Abstract
Background
The prevalence of obesity-associated insulin resistance (IR) is increasing along with the increase in obesity rates. In this study, we compared the predictive utility of four alternative indexes of IR [triglyceride glucose index (TyG index), metabolic score for insulin resistance (METS-IR), the triglyceride/high-density lipoprotein cholesterol (TG/HDL-C) ratio and homeostatic model assessment of insulin resistance (HOMA-IR)] for all-cause mortality and cardiovascular mortality in the general population based on key variables screened by the Boruta algorithm. The aim was to find the best replacement index of IR.
Methods
In this study, 14,653 participants were screened from the National Health and Nutrition Examination Survey (2001–2018). And TyG index, METS-IR, TG/HDL-C and HOMA-IR were calculated separately for each participant according to the given formula. The predictive values of IR replacement indexes for all-cause mortality and cardiovascular mortality in the general population were assessed.
Results
Over a median follow-up period of 116 months, a total of 2085 (10.23%) all-cause deaths and 549 (2.61%) cardiovascular disease (CVD) related deaths were recorded. Multivariate Cox regression and restricted cubic splines analysis showed that among the four indexes, only METS-IR was significantly associated with both all-cause and CVD mortality, and both showed non-linear associations with an approximate “U-shape”. Specifically, baseline METS-IR lower than the inflection point (41.33) was negatively associated with mortality [hazard ratio (HR) 0.972, 95% CI 0.950–0.997 for all-cause mortality]. In contrast, baseline METS-IR higher than the inflection point (41.33) was positively associated with mortality (HR 1.019, 95% CI 1.011–1.026 for all-cause mortality and HR 1.028, 95% CI 1.014–1.043 for CVD mortality). We further stratified the METS-IR and showed that significant associations between METS-IR levels and all-cause and cardiovascular mortality were predominantly present in the nonelderly population aged < 65 years.
Conclusions
In conjunction with the results of the Boruta algorithm, METS-IR demonstrated a more significant association with all-cause and cardiovascular mortality in the U.S. population compared to the other three alternative IR indexes (TyG index, TG/HDL-C and HOMA-IR), particularly evident in individuals under 65 years old.
Keywords: Metabolic score for insulin resistance, Triglyceride-glucose index, Mortality, Boruta algorithm, Insulin resistance, NHANES
Background
In recent years, obesity has increasingly become a widespread epidemic as quality of life continues to improve. Obesity rates [body mass index (BMI) > 30] and severe obesity rates (BMI > 35) are projected to rise to 50% and 25%, respectively, across the United States by 2030. Along with this increase in obesity rates, the prevalence of obesity-related insulin resistance (IR) and cardiovascular disease (CVD) will also increase [1, 2]. Clinically, IR, known as syndrome X, or insulin resistance syndrome, is a state of reduced sensitivity and responsiveness to the action of insulin that usually occurs several years before the onset of diabetes and has been shown to increase the risk of CVD [3–5]. Studies have shown that the state of hyperinsulinemia caused by IR can accelerate the production of fatty acids, impede the normal action of insulin and may trigger early atherosclerosis, atherosclerotic dyslipidemia, dysglycemia and blood pressure abnormalities [6]. Long-term metabolic disorder will increase the cardiovascular disease mortality and even all-cause mortality of patients. Therefore, early recognition of insulin resistance status is crucial for early treatment.
The hyperinsulinemic-euglycemic clamp technique is considered the gold standard for the assessment of IR, but due to its high assay cost and complex procedure, the method is limited to small studies and not used in large epidemiologic investigations [7]. The homeostasis model assessment of insulin resistance (HOMA-IR) is widely used in the assessment of IR due to its non-invasiveness and simplicity. However, the assessment efficacy of this model varies by population race and shows some limitations in patients treated with insulin or in patients with β-cell insufficiency [8, 9]. In addition, calculation of HOMA-IR relies on laboratory measurement of fasting insulin levels, which is often difficult to achieve in resource-limited countries, limiting its popularity in daily clinical practice. Therefore, there is a need for a less costly and more readily available indicator to assess IR more broadly and easily. Currently, the alternative indexes of IR commonly used in clinical practice are triglyceride glucose index (TyG index), metabolic score for Insulin resistance (METS-IR), the triglyceride /high-density lipoprotein cholesterol (TG/HDL-C) ratio and homeostatic model assessment of insulin resistance (HOMA-IR). The TyG index is inexpensive to calculate and easy to obtain, and is recognized as a time-saving and relatively simple marker of IR [10]. Multiple studies have shown that it performs consistently or better than HOMA-IR in assessing IR [11, 12]. Therefore, a large number of studies have been conducted to explore the relationship between TyG index and cardiovascular diseases and their prognosis. Some studies have reported that TyG index is significantly associated with all-cause mortality and cardiovascular mortality in the general population, especially in people under 65 years of age [13]. However, some studies have reported inconsistent results, stating that there is no significant relationship between the TyG index and all-cause or cardiovascular mortality [14]. The controversy surrounding the TyG index has somewhat limited its clinical application. In addition, there are fewer studies on other indexes of IR besides the TyG index. The existence of alternative indexes of IR that are better predictors of all-cause or cardiovascular mortality is an issue that needs to be focused on and resolved, which is essential to find the best alternative index of IR and to promote its clinical application.
Boruta algorithm is a supervised classification feature selection method based on random forests, which minimizes the error of the random forest model and ultimately forms a subset of the minimum optimal features [15, 16]. Most studies rely on clinical significance and experience in selecting variables for inclusion in multivariate Cox proportional risk models. In this study, we use the results of Boruta algorithm in conjunction with practical clinical significance to screen the variables to be included in multivariate Cox regression.
In this study, we included four replacement indexes of IR, TyG index, METS-IR, TG/HDL-C, and HOMA-IR, with the aim of comparing the predictive effects of these four indices on all-cause mortality and cardiovascular mortality in the general population, searching for the optimal replacement indices for insulin resistance, and identifying high-risk groups for insulin resistance.
Methods
Study population and design
The National Health and Nutrition Examination Survey (NHANES) is a major scientific program conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC). The survey is dedicated to assessing the health and nutritional status of the non-institutionalized population living in the United States, including adults and children, in order to provide a comprehensive picture of the health of Americans. Data are collected by NCHS every two years. NHANES received approval from the NCHS Research Ethics Review Board. Datasets generated and analyzed by NHANES are available on the NHANES website (http://www.cdc.gov/nchs/nhanes.htm). We downloaded NHANES data for nine cycles from 2001 to 2018 with the aim of investigating the association between the four alternative indexes of IR (TyG index, METS-IR, TG/HDL-C, and HOMA-IR) and all-cause and cardiovascular mortality. A total of 91,351 participants were surveyed. The following patients were excluded from the study: (1) Patients under 18 years of age or 85 years of age and older. (2) Pregnant patients. (3) Patients who lacked a calculated index related to the IR replacement indexes such as fasting triglycerides, high-density lipoprotein, fasting plasma glucose (FPG) and fasting serum insulin (FINS). (4) Patients lacking prognostic data. (5) Patients without important covariates such as gender, body mass index (BMI), race, poverty income ratio (PIR), education, smoking and drinking. (6) Patients with fasting subsample weights (WTSAF2YR) ≤ 0. The final study population consisted of 14,653 participants. The patient selection process is shown in Fig. 1.
Fig. 1.
Flow diagram of patient selection. TyG index triglyceride glucose index, METS-IR metabolic score for Insulin resistance, TG triglyceride, HDL-C high-density lipoprotein-cholesterol, HOMA-IR homeostatic model assessment of insulin resistance, BMI body mass index, PIR the ratio of family income to poverty
Insulin resistance replacement index
Four IR replacement indexes were included in this study, namely TyG Index, METS-IR, TG/HDL-C and HOMA-IR. TyG index = Ln[triglycerides (mg/dL) × glycemia (mg/dL)/2] [17]. METS-IR = Ln[2 × glycemia (mg/dL) + triglycerides (mg/dL)] × BMI/Ln HDL-C (mg/dL) [18]. TG/HDL-C calculated as triglycerides (mg/dL)/HDL-C (mg/dL) [19]. HOMA-IR was calculated using the formula FPG (mmol/L) × FINS (mIU/L) /22.5 [20].
Determination of mortality rates
The primary endpoint of this study was all-cause mortality and the secondary endpoint was cardiovascular mortality. To determine the mortality status of the follow-up population, we employed the NHANES public-use linked mortality file as of December 31, 2019, which was correlated to the NCHS and the National Death Index (NDI) through a probabilistic matching algorithm. Follow-up began on the date of the NHANES interview and ended on the date of death or the end of follow-up (December 31, 2019). In addition, cardiovascular death was defined as deaths due to cardiovascular disease according to the International Statistical Classification of Diseases, 10th Revision (ICD-10) and the NCHS classifying heart diseases (054-068).
Assessment of covariates
NHANES personnel used questionnaires in the household interviews, through which a variety of demographic and health-related information was collected, including age, gender, race, household income, education, smoking and drinking status. Race was categorized as Mexican American, other Hispanic, Non-Hispanic White, Non-Hispanic Black or Other. Household income was classified as low income, middle income and high income, based on the family PIR (< 1.0, 1.0–3.0, > 3.0), respectively. Education level was classified as less than high school, high school or equivalent, or college or above. Drinking status was recorded as never drinker (no more than 12 drinks in a lifetime), former drinker (no more than 12 drinks in the past year) and current drinker (greater than or equal to 12 drinks in the past year). Smoking status was recorded as never smoker (less than 100 cigarettes in a lifetime), former smoker (more than 100 cigarettes in a lifetime and had quit smoking for more than 1 year at the time of the survey) and current smoker (more than 100 cigarettes in lifetime and still smoking or quit less than 1 year ago at the time of the survey).
NHANES personnel measured participants’ height, weight, BMI, and blood pressure at the Mobile Examination Center (MEC). BMI was calculated as weight in kilograms divided by height in meters squared. BMI was divided into four groups based on the World Health Organization’s standardized thresholds, as follows Underweight: BMI < 18.5 kg/m2; Normal: 18.5 ≤ BMI < 25.0 kg/m2; Overweight: 25.0 ≤ BMI < 30.0 kg/m2; Obesity: BMI ≥ 30.0 kg/m2 [21]. In NHANES, serum specimens were collected as part of the laboratory tests to measure clinical markers such as glycosylated hemoglobin (HbA1C), FPG, low-density lipoprotein cholesterol (LDL-C), HDL-C, total cholesterol (TC), TG and serum creatinine (Scr) at baseline.
The comorbidities analyzed in this study comprised hypertension, diabetes, hyperlipidemia, CVD and metabolic syndrome (MetS). Participants’ blood pressures were obtained at the MEC, and details of the blood pressure measurements can be found on the NHANES website (http://www.cdc.gov/nchs/nhanes.htm, accessed on 11 November 2022). Hypertension was defined as the fulfillment of any of the following conditions: mean systolic blood pressure (SBP) ≥ 140 mmHg or mean diastolic blood pressure (DBP) ≥ 90 mmHg on three consecutive measurements in a calm state, self-reported hypertension, or the use of prescription antihypertensive medications. This criterion is based on the guidelines of the International Society of Hypertension, in which 140/90 mmHg is the threshold for determining hypertension [22]. The definition of diabetes includes the following criteria, one of which was sufficient for diagnosis: FPG ≥ 7.0 mmol/L (126 mg/dL), 2-h postprandial plasma glucose ≥ 11.1 mmol/L (200 mg/dL), HbA1C ≥ 6.5%, self-reported diabetes mellitus and insulin or oral hypoglycemic medication use [23]. Hyperlipidemia was defined in conjunction with the National Cholesterol Education Program (NCEP) Adult Treatment Third Edition (ATP 3) criteria: total cholesterol ≥ 200 mg/dL, triglycerides ≥ 150 mg/dL, HDL-C ≤ 40 mg/dL in men and ≤ 50 mg/dL in women, and LDL-C ≥ 130 mg/dL, self-reported cholesterol levels high or using cholesterol-lowering drugs [24]. The diagnosis of CVD was confirmed by asking participants “Has a doctor or other health expert ever informed you that you have congestive heart failure (CHF)/coronary heart disease (CHD)/angina pectoris/myocardial infarction (MI)/stroke?” If a person answered “yes” to any of these questions, they were considered to have CVD [4]. Metabolic syndrome was defined as follows: (1) waist circumference (WC) ≥ 94 cm in men or ≥ 80 cm in women; (2) SBP/DBP ≥ 130/85 mmHg or use of antihypertensive medications; (3) TG ≥ 150 md/dL or use of lipid-lowering medications; (4) HDL-C < 40 mg/dL in men or < 50 mg/dL in women; (5) FPG ≥ 100 mg/dL or diagnosed diabetes. Metabolic syndrome was diagnosed by meeting any two or more of conditions (2–5) on the basis of condition (1) [25, 26].
Statistical analysis
Due to the complexity of the NHANES sampling design, analyzing NHANES data requires that sample weights, clustering and stratification be incorporated into all analyses. Sampling weights were considered for all analyses in this study. According to the NHANES analysis guidelines, new weights were calculated by dividing the weights for the 2-year cycle by 9. We used the four alternative indexes of insulin resistance, TyG index, METS-IR, TG/HDL-C and HOMA-IR, as independent variables and stratified the study population characteristics and laboratory indexes according to their quartiles, and compared the baseline characteristics of the groups using one-way ANOVA for continuous variables and Pearson chi-square test for categorical variables. Continuous variables were expressed as mean and standard deviation (SD), and categorical variables were expressed as frequency and percentage. In addition, we divided the study cohort into survival and death groups based on patient prognosis and compared the baseline characteristics of the populations in each group with the same statistical methods as above.
