Abstract
Background
Pulmonary tuberculosis may result in haematogenous and lymphatic extension in case of failure of early detection, or immunocompromised status, leading to extrapulmonary tuberculosis. Rare sites of extrapulmonary tuberculosis include the gastrointestinal tract, musculoskeletal system, genital tract, middle ear and pericardium. Histopathological findings of macro-confluent granuloma with or without caseous necrosis, along with detection of acid-fast bacilli (AFB) on Ziehl-Neelsen (ZN) staining, and GeneXpert for detection of Mycobacterium tuberculosis DNA, are key in establishing a diagnosis of tuberculosis.
Methodology
Biopsy-proven extrapulmonary granulomatous lesions were included in this study. Histopathological evaluation of all extrapulmonary biopsy specimens sent to the Department of Pathology were done for the presence of granuloma and necrosis, and ZN staining for AFB was done in all the cases of granulomatous lesions with or without the presence of necrosis. The same cases, with biopsy specimens sent in normal saline, were re-evaluated in a molecular laboratory with the help of GeneXpert MTB to detect the DNA of Mycobacterium tuberculosis. All biopsy specimens from extrapulmonary sites which were sent to the Department of Pathology were used for DNA extraction.
Results
Out of the 10 cases of extrapulmonary granulomatous lesions, 8 showed caseous necrosis on microscopy, and 7 showed the presence of acid-fast bacilli on Ziehl-Neelsen staining. GeneXpert detected DNA of Mycobacterium tuberculosis in 9 cases.
Conclusion
Extrapulmonary tuberculosis rarely occurs as primary, and mostly spreads from lung parenchyma via a haematogenous route. Tuberculosis of the gastrointestinal tract, peritoneum, lymph nodes, and solid viscera are together termed abdominal tuberculosis. Entities like tuberculosis of the pericardium and ear are extremely rare. Extrapulmonary tuberculosis should be a differential in cases of chronic non-responding cases with diagnostic dilemmas. To avoid diagnostic delay, in cases of high suspicion, one should go for biopsy along with ZN staining for diagnostic confirmation as this is cost-effective, followed by GeneXpert for Mycobacterium tuberculosis in highly suspected cases with absent caseous necrosis and negative ZN staining.
Keywords: Extrapulmonary tuberculosis, granuloma, necrosis, Ziehl-Neelsen stain
Introduction
Tuberculosis affected 10.6 million people globally in 2021.[1] Its incidence is highest in developing countries. [2,3,4] Extrapulmonary tuberculosis (EPTB) occurs secondarily by haematogenous or lymphatic extension and comprises 20-25% of cases.[5] Late detection leads to increased mortality among EPTB patients.
Among diagnostic modalities, radioimaging is useful for screening and should be followed by microscopic confirmation. Histopathology reveals confluent macro-granuloma. Caseous necrosis is specific and may warrant anti-tubercular therapy. But its absence requires Ziehl-Neelsen (ZN) stain confirmation of acid-fast bacilli (AFB), with 5000-10,000 bacilli/cc. [6] Negative ZN cases are confirmed by GeneXpert for Mycobacterium tuberculosis DNA (GeneXpert MTB). Our study includes EPTB involving different systems and rare sites, presenting with non-classical symptoms that may pose a diagnostic challenge. The study aimed to establish and describe the rare cases of EPTB in terms of clinical presentation, radiological, histopathological, microbiological (ZN stain for AFB) and molecular (GeneXpert MTB) findings.
Methodology
Ethics
All procedures performed in the current study were approved by the Institutional Research Board (Patho/52 dated 08/02/2023) and were in accordance with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all individual participants included in the study and confidentiality of the subjects was maintained.
Study design
This study was a hospital-based prospective observational study.
Place of Study
The study was conducted in the Department of Pathology and the Department of Microbiology.
Study duration
The study period was from January 2022 to June 2022 (6 months).
Source of Study and Inclusion Criteria
All biopsy specimens received from extrapulmonary sites within the defined period which histopathologically showed the presence of granuloma were included in the study.
Exclusion Criteria
Non-granulomatous lesions and granulomatous lesions from the pulmonary site were excluded from our study.
Data Sources/ Methods of Assessment
After obtaining ethical approval, all biopsy specimens of extrapulmonary lesions were evaluated for the presence of granuloma and necrosis. During our study period, we received 10 such biopsy specimens from extrapulmonary sites which histomorphologically revealed granuloma formation, and these cases were included in our study.
