Abstract
The cerebellum has been involved in social abilities and autism. Given that the cerebellum is connected to the cortex via the cerebello‐thalamo‐cortical loop, the connectivity between the cerebellum and cortical regions involved in social interactions, that is, the right temporo‐parietal junction (rTPJ) has been studied in individuals with autism, who suffer from prototypical deficits in social abilities. However, existing studies with small samples of categorical, case–control comparisons have yielded inconsistent results due to the inherent heterogeneity of autism, suggesting that investigating how clinical dimensions are related to cerebellar–rTPJ functional connectivity might be more relevant. Therefore, our objective was to study the functional connectivity between the cerebellum and rTPJ, focusing on its association with social abilities from a dimensional perspective in a transdiagnostic sample. We analyzed structural magnetic resonance imaging (MRI) and functional MRI (fMRI) scans obtained during naturalistic films watching from a large transdiagnostic dataset, the Healthy Brain Network (HBN), and examined the association between cerebellum–rTPJ functional connectivity and social abilities measured with the social responsiveness scale (SRS). We conducted univariate seed‐to‐voxel analysis, multivariate canonical correlation analysis (CCA), and predictive support vector regression (SVR). We included 1404 subjects in the structural analysis (age: 10.516 ± 3.034, range: 5.822–21.820, 506 females) and 414 subjects in the functional analysis (age: 11.260 ± 3.318 years, range: 6.020–21.820, 161 females). Our CCA model revealed a significant association between cerebellum–rTPJ functional connectivity, full‐scale IQ (FSIQ) and SRS scores. However, this effect was primarily driven by FSIQ as suggested by SVR and univariate seed‐to‐voxel analysis. We also demonstrated the specificity of the rTPJ and the influence of structural anatomy in this association. Our results suggest that there is a complex relationship between cerebellum–rTPJ connectivity, social performance and IQ. This relationship is specific to the cerebellum–rTPJ connectivity, and is largely related to structural anatomy in these two regions.
Practitioner Points
We analyzed cerebellum–right temporoparietal junction (rTPJ) connectivity in a pediatric transdiagnostic sample.
We found a complex relationship between cerebellum and rTPJ connectivity, social performance and IQ.
Cerebellum and rTPJ functional connectivity is related to structural anatomy in these two regions.
Keywords: cerebellum, fMRI, functional connectivity, social abilities, temporo‐parietal cortex
The cerebellum and right temporoparietal junction (rTPJ) have been involved in autism spectrum disorders. In a transdiagnostic paediatric sample, we found a complex relationship between cerebellum–rTPJ connectivity, social performance and IQ, specific to the cerebellum–rTPJ connectivity, and related to structural anatomy in these two regions.
1. INTRODUCTION
The cerebellum has been involved in social abilities and autism (Laidi et al., 2017). The cerebellum contains more than 50% of the neurons of the brain (Li et al., 2013), and it plays an important role in a wide range of cognitive functions, i.e., social cognition. This makes it a potential target for impairments in individuals with autistic spectrum disorder (ASD) (Van Overwalle et al., 2020). Case–control studies of functional connectivity in ASD have reported cerebellar hypoconnectivity with the left inferior parietal lobule (IPL) and hyperconnectivity with sensory and motor networks (Oldehinkel et al., 2019; Stoodley et al., 2017). Given the abundant connections from the cerebellum to the cortex, it was proposed that the cerebellum may influence the maturation of neocortical neural circuitry through the cerebello‐thalamo‐cortical loops, in particular regions involved in social interactions, which could account for the relationship between autistic symptoms and cerebellar deficits (see review in Wang et al., 2014).
The posterior superior temporal sulcus (pSTS) or the temporo‐parietal junction (TPJ), particularly in the right hemisphere, has been shown to be largely implicated in social cognition, and its abnormalities have been observed in individuals with ASD (Zilbovicius et al., 2006, 2013). Activation of right pSTS or TPJ has been repeatedly reported in tasks of Theory of Mind (ToM), social processing, eye gaze perception, and attention (Dugué et al., 2018; Schurz et al., 2017). Accordingly, anatomical and functional abnormalities in this region have been identified in children and adults with ASD, including morphological alterations (such as pSTS length and sulcal depth), decreased cortical thickness, decreased gray matter volume, hypoperfusion and hypoconnectivity during rest (Alaerts et al., 2015; Hotier et al., 2017; Saitovitch et al., 2019). Some were associated with individual performance in social tasks.
Combining the aforementioned evidence, it has been hypothesized that in ASD the functional connectivity between the cerebellum and right TPJ (rTPJ) may be compromised, thus contributing to social impairments. Stoodley et al. (2017) have revealed hypoconnectivity between right Crus I and left IPL in children with ASD. Igelström et al. (2017) also reported for the first time disrupted functional connectivity between left Crus II and right dorsal TPJ in individuals with ASD as opposed to neurotypical controls.
Taken together, the evidence suggests that disrupted functional connectivity between the cerebellum and the rTPJ in ASD may be responsible for the typical social impairments observed in individuals with autism. However, significant challenges have hindered progress towards a reliable neuromarker of social communication deficits in this condition. First, as a spectrum disorder, individuals diagnosed with ASD can present a wide range of symptoms and severities. This variability can make it challenging to identify consistent neural markers or patterns across individuals. Second, ASD often co‐occurs with conditions such as ADHD, mood disorders, anxiety or learning disabilities (Lai et al., 2019). Furthermore, biological atypicalities identified in various psychiatric disorders are of relatively low specificity (Alexander et al., 2017; Insel & Cuthbert, 2015), questioning the use of categorical diagnosis in neuroimaging studies. Instead, we believe that it might be interesting to move away from studying disorders in isolation from one another and take a transdiagnostic approach (Alexander et al., 2017). Third, previous studies have used small sample sizes which may over or underestimate true effects in the population. Larger sample sizes can help capture this diversity and allow for more nuanced analyses that consider different subgroups within the ASD population. Moreover, large cohorts are essential to overcome sample size limitations.
Here, we perform brain‐wise analyses on large‐scale datasets in neuroimaging research on ASD to reach a replicable conclusion. The goal of our study is thus to investigate the associations between cerebellum–rTPJ gray matter density, functional connectivity and the severity of social impairments in a large‐scale transdiagnostic MRI dataset (Alexander et al., 2017). Based on the findings of Igelström et al. (2017), we expect to witness atypical cerebellum–rTPJ gray matter density and functional connectivity in individuals with severe impairments of social interaction.
2. MATERIALS AND METHODS
2.1. Dataset
The dataset we analyzed was obtained from the HBN initiative launched by the Child Mind Institute (New York, USA), available at http://fcon_1000.projects.nitrc.org/indi/cmi_healthy_brain_network/ (Alexander et al., 2017). The HBN initiative has a specific interest in establishing a large‐scale community‐based transdiagnostic biobank about mental health and learning disorders in children and adolescents, with comprehensive clinical assessments, behavioral and cognitive measurements, demographic information, and multiple sessions of multi‐modal neuroimaging. In our study, the HBN dataset is relevant as such a large‐scale transdiagnostic dataset can help avoid the long‐criticized limitations of small sample size and simple categorical dichotomy, especially in the highly heterogeneous field of ASD.
2.2. Participants
The HBN dataset used a community‐based model of recruitment, and collected a large amount of data from participants aged between 5 and 21 in the New York area. The inclusion criteria included: (1) between 5 and 21 years old; (2) fluent in English; and (3) capable of undergoing the clinical evaluation (Alexander et al., 2017). We excluded participants with excessive head motion during MRI scanning sessions, inadequate coverage of the rTPJ and cerebellum, and low IQ (quantified as IQ below 70). As a result, a total of 1404 participants (age: 10.52 ± 3.03 years, range: 5.822–21.820, 506 females) were considered in our analyses of structural MRI, in which 414 participants (age: 11.260 ± 3.318 years, range: 6.020–21.820, 161 females) were included for the following analyses of functional MRI (fMRI). Additional statistical testing showed no significant difference in SRS scores between the excluded (N = 990) and included (N = 414) groups, but the excluded group was significantly younger than the included (reported in Appendix S1).
