Abstract
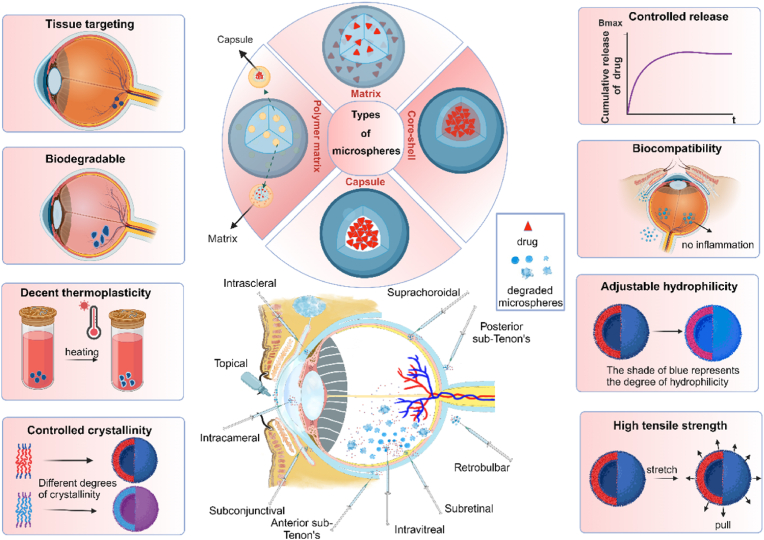
Posterior segment disease acts as a major cause of irreversible visual impairments. Successful treatment of posterior segment disease requires the efficient delivery of therapeutic substances to the targeted lesion. However, the complex ocular architecture makes the bioavailability of topically applied drugs extremely low. Invasive delivery approaches like intravitreal injection may cause adverse complications. To enhance the efficiency, several biomedical engineering systems have been developed to increase the penetration efficiency and improve the bioavailability of drugs at the posterior segments. Advantageously, biodegradable microspheres are found to deliver the therapeutic agents in a controlled fashion. The microspheres prepared from novel biomaterials can realize the prolonged release at the posterior segment with minimum side effects. Moreover, it will be degraded automatically into products that are non-toxic to the human body without the necessity of secondary operation to remove the residual polymer matrix. Additionally, biodegradable microspheres have decent thermoplasticity, adjustable hydrophilicity, controlled crystallinity, and high tensile strength, which make them suitable for intraocular delivery. In this review, we introduce the latest advancements in microsphere production technology and elaborate on the biomaterials that are used to prepare microspheres. We discuss systematically the pharmacological characteristics of biodegradable microspheres and compare their potential advantages and limitations in the treatment of posterior segment diseases. These findings would enrich our knowledge of biodegradable microspheres and cast light into the discovery of effective biomaterials for ocular drug delivery.
Keywords: Biomedical materials, Biodegradable microspheres, Ocular drug delivery, Controlled release
Graphical abstract
1. Introduction
Ocular diseases affecting the posterior segment of the eye constitute a leading cause of blindness. This type of disease encompasses age-related macular degeneration (AMD), retinitis pigmentosa (RP), diabetic retinopathy (DR), glaucoma, uveitis, macular edema, intraocular inflammation proliferative retinopathy, etc. These disorders typically exhibit chronic, degenerative characteristics, with the incidence increasing with age. Anatomically, the eyeball can be divided into anterior and posterior segments, with multiple biological barriers existing in them. Delivering drugs to the posterior segment of the eye is extremely thorny due to these barriers. Thus far, effectively traversing these barriers and delivering pharmacological agents to the ocular lesions remains a complex and challenging task. Generally, there are six routes for administering treatment to retinal diseases: topical drops, systemic administration, conjunctival-scleral administration, anterior chamber administration, intravitreal injection, and periocular injection (Fig. 1) [1] (Table 1). outlines the pros and cons of various drug delivery routes. However, topical administration is limited by the low bioavailability, while systemic administration carries a high risk of side effects. Intraocular and periocular injections require frequent invasive interventions and may induce severe complications. Encouragingly, the recent development of intraocular drug delivery systems (IDDS) has enabled the sustained release of pharmacologic molecules to the target region. IDDS includes implantable devices for controlled release, drug-targeting systemic administration (such as photodynamic therapy), and drug penetration approaches (such as iontophoresis). Microsphere is an implantable device used for controlled drug release and treating eye diseases. These microspheres achieve efficient drug delivery to the posterior segment by adjusting the biodegradation kinetics of the biomaterial. They can encapsulate a range of bioactive compounds, including antiproliferative agents, anti-inflammatory drugs, immunosuppressants, antibiotics, and biologics. In particular, there is growing interest in focusing on the biodegradable microspheres, as they enable sustained drug release and instant dissociation from the implantation site, holding promise for potential applications in ophthalmological practice.
Fig. 1.
Types and advantages of microspheres and their routes of administration.
Table 1.
Comparison of multiple routes of ocular drug administration.
| Route of Administration | Advantages | Disadvantages | Diseases | Loaded Active Substances |
|---|---|---|---|---|
| Topical drops | High patient compliance, non-invasive | Limited bioavailability, low dispersion | Glaucoma, Conjunctivitis, Dry eye | Antiglaucoma drugs, antibiotics, lubricants |
| Subconjunctival administration | Potential for both anterior and posterior drug delivery | Requires diffusion through multiple tissue to reach the posterior segment | Inflammatory conditions, Infections | Corticosteroids, antibiotics |
| Intravitreal Injection | High bioavailability, direct delivery to vitreous and retina | Poor patient compliance, complication caused by repeat injection | Retinal diseases, Macular degeneration, Diabetic retinopathy | Anti-VEGF agents, corticosteroids |
| Retrobulbar Injection | Selective delivery to anterior and posterior segments, longer duration of action | Poor patient compliance, complication caused by repeat injection | Glaucoma, Optic neuritis | Corticosteroids, Anesthetics |
| Systemic Administration | High patient compliance, non-invasive | Limited bioavailability, risk of systemic adverse effects at high doses | Ocular infections, Inflammation | Antibiotics, Antifungal, Antiviral |
| transscleral administration | Sustained delivery, non-invasive | Requires specialized drug delivery system | Glaucoma, Retinal diseases | Antiglaucoma drugs, Anti-inflammatory drugs |
| subretinal administration | Direct impact on retinal cells, high bioavailability | Invasive, technical challenges | Macular degeneration, Retinitis pigmentosa | Gene Therapy, Vitamins |
| intrascleral administration | Localized treatment, Avoids systemic side-effects | Difficulty in accurately placing the medication, potential for damage | Glaucoma | Corticosteroids, Antibiotics |
| anterior sub-tenon's | Allows for targeted delivery, less invasive than intraocular injections | Potential complications such as perforation and infection | Allergic conjunctivitis | Corticosteroids, anesthetic agents |
| posterior sub-tenon's | Allows for targeted delivery to posterior segment, less invasive than intraocular injections | Potential complications such as perforation and infection | Posterior uveitis, Retinal disorders | Anti-VEGF agents, corticosteroids |
Abbreviations:VEGF, vascular endothelial growth factor.
In this review, we introduce the latest advancements in microsphere production technology and elaborate on the various characteristics of biomaterials used to prepare microspheres. We discuss systematically the pharmacological characteristics of biodegradable microspheres and discuss their advantages and limitations in the treatment of posterior segment diseases. These findings would enrich our knowledge of biodegradable microspheres and cast light the discovery of an effective biomaterial for ocular drug delivery.
2. Biological barriers to ocular drug delivery
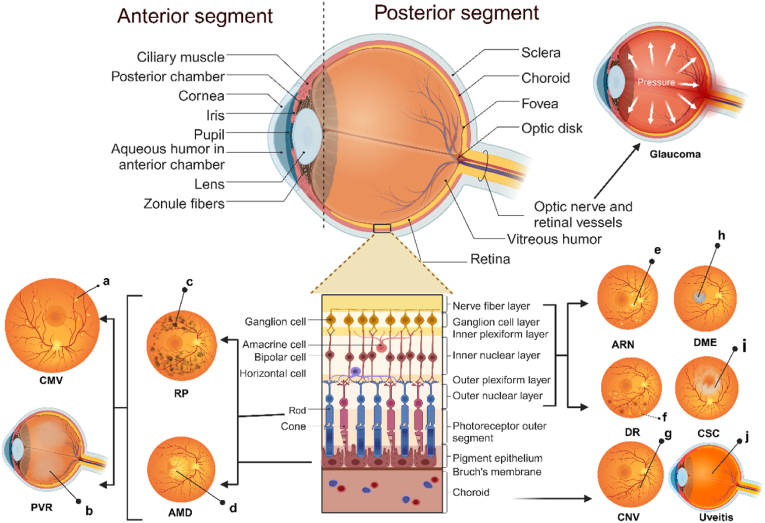
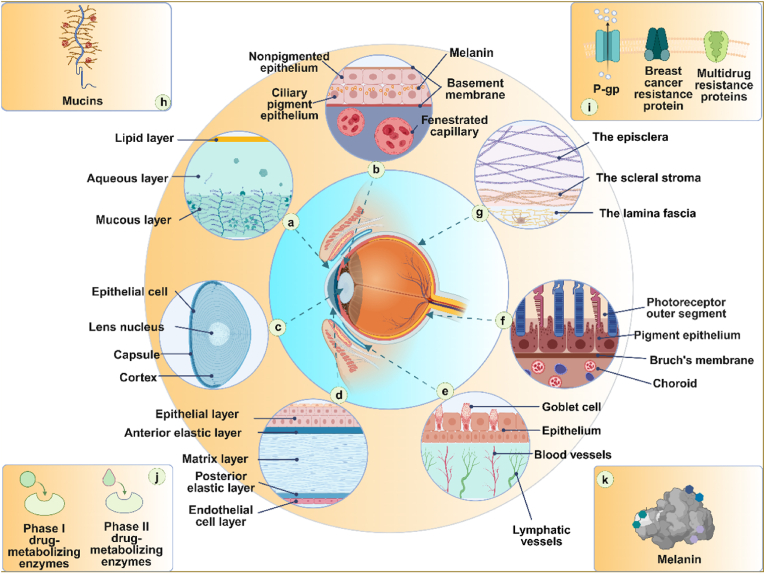
The eyeball can be divided into the anterior segment and the posterior segment, with the crystalline lens as the dividing point (Fig. 2). The anterior segment extends from the inner side of the cornea to the anterior surface of the crystalline lens, accounting for approximately one-third of the eyeball. On the other hand, the posterior segment extends from the posterior surface of the crystalline lens to the retina. It includes the retina, choroid membranes, and the posterior portion of the sclera, accounting for approximately two-thirds of the eyeball. The gel-like substance within this segment is called the vitreous humor. In ophthalmological practice, delivery of drugs to this location is extremely challenging due to various biological barriers within the eyeball. The biological barriers within the eyeball can be categorized into physiological barriers, anatomical barriers, and molecular barriers (Fig. 3). Physiological barriers include tear flow, nasolacrimal drainage, and the blink reflex. Anatomical barriers consist of the corneal barrier and the blood-aqueous barrier (BAB) in the anterior segment, as well as the scleral barrier, choroidal barrier, and blood-retinal barrier (BRB) in the posterior segment. Other barriers include ocular mucus, melanin, drug efflux transporters, and ocular metabolic enzymes, among others. The biodegradable microsphere sustained release system can break through most of the biological barriers and enable drug release continuously in the posterior segment.
Fig. 2.
Structures of the eye and posterior ophthalmic diseases.
a, Yellow and white lesions with patchy bleeding; b, Vitreous liquefaction; c, Pigmentation; d, White plaque; e, Yellow and white necrotic focus; f, "Cotton-wool" spots; g, neovascularization; h, Liquid accumulation; i, Liquid leakage; j, Inflammation.
Abbreviations:CMV, cytomegalovirus retinitis; PVR, proliferative vitreoretinopathy; RP, retinitis pigmentosa.. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Ocular barriers for drug delivery.
a, Tear Film Barrier; b, Blood-Aqueous Barrier; c, Lens Barrier; d, Corneal Barrier; e, Conjunctival Barrier; f, Blood-Retinal Barrier; g, Scleral Barrier; h, Mucus Barrier; i, Drug Efflux Transport Protein Barrier; j, Metabolic Barrier, k, Drug Binding to Melanin.
Abbreviations:P-gp, P-glycoprotein.
2.1. Physiological barriers (Tear Film Barrier)
Tear film is the outermost film that is composed of lipid layer, aqueous layer, and mucin layer, with a total thickness of about 6 μm. The lipid layer is made up of substances such as free fatty acids, triacylglycerols, phospholipids, and cholesterol, which seals tears and prevents the aqueous layer from evaporating. It prohibits the transportation of hydrophilic substances, while the aqueous layer resists lipophilic substances [2]. The aqueous layer accounts for 90 % of the total volume of the tear film. It can moisturize and lubricate the ocular surface, wash away foreign particles, and help prevent microorganism infections. The mucin layer provides a protective barrier to prevent the intrusion of harmful external substances through electrostatic attraction [3]. Non-specific binding of the drug to tear enzymes (such as lysozyme), mucins, and protein (such as albumin) prevents the drug from contacting the cornea and anterior chamber [4]. Additionally, tears can swiftly drain from the ocular surface into the nasal cavity through the nasolacrimal duct. This rapid drainage may lead to the quick absorption of ocular drugs into the bloodstream rather than retained in the eye.
2.2. Anatomical barriers
2.2.1. Corneal barrier
The Cornea, located at the anterior part of the eyeball, is the largest static barrier without any blood vessels and lymph nodes. Anatomically, the cornea is divided into an epithelial layer, anterior elastic layer, matrix layer, posterior elastic layer, and endothelial cell layer, in which the corneal epithelial layer and stroma layer act as the main barriers against drug transportation. The corneal epithelial layer is composed of 3–6 layers of lipid-rich and tightly connected epithelial cells, which prohibit the penetration of most hydrophilic drugs and a few lipophilic drugs. The stromal layer is composed of water, collagen, proteoglycan, and corneal stromal cells. This layer accounts for 90 % of the corneal thickness and is the main barrier for lipophilic drugs to pass through the cornea [5]. The endothelial layer consists of a pore-like single-cell layer that is more permeable than the epithelial layer, allowing the passage of macromolecules. Based on the dual barrier effect of the corneal epithelial cell layer and the stromal layer, only drug molecules with appropriate oil-water partition coefficients and dissociation constants have good corneal permeability.
2.2.2. Conjunctival Barrier
Conjunctiva is a transparent thin layer of mucosa with abundant blood vessels. The conjunctiva is composed of the palpebral conjunctiva and bulbar conjunctiva, as well as the fornix conjunctiva at the junction of the two. The conjunctival sac can hold less fluid under the blink action of the orbicularis oculi muscle. Conjunctival Barrier results in the short maintenance time and reduces the concentration of drug on the ocular surface. Tear film and mucus produced by conjunctiva can lubricate and protect the ocular surface. Drugs with hydrophilic and relative molecular weight (Mw) less than 20000 can easily penetrate the mucus layer of the conjunctiva and are carried away by conjunctival lymph and blood circulation, resulting in a reduced number of drug molecules in the eye tissue [6].
2.2.3. Scleral barrier
The sclera is the opaque protective membrane formed by collagen and elastic fibers. The sclera can be divided into the surface layer, the matrix layer, and the brown-black lamina. The surface layer of the sclera is composed of loose fibrous tissue. The scleral matrix layer is an opaque dense connective tissue layer with uneven thickness and oblique tight arrangement. The brown-black lamina is composed of fine elastic fibers containing a large number of pigment cells. The complex fiber network within the sclera makes the permeability of hydrophilic molecules much easier than the lipophilic molecules [7]. Additionally, the proteoglycan of the scleral extracellular matrix (ECM) is negatively charged under physiological conditions, so the permeability of positively charged molecules in the sclera is extremely low [8].
2.2.4. Choroidal barrier
The choroid is a highly vascularized layer between the retinal pigment epithelium (RPE) and the sclera. It is responsible for transporting oxygen and nutrients from the blood to the retina. It consists of five layers from inside toward outside: the Bruch's membrane, the choroidal capillary, the Sattler, the Haller layers, and the suprachoroidal space. The pores of choroidal capillaries allow macromolecules such as nutrients and proteins to pass through. High-speed choroidal blood circulation provides essential oxygen and nutrients for the retinal pigment epithelial cells with high metabolic activity. After hydrophilic drugs reach the suprachoroidal cavity, they are partially cleared in choroidal circulation. On the other hand, the matrix of the choroid combines with the lipophilic drugs and hinders the direct movement of the drugs to the choroidal stroma. Therefore, the choroidal barrier hinders the delivery of hydrophilic compounds to the posterior segment of the eye [9].
2.2.5. Lens Barrier
The lens is located between the posterior surface of the iris and the frontier surface of the vitreous body. It is made up of the lens capsule and lens fibers without any nerve and blood vessels. The crystalline capsule is mainly composed of collagen, chondroitin sulfate, and fibrin. On the other hand, the crystalline fiber is composed of proteins and proteins, mainly including α−, β− and γ-crystallins. The molecular structure of each protein contains a different assembly of sulfhydryl groups. Since the lens is highly filled with fibrous cells, the diffusion of drugs in the lens is very slow. Besides, the drug is only distributed in the loose capsule, epithelium, and cortex in the lens, but not in the lens nucleus with dense structure. Thus, the distribution of the drug to the lens is generally low [10,11].
2.2.6. Vitreous barrier
The vitreous body is the clear gel that fills the space between the lens and the retina of the eyeball in humans and other vertebrates, which consists of 98 % water, 2 % collagen, and a small amount of hyaluronic acid. Most of the proteins in vitreous are structural proteins, such as collagen, fibrin, and cartilage oligomeric matrix proteins. The structural proteins are arranged in a three-dimensional structure to form a grid, which is attached with hyaluronic acid mucopolysaccharide. Hyaluronic acid mucopolysaccharide is an anionic hydrophilic polymer with a relative Mw of 3 × 106–5.8 × 106. It can attract and combine positive ions or water since its overall net charge is negative. Hyaluronic acid is unevenly distributed in the vitreous body and its swelling pressure helps separate fibrin and support the retina. The static barrier formed by the vitreous tissue itself and the dynamic barrier formed by the rheological properties of its gel state collectively constitute the vitreous barrier. Generally speaking, positively charged biomedical materials interact with the negatively charged components of vitreous grids, resulting in the obstruction of molecular diffusion. However, these negatively charged particles, such as polylactic-glycolic acid (PLGA) or human serum albumin, can pass through the vitreous grids, and be successfully distributed in each portion of the vitreous body [12]. In this context, diverse hydrophilic, negatively charged, or uncharged particles have been used for intravitreal administration in ophthalmologic practice.
2.2.7. Blood-aqueous barrier
BAB is an anterior segment barrier that consists of the epithelial barrier and the endothelial barrier. The epithelial barrier is made up of tight junctions between unpigmented ciliary epithelial cells, forming a pathway for free molecular diffusion. The endothelial barrier is made up of Iris vessels containing diverse connexins (such as laminin, integrin, and tight junction protein, etc.) similar to epithelial tight junctions [13]. Its functions are primarily to protect the eyeball from harmful substances and to maintain the stability of the internal environment. In order to penetrate the BAB, substances in the blood must pass through the vascular endothelial cells, traverse the stroma, cross the epithelial cells of the ciliary body, and ultimately enter the anterior chamber. The permeability of drugs through BAB depends on the osmotic pressure and the physicochemical properties of the drug molecules. For instance, the lipid-soluble drugs can cross the BAB more readily than the hydrophilic drugs [13,14].
2.2.8. Blood-retinal barrier
BRB is a posterior segment barrier that can prevent diffusion of the drugs in the posterior segment and maintain the homeostasis of the neuroretina. It can regulate the flux of protein, ion, and water into and out of the retina tissue. Typically, BRB is divided into the inner BRB and the outer BRB. The outer BRB is formed by close junctions between retinal pigment epithelial cells. It is the rate-limiting factor for the transportation of hydrophilic drugs and macromolecules through the transscleral route to the retina. On the other hand, tight junctions at the top of the retinal capillary endothelium form the inner BRB with a transmembrane resistance comparable to that of the blood-brain barrier. The inner BRB can restrict the penetration of macromolecules, while some small molecules can penetrate into the BRB to a certain extent [15].
2.3. Other barriers
2.3.1. Mucus Barrier
The cornea and conjunctiva are covered by a layer of mucus, which is densest at the top of the epithelial apex and becomes more dilute as it extends outward into the tear fluid [16]. About 95 % of ocular mucus is moisture and other components are mucins and non-mucins, salts, and lipids. It plays a significant role in lubrication, cell signaling, and protecting the epithelium from external insults. Mucins are polypeptide chains rich in proline, threonine, and serine repeat domains that are highly glycosylated and overall negatively charged. Mucins assemble cross-linked networks through disulfide bonds, electrostatics, hydrogen bonds, and hydrophobic forces to protect underlying tissues from foreign particles [16]. Moreover, mucin not only interacts with each other to form a three-dimensional network structure but also interacts with drug particles via electrostatic attraction or repulsion to affect the diffusion efficiency. Through these mechanisms, mucins form a tight barrier against drug delivery into the eye [3].
