Abstract
Background
The prevalence of obesity (body mass index (BMI) > 30 kg/m2) is increasing in both developed and developing countries, leading to a rise in the numbers of obese patients requiring general anaesthesia. Obese patients are at increased risk of anaesthetic complications, and tracheal intubation can be more difficult. Flexible intubation scopes (FISs) are recommended as an alternative method of intubation in these patients. Intubation with an FIS is considered an advanced method, requiring training and experience; therefore it may be underused in clinical practice. Patient outcomes following intubation with these scopes compared with other devices have not been systematically reviewed.
Objectives
We wished to compare the safety and effectiveness of a flexible intubation scope (FIS) used for tracheal intubation in obese patients (BMI > 30 kg/m2) with other methods of intubation, including conventional direct laryngoscopy, non‐standard laryngoscopy and the use of intubating supraglottic airway devices. We aimed to compare the frequency of complications, as well as process indicators, such as time taken for intubation and the proportion of first attempts that were successful, between groups using the different methods of intubation.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and two trial registers on 18 January 2013, and performed reference checking and citation searching and contacted study authors to identify additional studies.
Selection criteria
We included randomized controlled trials (RCTs) of participants aged 16 years and older with a BMI > 30 kg/m2 that had compared the use of an FIS for tracheal intubation with any one of three comparison groups: direct laryngoscopy; non‐standard laryngoscopy (including indirect laryngoscopy using a videolaryngoscope (VLS) or a rigid or semi‐rigid stylet); or intubation of supraglottic airway devices (SADs).
Data collection and analysis
We used standard methodological approaches expected by The Cochrane Collaboration, including independent review of titles, data extraction and risk of bias assessment by two investigators.
Main results
Three eligible studies were identified, all comparing the use of an FIS with a VLS. All studies were small, with only 131 participants in total across all trials. It was impossible for the intubators to be unaware of the device used, so all studies were at high risk of performance and detection bias for outcomes related to intubation. Because of substantial differences in design between the studies, we did not combine their results in meta‐analyses. The results for all outcomes were inconclusive, with no differences noted between FIS and VLS. Two studies with experienced intubators reported first attempt success rates greater than 70% in both groups and less than 5% of participants requiring a change of intubation device. No evidence was found of any difference in difficulty or time taken between FIS and VLS intubation. No serious complications or airway trauma was reported, so we were unable to address these outcomes. Bleeding was uncommon, occurring in less than 5% of participants, and we found no evidence that it was more likely in the FIS group. One small study with a novice intubator reported no successful intubations using an FIS and compared with the use of an intubating SAD and stylet, as well as with a VLS. With only five participants in each group, no conclusions can be drawn from these additional comparisons.
Authors' conclusions
The evidence base is sparse, and the existing literature does not address the clinical questions of patient safety posed by this review. We are therefore unable to draw any conclusions on safety or effectiveness. More primary research is needed to investigate optimal intubation techniques in obese patients, and new studies should be powered to detect differences in complications and in success rates rather than process measures such as speed, which are of limited clinical importance.
Keywords: Humans; Anesthesia, General; Obesity; Intubation, Intratracheal; Intubation, Intratracheal/instrumentation; Intubation, Intratracheal/methods; Randomized Controlled Trials as Topic
Plain language summary
Intubation methods for obese patients requiring general anaesthesia
Patients requiring general anaesthesia need assistance with breathing during the operation. To provide this, the anaesthetist may insert a tube through the mouth or nose and down the trachea (windpipe) into the lungs. This is known as tracheal intubation, and usually the intubator uses a metal instrument called a laryngoscope to position the patient so s/he can see the vocal cords directly (direct laryngoscopy). This can be a difficult procedure in obese men and women for various reasons, including fatty tissue in the neck and throat. Guidelines suggest the use of flexible intubation scopes (FISs) for tracheal intubation in obese people. These scopes allow the intubator to see the airway via a camera, but no reviews have examined the use of an FIS in this situation. Intubation with an FIS is considered an advanced method, requiring training and experience; therefore it may be underused in clinical practice. We aimed to compare the safety and effectiveness of an FIS used for tracheal intubation in obese patients with direct laryngoscopy and other intubation methods that give the intubator an indirect view of the larynx. These other methods include videolaryngoscopes (VLSs)—metal laryngoscopes that contain a camera. We found three small studies, with a total of 131 patients, that compared an FIS with a VLS. The results for all patient safety outcomes were inconclusive, and no differences were noted between intubation with a flexible scope and intubation with a videolaryngoscope. We are unable to make any recommendations for practice based on this review. More research is needed to identify the technique for intubating obese people that would offer the best success rate with the fewest complications.
Summary of findings
Summary of findings for the main comparison. Intubation with FIS versus videolaryngoscope for obese patients requiring general anaesthesia.
| Intubation with FIS versus videolaryngoscope for obese patients requiring general anaesthesia | ||||||
| Patient or population: obese patients requiring general anaesthesia Settings: Intervention: intubation with FIS versus videolaryngoscope | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Intubation with FIS versus videolaryngoscope | |||||
| Change of intubation method | See comment | See comment | Not estimable | 131 (3) | See comment | Results from all studies were inconclusive and consistent with an increased or decreased risk of change of device in the FIS group1 |
| Patients with episodes of desaturation | See comment | See comment | Not estimable | 121 (2) | See comment | Results from all studies were inconclusive and consistent with an increased or decreased risk of hypoxia in the FIS group1 |
| Bleeding during/after intubation | See comment | See comment | Not estimable | 131 (3) | See comment | Results from all studies were inconclusive and consistent with an increased or decreased risk of bleeding in the FIS group1 |
| Sore throat | See comment | See comment | Not estimable | 85 (2) | See comment | Results from all studies were inconclusive and consistent with an increased or decreased risk of sore throat in the FIS group |
| Successful first intubation | See comment | See comment | Not estimable | 131 (3) | See comment | Results from all studies were inconclusive and consistent with an increased or decreased risk of successful first attempt in the FIS group |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Because of substantial differences in study design, these studies were not suitable for data synthesis.
Background
Description of the condition
Obesity prevalence and increase in surgery
The global prevalence of obesity is increasing. The World Health Organization (WHO) in 2008 estimated that more than 10% of the global adult population was obese, with a body mass index (BMI) > 30 kg/m2 (WHO obesity fact sheet 2012). Obesity rates vary greatly between countries, with prevalence rates ranging from USA 33%, Canada 24%, UK 23% and Greece 18% to Norway 10% and Japan and Korea both 4%, all in 2009 (OECD 2010; OECD 2012). Obesity is becoming more common in developed countries and in countries in which the current prevalence is lower than in the developed world, such as India and China. If these trends continue, projections are that the global prevalence of obesity may be 38% in 2030, with highest prevalences in China and Latin America (Kelly 2008).
Anaesthetic challenges of obesity
Obesity is a risk factor for many chronic health conditions such as diabetes, cancers and cardiovascular disease. The numbers of obese patients requiring a general anaesthetic for surgery can be expected to increase, reflecting the rising prevalence of obesity, frequent comorbidities in these patients and the use of bariatric surgery as treatment.
Obese patients pose considerable challenges for the anaesthetic team. Intubation may be more difficult because of upper airway narrowing, distorted anatomy, obstructive sleep apnoea and poorly defined external landmarks (Juvin 2003; Karkouti 2000; Lundstrom 2009). In the UK, the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society (NAP4) showed that obese patients accounted for 42% of patients who experienced a major airway complication during anaesthesia (leading to death, brain damage, emergency surgical airway or intensive care unit (ICU) admission) (Cook 2011). There is probably an increased risk of aspiration of gastric contents into the lungs of obese patients due to the presence of hiatus hernia and increased abdominal pressure (Smith 2003). The heavy chest wall, splinted diaphragm and reduced chest wall compliance alter lung volumes and gas exchange, so higher inflation pressures are required to ventilate the lungs of obese patients. Functional residual capacity (FRC), which is the volume of air left in the lungs at the end of normal expiration, is reduced in obese patients. This decreased respiratory reserve makes obese patients vulnerable to hypoxia, particularly during periods when a patient is not being ventilated, making airway management more time‐critical and increasing the risk of postoperative chest infection and other complications (Adams 2000; Malhotra 2008; Marley 2005). When tracheal intubation is difficult and multiple attempts are needed, obesity is strongly associated with difficulty with mask ventilation and other rescue techniques (Cook 2011; Cook 2012; Kheterpal 2013; Langeron 2000). There is uncertainty about the optimal anaesthetic techniques to minimize the risks in this population; this review will examine evidence comparing techniques for tracheal intubation in obese patients.
Description of the intervention
The standard anaesthetic practice of routine or rapid sequence induction, followed by tracheal intubation with direct laryngoscopy (i.e. using a device that enables the operator to gain a direct view of the laryngeal inlet), is often used in obese patients. Difficulties in visualizing the vocal cords and unsuccessful intubation may lead to a 'cannot intubate, cannot ventilate' situation. Obese patients therefore are often managed according to guidelines for the difficult airway (ASA 2003). Management options include awake intubation and alternative methods of intubation if difficulties with direct laryngoscopy are anticipated.
How the intervention might work
Potential contribution of intubation using a flexible scope
Fibreoptic intubation involves the use of an FIS, which may be a flexible bronchoscope or a flexible laryngoscope. These flexible scopes originally were all fibreoptic but may now use digital video technology. They allow a view of the airway transmitted from the tip of the flexible scope to be displayed at an eyepiece or on a screen. The selected tracheal tube (TT) is placed over the FIS, and once the flexible scope has been passed through the larynx into the trachea, the TT is moved into position and the scope removed. Unlike direct laryngoscopy, this procedure does not require head positioning (with cervical spine movement) to permit direct vision between the mouth and the vocal cords. Awake fibreoptic intubation is considered by many the "gold standard technique for difficult airway management" (Cook 2012). It is generally considered a more technically challenging method that requires training and experience (Cook 2011; Mason 1992; Smith 1997); it may therefore be underused in clinical practice. Possible complications of awake or anaesthetized fibreoptic intubation include failure to intubate, nosebleeds, arrhythmias and hypoxia (Cook 2012). Concerns have also arisen about laryngeal trauma that occurs when the scope and the TT are passed through the vocal cords without neuromuscular blocking agents (Maktabi 2002). The need for topical airway anaesthesia can occasionally lead to airway compromise (Ho 2006).
Alternative methods
Apart from flexible scopes, other alternatives to direct laryngoscopy for intubation of the difficult airway include the following devices (Behringer 2011).
