ABSTRACT
Klebsiella spp. are causative agents of healthcare-associated infections in patients who are immunocompromised and use medical devices. The antibiotic resistance crisis has led to an increase in infections caused by these bacteria, which can develop into potentially life-threatening illnesses if not treated swiftly and effectively. Thus, new treatment options for Klebsiella are urgently required. Phage therapy can offer an alternative to ineffective antibiotic treatments for antibiotic-resistant bacteria infections. The aim of the present study was to produce a safe and effective phage cocktail treatment against Klebsiella pneumoniae and Klebsiella oxytoca, both in liquid in vitro culture and an in vivo Galleria mellonella infection model. The phage cocktail was significantly more effective at killing K. pneumoniae and K. oxytoca strains compared with monophage treatments. Preliminary phage cocktail safety was demonstrated through application in the in vivo G. mellonella model: where the phage cocktail induced no toxic side effects in G. mellonella. In addition, the phage cocktail significantly improved the survival of G. mellonella when administered as a prophylactic treatment, compared with controls. In conclusion, our phage cocktail was demonstrated to be safe and effective against Klebsiella spp. in the G. mellonella infection model. This provides a strong case for future treatment for Klebsiella infections, either as an alternative or adjunct to antibiotics.
IMPORTANCE
Klebsiella infections are a concern in individuals who are immunocompromised and are becoming increasingly difficult to treat with antibiotics due to their drug-resistant properties. Bacteriophage is one potential alternative therapy that could be used to tackle these infections. The present study describes the design of a non-toxic phage cocktail that improved the survival of Galleria mellonella infected with Klebsiella. This phage cocktail demonstrates potential for the safe and effective treatment of Klebsiella infections, as an adjunct or alternative to antibiotics.
KEYWORDS: bacteriophage therapy, bacteriophages, Klebsiella
INTRODUCTION
Klebsiella spp. are often found as causative agents of healthcare-associated infections, mainly causing urinary tract infections (UTIs) and pneumonia (1, 2). Klebsiella have previously been estimated to cause 8% of the total number of nosocomial bacterial infections across Europe and the United States, with Klebsiella pneumoniae and Klebsiella oxytoca the most prevalent clinically relevant species (3, 4). Of particular concern are Klebsiella infections of immunocompromised patients and those who require the use of medical devices such as catheters and ventilators, because infections in this group of patients have mortality rates as high as 80% (1, 5). Gram-negative ESKAPE pathogens, such as K. pneumoniae, are currently considered to present the greatest bacterial threat to healthcare, as the emergence of antimicrobial resistant strains, resistant to most, or all, available antibiotics is increasing globally (6). Klebsiella, particularly K. pneumoniae, accumulate and disseminate multi-drug resistance determinants and are resistant to a wide range of antibiotic classes (7). Over the past two decades, the prevalence of multi-drug resistant strains of Klebsiella has increased throughout the healthcare system, which has led to UTIs, pneumonia, and sepsis which are increasingly difficult to treat using the antibiotics currently available (8, 9). Current approaches used to tackle such infections often focus on the use of a combinatorial approach of antibiotic therapy; however, such treatment has reported a limited efficacy in clinical trials and may be nephrotoxic (10, 11).
Preventing the spread and treating infections caused by antibiotic-resistant strains of Klebsiella are challenging prospects; therefore, alternative treatments to traditional antibiotic therapy, such as bacteriophage (phage) therapy, may provide a promising strategy to target these bacteria. A previously published systematic review of multiple phage therapy studies against ESKAPE pathogens reported that phage therapy was safe and effective for treatments caused by these pathogens (12). The aforementioned study reported that phage therapy was effective at degrading biofilms, reducing bacterial burden, encouraging wound healing, and improving patient outcomes. However, despite the urgent requirement for an alternative to antibiotic treatment for Klebsiella infections, there are no commercially available phage therapeutics to treat these bacteria.
Phage cocktails are a combination of phages administered as a mixture for phage therapy, as opposed to monophage therapy which uses a single phage (13). Phage cocktails present a number of advantages compared with monophage therapy, such as an increased host range and decreased resistance rates (13). Phage cocktails have demonstrated efficacy compared with monophage therapy in an in vivo study of Klebsiella infection, with the phage cocktail reducing the emergence of phage-resistant mutants and reducing overall bacterial load (14). A previous study reported the successful use of a phage cocktail to treat a patient with a long-term UTI caused by a multi-drug resistant strain of K. pneumoniae (15). The majority of the available data on the clinical efficacy of phage therapy against Klebsiella infections comes from administering phage as a last resort treatment, and these compassionate care cases have shown promise, eliminating a chronic UTI, removing Klebsiella from the gut, and enhancing wound healing (15–18). A meta-analysis conducted by Al-Anany et al. on the efficacy of phage therapy for UTIs reported ~72% of studies showed that patients improved following phage therapy, while ~99% of patients reported no adverse effects, which suggests that phage therapy is both effective and safe for the treatment of UTIs (19). However, these studies used personalized phage treatment, there is little currently known about how the efficacy of standardized phage cocktail treatment varies across a wider range of Klebsiella strains in in vivo models of infection, as most currently published studies focus on a single strain of bacteria.
The aim of the present study was to develop a safe and effective Klebsiella phage cocktail against a panel of K. pneumoniae and K. oxytoca strains and examine the efficacy of this treatment in an in vivo invertebrate model of Klebsiella infection, using Galleria mellonella (waxworm moth) larvae.
RESULTS
Virulence index
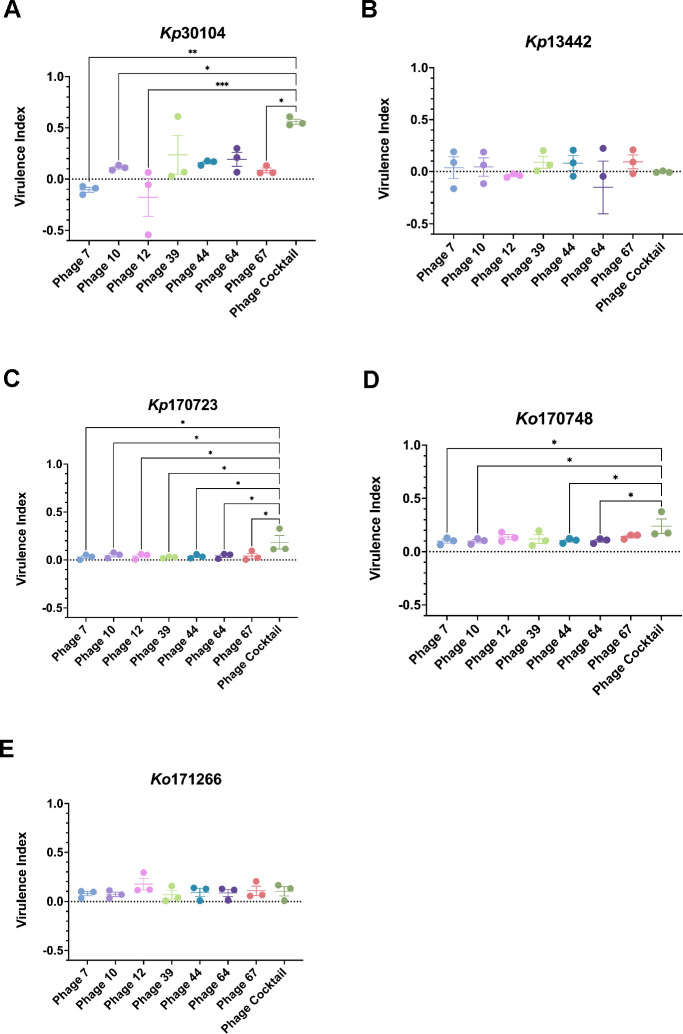
The virulence index (Vi) of each individual phage, in addition to the phage cocktail (PhC), was determined against the panel of Klebsiella strains, to analyze if the PhC was more effective at killing Klebsiella compared with monophage therapy treatment (Fig. 1). The Vi of the PhC against Kp30104 was significantly higher compared with phages 7, 10, 12, and 67. Furthermore, the PhC was significantly more effective at killing Kp170723 compared to all of the monophage treatments tested. The PhC was significantly more effective at killing Ko170748 compared with phages 7, 10, 44, and 64. There was no significant difference observed between the Vi of the PhC and single phage against Kp13442 and Kp171266. None of the phages tested in this study was previously shown to infect Kp13442 (20).
