Abstract
To infect and cause disease, bacterial pathogens must localize to specific regions of the host where they possess the metabolic and defensive acumen for survival. Motile flagellated pathogens exercise control over their localization through chemotaxis to direct motility based on the landscape of exogenous nutrients, toxins, and molecular cues sensed within the host. Here, we review advances in understanding the roles chemotaxis plays in human diseases. Chemotaxis drives pathogen colonization to sites of inflammation and injury and mediates fitness advantages through accessing host-derived nutrients from damaged tissue. Injury tropism may worsen clinical outcomes through instigating chronic inflammation and subsequent cancer development. Inhibiting bacterial chemotactic systems could act synergistically with antibacterial medicines for more effective and specific eradication.
Keywords: Chemotaxis, bacterial pathogenesis, motility, chronic inflammation
Connections between bacterial chemotaxis and human diseases
Bacterial chemotaxis is a widely conserved sensory system of ancient evolutionary origin that facilitates directed flagellar-based motility based on exogenous physicochemical gradients [1] (see glossary). Through chemotaxis bacterial populations rapidly alter their localization, on the time-scale of seconds, to enhance survival through attraction to nutrients and repulsion from toxins [1]–[3]. Many bacteria that cause disease in humans, especially gastrointestinal pathogens, dedicate large portions of their genomes to chemosensory systems that facilitate chemotaxis [2]. Bacterial pathogenesis is often enhanced through sensing of environmental cues that leads to the coordinated expression of virulence regulons. It has been proposed that serious sequelae resulting from bacterial infection could be prevented by disrupting chemotaxis networks and motility [4]. However, the relationship between bacterial pathogenesis and chemotaxis is complex, and knowledge of the fitness advantages chemotaxis provides for microbes within hosts remains incomplete. To date, no antibacterial therapeutics are commercially available that act through the mechanism of inhibiting bacterial chemotactic machinery.
In this review we synthesize the current understanding of bacterial chemotaxis at the host-pathogen interface in relation to human diseases. We discuss how these findings implicate the importance of chemotaxis for success in inflamed tissue and how bacterial localization, driven by chemotaxis, may factor into clinical outcomes.
Fundamentals of bacterial chemotaxis
Bacterial chemotaxis is a longstanding model system for studying cellular sensory transduction and recent reviews have described the underlying biophysics [1], [5], [6]. Here, we focus on the clinical perspective and will summarize the molecular pathways of chemotaxis with brevity to provide the basics for readers to understand how bacteria localize in response to chemotactic stimuli (Fig. 1).
Fig. 1. Bacterial chemotaxis across atomic, molecular, cellular, and population scales.
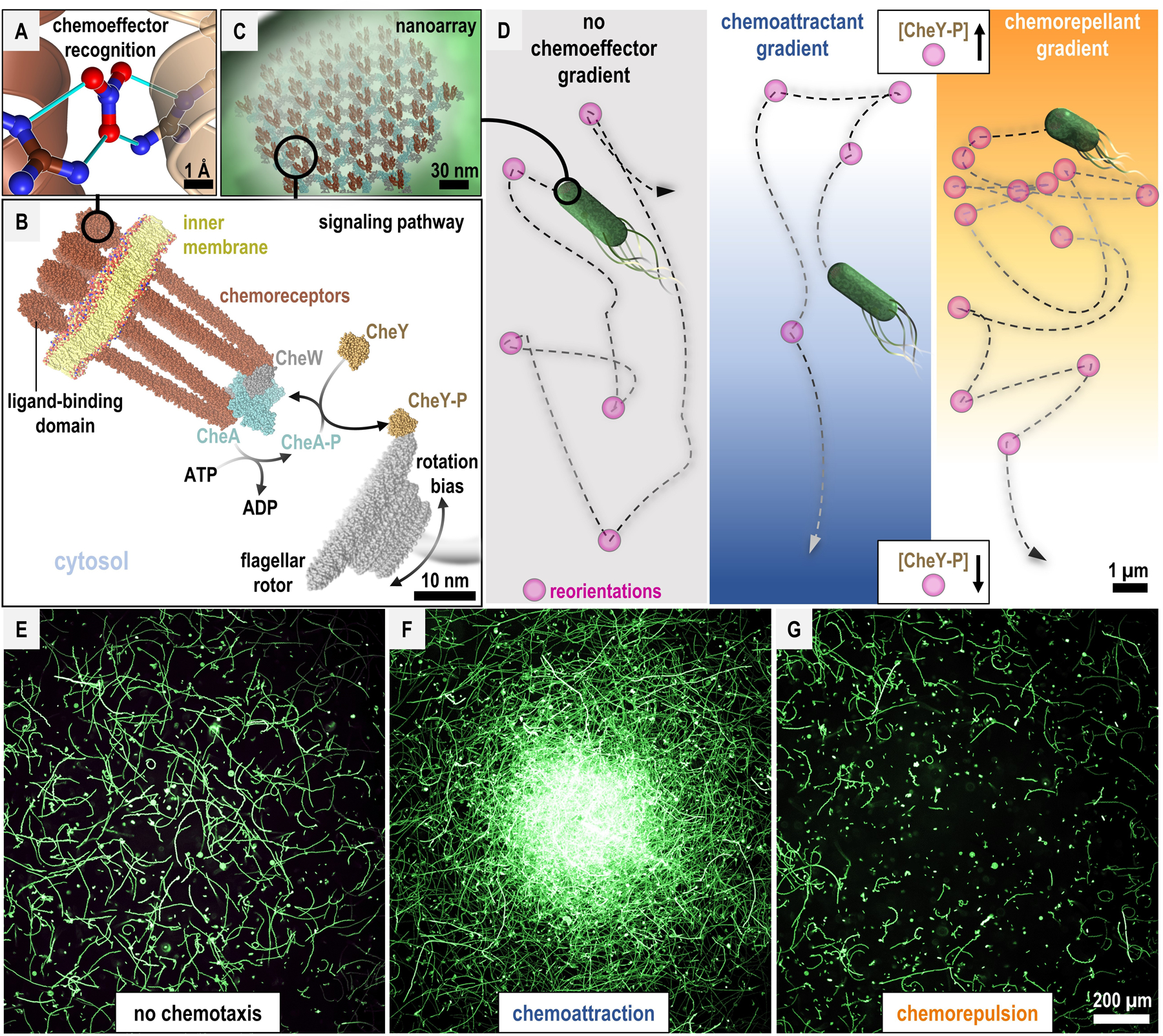
A. Chemoreceptors can recognize chemoeffectors through direct binding. McpN from Pseudomonas aeruginosa is shown binding nitrate at the interface between two chemoreceptor monomers (dark and light brown, PDB 6gcv [86]). Hydrogen bonds between the proteins and nitrate ligand are shown as cyan lines. B. Chemoreceptor core signaling unit [8], [104] and the canonical phosphorelay of bacterial chemotaxis. Distance between the chemoreceptor complex and flagellar rotor not to scale. C. Chemoreceptor nanoarrays amplify chemoeffector sensing [8], [104]. D. Bacterial swimming and reorientation bias in chemoeffector gradients. Hypothetical bacterium swimming trajectories are depicted as dashed lines. E-G. Chemotactic responses by motile bacterial populations absent chemoeffector, or to central sources of chemoattractant or chemorepellent. Size bars are indicated. See Figure S1 for in vitro methods of measuring chemotactic responses at the atomic, molecular, cellular, and population scales.
Chemotaxis imbues bacteria with the capability to localize, and relocalize, in response to chemical gradients, i.e. sources of chemicals. Chemical species that elicit chemotactic responses are referred to as chemoeffectors; chemicals that promote swimming up gradients are called chemoattractants and those that encourage swimming down gradients are called chemorepellents [1], [5]. Perception of chemoeffectors is facilitated by chemoreceptor proteins, which can directly bind chemoeffector ligands (Figure 1A) [7]. Chemoreceptors form trimers-of-dimers that further oligomerize into a large hexagonal lattice, known as a nanoarray, that serves to amplify ligand-sensing through highly-sensitive cooperativity [8], [9] (Fig. B,C). The chemoreceptor nanoarray complexes with the cytosolic histidine kinase chemotaxis protein A (CheA) and regulates its autophosphorylation based on chemoreceptor ligand occupancy. Phosphorylated CheA transfers the inorganic phosphate to chemotaxis protein Y (CheY). Phosphorylated CheY (CheY-Pi) can diffuse throughout the cell and directly bind the flagellar rotor to bias rotation, leading to changes in the direction of bacterial swimming. Chemorepellents elicit activation of this phosphorelay, raising CheY-Pi levels and increasing swimming reorientations, whereas chemoattractants inhibit phosphorylation and CheY-Pi production, leading to reduced swimming reorientations (Fig. 1D). Additional enzymes work in opposition to dephosphorylate CheA and CheY, and methyltransferases reversibly methylate chemoreceptors to dampen their signaling. These feedback pathways facilitate the logic necessary for adaptation to stimuli, enabling bacteria to either continue up or down chemoeffector gradients, or become desensitized to chemoeffectors [5].
Alongside sensing of exogenous stimuli via the canonical chemosensory pathway described above, bacteria integrate internal metabolic or energy status, i.e. energy-taxis, as well as redox-taxis and aerotaxis, into chemotactic behaviors [10]–[14]. We discuss these in further detail, and examples of their roles in virulence, in Box 1. Additionally, not all chemosensory pathways mediate chemotaxis (flagellum-mediated swimming motility), as some are involved in type-IV pili motility and control of secondary messengers [15].
Box 1.
Diversity of bacterial taxis behaviors.