Videolaryngoscopes (VLSs) or rigid fibreoptic scopes and other methods of indirect laryngoscopy. Examples include the McGrath Series 5, the Glidescope Video Laryngoscope and the Pentax Airway Scope. These devices generally consist of an anatomically shaped blade, fibreoptic bundles and a light source or a digital camera or video. Potential advantages include visualization of the airway without manipulation of the head and neck.
Rigid or semi‐rigid stylets that may be lighted, such as the Trachlight, which uses illumination of the anterior neck tissues to indicate placement, or optical, such as the Shikani Optical Stylet, which gives a view from the tip of the stylet.
Combined ventilation and intubation devices, such as an intubating supraglottic airway device (SAD), including the intubating laryngeal mask airway (ILMA), the LMA Fastrach or the LMA CTrach. These devices permit ventilation but facilitate intubation by channeling the TT. They can be used blindly or with a flexible scope.
Appendix 1 provides a list of some of the manufacturers of these devices.
Why it is important to do this review
The numbers of obese patients requiring general anaesthesia will continue to increase in both the developed world and the developing world. The advantages and disadvantages of the various intubation techniques in obese patients have not been systematically reviewed. A large body of literature compares the use of FISs with direct intubation in unselected or low‐risk populations (Adachi 2002; Aghdaii 2010; Barak 2003; Heidegger 2007; Li 2007), but studies have mainly focused on the haemodynamic response during intubation, rather than on serious airway complications. These alternative methods are sometimes used in conjunction with a flexible scope.
Other studies have compared intubation using an FIS with other non‐conventional techniques such as VLSs (Fridrich 1997; Shulman 2001), lighted stylets (Houde 2009) or intubating SADs (Joo 2001; Langeron 2001) in various patient populations, including obese patients and those with cervical spine disease. One review of non‐standard laryngoscopes and rigid fibreoptic intubation aids was limited by lack of data on patients with difficult airways (Mihai 2008). To our knowledge, no systematic reviews have examined the use of flexible intubation scopes.
Objectives
We wished to compare the safety and effectiveness of a flexible intubation scope used for tracheal intubation in obese patients (BMI > 30 kg/m2) with other methods of intubation, including conventional direct laryngoscopy, non‐standard laryngoscopy and the use of intubating supraglottic airway devices. We aimed to compare the frequency of complications as well as process indicators, such as time taken for intubation and the proportion of first attempts that were successful, between groups using the different methods of intubation.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomized controlled trials (RCTs) including quasi‐randomized studies and cluster‐randomized studies. We did not include simulation studies (i.e. those that were not set in actual clinical practice) in the review. We would have included cross‐over trials if the order of insertion had been randomly assigned. We included trials that were designed as equivalence or non‐inferiority trials and the more usual superiority trials.
Types of participants
We included studies with participants aged 16 years and older with a BMI > 30 kg/m2 requiring tracheal intubation before or during general anaesthesia. We considered both awake and asleep intubations. We included studies of planned flexible scope intubation but not studies of rescue intubation. We excluded studies of children because different anaesthetic considerations are involved in the treatment of obese children. Trials that include a mixed participant population, such as some younger than 16 years of age or non‐obese participants, were included only if the results on obese participants aged 16 years and older were reported separately.
Types of interventions
We considered studies that compared participants who underwent tracheal intubation using an FIS with any one of three comparison groups.
Direct laryngoscopy.
Non‐standard laryngoscopy (including indirect laryngoscopy using a VLS or use of a rigid or semi‐rigid stylet).
Intubating supraglottic airway devices.
Comparison groups could have used an FIS in combination with another method, for example, direct laryngoscopy used in conjunction with a flexible scope to clear the airway, but the flexible scope should not have been the primary technique. The intervention group had to use the FIS alone.
We had planned to use an amalgamated comparison group of all types of non‐standard laryngoscopy. If we had identified sufficient studies, we planned to undertake subgroup analyses for different types of devices within our main groups, for example, by comparing flexible scope tracheal intubation with the use of a VLS or even VLSs of particular designs.
Types of outcome measures
Our primary outcomes were the serious complications that underpin the clinical uncertainty about whether flexible scope intubation is the best choice in obese patients. We included failed intubation with first choice of device as a primary outcome. This is an important indicator of the success of an intubation technique. Failed intubation with first device may not always result in an adverse consequence for the patient, but it increases the risk of serious complications, especially in obese patients (Cook 2012). Other primary outcomes included serious complications and mortality. However, we anticipated that these outcomes might not be available in many eligible studies. Our secondary outcomes included surrogate process markers for airway problems, such as the numbers of attempts. We aimed to also assess the impact on patient‐reported measures of sore throat or hoarseness after surgery, as well as patient experiences of awake intubation.
Outcomes did not form part of the study eligibility assessment. We included in the review studies that met the participant, intervention and comparison criteria even if they reported no relevant outcomes. We attempted to contact study authors to find out whether data on outcomes were collected, but if such data were not available, these studies would have been recorded in a separate category of "eligible studies but no outcome data available".
Primary outcomes
Failed tracheal intubation with first device—change of intubation method required.
Hypoxia from induction to successful intubation, expressed as either dichotomous data (episodes of arterial oxygen saturation < 90%) or continuous data (lowest or mean arterial oxygen saturation).
Serious respiratory complications (including aspiration and lower respiratory tract infection) within 30 days of anaesthetic.
Mortality within 30 days of anaesthetic.
Secondary outcomes
Laryngeal or airway trauma, including any one of damage to vocal cords, bleeding or dental injury.
Participant‐reported sore throat or hoarseness, both early (within two hours of anaesthetic) and late (within 48 hours of anaesthetic).
Participant‐reported experience of awake intubation. We accept locally derived scales, as well as instruments used in other studies (Schnack 2011).
Proportion with successful first tracheal intubation.
Number of attempts for tracheal intubation,
Total time for tracheal intubation and commencement of ventilation.
Difficulty of tracheal intubation, as assessed by intubator or assessor.
Search methods for identification of studies
Electronic searches
We searched for eligible trials in the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL, 2012, Issue 12), MEDLINE (via Ovid) (from 1970 to 18 January 2013) and EMBASE (via Ovid) (from 1980 to 18 January 2013). We applied the Cochrane highly sensitive filter for RCTs in MEDLINE and EMBASE. Our search strategies for MEDLINE, EMBASE and CENTRAL are presented in the appendices (Appendix 2; Appendix 3; Appendix 4). We included all publications that reported study data, including abstracts, letters and articles. We did not restrict language of publication.
We searched the following trial registers in January 2012 for ongoing trials: www.clinicaltrials.gov and Current Controlled Trials (http://www.controlled‐trials.com/).
Searching other resources
We undertook forwards (January 2013) and backwards (March 2013) citation tracing for key review articles and eligible articles identified from the electronic resources.
We used four articles for forward citation tracing. These were decided after discussion between investigators and were studies or reviews of non‐standard airway management in obese patients or those with difficult airways (Abdelmalak 2011; Aikins 2010; Hagberg 2009; Mihai 2008). We used the Web of Science to identify all papers that had cited these articles and amalgamated the records with results derived from electronic databases.
One review author (AN) reviewed the reference lists (backwards citation tracing) of all three included articles (Abdelmalak 2011; Aikins 2010; Rosenstock 2012) and four additional articles that had been identified during title/abstract review as a source of useful references (Cullen 2012; Hagberg 2009; Hagberg 2010; Konrad 2011). AFS and AN reviewed all titles and abstracts from publications of potential interest.
We contacted investigators known to be involved in previous studies to enquire about ongoing or unpublished studies.
Data collection and analysis
Selection of studies
We collated the results of the searches and removed duplicates. The selection of eligible articles took place in two stages.
Two review authors (AN and AFS ) screened all titles and abstracts or clinical trial entries to remove studies that were very unlikely to be eligible. If no abstract was available but the title was possibly relevant, we obtained the full text of the article. We investigated clinical trial entries that were potentially eligible by searching for publications in MEDLINE and, if necessary, by contacting the investigator.
When all titles and abstracts had been screened, AN and AFS reviewed the full text of potentially relevant titles and recorded the details on the study eligibility form (a draft is included in Appendix 5) and met to compare results. Any differences that could not be resolved would have been referred to TMC. We recorded in a PRISMA flowchart the number of papers retrieved and exclusions at each stage, along with reasons given for those that were reviewed in full text. We summarized the details of ineligible papers that are well known or that might have appeared to be eligible in the Characteristics of excluded studies table.
Data extraction and management
AN and AFS extracted data from eligible studies using a paper extraction form (Appendix 5). If duplicate publications from the same study had been identified, we planned to create a composite dataset from all eligible publications.
If relevant information or data were not available in the paper, we contacted the lead author to request the additional details. We resolved disagreements by discussion and, if necessary, by consultation with TMC.
Assessment of risk of bias in included studies
We used the Cochrane risk of bias tool to assess the quality of the study design and the extent of potential bias (Higgins 2011a). We considered the following domains.
Sequence generation.
Allocation concealment.
Blinding of participants, personnel and outcomes assessors.
Incomplete outcome data.
Selective outcomes reporting.
It was not possible for the anaesthetist or the intubator to be blinded to the intervention in this research question; similarly, it was difficult for the assessors of outcomes during intubation to be unaware of the allocation of the participant. Outcomes assessed during or after the operation, such as airway trauma or respiratory complications, could be assessed by staff other than the intubator who were unaware of the intubation method used. Hypoxia during intubation may be influenced by the preoxygenation protocol, so we considered whether this was standardized between groups. It was feasible for asleep intubations for the participant to not know the intubation method used, but not for awake intubations, which may be important for participant‐reported outcomes such as sore throat.
Other sources of bias
We paid particular attention to sources of funding and the role of manufacturers and documented this information in the Characteristics of included studies table. If a study is sponsored or supported by a manufacturer, we would have attempted to determine the extent of the firm's involvement and whether any evidence of selective outcome reporting or other bias could be found. We reviewed the original protocol of the trial, if this was available, to identify any changes to procedure or missing outcome data that might indicate bias. However, sponsorship by a manufacturer was not necessarily equated with high risk of bias without other indications. We planned to undertake sensitivity analyses to assess whether overall results were altered when studies with industry support are omitted.
We completed a risk of bias table for each included study, and this was part of the data extraction form. For each outcome, risk of bias assessments were summarized for each domain in risk of bias graphs and figures and across all domains in Table 1.
Measures of treatment effect
Outcomes in this review were mainly dichotomous (mortality, complications, first attempt, failed intubation). For these outcomes, we entered into RevMan 5.2 totals and numbers of events from each randomization group and calculated risk ratios with 95% confidence intervals. For continuous measures, such as time for intubation, mean differences (MDs) were calculated if the data were presented as means and standard deviations. Continuous data reported as medians were included in the qualitative review only. Some outcomes were recorded in short ordinal scales, such as number of attempts, pain ratings for sore throat, experience of intubation. These were converted to dichotomous outcomes when appropriate.