Feature selection is an important step in model construction, and in this study, we used the Boruta algorithm, a supervised categorical feature selection method, to pinpoint all relevant features. Next, Cox proportional risk regression models were used to estimate hazard ratios (HRs) and their corresponding 95% confidence intervals (CIs) to test the relationship between the IR replacement indexes and all-cause and cardiovascular mortality. Model 1 represents a univariate Cox regression analysis. Model 2 was adjusted for age, sex and race. Model 3 was adjusted for all-cause mortality and cardiovascular mortality separately based on the specific results of the Boruta algorithm. Nonlinear correlations between the IR replacement index and mortality were explored using restricted cubic spline (RCS) regression models. If the relationship was linear, we selected the point at which the HR was 0 as the “inflection point”. We used two segmented Cox proportional risk models on either side of the inflection point to examine the relationship between the insulin resistance replacement indexes and mortality.
We further stratified the analysis of significant covariates to consider potential influences. In this study, we stratified analyses according to age (< 65 or ≥ 65 years), sex, BMI, education, smoking status, hypertension, diabetes mellitus and metabolic syndrome. R (V 4.3.1) was used for all statistical analyses. A P value of < 0.05 was determined to be statistically significant.
Results
Baseline characteristics of study participants
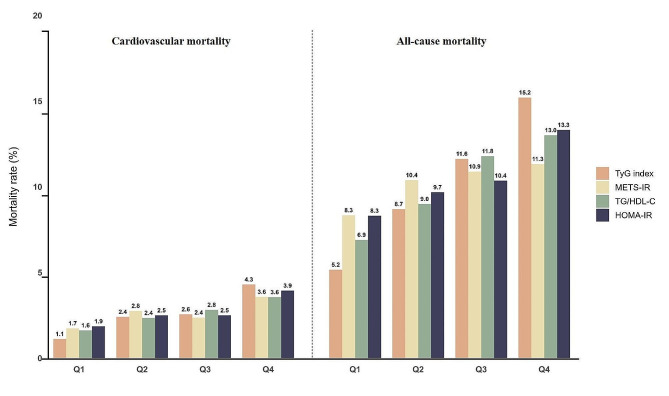
Table 1 shows the baseline characteristics of the cohort study participants (14,653) stratified by TyG index, METS-IR, TG/HDL-C and HOMA-IR quartiles. The mean age of the participants was 46 years, of which 49.82% were male. The mean TyG index, METS-IR, TG/HDL-C and HOMA-IR of the enrolled patients were 8.63 ± 0.66, 42.92 ± 12.54, 2.97 ± 3.83 and 3.39 ± 5.23, respectively. During 1,743,607 person-months of follow-up (median follow-up of 116 months), there were 2085 (10.23%) incident cases of all-cause mortality and 549 (2.61%) incident cases of cardiovascular mortality. Laboratory characteristics at baseline based on TyG index, METS-IR, TG/HDL-C and HOMA-IR quartiles are shown in Table 2. The baseline and laboratory characteristics of the enrolled patients showed approximately the same trend across the four indexes, with patients in the higher-scoring group tending to be older, male, obese, with a medium or lower household income, with a lower level of education, former alcohol drinkers and smokers compared to those in the lower-scoring group, and exhibiting a higher prevalence of comorbidities, including hypertension, diabetes mellitus, hyperlipidemia, CVD and metabolic syndrome (P < 0.001). In addition, the group with higher scores was associated with higher SBP, DBP and blood indices, including HbA1C, fasting serum insulin (FINS), FPG, TG, Uric acid, blood urea nitrogen (BUN), alanine transaminase (ALT), gamma glutamyl transferase (GGT), serum potassium, white blood cells (WBC) and blood platelet (PLT) (P < 0.001). Conversely, higher scores were associated with lower HDL-C (P < 0.001). Figure 2 shows bar graphs of the relationship between quartiles of the four indexes and mortality. It is easy to see that both all-cause mortality and cardiovascular mortality broadly show a gradual increase as the four indexes rise [TyG index: all-cause mortality (5.16% vs. 8.70% vs. 11.63% vs. 15.21%, P < 0.001) and cardiovascular mortality (1.12% vs. 2.41% vs. 2.56% vs. 4.30%, P < 0.001); METS-IR: all-cause mortality (8.34% vs. 10.39% vs. 10.89% vs. 11.33%, P < 0.001) and cardiovascular mortality (1.74% vs. 2.76% vs. 2.37% vs. 3.58%, P < 0.001); TG/HDL-C: all-cause mortality (6.85% vs. 9.00% vs. 11.79% vs. 13.01%, P < 0.001) and cardiovascular mortality (1.62% vs. 2.35% vs. 2.82% vs. 3.56%, P < 0.001); HOMA-IR: all-cause mortality (8.31% vs. 9.68% vs. 10.36% vs. 13.32%, P < 0.001) and cardiovascular mortality (1.86% vs. 2.50% vs. 2.45% vs. 3.94%, P < 0.001)]. Comparison of baseline characteristics between the survival group and death group is shown in Table 3, with statistical differences in all variables except BMI (P < 0.05).
Table 1.
Baseline characteristics according to TyG Index, METS-IR, TG/HDL-C and HOMA-IR quartiles, NHANES 2001–2018
| Overall | TyG index levels | METS-IR levels | TG/HDL-C levels | HOMA-IR levels | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | Overall, N = 14,653 | Q1, N = 3325 (22.69%) | Q2, N = 3658 (24.96%) | Q3, N = 3824 (26.10%) | Q4, N = 3846 (26.25%) | P Value | Q1, N = 3416 (23.31%) | Q2, N = 3719 (25.38%) | Q3, N = 3764 (25.69%) | Q4, N = 3754 (25.62%) | P Value | Q1, N = 3417 (23.32%) | Q2, N = 3684 (25.14%) | Q3, N = 3668 (25.03%) | Q4, N = 3884 (26.51%) | P Value | Q1, N = 3810 (26.00%) | Q2, N = 3643 (24.86%) | Q3, N = 3714 (25.35%) | Q4, N = 3486 (23.79%) | P Value |
| TyG index | 14,653 | 8.63 (0.66) | 7.83 (0.26) | 8.37 (0.12) | 8.79 (0.13) | 9.49 (0.46) | < 0.001 | 8.15 (0.47) | 8.47 (0.50) | 8.78 (0.55) | 9.11 (0.69) | < 0.001 | 7.90 (0.34) | 8.38 (0.27) | 8.76 (0.29) | 9.40 (0.51) | < 0.001 | 8.23 (0.53) | 8.51 (0.54) | 8.79 (0.57) | 9.12 (0.68) | < 0.001 |
| METS-IR | 14,653 | 42.92 (12.54) | 34.87 (9.14) | 39.51 (9.97) | 44.75 (11.28) | 52.26 (12.40) | < 0.001 | 29.41 (3.07) | 37.43 (2.06) | 45.13 (2.48) | 60.00 (9.81) | < 0.001 | 33.77 (8.48) | 39.50 (9.63) | 45.11 (11.08) | 52.44 (12.12) | < 0.001 | 33.63 (6.98) | 39.51 (8.28) | 45.90 (9.69) | 56.11 (13.00) | < 0.001 |
| TG/HDL-C | 14,653 | 2.97 (3.83) | 0.95 (0.35) | 1.68 (0.52) | 2.72 (0.89) | 6.49 (6.31) | < 0.001 | 1.31 (0.78) | 2.11 (1.38) | 3.23 (2.62) | 5.24 (6.38) | < 0.001 | 0.86 (0.23) | 1.59 (0.23) | 2.60 (0.38) | 6.55 (6.05) | < 0.001 | 1.77 (2.74) | 2.44 (2.89) | 3.45 (3.80) | 4.66 (5.17) | < 0.001 |
| HOMA-IR | 14,653 | 3.39 (5.23) | 1.76 (1.47) | 2.46 (2.99) | 3.46 (3.43) | 5.83 (8.79) | < 0.001 | 1.44 (1.19) | 2.16 (1.55) | 3.30 (2.54) | 6.69 (9.10) | < 0.001 | 1.86 (3.01) | 2.60 (3.15) | 3.56 (3.99) | 5.36 (8.05) | < 0.001 | 1.00 (0.32) | 1.92 (0.28) | 3.25 (0.53) | 8.54 (9.57) | < 0.001 |
| Age, years | 14,653 | 46 (16.61) | 40 (15.84) | 46 (16.70) | 48 (16.70) | 51 (15.20) | < 0.001 | 43 (17.41) | 47 (17.13) | 48 (16.19) | 47 (15.09) | < 0.001 | 44 (17.00) | 46 (17.02) | 48 (16.88) | 48 (15.31) | < 0.001 | 44 (16.26) | 46 (16.88) | 47 (16.79) | 49 (16.06) | < 0.001 |
| Gender, n (%) | 14,653 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||||||||||||||||
| Male | 7423 (49.82) | 1340 (38.67) | 1818 (47.97) | 2034 (53.26) | 2231 (58.84) | 1345 (35.78) | 2012 (53.39) | 2128 (56.79) | 1938 (53.70) | 1246 (34.47) | 1751 (45.53) | 1951 (53.43) | 2475 (64.45) | 1851 (45.41) | 1788 (47.50) | 1956 (53.33) | 1828 (54.47) | |||||
| Female | 7230 (50.18) | 1985 (61.33) | 1840 (52.03) | 1790 (46.74) | 1615 (41.16) | 2071 (64.22) | 1707 (46.61) | 1636 (43.21) | 1816 (46.30) | 2171 (65.53) | 1933 (54.47) | 1717 (46.57) | 1409 (35.55) | 1959 (54.59) | 1855 (52.50) | 1758 (46.67) | 1658 (45.53) | |||||
| BMI, kg/m2 | 14,653 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||||||||||||||||
| Underweight (< 18.5) | 216 (1.53) | 108 (3.41) | 67 (1.83) | 30 (0.72) | 11 (0.23) | 216 (6.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 126 (3.90) | 53 (1.47) | 25 (0.65) | 12 (0.25) | 156 (3.92) | 37 (1.08) | 17 (0.39) | 6 (0.15) | |||||
| Normal (18.5 to < 25) | 4142 (29.76) | 1537 (49.47) | 1226 (35.02) | 869 (23.11) | 510 (12.33) | 2955 (86.85) | 1106 (29.03) | 79 (1.57) | 2 (0.01) | 1597 (49.64) | 1220 (35.90) | 815 (22.83) | 510 (12.31) | 2185 (58.85) | 1208 (32.72) | 574 (14.65) | 175 (4.37) | |||||
| Overweight (25 to < 30) | 5006 (33.55) | 967 (28.19) | 1282 (35.45) | 1361 (35.10) | 1396 (35.10) | 244 (7.14) | 2457 (67.10) | 2038 (54.44) | 267 (6.34) | 1003 (29.27) | 1259 (33.23) | 1281 (34.91) | 1463 (36.44) | 1151 (30.12) | 1549 (42.40) | 1475 (38.84) | 831 (21.39) | |||||
|
Obesity (30 or greater) |
5289 (35.16) | 713 (18.93) | 1083 (27.70) | 1564 (41.07) | 1929 (52.34) | 1 (0.01) | 156 (3.87) | 1647 (43.99) | 3485 (93.65) | 691 (17.19) | 1152 (29.40) | 1547 (41.61) | 1899 (51.00) | 318 (7.11) | 849 (23.80) | 1648 (46.12) | 2474 (74.09) | |||||
| Race, n (%) | 14,653 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||||||||||||||||
| Mexican American | 2496 (7.78) | 379 (5.95) | 547 (7.06) | 729 (8.77) | 841 (9.25) | 361 (4.93) | 614 (7.37) | 790 (9.88) | 731 (9.03) | 367 (5.58) | 577 (7.18) | 704 (8.82) | 848 (9.37) | 440 (5.11) | 575 (7.02) | 729 (9.53) | 752 (10.27) | |||||
| Other Hispanic | 1172 (4.65) | 226 (4.31) | 281 (4.43) | 334 (5.07) | 331 (4.76) | 223 (3.84) | 310 (4.78) | 341 (5.53) | 298 (4.48) | 227 (3.91) | 284 (4.44) | 322 (5.11) | 339 (5.11) | 242 (3.72) | 297 (4.93) | 323 (5.02) | 310 (5.14) | |||||
| Non-Hispanic White | 7058 (70.84) | 1402 (65.87) | 1791 (71.70) | 1892 (71.61) | 1973 (73.92) | 1786 (73.18) | 1790 (70.51) | 1701 (69.41) | 1781 (70.19) | 1470 (66.57) | 1785 (71.64) | 1752 (70.46) | 2051 (74.25) | 2049 (74.82) | 1824 (71.95) | 1688 (67.92) | 1497 (67.51) | |||||