2.3. Phenotyping and clinical assessment
To evaluate the severity of social impairment in transdiagnostic population, we selected the Social Responsiveness Scale 2nd version (SRS‐2, https://www.wpspublish.com/srs-2-social-responsiveness-scale-second-edition) as our scale of interest, due to its wide applicability in both clinical and non‐clinical settings (Constantino et al., 2004). The SRS‐2 has a total of 65 items on a 4‐point Likert scale, and outputs one total score and five domain scores, including Awareness (AWR), Social Cognition (COG), Social Communication (COM), Social Motivation (MOT), Restricted/Repetitive Behavior (RRB). Since our focus was on social abilities, RRB was not included in the following analysis of clinical assessments. A high SRS T‐score usually indicates mild (60–65), moderate (66–75) or severe (76 or higher) deficits in social interactions and is correspondingly associated with clinically significant ASD diagnoses and severity.
To better control possible confounding factors such as IQ, we also included full‐scale IQ (FSIQ) score for each participant, measured by Wechsler Adult Intelligence Scale (WAIS, Wechsler, 2008), Wechsler Abbreviated Scale of Intelligence (WASI, Wechsler, 1999), or Wechsler Intelligence Scale for Children (WISC, Petermann, 2011).
2.4. MRI acquisition
The MRI data were collected in separate phases using different MRI scanners at three sites: a Siemens 3 Tesla Tim Trio MRI scanner at Rutgers University Brain Imaging Center (RUBIC), a Siemens 3 Tesla Prisma MRI scanner at Citigroup Biomedical Imaging Center (CBIC), and a Siemens 3 Tesla Prisma MRI Scanner at City University of New York (CUNY). T1‐weighted structural images were acquired with the following scan parameters: repetition time (TR) = 2500 ms, echo time (TE) = 3.15 ms, flip angle = 8°, slice number = 224, voxel size = 0.8 × 0.8 × 0.8 mm3. During naturalistic viewing task, functional images were acquired using a multiband echo‐planar imaging (EPI) pulse sequence with the following scan parameters: TR = 800 ms, TE = 30 ms, flip angle = 31°, slice number = 60, voxel size = 2.4 × 2.4 × 2.4 mm3, multi‐band acceleration factor = 6, field of view (FOV) = 204 × 204 × 144 mm3, bandwidth = 2290 Hz/Px, matrix size = 85 × 85 × 60; no field map correction was applied (see Alexander et al. (2017) for detailed parameters and protocols for structural and functional MRI scans).
2.5. Naturalistic viewing task
Participants were asked to passively view two film clips played during the fMRI scanning sessions. The first was a 10‐min clip from ‘Despicable Me’, the second was approximately 3.47 min from ‘The Present’. It has been proposed that perceiving naturalistic stimuli like film clips can be considered as resting state for fMRI experiments, yet with better signal quality and more reliability as opposed to conventional rests (Sonkusare et al., 2019). Studies have shown that a 10‐minute session of resting state is sufficient for functional connectivity analyses (Shehzad et al., 2009). Nevertheless, due to poor test–retest reliability of fMRI with short acquisition time as demonstrated by Noble et al. (2017), we do not include scanning sessions with the movie clip of ‘The Present’ in our functional analyses.
2.6. MRI preprocessing
The preprocessing steps, such as spatial normalization and brain segmentation, were completed on T1w images using CAT12 version 12.7 (https://neuro-jena.github.io/cat12-help/#Dahnke:2022, Gaser et al., 2022), an advanced tool for voxel‐based morphometry (VBM) analyses, with quality check (QC) reports for individual images. The detailed description of the pipeline is available in Gaser et al. (2022). Further analyses on structural images were only conducted on individuals with an image quality rating (IQR) equal and above 80 (or B−), corresponding to a description of ‘good’ or ‘excellent’ as described in Dahnke et al. (2022). We excluded 677 out of 2125 scans due to their low quality. All QC‐passed T1w images were smoothed with a 3 mm full‐width‐at‐half‐maximum (FWHM) using a Python library NiLearn 0.10.0 (Abraham et al., 2014).
The fMRI images in the HBN dataset were preprocessed using state‐of‐the‐art preprocessing pipeline fMRIPrep (https://fmriprep.org/en/stable/, Esteban et al., 2019, 2020). This pipeline performs preprocessing steps, including co‐registration, normalization, and segmentation. For the purpose of consistency in our study, only those with QC‐passed structural images were considered in the following functional analyses.
The dataset of fMRI images was then narrowed by excluding 611 participants because of excessive head motion (defined as mean framewise displacement (FD) > 0.3 mm, as in Frew et al., 2022), 102 participants because of a low coverage of the cerebellum (<90%) or rTPJ (<92%), and 16 participants because of IQ < 70. We also discarded one site of inclusion (CUNY) because there were only 8 participants left. Next, we processed the fMRI images by dropping the first five dummy scans, regressing out confounds including FD and 36 parameters (with Global Signal Regression, or GSR, Satterthwaite et al., 2013), filtering with a high‐pass of 0.01 Hz and a low‐pass of 0.1 Hz, and smoothing with a FWHM of 6 mm. All the above steps were achieved in the Python environment with NiLearn 0.10.0 package (Abraham et al., 2014).
2.7. Voxel‐based morphometry analysis
To understand if the structural anatomy could influence the functional connectivity part, we conducted a voxel‐based morphometry (VBM) analysis using FSL on the structural MRI images, with a special focus on the TPJ and the cerebellum. Throughout the whole study, we used the resampled TPJ mask from a previous study (Igelström et al., 2017) and the Buckner cerebellar atlas (Buckner et al., 2011) to define the rTPJ and the cerebellum. We applied general linear models to predict gray matter density in the region of interest (ROI), TPJ and cerebellum respectively, by demeaned variables including age, sex, SRS total score, FSIQ, scan location (coded as dummy variables), and IQR. To statistically test the significance of our models, permutation tests with 10,000 permutations each were conducted with FSL Randomise (Winkler et al., 2014). Results were FWE (family wise error) corrected for multiple comparison across space using TFCE (Threshold‐Free Cluster Enhancement).
The threshold for statistical significance was set as 0.05 throughout the study.
2.8. Univariate analysis: seed‐to‐voxel connectivity analysis
The seed‐to‐voxel connectivity analysis consists of two major steps. First, we used dual regression (Beckmann et al., 2009) with Buckner 7‐Network cerebellar atlas (Buckner et al., 2011) as seeds to extract subject‐specific spatial maps for each cerebellar ROI. The dual regression analysis, as indicated by its name, performs a spatial regression to extract subject‐specific time series in each ROI, and then a temporal regression to estimate subject‐specific spatial maps, one for each ROI. Thus, we obtained the ROI‐to‐voxel functional connectivity between ROIs in the cerebellum and voxels in the whole brain. Second, we used the FSL Randomise permutation‐testing tool (Winkler et al., 2014) with the mask of rTPJ to test the significance of general linear models similar to those in the VBM analyses. Specifically, we aimed to predict seed‐to‐voxel connectivity with the severity of social impairments—SRS total score and SRS communication domain score, respectively—while considering the effects of age, sex, FSIQ, scan location, and head motion (mean FD). All independent variables were demeaned, and p values were FWE‐corrected.
2.9. Multivariate analysis: canonical correlation analysis
The link between brain functional connectivity and social ability may result from complex interactions between multiple neuroanatomical and clinical variables. To further investigate in depth the association between cerebellum–rTPJ connectivity features and clinical measurements of social impairments, we conducted canonical correlation analysis (CCA), a mathematical approach to identifying latent patterns of correlation between two sets of variables, as opposed to only one dependent variable explained by linear mixed models.
To obtain ROI‐ROI functional connectivity, we parcellated the T1 whole brain images into 449 ROIs, using Schaefer's atlas (400 ROIs) for cortex (Kong et al., 2021; Schaefer et al., 2018), Buckner's atlas (17 ROIs) for cerebellum (Buckner et al., 2011), and Tian's atlas (32 ROIs) for subcortex (Tian et al., 2020). After resampling the atlases into our fMRI space, we extracted ROI‐level time series and used them to generate individuals' whole‐brain correlation matrices. By applying the rTPJ mask (Igelström et al., 2017) onto the cortical parcellation, we identified 61 ROIs with at least one voxel included in the mask. Again, as a sanity check, the rTPJ mask had no overlapping with any subcortical or cerebellar ROIs. So far, we had 1037 (17 × 61) features of cerebellum–rTPJ connectivity, and 414 subjects in total.
