Abstract
Pruritus is an uncomfortable sensation induced by various pruritogens, including serotonin. Serotonin, acting as an inflammatory mediator, can activate a histamine-independent pathway. Consequently, many anti-pruritus medications, such as antihistamines, are not effective in adequately relieving patient symptoms. Niclosamide, an anthelmintic drug, has recently demonstrated an affinity for Metabotropic glutamate receptors (mGluRs). mGluRs are a group of receptors activated by glutamate, and they are involved in regulating neuronal excitability. In this study, we utilized mouse models of serotonergic itch and administered different doses of Niclosamide to examine the expression of mGluR1, mGluR5, and 5-HT2. The administration of 5 mg/kg Niclosamide successfully suppressed pruritus in the mice. Additionally, the levels of mGluR1, mGluR5, 5-HT2, and TRPV1 were significantly reduced. These findings suggest that Niclosamide holds promise as a potential antipruritic drug.
Keywords: Serotonergic itch, mGluR, TRPV1, 5-HT2, Serotonin, Pruritus
Highlights
-
•
Niclosamide eases serotonin-induced itch in mice, demonstrating its potential as a therapeutic option.
-
•
Mice treated with niclosamide exhibited reduced mGluR1 and mGluR5 expression levels.
-
•
Niclosamide lowered TRPV1 and 5-HT2 expression levels in the mouse itch model's nervous system.
Abbreviations
- 5-HT
5-hydroxytryptamine, serotonin
- DAG
Diacylglycerol
- DRG
Dorsal root ganglia
- ER
Endoplasmic reticulum
- FDA
Food and Drug Administration
- IP3
Inositol triphosphate
- mGluRs
Metabotropic Glutamate Receptors
- NMDA
N-methyl-d-aspartate
- TRPV
Transient Receptor Potential Vanilloid
- TRPC
Transient receptor potential channels
1. Introduction
Pruritus, also known as itching, is an uncomfortable sensation that triggers the urge to scratch, influencing both mental and physical well-being in a detrimental way [1]. Pruritus could be categorized based on the duration of symptoms or the underlying causative factors. Pruritus is classified based on the duration of symptoms into two categories, acute (symptoms lasting less than 6 weeks) and chronic (symptoms lasting for 6 weeks or longer). Also, there are four categories of pruritus based on the underlying causative factor: neurogenic itch (This type of itch is induced by mediators, but in the absence of neural damage.), neuropathic itch (This category is associated with damaged neurons.), pruritoceptive itch (Itch in this category is linked to the activation of sensory fibers by pruritogens.), and psychogenic itch (This type of itch originates from psychosomatic or psychiatric factors) [2].
Serotonin, also known as 5-hydroxytryptamine (5-HT), is an inflammatory mediator and has been identified as a potent inducer of acute and chronic pruritus by histamine-independent pathways [3,4]. Antihistamines are ineffective in reducing serotonin-induced itch due to its non-histaminergic nature. Patients with chronic itch conditions, such as allergic contact dermatitis and atopic dermatitis, demonstrate elevated serotonin levels in their skin [5]. Additionally, an impaired 5-HT signaling has been linked to several systemic disorders characterized by itchiness, such as psoriasis, uremia, and cholestasis [6]. It is postulated that the induction of itch in rodents through subcutaneous (s.c.) and intradermal (i.d.) injections of serotonin occurs by directly affecting unmyelinated C-fibers [7,8].
The serotonin-induced itch pathway involves the participation of several subtypes of 5-HT receptors expressed in sensory nerves, such as 5-HT1, 5-HT2, 5-HT3, and 5-HT7 receptors [9]. Studies have demonstrated that selective serotonin re-uptake inhibitors (SSRIs) have a positive impact on non-dermatological pruritus by alleviating itch symptoms [10,11]. Serotonin can modulate pain and itch by regulating the release of other neurotransmitters, such as glutamate, or through the interaction of activated 5-HT receptors with other neurotransmitter receptors [12].
There is significant crosstalk between the itch and pain pathways, as they share several similar mechanisms. Both pain and itch are transmitted through small diameter C-fiber neurons, which can convey signals to the spinal cord and brain. Glutamate is a key neurotransmitter involved in these processes, functioning in both pain and itch signaling. It has been proposed as an excitatory neurotransmitter in the transmission of itch, acting at the synapse between C-fiber neurons and the spinal cord [13]. Metabotropic glutamate receptors (mGluRs), which are G-protein coupled receptors activated by glutamate, play roles in neuronal excitability and synaptic plasticity [14]. Group I mGluR (mGluR1 and mGluR5) leads to phosphoinositide hydrolysis and increase cellular excitability as a result [15]. Among the eight subtypes of mGluRs, the inhibition of mGluR1 and mGluR5 has shown potential in mitigating pain, suggesting a possible role for mGluR1 and mGluR5 in the signaling pathways of pain. mGluR1 and mGluR5 can have pronociceptive roles in the peripheral and central nervous systems, while the other subtypes mostly exhibit antinociceptive functions [[16], [17], [18], [19]]. Also, mGluR5, along with the N-methyl-d-aspartate (NMDA) receptor, plays a significant role in the transmission of pain sensation and its perception in the central nervous system (CNS) [14].
Niclosamide, an FDA-approved anthelmintic drug with a great safety record, is used to treat intestinal worms by disrupting the mitochondria of the parasitic worm. In recent years, an increasing amount of evidence has emerged indicating that niclosamide possesses numerous capabilities in inhibiting or regulating various signaling pathways and biological processes. Recent studies have shown niclosamide could be used as a treatment for cancers [[20], [21], [22], [23]], bacterial infections [24,25], viral infections [26,27], and type-2 diabetes [28]. Niclosamide exhibits high selectivity for Group I mGluRs, acting as a low-nanomolar allosteric antagonist. Also, it has been shown that mechanical hyperalgesia in neuropathic pain rats is downregulated by niclosamide [29].
