephrotoxic drugs (e.g., analgesics or antibiotics) and cautious regulation of fluids in CKD patients than in patients with previously normal kidney function, potentially leading to lower rates of mortality.
This study has several limitations. First, we did not identify overlapping causes of AKI since it was a retrospective review of medical charts and blood, urine, and imaging studies before and after the AKI. Therefore, in cases of multiple causes, one etiology was selected as the most likely cause. Second, within the surgical department, we did not distinguish between patient characteristics that vary by type of surgery, such as trauma or cancer surgery. Third, although the SOFA score is a reliable and relatively accurate indicator for assessing organ dysfunction and predicting the prognosis of patients admitted to ICU, indicators such as simplified acute physiology score and multiple organ dysfunction score are more commonly used to assess the severity of illness and should be further quantified and analyzed.
The incidence of AKI among critically ill patients was 38%, with a higher prevalence in medical patients compared to surgical patients. In medical patients, CAAKI was more common whereas in surgical patients, HAAKI was more frequent. This difference may be attributed to the higher prevalence of comorbidities or disease severity in medical patients and differences in reasons for AKI between medical and surgical patients. While the incidence and mortality of AKI itself were higher in medical patients, the impact on mortality was more pronounced in surgical patients. These findings emphasize the need for active monitoring of kidney function during hospital stays, regardless of a patient’s baseline kidney status. Furthermore, prevention of AKI should be prioritized in both medical and surgical departments.
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
Funding
The National Research Foundation of Korea (NRF) Grant funded by the Korean government (MIST) (No. 2022R1C1C1009362) supported this work.
Data sharing statement
The data presented in this study are available from the corresponding author upon reasonable request.
Authors’ contributions
Conceptualization: YL, DWK, HJK, EYS, SHS, HR
Data curation, Formal analysis, Investigation, Methodology: YL, TK, DEK, EMJ, HR
Funding acquisition: HR
Supervision: DWK, HJK, EYS, SHS, HR,
Writing–original draft: HR, YL
Writing–review & editing: HR, YL
All authors read and approved the final manuscript.
Supplementary Materials
Supplementary data are available at Kidney Research and Clinical Practice online (https://doi.org/10.23876/j.krcp.23.312).
References
- 1.Brivet FG, Kleinknecht DJ, Loirat P, Landais PJ. Acute renal failure in intensive care units: causes, outcome, and prognostic factors of hospital mortality; a prospective, multicenter study. French Study Group on Acute Renal Failure. Crit Care Med. 1996;24:192–198. doi: 10.1097/00003246-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Bagshaw SM, George C, Bellomo R, ANZICS Database Management Committee Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12:R47. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopes JA, Fernandes P, Jorge S, et al. Long-term risk of mortality after acute kidney injury in patients with sepsis: a contemporary analysis. BMC Nephrol. 2010;11:9. doi: 10.1186/1471-2369-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 5.Singbartl K, Kellum JA. AKI in the ICU: definition, epidemiology, risk stratification, and outcomes. Kidney Int. 2012;81:819–825. doi: 10.1038/ki.2011.339. [DOI] [PubMed] [Google Scholar]
- 6.Hoste EAJ, Kellum JA, Selby NM, et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol. 2018;14:607–625. doi: 10.1038/s41581-018-0052-0. [DOI] [PubMed] [Google Scholar]
- 7.Feest TG, Round A, Hamad S. Incidence of severe acute renal failure in adults: results of a community based study. BMJ. 1993;306:481–483. doi: 10.1136/bmj.306.6876.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X, Nie S, Liu Z, et al. Epidemiology and clinical correlates of AKI in Chinese hospitalized adults. Clin J Am Soc Nephrol. 2015;10:1510–1518. doi: 10.2215/CJN.02140215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:19–36. [Google Scholar]
- 10.Bagshaw SM, George C, Bellomo R, ANZICS Database Management Committee Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care. 2007;11:R68. doi: 10.1186/cc5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uchino S, Kellum JA, Bellomo R, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294:813–818. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 12.Rhee H, Park M, Kim IY. Nephrology consultation improves the clinical outcomes of patients with acute kidney injury. Kidney Res Clin Pract. 2023 Sep 8; doi: 10.23876/j.krcp.23.039. [Epub]. DOI: [DOI] [PubMed] [Google Scholar]
