ABSTRACT
Toll-like receptor 9 (TLR9) is an innate immune receptor that localizes to endosomes in antigen presenting cells and recognizes single stranded unmethylated CpG sites on bacterial genomic DNA (gDNA). Previous bioinformatic studies have demonstrated that the genome of the human pathogen Chlamydia trachomatis contains TLR9 stimulatory motifs, and correlative studies have implied a link between human TLR9 (hTLR9) genotype variants and susceptibility to infection. Here, we present our evaluation of the stimulatory potential of C. trachomatis gDNA and its recognition by hTLR9- and murine TLR9 (mTLR9)-expressing cells. Utilizing reporter cell lines, we demonstrate that purified gDNA from C. trachomatis can stimulate hTLR9 signaling, albeit at lower levels than gDNA prepared from other Gram-negative bacteria. Interestingly, we found that while C. trachomatis is capable of signaling through hTLR9 and mTLR9 during live infections in HEK293 reporter cell lines, signaling only occurs at later developmental time points. Chlamydia-specific induction of hTLR9 is blocked when protein synthesis is inhibited prior to the RB-to-EB conversion, exacerbated by the inhibition of lipooligosaccharide biosynthesis, and is significantly altered during the induction of aberrance/persistence. Our observations support the hypothesis that chlamydial gDNA is released during the conversion between the pathogen’s replicative and infectious forms and during treatment with antibiotics targeting peptidoglycan assembly. Given that C. trachomatis inclusions do not co-localize with TLR9-containing vacuoles in the pro-monocytic cell line U937, our findings also hint that chlamydial gDNA is capable of egress from the inclusion, and traffics to TLR9-containing vacuoles via an as yet unknown pathway.
KEYWORDS: Chlamydia, innate immunity, TLR9, pathoadaptation, persistence
INTRODUCTION
Chlamydia trachomatis is an obligate intracellular bacterium that utilizes a unique biphasic developmental cycle (1). The pathogen has three morphological forms; an Elementary Body (EB) that is infectious, but non-replicating, a Reticulate Body (RB) that is the replicative but non-infectious, and an Aberrant Body (AB) that is thought to be a defensive response by the bacterium to extracellular stressors. The microbe spends the majority of its existence in an intracellular pathogenic vesicle called an inclusion, where it is protected from a number of common immune responses to bacterial infections (2).
Chlamydia species are recognized by the innate immune system via Toll-like receptors (TLRs) and NOD-like receptors (NLRs), which respond to various components of bacterial and viral pathogens (3). This early interaction initiates a cytokine signaling cascade that results in the recruitment of immune cells to the area in order to fight off the infection and direct a subsequent, cell-mediated adaptive response (4). TLRs play an important role in numerous infections of the female lower genital tract (5), and a number of studies have investigated the importance of various immunostimulatory chlamydial components (lipids/lipoproteins/MOMP/HSP60, Lipooligosaccharide; LOS, peptidoglycan) and their cognizant innate immune receptors (TLR2, TLR4, NOD1/2), respectively (3). The predominantly held view is that TLR2 plays a major role during chlamydial infections, while TLR4 and NOD1/2 are accessory and play a more supportive role (6). This assessment is based largely on the fact that Chlamydia species produce less active/immunostimulatory LOS (7–9) and peptidoglycan (10–14) than similarly sized Gram-negative bacteria, supporting the hypothesis that these differences represent pathoadaptations by the organism that enable it to subvert the host’s innate immune system (2, 15).
Considerably less is known about the role of the innate immune receptor TLR9 during chlamydia infections. TLR9 recognizes single stranded, unmethylated cytosine-phosphate-guanosine (CpG) sites on bacterial genomic DNA (16). Unlike TLR2/4 and NOD1/2, which are broadly expressed by a large number of cell types, TLR9 is expressed almost exclusively in monocytes and dendritic cells (16–18). In addition to being expressed in only a small subset of cell populations, TLR9 is exclusively found within vesicles associated with the endosomal maturation pathway. When phagocytic cells ingest foreign, extracellular bacteria, TLR9 is translocated to the endosomal compartment (19) and signals after trafficking to the lysosome where it interacts with the DNA released from bacteria undergoing degradation (20). While C. trachomatis avoids fusing with vesicles in the endosomal maturation pathway in most cell types (21–27), this is not the case in many immune cells that actively express TLR9 (28–31).
Because many TLR9 stimulatory (and inhibitory) signaling motifs have been identified, it is possible to estimate the stimulatory potential of genomic DNA from any microbe whose genome has been sequenced (32). C. trachomatis stimulatory DNA CpG motifs have been calculated in the low-to-mid range (33, 34), indicating that its gDNA is likely recognizable by TLR9. However, in silico-based approaches rely entirely on previously characterized stimulatory and inhibitory CpG motifs, and as such, they can offer only a rough estimate of the stimulatory potential of a given microbe’s genome. Correlative studies have found that TLR9 gene polymorphisms are associated with increased risk of cervicitis (35) and pathology associated with chlamydia infections in human patients (36), as well as susceptibility to chlamydia infection in ruminants (37). Despite these observations, studies investigating TLR9’s role in responding to chlamydia infections in animal models have demonstrated little to no significant association with infectivity or disease presentation (34, 38, 39), leading us to question the degree to which TLR9 signaling occurs in chlamydia-infected cells.
Here, we present an analysis of the stimulatory potential of gDNA from C. trachomatis and through a series of experiments demonstrate its ability to induce TLR9 signaling.
RESULTS
Genomic DNA from C. trachomatis induces hTLR9 signaling in vitro
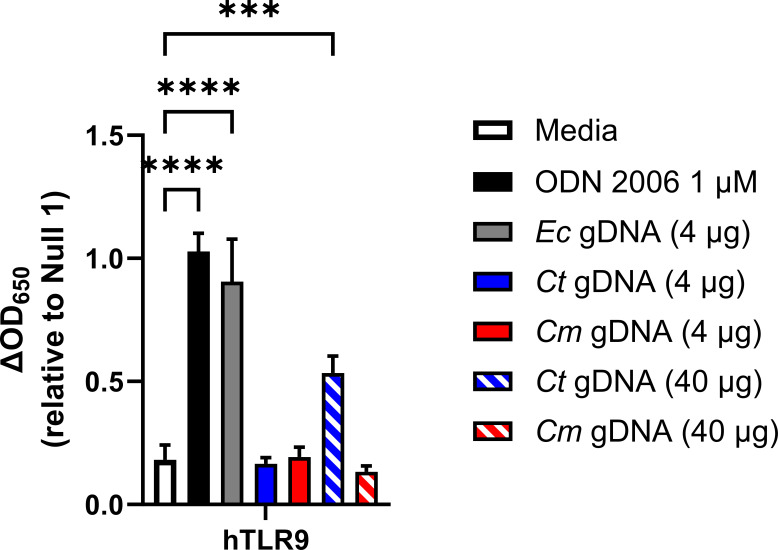
We began our study by first confirming that gDNA isolated from C. trachomatis was capable of initiating a signaling cascade through TLR9. Utilizing a human TLR9-expression reporter cell line (hTLR9-HEK-Blue) we examined secreted Embryonic Alkaline Phosphatase (SEAP) activity in the supernatants of reporter cells exposed to various concentrations of sonicated gDNA obtained from three different bacterial species; E. coli (strain MG1655), Chlamydia trachomatis (serovar L2, strain Bu/434), and Chlamydia muridarum (strain Nigg). We observed that sonicated E. coli gDNA was highly stimulatory, which we interpreted as validating our gDNA sonication procedure. Sonicated C. muridarum gDNA exhibited no measurable TLR9 stimulatory activity, and C. trachomatis gDNA was stimulatory (P < 0.0001), but only when x10 as much sonicated gDNA was used in the assay (Fig. 1). We took these results as confirmation that gDNA obtained from C. trachomatis EBs can act as a positive stimulatory ligand for hTLR9, and that C. trachomatis-specific TLR9 signaling is, as predicted by in silico analysis (33) more robust than that observed for C. muridarum.
Fig 1.
Genomic DNA from C. trachomatis induces hTLR9 signaling in vitro. A human TLR9 (hTLR9) HEK 293 reporter system was used to evaluate the stimulatory potential of sonicated genomic DNA isolated from E. coli (strain MG1655), C. trachomatis (strain L2 434/Bu), and Chlamydia muridarum (strain Nigg). Data presented are the mean of three independent, biological replicates and error bars represent standard error of the mean. Groups were compared via one-way ANOVA with multiple comparisons. ****; P < 0.0001, ***; P < 0.001. All comparisons to the media control not shown were not significant.