Unit of analysis issues
We had planned to use an amalgamated comparison group combining different devices in analyses. Studies that reported more than one comparison, for example, a group allocated to flexible scope intubation may be compared with both a direct laryngoscopy group, and a non‐standard laryngoscopy group would pose unit of analysis issues, so we planned to divide the intervention group if data allowed or to combine the control groups into a single pair‐wise comparison (Section 16.5.4, Higgins 2011).
Dealing with missing data
We contacted trial authors to request missing outcome data. If this was unsuccessful, we intended to perform sensitivity analyses to compare the effects of complete case analysis, worst case scenario and last observation carried forward options on the results of individual studies and any meta‐analyses performed.
Assessment of heterogeneity
We expected that the findings for any given outcome may differ between studies included in the review. This heterogeneity may be due to:
expertise of the intubator;
method of non‐standard intubation used (e.g. use of VLS);
device used (e.g. Glidescope, Pentax);
awake or asleep intubation and degree of sedation used;
anticipated difficulty of airway, assessed through measures such as Mallampti score or history of sleep apnoea;
extent of obesity (e.g. BMI > 40 kg/m2); or
type of operation and anaesthetic given.
We assessed heterogeneity using Chi2 and I2 statistics. Important heterogeneity (Chi2 P < 0.1 or I2 > 50%) will be investigated using subgroup analyses.
Assessment of reporting biases
We planned to examine funnel plots to assess the potential for publication bias if we had 10 or more studies reporting on a particular outcome, using visual assessment supplemented by Egger’s text for asymmetry. Heterogeneity between studies may lead to asymmetry, and we would have considered this possibility when reviewing results.
In addition to studies with no published results, reporting bias may be present within a study with data on some outcomes collected but not reported. When a report or the study protocol suggests that outcomes had not been reported, we planned to contact the study author to request outcome data.
Data synthesis
We had planned to perform meta‐analysis for outcomes for which we had comparable effect measures from more than one study and when measures of heterogeneity indicate that pooling of results is appropriate, with an I2 value of > 80% suggesting that an overall estimate was not appropriate.
We planned to use an amalgamated comparison group of non‐standard laryngoscopy initially in the main analyses. If we had sufficient studies, we then planned to undertake subgroup analyses for different types of devices within our main groups, for example, comparing flexible scope tracheal intubation with the use of a VLS or stylet, or even with particular designs of VLS. We had planned to consider studies of awake intubation separately initially, as the procedure differs considerably from asleep intubation. We planned to consider the extent of sedation used as a source of heterogeneity within this group.
As we anticipated substantial differences between studies in comparison devices and in the expertise of intubators, we planned to use random‐effects statistical models for any meta‐analysis, with Mantel‐Haenszel models for dichotomous outcomes.
Subgroup analysis and investigation of heterogeneity
If we had sufficient studies, we planned subgroup analyses to investigate the potential sources of heterogeneity described above.
Expertise of intubator.
Method of non‐standard intubation used (e.g. use of VLS);
Device used (e.g. Glidescope, Pentax).
Awake or asleep intubation and degree of sedation used.
Anticipated difficulty of airway, assessed through measures such as Mallampti score or history of sleep apnoea.
Extent of obesity (e.g. BMI > 40 kg/m2).
Type of operation and anaesthetic given.
Sensitivity analysis
We planned to undertake sensitivity analyses to explore the potential impact of missing data, as described in the section Dealing with missing data. We had also planned to carry out analyses stratified by risk of bias.
Summary of findings
We used the principles of the GRADE system to give an overall assessment of the evidence related to each of the following outcomes (Guyatt 2008).
Failed intubation with first device and change of intubation method required.
Hypoxia between induction and successful intubation.
Serious respiratory complications (including aspiration and lower respiratory tract infection) within 30 days of anaesthetic.
Mortality within 30 days of anaesthetic.
Participant‐reported sore throat or hoarseness, both early (within two hours of anaesthetic) and late (within 48 hours of anaesthetic).
Participant‐reported experience of awake intubation.
The GRADE approach incorporates risk of bias, directness of evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias to give an overall measure of how confident we could be that our estimates of effect were correct. AN and AFS each independently used GRADEPRO software to create a 'Summary of findings' table for each outcome. Any discrepancies were discussed and, if needed, referred to TMC for a final decision.
Results
Description of studies
Results of the search
The results are summarized in Figure 1. We found 76 potential publications through searches of the electronic databases and a further 70 through forwards citation and 11 through backwards citation.
1.
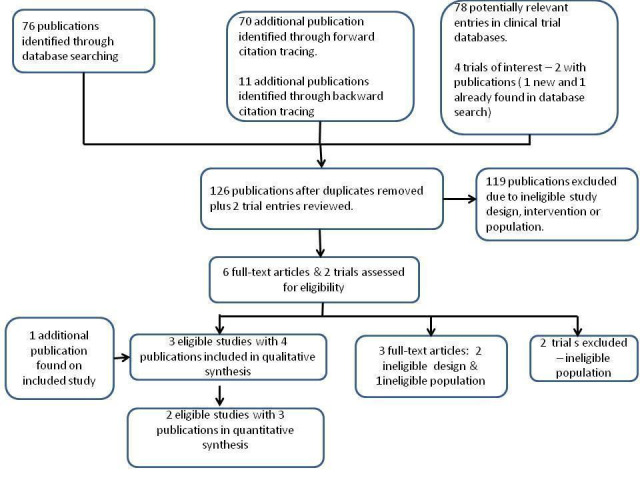
Flow chart.
We identified four trial entries of interest and linked two of these to publications (Abdelmalak 2011; Hames 2003). After removal of duplicates, we reviewed 126 titles and/or abstracts and two clinical trial entries. We identified three eligible studies, and we were notified of an additional recent publication on one study (Rosenstock 2012) during correspondence with the trial authors, so we included four publications from three studies in the review. We excluded two clinical trials, as their study populations had excluded patients with BMI > 35 kg/m2.
Included studies
The three eligible studies identified during the search (Abdelmalak 2011; Aikins 2010; Rosenstock 2012) are summarized in Characteristics of included studies.
Participants
Two studies were restricted to obese participants, 75 participants with BMI > 30 kg/m2 (Abdelmalak 2011) and 20 participants with BMI > 27.5 kg/m2 (Aikins 2010). Abdelmalak 2011 excluded patients with known difficult airways, who underwent awake intubation. Although the inclusion criterion in Aikins 2010 was < 30 kg/m2, the mean BMI in all intervention groups was > 30, and so we estimated that most participants were eligible and included the study. The study population in Rosenstock 2012 consisted of 93 participants with anticipated difficult laryngoscopy, of which 46 had BMI > 30 kg/m2.
Participants in Rosenstock 2012 had awake intubation before general anaesthesia for gynaecological, abdominal, urological and ENT procedures. In Aikins 2010 and Abdelmalak 2011, the intubation occurred after induction of anaesthesia. Anaesthesia was provided by rapid sequence induction in Aikins, and the reason for general anaesthesia was not given. In Abdelmalak, participants were undergoing unspecified elective surgery, and induction was by the standard method.
Interventions/comparisons
All three studies compared the use of an FIS with a VLS: Glidescope in Abdelmalak 2011; McGrath Series 5 in Rosenstock 2012 and a Bullard in Aikins 2010. Two additional comparison groups were included in Aikins 2010: an intubating SAD (Fastrach) and a stylet (Trachlight), but each group had only five participants. In Abdelmalak (Abdelmalak 2011), a flexible tip TT was used, but the other studies did not specify the TT model.
Expertise of intubator
The two intubators in Abdelmalak 2011 were described as experienced, and the six intubators in Rosenstock 2012 as “thoroughly trained in difficult airway management and also specifically experienced in using [both devices]”. In Aikins 2010, the study purpose was to assess the use of alternative methods by a novice anaesthesia physician. The intubator had 10 months' experience with direct laryngoscopy and had viewed instructional videos and received didactic instruction on four devices but no more than five practical experiences with devices.
Excluded studies
We excluded 119 titles/abstracts, as the design, study population or intervention did not meet our eligibility criteria. We reviewed six articles in full text. We excluded three of these because of inappropriate study design or population. We excluded two studies found via clinical trial registries, as their study populations had excluded patients with BMI > 35 kg/m2 (ISRCTN71888001; NCT01656967). The Characteristics of excluded studies table gives details of studies excluded after review in full text or trial entry.
Risk of bias in included studies
Figure 2 and Figure 3 summarize the risk of bias assessment across all three studies and for each individual study. Further details are given in Characteristics of included studies.
2.
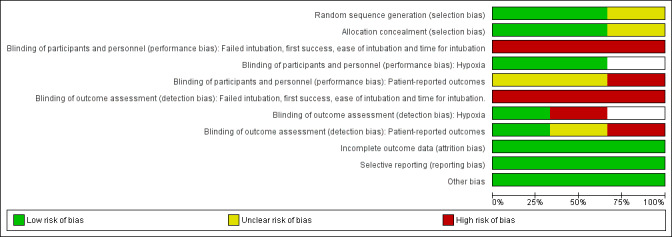
Risk of bias graph: review authors' judgements about all risk of bias items presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two studies gave sufficient detail about randomization and allocation concealment to be assessed as at low risk of selection bias (Abdelmalak 2011; Rosenstock 2012), but Aikins 2010 did not provide details.
Blinding
It is obviously not possible for the intubator to be unaware of the intubation method used, and so both performance bias and detection bias for outcomes directly related to the intubation are unavoidable. These outcomes include change of device, time taken for intubation, success rate and intubator's rating of difficulty. Hypoxaemia and episodes of desaturation during intubation are affected both by the intubation process and by preoxygenation protocols. Both studies (Abdelmalak 2011; Rosenstock 2012) reporting desaturation had standardized preoxygenation before the intubation and therefore were assessed as at low risk of performance bias. In Abdelmalak 2011, the investigator recording postintubation outcomes was blinded, but not in Rosenstock 2012. Participants in Abdelmalak 2011 were unaware of their allocation for the asleep intubations, but this was not clear in Aikins 2010. Participants undergoing awake intubations in Rosenstock 2012 were aware of their allocations, and hence participant‐reported outcomes were rated as at high risk of bias.
Incomplete outcome data
No losses to follow‐up were apparent in Abdelmalak 2011 or Aikins 2010. We were provided with intention‐to‐treat data on obese patients within Rosenstock 2012.
Selective reporting
All outcomes were prespecified in Methods and/or clinical trial entries. No outcomes were not reported.