Fig 1.
Virulence index of single phage and phage cocktail against (A) Kp30104, (B) Kp13442, (C) Kp170723, (D) Ko170748, and (E) Ko171266. *P < 0.05, **P < 0.01, ***P < 0.001 vs phage cocktail.
Endotoxin testing and removal
Endotoxin testing is an important step in the process of PhC preparation as endotoxin present in phage preparations is a highly immunogenic compound that can cause endotoxic shock when present at high quantities (21). The concentration of endotoxin present in the PhC before and after endotoxin removal is presented in Table 1. The maximum accepted level of endotoxin present in a medicinal product is 0.2 EU/kg/h for intrathecal administration or 5 EU/kg/h for intravenous administration, which is equivalent to 1.6 × 103 EU/109 PFU of phage treatment (22–24). As the level of endotoxin present in the PhC preparation after endotoxin removal was 70.41 EU/109 PFU, the PhC preparation was deemed safe for use in the G. mellonella model.
TABLE 1.
Concentration of endotoxin in phage cocktail preparation before and after endotoxin removal
| Endotoxin levels pre-endotoxin removal column (EU/109 PFU) | Endotoxin levels post-endotoxin removal column (EU/109 PFU) |
|---|---|
| 1.589 × 103 | 7.040 × 101 |
LD50 of Klebsiella in G. mellonella larvae
The survival of G. mellonella larvae injected with different Klebsiella strains was monitored for 5 days to determine the LD50 (lethal dose for 50% of the subjects) of each bacterial strain (Fig. 2). Based on these survival curves, the LD50 of each of the K. pneumoniae and K. oxytoca strains was calculated (Table 2). This showed that the optimal dose of K. pneumoniae was 2 × 104 to 5 × 105 and for K. oxytoca, it was 1 × 106 to obtain LD50.
Fig 2.
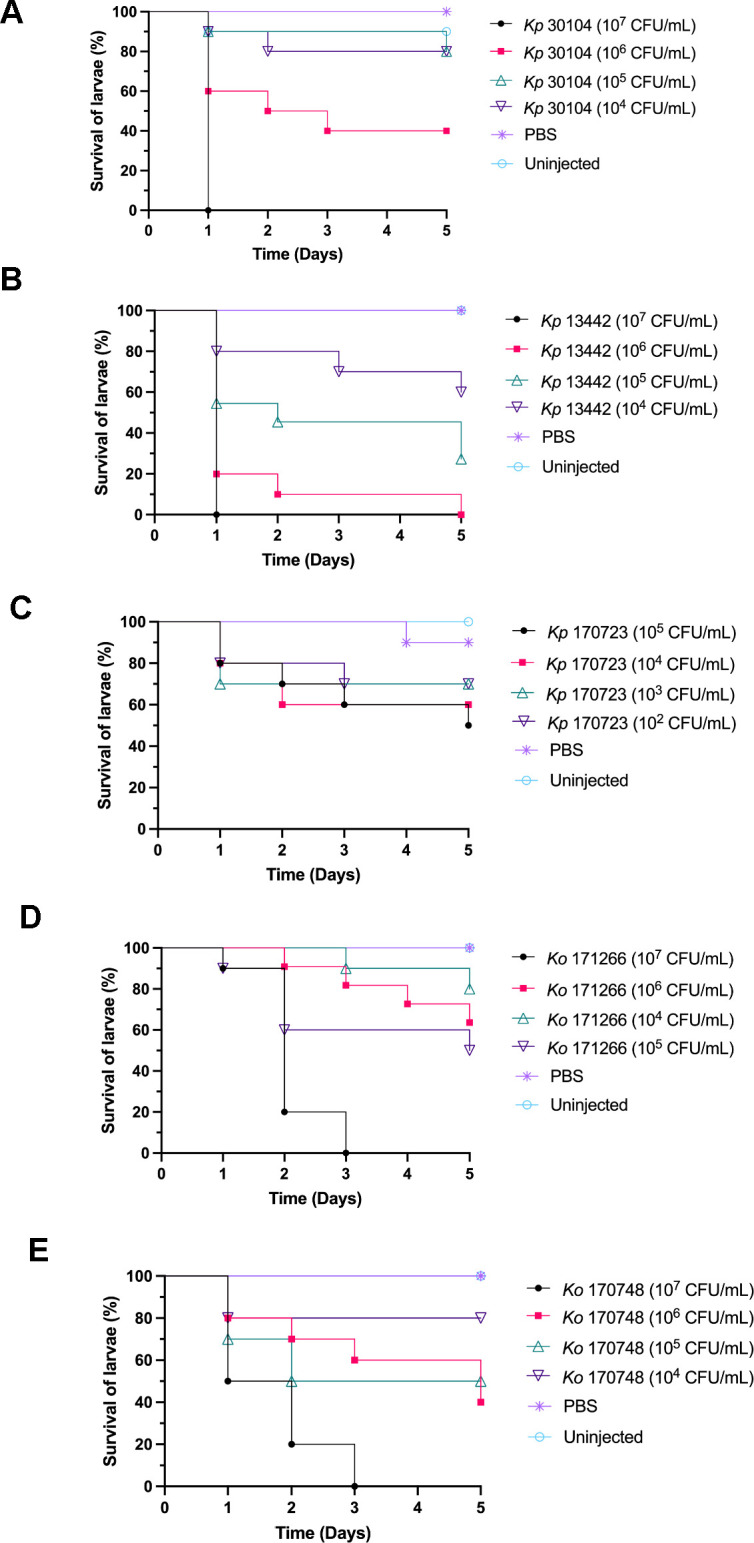
Survival curves of LD50 doses of (A) Kp30104, (B) Kp13442, (C) Kp170723, (D) Ko171266, and (E) Ko170748 in Galleria mellonella larvae after 5 days.
TABLE 2.
LD50 dose of different Klebsiella strains in Galleria mellonella
| Strain | LD50 (CFU/mL) |
|---|---|
| Kp30104 | 5 × 105 |
| Kp13442 | 2 × 104 |
| Kp170723 | 2 × 105 |
| Ko170748 | 1 × 106 |
| Ko171266 | 1 × 106 |
Survival of G. mellonella infected with Klebsiella with prophylactic phage therapy
Prophylactic PhC phage therapy was administered to larvae to determine if this treatment regime was effective at rescuing G. mellonella larvae from infection and death caused by Klebsiella infection. Prophylactic PhC was administered to the larvae 4 h before infection with Klebsiella, and survival of the larvae was monitored for 5 days (Fig. 3). The survival of larvae prophylactically treated with PhC (multiplicity of infection [MOI] = 1) and infected with Kp30104 was significantly increased, compared with bacteria-only controls. Prophylactic PhC (MOI = 1, 10, and 100) of Kp170723-infected and Ko170748-infected larvae significantly increased survival compared with bacteria-only controls. The survival of larvae prophylactically treated with PhC (MOI = 10 and 100) and infected with Ko171266 was significantly increased, compared with bacteria-only controls. There was no significant difference observed in the survival of phage-injected larvae compared with controls, which indicated that the PhC did not induce death in these larvae.
