Abstract
Derangements in the innate and adaptive immune responses observed in systemic inflammatory syndromes contributes to unique elevated atherosclerotic risk and incident cardiovascular disease. Novel multimodality imaging techniques may improve diagnostic precision for the screening and monitoring of disease activity. The integrated application of these technologies lead to earlier diagnosis and noninvasive monitoring of cardiac involvement in systemic inflammatory diseases that will aid in preclinical studies, enhance patient selection, and provide surrogate endpoints in clinical trials, thereby improving clinical outcomes. We review the common cardiovascular manifestations of immune-mediated systemic inflammatory diseases and address the clinical and investigational role of advanced multimodality cardiac imaging.
Keywords: cardiac magnetic resonance, computed tomography, echocardiography, PET, systemic inflammatory disease
There is mounting evidence of increased cardiovascular (CV) morbidity and mortality from autoimmune-mediated rheumatic and musculoskeletal diseases. Systemic inflammatory diseases (SIDs) encompass diverse conditions characterized by abnormal immune system activation. Although the specific mechanisms underlying each disease and their respective contributions to CV manifestations vary, a common etiologic pathway centers on systemic inflammation. Clinical and experimental therapeutic interventions1,2 designed to alter the primary progression of atherogenic factors have revealed additional and residual CV risk that further supports the inflammatory hypothesis.
Understanding the CV impact of immune-mediated inflammatory diseases has significant implications for early detection, risk stratification, prognostication, and implementing strategies to mitigate cardiometabolic risk and institute disease-modifying therapies that may alter the clinical course. Indeed, multimodality CV imaging plays a crucial role in assessing the extent of cardiac involvement across the SIDs by allowing for a more accurate diagnosis, phenotypic characterization, and monitoring of cardiac diseases.
In the present scientific statement, we provide a comprehensive overview on behalf of the American College of Cardiology (ACC) Cardiovascular Imaging Leadership Council to offer updated insights into frequently encountered SIDs (Table 1) and their most common CV manifestations (Central Illustration), and the integral role of integrated multimodality imaging approaches (Table 2).
TABLE 1.
Summary of Cardiovascular Manifestations in Systemic Inflammatory Disease
| Etiology | Type of Injury | Main Tissue Pathology |
Characteristic CV Findings |
Diagnostic Targets for Imaging |
Established Imaging Modalities |
Novel Imaging Modalities |
|
|---|---|---|---|---|---|---|---|
| Rheumatoid Arthritis | Adaptive immunity dysregulation, genetic + environmental factors leading to articular and systemic effects | Endothelial dysfunction from systemic inflammation, vascular injury | Atherosclerosis, microvascular dysfunction, vascular inflammation | Proatherogenic lipid profile, accelerated atherosclerosis (epicardial and microvascular disease), major ischemic events Coronary vasculitis Diastolic dysfunction preceding systolic dysfunction Myocarditis Pericardial effusion and acute pericarditis |
Atherosclerosis Coronary vasculature (epicardial and microvascular disease) |
First-line ASCVD imaging CAC ± coronary CTA SPECT-MPI Stress Echocardiography Structural imaging Echocardiography Second-line imaging CMR 13NH3-PET (CMD) |
DOTAtate PET 68Ga-FAPI PET/CT |
| Psoriatic arthritis | Immune dysregulation (innate and adaptive), IL-23/TH17 immune axis important component | Endothelial dysfunction from systemic inflammation, vascular injury | Atherosclerosis, microvascular dysfunction | Proatherogenic lipid profile, accelerated atherosclerosis, major ischemic events | Atherosclerosis Coronary vasculature (epicardial and microvascular disease) |
First-line ASCVD imaging CAC ± coronary CTA SPECT-MPI Stress Echocardiography Second-line imaging 13NH3-PET (CMD) |
|
| Spondyloarthritis | Multifactorial, genetic (HLA B27), environmental, dysregulated immune responses | Endothelial dysfunction, vascular injury Aortic root and aortic valvular disease in SpA-AS (AR) |
Atherosclerosis, microvascular dysfunction | Proatherogenic lipid profile, insulin resistance, accelerated atherosclerosis Increased risk for VTE Pericardial effusion SpA-AS associated with aortic root inflammation and dilatation, AR |
Atherosclerosis Coronary vasculature (mostly epicardial) Aortic root and proximal aorta Aortic valve disease |
First-line ASCVD imaging CAC ± coronary CTA SPECT-MPI Stress Echocardiography Structural imaging: 2DE for screening and detection of valvulopathy and aortopathy |
|
| Systemic lupus erythematosus | Dysregulated adaptive immunity, defective immune tolerance, and autoantibody production leading to systemic autoimmunity | Occlusive vasculopathy, vasculitis, immune complex deposition | Accelerated atherosclerosis Microvascular dysfunction Valvular disease Pericarditis Myocarditis |
Libman-Sacks nonbacterial thrombo-embolic endocarditis Regurgitant valvular lesions Myo/pericarditis often in setting of serositis Pericardial effusion, acute and chronic pericarditis are common |
Atherosclerosis Coronary vasculature (epicardial more common microvascular disease) |
Structural imaging Echocardiography CMR Second-line imaging 18FDG-PET 13NH3-PET (CMD) |
|
| Systemic vasculitis | Dysregulated immunity, type depends on type and vessel size affected | Predominantly vascular injury | Vessel wall inflammation and damage (precise pathology depending on vasculitis | Acute pericarditis Microvascular disease, vascular inflammation |
Coronary vasculature (microvascular disease more common than epicardial) Great vessels |
Structural imaging Echocardiography CMR Second-line imaging 13NH3-PET (CMD) 18FDG-PET |
DOTAtate PET 18F-FET-βAG-TOCA |
| Idiopathic inflammatory myopathies | Dysregulated immunity, genetic predisposition, environmental factors | Muscle damage, systemic inflammation, microvascular derangements | Lymphocytic and macrophagic infiltration of muscle tissues with muscle fiber damage, perifascicular atrophy Proliferation of satellite cells Vasculopathy in dermatomyositis Endomysial involvement in polymyositis |
Left ventricular systolic dysfunction more common than diastolic dysfunction Myocarditis |
Structural imaging Echocardiography CMR |
DOTAtate PET | |
| Systemic sclerosis | Vascular injury and endothelial damage leading to deranged proliferation of fibroblast and production of extracellular matrix components | Vascular injury, immune dysfunction, and adverse connective tissue remodeling | Microvascular disease Fibrosis |
Early diastolic dysfunction Systolic dysfunction in diffuse SSc subtype Right heart enlargement and dysfunction–RV hypertrophy AS>AR, MR, TR Pericardial effusion common, poor prognostic sign in PAH |
Microvascular disease Tissue fibrosis Adverse Remodeling |
First-line ASCVD imaging Stress Echocardiography CAC ± coronary CTA SPECT-MPI Structural imaging 2DE for screening and early detection of cardiopulmonary disease, diastolic dysfunction CMR for quantification of fibrosis, ECV quantification of inflammation |
Stress echo with STE for hemodynamic assessment, echo phenotyping, contractile reserve 4D flow DOTAtate PET |
| Sarcoidosis | Complex interplay of dysregulated immune responses, environmental factors, and genetic predisposition | Organ-level accumulation of inflammatory mononuclear phagocytes and T-lymphocytes into noncaseating granulomatous formation and disruption of adjacent tissue | Noncaseating granulomas, inflammatory macrophages | Systolic and diastolic dysfunction, nonischemic wall motion abnormalities, cardiomyopathy, and ventricular wall thinning Arrhythmia, (NSVT, MVT, SCD) Atrioventricular conduction disease (high-grade atrioventricular block in 42% of patients) Constrictive pericarditis |
Wall thinning, aneurysmal segments, myocardial replacement fibrosis in characteristic mid-wall or subepicardial distribution, commonly involving the basal septum, lateral and free wall of the LV |
Structural imaging Echocardiograph CMR Inflammatory imaging 18F-FDG-PET |
DOTAtate PET FMISO PET FLT PET |
2DE = 2-dimensional echocardiography; 4D = 4-dimensional; AR = aortic regurgitation; AS = aortic stenosis; ASCVD = atherosclerotic cardiovascular disease; CAC = coronary artery calcium; CMR = cardiac magnetic resonance; CTA = computed tomography angiography; CV = cardiovascular; DOTAtate = gallium 68 [68Ga]-DOTA-Tyr-octreotide; ECV = extracellular volume; FAPI = gallium 68 [68Ga]-labeled fibroblast activation protein inhibitor; FDG = fluorodeoxyglucose; FLT = 3′-Deoxy-3-[18F]-fluorothymidine; FMISO = [18F]-fluoromisonidazole; LV = left ventricle; MPI = myocardial perfusion imaging; MR = mitral regurgitation; MS = mitral stenosis; MVT = monomorphic ventricular tachycardia; NSVT = nonsustained ventricular tachycardia; PAH = pulmonary arterial hypertension; PET = positron emission tomography; PR = pulmonic regurgitation; RV = right ventricular; SCD = sudden cardiac disease; SpA = spondyloarthritis; SpA-AS = ankylosing spondylitis; SPECT = single-photon emission computed tomography; SSc = systemic sclerosis; STE = speckle-tracking echocardiography; TR = tricuspid regurgitation; VTE = venous thromboembolism.
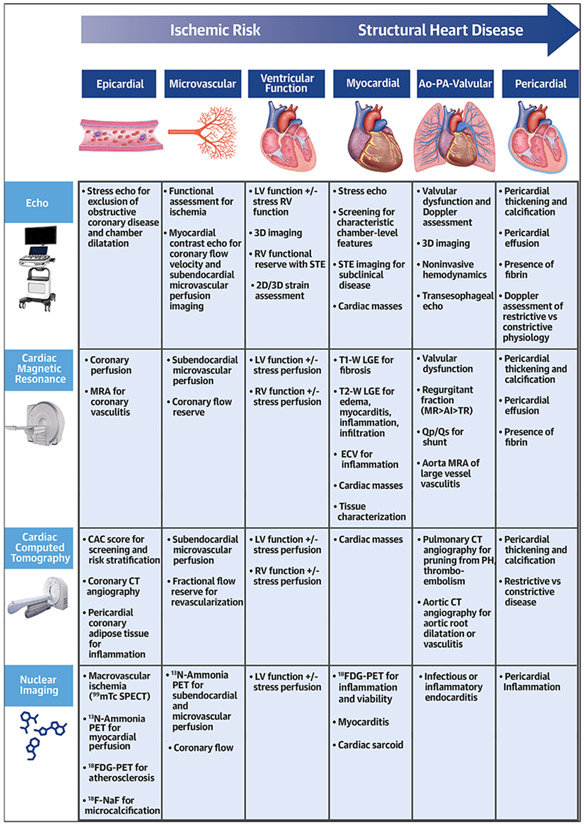
CENTRAL ILLUSTRATION. The Role of Multimodality Cardiac Imaging in Systemic Inflammatory Diseases.
The integrated application of multimodality imaging techniques may improve diagnostic precision for disease activity screening and monitoring of cardiac involvement in systemic inflammatory diseases. 2D = 2-dimensional; 3D = 3-dimensional; CAC = cardiac calcium calcification; CT = computed tomography; Echo = echocardiography; ECV = extracellular volume; FDG = fluorodeoxyglucose; LGE = late gadolinium enhancement; LV = left ventricle; MR = mitral regurgitation; MRA = magnetic resonance angiography; PET = positron emission tomography; RV = right ventricle; SPECT = single photon emission computed tomography; STE = speckle tracking echocardiograpy; TR = tricuspid regurgitation.
TABLE 2.
