Key Points
Question
Is it feasible to deploy prehospital zone 1 (supraceliac) partial resuscitative endovascular balloon occlusion of the aorta (Z1 P-REBOA) in the prehospital resuscitation of adult trauma patients at risk of cardiac arrest and death due to exsanguination?
Findings
In this cohort study of 16 patients with severe injuries and shock, prehospital Z1 P-REBOA was delivered successfully in 8 of the 11 patients who underwent Z1 REBOA. This strategy was associated with improved proximal blood pressure and early mortality.
Meaning
The findings indicate that in the prehospital resuscitation of adult trauma patients at risk of cardiac arrest and death due to exsanguination, Z1 P-REBOA is feasible and may enable early survival, but with a significant incidence of late death.
This cohort study evaluates the use of prehospital partial resuscitative endovascular balloon occlusion of the aorta in injured patients at risk of exsanguination.
Abstract
Importance
Hemorrhage is the most common cause of preventable death after injury. Most deaths occur early, in the prehospital phase of care.
Objective
To establish whether prehospital zone 1 (supraceliac) partial resuscitative endovascular balloon occlusion of the aorta (Z1 P-REBOA) can be achieved in the resuscitation of adult trauma patients at risk of cardiac arrest and death due to exsanguination.
Design, Setting, and Participants
This was a prospective observational cohort study (Idea, Development, Exploration, Assessment and Long-term follow-up [IDEAL] 2A design) with recruitment from June 2020 to March 2022 and follow-up until discharge from hospital, death, or 90 days evaluating a physician-led and physician-delivered, urban prehospital trauma service in the Greater London area. Trauma patients aged 16 years and older with suspected exsanguinating subdiaphragmatic hemorrhage, recent or imminent hypovolemic traumatic cardiac arrest (TCA) were included. Those with unsurvivable injuries or who were pregnant were excluded. Of 2960 individuals attended by the service during the study period, 16 were included in the study.
Exposures
ZI REBOA or P-REBOA.
Main Outcomes and Measures
The main outcome was the proportion of patients in whom Z1 REBOA and Z1 P-REBOA were achieved. Clinical end points included systolic blood pressure (SBP) response to Z1 REBOA, mortality rate (1 hour, 3 hours, 24 hours, or 30 days postinjury), and survival to hospital discharge.
Results
Femoral arterial access for Z1 REBOA was attempted in 16 patients (median [range] age, 30 [17-76] years; 14 [81%] male; median [IQR] Injury Severity Score, 50 [39-57]). In 2 patients with successful arterial access, REBOA was not attempted due to improvement in clinical condition. In the other 14 patients (8 [57%] of whom were in traumatic cardiac arrest [TCA]), 11 successfully underwent cannulation and had aortic balloons inflated in Z1. The 3 individuals in whom cannulation was unsuccessful were in TCA (failure rate = 3/14 [21%]). Median (IQR) pre-REBOA SBP in the 11 individuals for whom cannulation was successful (5 [46%] in TCA) was 47 (33-52) mm Hg. Z1 REBOA plus P-REBOA was associated with a significant improvement in BP (median [IQR] SBP at emergency department arrival, 101 [77-107] mm Hg; 0 of 10 patients were in TCA at arrival). The median group-level improvement in SBP from the pre-REBOA value was 52 (95% CI, 42-77) mm Hg (P < .004). P-REBOA was feasible in 8 individuals (8/11 [73%]) and occurred spontaneously in 4 of these. The 1- and 3-hour postinjury mortality rate was 9% (1/11), 24-hour mortality was 27% (3/11), and 30-day mortality was 82% (9/11). Survival to hospital discharge was 18% (2/11). Both survivors underwent early Z1 P-REBOA.
Conclusions and Relevance
In this study, prehospital Z1 P-REBOA is feasible and may enable early survival, but with a significant incidence of late death.
Trial Registration
ClinicalTrials.gov Identifier: NCT04145271
Introduction
Uncontrolled bleeding is the most common cause of preventable death after injury and is responsible for approximately one-third of all trauma-related mortality.1,2,3,4,5 Most of these deaths, both in civilian and military settings, occur rapidly from exsanguination—typically within 30 minutes of injury and well before most patients can reach the hospital.6,7,8 For those that do reach the hospital, mortality is highest in the first 3 to 72 hours, attributable to the consequences of early and profound hemorrhagic shock.8,9 Most deaths are due to noncompressible torso hemorrhage, and yet there are no established solutions for temporizing this bleeding in the prehospital or far-forward environment.
Resuscitative endovascular balloon occlusion of the aorta (REBOA) is a technique for the temporary proximal control of subdiaphragmatic torso hemorrhage.10 Balloon occlusion of the descending thoracic aorta (zone 1 [Z1], supraceliac) reduces distal blood loss while also improving coronary and cerebral perfusion.11,12 This intervention can temporarily delay cardiac arrest from exsanguination, potentially extending the time window to provide definitive treatment.13 However, in the UK, the average time taken to reach a hospital following major injury is 90 minutes, long after the window of potential benefit.3,14
In the prehospital setting, where there is potentially the most benefit from earlier, temporary truncal hemorrhage control, we have previously shown that it is possible to deploy REBOA.15,16 However, this was limited to infrarenal placement (Z3) to avoid prolonged visceral ischemia.15 To further investigate this resuscitation strategy in this setting, it is important to establish whether it can be delivered in patients with more proximal hemorrhage in the abdomen (Z1 REBOA) and to achieve maximal increase in proximal blood pressure (BP) and therefore myocardial perfusion in patients with profound shock and traumatic cardiac arrest (TCA) caused by subdiaphragmatic exsanguinating hemorrhage.10,13,17 However, this comes with an increased risk of visceral ischemic injury.
Partial REBOA (P-REBOA) describes allowing titrated low-volume aortic flow past the balloon. It can provide a balance by creating a region of distal permissive hypotension, thus reducing hemorrhage, while mitigating distal ischemia or reperfusion injury and simultaneously augmenting proximal hemodynamics.18,19 To our knowledge, Z1 P-REBOA has not previously been deployed in the prehospital environment.
The overall aim of this study was to explore the potential use of prehospital Z1 P-REBOA in patients with immediately life-threatening noncompressible torso hemorrhage. Our first objective was to determine whether it was feasible to deploy prehospital Z1 REBOA in critically bleeding trauma patients. The second objective was to determine the feasibility of delivering controlled P-REBOA in these patients and assess technical performance. Finally, we wished to explore the potential impact of Z1 P-REBOA on prehospital and in-hospital clinical outcomes and safety.
Methods
Study Design
We performed a prospective observational cohort study of prehospital Z1 P-REBOA delivered by a physician-led prehospital team in an advanced urban trauma system including injured patients with immediately life-threatening subdiaphragmatic hemorrhage. The study is a sequentially reported case series, adhering to the Idea, Development, Exploration, Assessment and Long-term follow-up (IDEAL) 2A framework for surgical innovation.20 Patients were followed up until discharge, death, or for 90 days from the date of the final patient’s admission to hospital. Due to the emergency context, enrollment proceeded without consent. Consent for continued participation was sought once patients were no longer in a life-threatening condition. Ethics approval was granted by the London–Southeast Research Ethics Committee and the Health Research Authority and sponsored by Barts Heath National Health Service Trust. All cases were reviewed by a multidisciplinary investigative committee, which provided oversight and guidance for the study management group.
