Abstract
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been spreading globally and threatening public health. Advanced in vitro models that recapitulate the architecture and functioning of specific tissues and organs are in high demand for COVID-19-related pathology studies and drug screening. Three-dimensional (3D) in vitro cultures such as self-assembled and engineered organoid cultures surpass conventional two-dimensional (2D) cultures and animal models with respect to the increased cellular complexity, better human-relevant environment, and reduced cost, thus presenting as promising platforms for understanding viral pathogenesis and developing new therapeutics. This review highlights the recent advances in self-assembled and engineered organoid technologies that are used for COVID-19 studies. The challenges and future perspectives are also discussed.
Keywords: Lung organoid, COVID-19, self-assembled organoid, engineered organoid
1. Introduction
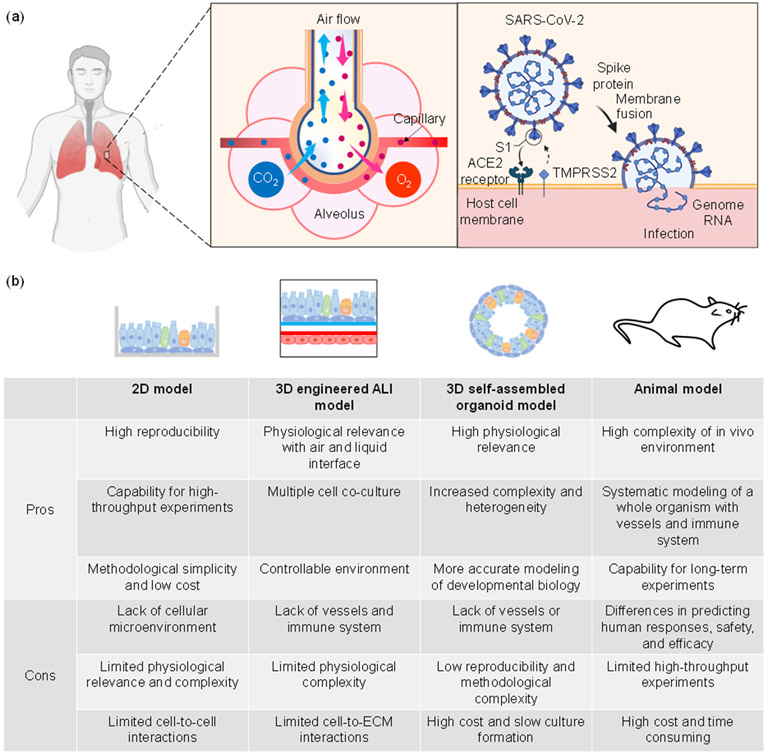
Since 2019, the coronavirus disease (COVID-19), which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, has spread rapidly around the world, affecting millions. The dominant site of infection for SARS-CoV-2 is the human lung, a major component of the respiratory system, which mediates gas exchange via the alveoli into the bloodstream. As shown in Figure 1(a), after invasion into the respiratory system, the virus attacks pulmonary cells, in particular alveolar epithelial type 2 cells (AT2), through binding of its viral spike (S) protein, which is activated by the proteolytic cleavage from the type 2 transmembrane serine protease (TMPRSS2), to the angiotensin-converting enzyme 2 (ACE2) receptor on the host cell membrane [Xu 2020]. This may result in a variety of symptoms, such as cough and fever (mild), respiratory failure, hypoxemia and pneumonia (severe), and even septic shock and organ failure (critical) [Cespedes 2020].
Figure 1.
(a) Illustration of the human alveolar structure and the SARS-CoV-2 infection process. (b) Summarized pros and cons of different models.
Given the severity of the COVID-19 pandemic, research models and platforms, from simple to advanced, are essential to enable studies of viral pathogenesis, prophylactics, and therapeutics. Two-dimensional (2D) cell culture systems are most commonly used and allow cells to grow as monolayers in flat culture flasks or Petri dishes [Kapalczynska 2018]. The simplicity and relatively modest cost have made 2D cell cultures an attractive option [Shankaran 2021]. The uniformity of 2D lung cultures has been deployed for studying cellular differentiation, tissue response, and disease modeling [Konar 2016, Shankaran 2021]. 2D culture models have been used to study the pathogenesis of COVID-19, including SARS-CoV-2 biology, replication, as well as cellular responses [Sungnak 2020, Zhu 2020a]. However, 2D cultures fail to fully recapitulate the physiologically relevant dynamics and complexity of SARS-CoV-2 infection. For example, cellular communications and outputs are not accurately represented in high-throughput screening. Thus, experiments with 2D cultures may lack translational and clinical correlations.