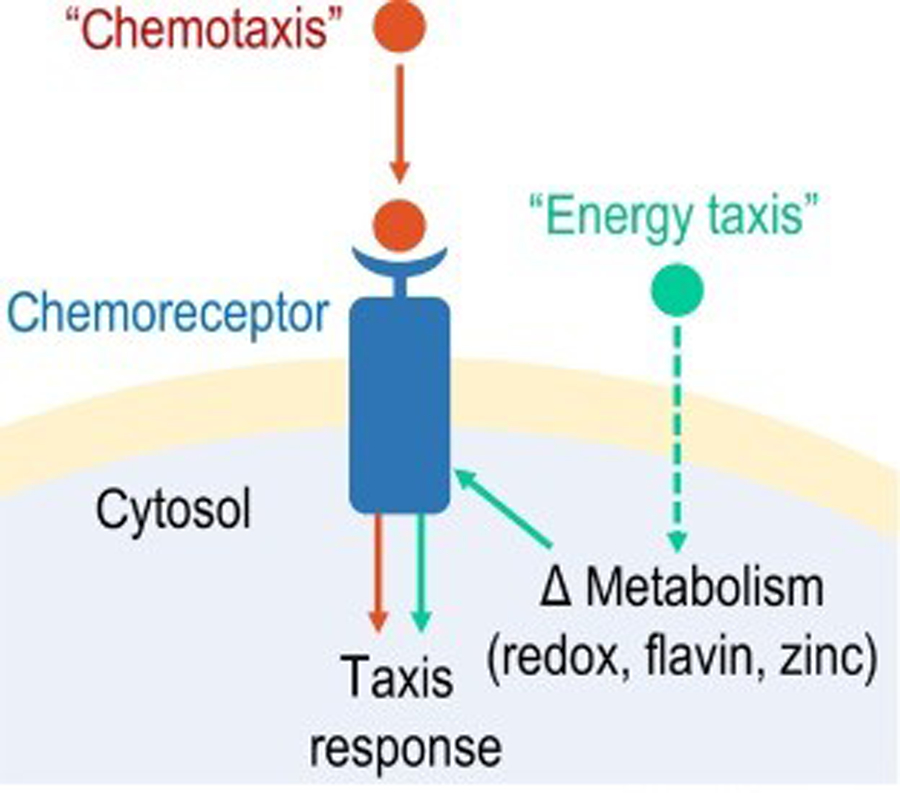
Bacteria exhibit a wide range of taxis behaviors across biology whereby bacterial populations control localization through stimuli that trigger swimming reorientations (see Figure 1 in main text). In this review we have used ‘chemotaxis’ as an umbrella term for these collective phenomena and to keep the concepts herein approachable to non-experts. However, the field utilizes more precise terminology to distinguish taxis behaviors based on mechanism and the type and source of stimuli. Bacterial swimming behaviors can be influenced by gravity (geotaxis), light (phototaxis), magnetic fields (magnetotaxis), fluid current (rheotaxis), pH conditions (pH taxis), temperature (thermotaxis), osmolarity (osmotaxis), oxygen concentrations (aerotaxis), redox potentials (energy taxis or redox taxis), and forces in vortices (gyrotaxis) [110]. The distinction between the mechanisms of chemotaxis and energy taxis is that the former relates to direct recognition of the chemoeffector ligand, typically originating from an exogenous source, whereas the latter relates to sensing internal metabolic changes induced by stimuli, such as through changes to pools of flavin redox potentials or zinc homeostasis (Figure I) [15]. Cases exist where a taxis behavior may fall into more than one of the aforementioned categories, or stimuli may elicit multiple taxis behaviors. An exemplar is the family of aerotaxis chemoreceptors (Aer), which are well documented as playing important roles in pathogenicity. Some Aer chemoreceptors mediate aerotaxis through direct sensing of O2 via heme [111,112], and others perform energy taxis by monitoring flavin adenine dinucleotide oxidoreduction [113]. S. enterica uses Aer and energy taxis for attraction to host-derived nitrate, which contributes to invasion of Peyer’s patches [11].
From the perspective of a motile bacterial pathogen, the environment of the human host is a dynamic landscape of complex overlapping chemoeffector gradients frequently perturbed by gut motility and hydrodynamic flow, nutrient influx, resident microbiota competitors, and inflammatory processes. Identifying what chemical species are chemoeffectors and measuring chemotactic behaviors of bacteria is non-trivial, and no single methodology or model system can fully recapitulate the circumstances in a human host (Fig. S1). An up-to-date compendium of purported chemoeffectors, with varying degrees of substantiation in terms of reproducibility, defined molecular mechanisms, and an established role in vivo, has been published [3]. Below, we focus our discussion on new and emerging evidence for the roles of chemoeffectors at the human host-pathogen interface for bacteria that pose substantial worldwide health burdens.
Chemotaxis systems of WHO priority pathogens
In 2017, the World Health Organization published its first list of antibiotic-resistant “priority pathogens” (WHOpp) for which the development of new antimicrobial medicines is urgently required [16]. Based on factors such as severity of infections and lack of effective treatment options, species from 19 bacterial genera were identified that pose imminent threats to human health, 12 of which appear to possess chemotaxis-driven swimming motility [17, p. 3]. Of these, chemotaxis systems in Shigella, Enterobacter, Morganella, Serratia, Proteus, Providencia, and Citrobacter remain poorly understood, whereas chemotaxis in Pseudomonas, Helicobacter, Escherichia, Salmonella, and Campylobacter have been studied extensively. Consequently, we focus on these five genera as models to understand what roles chemotaxis plays in human infections and disease outcomes (Fig. 1A). WHOpp that possess chemotaxis systems account for a significant number of deaths per year from infections or complications with underlying diseases such as cystic fibrosis (CF), environmental enteric dysfunction (EED), Guillain-Barré Syndrome (GBS), and chronic inflammation leading to the development of cancers (Fig. 2B–D). The latter include gastric cancer, bladder cancer, and colonic cancer, which are associated with infections by Helicobacter [18], Escherichia [19], and Salmonella/Escherichia/Campylobacter [20], [21], respectively (Fig. 2D).
Fig. 2. Disease and mortality associated with chemotactic WHOpp.
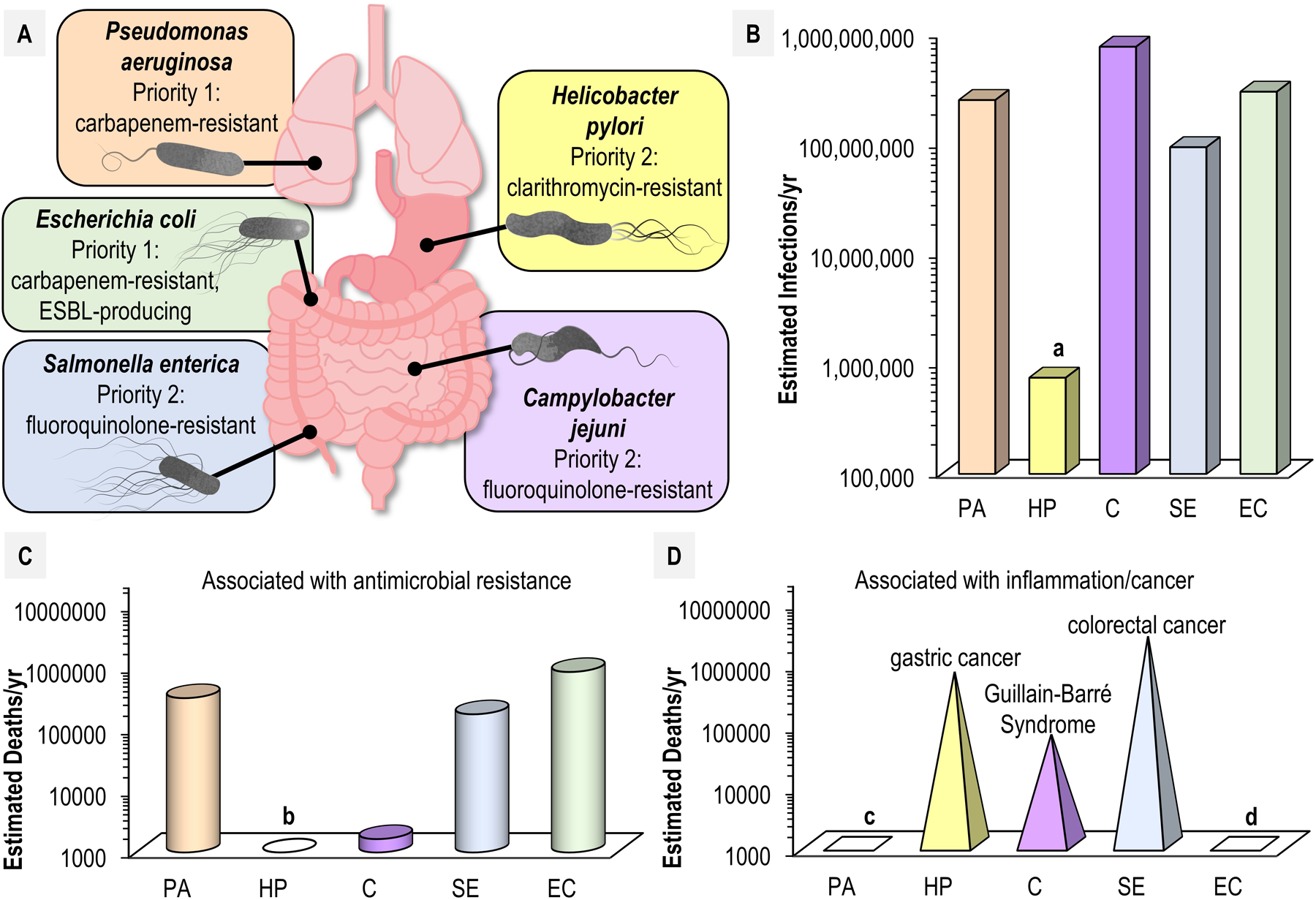
A. Typical infection sites associated with human disease for select chemotactic pathogens are indicated, along with their priority designation by WHO. B. Estimated annual infections worldwide by pathogen: PA; Pseudomonas aeruginosa, HP; Helicobacter pylori, C; Campylobacter spp., SE; Salmonella enterica (all serovars), EC; Escherichia coli. Estimate of H. pylori annual infections is based on that the bacteria infect approximately half of the world’s total population (a). C. Annual deaths associated with antimicrobial resistance worldwide. Estimates based on data from [105]. H. pylori is not typically associated with deaths from acute infection and so is omitted (b). D. Speculative estimates for deaths associated with select diseases of inflammation and cancer are shown. Estimates are based on risk factors associated with bacterial infections based on available data: “Antibiotic Resistance Threats in the United States” https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf, “WHO publishes list of bacteria for which new antibiotics are urgently needed” https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed, “Population-based Prospective Study of the Combined Influence of Cigarette Smoking and Helicobacter pylori Infection on Gastric Cancer Incidence: The Hisayama Study” https://academic.oup.com/aje/article/168/12/1409/155955, “Guillain-Barré Syndrome” https://www.cdc.gov/campylobacter/guillain-barre.html, and [106]. P. aeruginosa and E. coli are agents suspected to instigate diseases of chronic inflammation and cancers, but we did not identify literature describing specific risk factors associated with these diseases (c, d).