hTLR9 does not localize with C. trachomatis inclusions in U937 cells
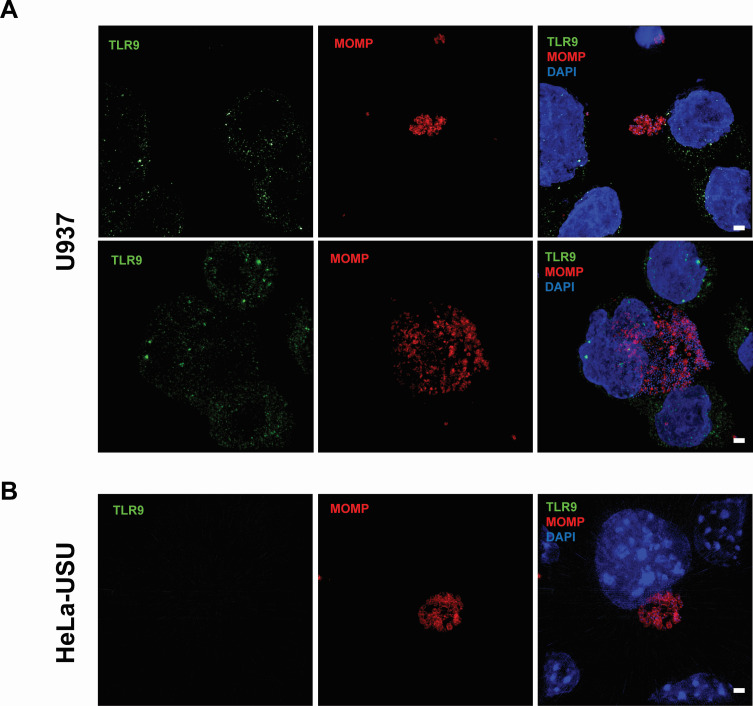
In unstimulated immune cells, TLR9 is maintained in the endoplasmic reticulum (20, 40). Upon stimulation, the receptor traffics through the Golgi Complex and primarily localizes to endolysosomes (41), where it can interact with any foreign bacterial DNA sampled from the environment. While chlamydial inclusions are generally thought to be non-fusogenic with endosomes and lysosomes in most cell types (21–27), the exception to the rule is macrophages and certain specialized dendritic cells (28–31). While TLR9 is thought to be expressed in only subsets of dendritic cells and B cells in humans (42), previous studies have demonstrated CpG-oligodeoxynucleotides (ODNs) signaling in the human monocyte-derived cell line U937 (43–45). To assess whether C. trachomatis resides within TLR9-containing compartments within U937 cells, we conducted an immunolabeling experiment. We found that U937 cells contained hTLR9-labeled vacuoles, which appeared equivalently spaced throughout the cytosol (Fig. 2A), and these labeled vacuoles were not present in our control cell line; HeLa-USU (Fig. 2B). When we examined cells infected with C. trachomatis at 40 hours post infection (hpi), we observed no direct co-localization with inclusions (Fig. 2; Fig. S1). While TLR9-labeled vacuoles were often visible within close proximity to inclusions, we conclude that C. trachomatis and hTLR9 do not appear to associate within the same intracellular vacuole within U937 cells.
Fig 2.
hTLR9-containing vacuoles do not co-localize with C. trachomatis inclusions in U937 cells. (A) U937 (pro-monocytic, human myeloid leukemia derived) and (B) HeLa cells were infected via rocking incubation with C. trachomatis at a MOI of ~5. At 40 hpi, cells were fixed in 10% PFA, blocked with 3% BSA, and labeled with polyclonal antibodies to the pathogen’s major outer membrane protein (MOMP) and TLR9. Images presented are Maximum Intensity Projections from zStacks acquired from a Zeiss PS.1 ELYRA imaging system and are representative of over 20 fields of view observed over two separate labeling experiments. Scale bar ~2 µm.
Pre-exposure to stimulatory TLR9 ligands does not impact the development of C. trachomatis in U-937 cells
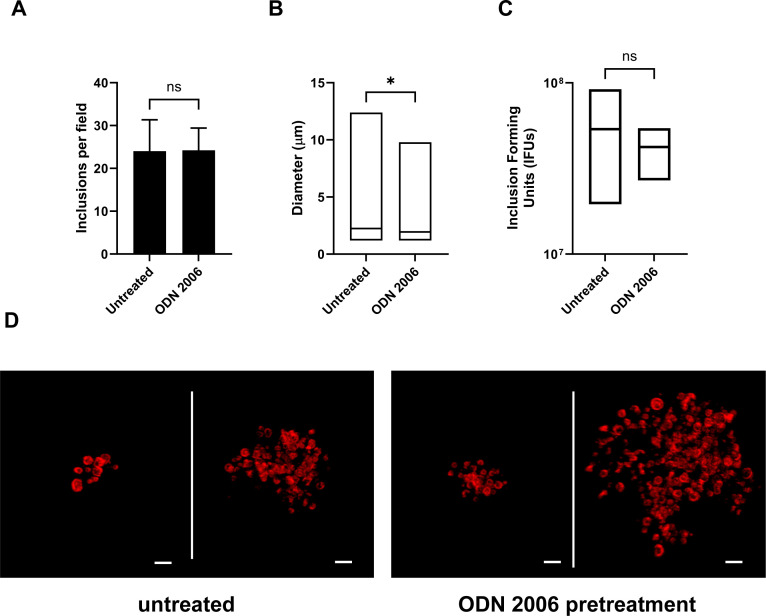
While C. trachomatis gDNA does stimulate signaling through hTLR9, it is significantly less immunostimulatory when compared with other bacteria (Fig. 1, 33). Previous researchers have proposed that this represents a shared pathoadaption by bacterial STIs, enabling them to suppress their immunogenicity and evade or delay cell-mediated immune responses (33). We sought to test this hypothesis by examining whether pre-treatment of U937 cells with a known hTLR9 agonist (ODN 2006) resulted in higher tolerance to subsequent infection by C. trachomatis. We found that cells pretreated with ODN 2006 appeared to be just as susceptible to infection as non-pretreated cells, as measured by inclusion forming unit counts at 24 hpi (Fig. 3A), however, there was a measurable difference (P < 0.05) in the overall size of inclusions between the two groups, with fewer large (> 5 µm diameter) inclusions present in the pretreated group (Fig. 3B). No significant difference was observed between untreated and pre-treated cells in terms of their capacity to generate new elementary bodies, as assessed at 40 hpi via inclusion forming unit (IFU) counts (Fig. 3C). The vast majority of cells under both conditions contained small inclusions with only a few bacteria present. Larger inclusions appeared oddly-shaped and contained RBs that appeared dispersed and not closely associated with inclusion walls (Fig. 3D).
Fig 3.
Pre-exposure to stimulatory TLR9 ligands does not impact the development of C. trachomatis in U-937 cells. (A) The average number of inclusions observed per field of view (from 20 fields) in U937 cells that were left untreated or pretreated with the TLR9 agonist ODN 2006 for 18 hours prior to infection with C. trachomatis. Cell monolayers were fixed and labeled at 24 hpi. (B) Inclusion size measurements in U937 cells that were untreated or pretreated with ODN 2006 prior to infection. Data from two separate experiments were pooled for the analysis, and groups were compared via unpaired t test with Welch’s correction. *; P < 0.05. (C) Inclusion forming unit (IFU) counts from C. trachomatis-infected U937 cells that were untreated or pretreated with ODN 2006 prior to infection. Data presented are the mean of three separate experiments (biological replicates) and groups were compared via unpaired t test with Welch’s correction. ns; not significant. (D) Representative images of larger (> 5 µm) inclusions containing multiple RB-sized bacteria found in C. trachomatis-infected U937 cells. All cells were fixed and imaged 24 hpi. Scale bar, ~2 µm.
Infection with live C. trachomatis, but not live C. muridarum, induces hTLR9 signaling in HEK293 reporter cells late in the pathogen’s developmental cycle
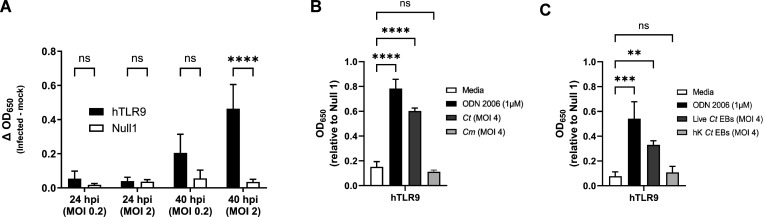
While we effectively demonstrated that chlamydial gDNA is immunostimulatory and recognized by TLR9 (Fig. 1), we reasoned that because the trafficking of extracellular gDNA to TLR9-containing vesicles is dependent on endosomes (19), this would likely be the case for chlamydia-specific TLR9 signaling as well. However, as chlamydial inclusions only associate with endosomes / lysosomes in some dendritic cells, and our hTLR9 reporter cell line is not of monocytic origin (46), we questioned whether direct infection of hTLR9-expressing HEK-293 cells with C. trachomatis would result in measurable, hTLR9-specific SEAP activity. To test this, reporter cells were infected with C. trachomatis at a MOI of either 2 or 0.2 and SEAP activity was assessed at 24 and 40 hpi. Surprisingly, direct infections did result in measurable, dose-dependent hTLR9 activity, albeit significant signaling did not occur until the later stages of the pathogen’s developmental cycle (Fig. 4A). Follow-up assays demonstrated that this signaling was unique to C. trachomatis, as live infections with C. muridarum did not result in similar hTLR9-specific activity (Fig. 4B). To account for the possibility that C. trachomatis-specific hTLR9 signaling was due to the presence of extracellular gDNA from killed/lysed bacteria present in the infection inoculum, we ran the assay comparing the signaling potential of live vs. heat-killed organisms, and found measurable TLR9 signaling activity only during live infections (Fig. 4C). These observations indicate that chlamydial viability is required for this late-stage signaling observed in our hTLR9 reporter cells.
Fig 4.
C. trachomatis-induced hTLR9 signaling occurs late in the pathogen’s developmental cycle in non-phagocytic cells. (A) SEAP activity was measured from the supernatants of hTLR9- and Null 1-HEK 293 reporter cells infected with C. trachomatis serovar L2 (strain Bu/434) at 24 and 40 hpi. (B) A comparison of the hTLR9-dependent SEAP activity present in supernatants obtained from reporter cells infected with either C. trachomatis or C. muridarum for 48 hours. (C) hTLR9 signaling assessed at 48 hpi for live and heat-killed C. trachomatis EBs. For all panels, columns represent the mean value calculated for data acquired from three separate experiments (biological replicates) and error bars represent standard error of the mean. Groups were compared via two-way and one-way ANOVA with multiple comparisons, respectively. ****; P < 0.0001, ***; P < 0.001, ns; not significant.