Setting
London’s Air Ambulance provides a 24-hour dedicated advanced trauma service to Greater London, working in conjunction with the London Ambulance Service. The physician-paramedic team attends approximately 2000 injured patients per year and can deliver advanced prehospital interventions, including prehospital anesthesia, blood transfusion, resuscitative thoracotomy, and REBOA (Z3, since 2014).15,21 Severely injured patients are transported to 1 of London’s 4 major trauma centers.
Inclusion and Exclusion Criteria
Eligible participants for enrollment were trauma patients aged 16 years and older and attended by London’s Air Ambulance with a clinical diagnosis of exsanguinating subdiaphragmatic hemorrhage, with recent or imminent risk of hypovolemic cardiac arrest thought to be amenable to treatment with Z1 REBOA. Exclusion criteria were patients suspected to be younger than 16 years, unsurvivable injuries, or pregnancy. Patients entered the study at the point an attempt was made at common femoral artery access for Z1 REBOA.
Prehospital REBOA
Z1 REBOA was performed as an adjunct to standard trauma care via an 8F access sheath (Avanti; Cordis) inserted percutaneously into the common femoral artery under ultrasound guidance and using the ER-REBOA catheter (Prytime Medical Devices). Arterial BP was measured distal to the balloon (common femoral artery, sidearm access sheath) and proximal to the balloon (thoracic aorta, catheter tip). Catheter insertion depth was determined by measuring external landmarks (cannulation site to mid sternum) targeting approximately 45 cm.22,23 The balloon was inflated using 0.9% saline until the distal pulsatile pressure trace (measured from the access sheath sidearm) became nonpulsatile and proximal pressure improved.19
Pressure measured distal to the site of balloon occlusion is directly proportional to flow, exhibiting a linear relationship.18 An increase in the distal mean arterial pressure of 5 to 10 mm Hg above the postinflation baseline is associated with an increase in distal flow of 250 to 500 mL/min.18,19 Therefore, when instituting P-REBOA, distal pressure can be targeted as a surrogate for flow.
Outcomes
Successful prehospital Z1 REBOA was defined as the composite end point of balloon insertion to 35 to 55 cm, proximal arterial BP transduced, and balloon inflation. Successful prehospital P-REBOA was defined as the composite end point of evidence of an increase in distal mean arterial pressure of at least 5 to 10 mm Hg above the postinflation baseline and/or a return of distal pulsatility, or distal pulsatility never absent postnitial balloon inflation.
Clinical end points included systolic BP (SPB) response to Z1 REBOA, incidence of prehospital traumatic cardiac arrest, mortality rate (1 hour, 3 hours, 24 hours, or 30 days postinjury), cause of death, and survival to hospital discharge. All adverse events related to patient injury, resulting critical illness, and treatment as well as femoral cannulation, REBOA catheter insertion, and/or the anticipated effects of aortic occlusion were predefined and recorded.
Data Collection
Temporal, physiological, and clinical data were prospectively collected and recorded using REDCap.24 Physiological data were downloaded directly from the patient monitor (ZOLL X-series; Zoll Medical). Other data were obtained from original sources, including standard prehospital and in-hospital patient records, blood tests, imaging, and postmortem data. Injuries were classified according to the Abbreviated Injury Scale 2005 and Injury Severity Score by certified coders.25,26
Statistical Analysis
Continuous data are reported as medians with IQRs and categorical data as frequency and percentage. Paired data were compared using the Wilcoxon matched-pairs signed rank test (PRISM version 10; GraphPad). Statistical significance was set as a 2-tailed P value less than .05.
Results
During the 21-month study period (June 2020 through to March 2022), of 2960 individuals attended by the service, arterial access for Z1 REBOA was attempted in 16 patients (median [range] age, 30 [17-76] years; 13 [81%] male and 3 [19%] female). Most injuries (13/16 [81%]) were caused by high-energy blunt trauma (Table 1). The median (IQR) time from injury to London’s Air Ambulance arrival on scene was 21 (16-28) minutes. Patients were critically injured, with a median (IQR) Injury Severity Score of 50 (39-57), and were profoundly hypotensive, with a median (IQR) initial SBP measured by London’s Air Ambulance of 58 (47-82) mm Hg and diastolic BP (DBP) of 35 (27-49) (Table 1). One patient had unrecordable noninvasive BP but a palpable central pulse. Three patients were in TCA (Tables 1 and 2).
Table 1. Baseline and Outcome Characteristics.
| Demographic characteristics | Other characteristics | Timing/trajectory | Outcome | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient No. (sequential order) | Mechanism of injury | ISS | First BP measurement (mean arterial pressure), mm Hg | First HR measurement, bpma | Initial GCS score | Injury to LAA arrival, min | TCA preintervention | Z1 P-REBOA | Discharge from hospital alive | |
| Patients who underwent Z1 REBOA | ||||||||||
| 1 (1) | Stabbing proximal thigh/flank | 32 | 57/12 (20) | 39b | 3 | 44 | Yes | Yes | Yes | |
| 2 (2) | Fall, 13-m bridge | 50 | 59/39 (48) | 170 | 9 | 18 | Yesc | No | No | |
| 3 (3) | Fall, 22-m building | 57 | 58/28 (40) | 141 | 14 | 12 | No | Yes | No | |
| 4 (4) | Motorcyclist collision with bus stop | 55 | 47/29 (35) | 131 | 9 | 25 | No | Yes | No | |
| 5 (9) | Motorcyclist collision with lamppost | 57 | 85/39 (56) | 78 | 14 | 19 | No | Yes | No | |
| 6 (10) | Crushed by 2 cars | 16 | 103/76 (85) | 136 | 9 | 29 | No | Yes | No | |
| 7 (11) | Pedestrian hit by train | 66 | 47/31 (38) | 153 | 3 | 43 | Yesd | Yes | No | |
| 8 (12) | Fall, 18-m building | PLE | TCA | 33b | 3 | 24 | Yes | No | No | |
| 9 (14) | Fall, 22-m building | 50 | 26/17 (20) | 156 | 11 | 17 | No | Yes | No | |
| 10 (15) | Motorcyclist collision with lamppost | 50 | 74/50 (60) | 120 | 15 | 21 | Yes | No | No | |
| 11 (16) | Fall, 48-m building | 50 | U/R NIBP | 161 | 4 | 13 | No | Yes | Yes | |
| Failed attempt CFA access; Z1 REBOA attempt | ||||||||||
| 12 (5) | Buttock stabbing | 16 | TCA | 155b | 3 | 16 | Yes | No | No | |
| 13 (6) | Fall, 18-m building | 43 | 32/27 (29) | 69b | 3 | 16 | Yes | No | No | |
| 14 (13) | Groin stabbing | PLE | TCA | 10b | 3 | 45 | Yes | No | No | |
| CFA access only; no Z1 REBOA attempt due to improvement in clinical condition, patient no longer met inclusion criteria | ||||||||||
| 15 (7) | Pedestrian hit by train | 59 | 58/46 (52) | 51 | 7 | 23 | No | No | Yes | |
| 16 (8) | Fall, 30-m building | 41 | 99/63 (75) | 80 | 14 | 21 | No | No | Yes | |
Abbreviations: BP, blood pressure; GCS, Glasgow Coma Scale; HR, heart rate; ISS, Injury Severity Score; LAA, London’s Air Ambulance; N/R, no REBOA—not attempted due to clinical improvement or not recorded; NIBP, noninvasive BP; PEA, pulseless electrical activity; PLE, pronounced life extinct; P-REBOA, partial REBOA; REBOA, resuscitative endovascular balloon occlusion of the aorta; TCA, traumatic cardiac arrest; U/R, unrecordable; Z, zone.