The specimens of clinically suspected extrapulmonary tuberculosis cases were sent in two parts: one in formalin and the other in normal saline solution. The formalin-fixed specimens were grossed as per standard protocol and slides were prepared and stained with Haematoxylin and Eosin. Microscopic evaluation of the Haematoxylin and Eosin-stained slides was done, and the presence of granuloma and necrosis were recorded. The granulomatous lesions, irrespective of the presence of necrosis, were subjected to ZN staining for AFB. AFB appearing as red-colored long narrow beaded rods were considered positive. Refractile bodies were considered as negative. Clinical and radiological findings of these cases were retrieved from the records of the patients.
The same cases which were sent as fresh biopsy specimens in normal saline solution were evaluated in the molecular laboratory with the help of GeneXpert MTB to detect DNA of Mycobacterium tuberculosis. We have used 16 modules GeneXpert from Cepheid India Pvt. Limited, with Serial no. 840271. All the tests and procedures were performed as per standard laboratory protocols and manufacturer’s instructions.
The clinical presentations of the cases, radiological, histopathological, microbiological (ZN Stain for AFB), molecular (GeneXpertMTB) and other laboratory findings were studied and compared.
All the fresh biopsy specimens from extrapulmonary sites sent in normal saline solution were used for GeneXpert.
All the biopsies from extrapulmonary sites were received in normal saline in the Department of Pathology, as per prior instructions. A part of the fresh tissue in normal saline was sent to the Department of Microbiology for GeneXpert and the rest of the specimen was fixed in formalin, and then sectioning was done as per protocol and slides prepared for histopathological examination.
Results
During our study period, we received ten cases of extrapulmonary granulomatous lesions. Out of those ten cases, two were from the omentum, and one was from the endometrium, one from the bilateral fallopian tubes, and one each from talus bone, pericardium, liver, spleen, testis, and ear, respectively. Fever, night sweats and weight loss were common in all the cases. In addition to that, cases of abdominal tuberculosis (involving the omentum, liver and spleen) presented with abdominal pain, while cases involving the genital tract (testis, endometrium and fallopian tube) presented with primary infertility. A granulomatous lesion involving the talus bone presented with pain in the ankle joint and discharging sinus, and the ear lesion presented with painful ear discharge. The patient having granulomatous pericardial lesion experienced chest pain, shortness of breath and pericardial friction rub. Radiological findings showed a hypointense lesion on the T1W image with minimal enhancement on CT in solid organs. Ultrasonography from the testis and tubo-ovarian complex showed heterogeneous and diffuse enlargement. CECT scan from the stomach showed diffuse irregular enhancing wall thickening. X-ray from the talus showed an osteolytic lesion with sclerotic margins and sequestrum.
Histomorphological findings suggested the presence of granuloma with necrosis in eight cases and 2 showed absence of necrosis. On microbiological examination, AFB was documented in 7 cases and not identified in 3 cases (Figure 1 – 3).
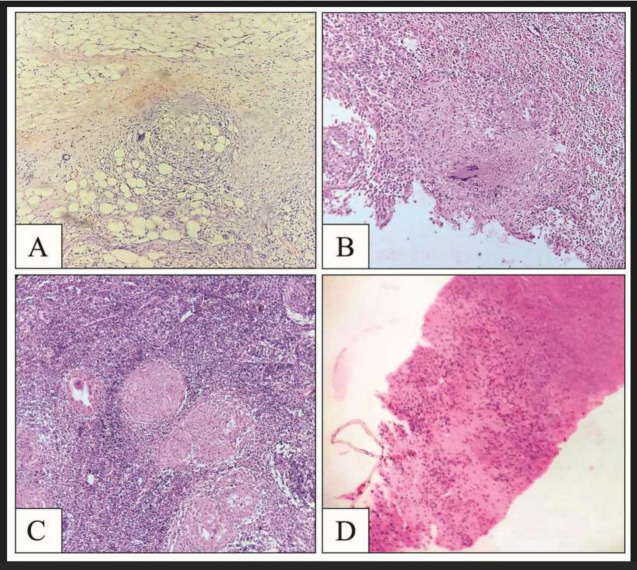
Figure 1.