Summary of Imaging Modalities
| Echocardiography | CMR Imaging | Cardiac CT | Nuclear/PET | |
|---|---|---|---|---|
| Diagnostic technique | 2DE: chamber-level systolic function, valvular disease, pericardial disease 3DE: complex valvular disease (eg, severe TR), function including volumetric assessment of RV ejection fraction STE: global and regional chamber-level function Doppler echo: diastolic dysfunction, estimation of chamber-level hemodynamics, constrictive pericarditis, tamponade TEE: valvulitis, valvular assessment Stress echo: risk stratification of CAD, exercise-induced PH, contractile reserve |
Precontrast /Postcontrast T1-diffuse fibrosis T2 mapping for assessment of edema, inflammation LGE for fibrosis/scar vs inflammation Cine/balanced SSFP for structure and function, pericardial assessment Stress imaging CMR-microvascular disease or epicardial CAD STIR-fat suppression for edema/inflammation MR tagging-constrictive pericarditis |
CAC score-atherosclerotic burden and risk stratification Coronary CTA-epicardial CAD, anomalous anatomy, vasculitis Coronary CTA + FFR-hemodynamic evaluation Pericardial assessment-pericarditis Valvular assessment |
SPECT-ischemia assessment NH13/82Rb PET-epicardial and microvasculature assessment FDG PET/CT-myocarditis, inflammatory cardiomyopathies (sarcoidosis) |
| Emerging Techniques | Stress with STE for assessment of occult diastolic dysfunction, RV contractile reserve Myocardial contrast for assessment microvascular dysfunction and inflammation |
4D flow-transvalvular, intracavitary flow MRA-vasculature (eg, vasculitis) Diffusion tension CMR (DT-CMR) |
PCAT/FAI-monitoring response to therapy CTP-flow limiting disease Photon-counting/Spectral-enhanced detection/resolution Nanoparticles molecular imaging (eg, N1177-activated Mo) |
18F-NaF-vascular inflammation ImmunoPET (eg, DOTA agents) inflammation, immune target specificity to SIDS |
| Strengths | First-line screening and diagnostic test for most SIDs No radiation Readily available, low cost Real-time interpretation Myocardial deformation analysis |
No radiation Superior spatial and temporal resolution Low interobserver variability |
Readily available Definitive evaluation of epicardial CAD (coronary CTA) CAC Cardiac CT: no contrast risk stratification (plaque characteristics) |
Stress SPECT readily available NH13/82Rb PET-gold standard for microvascular dysfunction Ability to integrate CAC score with scout CT |
| Limitations | Inability to detect inflammation (pericardium/myocardium) and tissue characterization Inadequate Doppler signal for hemodynamics Interobserver variability Difficult acoustic windows for RV visualization (patient habitus) Lack of more advanced techniques (ie, STE, 3D) at all clinical centers STE intervendor variability |
High signal to noise in inflammatory tissue Not available at many centers High cost Long scan time Exclusion of severe renal insufficiency (gadolinium and NSF) |
Contrast allergy requires premedication Exclusion of severe renal insufficiency Interobserver variability in HRP features Radiation exposure Prior PCI or severe CAC may preclude assessment |
High cost Radiation exposure Limited availability of perfusion PET SPECT attenuation artifacts, balanced ischemia missed FDG PET- Nonspecific FDG uptake and specific myocardial diet protocol |
3DE = 3-dimensional echocardiography; CT = computed tomography; CTP = computed tomography perfusion; FAI = fat attenuation index; FFR = fractional flow reserve; LGE = late gadolinium enhancement; MRA = magnetic resonance angiography; NSF = nephrogenic systemic fibrosis; PCAT = pericoronary adipose tissue; PH = pulmonary hypertension; SSFP = steady-state free precession; STE = speckle-tracking echocardiography; STIR = short-tau inversion recovery; TEE = transesophageal echocardiography; other abbreviations as in Table 1.
SYSTEMIC INFLAMMATORY ARTHRITIS
The most well-characterized autoimmune disease establishing the link between systemic inflammation and cardiovascular disease (CVD) is rheumatoid arthritis (RA), a polyarticular chronic inflammatory disease associated with markedly increased CV morbidity and mortality. Relevant disease-specific predictors of mortality in RA include older age, male sex, cumulative disease activity and duration, increased number of involved joints, presence of extra-articular manifestations, and rheumatoid factor positivity.3 Patients with RA have a 1.5- to 2.0-fold increased risk of developing coronary artery disease (CAD) (Figure 1) compared with the general population, likely attributable to derangements in lipid metabolism, endothelial dysfunction, and vascular inflammation.3,4
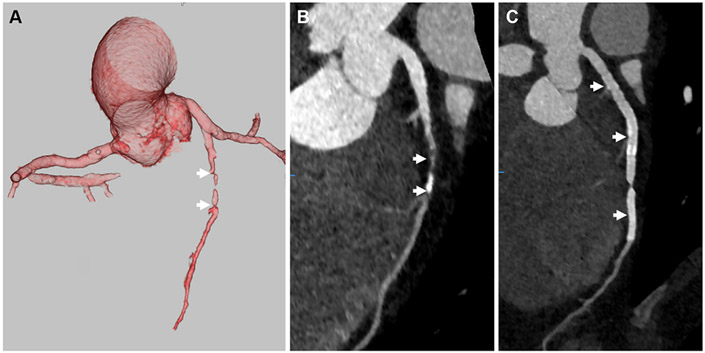
FIGURE 1. Coronary Computed Angiography in Rheumatoid Arthritis.
(A) 3-dimensional volume-rendered coronary computed tomography angiography image of a rheumatoid arthritis patient demonstrates occlusion of the mid left anterior descending artery (white arrows), and curved multiplanar reconstruction image (B) of the left anterior descending artery shows a large amount of plaque (white arrows) with an occluded (white asterisk) segment. In the curved multiplanar reconstruction image of the left circumflex (C), a small amount of plaque is present (white arrow).
RA is an important and independent risk factor for major ischemic events, even after accounting for traditional CV comorbidities. Although the immunopathologic mechanisms are incompletely understood, preclinical studies suggest an atherogenic role of circulating activated immune cells and elevated inflammatory cytokines (tumor necrosis factor [TNF]-α, interleukin [IL]-1β, IL-6).5 Approved RA therapies, including those that target IL-1 and IL-6, further support the notion of shared mechanistic immune pathways between RA and atherosclerosis.6
Derangements in lipid profiles are closely related to disease course. Early active RA, characterized by elevated TNF-α levels and proinflammatory cytokines, is inversely associated with depressed high-density lipoprotein levels resulting in a higher atherogenic index, a term coined the lipid paradox.7 In the late stages, the accelerated loss of skeletal muscle has significant adverse metabolic implications, including insulin resistance, worsening dyslipidemia, and a significant impact on prognosis and quality of life.8 CVD risk in RA is further affected by smoking, which has demonstrable effects on preclinical atherogenic markers such as carotid intimal media thickness.3 The heightened risk associated with these chronic inflammatory effects and traditional cardiometabolic risk factors have led the ACC/American Heart Association (AHA) guidelines to incorporate RA as a risk enhancing condition.9 At the same time, the European League Against Rheumatism (EULAR) recommends applying a 1.5 multiplier to existing CV risk scores to reflect the increased risk of heart disease in RA.8
Psoriatic arthritis (PsA) represents another significant immune-mediated inflammatory arthritis occurring in approximately one-third of patients with dermal psoriasis.9 Although typically a mild oligoarticular disease, aggressive erosive polyarticular disease is present in 20%.10 While underappreciated compared with RA, patients with PsA are at increased risk for major ischemic events, especially in women, those not on disease-modifying anti-rheumatic drugs (DMARDs), and those with aggressive polyarticular disease.11,12 PsA and RA both involve similar inflammatory pathways, leading to an atypical cardiometabolic profile, endothelial dysfunction, an expedited development of atherosclerosis, and plaque formation (Figure 2). Studies have also suggested that higher IL-17 concentration13 is a critical pathogenic contributor to increased CV risk in PsA. In addition to chronic inflammation, PsA patients have an increased prevalence of obesity and proatherogenic lipid profiles.14
FIGURE 2. Coronary Computed Angiography in Psoriatic Arthritis.
(A) 3-dimensional volume-rendered coronary computed tomography angiography image of a psoriatic arthritis patient demonstrating occlusion of the mid portion of the left anterior descending artery (white arrows), and curved multiplane reconstruction image (B) of the left anterior descending artery shows a moderate amount of plaque (white arrows) with an occluded segment. (C) Curved multiplanar reconstruction image after percutaneous intervention with a long segment stent (white arrows) throughout the left anterior descending artery.
Spondyloarthritis (SpA) is a group of chronic inflammatory rheumatic and musculoskeletal diseases with commonly shared characteristics frequently associated with increased CV risk. The hallmark features of SpA are axial inflammation of the sacroiliac joints and spine, peripheral arthritis and synovitis, enthesitis, inflammatory bowel disease, and anterior uveitis.11 There is also a common overlap with PsA, which is often considered one of the subtypes of SpA, along with reactive arthritis and ankylosing spondylitis (SpA-AS). Similar to other forms of systemic inflammatory arthritis, SpA is associated with an increased risk for major adverse CV events, caused by chronic inflammation leading to insulin resistance and dyslipidemia, and increased risk for venous thromboembolism.11
In addition to proatherogenic profiles, SpA-AS is commonly associated with aortic root inflammation and dilatation and aortic annular to commissural thickening resulting in aortic regurgitation, occurring in up to 80% of patients.15,16 Despite the high prevalence, there are no specific guidelines for incorporating 2-dimensional echocardiography (2DE) for screening and detecting subclinical valvular abnormalities. Identifying clinically applicable screening and early detection algorithms for valvular heart disease in SpA-AS allows for closer monitoring, timely intervention, and appropriate management strategies to prevent disease progression.
Across the systemic inflammatory arthritic diseases, accelerated atherosclerosis leads to a 2-fold higher incidence of adverse CV events, with or without concomitant traditional risk factors.4 These robust findings highlight that inflammatory arthritic diseases, primarily when active and poorly controlled, represent an independent and robust proatherogenic risk enhancer.9 Established immunosuppressive therapies that quell active inflammation can further accelerate atherosclerotic cardiovascular disease (ASCVD).17 Managing and mitigating the adverse metabolic effects of these conditions involves a comprehensive approach incorporating heightened surveillance. Although the ACC/AHA guidelines have acknowledged that 10-year ASCVD risk scores underestimate the actual CV risk in inflammatory arthritis, preventative therapies such as statins remain underused in patients with SIDs, given the lack of data supporting widespread implementation.18
Coronary artery calcification (CAC) is a hallmark of atherosclerosis, and an extensive body of data supports the value of CAC score as a predictor for significant CV outcomes in asymptomatic individuals.19 The CAC score is recommended for low- and intermediate-risk asymptomatic populations and has an ACC/AHA Class IIa recommendation for adults at intermediate or borderline risk based on 10-year ASCVD risk.9 Consistent with the known increased risk for premature CAD, studies in RA reveal a greater prevalence of CAC than control subjects.20,21 CAC imaging can screen young asymptomatic patients with systemic inflammatory arthritic diseases and identify those at the greatest risk for adverse CV events.
Given the complex interplay between inflammation and traditional CV risk factors within each SIDs, screening and prevention recommendations must be tailored to each specific disease, optimizing timing and frequency. Appropriate management includes healthy lifestyle modifications and optimizing the control of systemic inflammation with DMARDs and biological therapies. By targeting both inflammatory pathways and traditional risk factors, it may be possible to mitigate cardiometabolic abnormalities and associated risk of ASCVD, while also targeting other CV complications such as incident heart failure (HF), and valvular, myocardial, and pericardial disease. However, monitoring for a broad range of potential CV manifestations can be challenging due to significant disease variability, and essential management decisions may be affected by patient-level ASCVD risk. For example, anti-TNFα agents are contraindicated in RA patients with pre-existing heart failure,22 whereas intravenous immunoglobulin (IVIG) is avoided in RA patients with high thrombotic risk.23 More recent data on the potential CV risk of JAK inhibitors from the Oral Rheumatoid Arthritis Trial (ORAL) Surveillance study resulted in a black box warning based on uncertainty regarding the safety of these agents in patients at high CV risk or with known disease.24,25 Given these complexities, there is a critical need for a structured approach integrating multimodality cardiac imaging to guide diagnosis, prognostication, and monitoring to attenuate both short and long-term CV events.
