Abstract
The aim of this review is to raise awareness and knowledge among healthcare professionals and policymakers about late adverse effects in survivors of childhood leukemia. With contemporary treatment, over 90% of children with acute lymphoblastic leukemia (ALL) and over 60% with acute myeloid leukemia (AML) are cured. Large cohort studies demonstrate that 20% of ALL and most AML survivors have at least one chronic health condition by 20-25 years after diagnosis. These are life-changing or threatening in some survivors and contribute to increased premature mortality. We describe the frequency, causes, clinical features, and natural history of the most frequent and severe late adverse effects in childhood leukemia survivors, including subsequent malignant neoplasms, metabolic toxicity, gonadotoxicity and impaired fertility, endocrinopathy and growth disturbances, bone toxicity, central and peripheral neurotoxicity, cardiotoxicity, psychosocial late effects, accelerated ageing and late mortality. The wide range of late effects in survivors of haemopoietic stem cell transplant is highlighted. Recent developments informing the approach to long-term survivorship care are discussed, including electronic personalized patient-specific treatment summaries and care plans such as the Survivor Passport (SurPass), surveillance guidelines and models of care. The importance of ongoing vigilance is stressed given the increasing use of novel targeted drugs with limited experience of long-term outcomes.
Conclusion.
It is vital to raise awareness of the existence and severity of late effects of childhood leukemia therapy among parents, patients, health professionals, and policymakers. Structured long-term surveillance recommendations are necessary to standardize follow-up care.
Keywords: Child, Acute Leukemia, Late Effects, Survivor, Long-Term Follow-Up
Introduction
The improving cure rate for childhood acute leukemia has been one of the major success stories of contemporary pediatric oncology over recent decades. In high-income countries, over 90% of children with acute lymphoblastic leukemia (ALL), and 60-70% of those with acute myeloid leukemia (AML), are cured. However, this success has been accompanied by considerable long-term toxicity. Large cohort studies demonstrate that 20% of ALL and most AML survivors have at least one chronic health condition by 20-25 years after diagnosis (1, 2). These “late effects” may be life-changing or threatening and contribute to increased risk of premature mortality.
Many late effects seen in childhood leukemia survivors (CLS) are associated with the same treatments that have played an important role in improving cure rates. It is therefore vital to seek the right balance between increasing treatment intensity (aiming to cure more children) and reducing intensity (to decrease treatment-related late toxicity). Modern treatment regimens seek to use our burgeoning knowledge of leukemia cell and molecular biology to stratify treatment more optimally, reserving more intensive and toxic treatment for those children who need it most whilst avoiding it in those with good prognosis. Pediatric oncologists need a good understanding of the range and nature of late effects to allow them to develop strategies for improving the longevity and quality of survival from leukemia. In addition, it is very important that healthcare professionals (HCP) seeing long-term CLS are aware of the presentations and nature of potential late effects.
This article describes the most common and serious chronic toxicities occurring in CLS. Acute toxicities are not covered except where they lead directly to subsequent late effects. Long-term follow-up (LTFU) and survivorship care are also described.
Epidemiology
CLS represent a growing population, constituting over 30% of all childhood cancer survivors (CCS) (3). Thus, it is increasingly important to characterize the frequency and nature of antileukemic treatment-related late effects. All organ systems are at risk, with late effects including alterations in growth and development, neurocognitive impairment, psychosocial disorders, subsequent malignancies, and cardiovascular, reproductive, musculoskeletal and neurological damage, amongst other toxicities. The overall cumulative burden (expressed as a mean number of events per individual) has been reported as 4.10, 7.96 and 16.71 for childhood ALL survivors, and as 7.24, 11.42 and 18.68 for childhood AML survivors, at 25, 35 and 50 years attained age, respectively (3). CLS experience reduced overall health-related quality of life compared to healthy controls or siblings (4). Whilst many studies have examined long-term morbidity and premature mortality in large cohorts of CCS, few have systematically focused on those treated for childhood leukemia (2, 5, 6). Nevertheless, managing chronic toxicities is essential to provide optimal long-term care once pediatric leukemia has been cured.
Important Late Adverse Effects in Survivors of Childhood Leukemia
Treatment-related, personal and health behavior characteristics that may influence the risk of late effects are depicted in Figure 1. Table 1 lists the nature of and risk factors for late effects as well as recommended clinical evaluation or surveillance tests for at risk survivors.
Figure 1.
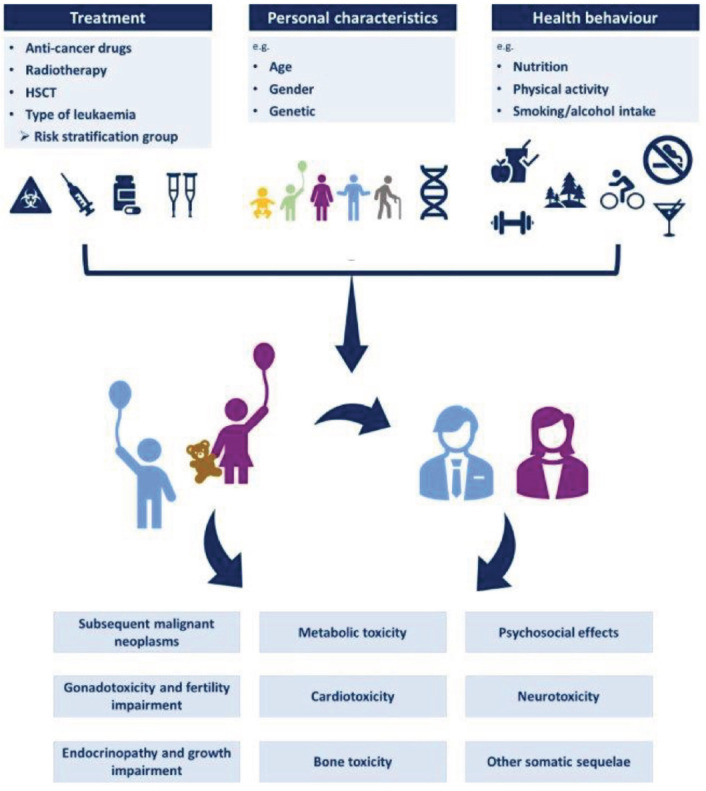
Treatment-related, personal and health behavior characteristics that may influence the risk of late effects in childhood leukemia survivors.
Table 1.
Late Adverse Effects, Risk Factors and Recommended Surveillance or Evaluation in Survivors of Childhood Leukaemia*
| Late adverse effect | Patient, disease and treatment-related risk factors | Recommended evaluation or surveillance test and frequency (Recommendations for survivors in absence of surveillance test) |
|---|---|---|
| Bone toxicity# | ||
|
| ||
| •Reduced bone mineral density | •Cranial or craniospinal radiotherapy •TBI •Prolonged corticosteroid treatment •Gonadal failure •GHD |
•DXA scan at entry into LTFU (2-5 years after completion of treatment), and again at about 25 years age •Additional DXA scans between entry into LTFU and 25 years age, and after 25 years age, may be appropriate as clinically indicated NB: Considered delaying first DXA-scan (until after puberty completed) in pre-pubertal and pubertal survivors |
|
| ||
| Cardiac toxicity | ||
|
| ||
| •Arrhythmia •Cardiomyopathy •Coronary artery disease (asymptomatic) •Pericardial disease •Valvular heart disease |
Arrhythmia | Arrhythmia |
|
| ||
| •Anthracyclinesa | •ECG once at entry into LTFU •Repeat ECG once after the age of 18 years if entry into LTFU was at a younger age |
|
|
| ||
| Cardiomyopathy | Cardiomyopathy | |
|
| ||
| •Anthracyclines | •Echocardiogram with specific attention to left ventricular systolic function, starting 2 years after treatment •If treated with a total cumulative anthracycline dose ≥250 mg/m2: at least every 2-3 years •If treated with a cumulative dose ≥100-250 mg/m2: at least every 5 years •Echocardiogram with specific attention to left ventricular function, prior to pregnancy or in the first trimester, if female and treated with anthracyclines •Screening for modifiable cardiovascular risk factors (hypertension, diabetes, dyslipidaemia, obesity, smoking and low levels of physical activity) |
|
|
| ||
| Central nervous system toxicity | ||
|
| ||
| •Cerebral vasculopathy (including cerebrovascular accident [stroke]) •Neurocognitive toxicity (including problems in the cognitive domains of academic and school performance, attention, executive functions, intelligence, language, memory, processing speed or visual-motor integration) |
Cerebral vasculopathy | Cerebral vasculopathy |
|
| ||
| •Radiotherapy to a volume exposing the head, brain or neck, including TBI | •Discuss importance of controlling cardiovascular and stroke risk factors (hypertension, diabetes, dyslipidaemia, obesity, smoking, low levels of physical activity) | |
|
| ||
| Neurocognitive toxicity | Neurocognitive toxicity | |
|
| ||
| •Radiotherapy to a volume exposing the brain, including TBI •High dose IV cytarabine •High dose IV methotrexate •Intrathecal chemotherapy especially if the survivor was treated at a young age |
•Medical history with specific attention to educational and/or vocational progress or decline ◆At least every 2 years in survivors ≤18 years of age ◆At least every 5 years in survivors >18 years of age Referral to (neuro)psychologist for formal neuropsychological evaluation as clinically indicated |
|
|
| ||
| Dental and oral problems | ||
|
| ||
| •Dental caries •Dental developmental problems (especially if treated at a young age or having suffered from poor nutritional condition) •Xerostomia •Periodontal disease |
•Radiotherapy to a volume exposing the oral cavity or salivary glands, including TBI •Allogeneic HSCT •Chemotherapy |
•Regular dental follow-up |
|
| ||
| Endocrinopathy and growth disturbance | ||
|
| ||
|
Endocrinopathy and growth disturbance
•HP axis problems (including GHD, TSHD, LH/FSHD and ACTHD) •Precocious puberty (central) (CPP) •Thyroid function problems (including hypothyroidism and hyperthyroidismb) |
HP axis problems; Precocious puberty (central) | HP axis problems |
|
| ||
| •Radiotherapy to a volume exposing HP region, including TBI |
Pre-pubertal and peri-pubertal survivors at risk:
•Height velocity in relation to parental height every 6 months •Tanner stage every 6 months •fT4, TSH, morning cortisol every year •Starting 6-12 months after completion of radiotherapy Post-pubertal survivors at risk: •fT4, TSH, morning cortisol, IGF-1 •Early morning testosterone, or free testosterone in survivors with overweight, and LH (males) •Estradiol, FSH and LH (females) •Every year, starting 6-12 months after completion of radiotherapy •Continue surveillance for at least 15 years from exposure. Continuation of surveillance should be a shared decision between survivor and HCP in the context of the available health care resources. If surveillance is discontinued, the survivor should be educated about possible signs and symptoms of HP axis problems. NB: An IGF-1 level even as high as 0 SDS does not exclude GHD. Precocious puberty (central) Pre- and peri-pubertal survivors at risk: •Height velocity in relation to parental height every 6 months •Tanner stage every 6 months •Starting 6-12 months after completion of radiotherapy •Use early morning testosterone (before 10:00 AM) as surveillance modality in pubertal boys who have been exposed to radiotherapy to the testes since testicular volume may be unreliable. •Continue surveillance until the age of 8 years (girls) and 9 years (boys). |