2.3.2. Drug Efflux Transport Protein Barrier
Drug efflux transporters mainly include the P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and multidrug resistance proteins (MRPs). They reduce intracellular drug concentration and bioavailability by expelling drug molecules from cells [17]. P-gp is recognized as a prototype ABC efflux transporter that is present in the human cornea and on the apical and basolateral surface of the RPE [[17], [18], [19]]. BCRP is present in human corneal epithelial cells, conjunctival epithelial cells, and retinal pigment epithelial cells and they are considered as half ABC transporters which function through homodimerization [17,20]. MRPs are present in human corneal epithelium and retinal pigment epithelial cells. They actively participate in the efflux of organic anions, glutathione, glucuronide, or sulfate conjugated compounds [17]. Corneal can express P-gp, MRP1, MRP2, and BCRP, which can pump drugs out of the cells into tears. RPE expresses P-gp, MRP1, MRP5, and BCRP, which can pump drugs out of the vitreous body into the blood, thereby affecting the drug concentration in the vitreous body.
2.3.3. Binding capacity of melanin
Melanin is a macromolecule present in pigmented cells, such as the uveal tract (iris, choroid, ciliary body) and the RPE. It consists of two categories, yellow-reddish pheomelanin and brown-black eumelanin [21]. Due to the hydrophobicity of eumelanin and the negatively charged 5,6-dihydroxyindole-2-carboxylic acid units, the binding activity of drug molecules to eumelanin is typically described as hydrophobic interactions along with electrostatic forces. In addition to the hydrophobic interactions and electrostatic, charge transfer interactions and hydrogen bonding may also contribute to the binding activities [22]. Several lines of evidence have shown that melanin can interact with drug molecules and influence their distribution in the posterior segment of the eye [23].
2.3.4. Metabolic Barrier
Drug-metabolizing enzymes mainly include the phase I drug-metabolizing enzymes and phase II drug-metabolizing enzymes, which play an important catalytic role in the drug metabolism of the eye. Phase I drug-metabolizing enzymes are part of the microsomal drug metabolic enzyme system, and cytochrome P450 enzyme is a crucial subclass among them. These are isozymes that contain heme, which participate in about 80 % of the oxidative metabolism of drugs and about 50 % of drug elimination [24]. Phase II drug-metabolizing enzymes are non-microsomal enzyme systems that mainly catalyze processes such as glucuronidation, sulfation, or acetylation [25]. Drug metabolic enzymes in ocular tissue can degrade the drug molecules or detoxify the toxicity of drugs. However, they will reduce the bioavailability and create a challenge for drug delivery.
3. Biodegradable microspheres
3.1. Introduction of microsphere
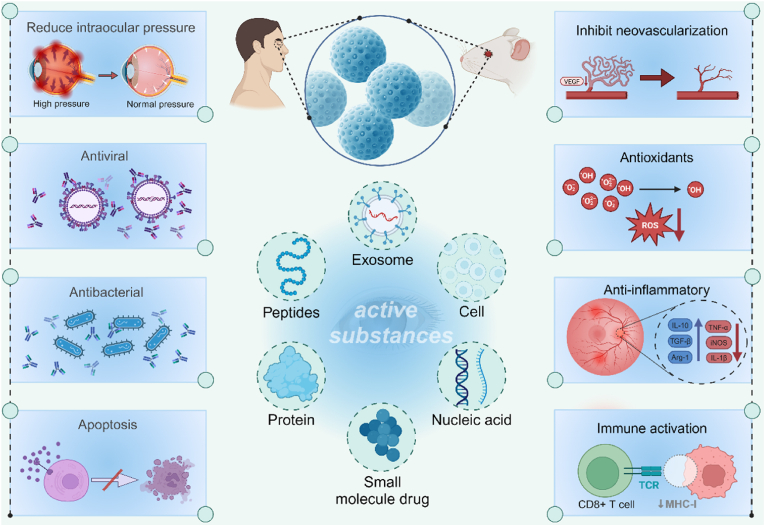
Microsphere is a type of particle dispersion system in which drug molecules are present in a polymer matrix through adsorption or dispersion, with particle sizes ranging from 1 to 1000 μm in diameter. Microspheres can encapsulate multiple pharmaceutical ingredients, such as proteins, peptides, nucleic acids, small molecule drugs, etc., and exert their corresponding pharmacological effects. (Fig. 4). They can be divided into three main types according to their spatial structure: the capsule-type, the matrix-type, and the composite type. In capsule microspheres, the drug is encapsulated within a core and surrounded by one or more layers of polymer. In matrix microspheres, the active ingredient is dispersed throughout the entire polymer matrix. Composite microspheres can be divided into two types, the Core-shell microsphere and the polymer matrix microsphere [26]. Typically, researchers use intraocular injection or periorbital injection to directly deliver drug to the site of action to enhance the passage through various eye barriers and reduce off-target effects [27]. However, direct administration is difficult to maintain therapeutic concentrations at the target site and requires repeated intraocular injections [28]. As a drug carrier, microspheres can encapsulate drugs and release them in a controlled manner, extending the half-life of the drug in the eye and achieving long-term sustained release, thereby reducing the frequency of dosing and improving patients' medication compliance. Therefore, injecting microspheres into the targeted site of the eyeball can help the drug better penetrate the ocular barrier. Microspheres can be administered through intravitreal injection and subsequently, they may either be degraded or remain at the administration site after an extended period of release. For chronic conditions requiring long-term treatment, biodegradable microspheres are always the optimum choice. The sustained release of active substances from microspheres can reduce the need for frequent dosing, and improve patient compliance. Therefore, this approach has garnered significant interest, especially for treating chronic retinal diseases that necessitate long-term delivery of low-concentration pharmacologic substances.
Fig. 4.
Active substances of microsphere encapsulation and their pharmacological effects.
Abbreviations:VEGF, vascular endothelial growth factor; ROS, reactive oxygen species; TCR, T-cell receptor; MHC-1, major histocompatibility complex-1. Created with BioRender.com.
3.2. Polymers used in biodegradable microspheres
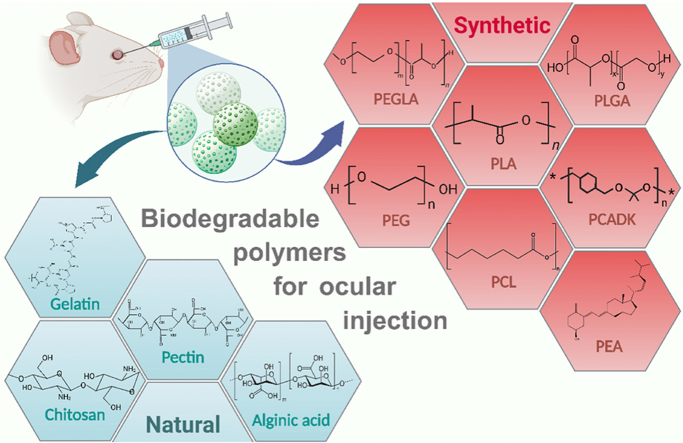
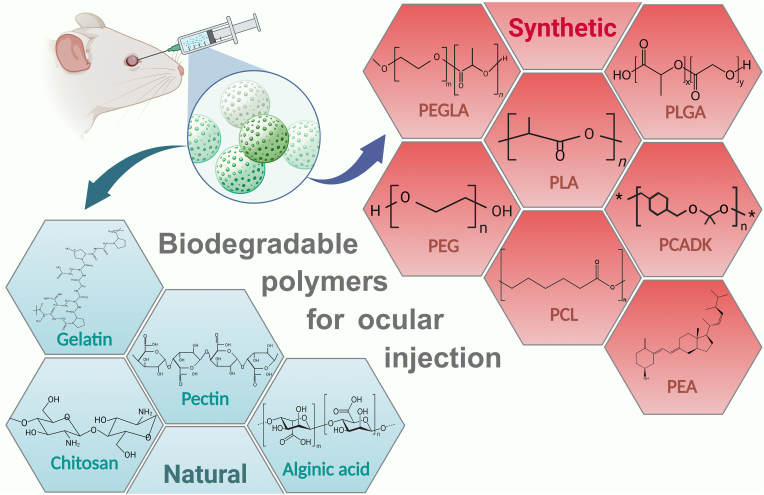
Various biodegradable polymers of natural and synthetic origins are utilized in the formulation of biodegradable microspheres. Different biodegradable polymers used in the preparation of microspheres are depicted in (Fig. 5). They are used as carriers of controlled release technology to achieve continuous intraocular drug release. In addition to satisfactory biocompatibility, these polymers have demonstrated other advantages including thermoplasticity, controlled crystallinity, regulable hydrophilicity, high tensile strength, and adjustable degradation rate. Currently, a variety of biodegradable polymers have been approved by the United States Food and Drug Administration (FDA) to prepare microspheres. However, they still have some limitations during ocular applications (Table 2) [29,30]. In addition, the biological environment of the eye is quite sensitive, and the toxicity of biodegradation products of microspheres to ocular function still needs to be further explored. By comparing the advantages and disadvantages of different polymers for ocular applications, we wish to develop biomaterials and provide ideas for further modifications to make them more suitable for ocular applications.
Fig. 5.
Classification of biodegradable polymers for ocular injection.
Abbreviations:PLA, polylactic-lactic acid; PCADK, poly (cyclohexane-1,4-diyl acetone dimethylene ketal); PEGLA, poly (ethylene glycol)-b-poly (D, l-lactic acid); PEA, polyester amide; PLGA, polylactic-glycolic acid; PCL, poly (ε-caprolactone); PEG, poly (ethylene glycol).
Table 2.
Advantages and disadvantages of polymers used in the preparation of biodegradable microspheres and their ophthalmic clinical application.
| Polymers used in biodegradable microspheres | polymers | FDA approved for eye use | advantages | disadvantages |
|---|---|---|---|---|
| Natural biodegradable polymers | Gelatin | Yes | Easily derived, biocompatible, less immunogenic, low cost | Strength depends on source and processing conditions, challenging to safely crosslink |
| Alginic acid | Yes | gel forming ability | Poor mechanical strength | |
| chitosan | No | mucosal adhesion, in-situ gelation, transfection, and permeation-enhancing capabilities | Insoluble in neutral or alkaline, solutions, strong electrostatic behavior | |
| Pectin | No | gel-forming properties, mucosal adhesion | Lack of clinical data | |
| Synthetic biodegradable polymers | PLA | Yes | Synthesized from natural sources, easily processed, slow degradation rate | Acidic degradation by products |
| PLGA | Yes | water soluble, tunable degradation rate | Acidic degradation by products | |
| PEG | Yes | water soluble, biocompatible | Fast degradation compared to other synthetic polymers | |
| PCL | No | easily modified, inexpensive | Poor mechanical properties | |
| PEA | No | Enhanced biocompatibility and excellent mechanical properties | Lack of clinical data | |
| PCADK | No | excellent biocompatibility and biodegradability properties | Degrades slowly in vivo |
Abbreviations:PLA, polylactic-lactic acid; PLGA, polylactic-glycolic acid; PEG, poly (ethylene glycol); PCL, poly (ε-caprolactone); PEA, polyester amide; PCADK, poly (cyclohexane-1,4-diyl acetone dimethylene ketal).
3.2.1. Natural biodegradable polymers
A variety of natural biodegradable polymers have been used to prepare microspheres. Chitosan is a highly promising natural biodegradable polymer with a multitude of advantageous properties, including stability, sustained release, mucosal adhesion, in-situ gelation, transfection, and permeation-enhancing capabilities [31]. These characteristics form a strong foundation for chitosan in the field of ocular drug delivery. The unique physical attributes of chitosan enable drug transport across cellular membranes without compromising cell integrity. Chitosan's innate advantage of enabling drug transport both beside and across cells opens up a new avenue for drug delivery to the eyes. Its non-toxicity, minimal ocular irritation, and capacity for sustained drug release make it an ideal polymer in ophthalmic formulations [32,33]. However, chitosan has a low solubility in aqueous solutions above pH 6.5. To overcome this challenge, researchers have developed various chitosan derivatives with improved hydrophilicity [34]. Additionally, one study has found that intravitreal injection of chitosan nanoparticles can cause inflammation in the eye [35]. Therefore, further research is needed to explore the biocompatibility of chitosan and its derivatives to accelerate their clinical application. Pectin is a naturally obtained complex polysaccharide, containing up to 65 % galacturonic acid. Due to its high molecular weight and high degree of branching, it can be transformed into an expandable hydrogel. The low pH conditions and high pectin concentration in the solution are conducive to curling within the structure, thereby forming a gel. Because of its gel-forming properties, pectin is a suitable drug delivery carrier for ophthalmic preparations [30].One research utilizing spray drying technology has prepared pectin microspheres for loading piroxicam. In vitro pharmacological experiments indicate that the piroxicam-loaded pectin microspheres are spherical with the diameter of less than 10 μm, and can release 90 % of the piroxicam content within approximately 2 h. Additionally, researchers evaluated the corneal pre-retention of microspheres loaded with fluorescein in albino rabbits. The vivo experiments showed that compared to fluorescein solution, the aqueous dispersion of fluorescein microspheres significantly increased the retention time in the eyes (2.5 vs. 0.5 h), and compared to commercial piroxicam eye drops, the bioavailability of piroxicam in the aqueous humor significantly increased (2.5-fold) [36]. Although pectin microspheres show good potential for ocular applications, more experiments are needed to study their ocular tolerance and accelerate their clinical application. Gelatin is a natural polymer obtained from partial acid or alkaline hydrolysis of animal collagen through thermal or enzymatic degradation, which can be degraded into amino acids in the body [29]. Due to its excellent biocompatibility, moderate biodegradability, non-toxicity, and non-antigenicity, gelatin-based microspheres have been developed into a versatile platform for ocular drug delivery [37]. Currently, an ab interno gelatin stent (XEN45 Gel Stent, Allergan Inc., Irvine, California, USA) has been approved by FDA for the treatment of glaucoma [38]. However, gelatin has poor mechanical properties and is difficult to safely cross-link, which limits its application to a certain extent [29]. Alginic acid is a biodegradable natural polysaccharide that is extracted from marine brown algae and has been approved by the FDA for eye use. It is composed of β-d-mannuronic acid (M unit) and α-l-guluronic acid (G unit) through β-1,4- It is formed by glycosidic bonds. Under acidic pH, alginic acid can absorb water to form a gel, but its mechanical properties deteriorate after gel formation. Its mechanical strength and compressive modulus increase with the proportion and length of the G units [30]. Researchers mixed retinoic acid with sodium alginate to create sodium alginate-retinoic acid microspheres. Under the microscope, these microspheres appeared as yellowish, semi-transparent, and spherical with a smooth surface. The size was relatively uniform, with an average diameter of 95.2443 ± 8.6265 μm. They could release the drug in vitro, maintaining a stable release for 28–30 days. When injected into the vitreous body of rabbits, the effective drug concentration was consistently detectable in the aqueous humor for up to 6 weeks [39].
3.2.2. Synthetic biodegradable polymers
Diverse synthetic biodegradable polymers have been used for the preparation of ocular microspheres in the past decades. Polylactic-lactic acid (PLA) is a hydrophobic polymer typically made from fermented plant starch that is easy to process and slowly degrades in the eye. while poly-glutamic acid (PGA) exhibits flexibility, relatively lower hydrophobicity, and faster degradation rate. PLGA, a copolymer of PLA and PGA, perfectly inherits some properties from both monomers. Generally, by altering the ratios of PGA and PLA within PLGA, it is possible to significantly improve its biocompatibility for ocular applications, as well as precisely control the degradation kinetics, crystallinity, and hydrophobic properties to meet specific medical requirements [29]. Previous studies have used PLGA microspheres for ocular drug delivery in humans, and the results indicate that PLGA microspheres have good biocompatibility within the eye and almost do not cause intraocular inflammation [40,41]. However, other studies suggest that the acidic degradation products of PLA and PLGA may lead to chronic inflammation in ocular tissues 38018313. Poly (ε-caprolactone) (PCL) is a synthetic aliphatic polyester its low cost, ease of modification and copolymerization, and processability that is low cost and easily modified. PCL can degrade into 6-hydroxyhexanoic acid in vivo by nonenzymatic random hydrolytic cleavage of ester linkages [42]. Since the acidity of 6-hydroxyhexanoic acid is lower than that of lactic acid, PCL nanoparticles are less toxic to ARPE-19 than PLGA nanoparticles at the same concentration [43]. It has considerable entrapment efficiency for both hydrophilic and hydrophobic drugs. In particular, the burst rate is higher, while the encapsulation efficiency is lower for hydrophilic drugs. Currently, tremendous research interest has been sparked by a type of core-shell microspheres with a PCL-based shell [26]. A pharmacokinetic study has demonstrated that the core-shell microspheres with PCL-based shells can slow down the degradation process of devices and provide effective protection for an extend period of time [26]. Polyester amide (PEA), an amino acid-based polymer, possesses biodegradable and good mechanical properties by incorporating ester and amide groups into its polymer chain. Furthermore, this material has demonstrated perfect biocompatibility with minimal inflammatory response in the eye [44]. However, there is a lack of clinical data on its ocular application, and further exploration is needed to verify the feasibility of its ocular drug delivery. Poly (ethylene glycol) (PEG) is a clear, colorless polymer based on ethylene oxide monomers with good hydrophilicity and biocompatibility which can be degraded into ethylene glycol and diethylene glycol in vivo 16343594. However, compared with other synthetic polymers, its degradation rate in vivo is too fast, which is not conducive to its ocular application [29]. Poly (ethylene glycol)-b-poly (D, l-lactic acid) (PEGLA) is a material widely used in the preparation of nanoparticles and micelles which consists of PEG and PLA [45]. The drug release rate can be adjusted by regulating the ratio of PEG to PLA in PEGLA. Theoretically, the higher the proportion of PEG in PEGLA, the greater the hydrophilicity and the faster the drug release rate. Poly (cyclohexane-1,4-diyl acetone dimethylene ketal) (PCADK), a novel acid-sensitive polymer with excellent biocompatibility and biodegradability properties. The degradation products of PCADK do not result in the formation of an acidic environment. Conversely, these degraded products can be excreted without exacerbating any existing inflammation. They have exhibited excellent biocompatibility and biodegradability in a series of ophthalmological researches. Nevertheless, PCADK degrades slowly at physiological pH, limiting its application in the field of drug delivery [46]. In this context, a novel PCADK/PLGA hybrid microsphere system has been currently developed. This system not only enhances drug encapsulation efficiency but also reduces ocular irritation, showcasing significant application potential [47].
3.3. Manufacturing process
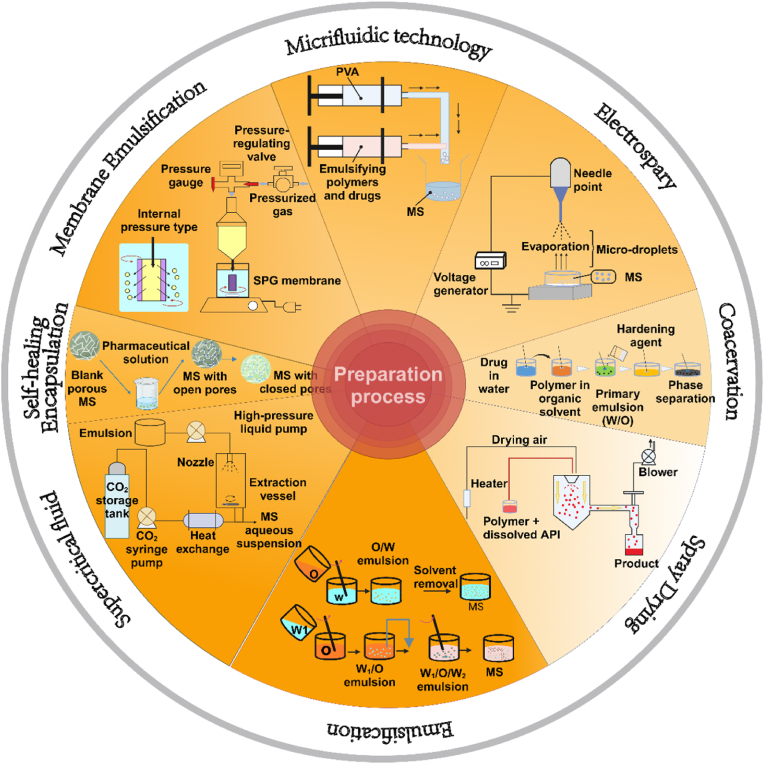
Currently, several methods have been adopted to prepare biodegradable microspheres (Fig. 6). However, they cannot be industrialized due to certain limitations. By discussing the advantages and disadvantages of the manufacturing processes of biodegradable microspheres, we wish to provide clues for future industrial preparation of microspheres.
Fig. 6.
Preparation method of biodegradable microspheres.
Abbreviations: PVA, polyvinyl alcohol; MS, microsphere; W/O, water in oil; O/W, oil in water; W/O/W, water in oil in water; SPG, shirasu porous glass.