SYSTEMIC LUPUS ERYTHEMATOSUS
In systemic lupus erythematosus (SLE), CV involvement is common and can include valvular, pericardial, myocardial, and vascular (aortitis, coronary arteritis, microvascular and epicardial CAD) manifestations. Although patients with SLE are susceptible to traditional CV risk factors, such as hypertension, dyslipidemia, obesity, and smoking, the risk of developing ASCVD is up to 48-fold higher than the general population.26
The exact mechanisms by which SLE accelerates atherosclerosis are complex and multifactorial, including immune-mediated inflammation, endothelial dysfunction, oxidative stress, and dyslipidemia. Several pathogenic mechanisms contribute to the residual risk of accelerated atherosclerosis and vascular injury in SLE with an appreciated role for a type I interferon-γ signature and dysregulation of adaptive immune responses.27 SLE pathogenesis is affected by the release of neutrophil extracellular traps; autoantibodies, such as antiphospholipid (aPL) antibodies; and immune complexes, which activate complement and subsequent tissue injury.28,29 The presence of aPL antibodies in SLE can lead to secondary antiphospholipid syndrome and an increased risk of thrombotic events. Many SLE patients will have a high titer of antinuclear antibodies and specific autoantibodies such as anti-double-stranded DNA, the latter of which associates with endothelial dysfunction, promotion of dyslipidemia, and accelerated atherosclerosis.30 In SLE, proatherogenic lipid profiles and hypertension are common, given the prevalence of glucocorticoid use and renal involvement.31
There is a growing body of evidence supporting the inclusion of SLE as a risk-enhancing condition in current ASCVD risk estimation models,9 representing an important area for future refinement and validation. The elevated atherogenic risk highlights the potential role of CAC screening to guide preventive therapies and refine ASCVD risk stratification. Additionally, nuclear molecular imaging techniques that quantify the integrated effects of myocardial blood flow or assess cellular inflammation processes in vivo can probe disease pathogenesis and the downstream effects of CV risk. Myocardial perfusion imaging (MPI) with single photon emission computed tomography (SPECT) remains the most widely available tool to identify the presence of inducible ischemia. Providing greater spatial resolution, 13N-ammonia or 82Rb (rubidium) perfusion positron emissions tomography (PET) with the incorporation of quantitative blood flow imaging have clinical and prognostic value in identifying impaired coronary flow reserve in SLE.32-34
CV disease is a major cause of mortality in patients with SLE, primarily driven by accelerated atherosclerosis in the setting of disease-specific pathophysiology confounded by traditional CV risk factors. Premature CAD and myocardial infarction (MI) can result in HF, which occurs in 1% to 10% of SLE patients.35 SLE-associated HF is further influenced by direct myocardial inflammation, and acute myocarditis occurs in 3% to 15% of patients, often manifesting concurrently with pericarditis during acute SLE disease flares.36 In cases of acute SLE myocarditis with associated HF or conduction abnormalities, potent immunosuppression may be required. Cyclophosphamide and corticosteroids remain the first-line treatment of life-threatening complications, including myocarditis, whereas more mild diseases may incorporate steroid-sparing options such as mycophenolate mofetil or azathioprine.31,37
Given the increased morbidity and mortality of CVD in this population, attention has increasingly focused on the early detection of subclinical myocardial diseases in SLE. Recent studies have elucidated the emerging role of speckle-tracking echocardiography (STE), a recent advancement that allows for chamber-level regional and global contractility estimates from the deformation of ultrasound-myocardial tissue interactions.38 Through the utilization of a software-based algorithm, chamber-level regional and global contractility are estimated from the deformation of ultrasound-myocardial tissue interactions, known as “speckles,” throughout the cardiac cycle. STE imaging can be performed on all 4 cardiac chambers, however strongly relies on adequate image quality at frame rates of 70 to 90 frames/sec, and also requires post-processing using dedicated equipment and interpretation by experienced operators.38 STE-derived contractile metrics are valuable in identifying subclinical myocardial dysfunction and impairment in SLE patients, even those with normal left ventricular (LV) ejection fraction, negatively correlating with disease activity.36 Cardiac magnetic resonance (CMR) is also an important clinical tool for diagnosing SLE myocarditis due to its ability to detect early tissue changes in subclinical and clinically overt cases (Figure 3).39 CMR is an effective and valuable modality for evaluating cardiac function, myocardial perfusion, valvular/vascular flow, and tissue characterization in the same examination without harmful ionizing radiation, which is an important consideration given the young female preponderance of SLE.4 One of the primary advantages of CMR is its ability to detect and quantify myocardial edema, an early and sensitive indicator of myocardial inflammation. T2-weighted imaging sequences are beneficial for identifying areas of edema within the myocardium,4 which, in the context of SLE myocarditis, can enable early detection of adverse CV involvement even in cases of minimal or absent symptoms. Late gadolinium enhancement (LGE) images generated from T1-W inversion recovery pulse sequence, applied 10 to 15 minutes after intravenous gadolinium administration, permit detection, and quantification of myocardial replacement fibrosis,4 and can detect disease-specific patterns of extracellular volume expansion. In the acute phase of myocarditis, LGE correlates with myocardial necrosis (associated with edema as assessed by T2 mapping), whereas in the chronic phase, LGE corresponds with fibrosis, with less or no edema. In SIDs, the patterns of LGE distribution can vary depending on the underlying disease and the specific pathophysiological processes involved. LGE patterns in SLE typically follow a noncoronary distribution and are patchy; however, when present in the basal inferolateral segments, may signify myocarditis or microvascular involvement.4
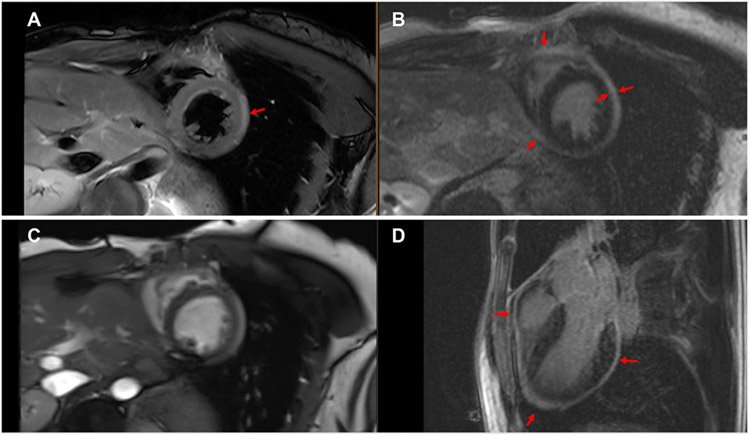
FIGURE 3. Cardiac Magnetic Resonance in Systemic Lupus Erythematosus.
(A) T2-weighted image is shown demonstrating pericardial thickening and edema in a patient with systemic lupus erythematosus. (B) Short-axis late gadolinium enhancement image of pericardial thickening and enhancement. (C) Cine steady-state free precession image demonstrates enhancement and thickening. (D) 3-chamber view late gadolinium enhancement image demonstrates circumferential pericardial thickening and enhancement, prominent in the apical region. Red arrows indicate pericardial thickening and enhancement.
Pericardial disease, most commonly acute pericarditis, is often the first manifestation of SLE, occurring anywhere from 16% to >50%.40 Although there are no societal recommendations for routine screening for pericardial disease, 2DE is the first-line imaging modality when pericardial disease is suspected,40 typically presenting as pericardial effusion or thickening. The addition of Doppler imaging allows for further interrogation of the physiological and hemodynamic effects of pericardial effusion. Although rare, SLE can also present with constrictive pericarditis and can be further characterized by 2DE, cardiac computed tomography (CT), and CMR imaging. In particular, CMR can define pericardial and myocardial anatomy, including pericardial thickening, an important feature differentiating constrictive pericarditis from restrictive cardiomyopathy. T1-weighted black blood and balanced steady-state free precession (SSFP) CMR sequences permit anatomical characterization of the pericardium, whereas native T1 and T2 mapping provide information on pericardial inflammation and edema. Postgadolinium enhancement of the pericardium represents a key marker of pericardial inflammation, and the persistence of this finding, despite standard medical treatment in symptomatic patients, should prompt intensification and/or prolongation of treatment.4 CMR can also be used to follow pericardial inflammation and guide treatment. Frequently, tagging sequences can demonstrate fibrotic adhesions and diagnose myocardial involvement in constrictive pericarditis.41 As an example, quantitative assessment of pericardial LGE can provide incremental prognostic information to predict clinical improvement in SLE patients with constrictive pericarditis treated with anti-inflammatory therapy.42
Last, valvular disease, such as Libman-Sacks endocarditis, nonbacterial thrombotic endocarditis resulting from immunoglobulin and complement deposition on valvular structures, is a severe manifestation of SLE, most commonly manifesting as valvular regurgitation.16,43 Valvular disease and sterile nonbacterial thrombotic endocarditis are much more prevalent in SLE patients with concomitant antiphospholipid syndrome and/or antibodies.43 The coexistence of antiphospholipid antibodies with SLE carries a 3-fold risk of valvular involvement and an increased risk of valvular thrombosis caused by hypercoagulability.
The diverse range of potential CV manifestations in patients with SLE could lend to standardized routine screening with multimodality imaging, although this is not currently in the standard of care. Integrated multimodality imaging modalities have the potential to detect subclinical CV involvement in SLE patients, a group with exceedingly high CVD risk, and aid in early detection, risk stratification, and implementation of preventive measures through close cardiorheumatologic collaboration.
SYSTEMIC VASCULITIS
Vasculitis is a systemic disease identified by noninfectious necrotizing inflammation of the vessels and may be idiopathic (primary) or secondary caused by an underlying condition. The pathogenesis is poorly understood, although perivascular inflammatory infiltrates, thrombosis, fibrosis, and scar formation characterize histologic features. Given that the etiology of idiopathic vasculitis is unknown, classification is based on the vessel size large, medium, small, and variable vessel vasculitis. This heterogeneous group of diseases represents a significant challenge in diagnosis and management. The most common subtypes include large vessel vasculitis as giant cell arteritis and Takayasu’s arteritis (TAK), medium vessel polyarteritis nodosa (PAN), Kawasaki disease (KD), and small vessel vasculitis, the latter divided into 2 major categories: antineutrophil cytoplasmic antibody-associated vasculitis and immune complex small vessel vasculitis. In variable vessel vasculitis, there is no predilection for the vessel size; however, 2 primary forms have been reported, including Behcet’s disease vasculitis and Cogan’s syndrome vasculitis. Secondary vasculitis is associated with vascular inflammation in the setting of an underlying disease or specific cause, such as RA, sarcoidosis, or IgG4-related disease.
Systemic vasculitides (SVs) can involve the myocardium, pericardium, valvular structures, and coronary arteries; however, the exact prevalence is unknown. Based on endomyocardial biopsy (EMB) findings, myocardial inflammation from microvascular and macrovascular disease may lead to ischemia and HF.44 Among the vasculitides, eosinophilic granulomatosis with polyangiitis, myocarditis can be fulminant and associated with high morbidity.45 The estimated annual incidence of coronary artery vasculitis is highest in PAN at 4 to 10 per million, followed by TAK and KD (1 to 2 per million).44 However, the involvement of the coronary arteries among SV is relatively high, affecting up to 50% of patients with PAN and 30% of TAK. In addition to coronary artery inflammation and epicardial arteritis, SVs are associated with accelerated atherosclerosis, which may manifest as premature CAD or cardiomyopathy. Valvular dysfunction can also occur, most commonly involving the aortic valve in up to 35% of TAK patients.46 Echocardiography plays a crucial role in the initial evaluation of cardiac involvement in vasculitis, including the detection of significant valvular disease, acute or chronic ventricular dysfunction, and concurrent pericardial involvement.
In SV, microvascular involvement can result in several CV manifestations ranging from myocarditis, LV dysfunction, and ischemia, which makes both screening and diagnosis challenging. Although EMB is the gold standard for definitive diagnosis, this is not often accessible, and multimodality imaging is critical for the rapid diagnosis and initiation of early treatment to prevent future morbidity. The 2018 EULAR guidelines recommend the use of CMR and magnetic resonance angiography (MRA) for diagnosis and monitoring of large vessel vasculitis to aid in clinical decision-making and assessment of disease activity and treatment response.47 For the evaluation of cranial involvement, Doppler ultrasound is the first-line imaging modality. The noncompressible “halo” sign is the most suggestive finding of giant cell arteritis. High-resolution CMR imaging is an alternative if the ultrasound is not available or inconclusive. PET and CT are not recommended for cranial artery inflammation, whereas coronary CT angiography (CTA) is ideal for assessing coronary artery inflammation, aneurysms, and plaque formation in SV patients (Figure 4).
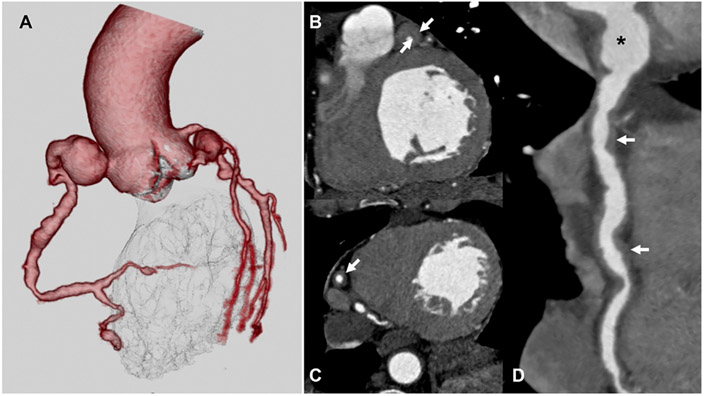
FIGURE 4. Coronary Computed Angiography in Vasculitis.
(A) 3-dimensional volume-rendered coronary computed tomography angiography image of a patient with IgG-4 vasculitis demonstrates Diffuse ectasia and multifocal aneurysms throughout the coronary arteries. Multiplanar reformat computed tomography angiography images through the left anterior descending artery (B), right coronary artery (C), and significant wall thickening (white arrows). Similarly, the curved MPR image (D) of the RCA shows diffuse ectasia, aneurysm (black asterisk) of the proximal segment, and marked wall thickening.
