ABSTRACT
Neisseria gonorrhoeae is the etiological agent of the sexually transmitted infection gonorrhea. The pathogen is a global health challenge since no protective immunity results from infection, and far fewer treatment options are available with increasing antimicrobial resistance. With no efficacious vaccines, researchers are exploring new targets for vaccine development and innovative therapeutics. The outer membrane TonB-dependent transporters (TdTs) produced by N. gonorrhoeae are considered promising vaccine antigens as they are highly conserved and play crucial roles in overcoming nutritional immunity. One of these TdTs is part of the hemoglobin transport system comprised of HpuA and HpuB. This system allows N. gonorrhoeae to acquire iron from hemoglobin (hHb). In the current study, mutations in the hpuB gene were generated to better understand the structure–function relationships in HpuB. This study is one of the first to demonstrate that N. gonorrhoeae can bind to and utilize hemoglobin produced by animals other than humans. This study also determined that when HpuA is absent, mutations targeting extracellular loop 7 of HpuB led to defective hHb binding and utilization. However, when the lipoprotein HpuA is present, these loop 7 mutants recovered their ability to bind hHb, although the growth phenotype remained significantly impaired. Interestingly, loop 7 contains putative heme-binding motifs and a hypothetical α-helical region, both of which may be important for the use of hHb. Taken together, these results highlight the importance of loop 7 in the functionality of HpuB in binding hHb and extracting and internalizing iron.
KEYWORDS: TonB-dependent transporter, hemoglobin–haptoglobin receptor, lipoprotein, hemoglobin, N. gonorrhoeae, N. meningitidis
INTRODUCTION
Gonorrhea, the common sexually transmitted infection that poses a serious global health issue, is caused by Neisseria gonorrhoeae, an obligate human pathogen. In 2020, the World Health Organization estimated that 82.4 million new gonococcal infections occurred worldwide (1). In 2021, the Centers for Disease Control and Prevention (CDC) noted that 710,151 cases of gonorrhea were reported in the United States, representing an increase of 118% since 2009 (2). In 2018, the direct medical costs associated with gonococcal infections in the United States were about $271 million (3). A gonococcal infection often presents as urethritis or epididymitis in men or as cervicitis in women (4, 5). Approximately 80% of the cases in women are asymptomatic; an untreated gonococcal infection can lead to severe secondary sequelae such as infertility, ectopic pregnancy, or pelvic inflammatory disease. Systemic infections in both sexes can lead to dissemination and, rarely, endocarditis and meningitis (5–9). One challenge with gonorrhea is that protective immunity is not conferred by infection (4, 10, 11). Also, there has been a steady rise in gonococcal antimicrobial resistance (AMR) worldwide over the past 50 years (12), making the infection increasingly difficult to treat. With resistance to all classes of antibiotics having emerged in 2010, the treatment option recommended by the CDC was a dual therapy with ceftriaxone plus azithromycin (13–15). However, in 2020, azithromycin use was discontinued due to the continuing increase in resistance, leaving ceftriaxone as the only treatment option even though the occurrence of ceftriaxone resistance has also been increasing (16–19). For this reason, researchers are now revisiting previously used antibiotics as possible alternatives to avert this impending AMR crisis (20). Thus far, there is also no effective vaccine against gonorrhea (21), and together with the declining availability of effective treatment options, it is anticipated that gonorrhea could ultimately become untreatable. We are, therefore, in dire need of new therapeutic options and preventive strategies against N. gonorrhoeae.
With no effective vaccines available, identifying promising targets for the development of vaccines and innovative therapeutics is crucial. The outer membrane TonB-dependent transporters (TdTs) are putative vaccine antigen targets as they are highly conserved among all pathogenic Neisseria strains (22), and many are not subject to high-frequency phase or antigenic variation (23). More importantly, the TdTs play a vital part in allowing N. gonorrhoeae to overcome nutritional immunity, the process by which essential metals are restricted by the human host to hinder the growth of invading bacteria (24–26).
The hemoglobin receptor system, comprised of HpuA and HpuB, allows N. gonorrhoeae to acquire iron from human hemoglobin (hHb) (27, 28). HpuA is the surface-exposed lipoprotein, and HpuB is the TdT. The HpuAB system is a phase variable (29, 30), and HpuAB-expressing variants are selected during gonococcal infection in women within the first half of the menstrual cycle (31). Both HpuA and HpuB are thought to be required for the utilization of hemoglobin–iron; in contrast, the transferrin iron utilization (TbpAB) system only requires the expression of the TdT TbpA for iron utilization (22, 30), but the presence of TbpB increases the efficiency of uptake (32).
Because the HpuAB system is produced in the outer membrane, is necessary for growth in the sole presence of hemoglobin, and is relatively well conserved in sequence, these proteins could be considered as part of a cocktail vaccine to protect against gonococcal infections. While wild-type (WT) gonococcal proteins are generally considered adequate antigens that could generate protective immunity, some studies using nonbinding mutant forms of the lipid-modified proteins TbpB and factor H binding protein (fHbp) as vaccine antigens have shown that mutagenesis enhanced immunogenicity (33–35). The precise molecular mechanism behind this observation is currently unknown, but preventing the vaccine antigen from being able to interact with its cognate human ligand following inoculation might enhance its immunogenicity and protective effect. While a similar phenomenon has not yet been described for the TdTs, it is possible that a cocktail of mutants derived from the TdTs could result in enhanced protection as was reported for the two lipoproteins. Since there seems to be a selective advantage to expressing HpuAB during menses, HpuB and/or HpuA mutants unable to bind and grow on hHb could potentially be considered as part of a protective vaccine cocktail against N. gonorrhoeae. We hypothesized that mutation of HpuB extracellular loops would reduce or abrogate Hb binding and consequently cause defects in growth with Hb as a sole iron source.
The current study aims to elucidate the interaction between gonococcal HpuB and hHb and to contribute a detailed structure–function analysis of the gonococcal HpuAB system. Loop deletion variants of HpuB were generated and analyzed for their ability to bind to hHb and grow on hHb as a sole iron source. In this current study, we found that mutations targeting extracellular loop 7, which contains conserved, putative heme-binding motifs, demonstrated impaired binding and growth, highlighting the importance of this loop in HpuB function. We also found that the lipoprotein, HpuA, can restore Hb binding and growth support, which was inhibited in the HpuB loop 7 mutants.
RESULTS
Hypothetical membrane topology of HpuB as a foundation for mutagenesis
Geneious was used to create a sequence alignment between meningococcal TbpA [for which the crystal structure is known (PDB 3V89)], gonococcal TbpA (WP_003693614), and HpuB (WP_003687084). Next, using the HpuB sequence in NetSurfP 2.0 and information about the structural features of TbpA, the beta strands of HpuB were predicted. Finally, TOPO2 was used to create a transmembrane protein 2D topology, and PHYRE2 was used to predict the folded structure of HpuB. A representation of the hypothetical HpuB topology model with extracellular loops, helices, and plug domain is shown in Fig. 1. Using this model, mutations were created to interrogate the role of each of the following predicted loop regions: Δ236–246 (loop 2), Δ306–311 (loop 3), Δ366–370 (loop 4), Δ538–544 (predicted helix in loop 7), ΔH548, and Δ555–559 (predicted conserved motifs in loop 7). While comparing the sequence of the gonococcal HpuB to that of other bacterial heme uptake systems, we identified conserved motifs (Fig. S1): a histidine located between a FRAP motif and a NXXL (NPEL in N. gonorrhoeae FA19) motif. Additional single-point mutants were generated by deleting either the histidine or the NPEL motifs but not the FRAP motif as it is predicted to be part of a transmembrane domain (36).
Fig 1.
Hypothetical topology map of HpuB as a foundation for mutagenesis. The putative loops are extracellularly located with loop helices represented by coil-like regions. The loop number is shown above each loop. Beta strands are shown in green within the outer membrane. The orange arrows indicate the locations of the deletion mutations generated in this study. Image created with BioRender.com.
