SUMMARY
Enterococci are a diverse group of Gram-positive bacteria that are typically found as commensals in humans, animals, and the environment. Occasionally, they may cause clinically relevant diseases such as endocarditis, septicemia, urinary tract infections, and wound infections. The majority of clinical infections in humans are caused by two species: Enterococcus faecium and Enterococcus faecalis. However, there is an increasing number of clinical infections caused by non-faecium non-faecalis (NFF) enterococci. Although NFF enterococcal species are often overlooked, studies have shown that they may harbor antimicrobial resistance (AMR) genes and virulence factors that are found in E. faecium and E. faecalis. In this review, we present an overview of the NFF enterococci with a particular focus on human clinical manifestations, epidemiology, virulence genes, and AMR genes.
KEYWORDS: Enterococcus, antimicrobial resistance, virulence factors
INTRODUCTION
The Enterococcus genus comprises a group of Gram-positive bacteria that are frequently found as commensals in the human and animal gastrointestinal (GI) tract. Although the term “entérocoque” was introduced by Thiercelin (1, 2) in 1899, the genus was regarded as a subgroup of Streptococcus until 1984. Most enterococcal human disease is caused by two major species—Enterococcus faecium and Enterococcus faecalis. However, in recent years, there has been an increasing number of infections caused by non-faecium non-faecalis (NFF) enterococci. The NFF enterococci predominantly affect immunocompromised patients and may cause a range of clinical manifestations. Treatment of NFF enterococcal infections may be clinically challenging as some species are intrinsically resistant to glycopeptides and/or can acquire resistance mechanisms to multiple antibiotics through horizontal gene transfer (HGT). In addition, NFF enterococci may host multiple virulence factors. This review provides an overview of NFF enterococci, with a focus on their clinical manifestations, virulence factors, and antimicrobial resistance (AMR) genes.
PHENOTYPIC CHARACTERISTICS
Enterococci are typically spherical or ovoid in shape and are arranged in pairs or short chains. They are sometimes described as coccobacillary when grown on solid media but are typically ovoid when grown in broth (2, 3). β-hemolysis is more frequently associated with E. faecalis, particularly in non-sheep blood, while the other enterococci are typically α-hemolytic or non-hemolytic (4–6). On blood agar after 24 hours of incubation, enterococci appear as smooth white colonies 1–2 mm in diameter, although for some species the colonies may be smaller. Enterococci are non-spore-forming facultative anaerobes and obligatory fermentative chemoorganotrophs. Members of the genus have a temperature growth range from 10°C to 45°C, with an optimum growth temperature of 35°C (7). Enterococci are usually homofermentative, producing lactic acid as the product of glucose fermentation, without the production of gas. Although most species are non-motile, motility has been observed in Enterococcus gallinarum, Enterococcus casseliflavus, Enterococcus massiliensis, and “Enterococcus timonensis” (7).
POPULATION STRUCTURE AND EVOLUTION
The Enterococcus genus is a member of the Enterococcaceae family, which consists of six other genera: Bavariicoccus, Catellicoccus, Melissococcus, Pilibacter, Tetragenococcus, and Vagococcus. While typically associated with the GI tract of humans and production animals, enterococci are ubiquitous and can be found in the GI tract of birds, reptiles, insects, and rats, as well as soil, plants, and water (8–10).
As of 2022, 74 enterococcal species are recognized within the genus (Table S1), 62 of which have validly published species names (11).
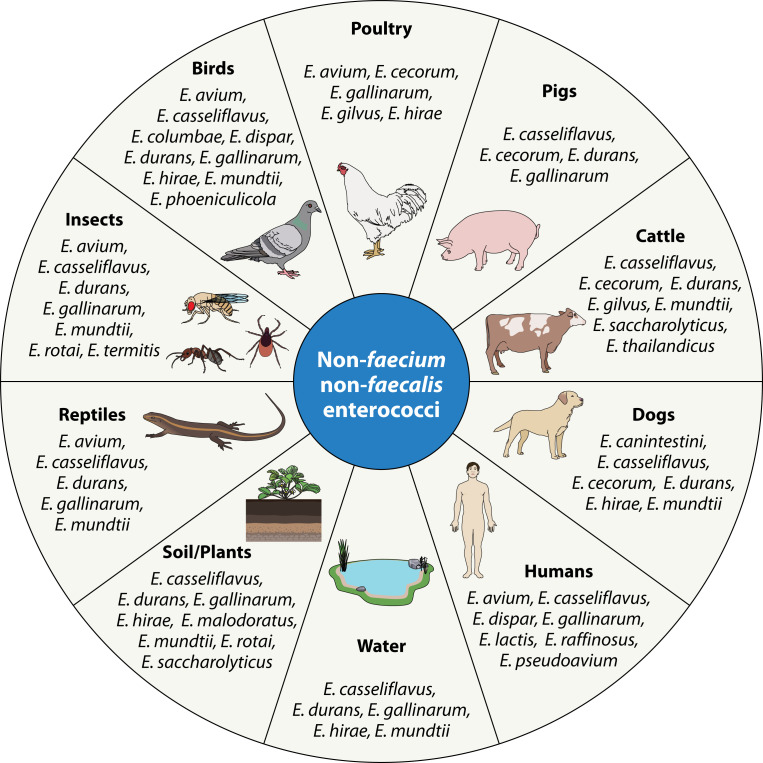
While both E. faecium and E. faecalis are the most frequently isolated species in the human GI tract, E. faecium predominates in the gut of production animals such as pigs, poultry, and cattle (9, 10). However, NFF enterococci, such as E. gallinarum, Enterococcus cecorum, Enterococcus durans, and Enterococcus avium, are also found in production animals (12–14). In the environment, Enterococcus mundtii and E. casseliflavus are typically found in plants (15) (Fig. 1).
Fig 1.
Non-faecium non-faecalis enterococci and the host/environments from which they are most frequently isolated.
16S rRNA gene sequencing has shown that enterococci are more closely related to Vagococcus, Tetragenococcus, and Carnobacterium than they are to Streptococcus and Lactococcus (16). The Enterococcus genome ranges from 2.7 to 3.5 Mb and has a low GC content with an average of 37.9% (17–19). Based on whole-genome sequencing of 37 enterococci, which included 34 species, Zhong et al. (18) recently reported that the core genome of Enterococcus consisted of 605 genes. Using a maximum likelihood phylogenetic tree based on the 605 core genes, six phylogenetic groups were identified: the E. faecium group, the E. faecalis group, the Enterococcus dispar group, the E. casseliflavus group, the Enterococcus pallens group, and the Enterococcus canis group (Table 1).
TABLE 1.
Phylogenetic groups of the genus Enterococcus based on 605 core genesa
| Branch name | Species in branch | |
|---|---|---|
| Group 1 | E. faecium branch | E. faecium, E. mundtii, E. durans, Enterococcus hirae, Enterococcus ratti, Enterococcus villorum, Enterococcus thailandicus, and Enterococcus phoeniculicola |
| Group 2 | E. faecalis branch | E. faecalis, Enterococcus termitis, Enterococcus quebecensis, Enterococcus moraviensis, Enterococcus caccae, Enterococcus haemoperoxidus, and Enterococcus silesiacus |
| Group 3 | E. dispar branch | E. dispar, Enterococcus canintestini, and Enterococcus asini |
| Group 4 | E. casseliflavus branch | E. casseliflavus, E. gallinarum, Enterococcus aquimarinus, Enterococcus saccharolyticus, Enterococcus italicus, Enterococcus sulfureus, Enterococcus cecorum, and Enterococcus columbae |
| Group 5 | E. pallens branch | E. pallens, Enterococcus hermanniensis, Enterococcus devriesei, Enterococcus gilvus, Enterococcus malodoratus, Enterococcus avium, and Enterococcus raffinosus |
| Group 6 | E. canis branch | E. canis |
Phylogenetic groups were based on a maximum likelihood tree from 605 core genes generated by Zhong et al. (18).
Evolutionary studies suggest that E. canis is the most ancient enterococcal species, while the enterococcal species found in mammals have evolved more recently. It is hypothesized that the mammalian enterococcal species expanded into the plant, bird, soil, and food environments (18).
EPIDEMIOLOGY OF NFF ENTEROCOCCI CAUSING BLOODSTREAM INFECTIONS
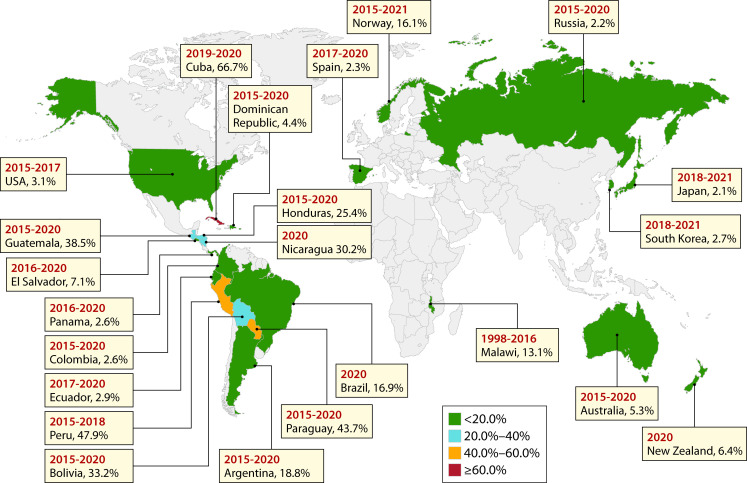
In humans, approximately 90% of enterococcal bloodstream infections are caused by E. faecium and E. faecalis (20, 21). The global prevalence of NFF enterococci associated with enterococcal bloodstream infections in humans is summarized in Fig. 2 (22–43).
Fig 2.
The prevalence of non-faecium non-faecalis Enterococcus infections among all enterococcal infections worldwide (16–37). Non-faecium non-faecalis Enterococcus infection data from all countries were collected from continuous national surveillance, except for Malawi (only from one health center). Four countries (Norway, Spain, New Zealand, and Australia) identified the isolates down to the species level. The remaining countries identified the isolates as enterococci not belonging to E. faecium or E. faecalis.
Except for Malawi in Africa (44), the NFF enterococcal infection data were obtained from continuous national surveillance programs. In some parts of the world, limited information is available with only one study cited from Africa and two studies each in Europe and Asia.
One of the largest ongoing national enterococcal bloodstream surveillance programs is performed in Australia by the Australian Group for Antimicrobial Resistance. Known as the Australian Enterococcus Surveillance Outcome Program (AESOP), the program collects continuous data on all enterococcal species from blood cultures from up to 30 laboratories servicing 48 hospitals from all Australian states and mainland territories. From 2013 to 2022, of the 11,681 unique episodes of enterococcal bacteriemia collected in the program, 5.6% were caused by NFF enterococci and include E. casseliflavus, E. gallinarum, and E. avium as the predominant species (Table 2) (21, 45–53).
TABLE 2.
Number of enterococcal isolates from human bloodstream infections collected as part of the AESOP from 2013 to 2022 (21, 45–53)c
| Species | Year | Total | 30-day all-cause mortality rate (%)a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |||
| E. avium | 5 | 9 | 11 | 8 | 9 | 18 | 13 | 12 | 6 | 16 | 107 | 10.1 |
| E. casseliflavus | 16 | 19 | 16 | 25 | 19 | 21 | 22 | 19 | 20 | 21 | 198 | 6.7 |
| E. cecorum | − | 1 | − | − | − | − | − | 1 | − | 1 | 3 | 0 |
| E. dispar | − | − | − | − | − | − | − | − | − | 1 | 1 | 100 |
| E. durans | 3 | 1 | 1 | − | 5 | 3 | 3 | 2 | 5 | 4 | 27 | 16.6 |
| E. faecalis | 478 | 523 | 562 | 595 | 602 | 676 | 699 | 667 | 705 | 812 | 6,319 | 14.5 |
| E. faecium | 325 | 380 | 402 | 413 | 481 | 491 | 596 | 488 | 523 | 613 | 4,712 | 25.2 |
| E. gallinarum | 11 | 13 | 7 | 11 | 14 | 29 | 21 | 20 | 23 | 17 | 166 | 13.3 |
| Enterococcus gilvus | 1 | − | 1 | − | − | − | − | − | − | 2 | 4 | 50 |
| Enterococcus hirae | 5 | 2 | 4 | 3 | 2 | 6 | 3 | 8 | 7 | 5 | 45 | 12.9 |
| E. mundtii | − | 1 | − | − | − | − | − | − | 1 | − | 2 | 0 |
| Enterococcus lactisb | − | − | − | − | − | − | − | − | − | 29 | 29 | 3.44 |
| Enterococcus raffinosus | 3 | 3 | 7 | 3 | 4 | 3 | 3 | 12 | 9 | 13 | 60 | 13.9 |
| Enterococcus saccharolyticus | − | − | − | − | 1 | − | − | − | − | − | 1 | 100 |
| Enterococcus thailandicus | − | − | − | − | − | − | 1 | − | − | − | 1 | NA |
| Enterococcus sp. | 2 | − | − | − | − | 1 | − | 1 | 1 | 1 | 6 | 66.6 |
| Total | 849 | 952 | 1,011 | 1,058 | 1,137 | 1,248 | 1,361 | 1,230 | 1,300 | 1,535 | 11,681 | |
30-day all-cause mortality was calculated only from patients in which the outcome was known.
Prior to 2022, E. lactis was classified as E. faecium clade B.
−, no isolates detected; NA, data not available.
CLINICAL MANIFESTATIONS
Enterococci are considered opportunistic pathogens, as they are observed as commensal gut colonizers up to 20 times more frequently than being noted as the causal agents of symptomatic infections (17). Although enterococci are associated with endocarditis, septicemia, urinary tract infections, and wound infections, microbiological cultures are often polymicrobial, and enterococci are frequently dismissed as the causal agent of disease.
Of the NFF enterococci, E. casseliflavus and E. gallinarum are the most frequently reported, each causing 1%–3% of enterococcal infections (54). NFF enterococci can cause a diverse range of infections. The most frequent NFF enterococcal clinical manifestations are catheter-associated bloodstream infections followed by biliary tract infections and intra-abdominal infections (55). Other types of infections such as urinary tract infections, soft tissue infections, infective endocarditis, and respiratory infections tend to be less frequent. In recent years, there have been sporadic case reports of NFF enterococci causing meningitis, multifocal joint infections, cholangitis, and endophthalmitis (56–59).
NFF enterococcal infections are often polymicrobial, with 40%–60% of infections caused by more than one organism (20, 60). In Australia, 59.8% of NFF enterococcal bloodstream infections are polymicrobial (55). In polymicrobial infections, NFF enterococci are almost always associated with Gram-negative bacilli, such as Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Klebsiella aerogenes (55, 60, 61). In rare cases, NFF enterococci are also isolated with E. faecium or E. faecalis.
When infections due to NFF enterococci occur, almost all patients (>95%) have underlying conditions (20, 61). The most frequently described conditions are malignancy, biliary disease, solid organ transplant, bone marrow transplant, and diabetes mellitus. Risk factors for bacteremia include biliary drainage catheters in place, history of prior hospital admission, leukopenia, and central venous catheters (20). Additionally, NFF enterococcal infections tend to be associated with the immunocompromised and the elderly (55).
Globally, the all-cause 60-day mortality incidence for NFF enterococcal infections ranges from 13% to 20% but can be significantly higher in immunocompromised patients (20, 60, 61). In bacteremia, mortality can be as low as 2.5% but as high as 42% in immunocompromised patients (20, 62). In Australia, the all-cause 30-day mortality incidence for enterococcal bloodstream infections is 14.2% (55).
NFF ENTEROCOCCI CAUSING INFECTIONS IN HUMANS
Several different species of NFF enterococci have been reported as the causative agent of infections in humans, and a summary of the infections caused by each species is listed in Table 3.
TABLE 3.
Non-faecium non-faecalis Enterococcus infections in humans
| Species | Meningitis | Endophthalmitis | Periodontitis | Pneumonia | Endocarditis | Bloodstream infection | Biliary tract infection | UTI | Osteomyelitis | Peritonitis |
|---|---|---|---|---|---|---|---|---|---|---|
| E. avium | x | x | x | x | x | x | x | x | ||
| Enterococcus canintestini | x | |||||||||
| E. casseliflavus | x | x | x | x | ||||||
| Enterococcus cecorum | x | x | x | x | ||||||
| E. dispar | x | x | ||||||||
| E. durans | x | x | x | x | x | |||||
| E. gallinarum | x | x | x | x | x | x | x | |||
| Enterococcus gilvus | x | x | ||||||||
| Enterococcus hirae | x | x | x | x | x | x | x | |||
| Enterococcus lactis | x | |||||||||
| E. mundtii | x | x | x | |||||||
| Enterococcus pseudoavium | x | |||||||||
| E. pallens | x | |||||||||
| Enterococcus raffinosus | x | x | x | x | ||||||
| Enterococcus saccharolyticus | x | |||||||||
| Enterococcus thailandicus | x | x | x |
E. avium
Originally known as group Q streptococci, E. avium was first recognized in 1984 and is a rare cause of human infections (3). The species is principally found in low abundance in the GI tract of poultry and mammals (including humans) (63). The first E. avium bloodstream infections were documented in 1992 and were related to ulcerative colitis (64). While bloodstream infections are the most frequently reported clinical manifestation, E. avium can be associated with a diverse range of infections, including surgical site infections (65, 66), abscesses (67–69), biliary tract infections (20), meningitis (70, 71), endocarditis (72, 73), osteomyelitis (74), periodontitis (75), sinusitis (76), and urinary tract infections (77). In Australia, 18% of NFF enterococcal bacteremia are caused by E. avium, 45.5% of which are polymicrobial (52).
Enterococcus canintestini
E. canintestini resides in the GI tract of healthy dogs (78). E. canintestini has been reported as a cause of infection in only one published case (20). Reported in Taiwan in 2004, the organism was isolated from the blood of a 72-year-old female patient with cervical cancer who was undergoing chemotherapy. The bacteremia was not polymicrobial. Although no additional human infections have been reported, the species is frequently detected in the vaginal swabs of mothers and the meconium of newborns (79).
E. casseliflavus
E. casseliflavus is the most frequently isolated NFF enterococcal species found in human infections. The organism resides in the GI tract of humans, cattle, birds, and pigs. Bacteremia is the most frequently reported clinical manifestation with the global prevalence of bloodstream infections ranging from 1.9% to 9% (45, 47, 80), and the mortality rate can be as high as 51.2% (60). E. casseliflavus has also been associated with endocarditis (81–84), eye infections (84–92), and meningitis (93–95). In Australia, 32.6% of NFF enterococcal bacteremia is caused by E. casseliflavus, with a 30-day all-cause mortality rate of 6.7% (52).
E. cecorum
E. cecorum was first isolated in 1983 from the GI tract of healthy chickens (96). While it mainly appears as a gut commensal in poultry, it can also be found in the GI tract of pigs, calves, ducks, cats, and dogs and may cause infections in these animals. In very rare circumstances, it may cause a diverse range of human infections and is responsible for 0.01% of NFF enterococcal bloodstream infections in Australia (52). Sporadic cases of human infections caused by E. cecorum have been reported over the last 30 years and include septicemia (97), peritonitis (98, 99), thoracic empyema (100), endocarditis (101, 102), meningitis (103), incisional hernia plate infections (104), and urinary tract infections (104). There is increasing concern over E. cecorum as an AMR reservoir, as detection of resistance to multiple classes of antibiotics is becoming more frequent in poultry isolates (105–113).
E. durans
Originally known as Streptococcus durans, E. durans was first isolated in 1937 and was reclassified to the Enterococcus genus in 1984 (114, 115). The species is predominantly found in the alimentary tract of foals, piglets, calves, and puppies and is a known cause of enteritis in these animals. It is frequently detected in a range of animal and dairy products (116–142). E. durans was first recognized as a cause of human infection in 1990 and was cultured from an infected wound(54) . It is now known to cause a range of infections, including bloodstream infections (21, 143), respiratory tract infections (144), endocarditis (62, 145–154), urinary tract infections (155), and brain infections (156, 157). In Australia, E. durans is responsible for 4% of NFF enterococcal bacteremia and has a 30-day-all-cause mortality rate of 16.6% (21).
E. gallinarum
Although E. gallinarum is predominantly a gut commensal of mammals and birds, it is one of the most prevalent NFF enterococcal species found in human disease. In humans, E. gallinarum has been associated with endocarditis (84, 158–163), bloodstream infections (60, 80, 164–172), brain infections (57, 173–175), cholangitis (58), arthritis (56), peritonitis (176), endocarditis (84, 158–163), ophthalmitis (84, 177), and urinary tract infections (84, 178–180). Globally, E. gallinarum accounts for 0.8%–3.5% of enterococcal bacteremia (47, 50), with a mortality rate of 26.7% (60). In Australia, E. gallinarum is the second most frequent cause of NFF enterococcal bacteremia (27.2%), with a 30-day-all-cause mortality rate of 13.3% (45, 46, 48–50, 52).
Enterococcus gilvus
Although E. gilvus is predominantly associated with dairy products, such as cheese and milk, the species can occasionally be found in poultry (134, 181, 182). The first human infection of E. gilvus was reported in 2002, in a bile sample from the biliary tract (183). E. gilvus is an extremely rare cause of infection in humans, with only two additional cases reported worldwide since its initial discovery, both being bloodstream infections in Australia (45, 47).
Enterococcus hirae
E. hirae was first detected in 1985 and was associated with growth defects in young chickens (184). There are sporadic case reports of the species causing a diverse range of human infections, including endocarditis (185–188), pneumonia (189), bloodstream infections (190–193), spondylitis (194), pyelonephritis (191, 195), gastritis (196), cystitis (197), periodontal infections (198, 199), and peritonitis (200). In Australia, E. hirae is responsible for 7% of NFF enterococcal bacteremia, with a 30-day-all-cause-mortality rate of 12.9% (45, 46, 48–50, 52, 55).
Enterococcus lactis
E. lactis was first isolated from raw milk and was previously considered to be rarely pathogenic (201). Studies on this bacterium primarily focus on its use in the food industry, particularly as a starter culture for cheese and milk products (202). Whilst E. lactis was not typically considered a cause of human infections, recent studies based on the core and pan-genome, multi-locus sequence typing, and digital DNA-DNA hybridization have found E. faecium clade B should be reclassified to E. lactis (203, 204). Based on the reclassification, E. lactis may be a more frequent cause of human infections than was previously thought. In the Australian AESOP 2022 report, 29 enterococci were identified as E. lactis based on the new classification, making up 1.9% of enterococcal bloodstream infections (53).
Additionally, based on DNA-DNA hybridization, Enterococcus xinjiangensis, which was first found in yogurt in 2016, has also recently been reclassified as E. lactis (201, 205).
E. mundtii
E. mundtii was initially isolated in cow teats and cow milk handlers (206). Having previously been misidentified as E. faecium and E. faecalis by commercial identification systems, E. mundtii was officially recognized as a species in 1984 (207). It has since been detected in numerous animal and environmental sources, including water (208, 209), poultry (137), horses (210), corn stovers (211, 212), dairy farms (213, 214), slaughterhouses (215, 216), fish (217), caterpillars (218), and other wildlife (219). Although E. mundtii rarely causes human infections, it has been found in thigh abscesses (207), sinusitis (207), endophthalmitis (220), bloodstream infections (170, 178), and urinary tract infections (221). Between 2013 and 2021, the AESOP has only reported two episodes of E. mundtii bacteremia in Australia (52).
Enterococcus raffinosus
E. raffinosus was first described in 1989 from patients with deep vein thrombosis, liver disease, and rheumatoid arthritis (222). Compared to other NFF enterococci, E. raffinosus is a relatively infrequent cause of human infections but has been found in cases of vertebral osteomyelitis (223), hematoma (224), vaginal infections (225), decubitus ulcers (226), endocarditis (227, 228), endophthalmitis (143, 229), sinusitis (143, 230), bloodstream infections (20, 61, 231, 232), and among hemodialysis patients (233). In Australia, E. raffinosus is responsible for 8.2% of NFF enterococcal bacteremia and has a 30-day-all-cause-mortality rate of 13.9% (21).
Enterococcus saccharolyticus
Originally grouped with Streptococcus bovis, E. saccharolyticus was first described as a species in 1984 (234). The organism is primarily isolated from animal and environmental sources, such as freshwater or sewage and is occasionally found as the causative agent of bovine mastitis (235). In rare circumstances, it is the cause of human infections and has been identified in cases of infected lymphocele (236) and bacteremia (49).
E. thailandicus
Initially isolated from fermented sausages in Thailand, E. thailandicus is an extremely rare cause of human disease (138). The species is infrequently isolated from the GI tract of animals, such as cows and beavers, and in environmental sources, such as farms and water bodies (216, 237–239). There is some debate as to whether E. thailandicus has been found in the human GI tract, as the species was previously grouped with E. sanguinicola, which has been detected in humans (238, 240, 241). To date, there have only been two recorded cases of E. thailandicus causing human infections, both of which were polymicrobial. The first case was a biliary tract infection reported in Australia (55) and the second case occurred in a patient in Belgium with an intra-abdominal infection (242).
Less common NFF enterococci causing human infections
While overwhelmingly detected in animals and in the environment, there are several less common NFF enterococci, which occasionally cause human infections. Species include E. dispar, which has caused urinary tract infections and septicemia (243–245), Enterococcus pseudoavium, which is responsible for 2.6% of enterococcal urinary tract infections in India (243), and E. pallens, which sporadically causes bacterial peritonitis (183, 246).
Additionally, there are NFF enterococci species isolated in humans, which are not known to cause infection: Enterococcus villorum, Enterococcus rivorum, and Enterococcus phoeniculicola have been found in the vagina of pregnant women and meconium of newborn infants (79, 247–249); Enterococcus caccae, “E. massiliensis,” and “Enterococcus mediterraneensis” have been found in the fecal samples of healthy individuals (250–252).
VIRULENCE FACTORS
As the majority of enterococcal infections are caused by E. faecalis and E. faecium, most studies involving virulence factors have focused on these two species. When the NFF enterococci species are isolated, the studies are often limited due to insufficient sample size. Additionally, the species are rarely studied in vitro, so it is unknown if these virulence genes are responsible for the same phenotypes observed in E. faecium and E. faecalis. Some genetic studies have shown NFF enterococci possess many virulence factors, even when isolated as a commensal (Table 4).
TABLE 4.
Virulence factors in enterococci
| Function | Virulence factor | Gene(s) | References |
|---|---|---|---|
| Adhesion | Aggregation substance | asa1 | (253–257) |
| Collagen-binding adhesin | ace, acm | (258–263) | |
| Enterococcal surface protein | esp | (264–270) | |
| Pili | pilA, pilB, ebpA, ebpB, ebpC, srtC | (271–275) | |
| Lysins | β-hemolysis | cylLL, cylLS, cylM, cylA, cylB | (4, 276–280) |
| Gelatinase | gelE | (270, 281) | |
| Serine protease | sprE | (282–284) | |
| Hyaluronidase | hyl | (257, 285, 286) | |
| Sex pheromones | Conjugative plasmid transfer, neutrophil chemotaxis, superoxide formation, inflammation | cpd, cob, ccf, cad | (263, 287–289) |
Adhesion
One of the major virulence determinants in enterococci is their ability to adhere to host tissue. When the first enterococcal genome was published, a series of 41 putative cell wall proteins were identified. The proteins belong to a family known as microbial surface component recognizing adhesive matrix molecule (MSCRAMM) (290). The process of adhesion is complex and involves at least four major proteins, including the two collagen-binding adhesins (Ace and Acm), enterococcal surface protein (Esp), and aggregation substance (AS). Additionally, pili have been shown to play a role in biofilm formation in some enterococcal isolates.