Accumulated evidences strongly suggest the transient receptor potential (TRP) channel superfamily as a pivotal mediator in somatosensory signaling, with particular emphasis on the role of transient receptor potential vanilloid (TRPV1) [30]. The TRPV1 is a polymodal receptor cations channel and has the most affinity to calcium ions. TRPV1 is directly stimulated by pain-inducing stimuli such as capsaicin, heat, acid [31]. TRPV1 serves as a central mediator for both histaminergic and non-histaminergic pruritus, while some aspects of non-histaminergic pruritus are also mediated by TRPA1 [32]. In addition, non-histaminergic pruritus, such as the pruritus observed in cholestasis, and serotonergic has also been associated with the sensitization of TRPV1 by pruritogens [33,34]. Metabotropic glutamate receptors of Group I (mGluRs), particularly mGluR5, exhibit co-expression with transient TRPV1 in dorsal root ganglion (DRG) neurons [35]. Recent investigations have revealed that TRPV1 and mGluR5 are associated in a membrane-delimited manner within the central presynaptic terminals of nociceptive neurons [36].
In this study, we evaluated the impact of niclosamide on the inhibition of serotonergic pruritus in a mouse model. Additionally, we examined its effects on the expression of 5-HT2, mGluR1, mGluR5, and TRPV1. While there are several studies on the role of mGluRs in pain, no study has investigated the potential role of mGluRs in itch or the interaction between serotonin receptors and glutamate receptors in neural signal transmission of pruritus. Our study also explores the potential for drug repositioning, highlighting an anthelmintic medication that demonstrates antipruritic effects in animal models.
2. Result
2.1. Scratching behavior
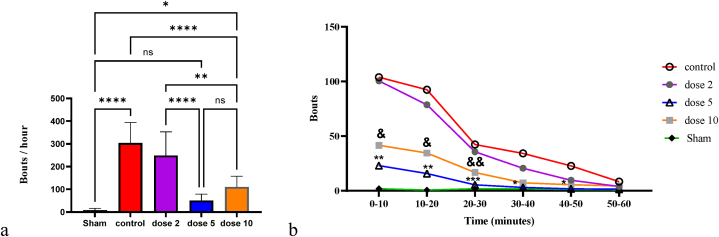
Fig. 1a presents the results of scratching behavior in each group. The number of bouts was significantly increased in the control group (Mean ± SD = 304.0 ± 89.96) compared to the sham group (Mean ± SD = 8.375 ± 7.671; p-value <0.0001). Treatment with 5 mg/kg (Mean ± SD = 50.50 ± 28.53; p-value <0.0001) and 10 mg/kg (Mean ± SD = 110 ± 47.34; p-value <0.0001) niclosamide resulted in a decrease in the number of bouts per hour in the serotonergic itch model compared to the controls. However, treatment with 2 mg/kg niclosamide (Mean ± SD = 248.9 ± 104.2) did not significantly decrease the number of bouts per hour compared to the control group (p-value = 0.4710). Among the treated groups, the number of bouts per hour in the 5 mg/kg niclosamide group was not significantly different from the sham group (p-value = 0.7118). As the 10 mg/kg dose showed no significant difference compared to the 5 mg/kg dose (p-value = 0.3944), and both doses demonstrated a reasonable response in suppressing itch, we did not increase the dose further.
Fig. 1.
Frequency of bouts per hour (a) and every 10 min (b) across all groups. */P < 0.05; **/P < 0.01; ***/P < 0.001; ****/P < 0.0001; ns/Not significant. Bars represent mean ± SD.