|
|
| ||
| Thyroid function problems | Thyroid function problems | |
|
| ||
| •Radiotherapy to a volume exposing the thyroid gland, including TBI •Allogeneic HSCT |
•TSH and fT4 measurement •Every year in survivors ≤18 years age and at least every 2-3 years in survivors >18 years age Female survivors at risk for hypothyroidism: ◆Measure TSH and fT4 prior to attempting pregnancy and periodically during pregnancy |
|
|
| ||
| Eye problems | ||
|
| ||
| ◆Cataract ◆Xerophthalmia ◆Glaucoma |
Cataract | •Medical history with specific attention to symptoms of visual or eye problems •Examination of eye •At least every 5 years |
|
| ||
| ◆Radiotherapy to a volume exposing the eye and orbit, including TBI ◆Prolonged corticosteroids | ||
|
| ||
| Xerophthalmia | ||
|
| ||
| ◆Chronic GvHD | ||
|
| ||
| Glaucoma | ||
|
| ||
| ◆Prolonged corticosteroids | ||
|
| ||
| Fatigue (cancer-related) | ||
|
| ||
| •All leukemia survivors are at risk for cancer-related fatigue, but the main risk factors are: ◆Psychological distress ◆Late effects or health problems ◆Pain ◆Older age at follow-up ◆Radiotherapy |
•Medical history focused on survivors’ feelings of tiredness and exhaustion •Regularly (at every LTFU visit or general medical check-up) |
|
|
| ||
| Gonadotoxicity and fertility impairment | ||
|
| ||
|
Gonadotoxicity and fertility impairment
•Male -Impaired spermatogenesis -Testosterone deficiency -Physical sexual dysfunction •Female -Premature ovarian insufficiency (including amenorrhea and premature menopause) Fertility impairment as a consequence of any of above manifestations of gonadotoxicity |
Male – impaired spermatogenesis | Male – impaired spermatogenesis |
|
| ||
| •Alkylating agents •Radiotherapy to a volume exposing the testes, including TBI |
Post-pubertal survivors at risk that desire assessment of potential for future fertility:
•Semen analysis |
|
|
| ||
| Male – testosterone deficiency | Male – testosterone deficiency | |
|
| ||
| •Radiotherapy ≥12 Gy to a volume exposing the testes, including TBI |
Post-pubertal survivors treated with radiotherapy ≥12 Gy to a volume exposing the testes, including TBI:
•Early morning testosterone at clinically appropriate time intervals •LH in addition to (early morning) testosterone if clinical signs of hypogonadism, previous low or borderline testosterone concentrations, or if an early morning testosterone sample cannot be obtained •At least every 2-3 years |
|
|
| ||
| Male – physical sexual dysfunction | Male – physical sexual dysfunction | |
|
| ||
| •Testosterone deficiency | •Relevant sexual history | |
|
| ||
| Female – premature ovarian insufficiency | Female – premature ovarian insufficiency (POI) | |
|
| ||
| •Alkylating agents •Radiotherapy to a volume exposing the ovaries, including TBI |
Pre- and peri-pubertal survivors at risk:
•FSH and estradiolc in case of failure to initiate or progress through puberty at least for girls ≥11 years of age, and for girls with primary amenorrhea (by 16 years of age) Post-pubertal survivors at risk: •FSH and estradiolc,d in case of menstrual cycle dysfunction suggesting POI, or if assessment of potential for future fertility is desired Post-pubertal survivors at risk: •FSH and estradiolc,d in case of menstrual cycle dysfunction suggesting POI, or if assessment of potential for future fertility is desired |
|
|
| ||
| Iron overload | ||
|
| ||
| •HSCT •Multiple red blood cell transfusions |
•Serum ferritin once at entry into LTFU | |
|
| ||
| Liver toxicity | ||
|
| ||
| •Radiotherapy to a volume exposing the liver, including TBI •HSCT (irrespective of GvHD) •Methotrexate •Mercaptopurine •Thioguanine •Busulfan •Sinusoidal obstruction syndrome •Chronic GvHD •Chronic viral hepatitis |
•Serum liver enzyme concentrations (ALT, AST, gGT, ALP) once at entry into LTFU | |
|
| ||
| Lower urinary tract toxicity | ||
|
| ||
| •Cyclophosphamide •Radiotherapy to a volume exposing the bladder, including TBI |
•Medical history with specific attention to urinary tract symptoms •At least every 5 years |
|
|
| ||
| Mental health problems | ||
|
| ||
| •Anxiety •Behavioural problems •Depression •Post-traumatic stress •Suicidal ideation |
•All leukemia survivors are at risk for mental health problems | •Medical history with focus on survivors’ mental health •Regularly (at every LTFU visit or general medical check-up) |
|
| ||
| Metabolic syndrome and its components | ||
|
| ||
| •Dyslipidemia •Hypertension •Impaired glucose metabolism and diabetes mellitus •Overweight and obesity |
Dyslipidemia | Dyslipidemia |
|
| ||
| •Cranial radiotherapy •TBI •HSCT |
•Fasting lipid profile starting no later than 40 years age, and at least every 5 years subsequently | |
|
| ||
| Hypertension | Hypertension | |
|
| ||
| •Radiotherapy to a volume exposing the kidneys, or to a volume exposing the heart and associated large vessels, including TBI •Immunosuppressives, eg cyclosporine, tacrolimus, prolonged corticosteroids |
•Blood pressure measurement at least every 2 years and at every LTFU visit | |
|
| ||
| Impaired glucose metabolism and diabetes mellitus | Impaired glucose metabolism and diabetes mellitus | |
|
| ||
| Psychosocial problems | ||
|
| ||
| •Dependent living •Educational problems •Relationship problems •Social withdrawal •Under-employment or unemployment |
•All leukemia survivors are at risk for psychosocial problems | •Social history focused on educational progress and/or vocational planning and employment status and social withdrawal •Regularly (at every LTFU visit or general medical check-up) •At least annually until education is completed |
|
| ||
| Pulmonary toxicity | ||
|
| ||
| •Busulfan •Radiotherapy to a volume exposing the lungs, including TBI •Allogeneic HSCT |
•Pulmonary function tests, including spirometry and diffusing capacity for carbon monoxide (DLCO), once at entry into LTFU | |
|
| ||
| Renal toxicity | ||
|
| ||
| •Radiotherapy to a volume exposing the kidney or urinary tract, including TBI •HSCT |
All survivors at risk:
•Glomerular function testing including blood testing (creatinine), urine testing (creatinine, proteinuria), eGFR calculation, at least every 5 years Other advice: - Education about caution with the use of NSAIDs |
|
|
| ||
| Spleen problems (overwhelming bacterial infections) | ||
|
| ||
| •Radiotherapy ≥10 Gy to a volume exposing the spleen, including TBI •Allogeneic HSCT (conditioned with or without TBI) |
•Patient education about events that necessitate immediate start of therapeutic antibiotics and prompt evaluation by a HCP (ensure antibiotics are readily available) •Advise use of medical bracelet or patient card |
|
|
| ||
| Subsequent malignant neoplasms | ||
|
| ||
| •Breast cancer (female) •CNS neoplasms (meningiomas, high-grade gliomas and other CNS neoplasms) •Colorectal cancer •Melanoma and non-melanoma skin cancer •Thyroid cancer |
Breast cancer (female) | Breast cancer (female) |
|
| ||
| •Radiotherapy ≥ 10 Gy to a volume exposing the breasts, including TBI | •Mammography and breast MRI, every year if ≥25 years of age or ≥8 years from radiotherapy, whichever occurs last | |
|
| ||
| CNS neoplasms | CNS neoplasms | |
|
| ||
| •Radiotherapy to a volume exposing the head or brain, including TBI | •A decision whether to undertake MRI surveillance should be made by the leukemia survivor and HCP after careful consideration of the potential harms and benefits of surveillance (there is insufficient evidence to make a recommendation for routine MRI surveillance for asymptomatic survivors) | |
|
| ||
| Colorectal cancer | Colorectal cancer | |
|
| ||
| •Radiotherapy to a volume exposing the colon and rectum, including TBI | Starting 5 years after radiotherapy or at the age of 30 years, whichever occurs last: •Fecal occult blood testing every 3 years •Alternatively, consider colonoscopy every 5 years |
|
|
| ||
| Melanoma and non-melanoma skin cancer | Melanoma and non-melanoma skin cancer | |
|
| ||
| •Any radiotherapy, including TBI, predominantly in the radiotherapy field •HSCT, especially with a history of skin GvHD |
•Self-examination for new skin lesions and changing moles, at least every 6 months •History at least every 2 years •Skin examination at least every 2 years |
|
|
| ||
| Thyroid cancer | Thyroid cancer | |
|
| ||
| Radiotherapy to a volume exposing the thyroid gland, including TBI | •Provide counselling regarding options for differentiated thyroid carcinoma surveillance, at least every 5 years •If a decision to commence surveillance is made, make a shared decision for one of these modalities: ◆Neck palpation, every 1-2 years, starting 5 years after radiotherapy, or ◆Thyroid ultrasound, every 3-5 years, starting 5 years after radiotherapy |
|
NB
1) This Table lists the more common and / or more serious late adverse effects occurring in childhood leukemia survivors. It is not intended to include all possible late effects.
2) Radiotherapy may be an additional risk factor for some late effects when administered at doses than those usually received by leukemia patients (ie doses than those delivered for cranial or craniospinal radiotherapy, or TBI, in leukemia), but these instances are not included as risk factors in this table due to the lack of evidence of their relevance to leukemia survivors.
Abbreviations: ACTHD = adrenocorticotropic hormone deficiency, ALP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, CNS = central nervous system, CPG = clinical practice guideline, DXA = dual-energy X-ray absorptiometry, ECG = electrocardiogram, FSH = follicle stimulating hormone, fT4 = free thyroxine, gGT = gamma-glutamyltransferase, GHD = growth hormone deficiency, GvHD = graft-versus-host disease, IGF-1 = insulin-like growth factor 1, IV = intravenous, HCP = health care provider, HP = hypothalamic-pituitary, HSCT = hematopoietic stem cell transplantation, LH = luteinising hormone, LH/FSHD = luteinising hormone/follicle stimulating hormone deficiency, LTFU = long-term follow-up, NSAIDs = non-steroidal anti-inflammatory drugs, SDS = standard deviation score, TBI = total body irradiation, TSH = thyroid stimulating hormone, TSHD = thyroid stimulating hormone deficiency.
