ABSTRACT
Strain-transcending antibodies against virulence-associated subsets of P. falciparum-infected erythrocyte surface antigens could protect children from severe malaria. However, the evidence supporting the existence of such antibodies is incomplete and inconsistent. One subset of surface antigens associated with severe malaria, rosette-mediating Plasmodium falciparum Erythrocyte Membrane Protein one (PfEMP1) variants, cause infected erythrocytes to bind to uninfected erythrocytes to form clusters of cells (rosettes) that contribute to microvascular obstruction and pathology. Here, we tested plasma from 80 individuals living in malaria-endemic regions for IgG recognition of the surface of four P. falciparum rosetting strains using flow cytometry. Broadly reactive plasma samples were then used in antibody elution experiments in which intact IgG was eluted from the surface of infected erythrocytes and transferred to heterologous rosetting strains to look for strain-transcending antibodies. We found that seroprevalence (percentage of positive plasma samples) against allopatric rosetting strains was high in adults (63%–93%) but lower in children (13%–48%). Strain-transcending antibodies were present in nine out of eleven eluted antibody experiments, with six of these recognizing multiple heterologous rosetting parasite strains. One eluate had rosette-disrupting activity against heterologous strains, suggesting PfEMP1 as the likely target of the strain-transcending antibodies. Naturally acquired strain-transcending antibodies to rosetting P. falciparum strains in humans have not been directly demonstrated previously. Their existence suggests that such antibodies could play a role in clinical protection and raises the possibility that conserved epitopes recognized by strain-transcending antibodies could be targeted therapeutically by monoclonal antibodies or vaccines.
KEYWORDS: severe malaria, PfEMP1, rosette formation, virulence, immunology, antibodies, epitopes
INTRODUCTION
During the blood stage of Plasmodium falciparum malaria, the parasite modifies the surface of its host erythrocyte by displaying variant surface antigens (VSAs) on the infected cell surface. These VSAs are derived from polymorphic protein families, the most well studied of which is P. falciparum erythrocyte membrane protein 1 (PfEMP1), which is a family of adhesion proteins that mediate sequestration of infected cells in the microvasculature (1). Other VSA families include the repetitive interspersed family (RIFIN), subtelomeric variant open reading frame (STEVOR), and surface-associated interspersed protein (SURFIN) families (2). Some PfEMP1 variants cause rosetting (3–7), where the infected erythrocyte binds to uninfected erythrocytes, and there is also some limited evidence for the involvement of RIFIN and STEVOR in rosetting interactions (8, 9). Rosetting enhances microvascular obstruction (10) and has been consistently associated with severe malaria in African children (11–14). For this reason, rosetting is considered a parasite virulence phenotype.
It has been hypothesized that antibodies that target VSAs (PfEMP1 in particular) are clinically protective (15, 16). In addition, the diversity of PfEMP1 variants, and the fact that each P. falciparum isolate encodes roughly 60 distinct variants, with minimal overlap between the PfEMP1 repertoires of different isolates (17), strongly suggests that PfEMP1 is an important immune target. As protection against severe malaria is acquired after just a few infections (18), naturally acquired strain-transcending antibodies against VSAs must exist if humoral responses to polymorphic parasite antigen families contribute to protection against severe malaria. Such strain-transcending antibodies, defined here as antibodies against the surface of live infected erythrocytes that recognize multiple genetically distinct P. falciparum strains, are thus targeting conserved rather than variant-specific epitopes.
Previous work shows that sera from semi-immune adults living in malaria-endemic regions have broad reactivity with the infected erythrocyte surface of diverse parasite strains, as demonstrated by agglutination assays or flow cytometry (15, 16, 19, 20). This broad serological recognition could be due to the presence of strain-transcending antibodies or due to the acquisition of an extensive pool of variant-specific antibodies, and most serological studies do not differentiate between these two possibilities. A small number of studies have identified human strain-transcending monoclonal and polyclonal antibodies to VAR2CSA, the PfEMP1 variant that causes infected erythrocyte adhesion to placental syncytiotrophoblasts (21–23), and broadly strain-transcending antibodies to RIFIN variants have been described (24). The only study that sought to identify human strain-transcending antibodies to rosetting parasites found only variant-specific responses (6). However, only three rosetting strains were studied (6), none of which had the dual rosetting and IgM Fc-binding adhesion phenotype that is most strongly associated with severe malaria (25–27), and which can be targeted by strain-transcending antibodies against PfEMP1 raised in rabbits (7).
We hypothesized that people living in malaria-endemic regions acquire strain-transcending IgG antibodies against virulence-associated VSAs, and sought to test this hypothesis by first identifying plasma samples from malaria-exposed humans that recognize multiple parasite strains displaying the rosetting IgM Fc-binding phenotype and then using the broadly reactive plasma in IgG elution experiments to test for homologous and heterologous infected erythrocyte recognition.
RESULTS
Detection of naturally acquired human IgG to rosetting P. falciparum strains
First, we investigated whether individuals living in malaria-endemic regions naturally acquire antibodies to multiple rosetting parasite strains. Archived plasma samples from 40 adults and 40 children were tested for IgG recognition of the surface of live infected erythrocytes by flow cytometry, using four rosetting IgM Fc-binding parasite strains (Table 1). Because of spontaneous var gene switching (28, 29), cultured parasites contain a heterogeneous mix of infected erythrocytes expressing different PfEMP1 types, even after selection for rosetting. Therefore, we used a dual human plasma/rabbit anti-PfEMP1 surface staining protocol to allow specific examination of human IgG binding to infected erythrocytes expressing the rosette-mediating PfEMP1 variants of interest. For each parasite strain, the PfEMP1-positive infected erythrocytes were identified using rabbit polyclonal IgG against the rosette-mediating PfEMP1 N-terminal domain and an Alexa Fluor 647-conjugated anti-rabbit IgG secondary antibody. The human antibodies bound to this infected erythrocyte population were detected with an Alexa Fluor 488-conjugated anti-human gamma chain-specific secondary antibody (Fig. S1). Any background staining of infected or uninfected erythrocytes, which occurs commonly with human sera and plasma (30), was corrected for as described in Materials and Methods and Fig. S2. Examples of human plasma samples showing negative or strong positive IgG staining are shown in Fig. S3.
TABLE 1.
Geographical origin and adhesion phenotype of the P. falciparum strains studied
| Genotype [alternative name] (reference) | Origin | Parasite strain [alternative names] (reference) | Var gene transcribed/DCa [alternative name] (reference) | Adhesion phenotypeb |
|---|---|---|---|---|
| IT [IT4, IT04, FCR3] (31) | South-East Asia (32) | ITvar60R+ [R + PA1, PAR+, FCR3S1] (25, 33) | ITvar60/DC11 [FCR3S1.2var2] (5, 7) | IgM-positive rosetting (25, 26) |
| IT/R29 [R29, R29R+](28) | ITvar9/DC16 [R29var1] (3) | IgM-negative rosetting (26) | ||
| IT-HBEC (34) | ITvar19/DC8 (35) | HBECc-binding (35) | ||
| TM284 (25) | Thailand (25) | TM284R+ (25) | TM284var1/DC11 (7, 27) | IgM-positive rosetting (25, 26) |
| HB3 (36, 37) | Honduras (36, 37) | HB3R+(7) | HB3var6/DC16 (7) | IgM-positive rosetting (7) |
| 11019 [PFKE10] (24) | Kenya (24) | 11019R+ | 11019varR1/DC11 [PX0203.g54)] (UP)d | IgM-positive rosetting (UP) |
| 9197 [PC0053-C] (UP) | Kenya (24) | 9197R+ | PC0053-C.g687 (UP) | IgM-negative rosetting (UP) |
| 9197varUNR+ | Unknown | |||
| 9605 [PFKE08] (24) | Kenya (24) | 9605R+ | Unknown | IgM-negative rosetting (UP) |
| NF54 (38) | Africa (32) | 3D7-HBEC (34) | 3D7_PFD0020c/DC8-like (35) | HBECc-binding (35) |
DC: domain cassette, a characteristic set of adhesion domains arranged in tandem, used to classify PfEMP1 variants into functionally related types (17). The PfEMP1 architecture and amino acid sequence identities between domains for these variants are given in references (7) and (35).