Chemotaxis drives specific colonization topography based on chemoeffector gradients within the host [2]. However, the niche a pathogen colonizes is not static. Changes to chemoeffector gradients may occur due to aging, diet, or health. Pathogens themselves shape the human host environment as the infection proceeds through incubation, prodromal, illness, and chronic stages (Fig. 3) [22], [23]. These evolving circumstances pose discrete challenges and opportunities for motile chemotactic pathogens. Below, we discuss results from recent chemotaxis studies in this context to infer the ways in which chemotaxis provides fitness advantages for pathogens within the dynamic environment of the human host.
Fig. 3. Evolution of chemoeffector gradients during disease progression.
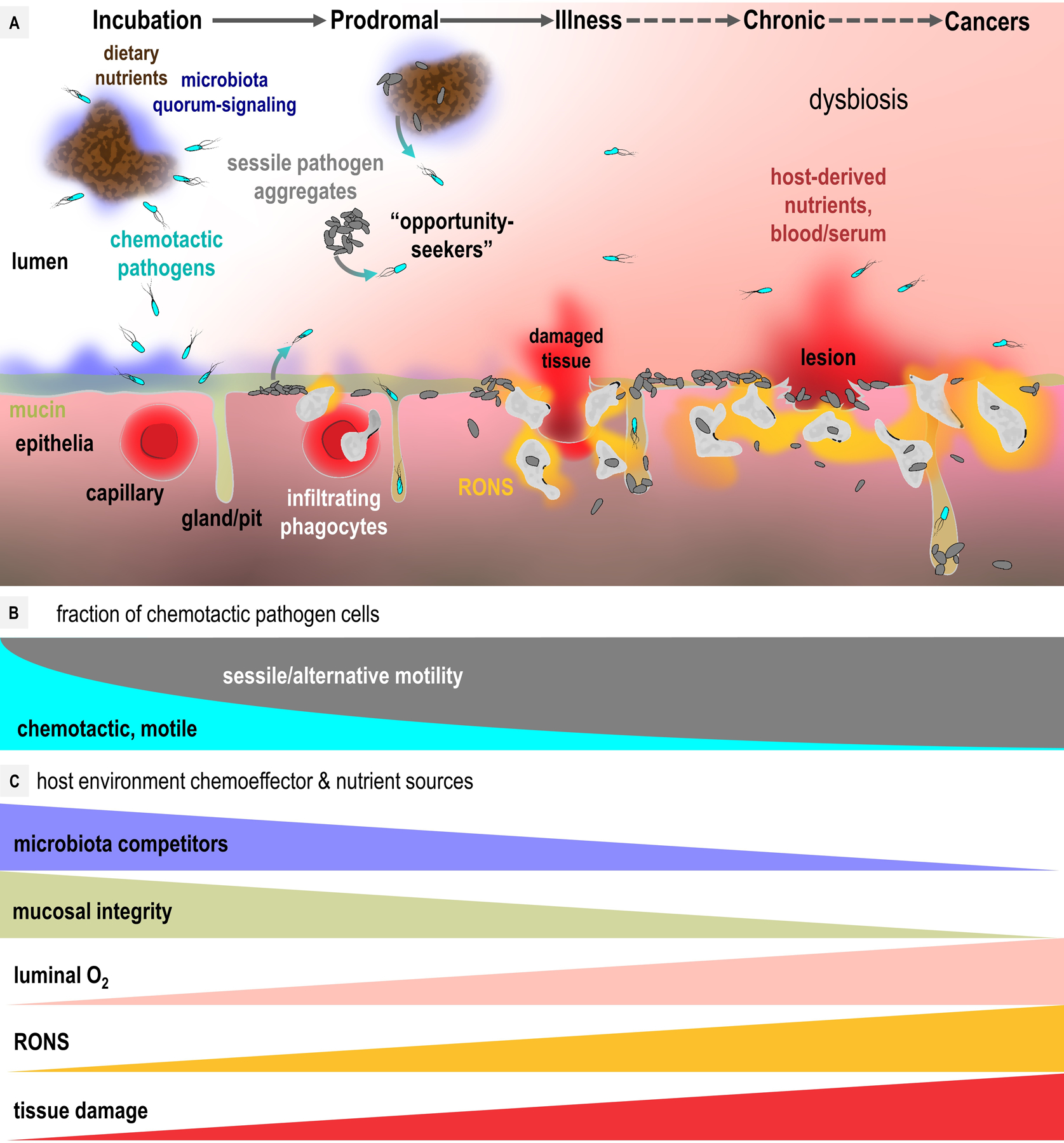
A. A generalized model of the host environment during infection. Chemotactic pathogens (cyan cells) localize to nutrient sources and host tissue. After initial colonization pathogens shift toward sessility (gray cells), with some cells departing aggregates and biofilms to act as chemotactic “opportunity seekers” to colonize new regions (gray to cyan arrows). Host inflammatory responses and RONS generation (orange) can result in tissue damage (red) through phagocyte transmigration, and disrupt luminal O2 gradients (pink). Most infections resolve, but some may progress to chronic or cancerous stages (dashed lines). B. Motile and chemotactic versus sessile fraction of pathogen populations as a function of disease progression. C. Gradients of chemoeffectors, nutrients, and toxins relevant to pathogen colonization as a function of disease progression.
Roles for chemotaxis at different stages of infection
Initial colonization: incubation stage
Motility is a prerequisite for chemotaxis, so the magnitude of the role of chemotaxis for any infection stage relates to the prevalence of motile cells in the bacterial population (Fig. 3). A current paradigm is that the motile fraction decreases after initial colonization, presumably due to the high energetic costs of motility and chemotaxis [2], [24]. There is variability amongst pathogens in this regard, perhaps owing to the challenges of colonizing certain host environments. For instance, H. pylori maintains motile populations long-term that migrate between stomach regions and seed colonization of new gastric glands [25]–[27]. In contrast, P. aeruginosa infections of the lung, and C. jejuni infection of the intestine, show strong shifts toward sessility through biofilm formation, and strains isolated from patients often show genes associated with motility and chemotaxis to be downregulated or lost [28]–[31]. Even if chemotaxis confers the greatest fitness advantages early in infection, thereafter even relatively small numbers of chemotactic cells imbue the bacterial population with the versatility to spread and relocate based on changing host circumstances (Fig. 3).
To colonize a naïve host, a pathogen often needs to outcompete native obligate fermenters of the microbiome within a mostly anaerobic environment (Fig. 3). To assist in this, WHOpp chemotaxis systems interpret gradients of quorum-sensing molecules like indole [32] and autoinducer-2 [33] to regulate pathogen expansion and detect bacterial competitors (Fig. 3). In combination with chemoattraction to host-secreted factors such as urea [34] and mucin [35], chemotaxis drives pathogens from the lumen into contact with host tissue, to facilitate adherence and/or invasion of host cells [10], [36]–[38]. Roles of chemotaxis for pathogens colonizing naïve hosts is demonstrated by experiments with healthy conventional (i.e. intact microbiome) mammalian models inoculated with wildtype (WT) versus chemotactic-deficient strains. In such experiments P. aeruginosa [39], H. pylori [26], C. jejuni [40], S. enterica [38], and E. coli [41] show chemotaxis contributes to fitness and virulence in the range of 5–1000-fold. Notably, the roles of chemotaxis at early stages of infection are not captured in toto by experiments with gnotobiotic models, or animals pretreated with antibiotics to enhance pathogen colonization, because these systems have eliminated, or undermined, competition with the microbiome.
Briefly, we note there is variability amongst naïve host environments that may profoundly affect chemoeffector gradients, and thus, also, pathogen localization and colonization. One example is host age. The enteric pathogen C. jejuni, a prevalent cause of diarrhea in infants, exhibits chemoattraction to fucose derived from human breastmilk oligosaccharides, resulting in the expulsion of the pathogen into the feces of infants, where free fucose levels reach 4–5 mg/gram [42]–[45]. In this context breastmilk oligosaccharides ingested by infants confer protection against infection. In contrast, in the absence of dietary colostrum, C. jejuni scavenges fucose via gut microbiota that cleave mucins, such as Bacteroides vulgatus, resulting in increased C. jejuni colonization [46], [47]. This demonstrates the same chemotactic machinery operating in diverse host environments can lead to very different infection outcomes.
Post-inflammation: prodromal, illness, and chronic stages
As infection persists through prodromal and illness stages, inflammatory responses dramatically transform the host environment (Fig. 3) [23], [48]. Antibacterial processes perturb native microbiota, reactive oxygen and nitrogen species (RONS) are catalyzed by infiltrating phagocytes, and luminal oxygenation gradients are disrupted, permitting aerobic respiration (Fig. 3) [49]. Chemotaxis provides pathogen populations a means to capitalize on these new opportunities. Benefits from chemotaxis for pathogens beyond initial colonization is demonstrated unequivocally by detailed examination of colonization and expansion over extended intervals. For example, a comparison of the spatial and temporal colonization dynamics of WT H. pylori and chemotaxis-null (Che−) strains in mice showed chemotaxis is essential for spreading to new gastric glands over a 180 day period; a low inoculum (106 CFU) was sufficient for robust WT colonization of gastric mucus and glands within six days, whereas the Che− population collapsed [25], [50]. WT H. pylori exhibit “priority effects” and resist challenges from invaders, but chemotaxis-deficient strains are displaced [25], [26].
Animal dysbiosis models give further insight into how chemotaxis advances infection after initial colonization in which the host environment has become inflamed. The mouse colitis model is a widely-used system in which animals are primed with streptomycin that induces inflammation and disrupts the microbiota. In this host background competitive indices show S. enterica serovar Typhimurium to exhibit 10-fold greater fitness versus chemotaxis-null (Che−) strains, or those with chemoreceptor deletions such as tsr and aer [51] or mcpC (4-fold) [38].
Much of the health burden of WHOpp stems from pro-inflammatory colonization strategies in which pathogens instigate, and thrive, under conditions of severe inflammation (Fig. 2D). Studies with S. Typhimurium, H. pylori, and P. aeruginosa indicate motility and chemotaxis are among the mechanisms that drive aggressive inflammation responses [52]–[54], which can be counterproductive to pathogen eradication and cause lasting damage. One mechanism is through infiltrating phagocytes that disrupt the integrity of the epithelial barrier and cause tissue necrosis and bleeding [55] (Fig. 3). An emerging body of research reveals inflammation and host injuries present opportunities for pathogens to pirate host-derived nutrients [48], [51], [56]–[59] (Fig. 4). In particular, there is mounting evidence H. pylori induces gastric injuries through epithelial metaplasia to expand access to new stomach regions, and then preferentially colonizes injured tissue [60]. Experiments with gastric tissue injured by two-photon microscopy show H. pylori uses motility and chemotaxis to localize to lesions within minutes, with similar responses observed in injured organoid models [61], [62] (Fig. 4A,B, Movie S1).
