Abstract
Background
Human immunodeficiency virus (HIV) infection is an important risk factor for Coronavirus Disease 2019 (COVID‐19), but data on the prevalence of COVID‐19 among people living with HIV (PLWH) is limited in low‐income countries. Our aim was to assess the seroprevalence of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) specific antibodies and associated factors among PLWH in Sierra Leone.
Methods
We conducted a cross‐sectional survey of PLWH aged 18 years or older in Sierra Leone between August 2022 and January 2023. Participants were tested for SARS‐CoV‐2 antibodies using a rapid SARS‐CoV‐2 antibody (immunoglobulin M/immunoglobulin G [IgG]) kits. Stepwise logistic regression was used to explore factors associated with SARS‐CoV‐2 antibody seroprevalence with a significance level of p < .05.
Results
In our study, 33.4% (1031/3085) participants had received a COVID‐19 vaccine, and 75.7% were SARS‐CoV‐2 IgG positive. Higher IgG seroprevalence was observed in females (77.2% vs. 71.4%, p = .001), adults over 60 years (88.2%), those with suppressed HIV RNA (80.7% vs. 51.7%, p < .001), antiretroviral therapy (ART)‐experienced individuals (77.9% vs. 44.6%, p < .001), and vaccinated participants (80.7% vs. 73.2%, p < .001). Patients 60 years or older had the highest odds of IgG seroprevalence (adjusted odds ratio [aOR] = 2.73, 95% CI = 1.68–4.65). Female sex (aOR = 1.28, 95%CI = 1.05–1.56), COVID‐19 vaccination (aOR = 1.54, 95% CI = 1.27–1.86), and ART (aOR = 2.20, 95% CI = 1.56–3.11) increased the odds, whereas HIV RNA ≥ 1000 copies/mL (aOR = 0.32, 95% CI = 0.26–0.40) reduced the odds of IgG seroprevalence.
Conclusions
We observed a high seroprevalence of SARS‐CoV‐2 antibody among PLWH in Sierra Leone. We recommend the introduction of targeted vaccination for PLWH with a high risk of severe COVID‐19, especially those with an unsuppressed HIV viral load.
Keywords: HIV infection, immunoglobulin G, SARS‐CoV‐2, seroepidemiologic studies
1. INTRODUCTION
In May 2023, the World Health Organization (WHO) declared the end of the Coronavirus disease 2019 (COVID‐19) pandemic, marking a critical step toward ending the COVID‐19 pandemic. 1 Although COVID‐19 is no longer a global public health emergency, the spread of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the pathogen that causes COVID‐19, is still ongoing. 1 , 2 Therefore, in the postpandemic era, countries and regions should establish routine surveillance systems to detect the SARS‐CoV‐2 virus, paying special attention to high‐risk/vulnerable populations, including people living with human immunodeficiency virus (HIV) (PLWH). 2 , 3 , 4 HIV infection is an important risk factor for COVID‐19. Consequently, PLWH experienced adverse outcomes related to COVID‐19 infection, including severe illness and deaths compared to the general population. 5 , 6 , 7
HIV is a public health problem in Africa, with approximately 67% of the global HIV population living in Africa by the end of 2022, with the majority not on treatment. 8 Sierra Leone, a low‐income country in sub‐Saharan Africa, had an HIV prevalence of 1.7% in 2019. 9 Despite the low HIV prevalence, the country still faces challenges in the HIV response, such as late‐stage HIV diagnosis and advanced HIV disease. 10 , 11 Moreover, due to the challenges in rollout and acceptance, the COVID‐19 vaccine coverage in the general population of Sierra Leone was only 33.5% at the start of the study in August 2022, rising to 52.4% by January 2023. 12 This rate may be even lower in PLWH. For example, only 17% of PLWH in a tertiary hospital in Sierra Leone received a COVID‐19 vaccine between April and June 2022. 13
Seroepidemiologic assessment is a powerful method to determine the prevalence of infection, estimate the burden of disease, identify associated factors, and evaluate control and immunization programs. In Sierra Leone, serological data on endemic SARS‐CoV‐2 infection are scarce, and the burden of COVID‐19 remains unknown, especially in PLWH. To our knowledge, only two studies have reported the seroprevalence of COVID‐19 antibodies in Sierra Leone. One national survey in March 2021 reported an overall immunoglobulin G (IgG) antibody seroprevalence of 2.3% and immunoglobulin M (IgM) of 0.6%. 14 Another study between March and July 2021 reported an IgG/IgM antibodies seroprevalence of 89% among the unvaccinated health facility staff. 15 As of the time of writing, there are no reports on the seroprevalence of SARS‐CoV‐2 IgG/IgM antibodies in PLWH in Sierra Leone. Understanding the seroprevalence for SARS‐CoV‐2 in PLWH has important policy implications for preventing and mitigating the impact of future public emergencies in this population. Additionally, the findings from this study will add to the global body of evidence on SARS‐CoV‐2 antibodies among PLWH.