Parasite strains that have the dual rosetting and IgM Fc-binding phenotype are here designated “IgM-positive rosetting” and those that form rosettes but do not bind IgM-Fc are designated “IgM-negative rosetting.”
HBEC: human brain endothelial cell.
UP: unpublished.
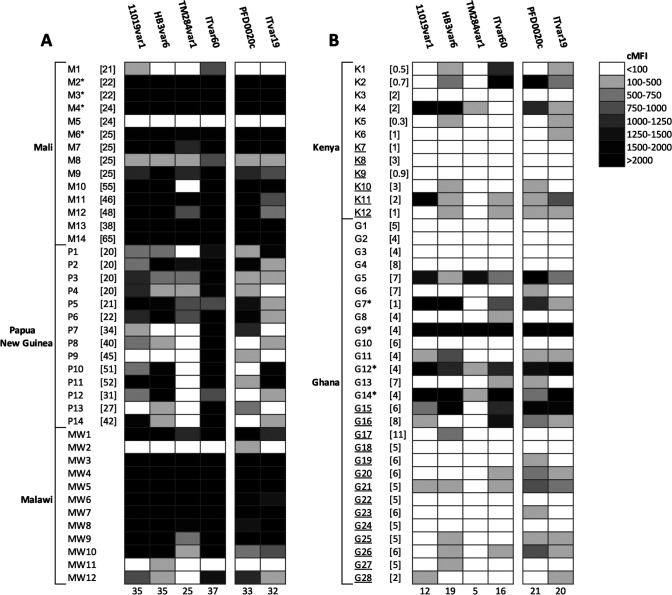
The four rosetting parasite strains, which originated from Kenya, Honduras, Thailand, and SE Asia, were well recognized by adult plasma samples from Mali, Papua New Guinea, and Malawi (Fig. 1A). Seroprevalence values, defined here as the percentage of plasma samples positive for recognition of each strain, were calculated and 95% (38/40) of the adult samples recognized at least one rosetting strain, with 62.5% (25/40) recognizing all four rosetting strains. For the childrens’ plasma samples, which came from donors in Kenya and Ghana aged 4 months to 11 years, 60% (24/40) recognized at least one rosetting strain, but only 4/40 (10%) recognized all four rosetting strains (Fig. 1B). For comparison, IgG responses to two P. falciparum strains previously selected for binding to human brain endothelial cells, which express PfEMP1 variants associated with virulence, composed of a characteristic set of tandem adhesion domains known as “domain cassette 8” (DC8) (35), were tested for recognition with the same plasma set (Fig. 1). 92.5% (37/40) of adults’ samples and 62.5% (25/40) of childrens’ samples recognized at least one of the DC8-expressing parasite strains, and 70% (28/40) of adults and 40% (16/40) of children recognized both strains.
Fig 1.
Recognition of infected erythrocytes expressing rosetting or DC8 PfEMP1 variants by human IgG. (A) Adults’ plasma samples. (B) Childrens’ plasma samples. Four rosetting parasite strains (expressing the PfEMP1 variants 11019VAR1, HB3VAR6, TM284VAR1, and ITVAR60) and two DC8-expressing human brain endothelial cell-binding parasite strains (expressing 3D7_PFD0020c and ITVAR19) were incubated with human plasma at 1/10 dilution. The infected erythrocytes expressing the specific variant of interest were detected with rabbit anti-PfEMP1 IgG as described in Fig. S1, and the human IgG bound to those cells was detected with an Alexa Fluor 488-conjugated anti-human IgG (gamma chain) antibody at 1/1,000 dilution. The corrected median fluorescence intensity (cMFI) was determined by subtracting non-specific background staining as described in the methods and Fig. S2. Each plasma sample was tested in duplicate in each experiment, and the mean of the corrected MFI from two independent experiments for each parasite strain is shown. The number of positive plasma samples (cMFI >100) for each parasite strain is shown at the base of each column. The age in years of each individual at the time of plasma donation is shown in square brackets if known. Samples from children with severe malaria are underlined. *Samples used in eluted antibody experiments.
Detection of strain-transcending activity against rosetting strains using eluted antibodies
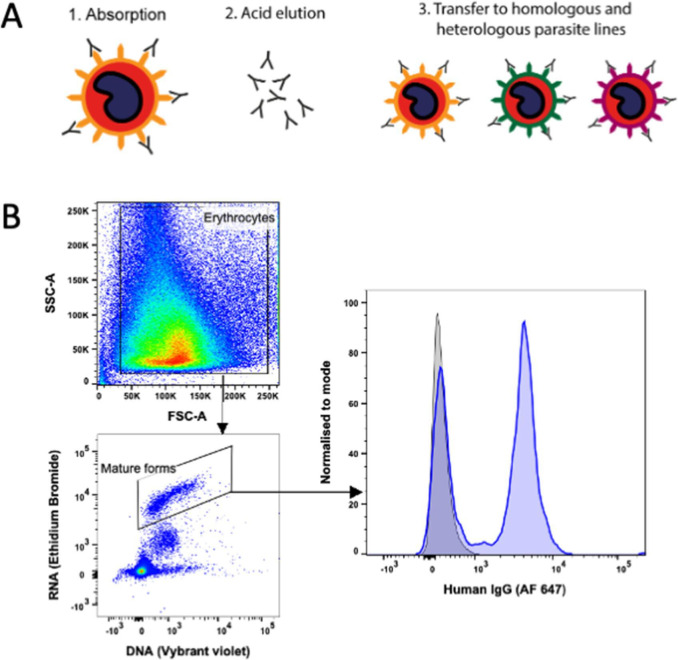
Plasma from four adults (M2, M3, M4, and M6) and four children (G7, G9, G12, and G14) were chosen for eluted antibody experiments, based on broad recognition (positive with three or four rosetting strains) and plasma availability. Each plasma sample was incubated with purified infected erythrocytes and the bound immunoglobulin was eluted from the infected erythrocyte surface using cold acid elution (19, 39). ITvar60R+ and TM284R+ were selected as the adsorbing parasite strains due to the relative ease of growing large culture volumes and maintaining the rosetting phenotype in these strains. The eluates were tested for their ability to stain homologous and heterologous live infected erythrocytes as shown in Fig. 2A. As the IgG in the eluates was present at low concentrations, co-staining with rabbit IgG to the PfEMP1 variant of interest was not included in the staining strategy in this case, to prevent the possibility of the rabbit IgG competing with the human IgG for PfEMP1. Instead, the mature PfEMP1-expressing infected erythrocyte population was identified by staining with both Vybrant DyeCycle Violet and Ethidium Bromide as previously described (40) (Fig. 2B), and the human IgG median fluorescence intensity values were corrected for non-specific background staining as described in Materials and Methods.
Fig 2.
Method of detection of strain-transcending IgG in eluates. (A) Schematic diagram of the acid elution method. (B) Successful detection of IgG in eluates. Left, dot-plots showing the gating strategy used to identify mature infected erythrocytes in whole cultures. Forward and side scatter were used to exclude debris and set a gate on all erythrocytes, then mature pigmented-trophozoite and schizont-infected erythrocytes were identified using Vybrant Dyecycle Violet (DNA stain, 1/2,500 dilution) and Ethidium Bromide (DNA/RNA stain, 20 µg/mL) as the DNA and RNA high population (40). Right, fluorescence intensity histogram of ITvar60R+ mature infected erythrocytes stained with neat eluate of plasma M2 from ITvar60R+ (blue), compared to a concentration-matched human IgG control (gray). Bound human IgG was detected with an Alexa Fluor 647-conjugated anti-human IgG (gamma chain) antibody at 1/1,000 dilution. A bimodal staining pattern is expected if the eluted antibodies recognize a VSA such as PfEMP1, which is expressed by some but not all of the infected cells in the culture.
