Abstract
The controlled environment ecosystem is a meticulously designed plant growing chamber utilized for cultivating biofortified crops and microgreens, addressing hidden hunger and malnutrition prevalent in the growing population. The integration of speed breeding within such controlled environments effectively eradicates morphological disruptions encountered in traditional breeding methods such as inbreeding depression, male sterility, self-incompatibility, embryo abortion, and other unsuccessful attempts. In contrast to the unpredictable climate conditions that often prolong breeding cycles to 10–15 years in traditional breeding and 4–5 years in transgenic breeding within open ecosystems, speed breeding techniques expedite the achievement of breeding objectives and F1–F6 generations within 2–3 years under controlled growing conditions. In comparison, traditional breeding may take 5–10 years for plant population line creation, 3–5 years for field trials, and 1–2 years for variety release. The effectiveness of speed breeding in trait improvement and population line development varies across different crops, requiring approximately 4 generations in rice and groundnut, 5 generations in soybean, pea, and oat, 6 generations in sorghum, Amaranthus sp., and subterranean clover, 6–7 generations in bread wheat, durum wheat, and chickpea, 7 generations in broad bean, 8 generations in lentil, and 10 generations in Arabidopsis thaliana annually within controlled environment ecosystems. Artificial intelligence leverages neural networks and algorithm models to screen phenotypic traits and assess their role in diverse crop species. Moreover, in controlled environment systems, mechanistic models combined with machine learning effectively regulate stable nutrient use efficiency, water use efficiency, photosynthetic assimilation product, metabolic use efficiency, climatic factors, greenhouse gas emissions, carbon sequestration, and carbon footprints. However, any negligence, even minor, in maintaining optimal photoperiodism, temperature, humidity, and controlling pests or diseases can lead to the deterioration of crop trials and speed breeding techniques within the controlled environment system. Further comparative studies are imperative to comprehend and justify the efficacy of climate management techniques in controlled environment ecosystems compared to natural environments, with or without soil.
1. Introduction
The controlled environment ecosystem (CEE) serves as a sophisticated plant growth chamber essential for speed breeding and cultivating horticultural or agricultural crops, microgreens, and various other plants. These crops and the speed breeding process find applications in protected cultivation, vertical farming, container farming, plant factories, and even specialized environments, such as National Aeronautics and Space Administration (NASA) biomass production chambers. Controlled environment technologies such as hydroponics, aeroponics, aquaculture, aquaponics, and genoponics play pivotal roles in these endeavors.1 Controlled environment agriculture (CEA), indoor farming (IF), and indoor agriculture (IA) are alternative terms commonly used to describe the controlled environment ecosystem. Speed breeding, a state-of-the-art plant breeding technique, enables the production of superior crops within shorter generation times under meticulously controlled environmental conditions. This method integrates various techniques including plant tissue culture, advanced breeding methods, genetic engineering, molecular approaches, utilization of light-emitting diodes (LEDs), growth regulators, immature germination, and micronutrient supplementation to achieve desired breeding objectives and cultivate improved or superior crops.2 Alternative scientific terms for speed breeding (SB) include rapid breeding, crop improvement, smart breeding, and crop improvement.
Controlled environment agriculture dates back to ancient times, with records showing that the Roman doctor Tiberius Caesar grew cucumbers under such conditions as early as 14–37 A.D.3 The design and structure of modern greenhouses were first documented in the 1670s, with European countries pioneering the construction of wood-framed structures covered with either glass or oiled paper. In 1780, during the American Revolution, the first American greenhouse was erected by George Washington at Mount Vernon’s Conservatory.4 The use of incandescent lamps for influencing plant growth was pioneered by Liberty Hyde Bailey at Cornell University in 1889. By 1900, improved heated water systems were being implemented in American greenhouses. In the 1960s, the Agricultural Research Service (ARS) Phyto-Engineering Laboratories of the U.S. Department of Agriculture (USDA) initiated the production of lettuce, tomato, and cucumber in plant growth chambers. During the mid-1980s to the late 1990s, the National Aeronautics and Space Administration (NASA) utilized the Kennedy Space Center’s biomass production chamber to grow wheat and barley for space missions. Commercial utilization of controlled environment agriculture began in 1999 when Cornell University embarked on mass production of lettuce.5−7 The evolution of applied research theories has paved the way for the development of speed breeding as a method to enhance crop production under controlled growing conditions. Renowned scientist Lee Hickey from the University of Queensland, Australia, introduced the concept of speed breeding. He first applied this technique to wheat, peanut, and barley, using the single seed descent (SSD) method in glasshouses to create disease-resistant varieties. With the advent of new plant growth room designs, such as controlled environment chambers, glasshouses, and homemade growth rooms, efforts to improve crops through speed breeding techniques have expanded. Plant production and speed breeding are influenced by various factors, including light quantity, light quality, photosynthetic active radiation (PAR), temperature, sterile/axenic environments, growing times, conditions, plant age, system types, growing media in closed systems, vertical farming, and protected cultivation. The controlled environment ecosystem holds significant potential for cultivating improved plants, transgenic crops, tissue culture plants, and employing vertical farming and protected cultivation methods, all within controlled environmental systems.8,9
The natural climate serves as a critical variable in accelerating the growth and development of plants. Various climatic factors, including water availability, light intensity, temperature, humidity levels, and atmospheric pressure, consistently impact the biological processes of crops.10 Nevertheless, fluctuations and disruptions in the natural cycle of climatic factors can significantly influence crop growth, development, and improvement. Irregularities in monsoon patterns and fluctuations in the temperature or light can disrupt soil properties and hinder plant growth. Climate change has led to the adaptation of pest species and altered the dynamics of the gaseous cycle, posing challenges to field trials and the process of plant hardening.11 Moreover, unstable climatic conditions exacerbate challenges related to pollination, self-incompatibility, seed shattering, male sterility, and breeding cycle. In open ecosystems, the impacts of these conditions prolong crop development in traditional breeding, requiring 10 to 15 years (5 to 10 years for plant population line creation, 3 to 5 years for field trials, and 1 to 2 years for variety release). Conversely, the development of transgenic crops through direct or indirect gene manipulation takes 4 to 5 years, further complicated by the interaction with unstable climatic factors in open fields. Conventional agriculture, known for its extensive land use and high water consumption, often leads to significant agrochemical leaching and soil erosion. The production and management practices associated with traditional agriculture contribute to yield losses, pest infestations, and weed emergence, exacerbated by the effects of climate change.
The controlled environment plant growth system is an artificially regulated chamber where crop or microgreen production and speed breeding take place, with climate factors meticulously controlled and manipulated.12 It conserves 80% of land usage and 90% of water consumption and efficiently manages nutrient runoff compared to traditional agriculture. By limiting eutrophication emergence and minimizing the stress impact, it establishes a stable artificial ecosystem conducive to quality growth and high yields. Additionally, it provides a more accurate delivery of nutrient ions to the plant system compared to soil-based agriculture. The controlled environment system holds vast potential for mitigating climate change effects and mitigating crop losses associated with soil-based agriculture.13 The controlled environment plant growth system employs recycled agrowaste and wastewater for plant production.14 The controlled environment plant growth chamber ensures the stability of carbon sequestration and minimizes the carbon footprint, effectively offsetting greenhouse gas emissions and potential yield losses. It enhances photosynthetic assimilation, metabolic efficiency, and maintains stable climatic conditions conducive to plant growth.15 The controlled environment plant growth chamber reduces sources of pollution and minimizes pest emergence compared to soil agriculture, enabling the successive growth of seasonal crops.
Speed breeding, within a controlled environment ecosystem, demonstrates the capability to yield high-performing and superior crops. Its versatility extends to agricultural or horticultural crops, offering opportunities for crop improvement at any stage within a controlled environment setup. Moreover, it facilitates the production of tissue-cultured crops, biofortified crops, and nanomodified crops within the controlled environment ecosystem.16 This method combines genomics and phenomics to analyze the structure and function of crops, utilizing sensor-based technologies to explore qualitative and quantitative traits in both crops and growing media within controlled growth conditions.17 Speed breeding mitigates crop loss and revenue challenges in conventional ecosystems while addressing hidden hunger, malnutrition, and food security concerns among expanding global populations.18 Speed breeding enhances both physical and chemical traits in crops or microgreens cultivated within controlled environment plant growth chambers. These improvements encompass plant height, leaf number, fruit size, fruit weight, and overall yield, alongside enhancements in chemical attributes like total soluble solids, biochemical content, color, flavors, water use efficiency, and nutrient use efficiency.19 Under a controlled environment plant growth chamber, completing the F1–F7 generations in crop development typically takes 2–3 years. Speed breeding fosters robust growth and nutrient-rich yields by reducing the toxicity and impurity levels in both crops and microgreens. With reduced manpower and time requirements, speed breeding yields high-quality crops and microgreens, meeting diverse physical targets as needed.20 Speed breeding enhances seed viability and vigor, accelerating morphological and anatomical growth rates compared to traditional soil agriculture. Furthermore, it enables stable phytoremediation, enhances resilience against biotic and abiotic stresses, supports biofortification efforts, facilitates improvements in tissue-cultured plants, and integrates nanotechnology within controlled environment plant growth chambers. Speed breeding creates ample opportunities for research, promotes the establishment of new industries, and contributes to employment growth.21 While many aspects of the controlled environment ecosystem have been extensively studied and reviewed, there remains a need for comprehensive compilations and reviews focusing on plant production, speed breeding for trait improvement, the integration of artificial intelligence (AI) in screening phenotypic characters, and the dynamics of nutrients, water, and climate within controlled environments. This review aims to emphasize crop and microgreen production, the intricacies of nutrient, water, and climate dynamics, technological interventions in speed breeding, and the utilization of AI for phenotypic characterization in crops grown under controlled environment ecosystems. Through this exploration, we seek to further advance both theoretical understanding and practical techniques in this field.
2. Crop and Microgreen Productions in CEE through Controlled Environment Technologies
A diverse range of crops and plants, spanning barley, rice, oats, wheat grass, mint, basil, rosemary, sage, oregano, amaranth, beets, chard, quinoa, spinach, and a plethora of vegetables, herbs, and flowers, have benefited from the successful implementation of a controlled environment ecosystem. This innovative approach fostered optimal growth conditions, enhancing yield and quality across various agricultural and horticultural species.22,23
2.1. Horticultural Crop Production in a Closed System under Controlled Environment Conditions
Vegetable crops were cultivated using both water-based techniques like the Nutrient Film Technique (NFT) and substrate-based methods such as container farming within controlled environment setups.24 The NFT has been utilized in several crops such as tomato, lettuce, and cucumber for obtaining high yield, pest resistance, and reduced toxic products in the greenhouse. This method utilizes substrates like rock wool, cocopeat, sawdust, peat moss, vermiculite, sand, and perlite.25 Furthermore, tomato seedlings gave superior morphological and anatomical growth in rockwool cubes containing specific nutrients under NFT.26 Besides, keeping tomatoes in slabs, buckets, black gunny bags, and lay-flat containing mixtures of rockwool + coconut coir + perlite + peat with the recommended dose of nutrient solution through the NFT method under greenhouse condition revealed significant morphological growth and yield.27
The beneficial growth and good yield in eggplant were obtained in polystyrene trays containing 1:1 (v/v) peat: vermiculite, using different growing media (sawdust, spent mushroom compost, volcanic tuff, perlite) with discharge of standard nutrient solutions comprising 135 mg N, 48 mg P, 283 mg K, 0.05 mg Mo, 128 mg Ca, 0.5 mg B through a drip system in a glass greenhouse.28,29 Additionally, the pest-free and biochemical-enriched onion seedlings were obtained in pot culture grown in oasis cubes, while robust quality growth and nutrient-enriched cucumber were harvested in cocopeat growing media with the application of Hoagland nutrient solution under controlled environmental conditions.30,31 In the case of aeroponics potato cultivation under controlled conditions, the qualitatively and quantitatively nutritious mini tubers were harvested after 2 weeks of transplanting in a test tube, magenta box, and styrofoam (thermocoal box) with the recommendation of nutrient solutions.32 The groovy biochemical content, growth, physiological response, and yield were achieved in chili through the fertigation method in a glass house.33 Utilizing full dose, half dose (with a 50% reduction in macronutrients), or pure water (devoid of nutrients) of the Hoagland nutrient solution in the floating method resulted in significantly enhanced growth parameters, biomass, and yield in spinach cultivated within a greenhouse environment.34
Carrots experienced enhanced growth and yield in a greenhouse setting using rockwool blocks enriched with vermiculite and supplied with nutrient solutions via subsurface irrigation.35 Melons were cultivated in an aeroponics system, and lettuce in a combination of polystyrene foam and pots with three nutrient solutions delivered through a floating system.36,37 Ginger was grown in pots containing various ratios of coir dust and burnt paddy husk, receiving MARDI nutrient solution through irrigation channels within a side-netted rain shelter.38 Green beans thrived in pots supplemented with peat, perlite, and sand, utilizing subsurface irrigation for nutrient supply.39 Broccoli flourished in rockwool with Hoagland nutrient solution in a multitunnel greenhouse.40 Kohlrabi was cultivated using an NFT in a greenhouse.41 Rhododendrons were grown in containers filled with a mixture of bark, sphagnum peat moss, perlite, and vermiculite.42 Hybrid lilies were raised in plastic bags containing varying proportions of cocopeat, perlite, and palm trunk, with nutrient solution discharge.43 Mustard was cultivated using hydroponic and aquaponic systems.44 Various vegetables such as tomatoes, cucumbers, eggplants, chili peppers, carrots, lettuce, and spinach were grown in an open rooftop system.45,46 Different substrates, including tuff, perlite, and sawdust, were observed to affect cucumber production in varying proportions within the greenhouse.47 Broccoli, cauliflower, and cabbage achieved typical growth and yield when cultivated in various growing mediums such as commercial peat and paper waste in pot culture within a plastic greenhouse.48
Different container systems were employed for melon production in greenhouse settings, including fertigation systems using polybags and cocopeat, U-shaped troughs made of PVC tubes, double U-shaped troughs, and triangular containers constructed from polyethylene sheets. While melon production was observed in all containers, the fruit quality was found to be superior in the U-shaped troughs.49 Plastic pots filled with perlite and cocopeat were utilized for cucumber cultivation in a polyhouse.50 Pepper plants exhibited productive morphological growth and high-quality fruit when grown in gunny bags filled with peat or a mixture of peat, perlite, and sand (1:1:1 ratio) substrate under greenhouse conditions.51
Various substrate media have been noted to yield suboptimal production outcomes for several crops. Diverse combinations of cocopeat and vermiculite in differing proportions were employed for cultivating strawberry cv. Chandler within polybags placed in a passively ventilated greenhouse.52 Using vermiculite: erlite culture medium in a 1:1 ratio, gerbera plant production is recommended in a controlled environment ecosystem.53 The utilization of coconut fiber and peat as a growing medium proved advantageous in the cultivation of Galanthus elwesii under soilless conditions, resulting in a notable improvement in bulb quality.54 Peat and a composite medium (consisting of peat, slag, and perlite in a 1:1:1 ratio) demonstrated enhanced growth for Limonium sinuatum.55 Besides, coconut fiber as a substrate media showed improvement in Lisianthus russellianus growth and flower quality.56 Furthermore, a blend of cocopeat, vermiculite, and FYM (in a 1:1:1 ratio) as a growing medium also contributed to improved flower production of the potted Chrysanthemum cv. Anmol, accompanied by enhanced plant morphological development.57 Additionally, a composition of 0.5 kg gravel with 1.2 kg coconut fiber along with four holes at the base in a pot showed the production of sunflowers under saline stress conditions in hydroponic conditions.30 The mustard spinach seedlings showed robust germination rates and dense seedling growth when cultivated in polystyrene trays filled with a vermiculite substrate. They were then transplanted into net pots filled with gravel within a shade net house covered with black and white shading material.58
2.2. Microgreen Plant Production in a Closed System under a Controlled Environment
The microgreen utilization traces back to 1930 when North American pharmacies began harnessing the medicinal properties of wheatgrass. By the 1970s, this trend expanded with the cultivation of microgreens like buckwheat, sunflower, and radish during the winter months, aptly dubbed as winter greens.59 Microgreens like basil, arugula, kale, beets, and cilantro were utilized for serving in food items later in San Francisco in the 1980s;60 however, in the mid-90s, lettuce microgreens were served as salad and garnishings, which led to its terminology in 1992 as “Microgreen and Babygreen” by Craig Hartman. These contain 4–40% higher concentrations of micronutrients than matured vegetables and herbal plants.61
Microgreen plants from diverse families such as Alliaceae, Amaranthaceae, Apiaceae, Asteraceae, Boraginaceae, Brassicaceae, Cucurbitaceae, Fabaceae, Lamiaceae, Oxalidaceae, Poaceae, Leguminoceae, Polygonaceae, and Portulacaceae were cultivated for consumption, the production of high-value products, and revenue generation.4 The delicate leaves of microgreen plants boast rich flavors and bioactive compounds, including vitamins, antioxidants, and minerals, surpassing those found in mature leafy greens.62 These portions of the plants have much higher nutrient content than their fully grown portions. Due to this, microgreen plants having tender leaves have more potential to confine microbial contamination than seedlings.63 The intake of microgreens is responsible for the regulation of body weight, lowers cholesterol levels, and protects against heart disease. They also contribute to detoxification, purifying, and fortifying the blood, acting as diuretics to support kidney function.64 Besides, microgreens help with anemia, reduce the risk of eye diseases, help maintain strong and healthy bones, and promote blood clotting. Microgreens synthesize abundant ascorbic acid, neoxanthin, violaxanthin, phylloquinone, α-carotene, tocopherols, lutein, and carotenoids, along with higher levels of zinc, iron, and protein.65 Additionally, they reduce the generation of antinutritional components. Microgreens are abundant in essential macronutrients and vital micronutrients.65
Furthermore, microgreen and crop production were initiated by utilizing substrate-growing media such as vermiculite, bagasse, sawdust, and rice hulls, which possess natural physical and chemical properties for their production. Bagasse and sawdust exhibit a high water retention capacity, coupled with low porosity and a notably high saturation point, respectively.66 Redwood, pine bark, and fir bark serve as ideal substrates for fostering the safe growth of microgreens. Conversely, cedar and walnut are best avoided due to potential toxicity concerns associated with these substrates. Notably, the combination of sphagnum peat and vermiculite, commonly termed peat-lite, has emerged as a highly effective growing medium for promoting the cultivation of both microgreens and crops alike. Nutrient-rich substrates such as rock wool, cocopeat, sawdust, peat moss, vermiculite, sand, and perlite were employed for greenhouse vegetable cultivation.66 Cucumbers and tomatoes have thrived when cultivated with bark from pine, cypress, redwood, and fir in greenhouse settings.66 Optimal fruit weight and yield were achieved through the utilization of perlite and a cocopeat-perlite nutrient mixture for greenhouse tomato production.66
Indoor production of microgreen plants such as fenugreek, chickpea, mung bean, and coriander demonstrated superior growth and yield responses when cultivated with nutrient-rich media like cocopeat and peat, compared to outdoor environments.67 Using substrate growing media of mushroom, vermicompost, perlite, sawdust, peat moss, and compost, crops like kale, arugula, pak choi, and Swiss chard in the greenhouse were grown to find the root area, chlorophyll contents, carotenoid contents, shoot length, total phenolics, high yield, flavonoids, and antioxidant enzymes.68Brassica rapa var. chinensis and Brassica oleracea L. var. acephala had the highest sugar and protein contents with growing media which includes sawdust at 20%, vermicast at 30%, mushroom compost at 30%, and perlite at 20% in a greenhouse.69 The significant yield and biomass in nutrient media consisting of peat and coco coir were observed in various crops, such as radish, microgreens, kale, basil, etc. in the climate-controlled greenhouse than in indoor farming.70 The successful emergence of spinach, radish, and carrot was observed in nutrient media having cocopeat and rice husk in the ratio of 1:1 in a bagasse container.71
Cocopeat, utilized as a growing medium, has demonstrated the ability to enhance micronutrient content, including zinc (Zn2+), magnesium (Mg2+), and potassium (K+), as well as increased levels of ascorbic acid, chlorophyll, carotenoids, and overall growth response in leguminous microgreens such as cowpea, mungbean, fenugreek, and horse gram, as well as cereal microgreens like wheat and sorghum when cultivated within closed systems.72 Even superior morphological growth and yield have been observed in lettuce, broccoli, turnip, and kale varieties when grown on a nutrient medium composed of cocopeat, perlite, and vermiculite in a ratio of 3:1:1 within a closed system.73 The growths of shoot length and stem diameter of tobacco were increased in a substrate composition of pine bark (50%), sand (50%), and compost (100%) under an open system. However, cattle manure was not recommended with the same media composition in tobacco plants.74 Green bean microgreens displayed enhanced morphological growth and prolonged shelf life when grown using straw nanofiber and hydrogel derived from diaper waste within a closed system.74
On the other hand, various hydroponic pads like hemp mats, biostrate, jute mats, and micromats gave positive results in microgreen production in the greenhouse. Excluding a hemp mat, radish plants have recorded the highest nitrogen concentration, while mustard recorded the highest phosphorus content. Variations in the fresh and dry weights of shoots as well as the mineral nutrient content in radish, broccoli, kale, mustard, and cabbage microgreens were even observed in diverse hydroponic pads. Microgreens cultivated on hemp mats exhibited the greatest shoot height, fresh and dry shoot weights, and concentration of potassium.75 The physical development, weight gain, and productivity were observed in mustard, arugula, basil, and radish microgreens grown in a controlled environment system using NFT with coarse burlap fabric.76 In kale cultivation, achieving optimal levels of proline content, plant canopy temperature regulation, and fresh yield was accomplished by utilizing a 17.5% irrigation threshold within a hydroponic wick system, managed through an EVC container, within a controlled environment vertical farming setup.77 Furthermore, favorable plant height and yield results were documented for broccoli, red sorrel microgreens, basil, mizuna, and sunflower, cultivated within a vertical hydroponic framework and an ebb-flow bench system. These systems utilized a growing medium comprised of perlite mix, peat, and cellulose within a double-layered greenhouse equipped with a heating system. Additionally, rice grass and wheat grass, grown in a screen house, displayed similar positive results when grown in nutrient media containing sawdust, cocopeat, and husk charcoal.78
The preceding details confirm the significant potential of controlled environment systems for cultivating agricultural and horticultural crops, particularly for microgreen production, using soilless cultivation methods (Table 1).79 The synthetic ecosystem maintains consistent biotic and abiotic relationships to stimulate the growth and maturation of crops and microgreen vegetation. It ensures steady climatic conditions conducive to cultivating crops and microgreens rich in biochemical compounds and promoting high-quality growth.80 The constructed environment addresses issues such as crop yield reduction, climate-related damage, natural resource management or preservation, environmental considerations, and seasonal crop production declines in the natural environment.81 Investigation may be necessary to comprehend essential or consistent thresholds of climatic elements, carbon capture, carbon emissions, and suitability for crop and microgreen cultivation within a controlled environmental setup. Comparative research and analysis are essential to grasp the vital or stable levels of climatic factors, carbon capture, and carbon emissions associated with crop and microgreen production within predetermined conditions and open environments82,83 (Table 1) (Figure 1).