Adapted from van Kalsbeek, 2021 (63)
Taken from van Atteveld, 2021 (64)
Anthracyclines include doxorubicin, daunorubicin, epirubicin, idarubicin and mitoxantrone. The following formulas may be used to convert to doxorubicin isotoxic equivalents prior to calculating total cumulative anthracycline dose. Doxorubicin: multiply total dose x 1; Daunorubicin: multiply total dose x 0.6; Epirubicin: multiply total dose x 0.8; Idarubicin: multiply total dose x 5; Mitoxantrone: multiply total dose x 10.
Risk of hypothyroidism for all mentioned exposures. Risk of hyperthyroidism after radiotherapy to a volume exposing the thyroid gland, including TBI, or allogeneic HSCT.
If amenorrhea, measure FSH and estradiol randomly; if oligomenorrhea, measure during early follicular phase (day 2-5)
This assessment should be performed after ending oral contraceptive pill/sex steroid replacement therapy use, if applicable, ideally after two months discontinuation.
Subsequent Malignant Neoplasms
Subsequent malignant neoplasms (SMN) are one of the most serious late effects in long-term survivors of childhood ALL. The reported incidence has varied substantially between 1.8 and 18% in different survivor study populations and treatment eras (5, 7, 8). Compared to survivors treated in the 1970s, those from the 1990s had a lower rate of SMN that was comparable to that of the general population (standardized incidence ratio [SIR] [95% confidence intervals, CI], 1.0 [0.6 to 1.6]), although when stratified by time since leukemia diagnosis the SIR was higher in survivors between 5 and 15 years from diagnosis (SIR 2.3 [1.2-4.0]) (6).
A recent population-based analysis from the United States (US) based Surveillance, Epidemiology, and End Results (SEER-18) database (1973–2014) found that the commonest SMN after pediatric leukemia were thyroid carcinoma (18.3%), sarcoma (15.1%), astrocytoma (10.4%), lymphoma (9.6%), salivary gland carcinoma (7.2%), melanoma (4.4%) and breast cancer (4%) (8). Radiotherapy, chemotherapy and patient-related factors are potential risk factors. Radiotherapy-related SMN mostly occur within the radiotherapy field, and for leukemia survivors, the risk of central nervous system (CNS) tumors including benign or malignant meningiomas and gliomas is increased. Recent evidence suggests that alkylating agent and anthracycline chemotherapy increase the risk of developing certain solid SMN in addition to that of subsequent acute leukemia / myelodysplasia. These risks may be modified by other patient characteristics, such as age at exposure and inherited genetic susceptibility (9). Evidence suggests that the risk of subsequent CNS tumors is reduced in CLS following the elimination of cranial radiotherapy (CRT) for CNS-directed treatment in nearly all children with ALL (2, 5).
The mean duration from ALL diagnosis to development of a SMN has been reported to be about 5 years, and over 76% of SMN in CLS occurred within 20 years of the initial diagnosis However, some SMN (eg breast cancer) may occur 20 years or more after initial diagnosis (8).
SMN have poorer survival rates compared with a first malignancy at the same age, with 5-year survival being 33.1% lower for children (<15-year age at the time of diagnosis of SMN), 20.2% lower for adolescents and young adults (AYA, 15–39-year age), and 8.3% lower for older adults (10). It is uncertain whether this reflects differences in the biological characteristics of SMN compared to equivalent first malignancies, limitations in treatment imposed by therapy already received for the first diagnosis (eg limitations in radiotherapy or chemotherapy dosing due to toxicities) or a combination of these and other factors. This adverse impact on survival highlights the importance of surveillance (see Table 1).
Survivors of childhood AML have been found to have a small increased risk of SMN (SIR 10.6; [3.3-22.3]) and a cumulative incidence of 1.3% at 15 years (11).
Metabolic Syndrome Including Obesity
The metabolic syndrome (MetS) occurs in at least a third of adult survivors of childhood ALL and includes abdominal obesity (defined by increased waist circumference), hypertriglyceridemia, hypercholesterolemia, hypertension and impaired glucose metabolism (12). All these factors increase the risk of developing cardiovascular disease or type II diabetes mellitus (T2DM) leading to increased morbidity and premature mortality. The intrinsic and extrinsic risk factors that contribute the most to the development of these metabolic complications include radiotherapy (specifically CRT and total body irradiation [TBI]), older age at treatment, unhealthy diet, and minimal or no physical activity.
Whilst the prevalence of T2DM in ALL survivors is quite low, the risk in pediatric hematopoietic stem cell transplant (HSCT) survivors is higher and a prevalence of around 9% has been reported (12). Older age at diagnosis and starting puberty during treatment were found to be important risk factors. The influence of asparaginase toxicity with hyperglycemia and/or pancreatitis is not yet fully investigated. Importantly, hyperinsulinemia, impaired glucose tolerance, hypertriglyceridemia, low concentration of high-density lipoprotein (HDL) cholesterol, and abdominal obesity are more common among HSCT recipients than among non-HSCT leukemia patients.
Obesity, defined as an abnormal or excessive accumulation of body fat, is reported in 11-56% of adult CLS (13). The excess weight gain observed in children treated for ALL usually persists and 40-50% of young adult survivors remain obese (14). Importantly, abdominal obesity is a risk factor for chronic diseases despite normal body mass index (BMI). Risk factors for obesity related to the leukemia treatment are prolonged corticosteroid exposure and CRT leading to hypothalamic damage and potentially growth hormone deficiency (GHD) as well as younger age at diagnosis and female sex. Additional treatment-associated factors include longstanding immobility during treatment, muscle weakness or osteonecrosis (13).
In childhood ALL survivors the standardized mortality hazard ratio is 9.5 (95% CI 8.8–10.2) compared to healthy siblings, with non-cancer related mortality mostly attributed to cardiovascular causes (14). Obesity and other components of the MetS can increase the global risk for cardiovascular complications by 40-fold. Thus, prevention and treatment approaches have become increasingly important to decrease morbidity and mortality (14).
Gonadotoxicity and Fertility Impairment
Fertility impairment has been reported in 42-66% of male and 11-26% of female CCS (15-19) and is higher after alkylating agent treatment and/or radiation to fields involving the testes and ovaries, including TBI (5, 20). ALL survivors treated on older protocols (1962-79) experienced more reproductive system late effects than those treated more recently (1991-2007). Alkylating agents are the most gonadotoxic chemotherapeutic agents and the risk increases with higher cumulative doses, as estimated by the cyclophosphamide equivalent dose (CED) (21). In contrast, methotrexate and vinca alkaloids have minimal or no gonadotoxic effects (22).
Although fertility preservation procedures are seldom feasible at initial diagnosis of acute leukemia due to the child’s condition and need for urgent chemotherapy, as well as concerns about leukemic infiltration of gonadal tissue, it remains important that patients and their parents should be counselled about the level of risk of gonadotoxicity. Furthermore, their HCP should ensure timely identification and management of testosterone or estrogen deficiency in at-risk patients and refer survivors to reproductive medicine specialists when appropriate.
Males
Sertoli cells (involved in spermatogenesis) in the testicles are more sensitive to radiotherapy and chemotherapy than Leydig cells (responsible for androgen production).
Chemotherapy agents most often associated with impaired spermatogenesis in male CLS include cyclophosphamide, ifosfamide, busulfan and melphalan (23). Combinations of alkylating agents have an additive effect on gonadotoxicity. Although there is individual variability in the risk of gonadotoxicity after exposure, the cumulative dose likely to produce azoospermia has been established for most agents. Prepubertal status at diagnosis is not protective. Alkylating agent-associated azoospermia is usually permanent although recovery of normal spermatogenesis years after treatment has been described. The testicular germinal epithelium is particularly sensitive to radiotherapy. Testicular doses as low as 0.1 gray (Gy) impair spermatogenesis acutely, and recovery is unlikely after a single testicular dose exceeding 4-6 Gy (23). Testosterone deficiency occurs after testicular radiation of >20 Gy in prepubertal males and after >30 Gy in older boys (23). Subclinical dysfunction of androgen production may occur with lower doses of testicular radiotherapy of 12 Gy (24).
Females
Females are born with a fixed number of ovarian primordial follicles, which varies between individuals and declines with age. Radiotherapy at an older age is associated with greater dose-related risk of ovarian damage, as the result of a smaller oocyte pool at the time of treatment (25). Doses of 5 Gy can impair ovarian function in postpubertal girls (10 Gy in prepubertal), and doses ≥10 Gy (≥15 Gy in prepubertal age) are more likely to cause premature ovarian insufficiency (POI), presenting as amenorrhoea (primary or secondary) or premature menopause (22, 25, 26). Estrogen replacement treatment is required to induce puberty in younger girls with POI and to optimize future cardiac and bone health in post-pubertal individuals. Mathematical modelling of the rate of oocyte decline suggests that the sterilizing dose is 20.3 Gy in infants, 18.4 Gy at age 10 years, and 16.5 Gy at age 20 years. Doses as low as 2 Gy have been estimated to deplete the follicular pool by up to 50% (25). The risk of radiotherapy is increased by additional alkylating chemotherapy. Alkylating agent-induced POI correlates directly with cumulative dose and age at exposure [18]. TBI is a major risk factor for POI. CRT doses causing lower pregnancy rates varied significantly by study, but even low doses (18-24 Gy) used in historical ALL protocols have been reported to decrease fertility rates compared with sibling controls, whilst doses >30 Gy demonstrated the highest risk for fertility impairment due to central hypogonadotropic hypogonadism (22, 26, 27).
Most AML survivors treated with chemotherapy alone had normal pubertal development and fertility. However, anti-Mullerian hormone (AMH) levels were decreased in 13% of post-pubertal females, implying a potential risk of POI in female AML survivors (28).
Endocrinopathy and Growth Disturbances
Endocrine complications are frequently observed in CCS, with 50% of CCS experiencing at least one hormonal disorder during their lifetime (29). The cumulative incidence of grade 1-5 endocrine late effects in CCS is 62.6%, 81.9% and 91.6% at 30, 40 and 50 years attained age, respectively (3). The most common endocrine late effects include hypothalamic-pituitary dysfunction, primary thyroid dysfunction, primary gonadal injury, obesity, T2DM, MetS, and decreased bone mineral density (BMD) (30).
The main risk factors are previous exposure to radiotherapy that included key endocrine organs (hypothalamus/pituitary, thyroid, pancreas and gonads) and/or alkylating agents. The risk increases with the time interval since treatment, the total dose received, fraction size and number, and the method of radiotherapy delivery. In addition to alkylating agents (gonadotoxicity), chemotherapeutic agents associated with endocrine late effects include platinum drugs (gonadotoxicity), glucocorticoids (obesity, decreased BMD), and tyrosine kinase inhibitors (impaired linear growth, primary hypothyroidism) (29).