Animal models provide an alternative and can yield unique insight into complex biological systems, thereby elucidating viral pathogenesis and providing a way to evaluate pharmacologic responses [Amore 2010]. Fortunately, as the entire genome of the COVID-19 virus is more than 80% similar to that of the previous human SARS-like bat CoV, previously animal models used for SARS-CoV can be implemented to study the infectivity and pathogenicity of SARS-CoV-2 [Shereen 2020]. Animal models of human disease are expected to have routes of infection, disease severity, and morbidity and mortality levels similar to those observed in humans [Health 2021]. Rhesus macaques [Shan 2020], transgenic mice [Bao 2020], and ferrets [Kim 2020b] have been among the first animal models used for SARS-CoV-2 studies of infection, drug screening, and vaccine testing. Thus far, animals that are susceptible to or have been used as animal models to study SARS-CoV-2 include cats, ferrets, fruit bats, hACE2-expressing mice, hamsters, tree shrews, and nonhuman primates [Health 2021]. The types and pros/cons of various animal models have been well summarized, and these models have been further refined to explore several important aspects of COVID-19 such as the pathology of, transmission of, and host responses to SARS-CoV-2 as well as the safety and efficacy of potential therapeutics and vaccines [Cleary 2020, Munoz-Fontela 2020, Hu 2021, Shou 2021]. However, due to the genetic differences from humans, animal models are not capable of fully mimicking human physiological responses. To date, there is no perfect animal model that completely replicates the human response due to the anatomical differences between animal and human respiratory systems [Callaway 2020, Munoz-Fontela 2020].
The limitations of 2D and in vivo animal systems have motivated researchers to develop and advance three-dimensional (3D) in vitro culture systems. The use of an extracellular matrix (ECM) or scaffolding guides the formation of multiple cell types into a 3D architecture. Generally, 3D culture systems may include various biological system features such as cell-to-cell and cell-to-ECM interactions, tissue-specific stiffness, oxygen and nutrient gradients, and the cooperation of tissue-specific multiple cells, which resemble the in vivo cellular microenvironment [Griffith 2006, Langhans 2018]. With properly oriented cells, 3D models are more physiologically accurate for clinical applications. Extensive research has been performed, and biomaterials such as collagen and Matrigel have been utilized as scaffolds to successfully generate lung organoids [Ingber 2022]. 3D models can aid in the understanding of the mechanism of SARS-CoV-2 infection and promote therapeutics research. In particular, cellular self-assembled and engineered organoids have exhibited substantial benefits in viral disease studies and appear to be promising approaches for COVID-19 studies. For simplicity, such organoids are also collectively referred as lung organoid cultures. Pros and cons of various models are summarized in Figure 1(b).
While typical 3D cell culture models [Ni 2022, Plebani 2022] and various types of human stem cell-derived organoid models [Han 2022] have been reviewed for COVID-19-related applications, this report is uniquely different due to its classification of lung organoid cultures (cellular self-assembled organoids versus engineered organoids) and the articulation of state-of-the-art knowledge of the organoid culture design, cell sources, and extracellular matrix. The resulting information may help researchers across diverse disciplines communicate more effectively and create more advanced 3D organoid cultures for studying lung diseases in general. Furthermore, challenges and future perspectives are discussed in terms of the physiologically relevant complexity and functionality, advanced extracellular matrix, organoids-on-a-chip, viral infection effectiveness, and high-throughput drug screening.