Mutated hpuB genes were created and confirmed to be expressed from an inducible promoter in an ectopic site in the gonococcal chromosome
hpuA and hpuB belong to the same operon with phase variable hpuA located upstream of hpuB. These genes are natively expressed only during iron-restricted growth conditions (30, 37). To avoid fluctuations in protein expression levels under iron restriction, we constructed two strains mutated in the native locus; one was unable to produce either HpuA or HpuB (RSC150). The other mutant strain had a locked, phase-on hpuA gene and an inactivated hpuB gene in the native chromosomal site (RSC275) (Table 1). Plasmid pVCU234, a complementation vector with an isopropyl-β-D-1-thiogalactopyranoside (IPTG) inducible promoter followed by a strong ribosome-binding site, was used to ectopically insert WT or mutated versions of hpuB into the RSC150 (A−B−) or RSC275 (A+B−) backgrounds (see Fig. 2A and B for schematic representation). Using this approach allowed for better control over the production of HpuB, with the addition of 1 mM IPTG, rather than iron restriction.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli | ||
| TOP10 | F2 mcrA D(mrr-hsdRMS-mcrBC) f 80lacZDM15 DlacX74 nupG recA1 araD139 D(ara-leu)7697 galE15 galK16 rpsL(Strr) endA1 l2 | Invitrogen |
| Gonococcal | ||
| RSC150 | FA19 hpuA::Ω (hpuA− hpuB−) | This study |
| RSC151 | FA19 hpuA::kan (hpuA− hpuB+) | This study |
| RSC275 | FA19 hpuB::Ω (hpuA+ phase on, hpuB−) | This study |
| RSC276 | RSC150 + ectopic WT hpuB (hpuA− hpuB+) | This study |
| RSC277 | RSC150 + ectopic Δ236–246 mutant hpuB (loop2) | This study |
| RSC278 | RSC150 + ectopic Δ306–311 mutant hpuB (loop3) | This study |
| RSC279 | RSC150 + ectopic Δ366–370 mutant hpuB (loop4) | This study |
| RSC281 | RSC150 + ectopic Δ538–544 mutant hpuB (loop7 helix) | This study |
| RSC282 | RSC150 + ectopic Δ555–559 mutant hpuB (loop7 NPEL motif) | This study |
| RSC283 | RSC150 + ectopic ΔH548 mutant hpuB (loop7 histidine motif) | This study |
| RSC284 | RSC275 + ectopic WT hpuB (hpuA+ hpuB+) | This study |
| RSC285 | RSC275 + ectopic Δ236–246 mutant hpuB (loop2) | This study |
| RSC286 | RSC275 + ectopic Δ306–311 mutant hpuB (loop3) | This study |
| RSC287 | RSC275 + ectopic Δ366–370 mutant hpuB (loop4) | This study |
| RSC289 | RSC275 + ectopic Δ538–544 mutant hpuB (loop7 helix) | This study |
| RSC290 | RSC275 + ectopic Δ555–559 mutant hpuB (loop7 NPEL motif) | This study |
| RSC291 | RSC275 + ectopic ΔH548 mutant hpuB (loop7 histidine motif) | This study |
| Plasmids | ||
| pVCU234 | pKH37 1 ribosome-binding site | (38) |
| pVCU252DC | pVCU403 + hpuA | (38) |
| pVCU403 | pUC18 + GC uptake sequence | (39) |
| pVCU551 | pVCU550 + Ω cassette (znuA::Ω) | (40) |
| pGSU421 | pVCU252DC + Ω cassette (hpuA::Ω) | This study |
| pGSU422 | pVCU252DC + hpuA::kan | This study |
| pGSU442 | pVCU234 + WT hpuB | This study |
| pGSU443 | pVCU234 + Δ236–246 mutant hpuB | This study |
| pGSU444 | pVCU234 + Δ306–311 mutant hpuB | This study |
| pGSU445 | pVCU234 + Δ366–370 mutant hpuB | This study |
| pGSU447 | pVCU234 + Δ538–544 mutant hpuB | This study |
| pGSU448 | pVCU234 + Δ555–559 (NPEL motif) mutant hpuB | This study |
| pGSU449 | pVCU234 + ΔH548 (conserved histidine) mutant hpuB | This study |
| pGSU450 | pVCU403 + truncated hpuA and truncated hpuB | This study |
| pGSU451 | pGSU450 + Ω cassette (hpuB::Ω) | This study |
Fig 2.
Diagram demonstrating how hpuB mutations were moved into the gonococcal chromosome generating gonococcal strains that were either HpuA− (A) or HpuA+ (B). Wild-type and mutated versions of hpuB were cloned into a complementation vector, behind an IPTG-inducible promoter (lacPOPO) and ribosome-binding site. Linearized plasmids* (Table 1) containing either the wild-type (pGSU442) or mutated versions of hpuB* (pGSU443-449) were used to transform piliated recipient N. gonorrhoeae, resulting in the insertion into an ectopic site between the aspC and lctC loci. (A) The recipient for transformation was RSC150, which contains an Ω insertion in the upstream hpuA gene, resulting in a transformant strain that is hpuA− and hpuB− in the native locus, due to the polar nature of the transposon. Transformation resulted in strains with wild-type hpuB (RSC276) in the ectopic site or mutated versions of hpuB* (RSC277-283) in the ectopic site. (B) The recipient for transformation was RSC275, which contains an Ω insertion in the downstream hpuB gene, resulting in a transformant strain that is hpuA+ hpuB− in the native locus. Transformation resulted in strains with wild-type hpuB (RSC284) in the ectopic site or mutated versions of hpuB* (RSC285-291) in the ectopic site. The symbol 5′ to the hpuA gene indicates the promoter that drives the expression of the hpuAB operon, which is repressed by iron (Fe).
After growing the hpuB mutants with or without IPTG, a western blot was performed to confirm the production of HpuB with the addition of IPTG (Fig. 3). Strain RSC150 (A−B−) was used as a negative control whereas RSC284 (A+B+), with a native and locked phase-on hpuA gene and an ectopic hpuB gene, was used as a positive control. To confirm the equivalent loading of lanes, we utilized a stain-free gel (Bio-Rad), which contains a proprietary compound that causes proteins with tryptophan residues to fluoresce (Fig. S2). Immunoblot analysis using an HpuB peptide-specific polyclonal antiserum (Biosynth) confirmed that HpuB production was regulated by IPTG in all the mutant strains (Fig. 3). Following this confirmation, we progressed to the characterization of the binding and growth phenotypes of these mutants.
Fig 3.
Mutated hpuB genes were created and confirmed to be expressed from an inducible promoter in an ectopic site in the gonococcal chromosome. HpuB mutant strains were grown on GC medium base (GCB) plates containing DFO (Fe limiting), with (+) or without (−) 1 mM IPTG. Cells were collected from plates and resuspended into phosphate-buffered saline (PBS). From these suspensions, whole-cell lysates were prepared by standardizing the cell suspensions to an OD600 of 1, pelleting cells, and resuspending pellets in lysis buffer. Western blots were performed to characterize the production of HpuB in the presence or absence of IPTG using an anti-HpuB antibody. The strain with the WT hpuB gene in the ectopic site and native hpuA gene was used as a positive control (A+B+). The strain lacking both native hpuA and hpuB genes (A−B−) was used as a negative control. Data shown are representative of three biological replicates.
Loop 7 hpuB mutants were impaired for growth on hemoglobin as a sole iron source
We evaluated whether any of the mutations impacted the ability of N. gonorrhoeae to utilize hemoglobin and whether the presence or absence of the lipoprotein, HpuA, further affected utilization. We performed growth assays in metal-restricted conditions, with Desferal (DFO) added to derepress hpuA expression and IPTG to induce hpuB expression from the ectopic site. One-micromolar hHb was added as the sole source of iron and compared to conditions to which no hHb was added. The growth of all the mutant strains, under iron restriction and with the presence of IPTG, was compared to that of the positive control A+B+ (Fig. 4). An area under the curve (AUC) calculation was used to represent the total amount of growth over the entire assay (Fig. S3A and B). In the absence of any iron source (0 µM hHb), no growth was observed for any of the strains regardless of the expression of native hpuA (Fig. S4A and B). However, when 1 µM hHb was added, the hpuB mutants demonstrated different levels of growth. As expected, the negative control, RSC150 (A−B−), exhibited no detectable growth, and the positive control, RSC284 (A+B+), demonstrated the most robust growth.
Fig 4.
Loop 7 HpuB mutants are impaired for growth on hemoglobin as a sole iron source. Gonococci were grown on GCB/DFO/IPTG plates before being resuspended in Chelex (Bio-Rad)-treated defined medium (CDM). The cell suspensions were standardized to an OD600 of 0.002 before being added to a 96-well plate containing DFO, IPTG, and 1 µM hHb. Cells were grown for 21 hours, during which the OD600 was recorded in 30-minute intervals to assess the growth of strains in the mutant hpuA background (A) and the wild-type hpuA background (B). A−B− is used as a negative control and A+B+ as a positive control. A+B− as well as A−B+ also represent controls as both proteins are thought to be required for growth. From the growth curves, the AUC was calculated using GraphPad Prism then normalized to growth by the A+B+ strain. The data from three biological replicates were analyzed to generate the means and standard deviations shown. Statistically significant differences were identified by using Student’s t-test relative to the A+B+ strain (*P < 0.05; **P < 0.005) and the A−B− strain (#P < 0.05; ##P < 0.005).