Ace is a MSCRAMM and is one of the most well-studied adhesins in E. faecalis. It has a high sequence similarity to the A domain of the collagen adhesin (Cna) in Staphylococcus aureus. The cell surface protein mediates adherence of E. faecalis cells to bovine and rat collagen type I (CI), human collagen type IV (CIV), and mouse laminin (258, 291). In animal infection models, Ace has been shown to play a role in early endocarditis establishment (259). The ace gene is found in most clinical isolates of E. faecalis and tends to be found more frequently as a pseudogene in non-clinical isolates (258). Studies researching animal isolates of Enterococcus have identified ace in many NFF enterococci, including Enterococcus columbae, E. hirae, E. gallinarum, E. casseliflavus, E. cecorum, and E. durans (260).
Acm, a collagen adhesin, has 62% similarity to the S. aureus collagen adhesin Cna. The Acm protein consists of two domains (domains A and B) and is 60% and 75% similar to domains A and B of Cna, respectively (261). This adhesin was first identified in E. faecium, and while almost 100% of clinical isolates have the acm gene, not all exhibit binding to the Acm receptor, collagen type I (261). Introduction of the acm gene into acm deletion mutants showed an 11-fold increase in E. faecium adherence to collagen but not to fibronectin or fibrinogen (261). Acm has also been shown to play a role in endocarditis as acm deletion mutants were highly attenuated in mouse models of infection and had reduced valve colonization ability (262). Until recently, acm had not been identified in Enterococcus species other than E. faecium. However, a 2022 study found acm in isolates of E. gallinarum and E. casseliflavus in food products of animal origin (263).
Esp was initially identified in E. faecalis and was found to be significantly enriched in clinical isolates compared to isolates obtained from fecal samples of asymptomatic patients (264). Molecular studies have shown that, while Esp does not directly contribute to the histopathology of E. faecalis, it is associated with biofilm formation (265) and colonization and persistence in urinary tract infections (266). Esp is orthologous to Bap, a biofilm-associated protein in S. aureus. The Esp protein forms aggregates with an amyloid-like conformation, which is directly related to its ability to form biofilms (267). The presence of the esp gene in E. faecium appears to be restricted to hospital-associated isolates belonging to CC17 (268), although it has recently been detected in several NFF enterococci including E. casseliflavus, E. mundtii, E. durans, Enterococcus solitarius, and Enterococcus malodoratus (269, 270).
AS (encoded by asa1) is a plasmid-encoded enterococcal surface protein and was first discovered in relation to binding between bacterial cells, facilitating the transfer of the pAD1 plasmid between bacterial cells (253). However, in vitro studies have shown that the AS protein increases the adherence of bacteria to renal tubular cells (254) and protects E. faecalis from killing by polymorphonucleocytes (255). In E. faecalis, asa1 is highly associated with isolates from urine, blood, and wound sites (256). Reports of asa1 in NFF enterococci are rare, although the gene has been identified in E. gallinarum, E. raffinosus, and E. mundtii (257).
Pili have been well described in enterococci, being known primarily for their role in biofilm formation and endocarditis. Clinical isolates of E. faecium are significantly enriched for pilA and pilB genes compared to isolates from fecal swabs (271). Additionally, the endocarditis and biofilm-associated pilus, Ebp, has been well characterized. As its name suggests, Ebp plays a major role in endocarditis and biofilm formation and consists of ebpA, ebpB, ebpC, and an associated srtC (encoding sortase C) gene that also has an independent promoter (272). The protein complex has also been shown to play a role in animal models of urinary tract infection (273), adherence to human platelets (274), cell-cell aggregation, and intraspecies gene transfer (275). The epb locus is highly conserved between species of Enterococcus, including E. casseliflavus and E. gallinarum (274); the only notable difference is the absence of epbB in E. casseliflavus.
Lysins
β-hemolysis is identified in approximately one-third of E. faecalis isolates and has been associated with genes from the cyl operon that encodes cytolysin genes. The operon includes two cytolysin structural subunits (encoded by cylLL and cylLS), which are post-translationally modified intracellularly by the cylM gene product and transported out of the cell by an ATP-binding cassette transporter encoded by cylB; after externalization, cytolysin precursor components are activated by CylA, an extracellular activator serine protease (276, 277). The final gene in the operon cylI encodes the immunity protein for self-protection from cytolysin. Cytolysin has been studied in both animal and human models of infection and contributes to the severity of enterococcal disease (278, 279). While rare, β-hemolytic activity has been observed in E. avium, E. casseliflavus, E. cecorum, E. durans, Enterococcus flavescens, E. gallinarum, E. malodoratus, and E. raffinosus, and this has been associated with the presence of cylLL, cylA, and cylLS (4, 280).
E. faecalis is known to produce extracellular proteases, specifically gelatinase (encoded by gelE), serine protease (encoded by sprE), and hyaluronidase (encoded by hyl). Extracellular gelatinase is a matrix metalloproteinase that hydrolyzes gelatin, collagen, and other proteins. In E. faecalis, gelE plays a role in immune evasion by preventing both opsonization and the formation of membrane attack complex through the hydrolysis of C3a (281). The protease has also been shown to cleave the anaphylatoxin complement C5a, leading to decreased neutrophil migration in vitro (292). The sprE gene lies immediately downstream of gelE and encodes a secreted 26-kDa serine protease, which is homologous to the S. aureus V8 protease (282). The presence of both proteases is associated with biofilm formation (293). In vitro studies have shown that sprE deletion mutants exhibit increased autolysis, while gelE deletion prevents extracellular DNA release from the biofilm (294). The loss of one or both protease genes results in attenuated virulence in animal models of infection (295). The two genes are transcriptionally regulated by the fsr locus, and deletion of this locus prevents biofilm formation (294). Similar to other virulence genes in NFF enterococci, the presence of gelE and sprE is infrequent; however, gelE has been identified in E. casseliflavus, E. gallinarum, E. avium, E. durans, and E. raffinosus (270), and sprE has been identified in E. hirae, E. mundtii, and E. durans (283, 284).
Hyaluronidase is an enzyme produced by a variety of bacteria that breaks down hyaluronic acid. Hyaluronidase depolymerizes the mucopolysaccharide moiety of connective tissues, allowing bacteria to invade between cells (285). The gene encoding hyaluronidase has been identified in E. faecalis, particularly among oral strains associated with periodontal disease, and is more prevalent in clinical isolates of E. faecium (257, 285). The hyl gene is typically absent in most studies of NFF enterococci although it has been detected in E. casseliflavus, E. gallinarum, E. durans, E. malodoratus, and E. mundtii (257, 286).
Sex pheromones
E. faecalis possesses several chromosomally encoded genes that produce “sex pheromones.” These sex pheromones are small linear peptides that are produced by the recipient cells that stimulate (or inhibit) the transfer of conjugative plasmids between enterococci. When the peptide binds to the donor cells that carry a conjugative plasmid, the donor produces the AS adhesin, which results in the clumping of donor and recipient cells (287). This close contact facilitates the transfer of the conjugative plasmid. While these peptides have a role in plasmid transfer, they can play a role in neutrophil chemotaxis. The small peptides bind to formyl peptide receptors 1 and 2 (FPR1 and FPR2) and G protein-coupled receptor 41 or 4 (GPR41 or GPR4), which are found on the surface of neutrophils and other leukocytes (288). In addition to neutrophil chemotaxis, the binding of these peptides to the cell receptors can result in superoxide formation and the activation of inflammatory mediators such as IL-8 (289). There are several sex pheromones, and a single bacterium may produce multiple types. They are typically encoded by cpd, cob, ccf, and cad. Historically, the genes have been exclusively studied in E. faecalis, but they have recently been identified in E. gallinarum and E. casseliflavus (263). While these sex pheromones have been studied in mouse models and in vitro, their role in human infection and colonization remains unclear.
ANTIBIOTIC RESISTANCE
In the 1970s and 1980s, the continued use of antibiotics led to the evolution of antimicrobial-resistant E. faecium, which has become a frequent cause of hospital-acquired bacteremia (296). Antimicrobial resistance in E. faecium was particularly driven by the use of cephalosporins, which eradicated the intestinal bacterial microbiota and selected for vancomycin-resistant enterococci (VRE) (297, 298).
Antimicrobial-resistant NFF enterococci have been reported in several studies. The antimicrobial susceptibility of NFF enterococci against certain common antibiotics is summarized in Table 5 (57, 93, 100, 144, 178, 191, 299–319).
TABLE 5.
The antibiotic susceptibility of non-faecium non-faecalis enterococci in human clinical samples (57, 93, 100, 144, 178, 191, 299–319)a
| Antibiotics | Minimum inhibitory concentration (mg/L) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. avium | E. casseliflavus | E. cecorum | E. dispar | E. durans | E. gallinarum | E. hirae | E. mundtii | E. raffinosus | E. solitarius | E. sulfurous | E. thailandicus | |
| Aminoglycoside | ||||||||||||
| Amikacin | ≥512 | −b | − | − | − | − | − | − | − | − | − | − |
| Gentamycin | ≥2,048 | 8–1,024 | − | >500 | >500 | ≤50–2,000 | ≥500 | − | >2,048 | ≥500 | − | − |
| Netilmicin | − | − | − | − | − | − | − | − | − | − | − | − |
| Streptomycin | ≥2,048 | 64 | − | >2,000 | >2,000 | ≤125–250 | ≥2,048 | − | >2,048 | ≥2,000 | − | − |
| Beta-lactams | ||||||||||||
| Cephalosporins | ||||||||||||
| Cefotaxime | − | − | 0.25 | − | − | − | − | − | − | − | − | − |
| Carbapenems | ||||||||||||
| Imipenem | 0.5–128 | ≤0.06–64 | − | − | − | ≤1−≥16 | ≥1 | − | >16 | − | − | − |
| Penicillins | ||||||||||||
| Amoxicillin | − | − | − | − | − | − | 0.25 | − | − | − | − | − |
| Amoxicillin-clavulanic acid | − | >20/10 | − | >20/10 | >20/10 | − | − | − | − | − | − | − |
| Ampicillin | ≤2− ≥32 | ≤2−≥32 | 0.38 | >10 | >10 | ≤2−≥ 32 | ≥16 | − | >32 | ≥16 | − | 1 |
| Penicillin | 16–64 | 0.5–4 | 0.094 | >10 | ≥16 | 16–64 | ≥16 | 8 | 16–64 | − | − | 32 |
| Fluoroquinolones | ||||||||||||
| Ciprofloxacin | ≥1 | 8–12 | − | >5 | >5 | 1–8 | 0.5 | − | 4 | ≥4 | − | 0.5 |
| Levofloxacin | − | 4 | − | − | − | 1 | − | − | − | − | − | − |
| Moxifloxacin | − | 1 | − | − | − | 1 | − | − | − | − | − | − |
| Nemonoxacin | − | − | − | − | − | − | − | − | − | − | − | − |
| Norfloxacin | − | 8–16 | − | − | − | 2–8 | − | − | − | − | − | − |
| Ofloxacin | 0.5–64 | ≤0.06–128 | − | − | − | 0.25–128 | − | − | − | − | − | − |
| Glycopeptide | ||||||||||||
| Teicoplanin | ≤0.5 | ≤0.5–1 | − | ≥32 | 96 | ≤0.5–32 | ≤2 | − | 32–256 | 1–16 | 1–16 | − |
| Vancomycin | ≤0.5–1 | 1–4 | 1.5 | ≥32 | ≥256 | ≤0.5–32 | 1 | ≥128 | 128–1,024 | 128−≥256 | ≥256 | 0.5 |
| Glycylcyclines | ||||||||||||
| Tigecycline | <0.12 | <0.12 | − | − | 0.047 | <0.12 | − | − | <0.12 | − | − | |
| Lipopeptides | ||||||||||||
| Daptomycin | − | 0.5–2 | − | − | 32 | 0.5–8 | − | − | 1 | − | − | 1 |
| Lipoglycopeptides | ||||||||||||
| Dalbavancin | − | ≤0.015–0.25 | − | − | − | ≤0.015–0.25 | − | − | − | − | − | − |
| Macrolides, lincosamides, and streptogramins | ||||||||||||
| Clindamycin | − | 4 | − | − | − | ≥8 | − | − | >256 | − | − | − |
| Erythromycin | ≤0.06−> 128 | 0.125–32 | − | >15 | >15 | ≤0.06–128 | ≥8 | − | >256 | − | − | − |
| Roxithromycin | − | − | − | − | 0.25 | − | − | − | − | − | − | − |
| Quinupristin-dalfopristin | 1–8 | 1–4 | − | − | − | 1–>16 | − | − | >16 | − | − | |
| Oxazolidinones | ||||||||||||
| Linezolid | 16 | 2 | 0.75 | − | 1 | 1–4 | 2 | − | 1–2 | − | − | 2 |
| Tedizolid | 4 | − | − | − | − | 0.5 | − | − | − | − | − | 0.25 |
| Tetracyclines | ||||||||||||
| Minocycline | ≤0.06–16 | ≤0.06–16 | − | − | − | ≤0.06–32 | − | − | − | − | − | |
| Tetracycline | ≥16 | 2–16 | − | − | − | 2–16 | − | − | 64 | − | − | |
| Miscellaneous agents | ||||||||||||
| Chloramphenicol | − | 8–16 | − | − | − | 64 | − | − | − | − | − | 32 |
| Fosfomycin | − | 128 | − | − | − | 16–512 | − | − | − | − | − | |
| Fusidic acid | − | − | − | − | − | 2 | − | − | − | − | − | |
| Metronidazole | >128 | >50 | − | − | − | − | − | − | − | − | − | |
| Nitrofurantoin | − | 8–64 | − | − | − | 64–128 | − | − | − | − | − | |
| Rifampicin | − | 4–8 | − | − | − | 64 | − | − | 2 | − | − | |
| Trimethoprim-sulfamethoxazole | − | <0.008–0.5 | − | − | >32 | − | − | − | − | − | − | |
Only NFF enterococci for which antibiotic susceptibility data were available were included in this table.
−, no susceptibiltiy data available for a given species.
Beta-lactams
Beta-lactam antibiotics inhibit bacterial growth by binding to D,D-transpeptidases (penicillin-binding proteins), which catalyze cross-linking of peptidoglycan during cell wall synthesis. The inhibition of cell wall synthesis leads to defects in the bacterial cell wall structure and eventually cell lysis. Enterococci are typically considered to have intrinsic low-level non-susceptibility to beta-lactams. In enterococci, there are seven genes involved in beta-lactam resistance: pbp5, ponA, pbpF, murAA, croRS, asrR, ireK/ireP, and stk/stpA (Table 6).
TABLE 6.
Antibiotic resistance-associated genes in non-faecium non-faecalis enterococci
| Antibiotic | Resistance mechanism | Gene(s) | Genes detected in NFF enterococci | References |
|---|---|---|---|---|
| β-lactams | Low-affinity penicillin-binding proteins | pbp5, ponA, pbpF | −a | (320–323) |
| Peptidoglycan synthesis | murAA, murAB | − | (324) | |
| Two-component signal transduction systems | croRS, ireK | − | (325–329) | |
| Transcriptional regulators | asrR | asrR homologs have been detected in E. casseliflavus, E. durans, E. gallinarum, and E. hirae | (330, 331) | |
| β-lactamase production | blaZ | blaZ detected in E. casseliflavus, E. gallinarum, and E. mundtii | (128, 332–334) | |
| Aminoglycosides | Modification of antibiotic (intrinsic resistance) | aac(6′)-Ii, efmM | Genes not detected in NFF enterococci but high-level aminoglycoside resistance reported in E. avium, E. dispar, and E. pseudoavium | (243, 335, 336) |
| Modification of antibiotic (acquired resistance) | aph(3′)-IIIa | aph(3′)-IIIa detected in E. avium, E. dispar, E. durans, E. gallinarum, and E. hirae | (337–340) | |
| Modification of antibiotic (acquired resistance) | ant(6)-Ia | ant(6)-Ia detected in E. casseliflavus and E. hirae | (341–343) | |
| Modification of antibiotic (acquired resistance) | ant(9)-Ia | ant(9)-Ia detected in E. avium, E. durans, and E. gallinarum | (344–348) | |
| Modification of antibiotic (acquired resistance) | aac(6')-aph(2') | aac(6')-aph(2') detected in E. avium, E. casselflavus, E. durans, E. hirae, and E. raffinosus | (340, 349–351) | |
| Glycopeptides | Production of low vancomycin affinity peptidoglycan | vanC | vanC is intrinsically found in E. casseliflavus and E. gallinarum | (142, 316, 352) |
| vanA | vanA detected in E. cecorum, E. dispar, E. durans, E. gallinarum, and E. raffinosus | (61, 308, 353–355) | ||
| vanD | vanD detected in E. avium, E. gallinarum, and E. raffinosus | (310, 356–358) | ||
| vanN | vanN detected in E. lactis | (359–361) | ||
| Tetracyclines | Efflux pumps | tetK, tetL | tetK and tetL detected in E. avium, E. casseliflavus, E. cecorum, E. durans, E. gallinarum, and E. hirae | (105, 140, 362) |
| Ribosomal protection proteins | tetM, tetO, tetS | tetM, tetO, and tetS detected in E. avium, E. casseliflavus, E. cecorum, E. durans, E. gallinarum, and E. hirae | (105, 362, 363) | |
| Glycylcyclines | Target modification | rpsJ mutations | − | (364–366) |
| Ribosomal protection protein | tetM | tetM and tetL detected in E. avium, E. casseliflavus, E. cecorum, E. durans, E. gallinarum, and E. hirae but association with glycylcycline resistance has not been investigated | (105, 141, 362) | |
| Efflux pumps | tetL | |||
| Aminomethylcyclines | Target modification | 16s rRNA gene | − | (367) |
| Macrolide-lincosamide-streptogramin B class | ATP-binding cassette protein— mechanism unknown | lsaA, etaA | lsaA detected in E. casseliflavus E. gallinarum, and E. hirae | (368, 369) |
| Efflux pumps | mefA, mefE, msrA, msrC, mreA | mefA detected in E. durans | (370–374) | |
| Target modification | ermA, ermB, ermC | ermA detected in E. avium, ermB detected in E. avium, E. durans, E. gallinarum, and E. hirae | (375–379) | |
| Inactivation of antibiotic | mphC, ereA, lnuA, lnuB, linB |
ereA detected in NFF enterococci (species not identified), linB detected in E. gallinarum |
(380, 381) | |
| Inactivation of antibiotic | vatD, vatE | vatD and vatE detected in E. hirae | (382, 383) | |
| Fluoroquinolones | Protection proteins | qnr | qnr detected in E. casseliflavus | (384–387) |
| Target modification | Mutations in gyrA and parC | − | (388) | |
| Efflux pumps | emeA, erfAB | − | (389–391) | |
| Lipopeptides | Genetic mechanisms have yet to be identified | (392–394) | ||
| Oxazolidinones | Target modification | Mutations in 23S rRNA gene | G2576T point mutation detected in E. hirae | (395) |
| Target modification | cfrA, cfrB, cfrC, cfrD | cfr detected in E. avium, E. casseliflavus, and E. gallinarum | (396–398) | |
| Protection proteins | optrA, poxtA |
optrA detected in E. hirae, poxtA detected in E. hirae and E. lactis |
(343, 396–400) |
−, the gene/s has not been detected in any NFF species.
In bacteria, there is a large diversity of penicillin-binding proteins (PBPs), with each bacterium often possessing several PBPs (401–403). Enterococci possess multiple PBPs, three of which are associated with β-lactam resistance: PBP5, PonA, and PbpF. While all three proteins are associated with β-lactam resistance, the predominant mechanism through which E. faecium is intrinsically not susceptible to β-lactams is via the production of a low-affinity class B penicillin-binding protein 5 (PBP5). Acquired resistance to high levels of ampicillin in this species has been observed in isolates with increased production of PBP5 (320). pbp5 deletion mutants show significant reductions in cephalosporin resistance (321). Additionally, point mutations in PBP5 can result in reduced affinity for penicillins and increased resistance to these antibiotics (322). While all enterococci possess PBPs, pbp5 has not been identified in NFF Enterococcus species, possibly in part due to a lack of AMR studies.
In E. faecalis, resistance to extended-spectrum cephalosporins, such as ceftriaxone, can occur through the presence of two PBPs: PonA and PbpF. Although single deletion mutants of ponA and pbpF do not increase susceptibility to ceftriaxone, double deletion mutants result in sensitization to this antibiotic (323). Site-directed mutagenesis of ponA and pbpF has shown that they are both essential partners of PBP5 (323). Neither ponA nor pbpF have been identified in NFF enterococcal species.
Intrinsic resistance to cephalosporins in E. faecalis can also occur through a UDP-N-acetylglucosamine 1-carboxyvinyl transferase known as MurAA (324). The enzyme is responsible for the phosphoenolpyruvate-dependent conversion of UDP-N-acetylglucosamine to UDP-N-acetylglucosamine-enolpyruvate, which is the first step in peptidoglycan synthesis. Deletion of murAA leads to increased susceptibility to cephalosporins in E. faecalis. MurAA appears to only play a role in E. faecalis, as deletion of its homolog, murAB in E. faecium has no effect on its susceptibility to cephalosporins (324). Neither murAA nor murAB have been detected in NFF enterococci.
In addition to PBPs, two-component signal transduction systems (TCSs) are known to contribute to β-lactam resistance, particularly to cephalosporins. The TCSs cause changes in the expression of several genes, resulting in physiological changes in the bacterial cell, which lead to increased resistance to β-lactams. Enterococci possess several TCSs; for example, E. faecalis strain V583 has at least 17 TCSs (404). In enterococci, two TCSs have been associated with β-lactam resistance, CroRS and IreKP.
CroRS is a TCS that is found in E. faecalis and E. faecium. CroS, a sensor with histidine kinase activity, phosphorylates the cognate response regulator CroR, which alters transcription in the bacterial cell leading to increased expression of pbp4/pbp5 (405). Deletion of croRS results in a 4,000-fold reduction in MIC for ceftriaxone (325, 326). In E. faecalis, disruption of CroRS leads not only to increased susceptibility to cephalosporins but also to ampicillin, bacitracin, and vancomycin (325, 404).
IreK (intrinsic resistance of enterococci kinase) is another TCS that is reported exclusively in E. faecalis. IreK is a eukaryotic-type serine/threonine kinase that belongs to a family of transmembrane kinases defined by the presence of multiple extracellular penicillin-binding protein and serine/threonine kinase-associated domains (327). IreK has been shown to directly phosphorylate its substrate, IreB. While the function of IreK/B remains unknown, it is hypothesized that they regulate peptidoglycan biosynthesis, metabolism, and resistance to cell wall stress (328). While its function is yet to be elucidated, it is clear that IreK plays a role in cephalosporin resistance, as ireK deletion mutants are susceptible to cephalosporins (328). While IreK is involved in cephalosporin resistance, it is also reciprocally regulated by IreP, a PP2C-type protein phosphatase encoded immediately upstream of ireK (328). Mutants lacking IreP are hyper-resistant to cephalosporins, and this resistance is mediated by IreK. While E. faecium does not possess IreK/B, it possesses its own serine/threonine kinase, Stk, and its cognate phosphatase, StpA. Impaired activity of StpA results in increased activity of L,D-transpeptidation pathway in PBP synthesis, leading to a non-mutational increase in ampicillin activity (329). Neither of the serine/threonine kinases has been identified in NFF enterococci.
In addition to TCSs, there are transcriptional regulators that are associated with penicillin resistance in E. faecium such as asrR (antibiotic and stress response regulator). AsrR is part of the MarR family that regulates 181 genes, including pbp5 (330). Deletion of asrR resulted in increased expression of pbp5 and increased resistance to ampicillin and vancomycin (330). AsrR homologs have not been found in E. faecalis; however, they have been identified in several NFF species, including E. durans, E. hirae, E. gallinarum, and E. casseliflavus (331). The role of these homologs in NFF enterococci has yet to be determined.
Beta-lactamases are enzymes that confer resistance to bacteria by hydrolyzing the β-lactam ring of penicillins. In enterococci, the enzyme is encoded by blaZ and can be transferred horizontally. However, the expression of blaZ is constitutively low, and a high bacterial inoculum is required to result in phenotypic penicillin resistance (332). β-lactamase production was first reported in E. faecalis in 1981 (332). β-lactamase production is extremely rare and is infrequently reported, although this may be underreported due to its low expression. In NFF enterococci, blaZ-associated ampicillin resistance has been reported in E. casseliflavus, E. gallinarum, and E. mundtii (333, 334).
While clinical isolates of E. faecalis and E. faecium are typically intrinsically resistant to penicillin, bacteremia isolates of E. gallinarum and E. casseliflavus are typically penicillin susceptible (60). β-lactam resistance has also been reported phenotypically in E. avium (375) and E. raffinosus (406, 407), but the mechanism of resistance is unknown. In rare circumstances, penicillin resistance has been observed in E. durans, E. casseliflavus, E. gallinarum, and E. mundtii; however, these resistant isolates were obtained from animal or environmental sources and have not been reported in human infection (408, 409).
Aminoglycosides
The aminoglycosides are a group of broad-spectrum antibiotics that irreversibly bind to the bacterial 30S ribosome, thereby interfering with protein translation, and resulting in the inhibition of protein synthesis. All enterococci are intrinsically low-level resistant to aminoglycosides with MICs ranging from 4 to 256 mg/L, depending on the antibiotic used. Two genes are responsible for intrinsic aminoglycoside resistance in E. faecium only: aac(6′)-Ii and efmM (Table 6). The aac(6′)-Ii gene encodes a 6′-N-aminoglycoside acetyltransferase, which modifies aminoglycoside antibiotics, including tobramycin, sisomicin, kanamycin, and netilmicin (335). The efmM (E. faecium methyltransferase) gene encodes an S-adenosyl methionine-dependent m5C methyltransferase (MTase) that specifically targets nucleotide 1,404 in 16S rRNA. While 16s rRNA methylation due to this enzyme imparts a smaller contribution to aminoglycoside resistance compared to aac(6′)-Ii, knockout mutants of the gene still contribute a twofold increase in susceptibility to kanamycin and tobramycin (336). High-level resistance to aminoglycosides has been reported in several NFF enterococcal species, including E. avium (376), E. dispar, and E. pseudoavium (243).
Acquired resistance to aminoglycosides in enterococci can occur via aph(3′)-IIIa, which encodes a 3′-aminoglycoside O-phosphotransferase type IIIa (Table 6). The gene is primarily associated with high-level spectinomycin resistance, and the enzyme can inactivate multiple aminoglycoside antibiotics, such as butirosin, amikacin, and isepamicin via the addition of a phosphate group (337). The gene is frequently detected in both E. faecalis and E. faecium, with prevalence being as low as 37% and as high as 50% (338, 339). In the few studies in which aph(3′)-IIIa has been investigated in NFF enterococci, the prevalence ranged from 28% to 100%, although the prevalence is likely skewed due to exceedingly small study sample sizes. Nevertheless, aph(3′)-IIIa has been detected in E. durans, E. avium, E. hirae, E. dispar, and E. gallinarum (338, 340).
The second major mechanism of acquired aminoglycoside resistance in enterococci is via ant(6)-Ia. The gene encodes an aminoglycoside nucleotidyltransferase, which modifies aminoglycoside antibiotics resulting in high-level resistance to gentamicin and streptomycin. The ant(6)-Ia gene has been identified in E. faecium and E. faecalis isolated from human and animal origin (341, 342). It has also been detected in NFF enterococci, such as E. casseliflavus and E. hirae, isolated from meat products (343, 408).