In childhood ALL survivors the cumulative burden for endocrine late effects was 1.09 at age 30 years and 2.62 at age 50 years, while for childhood AML survivors it was 1.65 at age 30 years and 2.66 at age 50 years (3). All leukemia survivors treated in the 1970s received CRT or (less commonly) craniospinal radiotherapy as part of CNS-directed treatment, mostly with 18-24 Gy. Currently, less than 5% of children with ALL receive CRT. Nevertheless, CRT is a potent cause of hypopituitarism, and the frequency, nature and extent of pituitary hormone deficit is related to dose, the fraction schedule, and the time interval since radiotherapy (30, 31).
The hypothalamus is more radiosensitive than the anterior pituitary gland, so GHD is usually the first endocrinopathy to be diagnosed (32). Survivors who received doses greater than 18 Gy are at highest risk, but even TBI conditioning prior to HSCT with doses of 10 Gy as a single fraction or 12 Gy in 6 fractions may partially reduce growth hormone secretion in 35-50% survivors (30-32). Both younger age and female sex were significantly associated with a greater risk of lower final height in patients treated with CRT (30). Prolactin and thyroid stimulating hormone (TSH) insufficiencies have also been reported after longer follow-up (30,32,33). It was previously considered that higher radiotherapy doses are needed to cause life-threatening adrenocorticotropic hormone (ACTH) insufficiency, but ACTH insufficiency has been reported in a homogenous group of ALL survivors treated with moderate dose CRT (18-24 Gy) (33). Precocious puberty or rapid progression of puberty can also occur after CRT ≥18 Gy, with female sex and lower age at the time of treatment being additional risk factors (33).
Although the evolution of therapy for childhood ALL has not changed the cumulative burden involving the endocrine system, the specific types of endocrinopathy observed have changed markedly. Endocrinopathies among survivors treated on earlier protocols largely involved adrenal insufficiency and GHD due to chemoradiotherapy-induced hypothalamic-pituitary dysfunction, while impairments in glucose metabolism and body composition and overweight or obesity have become prominent among survivors treated with more recent protocols (5).
Bone Toxicity
Osteonecrosis (avascular necrosis) and low BMD are the two most common bone problems in ALL survivors and may seriously impact quality of life (34). Osteonecrosis is described during and after treatment with high cumulative doses of steroids, HSCT and radiotherapy.
In young adults, BMD is dependent on peak bone mass (PBM). ALL survivors may not have undergone optimal bone growth at puberty due to their illness and treatment (35). Inadequate lean mass acquisition, weight-bearing physical activity, diet, as well as the presence of endocrinopathies, may also impair attainment of PBM (34-36). The prevalence of osteopenia and osteoporosis in CLS is not yet well documented. Moreover, the occurrence of fractures is still insufficiently characterized (37). ALL survivors have the potential highest risk of developing low BMD amongst all CCS as the disease and its specific treatments are the most important risk factors for BMD deficits, namely high cumulative steroid doses, methotrexate, HSCT, CRT and testicular radiotherapy (38).
Low BMD is commonly described during treatment of pediatric ALL, as well as reduced levels of bone formation markers, and this may lead to an increase of fracture incidence (39).
In a study assessing longitudinal BMD and bone structure in ALL survivors who had not received CRT, the findings suggested that ALL treatment in childhood without CRT may not result in long-term detrimental effects on bone development (40). The whole-body bone mass tended to be only marginally lower in long-term allogeneic HSCT survivors than in ALL survivors treated without HSCT, and the size-adjusted bone mass (bone mineral content for bone area) remained normal (41).
Neurotoxicity
Central Nervous System Toxicity
CLS are at risk of long-term neurologic sequelae. One of the most serious consequences is neurocognitive morbidity. Cognitive deficits in CLS treated in the 1970s and 1980s with CRT were frequent, dose-dependent, progressive with duration of follow-up and more pronounced in girls or those treated at a younger age (42). Long-term CLS treated with CRT were found to have significant impairments in memory, task efficiency, and emotional regulation. It was reported that doses of 24 Gy are associated with an average decline in intelligence of about 10 intelligence quotient (IQ) points (43). The most severe delayed neurotoxicity is observed in patients treated with a combination of CRT and neurotoxic systemic and intrathecal chemotherapy. This is probably due to radiation-induced increased permeability of the blood-brain barrier to neurotoxic chemotherapy (44). In more recent decades, leukemia treatment mostly incorporates intensified systemic and intrathecal chemotherapy with methotrexate instead of CRT. Many studies have confirmed that treatment containing chemotherapy alone is still associated with cognitive deficits and other neuropsychologic impairments as well, but to a lesser degree than after CRT (45). Neurocognitive deficits in ALL survivors include attention, memory, and executive function. These survivors are at higher risk for increased utilization of special education services, poorer academic achievement, higher rates of unemployment, and lower socioeconomic status in adulthood. Adult CLS are at increased risk for late-onset auditory-vestibular-visual sensory deficits, coordination problems, motor problems, seizures and headaches (42). They are also at an increased risk of cerebrovascular vasculopathy, with a relative risk of 6.4 for a cerebrovascular accident (stroke) compared with sibling controls, if treated with CRT (46).
Peripheral Nervous System Toxicity
Clinical manifestations of chemotherapy-induced peripheral neuropathy (CIPN) have been reported in up to 35% of children treated for ALL (47). Vincristine has been identified as the main causative agent. Clinical symptoms include a mixed sensorimotor neuropathy (loss of deep tendon reflexes, paresthesia, neuropathic pain, wrist or foot drop, sensory loss to pain, temperature and proprioception, difficulty with balance and coordination) or autonomic neuropathy (constipation, abdominal pain, paralytic ileus, bladder atony with urinary retention, and orthostatic hypotension). Neurotoxicity can be acute or chronic. Acute neurotoxicity had been thought to be essentially reversible for many years. However, chronic neurotoxicity can occur in a peripheral “stocking and glove” pattern and is an axonal sensory neuropathy that increases with repeated dosing of vincristine. It is often irreversible, with high morbidity and decreased quality of life for many years after treatment completion (48).
Cardiotoxicity
Cardiovascular-related morbidity is a substantial health burden in CCS, and major cardiac events are the leading cause of non-cancer mortality in this population (49). Within 5-10 years after treatment, over 50% of CCS have subclinical evidence of cardiac damage and there is a striking increase in the cumulative incidence of late cardiotoxicity in survivors older than 35 years relative to other health outcomes (49, 50). Late-onset dose-dependent cardiotoxicity is largely due to anthracycline and related agents and chest-directed radiotherapy. Other risk factors include younger age at diagnosis, female gender, longer time since treatment, the presence of pre-existing cardiovascular disease and comorbidities, hyperlipidemia, obesity, diabetes, smoking, and genetic factors (51, 52).
Common cardiovascular adverse events are cardiomyopathy/congestive heart failure, coronary artery disease, stroke, arrhythmias, valvular abnormalities, systemic and pulmonary hypertension, pericardial disease and vascular dysfunction (53). Compared with the general population, CCS were eight times more likely to die from cardiovascular-related disease (54).
CLS had a 4.2-fold increased risk for congestive heart failure, a 3.3-fold increased risk for myocardial infarction, a 2.6-fold increased risk for pericardial disease, and a 2.6-fold increased risk for valvular abnormalities compared with sibling controls (55). In childhood AML survivors, the cumulative incidence of cardiotoxicity was 16% and 27% at 10 and 15 years, respectively (56).
The St Jude Lifetime Cohort Study reported 980 childhood ALL survivors with median time from diagnosis of 30.0 years. The frequency of hypertension was higher among survivors than age- and sex-matched community controls (53.8% versus 43.7%), though not statistically significant, with 18% of survivors having hypertension grade 2 or worse. No difference was found in the frequency of cardiomyopathy or metabolic problems (and modifiable cardiovascular factors) including dyslipidemia, overweight or obesity, and impaired glucose metabolism, between survivors and controls (5).
Psychosocial Late Effects
The experience of leukemia in childhood can have an impact on mental health and social aspects of life long into survivorship. Frequent problems in CCS include depression or anxiety, symptoms of post-traumatic stress, including intrusion (eg flashbacks), avoidance of situations related to the trauma (eg hospitals), and arousal (eg irritability or concentration difficulties) (57-59). In addition, CCS are at increased risk of suicide ideation as well as completion (57). On the positive side, many CCS, especially after leukemia, also experience positive changes such as post-traumatic growth or benefit finding (57, 59). CCS report an improved experience of relating to others and they find that they also have new possibilities and experience an overall appreciation of life. During the treatment phase, many young leukemia patients miss school for a lengthy period of time and often need educational support thereafter, with survivors of ALL at particular need of further assistance (60). In the long-term, CLS are at higher risk of lower educational achievement compared to their peers (61). In addition, many survivors also experience problems with employment and CLS have a higher risk of unemployment (62).
The high dependency on parents, family and HCP during their diagnosis and treatment generates additional stress when survivors become independent and engage in romantic relationships. Fewer CCS compared to general population are married, and many experience psychosexual problems (61).
Others
Several other late effects such as chronic dental and oral problems, fatigue and pain are well described in CLS. In addition, liver and lower urinary tract toxicity, splenic dysfunction and obstetric problems may occur occasionally in some CLS, most commonly after HSCT. Further information about most frequent and/or more serious late effects, risk factors and recommended evaluation or surveillance test is provided in Table 1 (63, 64).
Accelerated Ageing
There is increasing awareness of the occurrence of accelerated ageing in CCS. In the general population, ageing is characterized by a progressive reduction in physiological reserve capacity clinically manifest as frailty, a syndrome comprising at least three out of the following five markers of low physiological reserve, specifically exhaustion, weakness, low physical activity, slow walking speed and unintentional weight loss. A Childhood Cancer Survivor Study (CCSS) report showed that the prevalence of frailty was three times higher in survivors (6.4% at a mean age of 37.6 years) than in their siblings (2.2% at mean age 42.9 years), and that the risk factors included CRT (65). In addition, a Bone Marrow Transplant Survivor Study (BMTSS) showed that young adult HSCT survivors were more likely than their siblings to be frail at a mean of 8.7 years post-transplant, with those with active chronic graft-versus-host disease (cGvHD) showing a 15-fold increased risk compared to siblings. Furthermore, frail HSCT survivors had a significantly higher risk of subsequent non-relapse mortality over the next 10 years compared to those without frailty (23.9% vs 10.2%) (66). A study in 87 asymptomatic young adult survivors of childhood ALL (median age, 25 years) showed a pattern of chronic inflammation and telomere attrition consistent with early development of the cellular processes driving accelerated ageing (67).