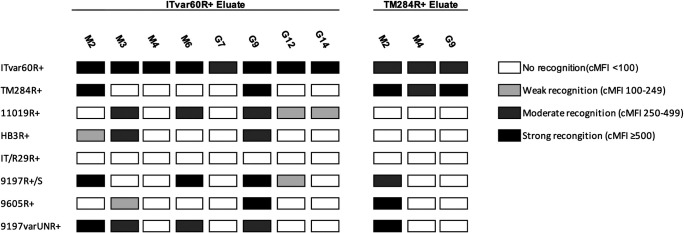
Using ITvar60R+ as the adsorbing strain, all eight eluates recognized the homologous parasites, showing successful elution of IgG from the infected erythrocyte surface (Fig. 2B and 3; Fig. S4). For TM284R+, five eluates did not stain homologous parasites; therefore, these samples were excluded, as this indicated a failure to elute sufficient IgG for detection. The other three positive TM284R+ eluates and the eight positive ITvar60R+ eluates were used to stain geographically diverse rosetting strains expressing distinct PfEMP1 variants (Table 1), to look for strain-transcending activity. Nine of the eleven eluates had heterologous activity against at least one other rosetting strain, with six of these reacting with multiple heterologous strains (Fig. 3; Fig. S4). The strain-transcending activity of eluates from ITvar60R + was most frequent against 11019R+, with five of the eight eluates recognizing this strain. The other rosetting strains were recognized by between two and four of the ITvar60R+ eluates, except for rosetting strain IT/R29R+, which was not recognized. Given that ITvar60R+ and IT/R29R+ are different variants from the same parasite genotype (IT/FCR3), it is perhaps not surprising that they are antigenically distinct, because shared epitopes within the same repertoire would reduce the number of immunologically novel variants available to facilitate parasite survival and prolong infection in the human host. For the TM284R+ eluates, strain-transcending activity against ITvar60R+ was most frequent, as all three eluates recognized this rosetting strain. The relatively high sequence similarity between the N-terminal DBLα domain (63% amino acid identity) and Cysteine-Rich Interdomain Region (CIDRβ, 82% amino acid identity) of these two variants has been noted previously (7).
Fig 3.
Summary of recognition of homologous and heterologous rosetting infected erythrocytes by eluted antibodies using flow cytometry. Eight rosetting parasite strains were stained with antibodies eluted from ITvar60R+ (eight individual donors; four adults M2, M3, M4, M6, and 4 children G7, G9, G12, and G14) or TM284R+ (three individual donors; two adults M2 and M4 and 1 child G9). Mature infected erythrocytes were identified using Vybrant Dyecycle Violet at 1/2,500 dilution (DNA stain) and ethidium bromide at 20 µg/mL (DNA/RNA stain) (40). Eluates were used neat, and human IgG bound to mature infected erythrocytes was detected with an Alexa Fluor 647-conjugated anti-human IgG (gamma chain) antibody at 1/1,000 dilution. Corrected median fluorescence intensities (cMFI) were adjusted for background staining as described in the methods. Due to the limited availability of eluate, the experiments were performed once.
The pattern of staining with the eluted antibodies allowed us to draw inferences about the nature of the conserved determinant(s) on the surface of infected erythrocytes. The entire mature-infected erythrocyte population was examined, which (as described above) consists of a mixture of cells expressing the rosetting PfEMP1 variant of interest and cells that have switched to express other PfEMP1 types. If the eluted antibodies were specifically recognizing the rosette-mediating PfEMP1 variant-expressing cells, then some but not all infected erythrocytes should be recognized, resulting in a bimodal distribution on the fluorescence intensity histograms. If, on the other hand, the eluted antibodies were recognizing a conserved epitope or protein present on all infected erythrocytes, then a single population of positively stained infected cells would be seen. Examination of the data shows a clear bimodal pattern of IgG reactivity in many cases, as would be expected for antibodies against a VSA such as PfEMP1 (Fig. 2B; Fig. S4).
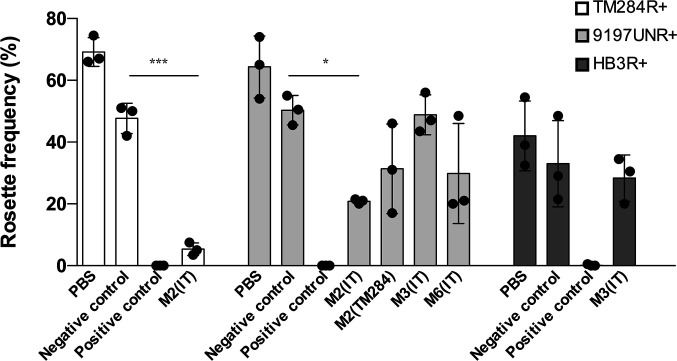
Eluate M2(ITvar60R+) can disrupt rosettes
As well as being an immune target, PfEMP1 is the adhesion molecule that mediates rosetting in many P. falciparum strains (3–7). We, therefore, investigated whether the eluted antibodies had rosette-disrupting activity against heterologous strains, as this might be detected if they are targeting PfEMP1. Due to limited eluate availability, only some eluate/parasite strain combinations were tested. Eluate M4 (eluted from ITvar60R+) was used as a negative control, as it only recognized the homologous strain by flow cytometry, so it should not show any heterologous rosette-disrupting activity. Rabbit IgG raised to the DBLα domain of the rosette-mediating PfEMP1 variants for each strain was used as a positive control for rosette disruption, except for strain 9197varUNR+, where heparin was used. When incubated at a final dilution of 1 in 2, eluate M2 (eluted from ITvar60R+) disrupted rosettes in both TM284R+ and 9197UNR+ (Fig. 4). This suggests that in the M2 plasma sample (eluted from IT parasites), PfEMP1 could be the target of the strain-transcending antibodies. The other eluates tested did not have statistically significant rosette-disrupting activity on the parasite strains tested, either because they recognize epitopes or antigens distinct from the rosetting binding site, or because the concentration of IgG was too low to disrupt rosettes.
Fig 4.
Rosette-disrupting activity of eluates. Rosette frequency of parasite strains TM284R+, 9197UNR+, and HB3R+ (indicated in the key) incubated in the presence of a negative control eluate that does not recognize mature infected erythrocytes, a positive control (antisera to the relevant PfEMP1 DBLα domain or heparin), or selected eluates which showed surface recognition against the tested parasite strain. The plasma and eluting strain for each eluate is indicated on the X-axis. Results from three independent experiments are shown as individual data points. Bar heights show the mean of the three experiments, and error bars indicate the SD. Significant differences between the negative control and tested eluate by student’s t-test or one-way ANOVA are shown, ***P < 0.001, *P < 0.05. All differences between the negative and positive controls were statistically significant (P < 0.0001).
DISCUSSION
Here we have shown that both adults and children living in P. falciparum endemic regions have naturally acquired IgG responses to allopatric rosetting parasite strains. In adults, these responses were common, with seroprevalence ranging from 63% to 93%, whereas in children, the seroprevalence was lower, ranging from 13% to 48%. These findings build on previous studies that have described the age-related build-up of anti-rosetting immunity and a high seroprevalence in adults of antibodies that recognize rosetting parasite strains (4, 6, 20, 41, 42). The results imply that P. falciparum isolates that share antigenic determinants with the culture-adapted rosetting strains used in this study circulate in the regions from which the plasma samples were derived and that exposure to such isolates induces antibodies in the majority of the adult population. Given the diverse geographical origins of the plasma and parasite strains tested, this is in keeping with the existence of globally conserved epitopes on the surface of rosetting infected erythrocytes (7).
Using the eluted antibody technique, we went on to demonstrate the presence of strain-transcending antibodies in both adults and children that recognize the infected erythrocyte surface of genetically distinct rosetting strains. The use of acid elution to show the existence of strain-transcending antibodies against epitopes on live infected erythrocytes was first reported by Marsh and Howard in the 1980s (19). Surprisingly, in the four decades since then, this result has not to our knowledge been replicated until the work reported here. A more recent study by Tan et al. (24) used the mixed agglutination technique to show the existence of rare strain-transcending antibodies in Kenyan adults, which target some RIFIN family members. Mixed agglutination relies on the ability of bivalent IgG to cause mixed color agglutinates if surface epitopes are shared between genetically distinct infected erythrocytes pre-stained with different colors (43). Another previous study using mixed agglutination sought to identify strain-transcending antibodies to three rosetting P. falciparum strains, Palo Alto VarO, IT4 R29, and 3D7 PF13 in Senegalese sera, but found only variant-specific responses (6). However, a study in Pakistan identified agglutinating and rosette-disrupting responses to heterologous parasites in convalescent serum from children recovering from malaria, suggesting the presence of strain-transcending responses to rosetting strains (44). Our results unequivocally demonstrate that human IgG eluted from the infected erythrocyte surface of one rosetting strain can recognize multiple heterologous rosetting strains, and therefore prove the existence of naturally acquired strain-transcending antibodies to rosetting infected erythrocytes in humans. This, in turn, implies the presence of conserved epitopes on infected erythrocyte surface antigens displayed by rosetting parasite strains.