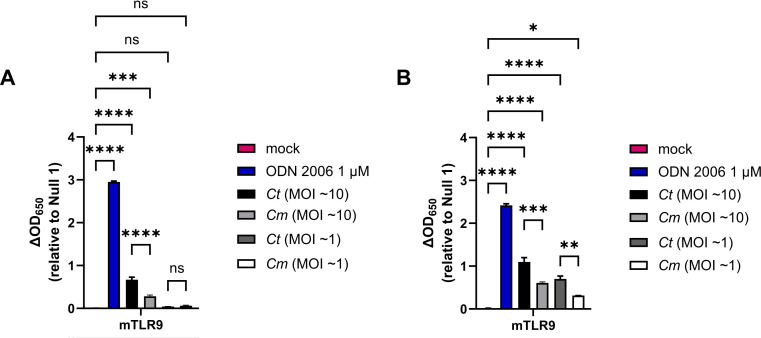
C. trachomatis and C. muridarum signal through mTLR9 in infected HEK293 reporter cells
We found it curious that C. trachomatis appeared to signal more robustly through hTLR9 than C. muridarum, as we had hypothesized that each pathogen would have evolved to minimize the immunogenicity to the TLRs of their respective host species. To examine this question of cross-species recognition further, we conducted an additional strain comparison using a reporter cell line expressing the murine allele of TLR9 (mTLR9). mTLR9 has been shown to exhibit structural differences from hTLR9 and recognizes different CpG motifs (42, 47). We examined two different MOIs for each organism (10 and 1), and observed mTLR9 signaling at the 24 hpi time point for both C. trachomatis or C. muridarum when our highest MOI was used (Fig. 5A). At the 48 hpi time point, mTLR9 signaling was observable at MOIs as low as 1 (Fig. 5B), and when compared directly, C. trachomatis signaling was approximately twice that of C. muridarum at both time points examined (Fig. 5A and B). When combined with the purified gDNA data, these observations demonstrate that C. muridarum is significantly less stimulatory than C. trachomatis as assessed by both the human and murine TLR9 receptors.
Fig 5.
C. trachomatis and C. muridarum signal through mTLR9 during live infections of HEK293 reporter cells. SEAP activity was measured from the supernatants of mTLR9-HEK 293 reporter cells infected with C. trachomatis and C. muridarum at 24 (A) and 48 hpi (B). For all panels, columns represent the mean value calculated for data acquired from three biological replicates and error bars represent standard error of the mean. Groups were compared via one-way ANOVA with multiple comparisons. ****; P < 0.0001, ***; P < 0.001, **; P < 0.01, *; P < 0.05, ns; not significant.
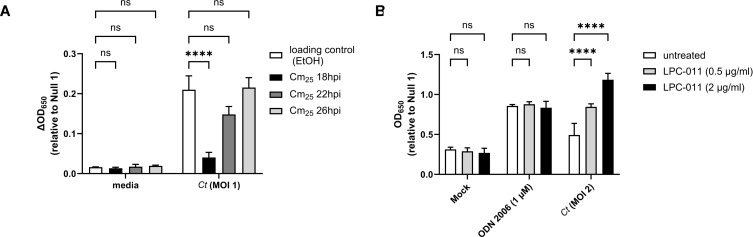
Chlamydia-specific TLR9 signaling in HEK293 cells occurs as a result of the RB-to-EB conversion
Given that we did not observe chlamydia-specific hTLR9 signaling at 24 hpi (Fig. 4A), we reasoned that this could be due to one of two possibilities: 1) that not enough intracellular bacterial replication had occurred to meet the threshold needed for detection by TLR9 and/or 2) that chlamydial gDNA availability varied at different time points post infection. The conversion between the chlamydial replicative and infectious forms begins to occur (in cultured epithelial cells) between ~20 and 24 hpi (48, 49). To determine whether the interruption of EB development affects chlamydia-specific TLR9 signaling, we measured signaling in cells infected with C. trachomatis that were treated with chloramphenicol (Cm25) at various time points post-infection. We observed that signaling only occurred in untreated cells and cells treated with Cm25 at or later than 22 hpi (Fig. 6A), roughly coinciding with the initiation of RB-to-EB conversion.
Fig 6.
Chlamydia-specific TLR9 signaling in HEK293 cells occurs as a result of the RB-to-EB conversion. (A) C. trachomatis-infected hTLR9-HEK293 reporter cells were treated with chloramphenicol (25 ug/ml; Cm25) at the time points indicated and SEAP activity was measured at 44hpi. (B) The effects of the LOS inhibitor LPC-011 on C. trachomatis-induced hTLR9 signaling. All columns represent mean values calculated for data acquired from three biological replicates and error bars represent standard error of the mean. Groups were compared via two-way ANOVA with multiple comparisons. ****; P < 0.0001, ns; not significant.
The autolysis of C. trachomatis RBs has been described previously (50), and it is commonly thought that a small subset of RBs within the inclusion will undergo lysis during the developmental cycle. We hypothesized that such lytic events are likely the cause of gDNA release within inclusions. Given the delay we observed in TLR9 signaling in our HEK293 report cells (Fig. 4A), we also hypothesized that the most likely time when gDNA is released from the microbe would be during the transition state between replicative and infectious forms. To examine the degree to which gDNA release impacted our chlamydia-specific TLR9 signaling, we examined the effects of the LpxC inhibitor LPC-011 (51) on C. trachomatis-induced TLR9 signaling. LpxC carries out the 2nd step in the lipooligosaccharide (LOS) biosynthesis pathway, and while LOS biosynthesis is not required for the transition of chlamydial EBs to RBs, nor for RB replication, it is required for the conversion of RBs into EBs at the end of the pathogen’s developmental cycle (51, 52). In the absence of LOS, RBs lyse during the conversion process. When infected HEK293 cells were treated with the LOS inhibitor LPC-011, a dose-respondent increase in chlamydia-specific hTLR9 signaling was detected (Fig. 6B). Together, these observations support the hypothesis that C. trachomatis gDNA release in epithelial cells is the result of the process of RB-to-EB conversion.
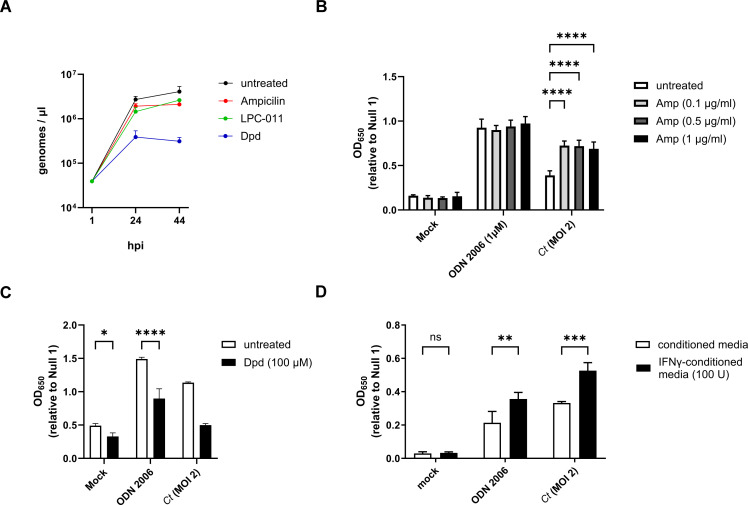
Persistence alters C. trachomatis-induced hTLR9 signaling
We have previously reported that stress-inducing conditions linked to the aberrant/persistent phenotype in C. trachomatis can directly affect the recognition of the pathogen by the Nod-like Receptor (NLR) NOD1 (53). Prior studies have also found that these persistence-inducing conditions can affect the ability of chlamydia species and chlamydia-related organisms to regulate DNA replication. While cell division is inhibited during persistence, some persistence-inducing conditions result in a marked reduction in chlamydial DNA replication (54, 55) while others result in the accumulation of 100 s of genome copies residing within a single, enlarged bacterium (55–59). Given that many of these “aberrant” chlamydial forms exhibit evidence of membrane disruptions and structural defects (60–62), we reasoned that DNA could likely be shed by these aberrant forms into the inclusion space. We also hypothesized that continued DNA replication would directly impact TLR9-stimulation by C. trachomatis, as the abundance of available ligand (gDNA) would likely correspond to the signaling potential of the microbe. We began by examining genome replication in C. trachomatis under aberrance induction by ampicillin and the iron chelator 2,2′-Dipyridyl (Dpd) over the course of the microbe’s developmental cycle (~44 hpi). Similar to previously published results (48), we found that the majority of C. trachomatis genome replication occurs within the first 24 hours post-infection under all the conditions tested, and that persistence induced by iron chelation resulted in ~1 log10 reduction in genome counts (Fig. 7A).
Fig 7.
Persistence alters chlamydia-induced hTLR9 signaling. (A) C. trachomatis genome copies were measured in untreated cells as well as in cells infected in the presence of ampicillin, the iron chelator 2,2′-Dipyridyl (Dpd), and the LpxC inhibitor (LPC-011) over the span of 44 hours. Measurements were taken at 1, 24, and 44 hpi. (B–D) The effects of (B) ampicillin; Amp, (C) 2,2′-Dipyridyl; Dpd, and (D) tryptophan-depletion via interferon gamma (IFNγ) were assessed on hTLR9-stimulatory activity of ODN 2006 and infection by C. trachomatis. All columns represent mean values calculated for data acquired from three biological replicates and error bars represent standard error of the mean. groups were compared via two-way ANOVA with multiple comparisons. ****; P < 0.0001, ***; P < 0.001, **; P < 0.01, *; P < 0.05, ns; not significant.
Having assessed the relative differences in genome replication under a variety of persistence-inducing conditions, we next tested chlamydia-infected cells under these same conditions over a 44 hour period in our hTLR9 HEK293 reporter assay. Antibiotics that target peptidoglycan are known to result in high levels of “temporary polyploidy” in the resulting chlamydial aberrant bodies (55–59) and we observed that in the presence of ampicillin, C. trachomatis-induced TLR9 activity was significantly enhanced (Fig. 7B). Conversely, iron restriction is associated with a reduction in genome replication in chlamydia species and chlamydia-like organisms (Fig. 7A ) (54, 55), and we observed a marked decrease in TLR9 activity when infected cells were treated with the iron chelator 2,2-dipyridyl (Dpd) (Fig. 7C). However, we also observed that the condition impacted our positive control (ODN 2006), indicating that the effects on signaling are likely indirect. Tryptophan starvation resulting from treatment of primate-derived cells with interferon gamma (IFNγ) also results in the accumulation of large numbers of genome copies within single aberrant chlamydial bodies (63, 64), and we found that this condition significantly enhanced hTLR9 signaling (Fig. 7D), however, this was also not unique to chlamydia-induced signaling as we saw significant enhancement in our positive control. Taken together, these data indicate that TLR9 signaling results from the release of gDNA from C. trachomatis within its inclusion and that, similar to NOD1 signaling (53), “persistence”-inducing conditions can affect both chlamydia-specific and chlamydia non-specific TLR9 signaling.