Fig. 1b also shows the number of bouts over time. The number of bouts decreased in all groups over time. The number of bouts was significantly lower in the 5 mg/kg and 10 mg/kg niclosamide-treated groups during the first 30 min compared to the control group. The mean number of bouts in the first 10 min was 23.00 in the 5 mg/kg niclosamide group (p-value = 0.0015 compared to controls), 41.63 in the 10 mg/kg group (p-value = 0.0121 compared to controls), and 103.90 in the control group. Fig. 1b indicates that the highest reduction in itch occurred in the initial minutes after serotonin injection in the 5 mg/kg and 10 mg/kg groups compared to controls. In contrast, in the 2 mg/kg group, the mean number of bouts was 100.5, indicating that this dosage was less effective and slower at reducing serotonin-induced itching compared to the other two doses.
2.2. Molecular analysis
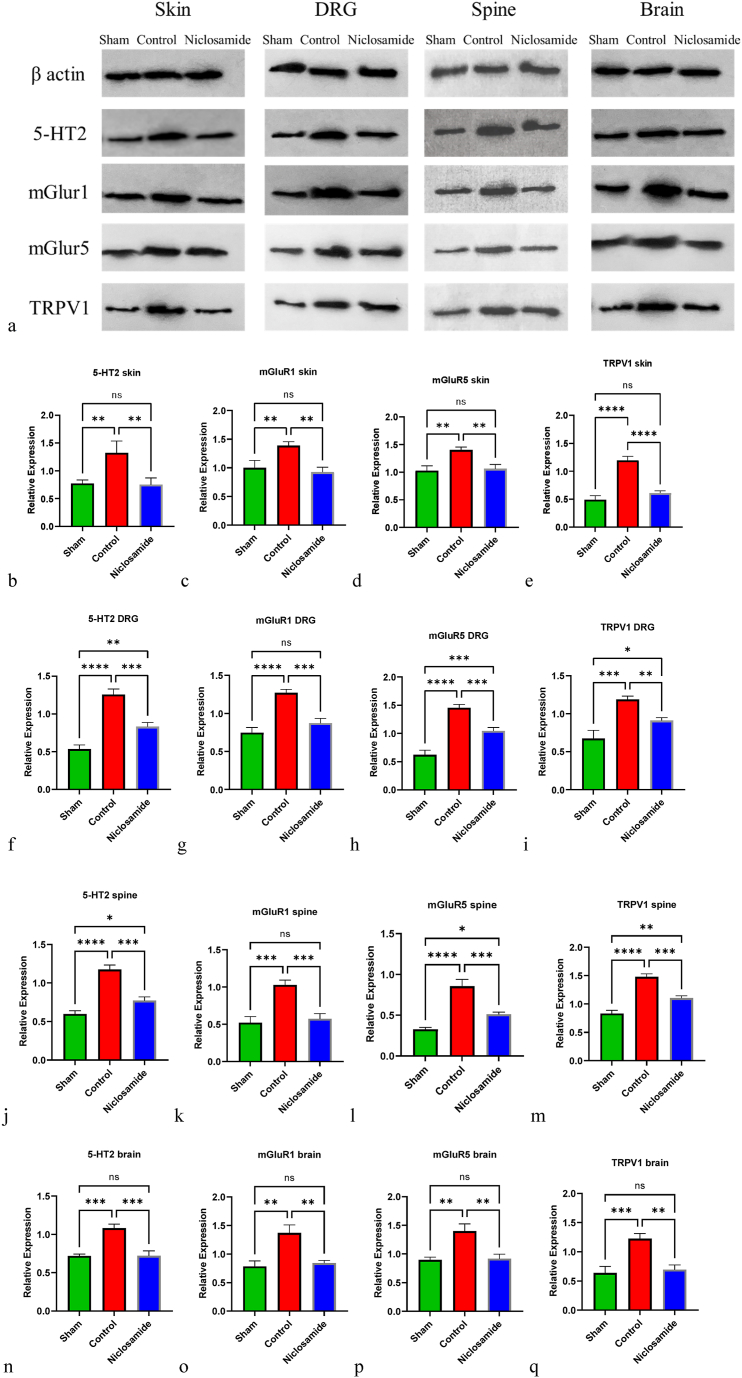
The levels of 5-HT1, mGluR1, mGluR5, and TRPV1 were measured in skin (Fig. 2b–e), DRG (Fig. 2f–i), spine (Fig. 2j-m), and brain samples (Fig. 2n-q). In the control group, the levels of all four molecules were significantly increased compared to the sham group in skin, DRG, spine, and brain samples. However, after treatment with 5 mg/kg niclosamide, the levels of 5-HT1, mGluR1, mGluR5, and TRPV1 were significantly decreased in skin (p-value = 0.0073, 0.0029, 0.0032, <0.0001 respectively), DRG (p-value = 0.0003, 0.0004, 0.0009, 0.0073 respectively), spine (p-value = 0.0002, 0.0007, 0.0005, 0.0002 respectively), and brain samples (p-value = 0.0003, 0.0018, 0.0016, 0.0012 respectively) compared to the control group. The levels of 5-HT1, mGluR1, mGluR5, and TRPV1 of 5 mg/kg treated group and sham group in skin and brain do not show significant difference. Further details are provided in Fig. 2.
Fig. 2.
Western blot analysis of 5-HT2, mGluR1, mGluR5, and TRPV1 in skin, DRG, Spine, and brain tissues of sham, control, and 5 mg/kg niclosamide groups (a). The relative expression levels are depicted in (b–p). */P < 0.05; **/P < 0.01; ***/P < 0.001; ****/P < 0.0001; ns/Not significant. Bars represent mean ± SD. Original and non-adjusted images of the Western bolt (a) are presented in the supplementary file.
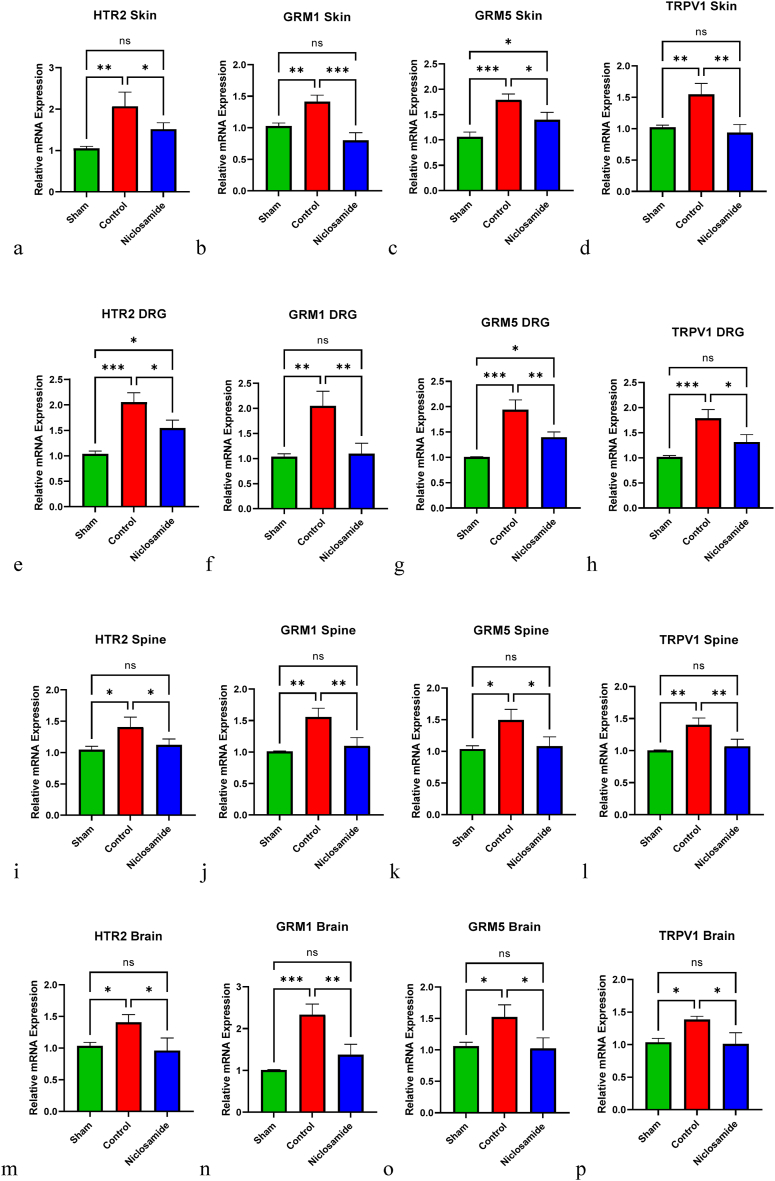
The mRNA expression levels of HT2R, GMR1, GMR5, and TRPV1 were quantified in skin (Fig. 3a–d), DRG (Fig. 3e–h), spine (Fig. 3i-l), and brain tissue samples (Fig. 3m-p). In the control group, all four mRNA levels were significantly higher than those in the sham group across all tissue types. However, following treatment with 5 mg/kg niclosamide, the expression levels of HT2R, GMR1, GMR5, and TRPV1 were markedly reduced in skin (p-value = 0.0494, 0.0006, 0.0182, 0.0026 respectively), DRG (p-value = 0.0113, 0.0035, 0.0045, 0.0115 respectively), spine (p-value = 0.0472, 0.0061, 0.0216, 0.0081 respectively), and brain (p-value = 0.0178, 0.0032, 0.0166, 0.0134 respectively) samples compared to the control group.