3.3.1. Emulsification
Emulsification solvent evaporation is a common technique for preparing microspheres. It can be used for the encapsulation of temperature-sensitive drugs, and the operation process is simple. During the preparation process, the particle size of drugs can be controlled accurately. The emulsification solvent evaporation method can be divided into two categories: single emulsification solvent evaporation and double emulsification solvent evaporation methods. Single emulsification solvent evaporation method includes the oil in water (O/W), water in oil (W/O), and oil in oil (O/O), thereinto, O/W method is suitable for encapsulation of hydrophobic drug molecules. The double emulsification solvent evaporation method includes the water in oil in water (W/O/W), solid in oil in water (S/O/W), and water in oil in oil (W/O/O). Among them, W/O/W is suitable for encapsulating hydrophilic drug molecules [48]. However, the emulsion solvent evaporation method is difficult to scale up due to multiple factors such as high preparation cost, complex operation technique, strict quality control, and poor safety.
3.3.2. Spray drying
Spray drying is a preparation technique that involves the sequential process of atomizing a solution or emulsion containing drugs and polymers, injecting them into hot air, and rapidly evaporating the solvent to transform the atomized droplets into solid particles. This method has many advantages. Firstly, it can reduce drug losses since there is minimal loss of the external aqueous phase, and enhance the encapsulation efficiency. Moreover, spray drying can effectively encapsulate a wide range of drugs, including biologically sensitive proteins and peptides. It helps reduce microsphere aggregation by adjusting the parameters of the atomizer, selecting the appropriate solvent, and controlling the inlet and outlet air temperature [49]. Another significant advantage is that particles prepared by spray drying typically do not contain organic solvents, whereas other methods may potentially introduce organic solvent contamination to the particles [50]. However, this technique also has some limitations. The choice of shell materials that can be used is limited, which may restrict its applicability. Additionally, a significant amount of undried excipients may adhere to the inner walls of the spray drying apparatus, leading to a waste of materials [51]. The drying temperature has a significant impact on the properties of microspheres, where high temperatures can cause deformation and aggregation of microspheres, while low temperatures may result in increased residual solvent levels, making it challenging to control the particle size.
3.3.3. Microfluidic technology
Microfluidic technology is a method for producing microspheres by generating droplets through microchannels. This process involves two immiscible liquids that flow within microchannels, one as the continuous phase and the other as the dispersed phase. When the two phases meet at the intersection of the channel, the interface becomes unstable and eventually breaks, forming dispersed droplets. Microfluidic technology has several advantages: it allows for the control of microsphere size by adjusting flow rates and enables the design of microfluidic devices with specific channel shapes to meet the functional and structural requirements of different microspheres, thus achieving personalized customization for preparing microspheres [52].
3.3.4. Membrane emulsification
Membrane emulsification technology can be categorized into the direct membrane emulsification technology and the rapid membrane emulsification technology. Its main principle refers to the process of pressurizing the dispersed phase liquid through a microporous membrane of the same pore size to form a monodisperse emulsion. When the droplet size reaches a suitable range, it breaks away from the membrane pores and enters the continuous phase under the action of transmembrane pressure. The core of the membrane emulsification method is to cover the membrane surface with pores. The main types of membranes include stainless steel membranes, polymer membranes, shirasu porous glass (SPG) membranes, and so on. Among them, the SPG membranes are the most prevalent type [53]. Compared with the traditional emulsification method, the membrane emulsification method has the advantages of uniform particle size, milder conditions, simple preparing equipment, and energy-efficient processes. However, its small dispersion flux leads to low yield, limiting its applicability [54].
3.3.5. Interfacial polycondensation
The Interfacial polycondensation technique involves an emulsification process, which seeks to place two or more immiscible monomers in an immiscible solvent and then prepare the microspheres through coacervation reactions occurring at the interface between the two phases. According to the solubility of the emulsion droplets acting as soft templates, emulsions can be classified into three types: O/O, W/O, and O/W. This method can protect and encapsulate fragile active ingredients, which aids in the handling and controlled release of fragile active ingredients [55]. However, there is still a need to develop new shell materials and expand its range of applications.
3.3.6. Coacervation
Coacervation or phase separation is a technique that involves the formation of three immiscible chemical phases. Firstly, inorganic salts or non-solvent substances are added as coagulants to the mixture of drug and polymer carrier (emulsion or suspension) to reduce suddenly the solubility of the polymer, so that it can precipitate from the mixed solution and wrap on the surface of the drug to form a protective layer. Then curing the protective layer through a certain method to obtain microspheres with high encapsulation efficiency [56]. One significant advantage of coacervation is its versatility, as it can be applied to any core material dispersible in a liquid. However, one potential drawback is the agglomeration of the coated product, which may impact its overall quality and performance.
3.3.7. Electrospray
Electrospray technology, also known as electrostatic spray coating, is a method for producing polymer microspheres and composite microspheres by utilizing electrostatic forces under high pressure. Compared with traditional methods for preparing polymer microspheres, electrospray offers significant advantages such as the ability to produce high-purity microspheres, the relatively simple operation procedures, and the suitability for loading various hydrophilic and hydrophobic drugs [57].
3.3.8. Supercritical fluid technology
Supercritical fluid (SCF) method includes the rapid expansion of supercritical solutions, supercritical anti-solvent, and solution-enhanced dispersion by SCF. Its preparation procedures include the following: firstly, the drug, polymer, and SCF solution are mixed uniformly and then the whole system is saturated by adjusting temperature and pressure; reducing the saturation degree of the whole system by changing temperature and pressure; eventually the solute is precipitated from the whole system and the encapsulation of the drug by the polymer is realized to form the microspheres with homogeneous particle size. SCF method has an efficient mass transfer ability which can quickly remove organic solvents. Moreover, the preparation process is mild and there is no need for heating. Because of these advantages, the SCF method is considered as the optimum method for biomedical microspheroidization with promising development prospects. However, the commercialization process is slow due to the high cost and complex process conditions [58].
3.3.9. Self-healing encapsulation technology
Self-healing encapsulation means that the pre-prepared blank porous microspheres are mixed with the drug solution so that the drug spontaneously spreads into the microspheres, and then raise the temperature above the glass transition temperature of the polymer or seal the pores of the microspheres using a solvent. Self-healing encapsulation can reduce the effects of shear stress and dampen the exposure to the oil/water interface. Microspheres are sterilized before loading to prevent protein/peptide from losing activity when they are exposed to the sterilization process [59]. Therefore, self-healing encapsulation technology is very friendly to sensitive biomolecules and the preparation process is relatively simple. The only deficiency of this technology is the low loading capacity. However, a better entrapment effect can be achieved by adjusting the microsphere porosity, opening/closing conditions, drug solution concentrations, sealing conditions, and other process parameters.
3.4. Factors affecting the release of microspheres
In recent years, personalized medicine has garnered widespread attention, with drug therapy being tailored to the specific characteristics of individual patients. In personalized medicine, microspheres can be used for precise drug delivery by controlling drug release rate and time to maximize therapeutic effects while reducing side effects. Therefore, there is an urgent need to regulate the drug release rate of microspheres in the eye for treating posterior segment diseases. By summarizing the factors affecting drug release from biodegradable microspheres, it will be helpful for developing microspheres with advantageous release characteristics, thereby accelerating the progress of their clinical application.
3.4.1. Effect of additives on release
Several lines of evidences suggest that adding different types of additives to the microspheres can increase the drug release efficiency and prolong the administration time. According to the different characteristics of additives, they can be divided into hydrophilic additives, lipophilic additives, and additives with pathological characteristics.
The addition of hydrophilic additives to the microspheres can increase the release efficiency of the drug [60]. Polyethylene glycol, pluronic F68, and gelatin are all hydrophilic additives. Addition of PEG 1000 (30 %) or pluronic F68 (3 %) accelerates the release course of cyclosporine (CyS) from PLGA microspheres. Thereinto, pluronic F68 increases the release efficiency of CyS more significantly and maintains therapeutic dosage in the disease-related tissue such as choroid-retina, iris, and ciliary body for at least 65 days [61]. Another study shows that incorporating gelatin into the aqueous phase of the O/W emulsion during the preparation of acyclovir-loaded PLGA microsphere can promote the pore formation of the microspheres, and increase the drug diffusion, thus accelerating the release rate of acyclovir at a constant rate for 63 days [62].
Adding Lipophilic additives to the microspheres can control the drug release, improve the stability of the microspheres, increase the drug entrapment efficiency, and prolong the release time of active substances [63]. PCL is a hydrophobic, slowly biodegradable, and drug-permeable polymer. A study shows that coating PCL as the shell of microparticle on the chitosan core can effectively increase the capacity of drug loading to 25 %, reduce burst release by nearly 30 %, and prolong the release time of bevacizumab from 3 months to 6 months [26]. In a study, researchers utilize biodegradable tributyrin as an additive, effectively controlling the in vitro release rate of ganciclovir (GCV) within PLGA microsphere, thereby achieving prolonged release durations [64]. This innovative technology offers a novel avenue for optimizing drug delivery systems and enhancing drug stability.
Sometimes the use of additives isn't just about altering the release properties of microspheres. It can also enhance the microspheres' therapeutic response to various diseases by adding pharmacologically active agents. Since most conditions affecting the posterior segment of the eyeball are usually multifactorial, incorporating pharmacological additives into microspheres might offer better treatment outcomes compared to single-drug administration. In a pharmacokinetic study, researchers employed biodegradable α-tocopherol as an additive, effectively modulating the in vitro release rate of GCV within PLGA microsphere. Additionally, α-tocopherol exhibits anti-proliferative advantages, which can reduce the adverse risks associated with intravitreal administration [64]. Vitamin A has the capacity to inhibit cell proliferation. The inclusion of Vitamin A palmitate in acyclovir microspheres results in a more prolonged release course of the drug and inhibits cell proliferation [65]. Moreover, the combined use of Vitamin E (VitE) and glial cell-derived neurotrophic factor (GDNF) enabled glaucoma treatment. The incorporation of VitE not only heightens the encapsulation efficiency but also safeguards the biotechnological products from degradation, thereby extending the release course of active substance. Another animal study shows that GDNF-loaded PLGA/VitE particles and GDNF/melatonin-loaded PLGA/VitE particles produce a similar effect in restoring photoreceptor function. Furthermore, encapsulation of solid melatonin results in a reduced initial burst of GDNF release, prolonged duration of release, and a slower rate of neurotrophic factor delivery observed during the delayed release phase. By employing pharmacologically active additives, microsphere drug delivery effectiveness can be optimized, thereby enhancing their responsiveness in therapeutic applications [66].
3.4.2. Effect of radiation sterilization on release
Gamma irradiation is one of the most widely used sterilization procedures. It can induce structural changes in polymers and encapsulated drugs, especially if the active molecule is a protein. A recent study demonstrates that microsphere sterilization can accelerate the initial drug release [67]. The time required to release 50 % of loaded dexamethasone (DEX) is reduced to 12 days for gamma-irradiated (25KGY) PLGA microspheres as compared with non-sterilized microspheres. The researchers speculate that the accelerated initial release of DEX after γ-irradiation might be caused by inducing the ester bond cleavage and reducing the polymer Mw. However, other studies suggest that sterilization has little effect on the release of microspheres. Under the same sterilization process, the release curves of sterilized DEX-loaded biodegradable PEA microspheres and unsterilized microspheres show similar results and the similarity factor(f2) is 98.3 [68]. Furthermore, Barcia E et al. compared the in vitro release profiles of unsterilized and sterilized drug-loaded PLGA microspheres and obtained an f2 parameter of 98.27 [69]. Compared with the previous experiment, the experimental process of the two is roughly similar, but the results are biased. A study has shown that rippled surfaces and air movement created by the high speed of a mechanical stirrer could cause an increased evaporation rate and a fast shell formation [70]. Therefore, the researchers speculate that a higher mechanical stirring rate led to more dimpled-surface microspheres, and the Mw of dimpled-surface microspheres change more profoundly after gamma irradiation compared with the smooth microspheres. Agreed well with this speculation, another study shows that the drug release behavior of sterilized Biodegradable PLGA microsphere loaded with GCV is similar to that of unsterilized microspheres, and the f2 value falls in the range of 51–55 [71].
3.4.3. Effects of preparation method on release
The preparation method of microspheres can affect the release curve of active substances in microspheres. There are many processes for the preparation of microspheres. Different preparation processes will affect the crystal form of the polymer, the distribution, and stability of drugs in the carrier materials, as well as the surface morphology, particle size, and internal structure of the microspheres, thus affecting the degree of drug release [72]. Single emulsification and double emulsification are commonly used in the preparation of microspheres. Researchers use the O/W and W/O/W methods to prepare PLGA (Mw 64000-10000) microspheres loaded with water-soluble drugs. The results show that the curve of drug release is different according to the preparation methods. W/O/W method can form porous microspheres and the initial drug release is serious. In the process of microsphere preparation, the rate of solvent evaporation, the type of solvent (dichloromethane/acetone, ethyl acetate, dichloromethane), and drug content can affect the porosity. In addition, vacuum drying will increase the porosity and accelerate the initial burst release. The O/W method can form matrix microspheres, the drug release mechanism is a three-phase release (the burst release phase, the lag phase, and sustained release phase) [73]. Similar to PLGA microsphere, PLA microspheres prepared by the double-emulsion method have higher loading and encapsulation values than the single-emulsion microspheres, and their initial burst release is also higher [74]. In the process of S/O/W emulsion evaporation, the change of external aqueous phase and organic phase solvent will also affect the average particle size of the microspheres and reduce the initial release of the microspheres, thereby prolonging the administration time [63]. For instance, water-soluble co-solvent ethanol is added to the internal organic phase, while a viscosity agent (hydroxypropyl methylcellulose-HPMC) is used in the external aqueous phase. The release properties of Chitosan-tripolyphosphate microspheres prepared by spray drying and emulsification are the same, but compared with spray drying, emulsification has advantages in terms of microsphere stability [75]. Compared with emulsion solvent evaporation and spray drying, PLGA microsphere prepared by microfluidic technology showed a slower drug release rate [76]. Moreover, the size of the microsphere produced by the membrane emulsification method is uniform, which can be controlled in a low-shear environment during the droplet formation process. In particular, the drug release in the microsphere can be adjusted by selecting the size of the microsphere [77,78]. Similar to the membrane emulsification method, the electrospray method can also adjust the release curve of microspheres by adjusting different experimental parameters (such as voltage and velocity) [79]. A recent study shows that the entrapment efficiency of porous loaded PLGA microspheres prepared by the phase separation method (58 %) is higher than that of the W/O/W solvent evaporation method (16 %) [80]. SCF technology increases the porosity of the microspheres, distributes the drugs more evenly in the microspheres, and reduces the initial drug release [81].
3.4.4. Effect of drug properties on release
Different properties of the loaded drug can affect the release curve of the microsphere. It has been demonstrated that the quantity and the interaction between drugs and polymers can affect the release of microspheres. Excessive drug loading in microspheres leads to drug accumulation on or near the surface of microspheres, resulting in high initial burst release [82]. Besides, Miyajima et al. found that basic drugs can catalyze the hydrolysis of ester bonds, thereby accelerating polymer degradation and drug release. Moreover, basic drugs may also shield the terminal carboxyl residues, thus interfering with the autocatalytic effect and slowing down the process of polymer degradation and water penetration into the matrix [83,84]. Compared to hydrophobic compounds, loading hydrophilic compounds presents greater challenges due to their faster release rates [79]. This occurs because hydrophobic substances impede the entry of water molecules into the microspheres, thereby slowing down the polymer degradation rate. Therefore, we should take into consideration of the properties of the loaded substance when selecting carrier materials. Additionally, the electric charge of drugs and polymer microspheres greatly also affects the release process. The interaction between the charges of the polymer in the microspheres and the carried drugs can effectively decide the release rate of the loaded drugs. This leads to a situation: when the electric charges of the polymer microspheres match those of the enclosed drugs, the electrostatic interaction between the negatively charged drug and the microsphere can slow down the diffusion rate [26]. Conversely, if their charges are the same, the microspheres release can be less efficient due to their mutual repulsion, which also shortens the release time. Differences in electric charge between drugs and polymers could trigger charge-dipole interactions, influencing the shape of microspheres. At certain polymer concentrations, higher drug dosage might lead to more molecules with opposite charges. This could accelerate the solidification and particle formation process of microspheres, resulting in smaller particles and a shorter release time. In contrast, lower drug dosage could inhibit or slow down the solidification and particle formation process [85]. Therefore, we should take into consideration of the electric charge of drugs and microspheres during the microsphere-making process [26].
3.4.5. Effect of polymer properties on release
The concentration of polymers influences significantly the morphology of particles. It's well known that these irregularly shaped microspheres can lead to unstable release rates [86]. Relevant experiments indicate that when polymer concentration reaches a certain level, it might disrupt the microsphere formation process, resulting in irregular particle shapes of microspheres. This phenomenon may be caused by the imbalance in polar interactions and aggregation among polar molecules [87]. Furthermore, the Mw of microsphere polymers also influences their release performance. Researchers have explored the release rates of different Mw microspheres loaded with 5-fluorouracil (5-FU), including the PLGA (3300 g/mol) microspheres, the PLA (3400 g/mol) microspheres and the PLA (4700 g/mol) microspheres. The PLGA microsphere with a Mw of 3300 g/mol exhibits the fastest in vitro release rate, with approximately 98 % of the drugs being released within 2 days. In contrast, the PLA microsphere with Mw of 3400 g/mol and 4700 g/mol released 85 % and 70 % of 5-FU, respectively, in nearly 7 days [88]. In the PLGA microsphere, different ratios of PLA and PGA also have an impact on the microsphere release. A study demonstrates that the polymer exhibits the fastest degradation rate when the monomer ratio is 50:50 [79]. This is because PLGA (50:50) has the lowest crystallinity and simultaneously higher hydrophilicity, which would facilitate the water penetration into the polymer matrix, and directly lead to an accelerated release rate. Furthermore, microspheres prepared from different polymers may also affect the release rate of microspheres.
3.4.6. Physical properties affect microspheres release
Accumulating evidences suggest that physical properties, such as the particle size, the surface morphology, and the internal structure of microspheres, can affect the drug release in the microspheres. Jing Z et al. have prepared Levofloxacin-loaded chitosan microspheres with different particle sizes and studied their release time in vitro. The results suggest that the smaller the size of the microspheres, the shorter the release time [86]. Furthermore, Zhang Z et al. prepared the paclitaxel-loaded PLGA microspheres by double emulsion solvent evaporation method and evaluated the in vitro release curve. They find that the smooth microspheres with internal scattered pores exhibit a slow linear release mode, while the rough microspheres with highly porous internal structures exhibit a faster S-curve release model [89]. Compared with the dense polymer matrix, some hollows in the microspheres can reduce the initial burst release of drugs in the microspheres [63]. By comparing the active substances released from PLA microsphere with different pore radii, researchers find that the external pores of microspheres are responsible for regulating the first-order release rate of microspheres [90]. In another study, two types of microspheres are prepared from PLGA polymers with different Mw (about 25 and 7 kDa). Pores are formed from the outside to the core in the two types of microspheres after drug release. In particular, the 7 kDa microspheres form pores earlier than the 25 kDa microspheres, resulting in a shorter drug release time [91].
3.4.7. Effect of intraocular environment on release
The release process of intraocular microspheres can be influenced by various factors, including temperature, pH, enzyme activity, and so on. To gain a better understanding of drug release in microspheres, researchers adjust some of the aforementioned factors in laboratory experiments to simulate in vivo conditions. A pioneering experiment has developed an in vitro strategy to enhance microsphere release. In this study, researchers place the PLGA microsphere in phosphate buffer saline buffer (pH 7.4) and then conduct experiments to release DEX at different temperatures: 37 °C, 53 °C, 60 °C and 70 °C, respectively. As the temperature increases gradually, the release time of DEX from the microspheres in vitro decreases progressively [92]. Another experiment examines the impact of acidic pH on the DEX-loaded PLGA microsphere release. Under a 25K formulation, as the pH drops from 7.4 to 2.4, the overall external release time decreases from roughly 30 days to approximately 19 days. For the 70K formulation, the time required to achieve 80 % drug release reduces from 84 days to 52 days [93]. Microsphere release behavior often differs between in vitro and in vivo conditions because the factors of the in vivo environment that cannot be accurately replicated in vitro, such as the enzymes and lipids [94]. In vitro release profiles typically consist of three main phases: an initial burst phase, a zero-order release phase, and a lag phase. However, the absence of a lag phase is a common phenomenon in the in vivo release profiles. This discrepancy may be attributed to distinct erosion behaviors between in vitro and in vivo environments. Enzymatic degradation can result in a "from the outside in" erosion, which differs from the "from the inside out" erosion that always occurs in vitro. Besides, lipids can also expedite the degradation of microspheres [94].