| Non-Hispanic Black | 2756 (10.54) | 1000 (17.20) | 761 (11.28) | 559 (7.78) | 436 (6.26) | 573 (9.03) | 682 (10.50) | 709 (10.44) | 792 (12.24) | 1027 (17.09) | 757 (11.10) | 590 (9.02) | 382 (5.52) | 696 (9.61) | 660 (10.16) | 678 (10.33) | 722 (12.53) | |||||
| Other/multiracial | 1171 (6.19) | 318 (6.67) | 278 (5.53) | 310 (6.77) | 265 (5.81) | 473 (9.02) | 323 (6.84) | 223 (4.74) | 152 (4.06) | 326 (6.85) | 281 (5.64) | 300 (6.59) | 264 (5.75) | 383 (6.74) | 287 (5.94) | 296 (7.20) | 205 (4.55) | |||||
| PIR, n (%) | 14,653 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||||||||||||||||
| < 1.0 | 2879 (13.50) | 646 (13.98) | 702 (13.22) | 749 (13.52) | 782 (13.32) | 652 (13.64) | 667 (11.78) | 721 (13.30) | 839 (15.28) | 635 (13.03) | 714 (13.34) | 693 (13.17) | 837 (14.40) | 681 (11.86) | 678 (12.99) | 752 (14.20) | 768 (15.53) | |||||
| 1.0–3.0 | 6121 (36.23) | 1302 (33.61) | 1466 (34.38) | 1640 (38.80) | 1713 (38.00) | 1289 (32.61) | 1570 (36.61) | 1605 (36.48) | 1657 (39.31) | 1321 (32.97) | 1522 (35.80) | 1590 (37.94) | 1688 (38.00) | 1503 (33.71) | 1504 (35.57) | 1555 (35.91) | 1559 (40.82) | |||||
| > 3.0 | 5653 (50.27) | 1377 (52.41) | 1490 (52.40) | 1435 (47.68) | 1351 (48.68) | 1475 (53.75) | 1482 (51.61) | 1438 (50.22) | 1258 (45.41) | 1461 (54.00) | 1448 (50.86) | 1385 (48.89) | 1359 (47.60) | 1626 (54.43) | 1461 (51.44) | 1407 (49.89) | 1159 (43.65) | |||||
| Education, n (%) | 14,653 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||||||||||||||||
| Below high school | 1528 (5.38) | 198 (3.31) | 332 (4.85) | 433 (5.97) | 565 (7.29) | 217 (3.39) | 381 (5.11) | 512 (7.09) | 418 (6.01) | 209 (3.39) | 371 (4.94) | 396 (5.98) | 552 (7.05) | 308 (4.18) | 332 (4.53) | 438 (6.22) | 450 (7.04) | |||||
| High school | 5558 (34.78) | 1127 (29.18) | 1363 (34.32) | 1519 (37.09) | 1549 (38.23) | 1173 (30.31) | 1390 (34.35) | 1463 (36.96) | 1532 (37.60) | 1151 (29.06) | 1355 (33.64) | 1494 (37.57) | 1558 (38.44) | 1318 (30.77) | 1406 (35.98) | 1410 (35.05) | 1424 (38.45) | |||||
| Above high school | 7657 (59.84) | 2000 (67.51) | 1963 (60.83) | 1872 (56.94) | 1732 (54.48) | 2026 (66.30) | 1948 (60.54) | 1789 (55.95) | 1804 (56.39) | 2057 (67.55) | 1958 (61.42) | 1778 (56.45) | 1774 (54.51) | 2184 (65.05) | 1905 (59.49) | 1866 (58.73) | 1612 (54.51) | |||||
| Drinking, n (%) | 14,653 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||||||||||||||||
| Never | 2060 (11.46) | 515 (12.99) | 495 (11.40) | 494 (9.73) | 556 (11.84) | 514 (11.73) | 475 (10.64) | 537 (11.51) | 534 (11.95) | 522 (12.60) | 525 (11.73) | 525 (11.29) | 488 (10.32) | 468 (9.60) | 506 (13.89) | 527 (11.61) | 559 (13.28) | |||||
| Former | 3923 (24.63) | 813 (21.28) | 974 (24.23) | 1086 (26.37) | 1050 (26.46) | 760 (19.73) | 936 (22.18) | 1007 (25.13) | 1220 (31.58) | 815 (21.09) | 960 (23.36) | 1057 (26.80) | 1091 (27.05) | 869 (20.48) | 939 (25.78) | 1003 (25.02) | 1112 (31.63) | |||||
| Current | 8670 (63.91) | 1997 (65.73) | 2189 (64.37) | 2244 (63.90) | 2240 (61.70) | 2142 (68.54) | 2308 (67.18) | 2220 (63.36) | 2000 (56.47) | 2080 (66.31) | 2199 (64.91) | 2086 (61.91) | 2305 (62.63) | 2473 (69.92) | 2198 (60.33) | 2184 (63.37) | 1815 (55.09) | |||||
| Smoking, n (%) | 14,653 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||||||||||||||||
| Never | 7867 (53.25) | 2126 (63.56) | 1986 (53.58) | 1976 (50.88) | 1779 (45.46) | 1944 (55.89) | 1993 (52.64) | 2005 (52.91) | 1925 (51.49) | 2145 (62.18) | 2076 (55.67) | 1876 (50.23) | 1770 (45.64) | 1985 (51.98) | 2039 (55.94) | 2008 (53.15) | 1835 (51.85) | |||||
| Former | 3400 (22.70) | 563 (16.51) | 802 (21.80) | 947 (24.78) | 1088 (27.42) | 599 (17.67) | 871 (22.83) | 966 (25.04) | 964 (25.40) | 659 (19.54) | 807 (21.51) | 905 (24.49) | 1029 (25.05) | 756 (19.37) | 789 (21.22) | 927 (24.50) | 928 (26.89) | |||||
| Current | 3386 (24.05) | 636 (19.93) | 870 (24.62) | 901 (24.34) | 979 (27.12) | 873 (26.44) | 855 (24.53) | 793 (22.05) | 865 (23.11) | 613 (18.28) | 801 (22.82) | 887 (25.28) | 1085 (29.31) | 1069 (28.65) | 815 (22.84) | 779 (22.35) | 723 (21.26) | |||||
| SBP, mmHg | 14,356 | 121 (17) | 116 (16) | 120 (17) | 123 (16) | 127 (17) | < 0.001 | 117 (17) | 121 (17) | 123 (16) | 125 (16) | < 0.001 | 118 (17) | 120 (17) | 122 (17) | 125 (17) | < 0.001 | 117 (17) | 121 (17) | 123 (16) | 126 (16) | < 0.001 |
| DBP, mmHg | 14,356 | 70 (12) | 67 (11) | 69 (12) | 71 (12) | 73 (13) | < 0.001 | 68 (11) | 68 (12) | 71 (12) | 73 (13) | < 0.001 | 68 (12) | 69 (12) | 70 (12) | 73 (12) | < 0.001 | 68 (11) | 69 (12) | 71 (12) | 72 (13) | < 0.001 |
| Hypertension, n (%) | 14,444 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||||||||||||||||
| Non-hypertension | 8315 (62.69) | 2413 (79.31) | 2224 (67.21) | 2011 (57.95) | 1667 (47.05) | 2491 (78.79) | 2268 (66.90) | 1998 (59.45) | 1558 (45.30) | 2276 (73.46) | 2182 (66.45) | 1932 (59.32) | 1925 (52.45) | 2633 (75.95) | 2288 (68.83) | 1973 (57.28) | 1421 (43.70) | |||||
| Hypertension | 6129 (37.31) | 859 (20.69) | 1379 (32.79) | 1759 (42.05) | 2132 (52.95) | 874 (21.21) | 1389 (33.10) | 1717 (40.55) | 2149 (54.70) | 1093 (26.54) | 1454 (33.55) | 1671 (40.68) | 1911 (47.55) | 1116 (24.05) | 1299 (31.17) | 1692 (42.72) | 2022 (56.30) | |||||
| Diabetes, n (%) | 14,613 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||||||||||||||||
| Non-diabetes | 11,863 (85.87) | 3149 (96.37) | 3271 (92.87) | 3141 (86.97) | 2302 (67.38) | 3207 (96.14) | 3252 (91.06) | 2970 (85.23) | 2434 (70.80) | 3062 (92.74) | 3140 (89.77) | 2887 (84.35) | 2774 (77.28) | 3552 (95.60) | 3270 (93.51) | 3064 (86.56) | 1977 (62.72) | |||||
| Diabetes | 2750 (14.13) | 171 (3.63) | 379 (7.13) | 672 (13.03) | 1528 (32.62) | 205 (3.86) | 457 (8.94) | 785 (14.77) | 1303 (29.20) | 349 (7.26) | 540 (10.23) | 766 (15.65) | 1095 (22.72) | 248 (4.40) | 362 (6.49) | 640 (13.44) | 1500 (37.28) | |||||
| Hyperlipidemia, n (%) | 14,653 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||||||||||||||||
| Non-hyperlipidemia | 3665 (26.31) | 1832 (56.98) | 1170 (33.22) | 599 (15.21) | 64 (1.26) | 1621 (49.04) | 1033 (28.24) | 616 (16.94) | 395 (10.43) | 1787 (54.13) | 1256 (35.66) | 607 (17.38) | 15 (0.43) | 1530 (42.02) | 1032 (28.50) | 699 (18.30) | 404 (11.72) | |||||
| Hyperlipidemia | 10,988 (73.69) | 1493 (43.02) | 2488 (66.78) | 3225 (84.79) | 3782 (98.74) | 1795 (50.96) | 2686 (71.76) | 3148 (83.06) | 3359 (89.57) | 1630 (45.87) | 2428 (64.34) | 3061 (82.62) | 3869 (99.57) | 2280 (57.98) | 2611 (71.50) | 3015 (81.70) | 3082 (88.28) | |||||
| CVD, n (%) | 14,458 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||||||||||||||||
| Non-CVD | 12,905 (91.58) | 3005 (93.67) | 3286 (90.85) | 3357 (88.46) | 3257 (84.86) | 3092 (93.36) | 3311 (90.00) | 3322 (88.80) | 3180 (85.35) | 3075 (92.54) | 3283 (90.47) | 3213 (88.24) | 3334 (86.26) | 3466 (92.38) | 3269 (91.16) | 3265 (89.13) | 2905 (84.03) | |||||
| CVD | 1553 (8.42) | 203 (6.33) | 331 (9.15) | 438 (11.54) | 581 (15.14) | 220 (6.64) | 368 (10.00) | 419 (11.20) | 546 (14.65) | 248 (7.46) | 346 (9.53) | 428 (11.76) | 531 (13.74) | 286 (7.62) | 317 (8.84) | 398 (10.87) | 552 (15.97) | |||||
| MetS, n (%) | 14,653 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |||||||||||||||||
| Non-MetS | 8095 (58.77) | 2875 (86.47) | 2613 (71.43) | 1938 (50.68) | 669 (17.39) | 3104 (90.87) | 2560 (68.84) | 1619 (43.01) | 812 (21.63) | 2790 (81.65) | 2573 (69.84) | 1905 (51.94) | 827 (21.29) | 3242 (85.09) | 2436 (66.87) | 1671 (44.99) | 746 (21.40) | |||||
| MetS | 6558 (41.23) | 450 (13.53) | 1045 (28.57) | 1886 (49.32) | 3177 (82.61) | 312 (9.13) | 1159 (31.16) | 2145 (56.99) | 2942 (78.37) | 627 (18.35) | 1111 (30.16) | 1763 (48.06) | 3057 (78.71) | 568 (14.91) | 1207 (33.13) | 2043 (55.01) | 2740 (78.60) | |||||
| All-cause mortality, n (%) | 14,653 | 2085 (10.23) | 249 (5.16) | 479 (8.70) | 611 (11.63) | 746 (15.21) | < 0.001 | 431 (8.34) | 551 (10.39) | 561 (10.89) | 542 (11.33) | < 0.001 | 337 (6.85) | 499 (9.00) | 592 (11.79) | 657 (13.01) | < 0.001 | 504 (8.31) | 487 (9.68) | 513 (10.36) | 581 (13.32) | < 0.001 |
| Cardiovascular mortality, n (%) | 14,653 | 549 (2.61) | 57 (1.12) | 129 (2.41) | 147 (2.56) | 216 (4.30) | < 0.001 | 86 (1.74) | 148 (2.76) | 146 (2.37) | 169 (3.58) | < 0.001 | 86 (1.62) | 119 (2.35) | 155 (2.82) | 189 (3.56) | < 0.001 | 127 (1.86) | 126 (2.50) | 124 (2.45) | 172 (3.94) | < 0.001 |
The data was shown as mean (SD) for continuous, n (%) for categorical
TyG index triglyceride glucose index, METS-IR metabolic score for Insulin resistance, TG triglyceride, HDL-C high-density lipoprotein-cholesterol, HOMA-IR homeostatic model assessment of insulin resistance, BMI body mass index, PIR the ratio of family income to poverty, SBP systolic pressure, DBP diastolic pressure, CVD cardiovascular disease, MetS metabolic syndrome
P values in bold meant significantly different (P < 0.05)
Table 2.