Fig 3.
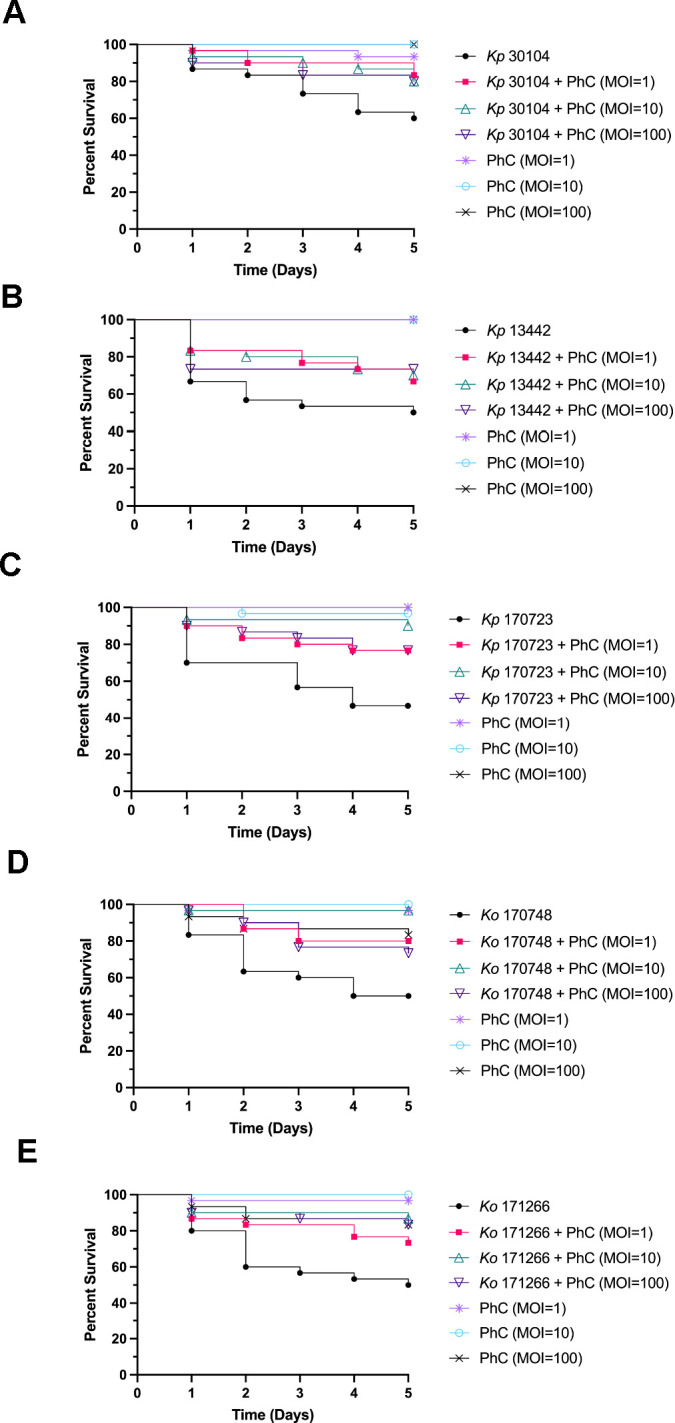
Survival curves of prophylactic phage cocktail treatment against Galleria mellonella infected with (A) Kp30104, (B) Kp13442, (C) Kp170723, (D) Ko170748, and (E) Ko171266.
Survival of G. mellonella infected with Klebsiella treated with co-injection phage therapy
Co-injection of Klebsiella and PhC was administered to larvae to determine if this treatment regime could prevent infection and death of larvae injected with Klebsiella. PhC and Klebsiella were combined and injected into larvae, and survival of G. mellonella was monitored for 5 days (Fig. 4). Co-injection of PhC (MOI = 1, 10, and 100) significantly improved survival of larvae injected with Kp30104, compared with bacteria-only controls. The survival of larvae treated with PhC (MOI = 100) and Ko170748 was significantly improved compared with bacteria-only controls. There was no significant difference observed in the survival of phage-injected larvae compared with controls, which indicated that the PhC did not induce death in these larvae.
Fig 4.
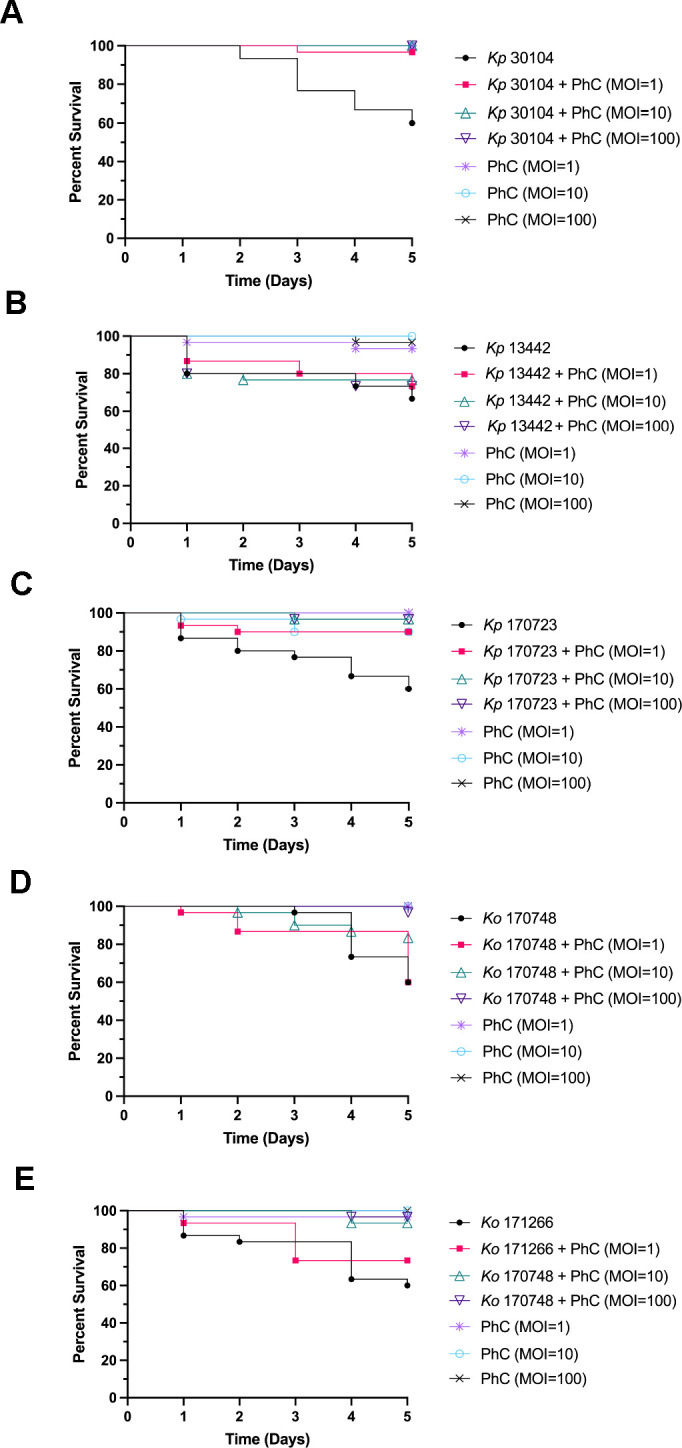
Survival curves of co-injection phage cocktail treatment against Galleria mellonella infected with (A) Kp30104, (B) Kp13442, (C) Kp170723, (D) Ko170748, and (E) Ko171266.