As the inputs of CCA, we defined two canonical components, one with connectivity features “X”, and the other with clinical features “Y”. For connectivity features “X”, we included all the cerebellum–rTPJ connectomes (1037 in total), regressed out confounding effects of head motion, age, sex, and scan location (see Appendix S3 for the relationship among age, sex, and social abilities), normalized with Fisher's r‐to‐z transformation, and reduced the dimensionality to 100 using principal component analysis (PCA, explaining more than 80% of the variance). Four subjects were excluded as outliers (out of 3 standard deviations) at this step, resulting in 410 observations in total. For clinical features “Y”, we included FSIQ as well as normalized SRS sub‐scores for social communication impairment (AWR, COG, MOT, and COM). We then performed CCA and obtained two canonical components that maximized the correlation between a linear combination of “X” and that of “Y”. Both PCA and CCA were achieved using the Scikit‐Learn package in the Python environment (version 1.2.2, Pedregosa et al., 2011).
To validate the statistical significance of our model, we used 10,000 permutations to define the null distribution and threshold of statistical significance. We performed a train‐test validation analysis, as described in Smith et al. (2015), 80% of the observations were randomly selected as the train subset, leaving the remaining 20% as the test subset. The connectome weights yielded by the CCA on the train subset were applied on the test subset to calculate the new canonical variates and their correlation, followed by a permutation test (1000) to estimate the statistical significance of the canonical correlation within the test subset. Such train‐test analysis was run 10 times as Smith et al. (2015) did in their study.
The interpretation of CCA was achieved by simple correlation between canonical vectors and original variables in order to understand how much each variable contributes to the multivariate association between brain connectivity and clinical assessments of social abilities (Ilioska et al., 2022).
As a step forward, we tested if adding the whole cortex—in addition to the restricted rTPJ for which we had a hypothesis—would generally improve the canonical correlation. We performed the same CCA procedure, validation, and interpretation on the functional connectivity between cerebellum and the whole cortex. In the matrix of connectivity features “X”, a total of 6800 connectomes (17 × 400) were included, and then reduced to 100 principal components by a PCA, explaining 61.5% of the variance in the original data. Six subjects were excluded as outliers (out of 3 standard deviations) at this step, resulting in 408 observations in total.
Considering that structural and functional images may provide complementary information (Pang et al., 2023), we further combined functional connectivity and gray matter density into the same CCA procedure. Besides the above features of cerebellum–rTPJ connectivity, we also included 75 features of ROI‐level gray matter density, which was extracted in the rTPJ and cerebellum and averaged over the same ROIs defined by the Schaefer's atlas (Kong et al., 2021; Schaefer et al., 2018) and Buckner's atlas (Buckner et al., 2011). By adding the structural information, we expected to increase the overall canonical correlation between brain variables and clinical variables.
2.10. Support vector regression
Last, we used a classic machine learning approach, support vector regression (SVR), to predict social abilities (SRS total score) or FSIQ with functional connectivity between the cerebellum and rTPJ. As before, we used as predictors the ROI‐level cerebellum‐rTPJ connectomes after regressing out confounding effects (age, sex, scan location, and head motion) and normalizing the data by Fisher's z transformation. When predicting SRS total score by SVR model, we also regressed out the effects of FSIQ from connectivity measures in advance for an unconfounded effect of SRS score. SVR with a Radial Basis Function kernel was computed by the Scikit‐learn package (version 1.2.2, Pedregosa et al., 2011). To evaluate the predictability, the SVR models were examined by 10‐fold cross validation, with negative mean squared error (MSE) as the index—a higher index indicates less distance between predicted values and actual values, thus a better prediction. The threshold for statistical significance was estimated via permutation testing of 1000 iterations.
3. RESULTS
3.1. Study sample: demographics and social abilities
In the VBM analysis of structural MRI images, we included a total of 1404 participants, with an SRS total t‐score of 57.021 ± 11.212, and an FSIQ of 101.359 ± 16.315; in the analysis of fMRI data, the dataset was narrowed down to 414 participants, with an SRS total t‐score of 56.488 ± 10.882, an SRS communication t‐score of 56.290 ± 11.161, and an FSIQ of 103.626 ± 16.553 (as summarized in Table 1). The distributions and pairwise correlations of clinical measures (age, FSIQ, SRS total score, SRS‐AWR, SRS‐COG, SRS‐MOT, SRS‐COM) in the functional analyses are reported in Appendix S2.
TABLE 1.
Summary of demographic information.
| Variable | Count | Mean | Standard deviation | Min | Max | |
|---|---|---|---|---|---|---|
| Structural analysis | Age (years) | 1404 | 10.516 | 3.034 | 5.822 | 21.820 |
| Sex | Male = 898, female = 506 | |||||
| SRS Total T | 1404 | 57.021 | 11.212 | 37 | 90 | |
| FSIQ | 1404 | 101.359 | 16.315 | 70 | 157 | |
| Diagnosis | ADHD | 657 | ||||
| Specific learning disorder | 121 | |||||
| ASD | 88 | |||||
| Communication disorder | 32 | |||||
| Motor disorder | 12 | |||||
| Nonadherence to medical treatment | 7 | |||||
| Intellectual disability | 4 | |||||
| Other neurodevelopmental disorder | 2 | |||||
| Functional analysis | Age (years) | 414 | 11.260 | 3.318 | 6.020 | 21.820 |
| Sex | Male = 253, female = 161 | |||||
| SRS Total T | 414 | 56.488 | 10.882 | 38 | 90 | |
| SRS COM T | 414 | 56.290 | 11.161 | 38 | 90 | |
| FSIQ | 414 | 103.626 | 16.553 | 70 | 146 | |
| Diagnosis | ADHD | 192 | ||||
| Specific learning disorder | 37 | |||||
| ASD | 23 | |||||
| Communication disorder | 7 | |||||
| Motor disorder | 2 | |||||
| Nonadherence to medical treatment | 2 | |||||
| Intellectual disability | 1 | |||||
| Other neurodevelopmental disorder | 0 | |||||
Note: Demographic information was summarized for the subsets in structural analysis and functional analysis separately.
Abbreviations: ADHD, attention deficit hyperactivity disorder; ASD, autism spectrum disorder; FSIQ, full‐scale IQ; SRS COM T, SRS communication t‐score; SRS Total T, SRS total t‐score.
3.2. Significant canonical correlation between connectivity, SRS scores and FSIQ
The CCA analysis revealed a significant correlation between cerebellum‐rTPJ functional connectivity and clinical measures of social communication impairments and FSIQ (Figure 1a), Pearson's r = 0.654, p < .001 based on a null distribution provided by permutations (Figure 1b). The train‐test validation analyses showed significant correlation coefficients in all the 10 iterations of train‐test randomization, mean Pearson's r = 0.2743, mean threshold for significance (p < .05) r = 0.1820.
FIGURE 1.
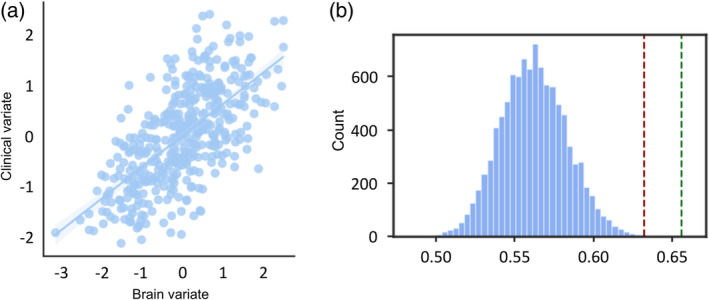
CCA results with cerebellum–rTPJ functional connectivity. (a) Brain–clinical correlation: the brain canonical variate (a linear combination of normalized cerebellum–rTPJ functional connectivities) is positively correlated to the clinical canonical variate (a linear combination of normalized SRS domain scores and FSIQ). Each dot represents one subject, and the line represents the fitted correlation between the brain canonical variate and the clinical canonical variate, Pearson's r = 0.654. (b) Permutation analysis results: to assess the statistical significance of the observed brain–clinical correlation, we conducted a permutation analysis with 10,000 iterations. We obtained a normal null distribution of the canonical correlations between brain and clinical variates. The red line on the plot corresponds to the 99.9 percentile of this null distribution, and the green line represents the true canonical correlation derived from the original data. The proximity of the green line to the red line indicates the extent to which the observed correlation is statistically significant.
The FSIQ and SRS cognition domain scores were the two main clinical variables associated with the clinical canonical variate (Figure 2a). As for the correlations between functional connectivity and brain canonical variate, we selected only significant correlations (p < .05) and averaged positive and negative coefficients separately over ROIs in either the cerebellum (Figure 2b) or the rTPJ (Figure 2c). It is worth noting in the cerebellar flat maps that bilateral Crus I and II—the cognitive parts of the cerebellum (Figure 2b)—contribute more to the overall canonical correlation than bilateral I–V and VIIIb, the somatomotor areas. However, we were not able to identify a clear pattern in the rTPJ plots.