Other potential sources of bias
In Rosenstock 2012, the VLSs used were loaned by the manufacturer, but no direct funding was provided to the study authors. No commercial involvement was reported in the other studies.
Effects of interventions
See: Table 1
Comparison of FIS with VLS
All three eligible studies reported on this comparison, with three different models of VLS, including a total of 131 participants. Other differences between the studies were awake (Rosenstock 2012) versus asleep (Abdelmalak 2011; Aikins 2010) intubation; novice (Aikins 2010) versus experienced (Abdelmalak 2011; Rosenstock 2012) intubator; and rapid sequence induction (Aikins 2010) versus standard sequence induction (Abdelmalak 2011; Rosenstock 2012). We had planned to consider awake intubation studies separately, but we found only one (Rosenstock 2012). Given the substantial differences in intubator expertise between Abdelmalak 2011 and Aikins 2010, we decided not to attempt data synthesis and described the results of individual studies.
Primary outcomes
Change of intubation method required
All studies reported the number of participants who required a change of intubation device because of failure of intubation with the allocated device, although the definition of failure varied. In Abdelmalak 2011, failed intubation was considered as longer than three minutes taken or more than four attempts with the allocated device. In Rosenstock 2012, the first technique was considered failed after three attempts, then optimal participant positioning was secured before an attempt at tracheal intubation was made with the alternative device. In both studies, these failures were crossed over to the alternative device. In Aikins 2010, for VLS and FIS two attempts were allowed with a maximum time of 120 seconds. The rescue method in this study was direct laryngoscopy.
The results from the three individual studies are summarized in Analysis 1.1. Failure rates were high in Aikins 2010, with none of the five participants successfully intubated in the FIS group and only two of five in the VLS group. Failure rates were much lower in Abdelmalak 2011 (3% in both groups) and Rosenstock 2012 (4% in the FIS group and 5% in the VLS group). The results from all studies were inconclusive and consistent with an increased or decreased risk of change of device in the FIS group.
1.1. Analysis.
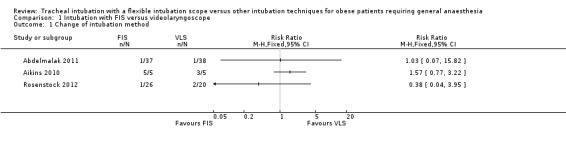
Comparison 1 Intubation with FIS versus videolaryngoscope, Outcome 1 Change of intubation method.
Hypoxia from induction to successful intubation
Both Abdelmalak 2011 and Rosenstock 2012 reported episodes of hypoxia (oxygen saturation < 90%; Analysis 1.2). In Abdelmalak 2011, the time period was anytime from one minute before intubation to 10 minutes afterwards, whereas in Rosenstock 2012, hypoxia was recorded during intubation. Hypoxia was more common in Rosenstock 2012, with 5 of 26 (19.2%) of the FIS group and 4 of 20 (20%) in the VLS group experiencing an episode of desaturation. In Abdelmalak 2011, 2 of 37 (5.4%) participants in the FIS group had an episode of desaturation compared with 4 of 38 (10.5%) in the VLS group. The results were inconclusive and consistent with an increased or decreased risk of hypoxia in the FIS group.
1.2. Analysis.
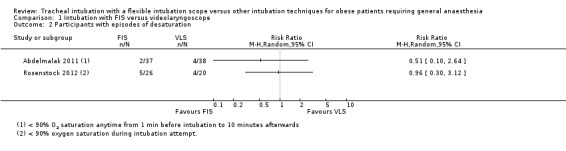
Comparison 1 Intubation with FIS versus videolaryngoscope, Outcome 2 Participants with episodes of desaturation.
Serious respiratory complications and mortality within 30 days of anaesthetic
No cases of serious complications or fatalities were reported in any study, so we were unable to study these outcomes.
Secondary outcomes
Laryngeal or airway trauma
All three studies recorded outcomes for trauma or damage (Analysis 1.3). Aikins 2010 monitored mucosal bleeding or dental damage. Rosenstock 2012 looked for tooth damage and signs of soft tissue damage, and Abdelmalak 2011 bleeding on intubation. Only bleeding occurred, with no dental injury or laryngeal trauma reported in any study. Bleeding was uncommon, occurring in 1 of 37 (2.7%) participants in the FIS group and in 1 of 38 (2.6%) in the VLS group in Abdelmalak 2011, and in 1 of 26 (3.8%) FIS participants and 1 of 20 (5%) VLS participants in Rosenstock 2012. No bleeding events were reported in Aikins 2010. The results were inconclusive and consistent with an increased or decreased risk of bleeding in the FIS group.
1.3. Analysis.
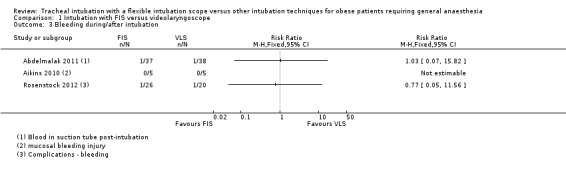
Comparison 1 Intubation with FIS versus videolaryngoscope, Outcome 3 Bleeding during/after intubation.
Participant–reported sore throat or hoarseness
This outcome was reported by Abdelmalak 2011 and Aikins 2010, both on the first postoperative day (POD1). Abdelmalak 2011 recorded symptoms as none, mild, moderate and severe, and we dichotomized this outcome to none or any (Analysis 1.4). In Aikins 2010, one of five participants in the VLS group reported mild oral discomfort but none of the five participants with failed FIS intubation. In Abdelmalak 2011, 17 of 37 (44.7%) FIS participants and 16 of 38 (42.1%) VLS participants reported a sore throat. The results were inconclusive and consistent with an increased or decreased risk of sore throat in the FIS group.
1.4. Analysis.
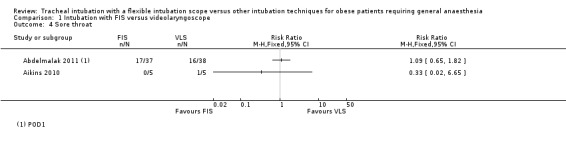
Comparison 1 Intubation with FIS versus videolaryngoscope, Outcome 4 Sore throat.
Participant‐reported experience of awake intubation
The only study of awake intubation (Rosenstock 2012) reported participants' assessment of discomfort on a visual analogue scale (0 to 10) for 25 participants in the FIS group and for 19 in the VLS group. No difference between groups was noted, with the median score of 2 (interquartile range (IQR) 1 to 3]) in the FIS group and 2 [IQR 0 to 4 in the VLS group.
Proportion with successful first intubation
All three studies reported the proportion of intubations that were successful at the first attempt (Analysis 1.5). Most participants were successfully intubated at the first attempt in Abdelmalak 2011 (32/37 (86.5%) in the FIS group and 36/38 (94.7%) in the VLS group) and in Rosenstock 2012 (20/26 (76.9%) in the FIS group and 14/20 (70.0%) in the VLS group). No FIS participants and only two of five VLS participants were successfully intubated in Aikins 2010. The results were inconclusive and consistent with an increased or decreased first attempt success rate in the FIS group.
1.5. Analysis.
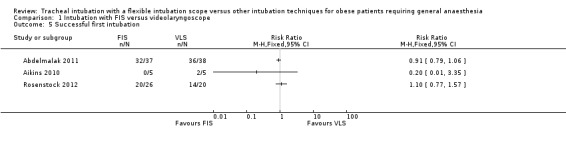
Comparison 1 Intubation with FIS versus videolaryngoscope, Outcome 5 Successful first intubation.
Number of attempts
We planned to analyse number of attempts as a continuous variable. However, as most participants required only one attempt and failure was considered after three or four attempts, this analysis did not add extra information.
Total time taken for tracheal intubation
All studies reported time to intubation, defined as time from insertion of the device to capnographic confirmation (Abdelmalak 2011; Rosenstock 2012) or manual ventilation (Aikins 2010). In Abdelmalak 2011, participants who required change of device were assigned longest successful intubation time for assigned method plus one second. Aikins 2010 reported times only for successful intubation, so did not report any times for the FIS groups. Rosenstock 2012 reported the total time including the time for intubation using the first device plus time for intubation using the alternative device, but not including the time spent preparing the alternative device.
Median times for successful intubation were similar in the two groups. In Abdelmalak 2011, the median time was 43 seconds (IQR 35 to 58) in the FIS group and 37 seconds (IQR 25 to 48) in the VLS group. In Rosenstock 2012, the median time was 65 seconds (IQR 27 to 678) in the FIS group and 65 seconds (IQR 33 to 424) in the VLS group. The results were inconclusive and consistent with an increased or decreased intubation time in the FIS group.
Difficulty of intubation as assessed by intubator or assessor
Abdelmalak 2011 and Rosenstock 2012 reported the intubator's assessment of difficulty as recorded on visual analogue scales (Analysis 1.6). FIS intubations were rated as more difficult, but the results were inconclusive and consistent with increased or decreased difficulty in the FIS group. Both of these studies used experienced intubators.
1.6. Analysis.
Comparison 1 Intubation with FIS versus videolaryngoscope, Outcome 6 Intubator's assessment of difficulty of intubation.
| Intubator's assessment of difficulty of intubation | ||||
|---|---|---|---|---|
| Study | Group (N) | Median | IQR | Significance test |
| Abdelmalak 2011 | FIS (37) | 20 | 11‐40 | |
| Abdelmalak 2011 | VLS (38) | 15 | 10‐21 | P=0.19 (Cox regression) |
| Abdelmalak 2011 | Measure : VAS 0‐100 (extremely difficult) | |||
| Rosenstock 2012 | FIS (26) | 2 | 1‐3 | |
| Rosenstock 2012 | VLS (20) | 1 | 1‐6.5 | |
| Rosenstock 2012 | Measure: VAS ‐10 (most difficult) | N/A | ||
Comparison of FIS with other devices
We found only one study that reportedcomparisons between FIS and other devices. Aikins 2010 reported comparisons between an FIS with an intubating SAD (Fastrach) and a stylet (Trachlight), but each group included only five participants. The success rate was highest in the intubating SAD group, with all five participants successfully intubated, all at the first blind attempt using a TT only. Only one of five participants assigned to the stylet was successfully intubated, also at the first attempt. The lack of data from the FIS group means that other outcomes cannot be assessed for these comparisons.
Subgroup analyses, investigation of heterogeneity and sensitivity analyses
Because of the small number of included studies, we were not able to undertake any of our planned subgroup or sensitivity analyses, nor were we able to investigate publication bias.