Current evidence for the commonness of strain-transcending antibodies against P. falciparum VSAs is contradictory. Most previous studies using mixed agglutination to look for strain-transcending antibodies on the surface of infected erythrocytes indicate that strain-transcending activity is rarely detected in plasma samples from malaria-endemic regions (24, 43, 45). Similarly, an extensive competition ELISA suggested that strain-transcending activity to PfEMP1 was very rare, only being detected in 1% of the 1245 heterologous protein combinations tested (46). By contrast, one study detected frequent mixed strain agglutinates in the presence of plasma from malaria-exposed Indian donors (47), and serological studies have demonstrated recognition of allopatric isolates, with which the donors could never have been infected (16, 19). Furthermore, people who have only ever been exposed to a single infecting parasite genotype, such as tourists returning from malaria-endemic areas, or participants in experimental malaria challenge studies, have been reported to develop antibodies to heterologous P. falciparum strains, or recombinant PfEMP1 domains from heterologous infected erythrocytes (48–50).
Some of the contradictory findings from prior studies may be explained if epitopes are only shared between functionally related PfEMP1 variants. In such a scenario, the choice of parasite strains examined in any study is crucial, and strain-transcending activity will only be detected if parasite strains expressing functionally related subsets of PfEMP1 are used. Prior studies have shown strain-transcending activity of human mAbs against the VAR2CSA PfEMP1 variant, which is responsible for infected erythrocyte adhesion to Chondroitin Sulfate A (CSA) in the placenta during pregnancy-associated malaria (21, 22). These mAbs recognize the infected erythrocyte surface of VAR2CSA-expressing culture-adapted parasite strains and clinical isolates but do not recognize parasite isolates expressing other PfEMP1 variant types. Our prior work shows that antibodies generated in rabbits against some rosette-mediating PfEMP1 variants have strain-transcending activity against culture-adapted parasite strains and clinical isolates, but only against parasites with the same dual rosetting IgM Fc-binding phenotype (7). Strain-transcending activity has also been described for antibodies against parasite strains showing adhesion to ICAM-1 (51) and EPCR (52), although much of the data for these functionally related subsets is based on recombinant protein assays. This is important because for PfEMP1, recognition and cross-reactivity of antibodies in ELISA-type assays using recombinant protein does not necessarily reflect recognition of native PfEMP1 on the infected erythrocyte surface (6, 7, 53). Hence, flow cytometry, agglutination assays, or other functional experiments with live infected erythrocytes are needed to prove the presence of strain-transcending antibodies against native surface antigens.
Although the target of the strain-transcending antibodies described here is unknown and will require further investigation, we provide preliminary evidence that the rosette-mediating adhesin PfEMP1 may be the target of antibodies in at least one of the eluates because the eluted IgG was able to disrupt the rosettes in two heterologous parasite strains. It is possible that some targets of the strain-transcending antibodies identified in this work are antigens other than PfEMP1. Recently, Tan and colleagues reported the existence of broadly strain-transcending antibodies to the surface of infected erythrocytes that recognized RIFINs (24). However, the strain-transcending activity observed here is unlikely to be due to the LAIR-1 insertions described by Tan et al., as such antibodies were rare, being found in only 3 out of 557 Kenyan adult plasma examined (24).
The work presented here has several limitations. In the eluted antibody experiments, strain-transcending activity could be missed due to incomplete elution of IgG from the surface of the adsorbing infected erythrocytes, which occurred commonly (data not shown). Furthermore, low concentrations of strain-transcending antibodies in plasma would be hard to detect using our methods, and our approach does not identify the target(s) of strain-transcending antibodies. This study does, however, confirm the biological relevance of strain-transcending antibodies, and by using live infected erythrocytes rather than recombinant proteins, it avoids the problems associated with serological assays in which recognition of recombinant PfEMP1 domains does not necessarily indicate recognition of native protein (53).
Despite the limitations of our study, the finding of high seroprevalence against allopatric rosetting isolates in adults from malaria-endemic regions, along with the demonstration of relatively common strain-transcending antibodies to the rosetting infected erythrocyte surface, are encouraging findings that warrant further investigation. Future experiments will aim to identify the targets of strain-transcending antibodies and to characterize the conserved epitopes that are recognized. Identification of cryptic conserved infected erythrocyte surface epitopes may be a useful approach for the development of interventions against severe malaria (54). For example, monoclonal antibodies that reverse rosetting could be a useful adjunctive therapy for severe malaria, or antigens containing conserved epitopes recognized by strain-transcending antibodies could be part of a vaccine to prevent severe disease (16). Similar approaches based on non-rosetting virulence mechanisms, such as DC8-associated adhesion (55), could generate a cocktail aimed at reversing or preventing dense sequestration in vital organs and reducing death and disability from malaria.
MATERIALS AND METHODS
Human plasma samples and research ethics
In all, 80 anonymized archived human plasma samples from residents of malaria-endemic regions (40 adults and 40 children) were used. The samples were collected after informed consent from donors or their parents/guardians, as part of previous studies in Mali (56), Malawi (57), Papua New Guinea (58), Kenya (59), and Ghana (Y. Azasi, G. Awandare and J.A. Rowe, unpublished). The number of samples studied from each location was Mali (n = 14), Malawi (n = 12), Papua New Guinea (n = 14), Kenya (n = 12), and Ghana (n = 28); the samples were selected randomly from those available from the above prior studies. The adults were healthy and/or asymptomatic, whereas the children had clinical P. falciparum infection at the time of plasma collection. The negative control human serum pool was made from 15 European donors at the Scottish National Blood Transfusion Service (SNBTS), Edinburgh, UK.
Rabbit antibodies to PfEMP1
The antibodies to specific PfEMP1 variants were generated as described previously by immunizing rabbits with recombinant DBLα protein produced in E. coli, then purifying total IgG from the rabbit serum on a protein A column (7, 35) (J.A. Rowe, unpublished data).
P. falciparum strains
The geographical origin, var gene/PfEMP1 expression, and adhesion phenotype of the culture-adapted parasite strains studied are shown in Table 1. In addition to the well-established laboratory strains IT, HB3, 3D7, and TM284, three recent culture-adapted Kenyan strains (24) were used. One of the Kenyan strains (11019R+) spontaneously showed high levels of rosetting, and the other two (9197 and 9605) were selected for rosetting using Percoll or gelatin sedimentation (60). The strain 9197R+ showed two distinct phenotypic forms in parallel selections, 9197R+ which expresses the rosette-mediating PfEMP1 variant 9197varR1 [encoded by PC0053-C.g687 in the Pf3k database (61)] (J.A. Rowe, unpublished data) and 9197varUNR+ which does not express 9197varR1, and whose predominant PfEMP1 type has not yet been identified.
P. falciparum culture
P. falciparum strains were cultured at 2% hematocrit in O+ erythrocytes and RPMI-1640 medium (Lonza, BE12-167F or Gibco, 31870-025) supplemented to give the following final concentrations: L-glutamine 2 mM (Gibco, 2530-081), glucose 16 mM, 4-(2-hydroxyethyl)−1-piperazineethanesulfonic acid (HEPES) 25 mM (Lonza, 17–737F), gentamicin 25 µg/mL (Lonza, 17-518L), AlbuMAX II Lipid-rich bovine serum albumin (BSA) (Gibco 11021037) 0.25%, and pooled human serum 5%, and the pH adjusted to 7.2–7.4 with sodium hydroxide. Both human serum and blood for culture were obtained from SNBTS, Edinburgh, UK. Cultures were gassed using 94% nitrogen, 1% oxygen, and 5% carbon dioxide (BOC, 280648L) and incubated at 37°C. Cultures were maintained at ~2% hematocrit and 2%–10% total parasitemia. Rosetting was maintained by selection with plasmagel flotation or Percoll selection approximately once a week (60).