Survival of G. mellonella infected with Klebsiella treated with remedial phage therapy
Remedial phage therapy with the PhC was administered to G. mellonella larvae in order to analyze if this treatment regime was effective at preventing death caused by Klebsiella infection. The PhC was administered 4 h following Klebsiella injection of the larvae, and survival of G. mellonella was monitored for 5 days (Fig. 5). Remedial PhC (MOI = 10) significantly improved the survival of larvae injected with Kp30104, compared with bacteria-only controls. There was no significant difference observed in the survival of phage-injected larvae compared with controls, which indicated that the PhC did not induce death in these larvae.
Fig 5.
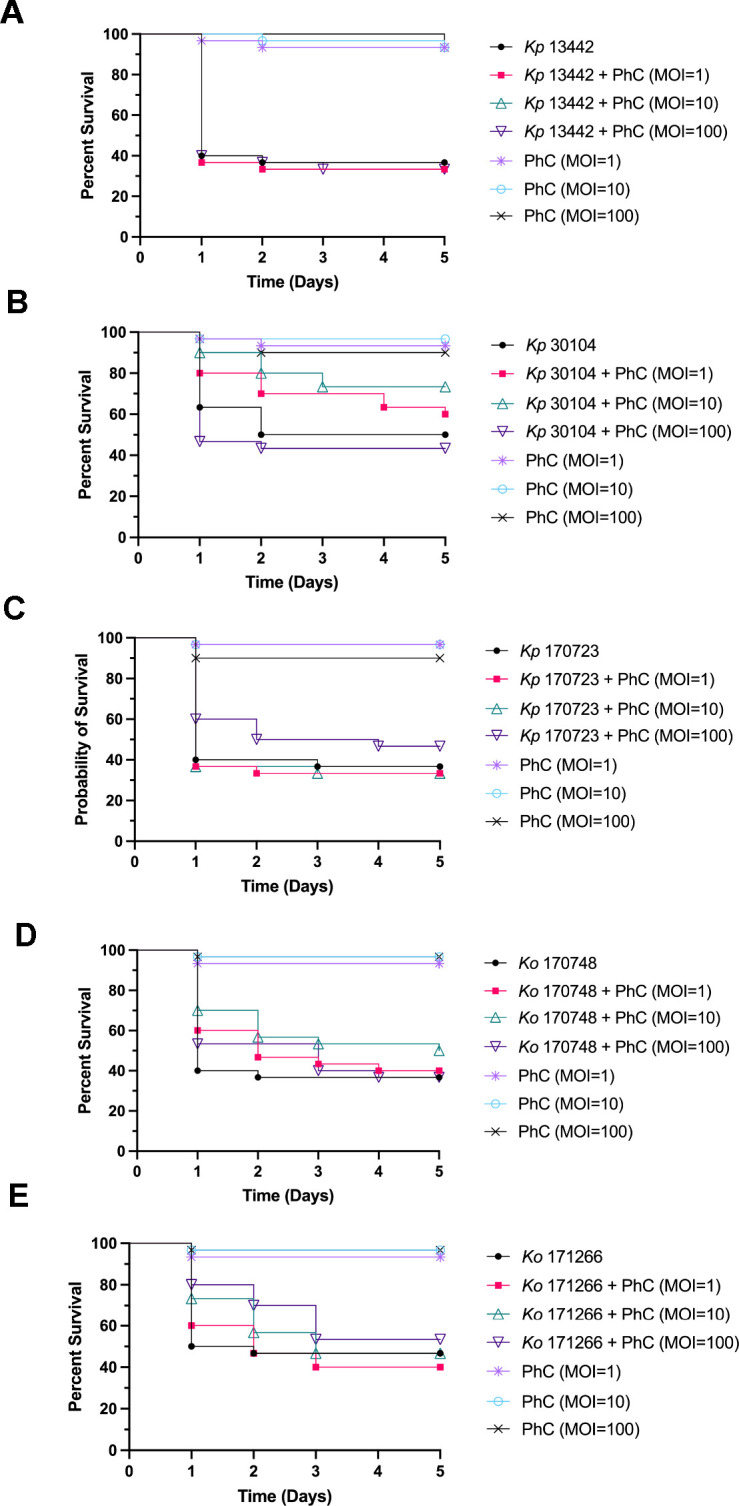
Survival curves of remedial phage cocktail treatment against Galleria mellonella infected with (A) Kp13442, (B) Kp30104, (C) Kp170723, (D) Ko170748, and (E) Ko171266.
DISCUSSION
Our study confirms the efficacy of a phage cocktail (PhC) treatment against K. pneumoniae and K. oxytoca strains in a G. mellonella infection model with prophylactic phage therapy, showing no adverse effects. A number of previously published studies have characterized effective monophage or PhC treatments against a single strain, sequence type, or capsule type of Klebsiella, but few have produced an effective and safe PhC treatment against multiple strains and species of Klebsiella (25–29). To the best of our knowledge, no studies have previously reported on the efficacy of a PhC treatment on both culture collection and clinically isolated strains of Klebsiella.
The phages selected for inclusion into the PhC treatment were genetically distinct and had the widest host range available from a panel of previously characterized phages (20). This PhC consisted of phages that spanned six phylogenetic groups, five families, three subfamilies, six genera, four sources of isolation, and six capsule type targets (20). This strategy was selected for the design of the PhC, as a combination of genetically distinct phages is more likely to be effective at removing target bacteria compared with monophage treatment, if a PhC contains phage with different host receptor targets, the host is unlikely to gain multiple mechanisms of resistance simultaneously without significantly impairing its own fitness (13). Our PhC was effective at killing four out of five Klebsiella strains tested, with the exception of Kp13442, a strain resistant to all individual phages tested (20). Kp13442 was tested in this study to determine if a PhC treatment would demonstrate synergy against a phage-resistant strain of bacteria under in vivo conditions; however, this was not the case. Despite the PhC demonstrating a lack of efficacy against Kp13442, there was some improvement observed in the survival rates of G. mellonella infected with this strain that received prophylactic PhC treatment. This may potentially be due to the time period between PhC injection and subsequent challenge with the bacteria being long enough to allow the PhC treatment to circulate throughout the larvae and adjust to the incubation temperature in time to optimally infect incoming bacteria (30, 31). Furthermore, whilse Kp13442 was insensitive to killing by the PhC, the PhC could potentially infect the bacteria and make the bacterium less virulent, which could consequently enable the larval immune system to more effectively tackle infection with Kp13442 (32).
The Vi results demonstrated that the PhC was more effective at killing Kp30104, Kp170723, and Ko170748 than a number of the monophage treatments tested, which suggested that the PhC has the potential to be a more effective treatment against these Klebsiella strains than monophage therapy. The PhC was the most effective against Kp170723 compared with the single phage treatments, which indicated synergistic killing effects between the phages in the cocktail, despite no monophage treatment demonstrating a high Vi against Kp170723.