The third mechanism of acquired aminoglycoside resistance is via Ant(9)-Ia. This protein is another aminoglycoside nucleotidyltransferase, which acts specifically on spectinomycin by binding to the 9-hydroxyl group (344). The introduction of plasmids carrying ant(9) from E. faecalis into E. coli have shown a 10,000-fold increase in the MIC of spectinomycin (345). In NFF enterococci, this gene has been detected in E. avium, E. gallinarum, and E. durans (346–348).
The fourth and final mechanism of acquired aminoglycoside resistance in enterococci is via the bifunctional enzyme Aac(6')-Aph(2') (349). This enzyme has both adenyltransferase and phosphotransferase activities and results in high-level gentamicin resistance (HLGR) (MIC > 1,000 mg/L) (349). This is the major mechanism through which HLGR occurs in enterococci. The aac(6')-aph(2') gene has been detected in several NFF enterococcal species, including E. avium, E. casseliflavus, E. durans, E. hirae, and E. raffinosus (340, 350, 351).
Glycopeptides
Glycopeptides are a class of antibiotics that inhibit peptidoglycan synthesis by binding with strong affinity to acyl-D-alanyl-D-alanine (a peptidoglycan precursor) on lipid II, preventing the addition of new peptidoglycan units (410). The most frequently used glycopeptides are vancomycin and teicoplanin.
In enterococci, vancomycin resistance can be either intrinsic or acquired. Acquired vancomycin resistance can be mediated by vanA, vanB, vanD, vanE, vanG, vanL, vanM, or vanN genes, while intrinsic resistance is mediated by vanC (411) (Table 6). A new van gene cluster, vanP, has recently been identified, but its role in vancomycin resistance has not been clearly established (412). The van gene clusters mediate resistance by synthesizing peptidoglycan precursors that do not end in the high vancomycin affinity D-alanine. Several NFF enterococci have developed resistance to vancomycin, such as E. casseliflavus (MIC 1,024 mg/L) (413), E. gallinarum (MIC 1,024 mg/L) (413), E. mundtii (MIC ≥ 128 mg/L) (414), and E. raffinosus (MIC 128–1,024 mg/L) (415). While vancomycin resistance can be mediated by any of the van genes in E. faecium and E. faecalis, resistance in NFF enterococci has only been associated with vanA, vanB, vanC, vanD, and/or vanN.
The vanA and vanB operons are historically the most prevalent in infections due to vancomycin-resistant enterococci and are typically associated with E. faecium. However, vancomycin resistance in NFF enterococci is most commonly caused by the chromosomally mediated vanC operon, which is intrinsic to E. gallinarum and E. casseliflavus only. Intrinsic resistance due to vanC was first reported in 1988 in the first case report of E. gallinarum, which was isolated from multiple blood cultures from a patient receiving prophylactic vancomycin (316). Many studies have reported almost all E. gallinarum and E. casseliflavus isolates as vancomycin resistant, but this is less frequently reported in other NFF enterococci (352). While initially believed to be non-transferrable, vanC has been identified in E. faecium and E. faecalis, indicating the potential for HGT (321, 416). The low-level vancomycin resistance caused by vanC is clinically important as isolates may be identified as vancomycin “susceptible” but could result in treatment failure (417).
Strains with the VanA phenotype possess inducible, high-level resistance to vancomycin (MIC ≥ 64 mg/L) and teicoplanin (MIC ≥ 16 mg/L). While not intrinsic to NFF enterococci, vanA has been identified in several NFF enterococcal species. A 2019 study in Japan identified vanA on a conjugative plasmid (pELF2), which was identified in E. faecium, E. gallinarum, and E. raffinosus and should be reported as resistant (418). Recently, there have been several reports of vanA-positive NFF enterococci causing outbreaks in hospitals, particularly E. raffinosus (61, 308, 353). Additionally, vanA-positive E. durans and E. dispar have been found in Belgium (354).
In the poultry industry, vanA-positive E. cecorum has been detected with a vancomycin MIC of > 256 mg/L and a teicoplanin MIC of 4 mg/L. The isolate also possessed Tn1546 on its chromosomal DNA (355). This case has raised concern about the possibility of poultry farms acting as a reservoir of antibiotic-resistant genes relevant to human health (419).
The vanD gene is chromosomally mediated and strongly associated with E. faecium. Isolates harboring vanD have moderate levels of vancomycin resistance (MIC range, 16–256 mg/L) and teicoplanin resistance (MIC range, 4–64 mg/L) (356). While rare, vanD has been found in E. avium, E. raffinosus, and E. gallinarum (310, 357, 358).
The vanN gene cluster has only recently been identified in E. faecium from both human and animal origin (359–361). However, these strains were identified as E. faecium clade B, which has now been reclassified as E. lactis (361), indicating that vanN is predominantly associated with E. lactis. VanN causes low-level vancomycin resistance (16 mg/L).
Tetracyclines
Tetracyclines have a broad spectrum of activity and low toxicity to the host and are therefore widely used to treat a range of infections. Tetracyclines are protein inhibitors and bind to the 30S ribosomal subunit, preventing the amino-acyl tRNA from binding to the ribosome. In enterococci, resistance to tetracycline can occur via two mechanisms: efflux pumps and ribosomal protection. Efflux pumps are encoded by plasmid-borne tetK and tetL and mediate the removal of tetracyclines (except for minocycline) from the bacterial cell (420). Ribosomal protection proteins are chromosomally encoded by tetM, tetO, and tetS and mediate the enzymatic alteration of ribosomal conformation and displace the tetracycline binding site (411) (Table 6). The three genes can be transferred via the Tn916 transposon and confer resistance to tetracycline, doxycycline, and minocycline (421, 422).
All five tet genes have been found in multiple NFF enterococci, including E. avium, E. casseliflavus, E. cecorum, E. durans, E. gallinarum, and E. hirae (105, 141, 362). Tetracycline resistance is frequently associated with NFF enterococci, with tetM being the most frequently detected AMR gene (105, 363) Tetracycline resistance has also been reported in E. dispar and E. pseudoavium, but the genetic resistance determinants were not investigated in the study (243).
Glycylcyclines
Glycylcyclines are derived from tetracycline and are designed to overcome resistance due to efflux pumps. Tigecycline is the most frequently used antibiotic in this class and is recommended for the treatment of VRE. Decreased susceptibility to tigecycline in enterococci is primarily related to target modification (rpsJ) and ribosomal protection proteins encoded by tetM and tetL (364–366). Tigecycline-resistant E. casseliflavus was initially isolated in Egypt with an MIC of 1.0 mg/L. The study investigated the effect of proton pump inhibitors (PPIs) on tigecycline resistance and showed that an increased dose of PPIs will substantially increase the tigecycline MICs by 4- to 128-fold (304). Another study has reported tigecycline non-susceptible isolates of E. gallinarum from poultry and of E. hirae from swine with MICs between 0.5 and 1.0 mg/L; however, the mechanism of resistance was not investigated (423). Similarly, the tetM and tetL genes have been detected in E. avium, E. casseliflavus, E. cecorum, E. durans, E. gallinarum, and E. hirae, but their association with glycylcycline resistance has not been investigated (105, 141, 362).
Aminomethylcyclines
Aminomethylcyclines are another tetracycline derivative and have a broad spectrum of activity against Gram-positive and Gram-negative bacteria, anaerobic bacteria, and atypical bacteria (424). Like tetracyclines, omadacycline inhibits bacterial protein synthesis by binding to the 30S ribosomal subunit; however, it still remains active against common tetracycline resistance mechanisms such as efflux pumps and ribosomal protection proteins. A recent study of 276 E. faecalis isolates showed that ribosomal protection protein gene, tetM, and the efflux pump genes, tetK and tetL, did not affect omadacycline (367). However, strains that had multiple mutations in the 16s rRNA genes demonstrated omadacycline resistance.
Among NFF enterococci, omadacycline susceptibility is rarely measured; however, antimicrobial susceptibility testing (AST) on NFF enterococcal isolates collected as part of the SENTRY antimicrobial surveillance program has not detected any omadacycline resistance (425, 426).
Macrolide-lincosamide-streptogramin B class antibiotics
The macrolide-lincosamide-streptogramin class (MLS) antibiotics are a class of structurally different but functionally similar class of antibiotics. Macrolides are characterized by a lactone ring with one or more attached deoxy sugars; lincosamides are alkyl derivatives of proline and consist of a pyrrolidine ring linked to a pyranose moiety via an amide bond, and streptogramins consist of naturally occurring cyclic peptide compounds that are grouped into two classes, group A and group B (427–429). MLS antibiotics bind to the 50S ribosomal subunit, preventing peptidyltransferase action and thereby blocking protein translation (430, 431). In enterococci, there are three mechanisms for the acquired resistance to MLS antibiotics: (i) modification of the drug target, (ii) inactivation/deactivation of the drug, and (iii) active efflux of the antibiotic (Table 6).
Target modification is the major mechanism of MLS resistance in enterococci. This primarily occurs via erythromycin methylases encoded by ermB or less commonly ermA and ermC, which act on specific residues in the 23S rRNA subunit. The modification of the target results in cross-resistance to all MLS antibiotics. In enterococci, ermB is responsible for approximately 80% of erythromycin resistance, followed by ermA and then ermC (432, 433). However, ermB is responsible for almost all high-level erythromycin resistance (MICs > 128 mg/L) and has been found in E. faecium, E. faecalis, E. durans, E. avium, E. hirae, and E. gallinarum (370). Quinupristin-dalfopristin (QD) (a streptogramin) resistance is also frequently associated with ermB carriage, and approximately 55% of all QD-resistant NFF enterococci are ermB positive (382). Macrolide resistance has been identified in E. avium and is associated with ermA and/or ermB (375–379). The erm genes are often found on conjugative plasmids, and efficient conjugative transfer of ermB from E. durans to E. faecium has been demonstrated in vitro (434).
Deactivation/inactivation of MLS antibiotics in enterococci occurs through phosphotransferases and nucleotidyltransferases. This mechanism of resistance is extremely rare in enterococci with only mphC (macrolide phosphotransferase), ereA (esterase), and lnuA/lnuB/linB (lincosamide nucleotidyltransferases) being infrequently detected in E. faecium. In recent studies, the genes have been prevalent among MLS-resistant strains of enterococci, with only ereA being identified in NFF species (380). Additionally, linB has been detected in E. gallinarum isolated from broiler chickens (381).
While target modification and drug inactivation usually result in resistance to only one antibiotic, the efflux pumps often confer resistance to multiple MLS antibiotics. Five genes are involved in the active efflux of MLS antibiotics: mefA, mefE, msrA, msrC, and mreA (370–374). The mefA, mefE, and mreA genes have been associated with macrolide resistance in enterococci, while msrA and mrsC have been associated with macrolide and streptogramin B resistance.
Additionally, MLS resistance can occur via vatD (formerly called satA) and vatE, which encode acetyltransferases that confer resistance to streptogramin A via an unknown mechanism (383). Although vatD and vatE are rarely found in NFF enterococci, the genes have been detected in three isolates of E. hirae, one isolate with vatD, and two isolates with vatE (382).
Finally, in enterococci, there exists a phenotype that exhibits cross-resistance to lincosamides, streptogramin A, and pleuromutilins (a class of protein synthesis inhibitors) known as the LSAP phenotype. This phenotype is shared with NFF enterococci, such as E. avium, E. gallinarum, and E. casseliflavus (435). In E. faecalis, intrinsic LSAP resistance is mediated by the ATP-binding cassette (ABC) protein LsaA. Disruption of the lsaA gene in E. faecalis is associated with a ≥40-fold decrease in MIC for QD, clindamycin, and dalfopristin. While not intrinsically resistant, E. faecium can acquire the LSAP phenotype via a point mutation in a lsaA homolog known as eatA (368, 369). Although it is infrequent, lsaA has been detected in some NFF enterococci, including E. gallinarum, E. hirae, and E. casseliflavus (436), while eatA appears to be unique to E. faecium (368).
Fluoroquinolones
Fluoroquinolones are broad-spectrum antibiotics that prevent DNA replication by inhibiting the ligase activity of the bacterial DNA gyrase and topoisomerase IV (437).
E. faecium and E. faecalis have low levels of intrinsic resistance to fluoroquinolones via the production of proteins in the Qnr family (encoded by qnr) (384, 385). The proteins protect the DNA gyrase and topoisomerase IV, thereby decreasing DNA binding of the quinolone and subsequently decreasing the formation of the quinolone–gyrase complex (386). In NFF enterococci, the qnr gene has only been reported in one study, in three isolates of E. casseliflavus (387) (Table 6).
High-level fluoroquinolone resistance can also occur through target modification and through the production of efflux pumps. Target modification can occur via mutations in the GyrA subunit of DNA gyrase (gyrA) and the ParC subunit of topoisomerase IV (parC), which reduce the antibiotic affinity for the target (388). Mutations in these two genes have not been reported in NFF enterococci.
The final mechanism of fluoroquinolone resistance is the production of efflux pumps. There are two efflux pumps that are associated with fluoroquinolone resistance in E. faecium and E. faecalis: the enterococcal multidrug resistance efflux pump (encoded by emeA) and an ABC family of efflux pumps encoded by erfAB (389–391). The two efflux pumps have not been identified in NFF enterococci.
While the genes associated with fluoroquinolone resistance have not been reported in NFF enterococci, ciprofloxacin resistance has been reported in NFF enterococcal species such as E. avium (375), E. dispar (243), and E. pseudoavium (243, 438, 439).
Lipopeptides
Lipopeptides are produced by other microorganisms and consist of a lipid connected to a peptide (440). While several lipopeptides are used as antibiotics, daptomycin is the only lipopeptide available for use in enterococci and is reserved as a last-line therapy for VRE. The mechanism of action of daptomycin remains unclear; however, it is known to disrupt the gram-positive bacterial cell membrane, leading to cell death (392). Genes associated with daptomycin resistance have not yet been defined, but potential candidates have been proposed in forced evolution studies (393, 394).
Although daptomycin resistance is rare in NFF enterococci (441), a study in 2019 found that 58.3% of E. hirae isolates (from a total of 84 isolates) obtained from water samples were resistant to daptomycin (MIC > 4 mg/L) (442). Daptomycin resistance in E. gallinarum and E. mundtii was also identified in the study.
Oxazolidinones
Oxazolidinones are synthetic molecules that bind to the bacterial 50S subunit and prevent protein translation. Linezolid was the first oxazolidinone to be approved in human health and is currently recommended for the treatment of Gram-positive infections, including VRE. Resistance to linezolid can occur through mutations in domain V of the 23S rRNA genes or through the acquisition of Cfr and Cfr-like methylases encoded by cfrA/B/C/D (443–445), and finally via ATP-binding cassette proteins encoded by optrA and poxtA (Table 6). The Cfr methylases confer resistance by post-translational methylation of the 23S rRNA, while OptrA and PoxtA confer resistance by a ribosomal protection mechanism (446, 447). In enterococci, these resistance genes have been found on mobile genetic elements and can be transferred between species via HGT (446, 448–450). Additionally, linezolid resistance can occur through mutations in the 23S rRNA, which reduces the affinity to the antibiotic; however, this has not been reported in NFF enterococci (451).
In recent years, linezolid resistance and linezolid-resistant genes have become increasingly prevalent among NFF enterococci. Resistance to linezolid has been reported in E. avium (314), E. casseliflavus (413), E. gallinarum, and E. hirae (413, 452, 453). Linezolid-resistant E. hirae isolated from animals have been reported with the G2576T point mutation in the V domain of the 23S rRNA (395). E. hirae harboring optrA and poxtA have also been detected in healthy humans (343). Similarly, poxtA2 (a poxtA ancestor) and optrA have been found in E. gallinarum isolated from swine (396, 399). The cfr gene has also been detected in E. casseliflavus, E. gallinarum, and E. avium (396–398). Additionally, a recently identified E. lactis isolated from pigs in China was found to be positive for a mobilizable poxtA (400).
CONCLUSION
The NFF enterococci include a diverse list of species that are ubiquitous in humans, animals, and the environment. While a less frequent cause of human infection compared to E. faecium and E. faecalis, NFF enterococci may cause a range of clinically significant diseases, which may not be routinely reported by the diagnostic microbiology laboratory. NFF enterococci may carry a range of virulence genes that are known to contribute to pathogenesis in E. faecium and E. faecalis; however, their role in NFF enterococci largely remains unstudied. Additionally, AMR genes for almost all known classes of antibiotics have been detected in NFF enterococci, and while it is not always clear if they result in phenotypic expression in these species, NFF enterococci may act as a reservoir for these genes. Historically, NFF enterococci have been overlooked, in part, due to the inability to characterize them in diagnostic microbiology laboratories. However, newer methods for identification, such as matrix-assisted laser desorption ionization–time of flight mass spectrometry, allow for classification at a species level. Consequently, the clinical significance and prevalence of previously unreported NFF enterococci species may alter in the future.
Biographies

Christopher A. Mullally is an early-career researcher at the School of Medical, Molecular, and Forensic Sciences at Murdoch University. He completed his PhD in Microbiology at the University of Western Australia in 2020. He currently holds two positions at Murdoch University, in the Neonatal Infection and Immunity group, and the Australian Group for Antimicrobial Resistance. His research focuses on sepsis pathogens with a special interest in diagnosis, prevention, and antimicrobial resistance.

Marhami Fahriani is a PhD candidate at the School of Medical, Molecular and Forensic Science, at Murdoch University. She received her medical degree at Universitas Syiah Kuala, Aceh, Indonesia and her Masters of Infectious Diseases at the School of Biomedical Science at The University of Western Australia, Perth, Australia. She is currently working on her doctoral thesis focusing on non-faecium non-faecalis enterococcal bloodstream infections.

Shakeel Mowlaboccus, Ph.D. is an early-career research scientist in the field of infectious diseases and antimicrobial resistance with over ten years of experience in microbiology, large-scale whole genome sequencing, bioinformatics analyses, molecular cloning, and protein expression. He currently works at Murdoch University in Western Australia where he specialises in understanding the molecular epidemiology and evolution of bacterial pathogens through state-of-the-art genomics. His research focuses on understanding the molecular mechanisms and the evolution of antimicrobial resistance in bacterial pathogens of public health concern including methicillin-resistant S. aureus, Enterococcus faecium, and Neisseria gonorrhoeae. Dr. Mowlaboccus is the curator of the Enterococcus faecium and Enterococcus faecalis sequence definition databases on the PubMLST website. He is also the current ASM International Young Ambassador representing Australia.

Geoffrey W. Coombs is the Chair of Public Health at the School of Veterinary and Life Sciences, Murdoch University and the Senior Clinical Scientist for PathWest Laboratory Medicine – WA, Fiona Stanley Hospital. At Murdoch University, he and his colleagues have established a three-million-dollar one health antimicrobial and infectious diseases research laboratory, which enables his group to perform basic and clinical antimicrobial resistance research on human and animal isolates. The laboratory has become the reference laboratory for the Australian Group on Antimicrobial Resistance (AGAR) Australian S. aureus (ASSOP) and Enterococcus (AESOP) Sepsis Onset Programs. During his professional career, he has received several awards including a Distinguished Service Award from the Australian Society for Microbiology (2016), a Royal College of Pathology of Australasia Faculty of Science Fellowship (2010), and a Chancellor’s Letter of Commendation for his PhD (2013).
Contributor Information
Christopher A. Mullally, Email: christopher.mullally@murdoch.edu.au.
Audrey N. Schuetz, Mayo Foundation for Medical Education and Research, Rochester, Minnesota, USA
Karen Carroll, Johns Hopkins University, Baltimore, Maryland, USA.
Vincent Cattoir, CHU de Rennes, Rennes, France.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/cmr.00121-23.
List of species in the Enterococcus genus.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Thiercelin M. 1899. Morphologie et modes de reproduction de l'enterocoque, p 551–553. In Comptes Rendus des Seances de la Societe de Biologie et des ses Filiales. Vol 11. [Google Scholar]
- 2. Schleifer KH, Kilpper-Balz R. 1984. Transfer of Streptococcus faecalis and Streptococcus faecium to the genus Enterococcus nom. rev. as Enterococcus faecalis comb. nov. and Enterococcus faecium comb. nov. Int J Syst Bacteriol 34:31–34. doi: 10.1099/00207713-34-1-31 [DOI] [Google Scholar]
- 3. Collins MD, Jones D, Farrow JAE, Kilpper-Balz R, Schleifer KH. 1984. Enterococcus avium nom. rev., comb. nov.; E. casseliflavus nom. rev., comb. nov.; E. durans nom. rev., comb. nov.; E. gallinarum comb. nov.; and E. malodoratus sp. nov. Int J Syst Evol Microbiol 34:220–223. doi: 10.1099/00207713-34-2-220 [DOI] [Google Scholar]
- 4. Semedo T, Almeida Santos M, Martins P, Silva Lopes MF, Figueiredo Marques JJ, Tenreiro R, Barreto Crespo MT. 2003. Comparative study using type strains and clinical and food isolates to examine hemolytic activity and occurrence of the cyl operon in enterococci. J Clin Microbiol 41:2569–2576. doi: 10.1128/JCM.41.6.2569-2576.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rogers DG, Zeman DH, Erickson ED. 1992. Diarrhea associated with Enterococcus durans in calves. J Vet Diagn Invest 4:471–472. doi: 10.1177/104063879200400423 [DOI] [PubMed] [Google Scholar]
- 6. Gomes BC, Esteves CT, Palazzo ICV, Darini ALC, Felis GE, Sechi LA, Franco B, De Martinis ECP. 2008. Prevalence and characterization of Enterococcus spp. isolated from Brazilian foods. Food Microbiol 25:668–675. doi: 10.1016/j.fm.2008.03.008 [DOI] [PubMed] [Google Scholar]
- 7. Teixeira LM, Carvalho MdGS, Facklam RR, Shewmaker PL, et al. 2023. Enterococcus, p 449–469. In Carroll KC, Jorgensen JH, Guido F, Pfaller MA, Landry ML, Richter SR, Warnock DW (ed), Manual of clinical Microbiology, 13th ed [Google Scholar]
- 8. Martin JD, Mundt JO. 1972. Enterococci in insects. Appl Microbiol 24:575–580. doi: 10.1128/am.24.4.575-580.1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mundt J. 1986. Enterococci, p 1063–1065. In Bergey's Manual of systematic Bacteriology [Google Scholar]
- 10. Mundt JO. 1963. Occurrence of enterococci in animals in a wild environment. Appl Microbiol 11:136–140. doi: 10.1128/am.11.2.136-140.1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schoch CL, Ciufo S, Domrachev M, Hotton CL, Kannan S, Khovanskaya R, Leipe D, Mcveigh R, O’Neill K, Robbertse B, Sharma S, Soussov V, Sullivan JP, Sun L, Turner S, Karsch-Mizrachi I. 2020. NCBI taxonomy: a comprehensive update on curation, resources and tools. Database (Oxford) 2020:baaa062. doi: 10.1093/database/baaa062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manero A, Blanch AR. 1999. Identification of Enterococcus spp. with a biochemical key. Appl Environ Microbiol 65:4425–4430. doi: 10.1128/AEM.65.10.4425-4430.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reuter G. 1992. Culture media for enterococci and group D-streptococci. Int J Food Microbiol 17:101–111. doi: 10.1016/0168-1605(92)90109-g [DOI] [PubMed] [Google Scholar]
- 14. Leclerc H, Devriese LA, Mossel DA. 1996. Taxonomical changes in intestinal (faecal) enterococci and streptococci: consequences on their use as indicators of faecal contamination in drinking water. J Appl Bacteriol 81:459–466. doi: 10.1111/j.1365-2672.1996.tb03533.x [DOI] [PubMed] [Google Scholar]
- 15. Müller T, Ulrich A, Ott EM, Müller M. 2001. Identification of plant‐associated enterococci. J Appl Microbiol 91:268–278. doi: 10.1046/j.1365-2672.2001.01373.x [DOI] [PubMed] [Google Scholar]
- 16. Gilmore MS, Clewell DB, Courvalin P, Dunny GM, Murray BE, Rice LB. 2002. The enterococci: pathogenesis, molecular biology, and antibiotic resistance. ASM press, Washington, DC. [Google Scholar]
- 17. Lebreton F, Willems RJ, Gilmore MS. 2014. Enterococcus diversity, origins in nature, and gut colonization. In Enterococci: from commensals to leading causes of drug resistant infection [PubMed] [Google Scholar]
- 18. Zhong Z, Zhang W, Song Y, Liu W, Xu H, Xi X, Menghe B, Zhang H, Sun Z. 2017. Comparative genomic analysis of the genus Enterococcus. Microbiol Res 196:95–105. doi: 10.1016/j.micres.2016.12.009 [DOI] [PubMed] [Google Scholar]
- 19. Paulsen IT, Banerjei L, Myers GSA, Nelson KE, Seshadri R, Read TD, Fouts DE, Eisen JA, Gill SR, Heidelberg JF, et al. 2003. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 299:2071–2074. doi: 10.1126/science.1080613 [DOI] [PubMed] [Google Scholar]
- 20. Tan C-K, Lai C-C, Wang J-Y, Lin S-H, Liao C-H, Huang Y-T, Wang C-Y, Lin H-I, Hsueh P-R. 2010. Bacteremia caused by non-faecalis and non-faecium Enterococcus species at a medical center in Taiwan, 2000 to 2008. J Infect 61:34–43. doi: 10.1016/j.jinf.2010.04.007 [DOI] [PubMed] [Google Scholar]
- 21. Coombs GW, Daley DA, Yee NWT, Shoby P, Mowlaboccus S. 2022. Australian group on antimicrobial resistance (AGAR) Australian enterococcal sepsis outcome programme (AESOP) annual report 2020. Commun Dis Intell (2018) 46. doi: 10.33321/cdi.2022.46.17 [DOI] [PubMed] [Google Scholar]
- 22. Weiner-Lastinger LM, Abner S, Benin AL, Edwards JR, Kallen AJ, Karlsson M, Magill SS, Pollock D, See I, Soe MM, Walters MS, Dudeck MA. 2020. Antimicrobial-resistant pathogens associated with pediatric healthcare-associated infections: summary of data reported to the National Healthcare Safety Network, 2015-2017. Infect Control Hosp Epidemiol 41:19–30. doi: 10.1017/ice.2019.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weiner-Lastinger LM, Abner S, Edwards JR, Kallen AJ, Karlsson M, Magill SS, Pollock D, See I, Soe MM, Walters MS, Dudeck MA. 2020. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: summary of data reported to the National Healthcare Safety Network, 2015-2017. Infect Control Hosp Epidemiol 41:1–18. doi: 10.1017/ice.2019.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heffernan H, Woodhouse R, Maitra A. 2020. Vancomycin-resistant enterococci. Wellington, New Zealand. https://www.esr.cri.nz/our-research/nga-kete/infectious-disease-intelligence/antimicrobial-resistance-amr/. [Google Scholar]
- 25. Palomar M, Alvarez F, Olaechea P, Gimeno R, Gracia M, Seijas I. 2017. Estudio nacional de vigilancia de infección nosocomial en servicios de medicina intensiva. Sociedad española de medicina intensiva crítica y unidades coronarias. https://hws.vhebron.net/envin-helics/Help/Informe%20ENVIN-UCI%202017.pdf. [Google Scholar]
- 26. Palomar M, Alvarez F, Olaechea P, Gimeno R, Gracia M, Seijas I. 2018. Estudio nacional de vigilancia de infección nosocomial en servicios de medicina intensiva. Sociedad española de medicina intensiva crítica y unidades coronarias. https://hws.vhebron.net/envin-helics/Help/Informe%20ENVIN-UCI%202018.pdf. [Google Scholar]
- 27. Palomar M, Alvarez F, Olaechea P, Gimeno R, Gracia M, Seijas I. 2019. Estudio nacional de vigilancia de infección nosocomial en servicios de medicina intensiva. Sociedad española de medicina intensiva crítica y unidades coronarias. https://hws.vhebron.net/envin-helics/Help/Informe%20ENVIN-UCI%202019.pdf. [Google Scholar]
- 28. Palomar M, Alvarez F, Olaechea P, Gimeno R, Gracia M, Seijas I. 2020. Estudio nacional de vigilancia de infección nosocomial en servicios de medicina intensiva. Sociedad española de medicina intensiva crítica y unidades coronarias. https://hws.vhebron.net/envin-helics/Help/Informe%20ENVIN-UCI%202020.pdf. [Google Scholar]
- 29. Palomar M, Alvarez F, Olaechea P, Gimeno R, Gracia M, Seijas I. 2021. Estudio nacional de vigilancia de infección nosocomial en servicios de medicina intensiva. Sociedad española de medicina intensiva crítica y unidades coronarias. https://hws.vhebron.net/envin-helics/Help/Informe%20ENVIN-UCI%202021.pdf. [Google Scholar]
- 30. Simonsen GS, Urdahl AM. 2015. NORM/NORM-VET 2015-usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway. https://munin.uit.no/handle/10037/19655.
