Abstract
Introduction:
This study aims to explore the predictive roles of echocardiographic parameters and biomarkers in determining outcomes among hospitalized COVID-19 patients experiencing cardiovascular events.
Methods:
A retrospective cohort study was conducted involving 49 COVID-19 patients who encountered cardiovascular events during hospitalization and underwent echocardiography. Our findings revealed notable associations between echocardiographic parameters and survival time.
Results:
A decrease in left ventricular ejection fraction (LVEF) of 10% was linked to a 20% reduction in survival time (TR: 0.80, 95% CI: 0.67 – 0.96, p = .017). Similarly, an increase in left ventricular (LV) volume by 10 mL was associated with a 9% decrease in survival time (TR: 0.91, 95% CI: 0.84 – 0.98, p = .011). Moreover, an increase in left atrial (LA) volume by 10 mL corresponded to an 8% decrease in survival time (TR: 0.92, 95% CI: 0.86 – 0.99, p = .026). Additionally, each 1 cm increase in right ventricular (RV) diameter was linked to a 22% reduction in survival time (TR: 0.78, 95% CI: 0.61 – 0.99, p = .043). Furthermore, a 10 mL increase in right atrial (RA) volume was associated with a 12% decrease in survival time (TR: 0.88, 95% CI: 0.78 – 0.98, p = .017). Notably, a tenfold rise in troponin levels was linked to a 33% decrease in survival time (TR: 0.67, 95% CI: 0.48 – 0.93, p = .014).
Conclusions:
Our study emphasizes the significant associations between various echocardiographic parameters and troponin levels with reduced survival time among COVID-19 patients experiencing cardiovascular events. These findings highlight the potential utility of echocardiography and troponin assessment in predicting outcomes and guiding management strategies in this patient population.
Keywords: COVID-19, SARS-CoV-2, Cardiovascular, Echocardiography, Troponin, Brain natriuretic peptide
Introduction
The SARS-CoV-2 virus has caused a worldwide pandemic since 2020, accounting for over 546 million infections and 6.3 million deaths as of June 29, 2022.1,2 Due to the novelty of the SARS-CoV-2 virus and new variants, our understanding of the epidemiology, trajectory, risk factors, and treatment of coronavirus disease 2019 (COVID-19) continues to evolve rapidly. Pre-existing cardiovascular pathology3 and/or new onset of cardiac injuries or events4,5 have been identified to be significant predictors of poor outcomes in hospitalized COVID-19 patients.6–9 Recent studies have associated specific results of laboratory tests10,11 and clinical diagnostic modalities, such as computerized tomography (CT),12,13 electrocardiography (ECG),14–16 and echocardiography,7,17,18 with poor outcomes in patients hospitalized with COVID-19.
Laboratory biomarkers, such as troponin,19,20 brain natriuretic peptide (BNP),21 n-terminal pro-hormone BNP (NT-proBNP),22–24 along with echocardiography,7,17,18 have been used to evaluate cardiac functions in COVID-19. However, there are limited data available to guide clinicians on how a combination of specific biomarkers with echocardiography3 may be used to risk-stratify and prognostically identify COVID-19 patients at risk for adverse events, longer lengths of stay (LOS), and death.
Due to the transmissibility and fatality rate of the SARS-CoV-2, leading societies only recommend the use of echocardiography if it is deemed to alter the management trajectory.25 However, the data regarding echocardiographic findings in COVID-19 remains sparse and inconclusive. In light of this, a retrospective, single-center, observational, exploratory cohort study was conducted on 87 SARS-CoV-2 polymerase chain reaction (PCR) positive patients who underwent a transthoracic echocardiography (TTE) during their inpatient admission between 1 March 2020 and 31 October 2020 in United Kingdom. The study showed 41.4% mortality in this cohort. A raised pulmonary artery systolic pressure (PASP) (36.8%) and right ventricle (RV) dysfunction (26.4%) were the most common echocardiographic features. In particular, the presence of RV dysfunction was significantly related to adverse outcomes.26 In addition, in hospitalized patients with COVID-19, pericardial effusion is prevalent, but rarely attributable to acute pericarditis. It is associated with myocardial dysfunction and mortality. A limited echocardiographic examination, including left ventricular ejection fraction (LVEF), tricuspid annular plane systolic excursion (TAPSE), and assessment for pericardial effusion, can contribute to outcome prediction.27 Another single-center prospective cohort study was conducted in Colombia, involving 153 patients hospitalized in intensive care for COVID-19 and confirmed via PCR who got an echo-cardiogram between May and October 2020. The study identified several echocardiographic variables associated with mortality within 60 days, including TAPSE, LVEF, PASP, acute cor pulmonale, right ventricle diastolic dysfunction, and right ventricular dilatation.28 Rodriguez et al. studied 38 patients in Spain and found LVEF < 50%, right ventricular dysfunction, pericardial effusion or segmental abnormalities of contractility was not associated with death or read-mission in the follow-up period.29 A study of 94 patients from Italy identified that the TAPSE/PASP ratio was an independent predictor of mortality.30 Three hospitals in New York (USA) with 510 patients demonstrated adverse right ventricular remodeling resulted in a greater than 2-fold increase in the risk of mortality.31 A Turkey study of 90 hospitalized patients documented that LVEF and right atrial diameter were independent predictors of right ventricular dilatation.32
However, although several studies have been performed in COVID-19 patients who received echocardiography, it should be taken into consideration that the majority of these studies had a small number of events, a situation that limits the power of their results. More studies are required to obtain more definitive conclusions. Particularly, there is no study to specifically evaluate echocardiography in the cohort of patients who actually suffered cardiovascular events. This is a critical area to investigate as these patients carry the worst prognosis.
We hypothesize that echocardiography performed before and after cardiovascular events in COVID-19 patients who suffered cardiovascular events correlates with myocardial injury markers and clinical outcomes. The primary purpose of this study is to investigate the role of echocardiographic parameters and biomarkers (troponin, BNP, NT pro-BNP) in predicting poor outcomes in hospitalized COVID-19 patients who suffered cardiovascular events.
Materials and methods
Study design, setting, and participants
This is an observational retrospective cohort study carried out within the Center for Excellence for Research in Infectious Disease (CERID) at University of Louisville, Louisville, KY, USA. The CERID team maintains an ongoing retrospective database of patients hospitalized with COVID-19 in Louisville, KY, USA.33,34 The purpose of this database is to investigate the multifaceted epidemiological hallmarks, clinical characteristics, and outcomes of patients hospitalized with COVID-19.