Table 1. Utilizing Controlled Environment Ecosystems for Crop and Microgreen Production.
| s. no. | crops and microgreens | types of enclosed environment systems | regulated environment infrastructure | references |
|---|---|---|---|---|
| 1 | Fodder maize | Hydroponic fodder system | Low-cost plastic house | (84) |
| 2 | Wheat | AB mix hydoponic media + polyethylene glycol, charcoal + cocopeat | Plastic house | (85) |
| 3 | Wheat | Modified nutrient solution | Artificial indoor lights | (86) |
| 4 | Sorghum | Hydroponic fodder system | Controlled conditions | (87) |
| 5 | Fodder maize, Fodder horse gram | Fabricated low-cost hydroponic fodder production unit | Controlled conditions | (88) |
| 6 | Rice, pearl millet | Nutrient solution + 0.09 mM (NH4)2SO4 + 0.05 mM KH2PO4 + 0.05 mM KNO3 + 0.03 mM K2SO4 + 0.06 mM Ca(NO3)2 + 0.07 mM MgSO4 + 0.11 mM Fe-EDTA + 4.6 μM H3BO3 + 1.8 μM MnSO4 + 0.3 μM ZnSO + 0.3 μM CuSO4 | Gas exchange chamber, root chamber | (89) |
| 7 | Fodder maize, grain maize, fodder bajra, grain bajra, barley, wheat, oat, fodder cowpea, grain cowpea, horse gram, soybean, lucerne | Fabricated hydroponics fodder production | Shed-net house | (90) |
| 8 | Barley | Fabricated hydroponic fodder system | Shed-net house | (91) |
| 9 | Maize, sorghum, bajra, barley, cowpea, lucerne, horse gram | Hydroponic fodder system | Low-cost greenhouse | (92) |
| 10 | Oat, wheat | Hydroponic green forage system | Controlled conditions | (93) |
| 11 | Wheat, barley, oats | Fabricated hydroponics system + aquaculture wastewater | Controlled conditions | (94) |
| 12 | Maize, oat, wheat | Hydroponic fodder system | Polyhouse | (95) |
| 13 | Maize | Hoagland nutrient solution | Controlled conditions | (96) |
| 14 | Capsicum, cucumber, tomato | Fertigation method | Greenhouse | (97) |
| 15 | Potato mini tubers | Wick system, drip system, deep-water cultivation, nutrient film technique, aeroponics | Controlled conditions | (98) |
| 16 | Potato mini tubers | Nutrient film technique, deep flow technique, aeroponics | Greenhouse | (99) |
| 17 | Potato mini tubers | Fertigation method | Greenhouse | (97) |
| 18 | Rose, carnation, gerbera, chrysanthemum, lilium | Fertigation method | Greenhouse | (97) |
| 19 | Strawberry | Cocopeat + vermiculite | Passive ventilated greenhouse | (100) |
| 20 | Sunflower | Nutrient solution containing gravel, coconut fiber | Greenhouse | (101) |
| 21 | Arugula, broccoli, red cabbage, sugar beet, amaranth, tendril pea (microgreens) | Coir fiber, peat-based mix | Greenhouse | (102) |
| 22 | Wheat, barley, oat (microgreens) | Sterile peat + cockpit + perlite | Controlled climate cabinet | (103) |
| 23 | Pearl millet, mungbean, lentil, red radish, mustard, red cabbage (microgreens) | Coco peat + vermiculite + sand | Controlled conditions | (104) |
| 24 | Wheat, kale, spinach (microgreens) | Perlite + peat + vermiculite | Pulsed electromagnetic field | (105) |
| 25 | Oat, wheat, rice, barley (microgreens) | Algae | Climatic chamber | (106) |
| 26 | Amaranthus, scallion, sunflower, borage, arugula, broccoli, red cabbage, cress, kale, mizuna, mustard, radish, sugar beet, red basil, green basil, shiso, lemon balm (microgreens) | Nutrient solutions | Polycarbonate-covered greenhouse with a cooling system and forced air ventilation | (107) |
| 27 | Jute (microgreens) | Half-strength Hoagland nutrient solution | Growth chamber | (108) |
| 28 | Sunflower, water spinach (microgreens) | Peat moss, coconut coir dust, leaf compost, food waste compost | Greenhouse | (109) |
| 29 | Barnyard millet (microgreens) | Wet cotton cloth | Room conditions | (110) |
| 30 | Peas, sunflower, radish (microgreens) | Nutrient film technique | Greenhouse | (111) |
| 31 | Mustard, kale, broccoli | Nutrient solution + sphagnum peat moss + bark + perlite + limestone | Conviron PGC flex growth chambers | (112) |
| 32 | Brassica rapa var. Japonica, Brassica rapavar. Chinensis, Raphanus sativus, Brassica juncea (microgreens) | Nutrient solution + sphagnum peat moss | Growth chambers | (113) |
| 33 | Red basil, rocket (microgreens) | Floating system + nutrient solution; coconut fiber, vermiculite, jute | Micro experimental growing cabinet | (114) |
| 34 | Radish, kale, cilantro, broccoli, pea (microgreens) | 1/2 strength Hoagland’s solution + sterile hemp mat | Percival environmental chamber | (115) |
| 35 | Amaranth, turnip (microgreens) | Vermiculite | Controlled environment growth chamber | (116) |
| 36 | Red cabbage, broccoli, mizuna, green mustard, pak choy (microgreens) | Sand + organic soil + coco coir; coco coir + rice husk; coco coir + calcium oxide + humic acid; white sphagnum peat + vermiculite | Greenhouse | (117) |
| 37 | Sunflower, kale, arugula, mustard (microgreens) | Peat + compost + coir + perlite | Indoor and greenhouse environments | (118) |
Figure 1.
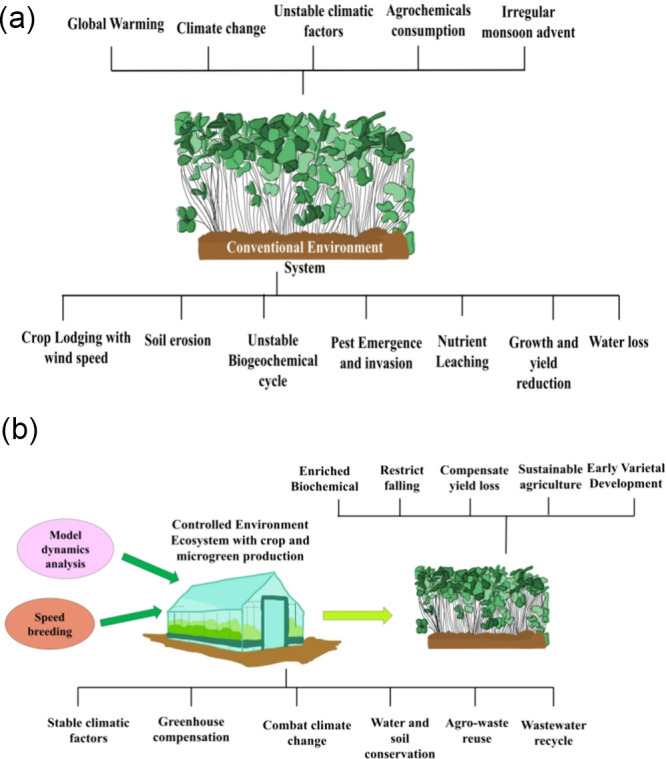
Stability of ecosystem in conventional and controlled environment agriculture systems. (a) Conventional Environment Ecosystem. (b) Controlled Environment Ecosystem.
3. Deciphering Models for Tracking Nutrients, Water, and Climate Dynamics in Controlled Environment Ecosystems using Advanced Technologies
In hydroponic systems with computer models, nutrient uptake in crops typically involves the precise control of nutrient solutions delivered directly to the plant roots. Computer models help optimize this process by monitoring and adjusting factors, such as nutrient concentration, pH levels, and water availability, to ensure optimal growth conditions. The plants absorb nutrients through their root systems in this soilless environment, facilitated by the nutrient-rich solution. The computer models help maintain the balance and provide real-time data for better management. The progress of greenhouse engineering with hydroponics has shown a heavy reliance on computational intelligence, especially through automated monitoring and control mechanisms. Advanced instrumentation and intelligent control systems employed in hydroponic setups hold the potential to enhance both the quality and quantity of production by efficiently managing diverse processes. These production systems are subject to continual monitoring and precise regulation. An essential aspect of these highly automated and computerized configurations is the accuracy and reliability of sensor-derived information, as well as the efficacy of decisions transmitted to actuators.119 The model involved in a controlled environment actively manages the interplay of nutrient, water, and climatic elements within a controlled environment framework (Table 2). It operates under the governance of an operating system, ensuring the provision of essential nutrients, water, and climate parameters to nurture plant growth within consistent standards.120 Effectively distributing nutrients, water, and climate variables regulates their crucial levels to sustain stability. Moreover, this model facilitates the creation of a controlled artificial ecosystem tailored for cultivating crops and microgreens, optimizing resource usage in the process.121 Additionally, it holds promise for mitigating crop loss and contributing to natural climate stabilization efforts. Within this model, distinct categories function according to specific principles and roles, all aimed at fostering plant growth within controlled environmental settings.122 An instrumental system was developed for monitoring and adjusting the pH and conductivity of the nutrient solution for hydroponic lettuce, which typically experiences significant fluctuations during cultivation.123 Furthermore, a Bayesian Network model has been developed to automate crop cultivation. Sensors and actuators are integrated to oversee and regulate farm parameters including light intensity, pH levels, electrical conductivity, water temperature, and relative humidity. Data collected from sensors is utilized to construct the Bayesian Network, which categorizes and forecasts optimal values for each actuator, enabling autonomous control of the hydroponics farm.124
Table 2. Diverse Models Involved in a Controlled Environment Ecosystem for Assessing Factors.
| s. no. | algorithm models | principles | assessing determinants | references |
|---|---|---|---|---|
| 1 | Decision-tree-based dosing algorithm | Real-time operating system | Carbon emission reduction, nutrient dynamics | (134) |
| 2 | Linear regression analysis | - | Electrical conductivity, pH | (135) |
| 3 | Nicolet model | Mechanistic | Crop growth, nitrate uptake | (136) |
| 4 | Fuzzy inference system | Real-time operating system | Control temperature, relative humidity, electrical conductivity, and pH | (137) |
| 5 | Plant talk | Python programs | Water dynamics, light emitting diodes monitor, plant care control | (138) |
| 6 | Machine learning, support vector regressor, extreme gradient boosting, random forest, deep neural network | Mechanistic | Crop growth | (139) |
| 7 | Artificial neural network, genetic algorithms | Real-time operating system | Electrical conductivity | (140) |
| 8 | Mechanistic and physiological model with machine learning (MPM-ml) | Michaelis-Menten enzyme kinetics | Nutrient quantity, nutrient flow rate, water-nutrient interaction, nutrient gradient, growth, yield | (141) |
| 9 | Recurrent neural network | Sensor | Crop growth | (142) |
| 10 | Light and shade system implementation | Sensor | High pressure sodium, light emitting diodes | (143) |
| 11 | Multiple linear regression | Sensor | Nutrient dynamics | (144) |
| 12 | Neural networks, genetic algorithms | Sensor | Water dynamics | (145) |
| 13 | Autoregressive moving-average model | Computer integrated system | Water status, light intensity, nutrient supply | (146) |
Another hydroponic system was designed with an advanced real-time operating system based on a microcontroller. ARM Cortex-M4 microcontroller (ARM) system was used to manage transmission signals. It oversees the measurement of crucial parameters such as electrical conductivity, pH levels, carbon dioxide concentration, temperature, and nutrient concentrations within a standardized environment. The system demonstrated its ability to maintain the desired concentration level within a narrow variation margin of less than 3%. Furthermore, the pH sensor used in this system exhibited commendable accuracy, with deviations of only 5.83% observed across pH values ranging from 3.23 to 10. The microcontroller interfaces with a digital temperature and humidity sensor (DHT) to monitor humidity levels and an MH-Z19 sensor to gauge carbon dioxide concentrations. Employing expert system-based automation (HES), in a controlled environment, is for regulating all aspects including temperatures, oxygen levels, nutrient supply, and operational settings for various components such as water heaters, fertilizer tubes, pH regulators, conditioners, moisture control systems, carbon dioxide generators, and artificial lighting. This synchronization ensures the creation of optimal conditions within the greenhouse system.125
Furthermore, the Arduino Uno microcontroller model examines the mobility and speed of the nutrient flow in solution. Arduino represents an electronic platform driven by open-source code, encompassing both hardware and software components. This versatile system possesses the capability to receive sensor input, trigger motor action, illuminate LEDs, and transmit data to the Internet and mobile devices, as well as receive and process incoming information, converting it into various applications. Users can dictate specific actions by programming instructions into the microcontroller embedded within the board. With its user-friendly interface, Arduino has found widespread use across a myriad of projects and applications. Its software caters to beginners with simplicity, while offering flexibility for advanced users. Compatible with Mac OS, Windows, and Linux, Arduino boasts cost-effectiveness in comparison to other microcontroller platforms. Additionally, the Arduino software can be expanded by proficient programmers through C++ libraries and is adaptable for circuit designers seeking to innovate.126 Moreover, an integrated hydroponic farm management system was developed to be capable of monitoring parameters like water temperature, water levels, nutrient solution concentrations, and acidity levels employing sensors linked to a microcontroller through an online platform.127
A neural network utilizing fault detection in a controlled environment is designed based on a quasi-network backpropagation algorithm, effectively discerned dynamic processes within the hydroponic system’s root zone, and accurately forecasted one-step-ahead values for pH and electrical conductivity.128 The PlantTalk system monitors water movement, regulates LED lighting, and manages plant care using Python programs. The Penman-Monteith and Stanghellini models analyze solar radiation, air temperature, humidity, and wind speed within a controlled environment. Moreover, alternative models such as the Priestley Taylor model and the Hargreaves & Samani model monitor air temperature and solar radiation within a controlled setting. The recurrent neural network and long short-term memory (RNN-LSTM) assess the levels of pH, temperature, humidity, and water diffusion, operating based on deep neural networks.
The mechanistic and physiological model with machine learning (MPM-ML) evaluates nutrient levels, nutrient transport, accessibility of water-soluble nutrients, substrate nutrient density, growth rate, productivity, and concentration gradients. Employing the Michaelis–Menten enzyme kinetics principle, this model functions by integrating with the Barber-Cushman model to calculate nutrient concentrations in the solution, mass transfer, diffusion, and Robin boundary conditions. Additionally, the Porter diffusion model is utilized to analyze substrate advection and diffusion equations.
The model provides insights into the operational dynamics and impacts of water, nutrient, and climatic variables within a controlled environment ecosystem. It regulates both critical and noncritical fluctuations in climatic factors, fostering stable plant growth under controlled conditions. Additionally, the model advocates for ecological reform and ecosystem preservation while conserving natural resources. With its potential to address and mitigate climate crises, it plays a crucial role in sustaining biogeochemical cycles and managing greenhouse gas emissions.125,129 Carbon dioxide concentrations in the surrounding environment, whether stable or fluctuating, are assessed using Carbon Enrichment for Plant Stimulation (CEPS) systems, implemented in both open and closed setups. Carbon sequestration in such systems is analyzed through models like the Rothamsted carbon model and Fourier or neural models, which explore carbon dynamics in both open and closed environments.130 Furthermore, Willits131 introduced a thermal model aimed at forecasting microclimate conditions within greenhouses equipped with mechanical ventilation and an evaporative cooling system. With the implementation of evaporative cooling, enlarging the canopy size holds greater significance in reducing air temperature. In the absence of evaporative cooling pads, the ratio of energy utilized for transpiration to incoming solar energy is estimated to vary between 1 and 75 for an outdoor air temperature of 36.8 °C and a humidity ratio of 3.3 g/kg, decreasing to 0.8 °C for an outdoor humidity ratio of 29.9 g/kg at the same air temperature. Max et al.132 also explored the impacts of different greenhouse cooling techniques, including mechanical ventilation and evaporative cooling, on the yield and quality of tomatoes in tropical climates.
Moreover, biochar can serve as a valuable resource for improving soil fertility, sequestering carbon, and enhancing the availability of essential nutrients for plants within open environment ecosystems. This method effectively enhances nutrient and carbon concentrations or availability under controlled conditions, while simultaneously preserving the integrity of the biogeochemical cycle.133 There is a need for investigation of the influence of biochar under controlled environment ecosystems using precise technologies such as speed breeding. Furthermore, the integration of artificial intelligence and machine learning in this approach will be further required for more significant results under such conditions.