FIGURE 2.
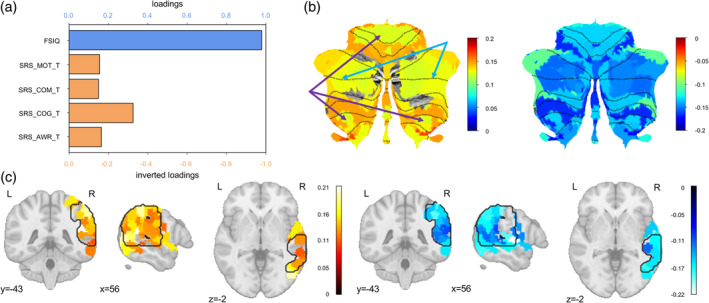
Interpretation of brain–clinical canonical correlation. (a) Loadings of clinical measurements: the bar plot demonstrates the loadings of each clinical measurement, that is, the correlation between the output clinical canonical variate and each clinical variable. FSIQ is highly related to the clinical canonical variate, thus FSIQ contributes the most to the overall brain‐clinical canonical correlation. SRS cognition domain score contributes the second most. It is worth noting that the red axes were inverted to facilitate interpretation because SRS scores reflect ASD severity. (b) Cerebellar flat maps of the loadings of rTPJ–cerebellum connectivity: the two flat maps of the cerebellum show the loadings of ROI–ROI rTPJ–cerebellum functional connectivities averaged over cerebellar parcellations. The left map shows significant positive loadings (p < .05), and the right map shows significant negative loadings (p < .05). The loading means the correlation between each ROI–ROI functional connectivity and the overall brain canonical variate. Therefore, the larger the absolute value of the loading is, the larger contribution this area has for the overall canonical correlation. Importantly, the somatomotor regions (pointed by the purple arrows) and the cognitive regions (pointed by the blue arrows) in the Buckner 17‐Network cerebellar atlas have clearly different amount of contributions to the overall canonical correlation. (c) Cortical maps of the loadings of rTPJ–cerebellum connectivity: the 3D plots summarize the loadings of ROI–ROI rTPJ–cerebellum functional connectivities averaged over rTPJ ROIs. The left map shows significant positive loadings (p < .05), and the right map shows significant negative loadings (p < .05). Again, the color bar indicates how much contribution an rTPJ ROI has for the overall canonical correlation. The black contours delineate the area of the rTPJ that we defined. It is worth noting that we identified all the ROIs with at least one voxel falling into the rTPJ mask, therefore, some ROIs (colored regions) also have voxels outside the rTPJ mask (black contours).
The linear models with seed‐to‐voxel connectivity showed that the cerebellum–rTPJ functional connectivity is related to age, sex, FSIQ, but not social abilities measured by either SRS total score or SRS communication domain score. We observed in all tested models the positive effect of age in Network3, the effect of sex in Network3 and Network7, the positive effect of FSIQ in Network2, Network4, and the negative effect of FSIQ in Network3 (Figure 3).
FIGURE 3.
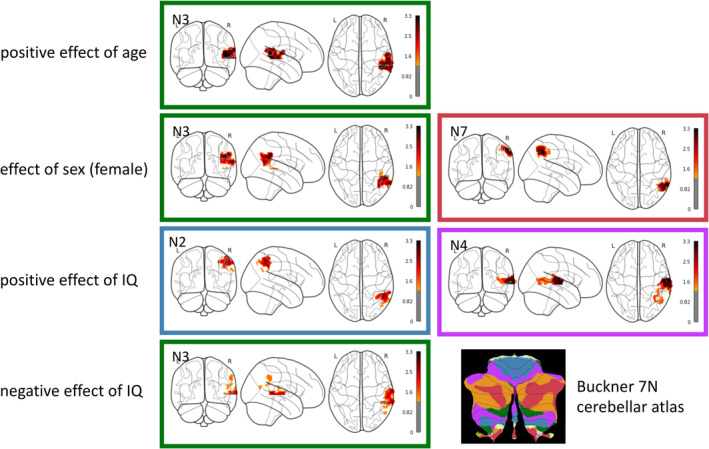
Effects of age, sex, IQ on seed‐to‐voxel connectivity between cerebellum and rTPJ. The linear models showed the positive effect of age on the connectivity between Network3 in the cerebellum and regions in the rTPJ, the effect of sex (female) in Network3 and Network7, the positive effect of IQ in Network2 and Network4, the negative effect of IQ in Network3. The color coding of frames is based on Buckner 7 N cerebellar atlas for reference. To better visualize the level of significance, colorbars represent the inverted log 10 of p value, so a higher position in the colorbar means a smaller p value. The threshold of significance 0.05 is converted to 1.3. N2, somatomotor network; N3, dorsal attention network; N4, ventral attention network; N7, default mode network.
3.3. The effect of structural anatomy on social abilities and IQ
Our VBM analyses did not show any significant effects of SRS total scores on gray matter density in either TPJ or the cerebellum. However, we found significant negative effects of age, significant effects of sex, and significant positive effects of FSIQ on the gray matter density in both TPJ and cerebellum (Figure 4). In summary, we discovered significant structural alterations in both TPJ and cerebellum that were related to age, sex, and FSIQ, but not to SRS total score.
FIGURE 4.
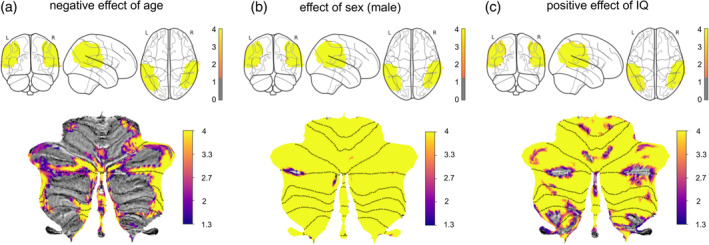
Effects of age, sex, and FSIQ on gray matter density in the rTPJ and cerebellum. (a) Significant negative effects of age are distributed in the rTPJ (top), and the cerebellum (bottom, flatmap). To better visualize the level of significance, colorbars represent the inverted log 10 of p value, so a higher position in the colorbar means a smaller p value. The threshold of significance 0.05 is converted to 1.3. (b) Significant effects of sex (male) on gray matter values in the rTPJ (top) and the cerebellum (bottom). (c) Positive effects of FSIQ on gray matter values in the rTPJ (top) and the cerebellum (bottom).
Since structural and functional information may provide complementary information, we included both cerebellum‐rTPJ connectivity and gray matter density as the inputs of the CCA to see if the canonical correlation can be increased. When taking both functional connectivity and structural values as brain variables, the CCA results showed a significant correlation (r = 0.657, p < .001), with a similar pattern of clinical loadings as before (Figure 5a). Overall, the structural variables are more correlated to the brain canonical variate in comparison to functional connectivity variables (Figure 5b).
FIGURE 5.
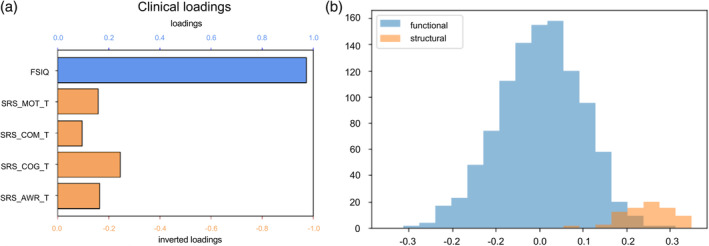
CCA results with cerebellum‐rTPJ functional connectivity and structural values. (a) The loadings of each clinical measurement, that is, the correlation between the output clinical canonical variate and each clinical variable. Since SRS reflects ASD severity, axes were inverted to facilitate interpretation. (b) Histogram of the loadings of structural and functional variables. Overall, structural variables are more correlated to the brain canonical variate.