When HpuA is not expressed, the Δ236–246 and Δ306–311 mutants demonstrated growth levels similar to the A+B+ strain, although that of the Δ236–246 mutant was significantly higher than that of the A+B+ strain. We postulate that these residues (236–246) normally block hemoglobin access to HpuB when HpuA is not expressed, while in the deletion, the need for HpuA is bypassed. Growth by the Δ366–370 mutant was both significantly lower than A+B+ and higher than A−B−. The loop 7 mutants Δ538–544, ΔH548, and Δ555–559 all demonstrated a significant growth defect, comparable to the A−B− strain (Fig. 4A). HpuA and HpuB are both thought to be required for hHb utilization (30). To assess the requirement for HpuA in the mutant strains generated in this study, we measured the growth of RSC275 (A+B−) and RSC276 (A−B+), and both strains grew similarly to the A−B− strain (Fig. 4A). This observation supports the importance of both HpuA and HpuB in hHb utilization.
In strains where HpuA was expressed, the Δ306–311 and Δ366–370 mutations resulted in growth levels similar to the A+B+ strain. In contrast, the Δ236–246 strain grew significantly below that of the A+B+ strain but still higher than A−B−. The loop 7 mutants, Δ538–544, Δ555–559, and ΔH548, demonstrated a significant decrease in growth, although they recovered some growth compared to when HpuA is not expressed (Fig. 4B).
Loop 7 hpuB mutants recover hHb binding ability when HpuA is produced
To determine the binding phenotypes of these hpuB mutants, we performed a whole-cell hHb binding enzyme-linked immunosorbent assay (ELISA). As shown in Fig. 5, various levels of hHb binding were observed for the mutant strains. As expected, the negative control, RSC150 (A−B−), showed the least detectable binding, and the positive control, RSC284 (A+B+), demonstrated the highest level of binding. When HpuA was absent, Δ236–246 and Δ306–311 mutants bound hHb to the level of the A+B+ strain, while Δ366–370 demonstrated significantly reduced hHb binding. The loop 7 mutants Δ538–544, Δ555–559, and ΔH548 exhibited profound hHb binding defects, comparable to that detected with the A−B− strain (Fig. 5A). RSC276 (A−B+) also bound hHb at the level demonstrated by the A+B+ strain, suggesting that HpuB alone can accomplish binding of hHb in this system.
Fig 5.
Loop 7 HpuB mutants recover hHb binding ability when HpuA is produced. Gonococcal HpuB mutants were grown on GCB/DFO/IPTG plates before being resuspended in PBS to an OD600 of 1. Cell suspensions were added to a 96-well ELISA plate and allowed to dry prior to blocking. The ELISA plate was then probed with horseradish peroxidase (HRP)-conjugated hHb before being washed and developed with a 3,3′,5,5′-tetramethylbenzidine (TMB) substrate. The binding ability of strains without (A) and with (B) native hpuA was assessed. The absorbance at 450 nm was read to quantify the signal. A−B− is used as a negative control and A+B+ as a positive control. A+B− and A−B+ are also used as controls since both HpuA and HpuB are thought to be required for binding. All the strains were normalized to A+B+ and showed as a percentage of A+B+. Three biological replicates are represented with their means and standard deviation shown. Student’s t-test was used to assess the statistically significant differences relative to A+B+ (*P < 0.005; **P < 0.0005).
When the lipoprotein HpuA was expressed in the native site, all mutants, except for Δ366–370, demonstrated hHb binding levels similar to the A+B+ strain. The Δ366–370 mutation resulted in a strain demonstrating a significant decrease in binding when compared to the A+B+ strain (Fig. 5B). RSC275 (A+B−) showed no detectable hHb binding, indicating that the production of HpuA alone does not allow hHb binding.
Mutated HpuB variants are expressed on the gonococcal cell surface and susceptible to trypsin digestion
To ensure that the mutations generated did not affect the surface exposure and the overall conformational fold of the resulting HpuB proteins, we conducted cell surface protease digestion. The mutated strains were grown on plates supplemented with both DFO and IPTG. Following overnight growth, colonies were recovered from plates and resuspended in PBS; standardized cell suspensions were subjected to a time course of trypsin digestion (41) before whole-cell lysates were prepared. The lysates were then subjected to SDS-PAGE and transferred to nitrocellulose for the subsequent detection of HpuB and proteolytic products using the HpuB peptide-specific polyclonal antiserum (Fig. 6). It should be noted that only the proteolytic products containing the peptide epitope, used to generate the antiserum (see Materials and Methods), were detected. A proper extracellular localization is confirmed by having a digestion pattern similar to the A+B+ wild-type strain. Over the time course utilized, the full-length HpuB protein (~89 kDa) was digested into ~65-, ~50-, and ~25-kDa fragments as seen in the A+B+ control strain. Mutations at locations Δ236–246, Δ306–311, and Δ366–370 resulted in HpuB-producing strains that displayed digestion patterns identical to that of the positive control, indicating proper folding and surface exposure. The loop 7 mutants, however, demonstrated more proteolytic products than the A+B+ strain, with additional fragments at ~55, ~44, and ~30 kDa, consistent with surface presentation but also additional exposure of trypsin-sensitive digestion sites (Fig. 6). Taken together, these data indicate that all the mutants generated in this study presented HpuB on the gonococcal cell surface.
Fig 6.
Mutated hpuB variants are expressed on the gonococcal cell surface and susceptible to trypsin digestion. Strains were grown on GCB/DFO/IPTG plates and resuspended in PBS to an OD600 of 0.4. Iron-starved whole cells were then treated with trypsin for 0, 10, 20, 30, and 40 minutes before the reaction was stopped with aprotinin. NT represents no treatment. Next, the cells were pelleted, and the lysates were subjected to SDS-PAGE and western blot. The blots were probed with anti-HpuB antibody followed by an AP-conjugated IgG secondary antibody. Nitro blue tetrazolium (NBT)/5-bromo-4-chloro-3-indolylphosphate (BCIP) was used to develop the blots. (A) A+B+ shows the positive control trypsin digestion pattern. (B) The digestion pattern of the mutants. The black arrows indicate the proteolytic products seen in the positive control.
Deletions in loop 7 moved histidine 548 further away from the closest heme group
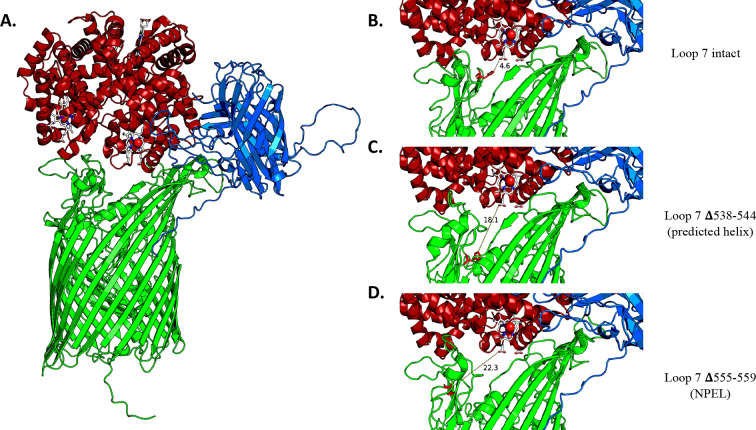
After obtaining the results described above, we sought to better understand the role of loop 7 and, in particular, the role of the conserved histidine and the NPEL motif, which are conserved among heme transporters of many Gram-negative bacteria. To analyze the interfaces in a HpuA-HpuB-Hb ternary complex, AlphaFold2-based ColabFold running in the AlphaFold-multimer mode (42–44) was used to generate predictive models of the three proteins in complex. A known Hb-heme crystal structure (PDB:1hho) was super-imposed onto the complex to correctly place the heme groups since ligands cannot be generated in the modeling software. Our predicted ternary structure shows HpuB in green, HpuA in blue, Hb in red, and the corresponding heme group in white with its Fe2+ shown as a red ball (Fig. 7A). A zoomed-in image of the interaction shows HpuB in green with residue H548 highlighted in red and the distance between the closest heme group and H548 measured in angstroms (Å). The distance between the closest heme group and H548 is 4.6 Å (Fig. 7B). However, this distance increases to 18.1 Å when the predicted loop helix Δ538–544 is deleted (Fig. 7C) and to 22.3 Å when the NPEL motif Δ555–559 is deleted (Fig. 7D). Interestingly, in the predicted binary complex of HpuB and Hb only, the distance between residue H548 and the heme group is 5.7 Å. In the mutants, this distance increases to 14.2 Å when Δ538–544 is deleted and 10.5 Å when Δ555–559 is deleted (Fig. S5). The relative position of the histidine, hypothesized to be essential for function (45), changes with the Δ538–544 and Δ555–559 deletions, pushing the histidine further away from the surface and from interacting with the heme group in the hHb.
Fig 7.