Participants in this study consisted of 700 adult patients hospitalized with a diagnosis of COVID-19 from March 7, 2020, to July 1, 2020. The study included all patients admitted to one of nine distinct adult acute care hospitals located within the Louisville, KY, USA metropolitan area, diagnosed with COVID-19 confirmed either by positive reverse transcriptase-polymerase chain reaction (RT-PCR) testing or by the presence of ground glass opacities on chest computerized tomography (CT). The study excluded any patients younger than 18 years and patients with COVID-19 who were not admitted. For this analysis, a cardiovascular (CV) event during hospital was defined as any of the following conditions: development of cardiogenic shock, heart failure, acute myocardial infarction, cardiomyopathy, myocarditis, a new, serious arrhythmia, acute worsening of long-term arrhythmia, cere-brovascular accident, pulmonary embolism, deep vein thrombosis (DVT), pulmonary edema, or cardiac arrest occurring after hospital admission confirmed by an attending physician at each participating hospital. One patient was excluded due to age (less than 18) and two patients were excluded due to missing data.
Human subject protection was ensured through the approval of the University of Louisville Institutional Review Board (IRB # 20.0257) as well as the IRBs from hospitals in which patients were hospitalized. The study was exempt from individual patient informed consent. Information for data analysis was gathered from the electronic health records (EHRs) of hospitalized COVID-19-positive patients and entered CERID’s secure Research Electronic Data Capture (REDCap) database.35 This database is compliant with the Health Insurance Portability and Accountability Act (HIPAA) and safeguards all private patient information through standard security measures approved by the IRBs listed above.
Data collection
A data abstraction instrument was developed by a team of healthcare professionals, including physicians, nurses, epidemiologists, biostatisticians, and researchers who were members of the CERID team. Information collected from the EHR included SARS-CoV-2 test results, demographic and hospitalization data, medical history, current medi-cations, signs and symptoms, physical examination, laboratory test results, radiographic findings, therapies utilized in treating patients, clinical course and complications of hospitalization, and outcomes. Data collection and entry were done by trained researchers in the University of Louisville School of Medicine to ensure consistency among data variables. Data were verified by a dedicated quality team at CERID, and discrepancies were resolved by reviewing the EHR.
After collecting echocardiographic parameters from COVID-19 patients who experienced at least one cardiovascular event and underwent echocardiography, we hypothesize that certain echocardiographic parameters correlate with clinical outcomes such as hospital length of stay and mortality.
Variables
Demographic variables
Demographic data, including age, sex, height, body mass index (BMI), and race or ethnicity of each patient, were gathered in order to fully evaluate and characterize the population sample.
Predictor variables
Predictor variables included measurements from echocardiography as well as troponin, BNP, and NT pro-BNP levels as measured in the laboratory. Echocardiography measurements included left ventricle (LV) ejection fraction (EF); LV volume, presence of LV dysfunction; right ventricular (RV) EF; RV diameter; left atrial (LA) volume; right atrial (RA) volume; size of ascending aorta; measurement of peak diastolic E-wave (in m/s); tricuspid annular plane systolic excursion (TAPSE, mm), estimated RV systolic pressure (RVSP); estimated RA pressure (RAP); mitral lateral annular tissue velocity (MV E’ in m/s); MV inflow early diastolic E-wave (m/s) to mitral lateral annular early diastolic velocity (E’) ratio; mitral, aortic, tricuspid, and pulmonic re-gurgitation and stenosis classified as moderate or higher; patent foramen ovale (PFO); and percentage of pericardial effusion if present at mild or higher levels. Laboratory variables included first troponin level in nanograms per milliliter (ng/mL) and event-triggered peak troponin level within 24 h of the cardiac event. In this study, we converted BNP and NT-pro BNP to one standard unit of measure, picograms per milliliter (pg/mL). using the following formulae: BNP measurement ratio of 0.289 picomoles per liter (pmol/l) is equivalent to 1 pg/mL, and NT-pro BNP measurement ratio of 0.118 pmol/l is equivalent to 1 pg/mL. Troponin values and BNP values were log-transformed for analysis.
Outcome variables
The outcomes variables were mortality during hospitalization, survival time, hospital length of stay (LOS), need for intensive care, and intensive care unit1 LOS. Hospital LOS was measured by total hours of care divided by 24 h to accurately measure partial days. Patients’ LOS was right-truncated to a maximum of 30 days (720 h). Patients’ survival time was measured by hours from the start of admission to death. If a patient did not expire during hospitalization, they were given a censored survival time equal to their hospital length of stay. Survival time was also right-truncated to a maximum of 30 days; if a patient expired during a hospitalization period extending beyond 30 days, calculations were conducted using a censored survival time of 30 days. The decision to truncate LOS data was influenced by several factors. Given that COVID-19 hospitalizations frequently extended over multiple weeks and all patients in the study experienced a cardiovascular complication, a cutoff of 30 days was deemed appropriate to attribute hospitalization duration to the disease and its associated complication. Only one patient had length of stay longer than 30 days. Additionally, any patients who expired were given a censored length of stay of 30 days (the worst LOS value) to prevent early mortality from appearing as short length of stay. In addition, we collected data on complications such as development of septic shock and acute respiratory distress syndrome (ARDS) diagnosed by attending physicians during hospitalization.
Statistical analysis
Sample Size and Diversity: This is a multiple center study with patients from Norton Healthcare, University of Louisville Hospital and Jewish Hospital in Louisville, KY, USA. A post-hoc power analysis for Pearson correlations with the sample size of 49 was performed, and a Pearson correlation of 0.39 or greater in magnitude with 80% power and a two-sided 5% type 1 error rate could be detected. A Pearson correlation of 0.39 is generally considered a weak correlation, so the sample size of 49 is adequate to identify reasonable correlations among BNP, Troponin, and Echo variables.
Baseline continuous variables were reported as medians and inter-quartile ranges (IQRs) and compared using Mann-Whitney U tests. Baseline categorical data were reported as frequencies and percentages and compared using Fisher’s Exact test. Pearson correlations were calculated to assess the relationship between continuous echocardiographic parameters and troponin/BNP values. Accelerated failure models using log-logistic distributions were created to compare both survival time and LOS for continuous echocardiographic parameters and troponin and BNP values. Time ratios (TRs), 95% confidence in-tervals, scale parameter values, and pseudo R2 were reported. Patients with missing laboratory values or echocardiographic parameters were excluded from analysis of the same values and parameters. P-values of less than 0.05 were considered statistically significant. All statistical analyses were performed using R version 3.5 (R Foundation for Statistical Computing).
Results
Study participants
A total of 49 hospitalized COVID-19 patients suffered at least one cardiovascular event during hospitalization and received echocardiography. Patients were predominantly male (n = 32, 65%) and of white race (n = 31, 63%); the median age was 66 years (IQR: 60–75). Hypertension (n = 30, 61%), obesity (n = 25, 51%), and hyperlipi-demia (n = 24, 49%) were the most common comorbidities. In addition, 7 (14%) had prior history of stroke, 9 (18%) had prior history of heart failure, and 14 (29%) had prior history of coronary artery disease, 11 (22%) had history of atrial fibrillation, 9 (18%) had prior MI, and 3 (6%) had prior DVT. A total of 27 patients (55%) were either current or previous smokers. The most frequent cardiovascular events were development of new arrhythmias (n = 23, 47%) and heart failure (n = 11, 22%). A total of 19 patients (39%) experienced multiple cardiac events during hospitalization.