Discussion
Summary of main results
We found only three eligible studies that compared tracheal intubation using an FIS with a VLS in obese participants. Because of substantial differences in design between the studies, we thought it was inappropriate to combine their results. All of the studies were small, with a total of 131 participants, and the results for all outcomes were inconclusive, with no differences demonstrated between FIS and VLS (Table 1). Two studies with experienced intubators reported first attempt success rates greater than 70% in both groups and less than 5% of participants requiring a change of intubation device. No evidence was found of any difference in difficulty or time taken between FIS and VLS intubation reported in two studies, both with experienced intubators. No serious complications or airway trauma was reported, so we were unable to address these outcomes. Bleeding was uncommon, occurring in less than 5% of participants, and we found no evidence that it was more likely in the FIS group. One small study with a novice intubator reported no successful intubations with an FIS and compared this with the use of a VLS, intubating SAD and stylet. With only five participants in each group, no conclusions can be drawn from these results, although they are consistent with FIS requiring significant training and experience.
Overall completeness and applicability of evidence
Data were sparse, and the few studies conducted were too small to investigate most of our outcomes. We were unable to pool estimates because of differences between studies, but even pooled estimates would not have given conclusive results. Both Abdelmalak 2011 and Rosenstock 2012 were designed and powered to investigate differences in time taken for intubation, but the differences used in power calculations were large (45 seconds in Abdelmalak 2011 and 30 seconds in Rosenstock 2012), and the differences found in these studies were much smaller. Observational data are also sparse, with Hagberg 2009 reporting on 12 awake FIS intubations in obese participants and Ezri 2004 reporting on seven FIS intubations, but no outcome data reported in either study.
To investigate serious adverse events such as the need for change of intubation device or airway injury, much larger studies would be required. To detect an increase in the incidence of serious adverse events from 5% (based on event rates in the included studies) to 10%, with 5% significance and 80% power, 435 participants would be required in each group. This estimate of study size is based on a 100% increase in incidence and detection of a smaller difference would require even larger studies.
Quality of the evidence
Two of the three included studies were well conducted and randomized. Studies of different methods of intubation will have unavoidable performance bias.
Potential biases in the review process
We are confident that we have identified all published studies that were designed to study the use of FIS for tracheal intubation in obese patients. We did, however, restrict our search to studies specifically mentioning obesity or BMI. Although we obtained some data for obese patients from mixed‐population studies, obese participants included in other randomized studies with mixed or non‐selected populations are missing from our review.
Agreements and disagreements with other studies or reviews
Numerous studies have examined FIS intubation in unselected populations, comparing this method with conventional laryngoscopy and with other methods such as VLS. These studies have concentrated on process measures and the haemodynamic response to intubation rather than serious complications. We are not aware of any reviews or meta‐analyses that have specifically reviewed the use of FIS in either non‐selected or obese participants. FIS has been recommended as standard care when difficult tracheal intubation is anticipated (ASA 2003). Concerns about its use include the risk of laryngeal trauma (Maktabi 2002) and the need for a relatively large TT (Ovassapian 1990). It is generally considered a more technically challenging method that requires training and experience (Cook 2011; Mason 1992; Smith 1997). We found no differences in reported difficulty in studies with experienced intubators (Abdelmalak 2011; Rosenstock 2012). In the small study (Aikins 2010) with a novice intubator, no successful FIS intubations were achieved.
We aimed to review and summarize the evidence for the safety and effectiveness of using an FIS for tracheal intubation in obese patients with other methods of intubation, but because of lack of data, we have not been able to address this important issue.
Authors' conclusions
Implications for practice.
The evidence base is sparse, and the existing literature does not address the clinical questions of patient safety posed by this review. We are therefore unable to draw any conclusions on safety or effectiveness.
Implications for research.
This review highlights the lack of available data. More primary research is needed to investigate optimal intubation techniques in obese patients, including comparisons of FIS with different models of VLS, intubating stylet and intubating SAD. Subsequent questions to be addressed are whether results found in studies of elective intubation transfer to emergency situations and whether results for obese patients with predicted easy airways differ from those for obese patients with predicted difficult intubation. Future studies could also usefully examine whether the optimal intubation technique is independent of the degree of obesity (e.g. mild obesity BMI 30 to 35 kg/m2, morbid obesity BMI > 40 kg/m2 and super‐morbid obesity BMI > 50 kg/m2).
Our outcome measures were chosen to be important to patients and anaesthetists. Definitions of outcomes varied between studies, which impacts on the ease of combining data from more than one study. Improved coherence in outcomes definitions in studies of airway interventions would improve the quality of evidence that can be extracted by systematic review and meta‐analysis. No study examined longer‐term outcomes such as chest infection, long‐term tissue injury or mortality. New studies should be powered to detect differences in success rates, patient experiences and complications rather than small differences in speed, which are easier to detect but of limited clinical importance.
What's new
| Date | Event | Description |
|---|---|---|
| 16 April 2014 | Amended | Contact details updated. |
Acknowledgements
We would like to thank Mathew Zacharias and Rodrigo Cavallazzi (content editors); Cathal Walsh (statistical editor); Martin Kryspin Sørensen, Jaideep J Pandit, Aparna Sinha, Chris Frerk and Lars Lundstrom (peer reviewers); and Anne Lyddiatt (consumer reviewer) for their help and editorial advice during the preparation of this systematic review. We would also like to thank Drs Basem Abdelmalak, Charlotte Rosenstock and Carin Hagberg for their helpful replies to our enquiries about their studies.
Appendices
Appendix 1. List of example manufacturers
McGrath Series 5 (Aircraft Medical Limited, Edinburgh, UK).
Glidescope Video Laryngoscope (Verathon Medical Inc, Bothell, WA, USA).
Pentax Airway Scope (Pentax_AWS, Ambu A/S, Ballerup, Denmark),
Trachlight (Laedal Medical, Armonk, NY, USA).
Shikani Optical Stylet (Clarus Medical, LLC, Minneapolis, MN, USA).
Intubating laryngeal mask airway (ILMA) (Intavent Direct, Maidenhead, UK).
LMA Fastrach (LMA North America Inc, San Deigo, CA, USA).
LMA CTrach (Intavent Direct Maidenhead, UK).
Appendix 2. MEDLINE search strategy via Ovid
|
Search strategy for Ovid MEDLINE(R) In‐Process and Other Non‐Indexed Citations and Ovid MEDLINE(R) 1946 to Present |
|
| 1 | (gastric adj3 band*).mp. |
| 2 | exp overweight/ or exp obesity/ |
| 3 | exp bariatric surgery/ |
| 4 | (obes* or overweight* or bariatric or BMI or body mass index).mp. |
| 5 | or/1‐4 |
| 6 | ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. |
| 7 | exp Fiber Optic Technology/ |
| 8 | (fibreoptic or fiberoptic or fiberscope or fibrescope).mp. |
| 9 | ((fibre or fiber) adj3 (optic or scope$)).mp. |
| 10 | exp intubation, intratracheal/ or exp airway management/ |
| 11 | (intub$ or ((airway or respiratory tract) adj3 manage$)).mp. |
| 12 | 10 or 11 |
| 13 | 7 or 8 or 9 |
| 14 | exp bronchoscopes/ or exp laryngoscopes/ |
| 15 | (bronchoscop* or laryngoscop*).mp. |
| 16 | (14 or 15) and flexible.ti,ab. |
| 17 | (flex$ adj3 scop$).mp. |
| 18 | (Ambu adj3 (Ascope or scop$)).mp. |
| 19 | or/16‐18 |
| 20 | 13 or 19 |
| 21 | 5 and 20 and 6 and 12 |
Appendix 3. EMBASE search strategy via Ovid
| Search strategy for Ovid EMBASE 1974 to 2013 January 18 | |
| 1 | randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ |
| 2 | (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] |
| 3 | ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. |
| 4 | (latin adj square).mp. |
| 5 | (animals not (humans and animals)).sh. |
| 6 | or/1‐4 |
| 7 | 6 not 5 |
| 8 | (gastric adj3 band*).mp. |
| 9 | exp overweight/ or exp obesity/ |
| 10 | exp gastroplasty/ or exp bariatric surgery/ |
| 11 | (obes* or overweight* or bariatric or BMI or body mass index).mp. |
| 12 | or/8‐11 |
| 13 | exp fiber optics/ |
| 14 | (fibreoptic or fiberoptic or fiberscope or fibrescope).mp. |
| 15 | exp fiberoptic bronchoscopy/ or exp fiberoptic laryngoscope/ |
| 16 | ((fibre or fiber) adj3 (optic or scope$)).mp. |
| 17 | exp respiratory tract intubation/ |
| 18 | (intub$ or ((airway or respiratory tract) adj3 manage$)).mp. |
| 19 | 17 or 18 |
| 20 | or/13‐16 |
| 21 | exp bronchoscopes/ or exp laryngoscopes/ |
| 22 | (bronchoscop* or laryngoscop*).mp. |
| 23 | (21 or 22) and flexible.ti,ab. |
| 24 | exp flexible bronchoscope/ |
| 25 | (flex$ adj3 scop$).mp. |
| 26 | (Ambu adj3 (Ascope or scop$)).mp. |
| 27 | or/23‐26 |
| 28 | 20 or 27 |
| 29 | 7 and 12 and 28 and 19 |
Appendix 4. CENTRAL search strategy
ID Search
#1 gastric near/3 band in Trials
#2 MeSH descriptor: [Overweight] explode all trees
#3 MeSH descriptor: [Obesity] explode all trees
#4 MeSH descriptor: [Bariatric Surgery] explode all trees
#5 obes* or overweight* or bariatric or BMI or body mass index
#6 #1 or #2 or #3 or #4 or #5
#7 MeSH descriptor: [Fiber Optic Technology] explode all trees
#8 fibreoptic or fiberoptic or fiberscope or fibrescope
#9 (fibre or fiber) near/3 (optic or scop*)
#10 #7 or #8 or #9
#11 MeSH descriptor: [Airway Management] explode all trees
#12 intub*
#13 (airway or respiratory tract) near/3 manage*
#14 #11 or #12 or #13
#15 MeSH descriptor: [Bronchoscopes] explode all trees
#16 MeSH descriptor: [Laryngoscopes] explode all trees
#17 bronchoscop* or laryngoscop*
#18 #15 or #16 or #17
#19 FLEXIBLE:ti,ab,kw (Word variations have been searched)
#20 #18 and #19
#21 flex* near/3 scop*
#22 Ambu near/3 (Ascope or scop*)
#23 #20 or #21 or #22
Appendix 5. Study eligibility and data extraction form
1. General information
| Date form completed(dd/mm/yyyy) | |
| Name/ID of person extracting data | |
|
Report ID (ID for this paper/ abstract/ report) |
|
| Other reports from same study | |
|
Publication type (e.g. full report, abstract, letter) |
2. Study eligibility
| Study Characteristics | Eligibility criteria | Yes/No/Unclear | Location in text | ||
| Type of study | Randomized Controlled Trial | ||||
| Controlled Clinical Trial (quasi‐randomized trial & cluster‐randomised) |
|||||
| Cross‐over trial (both interventions in patients‐ order randomised) |
|||||
| Participants | Adults > 16 years with BMI > 25 kg/m2 undergoing GA | ||||
| Types of intervention and comparison | Comparison of | ||||
| fibreoptic tracheal intubation (using either a flexible bronchoscope or flexible laryngoscope alone) | |||||
| With one of | |||||
| Direct laryngoscopy | |||||
| Non‐standard laryngoscopy (including indirect laryngoscopy using a videolaryngoscope or use of rigid/semi‐rigid stylet) | |||||
| Intubating supraglottic airway devices | |||||
| Types of outcome measures | Details of outcomes & location in text | ||||
| Failed intubation or change of intubation method required. | |||||
| Hypoxia between induction and full recovery. | |||||
| Serious respiratory complications (including lower respiratory tract infection) within 30 days of anaesthetic. | |||||
| Mortality within 30 days of anaesthetic | |||||
| Patient –reported sore throat/ hoarseness | |||||
| Patient satisfaction | |||||
| Laryngeal / airway Trauma | |||||
| Number of attempts at intubation | |||||
| Time to secure airway | |||||
| Outcomes are not part of the eligibility criteria – so a study which meets design, participant and intervention criteria is included. | |||||
| INCLUDE/ EXCLUDE/ UNCLEAR | |||||
| Reason for exclusion | |||||
DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW
3. Population and setting
|
Description Include comparative information for each group (i.e. intervention and controls) if available |
Location in text | |
|
Population description (Type of surgical procedures included) |
||
|
Definition of obesity (BMI ranges and means, medians) |
||
|
Setting (including location and social context) |
||
| Inclusion criteria | ||
| Exclusion criteria | ||
| Method/s of recruitment of participants | ||
| Informed consent obtained | Yes/No/Unclear |
4. Methods
| Descriptions as stated in report/paper | Location in text | |
| Aim of study | ||
| Design(e.g. parallel, crossover, cluster) | ||
|
Unit of allocation (by individuals, cluster/ groups or body parts) |
||
| Start date | ||
| End date | ||
| Total study duration | ||
| Ethical approval needed/ obtained for study | Yes/No/Unclear |
5. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group.