Fig. 3.
The PCR analysis of mRNA expression levels of HTR2, GRM1, GRM5, and TRPV1 genes in skin, DRG, Spine, and brain tissues of sham, control, and 5 mg/kg niclosamide groups. The relative expression levels are depicted in (a–o). */P < 0.05; **/P < 0.01; ***/P < 0.001; ****/P < 0.0001; ns/Not significant. Bars represent mean ± SD.
3. Discussion
Itch is characterized as an unfavorable sensation linked to the inclination to scratch. The presence of chronic itch has been observed to significantly diminish the overall quality of life [37] Consequently, numerous investigations have established a correlation between stress, anxiety, and depression with chronic pruritus in individuals diagnosed with dermatological and chronic diseases [38,39]. Itching is generated through various pathways that are generally classified into two categories: histaminergic and non-histaminergic. One of the stimuli for non-histaminergic pathways is serotonin. In patients with chronic and inflammatory diseases, serotonin is one of the substances that contributes to the induction of pruritus through its involvement in various pathways [40,41]. In this study we have induced pruritus by serotonin intradermal injection and have shown niclosamide reduce pruritus significantly in 5 mg/kg and 10 mg/kg furthermore we investigated the protein and gen expressions in skin, DRG, spine and brain tissues. The results indicates that mGluR1, mGluR5, 5-HT2 and TRPV1 were suppressed significantly in protein and gene expression in all assessed tissues in group which received niclosamide compared to the control group.
Niclosamide is an FDA approved anthelmintic drug to treat tapeworm infection in humans and other animals by uncoupling oxidative phosphorylation in the parasites. Recently, this drug demonstrated beneficial effects against obesity-related type 2 diabetes through the same mechanism in the mitochondria of the mouse liver [28]. One of recent studies demonstrated that niclosamide acts as an allosteric modulator of mGluR I and reduces neuropathic pain [29]. The phenolic hydroxyl group of niclosamide plays a pivotal role in establishing a crucial hydrogen bond with mGluR1 and 5 [29]. However, there is no previous study on investigating the effectiveness of niclosamide in relieving pruritus.
In this study, following the administration of niclosamide, itch-related behaviors decreased, and the expression levels of mGluR 1,5 also decreased. mGluRs are members of the class C G-protein-coupled receptors activated by the binding of glutamate. mGluRs are widely distributed throughout the nervous system on both neurons and glial cells. They can be classified into three general groups, where group I predominantly has a stimulatory role in pain-related signals, and group II and III have inhibitory roles [16]. Group I mGluRs are mainly found at the postsynaptic level, where they play a role in regulating neuronal excitability. They achieve this by interacting with Gαq/11 proteins and activating phospholipase C (PLC). The primary effect of group I mGluRs is to stimulate the breakdown of phosphoinositide, leading to the production of 2 s messengers known as inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). This triggers a cascade of intracellular events, starting with the release of calcium from intracellular stores due to IP3 [42].
The functions of mGluRs are widely studied in the pain pathway. Aira et al. demonstrated that an mGluR1 antagonist can prevent C-fiber-evoked dorsal horn potentials following activation by 5-HT2 receptors in nerve injury. They found that mGluR1 is coexpressed with 5-HT2 in dorsal horn neurons, contributing to 5-HT2-mediated pain [43]. Another study showed that a selective mGluR5 antagonist reduced serotonin levels and thermal hyperalgesia by suppressing dorsal raphe serotonergic neurons [44]. Additionally, research indicated that mGluR5 expression increases in the prefrontal cortex of chronic neuropathic pain models [45]. mGluR1 expression also increases in neuropathic pain, causing neuronal hyperexcitability in the anterior cingulate cortex [46].
Serotonin serves as an established neurotransmitter in both acute and chronic itch through the histamine-independent pathway [47]. Following intradermal injection of serotonin, itch was induced and the frequency of itch episodes increased compared to the sham group. Various molecular pathways and different receptors have been implicated in the mediation of the itch pathway via 5-HT. Researchers had been identified three distinct mechanisms involved in the initiation of itch through the serotonin pathway in mice. The first mechanism entails the activation of TRPV4 channels downstream of 5-HT2 receptors [48]. The second mechanism involves the sequential activation of the 5-HT7 receptor and subsequent activation of TRPA1 [6]. Lastly, the third mechanism involves the activation of the 5-HT2B receptor (via α-methyl-5-HT), which is linked to TRPC4 [49].
The 5-HT2 receptor interacts specifically with the Gαq isotype, one of the various G-protein alpha subunits. Upon binding of serotonin to the receptor, the G-protein complex is activated, leading to a conformational change that allows GTP to replace GDP in the alpha subunit [50]. The activated Gαq unit then associates with PLC, causing the conversion of phosphatidylinositol-4,5-bisphosphate (PIP2) into IP3 and DAG. IP3 promotes the release of calcium (Ca2+), while DAG activates protein kinase C (PKC) [50]. In another study, it has been demonstrated that activation of the 5-HT2 receptor leads to the activation of PKC, which in turn activates TRPV1. Consequently, the nociceptive neurons associated with cold sensation and hyperalgesia become hypersensitized [51]. A study conducted by Shim, W. et al. revealed that histamine, a potent pruritogenic agent, induces excitation of TRPV1 in sensory neurons through the downstream activation of phospholipase A2 and lipoxygenases [52]. Furthermore, mounting evidence indicates that capsaicin-sensitive primary afferent neurons, which express the TRPV1, are involved in the transmission of itch signals [36,53].
mGluR5 represents a class of metabotropic glutamate receptors that are coupled with Gαq/11 proteins and are associated with PLC signaling pathway. Upon activation, these receptors trigger the hydrolysis of phosphatidylinositol 4,5-bisphosphate into DAG and IP3 [54]. Previous studies have provided evidence that mGluR5 and TRPV1 are closely juxtaposed on the cell membrane, and mGluR5 activates TRPV1 through the involvement of DAG [55].