2. 3D Lung organoid cultures for COVID-19-related applications
2.1. Introduction of lung organoid cultures
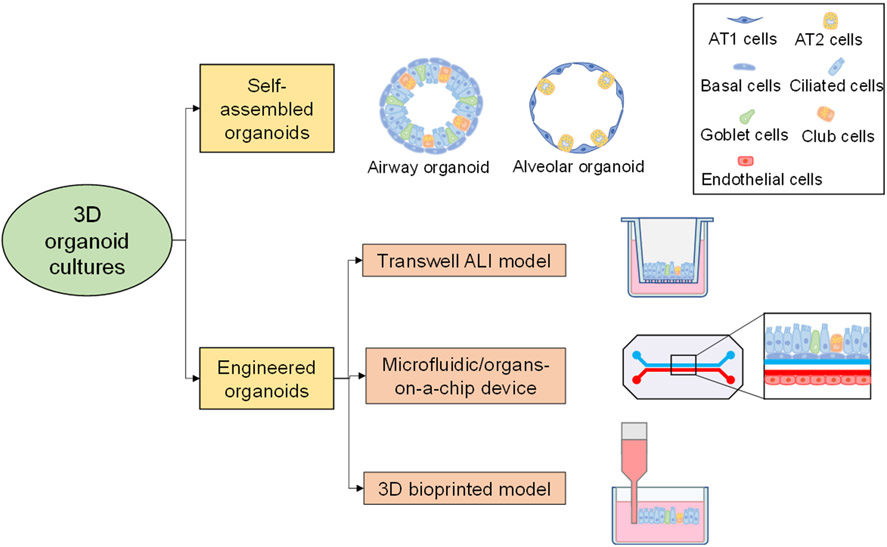
With the increasing need to screen and develop drugs to treat and prevent COVID-19, organoid cultures have been widely used as fast, efficient, and accurate systems to study the biology of SARS-CoV-2, cellular tropism, and candidate drug efficiency [Kim 2022]. Currently, refining 3D organoid culture systems is a major area of exploration. The “oid” in the term “organoid” originates from the Latin word “oides”, which means "to resemble." In general, a traditional organoid is defined as a 3D self-assembling organ-like miniature structure that grows usually from stem or progenitor cells and into an in vitro multi-cellular model with organ-specific cell types, structures, and functions. While having the same application objective, engineering strategies such as the organ-on-a-chip approach create multi-cellular 3D in vitro models directly without relying on cellular self-assembly. Herein, organoid cultures are categorized into cellular self-assembled or engineered organoids as shown in Figure 2. The term “self-assembled organoid” is used for the traditional organoid to distinguish it from other engineering efforts in making organoid cultures/models or mimics for COVID-19-related applications.
Figure 2.
Classification of typical 3D lung organoid cultures.
Self-assembled organoid cultures are 3D in vitro organ miniatures that are formed by multicellular self-organization, which derives from the characteristic proliferation and/or differentiation of human cells, mostly human stem cells [Kim 2020a, Hofer 2021]. Since fully mimicking the biochemical environment of in vivo systems is complex and challenging, organoid cultures are simplified models that accurately recapitulate the architecture and physiology of specific organs [Xu 2018]. Since the first development of organoids in 2009 [Sato 2009], organoids have been cultured to mimic a multitude of organs including the lung, and the future holds promise that any organ can be successfully cultured in vitro [Rauth 2021].
In addition to self-assembled organoids, engineered in vitro organoid models such as Transwell air-liquid interface (ALI) models, microfluidic/organ-on-a-chip models, and 3D-printed models have also been developed for lung-related studies [Baldassi 2021, da Silva da Costa 2021, Kabir 2021, Wang 2022]. Elaborate designs of lung-on-a-chip models have been reported in the last few years that successfully reconstituted the periodic breathing activity of the living lung and its microstructure as well as the dynamic microenvironment of the alveolar-capillary unit [Huh 2010, Stucki 2015, Khalid 2019, Shrestha 2019]. As a response to the serious global epidemic of COVID-19, microfluidic lung chips have been reported to model organ-level lung infection and immune responses [Zhang 2020, Huang 2021, Si 2021, Hashimoto 2022]. Compared to animal models, one of the advantages of such in vitro models is that a specific factor can be separated from the complex in vivo environment to clarify its single contribution.
Thus far, cellular self-assembled and engineered organoid culture models are deemed the most promising models for COVID-19 and future similar applications, yielding valuable information about the kinetics, pathogenesis, and host responses. Creating models that most accurately mimic the in vivo environment is valuable as these models would surpass the utility of traditional 2D cell cultures and animal models.