4. Application of biodegradable microspheres in posterior ophthalmic diseases
Posterior segment disease affects the back section of the eyeball, including the retina, choroid, and optic nerve, which may affect vision or even lead to blindness. There are a variety of treatment methods for posterior segment disease, including laser photocoagulation, photodynamic therapy, and medications. In recent years, biodegradable microspheres gradually come into sight with the progress of science and technology. Microspheres could deliver drugs more effectively to the back segment of the eye, improving treatment effectiveness while mitigating side effects. Both animal tests and clinical trials show that the biodegradable microspheres may act as good candidates for delivering drugs to the posterior segment disease (Table 3). However, the future researchers should verify the detailed pharmacologic profiles and safety in clinical work.
Table 3.
Experimental studies on the posterior ophthalmic diseases of biodegradable microspheres.
| Posterior ophthalmic diseases | Biodegradable microspheres | Preparation technology | Active substance(s) | Route of administration | Microsphere size | Drug release time | Model | REF |
|---|---|---|---|---|---|---|---|---|
| CSC | PLGA MS (50:50) | O/W emulsion solvent evaporation | Spironolactone | Intravitreal injection | 17.5 ± 5.0 μm | 31days (in vitro) | Wistar rats | [95] |
| DR | PLGA MS (85:15) | Modified emulsification solvent evaporation | Celecoxib | Subconjunctival injection | 1.11 ± 0.08 μm | 60 days (in vitro) | Sprague-Dawley rats | [96] |
| DR | PLA MS | O/W emulsion solvent evaporation | Budesonide | Subconjunctival injection | 3.60 ± 0.01 μm | 42 days (in vitro) | Sprague-Dawley rats | [97] |
| RP | PLGA MS | O/W emulsion solvent evaporation | hPI | Intravitreal injection | 10–32 μm | 10–11 days (in vivo) | rd10 mice | [98] |
| RP | PLGA MS (50:50) | O/W emulsion solvent evaporation | TUDCA | Intravitreal injection | 22.89 ± 0.04 μm | 28 days (in vitro) | Homozyg-ous P23H line 3 albino rats | [99] |
| RP | PLGA MS (37.5: 25) | W/O/W emulsion solvent evaporation | rhGDNF | Intravitreal injection | 27 ± 10 μm | 17 days (in vivo) | C3H/HeN mice | [100] |
| Uveitis | PLGA MS (50:50) | O/W emulsion solvent evaporation | DEX | Intravitreal injection | 20–53 μm | 33 days (in vivo) | Male New Zealand white rabbits | [69] |
| Uveitis | PLGA MS (75:25) | Emulsification solvent evaporation | Cyclosporine A | Intravitreal injection | ∼50 μm | 42 days (in vivo) | Japanese white rabbits | [101] |
| PVR | PLGA MS | Double emulsion phase separation | 5-FU | Intravitreal injection | 6.8 ± 0.9 μm | 168 days (in vivo) | Female New Zealand white rabbits | [102] |
| PVR | PLA MS | Emulsification solvent evaporation | Adriamycin | Intravitreal injection | ∼50 μm | 28 days (in vivo) | rabbits | [103] |
| PVR | PLGA MS (50:50) | Emulsification solvent evaporation | Retinoic acid | Intravitreal injection | ∼60 μm | 60 days (in vivo) | rabbits | [104] |
| PCR | alginate MS | – | Retinoic acid | Intravitreal injection | 95.2 ± 8.6 μm | 42 days (in vivo) | rabbits | |
| ARN | PLA MS | spray-drying | Acyclovir | Intravitreal injection | ∼25 μm | 14 days (in vivo) | rabbits | [105] |
| ARN | PLGA MS (50:50) | O/W emulsion solvent evaporation | Guanosine | Intravitreal injection | ∼80 μm | 7 days (in vitro) | rabbits | [106] |
| ARN | PLGA MS (85:15) | O/W emulsion solvent evaporation | Guanosine | Intravitreal injection | ∼120 μm | 7 days (in vitro) | rabbits | [106] |
| CMV | PLGA MS | Emulsification solvent evaporation | Ganciclovir | Intravitreal injection | ∼242 μm | 14 days (in vivo) | rabbits | [107] |
| CMV | PLGA MS (50:50) | O/O emulsion solvent evaporation | Ganciclovir | Intravitreal injection | 300–500 μm | 14 days (in vivo) | rabbits | [108] |
| CMV | Chitosan MS | W/O emulsion solvent evaporation | Ganciclovir | Subconjunctival injection | ∼20.28 μm | – | Wistar rats | [109] |
| AMD | PLGA MS (75:25) | W/O/W emulsion solvent evaporation | Ranibizumab biosimilar | – | ∼20 μm | 21 days (in vitro) | – | [110] |
| AMD | PLGA MS (50:50) | S/O/W emulsion solvent evaporation | BDNF, GDNF and VitE | Intravitreal injection | 20–38 μm | 49 days (in vivo) | C57BL/6J mice | [111] |
| AMD | Chitosan–PCL MS | SPG membrane emulsification | Bevacizumab | – | 12.7 ± 5.9 μm | 6 months (in vitro) | – | [26] |
| AMD | PEGLA MS | Emulsification solvent evaporation | Bevacizumab | – | 2–10 μm | 91 days (in vitro) | – | [112] |
| AMD | PLGA/PCADK MS (8:2) | S/O/W emulsion solvent evaporation | Bevacizumab | Intravitreal injection | 13.70 ± 8.47 μm | 56 days (In vivo) | Male New Zealand White rabbits | [113] |
| CNV | PLGA MS | Emulsification solvent evaporation | PKC412 | Periocular injection | ∼42.5 μm | 20 days (In vivo) | Four-week-old female Yorkshire pigs | [114] |
| CNV | PLGA MS (75:25) | W/O/W emulsion solvent evaporation | Aflibercept | Intravitreal injection | 8.2 ± 2.2 μm | 6 months (In vivo) | Long-Evans male rats | [115] |
| CNV | PLGA MS (75:25) | W/O/W emulsion solvent evaporation | Ranibizumab | – | 7.0 ± 1.3 μm | 176 days (In vitro) | – | [116] |
| CNV | PLGA MS (50:50) | O/W emulsion solvent evaporation | Rivoceranib | Intravitreal injection | 21.7 ± 4 μm | 50 days (In vitro) | C57BL/6 mice | [117] |
| Glaucoma | PLGA MS (50:50) | Modified spontaneous emulsion | GDNF | Intravitreal injection | ∼8 μm | 8 weeks (in vivo) | Adult male Brown Norway rats | [118] |
| Glaucoma | PLGA MS (50:50) | S/O/W emulsion solvent evaporation | GDNF and VitE | Intravitreal injection | 19.1 ± 9.4 μm | 11 weeks (In vivo) | Adult male Brown Norway rats | [119] |
| Glaucoma | PLGA MS (50:50) | Modified spontaneous emulsion | GDNF | Intravitreal injection | ∼10 μm | 71 days (In vitro) | DBA/2J (D2) mice | [120] |
| Glaucoma | PLGA MS (50:50) | S/O/W emulsion solvent evaporation | Fasudil | – | 66.9 ± 16 μm | 45 days (In vitro) | – | [121] |
| Glaucoma | PLGA MS (50:50) | W/O/W emulsion solvent evaporation (W1:rpm20000; W2:rpm10000) | Fasudil | – | W1: 3.4 ± 0.6 μm W2: 18.2 ± 2.7 μm |
45 days (In vitro) | – | [121] |
| Glaucoma | PLGA MS (50:50) | O/W emulsion solvent evaporation | Dexamethasone, Melatonin and Coenzyme Q10 | Intravitreal injection | 29.04 ± 1.89 μm | 21 days (In vivo) | Rats | [122] |
| Glaucoma | PCL MS | W/O/W emulsion solvent evaporation | Levobunolol HCl | – | 19.9 ± 0.371 μm | 6 h (In vitro) | – | 18608798 |
| Endophthalmitis | PLGA MS (50:50) | Emulsion-diffusion | Rifampicin | – | 1.04 ± 0.07 μm | 24 h (In vitro) | – | [123] |
| Cataract | PLGA MS (50:50) | Spray-drying | Triamcinolone acetonide and Ciprofloxacin | Sub-tenon's injection | 1.07 ± 0.35 μm | 28 days (In vivo) | Humans | [124] |
| Co-Transplantation with Retinal Progenitor Cells | PLGA MS | W/O/W emulsion solvent evaporation | MMP2 | Subretinal injection | 2–20 μm | 30 days (In vitro) | C57BL/6 mice | [125] |
| DME | PLGA MS | – | Triamcinolone acetonide | Intravitreal injection | – | 12 months (In vivo) | Humans | [126] |
Abbreviations:CSC, Central serous chorioretinopathy; PLGA MS, PLGA microspheres; O/W, oil in water; REF, reference; DR, diabetic retinopathy; PLA MS, polylactic-lactic acid microspheres; RP, retinitis pigmentosa; hPI, human recombinant proinsulin; TUDCA, Tauroursodeoxycholic Acid; W/O/W, water in oil in water; DEX, Dexamethasone; PVR, proliferative vitreoretinopathy; 5-FU, 5-fluorouracil.
ARN, acute retinal necrosis; CMV, Cytomegalovirus retinitis; O/O: oil in oil; AMD, age-related macular degeneration; BDNF, brain-derived neurotrophic factor; GDNF, glial cell-derived neurotrophic factor; VitE, Vitamin E; Chitosan–PCL MS, Chitosan–Poly (ε-caprolactone) microspheres; SPG, shirasu porous glass; PEGLA MS, Poly (ethylene glycol)-b-poly (D, l-lactic acid) microspheres; S/O/W: solid in oil in water; CNV, Choroidal neovascularization; PLGA/PCADK MS, polylactic-glycolic acid/Poly (cyclohexane-1,4-diyl acetone dimethylene ketal) microspheres; PKC412, protein kinase C142; MMP2: Matrix metalloproteinase 2; rhGDNF, recombinant human glial cell line-derived neurotrophic factor; DME, Diabetic macular edema.
4.1. Age-related macular degeneration
AMD is a degenerative retinopathy that causes a gradual loss of central vision. Typically, AMD can be classified into dry and wet types. It starts as dry or non-exudative, which might progress to wet or neovascular AMD at later stages. Although dry AMD accounts for approximately 90 % of cases, severe vision loss is predominantly associated with wet AMD [127]. Wet AMD is characterized by the growth of neovascular in the choroid layer of the eye, in which the vascular endothelial growth factor (VEGF) plays a crucial role [128]. Currently, intravitreal injection of anti-VEGF agents (such as bevacizumab, ranibizumab, and aflibercept) is the prevailing therapeutic strategy for AMD. Bevacizumab is a humanized monoclonal IgG antibody that specifically targets VEGF to inhibit neovascularization [129]. Previous experiments have shown that nanoparticles and microparticles made from PLGA and PEGLA could release Bevacizumab continuously for over 91 days in vitro [112]. Recent experiments have explored the use of chitosan-PCL-chitosan microspheres for the delivery of Bevacizumab, achieving a sustained release period of six months. These microspheres, approximately 10 μm in diameter, were capable of encapsulating 1.03 ± 0.17 mg of Bevacizumab with a drug loading efficiency of 25.77 %. In vitro release studies confirmed the microspheres' capability for prolonged drug release over six months. Additionally, 100 μL of 1 mg/mL core-shell microspheres were injected into porcine eyes, demonstrating the feasibility of intraocular injection of this delivery system [26]. Researchers have also prepared PLGA/PCADK microspheres loaded with bevacizumab. The microspheres have an average size of 13.70 ± 8.47 μm and an encapsulation efficiency of 35.32 ± 1.56 %. In an experimental setup, rabbits were divided into three groups, with each group consisting of 21 animals. One group received an intravitreal injection of 100 μL of a bevacizumab solution (final bevacizumab dose = 0.4 mg). The other two groups were injected intravitreally with bevacizumab-loaded microspheres (PLGA and PLGA/PCADK microspheres) to deliver the same final dose of bevacizumab. Data indicates that the PLGA/PCADK microsphere can better maintain the activity of bevacizumab compared to PLGA microsphere alone, thereby enhancing the drug's effectiveness. The addition of PCADK effectively increased the bioavailability of bevacizumab, which can be attributed to its unique composition that interacts with the non-enzymatic collagen in the eye tissue, leading to slower clearance of bevacizumab. By adding PCADK, the activity of bevacizumab is preserved while minimizing the irritation to the eyes [113]. Ranibizumab, a smaller fragment of an antibody known as an antigen-binding fragment, offers improved dispersion and penetration capacity compared with the larger Bevacizumab [130]. Due to lacking the antibody's Fc domain, Ranibizumab has a shorter half-life, thus reducing the risk of systemic complications for subjects [131]. In an experiment, PLGA microspheres, spherical in shape with an approximate diameter of 20 μm and an encapsulation efficiency of 89.0 ± 4.2 %, loaded with Ranibizumab biosimilar (RB), demonstrated excellent effectiveness in inhibiting VEGF function. These RB-loaded PLGA microspheres showed significant anti-angiogenic ability in vitro studies using HUVEC cells and tube formation assay. It was observed that RB released from the PLGA microspheres inhibited angiogenesis compared to untreated RB, indicating that the PLGA microspheres' fabrication process preserved the activity of RB. These findings suggest that RB-loaded PLGA microspheres could be an effective strategy for treating AMD and other VEGF-related diseases [110]. The neurotrophic factors (NTFs) are also the effective therapeutic agents for AMD. Injecting exogenous GDNF into the vitreous humor can reduce the death of retinal ganglion cells (RGCs) and their axon damage. GDNF can also preserve the function of photoreceptor cells in AMD mouse models. Thus, supplementing these molecules artificially could bring about intriguing therapeutic effects [118]. In a cellular experiment, GDNF, brain-derived neurotrophic factor (BDNF) and oily additive VitE are solidly incorporated into the PLGA microspheres. These microspheres (20–38 μm) are used to treat retinal diseases involving ongoing RPE and RGC apoptosis. Results show that the combined application of GDNF and BDNF effectively promoted RPE cell migration, a syngenetic effect not easily observed when applying GDNF alone. Furthermore, this combination demonstrates a positive impact on repairing RGCs and restoring photoreceptor function [111].
4.2. Acute retinal necrosis
Acute retinal necrosis (ARN) is a progressive necrotizing retinopathy caused by varicella zoster virus or herpes simplex virus, with retinal periarteritis progressing to diffuse necrotizing retinitis and retinal detachment [132]. ARN is a rare ophthalmic disease with an annual incidence of 0.63 per million population in the United Kingdom [133]. A study demonstrated the feasibility of intravitreal injection of PLA microsphere and PLGA microsphere for the long-term treatment of ARN. The researchers utilize the spray drying method to prepare acyclovir-loaded PLA and PLGA microspheres with different Mw and study the humoral drug concentration after intravitreal administration. They find that acyclovir-loaded PLA (28,000 g/mol) microspheres (150μg/0.1 ml) of 25 μm can be continuously released in the rabbit vitreous for at least 14 days. In addition, the researchers also find that the morphological characteristics of the microparticulate systems and their in vitro dissolution behavior depended on the polymer MW, with polymers with higher molecular weight and larger particle size obtaining slower drug release rates [105]. Chowdhury, D K et al. have prepared the PLGA (50:50, Mw75000) and PLGA (85:15, Mw140000) microspheres loaded with Guanosine, respectively. They find that both 75 kDa PLGA microspheres (average diameter 85 μm) and 140 kDa PLGA microspheres (average diameter 120 μm) are released in vitro for one week consistent with Higuchi diffusion release kinetics. Furthermore, initial release studies in rabbit vitreous humor showed good correlation between in vitro and in vivo release rates. Since the structure and solubility characteristics of guanosine are similar to those of acyclovir and GCV, similar microsphere technology based on biodegradable polymers can be used for long-term intraocular delivery of the two drugs [106].
4.3. Cytomegalovirus retinitis
Cytomegalovirus retinitis (CMV) retinitis is a common ocular viral infectious disease characterized by full-thickness retinal edema and necrosis or exudative detachment. The disease usually begins in the peripheral retina and spreads to the posterior pole, which may eventually lead to blindness. CMV retinitis mainly affects immunocompromised hosts, including bone marrow, neonates, and solid organ transplant recipients [134]. GCV has significant anti-human cytomegalovirus activity. In a recent study, PLGA (different ratios of lactic: glycolic acid) microspheres loaded with GCV are prepared and dispersed in PLGA-PEG-PLGA thermal gel solution, and subsequently injected into rabbit eyes at 3 mm below the limbus. Rabbits are administered 60 μL of the microparticle formulation (196 μg of GCV). This formulation maintains the average vitreal concentrations of GCV at nearly 0.8 μg/mL for 14 days, while direct injections only maintain the drug levels above 0.8 μg/mL for 54 h [107]. In another study, 10 mg of GCV-loaded PLGA (50:50) microspheres (300–500 μm, contain 864 μg GCV) are prepared and injected into the vitreous of rabbits inoculated with human cytomegalovirus. During the 14 days of the study, vitreous, retinitis, and optic neuritis are alleviated in the microsphere treated eyes compared with the vehicle treated controls. In particular, no immunofluorescence of viral antigen is observed in the microsphere treated eyes [108]. Furthermore, Chitosan microspheres can also be used to load GCV. The researchers have prepared ganciclovir chitosan (93 % deactylation) microspheres (GCMs) with an average diameter of 20.28 μm by W/O solvent evaporation method and inject them into the conjunctival sac of Wistar rats. They find that the eyes treated with GCMs exhibit in the increased GCV area under the curve (∼4.99-fold) the higher maximum peak concentration (2.69-fold) in aqueous humor, and delayed time to achieve maximum peak concentration compared with those GCV solution treated eyes. In vitro data show that GCV exhibits biphasic pattern from GCM, following Fickian (R2 = 0.9234, n value = 0.2329) type diffusion release mechanism that is different from the intraocular drug release mechanism of PLGA microspheres. But similar to PLGA microspheres, chitosan microspheres also have an initial burst release. Chitosan microspheres release the drug in an immediate burst (nearly 50 %) within the initial few minutes, and then slowly release the drug (up to 90 %) over several hours [109].
4.4. Choroidal neovascularization
Under pathological conditions, the neovascular originates beneath the choroid and extends into the macular area, leading to the formation of choroidal neovascularization (CNV). These new blood vessels are very fragile and prone to bleeding and leaking, which can disrupt the normal function of the retina, and ultimately resulting in irreversible visual impairments. One study has constructed CNV animal models by injecting 5 mg of VEGF (10 μg) impregnated gelatin microspheres into the subretinal space of monkeys. They found that subretinal injection of VEGF-impregnated gelatin microspheres resulted in the development of CNV and induced Early, disciform and reparative stages of CNV in monkeys' model similar to humans [135]. Previous studies have shown that inhibiting CNV growth can be achieved by blocking VEGF receptor signaling or using medications to lower VEGF levels [136]. Encouragingly, a kinase inhibitor called protein kinase C142 (PKC412) offers an effective way to treat CNV by simultaneously blocking signals from VEGF receptors 1 and 2 [137]. In recent research, microspheres containing PKC412 with particle size of 42.5 μm and the loading capacity of 45.9 % were injected into periocular space of pigs. These microspheres significantly suppressed the development of CNV at the site of Bruch's membrane rupture, demonstrating the potential of locally delivering PKC412 through the sclera to treat CNV. Following the injection of 100 mg of microspheres containing 50 % PKC412, the levels of PKC412 were undetectable in the plasma, vitreous, and retina after 10 days but were measurable in the choroid. After 20 days, high levels of PKC412 were observed in the choroid, vitreous, and retina. In contrast, eyes injected with 100 mg of 25 % PKC412 microspheres showed considerably lower PKC412 levels in all three compartments after 20 days compared to those with 50 % microspheres, though the levels were still significant. At neither dosage was PKC412 detected in the plasma, indicating a localized effect [114]. Another inhibitor, Rivoceranib can target the VEGFR2, a cell's internal ATP binding site, through selectively inhibiting the receptor tyrosine kinase. VEGFR2 plays a crucial role in VEGF-mediated endothelial cell proliferation, migration, and permeability. Hence, Rivoceranib can effectively reduce retinal vascular leakage and corneal neovascularization [138,139]. Successful vitreous delivery using PLGA delivery systems can inhibit neovascularization in the avascular zone of the fovea (subfoveal CNV) [117]. Previous experiments have demonstrated the therapeutic effect of inhibiting VEGF in a laser-induced CNV rat model using a microsphere hydrogel drug delivery system [140]. This approach showcases longer treatment duration and improved efficacy. However, the previous hydrogel utilized in these types of experiments is non-degradable, which somewhat limits its application. Recent studies indicate that a degradable PEG-PLLA-DA/NIPAAm hydrogel containing bevacizumab-loaded PLGA (8.2 ± 2.2 μm) microspheres, administered through a single intraocular injection, effectively treats the laser-induced CNV in rats. No persistent functional or morphological abnormalities were observed until 6 months after treatment. Moreover, this microsphere hydrogel drug delivery system is found to be safe, reliable, and biocompatible within the body [115]. Similar therapeutic effects are achieved by delivering ranibizumab using microsphere hydrogels [116].