- 31. Simonsen GS, Urdahl AM. 2016. NORM/NORM-VET 2016-usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway. https://www.vetinst.no/en/surveillance-programmes/norm-norm-vet-report.
- 32. Simonsen GS, Urdahl AM. 2017. NORM/NORM-VET 2017-usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway. https://www.vetinst.no/en/surveillance-programmes/norm-norm-vet-report.
- 33. Simonsen GS, Urdahl AM. 2018. NORM/NORM-VET 2018-usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway. https://www.vetinst.no/en/surveillance-programmes/norm-norm-vet-report.
- 34. Simonsen GS, Urdahl AM. 2019. NORM/NORM-VET 2019-usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway. https://www.vetinst.no/en/surveillance-programmes/norm-norm-vet-report.
- 35. Simonsen GS, Urdahl AM. 2020. NORM/NORM-VET 2020-usage of antimicrobial agents and occurrence of antimicrobial resistance in Norway. https://www.vetinst.no/en/surveillance-programmes/norm-norm-vet-report.
- 36. Ministry of Health LaW . 2021. Japan Nosocomial Infections Surveillance (JANIS). Japan. https://janis.mhlw.go.jp/english/report/open_report/2018/3/1/ken_Open_Report_Eng_201800_clsi2012.pdf. [Google Scholar]
- 37. Ministry of Health LaW . 2018. Japan Nosocomial Infections Surveillance (JANIS). Japan. https://janis.mhlw.go.jp/english/report/open_report/2019/3/1/ken_Open_Report_Eng_201900_clsi2012.pdf. [Google Scholar]
- 38. Ministry of Health LaW . 2019. Japan Nosocomial Infections Surveillance (JANIS). Japan. https://janis.mhlw.go.jp/english/report/open_report/2020/3/1/ken_Open_Report_Eng_202000_clsi2012.pdf. [Google Scholar]
- 39. Ministry of Health LaW . 2020. Japan Nosocomial Infections Surveillance (JANIS). Japan. https://janis.mhlw.go.jp/english/report/open_report/2021/3/1/ken_Open_Report_Eng_202100_clsi2012.pdf. [Google Scholar]
- 40. Kim EJ, Kwak YG, Kim TH, Lee MS, Lee S-O, Kim SR, Park SH, Ahn JY, Yun NR, Ryu SY, Kim ES, Choi J, Yoo HM, Shin MJ, Yoo S-Y, Hong KH, Moon H-W, Cho N, Son HJ, Kim SH, Choi YH, Kim M-N. 2019. Korean national healthcare-associated infections surveillance system, intensive care unit module report: summary of data from July 2017 through June 2018. Korean J Healthc Assoc Infect Control Prev 24:69. doi: 10.14192/kjicp.2019.24.2.69 [DOI] [Google Scholar]
- 41. Kim EJ, Kwak YG, Kwak SH, Ko SH, Kim JH, Kim ES, Kim TH, Ryu SY, Moon H-W, Park SH, Ahn JY, Yoo S-Y, Yoo HM, Yun NR, Lee MS, Lee S-O, Cho N, Choi J, Hong KH, Choi YH, Kim M-N. 2020. Korean national healthcare-associated infections surveillance system, intensive care unit module report: summary of data from July 2018 to June 2019. Korean J healthc assoc Infect Control Prev 25:115–127. doi: 10.14192/kjicp.2020.25.2.115 [DOI] [Google Scholar]
- 42. Kim EJ, Kwak YG, Kwak SH, Ko SH, Kweon OM, Kim ES, Kim JH, Kim TH, Kim TS, Moon H-W, Park SH, Ahn JY, Yoo S-Y, Yoo HM, Lee S-O, Cho N-H, Choe PG, Hong KH, Lee Y-M, Lee MS. 2021. Korean national healthcare-associated infections surveillance system, intensive care unit module report: summary of data from July 2019 through June 2020. Korean J healthc assoc Infect Control Prev 26:115–128. doi: 10.14192/kjicp.2021.26.2.115 [DOI] [Google Scholar]
- 43. Kuzmenkov AY, Trushin IV, Vinogradova AG, Avramenko AA, Sukhorukova MV, Malhotra-Kumar S, Dekhnich AV, Edelstein MV, Kozlov RS. 2021. AMRmap: an interactive web platform for analysis of antimicrobial resistance surveillance data in Russia. Front Microbiol 12:620002. doi: 10.3389/fmicb.2021.620002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Musicha P, Cornick JE, Bar-Zeev N, French N, Masesa C, Denis B, Kennedy N, Mallewa J, Gordon MA, Msefula CL, Heyderman RS, Everett DB, Feasey NA. 2017. Trends in antimicrobial resistance in bloodstream infection isolates at a large urban hospital in Malawi (1998–2016): a surveillance study. Lancet Infect Dis 17:1042–1052. doi: 10.1016/S1473-3099(17)30394-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Coombs GW, Pearson JC, Daly DA, Le TT, Robinson JO, Gottlieb T, Howden BP, Johnson PDR, Bennett CM, Stinear TP, Turnidge JD, Australian Group on Antimicrobial Resistance . 2014. Australian enterococcal sepsis outcome programme annual report, 2013. Commun Dis Intell Q Rep 38:E320–E326. [DOI] [PubMed] [Google Scholar]
- 46. Coombs GW, Daley DA, Thin Lee Y, Pang S, Pearson JC, Robinson JO, Johnson PD, Kotsanas D, Bell JM, Turnidge JD, Australian Group on Antimicrobial Resistance . 2016. Australian group on antimicrobial resistance Australian enterococcal sepsis outcome programme annual report, 2014. Commun Dis Intell Q Rep 40:E236–E243. [DOI] [PubMed] [Google Scholar]
- 47. Coombs GW, Daley DA, Lee YT, Pang S, Bell JM, Turnidge JD, Australian Group on Antimicrobial Resistance . 2018. Australian group on antimicrobial resistance (AGAR) Australian enterococcal sepsis outcome programme (AESOP) annual report 2015. Commun Dis Intell (2018) 42:S2209-6051(18)00015-5. [DOI] [PubMed] [Google Scholar]
- 48. Coombs GW, Daley DA, Lee YT, Pang S, Australian Group on Antimicrobial Resistance . 2018. Australian group on antimicrobial resistance (AGAR) Australian enterococcal sepsis outcome programme (AESOP) annual report 2016. Commun Dis Intell (2018) 42:S2209-6051(18)00020-9. [DOI] [PubMed] [Google Scholar]
- 49. Coombs GW, Daley DA, Lee YT, Pang S. 2019. Australian group on antimicrobial resistance (AGAR) Australian enterococcal sepsis outcome programme (AESOP) annual report 2017. Commun Dis Intell (2018) 43. doi: 10.33321/cdi.2019.43.42 [DOI] [PubMed] [Google Scholar]
- 50. Coombs GW, Daley DA, Lee YT, Pang S, Bell JM, Turnidge JD, Australian Group on Antimicrobial Resistance . 2020. Australian group on antimicrobial resistance (AGAR) Australian enterococcal sepsis outcome programme (AESOP) annual report 2018. Commun Dis Intell (2018) 44. doi: 10.33321/cdi.2020.44.19 [DOI] [PubMed] [Google Scholar]
- 51. Coombs GW, Daley DA, Mowlaboccus S, Pang S, Australian Group on Antimicrobial Resistance . 2020. Australian group on antimicrobial resistance (AGAR) Australian enterococcal sepsis outcome programme (AESOP) annual report 2019. Commun Dis Intell (2018) 44. doi: 10.33321/cdi.2020.44.19 [DOI] [PubMed] [Google Scholar]
- 52. Coombs GW, Daley DA, Shoby P, Mowlaboccus S. 2018. Australian group on antimicrobial resistance (AGAR) Australian enterococcal surveillance outcome program (AESOP). Commun Dis Intell (2018) 46. doi: 10.33321/cdi.2022.46.77 [DOI] [PubMed] [Google Scholar]
- 53. Coombs GW, Daley DA, Shoby P, Mowlaboccus S. 2023. Australian group on antimicrobial resistance (AGAR) Australian enterococcal surveillance outcome program (AESOP) bloodstream infection annual report 2022. Commun Dis Intell (2018) 47. doi: 10.33321/cdi.2023.47.68 [DOI] [PubMed] [Google Scholar]
- 54. Ruoff KL, de la Maza L, Murtagh MJ, Spargo JD, Ferraro MJ. 1990. Species identities of enterococci isolated from clinical specimens. J Clin Microbiol 28:435–437. doi: 10.1128/jcm.28.3.435-437.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Coombs GW, Daley DA, Yee NW, Mowlaboccus S. 2020. Australian group on antimicrobial resistance (AGAR) Australian enterococcal sepsis outcome programme (AESOP) annual report 2020. Commun Dis Intell (2018) 46. doi: 10.33321/cdi.2022.46.17 [DOI] [PubMed] [Google Scholar]
- 56. Amaro P, Ferreira J, Viegas R, Cardoso A, Correia J, Maurício H. 2021. Multifocal joint infection caused by Enterococcus gallinarum. Mod Rheumatol Case Rep 5:384–386. doi: 10.1080/24725625.2020.1847429 [DOI] [PubMed] [Google Scholar]
- 57. Zhao B, Ye MS, Zheng R. 2018. Enterococcus gallinarum meningitis: a case report and literature review. BMC Infect Dis 18:231. doi: 10.1186/s12879-018-3151-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Teng TZJ, Shelat VG. 2022. Enterococcus gallinarum causing acute cholangitis in an immunocompetent patient. Surg Infect (Larchmt) 23:93–94. doi: 10.1089/sur.2021.209 [DOI] [PubMed] [Google Scholar]
- 59. Shahin AV, Elkhayat AI, Greene JN. 2020. Endogenous vancomycin-resistant Enterococcus gallinarum endophthalmitis in hematologic malignancy. Infect Dis Clin Pract 28:301–304. doi: 10.1097/IPC.0000000000000856 [DOI] [Google Scholar]
- 60. Choi S-H, Lee S-O, Kim TH, Chung J-W, Choo EJ, Kwak YG, Kim M-N, Kim YS, Woo JH, Ryu J, Kim NJ. 2004. Clinical features and outcomes of bacteremia caused by Enterococcus casseliflavus and Enterococcus gallinarum: analysis of 56 cases. Clin Infect Dis 38:53–61. doi: 10.1086/380452 [DOI] [PubMed] [Google Scholar]
- 61. Lee YW, Lim SY, Jung J, Kim MJ, Chong YP, Kim S-H, Lee S-O, Kim YS, Choi S-H. 2022. Enterococcus raffinosus bacteremia: clinical experience with 49 adult patients. Eur J Clin Microbiol Infect Dis 41:415–420. doi: 10.1007/s10096-021-04389-x [DOI] [PubMed] [Google Scholar]
- 62. Ryu B-H, Hong J, Jung J, Kim MJ, Sung H, Kim M-N, Chong YP, Kim S-H, Lee S-O, Kim YS, Woo JH, Choi S-H. 2019. Clinical characteristics and treatment outcomes of Enterococcus durans bacteremia: a 20-year experience in a tertiary care hospital. Eur J Clin Microbiol Infect Dis 38:1743–1751. doi: 10.1007/s10096-019-03605-z [DOI] [PubMed] [Google Scholar]
- 63. Nowlan SS, Deibel RH. 1967. Group Q streptococci I. Ecology, serology, physiology, and relationship to established enterococci. J Bacteriol 94:291–296. doi: 10.1128/jb.94.2.291-296.1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mellman RL, Spisak GM, Burakoff R. 1992. Enterococcus avium bacteremia in association with ulcerative colitis. Am J Gastroenterol 87:375–378. [PubMed] [Google Scholar]
- 65. Ablaza VJ, LaTrenta GS. 1998. Late infection of a breast prosthesis with Enterococcus avium. Plast Reconstr Surg 102:227–230. doi: 10.1097/00006534-199807000-00037 [DOI] [PubMed] [Google Scholar]
- 66. Shinagawa N, Taniguchi M, Hirata K, Furuhata T, Fukuhara K, Mizugucwi T, Osanai H, Yanai Y, Hata F, Kihara C, et al. 2014. Bacteria isolated from surgical infections and its susceptibilities to antimicrobial agents--special references to bacteria isolated between April 2010 and March 2011. Jpn J Antibiot 67:293–334. [PubMed] [Google Scholar]
- 67. Escribano JA, Solivera J, Vidal E, Rivin E, Lozano J. 2013. Otogenic cerebellar abscess by Enterococcus avium, a very rare infectious agent. J Neurol Surg A Cent Eur Neurosurg 74:e155–e158. doi: 10.1055/s-0032-1333418 [DOI] [PubMed] [Google Scholar]
- 68. Park SY, Park KH, Cho YH, Choi SH. 2013. Brain abscess caused by Enterococcus avium: a case report and review of the literature. Infect Chemother 45:335–338. doi: 10.3947/ic.2013.45.3.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yıldırmak T, Gedik H, Simşek F, Kantürk A. 2014. Community-acquired intracranial suppurative infections: a 15-year report. Surg Neurol Int 5:142. doi: 10.4103/2152-7806.141891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fujimoto C, Yazawa S, Matsuoka F, Ishihara A, Hayami M, Kawasaki S. 2002. Bacterial meningoencephalitis in patients undergoing chronic hemodialysis: two case reports. Clin Neurol Neurosurg 104:64–68. doi: 10.1016/s0303-8467(01)00182-2 [DOI] [PubMed] [Google Scholar]
- 71. Jones S, England R, Evans M, Soo SS, Venkatesan P. 2007. Microbiologically confirmed meningoencephalitis due to Enterococcus avium: a first report. J Infect 54:e129–e131. doi: 10.1016/j.jinf.2006.08.012 [DOI] [PubMed] [Google Scholar]
- 72. Pérez-Castrillon JL, Martín-Luquero M, Martín-Escudero JC, Pascual P, Casero A, Herreros V. 1997. Endocarditis caused by Enterococcus avium. Scand J Infect Dis 29:530. doi: 10.3109/00365549709011873 [DOI] [PubMed] [Google Scholar]
- 73. Swaminathan S, Ritter SB. 1999. Enterococcus avium endocarditis in an infant with tetralogy of fallot. Pediatr Cardiol 20:227–228. doi: 10.1007/s002469900449 [DOI] [PubMed] [Google Scholar]
- 74. Okada A, Hangai M, Oda T. 2015. Bacteremia with an iliopsoas abscess and osteomyelitis of the femoral head caused by Enterococcus avium in a patient with end-stage kidney disease. Intern Med 54:669–674. doi: 10.2169/internalmedicine.54.3576 [DOI] [PubMed] [Google Scholar]
- 75. Nakou M, Kamma J, Gargalianos P, Laskaris G, Mitsis F. 1997. Periodontal microflora of HIV infected patients with periodontitis. Anaerobe 3:97–102. doi: 10.1006/anae.1997.0081 [DOI] [PubMed] [Google Scholar]
- 76. Paju S, Bernstein JM, Haase EM, Scannapieco FA. 2003. Molecular analysis of bacterial flora associated with chronically inflamed maxillary sinuses. J Med Microbiol 52:591–597. doi: 10.1099/jmm.0.05062-0 [DOI] [PubMed] [Google Scholar]
- 77. Abo-Zed A, Hegazy S, Phan T. 2022. Detection of Enterococcus avium in a case of urinary tract infection and haematuria. Access Microbiol 4:acmi000349. doi: 10.1099/acmi.0.000349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Naser SM, Vancanneyt M, De Graef E, Devriese LA, Snauwaert C, Lefebvre K, Hoste B, Švec P, Decostere A, Haesebrouck F, Swings J. 2005. Enterococcus canintestini sp. nov., from faecal samples of healthy dogs. Int J Syst Evol Microbiol 55:2177–2182. doi: 10.1099/ijs.0.63752-0 [DOI] [PubMed] [Google Scholar]
- 79. Bhagwat A, Annapure US. 2019. In vitro assessment of metabolic profile of Enterococcus strains of human origin. J Genet Eng Biotechnol 17:11. doi: 10.1186/s43141-019-0009-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Suzuki H, Hase R, Otsuka Y, Hosokawa N. 2017. A 10-year profile of enterococcal bloodstream infections at a tertiary-care hospital in Japan. J Infect Chemother 23:390–393. doi: 10.1016/j.jiac.2017.03.009 [DOI] [PubMed] [Google Scholar]
- 81. Gascón F, Castaño MA, González A, Cordón MD. 2003. Endocarditis due to Enterococcus casseliflavus. Enferm Infecc Microbiol Clin 21:275–276. doi: 10.1016/s0213-005x(03)72937-1 [DOI] [PubMed] [Google Scholar]
- 82. Okumura N, Watanabe T, Teranishi S, Suzuki D, Hashimoto T, Takahashi K, Hara T. 2021. Successful treatment of aortic valve endocarditis caused by Enterococcus casseliflavus: a case report. BMC Infect Dis 21:447. doi: 10.1186/s12879-021-06160-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Motie I, Burns K, Thompson R, Friar E, Bermingham I, Ranasinghe U, Wiese-Rometsch W. 2022. Acinetobacter radioresistens and Enterococcus casseliflavus co-infection with endocarditis, bacteremia, and pneumonia. IDCases 30:e01622. doi: 10.1016/j.idcr.2022.e01622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Monticelli J, Knezevich A, Luzzati R, Di Bella S. 2018. Clinical management of non-faecium non-faecalis vancomycin-resistant enterococci infection. Focus on Enterococcus gallinarum and Enterococcus casseliflavus/flavescens. J Infect Chemother 24:237–246. doi: 10.1016/j.jiac.2018.01.001 [DOI] [PubMed] [Google Scholar]
- 85. Khurana RN, Leder HA, Nguyen QD, Do DV. 2009. Enterococcus casseliflavus endophthalmitis associated with a horse tail injury. Arch Ophthalmol 127:1551–1552. doi: 10.1001/archophthalmol.2009.282 [DOI] [PubMed] [Google Scholar]
- 86. Sambhav K, Mathai A, Reddy AK, Reddy BV, Bhatia K, Balne PK. 2011. Endogenous endophthalmitis caused by Enterococcus casseliflavus. J Med Microbiol 60:670–672. doi: 10.1099/jmm.0.016675-0 [DOI] [PubMed] [Google Scholar]
- 87. Berenger BM, Kulkarni S, Hinz BJ, Forgie SE. 2015. Exogenous endophthalmitis caused by Enterococcus casseliflavus: a case report and discussion regarding treatment of intraocular infection with vancomycin-resistant enterococci. Can J Infect Dis Med Microbiol 26:330–332. doi: 10.1155/2015/784910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Low JR, Teoh CS, Chien JMF, Huang EH. 2015. Enterococcus casseliflavus endophthalmitis due to metallic intraocular foreign body. Eye (Lond) 29:840–841. doi: 10.1038/eye.2014.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nguyen J, Hartnett ME. 2017. Successful management of post-traumatic vancomycin-resistant Enterococcus endophthalmitis. Am J Ophthalmol Case Rep 5:117–118. doi: 10.1016/j.ajoc.2016.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bao QD, Liu TX, Xie M, Tian X. 2019. Exogenous endophthalmitis caused by Enterococcus casseliflavus: a case report. World J Clin Cases 7:3904–3911. doi: 10.12998/wjcc.v7.i22.3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Dave VP, Pathengay A, Braimah IZ, Panchal B, Sharma S, Pappuru RR, Mathai A, Tyagi M, Narayanan R, Jalali S, Das T. 2020. Enterococcus endophthalmitis: clinical settings, antimicrobial susceptibility, and management outcomes. Retina 40:898–902. doi: 10.1097/IAE.0000000000002462 [DOI] [PubMed] [Google Scholar]
- 92. Stephens A, Sivapathasuntharam C, James HK. 2021. Monocular loss of vision following an open tibial fracture: a case of Enterococcus casseliflavus endogenous endophthalmitis. BMJ Case Rep 14:e241292. doi: 10.1136/bcr-2020-241292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Iaria C, Stassi G, Costa GB, Di Leo R, Toscano A, Cascio A. 2005. Enterococcal meningitis caused by Enterococcus casseliflavus. First case report. BMC Infect Dis 5:3. doi: 10.1186/1471-2334-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Li MC, Guo HC, Chen G, Kong F, Zhang QH. 2011. Meningitis caused by Enterococcus casseliflavus with refractory cerebrospinal fluid leakage following endoscopic endonasal removal of skull base chondrosarcoma. Chin Med J (Engl) 124:3440. doi: 10.3760/cma.j.issn.0366-6999.2011.20.045 [DOI] [PubMed] [Google Scholar]
- 95. Stefanetti V, Beccati F, Passamonti F, Sgariglia E, Coletti M, Vuerich M, Marenzoni ML. 2016. Detection and DNA quantification of Enterococcus casseliflavus in a foal with septic meningitis. J Am Vet Med Assoc 249:96–100. doi: 10.2460/javma.249.1.96 [DOI] [PubMed] [Google Scholar]
- 96. Devriese LA, Dutta GN, Farrow JAE, Van De Kerckhove A, Phillips BA. 1983. Streptococcus cecorum, a new species isolated from chickens. Int J Syst Bacteriol 33:772–776. doi: 10.1099/00207713-33-4-772 [DOI] [Google Scholar]
- 97. Greub G, Devriese LA, Pot B, Dominguez J, Bille J. 1997. Enterococcus cecorum septicemia in a malnourished adult patient. Eur J Clin Microbiol Infect Dis 16:594–598. doi: 10.1007/BF02447923 [DOI] [PubMed] [Google Scholar]
- 98. De Baere T, Claeys G, Verschraegen G, Devriese LA, Baele M, Van Vlem B, Vanholder R, Dequidt C, Vaneechoutte M. 2000. Continuous ambulatory peritoneal dialysis peritonitis due to Enterococcus cecorum. J Clin Microbiol 38:3511–3512. doi: 10.1128/JCM.38.9.3511-3512.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hsueh P-R, Teng L-J, Chen Y-C, Yang P-C, Ho S-W, Luh K-T. 2000. Recurrent bacteremic peritonitis caused by Enterococcus cecorum in a patient with liver cirrhosis. J Clin Microbiol 38:2450–2452. doi: 10.1128/JCM.38.6.2450-2452.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Woo PCY, Tam DMW, Lau SKP, Fung AMY, Yuen K-Y. 2004. Enterococcus cecorum empyema thoracis successfully treated with cefotaxime. J Clin Microbiol 42:919–922. doi: 10.1128/JCM.42.2.919-922.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ahmed FZ, Baig MW, Gascoyne-Binzi D, Sandoe JAT. 2011. Enterococcus cecorum aortic valve endocarditis. Diagn Microbiol Infect Dis 70:525–527. doi: 10.1016/j.diagmicrobio.2011.04.001 [DOI] [PubMed] [Google Scholar]
- 102. Hsu H, Seah V, Marriott D, Moore J. 2022. Enterococcus cecorum infective endocarditis in a patient with chronic myeloid leukaemia and cirrhosis. Pathology 54:495–497. doi: 10.1016/j.pathol.2021.07.004 [DOI] [PubMed] [Google Scholar]
- 103. Perdigão Neto LV, Medeiros M, Ferreira SC, Nishiya AS, de Assis DB, Boszczowski Í, Costa SF, Levin AS. 2021. Polymerase chain reaction targeting 16S ribosomal RNA for the diagnosis of bacterial meningitis after neurosurgery. Clinics (Sao Paulo) 76:e2284. doi: 10.6061/clinics/2021/e2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Delaunay E, Abat C, Rolain J-M. 2015. Enterococcus cecorum human infection, France. New Microbes New Infect 7:50–51. doi: 10.1016/j.nmni.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cauwerts K, Decostere A, De Graef EM, Haesebrouck F, Pasmans F. 2007. High prevalence of tetracycline resistance in Enterococcus isolates from broilers carrying the erm(B) gene. Avian Pathol 36:395–399. doi: 10.1080/03079450701589167 [DOI] [PubMed] [Google Scholar]
- 106. Jackson CR, Kariyawasam S, Borst LB, Frye JG, Barrett JB, Hiott LM, Woodley TA. 2015. Antimicrobial resistance, virulence determinants and genetic profiles of clinical and nonclinical Enterococcus cecorum from poultry. Lett Appl Microbiol 60:111–119. doi: 10.1111/lam.12374 [DOI] [PubMed] [Google Scholar]
- 107. Borst LB, Suyemoto MM, Sarsour AH, Harris MC, Martin MP, Strickland JD, Oviedo EO, Barnes HJ. 2017. Pathogenesis of enterococcal spondylitis caused by Enterococcus cecorum in broiler chickens. Vet Pathol 54:61–73. doi: 10.1177/0300985816658098 [DOI] [PubMed] [Google Scholar]
- 108. Stępień-Pyśniak D, Marek A, Banach T, Adaszek Ł, Pyzik E, Wilczyński J, Winiarczyk S. 2016. Prevalence and antibiotic resistance of Enterococcus strains isolated from poultry. Acta Vet Hung 64:148–163. doi: 10.1556/004.2016.016 [DOI] [PubMed] [Google Scholar]
- 109. Suyemoto MM, Barnes HJ, Borst LB. 2017. Culture methods impact recovery of antibiotic-resistant enterococci including Enterococcus cecorum from pre- and postharvest chicken. Lett Appl Microbiol 64:210–216. doi: 10.1111/lam.12705 [DOI] [PubMed] [Google Scholar]
- 110. Jung A, Chen LR, Suyemoto MM, Barnes HJ, Borst LB. 2018. A review of Enterococcus cecorum infection in poultry. Avian Dis 62:261–271. doi: 10.1637/11825-030618-Review.1 [DOI] [PubMed] [Google Scholar]
- 111. Sharma P, Gupta SK, Barrett JB, Hiott LM, Woodley TA, Kariyawasam S, Frye JG, Jackson CR. 2020. Comparison of antimicrobial resistance and pan-genome of clinical and non-clinical Enterococcus cecorum from poultry using whole-genome sequencing. Foods 9:686. doi: 10.3390/foods9060686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Avberšek J, Mićunović J, Šemrov N, Ocepek M. 2021. Surveillance of the source of poultry infections with Enterococcus hirae and Enterococcus cecorum in Slovenia and E. hirae antibiotic resistance patterns. New Microbiol 44:210–216. [PubMed] [Google Scholar]
- 113. Hankel J, Bodmann B, Todte M, Galvez E, Strowig T, Radko D, Antakli A, Visscher C. 2021. Comparison of chicken cecal microbiota after metaphylactic treatment or following administration of feed additives in a broiler farm with enterococcal spondylitis history. Pathogens 10:1068. doi: 10.3390/pathogens10081068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Collins MD, Jones D, Farrow JAE, Kilpper-Balz R, Schleifer KH. 1984. Enterococcus avium nom. rev., comb. nov.; E. casseliflavus nom. rev., comb. nov.; E. durans nom. rev., comb. nov.; E. gallinarum comb. nov.; and E. malodoratus sp. nov. Int J Syst Bacteriol 34:220–223. doi: 10.1099/00207713-34-2-220 [DOI] [Google Scholar]
- 115. Sherman JM, Wing HU. 1937. Streptococcus durans n. sp. J Dairy Sci 20:165–167. doi: 10.3168/jds.S0022-0302(37)95679-0 [DOI] [Google Scholar]
- 116. Li J, Ren F, Gu H, Li X, Gan B. 2011. Safety evaluation in vitro of Enterococcus durans from Tibetan traditional fermented yak milk. J Microbiol 49:721–728. doi: 10.1007/s12275-011-1062-9 [DOI] [PubMed] [Google Scholar]
- 117. Pieniz S, de Moura TM, Cassenego APV, Andreazza R, Frazzon APG, Camargo FA de O, Brandelli A. 2015. Evaluation of resistance genes and virulence factors in a food isolated Enterococcus durans with potential probiotic effect. Food Control 51:49–54. doi: 10.1016/j.foodcont.2014.11.012 [DOI] [Google Scholar]
- 118. Butaye P, Devriese LA, Haesebrouck F. 1999. Comparison of direct and enrichment methods for the selective isolation of vancomycin-resistant enterococci from feces of pigs and poultry. Microb Drug Resist 5:131–134. doi: 10.1089/mdr.1999.5.131 [DOI] [PubMed] [Google Scholar]
- 119. Del Grosso M, Caprioli A, Chinzari P, Fontana MC, Pezzotti G, Manfrin A, Giannatale ED, Goffredo E, Pantosti A. 2000. Detection and characterization of vancomycin-resistant enterococci in farm animals and raw meat products in Italy. Microb Drug Resist 6:313–318. doi: 10.1089/mdr.2000.6.313 [DOI] [PubMed] [Google Scholar]