Includes PEA.
Pulseless electrical activity.
Described immediately postfall from height, responded to initial cardiopulmonary resuscitation, then rearrested pre-REBOA.
Loss of central pulse immediately pre-REBOA.
Table 2. Clinical Outcomes.
| Patient No. (sequential order) | Proximal hemodynamic response (mean arterial pressure) | Cardiac arrest | MODS, SOFA score at admission | Length of stay, d | Survival | CPC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-REBOA BP | Post-REBOA BP | Arrival at ED BP | Prehospital TCA | RT | Critical care | Hospital | 3 h | 24 h | 30 d | 90 d or Discharge | |||
| Patients who underwent Z1 REBOA | |||||||||||||
| 1 (1) | 53/10 (21) | 96/29 (51) | 105/85 (94) | Yes | No | 13 | 38 | 62 | Yes | Yes | Yes | Yes | 1 |
| 2 (2) | 24/16 (20) | TCA, Ma | 66/41 (49) | Yesb,c | Yesd,e | 13 | <1 | <1 | Yes | No | No | No | NA |
| 3 (3) | 52/37 (30) | 105/82 (89) | 106/57 (90) | No | No | 12 | 2 | 2 | Yes | Yes | No | No | NA |
| 4 (4) | 42/13 (27) | 79/53 (62) | 110/66 (78) | No | No | 4 | 12 | 12 | Yes | Yes | No | No | NA |
| 5 (9) | 25/15 (19) | 54/28 (37) | 102/73 (79) | No | No | 17 | 2 | 2 | Yes | Yes | No | No | NA |
| 6 (10) | 40/28 (32) | 67/48 (58) | 131/71 (94) | No | No | 12 | 2 | 2 | Yes | Yes | No | No | NA |
| 7 (11) | 47/31 (38) | 111/71 (84)f | 99/46 (65)f | Yesb | No | 13 | 11 | 11 | Yes | Yes | No | No | NA |
| 8 (12) | TCA, Mg | 114/38 (38)h | PLE | Yesb,c | No | PLE | 0i | 0 | No | No | No | No | NA |
| 9 (14) | 51/29 (34) | 56/46 (52) | 80/67 (75) | No | No | 13 | 2 | 2 | Yes | Yes | No | No | NA |
| 10 (15) | TCA, Ma | 74/54 (64) | 69/52 (58) | Yesb,c | Yesd,j | 13 | <1 | <1 | Yes | No | No | No | NA |
| 11 (16) | 48/39 (43) | 104/78 (88) | 100/81 (90) | No | No | 12 | 39 | 76 | Yes | Yes | Yes | Yes | 1 |
| Failed CFA access | |||||||||||||
| 12 (5) | F/A | F/A | 99/60 (73) | Yes | Yese,k | 17 | <1 | <1 | Yes | No | No | No | NA |
| 13 (6) | F/A | F/A | 52/27 (37) | Yes | Yese,k | 15 | 0l | 0 | Yes | No | No | No | NA |
| 14 (13) | F/A | F/A | PLE | Yes | Yese,k | PLE | 0m | 0 | No | No | No | No | NA |
| CFA access only | |||||||||||||
| 15 (7) | N/R | N/R | 114/71 (81) | No | No | 13 | 46 | 246 | Yes | Yes | Yes | Yes | 1 |
| 16 (8) | N/R | N/R | 143/69 (82) | No | No | 4 | 14 | 29 | Yes | Yes | Yes | Yes | 1 |
Abbreviations: BP, blood pressure; CPC, cerebral performance category score at hospital discharge; ED, emergency department; F/A, failed arterial access, M, missing data; MODS, multiple organ dysfunction syndrome; NA, not applicable; N/R, no REBOA—not attempted due to improvement; PHRT, resuscitative thoracotomy; PLE, pronounced life extinct; REBOA, resuscitative endovascular balloon occlusion of the aorta; RT, resuscitative thoracotomy; SOFA, sequential organ failure assessment; TCA, traumatic cardiac arrest; Z, zone.
Proximal arterial BP transduced post-REBOA, monitoring setup issue (failed transducer zero), patient in TCA.
TCA occurred pre-REBOA.
TCA persisted or recurred transiently post-REBOA.
RT indication: predicted intrathoracic pathology.
Location of RT intervention: prehospital.
Noninvasive BP value, proximal transducer failure.
Monitoring issue, proximal intra-aortic balloon pump transduced post-REBOA (transducer cable disconnection), patient in TCA.
External cardiac massage, established TCA.
Died prehospital.
Location of RT intervention: operating theater.
RT indication: failed femoral arterial access, exsanguination, and aortic control.
Died in operating theater.
Died in ED.
Feasibility
Of the 16 patients, the clinical condition of 2 improved and REBOA was not attempted. In the other 14 patients who had an attempt at Z1 REBOA, all demonstrated ongoing hemodynamic deterioration despite blood product transfusion, with 8 patients in TCA by the time of intervention (57%). Three patients (all in TCA) had failed attempts at femoral arterial access (21%) due to an inability to visualize the common femoral artery with ultrasonography. All 3 underwent immediate resuscitative thoracotomy for open aortic control. The 11 remaining patients (5 of 11 in TCA [46%]) had REBOA catheters inserted in Z1. There were initial, technical arterial BP-monitoring issues in 2 of these patients (both in TCA from the point of clinical contact), leaving 9 patients with a measured pre-REBOA median (IQR) SBP of 47 (33-52) mm Hg and DBP of 28 (14-34) mm Hg.