Based on the molecular pathways of mGluR Group 1 and 5-HT2, there are several potential points of interaction or cross-talk between these two receptor systems. Both mGluR Group 1 and 5-HT2 receptors activate PLC and lead to the production of IP3 and DAG. This shared signaling pathway suggests that activation of one receptor system could potentially influence the downstream signaling of the other receptor system, leading to cross-talk and modulation of cellular responses. Both mGluR Group 1 and 5-HT2 receptors can result in the release of intracellular calcium ions (Ca2+), although through different mechanisms. Activation of mGluR Group 1 leads to the release of Ca2+ from the endoplasmic reticulum (ER) through IP3-mediated signaling. Activation of 5-HT2 receptors can also result in the release of Ca2+ from intracellular stores. The shared modulation of intracellular calcium levels by these receptors may have synergistic or opposing effects on downstream signaling pathways and cellular responses.
In this study, the injection of niclosamide significantly reduced the expression levels of mGluR1, mGluR5, 5-HT2, and TRPV1 in skin, brain, spinal cord, and dorsal root ganglia tissues of serotonergic itch mice model. Niclosamide appears to suppress mGluR1, mGluR5, 5-HT2, and TRPV1 by downregulating the mRNA expression of these receptors in the nervous system, although the exact mechanisms remain to be elucidated in future studies. Given the high overlap between the receptor pathways of 5-HT2 and mGluR1/5, which both increase neuronal excitability, it is likely that niclosamide's inhibition of mGluR1 and mGluR5 has reduced pruritus induction and subsequently decreased the expression of 5-HT2 receptors.
4. Conclusion
Itch can have a detrimental impact on the overall quality of life for individuals experiencing it. Itch can be triggered through histaminergic and non-histaminergic pathways, with serotonin being one of the stimuli for the non-histaminergic pathway. This study successfully induced pruritus in mice through intradermal injection of serotonin and demonstrated that niclosamide, an FDA-approved antihelmintic drug, significantly reduced pruritus at dose of 5 mg/kg. Prior to this study, there was no investigation into the effectiveness of niclosamide in relieving itchiness. The injection of niclosamide in this study significantly reduced the expression levels of mGluR1, mGluR5, 5-HT2, and TRPV1 in various tissues, indicating its potential inhibitory effects on neuronal excitability and pruritus induction. The overlapping receptor pathways of 5-HT2 and mGluR5 and 1 suggest that niclosamide's inhibition of mGluR5 may contribute to the reduction in pruritus and subsequent decrease in 5-HT2 receptor expression. Overall, these findings suggest that niclosamide holds promise as a potential therapeutic option for the treatment of pruritus, and further research is warranted to explore its mechanisms of action and clinical efficacy in relieving itchiness in humans.
5. Method and materials
5.1. Animal and ethics
Male Balb/c mice weighing 20–25 g and aged 6 weeks were housed in the Experimental Medicine Research Center. Two to three mice were kept in each cage with free access to food and water. All animal studies were conducted following the guidelines outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication No. 86-23, Eighth Ed.). Ethical approval was obtained from the affiliated Medical university (Ethics number: 1402-2-101-67438).
5.2. Study design
Forty male Balb/C mice were randomly assigned to five equal groups: 1. Sham, 2. Control, 3. Mice treated with 2 mg/kg niclosamide, 4. Mice treated with 5 mg/kg niclosamide, 5. Mice treated with 10 mg/kg niclosamide. Three days before the test day, mice were placed in the test chambers for 1 h each day for habituation. On the test day, all mice except the sham group received 141 nmol/site serotonin via intradermal administration into the neck skin and were immediately placed into the test chambers. The sham group received 0.1 ml of normal saline intradermally. Niclosamide (Sigma, USA) was injected via intraperitoneal administration into the treated groups 1 h before the tests.
5.3. Scratching behavior test
For the scratching behavior test, after the administration of serotonin (Sigma, USA) (or normal saline in the sham group), each mouse was placed into a 10 × 10 × 10 cm chamber with one transparent wall facing the camera. Their behavior was recorded for 1 h. After the test, two blinded operators reviewed the recordings and counted the number of bouts. The mean of the scores was recorded. The number of bouts was counted when the mouse raised its hindlimb to its back and then returned down.
5.4. Sampling
After the scratching tests, mice were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine. Samples from the neck skin, dorsal root ganglia (DRG), brain, and spine were harvested and stored at −80 °C.
5.5. Western blot
To extract proteins from the samples, 100 μl of Pro-PRETM lysis buffer was added, and the samples were homogenized. The samples were kept at −20 °C for 20 min and then centrifuged at 4 °C at 13,000 rpm for 15 min. The supernatant was measured using the BCA protein assay method (iNtRON Biotechnology, Korea) and a spectrophotometer (Smartspec Plus spectrophotometer, Bio-Rad). The TV100 vertical system (Scie-Plas Ltd, UK) with 10 × 10 cm gel units and a Consort-EV202 power generator (Sigma, USA) were used for western blotting in this study. The proteins on the SDS-PAGE gel were transferred onto a PVDF membrane (Bio-Rad) with a pore size of 0.45 μm. The membrane was then blocked with 5 % bovine serum albumin (BSA, Sigma, USA) for 1 h before being washed with 1 % TBST buffer. Samples were incubated with β-actin antibody (1:5000, ab8227, Abcam, Cambridge, UK), anti-5-HT2 receptor antibody (1/1000, ab216959, Abcam, Cambridge, UK), recombinant anti-mGlur1 antibody (1:1000, ab259888, Abcam, Cambridge, UK), anti-mGlur5 antibody (1:1000, ab53090, Abcam, Cambridge, UK), and anti-TRPV1 antibody (1:1000, ab305299, Abcam, Cambridge, UK). The relative expressions were calculated by dividing 5-HT1, mGluR1, mGluR5, and TRPV1 by the housekeeping gene (β-actin).