Given the variability in clinical involvement and presentation, there are no standardized imaging protocols for evaluating coronary vasculature in patients with SV. In TAK, classic imaging features on coronary CTA are coronary artery ostial/proximal stenosis (>70%), followed by diffuse/focal distal narrowing or coronary artery aneurysms.48 In patients with TAK, there is higher pericoronary adipose tissue (PCAT) density compared with atherosclerotic CAD or control subjects.49 In PAN, coronary CTA demonstrates sequential areas of stenosis and aneurysms (beads on a string) throughout the coronary arteries. KD may have similar coronary imaging features as in PAN; however, KD should be preferentially considered when there is a single coronary artery aneurysm.41 18F-fluorodeoxyglucose (FDG)-PET is a vital imaging modality that relies on radionuclide-labeled glucose to detect areas of inflammation through its uptake by inflammatory cells–predominantly macrophages. Given improved availability, hybrid PET-CT imaging has emerged as an essential technique for imaging SIDs and SV through precise vascular localization and quantification of pathologic FDG uptake as a marker of inflammation.50
Although EULAR has provided recommendations for the use of multimodality imaging for both diagnosis and long-term monitoring, ultimately, the choice of the imaging method depends on both clinical availability and the predominant clinical symptoms to guide diagnosis, appropriate testing, and management. Unfortunately, there is no current standardized frequency of imaging assessments based on insufficient data and patient-level heterogeneity, and thus individualized decisions regarding serial surveillance are essential to monitoring for clinical improvement.
IDIOPATHIC INFLAMMATORY MYOPATHIES
Myositis or idiopathic inflammatory myopathies (IIMs) are a group of systemic autoimmune rheumatic diseases characterized by skeletal muscle inflammation and weakness that, when severe, can result in pharyngeal, respiratory, or myocardial involvement. The main subgroups of IIM include polymyositis, dermatomyositis, immune-mediated necrotizing myopathy, antisynthetase syndrome, and inclusion body myositis. The immunopathogenesis of IIM is not fully understood, but evidence suggests the involvement of both innate and adaptive immunity and nonimmune mechanisms, such as mitochondrial damage, cell stress, and altered energy metabolism.51 The HLA 8.1 haplotype has been identified as an important pathogenic risk factor for IIM and is the strongest known genetic risk factor.51 Similar to other SIDs, high-dose glucocorticoids that suppress the inflammatory response in IIM further contribute to cardiometabolic derangements, including hyperglycemia, dyslipidemia, and hypertension.
Although largely understudied, small cohort studies have revealed excess ASCVD risk in IIM similar to RA,52 and clinically evident cardiac disease has been reported in up to 9% of IIM patients in a EuroMyositis registry.53 In IIM, the prevalence of CVD widely varies between 6% to 75%, highlighting the heterogeneity of disease presentation, and typically manifests as acute myocarditis and/or HF.54 IIM-associated CVD is likely multifactorial due to direct myocardial inflammation, cofounded by traditional CV risk factors, and is clinically overt at diagnosis or may become clinically overt after initiation of treatment, or even during remission. However, a recent systemic review noted subclinical heart involvement in up to 50% of IIM patients on cardiac imaging.55 When present, cardiac involvement in IIM is associated with increased morbidity and mortality,52 emphasizing the importance of early detection and screening strategies to improve CV outcomes.
Similar to other SIDs, EMB is the gold standard for diagnosing cardiac involvement in IIM but is limited in clinical practice for routine screening and early detection, given its invasive nature. The Myositis Disease Activity Assessment Tool (MDAAT) assesses IIM disease activity by assessing muscle strength, skin involvement, extramuscular manifestations, and serologic testing.56 Although there is some evaluation of cardiac involvement incorporated in MDAAT, such as the presence or absence of symptoms suggestive of cardiac disease, it may not be sensitive enough to detect clinically silent subclinical involvement. Recent studies have shown that CMR imaging, using multiparametric scores derived from native T1 and T2 mapping, can outperform MDAAT in detecting early CV involvement in IIM.57 In one study of histologically proven IIM, CMR-derived circumferential, radial, and longitudinal strain measures were significantly lower in IIM patients, particularly when LGE was also present, despite normal LV ejection fraction.58 Specific to IIM, LGE is predominantly observed in the LV midventricular segments and the subepicardial layer of the interventricular septum and insertion points in a pattern similar to systemic sclerosis (SSc) patients.54 These findings highlight a common pathway of microvascular dysfunction, fibrosis, and myocardial inflammation between these 2 diseases.
Although the independent risk for cardiometabolic disease is not as established, mortality in IIM is up to 8 times higher than the general population and has not substantially changed over the past 25 years.59 These findings highlight the critical unmet need for rigorous studies to characterize myocardial disease and emphasize the importance of close clinical evaluation, electrocardiography, 2DE, and cardiac biomarker monitoring, to detect and manage CV complications at an early stage of the disease.
SYSTEMIC SCLEROSIS
SSc is a heterogeneous autoimmune disease characterized by widespread tissue fibrosis across multiple organ systems, vasculopathy, and dysregulation of the immune disease. Cardiac manifestations in SSc can be quite variable and are either caused by primary fibrotic effects on the myocardium, and/or secondary adverse remodeling caused by PH, interstitial lung disease, or scleroderma renal crisis. Often unrecognized until late in the disease course, previous studies have estimated a 15% to 35% prevalence depending on the diagnostic technique employed and when clinically overt, cardiac involvement in sSc is associated with a nearly 3-fold increase in mortality.60,61
The pathogenesis of SSc cardiac involvement centers on a vascular hypothesis where microvascular coronary disease results in recurrent ischemia-reperfusion injury, which in turn, causes myocardial inflammation, apoptosis of the cardiomyocytes, and replacement fibrosis.62 Myocardial perfusion defects can be seen in up to 82% of all SSc patients at rest, after exercise, or after cold stimulation.60 However, in the absence of angina, vasodilator imaging (dipyridamole, adenosine) is not currently indicated for routine screening of microvascular disease, but may help identify perfusion abnormalities in SSc patients presenting with anginal symptoms.62
In addition to microvascular ischemia leading to inflammation and replacement fibrosis, inherent myocardial contractile deficits63 and early diastolic impairments have been reported in SSc. Diastolic dysfunction may appear before clinical symptoms of HF, regardless of SSc disease subtype,64 and is an important and highly prevalent early manifestation of cardiac involvement in SSc. LV systolic dysfunction is far less common in SSc when compared with diastolic dysfunction, with an estimated incidence of 11% to 15%,60 and is thought to be secondary to focal ischemia from microvascular disease, leading to inflammation and myocardial fibrosis. However, studies have shown subclinical abnormalities in LV contractility in SSc patients, especially in SSc with concomitant CV risk factors such as hypertension.65
CMR-derived tissue characterization (Figure 5) may also be fundamental in detecting subclinical myocardial disease in SSc, which may aid in risk stratification. Native T1 mapping and extracellular volume quantification66 are more sensitive than conventional CMR techniques and can detect low-grade and diffuse myocardial inflammation, especially in SSc patients with aggressive disease, skeletal myositis, and conduction abnormalities.67 LGE quantification of myocardial fibrosis may demonstrate a diffuse or patchy distribution involving the LV midwall and subendocardium, related to microvascular dysfunction, fibrosis, and inflammation.4,67
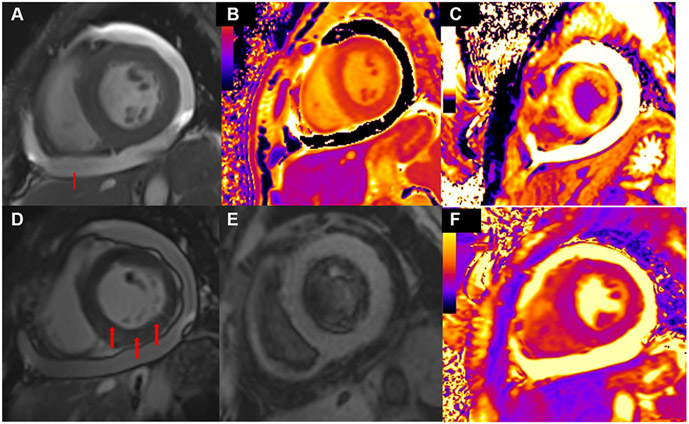
FIGURE 5. Cardiac Magnetic Resonance in Systemic Sclerosis.
(A) Moderate circumferential pericardial effusion seen in the short-axis view. (B) Native T1 mapping demonstrates myocardial inflammation. (C) Comparison of precontrast and postcontrast T1 mapping demonstrates diffuse myocardial inflammation (D) At the same level, late gadolinium enhancement demonstrates myocardial fibrosis. (E) T2-weighted short-tau inversion recovery imaging suppresses the signal of blood flow from fat thereby enhancing sensitivity to tissue fluid. Here, we see evidence of myocardial edema. (F) T2 mapping is more sensitive than qualitative and semiquantitative approaches to detect myocardial edema showing evidence of diffuse edema.
Pulmonary hypertension (PH) commonly occurs in SSc across WSPH (World Symposium of Pulmonary Hypertension) groups,68,69 and is associated with disproportionately poor outcomes, including diminished treatment response and functional status compared with other non-SSc PH etiologies.70 Recent advances in screening, risk stratification, and early initiation of directed therapies have marked improvement in mortality and clinical outcomes in SSc-associated pulmonary arterial hypertension (PAH)71; however, these benefits have not extended to WSPH group 2 and 3 disease in SSc.
Given the spectrum of possible cardiopulmonary involvement, echocardiography is a fundamental first step for screening, early detection, and prediction of adverse clinical events in SSc, given its high specificity and positive predictive value.68 As shown in Figure 6, 2DE provides crucial information on cardiac chamber size and function, diastolic dysfunction, valvular disease, and pericardial disease and can provide critical information regarding PH phenotype to distinguish precapillary vs postcapillary subtypes.72 In addition to detecting SSc-associated diastolic dysfunction and emerging HF with preserved ejection fraction,60 2DE is also integral for comprehensive imaging of the right heart. Several 2DE-based measures of right ventricular (RV) function predict mortality in SSc-PAH, including tricuspid annular plane systolic excursion, tissue Doppler S’ velocity, and fractional area change.60,73
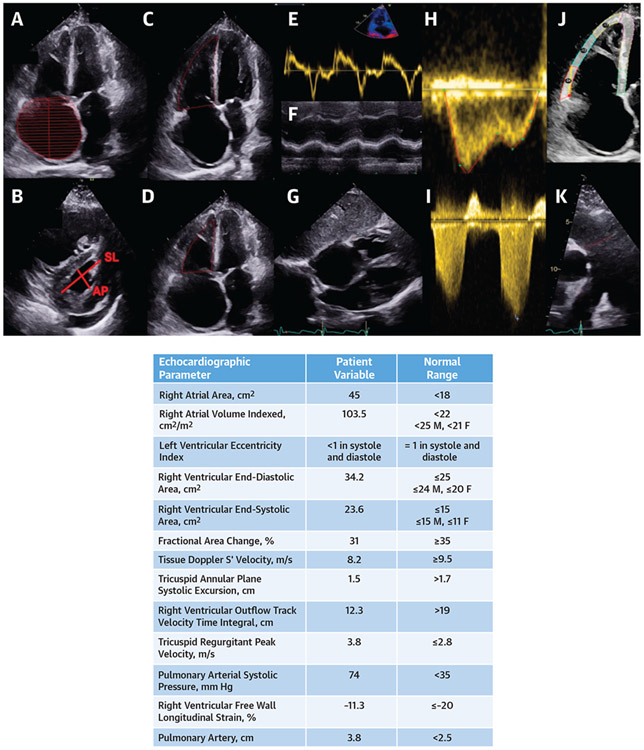
FIGURE 6. Echocardiography in Systemic Sclerosis With Pulmonary Arterial Hypertension.
(A) Severe right atrial enlargement with rightward bowing of the interatrial septum. Red hash marks represent end-diastolic volume of the right atrium. (B) Right ventricular enlargement, abnormal eccentricity index, and a small posterior pericardial effusion. (C and D) Severely dilated right heart chambers. Fractional area change is decreased at 31%. Red outline in C represent end-diastolic area of the right ventricle and represent end-systolic area of the right ventricle in D. (E) Tissue Doppler S’ velocity is reduced at 8.2 cm/s. (F) Tricuspid annular plane systolic excursion is reduced at 1.5 cm. (G) Increased right ventricular free wall thickness. (H) Right ventricular outflow track (RVOT) velocity time integral. (I) Pulmonary arterial systolic pressure is elevated at 74 mm Hg. (J) Right ventricular free wall strain is reduced at −11.3% (red indicates basal segment, blue indicates midventricular, and yellow indicates apical). (K) The main pulmonary artery is dilated at 3.8 cm (red line indicates end-diastolic diameter of the proximal pulmonary artery). AP = anteroposterior; SL = septolateral.