Baseline levels of laboratory characteristics according to TyG Index, METS-IR, TG/HDL-C and HOMA-IR quartiles, NHANES 2001–2018
| Overall | TyG index levels | METS-IR levels | TG/HDL-C levels | HOMA-IR levels | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | N | Overall, N = 14,653 | Q1, N = 3325 (22.69%) | Q2, N = 3658 (24.96%) | Q3, N = 3824 (26.10%) | Q4, N = 3846 (26.25%) | P Value | Q1, N = 3416 (23.31%) | Q2, N = 3719 (25.38%) | Q3, N = 3764 (25.69%) | Q4, N = 3754 (25.62%) | P Value | Q1, N = 3417 (23.32%) | Q2, N = 3684 (25.14%) | Q3, N = 3668 (25.03%) | Q4, N = 3884 (26.51%) | P Value | Q1, N = 3810 (26.00%) | Q2, N = 3643 (24.86%) | Q3, N = 3714 (25.35%) | Q4, N = 3486 (23.79%) | P Value |
| HbA1C, % | 14,622 | 5.57 (0.90) | 5.27 (0.43) | 5.40 (0.49) | 5.53 (0.65) | 6.06 (1.43) | < 0.001 | 5.28 (0.45) | 5.43 (0.66) | 5.59 (0.87) | 5.97 (1.27) | < 0.001 | 5.36 (0.57) | 5.47 (0.75) | 5.61 (0.90) | 5.80 (1.18) | < 0.001 | 5.30 (0.52) | 5.38 (0.56) | 5.56 (0.75) | 6.15 (1.40) | < 0.001 |
| FINS, pmol/L | 14,653 | 73.43 (80.65) | 45.30 (37.02) | 59.12 (50.71) | 79.26 (62.74) | 109.22 (126.05) | < 0.001 | 36.42 (24.52) | 52.01 (33.99) | 75.19 (49.25) | 130.85 (129.30) | < 0.001 | 44.50 (42.59) | 59.98 (50.90) | 78.74 (69.16) | 107.68 (118.35) | < 0.001 | 26.29 (8.34) | 47.89 (7.96) | 76.92 (15.23) | 163.96 (135.17) | < 0.001 |
| FPG, mmol/L | 14,653 | 5.80 (1.61) | 5.15 (0.54) | 5.46 (0.70) | 5.74 (0.97) | 6.83 (2.66) | < 0.001 | 5.23 (0.72) | 5.54 (0.97) | 5.87 (1.47) | 6.57 (2.40) | < 0.001 | 5.38 (0.97) | 5.59 (1.20) | 5.85 (1.52) | 6.32 (2.23) | < 0.001 | 5.17 (0.71) | 5.47 (0.79) | 5.82 (1.17) | 7.01 (2.66) | < 0.001 |
| LDL-C, mmol/L | 14,343 | 2.98 (0.91) | 2.60 (0.74) | 2.99 (0.83) | 3.18 (0.91) | 3.15 (1.01) | < 0.001 | 2.75 (0.85) | 3.03 (0.91) | 3.16 (0.92) | 3.00 (0.90) | < 0.001 | 2.66 (0.78) | 2.97 (0.85) | 3.12 (0.91) | 3.17 (0.99) | < 0.001 | 2.86 (0.88) | 3.05 (0.88) | 3.08 (0.94) | 2.96 (0.91) | < 0.001 |
| HDL-C, mmol/L | 14,653 | 1.39 (0.42) | 1.64 (0.45) | 1.47 (0.39) | 1.32 (0.34) | 1.13 (0.29) | < 0.001 | 1.74 (0.44) | 1.44 (0.34) | 1.25 (0.28) | 1.12 (0.28) | < 0.001 | 1.81 (0.43) | 1.47 (0.29) | 1.26 (0.25) | 1.04 (0.22) | < 0.001 | 1.60 (0.44) | 1.43 (0.40) | 1.30 (0.37) | 1.16 (0.29) | < 0.001 |
| TC, mmol/L | 14,653 | 5.04 (1.08) | 4.54 (0.88) | 4.92 (0.94) | 5.18 (1.00) | 5.51 (1.22) | < 0.001 | 4.92 (1.00) | 5.03 (1.05) | 5.15 (1.10) | 5.07 (1.14) | < 0.001 | 4.78 (0.95) | 4.91 (0.99) | 5.04 (1.03) | 5.41 (1.21) | < 0.001 | 4.94 (1.03) | 5.09 (1.05) | 5.14 (1.12) | 5.02 (1.11) | < 0.001 |
| TG, mmol/L | 14,653 | 1.50 (1.35) | 0.63 (0.15) | 1.01 (0.15) | 1.47 (0.25) | 2.86 (2.11) | < 0.001 | 0.93 (0.46) | 1.23 (0.66) | 1.62 (1.04) | 2.22 (2.16) | < 0.001 | 0.66 (0.19) | 1.01 (0.22) | 1.42 (0.31) | 2.79 (2.07) | < 0.001 | 1.06 (0.88) | 1.33 (1.05) | 1.69 (1.40) | 2.05 (1.83) | < 0.001 |
| Scr, umol/L | 14,638 | 78.71 (34.06) | 75.07 (34.42) | 78.36 (30.96) | 80.10 (33.53) | 81.14 (36.90) | < 0.001 | 75.38 (36.43) | 80.28 (37.93) | 79.85 (26.35) | 79.42 (33.93) | < 0.001 | 75.05 (33.07) | 77.62 (33.58) | 79.89 (32.78) | 81.99 (36.12) | < 0.001 | 78.55 (42.81) | 78.83 (36.97) | 78.62 (26.55) | 78.90 (23.01) | 0.011 |
| Uric acid, umol/L | 14,638 | 327.62 (82.37) | 290.17 (72.04) | 316.37 (75.90) | 341.18 (79.50) | 361.19 (84.08) | < 0.001 | 283.23 (69.26) | 318.32 (74.40) | 342.25 (77.56) | 367.79 (83.07) | < 0.001 | 288.44 (70.76) | 315.05 (74.99) | 338.11 (80.52) | 365.48 (82.04) | < 0.001 | 298.61 (73.66) | 317.57 (76.08) | 340.39 (80.29) | 364.17 (86.23) | < 0.001 |
| BUN, mmol/L | 14,639 | 4.72 (1.86) | 4.48 (1.64) | 4.65 (1.78) | 4.76 (1.92) | 4.97 (2.05) | < 0.001 | 4.45 (1.74) | 4.77 (1.80) | 4.78 (1.81) | 4.87 (2.07) | < 0.001 | 4.61 (1.73) | 4.66 (1.79) | 4.74 (1.94) | 4.84 (1.97) | < 0.001 | 4.54 (1.79) | 4.69 (1.86) | 4.76 (1.73) | 4.93 (2.08) | < 0.001 |
| Albumin, g/L | 14,639 | 42.66 (3.17) | 43.02 (3.18) | 42.66 (3.16) | 42.51 (3.15) | 42.48 (3.17) | < 0.001 | 43.55 (3.15) | 43.02 (3.00) | 42.58 (2.92) | 41.48 (3.24) | < 0.001 | 42.95 (3.11) | 42.73 (3.22) | 42.38 (3.24) | 42.60 (3.10) | < 0.001 | 43.16 (3.18) | 42.91 (3.07) | 42.65 (3.02) | 41.70 (3.26) | < 0.001 |
| LDH, IU/L | 14,619 | 127.49 (28.92) | 124.76 (28.15) | 127.81 (32.93) | 128.30 (26.44) | 128.96 (27.47) | < 0.001 | 123.02 (26.53) | 126.34 (25.68) | 128.56 (33.78) | 132.17 (28.42) | < 0.001 | 127.09 (27.25) | 126.65 (26.85) | 127.80 (33.43) | 128.41 (27.83) | 0.12 | 126.06 (29.73) | 126.23 (26.16) | 127.45 (32.45) | 131.00 (26.22) | < 0.001 |
| ALT, IU/L | 14,623 | 25.88 (26.36) | 21.62 (22.87) | 23.62 (15.34) | 26.84 (17.12) | 31.34 (41.24) | < 0.001 | 20.87 (22.26) | 24.60 (38.84) | 27.54 (18.68) | 30.65 (19.15) | < 0.001 | 21.48 (22.38) | 23.19 (15.83) | 26.69 (16.59) | 31.73 (40.39) | < 0.001 | 21.84 (20.60) | 23.25 (15.71) | 27.58 (40.29) | 32.54 (20.98) | < 0.001 |
| AST, IU/L | 14,621 | 25.39 (17.80) | 24.26 (22.97) | 24.61 (18.88) | 25.43 (12.74) | 27.23 (15.11) | < 0.001 | 24.34 (19.65) | 24.81 (12.50) | 26.04 (21.98) | 26.39 (15.54) | < 0.001 | 24.50 (20.45) | 24.31 (16.61) | 25.39 (16.24) | 27.23 (17.58) | < 0.001 | 24.86 (19.58) | 24.00 (13.08) | 25.27 (19.75) | 27.90 (17.51) | < 0.001 |
| TBil, umol/L | 14,633 | 12.74 (5.16) | 12.70 (5.30) | 13.01 (5.40) | 12.71 (5.10) | 12.54 (4.79) | 0.15 | 13.22 (5.26) | 13.15 (5.36) | 12.78 (5.02) | 11.82 (4.86) | < 0.001 | 12.72 (5.25) | 12.87 (5.22) | 12.73 (5.30) | 12.65 (4.87) | 0.80 | 13.47 (5.47) | 13.03 (5.14) | 12.36 (4.85) | 11.86 (4.90) | < 0.001 |
| GGT, IU/L | 14,639 | 28.21 (39.76) | 19.73 (23.78) | 23.34 (23.67) | 28.81 (31.76) | 40.78 (62.89) | < 0.001 | 20.87 (28.32) | 25.75 (30.61) | 30.08 (40.94) | 36.31 (52.87) | < 0.001 | 21.47 (31.50) | 23.46 (24.23) | 28.90 (31.87) | 38.27 (58.63) | < 0.001 | 22.21 (29.44) | 23.96 (27.02) | 29.73 (32.49) | 39.68 (63.08) | < 0.001 |
| Serum K, mmol/L | 14,638 | 4.03 (0.33) | 4.00 (0.32) | 4.03 (0.33) | 4.04 (0.34) | 4.07 (0.34) | < 0.001 | 4.01 (0.34) | 4.03 (0.34) | 4.04 (0.32) | 4.06 (0.32) | < 0.001 | 4.01 (0.33) | 4.03 (0.33) | 4.04 (0.34) | 4.06 (0.33) | < 0.001 | 4.02 (0.34) | 4.02 (0.33) | 4.05 (0.32) | 4.06 (0.34) | < 0.001 |
| Serum Na, mmol/L | 14,639 | 139.13 (2.16) | 139.23 (2.16) | 139.26 (2.07) | 139.21 (2.08) | 138.83 (2.29) | < 0.001 | 139.14 (2.19) | 139.33 (2.23) | 139.19 (2.05) | 138.87 (2.14) | < 0.001 | 139.22 (2.24) | 139.26 (2.05) | 139.15 (2.16) | 138.90 (2.18) | < 0.001 | 139.17 (2.19) | 139.26 (2.10) | 139.16 (2.07) | 138.88 (2.27) | < 0.001 |
| Serum Fe, umol/L | 14,631 | 16.22 (6.54) | 15.68 (6.90) | 16.39 (6.81) | 16.37 (6.30) | 16.38 (6.12) | < 0.001 | 17.33 (7.28) | 16.61 (6.63) | 16.26 (6.17) | 14.65 (5.66) | < 0.001 | 16.00 (6.95) | 16.41 (6.72) | 16.13 (6.47) | 16.29 (6.04) | 0.06 | 17.21 (7.20) | 16.61 (6.58) | 15.88 (6.07) | 14.78 (5.78) | < 0.001 |
| WBC, *10^9/L | 14,627 | 6.78 (2.19) | 6.13 (1.81) | 6.65 (2.36) | 6.95 (2.21) | 7.35 (2.14) | < 0.001 | 6.29 (1.95) | 6.57 (2.45) | 6.84 (2.00) | 7.42 (2.17) | < 0.001 | 6.09 (1.87) | 6.58 (2.08) | 7.03 (2.23) | 7.35 (2.32) | < 0.001 | 6.28 (2.34) | 6.62 (2.18) | 6.94 (2.00) | 7.45 (2.01) | < 0.001 |
| PLT, *10^9/L | 14,626 | 251.13 (66.10) | 242.04 (62.14) | 251.31 (65.92) | 254.86 (66.94) | 255.81 (68.22) | < 0.001 | 247.52 (62.97) | 246.04 (64.22) | 251.26 (64.76) | 259.79 (71.34) | < 0.001 | 241.84 (61.92) | 251.14 (65.30) | 256.04 (68.23) | 254.96 (67.67) | < 0.001 | 246.55 (64.40) | 250.52 (64.12) | 253.34 (64.61) | 255.51 (71.84) | < 0.001 |
| Hemoglobin, g/L | 14,627 | 144.44 (14.69) | 139.98 (14.17) | 143.55 (14.57) | 145.81 (14.44) | 148.23 (14.33) | < 0.001 | 141.35 (13.63) | 145.05 (14.46) | 146.01 (14.66) | 145.45 (15.52) | < 0.001 | 139.34 (13.83) | 143.33 (14.32) | 145.26 (14.72) | 149.37 (14.08) | < 0.001 | 142.43 (14.20) | 144.52 (14.31) | 145.68 (15.05) | 145.64 (15.08) | < 0.001 |
The data was shown as mean (SD)
TyG index triglyceride glucose index, METS-IR metabolic score for Insulin resistance, TG triglyceride, HDL-C high-density lipoprotein-cholesterol, HOMA-IR homeostatic model assessment of insulin resistance, HbA1C glycosylated hemoglobin, FINS fasting serum insulin, FPG fasting plasma glucose, LDL-C low-density lipoprotein-cholesterol, TC cholesterol, Scr serum creatinine, BUN blood urea nitrogen, LDH lactate dehydrogenase, ALT alanine transaminase, AST aspartate transaminase, TBil total bilirubin, GGT gamma glutamyl transferase, K potassium, Na sodium, Fe iron, WBC white blood cells, PLT blood platelet
P values in bold meant significantly different (P < 0.05)
Fig. 2.
All-cause mortality and cardiovascular mortality bar graphs based on quartiles of TyG index, METS-IR, TG/HDL-C and HOMA-IR stratification. TyG index triglyceride glucose index, METS-IR metabolic score for Insulin resistance, TG triglyceride, HDL-C high-density lipoprotein-cholesterol, HOMA-IR homeostatic model assessment of insulin resistance
Table 3.