5.6. RT-PCR
The expression of the genes for the 5-HT2 receptor (HTR2), mGluR1 (GRM1), mGluR5 (GRM5), and TRPV1 was measured in skin, DRG, spine, and brain tissues using the Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) technique. Initially, samples were treated with DNase I to eliminate genomic DNA contamination. The samples were then analyzed using the Applied Biosystems StepOnePlus™ RT-PCR system with SYBR® Green PCR Master Mix containing High ROX (Applied Biosystems, CA, USA). The primers designed using Primer3web version 4.1.0 for RT-PCR were as follows: 5-HTR2: forward sequence: CCTGATGTCACTTGCCATAGCTG, reverse sequence: CAGGTAAATCCAGACGGCACAG. GRM1: forward sequence: CTGGCATGAAGGAGTGCTGAAC, reverse sequence: AGCAGCTCACTTCTCCTTTCCG. GRM5: forward sequence: GATCTGTGTGCAGTGAACCGTG, reverse sequence: CACGCCTTGCAGGTGTACTCAT. TRPV1: forward sequence: CATCTTCACCACGGCTGCTTAC, reverse sequence: CAGACAGGATCTCTCCAGTGAC.
DNA polymerase activation was performed at 95 °C for 15 min, followed by 40 cycles of amplification, which included 94 °C for 15 s, 60 °C for 15 s, and 72 °C for 30 s. A melting curve analysis was conducted to ensure that all primers produced a single PCR product. The gene expression levels were analyzed using the 2−ΔCt method.
5.7. Statistical analysis
Western blots were measured using ImageJ software. Statistical analyses were conducted using GraphPad Prism (version 9.0.0) using the ANOVA test. A p-value <0.05 was considered significant.
Ethics
Ethical approval was obtained from the Ethics Committee of Tehran University of Medical Science (Ethics number: 1402-2-101-67438).
Funding source
This research received a grant from the Tehran University Medical Science (Grant No. 67438).
Data availability
The raw and analyzed datasets in this study are available upon reasonable request from corresponding author Dr. Ahmad Reza Dehpour.
CRediT authorship contribution statement
Zahra Ebrahim Soltani: Writing – review & editing, Writing – original draft, Supervision, Methodology, Funding acquisition, Formal analysis, Data curation, Conceptualization. Mohammad Elahi: Writing – review & editing, Writing – original draft, Project administration, Methodology, Investigation, Formal analysis, Data curation. Maziyar Askari Rad: Writing – original draft, Methodology, Data curation. Sara Farsio: Methodology, Data curation. Ahmad Reza Dehpour: Writing – review & editing, Validation, Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e33050.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Song J., Xian D., Yang L., Xiong X., Lai R., Zhong J. Pruritus: progress toward pathogenesis and treatment. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/9625936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cevikbas F., Lerner E.A. Physiology and pathophysiology of itch. Physiol. Rev. 2020;100(3):945–982. doi: 10.1152/physrev.00017.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haddadi N.S., Foroutan A., Shakiba S., Afshari K., Ostadhadi S., Daneshpazhooh M., et al. Attenuation of serotonin-induced itch by sumatriptan: possible involvement of endogenous opioids. Arch. Dermatol. Res. 2018;310(2):165–172. doi: 10.1007/s00403-018-1809-9. [DOI] [PubMed] [Google Scholar]
- 4.Hosogi M., Schmelz M., Miyachi Y., Ikoma A. Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain. 2006;126(1–3):16–23. doi: 10.1016/j.pain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Moser H.R., Giesler G.J., Jr. Itch elicited by intradermal injection of serotonin, intracisternal injection of morphine, and their synergistic interactions in rats. Neuroscience. 2014;274:119–127. doi: 10.1016/j.neuroscience.2014.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morita T., McClain S.P., Batia L.M., Pellegrino M., Wilson S.R., Kienzler M.A., et al. HTR7 mediates serotonergic acute and chronic itch. Neuron. 2015;87(1):124–138. doi: 10.1016/j.neuron.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostadhadi S., Haddadi N.S., Foroutan A., Azimi E., Elmariah S., Dehpour A.R. Development of resistance to serotonin-induced itch in bile duct ligated mice. Clin. Exp. Pharmacol. Physiol. 2017;44(6):680–685. doi: 10.1111/1440-1681.12752. [DOI] [PubMed] [Google Scholar]
- 8.Han L., Dong X. Itch mechanisms and circuits. Annu. Rev. Biophys. 2014;43(1):331–355. doi: 10.1146/annurev-biophys-051013-022826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahremany S., Hofmann L., Gruzman A., Cohen G. Advances in understanding the initial steps of pruritoceptive itch: how the itch hits the switch. Int. J. Mol. Sci. 2020;21(14) doi: 10.3390/ijms21144883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zylicz Z., Krajnik M., Sorge A.A., Costantini M. Paroxetine in the treatment of severe non-dermatological pruritus: a randomized, controlled trial. J. Pain Symptom Manag. 2003;26(6):1105–1112. doi: 10.1016/j.jpainsymman.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Mayo M.J., Handem I., Saldana S., Jacobe H., Getachew Y., Rush A.J. Sertraline as a first-line treatment for cholestatic pruritus. Hepatology. 2007;45(3):666–674. doi: 10.1002/hep.21553. [DOI] [PubMed] [Google Scholar]