2.2. Requirements of a physiologically relevant COVID-19 lung organoid culture
Depending on the region of the lung to be modeled, lung organoids can be designed in various ways, including both proximal and distal components. The starting cell type and culture microenvironment used to support proliferation and differentiation determine the eventual application of a lung organoid [Kong 2021]. To successfully recapitulate the physiological environment and simulate the functions of the human lung for COVID-19 research applications, certain physiological, cellular, and/or molecular features are required.
Descending into the trachea, which branches into the left and right bronchi, and down to the alveoli, the smallest component of the respiratory tract, the cellular compositions of these lung areas are distinct. The airways are composed of basal, club, ciliated, and goblet epithelial cells, while the alveoli contain type 1 (AT1) and type 2 (AT2) epithelial cells. The AT1 cells provide the surface area for the sacs to expand, while the AT2 cells secrete surfactants and proteins to lower surface tension and prevent alveolar collapse. The type of lung organoids created is thus dependent on the targeted location to be modeled. Each lung organoid models either a section of the conducting zone or the respiratory zone. Epithelial cells in both regions are the primary targets of SARS-CoV-19. The conducting zone includes all structures that form a passageway for the transport of gas, while the respiratory zone includes all structures that participate in gas exchange.
An ideal lung model used to study SARS-CoV-2 pathogenesis and evaluate candidate therapeutics is expected to be able to physiologically mimic the viral infection and replication processes. With regards to the choice of cell types, human stem cells such as human pluripotent stem cells (hPSCs) and adult stem cells (ASCs) are promising due to their unlimited proliferative capacity, their potential to yield all cell types, and their ability to be genetically modified using clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 editing [Kim 2022]. Aside from stem cells, immortalized human cell lines can also be used, such as BEAS-2B cells [Reddel 1988] or human bronchial epithelial cell lines (HBECs [Ramirez 2004]) to model the airway epithelium and immortalized human alveolar epithelial cell lines to model the alveolar epithelium [Tran 2022b]. Furthermore, epithelial cells can be combined with fibroblasts and/or endothelial cells to model mesenchyme and blood vessels. A potential benefit of using cell lines representing individual cell types is that each cell type could be individually genetically modified, facilitating studies of individual components. Since it has been demonstrated that ACE2 receptor and TMPRSS2 are required for SARS‑CoV-2 binding and successful replication following invasion into the host cells [Woodall 2021], a physiologically relevant lung organoid culture suitable for COVID-19 research should contain epithelial cells expressing the ACE2 receptor as well as TMPRSS2.
Thus, ideal lung organoids are expected to include certain epithelial cells (cellular requirements), have an ALI for gas exchange (physical requirements), and express ACE2 and TMPRSS2 (molecular requirements) that allow the infection of SARS-CoV-2. With these requirements in mind, organoid cultures are expected to lead to an improved understanding of the underlying mechanisms of SARS-CoV-2 infection and treatment.
3. Development of lung organoid cultures
3.1. Self-assembled lung organoids
To date, the most significant and widely studied lung organoids constructed for COVID-19 research include four main types: airway, alveolar, bronchial, and bronchioalveolar organoids, although the invasion of SARS-CoV-2 virus to other organs has also been modeled using organoids such as intestinal, kidney, liver, and brain organoids [Ramezankhani 2022]. Per the scope of the review, only the progress of airway, alveolar, bronchial, and bronchioalveolar organoids is discussed herein. Regarding the evaluation methods, immunofluorescence and scanning electron microscopes (SEM) can be used to study the cytopathic effects, whole-genome sequencing and real-time quantitative polymerase chain reaction (RT-qPCR) can determine viral replication kinetics and genetic alterations, transcriptomic profiling can reveal the differential expression of genes related to viral infection, and flow cytometry can help detect and quantify the cells before and after SARS-CoV-2 infection [Elbadawi 2020].