Recent studies have also elucidated the emerging role of STE in the prediction of adverse clinical outcomes in SSc. In SSc, STE-derived metrics of RV contractility may be diminished when conventional measures are otherwise normal74 and have been shown to predict mortality in SSc-associated PAH.75,76 Imaging of the RV is highly dependent on patient habitus as well as image acquisition, both of which can lead to potential variability in interobserver interpretation. Three-dimensional (3D) echocardiography, particularly in the volumetric assessment of RV ejection fraction, is being increasingly utilized clinically to monitor treatment response given the close correlation with CMR-derived techniques in PH.77
Stress echocardiography paired with STE is a promising novel application in screening SSc patients for PAH, valvular disease, and occult diastolic dysfunction. A recent study demonstrated that stress-STE revealed impairments in RV contractile reserve in SSc patients at risk for PAH,78 and was predictive of incident pulmonary vascular disease.79 The utilization of stress echocardiographic techniques in identifying emerging pulmonary vascular disease has exciting clinical implications in SSc for improved screening and early detection.
Last, SSc patients may be affected by the pericardial disease, ranging from 33% to 72%, and includes a broad spectrum of clinical manifestations, including acute and chronic pericarditis, pericardial effusions, and pericardial fibrosis leading to constrictive pericarditis. Although prevalent, symptomatic pericardial disease in SSc is relatively uncommon with frequency similar to the general population, and it can be treated with standard doses of Colchicine monotherapy or in conjunction with anti-inflammatory agents. However, in SSc-PAH, pericardial effusions are associated with considerable morbidity and mortality and are a poor prognostic sign.60
These findings highlight that cardiac involvement is common in SSc and can span pericardial, myocardial, and subendocardial microvascular disease, in addition to a maladaptive response to emerging pulmonary vascular disease. Often unrecognized until late in the disease course, SSc-associated cardiac disease is associated with marked increases in morbidity and mortality, and therefore, screening and early application of diagnostic tools are essential to improve long-term outcomes. Routine clinical evaluation, including symptom assessment, physical examination, and laboratory tests, should be combined with 2DE findings to provide a comprehensive cardiopulmonary assessment in SSc. Further advanced imaging modalities may also be indicated based on the initial 2DE findings and clinical context.
CARDIAC SARCOIDOSIS
Sarcoidosis is a multiorgan system disease characterized by the organ-level accumulation of inflammatory mononuclear phagocytes and T-lymphocytes into noncaseating granulomatous formation. The pathophysiology of sarcoidosis is multifactorial and involves a complex interplay of dysregulated immune responses, environmental factors, and genetic predisposition. Disease onset typically occurs around 25 to 45 years, although a second peak in women >50 years potentially triggered by postmenopausal hormonal changes has been described. Noncaseating granulomas can infiltrate any organ system resulting in a myriad of clinical presentations, and cardiac sarcoidosis (CS) has been reported in up to 5% to 10% of affected patients,80 manifesting as cardiomyopathy, arrhythmia, or atrioventricular conduction disease. High-grade atrioventricular block is a common presentation in up to 42% of patients81; however, direct granulomatous involvement of valvular structures, constrictive pericarditis, coronary artery granulomatous disease causing myocardial ischemia, and intracardiac masses have also been reported.
Despite significant diagnostic advances with integrated multimodality imaging approaches, CS remains challenging, particularly if extracardiac disease is not apparent.82 Screening is recommended in symptomatic patients with extracardiac sarcoidosis or distinct clinical scenarios in patients without known extracardiac sarcoidosis, including sustained monomorphic ventricular tachycardia, sudden cardiac death, or advanced conduction disease; in individuals younger than the age of 60 years; or in those with unexplained cardiomyopathy and/or HF.80 Diagnosis of CS traditionally requires histopathologic evidence of noncaseating granulomatous infiltration, either myocardial tissue or another afflicted organ, and characteristic clinical and imaging findings. Although lacking in sensitivity, 2DE is the only imaging modality recommended for screening patients with extracardiac sarcoidosis.80,83 Distinguishing 2DE findings in CS may include systolic and diastolic dysfunction, wall motion abnormalities, and ventricular wall thinning. Specifically, granulomatous infiltration of the basal septum can result in dyskinesis, akinesis, or aneurysmal formation, signifying the presence of myocardial inflammation, scarring, and impaired myocardial function. The sensitivity of 2DE measures of myocardial function in CS is amplified with adjunctive STE imaging adding prognostic value.84
CMR offers several advantages in assessing CS, including its ability to provide detailed information about myocardial structure, function, and tissue characterization (Figure 5).4 In addition to morphologic changes, including wall thinning and aneurysmal segments, LGE images generated from T1-W inversion recovery pulse sequence, applied 10 to 15 minutes after intravenous gadolinium administration, permit detection, and quantification of myocardial replacement fibrosis.4 In CS, LGE patterns demonstrate a characteristic midwall or subepicardial distribution, commonly involving the basal septum, lateral, and free wall of the LV, suggestive of granulomatous inflammation. Relative sparing of the subendocardium may help distinguish characteristic granulomatous infiltration from ischemic heart disease.4
18F-FDG-PET is an integral part of the multimodality approach in CS used for diagnosis and monitoring of treatment response, and relies on the utilization of radionuclide-labeled glucose to detect areas of inflammation through the uptake by inflammatory cells–predominantly macrophages. Consensus guidelines recommend the use of cardiac PET for cardiac sarcoidosis in specific clinical scenarios and include the following: 1) unexplained, advanced atrioventricular condition block in an individual <60 years of age; 2) idiopathic sustained ventricular tachycardia; 3) histologic evidence of extracardiac sarcoid; or 4) patients with a diagnosis of CS currently on treatment.85 A whole-body PET should be ideally performed simultaneously to detect extracardiac involvement.
Several limitations to the utilization of 18F-FDG-PET include the critical dietary preparation required to suppress normal FDG uptake of myocytes and access to specialized equipment and radiotracers, which may limit widespread utilization. The accurate interpretation of 18F-FDG-PET images also requires expertise and experience in recognizing and distinguishing areas of increased uptake indicative of inflammation from normal physiological uptake or other noninflammatory conditions. Although CMR and FDG-PET are both highly sensitive and comparable for detecting CS,4 the wider availability and lower cost may favor CMR as the first-choice modality in cardiac inflammatory and ischemic disease evaluation.
CLINICAL IMAGING FRAMEWORK FOR SIDs
2DE is an essential and widely used imaging modality in assessing cardiac involvement in SIDs because of its availability, real-time interpretation, low cost, and noninvasive nature. 2DE is pivotal in screening, diagnosing, assessing disease severity, and serial monitoring for disease progression and therapeutic effects. Regional and global wall motion abnormality may suggest ischemia from obstructive epicardial and/or microvascular disease, seen commonly in SIDs, and pathologic granulomatous infiltration seen in CS. ACC/AHA guidelines also reinforce the role of stress 2DE imaging for ischemia evaluation.86 Additionally, 2DE is the first line for evaluating pericardial disease and associated effusions, as frequently seen in SSc and SLE. Valvular dysfunction is assessed using 2DE with Doppler-flow analysis and adjunctive 3D echocardiography to assess valve morphology. Doppler-derived hemodynamics is also essential for chamber-level hemodynamic assessment, crucial for PH screening.69 Newer STE methodology enables the detection of subclinical myocardial involvement in SIDs,36,74-76,84 and can be coupled with stress imaging to assess contractile reserve and predict emerging pulmonary vascular disease.78,79,87
Coronary CTA is crucial in visualizing the coronary artery lumen for accurately detecting CAD and calculating the CAC score based on the number, areas, and peak Hounsfield units of the calcified lesions, reflecting the total ASCVD burden.88 One drawback, however, is the necessary administration of iodinated contrast, which can worsen renal impairment in chronic kidney disease frequently associated with SIDs. Compared with MRA, coronary CTA is more sensitive (85% vs 72%) and specific (95% vs 87%) for the noninvasive detection of CAD89; however, MRA may be more feasible in young, asymptomatic, and chronic kidney disease patients due to lack of contrast exposure.
Using diffusible radiotracers, SPECT is the most widely used and available technique to assess myocardial perfusion during stress and exercise. Myocardial perfusion will not increase appropriately in the stenotic artery territories, creating heterogeneous uptake depicting tissue affected by significant coronary stenoses. Although highly sensitive for detecting obstructive CAD, the specificity of SPECT remains low.90 The improved resolution and sensitivity of PET-MPI for coronary perfusion and coronary flow reserve promises to improve on these limitations. Coronary angiography remains the gold standard for diagnosing and treating CAD86; however, because of its invasive nature, cost, and exposure to radiation and contrast, it remains unattractive as a screening tool.
NOVEL AND EMERGING IMAGING TECHNIQUES
CORONARY CT AND FAT ATTENUATION INDEX.
Coronary CTA is a sensitive imaging modality for diagnosing obstructive and nonobstructive CAD, capable of identifying high-risk plaque features such as positive remodeling and low attenuation plaque.91 An important physiologic assessment modality–fractional flow reserve (FFR)-CT–has been developed and validated in addition to anatomy. FFR-CT is a physiologic simulation technique that utilizes coronary CTA data to estimate lesion-specific ischemia.92 Accompanying substantial technical advances in coronary CTA in the last decade, large prospective trials in stable chest pain patients suggest that coronary CTA could be an alternative to functional testing.93
The assessment of PCAT density or attenuation change using coronary CTA has emerged as a novel imaging biomarker for early-stage coronary artery inflammation. During coronary artery inflammation, changes occur in the lipid and water content of the pericoronary fat, leading to increased fat density in coronary CTA. PCAT density/attenuation changes are a potential early coronary artery inflammation imaging marker.94,95 Adjunctive methods such as the fat attenuation index (FAI) employ semiautomated plaque analysis tools to quantify PCAT using the coronary CTA data set and enable assessing PCAT density changes, providing a quantitative measure of coronary artery inflammation.95 In a recent post hoc analysis, PCAT assessment using FAI around the 3 coronaries predicted all-cause and cardiac-specific mortality.94 Moreover, PCAT attenuation quantification around coronary artery atherosclerosis can also identify vulnerable plaques, which could be a valuable tool to guide future prevention strategies,96 and could be used to track responses to anti-inflammatory therapies.97
CT PERFUSION.
CT perfusion is a novel and emerging method to assess myocardial ischemia. After detecting significant luminal stenosis on coronary CTA images, static or dynamic stress CT perfusion images are acquired to assess flow-limiting disease. Stress-induced myocardial perfusion defects appear as low attenuation areas in the myocardium. CT perfusion has high diagnostic accuracy, comparable to SPECT, in detecting myocardial ischemia. Dual-energy or newer technologies, such as spectral CT, can offer a higher signal-to-noise ratio in myocardial perfusion imaging.98 However, despite its diagnostic value and emerging CT-based technologies, CT perfusion remains an underutilized imaging tool.
MYOCARDIAL CONTRAST ECHOCARDIOGRAPHY.
Although conventional echocardiography provides crucial information regarding chamber quantification and function and the noninvasive assessment of chamber-level hemodynamics, myocardial contrast echocardiography (MCE) is an emerging technique for evaluating subendocardial myocardial blood flow.99 Coronary microvascular disease is a common feature of SIDs, and recent clinical practice guidelines86 demonstrate the feasibility and reproducibility of MCE-guided coronary flow velocity assessment of the distal left anterior descending and posterior descending arteries. The coupling of MCE with STE, global, regional, and even layer-specific strain may also demonstrate abnormalities in coronary blood flow in women presenting with ischemia and chest pain without obstructive epicardial coronary disease.87
CMR AND 4-DIMENSIONAL FLOW.
Diffusion tensor (DT)-CMR is a novel, noninvasive technique that can detect subclinical changes in myocardial function and, although still early in development, may assist with early diagnosis and risk prediction.100 Four-dimensional (4D) flow is a CMR method that allows 3D visualization of vascular flow over time and quantitative assessment of transvalvular or intracavity flow.101 In PH, 4D flow can assess the hemodynamic changes in the pulmonary circulation. The pulmonary artery is also less distensible in PH, which may predict mortality and greater retrograde blood flow through the pulmonary artery in patients with PH, thought to be secondary to a turbulent vortex. The length of time of which the vortex is present during the cardiac cycle correlates with pulmonary pressures.102
PET AND MOLECULAR IMAGING.
Cardiac PET is now a standard imaging test used to evaluate ischemia and coronary microvascular dysfunction with high sensitivity and specificity. Over the past several decades, multiple SIDs associated with a high burden of microvasculature dysfunction, such as RA, PsA and SLE, are detectable by cardiac PET MPI (Figure 7).103,104 Recent data demonstrates that coronary microvascular dysfunction has prognostic importance in SIDs with the degree of coronary vasomotor impairment associating with an increased risk of all-cause mortality and major adverse CV events.105 Whether specific treatment with anti-inflammatory therapies improves vasomotor dysfunction remains unclear and highlights the need for future mechanistic studies.106
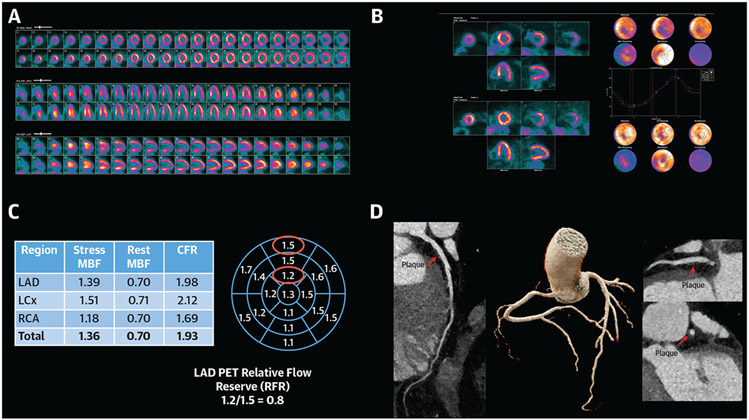
FIGURE 7. Coronary Microvascular Dysfunction in a Patient With Active Rheumatoid Arthritis Presenting With Dyspnea.