4.5. Central serous chorioretinopathy
Central serous chorioretinopathy (CSC) is a chorioretinal disease characterized by serous retinal detachment at the macula region. It is often associated with pigment epithelial detachments, RPE atrophy, and choroidal thickening [141,142]. CSC can occur frequently in the population between the ages of 20 and 60 [142]. At present, it is speculated that three possible pathogenesis may lead to CSC: RPE dysfunction, increased scleral thickness, and venous overload choroidopathy [143]. Researches have shown that both glucocorticoids and mineralocorticoids can activate the mineralocorticoid receptors on choroidal endothelial cells, which subsequently mediate the vascular dilation through upregulating the expression of the endothelial calcium-dependent potassium channel [144]. Systemic administration of Mineralocorticoid Receptors antagonist (MRA) can reduce subretinal effusion and choroidal vasodilation and improve the visual acuity of CSC patients, However, systemic administration of these drugs will induce some unwanted side effects [145]. Hence, intravitreal injection of biodegradable spironolactone (an MRA)-loaded PLGA (50:50) microspheres (17.5 ± 5.0 μm) can be used as an effective method for the treatment of CSC. Quantitative PCR is performed on rat retinas taken after 1 month of intravitreal injection. The results show that transcription of spironolactone target genes (like NOX2, NGAL, ICAM-1, and CCL2) is downregulated, indicating that the spironolactone released from PLGA microsphere reaches the retina and exerts biological effects on the rat retina. Furthermore, research also shows that high dosage (5 μL containing 0.5 mg of PLGA microspheres with a total dose of 53 μg of spironolactone) but not low dosage (5 μL containing 0.1 mg of PLGA microspheres with a total dose of 10.6 μg of spironolactone) of PLGA microsphere may induce retinal stress and alter rat visual function [95].
4.6. Diabetic macular edema
Diabetic macular edema (DME), defined as a retinal thickening involving the center of the macula, is characterized by the gradual leakage of fluid from blood vessels and the disruption of the BRB. It occurs in patients with DR, where abnormal fluid accumulates beneath or within the retina's macular region, severely affecting central vision [146]. Besides diabetes, hypertension can also elevate intravascular hydrostatic pressure, causing fluid to leak into the surrounding retina. In past decades, macular laser therapy acts as the main treatment option for DME, but with the introduction of anti-VEGF drugs, the use of macular laser treatment has decreased prominently [147]. In the treatment of DME, the use of intravitreal ranibizumab has proven effective [148]. However, despite its evident efficacy, its duration of action is short and side effects become noticeable due to repeated injections. To extend the therapeutic effects of ranibizumab and reduce side effects, biodegradable microspheres have been utilized to achieve sustained release of the drug. Corticosteroids can inhibit the formation of CNV and enhance the integrity of tight junctions. Triamcinolone acetonide (TA) is a synthetic corticosteroid endowed with anti-inflammatory properties and further actions like vasoconstriction and anti-proliferation. Clinical trials have been conducted in patients with diffuse macular edema to assess the effectiveness of TA-loaded microspheres. After treatment with 1 mg of PLGA microspheres containing TA, the retinal thickness of DME patients decreases significantly within 12 months, accompanied by the improved vision [126].
4.7. Diabetic retinopathy
DR is a common microvascular complication of diabetes with varying stages of progression that can lead to visual impairments [149]. The number of patients affected by DR is predicted to increase to 191 million by 2030 [150]. Typically, DR can be divided into two broad categories: the early stage of non-proliferative diabetic retinopathy (NPDR) and the advanced stage of proliferative diabetic retinopathy (PDR) [149]. The early stage of NPDR is characterized by special effects of the retinal vasculature, including altered blood flow, capillary occlusion, increased vascular permeability, the loss of pericytes, and disruption of endothelial cell tight junctions. The more advanced stage of PDR is characterized by the development of neovascular [151]. nox2 is a gene encoding NADPH oxidase 2 that highly expressed in retinal cells. This over activation of nox2 will increase the production of ROS and enhance the expression of VEGF [152]. VEGF can stimulate the growth and differentiation of vascular endothelial cells, thereby promoting the formation of neovascular in DR. Currently, inhibiting the VEGF expression is crucial for the treatment of DR. Budesonide is a small molecules corticosteroid with potent anti-inflammatory ability [153]. The subconjunctival delivery of 75 μg budesonide-loaded PLA (intrinsic viscosity 1.1 dL/g) microspheres (3.60 ± 0.01 μm) can reduce the VEGF expression in ARPE-19 cells [97]. Cyclooxygenase (Cox)-2 can enhance the prostaglandin E2 (PGE2) expression and disrupt the BRB in DR [154]. Celecoxib is an effective Cox-2 inhibitor that can reduce the level of prostaglandins in ocular tissue. Amrite, A C et al. have shown that subconjunctival injection of celecoxib-loaded PLGA (85:15; intrinsic viscosity 0.67 dL/g) microspheres (1.11 ± 0.08 μm) can inhibit effectively the PEG2 expression and reduce the BRB leakage caused by diabetes. In addition, in vitro studies showed that celecoxib-loaded PLGA microspheres released the drug in a biphasic manner within 60 days. Celecoxib was initially released abruptly for three days, then continued for the next 60 days. At the end of 60 days, about 50 % of the embedded drugs were released in vitro [96].
4.8. Glaucoma
Glaucoma is a chronic eye condition that can potentially damage the optic nerve and lead to gradual loss of peripheral vision. Glaucoma is the second leading cause of irreversible blindness worldwide, and it's estimated that the number of glaucoma cases will exceed 111 million by 2040 [155]. Generally, glaucoma is caused by the obstructed aqueous humor circulation and increased intraocular pressure. Although glaucoma is asymptomatic in its early stages, it's often accompanied by visual field loss and gradual worsening of visual acuity in later stages, eventually resulting in optic nerve damage and blindness [156]. Currently, most traditional treatments seek to reduce the intraocular pressure of glaucoma patients [157]. One study has used the double emulsion W/O/W solvent evaporation method to prepare PCL (Mw: 80000) microspheres (19.9 ± 0.371 μm) when the external aqueous phase pH is 12 for loading Levobunolol HC1. The study found that PCL microspheres loaded with Levobunolol HC1 can sustainably release Levobunolol HC1 for 6 h in vitro, and the release curve of Levobunolol HC1 is suitable for Higuchi diffusion release kinetics [158]. The new generation of glaucoma treatments, such as Rho-associated protein kinase (ROCK) inhibitors like netarsudil (Rhopressa) and ripasudil (Glanatec), also show promising potency in reducing eye pressure. Basically, these drugs are designed to target the trabecular meshwork and Schlemm's canal cells to enhance the outflow of aqueous humor [159]. Therefore, delivering ROCK inhibitors via microspheres from the vitreous body to anterior chamber cells may hold great potential. Experimental evidence has confirmed the feasibility of this approach, as fasudil-loaded PLGA microspheres can overcome biological barriers and induce the desired pharmacological effects. These microspheres have an average size of 66.9 ± 16 μm, facilitating targeted delivery and sustained release over 45 days in vitro. The drug content of these microspheres is 3.7 ± 1.3 μg/mg, with an encapsulation efficiency of 3.9 % ± 1.6 % and the drug loading of 0.4 % ± 0.2 %. This combination of properties enhances the potential of microspheres to deliver therapeutic agents effectively to the targeted cells in the anterior chamber [121]. These microspheres can reduce actin stress fiber formation in trabecular meshwork cells and schlemm's canal cells. Furthermore, ROCK inhibitors have shown neuroprotective effects on retinal cells and a vitreal delivery system could offer extra advantages, particularly for these glaucoma patients with degenerative retinopathy [160].In some insidious cases, the glaucomatous optic atrophy may progress despite the well-controlled eye pressure. Glaucoma is actually a manifestation of RGCs death, suggesting that neuroprotection, may act as an effective alternative therapy in this scenario [161]. In this context, delivering NTFs into the eye may promote the RGCs survival and influence visual function. GDNF has shown neuroprotective properties in the retina. A recent study has confirmed that biodegradable microspheres (∼10 μm) loaded with GDNF exert neuroprotective effects on RGCs in DBA/2J mice [120]. Another study shows that intravitreal injection of PLGA microsphere encapsulated GDNF significantly delays RGCs loss in a glaucoma rat model for at least 6 weeks. Additionally, microsphere-based GDNF therapy reduces the glial cell activation that is associated with the pressure-related eye damage [118]. In greater detail, VitE was incorporated into the formula as an oily additive. This compound is endowed with potent anti-proliferative properties and decent bioavailability. In a glaucoma rat model, PLGA microsphere (19.1 ± 9.4 μm) encapsulated GDNF and VitE demonstrates the efficacy for at least 11 weeks after intravitreal injection [119]. Furthermore, researchers propose an innovative combined therapy by encapsulating three neuroprotective agents (DEX, melatonin, and coenzyme Q10) into PLGA to form the microspheres (29.04 ± 1.89 μm). In a chronic ocular hypertension rodent model, the microsphere demonstrates potent neuroprotective effects on RGCs, providing an exciting therapeutic approach for glaucoma [122].
4.9. Proliferative vitreoretinopathy
As a serious complication of vitreoretinal surgery, proliferative vitreoretinopathy (PVR) is characterized by intraretinal fibrosis, the contracted cell membranes within the vitreous, and abnormal neovascular in the retina. The incidence of PVR is estimated to be 5–10 % of all RD cases [162]. Biodegradable microspheres have been used to deliver active substances to establish certain disease models. For instance, researchers found that subretinal injection of 50 μl of anionic gelatin (an isoelectric point of 5) microsphere suspension containing 2.5 μg of basic fibroblast growth factor (bFGF) and 50 μl of cationic gelatin (an isoelectric point of 9) microsphere suspension containing 0.125 MIU of interferon-beta (IFNβ) resulted in development and progression of intravitreal proliferative membranes and subsequent retinal traction in rabbits, whereas eyes with bFGF or IFNβ alone and eyes without bFGF and IFNβ showed no significant proliferation in the retina or vitreous cavity during follow-up [163]. Existed Anti-proliferation and antineoplastic drugs for PVR mainly include the 5-FU, taxol, daunorubicin, colchicine, retinoic acid (RA), ribozymes, cisplatin, vincristine, mitomycin, adriamycin and dactomycin [162]. Moritera, T et al. studied the therapeutic effect of PLA (Mw: 3400 g/mol) microspheres (∼50 μm) loaded with adriamycin on experimental PVR in rabbits. It is found that a single intravitreal injection of microspheres containing 10 μg of adriamycin significantly reduces the occurrence of traction RD from 50 % to 10 % after 28 days [103]. 5-FU can inhibit fibroblast proliferation and DNA synthesis, it is one of the most widely used drugs for PVR [164]. Yu, Z et al. perform vitrectomy on PVR rabbits, and then inject 3 % polyvinyl alcohol (PVA)/chitosan (deacetylation degree: 85 %, Mw: 200000) hydrogel and 5-FU loaded PLGA (50:50, Mw:25000) microspheres (∼60 μm) into the vitreous humor of animal models. These results show that 3 % PVA/chitosan hydrogel has similar properties to human vitreous. It can be used as a fresh in-situ cross-linked vitreous substitute for PVR. Furthermore, 5-FU loaded PLGA microspheres on the hydrogel may be an effective strategy to improve the prognosis of PVR [102]. Retinoic acid is a metabolite of vitamin A and plays an important role in the visual pathway. It exerts an anti-proliferation effect on RPE cells by preventing the overgrowth of confluent cell monolayers [104]. A small prospective randomized clinical trial shows that oral retinoic acid reduces significantly the incidence of retinal re-detachment and macular pucker formation by improving the visual acuity in grade C PVR patients post vitrectomy [165]. In another independent study, Giordano, G G et al. prepare the retinoic acid-loaded PLGA (50:50) microspheres (∼60 μm) and deliver them into in the eye of a PVR rabbit model. Two months after intravitreal injection, traction retinal detachment occur in 4 of 11 rabbits treated with 5 mg of PLGA microspheres containing 110 μg retinoic acid, while traction retinal detachment occurred in all 7 rabbits injected with blank microspheres [104]. FengxiangWang et al. prepared alginate (1.76 ± 0.05 μg/mg) microspheres (95.2 ± 8.6 μm) for loading retinoic acid. Injection of 15 mg of retinoic acid-loaded alginate microspheres containing 25 μg of retinoic acid into the vitreous body of rabbits revealed that the release rate of retinoic acid on days 1, 3, 7, 14, 21, 28, 36, and 42 was almost constant. Furthermore, as the residence time of retinoic acid-loaded alginate microspheres in the eye increases, the microspheres show changes from complete circles to partial defects, then to semicircles, to irregularities, and finally to flocculation. Importantly, no changes in the surrounding vitreous were observed throughout the process of microsphere disintegration [166].
4.10. Retinitis pigmentosa
RP is an inherited degenerative disease characterized by progressive loss of photoreceptors and visual field loss [167]. The global prevalence rate of RP is 1:4000 [168]. Clinical manifestations of RP typically include the pigmentary deposits resembling bone spicules, various degrees of retinal atrophy, retinal vessel narrowing, attenuation of the retinal vessels, and waxy pallor of the optic disc [167]. GDNF belongs to the transforming growth factor-b superfamily and its activity is mediated through the interaction with specific receptors and the tyrosine kinase receptor Ret [169]. In vivo and in vitro studies have demonstrated the potential of GDNF to rescue retinal photoreceptor and RGCs [170,171]. Andrieu-Soler et al. prepared PLGA (37.5: 25) microspheres (27 ± 10 μm) loaded with recombinant human glial cell line-derived neurotrophic factor (rhGDNF), and deliver it into the eye of animal model. Compared with untreated mice, the mice receiving rhGDNF-loaded microspheres exhibit enhanced photoreceptors survival and slower degeneration rate [100]. Tauroursodeoxycholic Acid (TUDCA), the most important component of bear bile, has shown potent anti-apoptotic effects in a rodent RP model [172]. Encapsulating TUDCA in microspheres has become an emerging strategy for intraocular delivery of TUDCA. A study shows that TUDCA-loaded PLGA (50:50) microspheres (22.89 ± 0.04 μm) can protect retinal function, slow down photoreceptor cell degeneration, and prevent the loss of synaptic contacts between photoreceptors and bipolar cells. The authors speculate that TUDCA-loaded PLGA microspheres are potent enough to prevents secondary neurodegenerative responses and avoided the remodeling of entire retinal circuits [99]. Proinsulin has shown its neuroprotective effect in a variety of RP models [173]. Researchers have designed a human recombinant proinsulin (hPI)-loaded PLGA microspheres (10–32 μm), and injected it into the vitreous body of rd10 mice. Compared with the 7 mg of blank microsphere treated controls, the amplitude of b-wave recorded in the 7 mg of hPI-loaded PLGA microspheres (3.2 μg hPI/mg) treated mice is significantly higher, the thickness of the outer nuclear layer (ONL) is thicker, and the cell density in the ONL layer is larger. Moreover, the authors speculate that proinsulin hPI- loaded PLGA microspheres exert these beneficial effects by activating the PI3–K/Akt pathway, in rd10 [98].
4.11. Uveitis
Uveitis is a group of choroidal diseases that lead to intraocular inflammation and severe visual impairment [174]. It can occur in any age group, but adults between the ages of 20 and 60 are most prone to affected. According to the main anatomical location of intraocular inflammation, uveitis is divided into four anatomical categories: anterior uveitis, intermediate uveitis, posterior uveitis, and panuveitis [175]. A study has shown that DEX-loaded PLGA (50:50) microspheres (53 μm) can effectively ameliorate the symptoms of panuveitis caused by lipopolysaccharide (LPS). One week after treatment with 10 mg of DEX-loaded PLGA microspheres containing 141 μg of DEX/mg microspheres, the intraocular inflammation caused by LPS injection is alleviated compared with the group receiving blank microspheres. In the same study, LPS is injected a second time to simulate secondary uveitis (30 days after microsphere injection). After the second injection of LPS, the animals treated with PLGA microsphere loaded with DEX do not exhibit any signs of inflammation [69]. CyS is a powerful immunosuppressant that is protective against several forms of chronic uveitis [101]. Previous studies have shown that CyS treatment has some limitations during topical, periocular, and systemic administration [101]. To this end, He Y et al. have prepared CyS-loaded PLGA (75:25, Mw: 15,000) microspheres for the treatment of uveitis. The results show that intravitreal injection of CyS-loaded PLGA microspheres significantly alleviate the inflammatory signs, reduces aqueous leukocyte counts, and inhibits the cellular infiltrate in the eyes of in rabbit uveitis model [101]. In addition, porous PLGA (Mw: 21000) microspheres (25.5 ± 0.5 μm) can be loaded with exosomes to treat primed mycobacterial uveitis (PMU). In mice and non-human primates with PMU, intravitreally injected microspheres loaded with exosomes from monkey regulatory T cells resulted in a substantial reduction in the levels of inflammatory cells. In addition, the researchers found that PLGA microspheres mainly precipitated and remained stable in the lower region of the vitreous cavity of mice, while the released exosomes tended to accumulate in the preretinal region [176].
4.12. Complications of cataract surgery
Endophthalmitis is a serious complication of cataract surgery. It results in severe symptoms such as blurry vision, eye pain, swollen eyelids, and redness of the conjunctiva. Owing to the prevalence of cataract surgeries, the incidence of endophthalmitis is increasing [177]. To prevent endophthalmitis, it's necessary to use antibiotics pre- and post-surgery. However, the antibiotics are readily cleared from the eye, and it is not feasible to administer via repeated injections. Therefore, it is crucial to develop antibiotic-releasing systems suitable for intraocular use. Rifampicin is a type of antibiotics, but it has several limitations like its short half-life (comprised between 1 and 5 h). Encouragingly, researchers have produced PLGA microspheres loaded with rifampicin, aiming to develop a solution for treating endophthalmitis. The rifampicin -loaded PLGA microspheres, with the diameter of 1.04 ± 0.07 μm, exhibit comparable antibacterial effects against Streptococcus epidermidis to free levofloxacin over 48 h. These microspheres also display highly effective anti-adhesive properties against artificial lenses. Due to their antibacterial and anti-adhesive potency, levofloxacin-loaded microspheres act as promising antibiotic molecules for preventing endophthalmitis [123]. Furthermore, a combination approach involving both steroids and antibiotics is also employed. In a clinical study, researchers have prepared ciprofloxacin-loaded PLGA (50:50) microspheres with an average particle size of 1.07 ± 0.35 μm and the drug/polymer ratio of 1:2, achieving an encapsulation efficiency of 99 %. A 0.3 mL of a combined solution containing 25 mg triamcinolone acetonide and 2.0 mg ciprofloxacin loaded PLGA microspheres was injected into the sub-Tenon space of patients after cataract surgery. Compared with these patients who receive the topical administration of prednisolone (1 %) and ciprofloxacin (3 %), the incidences of ocular inflammation symptoms, such as anterior chamber cell and flare, ciliary flush, and conjunctival erythema are not different among the two groups [124]. Furthermore, posterior capsule opacification (PCO) is another primary long-term complication of cataract surgery [178]. Methotrexate (MTX) is an antifolate antimetabolite with a promising prospect in treating PCO. Researchers have coated the acrylic intraocular lenses with MTX-loaded PLGA microspheres, and assess their proliferative inhibitory effects in vitro using anterior chamber models and human ex vivo capsular bags. They find that the MTX-encapsulated microspheres exhibit strong inhibitory effects on PCO without obvious toxicity to corneal cell lines. These findings suggest that MTX-encapsulated microspheres can potentially serve as a prospective method for modifying intraocular lenses [179].
4.13. Co-transplantation of microspheres with retinal progenitor cells
Retinal progenitor cells (RPCs) regeneration is a crucial strategy for treating terminal blindness. However, only a handful of grafted cells can integrate into the ONL and differentiate into new photoreceptors. The inhibitory ECM and cell adhesion molecule can prevent the integration of a sub-retinal graft into the host retina, and lead to the failure of retina regeneration. Notably, the matrix metalloproteinase 2 (MMP2) can facilitate the host-graft integration by degrading these molecules. Researchers have used the MMP2-loaded PLGA (50:50; Mw: 15,000 g/mol) microspheres (2–20 μm) to enhance RPCs regeneration and integration into the host retina. MMP2-loaded microspheres are co-transplanted with RPCs into the subretinal space of retinal degeneration mouse models. After the MMP2 are released from the microspheres, a notable degradation of CD44 and neural proteoglycans on the outer retinal surface are observed. During the whole process, no harming effects on the host retinal structure are detected. Moreover, the microspheres didn't affect cell differentiation characteristics. Therefore, co-transplantation of MMP2 microspheres with RPCs is a practical and safe strategy for promoting retinal regeneration [125].