The deletions in loop 7 moved histidine 548 further away from the closest heme group. HpuA-HpuB-Hb structure. AlphaFold2 was used to obtain predicted models of the HpuA-HpuB-Hb complexes. The AlphaFold2 models were generated with wild-type HpuB (A) or with the indicated mutations in HpuB (B, C, and D). A known Hb-heme crystal structure (PDB:1hho) was super-imposed on these models to illustrate the position of heme groups. HpuB is in green with H548 highlighted in red, HpuA in blue, Hb in red, and heme groups in white with Fe2+ as a red dot. The distance in angstroms (Å) between the heme group and histidine at position 548 is indicated.
N. gonorrhoeae can bind and utilize hemoglobins produced by animals other than humans, as a sole iron source
To date, N. gonorrhoeae has only been demonstrated to bind and utilize TdT ligands that are human in origin, for example, human transferrin (39, 46). To determine if this concept applies to the HpuAB system and whether N. gonorrhoeae only binds and utilizes hemoglobin of human origin, we performed a competition assay and a growth assay. A wavelength scan (data not shown) was also conducted to ensure that all of the purchased Hbs from the different species were in the reduced (ferrous iron) form.
A competition assay was conducted to establish whether N. gonorrhoeae can bind to Hb from a mouse (mHb), pig (pHb), and rat (rHb). RSC284 (A+B+) was grown as described above and tested by whole-cell Hb binding assay for hHb binding in the presence of other species of Hb as competitors. As expected, the lowest detectable level of binding was seen in the negative control (blocker only), and the highest level of binding was detected when hHb-HRP was allowed to bind without a competitor (Fig. 8). Unlabeled human, mouse, pig, and rat hemoglobin all competed with HRP-conjugated hHb for binding to the receptor system, as seen by a significant decrease in labeled hHb binding. N. gonorrhoeae can, therefore, bind mouse, pig, and rat Hb in addition to hHb.
Fig 8.

N. gonorrhoeae can bind hemoglobin produced by animals other than humans. A competition assay was used to establish whether N. gonorrhoeae can bind to Hb from mouse (mHb), pig (pHb), and rat (rHb). A+B+ was grown on GCB/DFO/IPTG plates before being resuspended in PBS to an OD600 of 1. The cell suspension was added to a 96-well ELISA plate and allowed to dry prior to blocking. The ELISA plate was then probed with either no Hb (i.e., blocker), 5 nm hHb-HRP without competitor, or 5 nm hHb-HRP + 20× (100 nm) excess competitor. The competitors used were unlabeled hHb, mHb, pHb, or rHb. The next step was to develop with TMB. 5 nm hHb-HRP + no competitor represents the positive control, and the blocker condition represents the negative control. Statistically significant differences were assessed by Student’s t-test relative to the no competitor condition (*P < 0.0005) and the blocker condition (#P < 0.0005). The mean and standard deviation of four biological replicates are shown.
Given this result, we next assessed the ability of the gonococcus to utilize Hb from different species by conducting a growth assay as described above but this time with either hHb, mHb, pHb, or rHb provided as the sole source of iron. The growth of RSC284 (A+B+) with Hb from different species was compared to the growth of A+B+ in the presence of hHb (Fig. 9). An AUC calculation was used to represent the total amount of growth over the entire assay (Fig. S6). The condition to which no iron source was added, as expected, demonstrated the least amount of growth while the highest amount of growth was detected when hHb was provided. The A+B+ strain was similarly able to utilize hHb, pHb, and rHb for growth in this assay. However, mHb supported the growth of A+B+ to some extent, with levels significantly higher than the no iron control, but growth was significantly lower than detected in the hHb condition. In contrast to the published observations with other gonococcal nutrient transport systems, these results indicate that N. gonorrhoeae can bind to and utilize Hb from different species, in addition to human.
Fig 9.

N. gonorrhoeae can grow on hemoglobins produced by species other than human, as a sole iron source. A growth assay was used to establish whether N. gonorrhoeae could grow on different Hb species, as their sole iron source. A+B+ was grown on GCB/DFO/ IPTG plates before being resuspended into CDM. The cell suspension was standardized to an OD600 of 0.002 before being added to a 96-well plate containing DFO and IPTG with no Hb or 1 µM human, mouse, pig, or rat Hb. Cells were grown for 21 hours while the OD600 was recorded in 30-minute intervals to assess the growth of A+B+ with hemoglobin of different species. The human Hb condition represents the positive control, and the no Hb condition represents the negative control. From the growth curves, the AUC was calculated using GraphPad Prism then normalized to growth on human Hb. Three biological replicates are represented with their means and standard deviation shown. Statistically significant differences were assessed by Student’s t-test relative to the human Hb condition (*P < 0.05) and no Hb (#P < 0.05).
DISCUSSION
The fight against gonorrhea, a serious public health concern, persists. There is no protective immunity elicited following infection; moreover, the number of gonococcal isolates resistant to all available antibiotics is dramatically increasing. The lack of an efficacious vaccine against N. gonorrhoeae does not help our odds of controlling this infection; therefore, there is an urgency to develop an effective vaccine that could significantly prevent and decrease disease incidence. Like other bacteria, N. gonorrhoeae requires metal inside the human host but is confronted with nutritional immunity imposed by the host. The human host restricts essential nutrients, like iron, to prevent bacteria from colonizing and causing disease. To overcome these metal-limited conditions imposed by the host, N. gonorrhoeae expresses eight outer membrane TdTs that bind to host metal-sequestering proteins to bypass nutritional immunity and access their metal stores (26). Of these TdTs, three are bipartite receptors consisting of a surface lipoprotein and a transmembrane protein. The first two are TbpAB and the lactoferrin binding protein receptor system (LbpAB), which enable iron uptake from transferrin and lactoferrin, respectively (41, 47–50). The third, HpuAB, the subject of the current investigation, facilitates iron acquisition from hemoglobin (30, 37).
In our study, we hypothesized that the deletion of select extracellular loops from HpuB would reduce or abrogate Hb binding and consequently cause defects in growth with Hb as a sole iron source. We expected that these deletion mutations would help identify domains of the HpuB transporter that are essential for ligand binding and iron uptake functions. Loops 2, 3, 4, and 7 of HpuB were targeted in the current study; of note, loop 7 contains conserved motifs found in many heme transporters. These loops were selected due to homology with similar ligand-binding domains in TbpA (32, 51–54) and the meningococcal Hb receptor, HmbR (27, 55). TbpA and HmbR are approximately 25% and 28% identical to HpuB, respectively.
Both HpuA and HpuB were thought to be required for Hb utilization (29, 30, 56). In our study, we generated HpuB loop deletion mutants, near the N-terminus between residues 236 and 559. Surprisingly, we observed that RSC275 (A+B−), expressing only the surface lipoprotein HpuA, could neither bind Hb nor utilize it for growth, whereas RSC276 (A−B+), expressing only transmembrane protein HpuB, could bind hHb but not utilize it as a sole iron source. This suggests a potential role for HpuA in increasing the efficiency of iron internalization by HpuB, as seen with the surface lipoprotein TbpB in the TbpAB system (57).
In the absence of the lipoprotein HpuA, mutant strains with deletions of Δ236–246 and Δ306–311 were able to bind to and utilize hHb at A+B+ control levels while Δ366–370 bound to and utilized Hb at levels significantly lower than that of the A+B+ positive control. This is interesting as RSC276, which also does not express HpuA, cannot utilize hHb when producing the WT version of HpuB, suggesting that these mutations allowed for the HpuA requirement to be bypassed during internalization.
In a study by Chen et al. (58), a strain lacking HpuA and producing WT HpuB was unable to grow on hHb (Hb−). However, Hb+ revertants were isolated at a low frequency when this strain was incubated on Hb as a sole source of iron. These revertants resulted from single point mutations in HpuB (clustered toward the C-terminus) and demonstrated restored growth on Hb as a sole iron source, even in the absence of HpuA. Chen et al. attributed this restored growth to the presence of free heme, released from Hb, as the addition of human serum albumin prevented the iron uptake phenotype. The authors suggested that free heme could be imported through the mutated forms of HpuB more readily than the wild-type versions. In the current study, we identified deletion mutations in HpuB that rendered the system competent for heme iron extraction from hHb and subsequent import in the absence of HpuA, suggesting that perhaps the function of HpuA is to enable easier access to Hb by HpuB to enhance the extraction and subsequent import of heme iron.
Our study demonstrated that when HpuA was expressed, Δ236–246, Δ306–311, and Δ366–370 were all able to bind and grow on hHb, with Δ236–246 growing significantly less and Δ366–370 binding significantly less than A+B+. Additionally, when looking closely at the AlphaFold2 models, the locations of the loop 2, 3, and 4 deletions all seemed to be near hHb and/or the HpuA interface. Therefore, a possible explanation for the ability of the strains lacking HpuA to grow on hHb could be that the mutations in HpuB circumvent the requirement for HpuA, by making the core of the beta-barrel more accessible and allowing for more efficient heme internalization. We speculate that a potential mechanism to explain our results is that, during hHb binding and metal internalization, HpuA acts on the HpuB extracellular loops by pulling them away from the orifice of the beta-barrel to facilitate more efficient heme uptake, as seen by the enhanced growth phenotypes when HpuA was present. The HpuB deletion mutants may demonstrate sufficient conformational change that there was a shift in the position of these extracellular loops to expose the beta-barrel and enable heme uptake even in the absence of HpuA.