Therefore, this study aimed to determine the seroprevalence of the SARS‐CoV‐2 specific antibodies and associated factors among PLWH in Sierra Leone after 2 years of the first confirmed case in the country.
2. MATERIALS AND METHODS
2.1. Study design and participants
We used a cross‐sectional hospital‐based design in recruiting PLHW aged 18 years or older from high HIV burden health facilities in Sierra Leone. Health facilities with more than 800 PLWH receiving antiretroviral therapy (ART) treatment in Sierra Leone were recruited in the study. However, four health facilities were excluded because of the distance or delay in obtaining consent to conduct the study. Finally, 10 health facilities were included in our study. Participants were recruited using convenience sampling as they visited health facilities from August 2022 to January 2023. Considering the unknown seroprevalence and 33.5% COVID‐19 vaccine coverage rate in the general population of Sierra Leone at the start of the study, we assumed that the overall SARS‐CoV‐2 seroprevalence would be about 40%, with 95% confidence level and 2% absolute error. We then calculated a minimum sample size of 2305 individuals.
2.2. Data collection
We consecutively recruited 3127 HIV patients from 10 health facilities in different regions of Sierra Leone. After obtaining written informed consent, demographic, HIV, and vaccine‐related information were collected through a patient interview or a clinical record by trained study staff members using a standardized data collection form to reduce information bias. About 10 mL of venous blood samples were aseptically collected in EDTA vacutainer tubes. We excluded patients with HIV viral load missing and other missing information from the detailed analysis. Therefore, 3085 adult patients were included in the final analysis (Supporting Information: Figure S1).
2.3. Laboratory testing
We used a rapid diagnostic test (Innovita) to detect SARS‐CoV‐2 IgM/IgG antibodies by a colloid gold immunochromatography competition assay with a sensitivity of 94.4% and a specificity of 98%, which has been prevalidated and approved to be used in Sierra Leone.
HIV viral load was quantified by real‐time quantitative PCR (Sansure Biotech Inc.) using a Roche lightcycler (Roche Corp.). The detection limit of HIV RNA was 20 IU/mL (2.08 IU/mL = 1 copies/mL).
2.4. Statistical analysis
Data analysis was performed using the R software version 4.3.0 (R Core Team, Vienna, Austria). The median (interquartile range [IQR]) and frequency (%) were used to summarize demographic information and HIV and COVID‐19 vaccine details.
We grouped the quantitative variable “age” into categorical variables, labeled as groups 18–30, 30–40, 40–50, 50–60, and 60–100. According to WHO guidelines, HIV viral load was categorized into suppressed (<1000 copies/mL) and unsuppressed (≥1000 copies/mL) groups. We determined the seroprevalence of antibodies as the positivity rate with its 95% confidence interval (95% CI) and Pearson's chi‐square test. Multivariable stepwise logistic regression (backward selection strategy) was used to explore factors associated with SARS‐CoV‐2 antibody seroprevalence and presented as adjusted odds ratios and its 95% CI. The level of significance was defined as p < .05.
3. RESULTS
3.1. Demographic characteristics and HIV details
Demographic characteristics and HIV details are shown in Table 1. Of the 3085 participants, 2299 (74.5%) were female. Median age was 36.0 (IQR 29.0–45.0) years. Up to 2882 (93.4%) were on ART for a median duration of 3.3 years (IQR 1.0–6.6). Among patients receiving ART, 1876 (60.8%) were using the fixed‐dose combination of tenofovir + lamivudine + dolutegravir (TLD). Viral suppression (HIV RNA < 1000 copies/mL) was achieved in 2553 (82.8%) participants.