Fig. 4. Pathogen chemoattraction to sites of host injury.
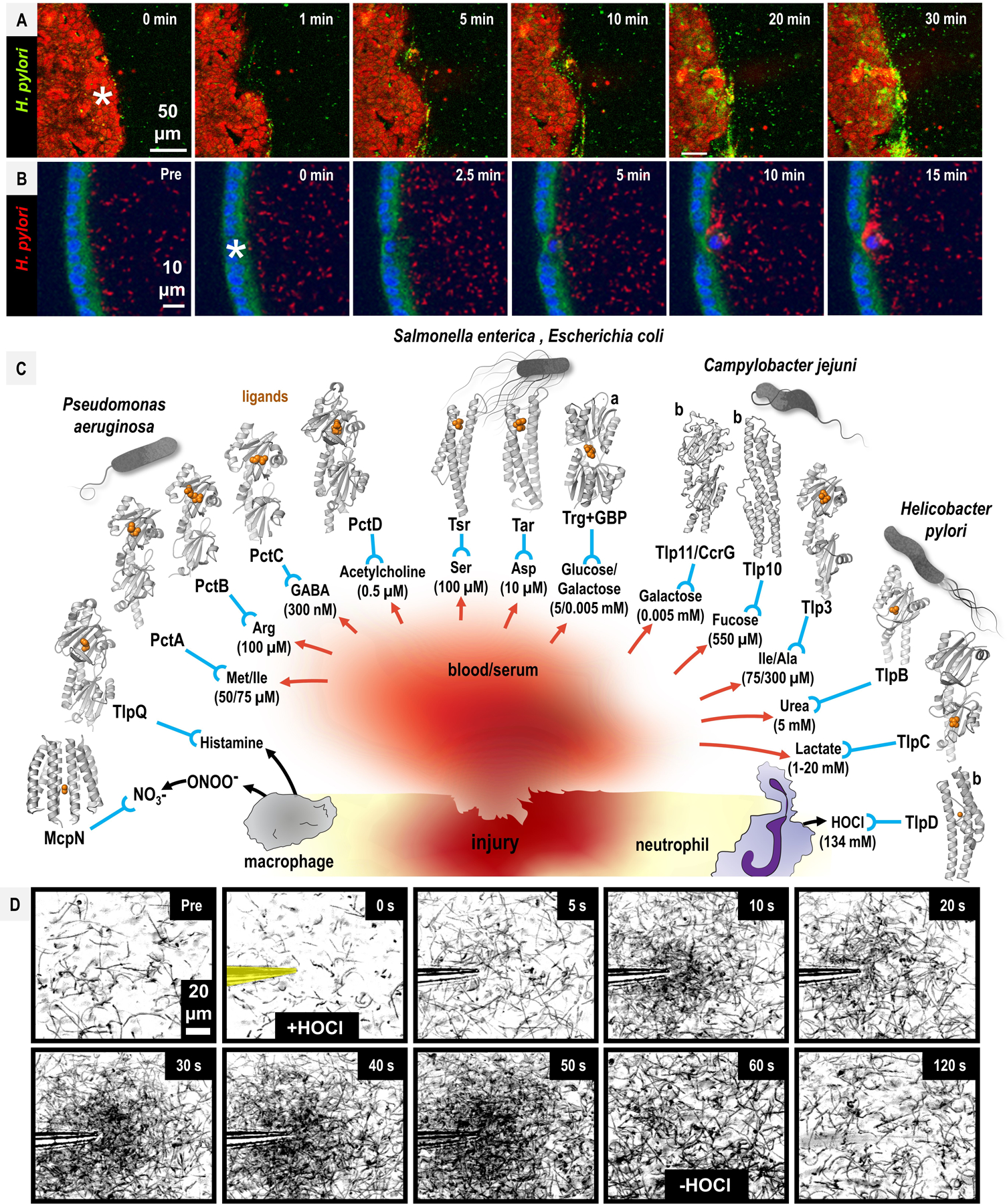
A-B. H. pylori exhibits chemoattraction to injured murine gastric tissue (A), and with murine gastric organoids (B). Injury was induced through single-cell photo-damage (asterisks). See also Movie S1 for the full video. Data from [61], [62], used with permission. C. Chemoattractants present at sites of injury and in human serum. The concentration of chemoattractants in blood/serum (red arrows), or produced through phagocyte oxidants (black arrows) are noted in parentheses, and the chemoreceptors involved in direct binding and sensing of the chemoattractants (blue) are indicated. Structures of chemoreceptor ligand-binding domains are shown for select WHOpp, with chemoeffector ligands in orange. For most of the interactions depicted it is unknown whether the presence of the chemoattractant within serum/damaged tissue mediates injury tropism. Trg senses glucose and galactose through galactose-binding protein (GBP); the structure shown is for GBP bound to galactose, denoted with “a.” Alphafold2 models are shown for Tlp11/CcrG and Tlp10 from C. jejuni, and TlpD from H. pylori, denoted with “b.” Chemoeffector concentrations are indicated. D. H. pylori chemoattraction to HOCl in vitro. A time-course of H. pylori chemotactic responses to the neutrophilic oxidant HOCl is shown pre-treatment (Pre) and at indicated timepoints. At time 0 s, a micropipette containing buffered 10 mM HOCl is inserted (yellow), and the motile bacteria in the field of view accumulate. At 60 s the HOCl source is removed and the bacteria disperse. Panels represent min-projections of 0.5 s at each time point. Data from [85], used with permission. See also Movie S2 for the full video.
One potential source of nutrients from damaged tissue is blood—rich in iron, amino acids, and sugars. Peptic ulcers caused by H. pylori are one of the most common sources of gastric bleeding and can result in anemia; when infection is cleared anemia can be alleviated [63], [64]. H. pylori can subsist on iron from blood hemoglobin in vitro, adheres to erythrocytes in capillaries of the lamina propria [65], [66], and reportedly exhibits chemoattraction to human blood plasma [67]. Bloody diarrhea is typical of infections by S. Typhimurium, C. jejuni, and enterohemorrhagic E. coli (EHEC), which may provide nutrients for expansion during colitis [68]–[70]. In the following section we consider the specific chemoattractant gradients present in inflamed and injured host tissue, including blood, that may aggravate inflammatory responses and contribute to chronic inflammation.
Chemoattractant gradients in diseases of chronic inflammation
Numerous metabolites and nutrients present in necrotic tissue and blood are sensed by WHOpp chemotaxis systems as chemoattractants. We mapped results from a collection of recent studies where small molecule chemoattractants present, or enriched, at sites of host injury are sensed through specific WHOpp chemoreceptors (Fig. 4C). We restricted our analysis to the subset of purported chemoeffectors that have experimental structures determined of the protein-ligand complex or well-defined direct-sensing mechanisms. The resulting network of host-pathogen interactions, described below, suggests chemotaxis systems of WHOpp are well-poised for opportunistic nutrient piracy at sites of injury.
Amino acids are released by damaged tissue, present at high concentrations in human serum, and serve as an excellent bacterial nutrient. For example, bacterial serine deaminases convert L-serine to pyruvate, effectively generating 15 ATP equivalents in a single enzymatic step. A striking number of WHOpp chemoreceptors are dedicated to directly sensing amino acids as chemoattractants (Fig. 4C). A suite of new crystal structures of Pseudomonas chemotactic transducer (Pct) chemoreceptor proteins were captured in complex with a broad range of amino acids: PctA with Trp, Met, and Ile, PctB with Arg and Gln, PctC with γ-aminobutyric acid (GABA) [71]. A pctABC deletion mutant was unable to localize to scratch-wounded CF epithelial cells, suggesting a direct linkage between chemoattraction to amino acids and pathogenesis [37]. Structures of transducer-like protein (Tlp) 3 from C. jejuni, also known as Campylobacter chemoreceptor for multiple ligands (CcmL), show the ligand-binding domain complexed with isoleucine, alanine, valine, phenylalanine, leucine and other hydrophobic derivatives [72, p.], [73]. C. jejuni chemoreceptor Tlp10 senses aspartate and isoleucine as chemoattractants, among other ligands [74]. The Tsr and Tar chemoreceptors of Enterobacteriaceae mediate strong chemoattractant responses through direct sensing of Ser and Asp, respectively [2].
Sugars and other metabolites plentiful in human serum are perceived as chemoattractants by WHOpp species (Fig. 4C). The Enterobacteriaceae chemoreceptor Trg facilitates chemoattraction to glucose and galactose, present in the blood at 5 mM and 5 µM, respectively [75], [76]. C. jejuni Tlp11 reportedly mediates chemoattraction to galactose and has thus been renamed as Campylobacter chemoreceptor for galactose (CcrG), and is associated with invasive isolates [77], and Tlp10 is involved in chemoattraction to fucose [74]. Serum urea levels are approximately 5 mM [78], which is a key host-derived molecule H. pylori utilizes as a substrate for urease to buffer against the acidic stomach environment. H. pylori chemoreceptor TlpB directly binds urea and mediates chemoattraction to sources as low as 50 nM [34], [79], and was demonstrated as a chemoattractant for injured tissue [61]. The structure of TlpC from H. pylori was captured in complex with lactate, a molecule present at millimolar concentrations in blood [80]. There has been substantial interest in the possibility that certain neurotransmitters are sensed as chemoattractants. A structure of such a complex was recently determined of P. aeruginosa chemoreceptor PctD bound with the eukaryotic signaling molecule acetylcholine, an integral inflammation signal that recruits T-cells to sites of infection [81], [82].
RONS catalyzed by phagocytes during inflammation have now been shown to serve important roles in directing bacterial localization (Fig. 4C). Despite the ability of neutrophils and macrophages to catalyze millimolar microgradients of antimicrobial oxidants like hypochlorous acid (HOCl) and peroxynitrite (ONOO−), bacterial pathogens utilize robust antioxidant enzymes to eliminate RONS and persist [83]–[85]. These host-generated oxidants can also supply inadvertent metabolic advantages for pathogenic bacteria. HOCl and ONOO− form tetrathionate and nitrate, which facultative anaerobes possessing nitrate- and tetrathionate-reductases, respectively, can use as terminal electron acceptors to outcompete native obligate anaerobes, as exemplified by S. Typhimurium and E. coli [10], [48], [51] (Fig. 4). P. aeruginosa McpN represents the first example of a chemoreceptor sensing nitrate directly [86] (Fig. 1A, Fig. 4C).