In contrast, the HpuB loop 7 deletion mutants demonstrated abrogated binding and growth on hHb when HpuA was not expressed. Interestingly, when HpuA was expressed, these loop 7 mutants completely recovered their hHb binding abilities; we observed partially recovered growth on hHb although this was still significantly reduced compared to the control A+B+ strain. This points to the importance of loop 7 residues, 538–544 (hypothetical helix), H548 (histidine motif), and 555–559 (NPEL motif), for growth on hHb, but also suggests that HpuA can facilitate the binding of hHb to the mutated transporter.
Many heme uptake systems in Gram-negative bacteria contain heme-binding motifs, including two conserved histidine residues located between a FRAP and a NXXL motif (45). The histidine residues are not found in all Gram-negatives, and examining the sequence from N. gonorrhoeae strain FA19, we identified only one histidine residue between a FRAP and a NPEL motif. AlphaFold2 prediction models of Hb-HpuB complexed with and without HpuA were also generated in this study. The distance between the identified histidine at position 548 (H548) and the heme group of the closest Hb subunit, thought to be the heme source, was measured in the models. In the presence of HpuA, the distance between H548 and the heme group is shorter than when HpuA is absent. This could explain a possible role for HpuA, which is to help stabilize HpuB in a conformation that favors a better binding affinity and shortens the distance between H548 and the heme to be transported across the barrel. This would be different from the other bipartite systems (TbpAB and LbpAB) where the anchored surface lipoprotein directly captures its cognate substrate and facilitates transfer to the barrel protein. Interestingly, regardless of HpuA expression, when comparing the distance between H548 and the heme in intact HpuB to that of HpuB Δ538–544 (hypothetical helix) or HpuB Δ555–559 (NPEL motif), the distance is longer in the deletion mutants where H548 seems to be pointing away from the surface and into the barrel. This could explain the growth defect seen in the loop 7 mutants as H548 would be too far away to interact with hHb, possibly preventing uptake. Finally, as the presence of HpuA restored the binding capability of the loop 7 mutants, another possible role for HpuA is to aid in ligand binding. One possible mechanism of function could be that HpuA helps orient hHb to stabilize its binding with HpuB, thereby bringing the heme group close enough to the putative heme-binding motif, H548, to initiate heme uptake.
In a previous study, Rohde et al. noted that heme did not compete with 125I-labelled hHb for binding and that the meningococcal HpuAB system did not discriminate between Hb of different species, possibly suggesting that HpuAB specifically recognizes the heme moiety of Hb (54). A study of meningococcal HmbR also showed a significant difference in the abilities of some animal Hbs to serve as iron sources or to enable the growth stimulation accomplished by hHb (59). In our gonococcal study, the wild-type A+B+ strain was able to bind HRP-conjugated hHb, and the binding was also inhibited by unlabeled human, mouse, pig, or rat Hb species, indicating that the gonococcal receptor system, like that described for Neisseria meningitidis, recognized other species of Hb other than that from humans. Subsequently, we determined that Hb from these different species also allowed the growth of the wild-type strain at levels lower than that generated with hHb, but the growth with the alternative forms of Hb still resulted in growth significantly above the background. These results are interesting as precedent suggested that N. gonorrhoeae exclusively bound to the human forms of the nutritional immunity proteins, including human transferrin, calprotectin, and S100A7 (39, 40, 46). However, the sequence conservation of Hbs from the different species is very high, consistent with the ability of the HpuAB system to recognize multiple forms of Hb from distinct animal species. While transgenic mouse models of disease are being developed (60, 61), to assess the contributions of TbpAB and the zinc transport systems to virulence, this may not be necessary for the HpuAB system as the mouse Hb can presumably be employed as an iron and heme source if it is available during experimental infections.
Although most of the gonococci isolated from humans do not express the hemoglobin receptor, the genes encoding the HpuAB system are maintained (30, 37) and could have a role in pathogenesis. One study concluded that the hemoglobin receptors facilitate the invasion and dissemination of N. meningitidis in the vascular system (62). Moreover, during infection in the early phases of a woman’s menstrual cycle, the expression of the gonococcal Hb receptor appears to provide a selective advantage (31). Therefore, elucidating the benefits of expressing these hemoglobin receptors would be crucial in determining their potential as vaccine components.
In summary, this study characterized the structure–function relationships in the gonococcal TonB-dependent transporter, HpuB. We showed that some mutations in HpuB bypassed the need for the surface lipoprotein, HpuA for binding and uptake. We also demonstrated the particular importance of the putative heme motifs in loop 7 for the internalization of heme. Our findings also highlighted the importance of HpuA for binding hHb, as the lipoprotein completely rescued the binding defects of the loop 7 mutants. We demonstrated the importance of residues 538–544, which constitute a hypothetical α-helical region in loop 7. This region could have a significant role in the interaction of HpuB with hHb and in ensuring heme extraction from Hb as seen previously with loop 3 helices of both TbpA and TdfJ (39, 63, 64). Therefore, acquiring a high-resolution structure of HpuB, either by protein x-ray crystallography or cryo-electron microscopy, will help better inform the choice of future mutational targets on the extracellular loops as in other studies (39, 63, 64). As previously published, transferrin-binding protein B (TbpB) and factor H binding protein (fHbp) mutants that are unable to bind host ligands demonstrated better vaccine-based protection (33–35). Although a similarly enhanced immunogenicity has not yet been described for the TdTs following mutagenesis, it is hypothesized that a cocktail of nonbinding mutants of the TdTs could enhance vaccine potential and offer better protection. Therefore, further characterizing HpuB and other TdTs for vulnerable surface-exposed, ligand-binding motifs is an important step toward potentially developing a protective antigonococcal vaccine.
MATERIALS AND METHODS
Gonococcal strains and growth conditions
Gonococci were propagated on GCB (Difco) agar containing Kellogg’s supplement I (65) and 12.4 µM Fe(NO3)3 at 36°C with 5% atmospheric CO2. In this study, most GCB plates were supplemented with 1 mM IPTG and 12.5 µM deferoxamine mesylate (Desferal/DFO) lacking Fe(NO3)3, to induce the ectopic hpuB gene and derepress hpuA in the native site. The GCB/DFO/IPTG conditions were used to culture GC unless otherwise noted. For growth experiments, cells cultured from GCB/DFO/IPTG plates were inoculated into CDM at a starting OD600 of 0.002. Then, the cells were grown, for up to 24 hours, with additional DFO, IPTG, and Hb, and optical density readings were taken every hour in a Cytation 5 BioTek microplate reader.
Gonococcal strain construction
Table 1 summarizes the different strains used and plasmids generated in this study. The hpuB mutants, with or without native hpuA, were generated from WT or mutated hpuB gene sequences (from strain FA19) synthesized by Genewiz Inc. using de novo synthesis. The regions mutated were in putative loops 2, 3, 4, and 7, the latter of which contained conserved motifs found in all hemoglobin receptors. These genes were subsequently subcloned into the SmaI site of pVCU234, using restriction endonucleases from New England BioLabs. Sequences of primers and plasmid maps are available upon request. The resulting plasmids were then linearized with PciI and used to transform piliated strains RSC150 (hpuA− hpuB−) and RSC275 (hpuA+ hpuB−). GCB plates supplemented with 1 µg/mL chloramphenicol were used to select transformants. PCR amplification of lysed chloramphenicol-resistant transformants, followed by sequencing of the resulting amplicons, confirmed the sequences of the integrated hpuB genes. The resulting gonococcal strains (Fig. 2) contained an omega cassette (Ω) in either hpuA or hpuB, which are co-transcribed in a bicistronic operon. The cassette contains transcriptional and translational stop signals and, therefore, prevents any expression downstream of the insertion point. RSC150 (hpuA− hpuB−) was generated by transforming FA19 with linearized pGSU421, which was generated by inserting the omega cassette from pVCU551 at the PpuMI site of pVCU252DC, within hpuA. Transformants were confirmed by PCR and sequencing.