- 120. Robredo B, Singh KV, Baquero F, Murray BE, Torres C. 2000. Vancomycin-resistant enterococci isolated from animals and food. Int J Food Microbiol 54:197–204. doi: 10.1016/s0168-1605(99)00195-6 [DOI] [PubMed] [Google Scholar]
- 121. Andrighetto C, Knijff E, Lombardi A, Torriani S, Vancanneyt M, Kersters K, Swings J, Dellaglio F. 2001. Phenotypic and genetic diversity of enterococci isolated from Italian cheeses. J Dairy Res 68:303–316. doi: 10.1017/s0022029901004800 [DOI] [PubMed] [Google Scholar]
- 122. Delgado S, Delgado T, Mayo B. 2002. Technological performance of several Lactococcus and Enterococcus strains of dairy origin in milk. J Food Prot 65:1590–1596. doi: 10.4315/0362-028x-65.10.1590 [DOI] [PubMed] [Google Scholar]
- 123. Ong CHS, Asaad M, Lim KC, Ngeow YF. 2002. Infrequent occurrence of vancomycin-resistant enterococci in poultry from Malaysian wet markets. Malays J Pathol 24:91–94. [PubMed] [Google Scholar]
- 124. Bustamante W, Alpízar A, Hernández S, Pacheco A, Vargas N, Herrera ML, Vargas A, Caballero M, García F. 2003. Predominance of vanA genotype among vancomycin-resistant Enterococcus isolates from poultry and swine in Costa Rica. Appl Environ Microbiol 69:7414–7419. doi: 10.1128/AEM.69.12.7414-7419.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Chou CC, Lin YC, Su JJ. 2004. Microbial indicators for differentiation of human- and pig-sourced fecal pollution. J Environ Sci Health A Tox Hazard Subst Environ Eng 39:1415–1421. doi: 10.1081/ese-120037842 [DOI] [PubMed] [Google Scholar]
- 126. Huys G, D’Haene K, Collard JM, Swings J. 2004. Prevalence and molecular characterization of tetracycline resistance in Enterococcus isolates from food. Appl Environ Microbiol 70:1555–1562. doi: 10.1128/AEM.70.3.1555-1562.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Martin B, Garriga M, Hugas M, Aymerich T. 2005. Genetic diversity and safety aspects of enterococci from slightly fermented sausages. J Appl Microbiol 98:1177–1190. doi: 10.1111/j.1365-2672.2005.02555.x [DOI] [PubMed] [Google Scholar]
- 128. Rizzotti L, Simeoni D, Cocconcelli P, Gazzola S, Dellaglio F, Torriani S. 2005. Contribution of enterococci to the spread of antibiotic resistance in the production chain of swine meat commodities. J Food Prot 68:955–965. doi: 10.4315/0362-028x-68.5.955 [DOI] [PubMed] [Google Scholar]
- 129. Tejedor-Junco MT, Afonso-Rodríguez O, Martín-Barrasa JL, González-Martín M. 2005. Antimicrobial susceptibility of Enterococcus strains isolated from poultry faeces. Res Vet Sci 78:33–38. doi: 10.1016/j.rvsc.2004.05.008 [DOI] [PubMed] [Google Scholar]
- 130. Lopes M de FS, Simões AP, Tenreiro R, Marques JJF, Crespo MTB. 2006. Activity and expression of a virulence factor, gelatinase, in dairy enterococci. Int J Food Microbiol 112:208–214. doi: 10.1016/j.ijfoodmicro.2006.09.004 [DOI] [PubMed] [Google Scholar]
- 131. Poeta P, Costa D, Rodrigues J, Torres C. 2006. Detection of genes encoding virulence factors and bacteriocins in fecal enterococci of poultry in Portugal. Avian Dis 50:64–68. doi: 10.1637/7394-061505R.1 [DOI] [PubMed] [Google Scholar]
- 132. Psoni L, Kotzamanides C, Andrighetto C, Lombardi A, Tzanetakis N, Litopoulou-Tzanetaki E. 2006. Genotypic and phenotypic heterogeneity in Enterococcus isolates from Batzos, a raw goat milk cheese. Int J Food Microbiol 109:109–120. doi: 10.1016/j.ijfoodmicro.2006.01.027 [DOI] [PubMed] [Google Scholar]
- 133. Belicová A, Krzková L, Krajcovic J, Jurkovic D, Sojka M, Ebringer L, Duinský R. 2007. Antimicrobial susceptibility of Enterococcus species isolated from Slovak Bryndza cheese. Folia Microbiol (Praha) 52:115–119. doi: 10.1007/BF02932149 [DOI] [PubMed] [Google Scholar]
- 134. Fracalanzza SAP, Scheidegger EMD, Santos P dos, Leite PC, Teixeira LM. 2007. Antimicrobial resistance profiles of enterococci isolated from poultry meat and pasteurized milk in Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz 102:853–859. doi: 10.1590/s0074-02762007005000120 [DOI] [PubMed] [Google Scholar]
- 135. Miranda JM, Guarddon M, Mondragon A, Vázquez BI, Fente CA, Cepeda A, Franco CM. 2007. Antimicrobial resistance in Enterococcus spp. strains isolated from organic chicken, conventional chicken, and Turkey meat: a comparative survey. J Food Prot 70:1021–1024. doi: 10.4315/0362-028x-70.4.1021 [DOI] [PubMed] [Google Scholar]
- 136. Pimentel LL, Semedo T, Tenreiro R, Crespo MTB, Pintado MME, Malcata FX. 2007. Assessment of safety of enterococci isolated throughout traditional Terrincho cheesemaking: virulence factors and antibiotic susceptibility. J Food Prot 70:2161–2167. doi: 10.4315/0362-028x-70.9.2161 [DOI] [PubMed] [Google Scholar]
- 137. Ghidán A, Kaszanyitzky EJ, Dobay O, Nagy K, Amyes SGB, Rozgonyi F. 2008. Distribution and genetic relatedness of vancomycin-resistant enterococci (VRE) isolated from healthy slaughtered chickens in Hungary from 2001 to 2004. Acta Vet Hung 56:13–25. doi: 10.1556/AVet.56.2008.1.3 [DOI] [PubMed] [Google Scholar]
- 138. Tanasupawat S, Sukontasing S, Lee JS. 2008. Enterococcus thailandicus sp. nov., isolated from fermented sausage ('mum') in Thailand. Int J Syst Evol Microbiol 58:1630–1634. doi: 10.1099/ijs.0.65535-0 [DOI] [PubMed] [Google Scholar]
- 139. Martín B, Corominas L, Garriga M, Aymerich T. 2009. Identification and tracing of Enterococcus spp. by RAPD-PCR in traditional fermented sausages and meat environment. J Appl Microbiol 106:66–77. doi: 10.1111/j.1365-2672.2008.03976.x [DOI] [PubMed] [Google Scholar]
- 140. Bekele B, Ashenafi M. 2010. Distribution of drug resistance among enterococci and Salmonella from poultry and cattle in Ethiopia. Trop Anim Health Prod 42:857–864. doi: 10.1007/s11250-009-9499-0 [DOI] [PubMed] [Google Scholar]
- 141. Nam HM, Lim SK, Moon JS, Kang HM, Kim JM, Jang KC, Kim JM, Kang MI, Joo YS, Jung SC. 2010. Antimicrobial resistance of enterococci isolated from mastitic bovine milk samples in Korea. Zoonoses Public Health 57:e59–64. doi: 10.1111/j.1863-2378.2009.01307.x [DOI] [PubMed] [Google Scholar]
- 142. Fard RMN, Heuzenroeder MW, Barton MD. 2011. Antimicrobial and heavy metal resistance in commensal enterococci isolated from pigs. Vet Microbiol 148:276–282. doi: 10.1016/j.vetmic.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 143. Toc DA, Pandrea SL, Botan A, Mihaila RM, Costache CA, Colosi IA, Junie LM. 2022. Enterococcus raffinosus, Enterococcus durans and Enterococcus avium iolated from a tertiary care hospital in Romania-retrospective study and brief review. Biology 11:598. doi: 10.3390/biology11040598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Kaufhold A, Klein R. 1995. Species identification and antibiotic susceptibility of enterococci isolated from clinical specimens of hospitalized patients. Zentralbl Bakteriol 282:507–518. doi: 10.1016/s0934-8840(11)80723-1 [DOI] [PubMed] [Google Scholar]
- 145. Tripodi MF, Locatelli A, Adinolfi LE, Andreana A, Utili R. 1998. Successful treatment with ampicillin and fluoroquinolones of human endocarditis due to high-level gentamicin-resistant enterococci. Eur J Clin Microbiol Infect Dis 17:734–736. doi: 10.1007/s100960050171 [DOI] [PubMed] [Google Scholar]
- 146. Stepanović S, Jovanović M, Lavadinović L, Stošović B, Pelemiš M. 2004. Enterococcus durans endocarditis in a patient with transposition of the great vessels. J Med Microbiol 53:259–261. doi: 10.1099/jmm.0.05382-0 [DOI] [PubMed] [Google Scholar]
- 147. Vijayakrishnan R, Rapose A. 2012. Fatal Enterococcus durans aortic valve endocarditis: a case report and review of the literature. BMJ Case Rep 2012:bcr0220125855. doi: 10.1136/bcr-02-2012-5855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kenzaka T, Takamura N, Kumabe A, Takeda K. 2013. A case of subacute infective endocarditis and blood access infection caused by Enterococcus durans. BMC Infect Dis 13:594. doi: 10.1186/1471-2334-13-594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Radovanovic M, Jevtic D, Barsoum MK, Patel J, Dumic I. 2022. Enterococcus durans cardiac implantable electronic device lead infection and review of Enterococcus durans endocarditis cases. Medicina (Kaunas) 58:307. doi: 10.3390/medicina58020307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Fallavollita L, Di Gioacchino L, Balestrini F. 2016. Bioprosthetic aortic valve endocarditis in association with Enterococcus durans. Tex Heart Inst J 43:165–167. doi: 10.14503/THIJ-14-4980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zala A, Collins N. 2016. Enterococcus durans prosthetic valve endocarditis: a previously unreported clinical entity. Heart Lung Circ 25:e133–e136. doi: 10.1016/j.hlc.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 152. Al Shehri M, Zarak MS, Sarwari AR. 2020. Late prosthetic valve infective endocarditis by Enterococcus durans. J Global Infect Dis 12:152. doi: 10.4103/jgid.jgid_99_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Mackowiak PA. 1989. The enterococci: evidence of species-specific clinical and microbiologic heterogeneity. Am J Med Sci 297:238–243. doi: 10.1097/00000441-198904000-00008 [DOI] [PubMed] [Google Scholar]
- 154. Mejàre B. 1975. Streptococcus faecalis and Streptococcus faecium in infected dental root canals at filling and their susceptibility to azidocillin and some comparable antibiotics. Odontol Revy 26:193–204. [PubMed] [Google Scholar]
- 155. Miskeen PA, Deodhar L. 2002. Antimicrobial susceptibility pattern of Enterococcus species from urinary tract infections. J Assoc Physicians India 50:378–381. [PubMed] [Google Scholar]
- 156. Guardado R, Asensi V, Torres JM, Pérez F, Blanco A, Maradona JA, Cartón JA. 2006. Post-surgical enterococcal meningitis: clinical and epidemiological study of 20 cases. Scand J Infect Dis 38:584–588. doi: 10.1080/00365540600606416 [DOI] [PubMed] [Google Scholar]
- 157. Abe Y, Nakamura K, Yamada M, Yamamoto Y. 2006. Encephalomalacia with Enterococcus durans infection in the brain stem and cerebral hemisphere in chicks in Japan. Avian Dis 50:139–141. doi: 10.1637/7419-080805R.1 [DOI] [PubMed] [Google Scholar]
- 158. Angelos T, Nikolaos S, Nikolaos K, Nektarios P, Evdokia P. 2018. A rare case of tricuspid valve endocarditis caused by Enterococcus gallinarum in a patient with recurrent urinary tract infection. Cardiovasc Hematol Disord Drug Targets 18:162–164. doi: 10.2174/1871529X18666180302113428 [DOI] [PubMed] [Google Scholar]
- 159. Dargere S, Vergnaud M, Verdon R, Saloux E, Le Page O, Leclercq R, Bazin C. 2002. Enterococcus gallinarum endocarditis occurring on native heart valves. J Clin Microbiol 40:2308–2310. doi: 10.1128/JCM.40.6.2308-2310.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ortu M, Gabrielli E, Caramma I, Rossotti R, Gambirasio M, Gervasoni C. 2008. Enterococcus gallinarum endocarditis in a diabetic patient. Diabetes Res Clin Pract 81:e18–e20. doi: 10.1016/j.diabres.2008.03.016 [DOI] [PubMed] [Google Scholar]
- 161. Swampillai J, Liang M, Fisher R, Devlin G. 2012. Enterococcus gallinarum causing native valve endocarditis and aorto-atrial fistula: a case report and literature review. Echocardiography 29:873–875. doi: 10.1111/j.1540-8175.2012.01685.x [DOI] [PubMed] [Google Scholar]
- 162. Raja K, Antony M, Harikrishnan S. 2018. Infective endocarditis due to streptococci and enterococci: a 3-year retrospective study. Indian J Pathol Microbiol 61:545. doi: 10.4103/IJPM.IJPM_205_17 [DOI] [PubMed] [Google Scholar]
- 163. Dias T, de Almeida J, Santos A, Santos RM, Carvalho A. 2019. Enterococcus gallinarum causing native valve endocarditis. Eur J Case Rep Intern Med 6:001054. doi: 10.12890/2019_001054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Merquior VLC, Gonçalves Neves FP, Ribeiro RL, Duarte RS, de Andrade Marques E, Teixeira LM. 2008. Bacteraemia associated with a vancomycin-resistant Enterococcus gallinarum strain harbouring both the vanA and vanC1 genes. J Med Microbiol 57:244–245. doi: 10.1099/jmm.0.47429-0 [DOI] [PubMed] [Google Scholar]
- 165. Zheng J-X, Li H, Pu Z-Y, Wang H-Y, Deng X-B, Liu X-J, Deng Q-W, Yu Z-J. 2017. Bloodstream infections caused by Enterococcus spp: a 10-year retrospective analysis at a tertiary hospital in China. J Huazhong Univ Sci Technolog Med Sci 37:257–263. doi: 10.1007/s11596-017-1725-9 [DOI] [PubMed] [Google Scholar]
- 166. Reid KC, Cockerill III FR, Patel R. 2001. Clinical and epidemiological features of Enterococcus casseliflavus/flavescens and Enterococcus gallinarum bacteremia: a report of 20 cases. Clin Infect Dis 32:1540–1546. doi: 10.1086/320542 [DOI] [PubMed] [Google Scholar]
- 167. Friedman G, Stepensky P, Abu Ahmad W, Masarwa R, Temper V, Oster Y, Amit S, Averbuch D. 2020. Enterococcal bacteremia in children with malignancies and following hematopoietic stem cell transplantation: a 15-year single-center experience. Pediatr Infect Dis J 39:318–324. doi: 10.1097/INF.0000000000002579 [DOI] [PubMed] [Google Scholar]
- 168. Barber GR, Lauretta J, Saez R. 2007. A febrile neutropenic patient with Enterococcus gallinarum sepsis treated with daptomycin and gentamicin. Pharmacotherapy 27:927–932. doi: 10.1592/phco.27.6.927 [DOI] [PubMed] [Google Scholar]
- 169. Corso A, Faccone D, Gagetti P, Togneri A, Lopardo H, Melano R, Rodríguez V, Rodriguez M, Galas M. 2005. First report of VanA Enterococcus gallinarum dissemination within an intensive care unit in Argentina. Int J Antimicrob Agents 25:51–56. doi: 10.1016/j.ijantimicag.2004.07.014 [DOI] [PubMed] [Google Scholar]
- 170. de Perio MA, Yarnold PR, Warren J, Noskin GA. 2006. Risk factors and outcomes associated with non-Enterococcus faecalis, non-Enterococcus faecium enterococcal bacteremia. Infect Control Hosp Epidemiol 27:28–33. doi: 10.1086/500000 [DOI] [PubMed] [Google Scholar]
- 171. Contreras GA, DiazGranados CA, Cortes L, Reyes J, Vanegas S, Panesso D, Rincón S, Díaz L, Prada G, Murray BE, Arias CA. 2008. Nosocomial outbreak of Enteroccocus gallinarum: untaming of rare species of enterococci. J Hosp Infect 70:346–352. doi: 10.1016/j.jhin.2008.07.012 [DOI] [PubMed] [Google Scholar]
- 172. Koganemaru H, Hitomi S. 2008. Bacteremia caused by VanC-type enterococci in a university hospital in Japan: a 6-year survey. J Infect Chemother 14:413–417. doi: 10.1007/s10156-008-0644-x [DOI] [PubMed] [Google Scholar]
- 173. Takayama Y, Sunakawa K, Akahoshi T. 2003. Meningitis caused by Enterococcus gallinarum in patients with ventriculoperitoneal shunts. J Infect Chemother 9:348–350. doi: 10.1007/s10156-003-0268-0 [DOI] [PubMed] [Google Scholar]
- 174. Praharaj I, Sujatha S, Parija SC, Gopalakrishnan MS. 2012. Fatal meningitis caused by vancomycin-resistant enterococci: report of two cases from south India. Indian J Med Microbiol 30:242–245. doi: 10.4103/0255-0857.96713 [DOI] [PubMed] [Google Scholar]
- 175. Antonello VS, Zenkner F de M, França J, Santos BR. 2010. Enterococcus gallinarum meningitis in an immunocompetent host: a case report. Rev Inst Med Trop Sao Paulo 52:111–112. doi: 10.1590/s0036-46652010000200009 [DOI] [PubMed] [Google Scholar]
- 176. Narciso-Schiavon JL, Borgonovo A, Marques PC, Tonon D, Bansho ETO, Maggi DC, Dantas-Corrêa EB, de Lucca Schiavon L. 2015. Enterococcus casseliflavus and Enterococcus gallinarum as causative agents of spontaneous bacterial peritonitis. Ann Hepatol 14:270–272. doi: 10.1016/S1665-2681(19)30791-4 [DOI] [PubMed] [Google Scholar]
- 177. Hillier RJ, Arjmand P, Rebick G, Ostrowski M, Muni RH. 2013. Post-traumatic vancomycin-resistant enterococcal endophthalmitis. J Ophthalmic Inflamm Infect 3:42. doi: 10.1186/1869-5760-3-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Green M, Barbadora K, Michaels M. 1991. Recovery of vancomycin-resistant Gram-positive cocci from pediatric liver transplant recipients. J Clin Microbiol 29:2503–2506. doi: 10.1128/jcm.29.11.2503-2506.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Orłowska A, Majdan M, Kozioł-Montewka M, Grzebalska A, Swatowski A. 2002. Asymptomatic bacteriuria in patients on continuous ambulatory peritoneal dialysis. Ann Univ Mariae Curie Sklodowska Med 57:285–289. [PubMed] [Google Scholar]
- 180. Sharifzadeh Peyvasti V, Mohabati Mobarez A, Shahcheraghi F, Khoramabadi N, Razaz Rahmati N, Hosseini Doust R. 2020. High-level aminoglycoside resistance and distribution of aminoglycoside resistance genes among Enterococcus spp. clinical isolates in Tehran, Iran. J Glob Antimicrob Resist 20:318–323. doi: 10.1016/j.jgar.2019.08.008 [DOI] [PubMed] [Google Scholar]
- 181. Ohki S, Hagi T, Nakano K, Shiroma A, Tamotsu H, Shimoji M, Shinzato M, Ashimine N, Minami M, Nakanishi T, Teruya K, Satou K, Moriya N, Kobayashi M, Nomura M, Suzuki C, Hirano T. 2018. Complete genome sequence of carotenoid-producing Enterococcus gilvus CR1, isolated from raw cow’s milk. Microbiol Resour Announc 7:e00988-18. doi: 10.1128/MRA.00988-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Zago M, Bonvini B, Carminati D, Giraffa G. 2009. Detection and quantification of Enterococcus gilvus in cheese by real-time PCR. Syst Appl Microbiol 32:514–521. doi: 10.1016/j.syapm.2009.07.001 [DOI] [PubMed] [Google Scholar]
- 183. Tyrrell GJ, Turnbull L, Teixeira LM, Lefebvre J, Carvalho M da GS, Facklam RR, Lovgren M. 2002. Enterococcus gilvus sp. nov. and Enterococcus pallens sp. nov. isolated from human clinical specimens. J Clin Microbiol 40:1140–1145. doi: 10.1128/JCM.40.4.1140-1145.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Farrow JAE, Collins MD. 1985. Enterococcus hirae, a new species that includes amino acid assay strain NCDO 1258 and strains causing growth depression in young chickens. Int J Syst Bacteriol 35:73–75. doi: 10.1099/00207713-35-1-73 [DOI] [Google Scholar]
- 185. Poyart C, Lambert T, Morand P, Abassade P, Quesne G, Baudouy Y, Trieu-Cuot P. 2002. Native valve endocarditis due to Enterococcus hirae. J Clin Microbiol 40:2689–2690. doi: 10.1128/JCM.40.7.2689-2690.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Talarmin JP, Pineau S, Guillouzouic A, Boutoille D, Giraudeau C, Reynaud A, Lepelletier D, Corvec S. 2011. Relapse of Enterococcus hirae prosthetic valve endocarditis. J Clin Microbiol 49:1182–1184. doi: 10.1128/JCM.02049-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Pinkes ME, White C, Wong CS. 2019. Native-valve Enterococcus hirae endocarditis: a case report and review of the literature. BMC Infect Dis 19:891. doi: 10.1186/s12879-019-4532-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Winther M, Dalager-Pedersen M, Tarpgaard IH, Nielsen HL. 2020. First Danish case of infective endocarditis caused by Enterococcus hirae. BMJ Case Rep 13:12. doi: 10.1136/bcr-2020-237950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Merlo J, Bustamante G, Llibre JM. 2020. Bacteremic pneumonia caused by Enterococcus hirae in a subject receiving regorafenib. Enferm Infecc Microbiol Clin (Engl Ed) 38:226–229. doi: 10.1016/j.eimc.2019.06.002 [DOI] [PubMed] [Google Scholar]
- 190. Gilad J, Borer A, Riesenberg K, Peled N, Shnaider A, Schlaeffer F. 1998. Enterococcus hirae septicemia in a patient with end-stage renal disease undergoing hemodialysis. Eur J Clin Microbiol Infect Dis 17:576–577. doi: 10.1007/BF01708623 [DOI] [PubMed] [Google Scholar]
- 191. Brulé N, Corvec S, Villers D, Guitton C, Bretonnière C. 2013. Life-threatening bacteremia and pyonephrosis caused by Enterococcus hirae. Med Mal Infect 43:401–402. doi: 10.1016/j.medmal.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 192. Brayer S, Linn A, Holt S, Ellery K, Mitchell S, Williams J. 2019. Enterococcus hirae bacteremia in an infant: case report and review of the literature. J Pediatric Infect Dis Soc 8:571–573. doi: 10.1093/jpids/piz028 [DOI] [PubMed] [Google Scholar]
- 193. Nakamura T, Ishikawa K, Matsuo T, Kawai F, Uehara Y, Mori N. 2021. Enterococcus hirae bacteremia associated with acute pyelonephritis in a patient with alcoholic cirrhosis: a case report and literature review. BMC Infect Dis 21:999. doi: 10.1186/s12879-021-06707-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Canalejo E, Ballesteros R, Cabezudo J, García-Arata MI, Moreno J. 2008. Bacteraemic spondylodiscitis caused by Enterococcus hirae. Eur J Clin Microbiol Infect Dis 27:613–615. doi: 10.1007/s10096-008-0476-8 [DOI] [PubMed] [Google Scholar]
- 195. Chan T-S, Wu M-S, Suk F-M, Chen C-N, Chen Y-F, Hou Y-H, Lien G-S. 2012. Enterococcus hirae-related acute pyelonephritis and cholangitis with bacteremia: an unusual infection in humans. Kaohsiung J Med Sci 28:111–114. doi: 10.1016/j.kjms.2011.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. El-Zimaity HMT, Ramchatesingh J, Clarridge JE, Abudayyeh S, Osato MS, Graham DY. 2003. Enterococcus gastritis. Hum Pathol 34:944–945. doi: 10.1016/s0046-8177(03)00287-9 [DOI] [PubMed] [Google Scholar]
- 197. Bilek HC, Deveci A, Ünal S, Tanrıverdi Çaycı Y, Tanyel E. 2020. Enterococcus hirae as a cause of bacteremic urinary tract infection: first case report from Turkey. J Infect Dev Ctries 14:1780–1482. doi: 10.3855/jidc.12522 [DOI] [PubMed] [Google Scholar]
- 198. Bicego-Pereira EC, Barbosa-Ribeiro M, de-Jesus-Soares A, Zaia AA, Ferraz CCR, Almeida JFA, Marciano MA, Feres M, Gomes BPFA. 2020. Evaluation of the presence of microorganisms from root canal of teeth submitted to retreatment due to prosthetic reasons and without evidence of apical periodontitis. Clin Oral Investig 24:3243–3254. doi: 10.1007/s00784-020-03200-z [DOI] [PubMed] [Google Scholar]
- 199. Merei F, Shapiro G, Abu Shakra I, Bickel A, Ganam S, Bez M, Kakiashvili E. 2020. A unusual case of multifocal pyogenic abscess formation following ERCP procedure. BMC Surg 20. doi: 10.1186/s12893-020-00759-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Sim JS, Kim HS, Oh KJ, Park MS, Jung EJ, Jung YJ, Kang DG, Seo SI, Kim WJ, Jang MK. 2012. Spontaneous bacterial peritonitis with sepsis caused by Enterococcus hirae. J Korean Med Sci 27:1598–1600. doi: 10.3346/jkms.2012.27.12.1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Li YQ, Gu CT. 2019. Proposal of Enterococcus xinjiangensis Ren et al. 2020 as a later heterotypic synonym of Enterococcus lactis Morandi et al. 2012. Int J Syst Evol Microbiol 71. doi: 10.1099/ijsem.0.004716 [DOI] [PubMed] [Google Scholar]
- 202. Morandi S, Silvetti T, Brasca M. 2013. Biotechnological and safety characterization of Enterococcus lactis, a recently described species of dairy origin. Antonie Van Leeuwenhoek 103:239–249. doi: 10.1007/s10482-012-9806-z [DOI] [PubMed] [Google Scholar]
- 203. Belloso Daza MV, Cortimiglia C, Bassi D, Cocconcelli PS. 2021. Genome-based studies indicate that the Enterococcus faecium clade B strains belong to Enterococcus lactis species and lack of the hospital infection associated markers. Int J Syst Evol Microbiol 71. doi: 10.1099/ijsem.0.004948 [DOI] [PubMed] [Google Scholar]
- 204. Raven KE, Reuter S, Reynolds R, Brodrick HJ, Russell JE, Török ME, Parkhill J, Peacock SJ. 2016. A decade of genomic history for healthcare-associated Enterococcus faecium in the United Kingdom and Ireland. Genome Res 26:1388–1396. doi: 10.1101/gr.204024.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Ren X, Li M, Guo D. 2016. Enterococcus xinjiangensis sp. nov., isolated from Yogurt of Xinjiang, China. Curr Microbiol 73:374–378. doi: 10.1007/s00284-016-1065-1 [DOI] [PubMed] [Google Scholar]
- 206. Collins MD, Farrow JAE, Jones D. 1986. Enterococcus mundtii sp. nov. Int J Syst Evol Microbiol 36:8–12. doi: 10.1099/00207713-36-1-8 [DOI] [Google Scholar]
- 207. Kaufhold A, Ferrieri P. 1991. Isolation of Enterococcus mundtii from normally sterile body sites in two patients. J Clin Microbiol 29:1075–1077. doi: 10.1128/jcm.29.5.1075-1077.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Ferguson DM, Moore DF, Getrich MA, Zhowandai MH. 2005. Enumeration and speciation of enterococci found in marine and intertidal sediments and coastal water in southern California. J Appl Microbiol 99:598–608. doi: 10.1111/j.1365-2672.2005.02660.x [DOI] [PubMed] [Google Scholar]