Z1 Balloon Occlusion
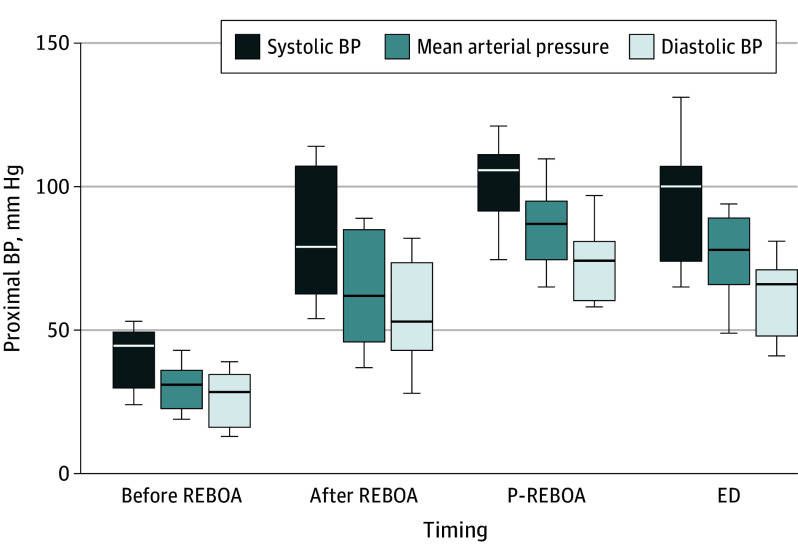
All catheters were inserted to 45 cm except 1 (47 cm). Proximal arterial (aortic) pressure was transduced in 10 of the 11 patients undergoing REBOA at the point of balloon inflation and all balloons were inflated. Therefore, the proportion of patients in whom Z1 REBOA was delivered as per definition was 91% (10 of 11). The median (IQR) time from patient injury (emergency call) to balloon inflation in Z1 was 57 (54-67) minutes. All procedures were performed at the scene of the injury except 1, which was performed during ambulance transfer to the hospital. Balloon occlusion was associated with significant improvement in BP; the 10 patients in whom BP was successfully measured, the median (IQR) post-REBOA SBP was 88 (64-107) mm HG and DBP was 51 (36-73) mm Hg (Figure 1). Median (IQR) group-level improvements in SBP and DBP were 40 mm Hg (95% CI, 5-64; P = .008) and 30 mm Hg (95% CI, 13-45; P = .008), respectively (Figures 1 and 2; Table 2; eFigure 1 and eTable 1 in Supplement 1).
Figure 1. Group-Level Hemodynamic Response to Zone 1 (Z1)–Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) and Partial (P)–REBOA.
The horizontal lines indicate medians; boxes indicate IQRs, and whiskers show maximum values. BP indicates blood pressure; ED, emergency department.
Figure 2. Individual Hemodynamic Responses to Zone 1 (Z1)–Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) and Partial (P)-REBOA.
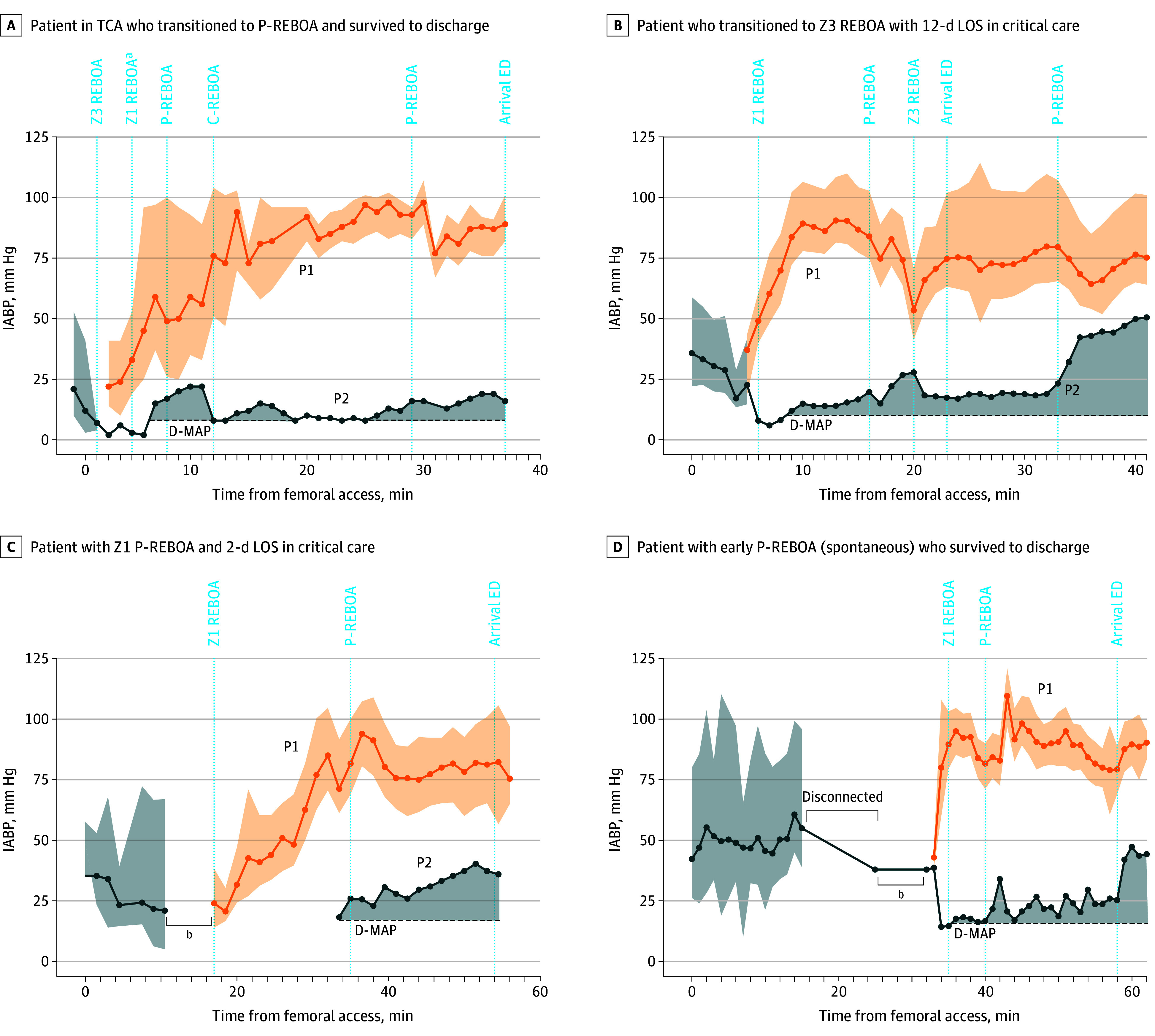
C-REBOA indicates complete REBOA; LOS, length of stay; D-MAP, postinflation distal mean arterial pressure baseline; P1, proximal (aortic) mean arterial blood pressure and pulse pressure measured from the tip of the REBOA catheter; P2, distal (common femoral artery) mean arterial pressure measured from the side arm of the 8F access sheath; TCA, traumatic cardiac arrest.
aExternal cardiac massage epinephrine, 100 μg.
bSheath upsize 4F (early femoral access for arterial blood pressure measurement) to 8F (REBOA arterial access sheath).