5. Potential drawbacks and improvement direction
Intraocular delivery of microspheres can potentially trigger immune reactions, which manifest as foreign body responses or ocular inflammation. Giordano and colleagues have observed the foreign body reactions in rabbits lasting up to six months after injecting PLGA microsphere into the vitreous humor [180]. Similar foreign body reactions have been reported with the use of microspheres and nanospheres made from Poly(l-valine-l-proline-l-alanine-l-valine-l-glycine) for intraocular delivery. In another study conducted by Thackaberry and colleagues, they prepared the PLGA microsphere and PLGA rods and evaluated the foreign body responses in both rabbits and non-human primates. Results suggest that the PLGA microsphere triggers innate immune responses, leading to increased expression of IL-8, MCP-1, MIP-1a, and TNF-α in the vitreous body. In contrast, PLGA rods of similar mass do not induce noticeable immune reactions. Therefore, researchers speculate that the size, shape, and surface area of PLGA microsphere may act as critical attributes that determine their toxicity [181]. These findings provide new insights for optimizing microsphere design to reduce their risk in the future. Notably, a recent clinical study shows that microsphere drug systems can rapidly disperse throughout the vitreous humor, leading to vitreous haze and blurred vision for at least 2–3 weeks post-injection [182]. To address this problem, Duvvuri and colleagues developed a thermogel carrier containing GCV microspheres (with a 23 % W/W PLGA-PEG-PLGA aqueous solution). The thermogelling PLGA-PEG-PLGA solution can immobilize the microspheres inside, thereby preventing them from migrating within the vitreous during administration [107]. In this context, the gel preparation presents a novel solution for alleviating the vitreous haze and blurred vision associated with microsphere injections. Additionally, acidic oligomers and monomers in PLGA may lower the pH of the solution, potentially affecting the stability of large-molecule drugs [183]. Therefore, it is essential to minimize these components in the final formulation to meet quality requirements, as they may induce toxic effects on intraocular tissue. Efforts should be made to improve the efficiency and safety of microspheres in several perspectives: Firstly, extending the release period can be achieved according to the drug release mechanism. Currently, microspheres have been used as a laser-controlled drug carrier. A specific wavelength laser is applied to the carrier when the intraocular drug concentration falls below a certain threshold, and then an appropriate amount of loaded drug is released in response to laser irradiation. Once the desired drug concentration is achieved, the laser irradiation stops immediately, enabling a controlled and stepwise drug release [184]. Secondly, dispersing microspheres within gels can prevent the spread of microspheres and prolong the administration time of microspheres. After being injected into the vitreous humor, the microspheres within the gel exhibit a significantly longer release curve compared to regular microspheres. Moreover, injectable gel microspheres cause less trauma to the eye compared to surgically implanted gels, offering marvelous advantages in improving patient compliance. At present, a variety of gel materials have shown good biocompatibility in other parts of the human body and hold promise as novel materials for ocular drug delivery. Thirdly, advancing toward biologic therapies is a promising direction. This includes implanting the genetically modified cells into the retina to secret therapeutic proteins or replacing the damaged retinal cells with healthy photosensitive cells through stem cell technology. Encapsulating them in microspheres can help overcome immune challenges during cell transplantation [125]. These methodology improvements may hold promise for developing an effective and long-lasting therapeutic molecule for posterior ophthalmic diseases.
6. Summary and prospect
With the increasing incidence of posterior ophthalmic diseases in the aged population, it is emergent to develop drug delivery systems with potent release efficiency and satisfactory safety. Accumulating evidences suggest that biodegradable microspheres hold the potential to deliver pharmacologic agents to the posterior ophthalmic diseases, and are expected to be used in ophthalmic practice. Currently, multiple hydrogel materials have shown good prospects for eye applications, such as gelma, hama, chitosan, etc. In the future, hydrogel microspheres are expected to be studied and improved for better clinical applications. Since most diseases affecting the posterior part of the eye are chronic and multifactorial, microspheres are particularly promising in this field. They can load different active substances and supplement additives with diverse pharmacological properties. Individualized treatment can be easily achieved by changing the type and dosage of microspheres. Notably, clinical trials suggest that the microspheres prepared from the PLGA biomaterials have decent tolerance after periocular and intravitreal injection in human patients. Moreover, biodegradable microspheres also act as a potential tool for retinal repair and regenerative cell medicine. Therefore, it is feasible to assume that diverse microparticle preparations suitable for ophthalmic use will be available for clinical practice in the future.
CRediT authorship contribution statement
Rongyue Xue: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. Hao Wu: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. Siyu Li: Methodology, Formal analysis. Ning Pu: Formal analysis. Dong Wei: Formal analysis. Na Zhao: Formal analysis. Yongheng Cui: Formal analysis. Haoyan Li: Formal analysis. Zongming Song: Writing – review & editing, Supervision, Methodology. Ye Tao: Writing – review & editing, Writing – original draft, Supervision, Conceptualization.
Declaration of competing interest
The authors declare no interest conflicts. They have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was funded by the Science and Technology Major Project of Henan Province (grant number 221100310200), and the Zhongyuan Science and Technology Leading Talent Project (grant number 224200510013). All diagrams have been created with BioRender.com.
Contributor Information
Zongming Song, Email: smeyes@zzu.edu.cn.
Ye Tao, Email: panghtoyezz@zzu.edu.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Kang-Mieler J.J., Osswald C.R., Mieler W.F. Advances in ocular drug delivery: emphasis on the posterior segment. Expet Opin. Drug Deliv. 2014;11(10):1647–1660. doi: 10.1517/17425247.2014.935338. [DOI] [PubMed] [Google Scholar]
- 2.Pflugfelder S.C., Stern M.E. Biological functions of tear film. Exp. Eye Res. 2020;197 doi: 10.1016/j.exer.2020.108115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imperiale J.C., Acosta G.B., Sosnik A. Polymer-based carriers for ophthalmic drug delivery. J. Contr. Release. 2018;285:106–141. doi: 10.1016/j.jconrel.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 4.Wels M., Roels D., Raemdonck K., De Smedt S.C., Sauvage F. Challenges and strategies for the delivery of biologics to the cornea. J. Contr. Release. 2021;333:560–578. doi: 10.1016/j.jconrel.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Bachu R.D., Chowdhury P., Al-Saedi Z., Karla P.K., Boddu S. Ocular drug delivery barriers-role of nanocarriers in the treatment of anterior segment ocular diseases. Pharmaceutics. 2018;10(1) doi: 10.3390/pharmaceutics10010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J.J., Kim K.J., Lee V.H. Role of P-glycoprotein in restricting propranolol transport in cultured rabbit conjunctival epithelial cell layers. Pharm. Res. (N. Y.) 2000;17(5):533–538. doi: 10.1023/a:1007508714259. [DOI] [PubMed] [Google Scholar]
- 7.Wang L., Zhou M.B., Zhang H. The emerging role of topical ocular drugs to target the posterior eye, OPHTHALMOL. THER. 2021;10(3):465–494. doi: 10.1007/s40123-021-00365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S.H., Lutz R.J., Wang N.S., Robinson M.R. Transport barriers in transscleral drug delivery for retinal diseases. Ophthalmic Res. 2007;39(5):244–254. doi: 10.1159/000108117. [DOI] [PubMed] [Google Scholar]
- 9.Abarca E.M., Salmon J.H., Gilger B.C. Effect of choroidal perfusion on ocular tissue distribution after intravitreal or suprachoroidal injection in an arterially perfused ex vivo pig eye model. J. Ocul. Pharmacol. Therapeut. 2013;29(8):715–722. doi: 10.1089/jop.2013.0063. [DOI] [PubMed] [Google Scholar]
- 10.Heikkinen E.M., Auriola S., Ranta V.P., Demarais N.J., Grey A.C., Del A.E., Toropainen E., Vellonen K.S., Urtti A., Ruponen M. Distribution of small molecular weight drugs into the porcine lens: studies on imaging mass spectrometry, partition coefficients, and implications in ocular pharmacokinetics. Mol. Pharm. 2019;16(9):3968–3976. doi: 10.1021/acs.molpharmaceut.9b00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sigurdsson H.H., Konraethsdottir F., Loftsson T., Stefansson E. Topical and systemic absorption in delivery of dexamethasone to the anterior and posterior segments of the eye. Acta Ophthalmol. Scand. 2007;85(6):598–602. doi: 10.1111/j.1600-0420.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- 12.Kim H., Robinson S.B., Csaky K.G. Investigating the movement of intravitreal human serum albumin nanoparticles in the vitreous and retina. Pharm. Res. (N. Y.) 2009;26(2):329–337. doi: 10.1007/s11095-008-9745-6. [DOI] [PubMed] [Google Scholar]
- 13.Dubald M., Bourgeois S., Andrieu V., Fessi H. Ophthalmic drug delivery systems for antibiotherapy-A review. Pharmaceutics. 2018;10(1) doi: 10.3390/pharmaceutics10010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh M., Bharadwaj S., Lee K.E., Kang S.G. Therapeutic nanoemulsions in ophthalmic drug administration: concept in formulations and characterization techniques for ocular drug delivery. J. Contr. Release. 2020;328:895–916. doi: 10.1016/j.jconrel.2020.10.025. [DOI] [PubMed] [Google Scholar]
- 15.Diaz-Coranguez M., Ramos C., Antonetti D.A. The inner blood-retinal barrier: cellular basis and development. Vis. Res. 2017;139:123–137. doi: 10.1016/j.visres.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popov A. Mucus-penetrating particles and the role of ocular mucus as a barrier to micro- and nanosuspensions. J. Ocul. Pharmacol. Therapeut. 2020;36(6):366–375. doi: 10.1089/jop.2020.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vadlapatla R.K., Vadlapudi A.D., Pal D., Mitra A.K. Role of membrane transporters and metabolizing enzymes in ocular drug delivery. Curr. Drug Metabol. 2014;15(7):680–693. doi: 10.2174/1389200215666140926152459. [DOI] [PubMed] [Google Scholar]
- 18.Constable P.A., Lawrenson J.G., Dolman D.E., Arden G.B., Abbott N.J. P-Glycoprotein expression in human retinal pigment epithelium cell lines. Exp. Eye Res. 2006;83(1):24–30. doi: 10.1016/j.exer.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy B.G., Mangini N.J. P-glycoprotein expression in human retinal pigment epithelium. Mol. Vis. 2002;8:422–430. [PubMed] [Google Scholar]
- 20.Xu J., Liu Y., Yang Y., Bates S., Zhang J.T. Characterization of oligomeric human half-ABC transporter ATP-binding cassette G2. J. Biol. Chem. 2004;279(19):19781–19789. doi: 10.1074/jbc.M310785200. [DOI] [PubMed] [Google Scholar]
- 21.Rimpela A.K., Reinisalo M., Hellinen L., Grazhdankin E., Kidron H., Urtti A., Del A.E. Implications of melanin binding in ocular drug delivery. Adv. Drug Deliv. Rev. 2018;126:23–43. doi: 10.1016/j.addr.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Raghavan P.R., Zane P.A., Tripp S.L. Calculation of drug-melanin binding energy using molecular modeling. Experientia. 1990;46(1):77–80. doi: 10.1007/BF01955422. [DOI] [PubMed] [Google Scholar]
- 23.Patel C., Pande S., Sagathia V., Ranch K., Beladiya J., Boddu S., Jacob S., Al-Tabakha M.M., Hassan N., Shahwan M. Nanocarriers for the delivery of neuroprotective agents in the treatment of ocular neurodegenerative diseases. Pharmaceutics. 2023;15(3) doi: 10.3390/pharmaceutics15030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manikandan P., Nagini S. Cytochrome P450 structure, function and clinical significance: a review. Curr. Drug Targets. 2018;19(1):38–54. doi: 10.2174/1389450118666170125144557. [DOI] [PubMed] [Google Scholar]
- 25.Fujiwara R., Yoda E., Tukey R.H. Species differences in drug glucuronidation: humanized UDP-glucuronosyltransferase 1 mice and their application for predicting drug glucuronidation and drug-induced toxicity in humans. Drug Metabol. Pharmacokinet. 2018;33(1):9–16. doi: 10.1016/j.dmpk.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang P., Jacobs K.M., Ohr M.P., Swindle-Reilly K.E. Chitosan–polycaprolactone core–shell microparticles for sustained delivery of bevacizumab. Mol. Pharm. 2020;17(7):2570–2584. doi: 10.1021/acs.molpharmaceut.0c00260. [DOI] [PubMed] [Google Scholar]
- 27.Iyer S., Radwan A.E., Hafezi-Moghadam A., Malyala P., Amiji M. Long-acting intraocular Delivery strategies for biological therapy of age-related macular degeneration. J. Contr. Release. 2019;296:140–149. doi: 10.1016/j.jconrel.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Varela-Fernandez R., Diaz-Tome V., Luaces-Rodriguez A., Conde-Penedo A., Garcia-Otero X., Luzardo-Alvarez A., Fernandez-Ferreiro A., Otero-Espinar F.J. Drug delivery to the posterior segment of the eye: biopharmaceutic and pharmacokinetic considerations. Pharmaceutics. 2020;12(3) doi: 10.3390/pharmaceutics12030269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allyn M.M., Luo R.H., Hellwarth E.B., Swindle-Reilly K.E. Considerations for polymers used in ocular drug delivery. Front. Med. 2021;8 doi: 10.3389/fmed.2021.787644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandra N.S., Gorantla S., Priya S., Singhvi G. Insight on updates in polysaccharides for ocular drug delivery. Carbohydr. Polym. 2022;297 doi: 10.1016/j.carbpol.2022.120014. [DOI] [PubMed] [Google Scholar]
- 31.Robla S., Prasanna M., Varela-Calvino R., Grandjean C., Csaba N. A chitosan-based nanosystem as pneumococcal vaccine delivery platform. Drug Deliv. Transl. Res. 2021;11(2):581–597. doi: 10.1007/s13346-021-00928-3. [DOI] [PubMed] [Google Scholar]
- 32.Alonso M.J., Sanchez A. The potential of chitosan in ocular drug delivery. J. Pharm. Pharmacol. 2003;55(11):1451–1463. doi: 10.1211/0022357022476. [DOI] [PubMed] [Google Scholar]
- 33.Dalpiaz A., Fogagnolo M., Ferraro L., Capuzzo A., Pavan B., Rassu G., Salis A., Giunchedi P., Gavini E. Nasal chitosan microparticles target a zidovudine prodrug to brain HIV sanctuaries. Antiviral Res. 2015;123:146–157. doi: 10.1016/j.antiviral.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Wang W., Meng Q., Li Q., Liu J., Zhou M., Jin Z., Zhao K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020;21(2) doi: 10.3390/ijms21020487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prow T.W., Bhutto I., Kim S.Y., Grebe R., Merges C., McLeod D.S., Uno K., Mennon M., Rodriguez L., Leong K., Lutty G.A. Ocular nanoparticle toxicity and transfection of the retina and retinal pigment epithelium. Nanomedicine. 2008;4(4):340–349. doi: 10.1016/j.nano.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giunchedi P., Conte U., Chetoni P., Saettone M.F. Pectin microspheres as ophthalmic carriers for piroxicam: evaluation in vitro and in vivo in albino rabbits. Eur. J. Pharmaceut. Sci. 1999;9(1):1–7. doi: 10.1016/s0928-0987(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 37.Milano F., Masi A., Madaghiele M., Sannino A., Salvatore L., Gallo N. Current trends in gelatin-based drug delivery systems. Pharmaceutics. 2023;15(5) doi: 10.3390/pharmaceutics15051499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong K., Lind J., Sheybani A. Safety and efficacy outcomes of the Xen45 Gel Stent use for refractory glaucoma: a surgery series from surgeon trainees at a tertiary teaching hospital. Eye Vis. 2020;7:5. doi: 10.1186/s40662-019-0171-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang F.X., He S.Z., Li X.J., Liang Q.L., Chen B. [The dynamic study of retinoic acid and its alginate sodium microspheres in rabbit eye] Zhonghua Yan Ke Za Zhi. 2006;42(9):814–817. [PubMed] [Google Scholar]
- 40.Cardillo J.A., Souza-Filho A.A., Oliveira A.G. Intravitreal Bioerudivel sustained-release triamcinolone microspheres system (RETAAC). Preliminary report of its potential usefulnes for the treatment of diabetic macular edema. Arch. Soc. Esp. Oftalmol. 2006;81(12):679–681. 675-7. [PubMed] [Google Scholar]
- 41.Paganelli F., Cardillo J.A., Melo L.J., Lucena D.R., Silva A.J., Oliveira A.G., Hofling-Lima A.L., Nguyen Q.D., Kuppermann B.D., Belfort R.J. A single intraoperative sub-tenon's capsule injection of triamcinolone and ciprofloxacin in a controlled-release system for cataract surgery. Invest. Ophthalmol. Vis. Sci. 2009;50(7):3041–3047. doi: 10.1167/iovs.08-2920. [DOI] [PubMed] [Google Scholar]
- 42.Pitt C.G., Chasalow F.I., Hibionada Y.M., Klimas D.M., Schindler A. Aliphatic polyesters. I. The degradation of poly(ϵ‐caprolactone)in vivo. J. Appl. Polym. Sci. 1981;26(11):3779–3787. doi: 10.1002/app.1981.070261124. [DOI] [Google Scholar]
- 43.Lin H., Yue Y., Maidana D.E., Bouzika P., Atik A., Matsumoto H., Miller J.W., Vavvas D.G. Drug delivery nanoparticles: toxicity comparison in retinal pigment epithelium and retinal vascular endothelial cells. Semin. Ophthalmol. 2016;31(1–2):1–9. doi: 10.3109/08820538.2015.1114865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andres-Guerrero V., Zong M., Ramsay E., Rojas B., Sarkhel S., Gallego B., de Hoz R., Ramirez A.I., Salazar J.J., Trivino A., Ramirez J.M., Del A.E., Cameron N., De-Las-Heras B., Urtti A., Mihov G., Dias A., Herrero-Vanrell R. Novel biodegradable polyesteramide microspheres for controlled drug delivery in Ophthalmology. J. Contr. Release. 2015;211:105–117. doi: 10.1016/j.jconrel.2015.05.279. [DOI] [PubMed] [Google Scholar]
- 45.Li F., Hurley B., Liu Y., Leonard B., Griffith M. Controlled release of bevacizumab through nanospheres for extended treatment of age-related macular degeneration. Open Ophthalmol. J. 2012;6:54–58. doi: 10.2174/1874364101206010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang C., Yu C., Liu J., Teng L., Sun F., Li Y. Preparation and in vivo evaluation of PCADK/PLGA microspheres for improving stability and efficacy of rhGH. Int. J. Pharm. 2015;495(2):924–931. doi: 10.1016/j.ijpharm.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Wang C., Yu C., Yu K., Teng L., Liu J., Wang X., Sun F., Li Y. Improving protein stability and controlling protein release by adding poly (Cyclohexane-1, 4-diyl acetone dimethylene ketal) to PLGA microspheres. Curr. Drug Deliv. 2015;12(6):726–735. doi: 10.2174/1567201812666150316112635. [DOI] [PubMed] [Google Scholar]
- 48.Lee P.W., Pokorski J.K. Poly(lactic-co-glycolic acid) devices: production and applications for sustained protein delivery. Wiley Interdiscip. Rev.-Nanomed. Nanobiotechnol. 2018;10(5):e1516. doi: 10.1002/wnan.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi N.Q., Zhou J., Walker J., Li L., Hong J., Olsen K.F., Tang J., Ackermann R., Wang Y., Qin B., Schwendeman A., Schwendeman S.P. Microencapsulation of luteinizing hormone-releasing hormone agonist in poly (lactic-co-glycolic acid) microspheres by spray-drying. J. Contr. Release. 2020;321:756–772. doi: 10.1016/j.jconrel.2020.01.023. [DOI] [PubMed] [Google Scholar]