- 209. Moore DF, Guzman JA, McGee C. 2008. Species distribution and antimicrobial resistance of enterococci isolated from surface and ocean water. J Appl Microbiol 105:1017–1025. doi: 10.1111/j.1365-2672.2008.03828.x [DOI] [PubMed] [Google Scholar]
- 210. Lauková A, Simonová M, Strompfová V, Styriak I, Ouwehand AC, Várady M. 2008. Potential of enterococci isolated from horses. Anaerobe 14:234–236. doi: 10.1016/j.anaerobe.2008.04.002 [DOI] [PubMed] [Google Scholar]
- 211. Pang H, Zhang M, Qin G, Tan Z, Li Z, Wang Y, Cai Y. 2011. Identification of lactic acid bacteria isolated from corn stovers. Anim Sci J 82:642–653. doi: 10.1111/j.1740-0929.2011.00894.x [DOI] [PubMed] [Google Scholar]
- 212. Li D, Ni K, Pang H, Wang Y, Cai Y, Jin Q. 2015. Identification and antimicrobial activity detection of lactic acid bacteria isolated from corn stover silage. Asian-Australas J Anim Sci 28:620–631. doi: 10.5713/ajas.14.0439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Magni C, Espeche C, Repizo GD, Saavedra L, Suárez CA, Blancato VS, Espariz M, Esteban L, Raya RR, Font de Valdez G, Vignolo G, Mozzi F, Taranto MP, Hebert EM, Nader-Macías ME, Sesma F. 2012. Draft genome sequence of Enterococcus mundtii CRL1656. J Bacteriol 194:550. doi: 10.1128/JB.06415-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Farah N, Mehdi A, Soomro SI, Soomro NI, Tareb R, Desmasures N, Vernoux JP, Bakhtiar SM, Imran M. 2016. Draft genome sequence of Enterococcus mundtii QAUEM2808, isolated from dahi, a fermented milk product. Genome Announc 4:e00995-16. doi: 10.1128/genomeA.00995-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Wambui J, Stevens M, Njage PMK, Wüthrich D, Egli A, Stephan R, Tasara T. 2018. Draft genome sequences of Enterococcus mundtii strains isolated from beef slaughterhouses in Kenya. Genome Announc 6:21. doi: 10.1128/genomeA.00446-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Wambui J, Tasara T, Njage PMK, Stephan R. 2018. Species distribution and antimicrobial profiles of Enterococcus spp. isolates from Kenyan small and medium enterprise slaughterhouses. J Food Prot 81:1445–1449. doi: 10.4315/0362-028X.JFP-18-130 [DOI] [PubMed] [Google Scholar]
- 217. Bourouni OC, El Bour M, Calo-Mata P, Mraouna R, Abedellatif B, Barros-Velàzquez J. 2012. Phylogenetic analysis of antimicrobial lactic acid bacteria from farmed seabass Dicentrarchus labrax. Can J Microbiol 58:463–474. doi: 10.1139/w2012-014 [DOI] [PubMed] [Google Scholar]
- 218. Pilon FM, Visôtto LE, Guedes RNC, Oliveira MGA. 2013. Proteolytic activity of gut bacteria isolated from the velvet bean caterpillar Anticarsia gemmatalis. J Comp Physiol B 183:735–747. doi: 10.1007/s00360-013-0744-5 [DOI] [PubMed] [Google Scholar]
- 219. Dec M, Stępień-Pyśniak D, Gnat S, Fratini F, Urban-Chmiel R, Cerri D, Winiarczyk S, Turchi B. 2020. Antibiotic susceptibility and virulence genes in Enterococcus isolates from wild mammals living in Tuscany, Italy. Microb Drug Resist 26:505–519. doi: 10.1089/mdr.2019.0052 [DOI] [PubMed] [Google Scholar]
- 220. Higashide T, Takahashi M, Kobayashi A, Ohkubo S, Sakurai M, Shirao Y, Tamura T, Sugiyama K. 2005. Endophthalmitis caused by Enterococcus mundtii. J Clin Microbiol 43:1475–1476. doi: 10.1128/JCM.43.3.1475-1476.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Sharifi-Rad M, Shadanpour S, van Belkum A, Soltani A, Sharifi-Rad J. 2016. First case of vanA-positive Enterococcus mundtii in human urinary tract infection in Iran. New Microbes New Infect 11:68–70. doi: 10.1016/j.nmni.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Sapico FL, Canawati HN, Ginunas VJ, Gilmore DS, Montgomerie JZ, Tuddenham WJ, Facklam RR. 1989. Enterococci highly resistant to penicillin and ampicillin: an emerging clinical problem? J Clin Microbiol 27:2091–2095. doi: 10.1128/jcm.27.9.2091-2095.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Sandoe JAT, Witherden IR, Settle C. 2001. Vertebral osteomyelitis caused by Enterococcus raffinosus. J Clin Microbiol 39:1678–1679. doi: 10.1128/JCM.39.4.1678-1679.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Freyaldenhoven BS, Schlieper G, Lütticken R, Reinert RR. 2005. Enterococcus raffinosus infection in an immunosuppressed patient: case report and review of the literature. J Infect 51:e121–e124. doi: 10.1016/j.jinf.2004.10.012 [DOI] [PubMed] [Google Scholar]
- 225. Savini V, Manna A, D’Antonio F, Talia M, Catavitello C, Balbinot A, Febbo F, Carlino D, Fioritoni F, Di Bonaventura G, D’Antonio D. 2008. First report of vaginal infection caused by Enterococcus raffinosus. J Med Microbiol 57:672–673. doi: 10.1099/jmm.0.47773-0 [DOI] [PubMed] [Google Scholar]
- 226. Savini V, Manna A, Di Bonaventura G, Catavitello C, Talia M, Balbinot A, Febbo F, D’Antonio D. 2008. Multidrug-resistant Enterococcus raffinosus from a decubitus ulcer: a case report. Int J Low Extrem Wounds 7:36–37. doi: 10.1177/1534734607313757 [DOI] [PubMed] [Google Scholar]
- 227. Mastroianni A. 2009. Enterococcus raffinosus endocarditis. first case and literature review. Infez Med 17:14–20. [PubMed] [Google Scholar]
- 228. Jasovich A, Ganaha MC, Ebi C, García RD, Blanco MA, Lopardo H. 2008. Endocarditis due to vancomycin-resistant Enterococcus raffinosus successfully treated with linezolid: case report and review of literature. Rev Argent Microbiol 40:204–207. [PubMed] [Google Scholar]
- 229. Chen KJ, Yang KJ, Sun CC, Yeung L. 2009. Traumatic endophthalmitis caused by Enterococcus raffinosus and Enterobacter gergoviae. J Med Microbiol 58:526–528. doi: 10.1099/jmm.0.008482-0 [DOI] [PubMed] [Google Scholar]
- 230. Savini V, Catavitello C, Favaro M, Masciarelli G, Astolfi D, Balbinot A, Bianco A, Mauti A, Dianetti J, Fontana C, D’Amario C, D’Antonio D. 2010. Enterococcus raffinosus sinusitis post-Aspergillus flavus paranasal infection, in a patient with myelodysplastic syndrome: report of a case and concise review of pertinent literature. J Clin Pathol 63:264–265. doi: 10.1136/jcp.2009.070177 [DOI] [PubMed] [Google Scholar]
- 231. Harrington SM, Ross TL, Gebo KA, Merz WG. 2004. Vancomycin resistance, ESP, and strain relatedness: a 1-year study of enterococcal bacteremia. J Clin Microbiol 42:5895–5898. doi: 10.1128/JCM.42.12.5895-5898.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Kim YJ, Jun YH, Choi HJ, You Y-K, Kim DG, Choi JY, Yoon SK, Kim SI. 2019. Impact of enterococcal bacteremia in liver transplant recipients. Transplant Proc 51:2766–2770. doi: 10.1016/j.transproceed.2019.02.064 [DOI] [PubMed] [Google Scholar]
- 233. Correia S, Ponce P, Jones-Dias D, Caniça M, Igrejas G, Poeta P. 2014. Vancomycin-resistant enterococci among haemodialysis patients in Portugal: prevalence and molecular characterization of resistance, virulence and clonality. Enferm Infecc Microbiol Clin 32:174–176. doi: 10.1016/j.eimc.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 234. Farrow JAE, Kruze J, Phillips BA, Bramley AJ, Collins MD. 1984. Taxonomic studies on Streptococcus bovis and Streptococcus equinus: description of Streptococcus alactolyticus sp. nov. and Streptococcus saccharolyticus sp. nov. Syst Appl Microbiol 5:467–482. doi: 10.1016/S0723-2020(84)80004-1 [DOI] [Google Scholar]
- 235. Juliano LCB, Gouvêa FLR, Latosinski GS, Fabri FHH, Salvador TB, Guimaraes FF, Ribeiro MG, Langoni H, Rall VLM, Hernandes RT, Leite DS, Pantoja JCF. 2022. Species diversity and antimicrobial resistance patterns of Enterococcus spp. isolated from mastitis cases, milking machine and the environment of dairy cows. Lett Appl Microbiol 75:924–932. doi: 10.1111/lam.13768 [DOI] [PubMed] [Google Scholar]
- 236. Nogami Y, Banno K, Adachi M, Kunitomi H, Kobayashi Y, Tominaga E, Aoki D. 2019. Profiling of the causative bacteria in infected lymphocysts after lymphadenectomy for gynecologic cancer by pyrosequencing the 16S ribosomal RNA gene using next-generation sequencing technology. Infect Dis Obstet Gynecol 2019:9326285. doi: 10.1155/2019/9326285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Lauková A, Kandričáková A, Imrichová J, Strompfová V, Miltko R, Kowalik B, Belzecki G. 2013. Properties of Enterococcus thailandicus isolates from beavers. Afr J Microbiol Res 7:3569–3574. doi: 10.5897/AJMR2013.5654 [DOI] [Google Scholar]
- 238. Shewmaker PL, Steigerwalt AG, Nicholson AC, Carvalho M da G, Facklam RR, Whitney AM, Teixeira LM. 2011. Reevaluation of the taxonomic status of recently described species of Enterococcus: evidence that E. thailandicus is a senior subjective synonym of "E. sanguinicola" and confirmation of E. caccae as a species distinct from E. silesiacus. J Clin Microbiol 49:2676–2679. doi: 10.1128/JCM.00399-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Wu X, Wu B, Li Y, Jin X, Wang X. 2021. Identification and safety assessment of Enterococcus thailandicus TC1 isolated from healthy pigs. PLoS ONE 16:e0254081. doi: 10.1371/journal.pone.0254081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Carvalho M da GS, Steigerwalt AG, Morey RE, Shewmaker PL, Falsen E, Facklam RR, Teixeira LM. 2008. Designation of the provisional new Enterococcus species CDC PNS-E2 as Enterococcus sanguinicola sp. nov., isolated from human blood, and identification of a strain previously named Enterococcus CDC PNS-E1 as Enterococcus italicus Fortina, Ricci, Mora, and Manachini 2004. J Clin Microbiol 46:3473–3476. doi: 10.1128/JCM.00603-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Carvalho M da G, Steigerwalt AG, Morey RE, Shewmaker PL, Teixeira LM, Facklam RR. 2004. Characterization of three new enterococcal species, Enterococcus sp. nov. CDC PNS-E1, Enterococcus sp. nov. CDC PNS-E2, and Enterococcus sp. nov. CDC PNS-E3, isolated from human clinical specimens. J Clin Microbiol 42:1192–1198. doi: 10.1128/JCM.42.3.1192-1198.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Mbouche P, Blairon L, Cupaiolo R, Zaouak Y, Hainaux B, Beukinga I, Tré-Hardy M. 2023. Enterococcus thailandicus, an unusual pathogen in humans encountered in an intra-abdominal infection. New Microbes New Infect 53:101137. doi: 10.1016/j.nmni.2023.101137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Goel V, Kumar D, Kumar R, Mathur P, Singh S. 2016. Community acquired enterococcal urinary tract infections and antibiotic resistance profile in north India. J Lab Physicians 8:50–54. doi: 10.4103/0974-2727.176237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Adhikari L. 2010. High-level aminoglycoside resistance and reduced susceptibility to vancomycin in nosocomial enterococci. J Glob Infect Dis 2:231–235. doi: 10.4103/0974-777X.68534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Jain S, Kumar A, Kashyap B, Kaur IR. 2011. Clinico-epidemiological profile and high-level aminoglycoside resistance in enterococcal septicemia from a tertiary care hospital in east Delhi. Int J Appl Basic Med Res 1:80–83. doi: 10.4103/2229-516X.91149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Lévesque S, Longtin Y, Domingo M-C, Massé C, Bernatchez H, Gaudreau C, Tremblay C. 2016. Enteroccocus pallens as a potential novel human pathogen: three cases of spontaneous bacterial peritonitis. JMM Case Rep 3:e005024. doi: 10.1099/jmmcr.0.005024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Bhagwat A2019. In vitro assessment of physiological properties of Enterococcus strains of human origin for possible probiotic use. Asian J Pharm Clin Res 12:194–203. doi: 10.22159/ajpcr.2019.v12i6.33294 [DOI] [Google Scholar]
- 248. Gaur SS, Annapure US. 2022. Untargeted metabolite profiling of Enterococcus villorum SB2, isolated from the vagina of pregnant women, by HR-LCMS. World J Microbiol Biotechnol 38:219. doi: 10.1007/s11274-022-03404-3 [DOI] [PubMed] [Google Scholar]
- 249. Bhagwat A, Annapure US. 2019. Maternal-neonatal transmission of Enterococcus strains during delivery. Beni-Suef Univ J Basic Appl Sci 8:1–9. doi: 10.1186/s43088-019-0029-5 [DOI] [Google Scholar]
- 250. Carvalho M da G, Shewmaker PL, Steigerwalt AG, Morey RE, Sampson AJ, Joyce K, Barrett TJ, Teixeira LM, Facklam RR. 2006. Enterococcus caccae sp. nov., isolated from human stools. Int J Syst Evol Microbiol 56:1505–1508. doi: 10.1099/ijs.0.64103-0 [DOI] [PubMed] [Google Scholar]
- 251. Takakura T, Francis R, Anani H, Bilen M, Raoult D, Bou Khalil JY. 2019. Enterococcus mediterraneensis sp. nov., a new bacterium isolated from the stool of a 39-year-old Pygmy. New Microbes New Infect 32:100599. doi: 10.1016/j.nmni.2019.100599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Le Page S, Cimmino T, Togo A, Million M, Michelle C, Khelaifia S, Lagier J-C, Raoult D, Rolain J-M. 2016. Noncontiguous finished genome sequence and description of Enterococcus massiliensis sp. nov. New Microbes New Infect 12:90–95. doi: 10.1016/j.nmni.2016.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Dunny GM, Leonard BA, Hedberg PJ. 1995. Pheromone-inducible conjugation in Enterococcus faecalis: interbacterial and host-parasite chemical communication. J Bacteriol 177:871–876. doi: 10.1128/jb.177.4.871-876.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Kreft B, Marre R, Schramm U, Wirth R. 1992. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect Immun 60:25–30. doi: 10.1128/iai.60.1.25-30.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Rakita RM, Vanek NN, Jacques-Palaz K, Mee M, Mariscalco MM, Dunny GM, Snuggs M, Van Winkle WB, Simon SI. 1999. Enterococcus faecalis bearing aggregation substance is resistant to killing by human neutrophils despite phagocytosis and neutrophil activation. Infect Immun 67:6067–6075. doi: 10.1128/IAI.67.11.6067-6075.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Coque TM, Patterson JE, Steckelberg JM, Murray BE. 1995. Incidence of hemolysin, gelatinase, and aggregation substance among enterococci isolated from patients with endocarditis and other infections and from feces of hospitalized and community-based persons. J Infect Dis 171:1223–1229. doi: 10.1093/infdis/171.5.1223 [DOI] [PubMed] [Google Scholar]
- 257. Biswas PP, Dey S, Sen A, Adhikari L. 2016. Molecular characterization of virulence genes in vancomycin-resistant and vancomycin-sensitive enterococci. J Glob Infect Dis 8:16–24. doi: 10.4103/0974-777X.176141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Nallapareddy SR, Qin X, Weinstock GM, Höök M, Murray BE. 2000. Enterococcus faecalis adhesin, ace, mediates attachment to extracellular matrix proteins collagen type IV and laminin as well as collagen type I. Infect Immun 68:5218–5224. doi: 10.1128/IAI.68.9.5218-5224.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Singh KV, Nallapareddy SR, Sillanpää J, Murray BE. 2010. Importance of the collagen adhesin ace in pathogenesis and protection against Enterococcus faecalis experimental endocarditis. PLoS Pathog. 6:e1000716. doi: 10.1371/journal.ppat.1000716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Dolka B, Czopowicz M, Chrobak-Chmiel D, Ledwoń A, Szeleszczuk P. 2020. Prevalence, antibiotic susceptibility and virulence factors of Enterococcus species in racing pigeons (Columba livia f. domestica). BMC Vet Res 16:7. doi: 10.1186/s12917-019-2200-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Nallapareddy SR, Weinstock GM, Murray BE. 2003. Clinical isolates of Enterococcus faecium exhibit strain‐specific collagen binding mediated by Acm, a new member of the MSCRAMM family. Mol Microbiol 47:1733–1747. doi: 10.1046/j.1365-2958.2003.03417.x [DOI] [PubMed] [Google Scholar]
- 262. Nallapareddy SR, Singh KV, Murray BE. 2008. Contribution of the collagen adhesin Acm to pathogenesis of Enterococcus faecium in experimental endocarditis. Infect Immun 76:4120–4128. doi: 10.1128/IAI.00376-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Akpınar Kankaya D, Tuncer Y. 2022. Detection of virulence factors, biofilm formation, and biogenic amine production in vancomycin‐resistant lactic acid bacteria (VRLAB) isolated from foods of animal origin. J Food Process Preserv 46. doi: 10.1111/jfpp.16423 [DOI] [Google Scholar]
- 264. Shankar V, Baghdayan AS, Huycke MM, Lindahl G, Gilmore MS. 1999. Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infect Immun 67:193–200. doi: 10.1128/IAI.67.1.193-200.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Toledo-Arana A, Valle J, Solano C, Arrizubieta MJ, Cucarella C, Lamata M, Amorena B, Leiva J, Penadés JR, Lasa I. 2001. The enterococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Appl Environ Microbiol 67:4538–4545. doi: 10.1128/AEM.67.10.4538-4545.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Shankar N, Lockatell CV, Baghdayan AS, Drachenberg C, Gilmore MS, Johnson DE. 2001. Role of Enterococcus faecalis surface protein Esp in the pathogenesis of ascending urinary tract infection. Infect Immun 69:4366–4372. doi: 10.1128/IAI.69.7.4366-4372.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Taglialegna A, Matilla-Cuenca L, Dorado-Morales P, Navarro S, Ventura S, Garnett JA, Lasa I, Valle J. 2020. The biofilm-associated surface protein Esp of Enterococcus faecalis forms amyloid-like fibers. NPJ Biofilms Microbiomes 6:15. doi: 10.1038/s41522-020-0125-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Willems RJ, Homan W, Top J, van Santen-Verheuvel M, Tribe D, Manzioros X, Gaillard C, Vandenbroucke-Grauls CM, Mascini EM, van Kregten E, van Embden JD, Bonten MJ. 2001. Variant esp gene as a marker of a distinct genetic lineage of vancomycinresistant Enterococcus faecium spreading in hospitals. Lancet 357:853–855. doi: 10.1016/S0140-6736(00)04205-7 [DOI] [PubMed] [Google Scholar]
- 269. Trivedi K, Cupakova S, Karpiskova R. 2011. Virulence factors and antibiotic resistance in enterococci isolated from food-stuffs. Vet Med 56:352–357. doi: 10.17221/1584-VETMED [DOI] [Google Scholar]
- 270. Semedo T, Santos MA, Lopes MFS, Figueiredo Marques JJ, Barreto Crespo MT, Tenreiro R. 2003. Virulence factors in food, clinical and reference enterococci: a common trait in the genus? Syst Appl Microbiol 26:13–22. doi: 10.1078/072320203322337263 [DOI] [PubMed] [Google Scholar]
- 271. Hendrickx APA, Bonten MJM, van Luit-Asbroek M, Schapendonk CME, Kragten AHM, Willems RJL. 2008. Expression of two distinct types of pili by a hospital-acquired Enterococcus faecium isolate. Microbiology (Reading) 154:3212–3223. doi: 10.1099/mic.0.2008/020891-0 [DOI] [PubMed] [Google Scholar]
- 272. Nallapareddy SR, Singh KV, Sillanpää J, Garsin DA, Höök M, Erlandsen SL, Murray BE. 2006. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J Clin Invest 116:2799–2807. doi: 10.1172/JCI29021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Singh KV, Nallapareddy SR, Murray BE. 2007. Importance of the ebp (endocarditisand biofilm-associated pilus) locus in the pathogenesis of Enterococcus faecalis ascending urinary tract infection. J Infecr Dis 195:1671–1677. doi: 10.1086/517524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Nallapareddy SR, Sillanpää J, Mitchell J, Singh KV, Chowdhury SA, Weinstock GM, Sullam PM, Murray BE. 2011. Conservation of Ebp-type pilus genes among enterococci and demonstration of their role in adherence of Enterococcus faecalis to human platelets. Infect Immun 79:2911–2920. doi: 10.1128/IAI.00039-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. La Rosa SL, Montealegre MC, Singh KV, Murray BE. 2016. Enterococcus faecalis Ebp pili are important for cell-cell aggregation and intraspecies gene transfer. Microbiology (Reading) 162:798–802. doi: 10.1099/mic.0.000276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Gilmore MS, Segarra RA, Booth MC, Bogie CP, Hall LR, Clewell DB. 1994. Genetic structure of the Enterococcus faecalis plasmid pAD1-encoded cytolytic toxin system and its relationship to lantibiotic determinants. J Bacteriol 176:7335–7344. doi: 10.1128/jb.176.23.7335-7344.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Haas W, Gilmore MS. 1999. Molecular nature of a novel bacterial toxin: the cytolysin of Enterococcus faecalis. Med Microbiol Immunol 187:183–190. doi: 10.1007/s004300050091 [DOI] [PubMed] [Google Scholar]
- 278. Huycke MM, Spiegel CA, Gilmore MS. 1991. Bacteremia caused by hemolytic, high-level gentamicin-resistant Enterococcus faecalis. Antimicrob Agents Chemother 35:1626–1634. doi: 10.1128/AAC.35.8.1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Jett BD, Jensen HG, Nordquist RE, Gilmore MS. 1992. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect Immun 60:2445–2452. doi: 10.1128/iai.60.6.2445-2452.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Poeta P, Costa D, Klibi N, Rodrigues J, Torres C. 2006. Phenotypic and genotypic study of gelatinase and β‐haemolysis activities in faecal enterococci of poultry in Portugal. J Vet Med B Infect Dis Vet Public Health 53:203–208. doi: 10.1111/j.1439-0450.2006.00941.x [DOI] [PubMed] [Google Scholar]
- 281. Park SY, Kim KM, Lee JH, Seo SJ, Lee IH. 2007. Extracellular gelatinase of Enterococcus faecalis destroys a defense system in insect hemolymph and human serum. Infect Immun 75:1861–1869. doi: 10.1128/IAI.01473-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Kawalec M, Potempa J, Moon JL, Travis J, Murray BE. 2005. Molecular diversity of a putative virulence factor: purification and characterization of isoforms of an extracellular serine glutamyl endopeptidase of Enterococcus faecalis with different enzymatic activities. J Bacteriol 187:266–275. doi: 10.1128/JB.187.1.266-275.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Lopes M de F, Simões AP, Tenreiro R, Marques JJF, Crespo MTB. 2006. Activity and expression of a virulence factor, gelatinase, in dairy enterococci. Int J Food Microbiol 112:208–214. doi: 10.1016/j.ijfoodmicro.2006.09.004 [DOI] [PubMed] [Google Scholar]
- 284. Amidi-Fazli N, Hanifian S. 2022. Biodiversity, antibiotic resistance and virulence traits of Enterococcus species in artisanal dairy products. Int Dairy J 129:105287. doi: 10.1016/j.idairyj.2021.105287 [DOI] [Google Scholar]
- 285. Kayaoglu G, Ørstavik D. 2004. Virulence factors of Enterococcus faecalis: relationship to endodontic disease. Crit Rev Oral Biol Med 15:308–320. doi: 10.1177/154411130401500506 [DOI] [PubMed] [Google Scholar]
- 286. Maasjost J, Lüschow D, Kleine A, Hafez HM, Mühldorfer K. 2019. Presence of virulence genes in Enterococcus species isolated from meat turkeys in Germany does not correlate with chicken embryo lethality. Biomed Res Int 2019:6147695. doi: 10.1155/2019/6147695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Chandler JR, Dunny GM. 2004. Enterococcal peptide sex pheromones: synthesis and control of biological activity. Peptides 25:1377–1388. doi: 10.1016/j.peptides.2003.10.020 [DOI] [PubMed] [Google Scholar]
- 288. Sannomiya P, Craig RA, Clewell DB, Suzuki A, Fujino M, Till GO, Marasco WA. 1990. Characterization of a class of nonformylated Enterococcus faecalis-derived neutrophil chemotactic peptides: the sex pheromones. Proc Natl Acad Sci U S A 87:66–70. doi: 10.1073/pnas.87.1.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Ember JA, Hugli TE. 1989. Characterization of the human neutrophil response to sex pheromones from Streptococcus faecalis. Am J Pathol 134:797–805. [PMC free article] [PubMed] [Google Scholar]
- 290. Sillanpää J, Xu Y, Nallapareddy SR, Murray BE, Höök M. 2004. A family of putative MSCRAMMs from Enterococcus faecalis. Microbiology 150:2069–2078. doi: 10.1099/mic.0.27074-0 [DOI] [PubMed] [Google Scholar]
- 291. Rich RL, Kreikemeyer B, Owens RT, LaBrenz S, Narayana SVL, Weinstock GM, Murray BE, Höök M. 1999. Ace is a collagen-binding MSCRAMM from Enterococcus faecalis. J Biol Chem 274:26939–26945. doi: 10.1074/jbc.274.38.26939 [DOI] [PubMed] [Google Scholar]