Table 1.
Demographic, COVID‐19 vaccine, and HIV details.
| Characteristic | N = 3085a |
|---|---|
| Age (years) | |
| 18–30 | 851 (27.6%) |
| 30–40 | 975 (31.6%) |
| 40–50 | 702 (22.8%) |
| 50–60 | 388 (12.6%) |
| 60–100 | 169 (5.5%) |
| Median (IQR) | 36.0 (29.0, 45.0) |
| Sex | |
| Female | 2299 (74.5%) |
| Male | 786 (25.5%) |
| Hospital | |
| Primary | 663 (21.5%) |
| Secondary | 200 (6.5%) |
| Tertiary | 2222 (72.0%) |
| HIV viral load (copies/mL) | |
| <1000 | 2553 (82.8%) |
| ≥1000 | 532 (17.2%) |
| ART | |
| Yes | 2882 (93.4%) |
| No | 203 (6.6%) |
| ART regimen | |
| TLE | 688 (22.3%) |
| TLD | 1876 (60.8%) |
| Unknown | 318 (10.3%) |
| No ART | 203 (6.6%) |
| ART duration (years) | 3.3 (1.0, 6.6) |
| COVID‐19 vaccination | |
| Yes | 1031 (33.4%) |
| No | 2054 (66.6%) |
| IgG against COVID‐19 | |
| Neg | 750 (24.3%) |
| Pos | 2335 (75.7%) |
| IgM against COVID‐19 | |
| Neg | 2940 (95.3%) |
| Pos | 145 (4.7%) |
Abbreviations: ART, antiretroviral therapy; IgG, immunoglobulin G; IQR, interquartile range; TLD, tenofovir + lamivudine + dolutegravir; TLE, tenofovir + lamivudine + efavirenz.
Median (IQR); n (%).
3.2. COVID‐19 vaccination
Of the 3085 participants, 1031 (33.4%) received the COVID‐19 vaccine, including Johnson & Johnson (158, 15.3%), Sinopharm (66, 6.4%), AstraZeneca (40, 3.9%), and Pfizer/BioNTech (25, 2.4%) vaccines. The type of COVID‐19 vaccines received by 742 (72.0%) participants were unknown (Supporting Information: Table S1). The median duration of vaccination was 272.0 (IQR 198.5–399.0) days (Supporting Information: Figure S2).
The common reasons for not being vaccinated in the 2054 unvaccinated participants were fear of side effects of the vaccines (1137, 55.4%), the belief that HIV is a contraindication to COVID‐19 vaccination (138, 6.7%), waiting to be scheduled for vaccination (135, 6.6%), no perceived need for COVID‐19 vaccination (64, 3.1%) and worries about the poor efficacy of the vaccines (52, 2.5%). In 391 (19.0%) participants, no reasons were provided (Supporting Information: Table S1).
3.3. Seroprevalence
The overall IgG seroprevalence in PLWH was 75.7% (95% CI, 74.1%–77.2%), whereas few PLWH were positive for SARS‐CoV‐2 IgM (Table 1). The changes in the seroprevalence of SARS‐CoV‐2 during the study period are shown in Figure 1. The IgG seroprevalence was 73.6% in participants enrolled at the start of the study in August 2022. The IgG seroprevalence increased from 77.1% among participants recruited in September 2022 to a peak of 78.9% in October 2022 before declining to 78.1%, 74.4%, and 71.1% for participants enrolled in November 2022, December 2022, and January 2023, respectively.
Figure 1.

The dynamic seropositivity of IgG antibody against SARS‐CoV‐2 in PLWH of Sierra Leone. IgG, immunoglobulin G; PLWH, people living with human immunodeficiency virus; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Female participants had a higher IgG seroprevalence than male participants (77.2% vs. 71.4%, p = .001). The IgG seroprevalence exponentially increased with age, with rates of 71.8% reported in participants below 30 years, and 75.0%, 77.5%, 77.3%, and 88.2% in those aged 30–39, 40–49, 50–59, and 60 years or older, respectively (Table 2). The seroprevalence was significantly higher among participants vaccinated against COVID‐19 than among unvaccinated participants (80.7% vs. 73.2%, p < .001) (Figure 2). Participants with HIV viral load less than 1000 copies/mL had a higher seroprevalence than those with HIV RNA levels ≥1000 copies/mL (80.7% vs. 51.7%, p < .001). Similarly, ART‐experienced participants had a higher seroprevalence than ART‐naive participants (77.9% vs. 44.6%, p < .001).