(A) 13N-ammonia Perfusion PET. Perfusion images demonstrate moderate ischemia in the left anterior descending (LAD) artery with vasodilator administration. (B) Functional imaging reveals normal LV volumes and systolic function at rest and stress (63% and 75%, respectively) without regional wall motion abnormalities. (C) Coronary flow reserve demonstrates globally reduced peak myocardial blood flow (normal >1.8) and associated reduction in coronary flow reserve (abnormal defined as CFR<2.0). The 17-segment model highlights the LAD relative flow reserve and a gradient suggestive of diffuse nonobstructive atherosclerosis. (D) Coronary CT was performed to rule out severe LAD stenosis which revealed evidence of a medium amount of predominantly noncalcified coronary plaque throughout the epicardial coronaries, resulting in minimal stenosis of the left main, proximal and mid-LAD, proximal left circumflex, and right coronary artery (shown is the LAD)
In atherosclerosis, 18F-FDG-PET is a common imaging marker of vascular inflammation. Initially, 18F-FDG-PET was used to differentiate culprit and high-risk atherosclerotic plaques in carotid artery disease; however, it later showed capability in identifying high-risk coronary plaques.107 Aortic 18F-FDG uptake is associated with noncalcified plaque burden in patients with PsA.108 FDG also distributes systemically to reflect global inflammation, with increased bone marrow and spleen uptake following acute ischemia.109 Despite its widespread availability, a significant clinical challenge is that FDG uptake is not limited to macrophages, and uptake by other cells that metabolize glucose, tissue hypoxia, and neovascularization contributes to signal intensity. 18F-FDG is also limited for coronary evaluation due to avid myocardial uptake that may prevent reliable interpretation. However, newer PET ligands with increased specificity for vascular inflammation may be more reliable for coronary artery imaging. 18F-NaF-PET, which detects osteogenic activity, is incorporated into the nascent hydroxyapatite matrix primarily to diagnose bone disease. Intense vascular inflammation can lead to early vascular calcification detected using 18F-NaF-PET. In patients with MI and stable angina, 18F-NaF was evident in culprit plaques and symptomatic carotid disease, whereas 18F-FDG-PET coronary uptake was obscured by myocardial uptake resulting in no appreciable differences between culprit and nonculprit plaques.110 Although promising, the role of 18F-NaF in refining risk stratification, monitoring disease progression, and guiding therapeutic decisions is unclear. Promising feasibility studies in RA suggest a potential role for 18F-NaF-PET to guide anti-inflammatory agent selection.111
Molecular imaging using highly selective PET probes extends applications beyond the current uses of PET-MPI for myocardial blood flow and 18F-FDG-PET for myocardial inflammation, with the potential to track cellular immunity for precision medicine applications. 68Ga or 64Cu-labeled DOTA agents targeting the somatostatin receptor 2 in activated macrophages show promising correlation with CV risk factors with high uptake in culprit coronary and carotid lesions,112 and more recently, in large vessel vasculitis.113 The advent of immunoPET, using monoclonal antibody (mAb) probes, has represented a paradigm-shifting advancement in oncology for monitoring tumor populations and responses to therapy.112 The development of numerous therapeutic agents, including small-molecule tyrosine receptor kinase inhibitors, mAb immunotherapies (eg, immune checkpoint inhibitors and CD20-specific mAb Rituxan) successfully applied to solid and liquid tumors are potential therapeutic and imaging targets in SIDs.114
In recent years, there has been growing interest in the development and application of novel radiotracers for PET imaging, including those that target fibroblast activation protein (FAP), an enzyme that is overexpressed in activated fibroblasts, a key component of the fibrotic process seen in various diseases, including atherosclerosis and RA. FAPI-PET imaging has shown promise in the detection of calcific arterial atherosclerosis, thereby providing important in vivo insights into the presence and extent of fibroblast activation within atherosclerotic plaques.32 FAPI-PET has also been used in RA to assess the degree of articular fibroblast activation and correlates with disease severity and activity.115
SIDs could serve as model systems for discovering immune pathogenesis in CV disease and may represent a new frontier of precision-based medicine in CAD. Although many other novel tracers are under active investigation, there is a persistent challenge because of limited availability.
FUTURE DIRECTIONS
Multimodality cardiac imaging is crucial in detecting and managing cardiac complications in SIDs. However, the complexity and heterogeneity of cardiac involvement within each disease state require careful selection of the appropriate imaging test. Different imaging modalities offer unique strengths and limitations, and choice is based on the specific question, clinical presentation, and the expertise and resources available.
A critical area of interest for future advancement of imaging in SIDs includes novel ways to visualize inflammation and characterize severity. The development of immunoPET, which combines PET with specific targeting agents (eg, radiolabeled antibodies), holds great promise for visualizing inflammation in SIDs. ImmunoPET enables the specific targeting and imaging of inflammatory cells and molecules, providing valuable insights into the extent and severity of inflammation. This technique can enhance our understanding of disease processes, monitor treatment response, and guide targeted therapies against specific leukocyte populations by quantifying and characterizing inflammatory activity. Research into imaging specific pathways or cells involved in innate or adaptive immunity is advancing rapidly and holds great promise for the future of precision medicine.116 As technology continues to evolve, these imaging methods may play a crucial role in diagnosing, monitoring, and treating various diseases by directly visualizing immune responses and pathways at the cellular level.
As CT technology and techniques evolve, there is also increasing interest in incorporating functional CT parameters, such as FFR-CT or CT-derived plaque characterization, in managing patients with SIDs and atherosclerosis. These techniques can provide detailed information about the extent and severity of CAD, guide treatment decisions, and monitor disease progression or treatment response. Functional CT techniques can potentially intensify therapies or guide immunosuppressive therapy in patients with SIDs at increased CV risk because of atherosclerosis. Incorporating FAI may also play a significant role in managing patients with SIDs for atherosclerosis in primary and secondary prevention, such as intensification and guiding immunosuppressive therapy and monitoring.
4D flow CMR is another evolving technique that enables the assessment of blood flow dynamics in all directions and throughout the cardiac cycle. By assessing the pulmonary artery and RV hemodynamics in response to treatment, 4D flow CMR could aid in prognostication, guide treatment selection, and facilitate treatment escalation when necessary. Echocardiography continues to evolve with the incorporation of advanced techniques such as myocardial deformation analysis (strain imaging) and 3D volumetric assessment. These techniques enable the early detection of subclinical myocardial dysfunction and the initiation of cardioprotective agents at an earlier stage. Myocardial deformation analysis provides valuable insights into cardiac mechanics, detecting subtle changes in contractile function before they become evident on conventional measures. 3D volumetric assessment allows for accurate and comprehensive cardiac structure and function evaluation. Recent advances in myocardial contrast echocardiography may also provide an exciting pathway to improve ultrasound-based imaging of disease-specific inflammation through the detection of targeted microbubbles or other gas-filled particle contrast agents.117
CONCLUSIONS
Multimodality imaging advancements are revolutionizing our understanding and management of cardiac involvement in SIDs. By providing precise and detailed information, imaging can guide personalized medicine approaches by tailoring treatment strategies to individual patients based on their specific disease characteristics and response to therapy. The concept of precision-based personalized medicine holds promise for optimizing patient outcomes in SIDs. Ultimately, the further refinement and advancement of these existing technologies have the potential to understand the biology of inflammation at an individual level and guide precision-based personalized medicine. Ongoing research and collaboration between imaging specialists and clinical cardio-rheumatology experts are crucial to continue advancing these technologies and translating them into clinical benefits for patients with SIDs.
HIGHLIGHTS.
Immune-mediated systemic inflammatory diseases are commonly associated with adverse CV manifestations.
Integrated multimodality imaging can improve screening, detection, and serial monitoring of cardiac function in patients with SIDs.
An evaluation and management approach that incorporates multimodality imaging is integral to improving clinical outcomes.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
Dr Weber has received funding support from the National Institutes of Health National Heart, Lung, and Blood Institute K23HL159276-02 and American Heart Association 21CDA851511; and has served on the Scientific Advisory Board of Novo Nordisk, Horizon Therapeutics, and Kiniksa. Dr Paik has received funding support from the National Institutes of Health NIAMS K23AR073927-03. Dr Klein has received research grants from and served on the scientific advisory board of Kiniksa and Cardiology Therapeutics. Dr Yu has received funding support from the National Institutes of Health National Heart, Lung, and Blood Institute R01HL159443-02 and National Institutes of Health NIAMS R01AR057374-09. Dr Mukherjee has received funding support from the National Institutes of Health National Heart, Lung, and Blood Institute R01HL162851 and National Scleroderma Foundation; and has served on the Data Safety Monitoring Board of Advarra, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- 2DE
2-dimensional echocardiography
- ASCVD
atherosclerotic cardiovascular disease
- CAC
coronary artery calcification
- CAD
coronary artery disease
- CMR
cardiac magnetic resonance
- CS
cardiac sarcoidosis
- CTA
computed tomography angiography
- CVD
cardiovascular disease
- EMB
endomyocardial biopsy
- FAI
fat attenuation index
- IIM
idiopathic inflammatory myopathies
- LGE
late gadolinium enhancement
- LV
left ventricle/ventricular
- KD
Kawasaki Disease
- mAb
monoclonal antibody
- MCE
myocardial contrast echocardiography
- MPI
myocardial perfusion imaging
- MRA
magnetic resonance angiography
- PAN
polyarteritis nodosa
- PAH
pulmonary arterial hypertension
- PCAT
pericoronary adipose tissue
- PET
positron emissions tomography
- PH
pulmonary hypertension
- PsA
psoriatic arthritis
- RA
rheumatoid arthritis
- RV
right ventricle
- SID
systemic inflammatory disease
- SLE
systemic lupus erythematosus
- SpA-AS
spondyloarthritis-ankylosing spondylitis
- SPECT
single photon emission computed tomography
- SSc
systemic sclerosis
- STE
speckle-tracking echocardiography
- SV
systemic vasculitides
- TAK
Takayasu’s arteritis
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
REFERENCES
- 1.Gasparyan AY. Cardiovascular risk and inflammation: pathophysiological mechanisms, drug design, and targets. Curr Pharm Des. 2012;18:1447–1449. 10.2174/138161212799504777 [DOI] [PubMed] [Google Scholar]
- 2.Zhao TX, Mallat Z. Targeting the immune system in atherosclerosis: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:1691–1706. 10.1016/j.jacc.2018.12.083 [DOI] [PubMed] [Google Scholar]
- 3.Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2012;71:1524–1529. 10.1136/annrheumdis-2011-200726 [DOI] [PubMed] [Google Scholar]
- 4.Mavrogeni S, Pepe A, Nijveldt R, et al. Cardiovascular magnetic resonance in autoimmune rheumatic diseases: a clinical consensus document by the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2022;23:e308–e322. 10.1093/ehjci/jeac134 [DOI] [PubMed] [Google Scholar]
- 5.Ohta H, Wada H, Niwa T, et al. Disruption of tumor necrosis factor-alpha gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis. 2005;180:11–17. 10.1016/j.atherosclerosis.2004.11.016 [DOI] [PubMed] [Google Scholar]