- 50.Motlekar N. Optimization of experimental parameters for the production of LMWH-loaded polymeric microspheres. Drug Des. Dev. Ther. 2009;2:39–47. [PMC free article] [PubMed] [Google Scholar]
- 51.Park J., Ye M., Park K. Biodegradable polymers for microencapsulation of drugs. Molecules. 2005;10(1):146–161. doi: 10.3390/10010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rezvantalab S., Keshavarz Moraveji M. Microfluidic assisted synthesis of PLGA drug delivery systems. RSC Adv. 2019;9(4):2055–2072. doi: 10.1039/C8RA08972H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akamatsu K., Ikeuchi Y., Nakao A., Nakao S. Size-controlled and monodisperse enzyme-encapsulated chitosan microspheres developed by the SPG membrane emulsification technique. J. Colloid Interface Sci. 2012;371(1):46–51. doi: 10.1016/j.jcis.2011.12.078. [DOI] [PubMed] [Google Scholar]
- 54.Li J., Feng T., Han L., Zhang M., Jiang T. Fabricating biopolymer-inorganic hybrid microspheres for enzyme immobilization: connect membrane emulsification with biomimetic mineralization. Particuology. 2022;64:171–177. doi: 10.1016/j.partic.2021.07.006. [DOI] [Google Scholar]
- 55.Zhang Y., Rochefort D. Characterisation and applications of microcapsules obtained by interfacial polycondensation. J. Microencapsul. 2012;29(7):636–649. doi: 10.3109/02652048.2012.676092. [DOI] [PubMed] [Google Scholar]
- 56.Butreddy A., Gaddam R.P., Kommineni N., Dudhipala N., Voshavar C. PLGA/PLA-Based long-acting injectable depot microspheres in clinical use: production and characterization overview for protein/peptide delivery. Int. J. Mol. Sci. 2021;22(16) doi: 10.3390/ijms22168884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dwivedi P., Han S., Mangrio F., Fan R., Dwivedi M., Zhu Z., Huang F., Wu Q., Khatik R., Cohn D.E., Si T., Hu S., Sparreboom A., Xu R.X. Engineered multifunctional biodegradable hybrid microparticles for paclitaxel delivery in cancer therapy. Mater. Sci. Eng., C. 2019;102:113–123. doi: 10.1016/j.msec.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 58.Park H., Kim J.S., Kim S., Ha E.S., Kim M.S., Hwang S.J. Pharmaceutical applications of supercritical fluid extraction of emulsions for micro-/nanoparticle formation. Pharmaceutics. 2021;13(11) doi: 10.3390/pharmaceutics13111928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scheiner K.C., Maas-Bakker R.F., van Steenbergen M.J., Schwendeman S.P., Hennink W.E., Kok R.J. Post-loading of proangiogenic growth factors in PLGA microspheres. Eur. J. Pharm. Biopharm. 2021;158:1–10. doi: 10.1016/j.ejpb.2020.10.022. [DOI] [PubMed] [Google Scholar]
- 60.Herrero-Vanrell R., Bravo-Osuna I., Andres-Guerrero V., Vicario-de-la-Torre M., Molina-Martinez I.T. The potential of using biodegradable microspheres in retinal diseases and other intraocular pathologies. Prog. Retin. Eye Res. 2014;42:27–43. doi: 10.1016/j.preteyeres.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 61.He Y., Liu Y., Liu Y., Wang J., Zhang X., Lu W., Ma Z., Zhu X., Zhang Q. Cyclosporine-loaded microspheres for treatment of uveitis: in vitro characterization and in vivo pharmacokinetic study. Invest. Ophthalmol. Vis. Sci. 2006;47(9):3983–3988. doi: 10.1167/iovs.05-1373. [DOI] [PubMed] [Google Scholar]
- 62.Martinez-Sancho C., Herrero-Vanrell R., Negro S. Optimisation of aciclovir poly(D,L-lactide-co-glycolide) microspheres for intravitreal administration using a factorial design study. Int. J. Pharm. 2004;273(1–2):45–56. doi: 10.1016/j.ijpharm.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 63.Arranz-Romera A., Esteban-Perez S., Molina-Martinez I.T., Bravo-Osuna I., Herrero-Vanrell R. Co-delivery of glial cell-derived neurotrophic factor (GDNF) and tauroursodeoxycholic acid (TUDCA) from PLGA microspheres: potential combination therapy for retinal diseases. Drug Deliv. Transl. Res. 2021;11(2):566–580. doi: 10.1007/s13346-021-00930-9. [DOI] [PubMed] [Google Scholar]
- 64.Emilia Barcia C.H.R.H. Biodegradable additives modulate ganciclovir release rate from PLGA microspheres destined to intraocular administration. Lett. Drug Des. Discov. 2005;2(2) [Google Scholar]
- 65.Martinez-Sancho C., Herrero-Vanrell R., Negro S. Vitamin A palmitate and aciclovir biodegradable microspheres for intraocular sustained release. Int. J. Pharm. 2006;326(1–2):100–106. doi: 10.1016/j.ijpharm.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 66.Checa-Casalengua P., Jiang C., Bravo-Osuna I., Tucker B.A., Molina-Martinez I.T., Young M.J., Herrero-Vanrell R. Retinal ganglion cells survival in a glaucoma model by GDNF/Vit E PLGA microspheres prepared according to a novel microencapsulation procedure. J. Contr. Release. 2011;156(1):92–100. doi: 10.1016/j.jconrel.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 67.Barbosa-Alfaro D., Andres-Guerrero V., Fernandez-Bueno I., Garcia-Gutierrez M.T., Gil-Alegre E., Molina-Martinez I.T., Pastor-Jimeno J.C., Herrero-Vanrell R., Bravo-Osuna I. Dexamethasone PLGA microspheres for sub-tenon administration: influence of sterilization and tolerance studies. Pharmaceutics. 2021;13(2) doi: 10.3390/pharmaceutics13020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andres-Guerrero V., Zong M., Ramsay E., Rojas B., Sarkhel S., Gallego B., de Hoz R., Ramirez A.I., Salazar J.J., Trivino A., Ramirez J.M., Del A.E., Cameron N., De-Las-Heras B., Urtti A., Mihov G., Dias A., Herrero-Vanrell R. Novel biodegradable polyesteramide microspheres for controlled drug delivery in Ophthalmology. J. Contr. Release. 2015;211:105–117. doi: 10.1016/j.jconrel.2015.05.279. [DOI] [PubMed] [Google Scholar]
- 69.Barcia E., Herrero-Vanrell R., Diez A., Alvarez-Santiago C., Lopez I., Calonge M. Downregulation of endotoxin-induced uveitis by intravitreal injection of polylactic-glycolic acid (PLGA) microspheres loaded with dexamethasone. Exp. Eye Res. 2009;89(2):238–245. doi: 10.1016/j.exer.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 70.Han C.S., Kang J.H., Kim Y.J., Kim D.W., Park C.W. Inhalable nano-dimpled microspheres containing budesonide-PLGA for improved aerodynamic performance. Int. J. Nanomed. 2022;17:3405–3419. doi: 10.2147/IJN.S372582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herrero-Vanrell R., Ramirez L., Fernandez-Carballido A., Refojo M.F. Biodegradable PLGA microspheres loaded with ganciclovir for intraocular administration. Encapsulation technique, in vitro release profiles, and sterilization process. Pharm. Res. (N. Y.) 2000;17(10):1323–1328. doi: 10.1023/a:1026464124412. [DOI] [PubMed] [Google Scholar]
- 72.Yu M., Yao Q., Zhang Y., Chen H., He H., Zhang Y., Yin T., Tang X., Xu H. Core/shell PLGA microspheres with controllable in vivo release profile via rational core phase design. Artif. Cell Nanomed. Biotechnol. 2018;46(sup1):1070–1079. doi: 10.1080/21691401.2018.1443940. [DOI] [PubMed] [Google Scholar]
- 73.O'Donnell P.B., McGinity J.W. Influence of processing on the stability and release properties of biodegradable microspheres containing thioridazine hydrochloride. Eur. J. Pharm. Biopharm. 1998;45(1):83–94. doi: 10.1016/S0939-6411(97)00126-4. [DOI] [PubMed] [Google Scholar]
- 74.Blatsios G., Tzimas A.S., Mattheolabakis G., Panagi Z., Avgoustakis K., Gartaganis S.P. Development of biodegradable controlled release scleral systems of triamcinolone acetonide. Curr. Eye Res. 2010;35(10):916–924. doi: 10.3109/02713683.2010.497599. [DOI] [PubMed] [Google Scholar]
- 75.Anal A.K., Stevens W.F., Remunan-Lopez C. Ionotropic cross-linked chitosan microspheres for controlled release of ampicillin. Int. J. Pharm. 2006;312(1–2):166–173. doi: 10.1016/j.ijpharm.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 76.Lim Y.W., Tan W.S., Ho K.L., Mariatulqabtiah A.R., Abu K.N., Abd R.N., Wong T.W., Chee C.F. Challenges and complications of poly(lactic-co-glycolic acid)-based long-acting drug product development. Pharmaceutics. 2022;14(3) doi: 10.3390/pharmaceutics14030614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ma G. Microencapsulation of protein drugs for drug delivery: strategy, preparation, and applications. J. Contr. Release. 2014;193:324–340. doi: 10.1016/j.jconrel.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 78.Vladisavljevic G.T. Structured microparticles with tailored properties produced by membrane emulsification. Adv. Colloid Interface Sci. 2015;225:53–87. doi: 10.1016/j.cis.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 79.Su Y., Zhang B., Sun R., Liu W., Zhu Q., Zhang X., Wang R., Chen C. PLGA-based biodegradable microspheres in drug delivery: recent advances in research and application. Drug Deliv. 2021;28(1):1397–1418. doi: 10.1080/10717544.2021.1938756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mandal T.K., Bostanian L.A., Graves R.A., Chapman S.R., Idodo T.U. Porous biodegradable microparticles for delivery of pentamidine. Eur. J. Pharm. Biopharm. 2001;52(1):91–96. doi: 10.1016/s0939-6411(01)00150-3. [DOI] [PubMed] [Google Scholar]
- 81.Kamali H., Atamanesh M., Kaffash E., Mohammadpour F., Khodaverdi E., Hadizadeh F. Elimination of residual solvent from PLGA microspheres containing risperidone using supercritical carbon dioxide. J. Drug Deliv. Sci. Technol. 2020;57 doi: 10.1016/j.jddst.2020.101702. [DOI] [Google Scholar]
- 82.Nan K., Ma F., Hou H., Freeman W.R., Sailor M.J., Cheng L. Porous silicon oxide-PLGA composite microspheres for sustained ocular delivery of daunorubicin. Acta Biomater. 2014;10(8):3505–3512. doi: 10.1016/j.actbio.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miyajima M., Koshika A., Okada J., Kusai A., Ikeda M. Factors influencing the diffusion-controlled release of papaverine from poly (L-lactic acid) matrix. J. Contr. Release. 1998;56(1–3):85–94. doi: 10.1016/s0168-3659(98)00076-5. [DOI] [PubMed] [Google Scholar]
- 84.Miyajima M., Koshika A., Okada J., Ikeda M. Effect of polymer/basic drug interactions on the two-stage diffusion-controlled release from a poly(L-lactic acid) matrix. J. Contr. Release. 1999;61(3):295–304. doi: 10.1016/s0168-3659(99)00149-2. [DOI] [PubMed] [Google Scholar]
- 85.Ivlev A.V., Morfill G.E., Konopka U. Coagulation of charged microparticles in neutral gas and charge-induced gel transitions. Phys. Rev. Lett. 2002;89(19) doi: 10.1103/PhysRevLett.89.195502. [DOI] [PubMed] [Google Scholar]
- 86.Zhou J., Chen Y., Luo M., Deng F., Lin S., Wu W., Li G., Nan K. Dual cross-linked chitosan microspheres formulated with spray-drying technique for the sustained release of levofloxacin. Drug Dev. Ind. Pharm. 2019;45(4):568–576. doi: 10.1080/03639045.2019.1569025. [DOI] [PubMed] [Google Scholar]
- 87.Rafat M., Cléroux C.A., Fong W.G., Baker A.N., Leonard B.C., O'Connor M.D., Tsilfidis C. PEG–PLA microparticles for encapsulation and delivery of Tat-EGFP to retinal cells. Biomaterials. 2010;31(12):3414–3421. doi: 10.1016/j.biomaterials.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 88.Moritera T., Ogura Y., Honda Y., Wada R., Hyon S.H., Ikada Y. Microspheres of biodegradable polymers as a drug-delivery system in the vitreous. Invest. Ophthalmol. Vis. Sci. 1991;32(6):1785–1790. [PubMed] [Google Scholar]
- 89.Zhang Z., Wang X., Li B., Hou Y., Yang J., Yi L. Development of a novel morphological paclitaxel-loaded PLGA microspheres for effective cancer therapy: in vitro and in vivo evaluations. Drug Deliv. 2018;25(1):166–177. doi: 10.1080/10717544.2017.1422296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ehtezazi T., Washington C., Melia C.D. First order release rate from porous PLA microspheres with limited exit holes on the exterior surface. J. Contr. Release. 2000;66(1):27–38. doi: 10.1016/s0168-3659(99)00255-2. [DOI] [PubMed] [Google Scholar]
- 91.Gu B., Sun X., Papadimitrakopoulos F., Burgess D.J. Seeing is believing, PLGA microsphere degradation revealed in PLGA microsphere/PVA hydrogel composites. J. Contr. Release. 2016;228:170–178. doi: 10.1016/j.jconrel.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zolnik B.S., Leary P.E., Burgess D.J. Elevated temperature accelerated release testing of PLGA microspheres. J. Contr. Release. 2006;112(3):293–300. doi: 10.1016/j.jconrel.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 93.Zolnik B.S., Burgess D.J. Effect of acidic pH on PLGA microsphere degradation and release. J. Contr. Release. 2007;122(3):338–344. doi: 10.1016/j.jconrel.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 94.Hu L., Zhang H., Song W. An overview of preparation and evaluation sustained-release injectable microspheres. J. Microencapsul. 2012;30(4):369–382. doi: 10.3109/02652048.2012.742158. [DOI] [PubMed] [Google Scholar]
- 95.Zhao M., Rodriguez-Villagra E., Kowalczuk L., Le Normand M., Berdugo M., Levy-Boukris R., El Z.I., Kaufmann B., Gurny R., Bravo-Osuna I., Molina-Martinez I.T., Herrero-Vanrell R., Behar-Cohen F. Tolerance of high and low amounts of PLGA microspheres loaded with mineralocorticoid receptor antagonist in retinal target site. J. Contr. Release. 2017;266:187–197. doi: 10.1016/j.jconrel.2017.09.029. [DOI] [PubMed] [Google Scholar]
- 96.Amrite A.C., Ayalasomayajula S.P., Cheruvu N.P., Kompella U.B. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Invest. Ophthalmol. Vis. Sci. 2006;47(3):1149–1160. doi: 10.1167/iovs.05-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kompella U.B., Bandi N., Ayalasomayajula S.P. Subconjunctival nano- and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expression. Invest. Ophthalmol. Vis. Sci. 2003;44(3):1192–1201. doi: 10.1167/iovs.02-0791. [DOI] [PubMed] [Google Scholar]
- 98.Isiegas C., Marinich-Madzarevich J.A., Marchena M., Ruiz J.M., Cano M.J., de la Villa P., Hernandez-Sanchez C., de la Rosa E.J., de Pablo F. Intravitreal injection of proinsulin-loaded microspheres delays photoreceptor cell death and vision loss in the rd10 mouse model of retinitis pigmentosa. Invest. Ophthalmol. Vis. Sci. 2016;57(8):3610–3618. doi: 10.1167/iovs.16-19300. [DOI] [PubMed] [Google Scholar]
- 99.Fernandez-Sanchez L., Bravo-Osuna I., Lax P., Arranz-Romera A., Maneu V., Esteban-Perez S., Pinilla I., Puebla-Gonzalez M., Herrero-Vanrell R., Cuenca N. Controlled delivery of tauroursodeoxycholic acid from biodegradable microspheres slows retinal degeneration and vision loss in P23H rats. PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0177998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Andrieu-Soler C., Aubert-Pouessel A., Doat M., Picaud S., Halhal M., Simonutti M., Venier-Julienne M.C., Benoit J.P., Behar-Cohen F. Intravitreous injection of PLGA microspheres encapsulating GDNF promotes the survival of photoreceptors in the rd1/rd1 mouse. Mol. Vis. 2005;11:1002–1011. [PubMed] [Google Scholar]
- 101.He Y., Wang J.C., Liu Y.L., Ma Z.Z., Zhu X.A., Zhang Q. Therapeutic and toxicological evaluations of cyclosporine a microspheres as a treatment vehicle for uveitis in rabbits. J. Ocul. Pharmacol. Therapeut. 2006;22(2):121–131. doi: 10.1089/jop.2006.22.121. [DOI] [PubMed] [Google Scholar]
- 102.Yu Z., Ma S., Wu M., Cui H., Wu R., Chen S., Xu C., Lu X., Feng S. Self-assembling hydrogel loaded with 5-FU PLGA microspheres as a novel vitreous substitute for proliferative vitreoretinopathy. J. Biomed. Mater. Res., Part A. 2020;108(12):2435–2446. doi: 10.1002/jbm.a.36995. [DOI] [PubMed] [Google Scholar]
- 103.Moritera T., Ogura Y., Yoshimura N., Honda Y., Wada R., Hyon S.H., Ikada Y. Biodegradable microspheres containing adriamycin in the treatment of proliferative vitreoretinopathy. Invest. Ophthalmol. Vis. Sci. 1992;33(11):3125–3130. [PubMed] [Google Scholar]
- 104.Giordano G.G., Refojo M.F., Arroyo M.H. Sustained delivery of retinoic acid from microspheres of biodegradable polymer in PVR. Invest. Ophthalmol. Vis. Sci. 1993;34(9):2743–2751. [PubMed] [Google Scholar]
- 105.Conti B., Bucolo C., Giannavola C., Puglisi G., Giunchedi P., Conte U. Biodegradable microspheres for the intravitreal administration of acyclovir: in vitro/in vivo evaluation. Eur. J. Pharmaceut. Sci. 1997;5(5):287–293. [Google Scholar]
- 106.Chowdhury D.K., Mitra A.K. Kinetics of a model nucleoside (guanosine) release from biodegradable poly(DL-lactide-co-glycolide) microspheres: a delivery system for long-term intraocular delivery. Pharmaceut. Dev. Technol. 2000;5(2):279–285. doi: 10.1081/pdt-100100542. [DOI] [PubMed] [Google Scholar]
- 107.Duvvuri S., Janoria K.G., Pal D., Mitra A.K. Controlled delivery of ganciclovir to the retina with drug-loaded Poly(d,L-lactide-co-glycolide) (PLGA) microspheres dispersed in PLGA-PEG-PLGA Gel: a novel intravitreal delivery system for the treatment of cytomegalovirus retinitis. J. Ocul. Pharmacol. Therapeut. 2007;23(3):264–274. doi: 10.1089/jop.2006.132. [DOI] [PubMed] [Google Scholar]
- 108.Veloso A.J., Zhu Q., Herrero-Vanrell R., Refojo M.F. Ganciclovir-loaded polymer microspheres in rabbit eyes inoculated with human cytomegalovirus. Invest. Ophthalmol. Vis. Sci. 1997;38(3):665–675. [PubMed] [Google Scholar]
- 109.Kapanigowda U.G., Nagaraja S.H., Ramaiah B., Boggarapu P.R. Improved intraocular bioavailability of ganciclovir by mucoadhesive polymer based ocular microspheres: development and simulation process in Wistar rats. Daru. 2015;23:49. doi: 10.1186/s40199-015-0132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tanetsugu Y., Tagami T., Terukina T., Ogawa T., Ohta M., Ozeki T. Development of a sustainable release system for a ranibizumab biosimilar using poly(lactic-co-glycolic acid) biodegradable polymer-based microparticles as a platform. Biol. Pharm. Bull. 2017;40(2):145–150. doi: 10.1248/bpb.b16-00437. [DOI] [PubMed] [Google Scholar]
- 111.Arranz-Romera A., Hernandez M., Checa-Casalengua P., Garcia-Layana A., Molina-Martinez I.T., Recalde S., Young M.J., Tucker B.A., Herrero-Vanrell R., Fernandez-Robredo P., Bravo-Osuna I. A safe GDNF and GDNF/BDNF controlled delivery system improves migration in human retinal pigment epithelial cells and survival in retinal ganglion cells: potential usefulness in degenerative retinal pathologies. Pharmaceuticals. 2021;14(1):50. doi: 10.3390/ph14010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li F. Controlled release of bevacizumab through nanospheres for extended treatment of age-related macular degeneration. Open Ophthalmol. J. 2012;6(1):54–58. doi: 10.2174/1874364101206010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu J., Li S., Li G., Li X., Yu C., Fu Z., Li X., Teng L., Li Y., Sun F. Highly bioactive, bevacizumab-loaded, sustained-release PLGA/PCADK microspheres for intravitreal therapy in ocular diseases. Int. J. Pharm. 2019;563:228–236. doi: 10.1016/j.ijpharm.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 114.Saishin Y., Silva R.L., Saishin Y., Callahan K., Schoch C., Ahlheim M., Lai H., Kane F., Brazzell R.K., Bodmer D., Campochiaro P.A. Periocular injection of microspheres containing PKC412 inhibits choroidal neovascularization in a porcine model. Investigative Opthalmology & Visual Science. 2003;44(11):4989. doi: 10.1167/iovs.03-0600. [DOI] [PubMed] [Google Scholar]