Table 2.
Seroprevalence of IgG antibody against SARS‐CoV‐2 by demographic, COVID‐19 vaccine and HIV status.
| Characteristic | Overall (N)a | Pos, n (%)b | 95% CIc | p Valued |
|---|---|---|---|---|
| Age | < .001 | |||
| 18–30 | 851 | 611 (71.8) | 68.6–74.8 | |
| 30–40 | 975 | 731 (75.0) | 72.1–77.6 | |
| 40–50 | 702 | 544 (77.5) | 74.2–80.5 | |
| 50–60 | 388 | 300 (77.3) | 72.8–81.3 | |
| 60–100 | 169 | 149 (88.2) | 82.1–92.4 | |
| Sex | .001 | |||
| Female | 2299 | 1774 (77.2) | 75.4–78.9 | |
| Male | 786 | 561 (71.4) | 68.1–74.5 | |
| COVID‐19 vaccination | <.001 | |||
| Yes | 1031 | 832 (80.7) | 78.1–83.0 | |
| No | 2054 | 1503 (73.2) | 71.2–75.1 | |
| Hospital | .9 | |||
| Primary | 663 | 505 (76.2) | 72.7–79.3 | |
| Secondary | 200 | 153 (76.5) | 69.9–82.1 | |
| Tertiary | 2222 | 1677 (75.5) | 73.6–77.2 | |
| HIV viral load (copies/mL) | <.001 | |||
| <1000 | 2553 | 2060 (80.7) | 79.1–82.2 | |
| ≥1000 | 532 | 275 (51.7) | 47.4–56.0 | |
| ART | <.001 | |||
| Yes | 2882 | 2245 (77.9) | 76.3–79.4 | |
| No | 203 | 90 (44.3) | 37.4–51.5 | |
| Month of sample collection | .2 | |||
| August 2022 | 163 | 120 (73.6) | 66.0–80.1 | |
| September 2022 | 619 | 477 (77.1) | 73.5–80.3 | |
| October 2022 | 175 | 138 (78.9) | 71.9–84.5 | |
| November 2022 | 613 | 479 (78.1) | 74.6–81.3 | |
| December 2022 | 1282 | 954 (74.4) | 71.9–76.8 | |
| January 2023 | 233 | 167 (71.7) | 65.3–77.3 | |
| Total | 3085 | 2335 (75.7) | 74.1–77.2 |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IgG, immunoglobulin G; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; TLD, tenofovir + lamivudine + dolutegravir; TLE, tenofovir + lamivudine + efavirenz.
n.
n (%).
95% CI = 95% confidence interval.
Pearson's chi‐squared test.
Figure 2.
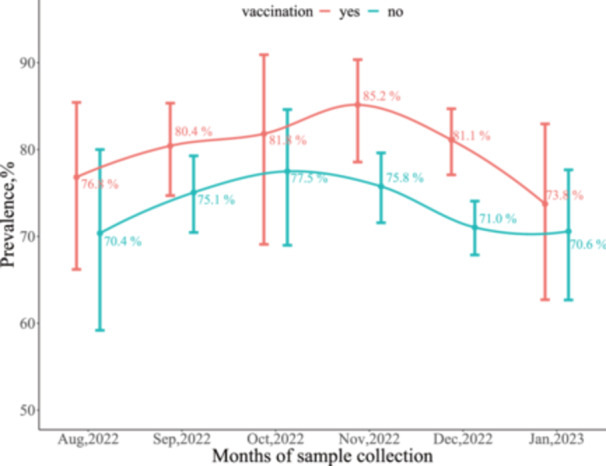
The dynamic seropositivity of IgG antibody against SARS‐CoV‐2 by vaccination in PLWH of Sierra Leone. IgG, immunoglobulin G; PLWH, people living with human immunodeficiency virus; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
3.4. Factors associated with seroprevalence
In multivariable analysis, patients 60 years or older had the highest odds of IgG seropositivity (aOR = 2.73, 95% CI = 1.68–4.65). Female participants (aOR = 1.28, 95% CI = 1.05–1.56), participants vaccinated against COVID‐19 (aOR = 1.54, 95% CI = 1.27–1.86), and those on ART (aOR = 2.20, 95% CI = 1.56–3.11) had increased odds of IgG seropositivity. In contrast, the odds of IgG seropositivity were lower in participants with HIV RNA levels ≥1000 copies/mL (aOR = 0.32, 95% CI = 0.26–0.40) (Table 3).