3.1. Impact of Light-emitting Diodes (LEDs) Spectrum on Photomorphogenesis and Crop Growth under a Controlled Environment Ecosystem
LEDs emit electromagnetic radiation across various wavelengths, which is absorbed by the plastidial photoreceptors of plants to facilitate biological and physiological processes. These photoreceptors selectively capture specific colors of light to trigger cellular, molecular, and biochemical responses. Far-red light (>730 nm)147 is predominantly captured, with occasional responsiveness to green wavelengths due to their broad absorption spectrum (530–700 nm) extending into the green range. Within the cryptochrome, phototropin, and zeitlupe family proteins, green, blue, and UV-A light are absorbed at wavelengths of 530–700 nm, 390–500 nm, and 320–390 nm, respectively.148,149 Similarly, ultraviolet resistance locus 8 (UVR8) responds to light at wavelengths of 290–315 nm. These processes intricately regulate various aspects of plant growth and development, including phytochrome-mediated responses, photoperiodism, flowering, photosynthesis, chlorophyll synthesis, responses to light stress, modulation of hormonal pathways, enzymatic functions, and the synthesis of secondary metabolites like alkaloids and terpenoids.150 Phytochrome, for instance, orchestrates flower opening and closure in response to redfar red light, while also governing processes such as germination, leaf development, metabolism, and flowering via perception of blue/green light. Phototropin regulates leaf orientation, phototropism, stomatal behavior, and chloroplast movement, while the zeitlupe protein family coordinates metabolic activity and flower development. Furthermore, UVR8 plays a role in regulating de-etiolation and flavonoid synthesis in plants cultivated under controlled artificial lighting environments.151
In controlled environments, crops utilize specific wavelengths of LED light to regulate their biological responses (Table 3). Both monochromatic and polychromatic emissions from LED lights influence the growth and physiological functions of crops in these controlled settings.152 For instance, in brinjal cultivation, monochromatic blue light was observed to promote growth in growth chambers. Compared with white light, blue light increased plant height by 2.16%, while red light led to a decrease in height by 0.38%. Additionally, a combination of red and blue light has shown benefits in enhancing the accumulation of photosynthetic pigments and advancing photosynthesis in eggplant seedlings, particularly when the blue:red light ratio was 1:3. Blue light increased ΦPSII (Photosystem II quantum yield) by 10.6%, whereas red light reduced it by 25.8% compared to white light.153 In the case of potato plantlets, exposure to blue light resulted in a shortened stature, characterized by larger leaves, well-developed roots, and abundant green foliage, along with noticeable changes in stomatal development. Furthermore, the combination of blue light with white light demonstrated a favorable response in enhancing growth and metabolic processes in onions by influencing photosynthesis-related genes, compared to using white light LEDs alone.154
Table 3. Impact of Diverse LED Light Spectrum on Crops Grown in a Controlled Environment Ecosystem.
| s. no. | LEDs parameters | crops | PPFD (μmol m–2 s–1) | type of system | nature of growth | impact | references |
|---|---|---|---|---|---|---|---|
| 1 | blue:red (1:3) | Brinjal | 300 | Phytotron chambers | Seedlings | • Rise in Chl a, Chl (a + b), Chl a/Chl b, and carotenoids contents by 20.3%, 18.8%, 5.9%, and 36.9%, respectively | (158) |
| • Enhanced contents of total sugar, sucrose, glucose, fructose, and starch by 0.93, 1.7, 0.97, 1.1, and 2.0-fold respectively | |||||||
| 2 | Red | Potato | - | Growth conditions | Single node | • Reduction in stem diameter by 0.26 mm, leaf area by 0.04 cm2 | (159) |
| • Rise in node number by 3.33 and node length 0.12 cm, dry weight by 38.04 mg, fresh weight by 527.55 mg, and health index by 0.03 units | |||||||
| 3 | Red:blue (3:1) | Potato | - | Growth conditions | Single node | • Rise in stem diameter by 0.25 mm, leaf area by 0.04 cm2, node length by 0.74 cm and number by 2.0, dry weight by 31.64 mg, fresh weight by 477.56 mg, and health index by 0.61 units | (159) |
| 4 | Red:blue (1:1) | Potato | - | Growth Conditions | Single Node | • Rise in stem diameter by 0.17 mm, leaf area by 0.14 cm2, node number by 0.5, fresh weight by 251.28 mg, and health index by 0.21 units | (159) |
| • Reduction in dry weight by 0.13 mg | |||||||
| 5 | Red:blue (1:3) | Potato | - | Growth Conditions | Single Node | • Rise in stem diameter by 0.10 mm, leaf area by 0.06 cm2, fresh weight by 199.61 mg, and health index by 0.36 units | (159) |
| • Reduction in node length by 0.83 cm | |||||||
| 6 | White:blue (3:1) | Green onion | 300 | Artificial climate room | Seedlings | • Increase in soluble sugar by 0.17%, crude cellulose by 0.03 mg/g, pyruvate content by 0.22 mg/g, soluble protein by 0.74 mg/g, free amino acid by 0.15 mg/g and volatile content by 0.05 mg/g than normal white light | (160) |
| • Rise in dipropyl disulfide (CAS: 629-19-6) by 5.80% than white light | |||||||
| 7 | Sodium pressure lamps (HPS) + red LEDs (87.5%) (630 to 660 nm) + blue LEDs (12.5%) (440 to 460 nm) | Cucumber | 220 | Greenhouse | Plant | • Increased Fs and Fm’ in both younger and older leaves. | (161) |
| • Increased the vitality index (PI) in both younger and older leaves of cucumber plants | |||||||
| • Highest commercial yields obtained throughout the harvest | |||||||
| 8 | Far red LEDs + red | Red leaf lettuce | 730; 300 | Greenhouse | Plant | • Increased total biomass, and leaf elongation | (162) |
| • Suppressed anthocyanin content and antioxidant potential | |||||||
| 9 | Far red LEDs | Red cross baby leaf lettuce | 160 | Greenhouse | Plant | • Decreased chlorophyll concentration by 14% as compared to white fluorescent lamps. The fresh weight, dry weight, stem length, leaf length and leaf width significantly increased by 28%, 15%, 14%, 44% and 15%, respectively, as compared to sole white fluorescent lamps. | (163) |
| • Decreased anthocyanins and carotenoids concentration by 40% and 11% as compared to sole white fluorescent lamps | |||||||
| 10 | Red (75%) + blue (25%) | Indian mustard + basil | ∼170 | Greenhouse | Plant | • Delayed or inhibited plant transition to flowering as compared to HPS or 460 nm + 635 nm LED combination effects | (164) |
| 11 | Red LEDs (660 nm) | Cabbages (red + green leaves) | 50 | Greenhouse | Plant | • Increased anthocyanin contents in red-leaf cabbages | (165) |
| 12 | Red LEDs (640 nm) applied 7 days before harvesting | Kale plants | 253.3 | controlled environment | Plant | • Enhanced chlorophyll a, b accumulation. | (166) |
| • Enhanced lutein accumulation | |||||||
| 13 | Red LEDs (658 nm) | Baby leaf lettuce | 130 | Greenhouse | Plant | • Phenolics concentration increased by 6% with supplemental red light | (167) |
| 14 | Red LEDs (638 nm) | Lettuce + Grand Rapids marjoram + green onions | ∼500 | Greenhouse | Plant | • Reduction of nitrate concentration | (168) |
| 15 | Red LEDs (638 nm) as 3 days preharvest treatment | Lettuce + green leaf + red leaf | ∼170 | Greenhouse | Plant | • Increased DPPH free radical scavenging activity. Increased phenolic compound and η tocopherol content | (169) |
| 16 | Red LEDs (638 nm) + HPS lighting for 3 days before harvesting | Green baby leaf lettuce + Thumper + Multibaby | 210; 300 | Greenhouse | Plant | • Increased concentration of total phenolics (28,5%), tocopherols (33,5% in ‘Multibaby’), sugars (52.0%) and antioxidant capacity (14.5%) but decreased concentration of ascorbic acid | (170) |
| 17 | Red LEDs (638 nm) + HPS lighting for 3 days before harvesting | Red leaf + green leaf + light green leaf lettuces | 300; 90 | Greenhouse | Plant | • Reduced content of nitrate in red (56.2%) and green (20.0%) leaf lettuce, but nitrate contents increased in light green leaf lettuce. | (171) |
| 18 | Red LEDs (638 nm) + HPS lighting for 3 days before | White mustard + spinach + rocket + dill + parsley + green onions + white lisbon | 300 | Greenhouse | Plant | Altered antioxidant activity, increased monosaccharide, and decreased nitrate accumulation in dill and parsley. Increase in vitamin C content in mustard, spinach, rocket, dill and green onion. | (172) |
| 19 | Green LEDs (510, 520, or 530 nm) | Baby leaf lettuce: (red leaf + green leaf + light green leaf) | 100, 200, and 300 | Greenhouse | Plant | Reduction of nitrate concentration and increase in saccharide contents in all baby leaf lettuce varieties. | (173) |
| 20 | Green LEDs (505, 535 nm) | Red leaf ‘Multired 4’ + green leaf ‘Multigreen 3’ + light green leaf ‘Multiblond 2’ + baby leaf lettuce | 30 | Natural and green house | Plant | 535 nm green LEDs had greater positive effect on ascorbic acid, tocopherol contents and DPPH freeradical scavenging capacity, when 505 nm LEDs had greater effect on total phenol and anthocyanin contents. | (174) |
| 21 | Blue LEDs (Sole 440 nm) applied 7 days before harvesting | Kale plants | 10.6 | Greenhouse | Plant | Enhanced N-carotene contents | (175) |
| 22 | Blue (468 nm) LEDs alone or in combination with red (655 nm) LEDs | Red leaf lettuce | ∼100 | Greenhouse | Seedling | Stimulated biomass accumulation in the roots; resulted in compact lettuce seedling morphology; Promoted the growth of lettuce after transplanting. | (176) |
| Greater polyphenol contents and total antioxidant status. | |||||||
| 23 | Blue LEDs (440 nm) + red (640 nm) | Red leaf lettuce | 30; 270 | Greenhouse | Plant | Leaf expansion | (177) |
| Increased concentration of anthocyanins, higher antioxidant potential | |||||||
| 24 | Blue LEDs (476 nm) | Baby leaf lettuce | 130 | Greenhouse | Plant | Anthocyanins concentration increased by 31%; carotenoids concentration increased by 12% | (163) |
| 25 | Blue LEDs (470 nm) | “Kinshun” (green leaves) + “Red Rookie” (red leaves) | 50 | Greenhouse | Seedling | Promoted petiole elongation in both cabbage varieties; higher chlorophyll contents in green leaf cabbages | (165) |
| 26 | Blue LEDs (460 nm) + red (660 nm) light (11,1% of blue light) | Nonheading Chinese cabbage | 80 | Greenhouse | Plant | • Higher chlorophyll concentration. Blue LEDs benefit vegetative growth, while red LEDs and blue plus red LEDs support reproductive growth | (178) |
| • Concentration of vitamin C was the greatest under blue LEDs | |||||||
| 27 | UV-A LEDs (373 nm) | Red cross baby leaf lettuce | 18 ± 2 | Greenhouse | Plant | Anthocyanin concentration increased by 11% | (163) |
Additionally, combining red and far-red light has been found to significantly hasten the growth of potato plants, resulting in heights of 30.3 and 27 cm for the Golden King and Chungang varieties, respectively.155 The use of simple and complex LED light emissions, including white, red, dark red, and a mixture of dark red, blue, and orange, has been shown to promote biological and physiological growth in jute (Corchorus capsularis) under controlled glasshouse conditions. Exposure to red light increased plant height by 12% compared to that with white light, while the stem diameter exhibited an 86% increase under blue light and an 82% increase under orange light. Similarly, the root diameter increased by 83% under blue light and 84% under orange light.156 Monochromatic LED light emissions, such as red, white, blue, and orange, have been effective in promoting morphological growth in Brassica napus within controlled glasshouse environments. For instance, exposure to red light increased the seedling length of Brassica napus by 29.2%, plant height by 13.2%, crown length by 32.2%, total chlorophyll by 17.5%, carotenoids by 20.4%, plant fresh weight by 18.6%, and dry weight by 20% compared to white light.157 Both monochromatic and polychromatic LED lighting have successfully supported cellular, biochemical, and molecular processes across various plant species. These investigations will further illuminate the effects of LED light emissions on millets, fiber crops, and oilseed crops under controlled environmental conditions, areas that have yet to be extensively explored.
3.2. Mechanism of Nutrient Translocation in Plants with Physiological Model and Machine Learning in a Controlled Environment Ecosystem
Mechanistic physiological models (MPMs) and machine learning (ML) algorithms are central in orchestrating the intricate interplay among water, light, nutrients, and growth parameters within controlled environments, thereby exerting profound effects on the plant growth rate and yield. Real-time sensors, outfitted with ion-selective electrodes (ISEs), collaborate seamlessly with a computerized database management system to scrutinize both practical observations and theoretical insights. Nestled within the root zones of plants, these sensors perpetually track the dynamics of nutrient levels and absorption, while artificial neural networks (ANNs) take charge of rectifying errors, refining accuracy, and mitigating disruptions during nutrient transportation. By amalgamating ANNs with two-point normalization techniques (TPNs), meticulous forecasting and regulation of nutrient movement within plants are accomplished with precision.179
3.3. Mechanism of Nutrient Translocation in the Root System of the Plant
In controlled environmental settings, the intricate interplay of root architecture, metabolism, and dynamics is discerned through the application of mechanistic physiological models (MPMs) and machine learning (ML). These sophisticated models not only govern the influx and dynamics of nutrients within the plant root system but also facilitate a nuanced understanding of substrate dynamics and root nutrient uptake. The integration of mechanistic physiological models, such as the Barber-Cushman model and Porter diffusion models, enables a comprehensive exploration of nutrient dynamics and uptake mechanisms within plant roots. The Barber-Cushman model, alongside the Porter diffusion model, operates independently, yet synergistically, in elucidating nutrition metabolism. This multifaceted model scrutinizes various facets, including nutrient concentrations, solution nutrient levels, spatial distributions, mass flow, diffusion processes (governed by the advection-diffusion equation), and Robin boundary conditions. Simultaneously, the Porter diffusion model meticulously examines substrate advection and diffusion equations within the intricate framework of plant roots, further enriching our understanding of nutrient dynamics and uptake processes. Furthermore, the Michaelis–Menten (MM) model regulates nutrient flow from the root cell to the plasma membrane, biomass growth, nutrient gradients, substrate dynamics, and root influx dynamics in the plant root system.180
Utilizing additive chemicals enhances biological growth, stimulates the production of phenolic compounds and flavonoids, improves nutrient availability, facilitates the synthesis of antioxidant compounds, fortifies processes, and bolsters stress resistance in plants.181 Under controlled conditions, the utilization of potassium nitrate (KNO3) elevates the levels of antioxidant enzymes, such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), and ascorbate peroxidase (APX) within both the root and leaf systems of radish plants. This augmentation mitigates salt stress in radishes. Additionally, it amplifies the presence of phenolic compounds, flavonoids, ascorbic acid, and anthocyanins.182 Under glasshouse conditions, the intake of synergic acid diminishes oxidative stress while concurrently boosting growth, biomass, gas exchange, and the presence of antioxidant compounds in either the root or shoot system of tomato plants.183 Furthermore, in Triticum aestivum L. plants, the use of taurine enhances growth, chlorophyll levels, and nutrient absorption by modulating the scavenging of reactive oxygen species (ROS), secondary metabolic pathways, and ion balance in response to stressors. Taurine’s efficacy mitigates the generation of nitric oxide, hydrogen sulfide, glutathione, and phenolic compounds, while also attenuating the impact of boron (B) and chromium (Cr) under regulated conditions.184 Moreover, employing advantageous additive chemicals in hydroponic systems, substrate-based cultivation, and accelerated breeding methodologies significantly boosts biological growth, stress resilience, reduction of reactive oxygen species (ROS), establishment of barriers against toxic metals, and enrichment of phytochemicals or phenolic compounds. Comprehensive studies may be necessary to grasp the intricate mechanisms and uptake dynamics of potassium nitrate, synergic acid, and taurine compounds within plant root systems by using mechanistic physiological models and machine learning techniques under controlled environmental settings.
4. Approaches of Technological Resources in Speed Breeding under a Controlled Environment Ecosystem
Speed breeding techniques encompass a diverse array of methodologies and tools aimed at accelerating the breeding process and enhancing desirable traits in crops under controlled conditions. These techniques include the utilization of various markers such as molecular, biochemical, and morphological markers as well as advanced breeding methods like Marker-Assisted Selection (MAS), Genome-Wide Association Studies (GWAS), Next-Generation Sequencing (NGS), Genomic Selection (GS), Targeted Induced Local Lesions IN Genomes (TILLING), mutation breeding, RNA interference (RNAi), and CRISPR/Cas gene editing. Additionally, tissue culture methods are employed to propagate plants rapidly.185,186 Speed breeding techniques have been effectively utilized to enhance several crops including Brassica species, Arabidopsis thaliana, various species of amaranthus, bread wheat, durum wheat, barley, chickpea, pea, grass pea, quinoa, oat, purple false brome, peanut, soybean, sorghum, broad beans, lentil, rice, and burrowing clover within controlled environment systems. These techniques have proven instrumental in improving traits essential for crop enhancement.187
4.1. Response of Speed Breeding Techniques in Crops for Trait Improvement in Controlled Environment Conditions
Recombinant inbred lines (RILs) with desired traits were successfully generated in soybean in five years using a temperature-controlled growth chamber and an incandescent lighting speed breeding technique, employing a single pod descent method.188 In a span of four years, early plant regeneration was achieved in groundnut through the utilization of a greenhouse, employing the photosynthetically active radiation (PAR) technique and gas heating speed breeding method, facilitated by the single seed descent approach.189 In a span of 7 to 8 years, early flowering and seed germination in broad beans and lentils were achieved using a speed breeding technique incorporating light emitting diodes (LEDs), precise temperature control, and growth regulators, all managed through the single seed descent method within an incubator. Similarly, in peas, recombinant inbred lines (RILs) were successfully developed within a time frame of 5 years by integrating light emitting diodes (LEDs), growth regulators, and speed breeding techniques using the single seed descent method within a growth chamber.190 For wheat, these lines were obtained within an eight-year time frame utilizing incandescent lighting, precise temperature control, or embryo culture in conjunction with a speed breeding approach, all implemented through the single seed descent method under carefully controlled conditions.191 Moreover, in chickpeas, these lines were produced in four years with LED and temperature-based speed breeding techniques through a single pod descent method under greenhouse.192 Similarly, the rapid productions of high-yielding varieties in rice were also produced in 4 years using the same technique under greenhouse and screen house facility.193 However, in soybeans, the germination rate with light effect was achieved in five years with light emitting diodes and speed breeding technique through a single seed descent method under climate-controlled chamber.194
Over a span of nine years, using LED, controlled temperature, growth regulators, and the embryo rescue speed breeding technique, segregated and pure lines were developed in wheat, barley, and sorghum populations via the single seed descent method within a controlled environment facility.195 Within a span of 6 to 7 years, the development of biotic stress-resistant traits and the establishment of pure line plant populations in bread wheat, durum wheat, or chickpea were demonstrated using a speed breeding technique based on LED lighting or temperature regulation. These methods were applied through the single seed descent approach under laboratory room conditions, temperature-controlled glasshouses, and controlled environments simulating glass homes, respectively.196,197 Moreover, employing analogous technologies, the breeding period was condensed, resulting in a complete development within five years, and early panicle emergence was achieved in oats within the Walnut Street Greenhouse.198 Conversely, for Arabidopsis thaliana, the breeding cycle was abbreviated, with a total development time of 10 years observed under Greenhouse.199 Furthermore, the generation of segregating plant populations in Amaranthus species was achieved within a six-year time frame utilizing these technologies under predefined conditions.200 In just six years, the rapid development of high-yielding sorghum varieties was accomplished using a speed breeding technique that incorporated light emitting diodes (LEDs), precise temperature control, and immature seed germination, all managed through the single seed descent method within a controlled greenhouse.201 Similarly, rapid biparental or multiparental plant populations in Trifolium subteraneum were established within the same time frame of six years, employing an expedited breeding method that involved incandescent lighting, temperature regulation, and growth regulators, all applied through the single seed descent method within a controlled plant growth facility202,203 (Table 4).