P-REBOA
P-REBOA was achieved in 8 of the 11 patients who underwent Z1 REBOA (73%). In 7 of these individuals, P-REBOA was instituted after a period of complete REBOA (C-REBOA) for a median (IQR) duration of 8 (3-18) minutes. The median (IQR) distal mean arterial pressure after institution of P-REBOA was 22 (20-25) mm Hg, from the immediate post–balloon inflation distal median (IQR) arterial pressure of 13 (7-15) mm Hg (median of differences, 10 mm Hg; 95% CI, 5-30; P = .02). In 1 patient, P-REBOA was used throughout the period of intervention. P-REBOA occurred spontaneously without the requirement for balloon deflation in 4 of the 8 patients undergoing P-REBOA. In the others, the median (IQR) volume removed to initiate P-REBOA was 1.0 (0.6-1.8) mL. In all 8 individuals who underwent P-REBOA, distal pulsatility on the arterial waveform was observed with the increase in distal mean arterial pressure (Table 1; Figures 1 and 2; eTable 1 and eFigure 1 in Supplement 1).
Proximal hemodynamic stability was maintained in 7 of the 8 patients during institution and maintenance of P-REBOA. The 3 patients who underwent Z1 REBOA in whom P-REBOA was not possible had associated severe ongoing proximal hypotension and therefore an inability to generate partial flow either spontaneously or by balloon deflation (Figures 1 and 2; Tables 1 and 2; eFigure 1 and eTable 1 in Supplement 1).
Prehospital Course
The median (IQR) time from injury (emergency call) to emergency department arrival was 89 (82-108) minutes. Continued improvement in proximal BP was observed compared with the pre-REBOA values, with a median (IQR) emergency department arrival SBP (in the 10 patients transported to the hospital) of 101 (77-107) mm Hg and DBP of 67 (51-75) mm Hg. Median group level improvements in SBP and DBP from preinflation values were 52 mm Hg (95% CI, 42-77; P = .004) and 42 mm Hg (95% CI, 20-58; P = .004), respectively. Three patients remained hypotensive (SBP <90 mm Hg), and 1 patient died on scene and therefore was not transported. External cardiac massage was performed post–Z1 REBOA in 3 individuals due to established TCA. This led to a sustained return of spontaneous circulation in 1 individual, with the patient surviving to hospital discharge (Figure 2). Four transient adverse events related to pressure transducer setup and operation and 1 to balloon deflation (5 of 11 cases [46%]) were reported (Figures 1 and 2; Table 2; eTables 1 and 2 in Supplement 1).
In-Hospital Management and Outcome
Definitive hemorrhage control was achieved with immediate damage-control surgery or angioembolization in 9 of the 11 patients undergoing Z1 REBOA. Of the remaining 2 patients, 1 underwent emergent orthopedic surgery and neurosurgery and 1 died on scene and therefore did not access in-hospital treatment. Ten of the 11 patients undergoing Z1 REBOA survived for more than 3 hours postinjury (1- and 3-hour mortality rate, 9%). Eight patients survived for 24 hours (24-hour mortality rate, 27%), and 2 patients survived for 30 days and to hospital discharge (30-day mortality rate, 82%; survival to hospital discharge, 18%). Both survivors underwent early Z1 P-REBOA and therefore survival to hospital discharge in the 8-patient Z1 P-REBOA cohort was 25% (2/8) (Tables 2 and 3; eFigure 2 in Supplement 1).
Table 3. In-Hospital Management, Cause, and Pathophysiological Mode of Death and Safety.
| Patient No. (sequential order) | Index hemorrhage-control intervention | Other procedure | Cause of deatha,b | Pathophysiological mode of deathb | Safety, morbidity, and complications |
|---|---|---|---|---|---|
| Patients who underwent Z1 REBOA | |||||
| 1 (1) | DCS laparotomy, packingc | Repeat laparotomy (multiple) | NA | NA | AKI, CRRT acute intestinal ischemia, bowel resection,d,e MODS |
| 2 (2) | Laparotomy, hepatic packing | Nephrectomy, hemicolectomy | 1a, Hemorrhage, hypovolemic shock; 1b, portal vein laceration/grade 4 liver laceration, renal artery injury | MODS, CS, EH | Acute intestinal ischemia,d bowel resection |
| 3 (3) | Laparotomy extraperitoneal pelvic packing; angioembolization IIAs bilaterally and lumbar artery | Repeat laparotomy/extraperitoneal pelvic packing, nasal packing epistaxis, ICP bolt insertion, debridement/stabilization bilateral open hindfoot fractures | 1a, Hemorrhage, hypovolemic shock; 1b, vertical shear pelvic fracture, bilateral internal iliac and left lumbar artery lacerations | MODS, CS, EH | AKI, CRRT, EIA cannulation, sheath removal, surgical closure arteriotomy |
| 4 (4) | Femoral traction pins, bilateral SI screws and anterior external fixation, laparotomy, surgical control gluteal arterial injury | Open forearm fracture ORIF; percutaneous lumbar spinal fracture fixation | 1a, ARDS, hypoxic ischemic encephalopathy; 1b, pneumonia, hemorrhage; 1c, comminuted unstable open pelvic fracture, kidney laceration/avulsion; 2, pulmonary contusions | Hypoxia, cerebral/edema, cerebellar tonsillar herniation | AKI, CRRT, MOD, right atrial thrombus |
| 5 (9) | Laparotomy and 4-quadrant packing | Repeat laparotomy (×2) and clamshell thoracotomy | 1a, Uncontrolled hemorrhage; 1b, grade 5 liver laceration | MODS, CS, EH | AKI, CRRT, distal arterial thrombus/embolism requiring intervention (CFA thrombectomy or embolectomy) |
| 6 (10) | DCS lower limbs bilaterally, completion traumatic AKIs | None | 1a, Hemorrhage (bilateral high SFA transections); 1b, severe bilateral crush injuries lower limbs, traumatic AKIs | MODS, vasoplegic shock | AKI, CRRT, liver dysfunction/failure, bilateral AKAs |
| 7 (11) | Laparotomy with CIA clamp | DCS lower limbs bilaterally, completion traumatic AKAs; ICP bolt: craniectomy, evacuation hematomaf | 1a, Hypoxic ischemic encephalopathy; 1b, TBI, hemorrhage-associated high bilateral traumatic lower limb amputations | TBI, exsanguination | Bilateral AKAs, MODS |
| 8 (12) | PLE | PLE | 1a, Hemorrhage, complex pelvic fracture, TBI | CS, EH, TBI | NA |
| 9 (14) | Open right distal femur, tibia fracture: external fixator/wound debridement; open right humerus fracture: external fixator/wound debridement | Decompressive craniectomy evacuation of SDH and ICP bolt | 1a, TBI and craniofacial disruption, hemorrhage—complex pelvic fracture, bilateral open femoral fractures | TBI, MODS, CS | AKI |
| 10 (15) | Thoracotomy | Nil | 1a, Hemorrhage; 1b, right renal artery laceration | MODS, CS, EH | Nil |
| 11 (16) | Laparotomy extra peritoneal pelvic packing | Angioembolization IIA and femoral arterial embolectomy; repeat laparotomy—bilateral IIA ligation | NA | NA | AKI, CRRT, MODS, distal arterial thrombus formation/embolism-thrombectomy, embolectomy |
| Failed attempt CFA access; Z1 REBOA attempt | |||||
| 12 (5) | Angioembolization IIA | Closure clamshell thoracotomy | 1a, Hemorrhage; 1b, stab wound left IIA | MODS, CS, EH | AKI, acute intestinal ischemia,d
CFA dissection |
| 13 (6) | Laparotomy and 4-quadrant packing, hepatic packing, extraperitoneal pelvic packing | In-hospital Z1 REBOA; VA ECMO cannulation | 1a, Hemorrhage; 1b, severe liver laceration, vertical shear fracture pelvis | MODS, CS, EH | AKI, CRRT |
| 14 (13) | PLE | PLE | 1a, Hemorrhage; 1b, stab wound right iliac vein | MODS, CS, EH | Nil |
| CFA access only; no Z1 REBOA attempt due to improvement in clinical condition | |||||
| 15 (7) | Angioembolization IIA bilaterally | ORIF comminuted pelvic fracture, ICP bolt; MUA nasal bones, ORIF mandible; multiple ORIF, LUL, LLL; surgical tracheostomy | NA | NA | Nil |
| 16 (8) | None | ORIF humerus and calcaneus | NA | NA | Nil |
Abbreviations: AKA, above-knee amputation; AKI, acute kidney injury; ARDS, acute respiratory distress syndrome; CFA, common femoral artery; CIA, common iliac artery; CRRT, continuous renal replacement therapy; CS, cardiogenic shock; DCS, damage-control surgery; EH, exsanguinating hemorrhage; EIA, external iliac artery; ICP, intracranial pressure; IIA, internal iliac artery; LLL, left lower lobe; LUL, left upper lobe; MODS, multiorgan dysfunction syndrome; MUA, manipulation under anesthetic; NA, not applicable; ORIF, open reduction internal fixation; PLE, pronounced life extinct; REBOA, resuscitative endovascular occlusion of the aorta; SFA, superficial femoral artery; SI, sacroiliac; TBI, traumatic brain injury; VA ECMO, venoarterial extracorporeal membrane oxygenation.