Of the three PhC phage therapy dosing regimes administered here, the most effective at killing a range of Klebsiella strains in the invertebrate model was prophylaxis, followed by co-injection. Prophylactic phage therapy was effective at increasing the survival of G. mellonella infected with Kp170723 and Ko170748, two clinically isolated strains of Klebsiella, which highlighted the potential for this PhC to be used to treat clinical infections caused by these strains. This phage therapy regime was also effective at increasing the survival of Kp30104. Prophylactic phage therapy could potentially act as a type of intervention therapy, preventing disease in patients when used prior to surgery or in patients who require the use of medical devices. Prophylactic phage therapy aims to prevent colonization and infection by bacteria before they can cause harm, as opposed to treating an established infection (33–35). This treatment regime presents an attractive option to tackle healthcare-associated Klebsiella infections, as these are often a result of the contamination of medical devices, such as catheters and ventilators (36). Co-injection of phage and bacteria is indicative of a clinical situation whereby phage therapy is administered to a patient at the same time the patient may potentially be colonized by Klebsiella, for example, upon admission to the hospital or the use of a medical device. Co-injection of the higher doses of PhC in the present study was effective at increasing the survival of G. mellonella injected with Kp30104 and Kp170723. The success of this co-injection regime of phage therapy may potentially be due to an increased frequency of bacteria-phage interactions, as the phage was combined directly with bacteria before injection into the larvae. This may enable the phage to more rapidly adhere to the invading bacteria, negating the need for chance encounters between phage and bacteria during systemic circulation in the larvae, to enable phage infection, proliferation, and killing of the target bacteria (37). Remedial phage therapy is a clinically relevant scenario for PhC administration to a patient with an ongoing Klebsiella infection. In the present study, remedial phage therapy was the least successful regime tested; however, one concentration of the PhC was effective at increasing the survival of larvae infected with Kp30104. Therefore, remedial PhC phage therapy shows potential but requires further optimization of the dose, timing, or PhC formulation to maximize efficacy. This observed lower efficacy of remedial phage therapy compared with prophylactic or co-injection phage therapy is consistent with previously published studies on the efficacy of different phage therapy regimes in G. mellonella (38, 39).
A previous study on the use of a prophylactic PhC against several bacterial species, including K. pneumoniae, was effective against infections in a mouse model (40). The pretreatment of medical devices has previously been reported to be highly effective at reducing colonization of devices by pathogens and preventing biofilm formation (41). The data presented in the present study add to a growing body of literature that supports the efficacy of prophylactic phage therapy for the prevention of bacterial infections. Furthermore, this demonstrates that phages can persist in an invertebrate host, were not degraded by the G. mellonella innate immune system, or were inactivated through binding to organic matter. Therefore, prophylactic phage therapy, at the treatment concentration provided in the present study, circulated throughout the G. mellonella system and encountered host bacteria.
The higher doses of PhC therapy administered were most effective at increasing the survival of G. mellonella compared with the lower doses of PhC. This association has also been reported in previous studies of phage therapy in G. mellonella (38, 42, 43). This is potentially due to increased bacteria-phage interactions that may occur as a result of an increased concentration of phage compared with low-dose phage therapy (13).
One of the aims of the present study was to design a safe PhC for use in an in vivo model of Klebsiella infection. The survival of the control phage-only groups of larvae was comparable to the untreated and injection trauma controls in each phage therapy regime, which demonstrated that the PhC was not innately toxic and could be carried forward safely into more complex infection models, such as mammalian models of Klebsiella infection. The acquisition of preliminary safety data is an important part of phage therapy design, as patient safety is an essential pillar for the introduction of phage therapy in human clinical trials (44).
Further development of this PhC treatment could include the testing of multiple doses of PhC therapy, as a single dose of the PhC was tested in the present study, as multiple doses of PhC may potentially increase the efficacy of the treatment. Additionally, the impact of PhC as an adjunct to antibiotic treatment is an important consideration for future phage therapy studies. Phage-antibiotic synergy has previously been reported and has shown promise in treating bacterial infections, including those caused by K. pneumoniae (45–47).
Our PhC is a step forward, it prevents infection by multiple Klebsiella strains with a pre-characterized, off-the-shelf formulation, compared with treating sick patients by first testing individual phages in expensive, time-consuming personalized phage therapy. As with any clinical measure, one of the major obstacles with this therapy is the insensitivity of an infecting pathogen to treatment. The PhC used in the present study was infective against Kp13442 (K110). Our previous work demonstrated the difficulty of isolating phages that infect a broad range of Klebsiella hosts and K-types, and also highlighted Kp13442 as the most phage-resistant strain tested, as it was insensitive to 29/30 phages (20). Fortunately, K110 is not a prevalent clinical Klebsiella K-type (48). For patients carrying an insensitive Klebsiella strain, the PhC treatment presented in the present study may be ineffective, and these bacteria may potentially need to be treated with antibiotics, or a Magisterial phage treatment (49).
To conclude, the present study demonstrates the design of a safe and effective PhC treatment against K. pneumoniae and K. oxytoca. The PhC was effective at increasing the survival of G. mellonella infected with both culture collection and clinically isolated strains of Klebsiella. Therefore, the PhC presented in this study shows potential for the future treatment of Klebsiella infections, as an alternative or adjunct to antibiotic treatments.
MATERIALS AND METHODS
Bacterial culture
The details of the bacterial strains used in this study are presented in Table 3. Klebsiella strains were maintained on Lysogeny broth (LB; Merck) agar plates. As required, liquid cultures were prepared by inoculating 10 mL of LB with a single colony of bacteria and incubating overnight at 37°C and 180 rpm shaking.
TABLE 3.
Details of Klebsiella strains used in the present study
| Strain name | Origin | Isolation source | Capsule type (KL) |
|---|---|---|---|
| Klebsiella pneumoniae 30104 (Kp30104) | DSMZ culture collection | Human blood | KL3 |
| Klebsiella pneumoniae 13442 (Kp13442) | NCTC culture collection | Hospital | KL110 |
| Klebsiella pneumoniae 170723 (Kp170723) | Clinical isolate | Urine | KL2 |
| Klebsiella oxytoca 170748 (Ko170748) | Clinical isolate | Catheter specimen urine | O1v1 |
| Klebsiella oxytoca 171266 (Ko171266) | Clinical isolate | Urostomy urine | OL104 |
Bacteriophage propagation
The details of the bacteriophage used in this study are presented in Tables 4 and 5, and the host range of these phages is presented in Table 6. A 10-µL volume of phage lysate was added to a log-phase culture of the Klebsiella host strain in LB supplemented with 5 mM CaCl2 (Thermo Fisher Scientific) and 5 mM MgCl2 (Thermo Fisher Scientific) and incubated at 37°C with 180 rpm shaking overnight. Phage lysate was centrifuged at 3,220 × g for 20 min at room temperature, and the supernatant was filtered through a 0.2-µm syringe filter (Sarstedt) to remove host cells. Phage was enumerated through serial dilution, then mixing 50 µL of the diluent with 125 µL of log-phase host bacteria, followed by incubation at room temperature for 10 min. Each dilution was mixed with 625 µL of LB top agar (0.4% agar) supplemented with 5 mM CaCl2 and 5 mM MgCl2 and immediately plated on six-well plates containing solidified 1% LB agar. The solidified overlay plates were incubated at 37°C overnight, and phage plaques were enumerated.
TABLE 4.