Outcomes
A total of 20 patients (41%) died in the hospital; of these patients, median survival time was 11.5 days (IQR: 8.7 – 14.0 days). Median hospital LOS for survivors was 8.8 days (IQR: 5.1 – 18.0 days). A total of 34 patients (69%) required admission to the ICU, with median ICU LOS of 5.7 days (IQR: 1.7 – 13.8 days) for survivors and a median ICU LOS of 11.1 days (IQR: 7.4 – 14.6 days) for non-survivors. A total of 14 patients (29%) experienced septic shock, and 24 patients (49%) developed ARDS. In the present study, development of a new arrhythmia was the most common cardiovascular complication, occurring in 47% of the patients, followed by acute heart failure, which occurred in 22% of the patients.
Correlations of echocardiographic parameters with troponin and BNP
Concerning the timing of echocardiography, 30 participants (61%) had echocardiography performed before their cardiovascular event, and 19 (39%) had it performed after. Median time before was 1 day (IQR: 1–4.5 days), and median time to echocardiography after event was 1 day (IQR 1–2.25 days).
Correlations of echocardiographic parameters with event-triggered peak troponin (or peak troponin, if event-triggered peak troponin was not available), as well as combined echocardiographic parameters with BNP, are shown in Table 1. Significant correlations were found between peak troponin and LVEF, LV volume, RA volume and mitral valve E/E’ ratio. Significant correlation was found between RV diameter and BNP.
Table 1.
Pearson correlations between echocardiographic parameters (both before and after event) and log peak troponin and log brain natriuretic peptide (BNP).
| Variable | log Troponin | log BNP | ||
|---|---|---|---|---|
| r a | P value | r a | P value | |
| LV ejection fraction, % | −0.58 | < .001 | −0.27 | .08 |
| LV volume, mL | 0.39 | .025 | 0.12 | .49 |
| LA volume, mL | −0.16 | .41 | −0.05 | .79 |
| RV diameter, cm | 0.33 | .07 | 0.48 | .008 |
| RA volume, mL | 0.36 | .037 | 0.18 | .33 |
| Ascending aorta size, cm | 0.14 | .69 | −0.04 | .92 |
| Peak E-wave, m/s | −0.13 | .39 | 0.28 | .07 |
| TV TAPSE | 0.06 | .75 | −0.04 | .82 |
| Estimated RVSP, mmHg | −0.10 | .64 | −0.05 | .82 |
| Estimated RAP, mmHg | 0.20 | .30 | −0.09 | .64 |
| MV E’ Lateral Velocity, m/s | −0.09 | .59 | −0.19 | .27 |
| MV E/E’ Lateral | 0.39 | .020 | 0.29 | .10 |
Abbreviations: LV, left ventricle; LA, left atrium; RV, right ventricle; RA, right atrium; TV, tricuspid valve; TAPSE, tricuspid annular plane systolic excursion; RVSP, right ventricle systolic pressure; RAP, right atrial pressure; MV, mitral valve; BNP, brain natriuretic peptide.
Pearson correlation coefficient.
Accelerated failure time models for hospital length of stay
Accelerated failure time models predicting hospital LOS and survival time for echocardiographic parameters, log troponin, and log BNP are shown in Tables 2 and 3. An increase in estimated RVSP of 5 mmHg was found to be non-significantly associated with a decrease in hospital LOS of 21% (TR: 0.79, 95% CI: 0.62 – 1.00, p = 0.051). No variables were found to be significantly associated with length of stay.
Table 2.
Accelerated failure time model estimates for length of stay for echocardiographic parameters, log troponin, and log BNP.
| Variable | TR | 95% CI LB |
UB | P value | b a | P value |
|---|---|---|---|---|---|---|
| LV ejection fractionb, % | 1.007 | 0.98 | 1.034 | .61 | 0.751 | .08 |
| LV volumec, mL | 1.003 | 0.993 | 1.012 | .85 | 0.717 | .09 |
| LA volumec, mL | 0.994 | 0.975 | 1.013 | .52 | 0.811 | .32 |
| RV diameterc, cm | 1.022 | 0.642 | 1.625 | .93 | 0.808 | .27 |
| RA volumec, mL | 1.006 | 0.987 | 1.025 | .56 | 0.774 | .19 |
| Ascending aorta sizec, cm | 0.297 | 0.06 | 1.464 | .14 | 0.703 | .29 |
| Peak E-wavec, m/s | 0.838 | 0.647 | 1.085 | .73 | 0.756 | .13 |
| TV TAPSEc | 1.022 | 0.964 | 1.083 | .47 | 0.768 | .19 |
| Estimated RVSPc, mmHg | 0.953 | 0.909 | 1.000 | .051 | 0.707 | .12 |
| Estimated RAPc, mmHg | 1.012 | 0.902 | 1.134 | .84 | 0.791 | .24 |
| MV E’ Lateral Velocityc, m/s | 1.013 | 0.979 | 1.048 | .47 | 0.718 | .07 |
| MV E/E’ Lateralc | 1.035 | 0.914 | 1.172 | .59 | 0.795 | .26 |
| Troponind, log ng/mL | 1.162 | 0.951 | 1.142 | .14 | 0.721‡ | .037 |
| BNPe, log pmol/L | 1.062 | 0.609 | 1.852 | .83 | 0.731 | .06 |
Abbreviations: TR, time ratio; CI, confidence interval; LB, 95% confidence interval lower bound; UB, 95% confidence interval upper bound; LV, left ventricle; LA, left atrium; RV, right ventricle; RA, right atrium; TV, tricuspid valve; TAPSE, tricuspid annular plane systolic excursion; RVSP, right ventricle systolic pressure; RAP, right atrial pressure; MV, mitral valve.
b is the scale parameter forthe log-logistic distribution. The shape parameter is calculated as 1/b.
Time ratio represents changes in length of stay due to a decrease in one unit.
Time ratio represents changes in length of stay due to an increase in one unit.
Time ratio represents changes in length of stay due to a one-unit increase in log troponin, or a 10-fold increase in troponin.
Time ratio represents changes in length of stay due to a one-unit increase in log BNP, or a 10-fold increase in BNP.
Table 3.