- 6.Weber BN, Giles JT, Liao KP. Shared inflammatory pathways of rheumatoid arthritis and atherosclerotic cardiovascular disease. Nat Rev Rheumatol. 2023;19:417–428. 10.1038/s41584-023-00969-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Venetsanopoulou AI, Pelechas E, Voulgari PV, Drosos AA. The lipid paradox in rheumatoid arthritis: the dark horse of the augmented cardiovascular risk. Rheumatol Int. 2020;40:1181–1191. 10.1007/s00296-020-04616-2 [DOI] [PubMed] [Google Scholar]
- 8.Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69:325–331. 10.1136/ard.2009.113696 [DOI] [PubMed] [Google Scholar]
- 9.Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74(10):1376–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2):ii14–ii17. 10.1136/ard.2004.032482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bengtsson K, Forsblad-d’Elia H, Lie E, et al. Are ankylosing spondylitis, psoriatic arthritis and undifferentiated spondyloarthritis associated with an increased risk of cardiovascular events? A prospective nationwide population-based cohort study. Arthritis Res Ther. 2017;19:102. 10.1186/s13075-017-1315-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326–332. 10.1136/annrheumdis-2014-205675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber B, Merola JF, Husni ME, Di Carli M, Berger JS, Garshick MS. Psoriasis and cardiovascular disease: novel mechanisms and evolving therapeutics. Curr Atheroscler Rep. 2021;23:67. 10.1007/s11883-021-00963-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonek K, Kuca-Warnawin E, Kornatka A, et al. Associations of IL-18 with altered cardiovascular risk profile in psoriatic arthritis and ankylosing spondylitis. J Clin Med. 2022;11(3):766. 10.3390/jcm11030766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grygiel-Górniak B, Oduah M-T, Olagunju A, Klokner M. Disorders of the aorta and aortic valve in connective tissue diseases. Curr Cardiol Rep. 2020;22:70. 10.1007/s11886-020-01314-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi E, Mathews LM, Paik J, et al. Multimodality evaluation of aortic insufficiency and aortitis in rheumatologic diseases. Front Cardiovasc Med. 2022;9:874242. 10.3389/fcvm.2022.874242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ocon AJ, Reed G, Pappas DA, Curtis JR, Kremer JM. Short-term dose and duration-dependent glucocorticoid risk for cardiovascular events in glucocorticoid-naive patients with rheumatoid arthritis. Ann Rheum Dis. 2021;80:1522–1529. 10.1136/annrheumdis-2021-220577 [DOI] [PubMed] [Google Scholar]
- 18.Garshick M, Underberg JA. The use of primary prevention statin therapy in those predisposed to atherosclerosis. Curr Atheroscler Rep. 2017;19:48. 10.1007/s11883-017-0685-7 [DOI] [PubMed] [Google Scholar]
- 19.Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. 10.1056/NEJMoa072100 [DOI] [PubMed] [Google Scholar]
- 20.Kiani AN, Magder LS, Post WS, et al. Coronary calcification in SLE: comparison with the Multi-Ethnic Study of Atherosclerosis. Rheumatology (Oxford). 2015;54:1976–1981. 10.1093/rheumatology/kev198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giles JT, Szklo M, Post W, et al. Coronary arterial calcification in rheumatoid arthritis: comparison with the Multi-Ethnic Study of Atherosclerosis. Arthritis Res Ther. 2009;11:R36. 10.1186/ar2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. 2002;91:988–998. 10.1161/01.res.0000043825.01705.1b [DOI] [PubMed] [Google Scholar]
- 23.Ramírez E, Romero-Garrido JA, López-Granados E, et al. Symptomatic thromboembolic events in patients treated with intravenous-immunoglobulins: results from a retrospective cohort study. Thromb Res. 2014;133:1045–1051. 10.1016/j.thromres.2014.03.046 [DOI] [PubMed] [Google Scholar]
- 24.Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386:316–326. 10.1056/NEJMoa2109927 [DOI] [PubMed] [Google Scholar]
- 25.Maqsood MH, Weber BN, Haberman RH, Lo Sicco KI, Bangalore S, Garshick MS. Cardiovascular and venous thromboembolic risk with janus kinase inhibitors in immune-mediated inflammatory diseases: a systematic review and meta-analysis of randomized trials. ACR Open Rheumatology. 2022;4(10):912–922. 10.1002/acr2.11479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miner JJ, Kim AH. Cardiac manifestations of systemic lupus erythematosus. Rheum Dis Clin North Am. 2014;40:51–60. 10.1016/j.rdc.2013.10.003 [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Kaplan MJ. Cardiovascular disease in systemic lupus erythematosus: an update. Curr Opin Rheumatol. 2018;30:441–448. 10.1097/BOR.0000000000000528 [DOI] [PubMed] [Google Scholar]
- 28.Lood C, Blanco LP, Purmalek MM, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;22:146–153. 10.1038/nm.4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doring Y, Libby P, Soehnlein O. Neutrophil extracellular traps participate in cardiovascular diseases: recent experimental and clinical insights. Circ Res. 2020;126:1228–1241. 10.1161/CIRCRESAHA.120.315931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patiño-Trives AM, Pérez-Sánchez C, Pérez-Sánchez L, et al. Anti-dsDNA antibodies increase the cardiovascular risk in systemic lupus erythematosus promoting a distinctive immune and vascular activation. Arterioscler Thromb Vasc Biol. 2021;41:2417–2430. 10.1161/atv-baha.121.315928 [DOI] [PubMed] [Google Scholar]
- 31.Tobin R, Patel N, Tobb K, Weber B, Mehta PK, Isiadinso I. Atherosclerosis in systemic lupus erythematosus. Curr Atheroscler Rep. 2023;25(11):819–827. [DOI] [PubMed] [Google Scholar]
- 32.Wu M, Ning J, Li J, et al. Feasibility of in vivo imaging of fibroblast activation protein in human arterial walls. J Nucl Med. 2022;63:948–951. 10.2967/jnumed.121.262863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorbala S, Di Carli MF. Cardiac PET perfusion: prognosis, risk stratification, and clinical management. Semin Nucl Med. 2014;44:344–357. 10.1053/j.semnuclmed.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Recio-Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J. 2009;30:1837–1843. 10.1093/eurheartj/ehp205 [DOI] [PubMed] [Google Scholar]
- 35.Souza DC, Santo AH, Sato EI. Mortality profile related to systemic lupus erythematosus: a multiple cause-of-death analysis. J Rheumatol. 2012;39:496–503. 10.3899/jrheum.110241 [DOI] [PubMed] [Google Scholar]
- 36.Kim CH Al, Kindi SG, Jandali B, Askari AD, Zacharias M, Oliveira GH. Incidence and risk of heart failure in systemic lupus erythematosus. Heart. 2017;103:227–233. 10.1136/heartjnl-2016-309561 [DOI] [PubMed] [Google Scholar]
- 37.Weber BN, Garshick M, Abbate A, et al. Acute cardiovascular complications of immune-mediated systemic inflammatory diseases. Eur Heart J Acute Cardiovasc Care. 2023. Aug 21:zuad096. Epub ahead of print. 10.1093/ehjacc/zuad096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geyer H, Caracciolo G, Abe H, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–369. 10.1016/j.echo.2010.02.015 [DOI] [PubMed] [Google Scholar]
- 39.Kadkhodayan A, Chareonthaitawee P, Raman SV, Cooper LT. Imaging of inflammation in unexplained cardiomyopathy. J Am Coll Cardiol Img. 2016;9:603–617. 10.1016/j.jcmg.2016.01.010 [DOI] [PubMed] [Google Scholar]
- 40.Klein AL, Abbara S, Agler DA, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr. 2013;26:965–1012.e1015. 10.1016/j.echo.2013.06.023 [DOI] [PubMed] [Google Scholar]
- 41.Akintoye E, Wang TKM, Nakhla M, et al. Quantitative echocardiographic assessment and optimal criteria for early intervention in asymptomatic tricuspid regurgitation. J Am Coll Cardiol Img. 2022;16(1):13–24. 10.1016/j.jcmg.2022.08.004 [DOI] [PubMed] [Google Scholar]
- 42.Cremer PC, Tariq MU, Karwa A, et al. Quantitative assessment of pericardial delayed hyper-enhancement predicts clinical improvement in patients with constrictive pericarditis treated with anti-inflammatory therapy. Circ Cardiovasc Imaging. 2015;8(5):e003125. 10.1161/circimaging.114.003125 [DOI] [PubMed] [Google Scholar]
- 43.Zuily S, Regnault V, Selton-Suty C, et al. Increased risk for heart valve disease associated with antiphospholipid antibodies in patients with systemic lupus erythematosus: meta-analysis of echocardiographic studies. Circulation. 2011;124:215–224. 10.1161/CIRCULATIONAHA.111.028522 [DOI] [PubMed] [Google Scholar]
- 44.Miloslavsky E, Unizony S. The heart in vasculitis. Rheum Dis Clin North Am. 2014;40:11–26. 10.1016/j.rdc.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 45.Dennert RM, van Paassen P, Schalla S, et al. Cardiac involvement in Churg-Strauss syndrome. Arthritis Rheum. 2010;62:627–634. 10.1002/art.27263 [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Yang K, Meng X, et al. Cardiac valve involvement in takayasu arteritis is common: a retrospective study of 1,069 patients over 25 years. Am J Med Sci. 2018;356:357–364. 10.1016/j.amjms.2018.06.021 [DOI] [PubMed] [Google Scholar]
- 47.Dejaco C, Ramiro S, Duftner C, et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018;77:636–643. 10.1136/annrheumdis-2017-212649 [DOI] [PubMed] [Google Scholar]
- 48.Kang EJ, Kim SM, Choe YH, Lee GY, Lee KN, Kim DK. Takayasu arteritis: assessment of coronary arterial abnormalities with 128-section dual-source CT angiography of the coronary arteries and aorta. Radiology. 2014;270:74–81. 10.1148/radiol.13122195 [DOI] [PubMed] [Google Scholar]
- 49.Wall C, Huang Y, Le EPV, et al. Pericoronary and periaortic adipose tissue density are associated with inflammatory disease activity in Takayasu arteritis and atherosclerosis. Eur Heart J Open. 2021;1:oeab019. 10.1093/ehjopen/oeab019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Geest KSM, Slijkhuis BGC, Tomelleri A, et al. Positron emission tomography imaging in vasculitis. Cardiol Clin. 2023;41:251–265. 10.1016/j.ccl.2023.01.012 [DOI] [PubMed] [Google Scholar]
- 51.Miller FW, Lamb JA, Schmidt J, Nagaraju K. Risk factors and disease mechanisms in myositis. Nat Rev Rheumatol. 2018;14:255–268. 10.1038/nrrheum.2018.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Párraga Prieto C, Ibrahim F, Campbell R, Chinoy H, Galloway J, Gordon P. Similar risk of cardiovascular events in idiopathic inflammatory myopathy and rheumatoid arthritis in the first 5 years after diagnosis. Clin Rheumatol. 2021;40:231–238. 10.1007/s10067-020-05237-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lilleker JB, Vencovsky J, Wang G, et al. The EuroMyositis registry: an international collaborative tool to facilitate myositis research. Ann Rheum Dis. 2018;77:30–39. 10.1136/annrheumdis-2017-211868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng C, Liu W, Sun X, et al. Myocardial involvement characteristics by cardiac MR imaging in patients with polymyositis and dermatomyositis. Rheumatology (Oxford). 2022;61:572–580. 10.1093/rheumatology/keab271 [DOI] [PubMed] [Google Scholar]
- 55.Fairley JL, Wicks I, Peters S, Day J. Defining cardiac involvement in idiopathic inflammatory myopathies: a systematic review. Rheumatology (Oxford). 2021;61:103–120. 10.1093/rheumatology/keab573 [DOI] [PubMed] [Google Scholar]
- 56.Lundberg IE, Tjarnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Arthritis Rheumatol. 2017;69:2271–2282. 10.1002/art.40320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang L, Tao Q, Zhao P, et al. Using multi-parametric quantitative MRI to screen for cardiac involvement in patients with idiopathic inflammatory myopathy. Sci Rep. 2022;12:9819. 10.1038/s41598-022-13858-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kersten J, Guleroglu AM, Rosenbohm A, et al. Myocardial involvement and deformation abnormalities in idiopathic inflammatory myopathy assessed by CMR feature tracking. Int J Cardiovasc Imaging. 2021;37:597–603. 10.1007/s10554-020-02020-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Silva KM, Li L, Lu N, Ogdie A, Avina-Zubieta JA, Choi HK. Persistent premature mortality gap in dermatomyositis and polymyositis: a United Kingdom general population-based cohort study. Rheumatology (Oxford). 2021;60:2653–2660. 10.1093/rheumatology/keaa661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hung G, Mercurio V, Hsu S, Mathai SC, Shah AA, Mukherjee M. Progress in understanding, diagnosing, and managing cardiac complications of systemic sclerosis. Curr Rheumatol Rep. 2019;21:68. 10.1007/s11926-019-0867-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elhai M, Meune C, Boubaya M, et al. Mapping and predicting mortality from systemic sclerosis. Ann Rheum Dis. 2017;76:1897–1905. 10.1136/annrheumdis-2017-211448 [DOI] [PubMed] [Google Scholar]
- 62.Faccini A, Agricola E, Oppizzi M, et al. Coronary microvascular dysfunction in asymptomatic patients affected by systemic sclerosis - limited vs. diffuse form. Circ J. 2015;79:825–829. 10.1253/circj.CJ-14-1114 [DOI] [PubMed] [Google Scholar]