Details of bacteriophage strains used in the present studya
| Phage name | Lab ID | Isolation source | Isolation strain | Capsule target (KL) | Accession no. |
|---|---|---|---|---|---|
| Klebsiella phage vB KaS-Ahsoka | 7 | Slurry, slurry tank, UK | Klebsiella aerogenes DSM 30053 | – | PRJEB40160 |
| Klebsiella phage vB KppS-Totoro | 10 | Estuary, Jelitkowo, Poland | Klebsiella pneumoniae DSM 30104 | KL3 | PRJEB40166 |
| Klebsiella phage vB KoM-Pickle | 12 | Estuary, Jelitkowo, Poland | Klebsiella oxytoca DSM 25736 | KL74 | PRJEB40176 |
| Klebsiella phage vB KqM-Weterburg | 39 | Raw sewage, Spernal sewage works, UK | Klebsiella quasipneumoniae DSM 28211 | KL35 | PRJEB40173 |
| Klebsiella phage vB KqP-Goliath | 44 | Raw sewage, Spernal sewage works, UK | Klebsiella quasipneumoniae DSM 700603 | KL53 | PRJEB40163 |
| Klebsiella phage vB KpM-Wobble | 64 | Mixed liquor sewage, Spernal sewage works, UK | Klebsiella pneumoniae 170958 | KL28 | PRJEB40182 |
| Klebsiella phage vB KpM-KalD | 67 | Mixed liquor sewage, Spernal sewage works, UK | Klebsiella pneumoniae DSM 13439 | KL14 | PRJEB40178 |
–, a capsule could not be resolved.
TABLE 5.
Taxonomy of phage used in the present study
| Phage name | Taxonomic group | Taxonomy | ||
|---|---|---|---|---|
| Family | Subfamily | Genus | ||
| Klebsiella phage vB KaS-Ahsoka | C | Drexlerviridae | Tunavirinae | Unclassified |
| Klebsiella phage vB KppS-Totoro | F | Demerecviridae | – | Sugarlandvirus |
| Klebsiella phage vB KoM-Pickle | H | Straboviridae | – | Slopekvirus |
| Klebsiella phage vB KqM-Weterburg | G | Ackermannviridae | – | Taipeivirus |
| Klebsiella phage vB KqP-Goliath | E | Autographiviridae | Slopekvirinae | Drulisvirus |
| Klebsiella phage vB KpM-Wobble | I | Straboviridae | Tevenvirinae | Jiaodavirus |
| Klebsiella phage vB KpM-KalD | H | Straboviridae | Tevenvirinae | Slopekvirus |
TABLE 6.
Host range of phage used in the present studya
| Klebsiella strain | Phage 7 | Phage 10 | Phage 12 | Phage 39 | Phage 44 | Phage 64 | Phage 67 |
|---|---|---|---|---|---|---|---|
| Kp30104 | Yes | Yes | Yes | Yes | – | Yes | Yes |
| Kp13442 | – | – | – | – | – | – | – |
| Kp170723 | – | – | Yes | Yes | Yes | Yes | Yes |
| Ko170748 | – | Yes | Yes | – | – | – | Yes |
| Ko171266 | – | Yes | Yes | – | – | – | Yes |
–, no infection of a host was detected in single-phage tests.
Bacteria-phage growth curves
The virulence index (Vi) was used to quantify the efficacy of a single phage and the phage cocktail against the Klebsiella strains in this study (50). An overnight culture of Klebsiella was refreshed 1:100 in LB and incubated at 37°C with 180 rpm shaking until log-phase growth was achieved. Phage was serially diluted from an MOI of 1 to 10−7 and 10 µL of each phage dilution was added to 190 µL of Klebsiella in a 96-well plate. The optical density of the samples was measured at 600 nm every 10 min for 18 h at 37°C using a FLUOstar Omega Microplate Reader (BMG Labtech). The Vi of each sample was then calculated.
Endotoxin testing and removal
Endotoxin testing of phage lysates was performed using the Pierce Chromogenic Endotoxin Quant Kit (Thermo Fisher Scientific, Inc.) according to the manufacturer’s instructions. The Pierce High Capacity Endotoxin Removal Spin Columns (Thermo Fisher Scientific, Inc.) were used to remove endotoxin from phage lysates.
Maintenance of G. mellonella
G. mellonella larvae (LiveFood UK Ltd.) were obtained, stored at 4°C immediately upon arrival, and used within 2 days of delivery. Larvae were individually weighed and those with a weight between 0.20 and 0.30 g were selected for further experiments. Groups of 10 larvae were randomly assigned to each treatment condition and stored in Petri dishes throughout the experiment.
Determining the LD50 of Klebsiella strains in G. mellonella
The LD50 of each Klebsiella strain was calculated over a 5-day period. An overnight culture of Klebsiella was diluted 1:100 in LB and incubated at 37°C with 180 rpm shaking until log-phase growth was achieved. Cultures were centrifuged twice at 3,220 × g for 15 min at room temperature, cell pellets were washed with PBS, and the final pellet was re-suspended and serially diluted in PBS. G. mellonella injection sites were sterilized using 70% ethanol. A 10-µL dose of Klebsiella was injected into the last left proleg of the larvae using a 30 G needle on a Hamilton 500 µL gastight syringe fitted with a Hamilton PB600-1 repeating dispenser. The Kp170723 doses used were 105, 104, 103, and 102 CFU/larvae. The doses for the remaining four Klebsiella strains were 107, 106, 105, and 104 CFU/larvae. Negative controls of PBS-injected and untreated larvae were included in each experiment. Larvae were incubated at 37°C in the dark for 5 days and remained unfed. Larval survival was monitored daily, and larvae were scored as either live or dead every 24 h, with larvae classified as dead once they turned black and immobile. Dead larvae were removed each day. At the end of the experiments, larvae were euthanized by placing them in a −20°C freezer for <2 h, then moved to a −80°C freezer for overnight storage before disposal. The LD50 dose calculated was used to infect larvae in subsequent experiments.
Phage therapy of Klebsiella-infected G. mellonella
Three different PhC phage therapy regimens (prophylaxis, co-injection, and remedial treatment), along with bacteria-only and phage-only controls, and PBS-injected and untreated larvae were prepared. A 10-µL dose of Klebsiella was used in each of the bacteria-treated larvae. PhC dilutions were prepared in PBS to an MOI of 1, 10, or 100 for each Klebsiella strain to be tested. A 10-µL dose of PhC was used in each of the phage-treated larvae.
The phage therapy regimens were designed as follows: (i) prophylaxis, PhC injected at 0 h and Klebsiella injected at 4 h; (ii) co-injection, PhC, and Klebsiella were mixed and immediately injected at 0 h; and (iii) remedial, Klebsiella injected at 0 h and PhC injected at 4 h. In the treatment groups where two injections were required (prophylaxis and remedial therapy), the first injection was in the last left proleg and the second injection was in the last right proleg. The survival of larvae was monitored over 5 days.
Statistical analysis
Statistical analysis and data visualization were performed using GraphPad Prism (version 9.5.1; Dotmatics). One-way analysis of variance with Tukey’s post-hoc test was used to determine statistically significant differences between multiple groups. Kaplan-Meier survival curves were produced, and a log-rank Mantel-Cox test was performed to determine the statistical significance between survival curves. Data are presented as the mean ± standard deviation of three independent experiments. P < 0.05 was considered to indicate a statistically significant difference.
ACKNOWLEDGMENTS
The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication. This work was partially supported by the MIBTP DTP PhD studentship awarded to L.K. and by a Warwick Integrative Synthetic Biology (WISB) early career fellowship, funded jointly by BBSRC/EPSRC to E.J., grant ref: BB/M017982/1.
Contributor Information
Eleanor Jameson, Email: e.jameson@bangor.ac.uk.
Kristin N. Parent, Michigan State University, East Lansing, Michigan, USA
DATA AVAILABILITY
All phage sequence data have previously been made available, and accession numbers are provided in Table 4.
These accession numbers can be retrieved at the European Nucleotide Archive at https://www.ebi.ac.uk/ena/browser/search.