Accelerated failure time model estimates for time to death for echocardiographic parameters, log troponin, and log BNP.
| Variable | TR | 95% CI LB |
UB | P value | b a | P value |
|---|---|---|---|---|---|---|
| LV ejection fractionb, % | 0.978 | 0.960 | 0.996 | .017 | 0.466 | < 0.001 |
| LV volumec, mL | 0.991 | 0.983 | 0.998 | .011 | 0.481 | < 0.001 |
| LA volumec, mL | 0.992 | 0.985 | 0.999 | .026 | 0.297 | < 0.001 |
| RV diameterc, cm | 0.776 | 0.608 | 0.991 | .043 | 0.381 | < 0.001 |
| RA volumec, mL | 0.987 | 0.976 | 0.998 | .017 | 0.445 | < 0.001 |
| Ascending aorta sizec, cm | 0.723 | 0.073 | 7.135 | .78 | 0.708 | 0.38 |
| Peak E-wavec, m/s | 0.938 | 0.670 | 1.314 | .31 | 0.493 | < 0.001 |
| TV TAPSEc | 0.992 | 0.953 | 1.033 | .69 | 0.514 | 0.001 |
| Estimated RVSPc, mmHg | 0.999 | 0.980 | 1.019 | .92 | 0.343 | < 0.001 |
| Estimated RAPc, mmHg | 0.970 | 0.913 | 1.030 | .33 | 0.360 | < 0.001 |
| MV E’ Lateral Velocityc, m/s | 0.997 | 0.983 | 1.012 | .71 | 0.509 | 0.001 |
| MV E/E’ Lateralc | 0.949 | 0.881 | 1.022 | .16 | 0.463 | < 0.001 |
| Troponind, log ng/mL | 0.826 | 0.710 | 0.960 | .014 | 0.488 | < 0.001 |
| BNPe, log pmol/L | 0.876 | 0.727 | 1.056 | .17 | 0.526 | .002 |
Abbreviations: TR, time ratio; CI, confidence interval; LB, 95% confidence interval lower bound; UB, 95% confidence interval upper bound; LV, left ventricle; LA, left atrium; RV, right ventricle; RA, right atrium; TV, tricuspid valve; TAPSE, tricuspid annular plane systolic excursion; RVSP, right ventricle systolic pressure; RAP, right atrial pressure; MV, mitral valve.
b is the scale parameter for the log-logistic distribution. The shape parameter is calculated as 1/b.
Time ratio represents changes in time to death due to a decrease in one unit.
Time ratio represents changes in time to death due to an increase in one unit.
Time ratio represents changes in time to death due to a one-unit increase in log troponin, or a 10-fold increase in troponin.
Time ratio represents changes in time to death due to a one-unit increase in log BNP, or a 10-fold increase in BNP.
Accelerated failure time models for survival time
LVEF, LV volume, LA volume, RV diameter, and RA volume were all significantly associated with survival time. A decrease in LVEF of 10% was associated with a decrease in survival time by 20% (TR: 0.80, 95% CI: 0.67 – 0.96, p = .017). For example, the median survival time predicted for a patient with an LVEF of 60% was 22.1 days; for a patient with an LVEF of 50% the median predicted survival time was 17.7 days. An increase in LV volume of 10 mL was found to yield a reduction in survival time of 9% (TR: 0.91, 95% CI: 0.84 – 0.98, p = .011). Thus, an increase in LV volume from 90 mL to 100 mL would cause predicted median survival time to decrease from 18.6 days to 16.9 days. An increase in left atrium volume of 10 mL was associated with a decrease in survival time of 8% (TR: 0.92, 95% CI: 0.86 – 0.99, p = .026). Thus, an increase in LA volume from 45 mL to 55 mL would cause predicted median survival time to decrease from 18.0 days to 16.6 days. A 1 cm increase in RV diameter was associated with a 22% reduction in survival time (TR: 0.78, 95% CI: 0.61 – 0.99, p = .043), and a 10 mL increase in RA volume was associated with a 12% reduction in survival time (TR: 0.88, 95% CI: 0.78 – 0.98, p = .017). Additionally, a 10-fold increase in troponin was associated with a reduction in survival time of 33% (TR: 0.67, 95% CI: 0.48 – 0.93, p = .014). Predicted survival curves for these parameters are shown in Fig. 1.
Fig. 1.
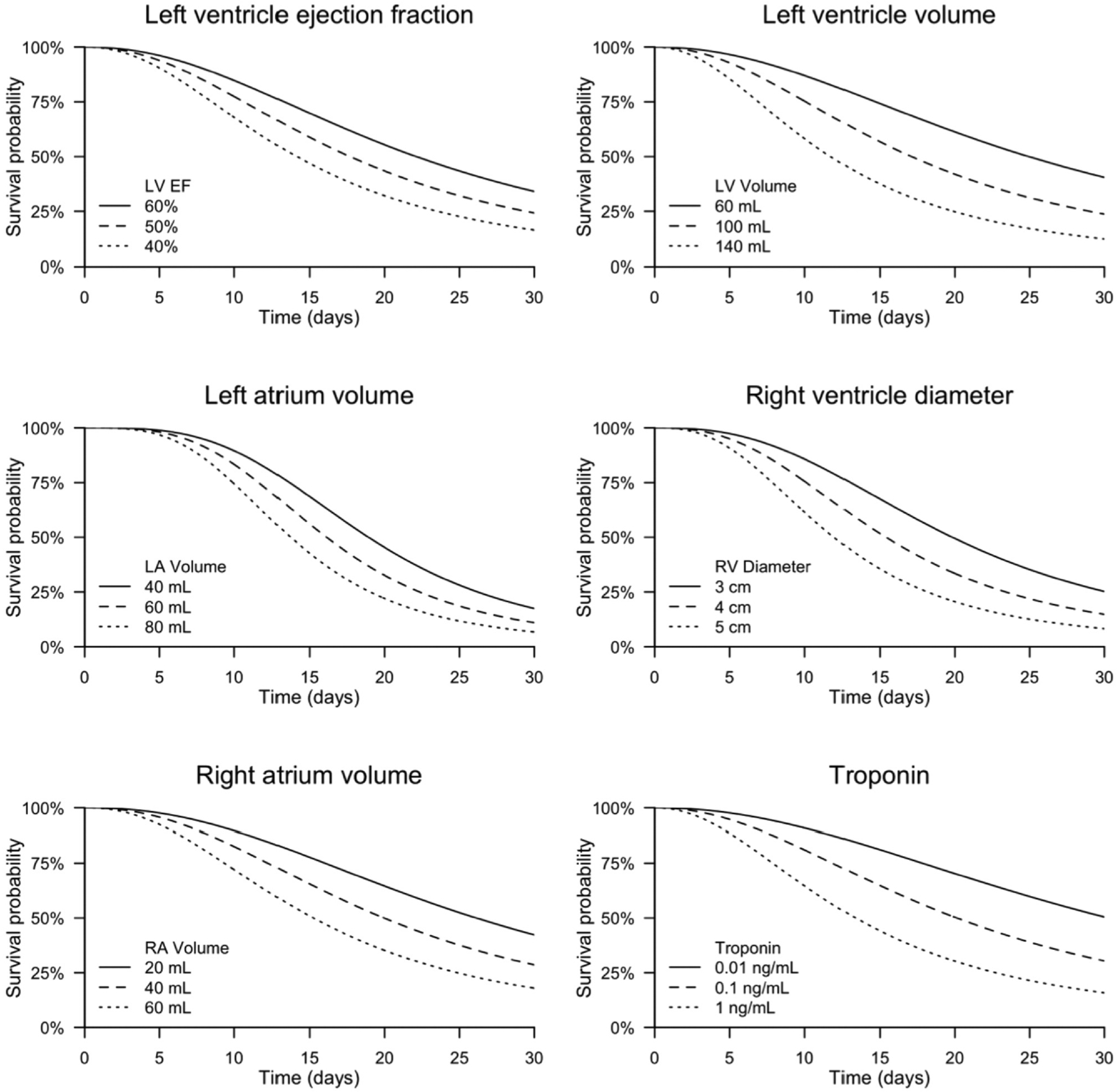
Estimated survival curves from accelerated failure time models. Abbreviations: LV, left ventricle.; EF, ejection fraction; LA, left atrium; RV, right ventricle; RA, right atrium.