Baseline characteristics according to different prognoses, NHANES 2001–2018
| Overall | All-cause mortality | Cardiovascular mortality | ||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | N | Overall N = 14,653 |
Survival group N = 12,568 (89.77%) |
Death group N = 2085 (10.23%) |
P Value | Non-cardiovascular death group N = 14,104 (97.39%) |
Cardiovascular death group N = 549 (2.61%) |
P Value |
| Age, years | 14,653 | 46 (17) | 44 (15) | 65 (14) | < 0.001 | 46 (16) | 66 (14) | < 0.001 |
| Gender, n (%) | 14,653 | < 0.001 | < 0.001 | |||||
| Male | 7423 (49.82) | 6199 (49.24) | 1224 (54.88) | 7080 (49.57) | 343 (59.06) | |||
| Female | 7230 (50.18) | 6369 (50.76) | 861 (45.12) | 7024 (50.43) | 206 (40.94) | |||
| BMI, kg/m2 | 14,653 | 0.10 | 0.009 | |||||
| Underweight (< 18.5) | 216 (1.53) | 177 (1.46) | 39 (2.09) | 210 (1.54) | 6 (1.15) | |||
| Normal (18.5 to < 25) | 4142 (29.76) | 3556 (30.02) | 586 (27.48) | 4012 (29.92) | 130 (24.06) | |||
| Overweight (25 to < 30) | 5006 (33.55) | 4255 (33.44) | 751 (34.52) | 4813 (33.60) | 193 (31.48) | |||
| Obesity (30 or greater) | 5289 (35.16) | 4580 (35.08) | 709 (35.91) | 5069 (34.94) | 220 (43.31) | |||
| Race, n (%) | 14,653 | < 0.001 | 0.002 | |||||
| Mexican American | 2496 (7.78) | 2258 (8.25) | 238 (3.67) | 2432 (7.87) | 64 (4.24) | |||
| Other Hispanic | 1172 (4.65) | 1082 (4.85) | 90 (2.91) | 1153 (4.72) | 19 (2.13) | |||
| Non-Hispanic White | 7058 (70.84) | 5725 (69.90) | 1333 (79.11) | 6720 (70.68) | 338 (76.71) | |||
| Non-Hispanic Black | 2756 (10.54) | 2393 (10.54) | 363 (10.61) | 2643 (10.48) | 113 (12.93) | |||
| Other/multiracial | 1171 (6.19) | 1110 (6.46) | 61 (3.70) | 1156 (6.25) | 15 (3.99) | |||
| PIR, n (%) | 14,653 | < 0.001 | < 0.001 | |||||
| < 1.0 | 2879 (13.50) | 2479 (13.33) | 400 (15.03) | 2769 (13.47) | 110 (14.79) | |||
| 1.0–3.0 | 6121 (36.23) | 5048 (34.93) | 1073 (47.65) | 5840 (35.88) | 281 (49.40) | |||
| > 3.0 | 5653 (50.27) | 5041 (51.74) | 612 (37.32) | 5495 (50.65) | 158 (35.81) | |||
| Education, n (%) | 14,653 | < 0.001 | < 0.001 | |||||
| Below high school | 1528 (5.38) | 1163 (4.69) | 365 (11.47) | 1428 (5.21) | 100 (11.92) | |||
| High school | 5558 (34.78) | 4644 (33.69) | 914 (44.32) | 5318 (34.49) | 240 (45.57) | |||
| Above high school | 7567 (59.84) | 6761 (61.62) | 806 (44.21) | 7358 (60.30) | 209 (42.51) | |||
| Drinking, n (%) | 14,653 | 0.004 | 0.012 | |||||
| Never | 2060 (11.46) | 1751 (11.21) | 309 (13.61) | 1972 (11.39) | 88 (14.12) | |||
| Former | 3923 (24.63) | 3355 (24.38) | 568 (26.85) | 3762 (24.51) | 161 (29.11) | |||
| Current | 8670 (63.91) | 7462 (64.41) | 1208 (59.54) | 8370 (64.10) | 300 (56.77) | |||
| Smoking, n (%) | 14,653 | < 0.001 | < 0.001 | |||||
| Never | 7867 (53.25) | 7079 (55.15) | 788 (36.57) | 7631 (53.53) | 236 (42.70) | |||
| Former | 3400 (22.70) | 2596 (21.11) | 804 (36.62) | 3199 (22.41) | 201 (33.51) | |||
| Current | 3386 (24.05) | 2893 (23.74) | 493 (26.81) | 3274 (24.06) | 112 (23.79) | |||
| SBP, mmHg | 14,356 | 121 (17) | 120 (16) | 132 (22) | < 0.001 | 121 (17) | 134 (24) | < 0.001 |
| DBP, mmHg | 14,356 | 70 (12) | 70 (12) | 67 (16) | < 0.001 | 70 (12) | 66 (17) | < 0.001 |
| Hypertension, n (%) | 14,444 | < 0.001 | < 0.001 | |||||
| Non-hypertension | 8315 (62.69) | 7738 (66.33) | 577 (30.79) | 8186 (63.70) | 129 (24.74) | |||
| Hypertension | 6129 (37.31) | 4641 (33.67) | 1488 (69.21) | 5714 (36.30) | 415 (75.26) | |||
| Diabetes, n (%) | 14,613 | < 0.001 | < 0.001 | |||||
| Non-diabetes | 11,863 (85.87) | 10,537 (88.13) | 1326 (66.04) | 11,542 (86.60) | 321 (58.78) | |||
| Diabetes | 2750 (14.13) | 1997 (11.87) | 753 (33.96) | 2523 (13.40) | 227 (41.22) | |||
| Hyperlipidemia, n (%) | 14,653 | < 0.001 | < 0.001 | |||||
| Non-hyperlipidemia | 3665 (26.31) | 3307 (27.43) | 358 (16.49) | 3591 (26.66) | 74 (13.39) | |||
| Hyperlipidemia | 10,988 (73.69) | 9261 (72.57) | 1727 (83.51) | 10,513 (73.34) | 475 (86.61) | |||
| CVD, n (%) | 14,458 | < 0.001 | < 0.001 | |||||
| Non-CVD | 12,905 (91.58) | 11,475 (92.73) | 1430 (68.62) | 12,579 (90.44) | 326 (59.38) | |||
| CVD | 1553 (8.42) | 899 (7.27) | 654 (31.38) | 1330 (9.56) | 223 (40.62) | |||
| MetS, n (%) | 14,653 | < 0.001 | < 0.001 | |||||
| Non-MetS | 8095 (58.77) | 7307 (58.14) | 788 (37.79) | 7913 (56.10) | 182 (33.15) | |||
| MetS | 6558 (41.23) | 5261 (41.86) | 1297 (62.21) | 6191 (43.90) | 367 (66.85) | |||
| TyG index | 14,653 | 8.63 (0.66) | 8.60 (0.66) | 8.87 (0.66) | < 0.001 | 8.62 (0.66) | 8.91 (0.69) | < 0.001 |
| METS-IR | 14,653 | 42.92 (12.54) | 42.80 (12.51) | 43.97 (12.74) | < 0.001 | 42.83 (12.49) | 46.01 (13.71) | < 0.001 |
| TG/HDL-C | 14,653 | 2.97 (3.83) | 2.92 (3.90) | 3.33 (3.08) | < 0.001 | 2.95 (3.85) | 3.47 (3.16) | < 0.001 |
| HOMA-IR | 14,653 | 3.39 (5.23) | 3.29 (5.01) | 4.24 (6.81) | < 0.001 | 3.35 (5.16) | 4.68 (7.34) | < 0.001 |
The data was shown as mean (SD) for continuous, n (%) for categorical
BMI body mass index, PIR the ratio of family income to poverty, SBP systolic pressure, DBP diastolic pressure, CVD cardiovascular disease, MetS metabolic syndrome, TyG index triglyceride glucose index, METS-IR metabolic score for Insulin resistance, TG triglyceride, HDL-C high-density lipoprotein-cholesterol, HOMA-IR homeostatic model assessment of insulin resistance
P values in bold meant significantly different (P < 0.05)
Quartile 1: 5.65 ≤ TyG index ≤ 8.19; 18.80 ≤ METS-IR ≤ 34.32; 0.16 ≤ TG/HDL-C ≤ 1.26; 0.03 ≤ HOMA-IR ≤ 1.45.
Quartile 2: 8.19 < TyG index ≤ 8.61; 34.32 < METS-IR ≤ 41.43; 1.26 < TG/HDL-C ≤ 2.07; 1.45 < HOMA-IR ≤ 2.42.
Quartile 3: 8.61 < TyG index ≤ 9.05; 41.43 < METS-IR ≤ 49.95; 2.07 < TG/HDL-C ≤ 3.50; 2.42 < HOMA-IR ≤ 4.18.
Quartile 4: 9.05 < TyG index ≤ 13.40; 49.95 < METS-IR ≤ 193.33; 3.50 < TG/HDL-C ≤ 141.61; 4.18 < HOMA-IR ≤ 269.41.
Feature selection
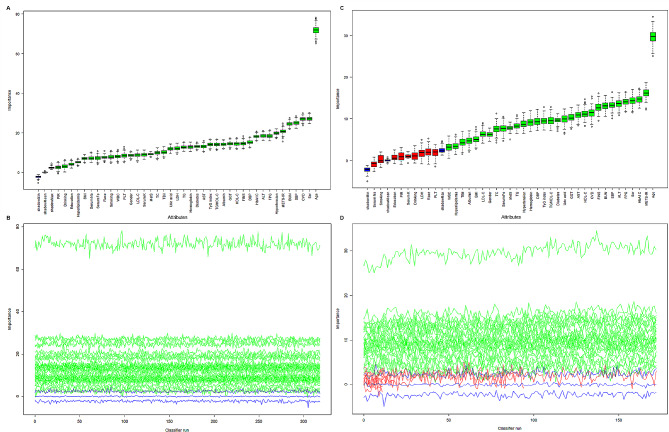
The results of feature screening based on Boruta’s algorithm are shown in Fig. 3. After 500 iterations it was determined that the six variables most closely associated with all-cause mortality (in order of z-value, excluding the insulin resistance replacement index) were age, CVD, Scr, SBP, BUN and hypertension, and the six variables most closely associated with cardiovascular mortality were age, HbA1C, FPG, Scr, ALT and SBP. Although several important characteristics such as gender, race, BMI, PIR, education, drinking and smoking were omitted due to low z-values compared with the most strongly associated or shaded characteristics, they were still included in the subsequent analyses on the basis of previous studies and clinical experience.
Fig. 3.
Feature selection process for all-cause mortality based on Boruta’s algorithm (A) and the value evolution of Z-score in the screening process (B). Feature selection process for cardiovascular mortality based on Boruta’s algorithm (C) and the value evolution of Z-score in the screening process (D). In A and C, the horizontal axis represents the variable name and the vertical axis represents the Z-values of each variable. In B and D, the horizontal axis represents the number of iterations, and the vertical axis represents the change in Z-values during the screening process. The blue boxes and lines correspond to the minimum, average, and maximum Z-scores for a shadow feature. The green boxes and lines represent the confirmed variables and the red ones represent the rejected variables in the model calculation. CVD cardiovascular disease, Scr serum creatinine, SBP systolic pressure, BUN blood urea nitrogen, METS-IR metabolic score for Insulin resistance, FPG fasting plasma glucose, ALT alanine transaminase, HbA1C glycosylated hemoglobin, DBP diastolic pressure, HDL-C high-density lipoprotein-cholesterol, GGT gamma glutamyl transferase, HOMA-IR homeostatic model assessment of insulin resistance, TG triglyceride, TyG index triglyceride glucose index, FINS fasting serum insulin, AST aspartate transaminase, LDH lactate dehydrogenase, TBil total bilirubin, MetS metabolic syndrome, TC cholesterol, K potassium, LDL-C low-density lipoprotein-cholesterol, PLT blood platelet, WBC white blood cells, Fe iron, Na sodium, BMI body mass index, PIR the ratio of family income to poverty
Relationship between the IR replacement index and mortality
We fitted three Cox regression models to investigate the independent correlations between TyG index, METS-IR, TG/HDL-C and HOMA-IR levels and risk of mortality, respectively (Table 4). In conjunction with the feature selection outcomes of the Boruta algorithm, for all-cause mortality, the variables adjusted in Model 3 included age, gender, race, BMI, PIR, education, drinking, smoking, CVD, Scr, SBP, BUN, hypertension. For cardiovascular mortality, the adjusted variables in Model 3 were age, gender, race, BMI, PIR, education, drinking, smoking, HbA1C, FPG, Scr, ALT, SBP. The analysis shows that in Model 1 (unadjusted) and Model 2 (adjusted for age, gender, race), when treated as a continuous variable, the TyG index exhibits a significant correlation with both all-cause mortality and cardiovascular mortality [Model 1: all-cause mortality: HR (95% CI) 1.595 (1.484–1.714), P < 0.001; cardiovascular mortality: HR (95% CI) 1.702 (1.514–1.913), P < 0.001; Model 2: all-cause mortality: HR (95% CI) 1.248 (1.150–1.355), P < 0.001; cardiovascular mortality: HR (95% CI) 1.375 (1.173–1.612), P < 0.001]; similarly, METS-IR demonstrates a significant correlation with both all-cause mortality and cardiovascular mortality [Model 1: all-cause mortality: HR (95% CI) 1.008 (1.004–1.013), P < 0.001; cardiovascular mortality: HR (95% CI) 1.019 (1.012–1.026), P < 0.001; Model 2: all-cause mortality: HR (95% CI) 1.009 (1.004–1.014), P < 0.001; cardiovascular mortality: HR (95% CI) 1.024 (1.015–1.032), P < 0.001]; there was also a correlation between TG/HDL-C and all-cause and cardiovascular mortality [Model 1: all-cause mortality: HR (95% CI) 1.011 (1.002–1.019), P = 0.012; cardiovascular mortality: HR (95% CI) 1.015 (1.004–1.025), P = 0.008; Model 2: all-cause mortality: HR (95% CI) 1.013 (1.003–1.024), P = 0.009; cardiovascular mortality: HR (95% CI) 1.019 (1.005–1.033), P = 0.007]; finally, HOMA-IR was similarly significantly correlated with both all-cause and cardiovascular mortality [Model 1: all-cause mortality: HR (95% CI) 1.018 (1.012–1.023), P < 0.001; cardiovascular mortality: HR (95% CI) 1.019 (1.013–1.026), P < 0.001; Model 2: all-cause mortality: HR (95% CI) 1.016 (1.012–1.021), P < 0.001; cardiovascular mortality: HR (95% CI) 1.019 (1.014–1.025), P < 0.001]. In model 3, only METS-IR as a continuous variable was associated with both all-cause and cardiovascular mortality [all-cause mortality: HR (95% CI) 1.015 (1.008–1.022), P < 0.001; cardiovascular mortality: HR (95% CI) 1.018 (1.004–1.032), P = 0.012]. When considered as a categorical variable, all four indexes show a significant correlation with both all-cause mortality and cardiovascular mortality in the three Cox regression models (P < 0.001), although not all models demonstrate higher HR for patients with moderate and high scores.