A – Omentum showing epithelioid cell granuloma, HE1 stain, 10x2
B – D1 region showing granuloma with Langhans giant cells, HE stain, 10x
C – Lymph node depicting necrotizing epithelioid cell granuloma, HE stain, 10x
D – Liver parenchyma showing ill-formed granuloma with necrosis, HE stain, 10x
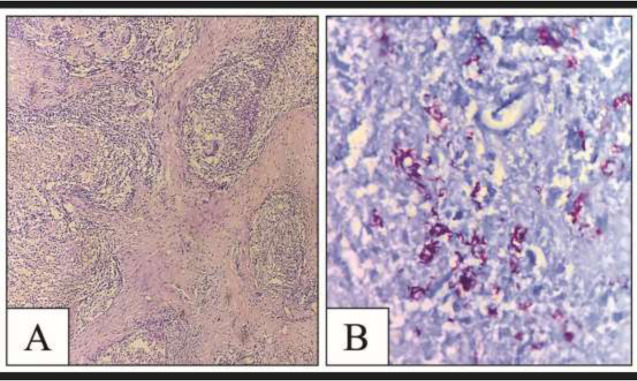
Figure 3.
A – Pericardial tissue showing epithelioid cell granuloma, HE stain, 10x
B – ZN stain demonstrating AFB in middle ear tissue as slender beaded red-colored rods
The AFB appeared as long slender beaded, red-colored rods. The presence of AFB did not correlate with the presence of necrosis, and in the 2 cases where necrosis was absent, AFB was detected by ZN stain. Conversely, the 3 cases with the absence of AFB showed the presence of necrosis.
GeneXpert MTB was performed for all ten cases of granulomatous lesions, and 9 showed GeneXpert MTB positive, thus having a sensitivity of 90% in our study.
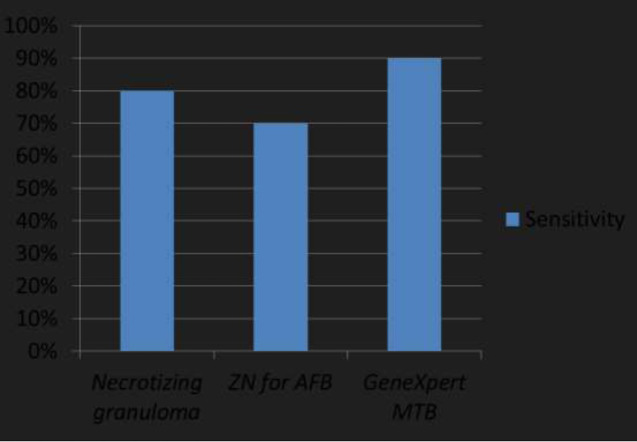
From our results, we found that the presence of necrotizing granuloma in extrapulmonary specimens had a high sensitivity of 80% when compared to a sensitivity of 70% for ZN stain on biopsy specimens. However, GeneXpert MTB on fresh biopsy specimens had the highest sensitivity of 90% among all diagnostic modalities. (Figure 4). Fresh biopsy specimens from extrapulmonary sites sent in normal saline solution were used for GeneXpert.
Figure 4.
Bar chart comparing the sensitivity of different diagnostic modalities in EPTB.
Discussion
Abdominal tuberculosis (AbTB) is the most common site for EPTB, accounting for about 12% of cases.[7] AbTB comprises tubercular lesions ofthe gastrointestinal tract, peritoneum, mesenteric lymph nodes, pancreas, spleen, etc. AbTB usually occurs by haematogenous extension or by ingestion of sputum infected with bacilli.[8] Ileo-caecum is the most commonly occurring site in AbTB involving the gastrointestinal tract. Abdominal pain, fever, anorexia, nausea, vomiting, and diarrhoea are the common presenting symptoms.At times, AbTB involving the gastrointestinal tract may present only with lymphadenopathy of mesenteric, omental, porta hepatic, celiac axis and peripancreatic lymph nodes.[9]In our study, we received AbTB cases from omentum (Figure 1A), spleen, pancreas, stomach, duodenum (Figure 1B) and retropancreatic lymph node (Figure 1C) presenting with similar symptoms and pathology as documented in literature.
Tuberculosis involving the hepatobiliary system occurs in isolation in about 21% of cases. It usually presents as miliary tuberculosis. The secondary involvement in this form of EPTB occurs through haematogenous extension. There is diffuse seeding of liver parenchyma in miliary tuberculosis in the form of millet-like tubercles, smaller than 2mm in size, whereas in local hepatic tuberculosis, the tubercles are greater than 2mm in diameter.[10] In our study we only received one case of local hepatic tuberculosis, presenting with ill-defined necrotizing granuloma (Figure 1D).