| Description as stated in report/paper | Location in text | |
|
Total no. randomized (or total pop. at start of study for NRCTs) |
||
|
Clusters (if applicable, no., type, no. people per cluster) |
||
| Baseline imbalances | ||
|
Withdrawals and exclusions (if not provided below by outcome) |
||
| Age | ||
| Sex | ||
| Race/Ethnicity | ||
|
Type and duration of surgery (e.g. peripheral or abdominal) |
||
|
Type of ventilation (spontaneous or mechanical, airway pressures used) |
||
|
Details of anaesthetic given (including position, premed, preoxygenation, induction & maintenance agents ) |
||
|
Neuromuscular blockade given (agents used) |
||
| Training and seniority of intubator | ||
| Other relevant sociodemographics | ||
| Subgroups measured | ||
| Subgroups reported |
6. Intervention groups
6.1 Intervention group
| Description as stated in report/paper | Location in text | |
| Group name | Fibreoptic intubation | |
| No. randomized to group | ||
|
Description of device (name and manufacturer ) |
||
| Method of insertion | ||
| Co‐interventions |
6.2 Comparison group ‐ repeated as required
| Description as stated in report/paper | Location in text | |
|
Group name (direct laryngoscopy, non standard laryngoscopy or intubating SAD) |
||
| No. randomized to group | ||
|
Description of device (name and manufacturer ) |
||
| Method of insertion | ||
| Co‐interventions |
7. Outcomes
| TYPES OF OUTCOME MEASURES | MEASURED | REPORTED | FORM COMPLETED |
| Primary outcomes | |||
| Failed intubation or change of airway device required. | |||
| Hypoxia between induction and full recovery. | |||
| Serious respiratory complications (including lower respiratory tract infection) within 30 days of anaesthetic. | |||
| Mortality within 30 days of anaesthetic | |||
| Secondary outcomes | |||
| Laryngeal / airway trauma | |||
| Patient‐reported sore throat or hoarseness | |||
| Patient‐reported experience of awake intubation | |||
| Placement – proportion successful 1st | |||
| Number of attempts | |||
| Placement –total time for securing airway device and commencing ventilation | |||
| Difficulty of tracheal intubation, assessed by intubator or assessor |
For each outcome ticked please complete a separate outcome form.
| Description as stated in report/paper | Location in text | |
|
Outcome name (number of attempts, pain) |
||
| Time points measured | ||
| Time points reported | ||
| Outcome definition(with diagnostic criteria if relevant) | ||
| Person measuring/reporting | ||
|
Unit of measurement (if relevant) |
||
| Scales: levels, upper and lower limits(indicate whether high or low score is good) | ||
| Is outcome/tool validated? | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | ||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
||
| Power | ||
| RESULTS | Description as stated in report/paper | Location in text |
| Comparison | ||
| Outcome | ||
| Subgroup | ||
| Timepoint (specify whether from start or end of intervention) | ||
| Post‐intervention or change from baseline? | ||
| Results: Intervention* | ||
| Results: Comparison* | ||
| No. missing participants and reasons | ||
| No. participants moved from other group and reasons | ||
| Any other results reported | ||
|
Unit of analysis (individuals, cluster/ groups or body parts) |
||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||
| Reanalysis required?(specify) | ||
| Reanalysed results |
*Results for continuous outcome : Mean : SD (or other variance): Total number of participants
Results for dichotomous outcome : Number participants with outcome: Total number of participants
8. Risk of Bias assessment
| Domain |
Risk of bias : high/low /unclear |
Support for judgement | Location in text |
|
Random sequence generation (selection bias) |
|||
|
Allocation concealment (selection bias) |
|||
|
Blinding of participants and personnel (performance bias) |
|||
|
Blinding of outcome assessment (detection bias) |
|||
|
Incomplete outcome data (attrition bias) |
|||
|
Selective outcome reporting? (reporting bias) |
|||
|
Other bias (baseline characteristics for cluster‐randomised, carryover for crossover trials) |
9. Applicability
| Yes/No/Unclear | Support for Judgment | |
| Have important populations been excluded from the study?(consider disadvantaged populations, and possible differences in the intervention effect) | ||
| Is the intervention likely to be aimed at disadvantaged groups?(e.g. lower socioeconomic groups) | ||
|
Does the study directly address the review question? (any issues of partial or indirect applicability) |
10. Other information
| Description as stated in report/paper | Location in text | |
| Key conclusions of study authors | ||
| References to other relevant studies | ||
| Correspondence required for further study information(from whom, what and when) | ||
Data and analyses
Comparison 1. Intubation with FIS versus videolaryngoscope.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change of intubation method | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Participants with episodes of desaturation | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Bleeding during/after intubation | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Sore throat | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Successful first intubation | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Intubator's assessment of difficulty of intubation | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdelmalak 2011.
| Methods | Single‐centre RCT, University hospital, Cleveland, Ohio, USA | |
| Participants | 75 participants > 18 years requiring orotracheal intubation for elective surgery. No details given of type of surgical procedure. BMI > 30 kg /m2 Exclusions: known difficult airway needing awake intubation; loose teeth; pregnant; patient required rapid sequence induction; attending anaesthesiologist required non‐standard TT Median BMI (IQR): FIS 37 (33 to 49); Glidescope 36 (34 to 44) Mean age (SD): FIS 54 years (11); Glidescope 52 years (16) % female: FIS 49%; Glidescope 68% % Caucasian: FIS 97%; Glidescope 87% |
|
| Interventions | 37 participants randomly assigned to fibreoptic intubation (FIS). Model of FIS not given. Sniffing position used, with ramp positioned under shoulders. Permitted to use external laryngeal manipulation or to change the position of the participant's head to improve the glottis view or to facilitate intubation 38 participants randomly assigned to videolaryngoscope (VLS). Glidescope: Verathon Medical. Sniffing position used, with ramp positioned under shoulders. Used with Mallinckrodt Satin‐Slip intubating stylet. Permitted to use external laryngeal manipulation or to change the position of the participant's head to improve the glottis view or to facilitate intubation Both groups used Flex‐Tip TT (Parker), size 7.5 for men and 7.0 for women |
|
| Outcomes | Failed intubation or change of airway device required. Failed intubation considered as > than three minutes taken or > four attempts Hypoxia < 90% at any time 1 minute before intubation to 10 minutes after Participant‐reported sore throat or hoarseness: rated as mild/ moderate or severe on POD1 Number of attempts for intubation Bleeding on intubation Placement: total time for securing airway device from insertion of Glidescope or for FIS insertion of Williams airway to when end‐tidal PCO2 > 2.7 kPa (given as medians) Difficulty of tracheal intubation, as assessed by intubator on VAS 0 to 100 (given as medians) |
|
| Details of anaesthetic induction and intubation | Preoxygenated to end‐tidal O2 conc 80% Standard IV induction. Induction and maintenance of anaesthesia overseen by anaesthesiologist who chose NMBA agent. When paralysed, randomization revealed Used Flex‐Tip TT (Parker), size 7.0 for men and 7.5 for women After induction, ventilated with 100% oxygen until confirmed NMB Mask ventilation permitted between intubation attempts |
|
| Training and seniority of intubator | Two “experienced” intubators | |
| Notes | Study supported through internal funding from Cleveland Clinic. Statement indicating no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated codes |
| Allocation concealment (selection bias) | Low risk | "Maintained in sequentially numbered opaque envelopes" |
| Blinding of participants and personnel (performance bias) Failed intubation, first success, ease of intubation and time for intubation | High risk | Intubators not blinded |
| Blinding of participants and personnel (performance bias) Hypoxia | Low risk | Standardized preoxygenation protocol. Randomization not revealed until participant paralysed |
| Blinding of participants and personnel (performance bias) Patient‐reported outcomes | Unclear risk | Participants were blinded to allocation. No details of how participants were treated after surgery and pain relief, etc, given. Unclear whether recovery staff were blinded |
| Blinding of outcome assessment (detection bias) Failed intubation, first success, ease of intubation and time for intubation. | High risk | Intubators not blinded |
| Blinding of outcome assessment (detection bias) Hypoxia | Low risk | Observer collecting postintubation records was blinded |
| Blinding of outcome assessment (detection bias) Patient‐reported outcomes | Low risk | Participants were blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes prespecified in Methods section and on trial register. No outcomes not reported |
| Other bias | Low risk | More men in FIS group. Article gives effect estimates adjusted for age |
Aikins 2010.
| Methods | Single‐centre RCT. University Hospital, Texas, USA | |
| Participants | 20 obese patients BMI > 27.5 kg/m2. Unclear whether patients undergoing surgery. Inclusion criteria: ASA II to III. Exclusion criteria: ASA IV to V Mean BMI (SD): FIS 32 (2.5); intubating SAD 34 (7); VLS 35 (4.5); stylet 34 (7) Mean age (SD): FIS 48.4 (16); intubating SAD 46.8 (9); VLS 40 (17); stylet 43 (14) |
|
| Interventions | Four intervention groups. Five participants randomly assigned to each of the following.