- 13.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 14.Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–1247. doi: 10.1007/s00134-021-06506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Association for the Study of the Liver EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 16.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 17.Pickkers P, Darmon M, Hoste E, et al. Acute kidney injury in the critically ill: an updated review on pathophysiology and management. Intensive Care Med. 2021;47:835–850. doi: 10.1007/s00134-021-06454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naicker S, Omar O, Gharbi MB. Epidemiology of Acute Kidney Injury in Africa. Semin Nephrol. 2008;28:348–353. doi: 10.1016/j.semnephrol.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Hoste EA, Kellum JA. Acute kidney injury: epidemiology and diagnostic criteria. Curr Opin Crit Care. 2006;12:531–537. doi: 10.1097/MCC.0b013e3280102af7. [DOI] [PubMed] [Google Scholar]
- 20.Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292–1298. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 21.Gammelager H, Christiansen CF, Johansen MB, Tønnesen E, Jespersen B, Sørensen HT. One-year mortality among Danish intensive care patients with acute kidney injury: a cohort study. Crit Care. 2012;16:R124. doi: 10.1186/cc11420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howell M, Walker RC, Howard K. Cost Effectiveness of Dialysis Modalities: A Systematic Review of Economic Evaluations. Appl Health Econ Health Policy. 2019;17:315–330. doi: 10.1007/s40258-018-00455-2. [DOI] [PubMed] [Google Scholar]
- 23.Fischer MJ, Brimhall BB, Lezotte DC, Glazner JE, Parikh CR. Uncomplicated acute renal failure and hospital resource utilization: a retrospective multicenter analysis. Am J Kidney Dis. 2005;46:1049–1057. doi: 10.1053/j.ajkd.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Fox CS, Muntner P, Chen AY, Alexander KP, Roe MT, Wiviott SD. Short-term outcomes of acute myocardial infarction in patients with acute kidney injury: a report from the national cardiovascular data registry. Circulation. 2012;125:497–504. doi: 10.1161/CIRCULATIONAHA.111.039909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bagshaw SM, George C, Bellomo R, ANZICS Database Management Committe A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1569–1574. doi: 10.1093/ndt/gfn009. [DOI] [PubMed] [Google Scholar]
- 26.Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park WY, Hwang EA, Jang MH, Park SB, Kim HC. The risk factors and outcome of acute kidney injury in the intensive care units. Korean J Intern Med. 2010;25:181–187. doi: 10.3904/kjim.2010.25.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med. 2006;34:1913–1917. doi: 10.1097/01.CCM.0000224227.70642.4F. [DOI] [PubMed] [Google Scholar]
- 29.Klahr S, Miller SB. Acute oliguria. N Engl J Med. 1998;338:671–675. doi: 10.1056/NEJM199803053381007. [DOI] [PubMed] [Google Scholar]
- 30.Peerapornratana S, Manrique-Caballero CL, Gómez H, Kellum JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96:1083–1099. doi: 10.1016/j.kint.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez H, Ince C, De Backer D, et al. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock. 2014;41:3–11. doi: 10.1097/SHK.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho E, Cha I, Yoon K, et al. Clinical characteristics and outcomes of septic acute kidney injury in critically ill patients. Korean J Nephrol. 2011;30:253–259. [Google Scholar]
- 33.Abebe A, Kumela K, Belay M, Kebede B, Wobie Y. Mortality and predictors of acute kidney injury in adults: a hospital-based prospective observational study. Sci Rep. 2021;11:15672. doi: 10.1038/s41598-021-94946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Søvik S, Isachsen MS, Nordhuus KM, et al. Acute kidney injury in trauma patients admitted to the ICU: a systematic review and meta-analysis. Intensive Care Med. 2019;45:407–419. doi: 10.1007/s00134-019-05535-y. [DOI] [PubMed] [Google Scholar]
- 35.Lingsma HF, Bottle A, Middleton S, Kievit J, Steyerberg EW, Marang-van de Mheen PJ. Evaluation of hospital outcomes: the relation between length-of-stay, readmission, and mortality in a large international administrative database. BMC Health Serv Res. 2018;18:116. doi: 10.1186/s12913-018-2916-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 37.Parmar A, Langenberg C, Wan L, May CN, Bellomo R, Bagshaw SM. Epidemiology of septic acute kidney injury. Curr Drug Targets. 2009;10:1169–1178. doi: 10.2174/138945009789753183. [DOI] [PubMed] [Google Scholar]
- 38.Oppert M, Engel C, Brunkhorst FM, et al. Acute renal failure in patients with severe sepsis and septic shock: a significant independent risk factor for mortality. Results from the German Prevalence Study. Nephrol Dial Transplant. 2008;23:904–909. doi: 10.1093/ndt/gfm610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.