REFERENCES
- 1. Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK, Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team . 2014. Multistate point-prevalence survey of health care–associated infections. N Engl J Med 370:1198–1208. doi: 10.1056/NEJMoa1306801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Podschun R, Pietsch S, Höller C, Ullmann U. 2001. Incidence of Klebsiella species in surface waters and their expression of virulence factors. Appl Environ Microbiol 67:3325–3327. doi: 10.1128/AEM.67.7.3325-3327.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Janda JM. 2015. The genus Klebsiella: an ever-expanding panorama of infections, disease-associated syndromes, and problems for clinical microbiologist. Clin Microbiol Case Rep 1:2. [Google Scholar]
- 4. Podschun R, Ullmann U. 1998. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11:589–603. doi: 10.1128/CMR.11.4.589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shankar C, Nabarro LE, Anandan S, Ravi R, Babu P, Munusamy E, Jeyaseelan V, Rupali P, Verghese VP, Veeraraghavan B. 2018. Extremely high mortality rates in patients with carbapenem-resistant, hypermucoviscous Klebsiella pneumoniae blood stream infections. J Assoc Physicians India 66:13–16. [PubMed] [Google Scholar]
- 6. de Man TJB, Lutgring JD, Lonsway DR, Anderson KF, Kiehlbauch JA, Chen L, Walters MS, Sjölund-Karlsson M, Rasheed JK, Kallen A, Halpin AL. 2018. Genomic analysis of a pan-resistant isolate of Klebsiella pneumoniae, United States 2016. mBio 9:10. doi: 10.1128/mBio.00440-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pendleton JN, Gorman SP, Gilmore BF. 2013. Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther 11:297–308. doi: 10.1586/eri.13.12 [DOI] [PubMed] [Google Scholar]
- 8. Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! an update from the infectious diseases society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011 [DOI] [PubMed] [Google Scholar]
- 9. Kuehn BM. 2013. Nightmare” bacteria on the rise in US hospitals, long-term care facilities. JAMA 309:1573. doi: 10.1001/jama.2013.2922 [DOI] [PubMed] [Google Scholar]
- 10. Kaur CP, Vadivelu J, Chandramathi S. 2018. Impact of Klebsiella pneumoniae in lower gastrointestinal tract diseases. J Dig Dis 19:262–271. doi: 10.1111/1751-2980.12595 [DOI] [PubMed] [Google Scholar]
- 11. Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M. 2012. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase–producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 55:943–950. doi: 10.1093/cid/cis588 [DOI] [PubMed] [Google Scholar]
- 12. El Haddad L, Harb CP, Gebara MA, Stibich MA, Chemaly RF. 2019. A systematic and critical review of bacteriophage therapy against multidrug-resistant ESKAPE organisms in humans. Clin Infect Dis 69:167–178. doi: 10.1093/cid/ciy947 [DOI] [PubMed] [Google Scholar]
- 13. Chan BK, Abedon ST. 2012. Phage therapy pharmacology: phage cocktails, p 1–23. In Advances in applied Microbiology. Elsevier. [DOI] [PubMed] [Google Scholar]
- 14. Chadha P, Katare OP, Chhibber S. 2016. In vivo efficacy of single phage versus phage cocktail in resolving burn wound infection in BALB/c mice. Microb Pathog 99:68–77. doi: 10.1016/j.micpath.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 15. Qin J, Wu N, Bao J, Shi X, Ou H, Ye S, Zhao W, Wei Z, Cai J, Li L, Guo M, Weng J, Lu H, Tan D, Zhang J, Huang Q, Zhu Z, Shi Y, Hu C, Guo X, Zhu T. 2020. Heterogeneous Klebsiella pneumoniae co-infections complicate personalized bacteriophage therapy. Front Cell Infect Microbiol 10:608402. doi: 10.3389/fcimb.2020.608402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuipers S, Ruth MM, Mientjes M, de Sévaux RGL, van Ingen J. 2019. A Dutch case report of successful treatment of chronic relapsing urinary tract infection with bacteriophages in a renal transplant patient. Antimicrob Agents Chemother 64:10. doi: 10.1128/AAC.01281-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Corbellino M, Kieffer N, Kutateladze M, Balarjishvili N, Leshkasheli L, Askilashvili L, Tsertsvadze G, Rimoldi SG, Nizharadze D, Hoyle N, Nadareishvili L, Antinori S, Pagani C, Scorza DG, Romanò ALL, Ardizzone S, Danelli P, Gismondo MR, Galli M, Nordmann P, Poirel L. 2020. Eradication of a multidrug-resistant, carbapenemase-producing Klebsiella pneumoniae isolate following oral and intra-rectal therapy with a custom made, lytic bacteriophage preparation. Clin Infect Dis 70:1998–2001. doi: 10.1093/cid/ciz782 [DOI] [PubMed] [Google Scholar]
- 18. Cano EJ, Caflisch KM, Bollyky PL, Van Belleghem JD, Patel R, Fackler J, Brownstein MJ, Horne B, Biswas B, Henry M, Malagon F, Lewallen DG, Suh GA. 2021. Phage therapy for limb-threatening prosthetic knee Klebsiella pneumoniae infection: case report and in vitro characterization of anti-biofilm activity. Clin Infect Dis 73:e144–e151. doi: 10.1093/cid/ciaa705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Al-Anany AM, Hooey PB, Cook JD, Burrows LL, Martyniuk J, Hynes AP, German GJ. 2023. Phage therapy in the management of urinary tract infections: a comprehensive systematic review. Phage (New Rochelle) 4:112–127. doi: 10.1089/phage.2023.0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Townsend EM, Kelly L, Gannon L, Muscatt G, Dunstan R, Michniewski S, Sapkota H, Kiljunen SJ, Kolsi A, Skurnik M, Lithgow T, Millard AD, Jameson E. 2021. Isolation and characterization of Klebsiella phages for phage therapy. Phage (New Rochelle) 2:26–42. doi: 10.1089/phage.2020.0046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raetz CRH, Whitfield C. 2002. Lipopolysaccharide endotoxins. Annu Rev Biochem 71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Europe, C.o . 2005. Bacterial endotoxins. 5th ed. Vol. 5. Council of Europe, Strasbourg. [Google Scholar]
- 23. Schooley RT, Biswas B, Gill JJ, Hernandez-Morales A, Lancaster J, Lessor L, Barr JJ, Reed SL, Rohwer F, Benler S, et al. 2017. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother 61:10. doi: 10.1128/AAC.00954-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hietala V, Horsma-Heikkinen J, Carron A, Skurnik M, Kiljunen S. 2019. The removal of endo-and enterotoxins from bacteriophage preparations. Front Microbiol 10:1674. doi: 10.3389/fmicb.2019.01674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singh S, Wilksch JJ, Dunstan RA, Mularski A, Wang N, Hocking D, Jebeli L, Cao H, Clements A, Jenney AWJ, Lithgow T, Strugnell RA. 2022. LPS O antigen plays a key role in Klebsiella pneumoniae capsule retention. Microbiol Spectr 10:e0151721. doi: 10.1128/spectrum.01517-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zurabov F, Zhilenkov E. 2021. Characterization of four virulent Klebsiella pneumoniae bacteriophages, and evaluation of their potential use in complex phage preparation. Virol J 18:9. doi: 10.1186/s12985-020-01485-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Manohar P, Tamhankar AJ, Lundborg CS, Nachimuthu R. 2019. Therapeutic characterization and efficacy of bacteriophage cocktails infecting Escherichia coli, Klebsiella pneumoniae, and enterobacter species. Front. Microbiol 10:574. doi: 10.3389/fmicb.2019.00574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Martins W, Li M, Sands K, Lenzi MH, Portal E, Mathias J, Dantas PP, Migliavacca R, Hunter JR, Medeiros EA, Gales AC, Toleman MA. 2022. Effective phage cocktail to combat the rising incidence of extensively drug-resistant Klebsiella pneumoniae sequence type 16. Emerg Microbes Infect 11:1015–1023. doi: 10.1080/22221751.2022.2051752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tan D, Zhang Y, Cheng M, Le S, Gu J, Bao J, Qin J, Guo X, Zhu T. 2019. Characterization of Klebsiella pneumoniae ST11 isolates and their interactions with lytic phages. Viruses 11:1080. doi: 10.3390/v11111080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Taj M. 2014. Effect of dilution, temperature and pH on the lysis activity of T4 phage against E. coli Bl21