“Following insertion the cuff was inflated with 25‐40 ml of air and manual ventilation attempted. Tidal volumes >10 ml/kg, adequate movement of the chest wall and over 15 cm H2O airway pressure were judged acceptable ventilation. Only one attempt at blind intubation through the Fastrach™ with the silicone ett was undertaken. If unsuccessful, one attempt at fibreoptic bronchoscope guided tracheal intubation through the Fastrach™ was allowed”
|
|
| Outcomes | Failed intubation.
Complications such as mucosal bleeding injury, hoarseness, dental injury, sore throat, difficult or painful swallowing assessed by participant review on POD0 and POD1 Time of insertion of test device into oropharynx to manual ventilation through TT |
|
| Details of anaesthetic induction and intubation | Rapid sequence induction Preoxygenation for three to five minutes; general anaesthesia was induced intravenously with 1 to 2 mg/kg propofol, 1 to 3 mcg/kg fentanyl and 1 mg/kg succinylcholine IV. After induction, cricoid pressure was maintained and mask ventilation verified. In all groups, an appropriately sized tracheal tube (TT) for oral intubation was chosen (inner diameter 7.5 to 8.0 mm in males and 7.0 to 7.5 mm in females) NBMA: 1 mg/kg succinylcholine |
|
| Training and seniority of intubator | Novice intubator (physician anaesthetist). Ten months' experience with direct laryngoscopy Viewed instructional videos and received didactic instruction on four devices. No more than five practical experience with devices |
|
| Notes | Statement indicating no sources of support and no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomly assigned—no further details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) Failed intubation, first success, ease of intubation and time for intubation | High risk | No mention of blinding |
| Blinding of participants and personnel (performance bias) Patient‐reported outcomes | Unclear risk | No details of how participants were treated after surgery and of pain relief, etc, given. Unclear whether recovery staff were blinded |
| Blinding of outcome assessment (detection bias) Failed intubation, first success, ease of intubation and time for intubation. | High risk | No mention of blinding |
| Blinding of outcome assessment (detection bias) Patient‐reported outcomes | Unclear risk | Not clear whether participant blinded. No standardized instruments described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes prespecified in Methods |
| Other bias | Low risk | |
Rosenstock 2012.
| Methods | Multi‐centre RCT. Three university hospitals, Copenhagen, Denmark | |
| Participants | 93 adult elective participants with anticipated difficult laryngoscopy requiring GA and awake oral intubation. Scheduled for gynaecological, abdominal, urological and ENT procedures No obesity inclusion, but 46 participants with BMI > 30 kg/m2 presented as subgroup. Outcome data (ITT analysis) obtained in personal communication with study authors Inclusion criteria: age > 18 years; ASA I to III; simplified airway risk index (SARI) > =4. Exclusion criteria: mouth opening < 15 mm; poor dental status; surgeon request of nasal intubation; contraindication to transtracheal injection 9 of total 93 participants did not complete protocol; 7 transtracheal injection impossible & 2 lack of co‐operation. Unclear how many of these were obese Mean BMI (SD): FIS 36.7 (6.1); VLS 38.2 (5.3) Mean age (SD): FIS 59.5 years (8.9); VLS 65.0 years (11.2) |
|
| Interventions | 26 obese participants randomly assigned to FIS. Scope make not given. Berman II intubation airway size 8 or 9 for women size 9 or 10 for men Assistant performed jaw thrust to expand oropharyngeal space. TT model not given. Participant position left to discretion of intubator 20 obese participants randomly assigned to VLS (McGrath series 5). Sniffing position used. A stylet was used to bend the tip of the tube 80 to 100 degrees |
|
| Outcomes | Failed intubation: "in the case the first technique failed after three attempts, then optimal patient positioning was secured before an attempt at tracheal intubation with the alternative device" Hypoxia of < 90% oxygen saturation during intubation attempt Participant report of discomfort during awake intubation measured on VAS score 0 (none) to 10 (worst possible) on discharge from recovery Tooth damage and signs of soft tissue damage. Bleeding reported Number of attempts at intubation Total time for intubation (from advancement of FIS or VLS behind teeth until appearance of a capnography curve) Intubator's evaluation of ease of technique VAS 0 to 10 (presented as medians) |
|
| Details of anaesthetic induction and intubation |
Awake intubation Participants given glycopyrrolate 4 to 5 mcg/kg after IV cannula placed Nasal catheter giving 2 to 4 litres O2 Continuous remifentanil infusion 0.1 to 0.15 mcg/kg/min with bolus dose of 0.75 mcg/kg as needed to keep participant sedation to Ramsay score of 2 to 4. Topical analgesia: lidocaine 10% to oropharynx 50 to 100 mg lidocaine given by transtracheal injection Sufficient analgesia given to avoid coughing and achieve acceptance of TT Make of TT used not standardized—size and make chosen before randomization |
|
| Training and seniority of intubator | Six investigators all “thoroughly trained in difficult airway management and also specifically experienced in using [both devices]" | |
| Notes | Two McGrath VLSs were provided by SECMA (Skaevinge, Denmark) to two hospitals for the duration of the trial. Statement that authors had no conflicts of interest and were not provided with any funding from the manufacturers | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Variable block‐size randomization, computer‐generated random numbers. First block included 20 patients and second block 15 patients" |
| Allocation concealment (selection bias) | Low risk | "Number assignment kept in sealed envelopes" Personal communication from authors—also numbered and opaque |
| Blinding of participants and personnel (performance bias) Failed intubation, first success, ease of intubation and time for intubation | High risk | Participants, investigators and care providers knew allocation |
| Blinding of participants and personnel (performance bias) Hypoxia | Low risk | All participants given 2to 4 litres of oxygen |
| Blinding of participants and personnel (performance bias) Patient‐reported outcomes | High risk | Participants, investigators and care providers knew allocation |
| Blinding of outcome assessment (detection bias) Failed intubation, first success, ease of intubation and time for intubation. | High risk | Participants, investigators and care providers knew allocation |
| Blinding of outcome assessment (detection bias) Hypoxia | High risk | Participants, investigators and care providers knew allocation |
| Blinding of outcome assessment (detection bias) Patient‐reported outcomes | High risk | Participants, investigators and care providers knew allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses to follow‐up in obese participants. ITT analyses in personal correspondence from study author |
| Selective reporting (reporting bias) | Low risk | All outcomes prespecified in Methods are reported |
| Other bias | Low risk | |
ENT: ear, nose and throat; FIS: flexible intubation scope; ITT: intention‐to‐treat; IV: intravenous; NMB: neuromuscular blockade; NMBA: neuromuscular blocking agent; POD: postoperative day; SAD: supraglottic airway device; TT: tracheal tube; VAS: visual analogue scale; VLS: videolaryngoscope.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ezri 2004 | Observational study of methods of intubation in obese participants undergoing bariatric surgery |
| Gercek 2008 | RCT of different methods of intubation in non‐obese participants to assess cervical spine motion |
| ISRCTN71888001 | RCT of nasotracheal intubation using FIS or Macintosh laryngoscope in maxillofacial surgery participants. BMI > 35 kg/m2 excluded |
| Liang 2010 | Letter with no data presented |
| NCT01656967 | RCT of use of AMBU AScope2 Fiberoptic Intubation versus Fastrach Intubating LMA. BMI > 35 kg/m2 excluded. Personal communication from study authors—only five participants with BMI > 30 kg/m2 |
FIS: flexible intubation scope; LMA: laryngeal mask airway; TT: tracheal tube: VLS: videolaryngoscope.
Differences between protocol and review
With only three eligible studies, which we decided not to pool, there are a number of differences between methods that were described in the protocol (Nicholson 2013) and those used in the review. These are listed below.
Objectives
In the protocol, we wrote, "We will consider the influence of factors such as the expertise of the intubator and degree of obesity of the patient on the safety and effectiveness of different methods". This was not possible during the review, as we did not amalgamate study results.
Assessment of risk of bias in included studies
In the protocol, we described how we would deal with cluster or cross‐over trials.
"Cluster designs may be used in this topic, with anaesthetist, operating theatre or hospital being the unit of randomization. For any cluster‐randomized trials that we include, we will pay particular attention to baseline characteristics of the patients and the expertise of the anaesthetist. Skill of the intubator is an important confounder and needs to be addressed by randomization.
Cross‐over trials, if we find any, will be included only for certain outcomes related to the success rate of intubation. These can be assessed in a cross‐over design in which an intubator uses both methods sequentially. Order of insertion must be randomized to prevent familiarity with the airway with the second method affecting results. Since many of our outcomes are measured after the operation is completed they cannot be assessed in a cross‐over design."
We did not find any eligible cross‐over or cluster trials and therefore did not use these methods.
In our protocol (Nicholson 2013), we wrote, "We will perform a pilot of 100 titles before all titles are reviewed in order to clarify criteria for discarding articles at this stage." However, in the review, we did not undertake a pilot of title review or data extraction because of the small numbers of studies found.
Outcome number 2: The time period for hypoxia had been defined in the protocol (Nicholson 2013) as from induction to full recovery. Given that the intervention was concerned only with methods to place a TT, on reflection this seemed too long. We modified the time period for hypoxia to run from induction to after successful intubation.
Measures of treatment effect
In the protocol, we stated, "For time‐to‐event data, such as mortality, hazard ratios may be the most appropriate effect measure so we may use the generic variance option in RevMan 5.1. We may also need to use odds or risk ratios if we are unable to extract or obtain the raw data of numbers and totals from the study but we will not combine different outcome measures in the same meta‐analysis". All eligible studies reported numbers of events for dichotomous outcomes; therefore we did not need to use these other methods.
Unit of analysis issues
In the protocol, we stated, "For any cluster trials included in the review, we will extract data directly from the publication only if the analysis used accounts for the cluster design with a method such as multi‐level modelling or generalized estimating equations. If these adjustments are not made within the report, we will undertake approximate analyses by recalculating standard errors or sample sizes based on the design effect. The resulting effect estimates and their standard errors will be analysed using the generic inverse variance method in RevMan." We found no eligible cluster trials and therefore did not need to use these methods.