RSC275 (hpuA+ hpuB−) was created to lock hpuA expression on and prevent slipped strand mispairing. This was accomplished by mutating the poly-G tract, near the mature start codon of hpuA from 9 residues to 10 residues. This repeat sequence is located between nucleotides 7 and 15 and, when phase on, encodes three glycine residues in the mature protein. Furthermore, to prevent subsequent phase variation, the wobble nucleotide of each glycine codon in the poly-G tract was changed to a different nucleotide that still allowed for the incorporation of the same amino acid. RSC275 was generated in a multistep process. First, an hpuA::Kan phase on mutant (RSC151) was generated. To do this, a kanamycin resistance gene was inserted near the middle of hpuA (phase locked on) in pVCU252 using the EZ-Tn5<Kan-2> Tnp Transposome kit (Lucigen), resulting in pGSU422. Linearized pGSU422 was used to transform FA19, and resulting transformants were screened via PCR and sequencing to verify the phase locked mutation was chromosomally located, resulting in RSC151. Second, to maintain the phase locked status in hpuA, pGSU450 was generated by cloning the region downstream of the mutation in hpuA through the first half of hpuB into the SmaI site of pVCU403. Subsequently, the omega cassette from pGSU421 was inserted into the BslwI site of pGSU450, disrupting hpuB and resulting in pGSU451. Finally, linearized pGSU451 was used to transform RSC151; resulting transformants were screened for kanamycin sensitivity, suggesting not disrupted hpuA, and subsequently confirmed by sequencing.
HpuB peptide-specific, polyclonal antiserum
Peptide and polyclonal antiserum were generated by Biosynth. The amino acid sequence of HpuB was analyzed to identify potential regions to target for antibody generation. Peptide 420NSDYSYFAKLYDPK433 was chosen based on its surface accessibility (in extracellular loop 5) and immunogenic profile using the Hopp–Woods algorithm. The HpuB peptide was synthesized using standard Fmoc solid-state peptide chemistry and conjugated to KLH, then emulsified 1:1 (volume) with Freund’s adjuvants for immunization. Two guinea pigs were immunized subcutaneously over a period of ~10 weeks, with terminal bleeds taken at the end.
Western blots
Gonococcal suspensions, in PBS, were normalized before being pelleted, resuspended in 2× Laemmli solubilizing buffer (Bio-Rad), and stored at −20°C. Subsequently, the whole-cell lysates were thawed, mixed with 5% β-mercaptoethanol, and boiled for 5 minutes. Precast 4%–20% gradient polyacrylamide gels (Bio-Rad) were used to separate the protein samples; then, a stain-free image was taken to ensure equal protein loading in each lane. Proteins were transferred onto a 0.45-µm nitrocellulose membrane (VWR), by electroblot. Next, the blots were blocked in 5% (wt/vol) bovine serum albumin dissolved in tris-buffered saline with 0.05% Tween 20 (TBST). The blots were then probed with the HpuB peptide-specific polyclonal antiserum described above (1:7,000 in blocker) for 1 hour at room temperature (RT). Next, the blots were washed three times with TBST and then probed with AP-conjugated anti-guinea pig IgG secondary antibody (1:10,000 in blocker) for 1 hour. Finally, the blots were washed again using TBST and developed using AP-reactive NBT/BCIP tablets (Sigma). A final image of the blots was taken on a Bio-Rad ChemiDoc gel imaging system using colorimetric detection.
Iron-restricted growth with hHb
Adapted from previously described methods (66), strains were streaked onto GCB/DFO/IPTG and incubated at 36°C with 5% CO2 for 16–19 hours. To test Hb utilization, iron-starved strains were inoculated at OD600 of 0.002 in 1× CDM and were added to a 96-well plate. Wells in the plate already contained 5 µM DFO, 1 mM IPTG, 5 mM mannitol, and 6 mM NaHCO3 with or without 1 µM hHb. This assay was also used to test the ability of N. gonorrhoeae to utilize and grow with Hb of different species; this time the wells in the plate contained 5 µM DFO, 1 mM IPTG, 5 mM mannitol, 6 mM NaHCO3, and either 1 µM hHb, mHb, pHb, or rHb. The BioTek Synergy plate reader was used to incubate the cultures grown at 36°C, shaking with 5% CO2, and to measure the OD600 every 30 minutes for up to 24 hours. The AUC of the resulting growth curves was calculated using GraphPad Prism to compare the growth of the strains. Student’s t-test on the AUC was performed for three biological replicates in Excel.
Whole-cell Hb binding determined by ELISA
Gonococci were iron-stressed by overnight growth on GC/DFO/IPTG agar plates. Whole gonococcal cells were collected from these plates, resuspended in PBS, and standardized to an optical density of 1 (OD600). Forty-three microliters of the cell suspension was applied in triplicate for each strain and allowed to dry in a MaxiSorp microtiter dish (Nunc) plate overnight. The dried microtiter plate was then blocked with 200 µL of 5% (wt/vol) nonfat dry milk in TBS, added for 1 hour of incubation. After the blocker was removed, 4 nM HRP-conjugated hHb in blocker was added for 1 hour of incubation at RT, after which the cells were washed five times with TBS. Later, 43 µL of TMB ELISA substrate solution (Thermo) was added to detect the amount of HRP-hHb bound to the cells in each well. After colorization, 43 µL of 0.18 M sulfuric acid (H2SO4) was added to each well to stop the reaction. Coloration was quantified by reading the absorbance at 450 nm using a Cytation 5 plate reader (BioTek). Statistical analysis was performed using Excel. Student’s t-test was performed on three biological replicates to assess statistical significance.
Surface exposure testing using trypsin digestion
Protease accessibility assays, following a protocol previously described (41), were conducted using trypsin. Iron-stressed, IPTG-treated gonococcal cells were suspended to an OD600 of ~0.4. For 0, 10, 20, 30, and 40 minutes at 36°C with 5% CO2, iron-stressed whole gonococcal cells were treated with 5 µg trypsin (Sigma) per milliliter of culture. At the appropriate time points, 0.6 trypsin-inhibiting units of aprotinin (Sigma) were used to stop the reaction. The resulting trypsin-digested whole cells were pelleted and resuspended in 2× Laemmli solubilizing buffer. As described above, western blotting was conducted to detect HpuB fragments.
Protein complex prediction by AlphaFold2 and ColabFold
For all protein complex predictions, the helix (42) software, ColabFold (44), was used with default settings (model type = alphafold2_multimer_v3, num_recycles = 3, num_relax = 0). Models were generated using the multimer mode (43) for the ternary complex of HpuAB and the tetrameric hemoglobin, the binary complex of HpuB and hemoglobin, and the different HpuB mutant variants generated in this study. Since ColabFold currently cannot incorporate ligands and co-factors into their structural predictions, the known crystal structure of the oxygenated hemoglobin (PDB:1hho) was super-imposed onto the model to correctly position the heme group within the hemoglobin. Visualization and images were prepared using PyMOL (Schrödinger LLC).
Competition Hb binding determination by ELISA
RSC284 was iron-stressed by overnight growth on DFO plates and resuspended as described above. Whole cells were standardized to an optical density of 0.4 (OD600). In the MaxiSorp microtiter dish (Nunc), 100 µL of the cell suspension was applied in quadruplets for each condition to be tested and allowed to dry in a plate for 2 days. The dried microtiter plate was also blocked with 200 µL of 5% (wt/vol) nonfat dry milk in TBS. One hundred microliters of each of the following conditions, incubated for 1 hour at RT, was added: blocker only as a negative control, 5 nM HRP-hHb as a positive control, and 5 nM HRP-hHb with 20× excess competitor. Unlabeled human, mouse, porcine, and rat Hbs were used as competitors. The wells were then washed and developed as described above. This time, the decrease in optical density (due to the presence of a competitor, unlabeled Hbs) was quantified by reading the absorbance at 450 nm using a Cytation 5 plate reader (BioTek). Statistical analysis was performed using Excel, and Student’s t-test was performed on four biological replicates.
ACKNOWLEDGMENTS
We acknowledge Scott Lewis and the immunology department at Biosynth for the generation of the HpuB antiserum.
This work was supported by funding from the National Health Service grants R01 A1 AI125421, R01 AI127793, and U19 AI144182 from the National Institute of Allergy and Infectious Diseases. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
This article is a direct contribution from Cynthia N. Cornelissen, a member of the Infection and Immunity Editorial Board, who arranged for and secured reviews by Ann E. Jerse, Uniformed Services University of the Health Sciences, and Joanna B. Goldberg, Emory University School of Medicine.
Contributor Information
Cynthia N. Cornelissen, Email: ccornelissen@gsu.edu.
Andreas J. Bäumler, University of California, Davis, Davis, California, USA
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/iai.00211-24.