Table 3.
Factors associated with a higher seroprevalence of IgG antibody against SARS‐CoV‐2 in PLWH of Sierra Leone.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | aORa | 95% CI | p Value | |
| Age | ||||||
| 18–30 | — | — | — | — | — | — |
| 30–40 | 1.18 | 0.96–1.45 | .125 | 1.12 | 0.90–1.40 | .3 |
| 40–50 | 1.35 | 1.07–1.71 | .011 | 1.20 | 0.94–1.54 | .2 |
| 50–60 | 1.34 | 1.01–1.78 | .041 | 1.15 | 0.86–1.55 | .4 |
| 60–100 | 2.93 | 1.83–4.91 | <.001 | 2.73 | 1.68–4.65 | <.001 |
| Sex | ||||||
| Male | — | — | — | — | — | — |
| Female | 1.36 | 1.13–1.63 | .001 | 1.28 | 1.05–1.56 | .015 |
| ART | ||||||
| No | — | — | — | — | — | — |
| Yes | 4.42 | 3.31–5.93 | <.001 | 2.20 | 1.56–3.11 | <.001 |
| HIV viral load | ||||||
| <1000 | — | — | — | — | — | — |
| ≥1000 | 0.26 | 0.21–0.31 | <.001 | 0.32 | 0.26, 0.40 | <.001 |
| COVID‐19 vaccination | ||||||
| No | — | — | — | — | — | — |
| Yes | 1.53 | 1.28–1.84 | <.001 | 1.54 | 1.27–1.86 | <.001 |
| Month of sampling collection | ||||||
| August 2022 | — | — | — | — | — | — |
| September 2022 | 1.2 | 0.80–1.78 | .358 | 1.34 | 0.88, 2.00 | .2 |
| October 2022 | 1.34 | 0.81–2.22 | .258 | 1.84 | 1.09, 3.12 | .024 |
| November 2022 | 1.28 | 0.85–1.9 | .222 | 2.02 | 1.32, 3.07 | .001 |
| December 2022 | 1.04 | 0.71–1.5 | .827 | 1.30 | 0.88, 1.89 | .2 |
| January 2023 | 0.91 | 0.58–1.42 | .670 | 1.10 | 0.68, 1.74 | .7 |
| Hospital | ||||||
| Primary | — | — | — | — | — | — |
| Secondary | 1.02 | 0.71–1.49 | .923 | NA | NA | NA |
| Tertiary | 0.96 | 0.78–1.18 | .714 | NA | NA | NA |
Abbreviations: aOR, adjusted odds ratio; ART, antiretroviral therapy; CI, confidence interval; IgG, immunoglobulin G; OR, odds ratio; PLWH, people living with human immunodeficiency virus; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
aOR, calculated by multivariable stepwise logistic regression using the backward selection strategy. After variable selection process, age, sex, ART, COVID‐19 vaccination, HIV viral load, and month of sampling collection were finally included in the regression model.
4. DISCUSSION
Owing to limited resources, and the lack of scientific research, the true burden of COVID‐19 in Sierra Leone in the postpandemic era is unknown. This study presents data on the seroprevalence of COVID‐19 in PLWH in the postpandemic era.