Discussion
The occurrence of cardiovascular events in hospitalized COVID-19 patients carries significant morbidity and mortality.6–9,36 In the present study, 41% of hospitalized patients with COVID-19 who suffered a CV event died. More interestingly, we demonstrated that an increase in LV volume, LA volume, RV diameter, RA volume, troponin levels, and decrease in LVEF dramatically reduced the survival time of hospitalized COVID-19 patients suffering cardiovascular events. Clinicians should pay special attention to these echocardiographic parameters so that these critically ill COVID-19 patients could be appropriately monitored and managed. The novelties of this current study include demonstration of echocardiographic parameters which affects survival time.
Cardiovascular system involvement in severe COVID-19 infection was recognized even in the early cases from China37 and continued to be a significant contributor to the mortality associated with COVID-19.9,36,38–46 The exact mechanism of cardiac involvement in COVID-19 remains under investigation. One potential mechanism is direct myocardial involvement mediated by angiotensin-converting enzyme-2 (ACE-2).47,48 Other suggested mechanisms include the creation of a cytokine storm from an imbalanced response among subtypes of T helper cells41 and hypoxia-mediated intracellular calcium accumulation leading to cardiac myocyte apoptosis.49 Direct myocardial injury, myocarditis, dysrhythmia, acute myocardial infarction, acute heart failure, cardiomyopathy, and venous thromboembolic events are among the cardiovascular events reported in hospitalized COVID-19 patients.39 The mortality associated with cardiovascular events in the present study was 41%. Other studies corroborate these findings and demonstrate that the mortality rate for hospitalized COVID-19 patients is significantly higher when cardiovascular events occur.9,36,38–41,50 Shi et al. reported mortality as high as 51% in hospitalized COVID-19 patients with cardiovascular events.8 They observed that after adjusting for age, preexisting cardiovascular diseases, and other comorbidities, the multivariable adjusted Cox proportional hazard regression model showed a significantly higher risk of death in patients with cardiac injury than in those without cardiac injury.8
Various studies have reported the echocardiographic features of cardiac involvement in COVID-19.7,18,48–68 A variety of findings have been described, including varying rates of abnormalities. Certain findings were indicative of poor outcomes, whereas others resolved on follow-up echocardiography. In previous studies, the most commonly reported findings in COVID-19 patients who received an echocardiography were RV dilatation and dysfunction.7,18,51,56,57,62 The reported rates ranged from 15% to 40% for RV dilatation, and from 13.9% to 41% for RV dysfunction.7,18,51,57,59,61,63 On repeat echocardiography for patients who suffered clinical deterioration, the most common feature was RV function deterioration.18 These findings corroborate those of the present study, where RV dysfunction was present in 18% of all patients who had CV events. The mean RV diameter was 3.6 cm across all echocardiography, providing further evidence of RV dilatation in these patients. RV dysfunction was present in 11% of ‘before-event’ echocardiography and in 28% of ‘after-event’ echocardiography, further implying RV functional deterioration, although this was not statistically significant. Suggested mechanisms for RV dilatation and dysfunction include pulmonary hypoxic vasoconstriction, thrombotic insults, and acute respiratory distress syndrome (ARDS).51,55,57 LV dysfunction, in the form of either wall motion abnormalities or reduced ejection fraction, has been reported in various studies.7,18,53–57,64 The wall motion abnormalities in some patients were found to resemble stress-induced cardiomyopathy7,54,57,58,61,64,66 Churchill et al. reported resolution of LV dysfunction in 82% of patients who underwent a follow-up echocardiography after a median 14 days.53 In the present study, LV diastolic dysfunction was a common echocardiographic finding, seen in 56% of the patients. These findings are corroborated by Szekely et al., who reported LV diastolic dysfunction as the most common LV echocardiographic feature in hospitalized COVID-19 patients.18 The pathophysiology behind COVID-19-associated myocardial dysfunction has not been elucidated; however, multiple factors have been implicated, including cytokine storm, procoagulant state, and direct viral-mediated effect.51,57
Cardiac biomarkers such a troponin, BNP, and NT-pro BNP have been used in various studies to evaluate cardiac function in hospitalized patients with COVID-19 infection.19–24 Mahajan et al. proposed an algorithm using troponin and natriuretic peptides for cardiac risk stratification and prognostication of patients with severe COVID-19.69 The presence of values above the upper reference limit for troponin in COVID-19 patients varies widely, ranging from 20% in cohorts of hospitalized patients to more than 50% in critically ill patients.8,21,23,70 Data about natriuretic peptides are more scarce, though up to 48% of critical COVID-19 patients present with elevated levels of NT-pro BNP, reflecting hemodynamic stress.71 In the present study, we aimed to identify correlations of certain echocardiographic findings and elevated biomarkers with unfavorable outcomes in hospitalized COVID-19 patients suffering cardiovascular events to aid in predicting these outcomes. We found that the decrease in LVEF and increase in LV volume and mitral valve E/E’ ratio (indicator of LV diastolic dysfunction) on a “before-event” echocardiography were correlated with increased first troponin levels in patients who would proceed to have cardiovascular events. This would suggest that the presence of both systolic and diastolic dysfunction of the LV on echocardiography is a reflection of direct myocardial injury in COVID-19. However, there was no correlation between the first BNP level and “before-event” echocardiographic parameters. Churchill et al. reported similar data in their institutional experience.39 They stated that among patients who had cardiac biomarkers assessed within 3 days prior to the echocardiographic exam, 48% of patients with high-sensitivity troponin (hs-troponin) ≥ 50 ng/L demonstrated LV dysfunction compared to 12% of patients with hstroponin < 50 ng/L.52
In the present study, cardiovascular event-related peak troponin levels were directly correlated with an increase in RV diameter in the “after-event” echocardiography. Plasma BNP levels were positively correlated with RV diameter on combined (both before- and after-event) echocardiography. Dweck et al. reported higher BNP as an independent predictor of LV dysfunction16; however, we did not find any correlation between LV function and BNP levels. The significant association observed between plasma BNP and RV dilatation in patients with COVID-19 is likely to reflect the occurrence of heart failure and its adverse sequelae in this group. These routine and relatively inexpensive biomarker tests (troponin and BNP/NT-pro BNP) might assist with the early diagnosis of cardiac dysfunction in this patient population and prompt the initiation of appropriate pharmacological and non-pharmacological therapies.