Cause of death classification: 1a indicates the disease or condition immediately causing death; 1b, the underlying cause of 1a; 1c, the underlying cause of 1b; 2, any disease or condition that did not cause death but contributed in some way.
Cause of death and pathophysiological mode of death agreed by a multidisciplinary consensus process (forensic pathologist, trauma surgeon, intensive care medicine specialist, anesthesiologist, emergency medicine physician, prehospital care physician).
Packing, not further specified.
Acute intestinal ischemia defined as evidence of gut necrosis at laparotomy or findings on computed tomography consistent with gut ischemia in the context of elevated blood lactate.
Iatrogenic bowel injury during initial procedure due to adhesions (previous laparotomy).
Iatrogenic injury ICP bolt insertion leading to acute intracranial hematoma and raised ICP.
Safety
Multiple organ dysfunction syndrome (≥2 organ system failures) was evident in 10 individuals (10/11 [91%]). Acute kidney injury (Kidney Disease: Improving Global Outcomes Criteria Stage 3) was present in 7 individuals (7/11 [64%]), with 6 (6/11 [55%]) requiring continuous renal replacement therapy. Both survivors remained free of continuous renal replacement therapy at hospital discharge. Acute intestinal ischemia was observed in 2 patients (2/11 [18%]) with both patients requiring bowel resection. Distal arterial thrombus formation requiring intervention (thrombectomy) was observed in 2 patients (2/11 [18%]), and 2 different patients received bilateral above-knee amputations (2/11, [8%]). In both cases, these procedures were completion traumatic above-knee amputations (Table 3).
Discussion
This cohort study reports the initial experience of ZI P-REBOA as a prehospital resuscitation strategy for patients with exsanguinating subdiaphragmatic hemorrhage following injury. Prehospital Z1 REBOA was feasible and associated with significant improvements in proximal BP. Z1 P-REBOA as a means of mitigating distal ischemia was implemented successfully in most cases and tolerated with proximal hemodynamic stability. The cohort of patients who met the inclusion criteria were more severely injured (median Injury Severity Score, 50) than in our previously reported observational study15 of prehospital Z3 REBOA (median Injury Severity Score, 34) and other in-hospital studies of REBOA.27,28
All patients displayed a positive hemodynamic response to Z1 REBOA. This response was gradual, with 45% remaining hypotensive (SBP <90 mm Hg) in the immediate post-REBOA phase with general recovery in proximal BP in most by emergency department arrival, and is in contrast to the often immediate BP response seen with REBOA in animal models of hypovolemic shock.17,18 This may reflect profound myocardial hypoperfusion in the immediate postinjury phase and the development of concurrent cardiogenic shock. This cohort had DBP levels below that required to allow effective coronary perfusion (median DBP preintervention, 28 mm Hg) and were therefore at high risk of myocardial ischemia, asystole, and TCA.29 Z1 REBOA was associated with an immediate increase in DBP to levels adequate to restore coronary perfusion.30 There were no instances of proximal hypertension in response to Z1 REBOA, which have been reported in large animal models to lead to hyperemic coronary blood flow, high left ventricular wall tension, and myocardial injury.12
P-REBOA was feasible in all patients who exhibited improved proximal BP post-REBOA, and the transition from C-REBOA to P-REBOA happened in less than 10 minutes. P-REBOA was observed to occur spontaneously in half of the patients. Several factors are likely to be important. First, procedurally, the balloon was inflated until the distal pulsatile trace disappeared, avoiding overinflation. Second, increased proximal BP (cardiac recovery, preload restoration, improved stroke volume and cardiac output) may have contributed. Increased aortic capacitance or diameter may have also been a factor. In individuals in whom a reduction of balloon volume was required to initiate P-REBOA, the volume removed was relatively small and again reflects the procedure (avoiding balloon overinflation). P-REBOA was not feasible in 3 individuals due to severe ongoing proximal hypotension, with 1 remaining in TCA postintervention. The prolonged period of complete aortic occlusion in 2 patients occurred due to an inability to generate a proximal pressure sufficient to deliver or establish P-REBOA or enable balloon deflation.
Three-fourths of patients survived for 24 hours postinjury despite more than half experiencing prehospital TCA due to exsanguination. However, survival to 30 days was low (2/11 [18%]), with most patients dying with clinical evidence of multiple organ dysfunction syndrome and cardiogenic shock. Early extracorporeal membrane oxygenation for patients with major injury and cardiogenic shock is now increasingly available.31,32 It is possible that earlier diagnosis of cardiogenic shock and early reperfusion with early extracorporeal membrane oxygenation could have led to survival in some cases.