Table 4. Intervention of Speed Breeding Methods in Phenotypic Traits Improvement under Controlled Environment Conditions.
| s. no. | crops | speed breeding methods | generation per annum | traits improvement | growing system | references |
|---|---|---|---|---|---|---|
| 1 | Soybean | Incandescent lights, temperature | 5 | Recombinant inbred lines (RILs) | Growth chamber | (188) |
| 2 | Groundnut | Photosynthetically active radiation (PAR) technique, gas heating | 4 | Early plant regeneration | Greenhouse | (189) |
| 3 | Broad bean, lentil | Light emitting diodes, temperature, growth regulators | 7 or 8 | Early flowering and seed germination | Incubator | (204) |
| 4 | Pea | Light emitting diodes, growth regulators | 5 | RIL | Growth chamber | (190) |
| 5 | Chickpea | Light emitting diodes, temperature | 4 | RIL | Greenhouse | (192) |
| 6 | Soybean | Light emitting diodes | 5 | Germination rate in light effect | Climate controlled chamber | (194) |
| 7 | Wheat, barley, sorghum | Light emitting diodes, temperature, growth regulators, embryo rescue | 9 | Segregated or pure lines plant populations | Controlled environment facility | (195) |
| 8 | Rice | Light emitting diodes, temperature | 4 | Rapid production of high-yielding variety | Screen house facility | (193) |
| 9 | Wheat | Incandescent light, temperature, embryo culture | 8 | RIL | controlled conditions | (191) |
| 10 | Bread wheat, durum wheat, chickpea | Light emitting diodes, temperature | 6–7 | Biotic stress resistance, pure line plant population | Laboratory room conditions, temperature controlled glasshouse, glass home | (196, 197) |
| 11 | Oat | Light emitting diodes, temperature, micronutrient | 5 | Short breeding time, early panicle emergence | Walnut street greenhouse | (198) |
| 12 | Sorghum | Light emitting diodes, temperature, immature seed germination | 6 | Rapid high yielding variety development | Controlled greenhouse | (201) |
| 13 | Arabidopsis thaliana | Light emitting diodes, temperature, growth regulators | 10 | Short breeding cycle | Greenhouse | (199) |
| 14 | Amaranthus spp. | Light emitting diodes, temperature, growth regulators | 6 | Segregating plant populations | Controlled growth chamber | (200) |
| 15 | Trifolium subteraneum | Incandescent light, temperature, growth regulators | 6 | Rapid biparental, multiparental plant populations | Controlled plant growth facility | (202, 203) |
Speed breeding techniques offer significant potential for enhancing oligogenic or qualitative traits while simultaneously reducing the generation time in crop breeding programs. Further research is warranted to explore the screening of quantitative traits, assess heritability, delve into the concept of plant ideotypes following Donald’s concept, investigate biofortification strategies, conduct biometric analyses, and undertake physiochemical characterization within the framework of existing breeding techniques and plant tissue culture methods under controlled environment conditions. Additionally, advancements in biometric analyses and physiochemical characterization techniques can provide valuable insights into the underlying genetic mechanisms and physiological processes governing trait expression. Moreover, the integration of plant tissue culture methods into speed breeding protocols offers opportunities for rapid propagation of elite genotypes, somaclonal variation screening, and the development of novel genetic variants. This approach can expedite the generation of diverse germplasm and accelerate the breeding cycle, ultimately enhancing the efficiency and effectiveness of crop improvement programs.204
4.2. Protocols of Speed Breeding in Rice, Sorghum, Chickpea, Soybean, and Groundnut in Controlled Environment Conditions
Speed breeding techniques significantly abbreviate the breeding cycle, enabling the swift creation of new varieties within a considerably reduced time frame.205 Parental selection, crossing, generation line development, multiplication, evaluation, adaptation, yield trials, and variety release can all be accomplished within a mere 2 to 3 years of generation time using speed breeding methodologies within a controlled environment ecosystem.206 The techniques and timelines for speed breeding and generation vary across crop development and enhancement within controlled environment settings.207 For instance, rice population lines can be achieved within a generation time of 80 days by using a rapid advancement method based on speed breeding. This method necessitates specific conditions, including an 11-h photoperiod, temperatures of 30 °C during the day and 25 °C at night, a photosynthetic photon flux density of 350 μmol m–2 s–1, 70% humidity, and a CO2 concentration of 475 ppm under controlled conditions. However, altering the photoperiod to 10 h and maintaining the same temperature, light intensity, and humidity, the generation time for rice population lines extends to 100 days under controlled conditions.208 For sorghum, the generation time was reduced to 77 days under controlled conditions when subjected to continuous light and maintained at a temperature of 30 °C.209 Additionally, the vegetative growth of soybean is observed within a generation time of 70 days under standardized conditions. These conditions include a 14-h photoperiod, temperatures of 30 °C during the day and 25 °C at night, a photosynthetic photon flux density of 220 μmol m–2 s–1 at canopy level, a CO2 concentration exceeding 440 ppm, and humidity ranging between 50 and 80%.210 Furthermore, in the context of chickpeas, a condensed breeding cycle was observed when population lines were subjected to specific conditions. This included exposure to a 22-h photoperiod, day temperatures of 22 °C and night temperatures of 17 °C, maintained humidity levels of 70%, and a photosynthetic photon flux density ranging between 440 and 650 μmol m–2 s–1 during the adult plant stage, all under predefined conditions.211 Furthermore, the integration of speed breeding with continuous light supply, day temperatures of 28 ± 3 °C and night temperatures of 17 ± 3 °C, along with 65% humidity, yielded a generation time of 89 days for groundnut population lines under standardized environmental conditions.212
5. Applications of Artificial Intelligence in Phenotypic Character Examination in Crops under a Controlled Environment Ecosystem
The real-time machine powered by artificial intelligence (AI) operates on neural network principles and algorithmic models. It is seamlessly incorporated into plant growth chambers, where predetermined environmental conditions facilitate the scrutiny of phenotypic traits and functional assessments in crops. This cutting-edge technology has demonstrated its efficacy across various crops, serving pivotal roles in screening for traits within controlled environments.213
Preharvest potential, yield performance, and identification of high-yield cultivars in soybean were evaluated through various advanced methodologies. These include the application of Best Linear Unbiased Prediction (BLUP), Neural Networks (NNs), kernel methods, and algorithmic models such as Multilayer Perceptron (MLP), Support Vector Machine (SVM), Ensemble-Stacking (E-S), Random Forest (RF), and Stochastic Gradient Descent (SGD) within an open field system.214,215 In soybeans, investigations into seed per pod and seed characteristics across diverse environments were conducted using Convolutional Neural Networks (CNNs) and algorithmic model Batch Normalization (BN) within a conventional field system.216 Similarly, French beans underwent scrutiny for average yield, adaptability under stress factors, and phenotypic stability, employing Artificial Neural Networks (ANNs) along with algorithmic models Mean Square Deviation (MSD) and Mean Square Residue (MSR) within a standard field scenario.217 Moreover, employing similar technologies, French beans were subjected to assessments of the oil content, callus physical characteristics, secondary metabolite synthesis, and somatic embryo development. These analyses were conducted in a research field218 and controlled environment room.219 Stress resistance and miRNA expression related to stress response were investigated in Arabidopsis thaliana utilizing deep learning techniques and algorithmic support vector machine (SVM) and naive Bayes classifiers within a growth room setting.220 In the case of sesamum, evaluations of seed yield, oil content, and identification of superior genotypes were carried out utilizing Artificial Neural Networks (ANNs) and algorithmic model Multiple Regression Analysis in a research field221 (Table 5).
Table 5. Intervention of Artificial Intelligence (AI) in Crops for Traits Identifications.
| s. no. | crops | artificial intelligence | algorithm models | traits identifications | growing system | references |
|---|---|---|---|---|---|---|
| 1 | Soybean | Best linear unbiased prediction (BLUP), neural networks (NNs), kernel methods | Multilayer perceptron (MLP), support vector machine (SVM), ensemble-stacking (E-S), random forest (RF), and stochastic gradient descent (SGD) | Preharvest potential, yield performance, high-yield cultivars | Open field | (214, 215) |
| 2 | Soybean | Convolutional neural networks (CNNs) | Model batch normalization (BN) | Seed per pod, seed character | Open field | (216) |
| 3 | French bean | Artificial neural networks (ANNs) | Mean square deviation (MSD), mean square of residue (MSR) | Average yield, high adaptation, and phenotypic stability | Open field | (217) |
| 4 | French bean | Artificial neural networks (anns) | Mean square deviation (MSD), mean square of residue (MSR) | Oil content, callus physical characters, secondary metabolite synthesis, somatic embryo | Research field, controlled room | (218, 219) |
| 5 | Arabidopsis thaliana | Deep learning | Algorithm support vector machine (svm), naive byes | Stress resistance, mirna expression in stress resistance | Growth room | (220) |
| 6 | Sesamum | Artificial neural networks (ANNs) | Multiple regression analysis | Seed yield, oil content, superior genotypes | Research field | (221) |
The integration of real-time machine learning presents significant opportunities for quantitative trait screening within speed breeding techniques implemented in controlled environment ecosystems. Exploring the intervention of speed breeding techniques in quantitative traits, biofortification, nutrient or water dynamics screening, tissue culture plant regeneration examination, biometric analysis, and physiochemical characterization using artificial intelligence becomes essential in studying crops under controlled growth conditions.
5.1. Intervention of Artificial Intelligence (AI) and Machine Learning (ML) in Phenotype Screening in Speed Breeding
The fundamentals of crop modeling, crop management, trait discovery, phenotype identification, quantification, diseases, and pest diagnosis, along with image processing, are integrated into the algorithmic models. These models leverage artificial intelligence, machine learning, automated machine learning, and information technology to screen, identify, classify, and assess various aspects such as growth, yield, disease, pest detection, plant-microbe relationships, environmental factors, climatic patterns, and phenotypes.222
Leveraging AI and ML streamlines the analysis and retrieval of data from vast populations, including quantitative traits, root phenotypes, environmental parameters, and multilocation field trials in field breeding. Moreover, these technologies serve as robust and concurrent tools for screening qualitative and quantitative data in plant systems within controlled environment ecosystems.223,224 Both methods are instrumental in facilitating high-throughput phenotyping of plants during speed breeding under controlled environmental conditions.
High-throughput phenotyping in plants is facilitated through the utilization of AI algorithm models, ML algorithm models, and automated ML algorithm models, which are integrated with sensor-based or satellite-based platforms.225 These advanced algorithms enable the identification of plant phenotypes and leaf morphology, measurement of growth parameters, detection of disease or pest symptoms, analysis of genomic and environmental data, assessment of genotype-phenotype-environment interactions, and investigation of relationships between phenotype and disease. Additionally, they facilitate the early detection of pathogenic hosts and the analysis of plant growth or disease images within the context of speed breeding in controlled environment ecosystems. The AI algorithm models excel in detecting leaf color, texture, specific gene features, crop traits, and weather patterns in plants under standardized conditions.226−228
In recent advancements, the fusion of AI with convolutional neural networks (CNNs) has revolutionized the precision of leaf segmentation and trait quantification.229 Similarly, the synergy of hyperspectral imaging with machine learning enables swift evaluations of crop performance.230 Moreover, the integration of unmanned aerial vehicles (UAVs) with deep learning facilitates the early detection of diseases in wheat fields.231 Additionally, the amalgamation of genomic data with machine learning techniques aids in predicting breeding outcomes with greater accuracy.232 Furthermore, the coupling of Bayesian optimization with genomic prediction enhances the selection process for high-yielding genotypes.233 Utilizing AI alongside UAV imagery, coupled with CNNs, efficiently identifies weed species or populations.234 Lastly, employing AI CNN gradient-weighted class activation mapping (Grad-CAM) offers a robust screening method for assessing chalkiness quality in rice.235
In addition to integrating crop models with AI algorithms to monitor plant development and performance, these models play a pivotal role in selecting key traits from plant population lines in breeding programs.236 Moreover, various crop models serve to gather empirical and theoretical data in plants. For instance, the AI system PHENOPSIS assesses plant responses to soil-water stress, while the crop model GROWSCREENFLUORO identifies phenotypic leaf growth and chlorophyll fluorescence to determine the abiotic stress tolerance. The artificial technology LemnaTec 3D Scanalyzer System comprehensively evaluates the salinity tolerance in rice. Additionally, the model technology PhenoBox identifies diseases such as head smut, corn smut, and responses to salt stress. The crop model technology PHENOVI-SION detects drought stress and recovery and analyzes stress responses. Furthermore, artificial intelligence through Pheno Field evaluates abiotic stress and related traits, while the AI model Plant Screen Robotic XYZ system examines diverse traits including stress tolerance mechanisms and drought tolerance.237
Machine learning techniques applied to plants under predefined conditions are utilized to decipher the transmission of information from DNA sequences to observable plant traits. In this realm, a plethora of ML algorithms have been introduced and honed to augment plant development.238 These ML-based algorithms delve into disease or pest diagnosis, yield response, the genotype-phenotype relationship, molecular events in biological systems, novel components in plants or pathogens, omics analysis, plant-microbe interaction, and disease identification in plants facilitated by speed breeding in controlled environment ecosystems. Various imaging modalities such as thermal or stereo visible light, remote sensing, Kinet RGB (red, green, blue) depth images, hyperspectral images, fluorescence imaging spectroscopy, unmanned aerial vehicles (UAVs) based RGB depth images, and multispectral images are employed to tackle disease identification problems in plants undergoing speed breeding under controlled environmental conditions.239,240
A plethora of machine learning algorithms, including support vector machine (SVM), successive approximation model (SAM), Gaussian processes classifier (GPC), Bayes factor, detection analysis resolution (DAR), object-based image analysis (OBIA) based classification, K-Nearest Neighbor (KNN), quadratic discriminant analysis (QDA), linear discriminant analysis (LDA), naïve Bayes (NB), simple logistic analysis (SL), library successive approximation model (Lib SVM), Library LINEAR (LINE), multilayer perceptron (MLP), binarized neural network (BNN), functional trees (FT), random forests (RF), quantitative trait loci (QTL), and genome-wide association studies (GWAS), have demonstrated success in species identification, classification, analyzing multiple traits, and identifying phenotypes or genotypes, as well as diseases or pests in various crops such as tomato, sugar beet, apple, and barley.241−243 Moreover, the integration of machine learning algorithm models with sensor-based technologies like light detection and ranging (LIDAR), hyperspectral imaging, thermal fluorescence, or 3D laser scanning, among others, is utilized to assess stress tolerance and yield in plants. These combined technologies are also employed to investigate stress and yield responses in crops such as cotton, triticale, maize, and citrus.244,245 Additionally, automated machine learning (AutoML) is recognized for its role in data preparation, extraction, selection, construction, model development, model evaluation, and image processing in plants, particularly in conjunction with speed breeding techniques under controlled environmental conditions.246,247
The fusion of AI and ML offers comprehensive screening of phenotypic or genotypic data, crop architecture and development, pest or disease symptoms or diagnosis, and image processing within speed breeding programs under controlled environmental conditions. These technologies necessitate standardized procedures for initiating speed breeding programs along with prominent features, reliable algorithm models, and physiological or biochemical-based traits. Effective utilization of algorithm models, AI, and ML demands expertise in their operation and maintenance within controlled environmental ecosystems. Furthermore, implementing sophisticated algorithm models and technologies is time-consuming and requires capital investment to establish physical targets.248,249,250
6. Intervention of Technologies in Speed Breeding under a Controlled Environment Ecosystem
The strategies for accelerating crop growth within controlled environment chambers encompass a variety of technologies.251 Utilizing biofortification approaches like the root/nutrient method, fertigation method, and foliar spray method, speed breeding techniques yield biofortified crops and microgreens in controlled ecosystems.252 Tissue culture methods, including cell culture, seed culture, endosperm culture, embryo culture, anther culture, and somaclonal variations, are integrated into speed breeding technology to enhance the development of superior crop varieties within controlled environments.253 The integration of speed breeding and tissue culture methods within controlled ecosystems substantially reduces issues such as loss of regenerated plantlets in protected cultivation, regeneration of albino plants, embryo abortion, pseudoseed formation, and ensures the production of disease-resistant crops.254 Additionally, this approach alters gene behavior, genetic makeup, gene expression, and inheritance patterns in agricultural or horticultural crops by leveraging techniques such as genomics, phenomics, proteomics, and transcriptomics.255 Moreover, incorporating genomics, phenomics, proteomics, and transcriptomics in speed breeding helps mitigate challenges like inbreeding depression, male sterility, self-incompatibility, embryo abortion, and enhances seed setting within controlled environment plant growth chambers.256
Furthermore, speed breeding expedites the development of genetically engineered crops using both direct and indirect genetic engineering methods, including gene editing techniques such as the CRISPR/Cas9 system.257 This approach can also be synergized with efforts to enhance biotic and abiotic stress tolerance through protein modification within controlled environment ecosystems. Additionally, it facilitates the advancement of phytoremediation crops by integrating phytoremediation technologies within controlled plant growth chambers.258 Moreover, speed breeding techniques can incorporate sensor-based architectures to assess various parameters such as biometric and stoichiometric parameters, cell cycle dynamics, genetic linkage, chromosomal inheritance theory, Donald’s crop ideotype concept, and Mendel’s genetic theories within controlled environments.259 Speed breeding practices also encompass optimizing light quality and intensity, particularly utilizing light-emitting diodes (LEDs), to enhance crop performance within controlled growth chambers.260
Moreover, it encompasses a variety of controlled growth systems aimed at enhancing crop and seed improvement within a single year. Healthy wheat and barley seeds can be harvested within 2.5–3 weeks in a controlled environment chamber. Local wheat and barley spikelets, on the other hand, are typically harvested in 2 and 4 weeks, respectively, in a glasshouse chamber with regulated lighting. Viable seeds of wheat, barley, oat, and triticale are harvested within 4 weeks in a homemade growth room. The speed breeding method attributes a shorter generation time compared to traditional breeding, resulting in accelerated variety development.261 This technique also reduces the timeline for plant population line development to 1–2 years, field trials to 3–5 years, and variety release to 1–2 years, as opposed to the 5–10 years typically required for plant population line development, 3–5 years for field trials, and 1–2 years for variety release in traditional breeding262 (Figure 2).
Figure 2.
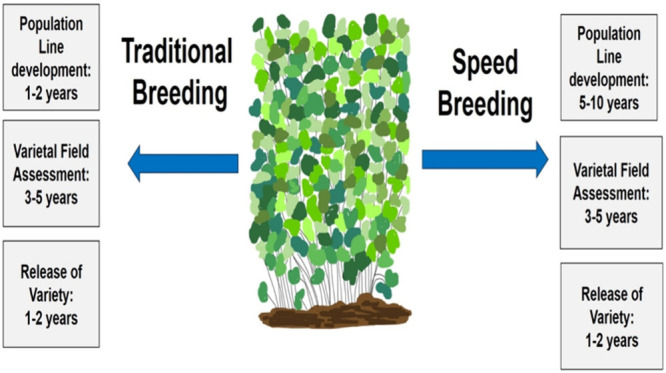
Generation time of variety release in speed and traditional breeding.
Furthermore, this method integrates with nanotechnology to foster the development of nanocrops within controlled environment ecosystems.263 Recycling wastewater technology and reclaimed wastewater can also be leveraged to enhance crop improvement under fast breeding conditions. The dynamics of wastewater can be examined using sensor-based technologies in conjunction with speed breeding within controlled environments.264
7. Challenges and Benefits of Speed Breeding in a Controlled Environment Ecosystem
Establishing crop production or speed breeding within a controlled environment ecosystem necessitates a well-equipped infrastructure. The process of developing parental lines, selection lines, and releasing varieties through speed breeding techniques demands various facilities and accessories. This includes a controlled environment plant growing chamber spanning 500–1000 square feet and essential equipment like carbon dioxide analyzers, temperature and relative humidity controllers, airflow/ventilation systems, as well as controls for light spectrum, intensity, and photoperiod. The capital investment for such infrastructure typically ranges from 20 to 30 lakh Indian rupees. Moreover, the implementation of speed breeding techniques incurs higher monetary costs at certain stages compared with traditional breeding, although the overall costs are generally lower (Table 6). A proficient subject expert is indispensable for comprehending the operation and upkeep of a controlled environment ecosystem. They must grasp the concept and approach of speed breeding techniques along with key trait selection under controlled environmental conditions.265 Furthermore, the scientist leading speed breeding research should possess a strong understanding of agronomy, crop physiology, genetics, plant breeding, and plant biotechnology. Alternatively, a multidisciplinary team with expertise in these areas should be actively engaged in the research. The costs associated with nutrient solutions, advanced infrastructure, and facilities are considerable, and a continuous supply of distilled water is indispensable for conducting crop trials using speed breeding techniques under controlled growing conditions. Even minor lapses or irregularities in maintaining photoperiodism, temperature, humidity, and control of pests or diseases can lead to the deterioration of crop trials and speed breeding techniques within the controlled environment system. Ensuring a regular power supply and maintaining an optimal temperature are essential for sustaining crop trials, research activities, and methodologies within the controlled environment. Throughout the cropping period in controlled environment technologies, it is imperative to uphold and monitor standard pH levels, total dissolved solids (TDS), and growing conditions to ensure the efficacy and success of the breeding programs.266
Table 6. Comparison of Approximately Monetary Costs Involved in Traditional and Speed Breeding of Peanuta.
| F2 generation |
F3 generation |
||||
|---|---|---|---|---|---|
| components | speed breeding (in INR) | traditional breeding (in INR) | speed breeding (in INR) | traditional breeding (in INR) | ref |
| Lights installment | 0 | 26981.29 | 0 | 23150.62 | (269, 270) |
| Gas heating | 0 | 19319.94 | 0 | 19819.59 | |
| Green house construction | 0 | 15405.99 | 0 | 15405.99 | |
| Labor cost | 11741.86 | 17404.60 | 26398.37 | 13907.03 | |
| Land and other equipment costs | 10242.90 | 0 | 5163.09 | 0 | |
| Total cost (without gas) | 21984.76 | 59791.88 | 30645.42 | 52463.63 | |
| Total cost (with gas) | NA | 79111.82 | NA | 71783.57 | |
| Time efficiency | 1 year | 2 year | 1 year | 3 year | |
NA: Not applicable.
Moreover, this method has proven to be highly effective in screening both key and broad-spectrum traits within early population lines. Speed breeding holds significant potential for minimizing genetic loss and morphological or reproductive disruptions while simultaneously enhancing selection intensity, heritability, and genetic gain and maintaining precise data records under controlled environmental conditions. It demonstrates competence in achieving desired breeding objectives and facilitating varietal development. This technique inherently accelerates the development of improved population lines and varieties within a shorter generation time per year, compared to conventional breeding methods. For example, in crops like wheat, pea, and chickpea, traits and line development typically occur over 4–6 generations per year using speed breeding, whereas in field breeding, it is limited to 1 generation per year. Similarly, in okra, phenotypic traits and selection lines manifest over 4–6 generations per year through speed breeding compared with 2–3 generations per year in field breeding. Likewise, for both qualitative and quantitative traits in tomatoes, the speed breeding technique enables 5–6 generations per year, while field breeding typically allows for only 2–3 generations per year. The adoption of speed breeding leads to reduced labor expenses, time savings, secured funding, and fewer field trials within a controlled environment ecosystem.267,268
8. Conclusions
The controlled environment setting proves to be advantageous for cultivating crops and microgreens, addressing food security concerns, and facilitating technology transfer. It ensures a consistent distribution of nutrients, water, and climatic conditions. Such environments play a crucial role in mitigating the effects of climate change and in sustainable use of natural resources. Speed breeding, an advanced plant breeding method, expedites crop improvement by achieving desired objectives within a shorter generation time, typically a year. This technique enhances both qualitative and quantitative traits across a range of crops, including rice, wheat, barley, sorghum, oat, soybean, peanut, chickpea, broad bean, and lentil, among others, within controlled environments.