The question of whether human pathogenic bacteria directly sense RONS as chemoeffectors, and whether that elicits chemoattraction or chemorepulsion, remains an area of active investigation [11], [50], [85], [87]–[89]. A putative HOCl-sensing chemoreceptor was first identified in H. pylori [85], and homologous proteins have been shown to be present in Salmonella, Campylobacter, Citrobacter, and other bacteria that cause disease in humans [88]. Real-time video microscopy of H. pylori shows rapid chemoattraction to HOCl sources in vitro, dependent upon chemoreceptor TlpD [85] (Fig. 4D, Movie S2). TlpD signaling is regulated through its chemoreceptor zinc-binding (CZB) domain, which mediates responses to HOCl through direct oxidation of a conserved zinc-cysteine redox switch [85], [88], [89]. The fraction of gastric glands colonized by H. pylori is decreased by about half for mice lacking phagocyte oxidase, which catalyzes O2−. and H2O2, precursors for HOCl generation by neutrophil myeloperoxidase [50]. More generally, bacteria may utilize CZBs to regulate chemotaxis based on cellular processes and stimuli that alter Zn2+ homeostasis, which could explain reports of responses to diverse stimuli such as H2O2, O2−., metals, and exogenous nutrients [88].
In summary, some intriguing evidence supports the notion chemotaxis mediates a sort of bacterial “vampirism” for advantages in late stages of infection. Spirochetes are one system in which chemoattraction responses to serum, and serum as a pathogen nutrient source, are well-documented—Borrelia burgdorferi and Treponema denticola exhibit chemoattraction to serum, and Leptospira interrogans toward hemoglobin [90], [91]. Chemoattraction to inflamed and damaged tissue, and potentially to human blood sources, could underly why certain pathogens aggregate at sites of injury and impair recovery [60], [62], [90], [91]. We note there are some components of human serum reported as chemorepellents [74], but our literature search did not reveal any examples of WHOpp chemorepulsion away from serum. Future investigations into the relationship of chemoattraction and host injury could provide important insights into the circumstances in which infections resolve or manifest into diseases of chronic inflammation (see Outstanding Questions Box).
Concluding Remarks
Is omeprazole a proof-of-concept chemotaxis-inhibiting therapeutic?
Antibacterial medicines generally act through inhibiting pathways essential for bacterial survival such as synthesis of cell walls, proteins, and nucleic acids. As discussed in the sections above, chemotaxis confers fitness advantages for bacteria within hosts, but is not essential for colonization and many pathogens lack chemotaxis systems altogether [2], [15]. Moreover, early stages of infection, when motility and chemotaxis is most utilized by pathogens, may not be a feasible window of opportunity for therapeutic intervention. So, are there clinical contexts that would justify designing new therapeutics that function through inhibiting bacterial chemotaxis? The proton pump inhibitor (PPI) drug omeprazole, a WHO Essential Medicine used in the treatment of H. pylori infection, could represent a proof-of-principle application of such a strategy (Fig. 5).
Fig. 5. Impact of omeprazole on H. pylori chemotaxis and colonization topography.
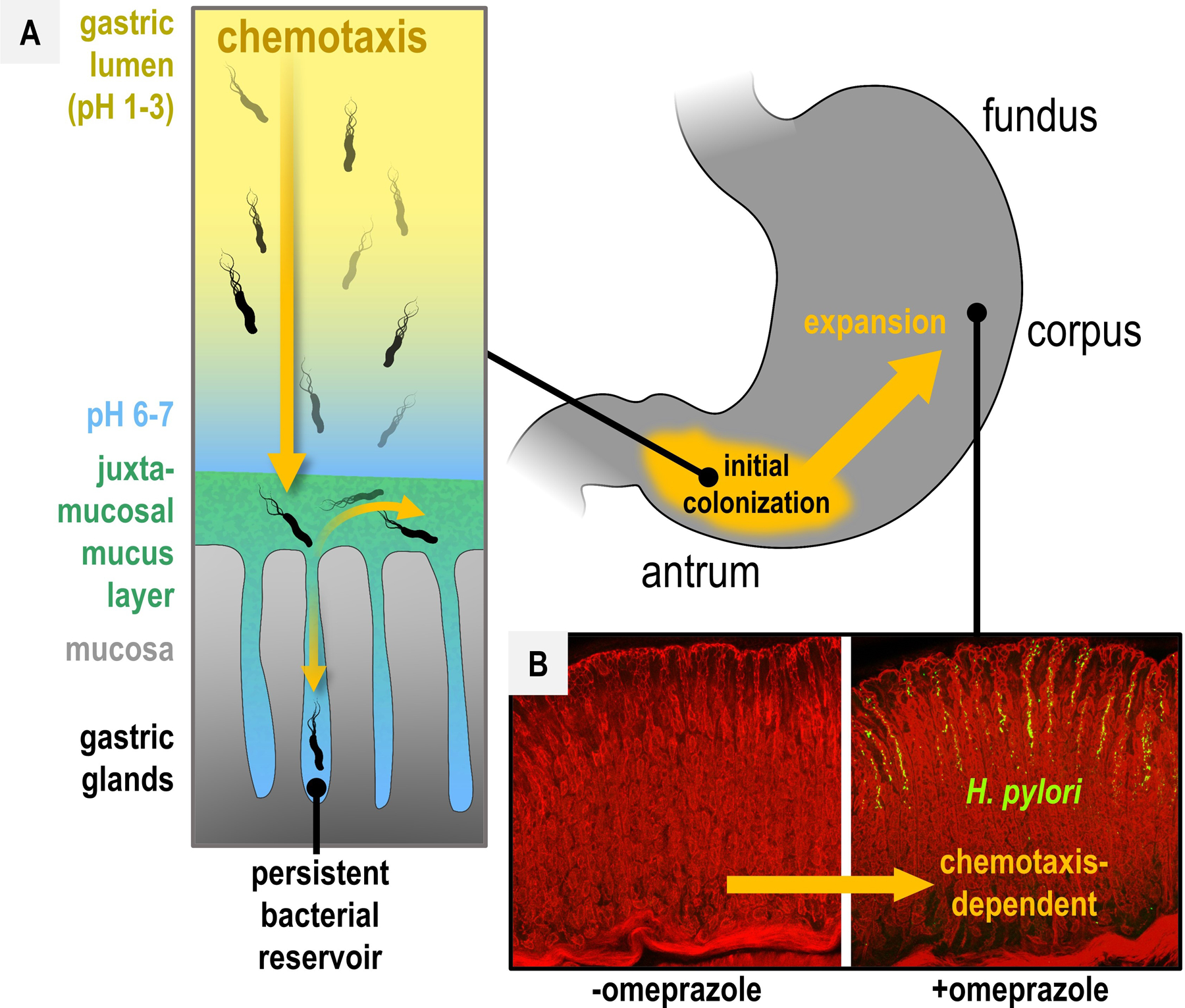
A. Chemotaxis-dependent localization is indicated by orange arrows. Chemotactic sensing of gastric pH gradients (yellow to blue) guides H. pylori to the neutral juxtamucosal mucus layer (green). Through chemotaxis, H. pylori invades gastric glands and establishes persistent bacterial reservoirs that seed expansion to new glands [26]. B. Omeprazole treatment inhibits parietal cell acid secretion in the corpus, enabling the bacteria to expand through chemotaxis to the corpus glands. Data from [94], used with permission.
Humans are one of only a few animals, and the only primates, that maintain a highly acidic stomach (pH 1.5–3.5), which is facilitated by parietal cells in the corpus gastric glands that secrete hydrochloric acid into the stomach lumen [92], [93]. The chemotaxis system of H. pylori is highly attuned to pH and multiple chemoreceptors coordinate to navigate the bacterium away from the deleterious acidic lumen toward the near-neutral mucosal lining and glands [94] (Fig. 5A). H. pylori exhibits antrum-dominant localization during initial colonization, a region of the stomach lacking parietal cells [26], [34], [94]–[96]. As infection persists, the bacterium inhibits parietal cell H+/K+ ATPase pumps that drive proton secretion through its type IV secretion system (T4SS) virulence factors, and other mechanisms, and spreads to the corpus and fundus [34], [94], [96]. Hence, the initial colonization, and subsequent expansion of H. pylori in the stomach, driven by chemotaxis, reflects avoidance of parietal cell-generated acid gradients [94] (Fig. 5).
In the 1990s the MACH1 and MACH2 clinical studies showed omeprazole boosted H. pylori eradication rates of combinatorial therapies of amoxicillin+clarithromycin from 25 to 95%, and metronidazole+clarithromycin from 72 to 91%, compared to a 1% elimination rate for omeprazole alone [97], [98]. Omeprazole upends the natural pH gradients of the stomach by inhibiting parietal cell H+/K+ ATPases, as well as sensitizes the bacteria to acid, and may increase the activity of pH-sensitive antibacterials [97]–[99]. Alongside these effects, the disrupted chemoeffector gradients induce a profound shift in the topography of H. pylori colonization, encouraging the bacteria to expand through chemotaxis to the glands and mucus of the corpus and fundus [94], [96], [100] (Fig. 5B). Some evidence links the dramatic PPI-driven disorientation of H. pylori to synergy with antibacterials [94], [101]. The inhibition of parietal cell function is short-lived and gastric pH resets within 24 hours after omeprazole treatment [101], [102]. Pulsed omeprazole administrations alone reduced bacterial load by 95% [101]. Thus, luring the bacteria to a temporarily neutral pH oasis, only to then regenerate the native bactericidal HCl gradients, may leave the bacteria stranded in an inhospitable niche and predispose them to elimination by antibacterial agents. Altered pH gradients could also encourage the bacteria to vacate sites of injury, thereby helping heal tissue.
Summary and outlook
The chemotactic portion of pathogen populations enable colonization of suitable niches early in infection and expansion to other suitable niches at later infection stages. Next-generation antimicrobials may benefit from adopting multifaceted strategies that better incorporate knowledge of the diversity of pathogen lifestyles within hosts [103]. The example of omeprazole suggests disorienting chemotactic pathogens can act synergistically with antibacterial therapies.
Supplementary Material
Glossary
- CF
cystic fibrosis, an inherited disorder affecting the lungs.
- CheA
histidine kinase chemotaxis protein A, a histidine kinase involved in relaying chemotactic signals.
- chemotaxis
directed flagellar-based motility toward and away from chemical gradients.
- chemoeffector
a chemical that elicits a chemotactic response from bacteria.
- chemoattractant
a chemical that elicits attraction mediated through chemotaxis.
- chemorepellent
a chemical that elicits repulsion mediated through chemotaxis.
- CheY
chemotaxis protein Y, the cellular phosphorylation status of CheY influences flagellar rotation.
