Italian pharmacologists Silvio Garattini and Vittorio Bertele' note that new anticancer drugs reaching the European market in 1995-2000 offered few or no substantial advantages over existing preparations, yet cost several times—in one case 350 times—as much
Though only an imperfect indicator of progress in cancer control,1 age standardised mortality in the European Union, for both sexes combined, had been increasing up to 1988; since then it has decreased from 147 to 136 per 100 000 inhabitants.2 Prevention is probably one of the main reasons for this drop, particularly the decrease in tobacco smoking; another reason is the use of screening for early diagnosis of cancers of the cervix and breast and possibly also of the colon and rectum..
The greatest changes have been 4500 fewer deaths from childhood tumours and 4000 fewer from lymphomas (Hodgkin's disease) each year over the past four decades. Among solid tumours, advances have been made in treating breast cancer, in which tamoxifen increases 10 year survival by 6% for node negative and 11% for node positive tumours,3 and chemotherapy increases survival by 7% and 11%, respectively.4 For most other common solid tumours such as those of lung, oesophagus, stomach, or pancreas, only limited survival gains have been achieved.2,5,6
On the whole, pharmacological treatments are credited with only a very small proportion of cures.7 However, some kind of pharmacological intervention is often considered as a last resort, particularly when cancer has already disseminated. We evaluated the progress made over the past few years in terms of new drugs approved for prescription in order to judge their likely impact on cancer mortality in the near future.
Summary points
Drugs approved in Europe in the first six years of activity of the European Medicines Evaluation Agency do not meet the expectations generated by the gains in basic knowledge on cancer cell proliferation and dissemination
To reach the market swiftly new drugs are often candidates for second or third line treatment of rare cancers, and they are evaluated in small phase II studies which assess their equivalence or non-inferiority (rather than superiority) to standard treatments
In spite of not improving survival, quality of life, or safety, these new drugs cost much more than the standard treatments
Clinical investigation must seek substantial advantages for patients in order to gain real benefit from future anticancer drugs
Methods
The European Medicines Evaluation Agency, set up in January 1995, has led to Europe-wide approval of the most important drugs, including anticancer medicines. Drugs approved by the agency are automatically marketable in 15 European countries. Despite their cost, new anticancer drugs will probably be used in a large proportion of patients.
We identified 12 anticancer drugs approved in the six years from 1995-2000 which contain new chemical entities or known active principles with new indications (table). The information on the new drugs was collected from several documents (available on www.emea.eu.int/index/indexh1.htm), including the European public assessment report, which describe the steps, reasons, and commitments for the approval of a given drug, and the summaries of product characteristics, the technical documents that report indications and adverse reactions for each drug. We calculated the costs of treatments on the basis of cost per cycle of therapy and compared costs, where possible, with those of reference drugs. Prices of drugs are those pertaining in Italy when available, converting the Italian lira at a rate of 1936.27 to the euro (£1.58, $1).
What defines a response to a drug?
To be of clinical interest a drug must provide measurable advantages to patients or to national health services
It should be more effective than placebo or any other available treatment; if there is no advantage in terms of efficacy, it should be at least safer, more tolerable, easier to use, or cheaper than active comparators
Outcome measures used should be objective, assessing survival or quality of life
Subjective end points such as the “time to progression” are liable to bias and should be avoided
New approvals
Analysis of the clinical trials reported in the European public assessment reports or summaries of product characteristics shows that anticancer agents are often approved on the basis of phase II trials. Few attempts are made to establish the value of the new drugs in relation to the reference drugs. The trials often look for “non-inferiority,” which entails recruiting only few patients and relatively short periods of observation. The end points tend to be subjective, such as the “time to progression”; seldom is there an evaluation of survival or quality of life. There is a tendency to seek the first approval for an indication for second or third line treatment in a relatively rare cancer in Europe: in three cases the indication was for Kaposi's sarcoma in patients with AIDS. There seem to be few innovative treatments for cancers of such sites as the colon and rectum, prostate, or pancreas.
As for safety, most drugs caused the usual signs of cytotoxicity, including neutropenia, thrombocytopenia, fever, infections, and gastrointestinal toxicity. In no instance did comparisons show a clear cut advantage, in terms of adverse reactions, over the reference drugs or analogous agents.