Late Mortality
Late mortality (LM) means death more than five years after diagnosis and reflects premature mortality which may affect CCS. The primary cause of LM is relapse or progression of the first primary cancer even several decades after initial diagnosis, but SMN and cardiovascular causes become the most frequent causes of death later than 20-30 years from diagnosis (68). A large study of children with ALL treated in Europe between 1982 and 2002 found an increased survival over time but also showed that a small excess risk of death persisted for up to 20 years after diagnosis (69). Data from the Italian Off-Therapy Registry found a standardized mortality ratio (SMR) of 11 after ALL and 16 after AML in patients treated between 1960 and 1999 (70). A CCSS study found a reduction in LM after standard-risk ALL comparing the 1970s to the 1990s, with the health-related LM in the 1990s comparable to US population in general. The twenty-year all-cause LM was 6.6 % (6). A separate CCSS study found a SMR of 6.5 for all leukemias treated between 1970 and 1999 (71).
Late Effects after HSCT
Survivors of childhood HSCT have a very high risk of chronic toxicities, with over 90% experiencing at least one and >70% at least three late effects (72). The risk is highest in those conditioned with TBI, but high-dose conditioning chemotherapy also causes considerable late toxicity. Numerous additional and potentially synergistic risk factors contribute to late effects in HSCT survivors, including chemotherapy or radiotherapy given months or years before transplant, sequelae from serious acute complications of HSCT, potentially toxic supportive care drugs, other longer-term complications (eg iron overload due to multiple blood transfusions), and especially cGvHD.
Some post-HSCT late effects, such as SMN, are potentially life-threatening. SMN are reported in up to 10–15% of HSCT survivors 15 years post-transplant, with skin, oral, thyroid and CNS tumors most frequently reported (73). Gonadotoxicity and consequent fertility impairment is frequently life-changing for HSCT survivors (74). Up to 30% of childhood allogeneic HSCT recipients develop cGvHD, with consequent tissue damage that has the potential to affect nearly any body organ or system (75). The range of possible manifestations is very large but commonly involves skin (lichenoid and/or sclerodermatous lesions), musculoskeletal system (sclerodermatous joint contractures), lungs (obstructive lung disease), gastrointestinal tract (oral lesions, gut strictures, malabsorption) and liver (cholestasis). Less commonly cGvHD may affect the kidneys (proteinuria), central (demyelination, vasculitis) and peripheral (myasthenia, neuropathy) nervous systems, and serosal surfaces (pleural, pericardial and peritoneal effusions). In addition, cGvHD may cause immunological impairment (delayed immune reconstitution and risk of late infections) and/or dysregulation (immune-mediated cytopenias), and may contribute to the development of SMN. Serious GvHD (acute or chronic) frequently leads to prolonged and intensive immunosuppressive treatment, contributing to or directly causing long-term toxicities (eg steroid toxicity leading to osteonecrosis and cataracts, renal toxicity due to calcineurin inhibitors). It is important to appreciate that although many of these late effects have multifactorial causes including previous or conditioning chemotherapy or radiotherapy, cGvHD can contribute significantly to adverse long-term outcomes and disability (76).
Survivorship and LTFU
Survivorship Passport
There are between 100-175,000 CLS in Europe, most of whom have already reached or are entering adulthood (77). It is therefore important that at the time of treatment completion or of transition to the LTFU clinic, survivors are provided with a detailed treatment summary containing information on the original leukemia and its treatment (78) as well as recommendations for LTFU ideally based on evidence-based clinical practice guidelines (CPG).
Several initiatives have been developed to provide all CCS with an electronic document containing, in a standardized format, the personal medical history and personalized care plan, both in the US with the Passport for Care (www.passportforcare.org) (79) and in Europe with the Survivorship Passport (SurPass - www.survivorshippassport.org) (80). The SurPass is a web-based tool developed with the support of several European Union (EU) funds and close collaboration amongst late effects experts, pediatric oncologists, survivor organizations and the information technology (IT) company Cineca. The recently released v1.2 of the SurPass combines an individual survivor’s health data with International Late Effects of Childhood Cancer Guideline Harmonization Group (IGHG) / Pan-European Network for Care of Survivors after Childhood and Adolescent Cancer (PanCare) evidence-based, international guidelines (63). The main pillars of SurPass are:
Treatment Summary (TS) - Using a common template and internationally approved coding systems, TS is personalized for each survivor. It includes in a standardized format all relevant information about diagnosis, cancer treatment, and any other relevant health data with potential long-term impact on organ function. This will help HCP to quickly understand the survivor’s medical history, and survivors themselves to provide access to their own health data.
Survivorship Care Plan (SCP) - Based on the TS and latest IGHG / PanCare CPG (63), algorithms in the SurPass platform generate personalized recommendations for follow-up based on the survivor’s risk profile. The SCP can be further tailored by HCP and CCS at each visit by shared decision-making.
The SurPass tool is based on a secure platform and is currently available in eight languages (English, Croatian, Dutch, French, German, Italian, Lithuanian, Spanish) and, if needed, each passport can be automatically translated.
Guidelines
Evidence-based CPG developed with rigorous methodology improve the consistency and quality of clinical care. When widely implemented in general medical practice they have been shown to improve health outcomes and to contribute to reductions in morbidity and mortality. In previous years several CPG for LTFU of CCS have been developed by several European and North American groups, providing surveillance recommendations to facilitate early detection and hence timely intervention to treat or ideally even prevent clinically overt late effects. However, several surveillance recommendations differ between these existing guidelines in terms of identifying groups at risk, as well as in recommended surveillance modalities and timing (81). To overcome these incongruences, IGHG was initiated in 2010 as a worldwide collaboration including researchers from Europe, North America, Australia and Japan to harmonize guidelines for LTFU of childhood and young adult cancer survivors (82). The team includes late effects experts, pediatric and radiation oncologists, other pediatric and medical subspecialists, primary care, nursing, psychology and CCS representatives. So far IGHG has published 15 evidence-based CPG for late effects surveillance (bone mineral density, cancer-related fatigue, cardiomyopathy, coronary artery disease, education / employment outcomes, male gonadotoxicity, hepatic toxicity, hypothalamic/pituitary surveillance, mental health problems, obstetric risks, ototoxicity, POI, subsequent breast and thyroid cancer, and subsequent CNS neoplasms) (82). Each guideline specifies i) who is at risk (needs surveillance) for the given late complication, ii) what surveillance modality should be used, iii), when (at what age or time) should be initiated and discontinued, if applicable, iv) at what frequency should be done, and v) what should be done when abnormalities are found. Further guidelines are under development or planned to be developed by IGHG as well as the update of previous ones in case new evidence from the literature becomes available.
Models of Care
To provide regular and well-organized LTFU for CCS, several models of care have been developed including cancer center-delivered care in a pediatric oncology clinic, medical oncology clinic, or LTFU clinic as the most common model. More recently, follow-up care led by a primary care physician or a shared care model between the treating hospital and the local hospital or primary care have been proposed (83). An important aspect for the choice of care is risk stratification. The risk of CCS suffering late adverse outcomes varies greatly depending on the diagnosis and treatment. CCS at low risk might profit from LTFU by a primary care provider, while those at high risk, such as many CLS, would profit most from attending a specialized LTFU clinic (83). LTFU services perform several important roles including the delivery of ongoing multidisciplinary care, including provision of a TS and SCP with accompanying education for each CCS, with transition of care from pediatric / adolescent to adult healthcare settings. In addition, late effects surveillance is an important component of care, starting no later than five years after treatment completion or from diagnosis, depending on the individual health care systems, and should be continued life-long, unless specified otherwise.
Future Developments
A great deal of published information is now available regarding the nature, causes and outcomes of late effects in CLS, but several important gaps in knowledge remain. The increasing use of novel immunotherapy and targeted therapies in children with high-risk and relapsed acute leukemia is not only offering these individuals a chance of long-term survival but also revealing their potential to cause a novel range of chronic toxicities many of which are poorly characterized and potentially under-recognized. The ACCELERATE Long-Term Follow-Up Working Group is developing an international LTFU registry to prospectively collect data regarding the chronic toxicities of these treatments (84). Preventive strategies are used increasingly to reduce the risk and severity of some late effects. Greater understanding of the cellular and molecular pathogenesis of late effects may inform the development of new approaches to prevent their occurrence or reduce their severity and may in turn be informed by increased knowledge of the contribution that genetic polymorphisms may play in determining susceptibility to particular toxicities. This information may also ultimately improve risk prediction, informing more accurate late effects counselling for patients and more precisely targeted use of surveillance investigations.
Conclusion
Whilst >90% of children with ALL and >60% with AML are now cured by contemporary treatment strategies, there is increasing recognition of the frequency, nature and potential severity of late adverse effects suffered by long-term survivors, some of which may be life-threatening, life-limiting or life-changing. Despite the rapidly growing body of published literature concerning the wide range of late effects and describing strategies for delivering long-term survivorship care, significant inequalities persist within and between European countries in the availability and quality of such care. It remains of the highest importance to raise awareness of the existence and severity of late effects of childhood leukemia therapy among parents and patients, improve knowledge amongst health professionals, and seek constructive dialogue with policymakers concerning provision and funding of long-term survivorship care.
Authors’ Contributions:
Conception and design: JR, RH and RS; Acquisition, analysis and interpretation of data: JR, RH, EB, LH, GM, VP, KS, HJHP, LZZ and RS; Drafting the article: JR, RH, EB, LH, GM, VP, KS, HJHP, LZZ and RS; Revising it critically for important intellectual content: JR, RH and RS; Approved final version of the manuscript: JR, RH, EB, LH, GM, VP, KS, HJHP, LZZ and RS.
Footnotes
What Is Already Known on This Topic:
Treatments for childhood acute leukemia have led to substantially improved cure rates, but these lifesaving therapies may also have a long-term impact on survivors’ health and quality of life. These late effects include alterations in growth and development, neurocognitive impairment, psychosocial disorders, subsequent malignancies, and organ system damage. All organ systems are at risk, including cardiovascular, endocrine, reproductive, musculoskeletal and neurological, amongst others. The entire landscape of long-term therapy-related complications emerging years or even decades after successful completion of treatment is currently being investigated in collaborative research settings. Many survivors are not aware of their personal risk, and it seems to be a general lack of information among healthcare professionals about potential delayed therapy-related complications. Structured risk-adapted long-term follow-up based on current surveillance guidelines and recommendations offers standardized and optimal health care for childhood leukemia survivors.
What This Study Adds:
Increasing attention is now being focused on issues relating to long-term morbidity and premature mortality associated with the treatments responsible for increased cure rates in the growing population of survivors from childhood acute leukemia. Our knowledge of the late treatment-related adverse events continues to improve through international collaborative research efforts. This review summarizes the most frequent and severe long-term consequences of childhood leukemia therapy. The review describes recent developments in survivorship care approaches, including survivorship passport, surveillance guidelines and models of care, and emphasizes the need for standardized life-long follow-up for childhood leukemia survivors.
Conflict of Interest: The authors declare that they have no conflict of interest.