3.4. Specificity of cerebellum–rTPJ functional connectivity
So far, we have shown that the cerebellum–rTPJ functional connectivity is significantly correlated with clinical measures, mainly driven by FSIQ and SRS cognition domain score. However, it is possible that our findings can be applied to the broader cerebellum‐cortical connectivity. To demonstrate the specificity of cerebellum‐rTPJ functional connectivity, we performed additional CCA in two scenarios: we replaced the cerebellum‐rTPJ functional connectivity with (1) functional connectivity between cerebellum and the whole cortex; (2) functional connectivity between cerebellum and the rest of the cortex while excluding rTPJ from the whole cortex. The CCA with the whole cortex showed again a significant and stable correlation (r = 0.652, p < .001). When we exclude the rTPJ from the CCA, the canonical correlation remained significant (r = 0.637, p < .001), but the results of the train‐test validation analysis (repeated 10 times as in Smith et al., 2015) were not stable anymore. This supported the hypothesis that the canonical correlation was significant and stable when the rTPJ was included in the brain variates; otherwise, the CCA validation became unstable. Taking all the CCA results together, we can conclude that the rTPJ plays a unique and crucial role in the canonical correlation between cerebellar connectivity and our clinical measures of interest.
3.5. Cerebellum–rTPJ connectivity predicts FSIQ and SRS cognition, but not SRS total score
We fitted three SVR models to predict FSIQ, SRS total score, and SRS cognition subscore respectively with functional connectivity between the cerebellum and rTPJ. The models were examined by 10‐fold cross validation, with an averaged negative MSE (of the 10 negative MSEs) as an overall index for predictability. The statistical significance was tested by 1000 permutations, and the threshold for statistical significance (p < .05) was defined as the 95th percentile of the null distribution of mean negative MSEs obtained in the permutation testing. The SVR model reliably predicted FSIQ, with an averaged negative MSE of −0.925 in 10‐fold cross validation, p < .001 based on permutation testing (Figure 6a). However, the model failed to predict SRS total score, with an averaged negative MSE of −1.027, p > .05 (Figure 6b). In terms of SRS cognition subscore, the SVR has a marginal performance, insignificant with only functional variables (averaged negative MSE = −1.015, p > .05), but marginally significant when taking both functional and structural variables into consideration (averaged negative MSE = −1.016, p < .05). Therefore, the cerebellum–rTPJ connectivity can predict FSIQ and SRS cognition, but not SRS total score.
FIGURE 6.
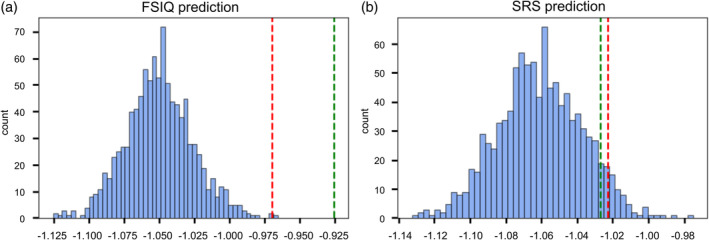
Permutation results to evaluate SVR models. (a) Permutation results with 1000 iterations of 10‐fold cross validation, using an SVR model to predict FSIQ. We obtained a normal null distribution of the mean negative MSEs from 10‐fold cross validation. The higher a mean negative MSE is, the better the model predicts. The red line represents the 99.9 percentile of the distribution, and the green line represents the true mean negative MSE we got using the original data. Here we can see the original SVR model can significantly predict FSIQ with cerebellum–rTPJ functional connectivity. (b) Permutation results with 1000 iterations of 10‐fold cross validation, using an SVR model to predict SRS total score. The red line represents the 95 percentile of the distribution, and the green line represents the true mean negative MSE we got using the original data. Here, the SVR model cannot predict SRS total score using cerebellum–rTPJ functional connectivity.
4. DISCUSSION
To summarize, we investigated the association between cerebellum‐rTPJ functional connectivity and social abilities in a transdiagnostic population, by a series of analyses—CCA, univariate linear models, and SVR. We observed a significant canonical correlation between the cerebellum‐rTPJ functional connectivities and clinical measures, which was primarily driven by FSIQ and SRS cognition domain score. We also showed that the structural anatomy in the cerebellum and rTPJ is highly related to FSIQ and contributes to the canonical correlation between functional connectivity and clinical measures. Then, we demonstrated the specificity of the rTPJ in the canonical correlation, by comparing the CCA results with functional connectivities between the cerebellum and the whole brain, and those between the cerebellum and the rest of the brain (without rTPJ). Last, the SVR models and their evaluation suggested that our SVR models could predict FSIQ, SRS cognition subscore, but not SRS total score. Overall, the variations in cerebellum–rTPJ functional connectivity are mostly explained by FSIQ and general cognition, instead of impairments in social interaction alone.
Our results suggest that there is a complex relationship between cerebellum–rTPJ functional connectivity, social abilities, and FSIQ, in which FSIQ and SRS cognition plays a more influential role than social communication performance. This conclusion is contrary to our hypothesis of disrupted cerebellum–rTPJ connectivity in people with severe social impairments. We believe that several factors could explain this lack of findings. First, cerebellar–rTPJ structural and functional atypicalities might be specific to ASD, which could explain the lack of findings in a large and heterogeneous transdiagnostic sample. Second, this could be related to an insufficient duration of the MRI acquisition (Ooi et al., 2024) or the relatively low sample size of our study (Marek et al., 2022). However, a recent large‐scale study on cerebellar structural alterations related to ASD has found the same pattern of strong effect of general functioning and IQ, but not specifically of social abilities (Elandaloussi et al., 2023). Our findings on functional connectivity are in line with those on structural alterations.
We demonstrate the specificity of cerebellum–rTPJ connectivity in the canonical correlation—it remains significant and stable with functional connectivities between the cerebellum and the whole brain as inputs, but becomes unstable after excluding the cerebellum–rTPJ connectivities. As reported in previous literature, rTPJ is involved in social cognition (Schurz et al., 2017) and witnesses both structural and functional changes in autistic population (Hotier et al., 2017; Saitovitch et al., 2019). Therefore, it is likely that the cerebellum–rTPJ connectivity plays a special role in cognitive and social functioning as opposed to cerebellar connections to the rest of the cortex.
When it comes to the effect of structural anatomy, we first observed significant structural alterations in both the cerebellum and the rTPJ related to age, sex and FSIQ, but not for SRS score. The absence of SRS‐correlated cerebellar changes replicated the conclusions in the latest large‐scale investigations—either comparing autistic and control subjects (Laidi et al., 2022), or analyzing transdiagnostic population (Elandaloussi et al., 2023). Both studies found no evidence of cerebellar abnormalities in individuals with ASD or severe social impairments. While the case–control study (Laidi et al., 2022) did not detect any effect of age, IQ, or sex, both the transdiagnostic investigation in structural anatomy (Elandaloussi et al., 2023) and our study on functional connectivity have supported the involvement of the cerebellum in general cognitive function (or IQ) as indicated by structural modifications.
As for the rTPJ, our findings in the structural analysis are generally in accordance with a few recent large‐scale research comparing autistic and neurotypical subjects (Bedford et al., 2020; Ecker et al., 2022; Haar et al., 2016; Khundrakpam et al., 2017; Van Rooij et al., 2018). Regardless of certain divergence, most of these studies revealed a negative relationship between age and the overall cortical thickness. Moreover, one of them identified the effect of male on cortical thickness in the superior temporal region (Bedford et al., 2020), and another unveiled a positive effect of IQ on overall gray matter volume. These effects of age, sex, and IQ are in line with our findings in the VBM analysis of rTPJ. However, we did not notice any effect of the severity of social deficits (measured by SRS score) on the gray matter densities in the rTPJ, as opposed to the general message of a positive correlation conveyed by the majority of these large‐scale studies.
When taking structural values into CCA together with cerebellum–rTPJ connectivity, we found that the structural variables are in general highly correlated with the brain variate, compared with functional variables. It suggests that the structure of the cerebellum and the rTPJ is also associated with IQ and social abilities, with even a larger contribution to the canonical correlation relationship.
Taken together, the above findings add to a growing body of evidence that the cerebellum may play a role in general cognitive functioning, rather than in specific social cognition. In autistic research, recent large‐scale investigations on the cerebellum have favored the strong effect of IQ, instead of social performance (Elandaloussi et al., 2023). Our findings on functional connectivity also support the postulated role of cerebellum in general cognition. Beyond the field of autism, atypicalities in the cerebellum, or more precisely, the cognitive part of the cerebellum (roughly bilateral Crus I and Crus II), have been shown in multiple mental health conditions, and also linked to cognitive functioning. For example, a recent large‐scale investigation on cerebellar structure in psychosis observed no effects of diagnosis (schizophrenia, bipolar disorder, or neurotypical controls), but an association between cognitive ability and cerebellar volumes (Moussa‐Tooks et al., 2022). Another indirect evidence for a ‘cognitive cerebellum’ may include the causal relationship between cerebellar–prefrontal network connectivity and negative symptoms in schizophrenia (Brady et al., 2019). Furthermore, cognitive deficits in autism may contribute to the impairments in social communication, as demonstrated by group comparison studies (Fong & Iarocci, 2020; Leung et al., 2016). These studies established a causative association between cognition and social abilities in autism. Overall, latest insights and our conclusion converge to a common ground, where the cerebellum is involved in general cognitive functioning that can further lead to socio‐communicative impairments in autism, and cerebellar deficits observed in autism and other psychiatric disorders may be explained by a decrease of overall cognitive ability.