Overall, we observed that 75.7% of PLWH in Sierra Leone had IgG antibodies against SARS‐CoV‐2. Lower seroprevalence of the SARS‐CoV‐2 IgG antibodies among PLWH was reported in Burkina Faso and South Africa. 16 , 17 Although we did not track participants longitudinally, we observed fluctuations in SARS CoV‐2 IgG antibody levels, with a peak level of 78.9% occurring in October 2022 and the lowest level of 71.7% occurring in January 2023. These fluctuations in the SARS‐CoV‐2 IgG antibodies could reflect on the fluctuations in COVID‐19 cases reported in Sierra Leone. 18
After stratifying the SARS‐CoV‐2 IgG antibodies by age and sex, we observed an increase in the levels of antibodies with age. Young adults below 30 years have lower SARS‐CoV‐2 antibody levels of 71.8% compared to 88.2% in older adults aged 60 years or older. These findings are consistent with the fact that older adults are at a higher risk of SARS‐CoV‐2 infection than young adults. 19 , 20 , 21 , 22 , 23 It is widely believed that men are more susceptible to SARS‐CoV‐2 infection and severe COVID‐19 disease than women. 24 In contrast to this observation, we found that HIV‐infected women in this study were more likely than men to be seropositive for SARS‐CoV‐2 IgG. The reason for this paradoxical finding is unknown but could be investigated in future research.
The IgG seroprevalence among the vaccinated was 80.7% compared to 73.2% among those unvaccinated participants. The high seroprevalence in the unvaccinated participants suggests that the SARS‐CoV‐2 virus may still be circulating in Sierra Leone. This finding should be interpreted with caution, as few PLHW were positive for SARS‐CoV‐2 IgM. On the other hand, the higher prevalence of SARS‐CoV‐2 antibodies in the vaccinated population may be attributed to the hybrid immunity from the natural infection and vaccination. At the end of the study (January 2023), the vaccine coverage (with at least one dose of any vaccine) rate in the general population of Sierra Leone was 52.4%, but the coverage reported in our study is only 33.4% in the same period. 11 The low number of vaccinated participants who received only one dose of the COVID‐19 vaccine may be due to previously reported vaccine hesitancy among PLWH, resulting in lower vaccination rates. 13 Given the high seroprevalence of COVID‐19 infection among unvaccinated PLWH and low vaccine coverage, primary and booster vaccinations are still strongly recommended for PLWH in Sierra Leone.
HIV viral load assessment is recommended by WHO and the national HIV program as the preferred method to monitor HIV treatment progress, with a viral threshold of <1000 copies/mL defined as viral suppression. 25 , 26 In our study, 82.8% of the participants were virally suppressed, in contrast to a viral suppression rate of 64.6% reported in 2019 and 2020. 27 It is generally accepted that PLWH receiving effective ART are able to achieve complete or nearly complete suppression of viral replication and maintain immune function comparable to that of the general population. 28 This statement supports findings from our study, which found that both viral suppression and ART were associated with a higher seroprevalence of SARS‐CoV‐2 IgG antibody after adjusting for demographic factors, vaccine status, and sample collection time.
Our study has strengths. To our knowledge, this is the first national study to determine the seroprevalence of SARS‐CoV‐2 antibody in PLWH in Sierra Leone. The study has a large sample size of 3085 participants included in the final analysis. Furthermore, benefiting from these 6‐month‐long durations of data collection, we were able to identify persistent patterns of fluctuations in the seroprevalence of SARS‐CoV‐2 antibody.
We acknowledge there are limitations of our study. Due to limited laboratory capacity, we only conducted a qualitative analysis of antibodies and were unable to perform a quantitative analysis. We did not establish a general population control group, which prevents us from determining whether the seroprevalence among PLWH is higher. Additionally, our study did not include private hospitals in Sierra Leone, which could be done in the future. Therefore, our results only represent the seroprevalence of SARS‐CoV‐2 antibodies among PLWH in public health facilities.
5. CONCLUSIONS
We observed a high seroprevalence of SARS‐CoV‐2 antibodies among PLWH in Sierra Leone between August 2022 and January 2023. We recommend the introduction of targeted vaccination for PLWH with a high risk of severe COVID‐19, especially those with an unsuppressed HIV viral load.