Both survivors underwent early and sustained P-REBOA (occurring spontaneously in 1 patient), and both had total REBOA times significantly longer than 60 minutes (majority P-REBOA). This is in contrast to previous reports suggesting that there are no survivors beyond 60 minutes of C-REBOA and is likely to reflect the impact of P-REBOA mitigating distal ischemia, albeit this is dependent on the recovery of proximal BP.33,34,35
The UK-REBOA trial represents the first in-hospital randomized clinical trial of REBOA. It reported that the addition of a REBOA strategy to standard care, incurring a 20-minute delay to definitively control hemorrhage, increased 90-day mortality with high probability.36 This finding is important: the time taken to perform REBOA at the point of injury is likely to increase prehospital time and therefore the time to definitively control hemorrhage, with potential negative impacts on survival.37 Conversely, it is possible that a significant proportion of the patients in the present study may have died prior to accessing definitive care in hospital without advanced intervention and will continue to do so without efforts to temporize exsanguinating hemorrhage in the prehospital phase of care.6,7,8,9,14,38 Further investigation into identifying specific pathophysiological signatures indicative of early death, to potentially improve patient selection, and to allow earlier targeted intervention in those most likely to benefit while avoiding delay in those who may not, is warranted.
Strengths and Limitations
Although the study has a small sample size, it represents most cases of this type performed worldwide. Arterial BP monitoring setup errors occurred in several cases, therefore limiting the number of complete datasets for analysis. Data pertaining to blood product transfusion volume were not collected. This was an early-stage, single-arm observational study of prehospital Z1 P-REBOA focused on technical, short-term clinical and safety-related outcomes only and therefore is not designed to evaluate the effectiveness of this strategy in comparison with standard, physician-delivered prehospital care.
Conclusions
In this study, prehospital Z1 P-REBOA using distal pressure as a surrogate for flow was feasible for the resuscitation of exsanguinating trauma patients at risk of imminent prehospital death. This strategy was associated with increased proximal BP, was tolerated with ongoing proximal hemodynamic stability, and may enable early survival, but with a significant incidence of multiple organ dysfunction syndrome and late death.
eFigure 1. Individual Hemodynamic responses to Z1-REBOA and P-REBOA
eFigure 2. Kaplan-Meier Survival Estimates Z1 REBOA, P-REBOA and Resuscitative Thoracotomy
eTable 1. Technical Outcomes – Z1 REBOA Patients
eTable 2. Key Timings (minutes)
Data sharing statement
References
- 1.Tien HC, Spencer F, Tremblay LN, Rizoli SB, Brenneman FD. Preventable deaths from hemorrhage at a level I Canadian trauma center. J Trauma. 2007;62(1):142-146. doi: 10.1097/01.ta.0000251558.38388.47 [DOI] [PubMed] [Google Scholar]
- 2.Davis JS, Satahoo SS, Butler FK, et al. An analysis of prehospital deaths: who can we save? J Trauma Acute Care Surg. 2014;77(2):213-218. doi: 10.1097/TA.0000000000000292 [DOI] [PubMed] [Google Scholar]
- 3.Marsden MER, Vulliamy PED, Carden R, Naumann DN, Davenport RA; National Trauma Research and Innovation Collaborative (NaTRIC) . Trauma laparotomy in the UK: a prospective national service evaluation. J Am Coll Surg. 2021;233(3):383-394.e1. doi: 10.1016/j.jamcollsurg.2021.04.031 [DOI] [PubMed] [Google Scholar]
- 4.Marsden M, Carden R, Navaratne L, et al. Outcomes following trauma laparotomy for hypotensive trauma patients: a UK military and civilian perspective. J Trauma Acute Care Surg. 2018;85(3):620-625. doi: 10.1097/TA.0000000000001988 [DOI] [PubMed] [Google Scholar]
- 5.Harvin JA, Maxim T, Inaba K, et al. Mortality after emergent trauma laparotomy: a multicenter, retrospective study. J Trauma Acute Care Surg. 2017;83(3):464-468. doi: 10.1097/TA.0000000000001619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holcomb JB. Transport time and preoperating room hemostatic interventions are important: improving outcomes after severe truncal injury. Crit Care Med. 2018;46(3):447-453. doi: 10.1097/CCM.0000000000002915 [DOI] [PubMed] [Google Scholar]
- 7.Alarhayem AQ, Myers JG, Dent D, et al. Time is the enemy: mortality in trauma patients with hemorrhage from torso injury occurs long before the “golden hour”. Am J Surg. 2016;212(6):1101-1105. doi: 10.1016/j.amjsurg.2016.08.018 [DOI] [PubMed] [Google Scholar]
- 8.Brohi K, Gruen RL, Holcomb JB. Why are bleeding trauma patients still dying? Intensive Care Med. 2019;45(5):709-711. doi: 10.1007/s00134-019-05560-x [DOI] [PubMed] [Google Scholar]
- 9.Bardes JM, Inaba K, Schellenberg M, et al. The contemporary timing of trauma deaths. J Trauma Acute Care Surg. 2018;84(6):893-899. doi: 10.1097/TA.0000000000001882 [DOI] [PubMed] [Google Scholar]
- 10.Perkins ZB, Lendrum RA, Brohi K. Resuscitative endovascular balloon occlusion of the aorta: promise, practice, and progress? Curr Opin Crit Care. 2016;22(6):563-571. https://journals.lww.com/co-criticalcare/pages/default.aspx. doi: 10.1097/MCC.0000000000000367 [DOI] [PubMed] [Google Scholar]
- 11.Benham DA, Calvo RY, Carr MJ, et al. Is cerebral perfusion maintained during full and partial resuscitative endovascular balloon occlusion of the aorta in hemorrhagic shock conditions? J Trauma Acute Care Surg. 2021;91(1):40-46. doi: 10.1097/TA.0000000000003124 [DOI] [PubMed] [Google Scholar]
- 12.Stonko DP, Edwards J, Abdou H, et al. The underlying cardiovascular mechanisms of resuscitation and injury of REBOA and partial REBOA. Front Physiol. 2022;13:871073. doi: 10.3389/fphys.2022.871073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marsden M, Lendrum R, Davenport R. Revisiting the promise, practice and progress of resuscitative endovascular balloon occlusion of the aorta. Curr Opin Crit Care. 2023;29(6):689-695. doi: 10.1097/MCC.0000000000001106 [DOI] [PubMed] [Google Scholar]
- 14.Thrailkill MA, Gladin KH, Thorpe CR, et al. Resuscitative endovascular balloon occlusion of the aorta (REBOA): update and insights into current practices and future directions for research and implementation. Scand J Trauma Resusc Emerg Med. 2021;29(1):8. doi: 10.1186/s13049-020-00807-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lendrum R, Perkins Z, Chana M, et al. Pre-hospital resuscitative endovascular balloon occlusion of the aorta (REBOA) for exsanguinating pelvic haemorrhage. Resuscitation. 2019;135:6-13. doi: 10.1016/j.resuscitation.2018.12.018 [DOI] [PubMed] [Google Scholar]