Speed breeding employs various effective methods such as biofortification, tissue culture, gene manipulation, and omics to develop healthier, disease-resistant crops and to overcome issues such as self-incompatibility, male sterility, poor seed setting, inbreeding depression, embryo abortions, and albino plant regeneration. Additionally, the recycling of wastewater can enhance crop growth through fast breeding in controlled environments. Sensor-based technology enables the investigation of wastewater dynamics, facilitating speed breeding under controlled conditions. Integration of algorithmic models and sensor-based technologies enhances the evaluation of crop characteristics, climatic factors, nutrient dynamics, and ambient gases within controlled environment ecosystems with precision and efficiency. Speed breeding ensures consistent environmental conditions for crop improvement and aids climate change mitigation within predefined ecosystems. It also minimizes input-output losses and damage from biotic or abiotic factors compared to conventional systems, thereby contributing to food security amidst growing populations. Further comparative studies are needed to comprehend and justify the mechanisms involved in climate resilience of major crops within controlled and natural environments. Investigations focusing on quantitative traits and omics in crops through speed breeding under controlled conditions are warranted. Additionally, exploring more breeding techniques to evaluate qualitative and quantitative traits and generation times in crops within controlled environments is essential.
Author Contributions
# H.P., V.A.S.N.D., and V.M. contributed equally.
No funding was received for this study from any sponsor and for the collection of information and preparation of data or the manuscript.
Declaration of generative AI and AI-assisted technologies in the writing process. During the preparation of this manuscript, the authors used generative artificial intelligence chatGPT, version 3.5 for language improvement of the manuscript. After using this tool, all authors reviewed and edited the content as required and take responsibility for the content of the publication.
The authors declare no competing financial interest.
References
- Zhao Z.; Xu T.; Pan X.; Susanti; White J. C.; Hu X.; Miao Y.; Demokritou P.; Ng K. W. Sustainable nutrient substrates for enhanced seedling development in hydroponics. ACS Sustainable Chem. Eng. 2022, 10, 8506–8516. 10.1021/acssuschemeng.2c01668. [DOI] [Google Scholar]
- Sharma A.; Pandey H.; Nampoothiri Devadas V. A. S.; Kartha B. D.; Jha R. Production of factors affecting, gene regulations, and challenges in tissue cultured plant through soilless culture. J. Agric. Food Chem. 2023, 71, 5804–5811. 10.1021/acs.jafc.2c08162. [DOI] [PubMed] [Google Scholar]
- Mitchell C. A. History of controlled environment horticulture: Indoor farming and its key technologies. HortScience 2022, 57, 247–256. 10.21273/HORTSCI16159-21. [DOI] [Google Scholar]
- Sharma A.; Hazarika M.; Heisnam P.; Pandey H.; Devadas V. S.; Wangsu M. Controlled Environment Ecosystem: A plant growth system to combat climate change through soilless culture. Crop Design 2024, 3, 100044. 10.1016/j.cropd.2023.100044. [DOI] [Google Scholar]
- Thomson A.; Price G. W.; Arnold P.; Dixon M.; Graham T. Review of the potential for recycling CO2 from organic waste composting into plant production under controlled environment agriculture. Journal of Cleaner Production 2022, 333, 130051. 10.1016/j.jclepro.2021.130051. [DOI] [Google Scholar]
- Neo D. C. J.; Ong M. M. X.; Lee Y. Y.; Teo E. J.; Ong Q.; Tanoto H.; Xu J.; Ong K. S.; Suresh V. Shaping and tuning lighting conditions in controlled environment agriculture: A review. ACS Agricultural Science & Technology 2022, 2, 3–16. 10.1021/acsagscitech.1c00241. [DOI] [Google Scholar]
- Sharma A.; Manpoong C.; Devadas V. S.; Kartha B. D.; Pandey H.; Wangsu M. Crop Hydroponics, Phyto-hydroponics, Crop Production, and Factors Affecting Soilless Culture. ACS Agricultural Science & Technology 2022, 2, 1134–1150. 10.1021/acsagscitech.2c00243. [DOI] [Google Scholar]
- Fussy A.; Papenbrock J. An overview of soil and soilless cultivation techniques-chances, challenges and the neglected question of sustainability. Plants 2022, 11, 1153. 10.3390/plants11091153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumisiriza M. S.; Kabirizi J. M.; Mugerwa M.; Ndakidemi P. A.; Mbega E. R. Can soilless farming feed urban East Africa? An assessment of the benefits and challenges of hydroponics in Uganda and Tanzania. Environmental Challenges 2022, 6, 100413. 10.1016/j.envc.2021.100413. [DOI] [Google Scholar]
- Pathak H.; Pal S.. Climate Smart Agriculture: Concepts, Challenges, and Opportunities; Green Energy Technology series; Springer, 2020; Vol. 1, pp 1865–3529, 10.1007/978-981-15-9132-7. [DOI] [Google Scholar]
- Lal R. Climate change and soil degradation mitigation by sustainable management of soils and other natural resources. Agricultural Research 2012, 1, 199–212. 10.1007/s40003-012-0031-9. [DOI] [Google Scholar]
- Wang X. Managing land carrying capacity: key to achieving sustainable production systems for food security. Land 2022, 11, 484. 10.3390/land11040484. [DOI] [Google Scholar]
- De Pascale S.; Arena C.; Aronne G.; De Micco V.; Pannico A.; Paradiso R.; Rouphael Y. Biology and crop production in Space environments: Challenges and opportunities. Life Sciences in Space Research 2021, 29, 30–37. 10.1016/j.lssr.2021.02.005. [DOI] [PubMed] [Google Scholar]
- Ainsworth E. A.; Yendrek C. R.; Sitch S.; Collins W. J.; Emberson L. D. The effects of tropospheric ozone on net primary productivity and implications for climate change. Annual review of plant biology 2012, 63, 637–661. 10.1146/annurev-arplant-042110-103829. [DOI] [PubMed] [Google Scholar]
- Blom T.; Jenkins A.; Pulselli R. M.; van den Dobbelsteen A. A. J. F. The embodied carbon emissions of lettuce production in vertical farming, greenhouse horticulture, and open-field farming in the Netherlands. Journal of Cleaner Production 2022, 377, 134443. 10.1016/j.jclepro.2022.134443. [DOI] [Google Scholar]
- Koc E.; Karayigit B. Assessment of biofortification approaches used to improve micronutrient-dense plants that are a sustainable solution to combat hidden hunger. Journal of Soil Science and Plant Nutrition 2022, 22, 475–500. 10.1007/s42729-021-00663-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan S.; Darvishzadeh R.; Boschetti M.; Pepe M.; Nelson A. Remote sensing-based crop lodging assessment: Current status and perspectives. ISPRS journal of photogrammetry and remote sensing 2019, 151, 124–140. 10.1016/j.isprsjprs.2019.03.005. [DOI] [Google Scholar]
- Zafar T.; Mehra A.; Das P.; Shaik B.; Malik A. A. Demystifying the advanced interventions of genetics and modern breeding approaches for nutritional security and sustainability of neglected and underused crop species (NUCS). Genetic Resources and Crop Evolution 2023, 71, 1–19. 10.1007/s10722-023-01823-1. [DOI] [Google Scholar]
- Savvas D.; Gruda N. Application of soilless culture technologies in the modern greenhouse industry-A review. European Journal of Horticultural Science 2018, 83, 280–293. 10.17660/eJHS.2018/83.5.2. [DOI] [Google Scholar]
- Alyokhin A.; Nault B.; Brown B. Soil conservation practices for insect pest management in highly disturbed agroecosystems-a review. Entomologia Experimentalis et Applicata 2020, 168, 7–27. 10.1111/eea.12863. [DOI] [Google Scholar]
- Gaikwad D. J.; Maitra S. Hydroponics cultivation of crops. Protected Cultivation and Smart Agriculture 2020, 1, 279–287. 10.30954/NDP-PCSA.2020.31. [DOI] [Google Scholar]
- Turner E. R.; Luo Y.; Buchanan R. L. Microgreen nutrition, food safety, and shelf life: A 503 review. J. Food Sci. 2020, 85, 870–882. 10.1111/1750-3841.15049. [DOI] [PubMed] [Google Scholar]
- Ebert A. W. Sprouts and Microgreens-Novel Food Sources for Healthy Diets. Plants 505 2022, 11, 571. 10.3390/plants11040571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruda N. S. Advances in soilless culture and growing media in today’s horticulture-An Editorial. Agronomy 2022, 12, 2773. 10.3390/agronomy12112773. [DOI] [Google Scholar]
- Kumar V.; Singh J. Trends in hydroponics practice/technology in horticultural crops: A review. International Journal of Plant & Soil Science 2023, 35, 57–65. 10.9734/ijpss/2023/v35i22759. [DOI] [Google Scholar]
- Bethke J. A.; Lieth H.. Controlled Environment Agriculture. White Paper, University of California, Division of Agriculture and Natural Resources, 2020; pp 1–35. [Google Scholar]
- Kılıc P.; Erdal I.; Aktas H. Effect of different substrates on yield and fruit quality of tomato grown in soilless culture. Infrastruktura i Ekologia Terenów Wiejskich 2018, 2, 249–261. 10.14597/INFRAECO.2018.2.1.016. [DOI] [Google Scholar]
- Erdal I.; Aktas H.; Kucukyumuk Z.; Daler S.; Ozen O.; Gencer K.. Effects of different growing mediums on the nutrient concentration of egg plant in soilless culture. In Technological Advancement for Vibrant Agriculture; Rakshid A., Ed.; Athens Institute for Education and Research, 2014; Vol. 2, pp 417–424. [Google Scholar]
- Makhadmeh I. M.; Al-Tawaha A.; Edaroyati P.; Al-Karaki G.; Tawaha A. R. A.; Hassan S. A. Effects of different growth media and planting densities on growth of lettuce grown in a closed soilless system. Research on Crops 2017, 18, 294–298. 10.5958/2348-7542.2017.00050.X. [DOI] [Google Scholar]
- Santos Junior J. A.; Gheyi H. R. G; Filho D. H. G.; Soares F. A.; Dias N. D. S. Efficiency of water use in sunflower grown in hydroponic system under saline stress. Engenharia Agricola 2013, 33, 718–729. 10.1590/S0100-69162013000400011. [DOI] [Google Scholar]
- Tunio M. H.; Gao J.; Shaikh S. A.; Lakhiar I. A.; Qureshi W. A.; Solangi K. A.; Chandio F. A. Potato production in aeroponics: An emerging food growing system in sustainable agriculture for food security. Chilean journal of agricultural research 2020, 80, 118–132. 10.4067/S0718-58392020000100118. [DOI] [Google Scholar]
- Zakaria N. I.; Ismail M. R.; Awang Y.; Megat Wahab P. E.; Berahim Z. Effect of root restriction on the growth, photosynthesis rate, and source and sink relationship of chilli (Capsicum annuum L.) grown in soilless culture. BioMed. research international 2020 2020, 2020, 1–18. 10.1155/2020/2706937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oztekin G. B.; Uludag T.; Tuzel Y. Growing spinach (Spinacia oleracea L.) in a floating system with different concentrations of nutrient solution. Applied Ecology and Environmental Research 2018, 16, 3333–3350. 10.15666/aeer/1603_33333350. [DOI] [Google Scholar]
- Tan A. X.; Jun Y. S. Opportunities for Emerging Wastewater Phosphorus Recovery Technologies to Enable Circular Phosphorus Usage in Nontraditional Hydroponic Agriculture. ACS Agricultural Science & Technology 2023, 3, 318–321. 10.1021/acsagscitech.3c00060. [DOI] [Google Scholar]
- Islam A. F. M. S.; Hirai H.; Kitaya Y. Hydroponic cultivation of carrots using modified rockwool blocks. Journal of Applied Horticulture 2008, 10 (2), 132–136. 10.37855/jah.2008.v10i02.28. [DOI] [Google Scholar]
- El-Tanahy A. M. M.; Mahmoud S. H.; Abou-Hussein S. D.; Bakry M. O. Recent trends in the cultivation of vegetable crops: A review. Current Science International 2022, 11 (4), 439–455. [Google Scholar]
- Sapkota S.; Sapkota S.; Liu Z. Effects of nutrient composition and lettuce cultivar on crop production in hydroponic culture. Horticulturae 2019, 5, 72. 10.3390/horticulturae5040072. [DOI] [Google Scholar]
- Suhaimi M. Y.; Mohamad A. M.; Mahamud S.; Khadzir D. Effects of substrates on growth and yield of ginger cultivated using soilless culture. J. Trop. Agric. Food Sci. 2012, 40, 159–168. [Google Scholar]
- El Youssfi L.; Choukr-Allah R.; Santamaria P.; Montesano F.. Soilless closed cycle production of green bean (Phaseolus vulgaris L.) using subirrigation: effects on yield, fruit quality, substrate and nutrient solution parameters. In XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010), International Symposium; International Society for Horticultural Science, 2010; Vol. 938, pp 383–390. [Google Scholar]
- Perez-Millan R.; Camara-Zapata J. M.; Fernandez-Zapata J. C.; Simon-Grao S.; Alfosea-Simon M.; Zavala-Gonzalez E. A.; Shahid M. A.; Garcia-Sanchez F. Application of Biocat G, selenium, and chitosan to counteract the negative effects of Cd in broccoli plants grown in soilless culture. Agronomy 2022, 12, 1327. 10.3390/agronomy12061327. [DOI] [Google Scholar]
- Oliveira F. A; Freitas R. S; Oliveira M. K.; Santos S. T; Costa J. P. B.; Morais Neta H. M; Marques I. C.; Cordeiro C. J. X Electrical conductivity of the nutrient solution for soilless cultivation of kohlrabi. Horticultura Brasileira 2022, 40, 129–135. 10.1590/s0102-0536-20220201. [DOI] [Google Scholar]
- Bi G.; Scagel C. F.; Bryla D. R. Nitrogen Rate, irrigation frequency and volume differentially influence growth, flowering, and nutrient uptake of container-grown rhododendron during the following growing season. Horticulturae 2022, 8, 647. 10.3390/horticulturae8070647. [DOI] [Google Scholar]
- Heidari S.; Mortazavi S. N.; Reezi S.; Nikbakht A. The possibility of replacing the cocopeat by palm substrate to the soilless culture of Lily. J. Ornamental Plants 2022, 12, 11–29. [Google Scholar]
- Rakhman A. The growth of mustard using hydroponics and aquaponics systems. Journal of Agricultural Engineering 2016, 4, 245–254. 10.23960/jtep-l.v4i4.%25p. [DOI] [Google Scholar]
- Subramani T.; Gangaiah B.; Baskaran V.; Swain S. Effect of soilless growing media on yield and quality of tomato (Solanum lycopersicum L.) under tropical island condition. International Journal of Current Microbiology and Applied Sciences 2020, 9, 2084–2090. 10.20546/ijcmas.2020.905.239. [DOI] [Google Scholar]
- Orsini F.; Mezzetti M.; Fecondini M.; Michelon N.; Gianquinto G. Simplified substrate soilless culture for vegetable production in Trujillo, Peru. In II International Conference on Landscape and Urban Horticulture 2010, 881, 163–167. 10.17660/ActaHortic.2010.881.19. [DOI] [Google Scholar]
- Al-Far A.; Tadros M. J.; Makhadmeh I. Evaluation of different soilless media on growth, quality, and yield of cucumber (Cucumis sativus L.) grown under greenhouse conditions. Australian Journal of Crop Science 2019, 13 (8), 1388–1401. 10.21475/ajcs.19.13.08.p2122. [DOI] [Google Scholar]
- Chrysargyris A.; Xylia P.; Akinci G.; Moustakas K.; Tzortzakis N. Printed paper waste as an alternative growing medium component to produce Brassica seedlings under nursery conditions. Sustainability 2020, 12, 5992. 10.3390/su12155992. [DOI] [Google Scholar]
- Fatahian V.; Halim R.A.; Ahmad I.; Chua K.; Teh C.B.S.; Awang Y. Melon production using four hydroponic systems. Acta Horticulturae 2013, 1004, 85–92. 10.17660/ActaHortic.2013.1004.8. [DOI] [Google Scholar]
- Mazahreh N.; Nejatian A.; Mousa M. Effect of different growing medias on cucumber production and water productivity in soilless culture under UAE conditions. Int. Res. J. Agric. Sci. Soil Sci. 2015, 3, 131–138. [Google Scholar]
- Gungor F.; Yildirim E. Effect of different growing media on quality, growth and yield of pepper (Capsicum annum L.) under greenhouse conditions. Pak. J. Bot. 2013, 45 (5), 1605–1608. [Google Scholar]
- Raja W. H.; Kumawat K. L.; Sharma O. C.; Sharma A.; Mir J. I.; Nabi S. U. N.; Lal S.; Quereshi I. Effect of different substrates on growth and quality of Strawberry cv. Chandler in soilless culture. Pharma Innovation J. 2018, 7 (12), 449–453. [Google Scholar]
- Kharrazi M.; Sharifi A.; Nejatizadeh S.; Khadem A.; Moradian M. Selection of optimal cultivation media for Gerbera (Gerbera jamesonii) growth in the hydroponic culture system. J. Hortic. Sci. 2020, 34 (2), 261–271. [Google Scholar]
- Kahraman Ö.; Özzambak M. E.. The effects of different media on Galanthus elwesii Hook bulb performance in soilless culture. Afyon Kocatepe Univ. J. Sci. Eng. 2014, 14 ( (1), ). [Google Scholar]
- Karagoz F. P.; Dursun A.; Karasal M. A review: use of soilless culture techniques in ornamental plants. Ornamental Horticulture 2022, 28, 172–180. 10.1590/2447-536x.v28i2.2430. [DOI] [Google Scholar]
- Domingues Salvador E.; Minami K.. Evaluation of different substrates on Lisianthus (Eustoma grandiflorum Shinn) growth. In International Symposium on Growing Media and Hydroponics; 2001; Vol. 644, pp 217–223. 10.17660/ActaHortic.2004.644.29 [DOI]
- Thakur T. A. N. Y. A. Effect of soilless growing media compositions on quality flower production of potted Chrysanthemum (Chrysanthemum morifolium). Annals of Plant and Soil Research 2022, 24, 278–281. 10.47815/apsr.2022.10161. [DOI] [Google Scholar]
- Maboko M. M. Effect of plant density and harvesting frequency on yield components of hydroponically grown mustard spinach (Brassica juncea). Acta Horticultrae 2013, 1007, 515–521. 10.17660/ActaHortic.2013.1007.59. [DOI] [Google Scholar]
- Prakash S. Study on the role of microgreens in human life. Int. J. Chem. Stud. 2019, SP6, 419–421. [Google Scholar]
- Thakur N.; Verma A. K.; Kaur J.; Thakur C.; Aakriti A review on microgreens as an emerging food for health benefits. Annals of Phytomedicine: An International Journal 2022, 11 (1), 68–77. 10.54085/ap.2022.11.1.7. [DOI] [Google Scholar]
- Ayeni A. Nutrient content of micro/baby-green and field-grown mature foliage of tropical spinach (Amaranthus sp.) and Roselle (Hibiscus sabdariffa L.). Foods 2021, 10 (11), 2546. 10.3390/foods10112546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S.; Jasmin L. B.; Saravaiya S. N.. Microgreens: A new beginning towards nutrition and livelihood urban per-urban and rural continuum. In Technologies and Sustainability of Protected Cultivation for High-Valued Vegetable Crops; Kumar S.; Patel N. B.; Saravaiya S. N.; Patel B. N., Eds.; 2018, 246–251. [Google Scholar]
- Sharma S.; Shree B.; Sharma D.; Kumar S.; Kumar V.; Sharma R.; Saini R. Vegetable microgreens: The gleam of next generation super foods, their genetic enhancement, health benefits and processing approaches. Food Research International 2022, 155, 1–19. 10.1016/j.foodres.2022.111038. [DOI] [PubMed] [Google Scholar]
- Mir S. A.; Shah M. A.; Mir M. M. Microgreens: Production, shelf life, and bioactive components. Critical reviews in food science and nutrition 2017, 57, 2730–2736. 10.1080/10408398.2016.1144557. [DOI] [PubMed] [Google Scholar]
- Sinha K.; Khare V. Review on: Antinutritional factors in vegetable crops. Pharma Innovation Journal 2017, 6, 353–358. [Google Scholar]
- Ghehsareh A. M.; Hematian M.; Kalbasi M. Comparison of date-palm wastes and perlite as culture substrates on growing indices in greenhouse cucumber. International Journal of Recycling of Organic Waste in Agriculture 2012, 1, 1–4. 10.1186/2251-7715-1-5. [DOI] [Google Scholar]
- Di Gioia F.; De Bellis P.; Mininni C.; Santamaria P.; Serio F. Physicochemical, agronomical and microbiological evaluation of alternative growing media for the production of rapini (Brassica rapa L.) microgreens. Journal of the Science of Food and Agriculture 2017, 97, 1212–1219. 10.1002/jsfa.7852. [DOI] [PubMed] [Google Scholar]
- Polash M. A. S.; Sakil A.; Sazia S.; Hossain A. Selection of suitable growing media and nutritional assessment of microgreens. Agricultural Research Journal 2019, 56, 752–756. 10.5958/2395-146X.2019.00116.9. [DOI] [Google Scholar]
- Saleh R.; Gunupuru L. R.; Lada R.; Nams V.; Thomas R. H.; Abbey L. Growth and Biochemical Composition of Microgreens Grown in Different Formulated Soilless Media. Plants 2022, 11, 3546. 10.3390/plants11243546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal D.; Mainani R.; Thakker R.; Solanki H. A study of selected microgreens in soilless media. International association of biologicals and computational digest 2022, 1, 228. 10.56588/iabcd.v1i2.74. [DOI] [Google Scholar]
- Dalal D.; Mainani R.; Thakker R.; Solanki H. A study of selected microgreens in soilless media. International association of biologicals and computational digest 2022, 1, 1–3. 10.56588/iabcd.v1i2.74. [DOI] [Google Scholar]
- Eswaranpillai U.; Murugesan P.; Karuppiah P. Assess the impact of cultivation substrates for growing sprouts and microgreens of selected four legumes and two grains and evaluation of its nutritional properties. Plant Science Today 2022, 10, 1–10. 10.14719/pst.2058. [DOI] [Google Scholar]
- Parul P.; Viveka K.; Anupama S.; Neelam S. Role of Substrate Media in Growth and Development of Selected Microgreens. Biol. Forum-An Int. J. 2022, 14, 1357–1361. [Google Scholar]
- Chandiposha M.; Takadini T. Effects of Different Soilless Growing Media on the Growth and Development of Tobacco Seedlings. International Journal of Agronomy 2022, 2022, 1–8. 10.1155/2022/9596945. [DOI] [Google Scholar]
- Li T.; Lalk G. T.; Arthur J. D.; Johnson M. H.; Bi G. Shoot production and mineral nutrients of five microgreens as affected by hydroponic substrate type and post-emergent fertilization. Horticulturae 2021, 7 (6), 129. 10.3390/horticulturae7060129. [DOI] [Google Scholar]
- Donielle A. N.Effects of seed density and other factors on the yield of microgreens grown hydroponically on burlap. Master of Agricultural and Life Sciences, Plant Science and Pest Management, School of Plant and Environmental Sciences, Virginia Tech, USA, 2018; pp 1–44. [Google Scholar]
- Tavan M.; Wee B.; Brodie G.; Fuentes S.; Pang A.; Gupta D. Optimizing sensor-based irrigation management in a soilless vertical farm for growing microgreens. Frontiers in Sustainable Food Systems 2021, 4, 1–21. 10.3389/fsufs.2020.622720. [DOI] [Google Scholar]
- Ciuta F.; Arghir L. D.; Tudor C. A.; Lagunovschi-Luchian V. Research on microgreens farming in vertical hydroponic system. J. Hortic., Forestry Biotechnol. 2020, 24, 27–34. [Google Scholar]
- Ritonga S.; Vinolina N. S.. Charloq Response of wheat grass and rice grass growth on various types of planting media. IOP Conference Series: Earth and Environmental Science; IOP, 2022; Vol. 977, pp 1–7.