- 31. Jończyk E, Kłak M, Międzybrodzki R, Górski A. 2011. The influence of external factors on bacteriophages. Folia Microbiol (Praha) 56:191–200. doi: 10.1007/s12223-011-0039-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. León M, Bastías R. 2015. Virulence reduction in bacteriophage resistant bacteria. Front Microbiol 6:343. doi: 10.3389/fmicb.2015.00343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prazak J, Valente L, Iten M, Grandgirard D, Leib SL, Jakob SM, Haenggi M, Que Y-A, Cameron DR. 2020. Nebulized bacteriophages for prophylaxis of experimental ventilator-associated pneumonia due to methicillin-resistant Staphylococcus aureus. Crit Care Med 48:1042–1046. doi: 10.1097/CCM.0000000000004352 [DOI] [PubMed] [Google Scholar]
- 34. SAYAMOV RM. 1963. Treatment and prophylaxis of cholera with bacteriophage. Bull World Health Organ 28:361–367. [PMC free article] [PubMed] [Google Scholar]
- 35. Kutateladze M, Adamia R. 2008. Phage therapy experience at the Eliava Institute. Med Mal Infect 38:426–430. doi: 10.1016/j.medmal.2008.06.023 [DOI] [PubMed] [Google Scholar]
- 36. Percival SL, Suleman L, Vuotto C, Donelli G. 2015. Healthcare-associated infections, medical devices and biofilms: risk, tolerance and control. J Med Microbiol 64:323–334. doi: 10.1099/jmm.0.000032 [DOI] [PubMed] [Google Scholar]
- 37. Abedon ST, Thomas-Abedon C. 2010. Phage therapy pharmacology. Curr Pharm Biotechnol 11:28–47. doi: 10.2174/138920110790725410 [DOI] [PubMed] [Google Scholar]
- 38. Beeton ML, Alves DR, Enright MC, Jenkins ATA. 2015. Assessing phage therapy against Pseudomonas aeruginosa using a Galleria mellonella infection model. Int J Antimicrob Agents 46:196–200. doi: 10.1016/j.ijantimicag.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 39. Nale JY, Chutia M, Carr P, Hickenbotham PT, Clokie MRJ. 2016. Get in early’; biofilm and wax moth (Galleria mellonella) models reveal new insights into the therapeutic potential of Clostridium difficile bacteriophages. Front Microbiol 7:1383. doi: 10.3389/fmicb.2016.01383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aleshkin AV, Volozhantsev NV, Svetoch EA, Kiseleva IA, Rubal’sky EO, Afanas’ev SS, Borzilov AI, Zatevalov AM, Vasil’ev DA, Zolotukhin SN, et al. 2016. Bacteriophages as probiotics: phage-based Probiotic dietary supplement in prophylaxis against foodborne infections. Infekc bolezni 14:31–40. doi: 10.20953/1729-9225-2016-2-31-40 [DOI] [Google Scholar]
- 41. Townsend EM, Moat J, Jameson E. 2020. CAUTI’s next top model–model dependent Klebsiella biofilm inhibition by bacteriophages and antimicrobials. Biofilm 2:100038. doi: 10.1016/j.bioflm.2020.100038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pu M, Li Y, Han P, Lin W, Geng R, Qu F, An X, Song L, Tong Y, Zhang S, Cai Z, Fan H. 2022. Genomic characterization of a new phage BUCT541 against Klebsiella pneumoniae K1-ST23 and efficacy assessment in mouse and Galleria mellonella larvae. Front Microbiol 13:950737. doi: 10.3389/fmicb.2022.950737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jeon J, Yong D. 2019. Two novel bacteriophages improve survival in Galleria mellonella infection and mouse acute pneumonia models infected with extensively drug-resistant Pseudomonas aeruginosa . Appl Environ Microbiol 85:e02900-18. doi: 10.1128/AEM.02900-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu D, Van Belleghem JD, de Vries CR, Burgener E, Chen Q, Manasherob R, Aronson JR, Amanatullah DF, Tamma PD, Suh GA. 2021. The safety and toxicity of phage therapy: a review of animal and clinical studies. Viruses 13:1268. doi: 10.3390/v13071268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bao J, Wu N, Zeng Y, Chen L, Li L, Yang L, Zhang Y, Guo M, Li L, Li J, Tan D, Cheng M, Gu J, Qin J, Liu J, Li S, Pan G, Jin X, Yao B, Guo X, Zhu T, Le S. 2020. Non-active antibiotic and bacteriophage synergism to successfully treat recurrent urinary tract infection caused by extensively drug-resistant Klebsiella pneumoniae. Emerg Microbes Infect 9:771–774. doi: 10.1080/22221751.2020.1747950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eskenazi A, Lood C, Wubbolts J, Hites M, Balarjishvili N, Leshkasheli L, Askilashvili L, Kvachadze L, van Noort V, Wagemans J, Jayankura M, Chanishvili N, de Boer M, Nibbering P, Kutateladze M, Lavigne R, Merabishvili M, Pirnay J-P. 2022. Combination of pre-adapted bacteriophage therapy and antibiotics for treatment of fracture-related infection due to pandrug-resistant Klebsiella pneumoniae. Nat Commun 13:302. doi: 10.1038/s41467-021-27656-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Qurat-ul-Ain H, Ijaz M, Siddique AB, Muzammil S, Shafique M, Rasool MH, Almatroudi A, Khurshid M, Chaudhry TH, Aslam B. 2021. Efficacy of phage-antibiotic combinations against multidrug-resistant Klebsiella pneumoniae clinical isolates. Jundishapur J Microbiol 14. doi: 10.5812/jjm.111926 [DOI] [Google Scholar]
- 48. Choi M, Hegerle N, Nkeze J, Sen S, Jamindar S, Nasrin S, Sen S, Permala-Booth J, Sinclair J, Tapia MD, et al. 2020. The diversity of lipopolysaccharide (O) and capsular polysaccharide (K) antigens of invasive Klebsiella pneumoniae in a multi-country collection. Front Microbiol 11:1249. doi: 10.3389/fmicb.2020.01249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Turner PE, Azeredo J, Buurman ET, Green S, Haaber JK, Haggstrom D, Kameda de Figueiredo Carvalho K, Kirchhelle C, Gonzalez Moreno M, Pirnay J-P, Portillo MA. 2024. Addressing the research and development gaps in modern phage therapy. Phage 5:30–39. doi: 10.1089/phage.2023.0045 [DOI] [Google Scholar]
- 50. Storms ZJ, Teel MR, Mercurio K, Sauvageau D. 2020. The virulence index: a metric for quantitative analysis of phage virulence. Phage (New Rochelle) 1:27–36. doi: 10.1089/phage.2019.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All phage sequence data have previously been made available, and accession numbers are provided in Table 4.
These accession numbers can be retrieved at the European Nucleotide Archive at https://www.ebi.ac.uk/ena/browser/search.