Table 4.
HRs (95% CIs) for mortality according to TyG Index, METS-IR, TG/HDL-C and HOMA-IR quartiles, NHANES 2001–2018
| All-cause mortality | Cardiovascular mortality | |||
|---|---|---|---|---|
| Model | HR (95%CI) | P Value | HR (95%CI) | P Value |
| TyG index | ||||
| Model 1 | ||||
| Continuous | 1.595 (1.485–1.714) | < 0.001*** | 1.702 (1.514–1.913) | < 0.001*** |
| Quartiles | ||||
| Q1 | 1 | 1 | ||
| Q2 | 1.571 (1.271–1.942) | < 0.001*** | 2.012 (1.290–3.140) | 0.002** |
| Q3 | 2.090 (1.744–2.505) | < 0.001*** | 2.128 (1.422–3.185) | < 0.001*** |
| Q4 | 2.711 (2.232–3.294) | < 0.001*** | 3.545 (2.462–5.104) | < 0.001*** |
| P for trend | < 0.001*** | < 0.001*** | ||
| Model 2 | ||||
| Continuous | 1.248 (1.150–1.355) | < 0.001*** | 1.375 (1.173–1.612) | < 0.001*** |
| Quartiles | ||||
| Q1 | 1 | 1 | ||
| Q2 | 0.959 (0.782–1.177) | 0.690 | 1.208 (0.787–1.854) | 0.387 |
| Q3 | 1.022 (0.860–1.215) | 0.810 | 1.029 (0.686–1.545) | 0.889 |
| Q4 | 1.218 (1.018–1.458) | 0.031* | 1.583 (1.093–2.292) | 0.015* |
| P for trend | < 0.001*** | < 0.001*** | ||
| Model 3 | ||||
| Continuous | 1.130 (1.039–1.230) | 0.004** | 0.959 (0.765–1.201) | 0.713 |
| Quartiles | ||||
| Q1 | 1 | 1 | ||
| Q2 | 0.930 (0.757–1.142) | 0.486 | 1.097 (0.715–1.681) | 0.672 |
| Q3 | 0.905 (0.752–1.089) | 0.290 | 0.836 (0.547–1.278) | 0.408 |
| Q4 | 1.038 (0.863–1.247) | 0.695 | 0.992 (0.660–1.492) | 0.971 |
| P for trend | < 0.001*** | < 0.001*** | ||
| METS-IR | ||||
| Model 1 | ||||
| Continuous | 1.008 (1.004–1.013) | < 0.001*** | 1.019 (1.012–1.026) | < 0.001*** |
| Quartiles | ||||
| Q1 | 1 | 1 | ||
| Q2 | 1.251 (1.098–1.425) | < 0.001*** | 1.597 (1.146–2.226) | 0.006** |
| Q3 | 1.315 (1.148–1.505) | < 0.001*** | 1.379 (0.995–1.910) | 0.054 |
| Q4 | 1.433 (1.246–1.648) | < 0.001*** | 2.181 (1.569–3.032) | < 0.001*** |
| P for trend | < 0.001*** | < 0.001*** | ||
| Model 2 | ||||
| Continuous | 1.009 (1.004–1.014) | < 0.001*** | 1.024 (1.015–1.032) | < 0.001*** |
| Quartiles | ||||
| Q1 | 1 | 1 | ||
| Q2 | 0.859 (0.758–0.974) | 0.017* | 1.046 (0.761–1.439) | 0.781 |
| Q3 | 0.890 (0.786–1.006) | 0.063 | 0.887 (0.644–1.221) | 0.462 |
| Q4 | 1.157 (1.008–1.328) | 0.038* | 1.721 (1.240–2.390) | 0.001** |
| P for trend | < 0.001*** | < 0.001*** | ||
| Model 3 | ||||
| Continuous | 1.015 (1.008–1.022) | < 0.001*** | 1.018 (1.004–1.032) | 0.012* |
| Quartiles | ||||
| Q1 | 1 | 1 | ||
| Q2 | 0.931 (0.776–1.117) | 0.441 | 0.957 (0.617–1.483) | 0.843 |
| Q3 | 1.034 (0.821–1.302) | 0.778 | 0.643 (0.382–1.084) | 0.098 |
| Q4 | 1.222 (0.933–1.601) | 0.146 | 0.868 (0.486–1.550) | 0.631 |
| P for trend | < 0.001*** | < 0.001*** | ||
| TG/HDL-C | ||||
| Model 1 | ||||
| Continuous | 1.011 (1.002–1.019) | 0.012* | 1.015 (1.004–1.025) | 0.008** |
| Quartiles | ||||
| Q1 | 1 | 1 | ||
| Q2 | 1.197 (0.988–1.450) | 0.067 | 1.319 (0.930–1.872) | 0.121 |
| Q3 | 1.551 (1.312–1.834) | < 0.001*** | 1.571 (1.135–2.174) | 0.006** |
| Q4 | 1.659 (1.403–1.961) | < 0.001*** | 1.917 (1.399–2.627) | < 0.001*** |
| P for trend | < 0.001*** | < 0.001*** | ||
| Model 2 | ||||
| Continuous | 1.013 (1.003–1.024) | 0.009** | 1.019 (1.005–1.033) | 0.007** |
| Quartiles | ||||
| Q1 | 1 | 1 | ||
| Q2 | 1.021 (0.841–1.240) | 0.834 | 1.117 (0.781–1.596) | 0.544 |
| Q3 | 1.143 (0.962–1.359) | 0.130 | 1.145(0.821–1.598) | 0.425 |
| Q4 | 1.310 (1.116–1.539) | < 0.001*** | 1.510 (1.091–2.090) | 0.013* |
| P for trend | < 0.001*** | < 0.001*** | ||
| Model 3 | ||||
| Continuous | 1.003 (0.991–1.014) | 0.630 | 0.996 (0.972–1.021) | 0.748 |
| Quartiles | ||||
| Q1 | 1 | 1 | ||
| Q2 | 0.935 (0.765–1.144) | 0.516 | 0.961 (0.666–1.387) | 0.832 |
| Q3 | 0.978 (0.807–1.184) | 0.816 | 0.894 (0.625–1.280) | 0.541 |
| Q4 | 1.075 (0.917–1.259) | 0.372 | 1.014 (0.714–1.441) | 0.937 |
| P for trend | < 0.001*** | < 0.001*** | ||
| HOMA-IR | ||||
| Model 1 | ||||
| Continuous | 1.018 (1.012–1.023) | < 0.001*** | 1.019 (1.013–1.026) | < 0.001*** |
| Quartiles | ||||
| Q1 | 1 | 1 | ||
| Q2 | 1.170 (1.009–1.357) | 0.037* | 1.355 (0.995–1.844) | 0.054 |
| Q3 | 1.320 (1.128–1.544) | < 0.001*** | 1.400 (1.023–1.918) | 0.036* |
| Q4 | 1.822 (1.555–2.135) | < 0.001*** | 2.424 (1.786–3.291) | < 0.001*** |
| P for trend | < 0.001*** | < 0.001*** | ||
| Model 2 | ||||
| Continuous | 1.016 (1.012–1.021) | < 0.001*** | 1.019 (1.014–1.025) | < 0.001*** |
| Quartiles | ||||
| Q1 | 1 | 1 | ||
| Q2 | 0.983 (0.857–1.128) | 0.808 | 1.129 (0.834–1.529) | 0.433 |
| Q3 | 0.954 (0.821–1.110) | 0.543 | 0.982 (0.715–1.349) | 0.910 |
| Q4 | 1.289 (1.117–1.488) | < 0.001*** | 1.677 (1.254–2.244) | < 0.001*** |
| P for trend | < 0.001*** | < 0.001*** | ||
| Model 3 | ||||
| Continuous | 1.016 (1.011–1.021) | < 0.001*** | 1.008 (0.999–1.018) | 0.071 |
| Quartiles | ||||
| Q1 | 1 | 1 | ||
| Q2 | 1.042 (0.901–1.207) | 0.578 | 1.132 (0.808–1.585) | 0.471 |
| Q3 | 0.984 (0.824–1.174) | 0.856 | 0.880 (0.580–1.335) | 0.548 |
| Q4 | 1.254 (1.041–1.511) | 0.017* | 1.128 (0.736–1.729) | 0.581 |
| P for trend | < 0.001*** | < 0.001*** | ||
Model 1: unadjusted
Model 2: adjusted for age, gender, race
Model 3 (All-cause mortality): adjusted for age, gender, race, BMI, PIR, education, drinking, smoking, CVD, Scr, SBP, BUN, hypertension
Model 3 (Cardiovascular mortality): adjusted for age, gender, race, BMI, PIR, education, drinking, smoking, HbA1C, FPG, Scr, ALT, SBP
TyG index triglyceride glucose index, METS-IR metabolic score for Insulin resistance, TG triglyceride, HDL-C high-density lipoprotein-cholesterol, HOMA-IR homeostatic model assessment of insulin resistance, BMI body mass index, PIR the ratio of family income to poverty, CVD cardiovascular disease, Scr serum creatinine, SBP systolic pressure, BUN blood urea nitrogen, HbA1C glycosylated hemoglobin, FPG fasting plasma glucose, ALT alanine transaminase, HR hazard ratio, CI confidence interval
*P < 0.05
**P < 0.01
***P < 0.001
P values in bold are < 0.05
The detection of nonlinear relationships
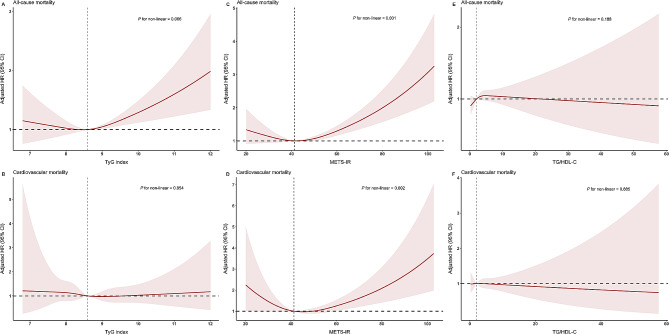
Considering that multivariate Cox proportional risk analyses indicated a potential nonlinear relationship between the three indexes and all-cause and cardiovascular mortality, we employed restricted cubic splines analysis to further investigate this correlation. Figure 4 shows adjusted restricted cubic spline plots of nonlinear associations between METS-IR and all-cause (Fig. 4C, nonlinear P < 0.001) and cardiovascular mortality (Fig. 4D, nonlinear P = 0.002). Although there was no significant nonlinear association between TyG index, TG/HDL-C, HOMA-IR and mortality, we chose the point at which the adjusted HR was 0 as the "inflection point". We fitted the associations between the four indexes and mortality using a standard Cox proportional risk regression model and a two-segmented Cox proportional risk regression model (Table 5). Although we incorporated different variables in exploring the inflection points for all-cause and cardiovascular mortality, through two-segmented Cox proportional risk regression models, we found that these four IR indexes had the same inflection points for all-cause mortality as they did for cardiovascular mortality. The inflection points of the TyG index, METS-IR, TG/HDL-C and HOMA-IR were 8.61, 41.33, 1.98 and 2.49, respectively. When the TyG index was higher than 8.61, for each unit increase in TyG index, the adjusted HR for all-cause mortality increased by 25.7% (HR 1.257; 95% CI 1.108, 1.426, P for log-likelihood ratio = 0.005), while there was no significant correlation with cardiovascular mortality. When METS-IR was higher than 41.33, for each unit increase in METS-IR, the adjusted HR for all-cause mortality and cardiovascular mortality increased by 1.9 and 2.8%, respectively (HR 1.019; 95% CI 1.011, 1.026 and HR 1.028; 95% CI 1.014, 1.043, respectively, P for log-likelihood ratio < 0.05). In the two-segmented Cox proportional risk regression models of TG/HDL-C, there was no significant correlation with either all-cause mortality or cardiovascular mortality (P for log-likelihood ratio > 0.05). Adjusted HR for all-cause mortality increased by 1.5% per unit increase in HOMA-IR when HOMA-IR was higher than 2.49 (HR 1.015; 95% CI 1.010, 1.020, P for log-likelihood ratio < 0.001), while there was no correlation with cardiovascular mortality.
Fig. 4.