- 292. Thurlow LR, Thomas VC, Narayanan S, Olson S, Fleming SD, Hancock LE. 2010. Gelatinase contributes to the pathogenesis of endocarditis caused by Enterococcus faecalis. Infect Immun 78:4936–4943. doi: 10.1128/IAI.01118-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Macovei L, Ghosh A, Thomas VC, Hancock LE, Mahmood S, Zurek L. 2009. Enterococcus faecalis with the gelatinase phenotype regulated by the fsr operon and with biofilm‐forming capacity are common in the agricultural environment. Environ Microbiol 11:1540–1547. doi: 10.1111/j.1462-2920.2009.01881.x [DOI] [PubMed] [Google Scholar]
- 294. Thomas VC, Thurlow LR, Boyle D, Hancock LE. 2008. Regulation of autolysis-dependent extracellular DNA release by Enterococcus faecalis extracellular proteases influences biofilm development. J Bacteriol 190:5690–5698. doi: 10.1128/JB.00314-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Engelbert M, Mylonakis E, Ausubel FM, Calderwood SB, Gilmore MS. 2004. Contribution of gelatinase, serine protease, and fsr to the pathogenesis of Enterococcus faecalis endophthalmitis. Infect Immun 72:3628–3633. doi: 10.1128/IAI.72.6.3628-3633.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. García-Solache M, Rice LB. 2019. The Enterococcus: a model of adaptability to its environment. Clin Microbiol Rev 32:e00058-18. doi: 10.1128/CMR.00058-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Donskey CJ, Hanrahan JA, Hutton RA, Rice LB. 2000. Effect of parenteral antibiotic administration on the establishment of colonization with vancomycin-resistant Enterococcus faecium in the mouse gastrointestinal tract. J Infect Dis 181:1830–1833. doi: 10.1086/315428 [DOI] [PubMed] [Google Scholar]
- 298. Rice LB. 2001. Emergence of vancomycin-resistant enterococci. Emerg Infect Dis 7:183–187. doi: 10.3201/eid0702.010205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Quiñones D, Goñi P, Rubio MC, Duran E, Gómez-Lus R. 2005. Enterococci spp. isolated from Cuba: species frequency of occurrence and antimicrobial susceptibility profile. Diagn Microbiol Infect Dis 51:63–67. doi: 10.1016/j.diagmicrobio.2004.08.018 [DOI] [PubMed] [Google Scholar]
- 300. Kapoor L, Randhawa VS, Deb M. 2005. Antimicrobial resistance of enterococcal blood isolates at a pediatric care hospital in India. Jpn J Infect Dis 58:101–103. doi: 10.7883/yoken.JJID.2005.101 [DOI] [PubMed] [Google Scholar]
- 301. Karlowsky JA, Zhanel GG, Hoban DJ, the Canadian VRE Surveillance Group . 1999. Vancomycin-resistant enterococci (VRE) colonization of high-risk patients in tertiary care Canadian hospitals. Diagn Microbiol Infect Dis 35:1–7. doi: 10.1016/s0732-8893(99)00045-0 [DOI] [PubMed] [Google Scholar]
- 302. Tuhina B, Anupurba S, Karuna T. 2016. Emergence of antimicrobial resistance and virulence factors among the unusual species of enterococci, from north India. Indian J Pathol Microbiol 59:50–55. doi: 10.4103/0377-4929.174795 [DOI] [PubMed] [Google Scholar]
- 303. Oguri T, Misawa S, Nakamura A, Igari J, Ishii Y, Yamaguchi K. 2001. Epidemiological study on vancomycin-resistant enterococci from fecal samples in the east area of Japan. Kansenshogaku Zasshi 75:541–550. doi: 10.11150/kansenshogakuzasshi1970.75.541 [DOI] [PubMed] [Google Scholar]
- 304. Hassan RM, Ghaith DM, Ismail DK, Zafer MM. 2018. Reduced susceptibility of Enterococcus spp. isolates from Cairo University hospital to tigecycline: highlight on the influence of proton pump inhibitors. J Glob Antimicrob Resist 12:68–72. doi: 10.1016/j.jgar.2017.12.005 [DOI] [PubMed] [Google Scholar]
- 305. Prakash VP, Rao SR, Parija SC. 2005. Emergence of unusual species of enterococci causing infections, south India. BMC Infect Dis 5:1–8. doi: 10.1186/1471-2334-5-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Cai J, Wang Y, Schwarz S, Lv H, Li Y, Liao K, Yu S, Zhao K, Gu D, Wang X, Zhang R, Shen J. 2015. Enterococcal isolates carrying the novel oxazolidinone resistance gene optrA from hospitals in Zhejiang, Guangdong, and Henan, China, 2010–2014. Clin Microbiol Infect 21:1095. doi: 10.1016/j.cmi.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 307. Gordon S, Swenson JM, Hill BC, Pigott NE, Facklam RR, Cooksey RC, Thornsberry C, Jarvis WR, Tenover FC. 1992. Antimicrobial susceptibility patterns of common and unusual species of enterococci causing infections in the United States. Enterococcal Study Group. J Clin Microbiol 30:2373–2378. doi: 10.1128/jcm.30.9.2373-2378.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Jolivet S, Fines-Guyon M, Nebbad B, Merle JC, Le Pluart D, Brun-Buisson C, Decousser J-W, Cattoir V. 2016. First nosocomial outbreak of vanA-type vancomycin-resistant Enterococcus raffinosus in France. J Hosp Infect 94:346–350. doi: 10.1016/j.jhin.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 309. Lee S-C, Wu M-S, Shih H-J, Huang S-H, Chiou M-J, See L-C, Siu L-K. 2013. Identification of vancomycin-resistant enterococci clones and inter-hospital spread during an outbreak in Taiwan. BMC Infect Dis 13:1–6. doi: 10.1186/1471-2334-13-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Tanimoto K, Nomura T, Maruyama H, Tomita H, Shibata N, Arakawa Y, Ike Y. 2006. First VanD-type vancomycin-resistant Enterococcus raffinosus isolate. Antimicrob Agents Chemother 50:3966–3967. doi: 10.1128/AAC.00607-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Kuzma J, Palcová L, Timko J, Bastová V, Janošcová V, Chmelař D. 2022. Detection and molecular characterization of VRE isolates in Slovakia from stool samples positive for Clostridioides difficile toxins. Folia Microbiol (Praha) 67:975–984. doi: 10.1007/s12223-022-01002-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Green MR, Anasetti C, Sandin RL, Rolfe NE, Greene JN. 2006. Development of daptomycin resistance in a bone marrow transplant patient with vancomycin-resistant Enterococcus durans. J Oncol Pharm Pract 12:179–181. doi: 10.1177/1078155206069165 [DOI] [PubMed] [Google Scholar]
- 313. Pfaller MA, Mendes RE, Duncan LR, Flamm RK, Sader HS. 2018. Activity of dalbavancin and comparator agents against Gram-positive cocci from clinical infections in the USA and Europe 2015–16. J Antimicrob Chemother 73:2748–2756. doi: 10.1093/jac/dky235 [DOI] [PubMed] [Google Scholar]
- 314. Gawryszewska I, Żabicka D, Hryniewicz W, Sadowy E. 2017. Linezolid-resistant enterococci in Polish hospitals: species, clonality and determinants of linezolid resistance. Eur J Clin Microbiol Infect Dis 36:1279–1286. doi: 10.1007/s10096-017-2934-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Pfaller M, Mendes RE, Streit JM, Carvalhaes CG. 2022. Tedizolid in vitro activity against Gram-positive clinical isolates causing bone and joint infections in hospitals in the USA, Europe, Latin America, and the Asia-Pacific region (2015-2019). Diagn Microbiol Infect Dis 104:115804. doi: 10.1016/j.diagmicrobio.2022.115804 [DOI] [PubMed] [Google Scholar]
- 316. Kaplan AH, Gilligan PH, Facklam RR. 1988. Recovery of resistant enterococci during vancomycin prophylaxis. J Clin Microbiol 26:1216–1218. doi: 10.1128/jcm.26.6.1216-1218.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Roche Y, Yoshimori RN. 1997. In-vitro activity of spiramycin and metronidazole alone or in combination against clinical isolates from odontogenic abscesses. J Antimicrob Chemother 40:353–357. doi: 10.1093/jac/40.3.353 [DOI] [PubMed] [Google Scholar]
- 318. Rafii F, Wynne R, Heinze TM, Paine DD. 2003. Mechanism of metronidazole-resistance by isolates of nitroreductase-producing Enterococcus gallinarum and Enterococcus casseliflavus from the human intestinal tract. FEMS Microbiol Lett 225:195–200. doi: 10.1016/S0378-1097(03)00513-5 [DOI] [PubMed] [Google Scholar]
- 319. Tripodi MF, Utili R, Rambaldi A, Locatelli A, Rosario P, Florio A, Ruggiero G. 1996. Unorthodox antibiotic combinations including ciprofloxacin against high-level gentamicin resistant enterococci. J Antimicrob Chemother 37:727–736. doi: 10.1093/jac/37.4.727 [DOI] [PubMed] [Google Scholar]
- 320. Rice LB, Carias LL, Hutton-Thomas R, Sifaoui F, Gutmann L, Rudin SD. 2001. Penicillin-binding protein 5 and expression of ampicillin resistance in Enterococcus faecium. Antimicrob Agents Chemother 45:1480–1486. doi: 10.1128/AAC.45.5.1480-1486.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Schwaiger K, Bauer J, Hörmansdorfer S, Mölle G, Preikschat P, Kämpf P, Bauer-Unkauf I, Bischoff M, Hölzel C. 2012. Presence of the resistance genes vanC1 and pbp5 in phenotypically vancomycin and ampicillin susceptible Enterococcus faecalis. Microb Drug Resist 18:434–439. doi: 10.1089/mdr.2011.0227 [DOI] [PubMed] [Google Scholar]
- 322. Fontana R, Cerini R, Longoni P, Grossato A, Canepari P. 1983. Identification of a streptococcal penicillin-binding protein that reacts very slowly with penicillin. J Bacteriol 155:1343–1350. doi: 10.1128/jb.155.3.1343-1350.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Arbeloa A, Segal H, Hugonnet J-E, Josseaume N, Dubost L, Brouard J-P, Gutmann L, Mengin-Lecreulx D, Arthur M. 2004. Role of class A penicillin-binding proteins in PBP5-mediated β-lactam resistance in Enterococcus faecalis. J Bacteriol 186:1221–1228. doi: 10.1128/JB.186.5.1221-1228.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Vesić D, Kristich CJ. 2012. MurAA is required for intrinsic cephalosporin resistance of Enterococcus faecalis. Antimicrob Agents Chemother 56:2443–2451. doi: 10.1128/AAC.05984-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Comenge Y, Quintiliani Jr R, Li L, Dubost L, Brouard J-P, Hugonnet J-E, Arthur M. 2003. The CroRS two-component regulatory system is requiredfor intrinsic β-lactam resistance in Enterococcus faecalis. J Bacteriol 185:7184–7192. doi: 10.1128/JB.185.24.7184-7192.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Miller WR, Munita JM, Arias CA. 2014. Mechanisms of antibiotic resistance in enterococci. Expert Rev Anti Infect Ther 12:1221–1236. doi: 10.1586/14787210.2014.956092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Pensinger DA, Schaenzer AJ, Sauer J-D. 2018. Do shoot the messenger: PASTA kinases as virulence determinants and antibiotic targets. Trends Microbiol 26:56–69. doi: 10.1016/j.tim.2017.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Kristich CJ, Little JL, Hall CL, Hoff JS. 2011. Reciprocal regulation of cephalosporin resistance in Enterococcus faecalis. mBio 2:e00199-11. doi: 10.1128/mBio.00199-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Sacco E, Cortes M, Josseaume N, Rice LB, Mainardi J-L, Arthur M. 2014. Serine/threonine protein phosphatase-mediated control of the peptidoglycan cross-linking L, D-transpeptidase pathway in Enterococcus faecium. mBio 5:e01446-14. doi: 10.1128/mBio.01446-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330. Lebreton F, van Schaik W, Sanguinetti M, Posteraro B, Torelli R, Le Bras F, Verneuil N, Zhang X, Giard J-C, Dhalluin A, Willems RJL, Leclercq R, Cattoir V. 2012. AsrR is an oxidative stress sensing regulator modulating Enterococcus faecium opportunistic traits, antimicrobial resistance, and pathogenicity. PLoS Pathog 8:e1002834. doi: 10.1371/journal.ppat.1002834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Gaca AO, Lemos JA. 2019. Adaptation to adversity: the intermingling of stress tolerance and pathogenesis in enterococci. Microbiol Mol Biol Rev 83:e00008-19. doi: 10.1128/MMBR.00008-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Murray BE, Mederski-Samaroj B. 1983. Transferable beta-lactamase. A new mechanism for in vitro penicillin resistance in Streptococcus faecalis. J Clin Invest 72:1168–1171. doi: 10.1172/JCI111042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Abouzaid A zahraa A, Abd Al-azeem MW, mohamed HMA. 2022. Molecular detection of virulence and resistance genes of Enterococcus species isolated from milk and milk products. SVU-Int J Vet Sci 5:1–17. doi: 10.21608/svu.2022.126055.1182 [DOI] [Google Scholar]
- 334. Di Cesare A, Vignaroli C, Luna GM, Pasquaroli S, Biavasco F. 2012. Antibiotic-resistant enterococci in seawater and sediments from a coastal fish farm. Microb Drug Resist 18:502–509. doi: 10.1089/mdr.2011.0204 [DOI] [PubMed] [Google Scholar]
- 335. Wennersten C, Moellering Jr R. 1980. Mechanism of resistance to penicillin-aminoglycoside synergism in Streptococcus faecium. Curr Chemother Infect Dis 1:710–712. [DOI] [PubMed] [Google Scholar]
- 336. Galimand M, Schmitt E, Panvert M, Desmolaize B, Douthwaite S, Mechulam Y, Courvalin P. 2011. Intrinsic resistance to aminoglycosides in Enterococcus faecium is conferred by the 16S rRNA m5C1404-specific methyltransferase EfmM. RNA 17:251–262. doi: 10.1261/rna.2233511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. McKay GA, Thompson PR, Wright GD. 1994. Broad spectrum aminoglycoside phosphotransferase type III from Enterococcus: overexpression, purification, and substrate specificity. Biochemistry 33:6936–6944. doi: 10.1021/bi00188a024 [DOI] [PubMed] [Google Scholar]
- 338. Kobayashi N, Alam MM, Nishimoto Y, Urasawa S, Uehara N, Watanabe N. 2001. Distribution of aminoglycoside resistance genes in recent clinical isolates of Enterococcus faecalis, Enterococcus faecium and Enterococcus avium. Epidemiol Infect 126:197–204. doi: 10.1017/s0950268801005271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Woegerbauer M, Zeinzinger J, Springer B, Hufnagl P, Indra A, Korschineck I, Hofrichter J, Kopacka I, Fuchs R, Steinwider J, Fuchs K, Nielsen KM, Allerberger F. 2014. Prevalence of the aminoglycoside phosphotransferase genes aph (3′)-IIIa and aph (3′)-IIa in Escherichia coli, Enterococcus faecalis, Enterococcus faecium, Pseudomonas aeruginosa, Salmonella enterica subsp. enterica and Staphylococcus aureus isolates in Austria. J Med Microbiol 63:210–217. doi: 10.1099/jmm.0.065789-0 [DOI] [PubMed] [Google Scholar]
- 340. Padmasini E, Padmaraj R, Ramesh SS. 2014. High level aminoglycoside resistance and distribution of aminoglycoside resistant genes among clinical isolates of Enterococcus species in Chennai, India. Sci World J 2014:329157. doi: 10.1155/2014/329157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Kim YB, Seo KW, Shim JB, Son SH, Noh EB, Lee YJ. 2019. Molecular characterization of antimicrobial-resistant Enterococcus faecalis and Enterococcus faecium isolated from layer parent stock. Poult Sci 98:5892–5899. doi: 10.3382/ps/pez288 [DOI] [PubMed] [Google Scholar]
- 342. Osuka H, Nakajima J, Oishi T, Funayama Y, Ebihara T, Ishikawa H, Saito K, Koganemaru H, Hitomi S. 2016. High-level aminoglycoside resistance in Enterococcus faecalis and Enterococcus faecium causing invasive infection: twelve-year surveillance in the Minami Ibaraki area. J Infect Chemother 22:61–63. doi: 10.1016/j.jiac.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 343. Nüesch-Inderbinen M, Biggel M, Zurfluh K, Treier A, Stephan R. 2022. Faecal carriage of enterococci harbouring oxazolidinone resistance genes among healthy humans in the community in Switzerland. J Antimicrob Chemother 77:2779–2783. doi: 10.1093/jac/dkac260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Kanchugal P S, Selmer M. 2020. Structural recognition of spectinomycin by resistance enzyme ANT (9) from Enterococcus faecalis. Antimicrob Agents Chemother 64:e00371-20. doi: 10.1128/AAC.00371-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. LeBlanc DJ, Lee LN, Inamine JM. 1991. Cloning and nucleotide base sequence analysis of a spectinomycin adenyltransferase AAD (9) determinant from Enterococcus faecalis. Antimicrob Agents Chemother 35:1804–1810. doi: 10.1128/AAC.35.9.1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Mahbub Alam M, Kobayashi N, Ishino M, Sumi A, Kobayashi K-I, Uehara N, Watanabe N. 2005. Detection of a novel aph (2") allele (aph [2"]-Ie) conferring high-level gentamicin resistance and a spectinomycin resistance gene ant (9)-Ia (aad9) in clinical isolates of enterococci. Microb Drug Resist 11:239–247. doi: 10.1089/mdr.2005.11.239 [DOI] [PubMed] [Google Scholar]
- 347. Yao T-G, Li B-Y, Luan R-D, Wang H-N, Lei C-W. 2020. Whole genome sequence of Enterococcus gallinarum EG81, a porcine strain harbouring the oxazolidinone-phenicol resistance gene optrA with chromosomal and plasmid location. J Glob Antimicrob Resist 22:598–600. doi: 10.1016/j.jgar.2020.06.012 [DOI] [PubMed] [Google Scholar]
- 348. Fatoba DO, Amoako DG, Akebe ALK, Ismail A, Essack SY. 2022. Genomic analysis of antibiotic-resistant Enterococcus spp. reveals novel enterococci strains and the spread of plasmid-borne tet(M), tet(L) and erm(B) genes from chicken litter to agricultural soil in South Africa. J Environ Manage 302:114101. doi: 10.1016/j.jenvman.2021.114101 [DOI] [PubMed] [Google Scholar]
- 349. Ferretti JJ, Gilmore KS, Courvalin P. 1986. Nucleotide sequence analysis of the gene specifying the bifunctional 6'-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol 167:631–638. doi: 10.1128/jb.167.2.631-638.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Watanabe S, Kobayashi N, Quiñones D, Nagashima S, Uehara N, Watanabe N. 2009. Genetic diversity of enterococci harboring the high-level gentamicin resistance gene aac (6′)-Ie-aph (2 ″)-Ia or aph (2 ″)-Ie in a Japanese hospital. Microb Drug Resist 15:185–194. doi: 10.1089/mdr.2009.0917 [DOI] [PubMed] [Google Scholar]
- 351. Chen Y-G, Qu T-T, Yu Y-S, Zhou J-Y, Li L-J. 2006. Insertion sequence IS Ecp1-like element connected with a novel aph (2 ″) allele [aph (2 ″)-Ie] conferring high-level gentamicin resistance and a novel streptomycin adenylyltransferase gene in Enterococcus. J Med Microbiol 55:1521–1525. doi: 10.1099/jmm.0.46702-0 [DOI] [PubMed] [Google Scholar]
- 352. Schouten MA, Hoogkamp-Korstanje JA, Meis JF, Voss A, European VRE Study Group . 2000. Prevalence of vancomycin-resistant enterococci in Europe. Eur J Clin Microbiol Infect Dis 19:816–822. doi: 10.1007/s100960000390 [DOI] [PubMed] [Google Scholar]
- 353. Kawalec M, Kedzierska J, Gajda A, Sadowy E, Wegrzyn J, Naser S, Skotnicki AB, Gniadkowski M, Hryniewicz W. 2007. Hospital outbreak of vancomycin-resistant enterococci caused by a single clone of Enterococcus raffinosus and several clones of Enterococcus faecium. Clin Microbiol Infect 13:893–901. doi: 10.1111/j.1469-0691.2007.01774.x [DOI] [PubMed] [Google Scholar]
- 354. Descheemaeker P, Ieven M, Chapelle S, Lammens C, Hauchecorne M, Wijdooghe M, Vandamme P, Goossens H. 2000. Prevalence and molecular epidemiology of glycopeptide-resistant enterococci in Belgian renal dialysis units. J Infect Dis 181:235–241. doi: 10.1086/315182 [DOI] [PubMed] [Google Scholar]
- 355. Harada T, Kawahara R, Kanki M, Taguchi M, Kumeda Y. 2012. Isolation and characterization of vanA genotype vancomycin-resistant Enterococcus cecorum from retail poultry in Japan. Int J Food Microbiol 153:372–377. doi: 10.1016/j.ijfoodmicro.2011.11.029 [DOI] [PubMed] [Google Scholar]
- 356. Depardieu F, Kolbert M, Pruul H, Bell J, Courvalin P. 2004. VanD-type vancomycin-resistant Enterococcus faecium and Enterococcus faecalis. Antimicrob Agents Chemother 48:3892–3904. doi: 10.1128/AAC.48.10.3892-3904.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Depardieu F, Foucault M-L, Bell J, Dubouix A, Guibert M, Lavigne J-P, Levast M, Courvalin P. 2009. New combinations of mutations in VanD-type vancomycin-resistant Enterococcus faecium, Enterococcus faecalis, and Enterococcus avium strains. Antimicrob Agents Chemother 53:1952–1963. doi: 10.1128/AAC.01348-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Boyd DA, Miller MA, Mulvey MR. 2006. Enterococcus gallinarum N04-0414 harbors a VanD-type vancomycin resistance operon and does not contain a d-alanine: d-alanine 2 (ddl2) gene. Antimicrob Agents Chemother 50:1067–1070. doi: 10.1128/AAC.50.3.1067-1070.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Nomura T, Tanimoto K, Shibayama K, Arakawa Y, Fujimoto S, Ike Y, Tomita H. 2012. Identification of VanN-type vancomycin resistance in an Enterococcus faecium isolate from chicken meat in Japan. Antimicrob Agents Chemother 56:6389–6392. doi: 10.1128/AAC.00747-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Boyd DA, Lévesque S, Picard A-C, Golding GR. 2015. Vancomycin-resistant Enterococcus faecium harbouring vanN in Canada: a case and complete sequence of pEfm12493 harbouring the vanN operon. J Antimicrob Chemother 70:2163–2165. doi: 10.1093/jac/dkv057 [DOI] [PubMed] [Google Scholar]
- 361. Lebreton F, Valentino MD, Schaufler K, Earl AM, Cattoir V, Gilmore MS. 2018. Transferable vancomycin resistance in clade B commensal-type Enterococcus faecium. J Antimicrob Chemother 73:1479–1486. doi: 10.1093/jac/dky039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Frazzon APG, Gama BA, Hermes V, Bierhals CG, Pereira RI, Guedes AG, d’Azevedo PA, Frazzon J. 2010. Prevalence of antimicrobial resistance and molecular characterization of tetracycline resistance mediated by tet (M) and tet(L) genes in Enterococcus spp. isolated from food in southern Brazil. World J Microbiol Biotechnol 26:365–370. doi: 10.1007/s11274-009-0160-x [DOI] [Google Scholar]
- 363. Ayeni FA, Odumosu BT, Oluseyi AE, Ruppitsch W. 2016. Identification and prevalence of tetracycline resistance in enterococci isolated from poultry in Ilishan, Ogun State, Nigeria. J Pharm Bioallied Sci 8:69–73. doi: 10.4103/0975-7406.171729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Fiedler S, Bender JK, Klare I, Halbedel S, Grohmann E, Szewzyk U, Werner G. 2016. Tigecycline resistance in clinical isolates of Enterococcus faecium is mediated by an upregulation of plasmid-encoded tetracycline determinants tet(L) and tet(M). J Antimicrob Chemother 71:871–881. doi: 10.1093/jac/dkv420 [DOI] [PubMed] [Google Scholar]
- 365. Cattoir V, Isnard C, Cosquer T, Odhiambo A, Bucquet F, Guérin F, Giard J-C. 2015. Genomic analysis of reduced susceptibility to tigecycline in Enterococcus faecium. Antimicrob Agents Chemother 59:239–244. doi: 10.1128/AAC.04174-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Niebel M, Quick J, Prieto AMG, Hill RLR, Pike R, Huber D, David M, Hornsey M, Wareham D, Oppenheim B, Woodford N, van Schaik W, Loman N. 2015. Deletions in a ribosomal protein-coding gene are associated with tigecycline resistance in Enterococcus faecium. Int J Antimicrob Agents 46:572–575. doi: 10.1016/j.ijantimicag.2015.07.009 [DOI] [PubMed] [Google Scholar]
- 367. Lin Z, Pu Z, Xu G, Bai B, Chen Z, Sun X, Zheng J, Li P, Qu D, Deng Q, Yu Z. 2020. Omadacycline efficacy against Enterococcus faecalis isolated in China: in vitro activity, heteroresistance, and resistance mechanisms. Antimicrob Agents Chemother 64:e02097-19. doi: 10.1128/AAC.02097-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Isnard C, Malbruny B, Leclercq R, Cattoir V. 2013. Genetic basis for in vitro and in vivo resistance to lincosamides, streptogramins A, and pleuromutilins (LSAP phenotype) in Enterococcus faecium. Antimicrob Agents Chemother 57:4463–4469. doi: 10.1128/AAC.01030-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Singh KV, Weinstock GM, Murray BE. 2002. An Enterococcus faecalis ABC homologue (Lsa) is required for the resistance of this species to clindamycin and quinupristin-dalfopristin. Antimicrob Agents Chemother 46:1845–1850. doi: 10.1128/AAC.46.6.1845-1850.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370. Portillo A, Ruiz-Larrea F, Zarazaga M, Alonso A, Martinez JL, Torres C. 2000. Macrolide resistance genes in Enterococcus spp. Antimicrob Agents Chemother 44:967–971. doi: 10.1128/AAC.44.4.967-971.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Clancy J, Petitpas J, Dib-Hajj F, Yuan W, Cronan M, Kamath AV, Bergeron J, Retsema JA. 1996. Molecular cloning and functional analysis of a novel macrolide‐resistance determinant, mefA, from Streptococcus pyogenes. Mol Microbiol 22:867–879. doi: 10.1046/j.1365-2958.1996.01521.x [DOI] [PubMed] [Google Scholar]