- 16.Sadek S, Lockey DJ, Lendrum RA, Perkins Z, Price J, Davies GE. Resuscitative endovascular balloon occlusion of the aorta (REBOA) in the pre-hospital setting: an additional resuscitation option for uncontrolled catastrophic haemorrhage. Resuscitation. 2016;107:135-138. doi: 10.1016/j.resuscitation.2016.06.029 [DOI] [PubMed] [Google Scholar]
- 17.Beyer CA, Johnson MA, Galante JM, DuBose JJ. Zones matter: hemodynamic effects of zone 1 vs zone 3 resuscitative endovascular balloon occlusion of the aorta placement in trauma patients. Injury. 2019;50(4):855-858. doi: 10.1016/j.injury.2019.03.013 [DOI] [PubMed] [Google Scholar]
- 18.Austin Johnson M, Davidson AJ, Russo RM, et al. Small changes, big effects: the hemodynamics of partial and complete aortic occlusion to inform next generation resuscitation techniques and technologies. J Trauma Acute Care Surg. 2017;82(6):1106-1111. doi: 10.1097/TA.0000000000001446 [DOI] [PubMed] [Google Scholar]
- 19.Johnson MA, Neff LP, Williams TK, DuBose JJ; EVAC Study Group . Partial resuscitative balloon occlusion of the aorta (P-REBOA): clinical technique and rationale. J Trauma Acute Care Surg. 2016;81(5)(Suppl 2 Proceedings of the 2015 Military Health System Research Symposium):S133-S137. doi: 10.1097/TA.0000000000001146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirst A, Philippou Y, Blazeby J, et al. No surgical innovation without evaluation: evolution and further development of the IDEAL framework and recommendations. Ann Surg. 2019;269(2):211-220. doi: 10.1097/SLA.0000000000002794 [DOI] [PubMed] [Google Scholar]
- 21.Lendrum RA, Perkins ZB, Davies GE. A training package for zone III resuscitative endovascular balloon occlusion of the aorta (REBOA). Scand J Trauma Resusc Emerg Med. 2017;22(suppl 1):18. doi: 10.1186/1757-7241-22-S1-P18 [DOI] [Google Scholar]
- 22.Pezy P, Flaris AN, Prat NJ, et al. Fixed-distance model for balloon placement during fluoroscopy-free resuscitative endovascular balloon occlusion of the aorta in a civilian population. JAMA Surg. 2017;152(4):351-358. doi: 10.1001/jamasurg.2016.4757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linnebur M, Inaba K, Haltmeier T, et al. Emergent non-image-guided resuscitative endovascular balloon occlusion of the aorta (REBOA) catheter placement: a cadaver-based study. J Trauma Acute Care Surg. 2016;81(3):453-457. doi: 10.1097/TA.0000000000001106 [DOI] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gennarelli TA, Wodzin E. AIS 2005: a contemporary injury scale. Injury. 2006;37(12):1083-1091. doi: 10.1016/j.injury.2006.07.009 [DOI] [PubMed] [Google Scholar]
- 26.Baker SP, O’Neill B, Haddon W Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187-196. doi: 10.1097/00005373-197403000-00001 [DOI] [PubMed] [Google Scholar]
- 27.Joseph B, Zeeshan M, Sakran JV, et al. Nationwide analysis of resuscitative endovascular balloon occlusion of the aorta in civilian trauma. JAMA Surg. 2019;154(6):500-508. doi: 10.1001/jamasurg.2019.0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenner M, Inaba K, Aiolfi A, et al. ; AAST AORTA Study Group . Resuscitative endovascular balloon occlusion of the aorta and resuscitative thoracotomy in select patients with hemorrhagic shock: early results from the American Association for the Surgery of Trauma’s Aortic Occlusion in Resuscitation for Trauma and Acute Care Surgery Registry. J Am Coll Surg. 2018;226(5):730-740. doi: 10.1016/j.jamcollsurg.2018.01.044 [DOI] [PubMed] [Google Scholar]
- 29.Madurska MJ, Abdou H, Leung LY, et al. The cardiac physiology underpinning exsanguination cardiac arrest: targets for endovascular resuscitation. Shock. 2021;55(1):83-89. doi: 10.1097/SHK.0000000000001607 [DOI] [PubMed] [Google Scholar]
- 30.Davies JE, Hadjiloizou N, Leibovich D, et al. Importance of the aortic reservoir in determining the shape of the arterial pressure waveform—the forgotten lessons of Frank. Artery Res. 2007;1(2):40-45. doi: 10.1016/j.artres.2007.08.001 [DOI] [Google Scholar]
- 31.Zonies D, Codner P, Park P, et al. AAST Critical Care Committee clinical consensus: ECMO, nutrition. Trauma Surg Acute Care Open. 2019;4(1):e000304. doi: 10.1136/tsaco-2019-000304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arlt M, Philipp A, Voelkel S, et al. Extracorporeal membrane oxygenation in severe trauma patients with bleeding shock. Resuscitation. 2010;81(7):804-809. doi: 10.1016/j.resuscitation.2010.02.020 [DOI] [PubMed] [Google Scholar]
- 33.Williams TK, Tibbits EM, Hoareau GL, et al. Endovascular variable aortic control (EVAC) versus resuscitative endovascular balloon occlusion of the aorta (REBOA) in a swine model of hemorrhage and ischemia reperfusion injury. J Trauma Acute Care Surg. 2018;85(3):519-526. doi: 10.1097/TA.0000000000002008 [DOI] [PubMed] [Google Scholar]
- 34.Brenner M, Teeter W, Hoehn M, et al. Use of resuscitative endovascular balloon occlusion of the aorta for proximal aortic control in patients with severe hemorrhage and arrest. JAMA Surg. 2018;153(2):130-135. doi: 10.1001/jamasurg.2017.3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brenner M, Bulger EM, Perina DG, et al. Joint statement from the American College of Surgeons Committee on Trauma (ACS COT) and the American College of Emergency Physicians (ACEP) regarding the clinical use of resuscitative endovascular balloon occlusion of the aorta (REBOA). Trauma Surg Acute Care Open. 2018;3(1):e000154. doi: 10.1136/tsaco-2017-000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jansen JO, Hudson J, Cochran C, et al. Emergency department resuscitative endovascular balloon occlusion of the aorta in trauma patients with exsanguinating hemorrhage: the UK-REBOA randomized clinical trial. JAMA. 2023;330(19):1862-1871. doi: 10.1001/jama.2023.20850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang R, Kerby JD, Kalkwarf KJ, et al. ; PROPPR Study Group . Earlier time to hemostasis is associated with decreased mortality and rate of complications: results from the Pragmatic Randomized Optimal Platelet and Plasma Ratio trial. J Trauma Acute Care Surg. 2019;87(2):342-349. doi: 10.1097/TA.0000000000002263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carroll SL, Dye DW, Smedley WA, et al. Early and prehospital trauma deaths: who might benefit from advanced resuscitative care? J Trauma Acute Care Surg. 2020;88(6):776-782. doi: 10.1097/TA.0000000000002657 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Individual Hemodynamic responses to Z1-REBOA and P-REBOA
eFigure 2. Kaplan-Meier Survival Estimates Z1 REBOA, P-REBOA and Resuscitative Thoracotomy
eTable 1. Technical Outcomes – Z1 REBOA Patients
eTable 2. Key Timings (minutes)
Data sharing statement