- 12.Hao S., Shi W., Liu W., Chen Q.-Y., Zhuo M. Multiple modulatory roles of serotonin in chronic pain and injury-related anxiety. Front. Synaptic Neurosci. 2023;15 doi: 10.3389/fnsyn.2023.1122381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koga K., Chen T., Li X.Y., Descalzi G., Ling J., Gu J., et al. Glutamate acts as a neurotransmitter for gastrin releasing peptide-sensitive and insensitive itch-related synaptic transmission in mammalian spinal cord. Mol. Pain. 2011;7:47. doi: 10.1186/1744-8069-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiechio S. Modulation of chronic pain by metabotropic glutamate receptors. Adv. Pharmacol. 2016;75:63–89. doi: 10.1016/bs.apha.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Hubert G.W., Paquet M., Smith Y. Differential subcellular localization of mGluR1a and mGluR5 in the rat and monkey Substantia nigra. J. Neurosci. 2001;21(6):1838–1847. doi: 10.1523/JNEUROSCI.21-06-01838.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzitelli M., Presto P., Antenucci N., Meltan S., Neugebauer V. Recent advances in the modulation of pain by the metabotropic glutamate receptors. Cells. 2022;11(16) doi: 10.3390/cells11162608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boye Larsen D., Ingemann Kristensen G., Panchalingam V., Laursen J.C., Nørgaard Poulsen J., Skallerup Andersen M., et al. Investigating the expression of metabotropic glutamate receptors in trigeminal ganglion neurons and satellite glial cells: implications for craniofacial pain. J. Recept. Signal Transduction. 2014;34(4):261–269. doi: 10.3109/10799893.2014.885049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osikowicz M., Mika J., Przewlocka B. The glutamatergic system as a target for neuropathic pain relief. Exp. Physiol. 2013;98(2):372–384. doi: 10.1113/expphysiol.2012.069922. [DOI] [PubMed] [Google Scholar]
- 19.Metabotropic glutamate receptor-5 and protein kinase C-epsilon increase in dorsal root ganglion neurons and spinal glial activation in an adolescent rat model of painful neck injury. J. Neurotrauma. 2010;27(12):2261–2271. doi: 10.1089/neu.2010.1460. [DOI] [PubMed] [Google Scholar]
- 20.Lu W., Lin C., Roberts M.J., Waud W.R., Piazza G.A., Li Y. Niclosamide suppresses cancer cell growth by inducing Wnt co-receptor LRP6 degradation and inhibiting the Wnt/β-catenin pathway. PLoS One. 2011;6(12) doi: 10.1371/journal.pone.0029290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin L., Gao Y., Zhang X., Wang J., Ding D., Zhang Y., et al. Niclosamide sensitizes triple-negative breast cancer cells to ionizing radiation in association with the inhibition of Wnt/β-catenin signaling. Oncotarget. 2016;7(27):42126–42138. doi: 10.18632/oncotarget.9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osada T., Chen M., Yang X.Y., Spasojevic I., Vandeusen J.B., Hsu D., et al. Antihelminth compound niclosamide downregulates Wnt signaling and elicits antitumor responses in tumors with activating APC mutations. Cancer Res. 2011;71(12):4172–4182. doi: 10.1158/0008-5472.CAN-10-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wieland A., Trageser D., Gogolok S., Reinartz R., Höfer H., Keller M., et al. Anticancer effects of niclosamide in human glioblastoma. Clin. Cancer Res. 2013;19(15):4124–4136. doi: 10.1158/1078-0432.CCR-12-2895. [DOI] [PubMed] [Google Scholar]
- 24.Sun Z., Zhang Y. Antituberculosis activity of certain antifungal and antihelmintic drugs. Tuber. Lung Dis. 1999;79(5):319–320. doi: 10.1054/tuld.1999.0212. [DOI] [PubMed] [Google Scholar]
- 25.Imperi F., Massai F., Ramachandran Pillai C., Longo F., Zennaro E., Rampioni G., et al. New life for an old drug: the anthelmintic drug niclosamide inhibits Pseudomonas aeruginosa quorum sensing. Antimicrob. Agents Chemother. 2013;57(2):996–1005. doi: 10.1128/AAC.01952-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu C.J., Jan J.T., Chen C.M., Hsieh H.P., Hwang D.R., Liu H.W., et al. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob. Agents Chemother. 2004;48(7):2693–2696. doi: 10.1128/AAC.48.7.2693-2696.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y.M., Lu J.W., Lin C.C., Chin Y.F., Wu T.Y., Lin L.I., et al. Antiviral activities of niclosamide and nitazoxanide against chikungunya virus entry and transmission. Antivir. Res. 2016;135:81–90. doi: 10.1016/j.antiviral.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao H., Zhang Y., Zeng X., Shulman G.I., Jin S. Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nat. Med. 2014;20(11):1263–1269. doi: 10.1038/nm.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ai N., Wood R.D., Yang E., Welsh W.J. Niclosamide is a negative allosteric modulator of group I metabotropic glutamate receptors: implications for neuropathic pain. Pharm. Res. (N. Y.) 2016;33(12):3044–3056. doi: 10.1007/s11095-016-2027-9. [DOI] [PubMed] [Google Scholar]
- 30.Li F., Wang F. TRPV1 in pain and itch. Adv. Exp. Med. Biol. 2021;1349:249–273. doi: 10.1007/978-981-16-4254-8_12. [DOI] [PubMed] [Google Scholar]
- 31.Clapham D.E. TRP channels as cellular sensors. Nature. 2003;426(6966):517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 32.Tsagareli M.G., Nozadze I., Tsiklauri N., Carstens M.I., Gurtskaia G., Carstens E. Thermal hyperalgesia and mechanical allodynia elicited by histamine and non-histaminergic itch mediators: respective involvement of TRPV1 and TRPA1. Neuroscience. 2020;449:35–45. doi: 10.1016/j.neuroscience.2020.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belghiti M., Estévez-Herrera J., Giménez-Garzó C., González-Usano A., Montoliu C., Ferrer-Montiel A., et al. Potentiation of the transient receptor potential vanilloid 1 channel contributes to pruritogenesis in a rat model of liver disease. J. Biol. Chem. 2013;288(14):9675–9685. doi: 10.1074/jbc.M113.455162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S.J., Park G.H., Kim D., Lee J., Min H., Wall E., et al. Analysis of cellular and behavioral responses to imiquimod reveals a unique itch pathway in transient receptor potential vanilloid 1 (TRPV1)-expressing neurons. Proc. Natl. Acad. Sci. U.S.A. 2011;108(8):3371–3376. doi: 10.1073/pnas.1019755108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker K., Reeve A., Bowes M., Winter J., Wotherspoon G., Davis A., et al. mGlu5 receptors and nociceptive function II. mGlu5 receptors functionally expressed on peripheral sensory neurones mediate inflammatory hyperalgesia. Neuropharmacology. 2001;40(1):10–19. doi: 10.1016/s0028-3908(00)00114-3. [DOI] [PubMed] [Google Scholar]