DISCUSSION
Our data indicates that C. trachomatis gDNA is a stimulatory ligand for both human and murine TLR9 and has a higher stimulatory potential than the murine pathogen C. muridarum. We found that TLR9 signaling from C. trachomatis was higher than that of C. muridarum in both in vitro testing of purified gDNA (Fig. 1) and live infections of reporter cells (Fig. 4B and 5 ). Given our data indicating that TLR9 signaling is impacted by the timing of the chlamydial developmental cycle (Fig. 4A and 6A ) and bacterial load (Fig. 4A and 5 ), this is a particularly significant finding. C. muridarum has a higher replication rate and a shorter developmental cycle than C. trachomatis (65), and we had initially predicted that it would release gDNA into the inclusion earlier and at a higher abundance than C. trachomatis. The fact that C. muridarum TLR9 signaling is lower than C. trachomatis may be due to its genome containing fewer CpG-stimulatory motifs than encoded in the genome of C. trachomatis. A prior in silico analysis based on stimulatory and inhibitory CpG motif sequences present in the genome of E. coli indicated that the C. muridarum genome has both a lower abundance of CpG sequences per kilo base and a lower CpG stimulatory index than C. trachomatis strains (34). This difference in the immunostimulatory potential of the gDNA from of these two pathogens may have arisen due to differences in tissue/host-specific prevalence of TLR9. TLR9 was originally thought to be expressed in a number of cell types (such as macrophages) in mice (66) and expressed in only a few specialized dendritic cell populations in humans (67). However, more recent studies have brought this general consensus into question, and TLR9 is now known to be highly expressed in multiple cell types in both human and murine lung tissue (68). Given its origin (69) and re-emergence (70) as a murine lung pathogen, C. muridarum likely evolved under heightened selective pressure in this environment and the reduced stimulatory potential of its gDNA may be evidence of a pathoadaptation to this niche. This is similar to what is predicted to have led to the loss in immunostimulatory potential of LOS present in a number of chlamydial species (9).
TLR9 haplotypes and polymorphisms have been previously shown to correlate with potential risk of cervicitis (35), cervical cancer (71), as well as susceptibility and the development of symptoms after HR-HPV and C. trachomatis infection (36, 72). TLR9-stimulatory adjuvants have also been used extensively in chlamydial vaccine development (73–77). Despite this, TLR9 has been deemed to play only a minor role in chlamydial recognition during active infections. This general consensus has been based on studies investigating pattern recognition molecules generated by C. muridarum that are recognized by oviduct epithelial cell lines (38) and assessing the impact of murine TLR9 on the clearance of C. trachomatis and C. pneumoniae infections utilizing a TLR9 −/− mouse infection model (34, 39). While these studies were well-powered and well-controlled, they did not directly explore the interaction of either pathogen with the receptor at early stages of infection. The relatively high doses required to generate stable C. trachomatis infections in the murine model are likely due to the presence of innate immunological factors (such as TLR9) that are thought to play a larger role in preventing infections from occurring (particularly in the lower genital tract), rather than controlling them once they are established. By comparison, TLR2 (38, 78–82) and TLR4 (7–9, 83) are thought to play a critical role in controlling chlamydial infections. While TLR2 and TLR4 have been shown to be highly expressed in the upper genital tract, TLR9 as well as NOD-like Receptors (NLRs) 1 and 2 are expressed more uniformly throughout the upper and lower genital tract (84). Similarly, the cGAS-STING signaling pathway, another innate DNA recognition system that grants heightened immunity to C. trachomatis infection, is notably restricted to the lower genital tract (85).
While we originally expected to observe co-localization of C. trachomatis inclusions with TLR9 in monocyte-derived cell lines (Fig. 2), we were unable to verify this in U937 cells. In macrophages and dendritic cells, the AP3 adaptor complex and the AP-3-interacting cation transporter (Slc15a4) are responsible for trafficking TLR9 from the early endosome to a specialized lysosome-related organelle where TLR9 then activates MYD88 signaling (86). In contrast, while a number of TLRs (as well as their adaptor protein MYD88) associate with the chlamydial inclusions in epithelial cells (78, 87), these inclusions avoid fusing with endosomes/lysosomes (88). We hypothesized that because prior studies observed fusion of C. trachomatis inclusions with vacuoles containing endosomal components (28–31), that the mechanism for TLR9 interacting with gDNA from C. trachomatis in U937 cells would be straightforward; bacterial lysis in endosomal-compartments containing TLR9. Our observations have led us to instead speculate that C. trachomatis-induced TLR9 signaling may occur via a mechanism independent of lysosomal fusion, which would explain why signaling is observable in non-phagocytic cell lines, such as our HEK293 report cells. Given that we observe signaling only on the second day postinfection (Fig. 4A), we believe that TLR9 signaling under these conditions is the result of gDNA being released from a subset of chlamydial RBs that fail to successfully convert into EBs. Our data demonstrating 1) prevention of the RB to EB conversion eliminates TLR9 signaling (Fig. 6A) and 2) artificially enhancing the frequency of lysis events during conversion (utilizing the LpxC inhibitor) enhances TLR9 signaling (Fig. 6B) supports this proposition. In the context of the observed differences in TLR9 signaling in C. trachomatis and C. muridarum-infected cells, variation in the incidence of lytic events during the RB-to-EB transition between species could also be a contributing factor.
It is presently unclear how the gDNA that is released during RB lysis traffics to TLR9-containing endosomes. Previous studies have indirectly demonstrated the presence of cytosolic chlamydial gDNA in infected cells in culture (89, 90), and we hypothesize that if chlamydia gDNA is capable of exiting the inclusion into the cell cytosol, this might enable it to eventually be trafficked to TLR9-containing compartments. To date, no direct mechanism has been proposed by which chlamydial DNA exits the inclusion. Evidence exists in multiple model systems that DNA transfer from a bacterium to a host cell can occur via type IV secretion systems (T4SS) (91–93), but there exists no example of DNA being successfully transferred via the secretion systems utilized by C. trachomatis (i.e., type II and type III secretion). Experiments investigating alternative trafficking route(s) of chlamydial gDNA from the inclusion to the TLR9 receptor are currently ongoing.
In addition to immune recognition, the presence of cytosolic chlamydial DNA in infected host cells has implications for other aspects of chlamydial biology, such as factors that influence genetic exchange between strains (94) and potential barriers to inter-species gene flow. Chlamydia species undergo conversion from RBs to EBs in a rather disjointed fashion, and the reasons for this are not well understood. C. trachomatis encodes ComEC homologs that enable DNA uptake and lateral gene transfer (95). These are presumably expressed predominantly in RBs, as most metabolic processes in chlamydial EBs are significantly reduced. Given our observations that C. trachomatis releases gDNA into its immediate environment during RB-to-EB transition events (Fig. 6), it stands to reason that asynchronous conversion between developmental forms may have arisen, in part, due to the potential for genetic exchange between replicating RBs and RBs lysing during conversion. C. trachomatis inclusions exhibit homotypic fusion, in that multiple inclusions within a cell can fuse into a single, enlarged inclusion. This process is largely dependent on IncA (96–98), a SNARE-like protein that is secreted by the chlamydial Type III secretion system and incorporates into the inclusion wall. Infections with different strains (99, 100) and mixed infections with different chlamydial species (101, 102) have been used to demonstrate the potential gene flow between chlamydia species that are capable of residing within shared inclusions. Interestingly, genetic exchange between non-fusogenic species has also been observed (102), suggesting that DNA trafficking can occur between inclusions. Studies exploring the mechanism(s) by which gDNA transits to and from the host cell cytosol are presently ongoing.
Conclusions
In this study, we demonstrate the stimulatory potential of chlamydial gDNA for recognition by hTLR9 and mTLR9, demonstrate that under normal growth conditions, chlamydia-specific TLR9 signaling occurs during the stage in the pathogen’s developmental cycle when developmental form conversion occurs, and that the induction of aberrance can either enhance or diminish this signaling. The disjoining of genome replication and cell division that occurs during chlamydial persistence has been previously suggested to be a beneficial adaptation, enabling the microbe to rapidly proliferate via numerous, simultaneous cell division events upon the removal of stress-inducing conditions. Our data indicates that any such benefit likely comes with inherent costs in TLR9-expressing immune cells, specifically the recognition of immunostimulatory chlamydial gDNA by a host’s innate immune defenses. We reason that persistence requires the evasion of immunological clearance mechanisms and as such, models of chlamydial persistence, including those independent of the “aberrant” phenotype (103), which demonstrate this characteristic in vitro are more likely to correspond to phenotypes associated with persistence in animal models and human patients.
MATERIALS AND METHODS
Reagents
Anti-TLR9 (AB134368) and Alexa Fluor secondary antibodies were purchased from Abcam and Invitrogen, respectively. Anti-MOMP (LS-C123239) was purchased from LSBio. LpxC inhibitor LPC-011 was graciously provided by Dr. Pei Zhou (Duke University).
Bacterial strains and cell lines
C. trachomatis serovar L2 strain 434/Bu, C. muridarum strain Nigg, and E. coli strain MG1655 were provided by Anthony Maurelli (University of Florida). C. trachomatis and C. muridarum stocks were generated utilizing HeLa-USU cells (also provided by Anthony Maurelli) unless otherwise noted. Whole cell lysate (“crude”) freezer stocks of C. trachomatis and C. muridarum EBs were generated from HeLa cells 40 hours post infection and stored at −80°C in sucrose phosphate glutamic acid buffer (7.5% wt/vol sucrose, 17 mM Na2HPO4, 3 mM NaH2PO4, 5 mM L-glutamic acid, pH 7.4) until use. Stocks were titered via inclusion forming unit (IFU) assay (described below). HEK-Blue-hTLR9, -mTLR9, and -Null1 cells were purchased from InvivoGen and propagated according to the manufacturer’s instructions. Cell lines were passaged in high-glucose Dulbecco’s modified Eagle medium (DMEM; Gibco) and 10% fetal bovine serum (FBS; HyClone). U937 cells were purchased from ATCC and propagated according to their instructions. All cell lines were checked for mycoplasma contamination two passages after the initial liquid nitrogen thaw, and every subsequent 10 passages.