Immunofluorescent staining of live infected erythrocytes with human plasma
Human plasma samples were tested against four IgM-binding rosetting parasite strains (ITvar60R+, TM284R+, HB3R+, and 11019R+) (7) and two DC8-expressing parasite strains that were previously selected for binding to human brain endothelial cells (ITvar19 and 3D7_PFD0020c)(35). Parasite culture in PBS/1% BSA at 1% hematocrit (Ht) was incubated for 45 min at 37°C with 1/10 dilution of human plasma from malaria-endemic region donors (or non-endemic European serum pool as a negative control) and 20 µg/mL of rabbit IgG raised against the DBLα region of the rosette-mediating PfEMP1 variant for each parasite strain (7) (or non-immunized rabbit IgG as a negative control). After washing three times with PBS, the cells were resuspended in PBS/1% BSA with 1/2,500 dilution of Vybrant DyeCycle Violet (Invitrogen, V35003), 1/1,000 Alexa Fluor 647-conjugated cross-absorbed goat anti-rabbit IgG (Invitrogen, A-21244), and 1/1,000 Alexa Fluor 488-conjugated cross-absorbed goat anti-human IgG (heavy chain specific) (Biotium, 20444). The plates were incubated for 30 min at 37°C in the dark then washed twice with PBS, and once with PBS/1% BSA. After the final wash, cells were fixed with PBS/0.5% paraformaldehyde (Thermo Fisher, 28906) for 15 min at room temperature. The fixative solution was removed and the cells were resuspended in PBS/0.1% BSA/0.01% sodium azide with 100 µg/mL fucoidan (Molekula, 9072-19-9) added to disrupt rosettes (7). The plates were protected from light and stored at 4°C until analysis by flow cytometry within 24 h.
Data acquisition and gating strategy for human plasma experiments
The 96-well plates were read on a BD LSRFortessa (BD Biosciences) at a flow rate of 2 µL/s until a maximum of 500,000 events were recorded, or 50 s had elapsed, whichever came first. The data were analyzed in FlowJo v.10 (BD Biosciences). All events were gated using forward and side scatter to exclude debris, followed by gating on all infected erythrocytes (Vybrant DyeCycle Violet high), followed by PfEMP1-positive cells (Alexa Fluor 647 high), to identify the rosetting PfEMP1 variant-expressing infected erythrocyte cell population of interest. Human IgG bound to these cells was detected in the 488 channel and the median fluorescence intensity (MFI) was recorded. An example of the gating strategy is shown in Fig. S1.
Data analysis and visualization for human plasma experiments
Because human plasma/sera sometimes show non-specific binding to infected and/or uninfected erythrocytes, controls were carried out and fluorescence intensity measurements were corrected for background staining (cMFI) as described (30). The cMFI = (MFI of the PfEMP1-positive infected erythrocytes stained with endemic plasma – MFI of the uninfected erythrocytes from the same sample) − (MFI of the PfEMP1-positive infected erythrocytes stained with the European negative control serum pool – MFI of the uninfected erythrocytes from the same sample). An example is shown in Fig. S2. To visualize the data, the cMFI values for all plasma samples against all parasite strains were summarized in a checkerboard. To quantify the seroprevalence (percentage of plasma samples giving a positive result) for each parasite strain, a mean cMFI of >100 fluorescence units (fu) from two independent experiments was considered positive. All cMFI values below 100 fu were considered negative, as recognition was not compelling when the cMFI was <100 (Fig. S3). This additional cut-off was applied to reduce the incidence of type 1 errors.
Magnetic-activated cell sorting (MACS) to purify infected erythrocytes
Prior to adsorption with human plasma, infected erythrocytes of strains ITvar60R+ or TM284R+ were purified by MACS as described previously (39), with the addition of either 1 mg/mL heparin (Sigma-Aldrich, H4784-1G) for ITvar60R+ or 50 µg/mL fucoidan (Molekula, 9072-19-9) for TM284R+, to disrupt rosettes. Purified infected erythrocytes were washed twice to remove heparin or fucoidan and then resuspended in RPMI 1640 containing 2 mM L-glutamine and 25 mM HEPES, but lacking bicarbonate (Gibco, 13018-015), supplemented to 16 mM glucose and 25 µg/mL gentamicin and adjusted to pH 7.2–7.4 with sodium hydroxide to make incomplete binding medium (ICBM). 20–50 μL PCV of rosetting infected erythrocytes were obtained, with a parasitemia of at least 60%.
Adsorption and antibody elution from the infected erythrocyte surface
Eight individual plasma samples (M2, M3, M4, M6, G7, G9, G12, and G14) showing broad recognition of rosetting strains were used in adsorption and antibody elution experiments. Prior to adsorption, any antibodies recognizing uninfected erythrocytes in each plasma sample were pre-adsorbed by incubation with an equal packed cell volume of O+ erythrocytes from a Scottish donor at 20 rpm on a rotating wheel for 1 h at room temperature, and the process repeated with fresh erythrocytes a total of four times. 20–50 μL PCV of MACS-purified infected erythrocytes of strains ITvar60R+ or TM284R+ were resuspended in 100 µL of ice-cold pre-absorbed plasma and incubated on ice for 90 min with gentle re-suspension every 10 min. The cells were washed five times in 1 mL of ice-cold 0.15 M NaCl then resuspended in 50 µL of ice-cold 0.15 M NaCl. Three rounds of elution were undertaken at successively lower pH points (39). First, 50 µL ice-cold glycine-HCl at pH 2.7 was added to the resuspended cells and placed on ice for 2 min. The cells were pelleted by centrifugation and the supernatant was transferred to a fresh tube and neutralized with 3 µL 2M ice-cold Tris-base. After re-suspension of the cell pellet in 50 µL ice-cold 0.15M NaCl, the elution was repeated with 50 µL glycine-HCl at pH 2.2, with the supernatant transferred into a tube containing 6 µL 2M Tris-base. This was repeated once more with glycine-HCl at pH 1.7 and transfer of the supernatant into a tube containing 8 µL 2M Tris-base. The pH of the eluted fractions was checked and adjusted to 7 by the addition of a further 2 µL of 2M Tris-base as required. All three eluted fractions were centrifuged at 9,447 × g for 5 min to pellet debris and the three fractions pooled into a clean tube. The eluted antibody was buffer-exchanged into PBS using a Millipore Amicon ultra 100K microcentrifuge concentration unit (Merck, UFC510024) with four rounds of buffer-exchange using 450 µL of PBS per round. The purified eluate was recovered in 20 µL total volume and stored at 4°C until use. For most samples, eluate volume was made up to a total of 150 µL with PBS to give sufficient volume for downstream experiments, resulting in protein concentrations up to 46 µg/mL. The exceptions were M2 eluates from both ITvar60R+ and TM284R+, which were adjusted to total volumes of 216 µL and 180 µL, respectively. This was to bring their protein concentrations to below 200 µg/mL, which was the concentration used for the human IgG negative control (Sigma, I2511) in subsequent experiments. These diluted eluates were used neat in the staining experiments.
Immunofluorescent staining of infected erythrocytes with eluted antibodies
Eight rosetting parasite strains (Table 1) were stained with eluted antibodies. Staining for recognition of the surface of live infected erythrocytes was carried out as described above for staining with human plasma, with modifications as follows: (i) the culture suspension at 1% Ht was resuspended in neat eluate or negative control purified human IgG (Sigma, I2511) at 200 µg/mL in PBS; (ii) the samples were not assayed in duplicate due to limited eluate availability and each parasite strain was tested once; and (iii) the staining strategy did not include variant specific rabbit polyclonal IgG. Instead, the secondary antibody 1/1000 Alexa Fluor 647-conjugated goat anti-human IgG (gamma chain specific) (Biotium, 20448) in PBS/1% BSA contained both 1/2,500 of Vybrant DyeCycle Violet and 20 µg/mL Ethidium Bromide (Sigma, 46067) to allow gating on mature (pigmented trophozoite and schizont)-infected erythrocytes (40).