- CZB domain
chemoreceptor zinc-binding domain, a sensory protein domain implicated in HOCl-sensing.
- RONS
reactive oxygen and nitrogen species, bactericidal oxidants catalyzed by the host immune system.
- Tlp
transducer-like protein, the historical name of chemoreceptors in some species like Helicobacter and Campylobacter.
References
- [1].Bi S and Sourjik V, “Stimulus sensing and signal processing in bacterial chemotaxis,” Curr. Opin. Microbiol, vol. 45, pp. 22–29, Oct. 2018, doi: 10.1016/j.mib.2018.02.002. [DOI] [PubMed] [Google Scholar]
- [2].Matilla MA and Krell T, “The effect of bacterial chemotaxis on host infection and pathogenicity,” FEMS Microbiol. Rev, vol. 42, no. 1, Jan. 2018, doi: 10.1093/femsre/fux052. [DOI] [PubMed] [Google Scholar]
- [3].Matilla MA, Velando F, Martín-Mora D, Monteagudo-Cascales E, and Krell T, “A catalogue of signal molecules that interact with sensor kinases, chemoreceptors and transcriptional regulators,” FEMS Microbiol. Rev, vol. 46, no. 1, p. fuab043, Jan. 2022, doi: 10.1093/femsre/fuab043. [DOI] [PubMed] [Google Scholar]
- [4].Erhardt M, “Strategies to Block Bacterial Pathogenesis by Interference with Motility and Chemotaxis,” in How to Overcome the Antibiotic Crisis : Facts, Challenges, Technologies and Future Perspectives, Stadler M and Dersch P, Eds. Cham: Springer International Publishing, 2016, pp. 185–205. doi: 10.1007/82_2016_493. [DOI] [PubMed] [Google Scholar]
- [5].Waite AJ, Frankel NW, and Emonet T, “Behavioral Variability and Phenotypic Diversity in Bacterial Chemotaxis,” Annu. Rev. Biophys, vol. 47, no. 1, pp. 595–616, May 2018, doi: 10.1146/annurev-biophys-062215-010954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Karmakar R, “State of the art of bacterial chemotaxis,” J. Basic Microbiol, vol. 61, no. 5, pp. 366–379, 2021, doi: 10.1002/jobm.202000661. [DOI] [PubMed] [Google Scholar]
- [7].Matilla MA and Krell T, “Chemoreceptor-based signal sensing,” Curr. Opin. Biotechnol, vol. 45, pp. 8–14, Jun. 2017, doi: 10.1016/j.copbio.2016.11.021. [DOI] [PubMed] [Google Scholar]
- [8].Cassidy CK et al. , “Structure and dynamics of the E. coli chemotaxis core signaling complex by cryo-electron tomography and molecular simulations,” Commun. Biol, vol. 3, no. 1, Art. no. 1, Jan. 2020, doi: 10.1038/s42003-019-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Muok AR et al. , “Atypical chemoreceptor arrays accommodate high membrane curvature,” Nat. Commun, vol. 11, no. 1, Art. no. 1, Nov. 2020, doi: 10.1038/s41467-020-19628-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rivera-Chávez F et al. , “Energy Taxis toward Host-Derived Nitrate Supports a Salmonella Pathogenicity Island 1-Independent Mechanism of Invasion,” mBio, vol. 7, no. 4, pp. e00960–16, Sep. 2016, doi: 10.1128/mBio.00960-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Behrens W et al. , “Localisation and protein-protein interactions of the Helicobacter pylori taxis sensor TlpD and their connection to metabolic functions,” Sci. Rep, vol. 6, no. 1, Art. no. 1, Apr. 2016, doi: 10.1038/srep23582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Starwalt-Lee R, El-Naggar MY, Bond DR, and Gralnick JA, “Electrolocation? The evidence for redox-mediated taxis in Shewanella oneidensis,” Mol. Microbiol, vol. 115, no. 6, pp. 1069–1079, Jun. 2021, doi: 10.1111/mmi.14647. [DOI] [PubMed] [Google Scholar]
- [13].Schweinitzer T and Josenhans C, “Bacterial energy taxis: a global strategy?,” Arch. Microbiol, vol. 192, no. 7, pp. 507–520, 2010, doi: 10.1007/s00203-010-0575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Taylor BL, Zhulin IB, and Johnson MS, “Aerotaxis and other energy-sensing behavior in bacteria,” Annu. Rev. Microbiol, vol. 53, pp. 103–128, 1999, doi: 10.1146/annurev.micro.53.1.103. [DOI] [PubMed] [Google Scholar]
- [15].Wuichet K and Zhulin IB, “Origins and Diversification of a Complex Signal Transduction System in Prokaryotes,” Sci. Signal, vol. 3, no. 128, pp. ra50–ra50, Jun. 2010, doi: 10.1126/scisignal.2000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tacconelli E et al. , “Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis,” Lancet Infect. Dis, vol. 18, no. 3, pp. 318–327, Mar. 2018, doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- [17].Gumerov VM, Ortega DR, Adebali O, Ulrich LE, and Zhulin IB, “MiST 3.0: an updated microbial signal transduction database with an emphasis on chemosensory systems,” Nucleic Acids Res, vol. 48, no. D1, pp. D459–D464, Jan. 2020, doi: 10.1093/nar/gkz988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Noto JM and Jr RMP, “The gastric microbiome, its interaction with Helicobacter pylori, and its potential role in the progression to stomach cancer,” PLOS Pathog, vol. 13, no. 10, p. e1006573, Oct. 2017, doi: 10.1371/journal.ppat.1006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chagneau CV et al. , “Uropathogenic E. coli induces DNA damage in the bladder,” PLOS Pathog, vol. 17, no. 2, p. e1009310, Feb. 2021, doi: 10.1371/journal.ppat.1009310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schultz BM et al. , “A Potential Role of Salmonella Infection in the Onset of Inflammatory Bowel Diseases,” Front. Immunol, vol. 8, p. 191, Feb. 2017, doi: 10.3389/fimmu.2017.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].He Z et al. , “Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin,” Gut, vol. 68, no. 2, pp. 289–300, Feb. 2019, doi: 10.1136/gutjnl-2018-317200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rivera-Chávez F, Lopez CA, and Bäumler AJ, “Oxygen as a driver of gut dysbiosis,” Free Radic. Biol. Med, vol. 105, pp. 93–101, Apr. 2017, doi: 10.1016/j.freeradbiomed.2016.09.022. [DOI] [PubMed] [Google Scholar]
- [23].Rogers AWL, Tsolis RM, and Bäumler AJ, “Salmonella versus the Microbiome,” Microbiol. Mol. Biol. Rev, vol. 85, no. 1, pp. e00027–19, Dec. 2020, doi: 10.1128/MMBR.00027-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Keegstra JM, Carrara F, and Stocker R, “The ecological roles of bacterial chemotaxis,” Nat. Rev. Microbiol, pp. 1–14, Mar. 2022, doi: 10.1038/s41579-022-00709-w. [DOI] [PubMed] [Google Scholar]
- [25].Keilberg D, Zavros Y, Shepherd B, Salama NR, and Ottemann KM, “Spatial and Temporal Shifts in Bacterial Biogeography and Gland Occupation during the Development of a Chronic Infection,” mBio, Oct. 2016, doi: 10.1128/mBio.01705-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fung C et al. , “High-resolution mapping reveals that microniches in the gastric glands control Helicobacter pylori colonization of the stomach,” PLOS Biol, vol. 17, no. 5, p. e3000231, May 2019, doi: 10.1371/journal.pbio.3000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ailloud F et al. , “Within-host evolution of Helicobacter pylori shaped by niche-specific adaptation, intragastric migrations and selective sweeps,” Nat. Commun, vol. 10, no. 1, Art. no. 1, May 2019, doi: 10.1038/s41467-019-10050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Faure E, Kwong K, and Nguyen D, “Pseudomonas aeruginosa in Chronic Lung Infections: How to Adapt Within the Host?,” Front. Immunol, vol. 9, 2018, Accessed: May 22, 2022. [Online]. Available: 10.3389/fimmu.2018.02416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Malhotra S, Hayes D, and Wozniak DJ, “Cystic Fibrosis and Pseudomonas aeruginosa: the Host-Microbe Interface,” Clin. Microbiol. Rev, vol. 32, no. 3, pp. e00138–18, Jun. 2019, doi: 10.1128/CMR.00138-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kalmokoff M et al. , “Proteomic Analysis of Campylobacter jejuni 11168 Biofilms Reveals a Role for the Motility Complex in Biofilm Formation,” J. Bacteriol, vol. 188, no. 12, pp. 4312–4320, Jun. 2006, doi: 10.1128/JB.01975-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dwivedi R et al. , “L-fucose influences chemotaxis and biofilm formation in Campylobacter jejuni,” Mol. Microbiol, vol. 101, no. 4, pp. 575–589, 2016, doi: 10.1111/mmi.13409. [DOI] [PubMed] [Google Scholar]
- [32].Yang J et al. , “Biphasic chemotaxis of Escherichia coli to the microbiota metabolite indole,” Proc. Natl. Acad. Sci, vol. 117, no. 11, pp. 6114–6120, Mar. 2020, doi: 10.1073/pnas.1916974117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Laganenka L, Colin R, and Sourjik V, “Chemotaxis towards autoinducer 2 mediates autoaggregation in Escherichia coli,” Nat. Commun, vol. 7, no. 1, Art. no. 1, Sep. 2016, doi: 10.1038/ncomms12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Huang JY et al. , “Chemodetection and Destruction of Host Urea Allows Helicobacter pylori to Locate the Epithelium,” Cell Host Microbe, vol. 18, no. 2, pp. 147–156, Aug. 2015, doi: 10.1016/j.chom.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Korolik V, “The role of chemotaxis during Campylobacter jejuni colonisation and pathogenesis,” Curr. Opin. Microbiol, vol. 47, pp. 32–37, Feb. 2019, doi: 10.1016/j.mib.2018.11.001. [DOI] [PubMed] [Google Scholar]