Genomic DNA isolation
Prior to the day of isolation, one 7 mL overnight culture of E. coli strain MG1655 was prepared. On the day of isolation, (4) 100 µL aliquot “crude” EB preparations of C. trachomatis serovar L2 strain 434/Bu and C. muridarum strain Nigg were thawed at room temperature. As stocks thawed, 6 mL of the overnight E. coli culture was centrifuged at 16,000 × g for 2 minutes. gDNA from C. trachomatis, C. muridarum, and E. coli were then isolated using a Promega Genomic DNA Purification Kit. gDNA from each species was rehydrated in 50 µL of sterile, deionized water (Fischer) and placed in a 4°C fridge overnight. On the following day, aliquots were placed on ice and the concentration of gDNA was assessed via nanodrop. To sufficiently shear gDNA for use in TLR9 signaling assays, gDNA stocks were then pulse-sonicated ~10 times (sonicating for 3 s then pausing for 1–2 s), and placed on ice immediately after for at least 10 minutes. If not used the same day, gDNA stocks were then stored at −20°C.
Quantification of inclusion forming units (IFU)
Ninety-six well tissue culture-treated plates (Fisher) were seeded 24 hours prior to IFU assays with 200 µL per well of a 200,000 L2 cells/mL suspension in DMEM/10% FBS (~40,000 cells per well). On the day of the assay, bacterial suspensions were thawed on ice and then serially diluted in infection medium [DMEM, 10% FBS, MEM Non-Essential Amino Acids (Sigma), 0.5 µg/mL cycloheximide]. Spent media was removed from the 96 well plate and 200 µL of each chlamydia dilution was added to each well, with each dilution conducted in duplicate. Plates were then spun in a tabletop centrifuge (Eppendorf) at 3,000 rpm at 35°C for 1 hour to synchronize infection and ensure that all infectious EBs came into contact with cell monolayers. Plates were then incubated for 24–28 hours at 37°C 5% CO2. At the desired hpi, infection medium was removed by suction, infected cells were fixed/permeabilized by the addition of 200 µL of ice-cold methanol and incubated at room temperature for ~10 minutes. The methanol was then aspirated and 1 drop of Pathfinder Chlamydia Culture Confirmation System (BioRad) was added to each well. Plates were incubated at room temperature in the dark for 30 minutes, after which time the antibody staining solution was removed, wells were gently washed three times with deionized water, and 1 drop of glycerol mounting medium was added to each well. Plates were inverted, stored at 4°C and examined under an epifluorescence microscope (Olympus) within 24 hours. Total inclusion forming units (IFU) were calculated by counting the number of visible inclusions for 20 different imaging fields using the 40× objective.
C. trachomatis infections of U937 cells
For infecting U937 cells, ~2.5 × 105 cells/mL were spun down and resuspended in infection medium containing ~1 × 106 IFU of C. trachomatis (MOI ~4). 2.5 × 105 cells were then added to each well of a 24 well plate and placed in the incubator for 24 or 44 hours. For pre-stimulation experiments, ~2.5 × 105 cells/mL were spun down and resuspended in infection medium ± 1 µM ODN 2006 and incubated overnight at 37°C. The next morning, cells were spun down and resuspended in infection medium containing ~1 × 106 IFU of C. trachomatis (MOI ~4), 2.5 × 105 cells were then added to each well of a 24 well plate and placed in the incubator (at 37°C) for an additional 24 or 44 hours.
Fluorescence microscopy/TLR9 and MOMP-labeling
Briefly, HeLa cells were infected with C. trachomatis L2 434/Bu as described above. At indicated time points infection medium was removed, and cells were washed three times with 1× PBS. Cells were fixed and permeabilized with methanol for 5 minutes and gently washed three times with 1× PBS. Cells were then further permeabilized and blocked as described above. Cells were then washed with 3% BSA and incubated with donkey anti-mouse or donkey anti-rabbit Alexa Fluor 488 (1:1,000 in 3% BSA). For visualizing cell nuclei, cells were incubated with Hoechst stain for ~5 minutes and then washed with 3% BSA and 1× PBS.
For labeling TLR9 in infected U937 and HeLa cells, cells were first washed three times in 1× PBS, fixed with 10% paraformaldehyde (PFA) for 10 minutes at room temperature, and subsequently washed three additional times with 1× PBS. Cells were then permeabilized with 100% Methanol for 2 minutes, washed three times with 1× PBS, and then blocked for 1 hour with 3% BSA. Cells were then incubated with anti-MOMP (1:500) and anti-TLR9 (1:100) for 1 hour at room temperature, followed by several washes in 3% BSA, and subsequent incubation with anti-goat and anti-rabbit conjugated antibodies (1:1000 Alexa594 and Alexa488, respectively). Cells were then further washed with 3% BSA, and finally PBS. Coverslips were mounted on slides with ProLong gold antifade mounting medium and stored in the dark at 4°C prior to imaging via structured-illumination (Elyra PS.1) microscopy.
HEK-Blue hTLR9 and Null1 NF-κB reporter assay
HEK-Blue cells expressing human or murine TLR9 and carrying the NF-κB SEAP (secreted embryonic alkaline phosphatase) reporter gene (InvivoGen) were used according to the manufacturer’s instructions and adapted to assess TLR-specific NF-κB activity induced via live C. trachomatis and isolated bacterial gDNA. Briefly, 3 × 105 cells/mL of HEK-Blue-hTLR9 or -Null1 cells were plated in 96-well plates (total reaction volume of 200 µL per well [∼6.0 × 104 cells per well]) and allowed to settle/adhere overnight at 37°C. Media was then removed and replaced with 200 µL of medium containing either C. trachomatis (at the MOIs indicated), purified genomic DNA, or the known TLR9-stimulating ligand ODN 2006. Plates were then centrifuged for 1 hour at 2,000 × g and subsequently incubated in a CO2 incubator at 37°C. Cell supernatants were collected at indicated time points for subsequent analysis of SEAP activity. A colorimetric reporter assay was then utilized to quantify the abundance of SEAP in cell supernatants. Twenty microliters of supernatant collected from infected cells was added to 180 µL of the SEAP detection solution (InvivoGen), followed by incubation at 37°C for ∼6 hours. SEAP enzymatic activity was then quantified using a plate reader set to 650 nm. Infected cells were compared with uninfected (media) controls and cells treated with ODN 2006 (positive control). To ensure that changes in alkaline phosphatase activity were TLR- dependent under each of the experimental conditions tested, all experiments were carried out in parallel in the HEK-Blue-Null1 cell line, which contains the empty expression vector but lacks TLR9. HEK-Blue TLR9 SEAP reporter assays were always carried out in three separate experiments, statistical analysis was conducted by either 1- or 2-way ANOVA, and significance values were analyzed by utilizing Sidak’s multiple-comparison test.
Assessing chlamydial genome copy number
L2 cells in 24-well plates were infected with C. trachomatis L2 at MOI of 0.1 as described above. The total content of the wells was harvested at 1, 24 and 44 hpi and DNA was extracted using DNAeasy blood and tissue kit (Qiagen). Genome copy number quantitation was done by quantitative polymerase chain reaction (qPCR) using C. trachomatis 16S rRNA primer-probe mix (Forward 5′-GTAGCGGTGAAATGCGTAGA-3′, Reverse 5′-CGCCTTAGCGTCAGGTATAAA-3′, Probe 5′-ATGTGGAAGAACACCAGTGGCGAA-3′) (Integrated DNA Technologies), iTaq universal probes supermix (Bio-Rad) and 1 µL DNA as template. PCR reaction (40 cycles) was done on a CFX96 qPCR machine (Bio-Rad) using following conditions: initial denaturation 95°C for 3 min, denaturation 95°C for 5 s and annealing/extension 60°C for 30 s. Genome copy number was determined using a standard curve generated from serial dilutions of genomic DNA from purified EBs.
ACKNOWLEDGMENTS
We would like to thank Dr. Anthony Maurelli (University of Florida) and his laboratory for providing us with the strains used in this work as well as helpful feedback during the development of this project and drafting of the manuscript. We would also like to thank Dr. Pei Zhou (Duke University) for graciously providing us with the LpxC inhibitor used in this work.
This work was supported by a MIRA ESI award (R35 GM138202) and a USU faculty start up award (HP73LIEC18) to G.W.L. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. The opinions and assertions expressed herein are those of the author(s) and do not necessarily reflect the official policy or position of the Uniformed Services University or the Department of Defense.
Conceptualization and Design, A.D. and G.W.L.; Data Curation and Formal analysis, A.D. and G.W.L.; Investigation, Methodology, Validation, Visualization, A.D., G.O., P.S., and G.W.L., Writing – original draft, G.W.L.; Writing – review & editing, A.D., G.O., P.S., and G.W.L.; Funding acquisition, Project administration and Supervision, G.W.L. All authors read and approved the final manuscript.
Contributor Information
George W. Liechti, Email: george.liechti@usuhs.edu.
Denise M. Monack, Stanford University School of Medicine, Stanford, California, USA
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/iai.00063-24.
hTLR9-containing vacuoles do not colocalize to C. trachomatis inclusions in U937 cells.