Data acquisition and gating strategy for eluted antibody experiments
Data acquisition was as described above for human plasma samples. All events were gated using forward and side scatter to exclude debris, followed by gating on mature infected erythrocytes (Vybrant DyeCycle Violet high, Ethidium bromide high). Human IgG bound to these cells was detected in the 647 channel and median fluorescence intensity was recorded.
Data analysis and visualization for eluted antibody experiments
For the eluted antibody experiments, cMFI = (MFI of the mature infected erythrocytes stained with eluted antibody − MFI of the uninfected erythrocytes from the same sample) − (MFI of mature infected erythrocytes stained with the commercial negative control human IgG – MFI of the uninfected erythrocytes from the same sample). To visualize the data, the cMFI values for each eluate against each parasite strain were summarized in a checkerboard.
Rosette disruption with eluates
Parasite culture at ~2% Ht was stained with 25 µg/mL Ethidium Bromide for 5 min at 37°C. The supernatant was removed and the cells resuspended at 4%–5% Ht in RPMI ICBM (as described above for MACS purification) supplemented with 15% heat-inactivated normal human serum from Scottish donors. 2 µL of PBS or eluate was added to 2 µL aliquots of the pre-stained culture suspension and incubated for 30 min at 37°C with resuspension after 15 min. The “eluate negative” sample M4 (IT) was chosen as a negative control because this eluate did not recognize any heterologous parasites by flow cytometry, so would not be expected to disrupt rosettes in heterologous strains. As a positive control, 2 µL of 1 mg/mL rabbit IgG against the specific PfEMP1 variant for each parasite strain (7) or 1 mg/mL heparin (Sigma-Aldrich, H4784-1G) was added. The aliquot identities were masked before counting to prevent observer bias. Wet preparations were made on multi-spot slides and at least 200 mature infected erythrocytes per spot were observed using a Leica DM2000 fluorescence microscope and 40× objective, with simultaneous brightfield/fluorescence to visualize both infected and uninfected erythrocytes. The rosette frequency (percentage of mature infected erythrocytes binding two or more uninfected erythrocytes) was recorded. Three independent experiments were done for each parasite strain/eluate combination tested. Not all parasite strain/eluate combinations could be tested due to limited eluate availability. The mean rosette frequency in the presence of eluted antibody was compared to that in the M4 “eluate negative” control using Student’s t-test or one-way ANOVA in GraphPad Prism v8.
ACKNOWLEDGMENTS
We are grateful to the donors from Ghana and the previous studies (Kenya, Malawi, Mali, and Papua New Guinea) who donated the plasma samples used in this work. We thank Dr. Eugene Martey (Komfo Anokye Teaching Hospital) and Amos Kotey (Agogo Presbyterian Hospital) for their help in collecting the Ghana plasma samples. We also thank Professor Kevin Marsh and Professor Stephen Rogerson and colleagues for help in collecting samples in prior studies and for comments on the manuscript.
This research was partly funded by the Wellcome Trust [grant number 204052/Z/16/Z] and by the Darwin Trust of Edinburgh (PhD studentship to Y.A.).
For open access, the author has applied a CC BY public copyright license to any Author Accepted Manuscript version arising from this submission.
Contributor Information
J. Alexandra Rowe, Email: alex.rowe@ed.ac.uk.
Jeroen P. J. Saeij, University of California Davis, Davis, California, USA
DATA AVAILABILITY
All raw data for flow cytometry and rosetting experiments are available from the authors on request.
ETHICS APPROVAL
Ethical approval was granted as described (56–59) and from the University of Edinburgh, School of Biological Sciences Ethical Review Panel (arowe002), Scottish National Blood Transfusion Service (SNBTS) Ethics Review Board (19 ~ 6), Ghana Health Service Review Committee ID No. GHS-ERC: 03/05/14 and Noguchi Memorial Institute for Medical Research-IRB study# 020/13–14.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/iai.00015-24.
Figures S1 to S4.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Smith JD, Rowe JA, Higgins MK, Lavstsen T. 2013. Malaria’s deadly grip: cytoadhesion of Plasmodium falciparum-infected erythrocytes. Cell Microbiol 15:1976–1983. doi: 10.1111/cmi.12183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wahlgren M, Goel S, Akhouri RR. 2017. Variant surface antigens of Plasmodium falciparum and their roles in severe malaria. Nat Rev Microbiol 15:479–491. doi: 10.1038/nrmicro.2017.47 [DOI] [PubMed] [Google Scholar]
- 3. Rowe JA, Moulds JM, Newbold CI, Miller LH. 1997. P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature 388:292–295. doi: 10.1038/40888 [DOI] [PubMed] [Google Scholar]
- 4. Vigan-Womas I, Guillotte M, Le Scanf C, Igonet S, Petres S, Juillerat A, Badaut C, Nato F, Schneider A, Lavergne A, Contamin H, Tall A, Baril L, Bentley GA, Mercereau-Puijalon O. 2008. An in vivo and in vitro model of Plasmodium falciparum rosetting and autoagglutination mediated by varO, a group A var gene encoding a frequent serotype. Infect Immun 76:5565–5580. doi: 10.1128/IAI.00901-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Albrecht L, Moll K, Blomqvist K, Normark J, Chen Q, Wahlgren M. 2011. var gene transcription and PfEMP1 expression in the rosetting and cytoadhesive Plasmodium falciparum clone FCR3S1.2. Malar J 10:17. doi: 10.1186/1475-2875-10-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vigan-Womas I, Guillotte M, Juillerat A, Vallieres C, Lewit-Bentley A, Tall A, Baril L, Bentley GA, Mercereau-Puijalon O. 2011. Allelic diversity of the Plasmodium falciparum erythrocyte membrane protein 1 entails variant-specific red cell surface epitopes. PLoS One 6:e16544. doi: 10.1371/journal.pone.0016544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghumra A, Semblat J-P, Ataide R, Kifude C, Adams Y, Claessens A, Anong DN, Bull PC, Fennell C, Arman M, Amambua-Ngwa A, Walther M, Conway DJ, Kassambara L, Doumbo OK, Raza A, Rowe JA. 2012. Induction of strain-transcending antibodies against Group A PfEMP1 surface antigens from virulent malaria parasites. PLoS Pathog 8:e1002665. doi: 10.1371/journal.ppat.1002665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goel S, Palmkvist M, Moll K, Joannin N, Lara P, Akhouri RR, Moradi N, Öjemalm K, Westman M, Angeletti D, Kjellin H, Lehtiö J, Blixt O, Ideström L, Gahmberg CG, Storry JR, Hult AK, Olsson ML, von Heijne G, Nilsson I, Wahlgren M. 2015. RIFINs are adhesins implicated in severe Plasmodium falciparum malaria. Nat Med 21:314–317. doi: 10.1038/nm.3812 [DOI] [PubMed] [Google Scholar]
- 9. Niang M, Bei AK, Madnani KG, Pelly S, Dankwa S, Kanjee U, Gunalan K, Amaladoss A, Yeo KP, Bob NS, Malleret B, Duraisingh MT, Preiser PR. 2014. STEVOR is a Plasmodium falciparum erythrocyte binding protein that mediates merozoite invasion and rosetting. Cell Host Microbe 16:81–93. doi: 10.1016/j.chom.2014.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaul DK, Roth EF, Nagel RL, Howard RJ, Handunnetti SM. 1991. Rosetting of Plasmodium falciparum-infected red blood cells with uninfected red blood cells enhances microvascular obstruction under flow conditions. Blood 78:812–819. doi: 10.1182/blood.V78.3.812.812 [DOI] [PubMed] [Google Scholar]
- 11. Carlson J, Helmby H, Hill AV, Brewster D, Greenwood BM, Wahlgren M. 1990. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet 336:1457–1460. doi: 10.1016/0140-6736(90)93174-n [DOI] [PubMed] [Google Scholar]