Accelerated failure time models were built for various echocardiographic parameters and biomarkers as predictors of survival time. Interestingly, a decrease in LVEF and increases in LV volume, left atrium volume, RV diameter, RA volume, and troponin exhibited strong correlations with a reduction in survival time in COVID-19 patients who suffered cardiovascular events. These predictors represented either direct myocardial injuries, strain on the right heart due to worsening lung functions, nervous system dysfunction, or inappropriate whole-body immune responses. An increase in atrial or ventricular volume and/or size or a decrease in ventricular function would represent progression of heart failure. The use of biomarkers as surrogate markers for cardiac risk stratification in hospitalized COVID-19 patients could be beneficial to enable early identification of patients at risk for adverse cardiovascular events and initiation of rapid treatment measures. Our findings could be used to build a risk prediction model for survival time in COVID-19 patients who suffer cardiovascular events. Changes in these echocardiographic parameters and troponin levels should alert physicians to imminent hemodynamic instability and mortality. Prompt treatment should be initiated along with point of care echocardiography and serial troponin assessment to follow the progression of disease.
Our study has several strengths. Firstly, to our knowledge, this is the first study to compare echocardiographic parameters before and after CV events to predict the clinical outcomes of patients with COVID-19. Secondly, the patient population in our study included the demographic characteristics, socioeconomic characteristics, and health behaviors of the general United States population.63 Thirdly, the range of clinical outcomes, including time-to-event outcomes, were evaluated in our study, thereby enhancing the internal validity of the results.
There are also several limitations to our study. Firstly, this is a retrospective study with inherent limitations, and multiple confounding factors could have affected the results. Secondly, the small sample size of 49 COVID-19 patients with cardiovascular events who received echocardiography could have limited our power to detect other significant correlations. Furthermore, given the small numbers, there is a possibility that the associations reported were due to chance, though they are biologically plausible and supported by the existing literature. Thirdly, the patient population was observed from March 2020 to June 2020 in one US city. Although Louisville, KY, USA correlates well with average USA demographics, results from this limited cohort might not be generalized for new SARS-CoV2 variants.72 Fourthly, multiple reasons contributed to the absence of follow-up echocardiography after the occurrence of cardiovascular events. Some patients died and some patients experienced recovery, leading treating physicians to deem follow-up echocardiography unnecessary. Our study aimed to depict the real-world situation of COVID-19 and cardiovascular events. Fifthly, nuanced interactions among these variables and their varied impacts on different cardiovascular event types could offer richer, more insightful conclusions. Due to the small sample size, these interactions could not be further analyzed in this cohort. We are planning to analyze a much larger cohort in the future.
Conclusion
In conclusion, increases in LV volume, LA volume, RV diameter, RA volume, troponin levels and decrease in LVEF, were significantly associated with decreases in the survival time of COVID-19 patients who suffered cardiovascular events. Future studies should evaluate echocardiographic features of COVID-19 patients in multicenter and larger cohorts to guide potential risk prediction tool development.
Acknowledgments
The authors acknowledge the excellent efforts of University of Louisville CERID group and Kornhauser Health Sciences Library to assist with this project.
Funding
This work was supported by the National Institute of Environmental Health Sciences [grant number P30 (P30ES030283)]; National Heart, Lung, and Blood Institute (R01HL158779); and National Institute of Allergy and Infectious Diseases (R01AI172873); Gilead Sciences COMMIT COVID-19 RFP Program [grant number IN-US-983–6063]; the American Heart Association (23CSA1052735). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, Gilead Sciences, or the American Heart Association.
Abbreviations:
- ARDS
acute respiratory distress syndrome
- AV
atrioventricular
- BNP
B-type natriuretic peptide
- CERID
Center of Excellence for Research in Infectious Diseases
- COVID-19
coronavirus disease 2019
- CT
computed tomography
- CV
cardiovascular
- ECG
electrocardiographic
- EHR
electronic health record
- ICU
intensive care unit
- LOS
length of stay
- LV
left ventricular
- LVH
left ventricular hypertrophy
- NT-pro BNP
N-terminal pro B-type natriuretic peptide
- OR
odds ratio
- RR
relative risk
- RT-PCR
reverse transcriptase-polymerase chain reaction
- RVH
right ventricular hypertrophy
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SD
standard deviation
Footnotes
Declaration of Competing Interest
Jiapeng Huang holds the position of Associate Editor for JATM, and was blinded from reviewing or making decisions for the manuscript. The authors declare that they have no conflicts of interest.
CRediT authorship contribution statement
Conceptualization, Stephen Furmanek, Vidyulata Salunkhe, Siddharth Pahwa, Harideep Samanapally, Pavani Nathala, Qian Xu, Yuchen Han, Emma C. Huang, T’shura Ali, Fnu Deepti, Alex Glynn, Trevor McGuffin, Justin J. Huang, Ian Farah, Christopher M Jones, Julio A Ramirez, Sean P Clifford, Forest W Arnold, Maiying Kong PhD, Lynn Roser, Jiapeng Huang;. Methodology, Stephen Furmanek, Qian Xu, Yuchen Han, T’shura Ali, Maiying Kong, and Jiapeng Huang;. Formal analysis, Stephen Furmanek, Qian Xu, Yuchen Han, Maiying Kong;. Data curation, Stephen Furmanek, Vidyulata Salunkhe, Harideep Samanapally, Pavani Nathala, T’shura Ali;. Writing—original draft preparation, Stephen Furmanek, Vidyulata Salunkhe, Siddharth Pahwa, Harideep Samanapally, Pavani Nathala, Qian Xu, Yuchen Han, Emma C. Huang, T’shura Ali, Fnu Deepti, Alex Glynn, Trevor McGuffin, Justin J. Huang, Ian Farah, Christopher M Jones, Julio A Ramirez, Sean P Clifford, Forest W Arnold, Maiying Kong PhD, Lynn Roser, Jiapeng Huang;. Writing—review and editing, Stephen Furmanek, Vidyulata Salunkhe, Siddharth Pahwa, Harideep Samanapally, Pavani Nathala, Qian Xu, Yuchen Han, Emma C. Huang, T’shura Ali, Fnu Deepti, Alex Glynn, Trevor McGuffin, Justin J. Huang, Ian Farah, Christopher M Jones, Julio A Ramirez, Sean P Clifford, Forest W Arnold, Maiying Kong PhD, Lynn Roser, Jiapeng Huang. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Ethical statement
The study was conducted by the Declaration of Helsinki, and approved by the University of Louisville Institutional Review Board (IRB # 20.0257)
Data availability statement
All study data are included in the article.