This study has several strengths. First, we overcame some often‐criticized issues in neuroimaging research of ASD, such as small sample size, and simplified case–control design. Previous research mostly adopted the design of case–control comparison to investigate the functional connectivity between cerebellum and TPJ in autism (Igelström et al., 2017; Stoodley et al., 2017). Our study, however, took a dimensional approach in a large transdiagnostic sample, and provided novel evidence and insights into the current debate about the role of the cerebellum in autism. Our findings also emphasized the importance of performing autistic research from a dimensional perspective. Second, we provided an advanced and progressive analysis pipeline, from conventional univariate analysis of seed‐to‐voxel connectivity to multivariate approach of canonical correlation, and from correlational linear models to predictive machine learning models. Similar pipelines can be applied in future brain‐wide association research. Third, we performed strict quality checks for both structural and functional images in the cohort, which ensured the quality of our data and the reliability of our findings.
This study has several limitations in the following aspects. First, we did not separate the left and right cerebellum in our cerebellar parcellations. Previous research has shown evidence of lateralization in the functional connectivity between cerebellum and TPJ (Igelström et al., 2017; Stoodley et al., 2017). However, we adopted bilateral cerebellar atlases derived from functional networks because of previous reports about non‐decussating fiber tracts from the cerebellum (Meola et al., 2016). Additional steps would help verify the complicated canonical correlations we identified between connectivity measures and clinical measures. Second, our structural results did rule out the possible contamination of structural changes related to social abilities, but new concerns are raised regarding the significant influence of FSIQ on both structural and functional modifications in the cerebellum and rTPJ. Further investigation is in need to clarify the relationship between structural and functional alterations. Third, our strict quality checks made the findings more reliable but at the cost of subject exclusion. Before the functional analyses, we excluded many participants (about 70%) mainly due to their excessive head motion and bad coverage of the cerebellum. The proportion of subject exclusion is larger than usual, because we have a pediatric and transdiagnostic sample. Larger samples with better quality of neuroimaging would be of great help in replicating and affirming our findings. Last, the cross‐sectional nature and the sample size of our study did not allow us to investigate the effect of age on cerebellar‐TPJ functional connectivity. We believe that the effect of age should be ideally modeled in large longitudinal cohorts.
Accordingly, future research could further explore the functional connectivity of the cerebellum based on a finer parcellation to account for potential lateralization of the connectivity network, that is, between the cerebellum and the rTPJ. Moreover, further steps should be taken to replicate the canonical correlations identified between connectivity and clinical measures. This could involve utilizing alternative validation techniques, or expanding the sample size. As for the overlapping between structural and functional results, further investigation is needed to assess how much effect we found in functional analyses actually comes from structural changes in the first place. By addressing these limitations and pursuing these future directions, researchers can better clarify the intricate relationships between cerebellum–rTPJ functional connectivity, cognitive function, and social abilities. These advancements can contribute to a deeper understanding of the neural basis of ASD, and shed light on innovative interventions or treatments for individuals with ASD.
5. CONCLUSION
In conclusion, our analyses in a transdiagnostic sample suggest that there is no specific association between social abilities and cerebellum–rTPJ connectivity, but that there is a complex relationship between cerebellum–rTPJ connectivity, social performance and IQ as indicated by our previous work on structural alterations in the cerebellum. Such a relationship is specific to cerebellum–rTPJ connectivity, and is related to their structural anatomy.
FUNDING INFORMATION
C.L. received funding from the Bettencourt Schueller Fondation (CCA INSERM Bettencourt Program).
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supporting information
APPENDIX S1: Supporting information.
ACKNOWLEDGEMENTS
We thank the participants of this study and would like to affirm our genuine respect for their contributions to the continued progress of medical science. We would like to thank Professor Kajsa Igelström (Linköping University) who provided the mask of the temporo‐parietal junction, based on her previous work.
Kong, Y. , Roser, M. , Bègue, I. , Elandaloussi, Y. , Neu, N. , Grigis, A. , Duchesnay, E. , Leboyer, M. , Houenou, J. , & Laidi, C. (2024). Cerebellum and social abilities: A structural and functional connectivity study in a transdiagnostic sample. Human Brain Mapping, 45(10), e26749. 10.1002/hbm.26749
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in Healthy Brain Network at https://data.healthybrainnetwork.org/main.php. These data were derived from the following resources available in the public domain: Healthy Brain Network, https://data.healthybrainnetwork.org/main.php.
REFERENCES
- Abraham, A. , Pedregosa, F. , Eickenberg, M. , Gervais, P. , Mueller, A. , Kossaifi, J. , Gramfort, A. , Thirion, B. , & Varoquaux, G. (2014). Machine learning for neuroimaging with scikit‐learn. Frontiers in Neuroinformatics, 8, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaerts, K. , Nayar, K. , Kelly, C. , Raithel, J. , Milham, M. P. , & Di Martino, A. (2015). Age‐related changes in intrinsic function of the superior temporal sulcus in autism spectrum disorders. Social Cognitive and Affective Neuroscience, 10(10), 1413–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, L. M. , Escalera, J. , Ai, L. , Andreotti, C. , Febre, K. , Mangone, A. , Vega‐Potler, N. , Langer, N. , Alexander, A. , Kovacs, M. , Litke, S. , O'Hagan, B. , Andersen, J. , Bronstein, B. , Bui, A. , Bushey, M. , Butler, H. , Castagna, V. , Camacho, N. , … Milham, M. P. (2017). An open resource for transdiagnostic research in pediatric mental health and learning disorders. Scientific Data, 4(1), 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann, C. F. , Mackay, C. E. , Filippini, N. , & Smith, S. M. (2009). Group comparison of resting‐state FMRI data using multi‐subject ICA and dual regression. NeuroImage, 47(Suppl 1), S148. [Google Scholar]
- Bedford, S. A. , Park, M. T. M. , Devenyi, G. A. , Tullo, S. , Germann, J. , Patel, R. , Anagnostou, E. , Baron‐Cohen, S. , Bullmore, E. T. , Chura, L. R. , Craig, M. C. , Ecker, C. , Floris, D. L. , Holt, R. J. , Lenroot, R. , Lerch, J. P. , Lombardo, M. V. , Murphy, D. G. M. , Raznahan, A. , … Chakravarty, M. M. (2020). Large‐scale analyses of the relationship between sex, age and intelligence quotient heterogeneity and cortical morphometry in autism spectrum disorder. Molecular Psychiatry, 25(3), 614–628. [DOI] [PubMed] [Google Scholar]
- Brady, R. O., Jr. , Gonsalvez, I. , Lee, I. , Öngür, D. , Seidman, L. J. , Schmahmann, J. D. , Eack, S. M. , Keshavan, M. S. , Pascual‐Leone, A. , & Halko, M. A. (2019). Cerebellar‐prefrontal network connectivity and negative symptoms in schizophrenia. American Journal of Psychiatry, 176(7), 512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner, R. L. , Krienen, F. M. , Castellanos, A. , Diaz, J. C. , & Yeo, B. T. (2011). The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of Neurophysiology, 106(5), 2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino, J. N. , Gruber, C. P. , Davis, S. , Hayes, S. , Passanante, N. , & Przybeck, T. (2004). The factor structure of autistic traits. Journal of Child Psychology and Psychiatry, 45(4), 719–726. [DOI] [PubMed] [Google Scholar]
- Dahnke, R. , Ziegler, G. , Großkreutz, J. , & Gaser, C. (2022). Retrospective quality assurance in T1 images. In preparation.