New approvals lead to expectations, fuelled by the pharmaceutical companies' direct and indirect promotion through the media, on the part of patients, their families, and their doctors, but these expectations may not be justified by the results of trials.7 At the time of approval the medicines agency may ask a company to carry out additional studies. The company may plead that off label use makes these difficult “for ethical reasons.” But it is for these ethical reasons that new drugs should be compared with existing drugs.
Costs
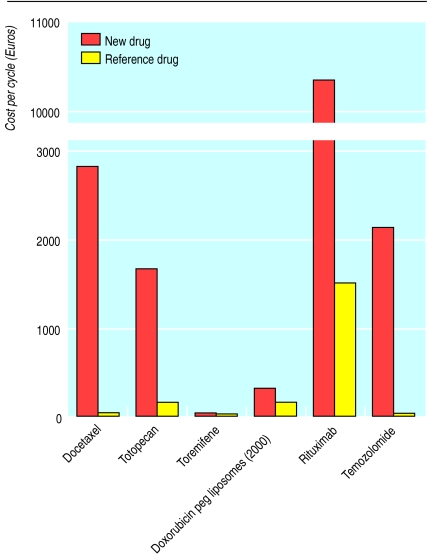
The approval of new drugs that offer no substantial advantages puts further burdens on national health services, insurers, and patients. The costs of the new preparations are several times—sometimes an order of magnitude—higher than those of existing drugs. This increase is difficult to justify, considering that the newer drugs are usually largely equivalent to the standard treatments in efficacy and safety. The lack of differences in activity makes any pharmacoeconomic evaluation virtually useless: it is difficult to explain why toremifene should cost more than twice as much as tamoxifen. Similarly, one temozolomide cycle costs about 350 times as much as a cycle of procarbazine, although there are serious doubts about the real efficacy of either treatment.9 In ovarian cancer, one cycle of topotecan costs over 10 times more than a cycle of cisplatin. The new drugs for advanced breast cancer cost 3-13 times as much as doxorubicin. Just to complicate matters further, pharmacoeconomic assessments of new anticancer drugs tend to be biased in their favour.10
None of the 12 drugs included in this list offers any significant improvement in activity. The liposomal preparation of doxorubicin may be less cardiotoxic, although cardiotoxicity does not seem to be an important limiting factor in the efficacy of doxorubicin.11 It has also been claimed that epirubicin is less cardiotoxic than doxorubicin.12
The monoclonal antibodies are completely new anticancer agents but their efficacy has yet to be confirmed by appropriate studies, while their safety seems unfavourable, contrary to all expectations.13 Perhaps the results will be better when some of these drugs are combined in new therapeutic schemes.
From these results over the past six years there is little to justify some of the promises made to the public. 7 It is widely expected that the general population of cancer patients not involved in clinical trials will gain no benefit from new anticancer drugs. It is to be hoped, however, that some new anticancer drugs, including resistant revertants, anti-angiogenic agents, pro-apoptotic drugs, and chemopreventive products, will soon undergo adequate clinical testing and show substantial benefits over current therapies.
Table.
Features of the 12 new anticancer drugs approved for marketing in the European Union from January 1995 to December 2000
| Active substance
|
Year of approval in EU
|
Indication
|
Mechanism of action
|
Basis of approval
|
Safety notes
|
Cost per cycle (€)*
|
||
|---|---|---|---|---|---|---|---|---|
| New drug
|
Reference drug
|
|||||||
| Docetaxel | 1995 | Advanced metastatic breast cancer (second line) | Increased microtubule assembly |
Response rate 56% (4.4% complete responses) in 117 patients from six pooled phase II studies Survival 15 months (v 14 months with doxorubicin) after alkylating agent failure in phase III trial in 326 patients Survival 11 months (v 9 months with doxorubicin-vinblastine) after anthracycline failure in 392 patients |
Mutagenicity positive Carcinogenicity not tested |
2836 | 15 (tamoxifen) |
|
| 1999 | Advanced metastatic non-small cell lung cancer |
Time to progression 12.3 weeks v 7 weeks with best supportive care; first year survival 40% v 16% (number of patients not reported in European public assessment report or the summary of product characteristics) | ||||||
| Totopecan | 1996 | Metastatic ovarian cancer (second line) | Inhibition of topoisomerase-1 | Response rates 10% (time to relapse <3months) and 16% (overall) in phase II non-comparative studies in 280 patients after cisplatin ± paclitaxel Response rate 20.5% (v 14% with paclitaxel), time to progression 19 weeks (v 15), and median survival 52 weeks (v 53) in a phase III open label study in 226 patients |
Mutagenicity positive Carcinogenicity not tested |
1674 | 158 (cisplatin) |
|
| Toremifene | 1996 | Advanced metastatic breast cancer (first line oestrogen receptor positive) | Anti-oestrogen | 40-60 mg toremifene was equivalent to 20-40 mg tamoxifen in 1869 patients (phase III trial and meta-analysis) | Mutagenicity negative Carcinogenicity negative |