- Benis K.; Ferrão P. Commercial farming within the urban built environment-taking stock of an evolving field in northern countries. Global food security 2018, 17, 30–37. 10.1016/j.gfs.2018.03.005. [DOI] [Google Scholar]
- Lazzarin M.; Meisenburg M.; Meijer D.; van Ieperen W.; Marcelis L.F.M.; Kappers I.F.; van der Krol A.R.; van Loon J.J.A.; Dicke M. LEDs make it resilient: effects on plant growth and defense. Trends in Plant Science 2021, 26, 496–508. 10.1016/j.tplants.2020.11.013. [DOI] [PubMed] [Google Scholar]
- Lei J. Reforming the natural resource auditing system from the ecological civilization perspective. Chinese Journal of Population, Resources and Environment 2020, 18, 87–96. 10.1016/j.cjpre.2019.01.001. [DOI] [Google Scholar]
- Murphy C. J.; Llort K. F.; Pill W. G. Factors affecting the growth of microgreen table beet. International journal of vegetable science 2010, 16, 253–266. 10.1080/19315261003648241. [DOI] [Google Scholar]
- Assefa G.; Urge M.; Animut G.; Assefa G. Effect of variety and seed rate on hydroponic maize fodder biomass yield, chemical composition, and water use efficiency. Biotechnology in Animal Husbandry 2020, 36, 87–100. 10.2298/BAH2001087A. [DOI] [Google Scholar]
- Bdr M. F. Growth and production of various wheat genotypes at various PEG concentration in hydroponic. Agrotech Journal 2018, 3, 21–26. 10.31327/atj.v3i1.521. [DOI] [Google Scholar]
- Grigas A.; Kemzuraitė A.; Steponavičius D.; Steponavičieṅė A.; Domeika R. Impact of slope of growing trays on productivity of wheat green fodder by a nutrient film technique system. Water 2020, 12, 3009. 10.3390/w12113009. [DOI] [Google Scholar]
- Chrisdiana R. Quality and quantity of sorghum hydroponic fodder from different varieties and harvest time. In IOP Conference Series: Earth and Environmental Science, IOP Publishing 2018, 119, 012014. 10.1088/1755-1315/119/1/012014. [DOI] [Google Scholar]
- Gunasekaran S.; Valli C.; Karunakaran R.; Gopi H.; Gnanaraj P. T. Evaluation of growth period and water and light requirement for optimum production of hydroponic maize and horse gram fodder. Organic Agriculture 2022, 12, 75–80. 10.1007/s13165-021-00373-z. [DOI] [Google Scholar]
- Iijima M.; Hirooka Y.; Kawato Y.; Watanabe Y.; Wada K. C.; Shinohara N.; Nanhapo P. I.; Wanga M. A.; Yamane K. Short-term evaluation of oxygen transfer from rice (Oryza sativa) to mixed planted drought-adapted upland crops under hydroponic culture. Plant Production Science 2017, 20, 434–440. 10.1080/1343943X.2017.1379882. [DOI] [Google Scholar]
- Jolad R.; Sivakumar S. D.; Babu C.; Sritharan N. Performance of Different Crops under Hydroponics Fodder Production System. Madras Agricultural Journal. 2018, 105 (1-3), 50. 10.29321/MAJ.2018.000101. [DOI] [Google Scholar]
- Kide W.; Abrha R. Yield and nutritive value of barley (Hordeum vilgari L.) fodder grown in soil and hydroponic system. Int. J. Life Sci. Res. 2016, 3, 194–197. [Google Scholar]
- Murthy A. K.; Dhanalakshmi G.; Chakravarthy K. Study on performance of different fodder crops under low cost green house hydroponic fodder production system. International Journal of Environment, Agriculture and Biotechnology 2017, 2, 951–953. 10.22161/ijeab/2.2.50. [DOI] [Google Scholar]
- Sinchire D. B. M.; Álvarez L. S. J.; Valdivieso J. I. B.; Mora E. D. C. Oat and wheat forage production under hydroponic and conventional systems. Cienc. Tecnol. Agropecu 2020, 21, 1–16. 10.21930/rcta.vol21_num3_art:1386. [DOI] [Google Scholar]
- Snow A. M.; Ghaly A. E.; Snow A. A comparative assessment of hydroponically grown cereal crops for the purification of aquaculture wastewater and the production of fish feed. American Journal of Agricultural and Biological Sciences 2008, 3, 364–378. 10.3844/ajabssp.2008.364.378. [DOI] [Google Scholar]
- Upreti S.; Ghimire R. P.; Banskota N. Comparison of different cereal grains for hydroponic fodder production in locally constructed polyhouse at Khumaltar, Lalitpur, Nepal. Journal of Agriculture and Natural Resources 2022, 5, 27–33. 10.3126/janr.v5i1.50378. [DOI] [Google Scholar]
- Zainab S. M.; Iram S.; Ahmad K. S.; Gul M. M. Nutritional composition and yield comparison between hydroponically grown and commercially available Zea mays L. fodder for a sustainable livestock production. Maydica 2019, 29, 1–10. [Google Scholar]
- Hasan M.; Sabir N.; Singh A. K.; Singh M. C.; Patel N.; Khanna M.; Pragnya P.. Hydroponics technology for horticultural crops. Technical Bulletin, Centre for Protected Cultivation Technology; ICAR-Indian Agricultural Research Institute, New Delhi, 2018; Vol. 188. pp 1–31.
- Rajendran S.; Domalachenpa T.; Arora H.; Li P.; Sharma A.; Rajauria G. Hydroponics: Exploring innovative sustainable technologies and applications across crop production, with Emphasis on potato mini-tuber cultivation. Heliyon 2024, 10, e26823. 10.1016/j.heliyon.2024.e26823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa R. M.; Pinto J. E. B. P.; Faquin V.; Pinto C. A. B. P.; Reis E. S. The production of seed potatoes by hydroponic methods in Brazil. Fruit, Vegetable Cereal Sci. Biotechnol. 2009, 3, 133–139. [Google Scholar]
- Raja W. H.; Kumawat K. L.; Sharma O. C.; Sharma A.; Mir J. I.; Nabi S. U. N.; Lal S.; Quereshi I. Effect of different substrates on growth and quality of strawberry cv. Chandler in soilless culture. Pharma Innovation J. 2018, 7 (12), 449–453. [Google Scholar]
- Santos Junior J. A.; Gheyi H. R. G; Filho D. H. G.; Soares F. A.; Dias N. D. S. Efficiency of water use in sunflower grown in hydroponic system under saline stress. Engenharia Agrícola 2013, 33, 718–729. 10.1590/S0100-69162013000400011. [DOI] [Google Scholar]
- Johnson S. A; Prenni J. E; Heuberger A. L; Isweiri H.; Chaparro J. M; Newman S. E; Uchanski M. E; Omerigic H. M; Michell K. A; Bunning M.; Foster M. T; Thompson H. J; Weir T. L Comprehensive evaluation of metabolites and minerals in 6 microgreen species and the influence of maturity. Current developments in nutrition 2021, 5, nzaa180. 10.1093/cdn/nzaa180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altuner F.; Tuncturk R.; Oral E.; Tuncturk M. Evaluation of pigment, antioxidant capacity and bioactive compounds in microgreens of wheat landraces and cereals. Chilean journal of agricultural research 2021, 81, 643–654. 10.4067/S0718-58392021000400643. [DOI] [Google Scholar]
- Dhaka A. S.; Dikshit H. K.; Mishra G. P.; Tontang M. T.; Meena N. L.; Kumar R. R.; Ramesh S. V.; Narwal S.; Aski M.; Thimmegowda V.; Gupta S.; Nair R. M.; Praveen S. Evaluation of growth conditions, antioxidant potential, and sensory attributes of six diverse microgreens species. Agriculture 2023, 13, 676. 10.3390/agriculture13030676. [DOI] [Google Scholar]
- Katsenios N.; Christopoulos M. V.; Kakabouki I.; Vlachakis D.; Kavvadias V.; Efthimiadou A. Effect of pulsed electromagnetic field on growth, physiology and postharvest quality of kale (Brassica oleracea), wheat (Triticum durum) and spinach (Spinacia oleracea) microgreens. Agronomy 2021, 11, 1364. 10.3390/agronomy11071364. [DOI] [Google Scholar]
- Drygaś B.; Depciuch J.; Puchalski C. Effect of Ascophyllum nodosum Alga Application on Microgreens, Yield, and Yield Components in Oats Avena sativa L. Agronomy 2021, 11, 1446. 10.3390/agronomy11071446. [DOI] [Google Scholar]
- Di Gioia F.; Hong J. C.; Pisani C.; Petropoulos S. A.; Bai J.; Rosskopf E. N. Yield performance, mineral profile, and nitrate content in a selection of seventeen microgreen species. Frontiers in Plant Science 2023, 14, 1–14. 10.3389/fpls.2023.1220691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puccinelli M.; Maggini R.; Angelini L. G.; Santin M.; Landi M.; Tavarini S.; Castagna A.; Incrocci L. Can Light Spectrum Composition Increase Growth and Nutritional Quality of Linum usitatissimum L. Sprouts and Microgreens?. Horticulturae 2022, 8, 98. 10.3390/horticulturae8020098. [DOI] [Google Scholar]
- Thepsilvisut O.; Sukree N.; Chutimanukul P.; Athinuwat D.; Chuaboon W.; Poomipan P.; Vachirayagorn V.; Pimpha N.; Chutimanukul P.; Ehara H. Efficacy of Agricultural and Food Wastes as the Growing Media for Sunflower and Water Spinach Microgreens Production. Horticulturae 2023, 9, 876. 10.3390/horticulturae9080876. [DOI] [Google Scholar]
- Durairajan M. B.; Sundararajan V. V.; Kannan G.; Paul B. M.; Muniyandi K.; Thangaraj P. Elicitation of nutritional, antioxidant, and antidiabetic potential of barnyard millet (Echinochloa esculenta (A. Braun) H. Scholz) sprouts and microgreens through in vitro bio-accessibility assessment. Food Chem. 2024, 441, 138282. 10.1016/j.foodchem.2023.138282. [DOI] [PubMed] [Google Scholar]
- Poudel P.; Connolly E. L.; Kwasniewski M.; Lambert J. D.; Di Gioia F. Zinc biofortification via fertigation using alternative zinc sources and concentration levels in pea, radish, and sunflower microgreens. Scientia Horticulturae 2024, 331, 113098. 10.1016/j.scienta.2024.113098. [DOI] [Google Scholar]
- Li Y.; Zhou B.; Teng Z.; Zhang M.; Yu L.; Luo Y.; Chen P.; Sun J. Improved metabolomic approach for evaluation of phytochemicals in mustard, kale, and broccoli microgreens under different controlled environment agriculture conditions. Journal of Agriculture and Food Research 2023, 14, 100719. 10.1016/j.jafr.2023.100719. [DOI] [Google Scholar]
- Alrifai O.The Effect of Light-Emitting Diodes on Phytochemical Synthesis in Brassica Microgreens Grown in Controlled Environments. Doctoral dissertation, Food Science, University of Guelph, Guelph, Canada, 2021, pp 1–260. [Google Scholar]
- Negri M.; Bulgari R.; Santoro P.; Ferrante A.. Evaluation of different growing substrates for microgreens production. In III International Symposium on Growing Media, Composting and Substrate Analysis; International Society for Horticultural Science, 2019; Vol. 1305, pp 109–114.
- Hummerick M.; Curry A.; Gooden J.; Spern C.; Spencer L.; Romeyn M.; Fischer J.. The microbiology of microgreens grown in controlled environment chambers under ISS conditions. 51st International Conference on Environmental Systems; International Conference on Environmental Systems, 2022; pp 1–11.
- Toscano S.; Cavallaro V.; Ferrante A.; Romano D.; Patane C. Effects of different light spectra on final biomass production and nutritional quality of two microgreens. Plants 2021, 10, 1584. 10.3390/plants10081584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang G. M.; Vu T. T. Selection of suitable growing substrates and quality assessment of Brassica Microgreens cultivated in greenhouse. Academia Journal of Biology 2022, 44 (2), 133–142. 10.15625/2615-9023/16833. [DOI] [Google Scholar]
- Jones-Baumgardt C.The Use of Light-Emitting Diodes for Microgreen Production in Controlled Environments. Masters dissertation 2019, Environmental Sciences; University of Guelph, Canada, pp 1–120. [Google Scholar]
- Ferentinos K. P.; Albright L. D. Fault detection and diagnosis in deep-trough hydroponics using intelligent computational tools. Biosystems Engineering 2003, 84 (1), 13–30. 10.1016/S1537-5110(02)00232-5. [DOI] [Google Scholar]
- Neo D. C. J.; Ong M. M. X.; Lee Y. Y.; Teo E. J.; Ong Q.; Tanoto H.; Xu J.; Ong K. S.; Suresh V. Shaping and tuning lighting conditions in controlled environment agriculture: A review. ACS Agricultural Science & Technology 2022, 2, 3–16. 10.1021/acsagscitech.1c00241. [DOI] [Google Scholar]
- Dungait J. A.; Hopkins D. W.; Gregory A. S.; Whitmore A. P. Soil organic matter turnover is governed by accessibility not recalcitrance. Global Change Biology 2012, 18, 1781–1796. 10.1111/j.1365-2486.2012.02665.x. [DOI] [Google Scholar]
- Shi K.; Zhang Y.; Zhou Y.; Liu X.; Zhu G.; Qin B.; Gao G. Long-term MODIS observations of cyanobacterial dynamics in Lake Taihu: Responses to nutrient enrichment and meteorological factors. Sci. Rep. 2017, 7, 1–16. 10.1038/srep40326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingues D. S.; Takahashi H. W.; Camara C. A. P.; Nixdorf S. L. Automated system developed to control pH and concentration of nutrient solution evaluated in hydroponic lettuce production. Computers and Electronics in Agriculture 2012, 84, 53–61. 10.1016/j.compag.2012.02.006. [DOI] [Google Scholar]
- Alipo M. I.; Cruz A. E. M. D.; Doria J. D. A.; Fruto R. M. S. On the design of nutrient film technique hydroponics farm for smart agriculture. Engineering in Agriculture, Environment and Food 2019, 12, 315–324. 10.1016/j.eaef.2019.02.008. [DOI] [Google Scholar]
- Maldonado A. I. L.; Reyes J. M. M.; Breceda H. F.; Fuentes H. R.; Contreras J. A. V.; Maldonado U. L.. Automation and robotics used in hydroponic system. Urban Horticulture-Necessity of the Future. IntechOpen 2019, 1–25. [Google Scholar]
- Arduino . Available online: http://www.Arduino.cc/en/Main/Products (accessed on 15 February 2023).
- Crisnapati P. N.; Wardana I. N. K.; Aryanto I. K. A. A.; Hermawan A.. Hommons: Hydroponic management and monitoring system for an IOT based NFT farm using web technology. In 5th International Conference on Cyber and IT Service Management (CITSM); IEEE, 2017; pp 1–6.
- Ferentinos K. P.; Albright L. D. Predictive neural network modeling of pH and electrical conductivity in deep-trough hydroponics. Transactions of ASAE 2002, 45 (6), 2007. 10.13031/2013.11412. [DOI] [Google Scholar]
- Ghiat I.; Mackey H. R.; Al-Ansari T. A review of evapotranspiration measurement models, techniques and methods for open and closed agricultural field applications. Water 2021, 13, 2523. 10.3390/w13182523. [DOI] [Google Scholar]
- Bao J.; Lu W. H.; Zhao J.; Bi X. T. Greenhouses for CO2 sequestration from atmosphere. Carbon Resources Conversion 2018, 1, 183–190. 10.1016/j.crcon.2018.08.002. [DOI] [Google Scholar]
- Willits D. S. Cooling fan ventilated greenhouses: A modelling study. Biosystems Engineering 2003, 84 (3), 315–329. 10.1016/S1537-5110(02)00270-2. [DOI] [Google Scholar]
- Max J. F.J.; Horst W. J.; Mutwiwa U. N.; Tantau H.-J. Effects of greenhouse cooling method on growth, fruit yield and quality of tomato (Solanum lycopersicum L.) in a tropical climate. Scientia Horticulturae 2009, 122 (2), 179–186. 10.1016/j.scienta.2009.05.007. [DOI] [Google Scholar]
- Elkhlifi Z.; Iftikhar J.; Sarraf M.; Ali B.; Saleem M. H.; Ibranshahib I.; Bispo M. D.; Meili L.; Ercisli S.; Torun Kayabasi E.; Alemzadeh Ansari N.; Hegedusova A.; Chen Z. Potential role of biochar on capturing soil nutrients, carbon sequestration and managing environmental challenges: a review. Sustainability 2023, 15, 2527. 10.3390/su15032527. [DOI] [Google Scholar]
- Cho W. J.; Gang M. S.; Kim D. W.; Kim J.; Jung D. H.; Kim H. J. Decision-tree-based ion-specific dosing algorithm for enhancing closed hydroponic efficiency and reducing carbon emissions. Frontiers in Plant Science 2023, 14, 1–12. 10.3389/fpls.2023.1301490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaewwiset T.; Yooyativong T.. Estimation of electrical conductivity and pH in hydroponic nutrient mixing system using Linear Regression algorithm. In 2017 International Conference on Digital Arts, Media and Technology; IEEE, 2017; pp 1–5.