- 36.Imamachi N., Park G.H., Lee H., Anderson D.J., Simon M.I., Basbaum A.I., et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc. Natl. Acad. Sci. U.S.A. 2009;106(27):11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halvorsen J.A., Dalgard F., Thoresen M., Bjertness E., Lien L. Itch and pain in adolescents are associated with suicidal ideation: a population-based cross-sectional study. Acta Derm. Venereol. 2012;92(5):543–546. doi: 10.2340/00015555-1251. [DOI] [PubMed] [Google Scholar]
- 38.Evers A.W.M., Peerdeman K.J., van Laarhoven A.I.M. What is new in the psychology of chronic itch? Exp. Dermatol. 2019;28(12):1442–1447. doi: 10.1111/exd.13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verhoeven E.W., Kraaimaat F.W., van de Kerkhof P.C., van Weel C., Duller P., van der Valk P.G., et al. Prevalence of physical symptoms of itch, pain and fatigue in patients with skin diseases in general practice. Br. J. Dermatol. 2007;156(6):1346–1349. doi: 10.1111/j.1365-2133.2007.07916.x. [DOI] [PubMed] [Google Scholar]
- 40.Tian B., Wang X.L., Huang Y., Chen L.H., Cheng R.X., Zhou F.M., et al. Peripheral and spinal 5-HT receptors participate in cholestatic itch and antinociception induced by bile duct ligation in rats. Sci. Rep. 2016;6 doi: 10.1038/srep36286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyahara Y., Funahashi H., Naono-Nakayama R., Haruta-Tsukamoto A., Muroi C., Kogoh Y., et al. Serotonin and noradrenaline modulate chronic itch processing in mice. Eur. J. Pharmacol. 2020;883 doi: 10.1016/j.ejphar.2020.173319. [DOI] [PubMed] [Google Scholar]
- 42.Niswender C.M., Conn P.J. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aira Z., Buesa I., Gallego M., García del Caño G., Mendiable N., Mingo J., et al. Time-dependent cross talk between spinal serotonin 5-ht2a receptor and mGluR1 subserves spinal hyperexcitability and neuropathic pain after nerve injury. J. Neurosci. 2012;32(39):13568–13581. doi: 10.1523/JNEUROSCI.1364-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palazzo E., Genovese R., Mariani L., Siniscalco D., Marabese I., de Novellis V., et al. Metabotropic glutamate receptor 5 and dorsal raphe serotonin release in inflammatory pain in rat. Eur. J. Pharmacol. 2004;492(2):169–176. doi: 10.1016/j.ejphar.2004.03.063. [DOI] [PubMed] [Google Scholar]
- 45.Chung G., Kim C.Y., Yun Y.-C., Yoon S.H., Kim M.-H., Kim Y.K., et al. Upregulation of prefrontal metabotropic glutamate receptor 5 mediates neuropathic pain and negative mood symptoms after spinal nerve injury in rats. Sci. Rep. 2017;7(1):9743. doi: 10.1038/s41598-017-09991-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao S.-H., Wen H.-Z., Shen L.-L., Zhao Y.-D., Ruan H.-Z. Activation of mGluR1 contributes to neuronal hyperexcitability in the rat anterior cingulate cortex via inhibition of HCN channels. Neuropharmacology. 2016;105:361–377. doi: 10.1016/j.neuropharm.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 47.Han L., Dong X. Itch mechanisms and circuits. Annu. Rev. Biophys. 2014;43:331–355. doi: 10.1146/annurev-biophys-051013-022826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akiyama T., Ivanov M., Nagamine M., Davoodi A., Carstens M.I., Ikoma A., et al. Involvement of TRPV4 in serotonin-evoked scratching. J. Invest. Dermatol. 2016;136(1):154–160. doi: 10.1038/JID.2015.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee S.H., Cho P.S., Tonello R., Lee H.K., Jang J.H., Park G.Y., et al. Peripheral serotonin receptor 2B and transient receptor potential channel 4 mediate pruritus to serotonergic antidepressants in mice. J. Allergy Clin. Immunol. 2018;142(4):1349. doi: 10.1016/j.jaci.2018.05.031. 52.e16. [DOI] [PubMed] [Google Scholar]
- 50.Lounsbury K. In: Pharmacology. Hacker M., Messer W., Bachmann K., editors. Academic Press; San Diego: 2009. Chapter 6 - signal transduction and second messengers; pp. 103–112. [Google Scholar]
- 51.Sugiuar T., Bielefeldt K., Gebhart G.F. TRPV1 function in mouse colon sensory neurons is enhanced by metabotropic 5-hydroxytryptamine receptor activation. J. Neurosci. 2004;24(43):9521–9530. doi: 10.1523/JNEUROSCI.2639-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shim W.S., Tak M.H., Lee M.H., Kim M., Kim M., Koo J.Y., et al. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J. Neurosci. 2007;27(9):2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kajihara Y., Murakami M., Imagawa T., Otsuguro K., Ito S., Ohta T. Histamine potentiates acid-induced responses mediating transient receptor potential V1 in mouse primary sensory neurons. Neuroscience. 2010;166(1):292–304. doi: 10.1016/j.neuroscience.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 54.Hermans E., Challiss R.A. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem. J. 2001;359(Pt 3):465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim Y.H., Park C.K., Back S.K., Lee C.J., Hwang S.J., Bae Y.C., et al. Membrane-delimited coupling of TRPV1 and mGluR5 on presynaptic terminals of nociceptive neurons. J. Neurosci. 2009;29(32):10000–10009. doi: 10.1523/JNEUROSCI.5030-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw and analyzed datasets in this study are available upon reasonable request from corresponding author Dr. Ahmad Reza Dehpour.