- Dugué, L. , Merriam, E. P. , Heeger, D. J. , & Carrasco, M. (2018). Specific visual subregions of TPJ mediate reorienting of spatial attention. Cerebral Cortex, 28(7), 2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker, C. , Pretzsch, C. M. , Bletsch, A. , Mann, C. , Schaefer, T. , Ambrosino, S. , Tillmann, J. , Yousaf, A. , Chiocchetti, A. , Lombardo, M. V. , Warrier, V. , Bast, N. , Moessnang, C. , Baumeister, S. , Dell'Acqua, F. , Floris, D. L. , Zabihi, M. , Marquand, A. , Cliquet, F. , … Murphy, D. G. (2022). Interindividual differences in cortical thickness and their genomic underpinnings in autism spectrum disorder. American Journal of Psychiatry, 179(3), 242–254. [DOI] [PubMed] [Google Scholar]
- Elandaloussi, Y. , Floris, D. L. , Coupé, P. , Duchesnay, E. , Mihailov, A. , Grigis, A. , Bègue, I. , Victor, J. , Frouin, V. , Leboyer, M. , Houenou, J. , & Laidi, C. (2023). Understanding the relationship between cerebellar structure and social abilities. Molecular Autism, 14(1), 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban, O. , Ciric, R. , Finc, K. , Blair, R. W. , Markiewicz, C. J. , Moodie, C. A. , Kent, J. D. , Goncalves, M. , DuPre, E. , Gomez, D. E. P. , Ye, Z. , Salo, T. , Valabregue, R. , Amlien, I. K. , Liem, F. , Jacoby, N. , Stojić, H. , Cieslak, M. , Urchs, S. , … Gorgolewski, K. J. (2020). Analysis of task‐based functional MRI data preprocessed with fMRIPrep. Nature Protocols, 15(7), 2186–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban, O. , Markiewicz, C. J. , Blair, R. W. , Moodie, C. A. , Isik, A. I. , Erramuzpe, A. , Kent, J. D. , Goncalves, M. , DuPre, E. , Snyder, M. , Oya, H. , Ghosh, S. S. , Wright, J. , Durnez, J. , Poldrack, R. A. , & Gorgolewski, K. J. (2019). fMRIPrep: A robust preprocessing pipeline for functional MRI. Nature Methods, 16(1), 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong, V. C. , & Iarocci, G. (2020). The role of executive functioning in predicting social competence in children with and without autism spectrum disorder. Autism Research, 13(11), 1856–1866. [DOI] [PubMed] [Google Scholar]
- Frew, S. , Samara, A. , Shearer, H. , Eilbott, J. , & Vanderwal, T. (2022). Getting the nod: Pediatric head motion in a transdiagnostic sample during movie‐and resting‐state fMRI. PLoS One, 17(4), e0265112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser, C. , Dahnke, R. , Thompson, P. M. , Kurth, F. , & Luders, E. (2022). CAT‐a computational anatomy toolbox for the analysis of structural MRI data. BioRxiv. 10.1101/2022.06.11.495736 [DOI] [Google Scholar]
- Haar, S. , Berman, S. , Behrmann, M. , & Dinstein, I. (2016). Anatomical abnormalities in autism? Cerebral Cortex, 26(4), 1440–1452. [DOI] [PubMed] [Google Scholar]
- Hotier, S. , Leroy, F. , Boisgontier, J. , Laidi, C. , Mangin, J. F. , Delorme, R. , Bolognani, F. , Czech, C. , Bouquet, C. , Toledano, E. , Bouvard, M. , Petit, J. , Mishchenko, M. , d'Albis, M.‐A. , Gras, D. , Gaman, A. , Scheid, I. , Leboyer, M. , Zalla, T. , & Houenou, J. (2017). Social cognition in autism is associated with the neurodevelopment of the posterior superior temporal sulcus. Acta Psychiatrica Scandinavica, 136(5), 517–525. [DOI] [PubMed] [Google Scholar]
- Igelström, K. M. , Webb, T. W. , & Graziano, M. S. (2017). Functional connectivity between the temporoparietal cortex and cerebellum in autism spectrum disorder. Cerebral Cortex, 27(4), 2617–2627. [DOI] [PubMed] [Google Scholar]
- Ilioska, I. , Oldehinkel, M. , Llera, A. , Chopra, S. , Looden, T. , Chauvin, R. , Van Rooij, D. , Floris, D. L. , Tillmann, J. , Moessnang, C. , Banaschewski, T. , Holt, R. J. , Loth, E. , Charman, T. , Murphy, D. G. M. , Ecker, C. , Mennes, M. , Beckmann, C. F. , Fornito, A. , & Buitelaar, J. K. (2022). Connectome‐wide mega‐analysis reveals robust patterns of atypical functional connectivity in autism. Biological Psychiatry, 94(1), 29–39. [DOI] [PubMed] [Google Scholar]
- Insel, T. R. , & Cuthbert, B. N. (2015). Brain disorders? Precisely. Science, 348(6234), 499–500. [DOI] [PubMed] [Google Scholar]
- Khundrakpam, B. S. , Lewis, J. D. , Kostopoulos, P. , Carbonell, F. , & Evans, A. C. (2017). Cortical thickness abnormalities in autism spectrum disorders through late childhood, adolescence, and adulthood: A large‐scale MRI study. Cerebral Cortex, 27(3), 1721–1731. [DOI] [PubMed] [Google Scholar]
- Kong, R. , Yang, Q. , Gordon, E. , Xue, A. , Yan, X. , Orban, C. , Zuo, X.‐N. , Spreng, N. , Ge, T. , Holmes, A. , Eickhoff, S. , & Yeo, B. T. (2021). Individual‐specific areal‐level parcellations improve functional connectivity prediction of behavior. Cerebral Cortex, 31(10), 4477–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, M. C. , Kassee, C. , Besney, R. , Bonato, S. , Hull, L. , Mandy, W. , Szatmari, P. , & Ameis, S. H. (2019). Prevalence of co‐occurring mental health diagnoses in the autism population: A systematic review and meta‐analysis. The Lancet Psychiatry, 6(10), 819–829. [DOI] [PubMed] [Google Scholar]
- Laidi, C. , Boisgontier, J. , Chakravarty, M. M. , Hotier, S. , d'Albis, M. A. , Mangin, J. F. , Devenyi, G. A. , Delorme, R. , Bolognani, F. , Czech, C. , Bouquet, C. , Toledano, E. , Bouvard, M. , Gras, D. , Petit, J. , Mishchenko, M. , Gaman, A. , Scheid, I. , Leboyer, M. , … Houenou, J. (2017). Cerebellar anatomical alterations and attention to eyes in autism. Scientific Reports, 7(1), 12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidi, C. , Floris, D. L. , Tillmann, J. , Elandaloussi, Y. , Zabihi, M. , Charman, T. , Wolfers, T. , Durston, S. , Moessnang, C. , Dell'Acqua, F. , Ecker, C. , Loth, E. , Murphy, D. , Baron‐Cohen, S. , Buitelaar, J. K. , Marquand, A. F. , Beckmann, C. F. , Frouin, V. , Leboyer, M. , … EU‐AIMS LEAP Group . (2022). Cerebellar atypicalities in autism? Biological Psychiatry, 92(8), 674–682. [DOI] [PubMed] [Google Scholar]
- Leung, R. C. , Vogan, V. M. , Powell, T. L. , Anagnostou, E. , & Taylor, M. J. (2016). The role of executive functions in social impairment in autism spectrum disorder. Child Neuropsychology, 22(3), 336–344. [DOI] [PubMed] [Google Scholar]
- Li, W. K. , Hausknecht, M. J. , Stone, P. , & Mauk, M. D. (2013). Using a million cell simulation of the cerebellum: Network scaling and task generality. Neural Networks, 47, 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek, S. , Tervo‐Clemmens, B. , Calabro, F. J. , Montez, D. F. , Kay, B. P. , Hatoum, A. S. , Donohue, M. R. , Foran, W. , Miller, R. L. , Hendrickson, T. J. , Malone, S. M. , Kandala, S. , Feczko, E. , Miranda‐Dominguez, O. , Graham, A. M. , Earl, E. A. , Perrone, A. J. , Cordova, M. , Doyle, O. , … Dosenbach, N. U. (2022). Reproducible brain‐wide association studies require thousands of individuals. Nature, 603(7902), 654–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meola, A. , Comert, A. , Yeh, F. C. , Sivakanthan, S. , & Fernandez‐Miranda, J. C. (2016). The nondecussating pathway of the dentatorubrothalamic tract in humans: Human connectome‐based tractographic study and microdissection validation. Journal of Neurosurgery, 124(5), 1406–1412. [DOI] [PubMed] [Google Scholar]