Financial Disclosure: This publishing was supported by Stanislaw Garwicz Fund.
References
- 1.Bhatt NS, Baassiri MJ, Liu W, Bhakta N, Chemaitilly W, Ehrhardt MJ, et al. Late outcomes in survivors of childhood acute myeloid leukemia:a report from the St. Jude Lifetime Cohort Study. Leukemia. 2021;35(8):2258–73. doi: 10.1038/s41375-021-01134-3. doi:10.1038/s41375-021-01134-3. Epub 2021 Jan 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mody R, Li S, Dover DC, Sallan S, Leisenring W, Oeffinger KC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia:a report from the Childhood Cancer Survivor Study. Blood. 2008;111(12):5515–23. doi: 10.1182/blood-2007-10-117150. doi:10.1182/blood-2007-10-117150. Epub 2008 Mar 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhakta N, Liu Q, Ness KK, Baassiri M, Eissa H, Yeo F, et al. The cumulative burden of surviving childhood cancer:an initial report from the St Jude Lifetime Cohort Study (SJLIFE) Lancet. 2017;390(10112):2569–82. doi: 10.1016/S0140-6736(17)31610-0. doi:10.1016/S0140-6736(17)31610-0. Epub 2017 Sep 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vetsch J, Wakefield CE, Robertson EG, Trahair TN, Mateos MK, Grootenhuis M, et al. Health-related quality of life of survivors of childhood acute lymphoblastic leukemia:a systematic review. Qual Life Res. 2018;27(6):1431–43. doi: 10.1007/s11136-018-1788-5. doi:10.1007/s11136-018-1788-5. Epub 2018 Jan 25. [DOI] [PubMed] [Google Scholar]
- 5.Mulrooney DA, Hyun G, Ness KK, Bhakta N, Pui CH, Ehrhardt MJ, et al. The changing burden of long-term health outcomes in survivors of childhood acute lymphoblastic leukaemia:a retrospective analysis of the St Jude Lifetime Cohort Study. Lancet Haematol. 2019;6(6):e306–16. doi: 10.1016/S2352-3026(19)30050-X. doi:10.1016/S2352-3026(19)30050-X. Epub 2019 May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon SB, Chen Y, Yasui Y, Pui CH, Hunger SP, Silverman LB, et al. Reduced Morbidity and Mortality in Survivors of Childhood Acute Lymphoblastic Leukemia:A Report From the Childhood Cancer Survivor Study. J Clin Oncol. 2020;38(29):3418–29. doi: 10.1200/JCO.20.00493. doi:10.1200/JCO.20.00493. Epub 2020 Jul 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bright CJ, Reulen RC, Winter DL, Stark DP, McCabe MG, Edgar AB, et al. Risk of subsequent primary neoplasms in survivors of adolescent and young adult cancer (Teenage and Young Adult Cancer Survivor Study):a population-based, cohort study. Lancet Oncol. 2019;20(4):531–45. doi: 10.1016/S1470-2045(18)30903-3. doi:10.1016/S1470-2045(18)30903-3. Epub 2019 Feb 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yavvari S, Makena Y, Sukhavasi S, Makena MR. Large Population Analysis of Secondary Cancers in Pediatric Leukemia Survivors. Children (Basel) 2019;6(12):130. doi: 10.3390/children6120130. doi:10.3390/children6120130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turcotte LM, Neglia JP, Reulen RC, Ronckers CM, van Leeuwen FE, Morton LM, et al. Risk, Risk Factors, and Surveillance of Subsequent Malignant Neoplasms in Survivors of Childhood Cancer:A Review. J Clin Oncol. 2018;36(21):2145–52. doi: 10.1200/JCO.2017.76.7764. doi:10.1200/JCO.2017.76.7764. Epub 2018 Jun 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keegan THM, Bleyer A, Rosenberg AS, Li Q, Goldfarb M. Second Primary Malignant Neoplasms and Survival in Adolescent and Young Adult Cancer Survivors. JAMA Oncol. 2017;3(11):1554–7. doi: 10.1001/jamaoncol.2017.0465. doi:10.1001/jamaoncol.2017.0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung W, Ribeiro RC, Hudson M, Tong X, Srivastava DK, Rubnitz JE, et al. Second malignancy after treatment of childhood acute myeloid leukemia. Leukemia. 2001;15(1):41–5. doi: 10.1038/sj.leu.2401948. doi:10.1038/sj.leu.2401948. [DOI] [PubMed] [Google Scholar]
- 12.Bielorai B, Pinhas-Hamiel O. Type 2 Diabetes Mellitus, the Metabolic Syndrome, and Its Components in Adult Survivors of Acute Lymphoblastic Leukemia and Hematopoietic Stem Cell Transplantations. Curr Diab Rep. 2018;18(6):32. doi: 10.1007/s11892-018-0998-0. doi:10.1007/s11892-018-0998-0. [DOI] [PubMed] [Google Scholar]
- 13.Gibson TM, Ehrhardt MJ, Ness KN. Obesity and metabolic syndrome among adult survivors of childhood leukemia. Curr Treat Options Oncol. 2016;17(4):17. doi: 10.1007/s11864-016-0393-5. doi:10.1007/s11864-016-0393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruzzi P, Bigi E, Predieri B, Bonvicini F, Cenciarelli V, Felici F, et al. Long-term effects on growth, development, and metabolism of ALL treatment in childhood. Expert Rev Endocrinol Metab. 2019;14(1):49–61. doi: 10.1080/17446651.2019.1561271. doi:10.1080/17446651.2019.1561271. Epub 2018 Dec 31. [DOI] [PubMed] [Google Scholar]
- 15.Green DM, Liu W, Kutteh WH, Ke RW, Shelton KC, Sklar CA, et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer:a report from the St Jude Lifetime Cohort Study. Lancet Oncol. 2014;15(11):1215–23. doi: 10.1016/S1470-2045(14)70408-5. doi:10.1016/S1470-2045(14)70408-5. Epub 2014 Sep 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chemaitilly W, Li Z, Krasin MJ, Brooke RJ, Wilson CL, Green DM, et al. Premature Ovarian Insufficiency in Childhood Cancer Survivors:A Report From the St. Jude Lifetime Cohort. J Clin Endocrinol Metab. 2017;102(7):2242–50. doi: 10.1210/jc.2016-3723. doi:10.1210/jc.2016-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine JM, Whitton JA, Ginsberg JP, Green DM, Leisenring WM, Stovall M, et al. Nonsurgical premature menopause and reproductive implications in survivors of childhood cancer:A report from the Childhood Cancer Survivor Study. Cancer. 2018;124(5):1044–52. doi: 10.1002/cncr.31121. doi:10.1002/cncr.31121. Epub 2018 Jan 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green DM, Kawashima T, Stovall M, Leisenring W, Sklar CA, Mertens AC, et al. Fertility of female survivors of childhood cancer:a report from the childhood cancer survivor study. J Clin Oncol. 2009;27(16):2677–85. doi: 10.1200/JCO.2008.20.1541. doi:10.1200/JCO.2008.20.1541. Epub 2009 Apr 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei C, Crowne E. The impact of childhood cancer and its treatment on puberty and subsequent hypothalamic pituitary and gonadal function, in both boys and girls. Best Pract Res Clin Endocrinol Metab. 2019;33(3):101291. doi: 10.1016/j.beem.2019.101291. doi:10.1016/j.beem.2019.101291. [DOI] [PubMed] [Google Scholar]
- 20.Skinner R, Wallace WH, Levitt GA UK Children's Cancer Study Group Late Effects Group. Long-term follow-up of people who have survived cancer during childhood. Lancet Oncol. 2006;7(6):489–98. doi: 10.1016/S1470-2045(06)70724-0. doi:10.1016/S1470-2045(06)70724-0. [DOI] [PubMed] [Google Scholar]
- 21.Green DM, Nolan VG, Goodman PJ, Whitton JA, Srivastava D, Leisenring WM, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure:a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61(1):53–67. doi: 10.1002/pbc.24679. doi:10.1002/pbc.24679. Epub 2013 Aug 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulder RL, Font-Gonzalez A, Hudson MM, van Santen HM, Loeffen EAH, Burns KC, et al. Fertility preservation for female patients with childhood, adolescent, and young adult cancer:recommendations from the PanCareLIFE Consortium and the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2021;22(2):e45–56. doi: 10.1016/S1470-2045(20)30594-5. doi:10.1016/S1470-2045(20)30594-5. [DOI] [PubMed] [Google Scholar]
- 23.Skinner R, Mulder RL, Kremer LC, Hudson MM, Constine LS, Bardi E, et al. Recommendations for gonadotoxicity surveillance in male childhood, adolescent, and young adult cancer survivors:a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. Lancet Oncol. 2017;18(2):e75–90. doi: 10.1016/S1470-2045(17)30026-8. doi:10.1016/S1470-2045(17)30026-8. Erratum in:Lancet Oncol. 2017;18(4):e196. [DOI] [PubMed] [Google Scholar]
- 24.Lopez R, Plat G, Bertrand Y, Ducassou S, Saultier P, Berbis J, et al. Testosterone deficiency in men surviving childhood acute leukemia after treatment with hematopoietic stem cell transplantation or testicular radiation:an L. E. A. study. Bone Marrow Transplant. 2021;56(6):1422–5. doi: 10.1038/s41409-020-01180-y. doi:10.1038/s41409-020-01180-y. Epub 2021 Jan 16. [DOI] [PubMed] [Google Scholar]
- 25.Wallace WH, Thomson AB, Saran F, Kelsey TW. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int J Radiat Oncol Biol Phys. 2005;62(3):738–44. doi: 10.1016/j.ijrobp.2004.11.038. doi:10.1016/j.ijrobp.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 26.van Dorp W, Mulder RL, Kremer LC, Hudson MM, den Heuvel-Eibrink van MM, den Berg van MH, et al. Recommendations for Premature Ovarian Insufficiency Surveillance for Female Survivors of Childhood, Adolescent, and Young Adult Cancer:A Report From the International Late Effects of Childhood Cancer Guideline Harmonization Group in Collaboration With the PanCareSurFup Consortium. J Clin Oncol. 2016;34(28):3440–50. doi: 10.1200/JCO.2015.64.3288. doi:10.1200/JCO.2015.64.3288. Epub 2016 Jul 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panasiuk A, Nussey S, Veys P, Amrolia P, Rao K, Krawczuk-Rybak M, et al. Gonadal function and fertility after stem cell transplantation in childhood:comparison of a reduced intensity conditioning regimen containing melphalan with a myeloablative regimen containing busulfan. Br J Haematol. 2015;170(5):719–26. doi: 10.1111/bjh.13497. doi:10.1111/bjh.13497. Epub 2015 May 14. [DOI] [PubMed] [Google Scholar]