References
- 1.Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19); 2020. Available at: 〈https://ourworldindata.org/coronavirus〉. [Accessed Jan 14, 2022]. [Google Scholar]
- 2.Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). COVID-19 Dashboard; 2020. Available at: 〈https://coronavirus.jhu.edu/map.html〉. [Accessed Jun 29, 2022]. [Google Scholar]
- 3.Li Y, Fang L, Zhu S, et al. Echocardiographic characteristics and outcome in patients with COVID-19 infection and underlying cardiovascular disease. Front Cardiovasc Med. 2021;8:642973. 10.3389/fcvm.2021.642973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pellegrino JL, Charlton NP, Carlson JN, et al. 2020 American Heart Association and American Red Cross focused update for first aid. Circulation. 2020;142(17):e287–e303. 10.1161/cir.0000000000000900. [DOI] [PubMed] [Google Scholar]
- 5.Li C, Jiang J, Wang F, et al. Longitudinal correlation of biomarkers of cardiac injury, inflammation, and coagulation to outcome in hospitalized COVID-19 patients. J Mol Cell Cardiol. 2020;147:74–87. 10.1016/j.yjmcc.2020.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inciarte A, Gonzalez-Cordon A, Rojas J, et al. Clinical characteristics, risk factors, and incidence of symptomatic coronavirus disease 2019 in a large cohort of adults living with HIV: a single-center, prospective observational study. Aids. 2020;34(12):1775–1780. 10.1097/qad.0000000000002643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishna H, Ryu AJ, Scott CG, Mandale DR, Naqvi TZ, Pellikka PA. Cardiac abnormalities in COVID-19 and relationship to outcome. Mayo Clin Proc. 2021;96(4):932–942. 10.1016/j.mayocp.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al Abbasi B, Torres P, Ramos-Tuarez F, et al. Cardiac troponin-I and COVID-19: a prognostic tool for in-hospital mortality. Cardiol Res. 2020;11(6):398–404. 10.14740/cr1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bansal A, Singh AD, Jain V, et al. The association of D-dimers with mortality, intensive care unit admission or acute respiratory distress syndrome in patients hospitalized with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Heart Lung. 2021;50(1):9–12. 10.1016/j.hrtlng.2020.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin GD, Ryerson CJ, Haramati LB, et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner society. Radiology. 2020;296(1):172–180. 10.1148/radiol.2020201365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haseeb S, Gul EE, Çinier G, et al. Value of electrocardiography in coronavirus disease 2019 (COVID-19). J Electro. 2020;62:39–45. 10.1016/j.jelectrocard.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He J, Wu B, Chen Y, et al. Characteristic electrocardiographic manifestations in patients With COVID-19. 966.e961–966.e964 Can J Cardiol. 2020;36(6) 10.1016/j.cjca.2020.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long B, Brady WJ, Bridwell RE, et al. Electrocardiographic manifestations of COVID-19. Am J Emerg Med. 2021;41:96–103. 10.1016/j.ajem.2020.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dweck MR, Bularga A, Hahn RT, et al. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020;21(9):949–958. 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szekely Y, Lichter Y, Taieb P, et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation. 2020;142(4):342–353. 10.1161/circulationaha.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arcari L, Luciani M, Cacciotti L, et al. Incidence and determinants of high-sensitivity troponin and natriuretic peptides elevation at admission in hospitalized COVID-19 pneumonia patients. Intern Emerg Med. 2020;15(8):1467–1476. 10.1007/s11739-020-02498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wibowo A, Pranata R, Akbar MR, Purnomowati A, Martha JW. Prognostic performance of troponin in COVID-19: a diagnostic meta-analysis and meta-regression. Int J Infect Dis. 2021;105:312–318. 10.1016/j.ijid.2021.02.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stefanini GG, Chiarito M, Ferrante G, et al. Early detection of elevated cardiac biomarkers to optimise risk stratification in patients with COVID-19. Heart. 2020;106(19):1512–1518. 10.1136/heartjnl-2020-317322. [DOI] [PubMed] [Google Scholar]
- 21.Gao L, Jiang D, Wen XS, et al. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir Res. 2020;21(1):83. 10.1186/s12931-020-01352-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):811–818. 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorrentino S, Cacia M, Leo I, et al. B-type natriuretic peptide as biomarker of COVID-19 disease severity-a meta-analysis. J Clin Med. 2020;9(9) 10.3390/jcm9092957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrico R, Balcom D, Johnson W, et al. Implementation of the Louisville COVID-19 surveillance protocol: experiences from the University of Louisville Center of Excellence for Research in Infectious Diseases [CERID]. Univ Louisville J Respir Infect. 2020;4(1):a10. 10.18297/jri/vol4/iss1/10 [DOI] [Google Scholar]
- 25.Skulstad H, Cosyns B, Popescu BA, et al. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging. 2020;21(6):592–598. 10.1093/ehjci/jeaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Babu A, Meng Z, Eden N, et al. Evaluating the role of transthoracic echocardiography in hospitalised patients with COVID-19 infection. Open Heart. 2022;9(1) 10.1136/openhrt-2021-001854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghantous E, Szekely Y, Lichter Y, et al. Pericardial involvement in patients hospitalized with COVID-19: prevalence, associates, and clinical implications. J Am Heart Assoc. 2022;11(7):e024363. 10.1161/jaha.121.024363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Díaz JJS, Rincon JM, López MAR, et al. Echocardiographic 60-day mortality markers in patients hospitalized in intensive care for COVID-19. Heart Lung. 2022;52:123–129. 10.1016/j.hrtlng.2021.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodríguez-Santamarta M, Minguito-Carazo C, Echarte-Morales JC, Del Castillo-García S, Valdivia-Ruiz J, Fernández-Vázquez F. Echocardiographic findings in critical patients with COVID-19. Rev Esp Cardiol. 2020;73(10):861–863. 10.1016/j.rec.2020.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Alto M, Marra AM, Severino S, et al. Right ventricular-arterial uncoupling in-dependently predicts survival in COVID-19 ARDS. Crit Care. 2020;24(1):670. 10.1186/s13054-020-03385-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J, Volodarskiy A, Sultana R, et al. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J Am Coll Cardiol. 2020;76(17):1965–1977. 10.1016/j.jacc.2020.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barman HA, Atici A, Tekin EA, et al. Echocardiographic features of patients with COVID-19 infection: a cross-sectional study. Int J Cardiovasc Imaging. 2021;37(3):825–834. 10.1007/s10554-020-02051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramirez JA, Carrico R. Healthcare workers in the time of COVID. Univ Louisville J Respir Infect. 2020;4(1):a14. 10.18297/jri/vol4/iss1/14 [DOI] [Google Scholar]