Association between TyG index and all-cause (A) and cardiovascular mortality (B) in the general population. Each hazard ratio was calculated with the TyG index level of 8.61 as reference. Association between METS-IR and all-cause (C) and cardiovascular mortality (D) in the general population. Each hazard ratio was calculated with the METS-IR level of 41.33 as reference. Association between TG/HDL-C and all-cause (E) and cardiovascular mortality (F) in the general population. Each hazard ratio was calculated with the TG/HDL-C level of 1.98 as reference. Association between HOMA-IR and all-cause (G) and cardiovascular mortality (H) in the general population. Each hazard ratio was calculated with the HOMA-IR level of 2.49 as reference. All-cause mortality was adjusted for age, gender, race, BMI, PIR, education, drinking, smoking, CVD, Scr, SBP, BUN, hypertension. Cardiovascular mortality was adjusted for age, gender, race, BMI, PIR, education, drinking, smoking, HbA1C, FPG, Scr, ALT, SBP. The solid line and red area represent the estimated values and their corresponding 95% CIs, respectively. TyG index triglyceride glucose index, METS-IR metabolic score for Insulin resistance, TG triglyceride, HDL-C high-density lipoprotein-cholesterol, HOMA-IR homeostatic model assessment of insulin resistance, BMI body mass index, PIR the ratio of family income to poverty, CVD cardiovascular disease, Scr serum creatinine, SBP systolic pressure, BUN blood urea nitrogen, HbA1C glycosylated hemoglobin, FPG fasting plasma glucose, ALT alanine transaminase, HR hazard ratio, CI confidence interval
Table 5.
Threshold effect analysis of TyG index, METS-IR, TG/HDL-C and HOMA-IR on all-cause and cardiovascular mortality, NHANES 2001–2018
| TyG index | Adjusted HR (95% Cl), P-Value | METS-IR | Adjusted HR (95% Cl), P-Value | TG/HDL-C | Adjusted HR (95% Cl), P-Value | HOMA-IR | Adjusted HR (95% Cl), P-Value |
|---|---|---|---|---|---|---|---|
| All-cause mortality | |||||||
| Fitting by the standard linear model | 1.130 (1.039–1.230) 0.004 | Fitting by the standard linear model | 1.015 (1.008–1.022) < 0.001 | Fitting by the standard linear model | 1.003 (0.991–1.014) 0.630 | Fitting by the standard linear model | 1.016 (1.011–1.021) < 0.001 |
| Fitting by the two-piecewise linear model | Fitting by the two-piecewise linear model | Fitting by the two-piecewise linear model | Fitting by the two-piecewise linear model | ||||
| Inflection point | 8.61 | Inflection point | 41.33 | Inflection point | 1.98 | Inflection point | 2.49 |
| TyG index < 8.61 | 0.925 (0.678–1.262) 0.622 | METS-IR < 41.33 | 0.972 (0.950–0.997) 0.030 | TG/HDL-C < 1.98 | 0.879 (0.686–1.127) 0.310 | HOMA-IR < 2.49 | 0.982 (0.833–1.157) 0.826 |
| TyG index ≥ 8.61 | 1.257 (1.108–1.426) < 0.001 | METS-IR ≥ 41.33 | 1.019 (1.011–1.026) < 0.001 | TG/HDL-C ≥ 1.98 | 1.000 (0.989–1.012) 0.979 | HOMA-IR ≥ 2.49 | 1.015 (1.010–1.020) < 0.001 |
| P for Log-likelihood ratio | 0.005 | P for Log-likelihood ratio | < 0.001 | P for Log-likelihood ratio | 0.630 | P for Log-likelihood ratio | < 0.001 |
| Cardiovascular mortality | |||||||
| Fitting by the standard linear model | 0.959 (0.765–1.201) 0.713 | Fitting by the standard linear model | 1.018 (1.004–1.032) 0.012 | Fitting by the standard linear model | 0.996 (0.972–1.021) 0.748 | Fitting by the standard linear model | 1.008 (0.999–1.018) < 0.001 |
| Fitting by the two-piecewise linear model | Fitting by the two-piecewise linear model | Fitting by the two-piecewise linear model | Fitting by the two-piecewise linear model | ||||
| Inflection point | 8.61 | Inflection point | 41.33 | Inflection point | 1.98 | Inflection point | 2.49 |
| TyG index < 8.61 | 1.025 (0.521–2.016) 0.943 | METS-IR < 41.33 | 0.964 (0.915–1.015) 0.162 | TG/HDL-C < 1.98 | 0.953 (0.595–1.525) 0.839 | HOMA-IR < 2.49 | 0.964 (0.691–1.345) 0.830 |
| TyG index ≥ 8.61 | 1.042 (0.758–1.432) 0.801 | METS-IR ≥ 41.33 | 1.028 (1.014–1.043) < 0.001 | TG/HDL-C ≥ 1.98 | 0.995 (0.973–1.018) 0.687 | HOMA-IR ≥ 2.49 | 1.010 (1.002–1.018) < 0.001 |
| P for log-likelihood ratio | 0.706 | P for log-likelihood ratio | 0.022 | P for log-likelihood ratio | 0.735 | P for log-likelihood ratio | 0.124 |
All-cause mortality was adjusted for age, gender, race, BMI, PIR, education, drinking, smoking, CVD, Scr, SBP, BUN, hypertension
Cardiovascular mortality was adjusted for age, gender, race, BMI, PIR, education, drinking, smoking, HbA1C, FPG, Scr, ALT, SBP
TyG index triglyceride glucose index, METS-IR metabolic score for Insulin resistance, TG triglyceride, HDL-C high-density lipoprotein-cholesterol, HOMA-IR homeostatic model assessment of insulin resistance, BMI body mass index, PIR the ratio of family income to poverty, CVD cardiovascular disease, Scr serum creatinine, SBP systolic pressure, BUN blood urea nitrogen, HbA1C glycosylated hemoglobin, FPG fasting plasma glucose, ALT alanine transaminase, HR hazard ratio, CI confidence interval
P values in bold are < 0.05
Stratified analyses
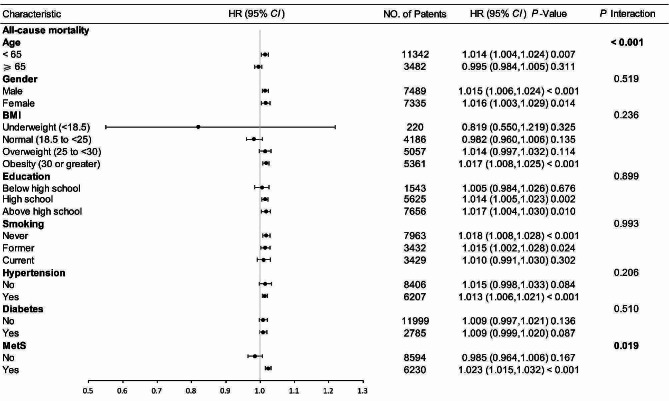
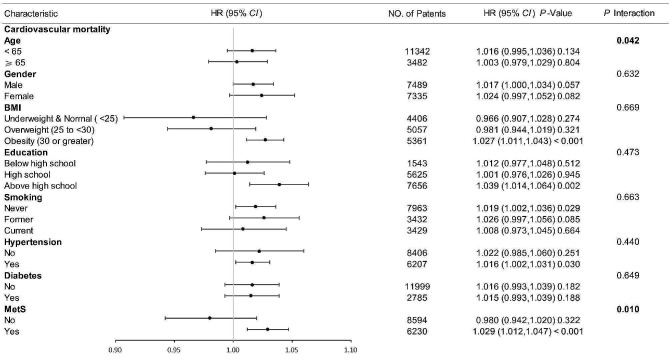
As shown in Figs. 5 and 6, to further assess the effect of METS-IR on outcome indicators, stratification was performed according to age, sex, BMI, education, smoking, hypertension, diabetes, and metabolic syndrome. There were no significant interactions in any of the subgroups except for the age subgroup (age subgroup: all-cause mortality: interaction P < 0.001, cardiovascular mortality: interaction P = 0.044) (other subgroups: all-cause mortality: interaction P = 0.241–0.937, cardiovascular mortality, interaction P = 0.363–0.679). METS-IR was strongly associated with all-cause mortality in patients < 65 years of age (HR (95% CI) 1.014 (1.004, 1.024), P = 0.008), but not in patients aged ≥ 65 years (HR (95% CI) 0.994 (0.983, 1.004), P = 0.247).
Fig. 5.
Subgroup analysis of the association between METS-IR and all-cause mortality. Adjusted for age, gender, race, BMI, PIR, education, drinking, smoking, CVD, Scr, SBP, BUN, hypertension, except the subgroup factors themselves. METS-IR metabolic score for insulin resistance, BMI body mass index, PIR the ratio of family income to poverty, CVD cardiovascular disease, Scr serum creatinine, SBP systolic pressure, BUN blood urea nitrogen, MetS metabolic syndrome, HR hazard ratio, CI confidence interval
Fig. 6.
Subgroup analysis of the association between METS-IR and cardiovascular mortality. Adjusted for age, gender, race, BMI, PIR, education, drinking, smoking, HbA1C, FPG, Scr, ALT, SBP, except the subgroup factors themselves. METS-IR metabolic score for insulin resistance, BMI body mass index, PIR the ratio of family income to poverty, HbA1C glycosylated hemoglobin, FPG fasting plasma glucose, Scr serum creatinine, ALT alanine transaminase, SBP systolic pressure, MetS metabolic syndrome, HR hazard ratio, CI confidence interval
Discussion
To our knowledge, this is the first study comparing the effect of TyG index with other surrogate indexes of IR (METS-IR, TG/HDL-C, HOMA-IR) on mortality in a large cohort. Our study found that TyG index, METS-IR, TG/HDL-C and HOMA-IR were positively correlated with age, BMI, blood pressure, HbA1C, FINS, FPG, TG, UA, BUN, ALT, GGT, serum potassium, WBC and PLT, and negatively correlated with HDL-C. This correlation may be attributed to the presence of traditional CVD risk factors, which have been reported previously [27]. In addition, in contrast to the previous approach of screening variables based on clinical experience alone, we combined the results of Boruta’s algorithm with traditional risk factors to include 13 clinical indicators in the multifactorial Cox regression analyses of all-cause mortality and cardiovascular mortality, respectively. After multivariate Cox regression and restricted cubic splines analysis, we unexpectedly found that the METS-IR performed better than the other three indexes, with significant correlations with both all-cause mortality and cardiovascular mortality, and both showed nonlinear associations that were similar to a “U-shaped” association. Furthermore, we conducted a threshold effect analysis and determined that the inflection point for METS-IR in both all-cause mortality and cardiovascular mortality was 41.33. After adjusting for confounders, each unit increase in the baseline METS-IR was associated with an increase in all-cause and cardiovascular mortality by 1.9 and 2.8%, respectively, for those with the baseline METS-IR above the inflection point. In contrast, TyG index and HOMA-IR were significantly associated with all-cause mortality only, whereas TG/HDL-C was not significantly associated with either mortality. Our study suggests that the METS-IR can be used as a predictor of all-cause and cardiovascular mortality in the general population when combined with important features derived from Boruta algorithm screening.
A large number of studies have been conducted on the relationship between TyG index and mortality in different populations. Chen et al. observed that there was a significant association between TyG index and all-cause and cardiovascular mortality in the general population, and that this association was most pronounced in the 45–64 year age group [13]. Liu et al. found that elevated TyG index can independently predict the mortality of ischemic stroke patients under 65 years old at 3 and 12 months [28]. And in patients with CHD, TyG index was shown to be positively associated with future cardiovascular events, suggesting that TyG index may be a useful predictor of clinical outcomes [29]. In a recent study, Zhang et al. demonstrated a U-shaped association between baseline TyG index and all-cause and cardiovascular disease mortality in patients with diabetes or prediabetic cardiovascular disease [4]. In addition, as early as 2010 Guerrero et al. found that the TyG index showed a high correlation with hyperinsulinemic-euglycemic clamp technique in IR assessment, with significant sensitivity and specificity [30]. Thus, although the exact biological mechanisms underlying the correlation between TyG index and mortality are unknown, the key pathways involved may be related to IR. In conclusion, the role of TyG index in the assessment of IR has been widely recognized and is regarded as a valuable indicator.
On the other hand, alternative estimates of insulin action have been widely used to study the relationship between IR and various clinical syndromes, and this has led to the development of a variety of IR replacement indexes, among which the METS-IR and TG/HDL-C are included. When calculating METS-IR, in addition to requiring fasting glucose and triglycerides, BMI and HDL-C are also needed. It has been shown that METS-IR is significantly associated with fat content in the liver and pancreas, and ectopic fat accumulation in muscle and liver tissues has been suggested as a mechanism for the development of IR [31, 32]. METS-IR, as a novel scoring system for screening insulin sensitivity, can effectively identify individuals at high risk of insulin resistance-associated pathological changes, thus saving costs associated with fasting insulin measurements [33]. It has been shown that the diagnostic performance of METS-IR was significantly higher than the TyG index and TG/HDL ratio, but did not differ from the TyG-BMI index [34]. Ramírez et al. assessed the relationship between obesity and IR by evaluating the abdominal volume index (AVI) and body fat index (BAI) using three risk scales (TyG index, METS-IR and TG/HDL-C), concluding that only in the case of the METS-IR, the AVI and BAI proved to be of value in the prediction of IR [35]. Furthermore, there have also been a number of studies on TG/HDL-C. One study of 449 healthy individuals found that TG/HDL provided an estimate of insulin sensitivity that was as accurate as using FPG and insulin concentration measures, and that the greater the TG/HDL ratio, the more insulin tolerant the patient was [36]. Karelis et al. further supported the significant correlation between TG/HDL-C and indicators of insulin action in a specific population by studying 131 overweight and obese menopausal women [37]. HOMA-IR, on the other hand, has been validated in the general population as early as 1999 and can be used as a common indicator for assessing IR [38]. Meta-analysis of prostate cancer patients by Somayeh et al. showed that HOMA-IR levels were positively correlated with fasting insulin levels, especially in patients older than 65 years [39]. However, due to the heterogeneity of study populations, sample sizes and follow-up times, the predictive effect of the four IR replacement indexes (TyG index, METS-IR, TG/HDL and HOMA-IR) on mortality in a large sample of the general population remains uncertain.