Figures S1 to S6.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. WHO . 2021. Gonorrhoea: latest antimicrobial global surveillance results and guidance for vaccine development published. Available from: https://www.who.int/news/item/22-11-2021-gonorrhoea-antimicrobial-resistance-results-and-guidance-vaccine-development#:~:text=WHO%20estimates%20that%2082.4%20million,curable%20when%20treated%20with%20antibiotics
- 2. CDC . 2023. National overview of STDs, 2021. Centers for Disease Control and Prevention. Available from: https://www.cdc.gov/std/statistics/2021/overview.htm#Gonorrhea [Google Scholar]
- 3. CDC . 2021. Sexually transmitted infections prevalence, incidence, and cost estimates in the United States. Available from: https://www.cdc.gov/std/statistics/prevalence-2020-at-a-glance.htm
- 4. Schmidt KA, Schneider H, Lindstrom JA, Boslego JW, Warren RA, Van de Verg L, Deal CD, McClain JB, Griffiss JM. 2001. Experimental gonococcal urethritis and reinfection with homologous gonococci in male volunteers. Sex Transm Dis 28:555–564. doi: 10.1097/00007435-200110000-00001 [DOI] [PubMed] [Google Scholar]
- 5. Walker CK, Sweet RL. 2011. Gonorrhea infection in women: prevalence, effects, screening, and management. Int J Womens Health 3:197–206. doi: 10.2147/IJWH.S13427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Portnoy J, Mendelson J, Clecner B, Heisler L. 1974. Asymptomatic gonorrhea in the male. Can Med Assoc J 110:169. [PMC free article] [PubMed] [Google Scholar]
- 7. Campos FPF de, Kawabata VS, Bittencourt MS, Lovisolo SM, Felipe-Silva A, Lemos APS de. 2016. Gonococcal endocarditis: an ever-present threat. Autops Case Rep 6:19–25. doi: 10.4322/acr.2016.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anan TJ, Culik DA. 1989. Neisseria gonorrhoeae dissemination and gonococcal meningitis. J Am Board Fam Pract 2:123–125. [PubMed] [Google Scholar]
- 9. Quillin SJ, Seifert HS. 2018. Neisseria gonorrhoeae host adaptation and pathogenesis. Nat Rev Microbiol 16:226–240. doi: 10.1038/nrmicro.2017.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu Y, Feinen B, Russell MW. 2011. New concepts in immunity to Neisseria gonorrhoeae: innate responses and suppression of adaptive immunity favor the pathogen, not the host. Front Microbiol 2:52. doi: 10.3389/fmicb.2011.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu Y, Liu W, Russell MW. 2014. Suppression of host adaptive immune responses by Neisseria gonorrhoeae: role of interleukin 10 and type 1 regulatory T cells. Mucosal Immunol 7:165–176. doi: 10.1038/mi.2013.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization . 2021. Gonorrhoea: latest antimicrobial global surveillance results and guidance for vaccine development published. Available from: https://www.who.int/news/item/22-11-2021-gonorrhoea-antimicrobial-resistance-results-and-guidance-vaccine-development#:~:text=WHO%20estimates%20that%2082.4%20million,curable%20when%20treated%20with%20antibiotics
- 13. Ohnishi M, Golparian D, Shimuta K, Saika T, Hoshina S, Iwasaku K, Nakayama S, Kitawaki J, Unemo M. 2011. Is Neisseria gonorrhoeae initiating a future era of untreatable gonorrhea?: detailed characterization of the first strain with high-level resistance to ceftriaxone. Antimicrob Agents Chemother 55:3538–3545. doi: 10.1128/AAC.00325-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Unemo M, Shafer WM. 2011. Antibiotic resistance in Neisseria gonorrhoeae: origin, evolution, and lessons learned for the future. Ann N Y Acad Sci 1230:E19–E28. doi: 10.1111/j.1749-6632.2011.06215.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Unemo M, Shafer WM. 2014. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 27:587–613. doi: 10.1128/CMR.00010-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. St. Cyr S, Barbee L, Workowski KA, Bachmann LH, Pham C, Schlanger K, Torrone E, Weinstock H, Kersh EN, Thorpe P. 2020. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morb Mortal Wkly Rep 69:1911–1916. doi: 10.15585/mmwr.mm6950a6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cámara J, Serra J, Ayats J, Bastida T, Carnicer-Pont D, Andreu A, Ardanuy C. 2012. Molecular characterization of two high-level ceftriaxone-resistant Neisseria gonorrhoeae isolates detected in Catalonia, Spain. J Antimicrob Chemother 67:1858–1860. doi: 10.1093/jac/dks162 [DOI] [PubMed] [Google Scholar]
- 18. Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. 2012. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful International clone causes treatment failure. Antimicrob Agents Chemother 56:1273–1280. doi: 10.1128/AAC.05760-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singh A, Turner JM, Tomberg J, Fedarovich A, Unemo M, Nicholas RA, Davies C. 2020. Mutations in penicillin-binding protein 2 from cephalosporin-resistant Neisseria gonorrhoeae hinder ceftriaxone acylation by restricting protein dynamics. J Biol Chem 295:7529–7543. doi: 10.1074/jbc.RA120.012617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fifer H, Livermore DM, Uthayakumaran T, Woodford N, Cole MJ. 2021. What’s left in the cupboard? Older antimicrobials for treating gonorrhoea. J Antimicrob Chemother 76:1215–1220. doi: 10.1093/jac/dkaa559 [DOI] [PubMed] [Google Scholar]
- 21. Russell MW, Jerse AE, Gray-Owen SD. 2019. Progress toward a gonococcal vaccine: the way forward. Front Immunol 10:2417. doi: 10.3389/fimmu.2019.02417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cornelissen CN, Hollander A. 2011. TonB-dependent transporters expressed by Neisseria gonorrhoeae. Front Microbiol 2:117. doi: 10.3389/fmicb.2011.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cornelissen CN, Anderson JE, Boulton IC, Sparling PF. 2000. Antigenic and sequence diversity in gonococcal transferrin-binding protein A. Infect Immun 68:4725–4735. doi: 10.1128/IAI.68.8.4725-4735.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weinberg ED. 1975. Nutritional immunity. Host’s attempt to withold iron from microbial invaders. JAMA 231:39–41. doi: 10.1001/jama.231.1.39 [DOI] [PubMed] [Google Scholar]
- 25. Kehl-Fie TE, Skaar EP. 2010. Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol 14:218–224. doi: 10.1016/j.cbpa.2009.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neumann W, Hadley RC, Nolan EM. 2017. Transition metals at the host-pathogen interface: how Neisseria exploit human metalloproteins for acquiring iron and zinc. Essays Biochem 61:211–223. doi: 10.1042/EBC20160084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lewis LA, Gray E, Wang YP, Roe BA, Dyer DW. 1997. Molecular characterization of hpuAB, the haemoglobin-haptoglobin-utilization operon of Neisseria meningitidis. Mol Microbiol 23:737–749. doi: 10.1046/j.1365-2958.1997.2501619.x [DOI] [PubMed] [Google Scholar]
- 28. Lee BC. 1992. Isolation of haemin-binding proteins of Neisseria gonorrhoeae. J Med Microbiol 36:121–127. doi: 10.1099/00222615-36-2-121 [DOI] [PubMed] [Google Scholar]
- 29. Lewis LA, Gipson M, Hartman K, Ownbey T, Vaughn J, Dyer DW. 1999. Phase variation of HpuAB and HmbR, two distinct haemoglobin receptors of Neisseria meningitidis DNM2. Mol Microbiol 32:977–989. doi: 10.1046/j.1365-2958.1999.01409.x [DOI] [PubMed] [Google Scholar]
- 30. Chen CJ, Elkins C, Sparling PF. 1998. Phase variation of hemoglobin utilization in Neisseria gonorrhoeae. Infect Immun 66:987–993. doi: 10.1128/IAI.66.3.987-993.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anderson JE, Leone PA, Miller WC, Chen C, Hobbs MM, Sparling PF. 2001. Selection for expression of the gonococcal hemoglobin receptor during menses. J Infect Dis 184:1621–1623. doi: 10.1086/324564 [DOI] [PubMed] [Google Scholar]
- 32. Noto JM, Cornelissen CN. 2008. Identification of TbpA residues required for transferrin-iron utilization by Neisseria gonorrhoeae. Infect Immun 76:1960–1969. doi: 10.1128/IAI.00020-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frandoloso R, Martínez-Martínez S, Calmettes C, Fegan J, Costa E, Curran D, Yu R-H, Gutiérrez-Martín CB, Rodríguez-Ferri EF, Moraes TF, Schryvers AB. 2015. Nonbinding site-directed mutants of transferrin binding protein B exhibit enhanced immunogenicity and protective capabilities. Infect Immun 83:1030–1038. doi: 10.1128/IAI.02572-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martínez-Martínez S, Frandoloso R, Rodríguez-Ferri E-F, García-Iglesias M-J, Pérez-Martínez C, Álvarez-Estrada Á, Gutiérrez-Martín C-B. 2016. A vaccine based on a mutant transferrin binding protein B of Haemophilus parasuis induces a strong T-helper 2 response and bacterial clearance after experimental infection. Vet Immunol Immunopathol 179:18–25. doi: 10.1016/j.vetimm.2016.07.011 [DOI] [PubMed] [Google Scholar]