- 115.Liu W., Tawakol A.P., Rudeen K.M., Mieler W.F., Kang-Mieler J.J. Treatment efficacy and biocompatibility of a biodegradable aflibercept-loaded microsphere-hydrogel drug delivery system. Transl. Vis. Sci. Technol. 2020;9(11):13. doi: 10.1167/tvst.9.11.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu W., Borrell M.A., Venerus D.C., Mieler W.F., Kang-Mieler J.J. Characterization of biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of ranibizumab. Transl. Vis. Sci. Technol. 2019;8(1):12. doi: 10.1167/tvst.8.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim E.S., Lee M.S., Jeong H., Lim S.Y., Kim D., Kim D., Jung J., Lyu S., Cho H.J., Kim D.M., Suh W., Jeong J.H. Sustained-release microspheres of Rivoceranib for the treatment of subfoveal choroidal neovascularization. Pharmaceutics. 2021;13(10):1548. doi: 10.3390/pharmaceutics13101548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jiang C., Moore M.J., Zhang X., Klassen H., Langer R., Young M. Intravitreal injections of GDNF-loaded biodegradable microspheres are neuroprotective in a rat model of glaucoma. Mol. Vis. 2007;13:1783–1792. [PubMed] [Google Scholar]
- 119.Checa-Casalengua P., Jiang C., Bravo-Osuna I., Tucker B.A., Molina-Martínez I.T., Young M.J., Herrero-Vanrell R. Retinal ganglion cells survival in a glaucoma model by GDNF/Vit E PLGA microspheres prepared according to a novel microencapsulation procedure. J. Contr. Release. 2011;156(1):92–100. doi: 10.1016/j.jconrel.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 120.Ward M.S., Khoobehi A., Lavik E.B., Langer R., Young M.J. Neuroprotection of retinal ganglion cells in DBA/2J mice with GDNF-loaded biodegradable microspheres. J. Pharmaceut. Sci. 2007;96(3):558–568. doi: 10.1002/jps.20629. [DOI] [PubMed] [Google Scholar]
- 121.Mietzner R., Kade C., Froemel F., Pauly D., Stamer W.D., Ohlmann A., Wegener J., Fuchshofer R., Breunig M. Fasudil loaded PLGA microspheres as potential intravitreal depot formulation for glaucoma therapy. Pharmaceutics. 2020;12(8):706. doi: 10.3390/pharmaceutics12080706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Arranz-Romera A., Davis B.M., Bravo-Osuna I., Esteban-Pérez S., Molina-Martínez I.T., Shamsher E., Ravindran N., Guo L., Cordeiro M.F., Herrero-Vanrell R. Simultaneous co-delivery of neuroprotective drugs from multi-loaded PLGA microspheres for the treatment of glaucoma. J. Contr. Release. 2019;297:26–38. doi: 10.1016/j.jconrel.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 123.Lee M.Y., Bourgeois S., Almouazen E., Pelletier J., Renaud F., Fessi H., Kodjikian L. Microencapsulation of rifampicin for the prevention of endophthalmitis: in vitro release studies and antibacterial assessment. Int. J. Pharm. 2016;505(1–2):262–270. doi: 10.1016/j.ijpharm.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 124.Paganelli F., Cardillo J.A., Melo L.A.S., Lucena D.R., Silva A.A., Oliveira A.G., Ho Fling-Lima A.L., Nguyen Q.D., Kuppermann B.D., Belfort R. A single intraoperative sub-tenon’s capsule injection of triamcinolone and ciprofloxacin in a controlled-release system for cataract surgery. Investigative Opthalmology & Visual Science. 2009;50(7):3041. doi: 10.1167/iovs.08-2920. [DOI] [PubMed] [Google Scholar]
- 125.Yao J., Tucker B.A., Zhang X., Checa-Casalengua P., Herrero-Vanrell R., Young M.J. Robust cell integration from co-transplantation of biodegradable MMP2-PLGA microspheres with retinal progenitor cells. Biomaterials. 2011;32(4):1041–1050. doi: 10.1016/j.biomaterials.2010.09.063. [DOI] [PubMed] [Google Scholar]
- 126.Cardillo J.A., Souza-Filho A.A., Oliveira A.G. Intravitreal Bioerudivel sustained-release triamcinolone microspheres system (RETAAC). Preliminary report of its potential usefulnes for the treatment of diabetic macular edema. Arch. Soc. Esp. Oftalmol. 2006;81(12):679–681. 675-7. [PubMed] [Google Scholar]
- 127.Fernandes A.R., Zielińska A., Sanchez-Lopez E., Dos Santos T., Garcia M.L., Silva A.M., Karczewski J., Souto E.B. Exudative versus nonexudative age-related macular degeneration: physiopathology and treatment options. Int. J. Mol. Sci. 2022;23(5):2592. doi: 10.3390/ijms23052592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ba J., Peng R.S., Xu D., Li Y.H., Shi H., Wang Q., Yu J. Intravitreal anti-VEGF injections for treating wet age-related macular degeneration: a systematic review and meta-analysis. Drug Des. Dev. Ther. 2015;9:5397–5405. doi: 10.2147/DDDT.S86269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Michels S., Rosenfeld P.J., Puliafito C.A., Marcus E.N., Venkatraman A.S. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005;112(6):1035–1047. doi: 10.1016/j.ophtha.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 130.Martin D.F., Maguire M.G., Ying G.S., Grunwald J.E., Fine S.L., Jaffe G.J. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011;364(20):1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bakri S.J., Snyder M.R., Reid J.M., Pulido J.S., Ezzat M.K., Singh R.J. Pharmacokinetics of intravitreal ranibizumab (Lucentis) Ophthalmology. 2007;114(12):2179–2182. doi: 10.1016/j.ophtha.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 132.Schoenberger S.D., Kim S.J., Thorne J.E., Mruthyunjaya P., Yeh S., Bakri S.J., Ehlers J.P. Diagnosis and treatment of acute retinal necrosis: a report by the American academy of ophthalmology. Ophthalmology. 2017;124(3):382–392. doi: 10.1016/j.ophtha.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 133.Heath G., Depledge D.P., Brown J.R., Hale A.D., Tutil H., Williams R., Breuer J. Acute retinal necrosis caused by the zoster vaccine virus. Clin. Infect. Dis. 2017;65(12):2122–2125. doi: 10.1093/cid/cix683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Port A.D., Orlin A., Kiss S., Patel S., D'Amico D.J., Gupta M.P. Cytomegalovirus retinitis: a review. J. Ocul. Pharmacol. Therapeut. 2017;33(4):224–234. doi: 10.1089/jop.2016.0140. [DOI] [PubMed] [Google Scholar]
- 135.Cui J.Z., Kimura H., Spee C., Thumann G., Hinton D.R., Ryan S.J. Natural history of choroidal neovascularization induced by vascular endothelial growth factor in the primate. Graefes Arch. Clin. Exp. Ophthalmol. 2000;238(4):326–333. doi: 10.1007/s004170050360. [DOI] [PubMed] [Google Scholar]
- 136.Amin R., Puklin J.E., Frank R.N. Growth factor localization in choroidal neovascular membranes of age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 1994;35(8):3178–3188. [PubMed] [Google Scholar]
- 137.Kwak N., Okamoto N., Wood J.M., Campochiaro P.A. VEGF is major stimulator in model of choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2000;41(10):3158–3164. [PubMed] [Google Scholar]
- 138.Lee J.E., Kim K.L., Kim D., Yeo Y., Han H., Kim M.G., Kim S.H., Kim H., Jeong J.H., Suh W. Apatinib-loaded nanoparticles suppress vascular endothelial growth factor-induced angiogenesis and experimental corneal neovascularization. Int. J. Nanomed. 2017;12:4813–4822. doi: 10.2147/IJN.S135133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Olsson A.K., Dimberg A., Kreuger J., Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat. Rev. Mol. Cell Biol. 2006;7(5):359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 140.Osswald C.R., Guthrie M.J., Avila A., Valio J.J., Mieler W.F., Kang-Mieler J.J. In vivo efficacy of an injectable microsphere-hydrogel ocular drug delivery system. Curr. Eye Res. 2017;42(9):1293–1301. doi: 10.1080/02713683.2017.1302590. [DOI] [PubMed] [Google Scholar]
- 141.Daruich A., Matet A., Marchionno L., De Azevedo J.D., Ambresin A., Mantel I., Behar-Cohen F. Acute central serous chorioretinopathy: factors influencing episode duration. retin.-J. Retin. Vitr. Dis. 2017;37(10):1905–1915. doi: 10.1097/IAE.0000000000001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.van Rijssen T.J., van Dijk E., Yzer S., Ohno-Matsui K., Keunen J., Schlingemann R.O., Sivaprasad S., Querques G., Downes S.M., Fauser S., Hoyng C.B., Piccolino F.C., Chhablani J.K., Lai T., Lotery A.J., Larsen M., Holz F.G., Freund K.B., Yannuzzi L.A., Boon C. Central serous chorioretinopathy: towards an evidence-based treatment guideline. Prog. Retin. Eye Res. 2019;73 doi: 10.1016/j.preteyeres.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 143.Fung A.T., Yang Y., Kam A.W. Central serous chorioretinopathy: a review. Clin. Exp. Ophthalmol. 2023;51(3):243–270. doi: 10.1111/ceo.14201. [DOI] [PubMed] [Google Scholar]
- 144.Zhao M., Celerier I., Bousquet E., Jeanny J.C., Jonet L., Savoldelli M., Offret O., Curan A., Farman N., Jaisser F., Behar-Cohen F. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J. Clin. Invest. 2012;122(7):2672–2679. doi: 10.1172/JCI61427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Daruich A., Matet A., Dirani A., Gallice M., Nicholson L., Sivaprasad S., Behar-Cohen F. Oral mineralocorticoid-receptor antagonists: real-life experience in clinical subtypes of nonresolving central serous chorioretinopathy with chronic epitheliopathy. Transl. Vis. Sci. Technol. 2016;5(2):2. doi: 10.1167/tvst.5.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sabanayagam C., Yip W., Ting D.S.W., Tan G., Wong T.Y. Ten emerging trends in the epidemiology of diabetic retinopathy. Ophthalmic Epidemiol. 2016;23(4):209–222. doi: 10.1080/09286586.2016.1193618. [DOI] [PubMed] [Google Scholar]
- 147.Sugimoto M., Tsukitome H., Okamoto F., Oshika T., Ueda T., Niki M., Mitamura Y., Ishikawa H., Gomi F., Kitano S., Noma H., Shimura M., Sonoda S., Sawada O., Ohji M., Harimoto K., Takeuchi M., Takamura Y., Kondo M., Sakamoto T. Clinical preferences and trends of anti-vascular endothelial growth factor treatments for diabetic macular edema in Japan. J. Diabetes Investig. 2019;10(2):475–483. doi: 10.1111/jdi.12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cardillo J.A., Melo L.A.S., Costa R.A., Skaf M., Belfort R., Souza-Filho A.A., Farah M.E., Kuppermann B.D. Comparison of intravitreal versus posterior sub–tenon’s capsule injection of triamcinolone acetonide for diffuse diabetic macular edema. Ophthalmology. 2005;112(9):1557–1563. doi: 10.1016/j.ophtha.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 149.Duh E.J., Sun J.K., Stitt A.W. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. 2017;2(14) doi: 10.1172/jci.insight.93751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee R., Wong T.Y., Sabanayagam C. Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis. 2015;2:17. doi: 10.1186/s40662-015-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Karan B.M., Little K., Augustine J., Stitt A.W., Curtis T.M. Aldehyde dehydrogenase and aldo-keto reductase enzymes: basic concepts and emerging roles in diabetic retinopathy. Antioxidants. 2023;12(7) doi: 10.3390/antiox12071466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rojas M., Zhang W., Xu Z., Lemtalsi T., Chandler P., Toque H.A., Caldwell R.W., Caldwell R.B. Requirement of NOX2 expression in both retina and bone marrow for diabetes-induced retinal vascular injury. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0084357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bandi N., Kompella U.B. Budesonide reduces vascular endothelial growth factor secretion and expression in airway (Calu-1) and alveolar (A549) epithelial cells. Eur. J. Pharmacol. 2001;425(2):109–116. doi: 10.1016/s0014-2999(01)01192-x. [DOI] [PubMed] [Google Scholar]
- 154.Saishin Y., Saishin Y., Takahashi K., Melia M., Vinores S.A., Campochiaro P.A. Inhibition of protein kinase C decreases prostaglandin-induced breakdown of the blood-retinal barrier. J. Cell. Physiol. 2003;195(2):210–219. doi: 10.1002/jcp.10238. [DOI] [PubMed] [Google Scholar]
- 155.Pan Y., Varma R. Natural history of glaucoma. Indian J. Ophthalmol. 2011;59(7):19. doi: 10.4103/0301-4738.73682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lee D.A., Higginbotham E.J. Glaucoma and its treatment: a review. Am. J. Health Syst. Pharm. 2005;62(7):691–699. doi: 10.1093/ajhp/62.7.691. [DOI] [PubMed] [Google Scholar]
- 157.Jassim A.H., Nsiah N.Y., Inman D.M. Ocular hypertension results in hypoxia within glia and neurons throughout the visual projection. Antioxidants. 2022;11(5) doi: 10.3390/antiox11050888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Karatas A., Sonakin O., Kilicarslan M., Baykara T. Poly (epsilon-caprolactone) microparticles containing Levobunolol HCl prepared by a multiple emulsion (W/O/W) solvent evaporation technique: effects of some formulation parameters on microparticle characteristics. J. Microencapsul. 2009;26(1):63–74. doi: 10.1080/02652040802141039. [DOI] [PubMed] [Google Scholar]
- 159.Tanna A.P., Johnson M. Rho kinase inhibitors as a novel treatment for glaucoma and ocular hypertension. Ophthalmology. 2018;125(11):1741–1756. doi: 10.1016/j.ophtha.2018.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Akaiwa K., Namekata K., Azuchi Y., Sano H., Guo X., Kimura A., Harada C., Mitamura Y., Harada T. Topical ripasudil suppresses retinal ganglion cell death in a mouse model of normal tension glaucoma. Investigative Opthalmology & Visual Science. 2018;59(5):2080. doi: 10.1167/iovs.17-23276. [DOI] [PubMed] [Google Scholar]
- 161.Levin L.A. Neuroprotection and regeneration in glaucoma. Ophthalmol Clin North Am. 2005;18(4):585–596. doi: 10.1016/j.ohc.2005.07.001. vii. [DOI] [PubMed] [Google Scholar]
- 162.Idrees S., Sridhar J., Kuriyan A.E. Proliferative vitreoretinopathy: a review. Int. Ophthalmol. Clin. 2019;59(1):221–240. doi: 10.1097/IIO.0000000000000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hirose F., Kiryu J., Tabata Y., Tamura H., Musashi K., Takase N., Usui H., Kuwayama S., Kato A., Yoshimura N., Ogura Y., Yasukawa T. Experimental proliferative vitreoretinopathy in rabbits by delivery of bioactive proteins with gelatin microspheres. Eur. J. Pharm. Biopharm. 2018;129:267–272. doi: 10.1016/j.ejpb.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 164.Blumenkranz M., Hernandez E., Ophir A., Norton E.W. 5-fluorouracil: new applications in complicated retinal detachment for an established antimetabolite. Ophthalmology. 1984;91(2):122–130. doi: 10.1016/s0161-6420(84)34318-4. [DOI] [PubMed] [Google Scholar]
- 165.Chang Y.C., Hu D.N., Wu W.C. Effect of oral 13-cis-retinoic acid treatment on postoperative clinical outcome of eyes with proliferative vitreoretinopathy. Am. J. Ophthalmol. 2008;146(3):440–446. doi: 10.1016/j.ajo.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 166.Wang F., He S., Chen B. Retinoic acid-loaded alginate microspheres as a slow release drug delivery carrier for intravitreal treatment. Biomed. Pharmacother. 2018;97:722–728. doi: 10.1016/j.biopha.2017.10.109. [DOI] [PubMed] [Google Scholar]
- 167.Hamel C., pigmentosa Retinitis, Orphanet J. Rare Dis. 2006;1:40. doi: 10.1186/1750-1172-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Verbakel S.K., van Huet R., Boon C., den Hollander A.I., Collin R., Klaver C., Hoyng C.B., Roepman R., Klevering B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018;66:157–186. doi: 10.1016/j.preteyeres.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 169.Treanor J.J., Goodman L., de Sauvage F., Stone D.M., Poulsen K.T., Beck C.D., Gray C., Armanini M.P., Pollock R.A., Hefti F., Phillips H.S., Goddard A., Moore M.W., Buj-Bello A., Davies A.M., Asai N., Takahashi M., Vandlen R., Henderson C.E., Rosenthal A. Characterization of a multicomponent receptor for GDNF. Nature. 1996;382(6586):80–83. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- 170.Klocker N., Braunling F., Isenmann S., Bahr M. In vivo neurotrophic effects of GDNF on axotomized retinal ganglion cells. Neuroreport. 1997;8(16):3439–3442. doi: 10.1097/00001756-199711100-00005. [DOI] [PubMed] [Google Scholar]
- 171.McGee S.L., Abel H., Hauswirth W.W., Flannery J.G. Glial cell line derived neurotrophic factor delays photoreceptor degeneration in a transgenic rat model of retinitis pigmentosa. Mol. Ther. 2001;4(6):622–629. doi: 10.1006/mthe.2001.0498. [DOI] [PubMed] [Google Scholar]
- 172.Fernandez-Sanchez L., Lax P., Pinilla I., Martin-Nieto J., Cuenca N. Tauroursodeoxycholic acid prevents retinal degeneration in transgenic P23H rats. Invest. Ophthalmol. Vis. Sci. 2011;52(8):4998–5008. doi: 10.1167/iovs.11-7496. [DOI] [PubMed] [Google Scholar]
- 173.Fernandez-Sanchez L., Lax P., Isiegas C., Ayuso E., Ruiz J.M., de la Villa P., Bosch F., de la Rosa E.J., Cuenca N. Proinsulin slows retinal degeneration and vision loss in the P23H rat model of retinitis pigmentosa. Hum. Gene Ther. 2012;23(12):1290–1300. doi: 10.1089/hum.2012.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Burkholder B.M., Jabs D.A. Uveitis for the non-ophthalmologist. BMJ Br. Med. J. (Clin. Res. Ed.) 2021;372:m4979. doi: 10.1136/bmj.m4979. [DOI] [PubMed] [Google Scholar]
- 175.de Smet M.D., Taylor S.R., Bodaghi B., Miserocchi E., Murray P.I., Pleyer U., Zierhut M., Barisani-Asenbauer T., LeHoang P., Lightman S. Understanding uveitis: the impact of research on visual outcomes. Prog. Retin. Eye Res. 2011;30(6):452–470. doi: 10.1016/j.preteyeres.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 176.Bao H., Tian Y., Wang H., Ye T., Wang S., Zhao J., Qiu Y., Li J., Pan C., Ma G., Wei W., Tao Y. Exosome-loaded degradable polymeric microcapsules for the treatment of vitreoretinal diseases. Nat. Biomed. Eng. 2023 doi: 10.1038/s41551-023-01112-3. [DOI] [PubMed] [Google Scholar]
- 177.Colleaux K.M., Hamilton W.K. Effect of prophylactic antibiotics and incision type on the incidence of endophthalmitis after cataract surgery. Can. J. Ophthalmol. 2000;35(7):373–378. doi: 10.1016/S0008-4182(00)80124-6. [DOI] [PubMed] [Google Scholar]
- 178.Kahraman G., Ferdinaro C., Wetzel B., Bernhart C., Prager F., Amon M. Intraindividual comparison of capsule behavior of 2 hydrophobic acrylic intraocular lenses during a 5-year follow-up. J. Cataract Refract. Surg. 2017;43(2):228–233. doi: 10.1016/j.jcrs.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 179.Kassumeh S.A., Wertheimer C.M., von Studnitz A., Hillenmayer A., Priglinger C., Wolf A., Mayer W.J., Teupser D., Holdt L.M., Priglinger S.G., Eibl-Lindner K.H. Poly(lactic-co-glycolic) acid as a slow-release drug-carrying matrix for methotrexate coated onto intraocular lenses to conquer posterior capsule opacification. Curr. Eye Res. 2018;43(6):702–708. doi: 10.1080/02713683.2018.1437455. [DOI] [PubMed] [Google Scholar]
- 180.Giordano G.G., Chevez-Barrios P., Refojo M.F., Garcia C.A. Biodegradation and tissue reaction to intravitreous biodegradable poly(D,L-lactic-co-glycolic)acid microspheres. Curr. Eye Res. 1995;14(9):761–768. doi: 10.3109/02713689508995797. [DOI] [PubMed] [Google Scholar]
- 181.Thackaberry E.A., Farman C., Zhong F., Lorget F., Staflin K., Cercillieux A., Miller P.E., Schuetz C., Chang D., Famili A., Daugherty A.L., Rajagopal K., Bantseev V. Evaluation of the toxicity of intravitreally injected PLGA microspheres and rods in monkeys and rabbits: effects of depot size on inflammatory response. Invest. Ophthalmol. Vis. Sci. 2017;58(10):4274–4285. doi: 10.1167/iovs.16-21334. [DOI] [PubMed] [Google Scholar]
- 182.Rahimy M.H., Peyman G.A., Chin S.Y., Golshani R., Aras C., Borhani H., Thompson H. Polysulfone capillary fiber for intraocular drug delivery: in vitro and in vivo evaluations. J. Drug Target. 1994;2(4):289–298. doi: 10.3109/10611869409015909. [DOI] [PubMed] [Google Scholar]
- 183.Brunner A., Mader K., Gopferich A. pH and osmotic pressure inside biodegradable microspheres during erosion. Pharm. Res. (N. Y.) 1999;16(6):847–853. doi: 10.1023/a:1018822002353. [DOI] [PubMed] [Google Scholar]
- 184.Takatsuka S., Kubota T., Kurashina Y., Onoe H. Near‐infrared‐triggered on‐demand controlled release of adeno‐associated virus from alginate hydrogel microbeads with heat transducer for gene therapy. Small. 2023;19(7) doi: 10.1002/smll.202204139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.