- 34.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an interna-tional community of software platform partners. J Biomed Inf. 2019;95:103208. 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oudit GY, Kassiri Z, Jiang C, et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Investig. 2009;39(7):618–625. 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Driggin E, Madhavan MV, Bikdeli B, et al. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75(18):2352–2371. 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 39.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Q, Samanapally H, Nathala P, et al. Outcomes and risk factors for cardiovascular events in hospitalized COVID-19 patients. J Cardiothorac Vasc Anesth. 2021;35(12):3581–3593. 10.1053/j.jvca.2021.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali AS, Sheikh D, Chandler TR, et al. Cardiovascular complications are the primary drivers of mortality in hospitalized patients with SARS-CoV-2 community-acquired pneumonia. Chest. 2023;163(5):1051–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furmanek S, Salunkhe V, Pahwa S, et al. Center of Excellence for Research in Infectious Diseases coronavirus study group on behalf of the COVID-19 CardioVascular Research Group. Correlations of before and after event echocardiographic parameters with troponin and BNP in hospitalized COVID-19 patients with cardiovascular events. J Cardiothorac Vasc Anesth. 2022;36(12):4553–4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nathala P, Salunkhe V, Samanapally H, et al. Center of Excellence for Research in Infectious Diseases (CERID) coronavirus study group on behalf of the COVID-19 Cardiovascular Research Group (COVID-CVRG). Electrocardiographic features and outcome: correlations in 124 hospitalized patients with COVID-19 and cardiovascular events. J Cardiothorac Vasc Anesth. 2022;36(8 Pt B): 2927–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Q, Samanapally H, Nathala P, et al. Center of Excellence for Research in Infectious Diseases (CERID) coronavirus study group on behalf of the COVID-19 CardioVascular Research Group (COVID-CVRG). Outcomes and risk factors for cardiovascular events in hospitalized COVID-19 patients. J Cardiothorac Vasc Anesth. 2021;35(12):3581–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu Q, Song K, Clifford SP, Kong M, Huang J. Meta-analysis of traditional chinese medicine Lianhua Qingwen in the treatment of coronavirus disease 2019. J Anesth Transl Med. 2023;2(2):20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Booth CM, Matukas LM, Tomlinson GA, et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801–2809. 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 48.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38(7):1504–1507. 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Argulian E, Sud K, Vogel B, et al. Right ventricular dilation in hospitalized patients with COVID-19 infection. JACC Cardiovasc Imaging. 2020;13(11):2459–2461. 10.1016/j.jcmg.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baycan OF, Barman HA, Atici A, et al. Evaluation of biventricular function in patients with COVID-19 using speckle tracking echocardiography. Int J Cardiovasc Imaging. 2021;37(1):135–144. 10.1007/s10554-020-01968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Churchill TW, Bertrand PB, Bernard S, et al. Echocardiographic features of COVID-19 illness and association with cardiac biomarkers. J Am Soc Echocardiogr. 2020;33(8):1053–1054. 10.1016/j.echo.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dabbagh MF, Aurora L, D’Souza P, Weinmann AJ, Bhargava P, Basir MB. Cardiac tamponade secondary to COVID-19. JACC Case Rep. 2020;2(9):1326–1330. 10.1016/j.jaccas.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deng Q, Hu B, Zhang Y, et al. Suspected myocardial injury in patients with COVID-19: Evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. 2020;311:116–121. 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hegde S, Khan R, Zordok M, Maysky M. Characteristics and outcome of patients with COVID-19 complicated by Takotsubo cardiomyopathy: case series with literature review. Open Heart. 2020;7(2) 10.1136/openhrt-2020-001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jain SS, Liu Q, Raikhelkar J, et al. Indications for and findings on transthoracic echocardiography in COVID-19. J Am Soc Echocardiogr. 2020;33(10):1278–1284. 10.1016/j.echo.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, Li H, Zhu S, et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. 2020;13(11):2287–2299. 10.1016/j.jcmg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahmoud-Elsayed HM, Moody WE, Bradlow WM, et al. Echocardiographic findings in patients with COVID-19 pneumonia. Can J Cardiol. 2020;36(8):1203–1207. 10.1016/j.cjca.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meyer P, Degrauwe S, Van Delden C, Ghadri JR, Templin C. Typical takotsubo syndrome triggered by SARS-CoV-2 infection. Eur Heart J. 2020;41(19):1860. 10.1093/eurheartj/ehaa306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pagnesi M, Baldetti L, Beneduce A, et al. Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID-19. Heart. 2020;106(17): 1324–1331. 10.1136/heartjnl-2020-317355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rath D, Petersen-Uribe Á, Avdiu A, et al. Impaired cardiac function is associated with mortality in patients with acute COVID-19 infection. Clin Res Cardiol. 2020;109(12): 1491–1499. 10.1007/s00392-020-01683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sollazzo V, Dibiase F, La Torre PP, et al. Echocardiographic features in patients with critical Covid-19 requiring endotracheal intubation. Int J Clin Skills. 2020;14(2):343–346. [Google Scholar]
- 63.Sud K, Vogel B, Bohra C, et al. Echocardiographic findings in patients with COVID-19 with significant myocardial injury. J Am Soc Echocardiogr. 2020;33(8):1054–1055. 10.1016/j.echo.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Taza F, Zulty M, Kanwal A, Grove D. Takotsubo cardiomyopathy triggered by SARS-CoV-2 infection in a critically ill patient. BMJ Case Rep. 2020;13(6) 10.1136/bcr-2020-236561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van den Heuvel FMA, Vos JL, Koop Y, et al. Cardiac function in relation to myocardial injury in hospitalised patients with COVID-19. Neth Heart J. 2020;28(7–8):410–417. 10.1007/s12471-020-01458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vera-Pineda R, Francisco Carrizales-Sepulveda E, Camacho-Ortiz A, et al. Echocardiographic characteristics of subjects with COVID-19: a case series. Cardiol Res. 2020;11(4):260–265. 10.14740/cr1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu H, Hou K, Xu R, et al. Clinical characteristics and risk factors of cardiac involvement in COVID-19. J Am Heart Assoc. 2020;9(18):e016807. 10.1161/jaha.120.016807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mahajan K, Chand Negi P, Ganju N, Asotra S. Cardiac biomarker-based risk stratification algorithm in patients with severe COVID-19. Diabetes Metab Syndr. 2020;14(5):929–931. 10.1016/j.dsx.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caro-Codón J, Rey JR, Buño A, et al. Characterization of myocardial injury in a cohort of patients with SARS-CoV-2 infection. Med Clin. 2021;157(6):274–280. 10.1016/j.medcli.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zwaenepoel B, Dhont S, Hoste E, Gevaert S, Schaubroeck H. The prognostic value of cardiac biomarkers and echocardiography in critical COVID-19. Front Cardiovasc Med. 2021;8:752237. 10.3389/fcvm.2021.752237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Furmanek S, Glick C, Chandler T, et al. The city of Louisville encapsulates the United States demographics. Univ Louisville J Respir Infect. 2020;4(2):a4. 10.18297/jri/vol4/iss2/4 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All study data are included in the article.