- Mathieu J.; Linker R.; Levine L.; Albright L.; Both A.J.; Spanswick R.; Wheeler R.; Wheeler E.; deVilliers D.; Langhans R. Evaluation of the Nicolet model for simulation of short-term hydroponic lettuce growth and nitrate uptake. Biosystems engineering 2006, 95, 323–337. 10.1016/j.biosystemseng.2006.07.006. [DOI] [Google Scholar]
- Pakzad A. E.; Pakzad A. A. Fuzzy ponics: A fuzzy based system to control temperature, relative humidity, electrical conductivity and PH of hydroponics. Int. J. Eng. Technol. 2018, 7, 308–312. [Google Scholar]
- Lakhiar I. A.; Gao J.; Syed T. N.; Chandio F. A.; Buttar N. A. Modern plant cultivation technologies in agriculture under controlled environment: A review on aeroponics. Journal of plant interactions 2018, 13, 338–352. 10.1080/17429145.2018.1472308. [DOI] [Google Scholar]
- Mokhtar A.; El-Ssawy W.; He H.; Al-Anasari N.; Sammen S. S.; Gyasi-Agyei Y.; Abuarab M. Using machine learning models to predict hydroponically grown lettuce yield. Frontiers in Plant Science 2022, 13, 1–10. 10.3389/fpls.2022.706042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhardiyanto H.; Arif C.; Setiawan B. I. Optimization of EC values of nutrient solution for tomato fruits quality in hydroponics system using artificial neural network and genetic algorithms. ITB Journal of Science 2009, 41, 38–49. 10.5614/itbj.sci.2009.41.1.3. [DOI] [Google Scholar]
- Saraswathy D. V.; Nithiesh C.; Kumaravel S. P.; Ruphasri S. Integrating Intelligence in Hydroponic Farms. International Journal of Electrical Engineering and Technology 2020, 11 (4), 150–158. 10.34218/IJEET.11.4.2020.017. [DOI] [Google Scholar]
- Lee J. W.; Moon T.; Son J. E. Development of growth estimation algorithms for hydroponic bell peppers using recurrent neural networks. Horticulturae 2021, 7, 284. 10.3390/horticulturae7090284. [DOI] [Google Scholar]
- Hernandez E.; Timmons M. B.; Mattson N. S. Quality, yield, and biomass efficacy of several hydroponic lettuce (Lactuca sativa L.) cultivars in response to high pressure sodium lights or light emitting diodes for greenhouse supplemental lighting. Horticulturae 2020, 6, 7. 10.3390/horticulturae6010007. [DOI] [Google Scholar]
- Helmi S.; Ariana S.; Supardin L. The role of brand image as mediation of the effect of advertising and sales promotion on customer purchase decisions. Journal of Economics and Sustainable Development 2022, 13 (8), 90–99. 10.7176/JESD/13-8-09. [DOI] [Google Scholar]
- Yumeina D.; Aji G. K.; Morimoto T. Dynamic optimization of water temperature for maximizing leaf water content of tomato in hydroponics using an intelligent control technique. In V International Symposium on Applications of Modelling as an Innovative Technology in the Horticultural SuActa Hortic.pply Chain-Model-ITActa Hortic.Acta Hortic.\\\\\\\\\\\\\\\\\\\\\\\ 2017, 1154, 55–64. 10.17660/ActaHortic.2017.1154.8. [DOI] [Google Scholar]
- Morimoto T.; Torii T.; Hashimoto Y. Computer integrated system for plant factory-Application of optimal regulator and genetic algorithm to the optimal control of physiological processes of plant. IFAC Proceedings 1993, 26, 1165–1168. 10.1016/S1474-6670(17)48655-1. [DOI] [Google Scholar]
- Oide M.; Nakasako M. Red light induced structure changes in phytochrome A from Pisum sativum. Sci. Rep. 2021, 11, 2827. 10.1038/s41598-021-82544-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann K. M. Phytochrome 730 (Pfr), the effector of the “high energy photomorphogenic reaction” in the far-red region. Naturwissenschaften 1967, 54, 544. 10.1007/BF00627231. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Folta K. M. Contributions of green light to plant growth and development. American Journal of Botany 2013, 100 (1), 70–78. 10.3732/ajb.1200354. [DOI] [PubMed] [Google Scholar]
- Ustin S. L.; Jacquemoud S.. How the optical properties of leaves modify the absorption and scattering of energy and enhance leaf functionality. In Remote Sensing of Plant Biodiversity; Springer, 2020; pp 349–384. [Google Scholar]
- Singh S.; Agrawal S. B.; Agrawal M. UVR8 mediated plant protective responses under low UV-B radiation leading to photosynthetic acclimation. Journal of Photochemistry and Photobiology B: Biology 2014, 137, 67–76. 10.1016/j.jphotobiol.2014.03.026. [DOI] [PubMed] [Google Scholar]
- Grishchenko O. V.; Subbotin E. P.; Gafitskaya I. V.; Vereshchagina Y. V.; Burkovskaya E. V.; Khrolenko Y. A.; Grigorchuk V. P.; Nakonechnaya O. V.; Bulgakov V. P.; Kulchin Y. N. Growth of micropropagated Solanum tuberosum L. plantlets under artificial solar spectrum and different mono-and polychromatic LED lights. Horticultural Plant Journal 2022, 8, 205–214. 10.1016/j.hpj.2021.04.007. [DOI] [Google Scholar]
- Di Q.; Li J.; Du Y.; Wei M.; Shi Q.; Li Y.; Yang F. Combination of red and blue lights improved the growth and development of eggplant (Solanum melongena L.) seedlings by regulating photosynthesis. Journal of Plant Growth Regulation 2021, 40, 1477–1492. 10.1007/s00344-020-10211-3. [DOI] [Google Scholar]
- Gao S.; Wang K.; Li N.; Lv Y.; Cao B.; Chen Z.; Xu K. The growth and photosynthetic responses of white LEDs with supplemental blue light in green onion (Allium fistulosum L.) unveiled by Illumina and single-molecule real-time (SMRT) RNA-sequencing. Environmental and Experimental Botany 2022, 197, 104835. 10.1016/j.envexpbot.2022.104835. [DOI] [Google Scholar]
- Rahman M. H.; Azad M. O. K.; Islam M. J.; Rana M. S.; Li K. H.; Lim Y. S. Production of Potato (Solanum tuberosum L.) Seed Tuber under Artificial LED Light Irradiation in Plant Factory. Plants 2021, 10, 297. 10.3390/plants10020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem M. H.; Rehman M.; Zahid M.; Imran M.; Xiang W.; Liu L. Morphological changes and antioxidative capacity of jute (Corchorus capsularis, Malvaceae) under different color light-emitting diodes. Brazilian Journal of Botany 2019, 42, 581–590. 10.1007/s40415-019-00565-8. [DOI] [Google Scholar]
- Saleem M.H.; Rehman M.; Fahad S.; Tung S.A.; Iqbal N.; Hassan A.; Ayub A.; Wahid M.A.; Shaukat S.; Liu L.; Deng G. Leaf gas exchange, oxidative stress, and physiological attributes of rapeseed (Brassica napus L.) grown under different light-emitting diodes. Photosynthetica 2020, 58 (3), 836. 10.32615/ps.2020.010. [DOI] [Google Scholar]
- Di Q.; Li J.; Du Y.; Wei M.; Shi Q.; Li Y.; Yang F. Combination of red and blue lights improved the growth and development of eggplant (Solanum melongena L.) seedlings by regulating photosynthesis. Journal of Plant Growth Regulation 2021, 40, 1477–1492. 10.1007/s00344-020-10211-3. [DOI] [Google Scholar]
- Chen L.; Xue X.; Yang Y.; Chen F.; Zhao J.; Wang X.; Khan A. T.; Hu Y. Effects of red and blue LEDs on in vitro growth and microtuberization of potato single-node cuttings. Frontiers of Agricultural Science and Engineering 2018, 5, 197–205. 10.15302/J-FASE-2018224. [DOI] [Google Scholar]
- Gao S.; Liu X.; Liu Y.; Cao B.; Chen Z.; Xu K. Comparison of the effects of LED light quality combination on growth and nutrient accumulation in green onion (Allium fistulosum L.). Protoplasma 2021, 258, 753–763. 10.1007/s00709-020-01593-y. [DOI] [PubMed] [Google Scholar]
- Gajc-Wolska J.; Kowalczyk K.; Przybysz A.; Mirgos M.; Orliński P. Photosynthetic efficiency and yield of cucumber (Cucumis sativus L.) grown under HPS and LED lighting in autumn-winter cultivation. Plants 2021, 10, 2042. 10.3390/plants10102042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutte G. W.; Edney S.; Skerritt T. Photoregulation of bioprotectant content of red leaf lettuce with light-emitting diodes. HortScience 2009, 44, 79–82. 10.21273/HORTSCI.44.1.79. [DOI] [Google Scholar]
- Li Q.; Kubota C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environmental and Experiment Botany 2009, 67, 59–64. 10.1016/j.envexpbot.2009.06.011. [DOI] [Google Scholar]
- Tarakanov I.; Yakovleva O.; Konovalova I.; Paliutina G.; Anisimov A. 2012. Light-emitting diodes: on the way to combinatorial lighting technologies for basic research and crop production. Acta Horticulturae 2012, 956, 171–178. 10.17660/ActaHortic.2012.956.17. [DOI] [Google Scholar]
- Mizuno T.; Amaki W.; Watanabe H. Effects of monochromatic light irradiation by LED on the growth and anthocyanin contents in leaves of cabbage seedlings. Acta Horticulturae 2011, 907, 179–184. 10.17660/ActaHortic.2011.907.25. [DOI] [Google Scholar]
- Lefsrud M. G.; Kopsell D. A.; Sams C. E. Irradiance from distinct wavelength light-emitting diodes affect secondary metabolites in kale. HortScience 2008, 43, 2243–2244. 10.21273/HORTSCI.43.7.2243. [DOI] [Google Scholar]
- Li Q.; Kubota C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environmental and Experiment Botany 2009, 67, 59–64. 10.1016/j.envexpbot.2009.06.011. [DOI] [Google Scholar]
- Samuolienė G.; Urbonaviciutė A.; Duchovskis P.; Bliznikas Z.; Vitta P.; Zukauskas A. Decrease in nitrate concentration in leafy vegetables under a solid-state illuminator. HortScience 2009, 44, 1857–1860. 10.21273/HORTSCI.44.7.1857. [DOI] [Google Scholar]
- Žukauskas A.; Bliznikas Z.; Breivė K.; Novičkovas A.; Samuolienė G.; Urbonavičiutė A.; Brazaitytė A.; Jankauskienė J.; Duchovskis P. Effect of supplementary pre-harvest LED lighting on the antioxidant properties of lettuce cultivars. Acta Horticulturae 2011, 907, 87–90. 10.17660/ActaHortic.2011.907.8. [DOI] [Google Scholar]
- Samuolienė G.; Sirtautas R.; Brazaitytė A.; Viršilė A.; Duchovskis P. Supplementary red-LED lighting and the changes in phytochemical content of two baby leaf lettuce varieties during three seasons. Journal of Food, Agriculture and Environment 2012, 10, 701–706. [Google Scholar]
- Samuolienė G.; Brazaitytė A.; Sirtautas R.; Novičkovas A.; Duchovskis P. Supplementary red-LED lighting affects phytochemicals and nitrate of baby leaf lettuce. Journal of Food, Agriculture and Environment 2011, 9, 271–274. [Google Scholar]
- Bliznikas Z.; Žukauskas A.; Samuolienė G.; Viršilė A.; Brazaitytė A.; Jankauskienė J.; Duchovskis P.; Novičkovas A. 2012. Effect of supplementary pre-harvest LED lighting on the antioxidant and nutritional properties of green vegetables. Acta Horticulturae 2012, 939, 85–91. 10.17660/ActaHortic.2012.939.10. [DOI] [Google Scholar]
- Samuolienė G.; Brazaitytė A.; Sirtautas R.; Novičkovas A.; Duchovskis P. The effect of supplementary LED lighting on the antioxidant and nutritional properties of lettuce. Acta Horticulturae 2012, 952, 835–841. 10.17660/ActaHortic.2012.952.106. [DOI] [Google Scholar]
- Samuolienė G.; Sirtautas R.; Brazaitytė A.; Duchovskis P. LED lighting and seasonality effects the antioxidant properties of baby leaf lettuce. Food Chem. 2012, 134, 1494–1499. 10.1016/j.foodchem.2012.03.061. [DOI] [PubMed] [Google Scholar]
- Lefsrud M. G.; Kopsell D. A.; Sams C. E. Irradiance from distinct wavelength light-emitting diodes affects secondary metabolites in kale. HortScience 2008, 43, 2243–2244. 10.21273/HORTSCI.43.7.2243. [DOI] [Google Scholar]
- Johkan M.; Shoji K.; Goto F.; Hashida S.-n.; Yoshihara T. 2010. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. 10.21273/HORTSCI.45.12.1809. [DOI] [Google Scholar]
- Stutte G. W.; Edney S.; Skerritt T. Photoregulation of bioprotectant content of red leaf lettuce with light-emitting diodes. HortScience 2009, 44, 79–82. 10.21273/HORTSCI.44.1.79. [DOI] [Google Scholar]
- Li H.; Tang C.; Xu Z.; Liu X.; Han X. Effects of different light sources on the growth of non-heading Chinese cabbage (Brassica campestris L.). Journal of Agricultural Science 2012, 4, 262–273. 10.5539/jas.v4n4p262. [DOI] [Google Scholar]
- Sharma A.; Hazarika M.; Heisnam P.; Pandey H.; Devadas V. S.; Wangsu M.; Kartha B. D. Factors affecting production, nutrient translocation mechanisms, and LED emitted light in growth of microgreen plants in soilless culture. ACS Agricultural Science & Technology 2023, 3, 701–719. 10.1021/acsagscitech.3c00260. [DOI] [Google Scholar]
- Cohen A. R.; Chen G.; Berger E. M.; Warrier S.; Lan G.; Grubert E.; Dellaert F.; Chen Y. Dynamically controlled environment agriculture: Integrating machine learning and mechanistic and physiological models for sustainable food cultivation. ACS ES&T Engineering 2022, 2, 3–19. 10.1021/acsestengg.1c00269. [DOI] [Google Scholar]
- Naikoo M. I.; Dar M. I.; Raghib F.; Jaleel H.; Ahmad B.; Raina A.; Khan F. A.; Naushin F. Role and regulation of plants phenolics in abiotic stress tolerance: An overview. Plant signaling molecules 2019, 157–168. 10.1016/B978-0-12-816451-8.00009-5. [DOI] [Google Scholar]
- Abeed A. H. A.; Saleem M. H.; Asghar M. A.; Mumtaz S.; Ameer A.; Ali B.; Alwahibi M. S.; Elshikh M. S.; Ercisli S.; Elsharkawy M. M.; Ali S.; Soudy F. A. Ameliorative effects of exogenous potassium nitrate on antioxidant defense system and mineral nutrient uptake in radish (Raphanus sativus L.) under salinity stress. ACS omega 2023, 8, 22575–22588. 10.1021/acsomega.3c01039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.; Saleem M. H.; Ali B.; Rasheed R.; Ashraf M. A.; Aziz H.; Ercisli S.; Riaz S.; Elsharkawy M. M.; Hussain I.; Alhag S. K.; Ahmed A. E.; Vodnar D. C.; Mumtaz S.; Marc R. A. Impact of foliar application of syringic acid on tomato (Solanum lycopersicum L.) under heavy metal stress-insights into nutrient uptake, redox homeostasis, oxidative stress, and antioxidant defense. Frontiers in Plant Science 2022, 13, 950120. 10.3389/fpls.2022.950120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M. A.; Rasheed R.; Hussain I.; Iqbal M.; Farooq M. U.; Saleem M. H.; Ali S. Taurine modulates dynamics of oxidative defense, secondary metabolism, and nutrient relation to mitigate boron and chromium toxicity in Triticum aestivum L. plants. Environmental Science and Pollution Research 2022, 29, 45527–45548. 10.1007/s11356-022-19066-5. [DOI] [PubMed] [Google Scholar]
- Wiltshire S.; Beckage B. Soil carbon sequestration through regenerative agriculture in the US state of Vermont. PLOS Climate 2022, 1, 1–23. 10.1371/journal.pclm.0000021. [DOI] [Google Scholar]
- Rai K. K. Integrating speed breeding with artificial intelligence for developing climate-smart crops. Molecular Biology Reports 2022, 49, 11385–11402. 10.1007/s11033-022-07769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brar D. S.; Khush G. S.. Cell and tissue culture for plant improvement. In Mechanisms of Plant Growth and Improved Productivity Modern Approaches; CRC Press, 2021; pp 229–278. [Google Scholar]
- Ghosh S.; Watson A.; Gonzalez-Navarro O. E.; Ramirez-Gonzalez R. H.; Yanes L.; Mendoza-Suarez M.; Hickey L. T. Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nature protocols 2018, 13, 2944–2963. 10.1038/s41596-018-0072-z. [DOI] [PubMed] [Google Scholar]
- Roumet P.; Morin F. Germination of immature soybean seeds to shorten reproductive cycle duration. Crop science 1997, 37, 521–525. 10.2135/cropsci1997.0011183X003700020035x. [DOI] [Google Scholar]
- Mobini S. H.; Warkentin T. D. A simple and efficient method of in vivo rapid generation technology in pea (Pisum sativum L.). In Vitro Cellular & Developmental Biology-Plant 2016, 52, 530–536. 10.1007/s11627-016-9772-7. [DOI] [Google Scholar]
- Yao Y.; Zhang P.; Liu H.; Lu Z.; Yan G. A fully in vitro protocol towards large scale production of recombinant inbred lines in wheat (Triticum aestivum L.). Plant Cell, Tissue and Organ Culture 2017, 128, 655–661. 10.1007/s11240-016-1145-8. [DOI] [Google Scholar]
- Samantara K.; Bohra A.; Mohapatra S. R.; Prihatini R.; Asibe F.; Singh L.; Reyes V. P.; Tiwari A.; Maurya A. K.; Croser J. S.; Wani S. H.; Siddique K. H. M.; Varshney R. K. Breeding more crops in less time: A perspective on speed breeding. Biology 2022, 11, 275. 10.3390/biology11020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard B. C. Y.; Beredo J. C.; Lenaerts B.; Mendoza R.; Santelices R.; Lopena V.; Verdeprado H.; Raghavan C.; Gregorio G. B.; Vial L.; Demont M.; Biswas P. S.; Iftekharuddaula K. M.; Rahman M. A.; Cobb J. N.; Islam M. R. Revisiting rice breeding methods-evaluating the use of rapid generation advance (RGA) for routine rice breeding. Plant Production Science 2017, 20, 337–352. 10.1080/1343943X.2017.1391705. [DOI] [Google Scholar]
- Jahne F.; Hahn V.; Wurschum T.; Leiser W. L. Speed breeding short-day crops by LED-controlled light schemes. Theor. Appl. Genet. 2020, 133, 2335–2342. 10.1007/s00122-020-03601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z.; Wang H. B.; Chen G. D.; Yan G. J.; Liu C. J. A procedure allowing up to eight generations of wheat and nine generations of barley per annum. Euphytica 2013, 191, 311–316. 10.1007/s10681-013-0909-z. [DOI] [Google Scholar]
- Watson A.; Ghosh S.; Williams M. J.; Cuddy W. S.; Simmonds J.; Rey M. D.; Hickey L. T. Speed breeding is a powerful tool to accelerate crop research and breeding. Nature plants 2018, 4, 23–29. 10.1038/s41477-017-0083-8. [DOI] [PubMed] [Google Scholar]
- Ghosh S.; Watson A.; Gonzalez-Navarro O. E.; Ramirez-Gonzalez R. H.; Yanes L.; Mendoza-Suarez M.; Hickey L. T. Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nature protocols 2018, 13, 2944–2963. 10.1038/s41596-018-0072-z. [DOI] [PubMed] [Google Scholar]
- Samineni S.; Sen M.; Sajja S. B.; Gaur P. M. Rapid generation advance (RGA) in chickpea to produce up to seven generations per year and enable speed breeding. The. Crop Journal 2020, 8, 164–169. 10.1016/j.cj.2019.08.003. [DOI] [Google Scholar]
- Sharma A.; Pandey H.; Devadas V. S.; Kartha B. D.; Vashishth A. Phytoremediation, stress tolerance and bio fortification in crops through soilless culture. Crop Design 2023, 2, 100027. 10.1016/j.cropd.2023.100027. [DOI] [Google Scholar]
- Ochatt S. J.; Sangwan R. S. Invitro shortening of generation time in Arabidopsis thaliana. Plant cell, tissue and organ culture 2008, 93, 133–137. 10.1007/s11240-008-9351-7. [DOI] [Google Scholar]
- Gonzalez-Barrios P.; Bhatta M.; Halley M.; Sandro P.; Gutierrez L. Speed breeding and early panicle harvest accelerates oat (Avena sativa L.) breeding cycles. Crop Science 2021, 61, 320–330. 10.1002/csc2.20269. [DOI] [Google Scholar]