- 12. Treutiger CJ, Hedlund I, Helmby H, Carlson J, Jepson A, Twumasi P, Kwiatkowski D, Greenwood BM, Wahlgren M. 1992. Rosette formation in Plasmodium falciparum isolates and anti-rosette activity of sera from gambians with cerebral or uncomplicated malaria. Am J Trop Med Hyg 46:503–510. doi: 10.4269/ajtmh.1992.46.503 [DOI] [PubMed] [Google Scholar]
- 13. Rowe A, Obeiro J, Newbold CI, Marsh K. 1995. Plasmodium falciparum rosetting is associated with malaria severity in Kenya. Infect Immun 63:2323–2326. doi: 10.1128/iai.63.6.2323-2326.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Doumbo OK, Thera MA, Koné AK, Raza A, Tempest LJ, Lyke KE, Plowe CV, Rowe JA. 2009. High levels of Plasmodium falciparum rosetting in all clinical forms of severe malaria in African children. Am J Trop Med Hyg 81:987–993. doi: 10.4269/ajtmh.2009.09-0406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bull PC, Marsh K. 2002. The role of antibodies to Plasmodium falciparum-infected-erythrocyte surface antigens in naturally acquired immunity to malaria. Trends Microbiol 10:55–58. doi: 10.1016/s0966-842x(01)02278-8 [DOI] [PubMed] [Google Scholar]
- 16. Bull PC, Abdi AI. 2016. The role of PfEMP1 as targets of naturally acquired immunity to childhood malaria: prospects for a vaccine. Parasitology 143:171–186. doi: 10.1017/S0031182015001274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rask TS, Hansen DA, Theander TG, Gorm Pedersen A, Lavstsen T. 2010. Plasmodium falciparum erythrocyte membrane protein 1 diversity in seven genomes--divide and conquer. PLoS Comput Biol 6:e1000933. doi: 10.1371/journal.pcbi.1000933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gonçalves BP, Huang C-Y, Morrison R, Holte S, Kabyemela E, Prevots DR, Fried M, Duffy PE. 2014. Parasite burden and severity of malaria in Tanzanian children. N Engl J Med 370:1799–1808. doi: 10.1056/NEJMoa1303944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marsh K, Howard RJ. 1986. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science 231:150–153. doi: 10.1126/science.2417315 [DOI] [PubMed] [Google Scholar]
- 20. Barragan A, Kremsner PG, Weiss W, Wahlgren M, Carlson J. 1998. Age-related buildup of humoral immunity against epitopes for rosette formation and agglutination in African areas of malaria endemicity. Infect Immun 66:4783–4787. doi: 10.1128/IAI.66.10.4783-4787.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barfod L, Bernasconi NL, Dahlbäck M, Jarrossay D, Andersen PH, Salanti A, Ofori MF, Turner L, Resende M, Nielsen MA, Theander TG, Sallusto F, Lanzavecchia A, Hviid L. 2007. Human pregnancy-associated malaria-specific B cells target polymorphic, conformational epitopes in VAR2CSA. Mol Microbiol 63:335–347. doi: 10.1111/j.1365-2958.2006.05503.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barfod L, Dobrilovic T, Magistrado P, Khunrae P, Viwami F, Bruun J, Dahlbäck M, Bernasconi NL, Fried M, John D, Duffy PE, Salanti A, Lanzavecchia A, Lim CT, Ndam NT, Higgins MK, Hviid L. 2010. Chondroitin sulfate A-adhering Plasmodium falciparum-infected erythrocytes express functionally important antibody epitopes shared by multiple variants. J Immunol 185:7553–7561. doi: 10.4049/jimmunol.1002390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Doritchamou JYA, Renn JP, Jenkins B, Mahamar A, Dicko A, Fried M, Duffy PE. 2022. A single full-length VAR2CSA ectodomain variant purifies broadly neutralizing antibodies against placental malaria isolates. Elife 11:e76264. doi: 10.7554/eLife.76264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tan J, Pieper K, Piccoli L, Abdi A, Perez MF, Geiger R, Tully CM, Jarrossay D, Maina Ndungu F, Wambua J, Bejon P, Fregni CS, Fernandez-Rodriguez B, Barbieri S, Bianchi S, Marsh K, Thathy V, Corti D, Sallusto F, Bull P, Lanzavecchia A. 2016. A LAIR1 insertion generates broadly reactive antibodies against malaria variant antigens. Nature 529:105–109. doi: 10.1038/nature16450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scholander C, Treutiger CJ, Hultenby K, Wahlgren M. 1996. Novel fibrillar structure confers adhesive property to malaria-infected erythrocytes. Nat Med 2:204–208. doi: 10.1038/nm0296-204 [DOI] [PubMed] [Google Scholar]
- 26. Rowe JA, Shafi J, Kai OK, Marsh K, Raza A. 2002. Nonimmune IgM, but not IgG binds to the surface of Plasmodium falciparum-infected erythrocytes and correlates with rosetting and severe malaria. Am J Trop Med Hyg 66:692–699. doi: 10.4269/ajtmh.2002.66.692 [DOI] [PubMed] [Google Scholar]
- 27. Ghumra A, Semblat J-P, McIntosh RS, Raza A, Rasmussen IB, Braathen R, Johansen F-E, Sandlie I, Mongini PK, Rowe JA, Pleass RJ. 2008. Identification of residues in the Cmu4 domain of polymeric IgM essential for interaction with Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1). J Immunol 181:1988–2000. doi: 10.4049/jimmunol.181.3.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roberts DJ, Craig AG, Berendt AR, Pinches R, Nash G, Marsh K, Newbold CI. 1992. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature 357:689–692. doi: 10.1038/357689a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peters JM, Fowler EV, Krause DR, Cheng Q, Gatton ML. 2007. Differential changes in Plasmodium falciparum var transcription during adaptation to culture. J Infect Dis 195:748–755. doi: 10.1086/511436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Williams TN, Newbold CI. 2003. Reevaluation of flow cytometry for investigating antibody binding to the surface of Plasmodium falciparum trophozoite-infected red blood cells. Cytometry A 56:96–103. doi: 10.1002/cyto.a.10088 [DOI] [PubMed] [Google Scholar]
- 31. Robson KJH, Walliker D, Creasey A, McBride J, Beale G, Wilson RJM. 1992. Cross-contamination of Plasmodium cultures. Parasitol Today 8:38–39. doi: 10.1016/0169-4758(92)90075-D [DOI] [Google Scholar]
- 32. Mu J, Awadalla P, Duan J, McGee KM, Joy DA, McVean GAT, Su X. 2005. Recombination hotspots and population structure in Plasmodium falciparum. PLoS Biol 3:e335. doi: 10.1371/journal.pbio.0030335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Helmby H, Cavelier L, Pettersson U, Wahlgren M. 1993. Rosetting Plasmodium falciparum-infected erythrocytes express unique strain-specific antigens on their surface. Infect Immun 61:284–288. doi: 10.1128/iai.61.1.284-288.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Claessens A, Rowe JA. 2012. Selection of Plasmodium falciparum parasites for cytoadhesion to human brain endothelial cells. J Vis Exp e3122:e3122. doi: 10.3791/3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Claessens A, Adams Y, Ghumra A, Lindergard G, Buchan CC, Andisi C, Bull PC, Mok S, Gupta AP, Wang CW, Turner L, Arman M, Raza A, Bozdech Z, Rowe JA. 2012. A subset of group A-like var genes encodes the malaria parasite ligands for binding to human brain endothelial cells. Proc Natl Acad Sci U S A 109:E1772–E1781. doi: 10.1073/pnas.1120461109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bhasin VK, Trager W. 1984. Gametocyte-forming and non-gametocyte-forming clones of Plasmodium falciparum. Am J Trop Med Hyg 33:534–537. doi: 10.4269/ajtmh.1984.33.534 [DOI] [PubMed] [Google Scholar]
- 37. Kyriacou HM, Steen KE, Raza A, Arman M, Warimwe G, Bull PC, Havlik I, Rowe JA. 2007. In vitro inhibition of Plasmodium falciparum rosette formation by Curdlan sulfate. Antimicrob Agents Chemother 51:1321–1326. doi: 10.1128/AAC.01216-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Geiman QM, Meagher MJ. 1967. Susceptibility of a new world monkey to Plasmodium falciparum from man. Nature 215:437–439. doi: 10.1038/215437a0 [DOI] [PubMed] [Google Scholar]