- 28.Molgaard-Hansen L, Skou AS, Juul A, Glosli H, Jahnukainen K, Jarfelt M, et al. Pubertal development and fertility in survivors of childhood acute myeloid leukemia treated with chemotherapy only:a NOPHO-AML study. Pediatr Blood Cancer. 2013;60(12):1988–95. doi: 10.1002/pbc.24715. doi:10.1002/pbc.24715. Epub 2013 Aug 23. [DOI] [PubMed] [Google Scholar]
- 29.Chemaitilly W, Cohen LE. Diagnosis of endocrine disease:Endocrine late effects of childhood cancer and its treatments. Eur J Endocrinol. 2017;176(4):R183–203. doi: 10.1530/EJE-17-0054. doi:10.1530/EJE-17-0054. [DOI] [PubMed] [Google Scholar]
- 30.Chemaitilly W, Cohen LE, Mostoufi-Moab S, Patterson BC, Simmons JH, Meacham LR, et al. Endocrine Late Effects in Childhood Cancer Survivors. J Clin Oncol. 2018;36(21):2153–9. doi: 10.1200/JCO.2017.76.3268. doi:10.1200/JCO.2017.76.3268. Epub 2018 Jun 6. [DOI] [PubMed] [Google Scholar]
- 31.Gebauer J, Higham C, Langer T, Denzer C, Brabant G. Long-Term Endocrine and Metabolic Consequences of Cancer Treatment:A Systematic Review. Endocr Rev. 2019;40(3):711–67. doi: 10.1210/er.2018-00092. doi:10.1210/er.2018-00092. [DOI] [PubMed] [Google Scholar]
- 32.Mulder RL, Kremer LC, van Santen HM, Ket JL, van Trotsenburg AS, Koning CC, et al. Prevalence and risk factors of radiation-induced growth hormone deficiency in childhood cancer survivors:a systematic review. Cancer Treat Rev. 2009;35(7):616–32. doi: 10.1016/j.ctrv.2009.06.004. doi:10.1016/j.ctrv.2009.06.004. Epub 2009 Jul 28. [DOI] [PubMed] [Google Scholar]
- 33.Follin C, Erfurth EM. Long-term effect of cranial radiotherapy on pituitary-hypothalamus area in childhood acute lymphoblastic leukemia survivors. Curr Treat Options Oncol. 2016;17(9):50. doi: 10.1007/s11864-016-0426-0. doi:10.1007/s11864-016-0426-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marcucci G, Beltrami G, Tamburini A, Body JJ, Confavreux CB, Hadji P, et al. Bone health in childhood cancer:review of the literature and recommendations for the management of bone health in childhood cancer survivors. Ann Oncol. 2019;30(6):908–20. doi: 10.1093/annonc/mdz120. doi:10.1093/annonc/mdz120. [DOI] [PubMed] [Google Scholar]
- 35.Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, et al. The National Osteoporosis Foundation's position statement on peak bone mass development and lifestyle factors:a systematic review and implementation recommendations. Osteoporos Int. 2016;27(4):1281–1386. doi: 10.1007/s00198-015-3440-3. doi:10.1007/s00198-015-3440-3. Epub 2016 Feb 8. Erratum in:Osteoporos Int. 2016 Apr;27(4):1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muszynska-Roslan K, Latoch E, Konstantynowicz J, Panasiuk A, Stewart A, Krawczuk-Rybak M. Bone mineral density in pediatric survivors of Hodgkin and non-Hodgkin lymphomas. Adv Med Sci. 2014;59(2):200–5. doi: 10.1016/j.advms.2014.02.004. doi:10.1016/j.advms.2014.02.004. Epub 2014 Jun 9. [DOI] [PubMed] [Google Scholar]
- 37.Han JW, Kim HS, Hahn SM, Jin SL, Shin YJ, Kim SH, et al. Poor bone health at the end of puberty in childhood cancer survivors. Pediatr Blood Cancer. 2015;62(10):1838–43. doi: 10.1002/pbc.25581. doi:10.1002/pbc.25581. Epub 2015 May 13. [DOI] [PubMed] [Google Scholar]
- 38.van der Sluis IM, van den Heuvel-Eibrink MM, Hählen K, Krenning EP, de Muinck Keizer-Schrama SM. Bone mineral density, body composition, and height in long-term survivors of acute lymphoblastic leukemia in childhood. Med Pediatr Oncol. 2000;35(4):415–20. doi: 10.1002/1096-911x(20001001)35:4<415::aid-mpo4>3.0.co;2-9. doi:10.1002/1096-911x(20001001)35:4<415::aid-mpo4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 39.Högler W, Wehl G, van Staa T, Meister B, Klein-Franke A, Kropshofer G. Incidence of skeletal complications during treatment of childhood acute lymphoblastic leukemia:comparison of fracture risk with the General Practice Research Database. Pediatr Blood Cancer. 2007;48(1):21–7. doi: 10.1002/pbc.20701. doi:10.1002/pbc.20701. [DOI] [PubMed] [Google Scholar]
- 40.Mostoufi-Moab S, Brodsky J, Isaacoff EJ, Tsampalieros A, Ginsberg JP, Zemel B, et al. Longitudinal assessment of bone density and structure in childhood survivors of acute lymphoblastic leukemia without cranial radiation. J Clin Endocrinol Metab. 2012;97(10):3584–92. doi: 10.1210/jc.2012-2393. doi:10.1210/jc.2012-2393. Epub 2012 Aug 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nysom K, Holm K, Michaelsen KF, Hertz H, Jacobsen N, Müller J, et al. Bone mass after allogeneic BMT for childhood leukaemia or lymphoma. Bone Marrow Transplant. 2000;25(2):191–6. doi: 10.1038/sj.bmt.1702131. doi:10.1038/sj.bmt.1702131. [DOI] [PubMed] [Google Scholar]
- 42.Goldsby RE, Liu Q, Nathan PC, Bowers DC, Yeaton-Massey A, Raber SH, et al. Late-occurring neurologic sequelae in adult survivors of childhood acute lymphoblastic leukemia:a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2010;28(2):324–31. doi: 10.1200/JCO.2009.22.5060. doi:10.1200/JCO.2009.22.5060. Epub 2009 Nov 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cousens P, Waters B, Said J, Stevens M. Cognitive effects of cranial irradiation in leukaemia:a survey and meta-analysis. J Child Psychol Psychiatry. 1988;29(6):839–52. doi: 10.1111/j.1469-7610.1988.tb00757.x. doi:10.1111/j.1469-7610.1988.tb00757.x. [DOI] [PubMed] [Google Scholar]
- 44.Copeland DR, Moore BD, 3rd, Francis DJ, Jaffe N, Culbert SJ. Neuropsychologic effects of chemotherapy on children with cancer:a longitudinal study. J Clin Oncol. 1996;14(10):2826–35. doi: 10.1200/JCO.1996.14.10.2826. doi:10.1200/JCO.1996.14.10.2826. [DOI] [PubMed] [Google Scholar]
- 45.Spiegler BJ, Kennedy K, Maze R, Greenberg ML, Weitzman S, Hitzler JK, et al. Comparison of long-term neurocognitive outcomes in young children with acute lymphoblastic leukemia treated with cranial radiation or high-dose or very high-dose intravenous methotrexate. J Clin Oncol. 2006;24(24):3858–64. doi: 10.1200/JCO.2006.05.9055. doi:10.1200/JCO.2006.05.9055. Erratum in:J Clin Oncol. 2006 Nov 10;24(32):5181. [DOI] [PubMed] [Google Scholar]
- 46.Bowers DC, Liu Y, Leisenring W, McNeil E, Stovall M, Gurney JG, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors:a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24(33):5277–82. doi: 10.1200/JCO.2006.07.2884. doi:10.1200/JCO.2006.07.2884. Epub 2006 Nov 6. [DOI] [PubMed] [Google Scholar]
- 47.Anghelescu DL, Tesney JM, Jeha S, Wright BB, Trujillo L, Sandlund JT, et al. Prospective randomized trial of interventions for vincristine-related neuropathic pain. Pediatr Blood Cancer. 2020;67(9):e28539. doi: 10.1002/pbc.28539. doi:10.1002/pbc.28539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aydin Köker S, Gözmen S, Demirağ B, Ünalp A, Karapinar TH, Oymak Y, et al. Effect of pyridoxine plus pyridostigmine treatment on vincristine-induced peripheral neuropathy in pediatric patients with acute lymphoblastic leukemia:a single-center experience. Neurol Sci. 2021;42(9):3681–6. doi: 10.1007/s10072-020-04970-w. doi:10.1007/s10072-020-04970-w. Epub 2021 Jan 13. [DOI] [PubMed] [Google Scholar]
- 49.Akam-Venkata J, Franco VI, Lipshultz SE. Late Cardiotoxicity:Issues for Childhood Cancer Survivors. Curr Treat Options Cardiovasc Med. 2016;18((7)):47. doi: 10.1007/s11936-016-0466-6. doi:10.1007/s11936-016-0466-6. [DOI] [PubMed] [Google Scholar]
- 50.Armstrong GT, Ross JD. Late Cardiotoxicity in Aging Adult Survivors of Childhood Cancer. Prog Pediatr Cardiol. 2014;36((1-2)):19–26. doi: 10.1016/j.ppedcard.2014.09.003. doi:10.1016/j.ppedcard.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franco VI, Lipshultz SE. Cardiac complications in childhood cancer survivors treated with anthracyclines. Cardiol Young. 2015;25(Suppl 2):107–16. doi: 10.1017/S1047951115000906. doi:10.1017/S1047951115000906. [DOI] [PubMed] [Google Scholar]
- 52.Bansal N, Amdani SM, Hutchins KK, Lipshultz SE. Cardiovascular disease in survivors of childhood cancer. Curr Opin Pediatr. 2018;30(5):628–38. doi: 10.1097/MOP.0000000000000675. doi:10.1097/MOP.0000000000000675. [DOI] [PubMed] [Google Scholar]
- 53.Armenian SH, Armstrong GT, Aune G, Chow EJ, Ehrhardt MJ, Ky B, et al. Cardiovascular Disease in Survivors of Childhood Cancer:Insights Into Epidemiology, Pathophysiology, and Prevention. J Clin Oncol. 2018;36(21):2135–44. doi: 10.1200/JCO.2017.76.3920. doi:10.1200/JCO.2017.76.3920. Epub 2018 Jun 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mertens AC, Yasui Y, Neglia JP, Potter JD, Nesbit ME, Jr, Ruccione K, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer:the Childhood Cancer Survivor Study. J Clin Oncol. 2001;19(13):3163–72. doi: 10.1200/JCO.2001.19.13.3163. doi:10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 55.Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer:retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. doi:10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barlogis V, Auquier P, Bertrand Y, Chastagner P, Plantaz D, Poiree M, et al. Late cardiomyopathy in childhood acute myeloid leukemia survivors:a study from the L. E. A. program. Haematologica. 2015;100(5):e186–9. doi: 10.3324/haematol.2014.116574. doi:10.3324/haematol.2014.116574. Epub 2015 Jan 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berkman AM, Robert RS, Roth M, Askins MA. A review of psychological symptoms and post-traumatic growth among adolescent and young adult survivors of childhood cancer. J Health Psychol. 2022;27(4):990–1005. doi: 10.1177/1359105320971706. doi:10.1177/1359105320971706. Epub 2020 Nov 6. [DOI] [PubMed] [Google Scholar]