37 | 15 (tamoxifen) |
|
| Doxorubicin peg liposomes |
1996 | Soft tissue sarcoma (second line) |
DNA intercalation | No difference compared with bleomycin-vincristine in 241 patients or bleomycin-vincristine-doxorubicin in 258 patients | Mutagenicity positive Carcinogenicity positive |
317 | ||
| 2000 | Metastatic ovarian cancer (second line) |
Not inferior to topotecan in a phase II study in 474 patients, but less active in patients refractory to platinum | 317 | 158 (cisplatin) |
||||
| Rituximab | 1998 | Follicular lymphomas (stages III and IV, resistant to previous chemotherapy) |
Binding to CD-20 | Response rate 48% (6% complete, 42% partial) in two phase II clinical trials (203 patients); effect similar to that of fludarabine or cladribine | Mutagenicity not tested Carcinogenicity not tested Cytokine release syndrome in over 50% of cases 39 fatalities and 66 serious adverse reactions in 12 000-14 000 treated patients |
10 359 | 1513 (fludarabine) |
|
| Temozolomide | 1998 | Glioblastoma and astrocytoma |
Alkylation of 06 and N7 guaniine |
Glioblastoma multiforme: median overall survival in 138 patients 5.4 months; median progression-free survival 2.9 months (v 1.9 with procarbazine); median overall survival in 225 patients 7.3 months (v 5.7) Anaplastic astrocytoma: median overall survival 14.6 months in phase II trial with 99 patients |
Mutagenicity positive Carcinogenicity positive |
2143 | 6 (procarbazine) |
|
| Tasonermin | 1999 | Soft tissue sarcoma (in combination with melphalan) |
Pleiotropic activity including inhibition of TNF-α-1α |
Equivalent to historical controls conventionally treated to prevent or delay amputation (phase II open label study in 126 patients, 13-17% complete responses, 50-69% partial) | Mutagenicity negative Carcinogenicity not tested |
|||
| Paclitaxel | 1999 | Soft tissue sarcoma (second line) |
Microtubule network reorganisation inhibition |
Response rate 57%, median time to progression 468 days, in 107 patients (67 resistant to doxorubicin liposomes) | Mutagenicity positive Carcinogenicity not tested |
1165 | ||
| Doxorubicin liposomes |
2000 | Advanced metastatic breast cancer (first line in combination with cyclophosphamide) |
DNA intercalation | No differences in response rate, median progression-free survival, or median overall survival v doxorubicin-cyclophosphamide (n=297) or epirubicin-cyclophosphamide (n=160) in comparative studies In another study (n=224) 75 mg doxorubicin liposomes were not inferior to 75 mg doxorubicin |
Mutagenicity positive Carcinogenicity positive Less cardiotoxic than doxorubicin Neutropenia, fever, gastrointestinal toxicity as with doxorubicin |
|||
| Trastuzumab | 2000 | Advanced metastatic breast cancer (second line) in combination with cyclophosphamide | Humanised mAb related to epidermal growth factor receptor 2 protein | Response rate 15%, with a median duration of 9.1 months in a “non-comparative open label” study in 222 patients resistant to anthracyclines or taxanes; survival at one year 55%, at two years 2% Progression-free survival prolonged by three months (7.4 v 4.6) in a randomised trial of trastuzumab + chemotherapy (anthracycline-cyclophosphamide) or paclitaxel (7.4 months) or chemotherapy alone (4.6 months) in 469 patients with metastatic breast cancer overexpressing HER-2; mortality not altered |
Mutagenicity negative Carcinogenicity not tested Cytokine release syndrome in 25% of cases Infections, leukopenia, gastrointestinal symptoms Heart failure, particularly in combination with anthracycline (24.5% v 7.4% with anthracycline alone) |
4384 | 1165 (paclitaxel) |
|
| Altretinoin | 2000 | Soft tissue sarcoma (second line) |
Activation of retinoic receptors |
Partial response rate 34% v 18% with placebo in a phase III study in 268 patients; modest cosmetic advantage as cutaneous lesions persist, although reduced in number or size | Mutagenicity negative Carcinogenicity not tested |
|||
| Capecitabine | 2000 | Metastatic colorectal cancer (first line) |
Fluorouracil prodrug: inhibition of DNA synthesis by prevention of deoxyuridylic acid conversion to thymidylic acid |
Not inferior to fluorouracil-leucovorin in two open label randomised phase III trials in 1207 patients; median time to progression 140 days (v 144 days), median overall survival rate 401 days (v 400 days), quality of life no different |
Mutagenicity positive carcinogenicity negative |
|||
Where no cost figure is shown, the drugs concerned were not available on the Italian market in December 2000.