- [36].Hoffmann S, Schmidt C, Walter S, Bender JK, and Gerlach RG, “Scarless deletion of up to seven methyl-accepting chemotaxis genes with an optimized method highlights key function of CheM in Salmonella Typhimurium,” PLOS ONE, vol. 12, no. 2, p. e0172630, Feb. 2017, doi: 10.1371/journal.pone.0172630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Schwarzer C, Fischer H, and Machen TE, “Chemotaxis and Binding of Pseudomonas aeruginosa to Scratch-Wounded Human Cystic Fibrosis Airway Epithelial Cells,” PLOS ONE, vol. 11, no. 3, p. e0150109, Mar. 2016, doi: 10.1371/journal.pone.0150109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cooper KG et al. , “Regulatory protein HilD stimulates Salmonella Typhimurium invasiveness by promoting smooth swimming via the methyl-accepting chemotaxis protein McpC,” Nat. Commun, vol. 12, no. 1, Art. no. 1, Jan. 2021, doi: 10.1038/s41467-020-20558-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Garvis S et al. , “Caenorhabditis elegans Semi-Automated Liquid Screen Reveals a Specialized Role for the Chemotaxis Gene cheB2 in Pseudomonas aeruginosa Virulence,” PLOS Pathog, vol. 5, no. 8, p. e1000540, Aug. 2009, doi: 10.1371/journal.ppat.1000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Day CJ et al. , “Variation of chemosensory receptor content of Campylobacter jejuni strains and modulation of receptor gene expression under different in vivo and in vitro growth conditions,” BMC Microbiol, vol. 12, no. 1, p. 128, Jun. 2012, doi: 10.1186/1471-2180-12-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lane MC et al. , “Role of Motility in the Colonization of Uropathogenic Escherichia coli in the Urinary Tract,” Infect. Immun, vol. 73, no. 11, pp. 7644–7656, Nov. 2005, doi: 10.1128/IAI.73.11.7644-7656.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Xu G, Amicucci MJ, Cheng Z, Galermo AG, and Lebrilla CB, “Revisiting monosaccharide analysis – quantitation of a comprehensive set of monosaccharides using dynamic multiple reaction monitoring,” Analyst, vol. 143, no. 1, pp. 200–207, Dec. 2017, doi: 10.1039/C7AN01530E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ruiz-Palacios GM, Cervantes LE, Ramos P, Chavez-Munguia B, and Newburg DS, “Campylobacter jejuni binds intestinal H(O) antigen (Fuc alpha 1, 2Gal beta 1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection,” J. Biol. Chem, vol. 278, no. 16, pp. 14112–14120, Apr. 2003, doi: 10.1074/jbc.M207744200. [DOI] [PubMed] [Google Scholar]
- [44].Bian X et al. , “Campylobacter Abundance in Breastfed Infants and Identification of a New Species in the Global Enterics Multicenter Study,” mSphere, vol. 5, no. 1, pp. e00735–19, Feb. 2020, doi: 10.1128/mSphere.00735-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Elgamoudi BA et al. , “The Campylobacter jejuni chemoreceptor Tlp10 has a bimodal ligand-binding domain and specificity for multiple classes of chemoeffectors,” Sci. Signal, Jan. 2021, doi: 10.1126/scisignal.abc8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Stahl M et al. , “l-Fucose utilization provides Campylobacter jejuni with a competitive advantage,” Proc. Natl. Acad. Sci, vol. 108, no. 17, pp. 7194–7199, Apr. 2011, doi: 10.1073/pnas.1014125108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Garber JM et al. , “The gastrointestinal pathogen Campylobacter jejuni metabolizes sugars with potential help from commensal Bacteroides vulgatus,” Commun. Biol, vol. 3, no. 1, pp. 1–11, Jan. 2020, doi: 10.1038/s42003-019-0727-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Winter SE et al. , “Gut inflammation provides a respiratory electron acceptor for Salmonella,” Nature, vol. 467, no. 7314, Art. no. 7314, Sep. 2010, doi: 10.1038/nature09415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Rivera-Chávez F et al. , “Depletion of Butyrate-Producing Clostridia from the Gut Microbiota Drives an Aerobic Luminal Expansion of Salmonella,” Cell Host Microbe, vol. 19, no. 4, pp. 443–454, Apr. 2016, doi: 10.1016/j.chom.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Collins KD, Hu S, Grasberger H, Kao JY, and Ottemann KM, “Chemotaxis Allows Bacteria To Overcome Host-Generated Reactive Oxygen Species That Constrain Gland Colonization,” Infect. Immun, Mar. 2018, doi: 10.1128/IAI.00878-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Rivera-Chávez F et al. , “Salmonella uses energy taxis to benefit from intestinal inflammation,” PLoS Pathog, vol. 9, no. 4, p. e1003267, 2013, doi: 10.1371/journal.ppat.1003267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Williams SM, Chen Y-T, Andermann TM, Carter JE, McGee DJ, and Ottemann KM, “Helicobacter pylori Chemotaxis Modulates Inflammation and Bacterium-Gastric Epithelium Interactions in Infected Mice,” Infect. Immun, vol. 75, no. 8, pp. 3747–3757, Aug. 2007, doi: 10.1128/IAI.00082-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Stecher B, Hapfelmeier S, Müller C, Kremer M, Stallmach T, and Hardt W-D, “Flagella and Chemotaxis Are Required for Efficient Induction of Salmonella enterica Serovar Typhimurium Colitis in Streptomycin-Pretreated Mice,” Infect. Immun, vol. 72, no. 7, pp. 4138–4150, Jul. 2004, doi: 10.1128/IAI.72.7.4138-4150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Markazi A, Bracci PM, McGrath M, and Gao S-J, “Pseudomonas aeruginosa Stimulates Inflammation and Enhances Kaposi’s Sarcoma Herpesvirus-Induced Cell Proliferation and Cellular Transformation through both Lipopolysaccharide and Flagellin,” mBio, vol. 11, no. 6, pp. e02843–20, Dec. 2020, doi: 10.1128/mBio.02843-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hillgruber C et al. , “Blocking neutrophil diapedesis prevents hemorrhage during thrombocytopenia,” J. Exp. Med, vol. 212, no. 8, pp. 1255–1266, Jul. 2015, doi: 10.1084/jem.20142076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Faber F and Bäumler AJ, “The impact of intestinal inflammation on the nutritional environment of the gut microbiota,” Immunol. Lett, vol. 162, no. 0, pp. 48–53, Dec. 2014, doi: 10.1016/j.imlet.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Anderson CJ et al. , “Microbes exploit death-induced nutrient release by gut epithelial cells,” Nature, vol. 596, no. 7871, Art. no. 7871, Aug. 2021, doi: 10.1038/s41586-021-03785-9. [DOI] [PubMed] [Google Scholar]
- [58].Yoo W and Byndloss MX, “How to thrive in the inflamed gut,” Nat. Microbiol, vol. 5, no. 1, Art. no. 1, Jan. 2020, doi: 10.1038/s41564-019-0642-z. [DOI] [PubMed] [Google Scholar]
- [59].Zeng MY, Inohara N, and Nuñez G, “Mechanisms of inflammation-driven bacterial dysbiosis in the gut,” Mucosal Immunol, vol. 10, no. 1, Art. no. 1, Jan. 2017, doi: 10.1038/mi.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sáenz JB, Vargas N, and Mills JC, “Tropism for Spasmolytic Polypeptide-Expressing Metaplasia Allows Helicobacter pylori to Expand Its Intragastric Niche,” Gastroenterology, vol. 156, no. 1, pp. 160–174.e7, Jan. 2019, doi: 10.1053/j.gastro.2018.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hanyu H, Engevik KA, Matthis AL, Ottemann KM, Montrose MH, and Aihara E, “Helicobacter pylori Uses the TlpB Receptor To Sense Sites of Gastric Injury,” Infect. Immun, vol. 87, no. 9, pp. e00202–19, Sep. 2019, doi: 10.1128/IAI.00202-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Aihara E et al. , “Motility and Chemotaxis Mediate the Preferential Colonization of Gastric Injury Sites by Helicobacter pylori,” PLoS Pathog, vol. 10, no. 7, p. e1004275, Jul. 2014, doi: 10.1371/journal.ppat.1004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lanas A et al. , “Non-variceal upper gastrointestinal bleeding,” Nat. Rev. Dis. Primer, vol. 4, no. 1, Art. no. 1, Apr. 2018, doi: 10.1038/nrdp.2018.20. [DOI] [PubMed] [Google Scholar]
- [64].De Franceschi L, Iolascon A, Taher A, and Cappellini MD, “Clinical management of iron deficiency anemia in adults: Systemic review on advances in diagnosis and treatment,” Eur. J. Intern. Med., vol. 42, pp. 16–23, Jul. 2017, doi: 10.1016/j.ejim.2017.04.018. [DOI] [PubMed] [Google Scholar]
- [65].Aspholm M et al. , “SabA Is the H. pylori Hemagglutinin and Is Polymorphic in Binding to Sialylated Glycans,” PLoS Pathog, vol. 2, no. 10, p. e110, Oct. 2006, doi: 10.1371/journal.ppat.0020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Senkovich O, Ceaser S, McGee DJ, and Testerman TL, “Unique Host Iron Utilization Mechanisms of Helicobacter pylori Revealed with Iron-Deficient Chemically Defined Media,” Infect. Immun, vol. 78, no. 5, pp. 1841–1849, May 2010, doi: 10.1128/IAI.01258-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Worku ML, Karim QN, Spencer J, and Sidebotham RL, “Chemotactic response of Helicobacter pylori to human plasma and bile,” J. Med. Microbiol, vol. 53, no. Pt 8, pp. 807–811, Aug. 2004, doi: 10.1099/jmm.0.45636-0. [DOI] [PubMed] [Google Scholar]
- [68].Duong VT et al. , “Genomic Serotyping, Clinical Manifestations, and Antimicrobial Resistance of Nontyphoidal Salmonella Gastroenteritis in Hospitalized Children in Ho Chi Minh City, Vietnam,” J. Clin. Microbiol, vol. 58, no. 12, pp. e01465–20, Nov. 2020, doi: 10.1128/JCM.01465-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Giallourou N et al. , “A novel mouse model of Campylobacter jejuni enteropathy and diarrhea,” PLOS Pathog, vol. 14, no. 3, p. e1007083, May 2018, doi: 10.1371/journal.ppat.1007083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Eichhorn I et al. , “Highly Virulent Non-O157 Enterohemorrhagic Escherichia coli (EHEC) Serotypes Reflect Similar Phylogenetic Lineages, Providing New Insights into the Evolution of EHEC,” Appl. Environ. Microbiol, vol. 81, no. 20, pp. 7041–7047, Oct. 2015, doi: 10.1128/AEM.01921-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gavira JA et al. , “How Bacterial Chemoreceptors Evolve Novel Ligand Specificities,” mBio, vol. 11, no. 1, pp. e03066–19, doi: 10.1128/mBio.03066-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Khan MF et al. , “Structure–Activity Relationship Study Reveals the Molecular Basis for Specific Sensing of Hydrophobic Amino Acids by the Campylobacter jejuni Chemoreceptor Tlp3,” Biomolecules, vol. 10, no. 5, Art. no. 5, May 2020, doi: 10.3390/biom10050744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Liu YC, Machuca MA, Beckham SA, Gunzburg MJ, and Roujeinikova A, “Structural basis for amino-acid recognition and transmembrane signalling by tandem Per–Arnt–Sim (tandem PAS) chemoreceptor sensory domains,” Acta Crystallogr. D Biol. Crystallogr, vol. 71, no. 10, pp. 2127–2136, Oct. 2015, doi: 10.1107/S139900471501384X. [DOI] [PubMed] [Google Scholar]