- 39. Moll K, Kaneko A, Scherf A, Wahlgren M. 2013. Methods in malaria research. 6th ed. EVIMalaR, Glasgow, UK. [Google Scholar]
- 40. Ch’ng J-H, Moll K, Quintana MDP, Chan SCL, Masters E, Moles E, Liu J, Eriksson AB, Wahlgren M. 2016. Rosette-disrupting effect of an anti-plasmodial compound for the potential treatment of Plasmodium falciparum malaria complications. Sci Rep 6:29317. doi: 10.1038/srep29317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vigan-Womas I, Lokossou A, Guillotte M, Juillerat A, Bentley G, Garcia A, Mercereau-Puijalon O, Migot-Nabias F. 2010. The humoral response to Plasmodium falciparum VarO rosetting variant and its association with protection against malaria in Beninese children. Malar J 9:267. doi: 10.1186/1475-2875-9-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Quintana MDP, Ch’ng J-H, Moll K, Zandian A, Nilsson P, Idris ZM, Saiwaew S, Qundos U, Wahlgren M. 2018. Antibodies in children with malaria to PfEMP1, RIFIN and SURFIN expressed at the Plasmodium falciparum parasitized red blood cell surface. Sci Rep 8:3262. doi: 10.1038/s41598-018-21026-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Newbold CI, Pinches R, Roberts DJ, Marsh K. 1992. Plasmodium falciparum: the human agglutinating antibody response to the infected red cell surface is predominantly variant specific. Exp Parasitol 75:281–292. doi: 10.1016/0014-4894(92)90213-t [DOI] [PubMed] [Google Scholar]
- 44. Iqbal J, Perlmann P, Berzins K. 1993. Serological diversity of antigens expressed on the surface of erythrocytes infected with Plasmodium falciparum. Trans R Soc Trop Med Hyg 87:583–588. doi: 10.1016/0035-9203(93)90097-a [DOI] [PubMed] [Google Scholar]
- 45. Giha HA, Staalsoe T, Dodoo D, Elhassan IM, Roper C, Satti GM, Arnot DE, Hviid L, Theander TG. 1999. Overlapping antigenic repertoires of variant antigens expressed on the surface of erythrocytes infected by Plasmodium falciparum. Parasitology 119:7–17. doi: 10.1017/s0031182099004485 [DOI] [PubMed] [Google Scholar]
- 46. Joergensen L, Turner L, Magistrado P, Dahlbäck MA, Vestergaard LS, Lusingu JP, Lemnge M, Salanti A, Theander TG, Jensen ATR. 2006. Limited cross-reactivity among domains of the Plasmodium falciparum clone 3D7 erythrocyte membrane protein 1 family. Infect Immun 74:6778–6784. doi: 10.1128/IAI.01187-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chattopadhyay R, Sharma A, Srivastava VK, Pati SS, Sharma SK, Das BS, Chitnis CE. 2003. Plasmodium falciparum infection elicits both variant-specific and cross-reactive antibodies against variant surface antigens. Infect Immun 71:597–604. doi: 10.1128/IAI.71.2.597-604.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krause DR, Gatton ML, Frankland S, Eisen DP, Good MF, Tilley L, Cheng Q. 2007. Characterization of the antibody response against Plasmodium falciparum erythrocyte membrane protein 1 in human volunteers. Infect Immun 75:5967–5973. doi: 10.1128/IAI.00327-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Elliott SR, Payne PD, Duffy MF, Byrne TJ, Tham W-H, Rogerson SJ, Brown GV, Eisen DP. 2007. Antibody recognition of heterologous variant surface antigens after a single Plasmodium falciparum infection in previously naive adults. Am J Trop Med Hyg 76:860–864. doi: 10.4269/ajtmh.2007.76.860 [DOI] [PubMed] [Google Scholar]
- 50. Turner L, Wang CW, Lavstsen T, Mwakalinga SB, Sauerwein RW, Hermsen CC, Theander TG. 2011. Antibodies against PfEMP1, RIFIN, MSP3 and GLURP are acquired during controlled Plasmodium falciparum malaria infections in naïve volunteers. PLoS One 6:e29025. doi: 10.1371/journal.pone.0029025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bengtsson A, Joergensen L, Rask TS, Olsen RW, Andersen MA, Turner L, Theander TG, Hviid L, Higgins MK, Craig A, Brown A, Jensen ATR. 2013. A novel domain cassette identifies Plasmodium falciparum PfEMP1 proteins binding ICAM-1 and is a target of cross-reactive, adhesion-inhibitory antibodies. J Immunol 190:240–249. doi: 10.4049/jimmunol.1202578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lau CKY, Turner L, Jespersen JS, Lowe ED, Petersen B, Wang CW, Petersen JEV, Lusingu J, Theander TG, Lavstsen T, Higgins MK. 2015. Structural conservation despite huge sequence diversity allows EPCR binding by the PfEMP1 family implicated in severe childhood malaria. Cell Host Microbe 17:118–129. doi: 10.1016/j.chom.2014.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lopez-Perez M. 2020. mSphere of influence: going native, or the risk of overreliance on recombinant antigens. mSphere 5:e00224-20. doi: 10.1128/mSphere.00224-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Good MF, Yanow SK. 2023. Hiding in plain sight: an epitope-based strategy for a subunit malaria vaccine. Trends Parasitol 39:929–935. doi: 10.1016/j.pt.2023.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Reyes RA, Raghavan SSR, Hurlburt NK, Introini V, Kana IH, Jensen RW, Martinez-Scholze E, Gestal-Mato M, Bau CB, Fernández-Quintero ML, Loeffler JR, Ferguson JA, Lee W-H, Martin GM, Theander TG, Ssewanyana I, Feeney ME, Greenhouse B, Bol S, Ward AB, Bernabeu M, Pancera M, Turner L, Bunnik EM, Lavstsen T. 2024. Broadly inhibitory antibodies against severe malaria virulence proteins. bioRxiv:2024.01.25.577124. doi: 10.1101/2024.01.25.577124 [DOI]
- 56. Moulds JM, Zimmerman PA, Doumbo OK, Kassambara L, Sagara I, Diallo DA, Atkinson JP, Krych-Goldberg M, Hauhart RE, Hourcade DE, McNamara DT, Birmingham DJ, Rowe JA, Moulds JJ, Miller LH. 2001. Molecular identification of Knops blood group polymorphisms found in long homologous region D of complement receptor 1. Blood 97:2879–2885. doi: 10.1182/blood.v97.9.2879 [DOI] [PubMed] [Google Scholar]
- 57. Duffy MF, Caragounis A, Noviyanti R, Kyriacou HM, Choong EK, Boysen K, Healer J, Rowe JA, Molyneux ME, Brown GV, Rogerson SJ. 2006. Transcribed var genes associated with placental malaria in Malawian women. Infect Immun 74:4875–4883. doi: 10.1128/IAI.01978-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cockburn IA, Mackinnon MJ, O’Donnell A, Allen SJ, Moulds JM, Baisor M, Bockarie M, Reeder JC, Rowe JA. 2004. A human complement receptor 1 polymorphism that reduces Plasmodium falciparum rosetting confers protection against severe malaria. Proc Natl Acad Sci U S A 101:272–277. doi: 10.1073/pnas.0305306101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Deans A-M, Lyke KE, Thera MA, Plowe CV, Koné A, Doumbo OK, Kai O, Marsh K, Mackinnon MJ, Raza A, Rowe JA. 2006. Low multiplication rates of African Plasmodium falciparum isolates and lack of association of multiplication rate and red blood cell selectivity with malaria virulence. Am J Trop Med Hyg 74:554–563. [PMC free article] [PubMed] [Google Scholar]
- 60. Handunnetti SM, Gilladoga AD, van Schravendijk MR, Nakamura K, Aikawa M, Howard RJ. 1992. Purification and in vitro selection of rosette-positive (R+) and rosette-negative (R-) phenotypes of knob-positive Plasmodium falciparum parasites. Am J Trop Med Hyg 46:371–381. doi: 10.4269/ajtmh.1992.46.371 [DOI] [PubMed] [Google Scholar]
- 61. Otto TD, Assefa SA, Böhme U, Sanders MJ, Kwiatkowski D, Berriman M, Newbold C, Pf3k consortium . 2019. Evolutionary analysis of the most polymorphic gene family in falciparum malaria. Wellcome Open Res 4:193. doi: 10.12688/wellcomeopenres.15590.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1 to S4.
Data Availability Statement
All raw data for flow cytometry and rosetting experiments are available from the authors on request.