This study explores the predictive value of four IR replacement indexes (TyG index, METS-IR, TG/HDL and HOMA-IR), in relation to mortality in the general population through multivariate Cox proportional risk analysis, incorporating the results of Boruta’s algorithm. The Boruta algorithm is a Random Forest-based feature selection method that identifies the most important features by comparing the Z-value of each feature with the Z-value of the “shaded features”. The Z-value of each attribute is obtained from the Random Forest model at each iteration by copying all the real features and destroying them sequentially, and the Z-value of the shadow is created by destroying the real features randomly. A feature is considered “important” if the Z-value of the real feature is greater than the maximum Z-value of the shaded feature in multiple independent trials [40, 41]. In contrast to previous multivariate regression analyses, which were based on clinical significance and experience, we evaluated the impact of the IR replacement indexes on all-cause and cardiovascular mortality more accurately by combining the most relevant characteristics of the dependent variable with the characteristics screened by Boruta’s algorithm. In our study, we found that among the four indexes, only METS-IR was significantly correlated with all-cause mortality and cardiovascular mortality, and all of them showed non-linear associations similar to a “U-shape”. Further stratified analysis of METS-IR showed significant interactions with age. Specifically, in the general population, significant associations between METS-IR levels and all-cause and cardiovascular mortality were observed mainly in the nonelderly population aged < 65 years. Exposure to insulin resistance resulting from the same disease duration may cause more severe complications in younger patients compared with older adults [13]. This is in part consistent with the study by Liu and Sharif et al. [28, 42].
Strengths and limitations
This study has several advantages. First, we pioneered the application of the Boruta algorithm to select the inclusion factors for multifactorial Cox regression on the basis of a large sample of data, which improved the confidence and accuracy of the study. In addition, our study confirmed the association between METS-IR and all-cause and cardiovascular mortality in the general population, which mainly existed in those aged < 65 years. This not only provides a theoretical basis for the application of METS-IR in non-elderly populations, but also provides new clues for research in the field of IR replacement indexes.
It is also important to point out some limitations of this study. First, this was an observational study and could not prove a cause-and-effect relationship between METS-IR and mortality. Second, relying only on self-reported data from the NHANES questionnaire to obtain smoking, alcohol consumption and comorbidities may have memory bias and subjective bias. Third, the IR replacement index should change dynamically. However, due to cost and other limitations, we were only able to obtain baseline data. Finally, the findings are based only on survey data from the general population in the United States, so careful consideration is needed when generalizing the results to other races and populations. Future studies should be conducted with these limitations in mind to further deepen understanding in this area.
Conclusion
This study retrospectively analyzed 14,653 participants from NHANES to compare the mortality-predicting effects of four IR replacement indexes (TyG index, METS-IR, TG/HDL and HOMA-IR) in the population. The results of the study showed that the METS-IR was significantly associated with all-cause and cardiovascular mortality in the general population, especially in those under 65 years of age.
Acknowledgements
We acknowledged the contributions of NHANES staff for creating and updating the NHANES database.
Abbreviations
- ATP 3
Adult treatment third edition
- AVI
Abdominal volume index
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- BAI
Body fat index
- BMI
Body mass index
- BUN
Blood urea nitrogen
- CDC
Centers for disease control and prevention
- CHD
Coronary heart disease
- CHF
Congestive heart failure
- CI
Confidence interval
- CVD
Cardiovascular disease
- DBP
Diastolic blood pressure
- FINS
Fasting serum insulin
- FPG
Fasting plasma glucose
- GGT
Gamma glutamyl transferase
- HbA1C
Glycosylated hemoglobin
- HDL-C
High-density lipoprotein cholesterol
- HOMA-IR
Homeostasis model assessment of insulin resistance
- HR
Hazard ratio
- IR
Insulin resistance
- LDH
Lactate dehydrogenase
- LDL-C
Low-density lipoprotein cholesterol
- MEC
Mobile examination center
- METS-IR
Metabolic score for insulin resistance
- MetS
Metabolic syndrome
- MI
Myocardial infarction
- NCEP
National cholesterol education program
- NCHS
National center for health statistics
- NDI
National death index
- NHANES
National health and nutrition examination survey
- PIR
Poverty income ratio
- PLT
Blood platelet
- SBP
Systolic blood pressure
- Scr
Serum creatinine
- SD
Standard deviation
- TC
Total cholesterol
- TBil
Total bilirubin
- TG
Triglycerides
- TyG index
Triglyceride glucose index
- WC
Waist circumference
- WBC
White blood cells
Author contributions
MXD and XYZ designed the study. MXD, XZ, SLL and GRM extracted and analyzed the data. MXD drafted the manuscript. LPB, QYZ and WXY revised the manuscript. The final version was approved by all authors.
Funding
This study was partially supported by the Basic Research Program of the Higher Education Key Research Program of the Henan Provincial Department of Education. (Project No. 24A320085, entitled "Study on RCas9-specific silencing of mutant alleles for the treatment of hypertrophic cardiomyopathy caused by MYH7 gene mutation").
Availability of data and materials
Data supporting the results of this study are available from the first author.
Declarations
Ethics approval and consent to participate
The National Center for Health Statistics and Ethics Review Board approved the protocol for NHANES, and all participants provided written informed consent.
Consent for publication
We confirm that we have obtained written informed consent to publish.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ward Z, Bleich S, Cradock A, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440–2450. doi: 10.1056/NEJMsa1909301. [DOI] [PubMed] [Google Scholar]
- 2.Hill MA, Yang Y, Zhang L, et al. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021;119:154766. doi: 10.1016/j.metabol.2021.154766. [DOI] [PubMed] [Google Scholar]
- 3.Tahapary DL, Pratisthita LB, Fitri NA, et al. Challenges in the diagnosis of insulin resistance: focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab Syndr: Clin Res Rev. 2022;16:102581. doi: 10.1016/j.dsx.2022.102581. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Q, Xiao S, Jiao X, et al. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: evidence from NHANES 2001–2018. Cardiovasc Diabetol. 2023;22:279. doi: 10.1186/s12933-023-02030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mancusi C, de Simone G, Best LG, et al. Myocardial mechano-energetic efficiency and insulin resistance in non-diabetic members of the strong heart study cohort. Cardiovasc Diabetol. 2019;18:56. doi: 10.1186/s12933-019-0862-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badmus OO, Hillhouse SA, Anderson CD, et al. Molecular mechanisms of metabolic associated fatty liver disease (MAFLD): functional analysis of lipid metabolism pathways. Clin Sci. 2022;136:1347–1366. doi: 10.1042/CS20220572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minh Huynh V, Tien Hoang A, Sinh Cao T, et al. Assessment of preferred methods to measure insulin resistance in Asian patients with hypertension. J Clin Hypertens. 2021;23:529–537. doi: 10.1111/jch.14155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tao L-C, Xu J-N, Wang T-T, et al. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022 doi: 10.1186/s12933-022-01511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhee EJ. Diabetes in Asians. Endocrinol Metab (Seoul, Korea) 2015;30:263–269. doi: 10.3803/EnM.2015.30.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Monte SM. Therapeutic targets of brain insulin resistance in sporadic Alzheimer’s disease. Front Biosci (Elite Ed) 2012;4:1582–1605. doi: 10.2741/e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chamroonkiadtikun P, Ananchaisarp T, Wanichanon W. The triglyceride-glucose index, a predictor of type 2 diabetes development: a retrospective cohort study. Prim Care Diabetes. 2020;14:161–167. doi: 10.1016/j.pcd.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Simental-Mendía L, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Wu K, Lin Y, et al. Association of triglyceride glucose index with all-cause and cardiovascular mortality in the general population. Cardiovasc Diabetol. 2023;22:320. doi: 10.1186/s12933-023-02054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Tan Z, Huang Y, et al. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022 doi: 10.1186/s12933-022-01546-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen W, Guo Y, Wang Y, et al. Deep differentiable random forests for age estimation. IEEE Trans Pattern Anal Mach Intell. 2021;43:404–419. doi: 10.1109/TPAMI.2019.2937294. [DOI] [PubMed] [Google Scholar]
- 16.Su Y, Yu G, Li D, et al. Identification of mitophagy-related biomarkers in human osteoporosis based on a machine learning model. Front Physiol. 2024 doi: 10.3389/fphys.2023.1289976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramdas Nayak V, Satheesh P, Shenoy M, et al. Triglyceride glucose (TyG) index: a surrogate biomarker of insulin resistance. JPMA J Pak Med Assoc. 2022;72:986–988. doi: 10.47391/JPMA.22-63. [DOI] [PubMed] [Google Scholar]
- 18.Widjaja N, Irawan R, Hanindita M, et al. METS-IR vs. HOMA-AD and metabolic syndrome in obese adolescents. J Med Invest: JMI. 2023;70:7–16. doi: 10.2152/jmi.70.7. [DOI] [PubMed] [Google Scholar]
- 19.Abbasi F, Reaven G. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: triglycerides × glucose versus triglyceride/high-density lipoprotein cholesterol. Metab: Clin Exp. 2011;60:1673–1676. doi: 10.1016/j.metabol.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Meyer C, Pimenta W, Woerle H, et al. Different mechanisms for impaired fasting glucose and impaired postprandial glucose tolerance in humans. Diabetes Care. 2006;29:1909–1914. doi: 10.2337/dc06-0438. [DOI] [PubMed] [Google Scholar]
- 21.WHO Consultation Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Org Tech Rep Ser. 2000;894:1–253. [PubMed] [Google Scholar]
- 22.Boutouyrie P, Chowienczyk P, Humphrey J, et al. Arterial stiffness and cardiovascular risk in hypertension. Circ Res. 2021;128:864–886. doi: 10.1161/CIRCRESAHA.121.318061. [DOI] [PubMed] [Google Scholar]
- 23.Qiu Z, Geng T, Wan Z, et al. Serum selenium concentrations and risk of all-cause and heart disease mortality among individuals with type 2 diabetes. Am J Clin Nutr. 2022;115:53–60. doi: 10.1093/ajcn/nqab241. [DOI] [PubMed] [Google Scholar]
- 24.(2002) Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation, 106: 3143–421 [PubMed]
- 25.James IC, Stephen RD, Karen AD, et al. Diagnosis and management of the metabolic syndrome: an American heart association/national heart, lung, and blood institute scientific statement. Circulation. 2005;112:2735. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 26.Alberti KGMM, Robert HE, Scott MG, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120:1640. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Cong H-l, Zhang J-X, et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol. 2020 doi: 10.1186/s12933-020-01054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu R, Li L, Wang L, et al. Triglyceride-glucose index predicts death in patients with stroke younger than 65. Front Neurol. 2023;14:1198487. doi: 10.3389/fneur.2023.1198487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin J-L, Cao Y-X, Wu L-G, et al. Triglyceride glucose index for predicting cardiovascular outcomes in patients with coronary artery disease. J Thorac Dis. 2018;10:6137–6146. doi: 10.21037/jtd.2018.10.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 31.Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371:2237–2238. doi: 10.1056/NEJMra1011035. [DOI] [PubMed] [Google Scholar]
- 32.Singh RG, Yoon HD, Poppitt SD, et al. Ectopic fat accumulation in the pancreas and its biomarkers: a systematic review and meta-analysis. Diabetes-Metab Res Rev. 2017 doi: 10.1002/dmrr.2918. [DOI] [PubMed] [Google Scholar]
- 33.Yaxmehen Bello-Chavolla O, Almeda-Valdes P, Gomez-Velasco D, et al. METS-IR, a novel score to evaluate insulin sensitivity, is predictive of visceral adiposity and incident type 2 diabetes. Eur J Endocrinol. 2018;178:533–544. doi: 10.1530/EJE-17-0883. [DOI] [PubMed] [Google Scholar]
- 34.Almeda-Valdes P, Herrera-Mercadillo R, Aguilar-Salinas C, et al. The role of diet in patients with metabolic syndrome. Curr Med Chem. 2019;26:3613–3619. doi: 10.2174/0929867324666170518095316. [DOI] [PubMed] [Google Scholar]
- 35.Ramírez-Manent JI, López-González ÁA, Tomás-Gil P, et al. Relationship between abdominal volume index and body adiposity index and scales of insulin resistance and metabolic syndrome. Diagnostics. 2023;13:3356. doi: 10.3390/diagnostics13213356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLaughlin T, Reaven G, Abbasi F, et al. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am J Cardiol. 2005;96:399–404. doi: 10.1016/j.amjcard.2005.03.085. [DOI] [PubMed] [Google Scholar]
- 37.Abbasi F, Reaven GM. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: triglycerides × glucose versus triglyceride/high-density lipoprotein cholesterol. Metab-Clin Exp. 2011;60:1673–1676. doi: 10.1016/j.metabol.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 39.Somayeh S, Esmaeil Yousefi R, Mehdi B, et al. Serum insulin level, HOMA-IR and prostate cancer risk: a systematic review and meta-analysis. Diabetes Metab Syndr. 2019;13:110. doi: 10.1016/j.dsx.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 40.Lei J, Sun T, Jiang Y, et al. Risk identification of bronchopulmonary dysplasia in premature infants based on machine learning. Front Pediatr. 2021;9:719352. doi: 10.3389/fped.2021.719352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li W, Zeng L, Yuan S, et al. Machine learning for the prediction of cognitive impairment in older adults. Front Neurosci. 2023;17:1158141. doi: 10.3389/fnins.2023.1158141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharif S, Groenwold RHH, van der Graaf Y, et al. Mediation analysis of the relationship between type 2 diabetes and cardiovascular events and all-cause mortality: findings from the SMART cohort. Diabetes Obes Metab. 2019;21:1935–1943. doi: 10.1111/dom.13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the results of this study are available from the first author.