- [74].Elgamoudi BA et al. , “The Campylobacter jejuni chemoreceptor Tlp10 has a bimodal ligand-binding domain that promotes host colonization,” Sci. Signal, vol. 14, no. 664, p. eabc8521, Jan. 2021, doi: 10.1126/scisignal.abc8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ning C and Segal S, “Plasma galactose and galactitol concentration in patients with galactose-1-phosphate uridyltransferase deficiency galactosemia: determination by gas chromatography/mass spectrometry,” Metabolism, vol. 49, no. 11, pp. 1460–1466, Nov. 2000, doi: 10.1053/meta.2000.9512. [DOI] [PubMed] [Google Scholar]
- [76].Vyas NK, Vyas MN, and Quiocho FA, “Sugar and Signal-Transducer Binding Sites of the Escherichia coli Galactose Chemoreceptor Protein,” Science, vol. 242, no. 4883, pp. 1290–1295, Dec. 1988, doi: 10.1126/science.3057628. [DOI] [PubMed] [Google Scholar]
- [77].Day CJ et al. , “A direct-sensing galactose chemoreceptor recently evolved in invasive strains of Campylobacter jejuni,” Nat. Commun, vol. 7, p. 13206, Oct. 2016, doi: 10.1038/ncomms13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Arihan O et al. , “Blood Urea Nitrogen (BUN) is independently associated with mortality in critically ill patients admitted to ICU,” PLOS ONE, vol. 13, no. 1, p. e0191697, Jan. 2018, doi: 10.1371/journal.pone.0191697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Goers Sweeney E et al. , “Structure and Proposed Mechanism for the pH-Sensing Helicobacter pylori Chemoreceptor TlpB,” Structure, vol. 20, no. 7, pp. 1177–1188, Jul. 2012, doi: 10.1016/j.str.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Machuca MA, Johnson KS, Liu YC, Steer DL, Ottemann KM, and Roujeinikova A, “Helicobacter pylori chemoreceptor TlpC mediates chemotaxis to lactate,” Sci. Rep, vol. 7, no. 1, Art. no. 1, Oct. 2017, doi: 10.1038/s41598-017-14372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Matilla MA et al. , “Chemotaxis of the Human Pathogen Pseudomonas aeruginosa to the Neurotransmitter Acetylcholine,” mBio, vol. 13, no. 2, pp. e03458–21, doi: 10.1128/mbio.03458-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Cox MA et al. , “Beyond neurotransmission: acetylcholine in immunity and inflammation,” J. Intern. Med, vol. 287, no. 2, pp. 120–133, 2020, doi: 10.1111/joim.13006. [DOI] [PubMed] [Google Scholar]
- [83].Perkins A, Nelson KJ, Parsonage D, Poole LB, and Karplus PA, “Peroxiredoxins: guardians against oxidative stress and modulators of peroxide signaling,” Trends Biochem. Sci, vol. 40, no. 8, pp. 435–445, Aug. 2015, doi: 10.1016/j.tibs.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Perkins A, Poole LB, and Karplus PA, “Tuning of Peroxiredoxin Catalysis for Various Physiological Roles,” Biochemistry, vol. 53, no. 49, pp. 7693–7705, Dec. 2014, doi: 10.1021/bi5013222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Perkins A, Tudorica DA, Amieva MR, Remington SJ, and Guillemin K, “Helicobacter pylori senses bleach (HOCl) as a chemoattractant using a cytosolic chemoreceptor,” PLOS Biol, vol. 17, no. 8, p. e3000395, Aug. 2019, doi: 10.1371/journal.pbio.3000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Martín-Mora D, Ortega Á, Matilla MA, Martínez-Rodríguez S, Gavira JA, and Krell T, “The Molecular Mechanism of Nitrate Chemotaxis via Direct Ligand Binding to the PilJ Domain of McpN,” mBio, vol. 10, no. 1, pp. e02334–18, Feb. 2019, doi: 10.1128/mBio.02334-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Collins KD et al. , “The Helicobacter pylori CZB Cytoplasmic Chemoreceptor TlpD Forms an Autonomous Polar Chemotaxis Signaling Complex That Mediates a Tactic Response to Oxidative Stress,” J. Bacteriol, vol. 198, no. 11, pp. 1563–1575, Jun. 2016, doi: 10.1128/JB.00071-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Perkins A et al. , “A Bacterial Inflammation Sensor Regulates c-di-GMP Signaling, Adhesion, and Biofilm Formation,” mBio, vol. 12, no. 3, pp. e00173–21, Jun. 2021, doi: 10.1128/mBio.00173-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zumwalt L, Perkins A, and Ogba OM, “Mechanism and Chemoselectivity for HOCl-Mediated Oxidation of Zinc-Bound Thiolates,” ChemPhysChem, vol. 21, no. 21, pp. 2384–2387, 2020, doi: 10.1002/cphc.202000634. [DOI] [PubMed] [Google Scholar]
- [90].Tanno-Nakanishi M, Kikuchi Y, Kokubu E, Yamada S, and Ishihara K, “Treponema denticola transcriptional profiles in serum-restricted conditions,” FEMS Microbiol. Lett, vol. 365, no. 16, p. fny171, Aug. 2018, doi: 10.1093/femsle/fny171. [DOI] [PubMed] [Google Scholar]
- [91].Lux R and Shi W, “Chemotaxis-guided Movements in Bacteria,” Crit Rev Oral Biol Med, p. 14, 2004. [DOI] [PubMed] [Google Scholar]
- [92].Dunn RR, Amato KR, Archie EA, Arandjelovic M, Crittenden AN, and Nichols LM, “The Internal, External and Extended Microbiomes of Hominins,” Front. Ecol. Evol, vol. 8, 2020, Accessed: Jun. 07, 2022. [Online]. Available: 10.3389/fevo.2020.00025 [DOI] [Google Scholar]
- [93].Beasley DE, Koltz AM, Lambert JE, Fierer N, and Dunn RR, “The Evolution of Stomach Acidity and Its Relevance to the Human Microbiome,” PLOS ONE, vol. 10, no. 7, p. e0134116, Jul. 2015, doi: 10.1371/journal.pone.0134116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Huang JY, Sweeney EG, Guillemin K, and Amieva MR, “Multiple Acid Sensors Control Helicobacter pylori Colonization of the Stomach,” PLOS Pathog, vol. 13, no. 1, p. e1006118, Jan. 2017, doi: 10.1371/journal.ppat.1006118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Sáenz JB and Mills JC, “Acid and the basis for cellular plasticity and reprogramming in gastric repair and cancer,” Nat. Rev. Gastroenterol. Hepatol, vol. 15, no. 5, Art. no. 5, May 2018, doi: 10.1038/nrgastro.2018.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Sigal M et al. , “Helicobacter pylori Activates and Expands Lgr5+ Stem Cells Through Direct Colonization of the Gastric Glands,” Gastroenterology, vol. 148, no. 7, pp. 1392–1404.e21, Jun. 2015, doi: 10.1053/j.gastro.2015.02.049. [DOI] [PubMed] [Google Scholar]
- [97].Lind T et al. , “The MACH2 study: Role of omeprazole in eradication of Helicobacter pylori with 1-week triple therapies,” Gastroenterology, vol. 116, no. 2, pp. 248–253, Feb. 1999, doi: 10.1016/S0016-5085(99)70119-8. [DOI] [PubMed] [Google Scholar]
- [98].Lind T et al. , “Eradication of Helicobacter pylori Using One-week Triple Therapies Combining Omeprazole with Two Antimicrobials: The MACH I Study,” Helicobacter, vol. 1, no. 3, pp. 138–144, 1996, doi: 10.1111/j.1523-5378.1996.tb00027.x. [DOI] [PubMed] [Google Scholar]
- [99].McGowan CC, Cover TL, and Blaser MJ, “The proton pump inhibitor omeprazole inhibits acid survival of Helicobacter pylori by a urease-independent mechanism,” Gastroenterology, vol. 107, no. 5, pp. 1573–1578, Nov. 1994, doi: 10.1016/0016-5085(94)90582-7. [DOI] [PubMed] [Google Scholar]
- [100].Logan RP, Walker MM, Misiewicz JJ, Gummett PA, Karim QN, and Baron JH, “Changes in the intragastric distribution of Helicobacter pylori during treatment with omeprazole.,” Gut, vol. 36, no. 1, pp. 12–16, Jan. 1995, doi: 10.1136/gut.36.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Azevedo-Vethacke M, Garten D, Groll C, and Schreiber S, “Specific Therapeutic Schemes of Omeprazole Affect the Orientation of Helicobacter pylori,” Antimicrob. Agents Chemother, vol. 53, no. 8, pp. 3511–3514, Aug. 2009, doi: 10.1128/AAC.00158-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Atanassoff PG, Brull SJ, Weiss BM, Landefeld K, Alon E, and Rohling R, “The Time Course of Gastric pH Changes Induced by Omeprazole and Ranitidine: A 24-Hour Dose-Response Study,” Anesth. Analg, vol. 80, no. 5, pp. 975–979, May 1995. [DOI] [PubMed] [Google Scholar]
- [103].Moradali MF, Ghods S, and Rehm BHA, “Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence,” Front. Cell. Infect. Microbiol, vol. 7, 2017, Accessed: Jun. 12, 2022. [Online]. Available: 10.3389/fcimb.2017.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Burt A et al. , “Complete structure of the chemosensory array core signalling unit in an E. coli minicell strain,” Nat. Commun, vol. 11, no. 1, Art. no. 1, Feb. 2020, doi: 10.1038/s41467-020-14350-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Murray CJ et al. , “Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis,” The Lancet, vol. 399, no. 10325, pp. 629–655, Feb. 2022, doi: 10.1016/S0140-6736(21)02724-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Mughini-Gras L et al. , “Increased colon cancer risk after severe Salmonella infection,” PLoS ONE, vol. 13, no. 1, p. e0189721, Jan. 2018, doi: 10.1371/journal.pone.0189721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