Genitourinary tuberculosis (GUTB) comprises around 20% of EPTB cases. It occurs mostly secondarily throughthe haematogenous spread of bacilli. GUTB mostly involves the kidneys. Tuberculosis involving the genitalia is very rare, among which tuberculosis ofthe testis accounts for only about 3% of the genital tuberculosis cases.[11]The other male genital organs infected with tuberculosis include prostate, seminal vesicles, vas deferens, epididymis, Cooper glands and penis, but these cases are extremely rare. The presenting complaint of male genital tuberculosis is mostly male infertility. In the female genital tract, it mostly involves the fallopian tubes (Figure 2A) but can also affect the ovaries (Figure 2B), endometrium (Figure 2C), and peritoneum. The presenting complaints among females of the reproductive age group include menstrual irregularity, abdominal pain, or lumpiness, and lastly infertility. In postmenopausal patients, it causes postmenopausal bleeding and may mimic endometrial malignancy. Mimickers of GUTB include genital malignancies, acute appendicitis, ovarian cysts, pelvic inflammatory disease, and ectopic pregnancy.[12] In our study, we received 3 cases of EPTB involving the genital tract and among those 3 cases, one female presented with primary infertility and abdominal pain, another female presented with abnormal uterine bleeding only, and the third male patient complained of testicular pain as the chief presenting symptom. This was in accordance with the data available in the literature. [11,12]
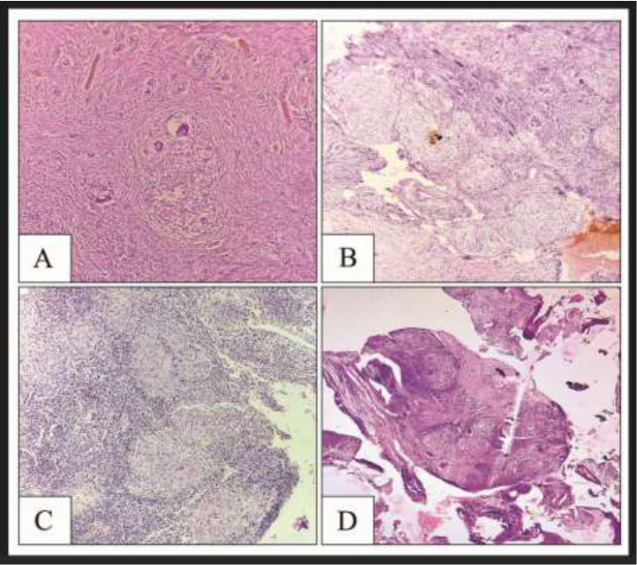
Figure 2.
A – Fallopian tube with epithelioid cell granuloma, HE stain, 10x
B – Ovarian stroma showing granuloma with Langhans giant cells, HE stain, 10x
C – Endometrium showing epithelioid cell granuloma, HE stain, 10x
D – Talus showing dead bony fragments and epithelioid cell granulomas, HE stain, 10x
The musculoskeletal system is another site involved in tuberculosis, in which bony and/or muscular tissue are affected. Bone and joint lesions occur secondarily due to haematogenous extension. 70-80% of musculoskeletal tuberculosis involves mainly weight-bearing bones and joints, with the spine, hip, and knee being the commonest sites to be involved.[13] Tuberculous arthritis is monoarticular and usually affects major joints including the hip and knee, although other joints such as the shoulder, ankle, elbow and wrist may also be involved. Tuberculous osteomyelitis mostly occurs in association with tuberculous arthritis, but it can occur even without arthritis. In our study, we received one case of tuberculosis involving the talus (Figure 2D), which presented with pain and a discharging sinus.
Pericardial tuberculosis is an uncommon form of tuberculosis and is associated with high mortality if diagnosed late with a delay in treatment. Secondary involvement is mostly through haematogenous and retrograde lymphatic extension. It can present as pericardial effusion, constrictive pericarditis, or a combination of both.[14] Definitive diagnosis of tuberculous pericarditisis made by histopathological examination of biopsy specimens (Figure 3A) and ZN staining for AFB identification. In our study, we found one case of tuberculous pericarditis who presented with chest pain and breathlessness, with radiological and histopathological features along with AFB and GeneXpert MTB confirming the presence of tuberculous pericarditis.
Tubercular otitis media (TOM) is also very rare, with an incidence of around 4% among head and neck tuberculosis cases. Sometimes, lack of suspicion due to the odd site leads to delayed diagnosis and treatment, giving rise to moderate to severe hearing loss.[15] In our study, one case showed TOM and the patient presented with discharge from the ear. Early diagnosis in this case with the presence of necrotizing granuloma along with AFB on ZN stain (Figure 3B) prevented the development of hearing loss in this patient.