Figure.
Comparison of some new drugs with existing drugs
Acknowledgments
SG and VB act as member and expert of the Committee for Proprietary Medical Products. The views presented here are those of the authors and should not be understood or quoted as being made on behalf of the European Medicines Evaluation Agency or its scientific committees.
Footnotes
Funding: None.
Competing interests: In the last five years VB has received fees for speaking from Schwarz Italia SpA and Instrumentation Laboratory, and for scientific advice from SmithKline Beecham and Aventis Pharma.
References
- 1.Quinn MJ, Babb PJ, Brock A, Kirby EA, Jones J. Cancer trends in England and Wales, 1950-1999. London: Stationery Office; 2001. pp. 206–207. . (Studies on medical and population subjects No 66.) [Google Scholar]
- 2.Levi F, Lucchini F, Negri E, La Vecchia C. The decline in cancer mortality in the European Union, 1988-1996. Eur J Cancer. 2000;36:1965–1968. doi: 10.1016/s0959-8049(00)00283-5. [DOI] [PubMed] [Google Scholar]
- 3.Early breast cancer trialists' collaborative group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 4.Early breast cancer trialists' collaborative group. Polychemotherapy for early breast cancer: an overview of the randomised trials. Lancet. 1998;352:930–942. [PubMed] [Google Scholar]
- 5.Berrino F, Gatta G, Chessa E, Valente F, Capocaccia R the Eurocare Working Group. Introduction: the Eurocare II study. Eur J Cancer. 1998;34:2139–2153. doi: 10.1016/s0959-8049(98)00334-7. [DOI] [PubMed] [Google Scholar]
- 6.Coleman MP. Opinion: why the variation in breast cancer survival in Europe? Breast Cancer Res. 1999;1:22–26. doi: 10.1186/bcr8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailar JC, Gornik HL. Cancer undefeated. N Engl J Med. 1997;336:1569–1574. doi: 10.1056/NEJM199705293362206. [DOI] [PubMed] [Google Scholar]
- 8.Garattini S, Bertele' V. Adjusting Europe's drug regulation to public health needs. Lancet. 2001;358:64–67. doi: 10.1016/s0140-6736(00)05258-2. [DOI] [PubMed] [Google Scholar]
- 9.Batchelor T. Temozolomide for malignant brain tumours. Lancet. 2000;355:1115–1116. doi: 10.1016/S0140-6736(00)02055-9. [DOI] [PubMed] [Google Scholar]
- 10.Friedberg M, Saffran B, Stinson TJ, Nelson W, Bennett CL. Evaluation of conflict of interest in economic analyses of new drugs used in oncology. JAMA. 1999;282:1453–1457. doi: 10.1001/jama.282.15.1453. [DOI] [PubMed] [Google Scholar]
- 11.Hortobagyi GN. Anthracyclines in the treatment of cancer. An overview. Drugs. 1997;54(suppl 4):S1–S7. doi: 10.2165/00003495-199700544-00003. [DOI] [PubMed] [Google Scholar]
- 12.Findlay BP, Walker-Dilks C. Epirubicin, alone or in combination chemotherapy, for metastatic breast cancer. Provincial breast cancer disease site group and the Provincial systemic treatment disease site group. Cancer Prev Control. 1998;2:140–146. [PubMed] [Google Scholar]
- 13.White CA, Weaver RL, Grillo-Lopez AJ. Antibody-targeted immunotherapy for treatment of malignancy. Annu Rev Med. 2001;52:25–45. doi: 10.1146/annurev.med.52.1.125. [DOI] [PubMed] [Google Scholar]