- Stetter M. G.; Zeitler L.; Steinhaus A.; Kroener K.; Biljecki M.; Schmid K. J. Crossing methods and cultivation conditions for rapid production of segregating populations in three grain amaranth species. Frontiers in plant science 2016, 7, 816. 10.3389/fpls.2016.00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmar S.; Gill R. A.; Jung K. H.; Faheem A.; Qasim M. U.; Mubeen M.; Zhou W. Conventional and molecular techniques from simple breeding to speed breeding in crop plants: recent advances and future outlook. International journal of molecular sciences 2020, 21, 2590. 10.3390/ijms21072590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobini S. H.; Lulsdorf M.; Warkentin T. D.; Vandenberg A. Plant growth regulators improve in vitro flowering and rapid generation advancement in lentil and faba bean. InVitro Cellular & Developmental Biology-Plant 2015, 51, 71–79. 10.1007/s11627-014-9647-8. [DOI] [Google Scholar]
- Kumar V.; Singh J. Trends in hydroponics practice/technology in horticultural crops: A review. International Journal of Plant & Soil Science 2023, 35, 57–65. 10.9734/ijpss/2023/v35i22759. [DOI] [Google Scholar]
- Bethke J. A.; Lieth H.. Controlled Environment Agriculture. White Paper, Division of Agriculture and Natural Resources, University of California; 2020; pp 1–35. [Google Scholar]
- Pandey S.; Singh A.; Parida S. K.; Prasad M. Combining speed breeding with traditional and genomics-assisted breeding for crop improvement. Plant breeding 2022, 141, 301–313. 10.1111/pbr.13012. [DOI] [Google Scholar]
- Rana M. M.; Takamatsu T.; Baslam M.; Kaneko K.; Itoh K.; Harada N.; Mitsui T. Salt tolerance improvement in rice through efficient SNP marker-assisted selection coupled with speed-breeding. International Journal of Molecular Sciences 2019, 20, 2585. 10.3390/ijms20102585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizal G.; Karki S.; Alcasid M.; Montecillo F.; Acebron K.; Larazo N.; Garcia R.; Slamet-Loedin I. H.; Quick W. P. Shortening the breeding cycle of sorghum, a model crop for research. Crop Science 2014, 54, 520–529. 10.2135/cropsci2013.07.0471. [DOI] [Google Scholar]
- Nagatoshi Y.; Fujita Y. Accelerating soybean breeding in a CO2-supplemented growth chamber. Plant and Cell Physiology 2019, 60, 77–84. 10.1093/pcp/pcy189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A.; Ghosh S.; Williams M. J.; Cuddy W. S.; Simmonds J.; Rey M. D.; Hickey L. T. Speed breeding is a powerful tool to accelerate crop research and breeding. Nature plants 2018, 4, 23–29. 10.1038/s41477-017-0083-8. [DOI] [PubMed] [Google Scholar]
- Ochatt S. J.; Sangwan R. S.; Marget P.; Ndong Y. A.; Rancillac M.; Perney P.; Röbbelen G. New approaches towards the shortening of generation cycles for faster breeding of protein legumes. Plant Breeding 2002, 121, 436–440. 10.1046/j.1439-0523.2002.746803.x. [DOI] [Google Scholar]
- Pazos-Navarro M.; Castello M.; Bennett R. G.; Nichols P.; Croser J. In vitro-assisted single-seed descent for breeding-cycle compression in subterranean clover (Trifolium subterraneum L.). Crop and Pasture Science 2017, 68, 958–966. 10.1071/CP17067. [DOI] [Google Scholar]
- Nabwire S.; Suh H. K.; Kim M. S.; Baek I.; Cho B. K. Application of artificial intelligence in phenomics. Sensors 2021, 21, 4363. 10.3390/s21134363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoosefzadeh-Najafabadi M.; Earl H. J.; Tulpan D.; Sulik J.; Eskandari M. Application of machine learning algorithms in plant breeding: predicting yield from hyperspectral reflectance in soybean. Frontiers in plant science 2021, 11, 1–16. 10.3389/fpls.2020.624273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier A. Technical nuances of machine learning: implementation and validation of supervised methods for genomic prediction in plant breeding. Crop Breeding and Applied Biotechnology 2021, 21, 1–18. 10.1590/1984-70332021v21sa15. [DOI] [Google Scholar]
- Uzal L. C.; Grinblat G. L.; Namias R.; Larese M. G.; Bianchi J. S.; Morandi E. N.; Granitto P. M. Seed-per-pod estimation for plant breeding using deep learning. Computers and electronics in agriculture 2018, 150, 196–204. 10.1016/j.compag.2018.04.024. [DOI] [Google Scholar]
- Correa A. M.; Teodoro P. E.; Goncalves M. C.; Barroso L. M. A.; Nascimento M.; Santos A.; Torres F. E. Artificial intelligence in the selection of common bean genotypes with high phenotypic stability. Genetics and Molecular Research 2016, 15, 1–7. 10.4238/gmr.15028230. [DOI] [PubMed] [Google Scholar]
- Niazian M.; Sadat-Noori S. A.; Abdipour M. Artificial neural network and multiple regression analysis models to predict essential oil content of ajowan (Carum copticum L.). Journal of applied research on medicinal and aromatic plants 2018, 9, 124–131. 10.1016/j.jarmap.2018.04.001. [DOI] [Google Scholar]
- Niazian M.; Sadat-Noori S. A.; Abdipour M.; Tohidfar M.; Mortazavian S. M. M. Image processing and artificial neural network-based models to measure and predict physical properties of embryogenic callus and number of somatic embryos in ajowan (Trachyspermum ammi (L.) Sprague). In Vitro Cellular & Developmental Biology-Plant 2018, 54, 54–68. 10.1007/s11627-017-9877-7. [DOI] [Google Scholar]
- Vakilian K. A. Machine learning improves our knowledge about miRNA functions towards plant abiotic stresses. Sci. Rep. 2020, 10, 1–13. 10.1038/s41598-020-59981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat J. A.; Feng X.; Mir Z. A.; Raina A.; Siddique K. H. Recent advances in artificial intelligence, mechanistic models, and speed breeding offer exciting opportunities for precise and accelerated genomics-assisted breeding. Physiologia Plantarum 2023, 175, e13969. 10.1111/ppl.13969. [DOI] [PubMed] [Google Scholar]
- Zhao C.; Zhang Y.; Du J.; Guo X.; Wen W.; Gu S.; Wang J.; Fan J. Crop phenomics: current status and perspectives. Frontiers in Plant Science 2019, 10, 1–19. 10.3389/fpls.2019.00714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.; Lee C.; Park J. E.; Mansoor S.; Chung Y. S.; Kim K. Drought stress restoration frequencies of phenotypic indicators in early vegetative stages of soybean (Glycine max L.). Sustainability 2023, 15, 4852. 10.3390/su15064852. [DOI] [Google Scholar]
- Gala de Pablo J.; Lindley M.; Hiramatsu K.; Goda K. High-throughput Raman flow cytometry and beyond. Acc. Chem. Res. 2021, 54, 2132–2143. 10.1021/acs.accounts.1c00001. [DOI] [PubMed] [Google Scholar]
- Shakoor N.; Lee S.; Mockler T. C. High throughput phenotyping to accelerate crop breeding and monitoring of diseases in the field. Current opinion in plant biology 2017, 38, 184–192. 10.1016/j.pbi.2017.05.006. [DOI] [PubMed] [Google Scholar]
- Xu R.; Li C. A review of high-throughput field phenotyping systems: Focusing on ground robots. Plant Phenomics 2022, 2022, 1–17. 10.34133/2022/9760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunathilake E. M. B. M.; Le A. T.; Heo S.; Chung Y. S.; Mansoor S. The path to smart farming: Innovations and opportunities in precision agriculture. Agriculture 2023, 13, 1593. 10.3390/agriculture13081593. [DOI] [Google Scholar]
- Minervini M.; Scharr H.; Tsaftaris S. A. Image analysis: The new bottleneck in plant phenotyping. IEEE Signal Processing Magazine 2015, 32, 126–131. 10.1109/MSP.2015.2405111. [DOI] [Google Scholar]
- Montes J. M.; Melchinger A. E.; Reif J. C. Novel throughput phenotyping platforms in plant genetic studies. Trends in Plant Science 2007, 12, 433–436. 10.1016/j.tplants.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Singh P.; Singh N.; Singh K. K.; Singh A.. Diagnosing of disease using machine learning. In Machine Learning and the Internet of Medical Things in Healthcare; Academic Press, 2021; pp 89–111. [Google Scholar]
- Crossa J.; Perez-Rodriguez P.; Cuevas J.; Montesinos-Lopez O.; Jarquin D.; de los Campos G.; Burgueno J.; Gonzalez-Camacho J. M.; Perez-Elizalde S.; Beyene Y.; Dreisigacker S.; Singh R.; Zhang X.; Gowda M.; Roorkiwal M.; Rutkoski J.; Varshney R. K. Genomic selection in plant breeding: methods, models, and perspectives. Trends in Plant Science 2017, 22, 961–975. 10.1016/j.tplants.2017.08.011. [DOI] [PubMed] [Google Scholar]
- Merrick L. F.; Herr A. W.; Sandhu K. S.; Lozada D. N.; Carter A. H. Optimizing plant breeding programs for genomic selection. Agronomy 2022, 12, 714. 10.3390/agronomy12030714. [DOI] [Google Scholar]
- Haq M. A. CNN based automated weed detection system using UAV imagery. Computer Systems Science & Engineering 2022, 42, 1–18. 10.32604/csse.2022.023016. [DOI] [Google Scholar]
- Wang C.; Caragea D.; Kodadinne N. N.; Hein N. T.; Bheemanahalli R.; Somayanda I. M.; Jagadish S. K. Deep learning based high-throughput phenotyping of chalkiness in rice exposed to high night temperature. Plant Methods 2022, 18, 1–19. 10.1186/s13007-022-00839-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York L. M. Functional phenomics: An emerging field integrating high-throughput phenotyping, physiology, and bioinformatics. Journal of Experimental Botany 2019, 70, 379–386. 10.1093/jxb/ery379. [DOI] [PubMed] [Google Scholar]
- Gill T.; Gill S. K.; Saini D. K.; Chopra Y.; De Koff J. P.; Sandhu K. S. A comprehensive review of high throughput phenotyping and machine learning for plant stress phenotyping. Phenomics 2022, 2, 156–183. 10.1007/s43657-022-00048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niazian M.; Niedbala G. Machine learning for plant breeding and biotechnology. Agriculture 2020, 10 (10), 436. 10.3390/agriculture10100436. [DOI] [Google Scholar]
- Mishra P.; Feller T.; Schmuck M.; Nicol A.; Nordon A.. Early Detection of Drought Stress in Arabidopsis Thaliana Utilizing a Portable Hyperspectral Imaging Setup. 10th Workshop on Hyperspectral Imaging and Signal Processing: Evolution in Remote Sensing (WHISPERS); IEEE, 2019.
- Mishra P.; Feller T.; Schmuck M.; Nicol A.; Nordon A.. Homogenising and Segmenting Hyperspectral Images of Plants and Testing Chemicals in a High-Throughput Plant Phenotyping Setup. 10th Workshop on Hyperspectral Imaging and Signal Processing: Evolution in Remote Sensing (WHISPERS); IEEE, 2019. [Google Scholar]
- Cobb J. N.; DeClerck G.; Greenberg A.; Clark R.; McCouch S. Next-generation phenotyping: Requirements and strategies for enhancing our understanding of genotype-phenotype relationships and its relevance to crop improvement. Theor. Appl. Genet. 2013, 126, 867–887. 10.1007/s00122-013-2066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.; Ganapathysubramanian B.; Singh A.; Sarkar S. Machine learning for high-throughput stress phenotyping in plants. Trends in Plant Science 2016, 21, 110–124. 10.1016/j.tplants.2015.10.015. [DOI] [PubMed] [Google Scholar]
- Atas M.; Yardimci Y.; Temizel A. A new approach to aflatoxin detection in chili pepper by machine vision. Computers and Electronics in Agriculture 2012, 87, 129–141. 10.1016/j.compag.2012.06.001. [DOI] [Google Scholar]
- Jafari M.; Shahsavar A. The application of artificial neural networks in modeling and predicting the effects of melatonin on morphological responses of citrus to drought stress. PLoS One 2020, 15, e0240427. 10.1371/journal.pone.0240427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A. R.; Olanrewaju R. O.; Chukwudum Q. C.; Olanrewaju S. A.; Fadugba S. E. Comparison study of generative and discriminative models for classification of classifiers. International Journal of Mathematics and Computers in Simulation 2022, 16, 76–87. 10.46300/9102.2022.16.12. [DOI] [Google Scholar]
- Tsaftaris S. A.; Minervini M.; Scharr H. Machine learning for plant phenotyping needs image processing. Trends in Plant Science 2016, 21, 989–991. 10.1016/j.tplants.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Koh J. C.; Spangenberg G.; Kant S. Automated machine learning for high-throughput image-based plant phenotyping. Remote Sensing 2021, 13, 858. 10.3390/rs13050858. [DOI] [Google Scholar]
- Sun D.; Cen H.; Weng H.; Wan L.; Abdalla A.; El-Manawy A. I.; Zhu Y.; Zhao N.; Fu H.; Tang J.; Li X.; Zheng H.; Shu Q.; Liu F.; He Y. Using hyperspectral analysis as a potential high throughput phenotyping tool in GWAS for protein content of rice quality. Plant Methods 2019, 15, 1–16. 10.1186/s13007-019-0432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzner R.; Eggert A.; Van Dusschoten D.; Pflugfelder D.; Gerth S.; Schurr U.; Uhlmann N.; Jahnke S. Direct comparison of MRI and X-ray CT technologies for 3D imaging of root systems in soil: potential and challenges for root trait quantification. Plant Methods 2015, 11, 1–11. 10.1186/s13007-015-0060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh M.; Iqra F.; Ambreen H.; Pravin K. A; Ikra M.; Chung Y. S. Integrating artificial intelligence and high-throughput phenotyping for crop improvement. Journal of Integrative Agriculture 2024, 23, 1787–1802. 10.1016/j.jia.2023.10.019. [DOI] [Google Scholar]
- Potts J.; Jangra S.; Michael V. N.; Wu X. Speed Breeding for Crop Improvement and Food Security. Crops 2023, 3, 276–91. 10.3390/crops3040025. [DOI] [Google Scholar]
- Consentino B. B.; Ciriello M.; Sabatino L.; Vultaggio L.; Baldassano S.; Vasto S.; Rouphael Y.; La Bella S.; De Pascale S. Current acquaintance on agronomic biofortification to modulate the yield and functional value of vegetable crops: A review. Horticulturae 2023, 9, 219. 10.3390/horticulturae9020219. [DOI] [Google Scholar]
- Brar D. S.; Khush G. S.. Cell and tissue culture for plant improvement. In Mechanisms of Plant Growth and Improved Productivity Modern Approaches; CRC Press, 2021; pp 229–278. [Google Scholar]
- Abdul Fiyaz R.; Ajay B. C.; Ramya K. T.; Aravind Kumar J.; Sundaram R. M.; Subba Rao L. V. Speed breeding: methods and applications, Accelerated Plant Breeding. Cereal Crops 2020, 1, 31–49. 10.1007/978-3-030-41866-3_2. [DOI] [Google Scholar]
- Mekonnen T. W.; Gerrano A. S.; Mbuma N. W.; Labuschagne M. T. Breeding of vegetable cowpea for nutrition and climate resilience in Sub-Saharan Africa: progress, opportunities, and challenges. Plants 2022, 11, 1583. 10.3390/plants11121583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai K. K. Integrating speed breeding with artificial intelligence for developing climate-smart crops. Molecular Biology Reports 2022, 49, 11385. 10.1007/s11033-022-07769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S.; Singh A.; Parida S. K.; Prasad M. Combining speed breeding with traditional and genomics-assisted breeding for crop improvement. Plant Breeding 2022, 141, 301. 10.1111/pbr.13012. [DOI] [Google Scholar]
- Demirer G. S.; Silva T. N.; Jackson C. T.; Thomas J. B. W.; Ehrhardt D. W.; Rhee S. Y.; Mortimer J. C.; Landry M. P. Nanotechnology to advance CRISPR-Cas genetic engineering of plants. Nat. Nanotechnol. 2021, 16, 243. 10.1038/s41565-021-00854-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat J. A.; Feng X.; Mir Z. A.; Raina A.; Siddique K. H. Recent advances in artificial intelligence, mechanistic models, and speed breeding offer exciting opportunities for precise and accelerated genomics-assisted breeding. Physiologia Plantarum 2023, 175, 1–19. 10.1111/ppl.13969. [DOI] [PubMed] [Google Scholar]
- Jahne F.; Hahn V.; Würschum T.; Leiser W. L. Speed breeding short-day crops by LED-controlled light schemes. Theor. Appl. Genet. 2020, 133, 2335–2342. 10.1007/s00122-020-03601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey L. T.; Hafeez N. A.; Robinson H.; Jackson S. A.; Leal-Bertioli S. C.; Tester M.; Gao C.; Godwin I. D.; Hayes B. J.; Wulff B. B. Breeding crops to feed 10 billion. Nature biotechnology 2019, 37, 744–754. 10.1038/s41587-019-0152-9. [DOI] [PubMed] [Google Scholar]
- Wanga M. A.; Shimelis H.; Mashilo J.; Laing M. D. Opportunities and challenges of speed breeding: A review. Plant Breeding 2021, 140, 185–194. 10.1111/pbr.12909. [DOI] [Google Scholar]
- Hameed A.; Poznanski P.; Noman M.; Ahmed T.; Iqbal A.; Nadolska-Orczyk A.; Orczyk W. Barley Resistance to Fusarium graminearum Infections: From Transcriptomics to Field with Food Safety Concerns. J. Agric. Food Chem. 2022, 70, 14571–14587. 10.1021/acs.jafc.2c05488. [DOI] [PubMed] [Google Scholar]
- Wang L.; Li Y.; Xu L.; Sun D.; Wang Y.; Wang Z. Rice straw pretreatment using cow breeding wastewater for methane production. Bioresour. Technol. 2022, 346, 126657. 10.1016/j.biortech.2021.126657. [DOI] [PubMed] [Google Scholar]
- Sajja S. B.; Mathew A.; Pasupuleti J.. Speed Breeding to Accelerate Crop Improvement.In Digital Agriculture: A Solution for Sustainable Food and Nutritional Security; Springer International Publishing: Singapore, 2024; pp 425–443. [Google Scholar]
- Sharma S.; Kumar A.; Dhakte P.; Raturi G.; Vishwakarma G.; Barbadikar K. M.; Das B. K.; Shivaraj S. M.; Sonah H.; Deshmukh R. Speed breeding opportunities and challenges for crop improvement. Journal of Plant Growth Regulation 2023, 42, 46. 10.1007/s00344-021-10551-8. [DOI] [Google Scholar]
- Cazzola F.; Bermejo C. J.; Gatti I.; Cointry E. Speed breeding in pulses: an opportunity to improve the efficiency of breeding programs. Crop and Pasture Science 2021, 72, 165–172. 10.1071/CP20462. [DOI] [Google Scholar]
- Temesgen B. Speed breeding to accelerate crop improvement. Int. J. Agric Sc Food Technol. 2022, 8, 178–186. 10.17352/2455-815X.000161. [DOI] [Google Scholar]
- O’connor D. J.; Wright G. C.; Dieters M. J.; George D. L.; Hunter M. N.; Tatnell J. R.; Fleischfresser D. B. Development and application of speed breeding technologies in a commercial peanut breeding program. Peanut Science 2013, 40 (2), 107–114. 10.3146/PS12-12.1. [DOI] [Google Scholar]
- Haroon M.; Wang X.; Afzal R.; Zafar M. M.; Idrees F.; Batool M.; Khan A. S.; Imran M. Novel plant breeding techniques shake hands with cereals to increase production. Plants 2022, 11 (8), 1052. 10.3390/plants11081052. [DOI] [PMC free article] [PubMed] [Google Scholar]