- 35. Beernink PT, Shaughnessy J, Braga EM, Liu Q, Rice PA, Ram S, Granoff DM. 2011. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J Immunol 186:3606–3614. doi: 10.4049/jimmunol.1003470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu X, Olczak T, Guo HC, Dixon DW, Genco CA. 2006. Identification of amino acid residues involved in heme binding and hemoprotein utilization in the Porphyromonas gingivalis heme receptor HmuR. Infect Immun 74:1222–1232. doi: 10.1128/IAI.74.2.1222-1232.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen CJ, Sparling PF, Lewis LA, Dyer DW, Elkins C. 1996. Identification and purification of a hemoglobin-binding outer membrane protein from Neisseria gonorrhoeae. Infect Immun 64:5008–5014. doi: 10.1128/iai.64.12.5008-5014.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cash DR. 2016. Drug and vaccine development for Neisseria gonorrhoeaea. PhD dissertation. Virginia Commonwealth University, Richmond, VA. [Google Scholar]
- 39. Cash DR, Noinaj N, Buchanan SK, Cornelissen CN. 2015. Beyond the crystal structure: insight into the function and vaccine potential of TbpA expressed by Neisseria gonorrhoeae. Infect Immun 83:4438–4449. doi: 10.1128/IAI.00762-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maurakis S, Keller K, Maxwell CN, Pereira K, Chazin WJ, Criss AK, Cornelissen CN. 2019. The novel interaction between Neisseria gonorrhoeae TdfJ and human S100A7 allows gonococci to subvert host zinc restriction. PLoS Pathog 15:e1007937. doi: 10.1371/journal.ppat.1007937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cornelissen CN, Sparling PF. 1996. Binding and surface exposure characteristics of the gonococcal transferrin receptor are dependent on both transferrin-binding proteins. J Bacteriol 178:1437–1444. doi: 10.1128/jb.178.5.1437-1444.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Žídek A, Potapenko A, et al. 2021. Highly accurate protein structure prediction with AlphaFold. Nature 596:583–589. doi: 10.1038/s41586-021-03819-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Evans R, O’Neill M, Pritzel A, Antropova N, Senior A, Green T, Žídek A, Bates R, Blackwell S, Yim J, Ronneberger O, Bodenstein S, Zielinski M, Bridgland A, Potapenko A, Cowie A, Tunyasuvunakool K, Jain R, Clancy E, Kohli P, Jumper J, Hassabis D. 2022. Protein complex prediction with AlphaFold-Multimer. bioRxiv. doi: 10.1101/2021.10.04.463034 [DOI]
- 44. Mirdita M, Schütze K, Moriwaki Y, Heo L, Ovchinnikov S, Steinegger M. 2022. ColabFold: making protein folding accessible to all. Nat Methods 19:679–682. doi: 10.1038/s41592-022-01488-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bracken CS, Baer MT, Abdur-Rashid A, Helms W, Stojiljkovic I. 1999. Use of heme-protein complexes by the Yersinia enterocolitica HemR receptor: histidine residues are essential for receptor function. J Bacteriol 181:6063–6072. doi: 10.1128/JB.181.19.6063-6072.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kammerman MT, Bera A, Wu R, Harrison SA, Maxwell CN, Lundquist K, Noinaj N, Chazin WJ, Cornelissen CN. 2020. Molecular insight into TdfH-mediated zinc piracy from human calprotectin by Neisseria gonorrhoeae. mBio 11:e00949-20. doi: 10.1128/mBio.00949-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cornelissen CN, Sparling PF. 1994. Iron piracy: acquisition of transferrin-bound iron by bacterial pathogens. Mol Microbiol 14:843–850. doi: 10.1111/j.1365-2958.1994.tb01320.x [DOI] [PubMed] [Google Scholar]
- 48. Biswas GD, Sparling PF. 1995. Characterization of lbpA, the structural gene for a lactoferrin receptor in Neisseria gonorrhoeae. Infect Immun 63:2958–2967. doi: 10.1128/iai.63.8.2958-2967.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Biswas GD, Anderson JE, Chen CJ, Cornelissen CN, Sparling PF. 1999. Identification and functional characterization of the Neisseria gonorrhoeae lbpB gene product. Infect Immun 67:455–459. doi: 10.1128/IAI.67.1.455-459.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pettersson A, Prinz T, Umar A, van der Biezen J, Tommassen J. 1998. Molecular characterization of LbpB, the second lactoferrin-binding protein of Neisseria meningitidis. Mol Microbiol 27:599–610. doi: 10.1046/j.1365-2958.1998.00707.x [DOI] [PubMed] [Google Scholar]
- 51. Boulton IC, Yost MK, Anderson JE, Cornelissen CN. 2000. Identification of discrete domains within gonococcal transferrin-binding protein A that are necessary for ligand binding and iron uptake functions. Infect Immun 68:6988–6996. doi: 10.1128/IAI.68.12.6988-6996.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Masri HP, Cornelissen CN. 2002. Specific ligand binding attributable to individual epitopes of gonococcal transferrin binding protein A. Infect Immun 70:732–740. doi: 10.1128/IAI.70.2.732-740.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yost-Daljev MK, Cornelissen CN. 2004. Determination of surface-exposed, functional domains of gonococcal transferrin-binding protein A. Infect Immun 72:1775–1785. doi: 10.1128/IAI.72.3.1775-1785.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rohde KH, Gillaspy AF, Hatfield MD, Lewis LA, Dyer DW. 2002. Interactions of haemoglobin with the Neisseria meningitidis receptor HpuAB: the role of TonB and an intact proton motive force. Mol Microbiol 43:335–354. doi: 10.1046/j.1365-2958.2002.02745.x [DOI] [PubMed] [Google Scholar]
- 55. Perkins-Balding D, Baer MT, Stojiljkovic I. 2003. Identification of functionally important regions of a haemoglobin receptor from Neisseria meningitidis. Microbiology (Reading) 149:3423–3435. doi: 10.1099/mic.0.26448-0 [DOI] [PubMed] [Google Scholar]
- 56. Biswas GD, Anderson JE, Sparling PF. 1997. Cloning and functional characterization of Neisseria gonorrhoeae tonB, exbB and exbD genes. Mol Microbiol 24:169–179. doi: 10.1046/j.1365-2958.1997.3421692.x [DOI] [PubMed] [Google Scholar]
- 57. Anderson JE, Sparling PF, Cornelissen CN. 1994. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J Bacteriol 176:3162–3170. doi: 10.1128/jb.176.11.3162-3170.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen C-J, Mclean D, Thomas CE, Anderson JE, Sparling PF. 2002. Point mutations in HpuB enable gonococcal HpuA deletion mutants to grow on hemoglobin. J Bacteriol 184:420–426. doi: 10.1128/JB.184.2.420-426.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stojiljkovic I, Larson J, Hwa V, Anic S, So M. 1996. HmbR outer membrane receptors of pathogenic Neisseria spp.: iron-regulated, hemoglobin-binding proteins with a high level of primary structure conservation. J Bacteriol 178:4670–4678. doi: 10.1128/jb.178.15.4670-4678.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zarantonelli M-L, Szatanik M, Giorgini D, Hong E, Huerre M, Guillou F, Alonso J-M, Taha M-K. 2007. Transgenic mice expressing human transferrin as a model for meningococcal infection. Infect Immun 75:5609–5614. doi: 10.1128/IAI.00781-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Levy M, Aouiti Trabelsi M, Taha MK. 2020. Evidence for multi-organ infection during experimental meningococcal sepsis due to ST-11 isolates in human transferrin-transgenic mice. Microorganisms 8:1456. doi: 10.3390/microorganisms8101456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Harrison OB, Bennett JS, Derrick JP, Maiden MCJ, Bayliss CD. 2013. Distribution and diversity of the haemoglobin-haptoglobin iron-acquisition systems in pathogenic and non-pathogenic Neisseria. Microbiology (Reading) 159:1920–1930. doi: 10.1099/mic.0.068874-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Maurakis SA, Stoudenmire JL, Rymer JK, Chazin WJ, Cornelissen CN. 2022. Mutagenesis of the loop 3 α-Helix of Neisseria gonorrhoeae TdfJ inhibits S100A7 binding and utilization. mBio 13:e0167022. doi: 10.1128/mbio.01670-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Greenawalt AN, Stoudenmire J, Lundquist K, Noinaj N, Gumbart JC, Cornelissen CN. 2022. Point mutations in TbpA abrogate human transferrin binding in Neisseria gonorrhoeae. Infect Immun 90:e0041422. doi: 10.1128/iai.00414-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kellogg DS, Peacock WL, Deacon WE, Brown L, Pirkle DI. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J Bacteriol 85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Maurakis S, Cornelissen CN. 2020. Metal-limited growth of Neisseria gonorrhoeae for characterization of metal-responsive genes and metal acquisition from host ligands. J Vis Exp 157:e60903. doi: 10.3791/60903 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1 to S6.