Legend for Fig. S1.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Stelzner K, Vollmuth N, Rudel T. 2023. Intracellular lifestyle of Chlamydia trachomatis and host-pathogen interactions. Nat Rev Microbiol 21:448–462. doi: 10.1038/s41579-023-00860-y [DOI] [PubMed] [Google Scholar]
- 2. Wong WF, Chambers JP, Gupta R, Arulanandam BP. 2019. Chlamydia and its many ways of escaping the host immune system. J Pathog 2019:8604958. doi: 10.1155/2019/8604958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yadav S, Verma V, Singh Dhanda R, Yadav M. 2021. Insights into the toll-like receptors in sexually transmitted infections. Scand J Immunol 93:e12954. doi: 10.1111/sji.12954 [DOI] [PubMed] [Google Scholar]
- 4. Murray SM, McKay PF. 2021. Chlamydia trachomatis: cell biology, immunology and vaccination. Vaccine 39:2965–2975. doi: 10.1016/j.vaccine.2021.03.043 [DOI] [PubMed] [Google Scholar]
- 5. Zhao X, Jiang W, Jin X, Wang W, Shao Q, Liu T, Huang C, Chen Z. 2023. Role of toll-like receptors in common infectious diseases of the female lower genital tract. Front Biosci (Landmark Ed) 28:232. doi: 10.31083/j.fbl2809232 [DOI] [PubMed] [Google Scholar]
- 6. Zou Y, Lei W, He Z, Li Z. 2016. The role of NOD1 and NOD2 in host defense against chlamydial infection. FEMS Microbiol Lett 363:fnw170. doi: 10.1093/femsle/fnw170 [DOI] [PubMed] [Google Scholar]
- 7. Heine Holger, Müller-Loennies S, Brade L, Lindner B, Brade H. 2003. Endotoxic activity and chemical structure of lipopolysaccharides from Chlamydia trachomatis serotypes E and L2 and Chlamydophila psittaci 6BC. Eur J Biochem 270:440–450. doi: 10.1046/j.1432-1033.2003.03392.x [DOI] [PubMed] [Google Scholar]
- 8. Heine H., Gronow S, Zamyatina A, Kosma P, Brade H. 2007. Investigation on the agonistic and antagonistic biological activities of synthetic Chlamydia lipid A and its use in in vitro enzymatic assays. J Endotoxin Res 13:126–132. doi: 10.1177/0968051907079122 [DOI] [PubMed] [Google Scholar]
- 9. Ingalls RR, Rice PA, Qureshi N, Takayama K, Lin JS, Golenbock DT. 1995. The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infect Immun 63:3125–3130. doi: 10.1128/iai.63.8.3125-3130.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jenkin HM. 1960. Preparation and properties of cell walls of the agent of meningopneumonitis. J Bacteriol 80:639–647. doi: 10.1128/jb.80.5.639-647.1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perkins HR, Allison AC. 1963. Cell-wall constituents of rickettsiae and psittacosis-lymphogranuloma organisms. J Gen Microbiol 30:469–480. doi: 10.1099/00221287-30-3-469 [DOI] [PubMed] [Google Scholar]
- 12. Garrett AJ, Harrison MJ, Manire GP. 1974. A search for the bacterial mucopeptide component, muramic acid, in Chlamydia. J Gen Microbiol 80:315–318. doi: 10.1099/00221287-80-1-315 [DOI] [PubMed] [Google Scholar]
- 13. Liechti GW, Kuru E, Hall E, Kalinda A, Brun YV, VanNieuwenhze M, Maurelli AT. 2014. A new metabolic cell-wall labelling method reveals peptidoglycan in Chlamydia trachomatis. Nature 506:507–510. doi: 10.1038/nature12892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liechti G, Kuru E, Packiam M, Hsu Y-P, Tekkam S, Hall E, Rittichier JT, VanNieuwenhze M, Brun YV, Maurelli AT. 2016. Pathogenic Chlamydia lack a classical sacculus but synthesize a narrow, mid-cell peptidoglycan ring, regulated by MreB, for cell division. PLoS Pathog 12:e1005590. doi: 10.1371/journal.ppat.1005590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen H, Wen Y, Li Z. 2019. Clear victory for Chlamydia: the subversion of host innate immunity. Front Microbiol 10:1412. doi: 10.3389/fmicb.2019.01412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. 2000. A toll-like receptor recognizes bacterial DNA. Nature 408:740–745. doi: 10.1038/35047123 [DOI] [PubMed] [Google Scholar]
- 17. Hemmi H, Kaisho T, Takeda K, Akira S. 2003. The roles of toll-like receptor 9, MyD88, and DNA-dependent protein kinase catalytic subunit in the effects of two distinct CpG DNAs on dendritic cell subsets. J Immunol 170:3059–3064. doi: 10.4049/jimmunol.170.6.3059 [DOI] [PubMed] [Google Scholar]
- 18. Klinman DM. 2004. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol 4:249–258. doi: 10.1038/nri1329 [DOI] [PubMed] [Google Scholar]
- 19. Latz E, Visintin A, Espevik T, Golenbock DT. 2004. Mechanisms of TLR9 activation. J Endotoxin Res 10:406–412. doi: 10.1179/096805104225006525 [DOI] [PubMed] [Google Scholar]
- 20. Latz E, Schoenemeyer A, Visintin A, Fitzgerald KA, Monks BG, Knetter CF, Lien E, Nilsen NJ, Espevik T, Golenbock DT. 2004. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat Immunol 5:190–198. doi: 10.1038/ni1028 [DOI] [PubMed] [Google Scholar]
- 21. Scidmore MA, Fischer ER, Hackstadt T. 2003. Restricted fusion of Chlamydia trachomatis vesicles with endocytic compartments during the initial stages of infection. Infect Immun 71:973–984. doi: 10.1128/IAI.71.2.973-984.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ronzone E, Paumet F. 2013. Two coiled-coil domains of Chlamydia trachomatis IncA affect membrane fusion events during infection. PLoS One 8:e69769. doi: 10.1371/journal.pone.0069769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paumet F, Wesolowski J, Garcia-Diaz A, Delevoye C, Aulner N, Shuman HA, Subtil A, Rothman JE. 2009. Intracellular bacteria encode inhibitory SNARE-like proteins. PLoS One 4:e7375. doi: 10.1371/journal.pone.0007375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heinzen RA, Scidmore MA, Rockey DD, Hackstadt T. 1996. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous Vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect Immun 64:796–809. doi: 10.1128/iai.64.3.796-809.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rzomp KA, Scholtes LD, Briggs BJ, Whittaker GR, Scidmore MA. 2003. Rab GTPases are recruited to chlamydial inclusions in both a species-dependent and species-independent manner. Infect Immun 71:5855–5870. doi: 10.1128/IAI.71.10.5855-5870.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cortina ME, Bishop RC, DeVasure BA, Coppens I, Derré I. 2022. The inclusion membrane protein IncS is critical for initiation of the Chlamydia intracellular developmental cycle. PLoS Pathog 18:e1010818. doi: 10.1371/journal.ppat.1010818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Del Balzo D, Capmany A, Cebrian I, Damiani MT. 2021. Chlamydia trachomatis infection impairs MHC-I intracellular trafficking and antigen cross-presentation by dendritic cells. Front Immunol 12:662096. doi: 10.3389/fimmu.2021.662096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sun HS, Eng EWY, Jeganathan S, Sin AT-W, Patel PC, Gracey E, Inman RD, Terebiznik MR, Harrison RE. 2012. Chlamydia trachomatis vacuole maturation in infected macrophages. J Leukoc Biol 92:815–827. doi: 10.1189/jlb.0711336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Al-Zeer MA, Al-Younes HM, Lauster D, Abu Lubad M, Meyer TF. 2013. Autophagy restricts Chlamydia trachomatis growth in human macrophages via IFNG-inducible guanylate binding proteins. Autophagy 9:50–62. doi: 10.4161/auto.22482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yasir M, Pachikara ND, Bao X, Pan Z, Fan H. 2011. Regulation of chlamydial infection by host autophagy and vacuolar ATPase-bearing organelles. Infect Immun 79:4019–4028. doi: 10.1128/IAI.05308-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gracey E, Lin A, Akram A, Chiu B, Inman RD. 2013. Intracellular survival and persistence of Chlamydia muridarum is determined by macrophage polarization. PLoS One 8:e69421. doi: 10.1371/journal.pone.0069421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lundberg P, Welander P, Han X, Cantin E. 2003. Herpes simplex virus type 1 DNA is immunostimulatory in vitro and in vivo. J Virol 77:11158–11169. doi: 10.1128/jvi.77.20.11158-11169.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Singer M, de Waaij DJ, Morré SA, Ouburg S. 2015. CpG DNA analysis of bacterial STDs. BMC Infect Dis 15:273. doi: 10.1186/s12879-015-1016-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ouburg S, Lyons JM, Land JA, den Hartog JE, Fennema JSA, de Vries HJC, Bruggeman CA, Ito JI, Peña AS, Lundberg PSJ, Morré SA. 2009. TLR9 KO mice, haplotypes and CPG indices in Chlamydia trachomatis infection. Drugs Today (Barc) 45 Suppl B:83–93. [PubMed] [Google Scholar]
- 35. Chauhan A, Pandey N, Desai A, Raithatha N, Patel P, Choxi Y, Kapadia R, Khandelwal R, Jain N. 2019. Association of TLR4 and TLR9 gene polymorphisms and haplotypes with cervicitis susceptibility. PLoS One 14:e0220330. doi: 10.1371/journal.pone.0220330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Verweij SP, Karimi O, Pleijster J, Lyons JM, de Vries HJC, Land JA, Morré SA, Ouburg S. 2016. TLR2, TLR4 and TLR9 genotypes and haplotypes in the susceptibility to and clinical course of Chlamydia trachomatis infections in Dutch women. Pathog Dis 74:ftv107. doi: 10.1093/femspd/ftv107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beishova I, Nurgaliyev B, Belaya A, Chuzhebayeva G, Ulyanov V, Ulyanova T, Kovalchuk A, Dushayeva L, Murzabayev K, Taipova A, Zholdasbekova A, Isabaev A. 2023. Marking of genetic resistance to Chlamydia, brucellosis and nastitis in Holstein cows by using polymorphic variants of LTF, MBK1 and TLR9 genes. Am J Anim Vet Sci 18:89–97. doi: 10.3844/ajavsp.2023.89.97 [DOI] [Google Scholar]
- 38. Derbigny WA, Kerr MS, Johnson RM. 2005. Pattern recognition molecules activated by Chlamydia muridarum infection of cloned murine oviduct epithelial cell lines. J Immunol 175:6065–6075. doi: 10.4049/jimmunol.175.9.6065 [DOI] [PubMed] [Google Scholar]