- 372. Tait-Kamradt A, Clancy J, Cronan M, Dib-Hajj F, Wondrack L, Yuan W, Sutcliffe J. 1997. mefE is necessary for the erythromycin-resistant M phenotype in Streptococcus pneumoniae. Antimicrob Agents Chemother 41:2251–2255. doi: 10.1128/AAC.41.10.2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Ross JI, Eady EA, Cove JH, Cunliffe WJ, Baumberg S, Wootton JC. 1990. Inducible erythromycin resistance in staphlyococci is encoded by a member of the ATP‐binding transport super‐gene family. Mol Microbiol 4:1207–1214. doi: 10.1111/j.1365-2958.1990.tb00696.x [DOI] [PubMed] [Google Scholar]
- 374. Clancy J, Dib-Hajj F, Petitpas JW, Yuan W. 1997. Cloning and characterization of a novel macrolide efflux gene, mreA, from Streptococcus agalactiae. Antimicrob Agents Chemother 41:2719–2723. doi: 10.1128/AAC.41.12.2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375. Na S, Park HJ, Park K-H, Cho O-H, Chong YP, Kim S-H, Lee S-O, Sung H, Kim M-N, Jeong J-Y, Kim YS, Woo JH, Choi S-H. 2012. Enterococcus avium bacteremia: a 12-year clinical experience with 53 patients. Eur J Clin Microbiol Infect Dis 31:303–310. doi: 10.1007/s10096-011-1311-1 [DOI] [PubMed] [Google Scholar]
- 376. Freitas AR, Novais C, Duarte B, Pereira AP, Coque TM, Peixe L. 2018. High rates of colonisation by ampicillin-resistant enterococci in residents of long-term care facilities in Porto, Portugal. Int J Antimicrob Agents 51:503–507. doi: 10.1016/j.ijantimicag.2017.11.007 [DOI] [PubMed] [Google Scholar]
- 377. Milanović V, Osimani A, Cardinali F, Litta-Mulondo A, Vignaroli C, Citterio B, Mangiaterra G, Aquilanti L, Garofalo C, Biavasco F, Cocolin L, Ferrocino I, Di Cagno R, Turroni S, Lazzi C, Pellegrini N, Clementi F. 2019. Erythromycin-resistant lactic acid bacteria in the healthy gut of vegans, ovo-lacto vegetarians and omnivores. PLoS One 14:e0220549. doi: 10.1371/journal.pone.0220549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Zerrouki H, Rebiahi S-A, Hadjadj L, Ahlem F, Elhabiri Y, Sedrati T, Rolain J-M, Diene SM. 2021. High frequency and diversity of vancomycin-resistant enterococci (VRE) in Algerian healthcare settings. Infect Genet Evol 92:104889. doi: 10.1016/j.meegid.2021.104889 [DOI] [PubMed] [Google Scholar]
- 379. Depardieu F, Foucault M-L, Bell J, Dubouix A, Guibert M, Lavigne J-P, Levast M, Courvalin P. 2009. New combinations of mutations in VanD-type vancomycin-resistant Enterococcus faecium, Enterococcus faecalis, and Enterococcus avium strains. Antimicrob Agents Chemother 53:1952–1963. doi: 10.1128/AAC.01348-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Abu Lila AS, Alharby TN, Alanazi J, Alanazi M, Abdallah MH, Rizvi SMD, Moin A, Khafagy E-S, Tabrez S, Al Balushi AA, Hegazy WAH. 2023. Clinical resistant strains of enterococci and their correlation to reduced susceptibility to biocides: phenotypic and genotypic analysis of macrolides, lincosamides, and streptogramins. Antibiotics (Basel) 12:461. doi: 10.3390/antibiotics12030461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Diarra MS, Rempel H, Champagne J, Masson L, Pritchard J, Topp E. 2010. Distribution of antimicrobial resistance and virulence genes in Enterococcus spp. and characterization of isolates from broiler chickens. Appl Environ Microbiol 76:8033–8043. doi: 10.1128/AEM.01545-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Jackson CR, Fedorka-Cray PJ, Barrett JB, Hiott LM, Woodley TA. 2007. Prevalence of streptogramin resistance in enterococci from animals: identification of vatD from animal sources in the USA. Int J Antimicrob Agents 30:60–66. doi: 10.1016/j.ijantimicag.2007.03.010 [DOI] [PubMed] [Google Scholar]
- 383. Soltani M, Beighton D, Philpott-Howard J, Woodford N. 2000. Mechanisms of resistance to quinupristin-dalfopristin among isolates of Enterococcus faecium from animals, raw meat, and hospital patients in Western Europe. Antimicrob Agents Chemother 44:433–436. doi: 10.1128/AAC.44.2.433-436.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. Arsène S, Leclercq R. 2007. Role of a qnr-like gene in the intrinsic resistance of Enterococcus faecalis to fluoroquinolones. Antimicrob Agents Chemother 51:3254–3258. doi: 10.1128/AAC.00274-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385. Sinel C, Cacaci M, Meignen P, Guérin F, Davies BW, Sanguinetti M, Giard J-C, Cattoir V. 2017. Subinhibitory concentrations of ciprofloxacin enhance antimicrobial resistance and pathogenicity of Enterococcus faecium. Antimicrob Agents Chemother 61:e02763-16. doi: 10.1128/AAC.02763-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Tran JH, Jacoby GA. 2002. Mechanism of plasmid-mediated quinolone resistance. Proc Natl Acad Sci U S A 99:5638–5642. doi: 10.1073/pnas.082092899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387. Cho S, Barrett JB, Frye JG, Jackson CR. 2020. Antimicrobial resistance gene detection and plasmid typing among multidrug resistant enterococci isolated from freshwater environment. Microorganisms 8:1338. doi: 10.3390/microorganisms8091338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. El Amin NA, Jalal S, Wretlind B. 1999. Alterations in GyrA and ParC associated with fluoroquinolone resistance in Enterococcus faecium. Antimicrob Agents Chemother 43:947–949. doi: 10.1128/AAC.43.4.947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Jonas BM, Murray BE, Weinstock GM. 2001. Characterization of emeA, a norA homolog and multidrug resistance efflux pump, in Enterococcus faecalis. Antimicrob Agents Chemother 45:3574–3579. doi: 10.1128/AAC.45.12.3574-3579.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Lee E-W, Huda MN, Kuroda T, Mizushima T, Tsuchiya T. 2003. EfrAB, an ABC multidrug efflux pump in Enterococcus faecalis. Antimicrob Agents Chemother 47:3733–3738. doi: 10.1128/AAC.47.12.3733-3738.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Mirzaii M, Alebouyeh M, Sohrabi MB, Eslami P, Fazli M, Ebrahimi M, HajiAsgarli P, Rashidan M. 2023. Antibiotic resistance assessment and multi-drug efflux pumps of Enterococcus faecium isolated from clinical specimens. J Infect Dev Ctries 17:649–655. doi: 10.3855/jidc.17304 [DOI] [PubMed] [Google Scholar]
- 392. Straus SK, Hancock REW. 2006. Mode of action of the new antibiotic for Gram-positive pathogens daptomycin: comparison with cationic antimicrobial peptides and lipopeptides. Biochim Biophys Acta 1758:1215–1223. doi: 10.1016/j.bbamem.2006.02.009 [DOI] [PubMed] [Google Scholar]
- 393. Palmer KL, Daniel A, Hardy C, Silverman J, Gilmore MS. 2011. Genetic basis for daptomycin resistance in enterococci. Antimicrob Agents Chemother 55:3345–3356. doi: 10.1128/AAC.00207-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Zeng W, Feng L, Qian C, Chen T, Wang S, Zhang Y, Zheng X, Wang L, Liu S, Zhou T, Sun Y. 2022. Acquisition of daptomycin resistance by Enterococcus faecium confers collateral sensitivity to glycopeptides. Front Microbiol 13:815600. doi: 10.3389/fmicb.2022.815600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395. Zarzecka U, Zakrzewski AJ, Chajęcka-Wierzchowska W, Zadernowska A. 2022. Linezolid-resistant Enterococcus spp. isolates from foods of animal origin—the genetic basis of acquired resistance. Foods 11:975. doi: 10.3390/foods11070975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396. Coccitto SN, Cinthi M, Fioriti S, Morroni G, Simoni S, Vignaroli C, Garofalo C, Mingoia M, Brenciani A, Giovanetti E. 2022. Linezolid-resistant Enterococcus gallinarum isolate of swine origin carrying cfr, optrA and poxtA genes. J Antimicrob Chemother 77:331–337. doi: 10.1093/jac/dkab408 [DOI] [PubMed] [Google Scholar]
- 397. Coccitto SN, Cinthi M, Simoni S, Vignaroli C, Massacci FR, Albini E, Garofalo C, Aquilanti L, Magistrali CF, Brenciani A, Giovanetti E. 2023. Identification of plasmids co-carrying cfr (D)/optrA and cfr (D2)/poxtA linezolid resistance genes in two Enterococcus avium isolates from swine brain. Vet Microbiol 282:109749. doi: 10.1016/j.vetmic.2023.109749 [DOI] [PubMed] [Google Scholar]
- 398. Liu Y, Wang Y, Dai L, Wu C, Shen J. 2014. First report of multiresistance gene cfr in Enterococcus species casseliflavus and gallinarum of swine origin. Vet Microbiol 170:352–357. doi: 10.1016/j.vetmic.2014.02.037 [DOI] [PubMed] [Google Scholar]
- 399. Cinthi M, Coccitto SN, Fioriti S, Morroni G, Simoni S, Vignaroli C, Magistrali CF, Albini E, Brenciani A, Giovanetti E. 2022. Occurrence of a plasmid co-carrying cfr(D) and poxtA2 linezolid resistance genes in Enterococcus faecalis and Enterococcus casseliflavus from porcine manure, Italy. J Antimicrob Chemother 77:598–603. doi: 10.1093/jac/dkab456 [DOI] [PubMed] [Google Scholar]
- 400. Shan X, Yang M, Wang N, Schwarz S, Li D, Du XD. 2022. Plasmid fusion and recombination events that occurred during conjugation of poxtA-carrying plasmids in enterococci. Microbiol Spectr 10:e0150521. doi: 10.1128/spectrum.01505-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Spratt BG. 1977. Properties of the penicillin‐binding proteins of Escherichia coli K12. Eur J Biochem 72:341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x [DOI] [PubMed] [Google Scholar]
- 402. Georgopapadakou NH. 1993. Penicillin-binding proteins and bacterial resistance to β-lactams. Antimicrob Agents Chemother 37:2045–2053. doi: 10.1128/AAC.37.10.2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403. Georgopapadakou NH, Liu FY. 1980. Penicillin-binding proteins in bacteria. Antimicrob Agents Chemother 18:148–157. doi: 10.1128/AAC.18.1.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Hancock LE, Perego M. 2004. Systematic inactivation and phenotypic characterization of two-component signal transduction systems of Enterococcus faecalis V583. J Bacteriol 186:7951–7958. doi: 10.1128/JB.186.23.7951-7958.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Timmler SB, Kellogg SL, Atkinson SN, Little JL, Djorić D, Kristich CJ. 2022. CroR regulates expression of pbp4(5) to promote cephalosporin resistance in Enterococcus faecalis. mBio 13:e0111922. doi: 10.1128/mbio.01119-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Chirurgi VA, Oster SE, Goldberg AA, Zervos MJ, McCabe RE. 1991. Ampicillin-resistant Enterococcus raffinosus in an acute-care hospital: case-control study and antimicrobial susceptibilities. J Clin Microbiol 29:2663–2665. doi: 10.1128/jcm.29.11.2663-2665.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Boyce JM, Opal SM, Potter-Bynoe G, LaForge RG, Zervos MJ, Furtado G, Victor G, Medeiros AA. 1992. Emergence and nosocomial transmission of ampicillin-resistant enterococci. Antimicrob Agents Chemother 36:1032–1039. doi: 10.1128/AAC.36.5.1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408. Chajęcka-Wierzchowska W, Zadernowska A, Łaniewska-Trokenheim Ł. 2016. Diversity of antibiotic resistance genes in Enterococcus strains isolated from ready‐to‐eat meat products. J Food Sci 81:M2799–M2807. doi: 10.1111/1750-3841.13523 [DOI] [PubMed] [Google Scholar]
- 409. Micallef SA, Goldstein RER, George A, Ewing L, Tall BD, Boyer MS, Joseph SW, Sapkota AR. 2013. Diversity, distribution and antibiotic resistance of Enterococcus spp. recovered from tomatoes, leaves, water and soil on US Mid-Atlantic farms. Food Microbiol 36:465–474. doi: 10.1016/j.fm.2013.04.016 [DOI] [PubMed] [Google Scholar]
- 410. Van Bambeke F. 2004. Glycopeptides in clinical development: pharmacological profile and clinical perspectives. Curr Opin Pharmacol 4:471–478. doi: 10.1016/j.coph.2004.04.006 [DOI] [PubMed] [Google Scholar]
- 411. Miller WR, Munita JM, Arias CA. 2014. Mechanisms of antibiotic resistance in enterococci. Expert Rev Anti Infect Ther 12:1221–1236. doi: 10.1586/14787210.2014.956092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412. Xavier BB, Coppens J, De Koster S, Rajakani SG, Van Goethem S, Mzougui S, Anantharajah A, Lammens C, Loens K, Glupczynski Y, Goossens H, Matheeussen V. 2021. Novel vancomycin resistance gene cluster in Enterococcus faecium ST1486, Belgium, June 2021. Euro Surveill 26. doi: 10.2807/1560-7917.ES.2021.26.36.2100767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413. Yasliani S, Mohabati Mobarez A, Hosseini Doust R, Satari M, Teymornejad O. 2009. Linezolid vancomycin resistant Enterococcus isolated from clinical samples in Tehran hospitals. Indian J Med Sci 63:297–302. doi: 10.4103/0019-5359.55117 [DOI] [PubMed] [Google Scholar]
- 414. Praharaj I, Sujatha S, Parija SC. 2013. Phenotypic & genotypic characterization of vancomycin resistant Enterococcus isolates from clinical specimens. Indian J Med Res 138:549–556. [PMC free article] [PubMed] [Google Scholar]
- 415. Kawalec M, Kedzierska J, Gajda A, Sadowy E, Wegrzyn J, Naser S, Skotnicki AB, Gniadkowski M, Hryniewicz W. 2007. Hospital outbreak of vancomycin-resistant enterococci caused by a single clone of Enterococcus raffinosus and several clones of Enterococcus faecium. Clin Microbiol Infect 13:893–901. doi: 10.1111/j.1469-0691.2007.01774.x [DOI] [PubMed] [Google Scholar]
- 416. Nishiyama M, Iguchi A, Suzuki Y. 2015. Identification of Enterococcus faecium and Enterococcus faecalis as vanC-type vancomycin-resistant enterococci (VRE) from sewage and river water in the provincial city of Miyazaki, Japan. J Environ Sci Health, Part A 50:16–25. doi: 10.1080/10934529.2015.964599 [DOI] [PubMed] [Google Scholar]
- 417. Fantin B, Leclercq R, Arthur M, Duval J, Carbon C. 1991. Influence of low-level resistance to vancomycin on efficacy of teicoplanin and vancomycin for treatment of experimental endocarditis due to Enterococcus faecium. Antimicrob Agents Chemother 35:1570–1575. doi: 10.1128/AAC.35.8.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418. Hashimoto Y, Kita I, Suzuki M, Hirakawa H, Ohtaki H, Tomita H. 2020. First report of the local spread of vancomycin-resistant enterococci ascribed to the interspecies transmission of a vanA gene cluster-carrying linear plasmid. mSphere 5:e00102-20. doi: 10.1128/mSphere.00102-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419. Chen M-Y, Lira F, Liang H-Q, Wu R-T, Duan J-H, Liao X-P, Martínez JL, Liu Y-H, Sun J. 2016. Multilevel selection of bcrABDR-mediated bacitracin resistance in Enterococcus faecalis from chicken farms. Sci Rep 6:34895. doi: 10.1038/srep34895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Chopra I, Roberts M. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev 65:232–260. doi: 10.1128/MMBR.65.2.232-260.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421. Pepper K, Horaud T, Le Bouguénec C, de Cespédès G. 1987. Location of antibiotic resistance markers in clinical isolates of Enterococcus faecalis with similar antibiotypes. Antimicrob Agents Chemother 31:1394–1402. doi: 10.1128/AAC.31.9.1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422. Bentorcha F, De Cespédès G, Horaud T. 1991. Tetracycline resistance heterogeneity in Enterococcus faecium. Antimicrob Agents Chemother 35:808–812. doi: 10.1128/AAC.35.5.808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423. Freitas AR, Novais C, Correia R, Monteiro M, Coque TM, Peixe L. 2011. Non-susceptibility to tigecycline in enterococci from hospitalised patients, food products and community sources. Int J Antimicrob Agents 38:174–176. doi: 10.1016/j.ijantimicag.2011.04.014 [DOI] [PubMed] [Google Scholar]
- 424. Zhanel GG, Esquivel J, Zelenitsky S, Lawrence CK, Adam HJ, Golden A, Hink R, Berry L, Schweizer F, Zhanel MA, Bay D, Lagacé-Wiens PRS, Walkty AJ, Lynch III JP, Karlowsky JA. 2020. Omadacycline: a novel oral and intravenous aminomethylcycline antibiotic agent. Drugs 80:285–313. doi: 10.1007/s40265-020-01257-4 [DOI] [PubMed] [Google Scholar]
- 425. Pfaller MA, Huband MD, Shortridge D, Flamm RK. 2021. Surveillance of omadacycline activity tested against clinical isolates from the USA: report from the SENTRY antimicrobial surveillance program, 2019. J Glob Antimicrob Resist 27:337–351. doi: 10.1016/j.jgar.2021.09.011 [DOI] [PubMed] [Google Scholar]
- 426. Carvalhaes CG, Huband MD, Reinhart HH, Flamm RK, Sader HS. 2019. Antimicrobial activity of omadacycline tested against clinical bacterial isolates from hospitals in mainland China, Hong Kong, and Taiwan: results from the SENTRY Antimicrobial Surveillance Program (2013 to 2016). Antimicrob Agents Chemother 63:e02262-18. doi: 10.1128/AAC.02262-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427. Bryskier A, Macrolides B-BE. 2005. Antimicrobial agents: antibacterials and antifungals, p 475–526 [Google Scholar]
- 428. Spížek J, Řezanka T. 2017. Lincosamides: chemical structure, biosynthesis, mechanism of action, resistance, and applications. Biochem Pharmacol 133:20–28. doi: 10.1016/j.bcp.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 429. Johnston NJ, Mukhtar TA, Wright GD. 2002. Streptogramin antibiotics: mode of action and resistance. Curr Drug Targets 3:335–344. doi: 10.2174/1389450023347678 [DOI] [PubMed] [Google Scholar]
- 430. Mankin AS. 2008. Macrolide myths. Curr Opin Microbiol 11:414–421. doi: 10.1016/j.mib.2008.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431. Leclercq R, Courvalin P. 1991. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob Agents Chemother 35:1267–1272. doi: 10.1128/AAC.35.7.1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432. Hummel A, Holzapfel WH, Franz C. 2007. Characterisation and transfer of antibiotic resistance genes from enterococci isolated from food. Syst Appl Microbiol 30:1–7. doi: 10.1016/j.syapm.2006.02.004 [DOI] [PubMed] [Google Scholar]
- 433. Jensen LB, Frimodt-Møller N, Aarestrup FM. 1999. Presence of erm gene classes in gram-positive bacteria of animal and human origin in Denmark. FEMS Microbiol Lett 170:151–158. doi: 10.1111/j.1574-6968.1999.tb13368.x [DOI] [PubMed] [Google Scholar]
- 434. Vignaroli C, Zandri G, Aquilanti L, Pasquaroli S, Biavasco F. 2011. Multidrug-resistant enterococci in animal meat and faeces and co-transfer of resistance from an Enterococcus durans to a human Enterococcus faecium. Curr Microbiol 62:1438–1447. doi: 10.1007/s00284-011-9880-x [DOI] [PubMed] [Google Scholar]
- 435. Cattoir V, Leclercq R. 2017. Resistance to macrolides, lincosamides, and streptogramins, p 269–280. In Antimicrobial drug resistance: mechanisms of drug resistance. Vol. 1. [DOI] [PubMed] [Google Scholar]
- 436. Rehman MA, Yin X, Zaheer R, Goji N, Amoako KK, McAllister T, Pritchard J, Topp E, Diarra MS. 2018. Genotypes and phenotypes of enterococci isolated from broiler chickens. Front Sustain Food Syst 2:83. doi: 10.3389/fsufs.2018.00083 [DOI] [Google Scholar]
- 437. Hooper DC. 2001. Emerging mechanisms of fluoroquinolone resistance. Emerg Infect Dis 7:337–341. doi: 10.3201/eid0702.010239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438. Petersen A, Jensen LB. 2004. Analysis of gyrA and parC mutations in enterococci from environmental samples with reduced susceptibility to ciprofloxacin. FEMS Microbiol Lett 231:73–76. doi: 10.1016/S0378-1097(03)00929-7 [DOI] [PubMed] [Google Scholar]
- 439. López M, Tenorio C, Del Campo R, Zarazaga M, Torres C. 2011. Characterization of the mechanisms of fluoroquinolone resistance in vancomycin-resistant enterococci of different origins. J Chemother 23:87–91. doi: 10.1179/joc.2011.23.2.87 [DOI] [PubMed] [Google Scholar]
- 440. Raaijmakers JM, De Bruijn I, Nybroe O, Ongena M. 2010. Natural functions of lipopeptides from Bacillus and Pseudomonas: more than surfactants and antibiotics. FEMS Microbiol Rev 34:1037–1062. doi: 10.1111/j.1574-6976.2010.00221.x [DOI] [PubMed] [Google Scholar]
- 441. Friedman DZP, Chesdachai S, Shweta FNU, Mahmood M. 2021. Enterococcus gallinarum endophthalmitis and meningitis in an allogeneic hematopoietic stem cell transplant patient: a case report and literature review. J Assoc Med Microbiol Infect Dis Can 6:313–318. doi: 10.3138/jammi-2021-0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442. Cho S, Hiott LM, McDonald JM, Barrett JB, McMillan EA, House SL, Adams ES, Frye JG, Jackson CR. 2020. Diversity and antimicrobial resistance of Enterococcus from the upper Oconee watershed, Georgia. J Appl Microbiol 128:1221–1233. doi: 10.1111/jam.14550 [DOI] [PubMed] [Google Scholar]
- 443. Long KS, Poehlsgaard J, Kehrenberg C, Schwarz S, Vester B. 2006. The Cfr rRNA methyltransferase confers resistance to phenicols, lincosamides, oxazolidinones, pleuromutilins, and streptogramin A antibiotics. Antimicrob Agents Chemother 50:2500–2505. doi: 10.1128/AAC.00131-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444. Deshpande LM, Ashcraft DS, Kahn HP, Pankey G, Jones RN, Farrell DJ, Mendes RE. 2015. Detection of a new cfr-like gene, cfr(B), in Enterococcus faecium isolates recovered from human specimens in the United States as part of the SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother 59:6256–6261. doi: 10.1128/AAC.01473-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445. Pang S, Boan P, Lee T, Gangatharan S, Tan SJ, Daley D, Lee YT, Coombs GW. 2020. Linezolid-resistant ST872 Enteroccocus faecium harbouring optrA and cfr(D) oxazolidinone resistance genes. Int J Antimicrob Agents 55:105831. doi: 10.1016/j.ijantimicag.2019.10.012 [DOI] [PubMed] [Google Scholar]
- 446. Wang Y, Lv Y, Cai J, Schwarz S, Cui L, Hu Z, Zhang R, Li J, Zhao Q, He T, Wang D, Wang Z, Shen Y, Li Y, Feßler AT, Wu C, Yu H, Deng X, Xia X, Shen J. 2015. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J Antimicrob Chemother 70:2182–2190. doi: 10.1093/jac/dkv116 [DOI] [PubMed] [Google Scholar]
- 447. Antonelli A, D’Andrea MM, Brenciani A, Galeotti CL, Morroni G, Pollini S, Varaldo PE, Rossolini GM. 2018. Characterization of poxtA, a novel phenicol–oxazolidinone–tetracycline resistance gene from an MRSA of clinical origin. J Antimicrob Chemother 73:1763–1769. doi: 10.1093/jac/dky088 [DOI] [PubMed] [Google Scholar]
- 448. Shen J, Wang Y, Schwarz S. 2013. Presence and dissemination of the multiresistance gene cfr in Gram-positive and Gram-negative bacteria. J Antimicrob Chemother 68:1697–1706. doi: 10.1093/jac/dkt092 [DOI] [PubMed] [Google Scholar]
- 449. Lazaris A, Coleman DC, Kearns AM, Pichon B, Kinnevey PM, Earls MR, Boyle B, O’Connell B, Brennan GI, Shore AC. 2017. Novel multiresistance cfr plasmids in linezolid-resistant methicillin-resistant Staphylococcus epidermidis and vancomycin-resistant Enterococcus faecium (VRE) from a hospital outbreak: co-location of cfr and optrA in VRE. J Antimicrob Chemother 72:3252–3257. doi: 10.1093/jac/dkx292 [DOI] [PubMed] [Google Scholar]
- 450. Morroni G, Brenciani A, Antonelli A, Maria D’Andrea M, Di Pilato V, Fioriti S, Mingoia M, Vignaroli C, Cirioni O, Biavasco F, Varaldo PE, Rossolini GM, Giovanetti E. 2018. Characterization of a multiresistance plasmid carrying the optrA and cfr resistance genes from an Enterococcus faecium clinical isolate. Front Microbiol 9:2189. doi: 10.3389/fmicb.2018.02189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451. Prystowsky J, Siddiqui F, Chosay J, Shinabarger DL, Millichap J, Peterson LR, Noskin GA. 2001. Resistance to linezolid: characterization of mutations in rRNA and comparison of their occurrences in vancomycin-resistant enterococci. Antimicrob Agents Chemother 45:2154–2156. doi: 10.1128/AAC.45.7.2154-2156.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452. Younus Z, Goyal SM, Singh V, Ikram A, Imran M. 2021. Genomic-based characterization of Enterococcus spp.: an emerging pathogen isolated from human gut. Mol Biol Rep 48:5371–5376. doi: 10.1007/s11033-021-06540-5 [DOI] [PubMed] [Google Scholar]
- 453. Baccani I, Antonelli A, Di Pilato V, Coppi M, Di Maggio T, Spinicci M, Villagran AL, Revollo C, Bartoloni A, Rossolini GM. 2021. Detection of poxtA2, a presumptive poxtA ancestor, in a plasmid from a linezolid-resistant Enterococcus gallinarum isolate. Antimicrob Agents Chemother 65:e0069521. doi: 10.1128/AAC.00695-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of species in the Enterococcus genus.