Conclusion
EPTB is a rare form of tuberculosis resulting from delayed diagnosis and delayed treatment of pulmonary tuberculosis cases. One must be aware right from the start and incorporate key elements in history-taking like family history and the presence or absence of symptoms like fever, night sweats and weight loss, apart from specific symptoms. This must be supplemented by clinical and radiological examination followed by histopathological identification of caseating macro-confluent granuloma on biopsy specimens. However, if caseation is absent, one should opt for ZN staining to detect AFB. Therefore, granuloma formation with caseation and/or detection of AFB on ZN staining is diagnostic for EPTB, and the cost-effective nature of this diagnostic approach has benefits, especially in resource-poor or developing nations. GeneXpert for MTB need not be done in all cases. But if the diagnostic dilemma persists in the absence of caseating necrosis or the absence of AFB in the presence of necrosis, GeneXpert MTB should be employed, as the sensitivity of this method is more than histopathological examination alone or ZN staining.
Footnotes
HE - Haematoxylin and Eosin
10x - Magnification
References
- [1].Ates Guler S, Bozkus F, Inci MF, Kokoglu OF, Ucmak H, Ozden S. et al. Evaluation of pulmonary and extrapulmonary tuberculosis in immunocompetent adults: a retrospective case series analysis. Medical Principles and Practice. 2015 Jan 1;24(1):75-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zumla A. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. The Lancet Infectious Diseases. 2010 May 1;10(5):303-4. [Google Scholar]
- [3].Lavadi RS, Sandeep BV, Banga MS, Halhalli S, Kishan A. Spinal intramedullary tuberculoma in a 3-year-old girl. Surgical Neurology International. 2021;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rivas-Garcia A, Sarria-Estrada S, Torrents-Odin C, Casas-Gomila L, Franquet E. Imaging findings of Pott’s disease. European Spine Journal. 2013 Jun; 22:567-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].García-Rodríguez JF, Álvarez-Díaz H, Lorenzo-García MV, Mariño-Callejo A, Fernández-Rial Á, Sesma-Sánchez P. Extrapulmonary tuberculosis: epidemiology and risk factors. Enfermedade sinfecciosasy microbiologia clinica. 2011 Aug 1;29(7):502-9. [DOI] [PubMed] [Google Scholar]
- [6].González-Martín J, García-García JM, Anibarro L, Vidal R, Esteban J, Blanquer R. et al. Documento de consenso sobre diagnóstico, tratamiento prevención de la tuberculosis. Enfermedades Infecciosasy Microbiología Clínica. 2010 May 1;28(5):297-e1. [DOI] [PubMed] [Google Scholar]
- [7].Udgirkar S, Jain S, Pawar S, Chandnani S, Contractor Q, Rathi P. Clinical profile, drug resistance pattern and treatment outcomes of abdominal tuberculosis patients in Western India. Arq Gastroenterol. 2019 Aug 13;56(2):178-83. [DOI] [PubMed] [Google Scholar]
- [8].Uzunkoy A, Harma M, Harma M. Diagnosis of abdominal tuberculosis: experience from 11 cases and review of the literature. World journal of gastroenterology: WJG. 2004 Dec 12;10(24):3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gopalaswamy R, Dusthackeer VA, Kannayan S, Subbian S. Extrapulmonary tuberculosis - an update on the diagnosis,treatment and drug resistance. Journal of Respiration. 2021 May 26;1(2):141-64. [Google Scholar]
- [10].Hickey AJ, Gounder L, Moosa MY, Drain PK. A systematic review of hepatic tuberculosis with considerations in human immunodeficiency virus co-infection. BMC Infectious Diseases. 2015 Dec; 15:1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rajabi MH, Gharaei HA, ArabAhmadi A, Yarmohammadi M. Isolated tuberculosis of testis: A case report. Caspian Journal of Internal Medicine. 2021;12(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Grace GA, Devaleenal DB, Natrajan M. Genital tuberculosis in females. The Indian journal of medical research. 2017 Apr;145(4):425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Leonard Jr MK, Blumberg HM. Musculoskeletal tuberculosis. Microbiology Spectrum. 2017 Jun. 1:371-92. [DOI] [PubMed] [Google Scholar]
- [14].Mayosi BM, Burgess LJ, Doubell AF. Tuberculous pericarditis. Circulation. 2005 Dec 6;112(23):3608-16. [DOI] [PubMed] [Google Scholar]
- [15].Sebastian SK, Singhal A, Sharma A, Doloi P. Tuberculous otitis media–series of 10 cases. Journal of otology. 2020 Sep 1;15(3):95-8. [DOI] [PMC free article] [PubMed] [Google Scholar]