- 39. Rothfuchs AG, Trumstedt C, Wigzell H, Rottenberg ME. 2004. Intracellular bacterial infection-induced IFN-γ is critically but not solely dependent on toll-like receptor 4-myeloid differentiation factor 88-IFN-αβ-STAT1 signaling. J Immunol 172:6345–6353. doi: 10.4049/jimmunol.172.10.6345 [DOI] [PubMed] [Google Scholar]
- 40. Leifer CA, Kennedy MN, Mazzoni A, Lee C, Kruhlak MJ, Segal DM. 2004. TLR9 is localized in the endoplasmic reticulum prior to stimulation. J Immunol 173:1179–1183. doi: 10.4049/jimmunol.173.2.1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chockalingam A, Brooks JC, Cameron JL, Blum LK, Leifer CA. 2009. TLR9 traffics through the golgi complex to localize to endolysosomes and respond to CpG DNA. Immunol Cell Biol 87:209–217. doi: 10.1038/icb.2008.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kayraklioglu N, Horuluoglu B, Klinman DM. 2021. CpG oligonucleotides as vaccine adjuvants. Methods Mol Biol 2197:51–85. doi: 10.1007/978-1-0716-0872-2_4 [DOI] [PubMed] [Google Scholar]
- 43. Shintani Y, Kapoor A, Kaneko M, Smolenski RT, D’Acquisto F, Coppen SR, Harada-Shoji N, Lee HJ, Thiemermann C, Takashima S, Yashiro K, Suzuki K. 2013. Tlr9 mediates cellular protection by modulating energy metabolism in cardiomyocytes and neurons. Proc Natl Acad Sci U S A 110:5109–5114. doi: 10.1073/pnas.1219243110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim SK, Choe JY, Park KY. 2021. Activation of CpG-ODN-induced TLR9 signaling inhibited by interleukin-37 in U937 human macrophages. Yonsei Med J 62:1023–1031. doi: 10.3349/ymj.2021.62.11.1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ward GA, Dalton RP, Meyer BS, McLemore AF, Aldrich AL, Lam NB, Onimus AH, Vincelette ND, Trinh TL, Chen X, Calescibetta AR, Christiansen SM, Hou H-A, Johnson JO, Wright KL, Padron E, Eksioglu EA, List AF. 2023. Oxidized mitochondrial DNA engages TLR9 to activate the NLRP3 inflammasome in myelodysplastic syndromes. Int J Mol Sci 24:3896. doi: 10.3390/ijms24043896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kavsan VM, Iershov AV, Balynska OV. 2011. Immortalized cells and one oncogene in malignant transformation: old insights on new explanation. BMC Cell Biol 12:23. doi: 10.1186/1471-2121-12-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bauer S, Kirschning CJ, Häcker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford GB. 2001. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci U S A 98:9237–9242. doi: 10.1073/pnas.161293498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miyairi I, Mahdi OS, Ouellette SP, Belland RJ, Byrne GI. 2006. Different growth rates of Chlamydia trachomatis biovars reflect pathotype. J Infect Dis 194:350–357. doi: 10.1086/505432 [DOI] [PubMed] [Google Scholar]
- 49. Lee JK, Enciso GA, Boassa D, Chander CN, Lou TH, Pairawan SS, Guo MC, Wan FYM, Ellisman MH, Sütterlin C, Tan M. 2018. Replication-dependent size reduction precedes differentiation in Chlamydia trachomatis. Nat Commun 9:45. doi: 10.1038/s41467-017-02432-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Matsumoto A. 1988. Structural characteristics of chlamydial bodies, p 21–45. In Barron AL (ed), Microbiology of Chlamydia. CRC Press, Inc., Boca Raton, FL. [Google Scholar]
- 51. Nguyen BD, Cunningham D, Liang X, Chen X, Toone EJ, Raetz CRH, Zhou P, Valdivia RH. 2011. Lipooligosaccharide is required for the generation of infectious elementary bodies in Chlamydia trachomatis. Proc Natl Acad Sci U S A 108:10284–10289. doi: 10.1073/pnas.1107478108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cram ED, Rockey DD, Dolan BP. 2017. Chlamydia spp. development is differentially altered by treatment with the LpxC inhibitor LPC-011. BMC Microbiol 17:98. doi: 10.1186/s12866-017-0992-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brockett MR, Liechti GW. 2021. Persistence alters the interaction between Chlamydia trachomatis and its host cell. Infect Immun 89:e0068520. doi: 10.1128/IAI.00685-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pokorzynski ND, Brinkworth AJ, Carabeo R. 2019. A bipartite iron-dependent transcriptional regulation of the tryptophan salvage pathway in Chlamydia trachomatis Elife 8:e42295. doi: 10.7554/eLife.42295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Scherler A, Jacquier N, Kebbi-Beghdadi C, Greub G. 2020. Diverse stress-inducing treatments cause distinct aberrant body morphologies in the Chlamydia-related bacterium, Waddlia chondrophila. Microorganisms 8:89. doi: 10.3390/microorganisms8010089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gordon FB, Quan AL. 1972. Susceptibility of Chlamydia to antibacterial drugs: test in cell cultures . Antimicrob Agents Chemother 2:242–244. doi: 10.1128/AAC.2.3.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gérard HC, Krausse-Opatz B, Wang Z, Rudy D, Rao JP, Zeidler H, Schumacher HR, Whittum-Hudson JA, Köhler L, Hudson AP. 2001. Expression of Chlamydia trachomatis genes encoding products required for DNA synthesis and cell division during active versus persistent infection. Mol Microbiol 41:731–741. doi: 10.1046/j.1365-2958.2001.02550.x [DOI] [PubMed] [Google Scholar]
- 58. Lambden PR, Pickett MA, Clarke IN. 2006. The effect of penicillin on Chlamydia trachomatis DNA replication. Microbiology 152:2573–2578. doi: 10.1099/mic.0.29032-0 [DOI] [PubMed] [Google Scholar]
- 59. Kintner J, Lajoie D, Hall J, Whittimore J, Schoborg RV. 2014. Commonly prescribed β-lactam antibiotics induce C. trachomatis persistence/stress in culture at physiologically relevant concentrations. Front Cell Infect Microbiol 4:44. doi: 10.3389/fcimb.2014.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang J, Frohlich KM, Buckner L, Quayle AJ, Luo M, Feng X, Beatty W, Hua Z, Rao X, Lewis ME, Sorrells K, Santiago K, Zhong G, Shen L. 2011. Altered protein secretion of Chlamydia trachomatis in persistently infected human endocervical epithelial cells. Microbiology (Reading) 157:2759–2771. doi: 10.1099/mic.0.044917-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Frohlich KM, Hua Z, Quayle AJ, Wang J, Lewis ME, Chou C, Luo M, Buckner LR, Shen L. 2014. Membrane vesicle production by Chlamydia trachomatis as an adaptive response. Front Cell Infect Microbiol 4:73. doi: 10.3389/fcimb.2014.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Matsumoto A, Manire GP. 1970. Electron microscopic observations on the effects of penicillin on the morphology of Chlamydia psittaci. J Bacteriol 101:278–285. doi: 10.1128/jb.101.1.278-285.1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lewis ME, Belland RJ, AbdelRahman YM, Beatty WL, Aiyar AA, Zea AH, Greene SJ, Marrero L, Buckner LR, Tate DJ, McGowin CL, Kozlowski PA, O’Brien M, Lillis RA, Martin DH, Quayle AJ. 2014. Morphologic and molecular evaluation of Chlamydia trachomatis growth in human endocervix reveals distinct growth patterns. Front Cell Infect Microbiol 4:71. doi: 10.3389/fcimb.2014.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Muramatsu MK, Brothwell JA, Stein BD, Putman TE, Rockey DD, Nelson DE. 2016. Beyond tryptophan synthase: identification of genes that contribute to Chlamydia trachomatis survival during gamma interferon-induced persistence and reactivation. Infect Immun 84:2791–2801. doi: 10.1128/IAI.00356-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lyons JM, Ito JI Jr, Peña AS, Morré SA. 2005. Differences in growth characteristics and elementary body associated cytotoxicity between Chlamydia trachomatis oculogenital serovars D and H and Chlamydia muridarum. J Clin Pathol 58:397–401. doi: 10.1136/jcp.2004.021543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. An H, Xu H, Yu Y, Zhang M, Qi R, Yan X, Liu S, Wang W, Guo Z, Qin Z, Cao X. 2002. Up-regulation of TLR9 gene expression by LPS in mouse macrophages via activation of NF-κB, ERK and P38 MAPK signal pathways. Immunol Lett 81:165–169. doi: 10.1016/s0165-2478(02)00010-x [DOI] [PubMed] [Google Scholar]
- 67. Kadowaki N, Ho S, Antonenko S, Malefyt RW, Kastelein RA, Bazan F, Liu YJ. 2001. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med 194:863–869. doi: 10.1084/jem.194.6.863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Schneberger D, Caldwell S, Kanthan R, Singh B. 2013. Expression of toll-like receptor 9 in mouse and human lungs. J Anat 222:495–503. doi: 10.1111/joa.12039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nigg C, Eaton MD. 1944. Isolation from normal mice of a pneumotropic virus which forms elementary bodies. J Exp Med 79:497–510. doi: 10.1084/jem.79.5.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mishkin N, Ricart Arbona RJ, Carrasco SE, Lawton S, Henderson KS, Momtsios P, Sigar IM, Ramsey KH, Cheleuitte-Nieves C, Monette S, Lipman NS. 2022. Reemergence of the murine bacterial pathogen Chlamydia muridarum in research mouse colonies. Comp Med 72:230–242. doi: 10.30802/AALAS-CM-22-000045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pandey NO, Chauhan AV, Raithatha NS, Patel PK, Khandelwal R, Desai AN, Choxi Y, Kapadia RS, Jain ND. 2019. Association of TLR4 and TLR9 polymorphisms and haplotypes with cervical cancer susceptibility. Sci Rep 9:9729. doi: 10.1038/s41598-019-46077-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang C, Yang Z, Luo P, Li T, Wang S, Sun F, Gong P, Mei B. 2023. Association of TLR4 and TLR9 gene polymorphisms with cervical HR-HPV infection status in Chinese Han population. BMC Infect Dis 23:152. doi: 10.1186/s12879-023-08116-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Pal S, Peterson EM, de la Maza LM. 2005. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect Immun 73:8153–8160. doi: 10.1128/IAI.73.12.8153-8160.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cong Y, Jupelli M, Guentzel MN, Zhong G, Murthy AK, Arulanandam BP. 2007. Intranasal immunization with chlamydial protease-like activity factor and CpG deoxynucleotides enhances protective immunity against genital Chlamydia muridarum infection. Vaccine 25:3773–3780. doi: 10.1016/j.vaccine.2007.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang J, Chen L, Chen F, Zhang X, Zhang Y, Baseman J, Perdue S, Yeh I-T, Shain R, Holland M, Bailey R, Mabey D, Yu P, Zhong G. 2009. A chlamydial type III-secreted effector protein (Tarp) is predominantly recognized by antibodies from humans infected with Chlamydia trachomatis and induces protective immunity against upper genital tract pathologies in mice. Vaccine 27:2967–2980. doi: 10.1016/j.vaccine.2009.02.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yu H, Jiang X, Shen C, Karunakaran KP, Jiang J, Rosin NL, Brunham RC. 2010. Chlamydia muridarum T-cell antigens formulated with the adjuvant DDA/TDB induce immunity against infection that correlates with a high frequency of gamma interferon (IFN-γ)/tumor necrosis factor alpha and IFN-γ/interleukin-17 double-positive CD4+ T cells. Infect Immun 78:2272–2282. doi: 10.1128/IAI.01374-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pal S, Cruz-Fisher MI, Cheng C, Carmichael JR, Tifrea DF, Tatarenkova O, de la Maza LM. 2020. Vaccination with the recombinant major outer membrane protein elicits long-term protection in mice against vaginal shedding and infertility following a Chlamydia muridarum genital challenge. NPJ Vaccines 5:90. doi: 10.1038/s41541-020-00239-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. O’Connell CM, Ionova IA, Quayle AJ, Visintin A, Ingalls RR. 2006. Localization of TLR2 and MyD88 to Chlamydia trachomatis inclusions. Evidence for signaling by intracellular TLR2 during infection with an obligate intracellular pathogen. J Biol Chem 281:1652–1659. doi: 10.1074/jbc.M510182200 [DOI] [PubMed] [Google Scholar]
- 79. Massari P, Toussi DN, Tifrea DF, de la Maza LM. 2013. Toll-like receptor 2-dependent activity of native major outer membrane protein proteosomes of Chlamydia tachomatis. Infect Immun 81:303–310. doi: 10.1128/IAI.01062-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang Y, Liu Q, Chen D, Guan J, Ma L, Zhong G, Shu H, Wu X. 2017. Chlamydial lipoproteins stimulate toll-like receptors 1/2 mediated inflammatory responses through MyD88-dependent pathway. Front Microbiol 8:78. doi: 10.3389/fmicb.2017.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Beckett EL, Phipps S, Starkey MR, Horvat JC, Beagley KW, Foster PS, Hansbro PM. 2012. TLR2, but not TLR4, is required for effective host defence against Chlamydia respiratory tract infection in early life. PLoS ONE 7:e39460. doi: 10.1371/journal.pone.0039460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Darville T, O’Neill JM, Andrews CW, Nagarajan UM, Stahl L, Ojcius DM. 2003. Toll-like receptor-2, but not toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J Immunol 171:6187–6197. doi: 10.4049/jimmunol.171.11.6187 [DOI] [PubMed] [Google Scholar]
- 83. Bulut Y, Faure E, Thomas L, Karahashi H, Michelsen KS, Equils O, Morrison SG, Morrison RP, Arditi M. 2002. Chlamydial heat shock protein 60 activates macrophages and endothelial cells through toll-like receptor 4 And MD2 in a MyD88-dependent pathway. J Immunol 168:1435–1440. doi: 10.4049/jimmunol.168.3.1435 [DOI] [PubMed] [Google Scholar]
- 84. Hafner LM, Cunningham K, Beagley KW. 2013. Ovarian steroid hormones: effects on immune responses and Chlamydia trachomatis infections of the female genital tract. Mucosal Immunol 6:859–875. doi: 10.1038/mi.2013.46 [DOI] [PubMed] [Google Scholar]
- 85. Su X, Xu H, French M, Zhao Y, Tang L, Li X-D, Chen J, Zhong G. 2022. Evidence for cGAS-STING signaling in the female genital tract resistance to Chlamydia trachomatis infection. Infect Immun 90:e0067021. doi: 10.1128/iai.00670-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Blasius AL, Arnold CN, Georgel P, Rutschmann S, Xia Y, Lin P, Ross C, Li X, Smart NG, Beutler B. 2010. Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for toll-like receptor signaling in plasmacytoid dendritic cells. Proc Natl Acad Sci U S A 107:19973–19978. doi: 10.1073/pnas.1014051107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yang C, Lei L, Collins JWM, Briones M, Ma L, Sturdevant GL, Su H, Kashyap AK, Dorward D, Bock KW, Moore IN, Bonner C, Chen C-Y, Martens CA, Ricklefs S, Yamamoto M, Takeda K, Iwakura Y, McClarty G, Caldwell HD. 2021. Chlamydia evasion of neutrophil host defense results in NLRP3 dependent myeloid-mediated sterile inflammation through the purinergic P2X7 receptor. Nat Commun 12:5454. doi: 10.1038/s41467-021-25749-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Elwell C, Mirrashidi K, Engel J. 2016. Chlamydia cell biology and pathogenesis. Nat Rev Microbiol 14:385–400. doi: 10.1038/nrmicro.2016.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang Y, Yeruva L, Marinov A, Prantner D, Wyrick PB, Lupashin V, Nagarajan UM. 2014. The DNA sensor, cyclic GMP-AMP synthase, is essential for induction of IFN-β during Chlamydia trachomatis infection. J Immunol 193:2394–2404. doi: 10.4049/jimmunol.1302718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bishop RC, Derré I. 2022. The Chlamydia trachomatis inclusion membrane protein CTL0390 mediates host cell exit via lysis through STING activation. Infect Immun 90:e0019022. doi: 10.1128/iai.00190-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bundock P, den Dulk-Ras A, Beijersbergen A, Hooykaas PJ. 1995. Trans-kingdom T-DNA transfer from Agrobacterium tumefaciens to Saccharomyces cerevisiae. EMBO J 14:3206–3214. doi: 10.1002/j.1460-2075.1995.tb07323.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hamilton HL, Dillard JP. 2006. Natural transformation of Neisseria gonorrhoeae: from DNA donation to homologous recombination. Mol Microbiol 59:376–385. doi: 10.1111/j.1365-2958.2005.04964.x [DOI] [PubMed] [Google Scholar]
- 93. Varga MG, Shaffer CL, Sierra JC, Suarez G, Piazuelo MB, Whitaker ME, Romero-Gallo J, Krishna US, Delgado A, Gomez MA, Good JAD, Almqvist F, Skaar EP, Correa P, Wilson KT, Hadjifrangiskou M, Peek RM. 2016. Pathogenic Helicobacter pylori strains translocate DNA and activate TLR9 via the cancer-associated CAG type IV secretion system. Oncogene 35:6262–6269. doi: 10.1038/onc.2016.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Demars R, Weinfurter J, Guex E, Lin J, Potucek Y. 2007. Lateral gene transfer in vitro in the intracellular pathogen Chlamydia trachomatis. J Bacteriol 189:991–1003. doi: 10.1128/JB.00845-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. LaBrie SD, Dimond ZE, Harrison KS, Baid S, Wickstrum J, Suchland RJ, Hefty PS. 2019. Transposon mutagenesis in Chlamydia trachomatis identifies CT339 as a ComEC homolog important for DNA. mBio 10:e01343-19. doi: 10.1128/mBio.01343-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hackstadt T, Scidmore-Carlson MA, Shaw EI, Fischer ER. 1999. The Chlamydia trachomatis IncA protein is required for homotypic vesicle fusion. Cell Microbiol 1:119–130. doi: 10.1046/j.1462-5822.1999.00012.x [DOI] [PubMed] [Google Scholar]
- 97. Suchland RJ, Rockey DD, Bannantine JP, Stamm WE. 2000. Isolates of Chlamydia trachomatis that occupy nonfusogenic inclusions lack IncA, a protein localized to the inclusion membrane. Infect Immun 68:360–367. doi: 10.1128/IAI.68.1.360-367.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Cingolani G, McCauley M, Lobley A, Bryer AJ, Wesolowski J, Greco DL, Lokareddy RK, Ronzone E, Perilla JR, Paumet F. 2019. Structural basis for the homotypic fusion of chlamydial inclusions by the SNARE-like protein IncA. Nat Commun 10:2747. doi: 10.1038/s41467-019-10806-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. DeMars R, Weinfurter J. 2008. Interstrain gene transfer in Chlamydia trachomatis in vitro: mechanism and significance. J Bacteriol 190:1605–1614. doi: 10.1128/JB.01592-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Jeffrey BM, Suchland RJ, Eriksen SG, Sandoz KM, Rockey DD. 2013. Genomic and phenotypic characterization of in vitro-generated Chlamydia trachomatis recombinants. BMC Microbiol 13:142. doi: 10.1186/1471-2180-13-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Suchland RJ, Carrell SJ, Wang Y, Hybiske K, Kim DB, Dimond ZE, Hefty PS, Rockey DD. 2019. Chromosomal recombination targets in Chlamydia interspecies lateral gene transfer. J Bacteriol 201:e00365-19. doi: 10.1128/JB.00365-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Suchland R. J., Sandoz KM, Jeffrey BM, Stamm WE, Rockey DD. 2009. Horizontal transfer of tetracycline resistance among Chlamydia spp. in vitro. Antimicrob Agents Chemother 53:4604–4611. doi: 10.1128/AAC.00477-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rockey DD, Wang X, Debrine A, Grieshaber N, Grieshaber SS. 2024. Metabolic dormancy in Chlamydia trachomatis treated with different antibiotics. Infect Immun 92:e0033923. doi: 10.1128/iai.00339-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
hTLR9-containing vacuoles do not colocalize to C. trachomatis inclusions in U937 cells.
Legend for Fig. S1.