- Moussa‐Tooks, A. B. , Rogers, B. P. , Huang, A. S. , Sheffield, J. M. , Heckers, S. , & Woodward, N. D. (2022). Cerebellar structure and cognitive ability in psychosis. Biological Psychiatry, 92(5), 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble, S. , Spann, M. N. , Tokoglu, F. , Shen, X. , Constable, R. T. , & Scheinost, D. (2017). Influences on the test–retest reliability of functional connectivity MRI and its relationship with behavioral utility. Cerebral Cortex, 27(11), 5415–5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldehinkel, M. , Mennes, M. , Marquand, A. , Charman, T. , Tillmann, J. , Ecker, C. , Dell'Acqua, F. , Brandeis, D. , Banaschewski, T. , Baumeister, S. , Moessnang, C. , Baron‐Cohen, S. , Holt, R. , Bölte, S. , Durston, S. , Kundu, P. , Lombardo, M. V. , Spooren, W. , Loth, E. , … EU‐AIMS LEAP Group . (2019). Altered connectivity between cerebellum, visual, and sensory‐motor networks in autism spectrum disorder: Results from the EU‐AIMS longitudinal European autism project. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(3), 260–270. [DOI] [PubMed] [Google Scholar]
- Ooi, L. Q. R. , Orban, C. , Nichols, T. , Zhang, S. , Tan, T. W. K. , Kong, R. , Marek, S. , Dosenbach, N. U. F. , Laumann, T. , Gordon, E. M. , Zhou, J. H. , Bzdok, D. , Eickhoff, S. B. , Holmes, A. J. , & Yeo, B. T. (2024). MRI economics: Balancing sample size and scan duration in brain wide association studies. bioRxiv. 10.1101/2024.02.16.580448 [DOI] [Google Scholar]
- Pang, J. C. , Aquino, K. M. , Oldehinkel, M. , Robinson, P. A. , Fulcher, B. D. , Breakspear, M. , & Fornito, A. (2023). Geometric constraints on human brain function. Nature, 618, 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedregosa, F. , Varoquaux, G. , Gramfort, A. , Michel, V. , Thirion, B. , Grisel, O. , Blondel, M. , Prettenhofer, P. , Weiss, R. , Dubourg, V. , Vanderplas, J. , Passos, A. , Cournapeau, D. , Brucher, M. , Perrot, M. , & Duchesnay, E. (2011). Scikit‐learn: Machine learning in Python. The Journal of Machine Learning Research, 12, 2825–2830. [Google Scholar]
- Petermann, F. (2011). Wechsler intelligence scale for children (WISC‐IV). Pearson. [Google Scholar]
- Saitovitch, A. , Rechtman, E. , Lemaitre, H. , Tacchella, J. M. , Vinçon‐Leite, A. , Douard, E. , Calmon, R. , Grévent, D. , Philippe, A. , Chabane, N. , Brunelle, F. , Boddaert, N. , & Zilbovicius, M. (2019). Superior temporal sulcus hypoperfusion in children with autism spectrum disorder: An arterial spin‐labeling magnetic resonance study. bioRxiv, 771584. 10.1101/771584 [DOI] [Google Scholar]
- Satterthwaite, T. D. , Elliott, M. A. , Gerraty, R. T. , Ruparel, K. , Loughead, J. , Calkins, M. E. , Eickhoff, S. B. , Hakonarson, H. , Gur, R. C. , Gur, R. E. , & Wolf, D. H. (2013). An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting‐state functional connectivity data. NeuroImage, 64, 240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer, A. , Kong, R. , Gordon, E. M. , Laumann, T. O. , Zuo, X. N. , Holmes, A. J. , Eickhoff, S. B. , & Yeo, B. T. (2018). Local–global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cerebral Cortex, 28(9), 3095–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurz, M. , Tholen, M. G. , Perner, J. , Mars, R. B. , & Sallet, J. (2017). Specifying the brain anatomy underlying temporo‐parietal junction activations for theory of mind: A review using probabilistic atlases from different imaging modalities. Human Brain Mapping, 38(9), 4788–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad, Z. , Kelly, A. C. , Reiss, P. T. , Gee, D. G. , Gotimer, K. , Uddin, L. Q. , Lee, S. H. , Margulies, D. S. , Roy, A. K. , Biswal, B. B. , Petkova, E. , Castellanos, F. X. , & Milham, M. P. (2009). The resting brain: Unconstrained yet reliable. Cerebral Cortex, 19(10), 2209–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Nichols, T. E. , Vidaurre, D. , Winkler, A. M. , Behrens, T. E. , Glasser, M. F. , Ugurbil, K. , Barch, D. M. , Van Essen, D. C. , & Miller, K. L. (2015). A positive–negative mode of population covariation links brain connectivity, demographics and behavior. Nature Neuroscience, 18(11), 1565–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonkusare, S. , Breakspear, M. , & Guo, C. (2019). Naturalistic stimuli in neuroscience: Critically acclaimed. Trends in Cognitive Sciences, 23(8), 699–714. [DOI] [PubMed] [Google Scholar]
- Stoodley, C. J. , D'Mello, A. M. , Ellegood, J. , Jakkamsetti, V. , Liu, P. , Nebel, M. B. , Gibson, J. M. , Kelly, E. , Meng, F. , Cano, C. A. , Pascual, J. M. , Mostofsky, S. H. , Lerch, J. P. , & Tsai, P. T. (2017). Altered cerebellar connectivity in autism and cerebellar‐mediated rescue of autism‐related behaviors in mice. Nature Neuroscience, 20, 1744–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, Y. , Margulies, D. S. , Breakspear, M. , & Zalesky, A. (2020). Topographic organization of the human subcortex unveiled with functional connectivity gradients. Nature Neuroscience, 23(11), 1421–1432. [DOI] [PubMed] [Google Scholar]
- Van Overwalle, F. , Manto, M. , Cattaneo, Z. , Clausi, S. , Ferrari, C. , Gabrieli, J. D. E. , Guell, X. , Heleven, E. , Lupo, M. , Ma, Q. , Michelutti, M. , Olivito, G. , Pu, M. , Rice, L. C. , Schmahmann, J. D. , Siciliano, L. , Sokolov, A. A. , Stoodley, C. J. , van Dun, K. , … Leggio, M. (2020). Consensus paper: Cerebellum and social cognition. Cerebellum (London, England), 19(6), 833–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rooij, D. , Anagnostou, E. , Arango, C. , Auzias, G. , Behrmann, M. , Busatto, G. F. , Calderoni, S. , Daly, E. , Deruelle, C. , Di Martino, A. , Dinstein, I. , Duran, F. L. S. , Durston, S. , Ecker, C. , Fair, D. , Fedor, J. , Fitzgerald, J. , Freitag, C. M. , Gallagher, L. , … Buitelaar, J. K. (2018). Cortical and subcortical brain morphometry differences between patients with autism spectrum disorder and healthy individuals across the lifespan: Results from the ENIGMA ASD Working Group. American Journal of Psychiatry, 175(4), 359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. S. H. , Kloth, A. D. , & Badura, A. (2014). The cerebellum, sensitive periods, and autism. Neuron, 83(3), 518–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, D. (1999). Wechsler abbreviated scale of intelligence.
- Wechsler, D. (2008). Wechsler adult intelligence scale‐fourth edition (WAIS‐IV) [Database record]. APA PsycTests. [Google Scholar]
- Winkler, A. M. , Ridgway, G. R. , Webster, M. A. , Smith, S. M. , & Nichols, T. E. (2014). Permutation inference for the general linear model. NeuroImage, 92, 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilbovicius, M. , Meresse, I. , Chabane, N. , Brunelle, F. , Samson, Y. , & Boddaert, N. (2006). Autism, the superior temporal sulcus and social perception. Trends in Neurosciences, 29(7), 359–366. [DOI] [PubMed] [Google Scholar]
- Zilbovicius, M. , Saitovitch, A. , Popa, T. , Rechtman, E. , Diamandis, L. , Chabane, N. , Brunelle, F. , Samson, Y. , & Boddaert, N. (2013). Autism, social cognition and superior temporal sulcus. Open Journal of Psychiatry, 3, 46–55. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1: Supporting information.
Data Availability Statement
The data that support the findings of this study are available in Healthy Brain Network at https://data.healthybrainnetwork.org/main.php. These data were derived from the following resources available in the public domain: Healthy Brain Network, https://data.healthybrainnetwork.org/main.php.