- 63.Hsu S, Kokkonen-Simon KM, Kirk JA, et al. Right ventricular myofilament functional differences in humans with systemic sclerosis-associated versus idiopathic pulmonary arterial hypertension. Circulation. 2018;137:2360–2370. 10.1161/circulationaha.117.033147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hinze AM, Perin J, Woods A, et al. Diastolic dysfunction in systemic sclerosis: risk factors and impact on mortality. Arthritis Rheumatol. 2022;74:849–859. 10.1002/art.42054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mercurio V, Hinze AM, Hummers LK, Wigley FM, Shah AA, Mukherjee M. Essential hypertension worsens left ventricular contractility in systemic sclerosis. J Rheumatol. 2021;48:1299–1306. 10.3899/jrheum.200873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moon JC, Messroghli DR, Kellman P, et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013;15:92. 10.1186/1532-429X-15-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pieroni M, De Santis M, Zizzo G, et al. Recognizing and treating myocarditis in recent-onset systemic sclerosis heart disease: potential utility of immunosuppressive therapy in cardiac damage progression. Semin Arthritis Rheum. 2014;43:526–535. 10.1016/j.semarthrit.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 68.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2022. 10.1183/13993003.00879-2022 [DOI] [PubMed] [Google Scholar]
- 69.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53:1801913. 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tedford RJ, Mudd JO, Girgis RE, et al. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circ Heart Fail. 2013;6:953–963. 10.1161/CIRCHEARTFAILURE.112.000008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hassan HJ, Naranjo M, Ayoub N, et al. Improved survival for patients with systemic sclerosis-associated pulmonary arterial hypertension: the Johns Hopkins Registry. Am J Respir Crit Care Med. 2023;207:312–322. 10.1164/rccm.202204-0731OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.D’Alto M, Romeo E, Argiento P, et al. Echocardiographic prediction of pre- versus postcapillary pulmonary hypertension. J Am Soc Echocardiogr. 2015;28:108–115. 10.1016/j.echo.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 73.Farrell C, Balasubramanian A, Hays AG, et al. A clinical approach to multimodality imaging in pulmonary hypertension. Front Cardiovasc Med. 2021;8:794706. 10.3389/fcvm.2021.794706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mukherjee M, Chung SE, Ton VK, et al. Unique abnormalities in right ventricular longitudinal strain in systemic sclerosis patients. Circ Cardiovasc Imaging. 2016;9:e003792. 10.1161/CIRCIMAGING.115.003792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mukherjee M, Mercurio V, Tedford RJ, et al. Right ventricular longitudinal strain is diminished in systemic sclerosis compared with idiopathic pulmonary arterial hypertension. Eur Respir J. 2017;50(5):1701436. 10.1183/13993003.01436-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sachdev A, Villarraga HR, Frantz RP, et al. Right ventricular strain for prediction of survival in patients with pulmonary arterial hypertension. Chest. 2011;139:1299–1309. 10.1378/chest.10-2015 [DOI] [PubMed] [Google Scholar]
- 77.Morikawa T, Murata M, Okuda S, et al. Quantitative analysis of right ventricular function in patients with pulmonary hypertension using three-dimensional echocardiography and a two-dimensional summation method compared to magnetic resonance imaging. Am J Cardiol. 2011;107:484–489. 10.1016/j.amj-card.2010.09.047 [DOI] [PubMed] [Google Scholar]
- 78.Mukherjee M, Mercurio V, Hsu S, et al. Assessment of right ventricular reserve utilizing exercise provocation in systemic sclerosis. Int J Cardiovasc Imaging. 2021;37(7):2137–2147. 10.1007/s10554-021-02237-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lu J, Jani V, Mercurio V, et al. Stress echocardiographic prediction of emerging pulmonary vascular disease in systemic sclerosis. J Am Soc Echocardiogr. 2023;36(2):259–261. 10.1016/j.echo.2022.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gilotra NA, Griffin JM, Pavlovic N, et al. Sarcoidosis-related cardiomyopathy: current knowledge, challenges, and future perspectives state-of-the-art review. J Card Fail. 2022;28:113–132. 10.1016/j.cardfail.2021.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ekström K, Lehtonen J, Nordenswan HK, et al. Sudden death in cardiac sarcoidosis: an analysis of nationwide clinical and cause-of-death registries. Eur Heart J. 2019;40:3121–3128. 10.1093/eurheartj/ehz428 [DOI] [PubMed] [Google Scholar]
- 82.Trivieri MG, Spagnolo P, Birnie D, et al. Challenges in cardiac and pulmonary sarcoidosis: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76:1878–1901. 10.1016/j.jacc.2020.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aggarwal NR, Snipelisky D, Young PM, Gersh BJ, Cooper LT, Chareonthaitawee P. Advances in imaging for diagnosis and management of cardiac sarcoidosis. Eur Heart J Cardiovasc Imaging. 2015;16:949–958. 10.1093/ehjci/jev142 [DOI] [PubMed] [Google Scholar]
- 84.Joyce E, Ninaber MK, Katsanos S, et al. Subclinical left ventricular dysfunction by echocardiographic speckle-tracking strain analysis relates to outcome in sarcoidosis. Eur J Heart Fail. 2015;17:51–62. 10.1002/ejhf.205 [DOI] [PubMed] [Google Scholar]
- 85.Chareonthaitawee P, Beanlands RS, Chen W, et al. Joint SNMMI-ASNC expert consensus document on the role of (18)F-FDG PET/CT in cardiacsarcoid detection and therapy monitoring. J Nucl Med. 2017;58:1341–1353. 10.2967/jnumed.117.196287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;78(22):e187–e285. [DOI] [PubMed] [Google Scholar]
- 87.Michelsen MM, Pena A, Mygind ND, et al. Coronary microvascular dysfunction and myocardial contractile reserve in women with angina and no obstructive coronary artery disease. Echocardiography. 2018;35:196–203. 10.1111/echo.13767 [DOI] [PubMed] [Google Scholar]
- 88.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827–832. 10.1016/0735-1097(90)90282-t [DOI] [PubMed] [Google Scholar]
- 89.Schuijf JD, Bax JJ, Shaw LJ, et al. Meta-analysis of comparative diagnostic performance of magnetic resonance imaging and multislice computed tomography for noninvasive coronary angiography. Am Heart J. 2006;151:404–411. 10.1016/j.ahj.2005.03.022 [DOI] [PubMed] [Google Scholar]
- 90.Underwood SR, Anagnostopoulos C, Cerqueira M, et al. Myocardial perfusion scintigraphy: the evidence. Eur J Nucl Med Mol Imaging. 2004;31:261–291. 10.1007/s00259-003-1344-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Puchner SB, Liu T, Mayrhofer T, et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol. 2014;64:684–692. 10.1016/j.jacc.2014.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tesche C, De Cecco CN, Albrecht MH, et al. Coronary CT angiography-derived fractional flow reserve. Radiology. 2017;285:17–33. 10.1148/radiol.2017162641 [DOI] [PubMed] [Google Scholar]
- 93.Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291–1300. 10.1056/NEJMoa1415516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oikonomou EK, Marwan M, Desai MY, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet. 2018;392:929–939. 10.1016/s0140-6736(18)31114-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Antonopoulos AS, Sanna F, Sabharwal N, et al. Detecting human coronary inflammation by imaging perivascular fat. Sci Transl Med. 2017;9(398):eaal42658. 10.1126/scitranslmed.aal2658 [DOI] [PubMed] [Google Scholar]
- 96.Goeller M, Achenbach S, Cadet S, et al. Pericoronary adipose tissue computed tomography attenuation and high-risk plaque characteristics in acute coronary syndrome compared with stable coronary artery disease. JAMA Cardiol. 2018;3:858–863. 10.1001/jamacardio.2018.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Elnabawi YA, Oikonomou EK, Dey AK, et al. Association of biologic therapy with coronary inflammation in patients with psoriasis as assessed by perivascular fat attenuation index. JAMA Cardiol. 2019;4:885–891. 10.1001/jamacardio.2019.2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Danad I, Fayad ZA, Willemink MJ, Min JK. New applications of cardiac computed tomography: dual-energy, spectral, and molecular CT imaging. J Am Coll Cardiol Img. 2015;8:710–723. 10.1016/j.jcmg.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lindner JR. Contrast echocardiography: current status and future directions. Heart. 2021;107:18–24. [DOI] [PubMed] [Google Scholar]
- 100.Khalique Z, Ferreira PF, Scott AD, Nielles-Vallespin S, Firmin DN, Pennell DJ. Diffusion tensor cardiovascular magnetic resonance imaging: a clinical perspective. J Am Coll Cardiol Img. 2020;13:1235–1255. 10.1016/j.jcmg.2019.07.016 [DOI] [PubMed] [Google Scholar]
- 101.Barker AJ, Roldan-Alzate A, Entezari P, et al. Four-dimensional flow assessment of pulmonary artery flow and wall shear stress in adult pulmonary arterial hypertension: results from two institutions. Magn Reson Med. 2015;73:1904–1913. 10.1002/mrm.25326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reiter G, Reiter U, Kovacs G, Olschewski H, Fuchsjager M. Blood flow vortices along the main pulmonary artery measured with MR imaging for diagnosis of pulmonary hypertension. Radiology. 2015;275:71–79. 10.1148/radiol.14140849 [DOI] [PubMed] [Google Scholar]
- 103.Weber B, Perez-Chada LM, Divakaran S, et al. Coronary microvascular dysfunction in patients with psoriasis. J Nucl Cardiol. 2022;29:37–42. 10.1007/s12350-020-02166-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weber BN, Stevens E, Barrett L, et al. Coronary microvascular dysfunction in systemic lupus erythematosus. J Am Heart Assoc. 2021;10:e018555. 10.1161/jaha.120.018555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weber BN, Stevens E, Perez-Chada LM, et al. Impaired coronary vasodilator reserve and adverse prognosis in patients with systemic inflammatory disorders. J Am Coll Cardiol Img. 2021;14:2212–2220. 10.1016/j.jcmg.2020.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weber B, Weisenfeld D, Seyok T, et al. Relationship between risk of atherosclerotic cardiovascular disease, inflammation, and coronary microvascular dysfunction in rheumatoid arthritis. J Am Heart Assoc. 2022;11:e025467. 10.1161/jaha.121.025467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Graebe M, Pedersen SF, Højgaard L, Kjaer A, Sillesen H. 18FDG PET and ultrasound echolucency in carotid artery plaques. J Am Coll Cardiol Img. 2010;3:289–295. 10.1016/j.jcmg.2010.01.001 [DOI] [PubMed] [Google Scholar]
- 108.Joshi AA, Lerman JB, Dey AK, et al. Association between aortic vascular inflammation and coronary artery plaque characteristics in psoriasis. JAMA Cardiol. 2018;3:949–956. 10.1001/jamacardio.2018.2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Emami H, Singh P, MacNabb M, et al. Splenic metabolic activity predicts risk of future cardiovascular events: demonstration of a cardiosplenic axis in humans. J Am Coll Cardiol Img. 2015;8:121–130. 10.1016/j.jcmg.2014.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Joshi NV, Vesey AT, Williams MC, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383:705–713. 10.1016/s0140-6736(13)61754-7 [DOI] [PubMed] [Google Scholar]
- 111.Mehdizadeh Seraj S, Raynor W, Rojulpote C, et al. Assessing the feasibility of NaF or FDG as PET probes to evaluate atherosclerosis in rheumatoid arthritis patients. J Nucl Med. 2019;60:1439–1439. [Google Scholar]
- 112.Tarkin JM, Joshi FR, Evans NR, et al. Detection of atherosclerotic inflammation by (68)Ga-DOTATATE PET compared to [(18)F]FDG PET imaging. J Am Coll Cardiol. 2017;69:1774–1791. 10.1016/j.jacc.2017.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ćorović A, Wall C, Nus M, et al. Somatostatin receptor PET/MR imaging of inflammation in patients with large vessel vasculitis and atherosclerosis. J Am Coll Cardiol. 2023;81:336–354. 10.1016/j.jacc.2022.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bruijnen S, Tsang ASM, Raterman H, et al. B-cell imaging with zirconium-89 labelled rituximab PET-CT at baseline is associated with therapeutic response 24 weeks after initiation of rituximab treatment in rheumatoid arthritis patients. Arthritis Res Ther. 2016;18:266. 10.1186/s13075-016-1166-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Luo Y, Pan Q, Zhou Z, et al. (68)Ga-FAPI PET/CT for rheumatoid arthritis: a prospective study. Radiology. 2023;307:e222052. 10.1148/radiol.222052 [DOI] [PubMed] [Google Scholar]
- 116.Jaffer FA, Libby P, Weissleder R. Molecular imaging of cardiovascular disease. Circulation. 2007;116:1052–1061. 10.1161/CIRCULATIONAHA.106.647164 [DOI] [PubMed] [Google Scholar]
- 117.Lindner JR. Contrast ultrasound molecular imaging of inflammation in cardiovascular disease. Cardiovasc Res. 2009;84:182–189. 10.1093/cvr/cvp302 [DOI] [PMC free article] [PubMed] [Google Scholar]