AUTHOR CONTRIBUTIONS
Wei Sun: Data curation; formal analysis; investigation; methodology; writing—original draft; writing—review & editing. Jinwen Song: Conceptualization; investigation; writing—review & editing. Sulaiman Lakoh: Resources; writing—review & editing. Jinquan Chen: Data curation; formal analysis. Abdulai T. Jalloh: Writing—review & editing. Foday Sahr: Writing—review & editing. Stephen Sevalie: Writing—review & editing. Darlinda F. Jiba: Investigation. Ibrahim F. Kamara: Investigation. Yingrong Xin: Validation. Zhongyang Ye: Validation. Feng Ding: Formal analysis. Li‐Zhong Dai: Funding acquisition. Ligui Wang: Conceptualization; funding acquisition; writing—original draft. Xishui Zheng: Funding acquisition; project administration; writing—original draft. Guang Yang: Conceptualization; investigation; writing—review & editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Ethics approval was obtained from the Sierra Leone Ethics and Scientific Review Committee (SLESRC) of the Ministry of Health and Sanitation, Government of Sierra Leone in accordance with the relevant guidelines and regulations and declaration of Helsinki. Approval to conduct this study was granted by SLESRC, dated June 21, 2022. Written informed consent had been obtained upon the enrollment of individual participants.
Supporting information
Supporting information.
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
The authors would like to express their gratitude to all those who have supported them throughout the research process. Authors also gratefully acknowledge all the participants enrolled in this study and healthcare workers in the different hospitals where the patients were recruited. This work was supported by the National Key Research and Development Program of China (grant number 2022YFC2602304), the National Natural Science Foundation of China (grant number 82101837), and the Beijing Natural Science Foundation (grant number 7222171).
Sun W, Song J, Lakoh S, et al. SARS‐CoV‐2 seroprevalence and associated factors among people living with HIV in Sierra Leone. Immun Inflamm Dis. 2024;12:e1338. 10.1002/iid3.1338
Wei Sun, Jinwen Song, and Sulaiman Lakoh contributed equally to this study.
Contributor Information
Li‐Zhong Dai, Email: dlzsansure@sansure.com.cn.
Ligui Wang, Email: wangligui1983@126.com.
Xishui Zheng, Email: ZXS73881@sina.com.
Guang Yang, Email: guang.23@163.com.
DATA AVAILABILITY STATEMENT
Data are available upon reasonable request.
REFERENCES
- 1. WHO . Statement on the fifteenth meeting of the IHR (2005) Emergency Committee on the COVID‐19 pandemic. WHO; 2023. Accessed July 15, 2023. https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic
- 2. Bertagnolio S, Thwin SS, Silva R, et al. Clinical features of, and risk factors for, severe or fatal COVID‐19 among people living with HIV admitted to hospital: analysis of data from the WHO Global Clinical Platform of COVID‐19. Lancet HIV. 2022;9(7):e486‐e495. 10.1016/s2352-3018(22)00097-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jassat W, Cohen C, Tempia S, et al. Risk factors for COVID‐19‐related in‐hospital mortality in a high HIV and tuberculosis prevalence setting in South Africa: a cohort study. Lancet HIV. 2021;8(9):e554‐e567. 10.1016/s2352-3018(21)00151-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu Y, Han J, Li X, et al. COVID‐19 vaccination in people living with HIV (PLWH) in China: a cross sectional study of vaccine hesitancy, safety, and immunogenicity. Vaccines. 2021;9(12):1458. 10.3390/vaccines9121458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang Y, Xie Y, Hu S, et al. Systematic review and meta‐analyses of the interaction between HIV infection and COVID‐19: two years' evidence summary. Front Immunol. 2022;13:864838. 10.3389/fimmu.2022.864838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hanson HA, Kim E, Badowski ME. A systematic review: impact of SARS‐CoV‐2 infection on morbidity, mortality, and viral suppression in patients living with HIV. SN Compr Clin Med. 2023;5(1):144. 10.1007/s42399-023-01480-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Basoulis D, Mastrogianni E, Voutsinas PM, Psichogiou M. HIV and COVID‐19 co‐infection: epidemiology, clinical characteristics, and treatment. Viruses. 2023;15(2):577. 10.3390/v15020577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. WHO . Global HIV Programme: HIV data and statistics. WHO; 2023. Acessed July 15, 2023. https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics
- 9.Sierra Leone demographic health survey. 2019. Accessed on May 24, 2023. https://dhsprogram.com/pubs/pdf/FR365/FR365.pdf
- 10. Yendewa GA, Poveda E, Lakoh S, et al. High prevalence of late‐stage disease in newly diagnosed human immunodeficiency virus patients in Sierra Leone. Open Forum Infect Dis. 2018;5(9):ofy208. 10.1093/ofid/ofy208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lakoh S, Jiba DF, Kanu JE, et al. Causes of hospitalization and predictors of HIV‐associated mortality at the main referral hospital in Sierra Leone: a prospective study. BMC Public Health. 2019;19(1):1320. 10.1186/s12889-019-7614-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Our World In Data . Coronavirus (COVID‐19) vaccinations. 2023. Accessed on July 13, 2023. https://ourworldindata.org/covid-vaccinations
- 13. Cummings PE, Lakoh S, Yendewa SA, et al. Understanding COVID‐19 vaccine uptake and hesitancy among people with HIV in Freetown, Sierra Leone: a cross‐sectional study. Vaccines. 2023;11(11):1685. 10.3390/vaccines11111685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barrie MB, Lakoh S, Kelly JD, et al. SARS‐CoV‐2 antibody prevalence in Sierra Leone, March 2021: a cross‐se ctional, nationally representative, age‐stratified serosurvey. BMJ Glob Health. 2021;6(11):e007271. 10.1136/bmjgh-2021-007271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lawal BJ, Gallagher KE, Kitonsa J, et al. Prevalence of immunoglobulin G and M to SARS‐CoV‐2 and other human coronaviruses in The Democratic Republic of Congo, Sierra Leone, and Uganda: a longitudinal study. Int J Infect Dis. 2023;131:183‐192. 10.1016/j.ijid.2023.03.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Francois KLA, Msomi N, Govender K, et al. Seroprevalence of SARS‐CoV‐2 immunoglobulin G in HIV‐positive and HIV‐negative individuals in KwaZulu‐Natal, South Africa. Afr J Lab Med. 2023;12(1):2065. 10.4102/ajlm.v12i1.2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaboré OD, Poda A, Ouattara CA, et al. Seroprevalence of SARS‐CoV‐2 IgG and associated factors among people living with HIV over the first 12 months following the outbreak of COVID‐19 in Burkina Faso, a sub‐Saharan African country. PLoS One. 2023;18(6):e0286665. 10.1371/journal.pone.0286665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu Z, Gao L, Xue C, et al. Epidemiological trends of coronavirus disease 2019 in Sierra Leone from March 2020 to October 2021. Front Public Health. 2022;10:949425. 10.3389/fpubh.2022.949425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Isho B, Abe KT, Zuo M, et al. Persistence of serum and saliva antibody responses to SARS‐CoV‐2 spike antigens in COVID‐19 patients. Sci Immunol. 2020;5(52):eabe5511. 10.1126/sciimmunol.abe5511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rodda LB, Netland J, Shehata L, et al. Functional SARS‐CoV‐2‐specific immune memory persists after mild COVID‐19. Cell. 2021;184(1):169‐183.e17. 10.1016/j.cell.2020.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS‐CoV‐2 and COVID‐19. Nat Rev Microbiol. 2021;19(3):141‐154. 10.1038/s41579-020-00459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tu B, Lakoh S, Xu B, et al. Risk factors for severity and mortality in adult patients confirmed with COVID‐19 in Sierra Leone: a retrospective study. Infect Dis Immun. 2022;2(2):83‐92. 10.1097/id9.0000000000000037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brodin P. Immune determinants of COVID‐19 disease presentation and severity. Nat Med. 2021;27(1):28‐33. 10.1038/s41591-020-01202-8 [DOI] [PubMed] [Google Scholar]
- 25. World Health Organization . Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach. WHO; 2021. Accessed July 20, 2023. https://www.who.int/publications/i/item/9789240031593 [PubMed]
- 26.Consolidated guidelines on HIV prevention, diagnosis, treatment and care in Sierra Leone. 2020. Accessed May 17, 2023. https://www.nas.gov.sl/publication/164-consolidated-hiv-guidelines-on-hiv-prevention-october-2020
- 27. Lakoh S, Jiba DF, Vandy AO, et al. Assessing eligibility for differentiated service delivery, HIV services utilization and virologic outcomes of adult HIV‐infected patients in Sierra Leone: a pre‐implementation analysis. Glob Health Action. 2021;14(1):1947566. 10.1080/16549716.2021.1947566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deeks SG, Overbaugh J, Phillips A, Buchbinder S. HIV infection. Nat Rev Dis Primers. 2015;1(1):15035. 10.1038/nrdp.2015.35 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Data Availability Statement
Data are available upon reasonable request.