- 58.Michel G, Brinkman TM, Wakefield CE, Grootenhuis M. Psychological Outcomes, Health-Related Quality of Life, and Neurocognitive Functioning in Survivors of Childhood Cancer and Their Parents. Pediatr Clin North Am. 2020;67(6):1103–34. doi: 10.1016/j.pcl.2020.07.005. doi:10.1016/j.pcl.2020.07.005. Epub 2020 Sep 23. [DOI] [PubMed] [Google Scholar]
- 59.Kosir U, Wiedemann M, Wild J, Bowes L. Psychiatric disorders in adolescent cancer survivors:A systematic review of prevalence and predictors. Cancer Rep (Hoboken) 2019;2(3):e1168. doi:10.1002/cnr2.1168. [Google Scholar]
- 60.Gilleland Marchak J, Devine KA, Hudson MM, Jacobson LA, Michel G, Peterson SR, et al. Systematic Review of Educational Supports of Pediatric Cancer Survivors:Current Approaches and Future Directions. J Clin Oncol. 2021;39(16):1813–23. doi: 10.1200/JCO.20.02471. doi:10.1200/JCO.20.02471. Epub 2021 Apr 22. [DOI] [PubMed] [Google Scholar]
- 61.Brinkman TM, Recklitis CJ, Michel G, Grootenhuis MA, Klosky JL. Psychological Symptoms, Social Outcomes, Socioeconomic Attainment, and Health Behaviors Among Survivors of Childhood Cancer:Current State of the Literature. J Clin Oncol. 2018;36(21):2190–7. doi: 10.1200/JCO.2017.76.5552. doi:10.1200/JCO.2017.76.5552. Epub 2018 Jun 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mader L, Michel G, Roser K. Unemployment Following Childhood Cancer. Dtsch Arztebl Int. 2017;114(47):805–12. doi: 10.3238/arztebl.2017.0805. doi:10.3238/arztebl.2017.0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Kalsbeek RJ, van der Pal HJH, Kremer LCM, Bardi E, Brown MC, Effeney R, et al. European PanCareFollowUp Recommendations for surveillance of late effects of childhood, adolescent, and young adult cancer. Eur J Cancer. 2021;154:316–28. doi: 10.1016/j.ejca.2021.06.004. doi:10.1016/j.ejca.2021.06.004. Epub 2021 Jul 29. [DOI] [PubMed] [Google Scholar]
- 64.van Atteveld JE, Mulder RL, van den Heuvel-Eibrink MM, Hudson MM, Kremer LCM, Skinner R, et al. Bone mineral density surveillance for childhood, adolescent, and young adult cancer survivors:evidence-based recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Diabetes Endocrinol. 2021;9(9):622–37. doi: 10.1016/S2213-8587(21)00173-X. doi:10.1016/S2213-8587(21)00173-X. Epub 2021 Jul 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ness KK, Krull KR, Jones KE, Mulrooney DA, Armstrong GT, Green DM, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer:a report from the St Jude Lifetime cohort study. J Clin Oncol. 2013;31(36):4496–503. doi: 10.1200/JCO.2013.52.2268. doi:10.1200/JCO.2013.52.2268. Epub 2013 Nov 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arora M, Sun CL, Ness KK, Teh JB, Wu J, Francisco L, et al. Physiologic Frailty in Nonelderly Hematopoietic Cell Transplantation Patients:Results From the Bone Marrow Transplant Survivor Study. JAMA Oncol. 2016;2(10):1277–86. doi: 10.1001/jamaoncol.2016.0855. doi:10.1001/jamaoncol.2016.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ariffin H, Azanan MS, Abd Ghafar SS, Oh L, Lau KH, Thirunavakarasu T, et al. Young adult survivors of childhood acute lymphoblastic leukemia show evidence of chronic inflammation and cellular aging. Cancer. 2017;123(21):4207–14. doi: 10.1002/cncr.30857. doi:10.1002/cncr.30857. Epub 2017 Jun 27. [DOI] [PubMed] [Google Scholar]
- 68.Armstrong GT, Chen Y, Yasui Y, Leisenring W, Gibson TM, Mertens AC, et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. N Engl J Med. 2016;374(9):833–42. doi: 10.1056/NEJMoa1510795. doi:10.1056/NEJMoa1510795. Epub 2016 Jan 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gatta G, Rossi S, Foschi R, Trama A, Marcos-Gragera R, Pastore G, et al. Survival and cure trends for European children, adolescents and young adults diagnosed with acute lymphoblastic leukemia from 1982 to 2002. Haematologica. 2013;98(5):744–52. doi: 10.3324/haematol.2012.071597. doi:10.3324/haematol.2012.071597. Epub 2013 Feb 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bagnasco F, Caruso S, Andreano A, Valsecchi MG, Jankovic M, Biondi A, et al. Late mortality and causes of death among 5-year survivors of childhood cancer diagnosed in the period 1960-1999 and registered in the Italian Off-Therapy Registry. Eur J Cancer. 2019;110:86–97. doi: 10.1016/j.ejca.2018.12.021. doi:10.1016/j.ejca.2018.12.021. Epub 2019 Feb 14. [DOI] [PubMed] [Google Scholar]
- 71.Suh E, Stratton KL, Leisenring WM, Nathan PC, Ford JS, Freyer DR, et al. Late mortality and chronic health conditions in long-term survivors of early-adolescent and young adult cancers:a retrospective cohort analysis from the Childhood Cancer Survivor Study. Lancet Oncol. 2020;21(3):421–35. doi: 10.1016/S1470-2045(19)30800-9. doi:10.1016/S1470-2045(19)30800-9. Epub 2020 Feb 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bresters D, van Gils IC, Kollen WJ, Ball LM, Oostdijk W, van der Bom JG, et al. High burden of late effects after haematopoietic stem cell transplantation in childhood:a single-centre study. Bone Marrow Transplant. 2010;45(1):79–85. doi: 10.1038/bmt.2009.92. doi:10.1038/bmt.2009.92. Epub 2009 May 4. [DOI] [PubMed] [Google Scholar]
- 73.Bomken S, Skinner R. Secondary Malignant Neoplasms Following Haematopoietic Stem Cell Transplantation in Childhood. Children (Basel) 2015;2(2):146–73. doi: 10.3390/children2020146. doi:10.3390/children2020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leiper A, Houwing M, Davies EG, Rao K, Burns S, Morris E, et al. Anti-Müllerian hormone and Inhibin B after stem cell transplant in childhood:a comparison of myeloablative, reduced intensity and treosulfan-based chemotherapy regimens. Bone Marrow Transplant. 2020;55(10):1985–95. doi: 10.1038/s41409-020-0866-9. doi:10.1038/s41409-020-0866-9. Epub 2020 Mar 30. Erratum in:Bone Marrow Transplant. 2020 Apr 24. [DOI] [PubMed] [Google Scholar]
- 75.Baird K, Cooke K, Schultz KR. Chronic graft-versus-host disease (GVHD) in children. Pediatr Clin North Am. 2010;57(1):297–322. doi: 10.1016/j.pcl.2009.11.003. doi:10.1016/j.pcl.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hamilton BK, Storer BE, Wood WA, Pidala JA, Cutler CS, Martin PJ, et al. Disability Related to Chronic Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2020;26(4):772–7. doi: 10.1016/j.bbmt.2019.10.019. doi:10.1016/j.bbmt.2019.10.019. Epub 2019 Oct 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.SIOP Europe. The SIOPE Strategic Plan - A European Cancer Plan for Children and Adolescents. 2015. [cited 2024 Jan 6] Available from: https://siope.eu/media/wp-content/uploads/2018/02/the-siope-strategic-plan.pdf .
- 78.Haupt R, Spinetta JJ, Ban I, Barr RD, Beck JD, Byrne J, et al. Long term survivors of childhood cancer:cure and care. The Erice statement. Eur J Cancer. 2007;43(12):1778–80. doi: 10.1016/j.ejca.2007.04.015. doi:10.1016/j.ejca.2007.04.015. Epub 2007 May 31. [DOI] [PubMed] [Google Scholar]
- 79.Poplack DG, Fordis M, Landier W, Bhatia S, Hudson MM, Horowitz ME. Childhood cancer survivor care:development of the Passport for Care. Nat Rev Clin Oncol. 2014;11(12):740–50. doi: 10.1038/nrclinonc.2014.175. doi:10.1038/nrclinonc.2014.175. Epub 2014 Oct 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haupt R, Essiaf S, Dellacasa C, Ronckers CM, Caruso S, Sugden E, et al. The 'Survivorship Passport'for childhood cancer survivors. Eur J Cancer. 2018;102:69–81. doi: 10.1016/j.ejca.2018.07.006. doi:10.1016/j.ejca.2018.07.006. Epub 2018 Aug 20. [DOI] [PubMed] [Google Scholar]
- 81.Mulder RL, van Kalsbeek RJ, Hudson MM, Skinner R, Kremer LCM. The Critical Role of Clinical Practice Guidelines and Indicators in High-Quality Survivorship After Childhood Cancer. Pediatr Clin North Am. 2020;67(6):1069–81. doi: 10.1016/j.pcl.2020.07.003. doi:10.1016/j.pcl.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 82.Kremer LC, Mulder RL, Oeffinger KC, Bhatia S, Landier W, Levitt G, et al. A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors:a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr Blood Cancer. 2013;60(4):543–9. doi: 10.1002/pbc.24445. doi:10.1002/pbc.24445. Epub 2012 Dec 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Michel G, Mulder RL, van der Pal HJH, Skinner R, Bárdi E, Brown MC, et al. Evidence-based recommendations for the organization of long-term follow-up care for childhood and adolescent cancer survivors:a report from the PanCareSurFup Guidelines Working Group. J Cancer Surviv. 2019;13(5):759–72. doi: 10.1007/s11764-019-00795-5. doi:10.1007/s11764-019-00795-5. Epub 2019 Aug 8. [DOI] [PubMed] [Google Scholar]
- 84.Kieran MW, Caron H, Winther JF, Henderson TO, Haupt R, Hjorth L, et al. ACCELERATE Long-Term Follow-Up Working Group. A global approach to long-term follow-up of targeted and immune-based therapy in childhood and adolescence. Pediatr Blood Cancer. 2021;68(7):e29047. doi: 10.1002/pbc.29047. doi:10.1002/pbc.29047. [DOI] [PubMed] [Google Scholar]