Contributions of authors
Conceiving of the review: Andrew F Smith (AFS).
Co‐ordinating the review: Amanda Nicholson (AN).
Undertaking manual searches: AN and Sharon R Lewis (SRL)—with support from CARG.
Screening search results: AN and AFS.
Organizing retrieval of papers: SRL and AN.
Screening retrieved papers against inclusion criteria: AN, SRL,Tim M Cook (TMC) and AFS.
Appraising quality of papers: AN and AFS. TMC resolving disagreements.
Abstracting data from papers: AN and AFS. TMC resolving disagreements.
Writing to authors of papers for additional information: AN and SRL.
Providing additional data about papers: AN and TMC.
Obtaining and screening data on unpublished studies: AFS and AN.
Providing data management for the review: AN.
Entering data into Review Manager (RevMan 5.1): SRL and AN.
Handling RevMan statistical data: AN.
Performing other statistical analysis not using RevMan: AN.
Interpreting data: SRL, AN, TMC and AFS.
Making statistical inferences: AN, TMC and AFS.
Writing the review: all review authors.
Securing funding for the review: AFS.
Performing previous work that was the foundation of the present study: N/A.
Serving as guarantor for the review (one review author): AFS.
Taking responsibility for reading and checking the review before submission: AN.
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR Cochrane Collaboration Programme Grant. Enhancing the safety, quality and productivity of perioperative care. Project Ref: 10/4001/04, UK.
This grant funds the work of AN, AS and SL on this review
Declarations of interest
From March to August 2011, AN worked for the Cardiff Research Consortium, which provided research and consultancy services to the pharmaceutical industry. The Cardiff Research Consortium has no connection with AN's work with The Cochrane Collaboration. AN's husband has small direct holdings in several drug and biotech companies as part of a wider balanced share portfolio. See Sources of support.
TMC was paid by Intavent Orthofix and the LMA company more than five years ago for lecturing, and his department has been given free or at cost airway equipment for evaluation or research from numerous airway companies. He (and his family) has no financial investments or ownership of any such company that he is aware of and reports no other sources of conflicts of interest.
AFS: See Sources of support.
SRL: See Sources of support.
Edited (no change to conclusions)
References
References to studies included in this review
Abdelmalak 2011 {published and unpublished data}
Aikins 2010 {published data only}
- Aikins NL, Ganesh R, Springmann KE, Lunn JJ, Solis‐Keus J. Difficult airway management and the novice physician. Journal of Emergencies Trauma & Shock 2010; Vol. 3, issue 1:9‐12. [20165715] [DOI] [PMC free article] [PubMed]
Rosenstock 2012 {published and unpublished data}
- Gatke MR, Rasmussen LS, Rosenstock CV. In reply. Anesthesiology 2013; Vol. 118, issue 2:463‐4. [PUBMED: 23340361] [DOI] [PubMed]
- Rosenstock CV, Thogersen B, Afshari A, Christensen A‐L, Eriksen C, Gatke MR. Awake fiberoptic or awake video laryngoscopic tracheal intubation in patients with anticipated difficult airway management: a randomized clinical trial. Anesthesiology 2012; Vol. 116, issue 6:1210‐6. [22487805] [DOI] [PubMed]
References to studies excluded from this review
Ezri 2004 {published data only}
- Ezri T, Muzikant G, Medalion B, Szmuk P, Charuzi I, Susmallian S. Anesthesia for restrictive bariatric surgery (gastric bypass not included): laparoscopic vs open procedures. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 2004; Vol. 28, issue 9:1157‐62. [15311219] [DOI] [PubMed]
Gercek 2008 {published data only}
ISRCTN71888001 {unpublished data only}
- ISRCTN71888001. Comparison of the incidence of epistaxis following nasotracheal intubation when using either the fibreoptic laryngoscope technique or the Macintosh laryngoscope technique. http://www.controlled‐trials.com/ISRCTN71888001 (accessed 11 June 2013).
Liang 2010 {published data only}
NCT01656967 {unpublished data only}
- NCT01656967. AMBU AScope2 Fiberoptic Intubation Versus Fastrach Intubating LMA. http://clinicaltrials.gov/show/NCT01656967 (accessed 11 June 2013).
Additional references
Adachi 2002
Adams 2000
- Adams JP, Murphy PG. Obesity in anaesthesia and intensive care. British Journal of Anaesthesia 2000;85(1):91‐108. [PUBMED: 10927998] [DOI] [PubMed] [Google Scholar]
Aghdaii 2010
- Aghdaii N, Azarfarin R, Yazdanian F, Faritus SZ. Cardiovascular responses to orotracheal intubation in patients undergoing coronary artery bypass grafting surgery. Comparing fiberoptic bronchoscopy with direct laryngoscopy. The Middle East Journal of Anesthesiology 2010; Vol. 20, issue 6:833‐8. [PUBMED: 21526669] [PubMed]
ASA 2003
Barak 2003
Behringer 2011
Cook 2011
- Cook TM, Woodall N, Frerk C. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: anaesthesia. British Journal of Anaesthesia. 2011/03/31 2011; Vol. 106, issue 5:617‐31. [PUBMED: 21447488] [DOI] [PubMed]
Cook 2012
- Cook TM, MacDougall‐Davis SR. Complications and failure of airway management. British journal of anaesthesia 2012;109 Suppl 1:i68‐85. [PUBMED: 23242753] [DOI] [PubMed] [Google Scholar]
Cullen 2012
Fridrich 1997
- Fridrich P, Frass M, Krenn CG, Weinstabl C, Benumof JL, Krafft P. The UpsherScope in routine and difficult airway management: a randomized, controlled clinical trial. Anesthesia and Analgesia 1997;85(6):1377‐81. [PUBMED: 9390612] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ. 2008/05/06 2008; Vol. 336, issue 7651:995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed]
Hagberg 2009
Hagberg 2010
Hames 2003
Heidegger 2007
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Higgins 2011a
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; Vol. 343:d5928. [PUBMED: 22008217] [DOI] [PMC free article] [PubMed]
Ho 2006
- Ho AM, Chung DC, Karmakar MK, Gomersall CD, Peng Z, Tay BA. Dynamic airflow limitation after topical anaesthesia of the upper airway. Anaesthesia and Intensive Care 2006;34(2):211‐5. [PUBMED: 16617642] [DOI] [PubMed] [Google Scholar]
Houde 2009
Joo 2001
Juvin 2003
Karkouti 2000
Kelly 2008
- Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. International Journal of Obesity (2005) 2008;32(9):1431‐7. [PUBMED: 18607383] [DOI] [PubMed] [Google Scholar]
Kheterpal 2013
- Kheterpal S, Healy D, Aziz MF, Shanks AM, Freundlich RE, Linton F, et al. Incidence, predictors, and outcome of difficult mask ventilation combined with difficult laryngoscopy: a report from the Multicenter Perioperative Outcomes Group. Anesthesiology 2013;Epub ahead of print. [PUBMED: 24071617] [DOI] [PubMed] [Google Scholar]
Konrad 2011
Langeron 2000
- Langeron O, Masso E, Huraux C, Guggiari M, Bianchi A, Coriat P, et al. Prediction of difficult mask ventilation. Anesthesiology 2000;92(5):1229‐36. [PUBMED: 10781266] [DOI] [PubMed] [Google Scholar]
Langeron 2001
Li 2007
- Li XY, Xue FS, Sun L, Xu YC, Liu Y, Zhang GH, et al. [Comparison of hemodynamic responses to nasotracheal intubations with Glide Scope video‐laryngoscope, Macintosh direct laryngoscope, and fiberoptic bronchoscope]. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2007/03/27 2007; Vol. 29, issue 1:117‐23. [PUBMED: 17380681] [PubMed]
Lundstrom 2009
- Lundstrom LH, Moller AM, Rosenstock C, Astrup G, Wetterslev J. High body mass index is a weak predictor for difficult and failed tracheal intubation: a cohort study of 91,332 consecutive patients scheduled for direct laryngoscopy registered in the Danish Anesthesia Database. Anesthesiology. 2009/02/06 2009; Vol. 110, issue 2:266‐74. [PUBMED: 19194154] [DOI] [PubMed]
Maktabi 2002
Malhotra 2008
- Malhotra A, Hillman D. Obesity and the lung: 3. Obesity, respiration and intensive care. Thorax 2008;63(10):925‐31. [PUBMED: 18820119] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marley 2005
- Marley RA, Hoyle B, Ries C. Perianesthesia respiratory care of the bariatric patient. Journal of Perianesthesia Nursing 2005;20(6):404‐31; quiz 432‐4. [PUBMED: 16387272] [DOI] [PubMed] [Google Scholar]
Mason 1992
Mihai 2008
OECD 2010
- Obesity and the Economics of Prevention: Fit not Fat. http://www.oecd.org/health/healthpoliciesanddata/obesityandtheeconomicsofpreventionfitnotfat.htm (accessed 9 October 2012).
OECD 2012
- OECD. Obesity Update. http://www.oecd.org/health/49716427.pdf (accessed 22 May 2013).
Ovassapian 1990
- Ovassapian A. Fiberoptic Airway Endoscopy in Anesthesia and Intensive Care. New York: Raven Press, 1990. [Google Scholar]
RevMan 5.1 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Schnack 2011
- Schnack DT, Kristensen MS, Rasmussen LS. Patients' experience of awake versus anaesthetised orotracheal intubation: a controlled study. European Journal of Anaesthesiology 2011;28(6):438‐42. [PUBMED: 21544021] [DOI] [PubMed] [Google Scholar]
Shulman 2001
Smith 1997
- Smith JE, Jackson AP, Hurdley J, Clifton PJ. Learning curves for fibreoptic nasotracheal intubation when using the endoscopic video camera. Anaesthesia 1997;52(2):101‐6. [PUBMED: 9059089] [DOI] [PubMed] [Google Scholar]
Smith 2003
- Smith G, Ng A. Gastric reflux and pulmonary aspiration in anaesthesia. Minerva Anestesiologica 2003;69(5):402‐6. [PUBMED: 12768174] [PubMed] [Google Scholar]
WHO obesity fact sheet 2012
- WHO fact sheet: No. 311: Obesity and overweight. http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed 9 October 2012).
References to other published versions of this review
Nicholson 2013
- Nicholson A, Smith AF, Cook TM, Lewis SR. Tracheal intubation with a flexible intubation scope versus other intubation techniques for obese patients requiring general anaesthesia. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD010320] [DOI] [PMC free article] [PubMed] [Google Scholar]