3.1.1. Self-assembled organoid cultures
3.1.1.1. Airway and bronchial organoids
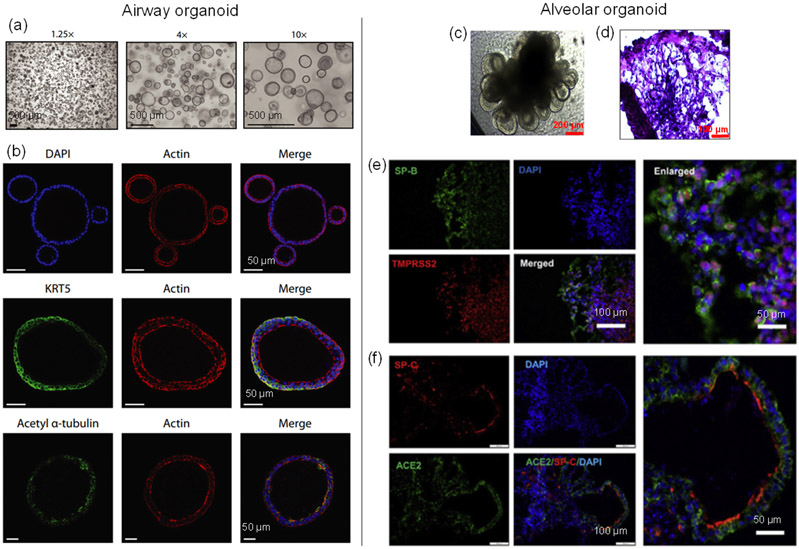
Airway organoids are differentiated 3D epithelial structures that mimic the cellular environment and organization of the human lung upper airways, and can serve as a useful tool for studying human lung diseases and COVID-19 [van der Vaart 2021a, van der Vaart 2021b]. The airway epithelium, containing mainly goblet, basal, club, and ciliated cells, is the first line of defense against airborne pathogens and protects the alveoli, which provides a vulnerable surface area about the size of half a tennis court in an adult human [Haefeli-Bleuer 1988, Ochs 2004]. Other cell types, including pulmonary neuroendocrine cells and ionocytes, exist in this region as well, but they are rare. Airway organoids derived from human lung tissues could morphologically and functionally simulate the human airway epithelium, serving to assess the infectivity of emerging influenza viruses [Zhou 2018]. For typical airway organoids, basal cells were on the outer layer of the organoids while the multi-ciliated cells were found on the luminal side (Figure 3 (a-b)) [van der Vaart 2021a]. Since the onset of the COVID-19 pandemic, airway organoid models infected with SARS-CoV-2 have been used to study the viral replication kinetics, tropism, and host response [Elbadawi 2020]. For example, airway organoids, which were generated from human embryonic stem cells (hESCs), contained basal cells, ciliated cells, club cells, and goblet cells, and the cellular constituents of ciliated and club cells were susceptible to SARS-CoV-2 infection [Pei 2021]. Using this model, the anti-viral performance of drugs, such as remdesivir, was evaluated after the infection, demonstrating its potential as a drug screening platform. Moreover, airway organoid models are also expected to be used to study the immunological responses to SARS-CoV-2 when co-cultured with different immune cells [Elbadawi 2020].
Figure 3.
Lung organoid cultures. Left column: airway organoid [van der Vaart 2021a]. (a) Brightfield images of airway organoids. (b) Confocal images showing Cytokeratin 5 (KRT5, basal cell marker) and Acetyl α-tubulin (ciliated cell marker) of the organoid. Right column: alveolar organoid [Tiwari 2021]. (c) Phase-contrast image of lung alveolar organoids. (d) Hematoxylin & eosin (H&E) staining of alveolar-like morphology. (e-f) Confocal images showing AT2 cells ((e) SP-B and (f) SP-C) co-labeled with (e) TMPRSS2 and (f) ACE2.
As severe acute bronchopneumonia is frequently observed in COVID-19 patients, bronchial organoids, mimicking the small airways of the respiratory tract, have been used to clarify the mechanism by which the bronchial epithelial layer is destroyed [Deguchi 2021, Sano 2022]. To reproduce the infection of SARS-CoV-2 in the bronchi, Sano et al. [Sano 2022] used cryopreserved normal human bronchial epithelial cells (NHBEs) to generate human bronchial organoids composed of basal, ciliated, goblet, and club cells, and found that ciliated cells were infected with the virus and died, while the basal cells survived after viral infection and differentiated into ciliated cells, possibly under the regulation of fibroblast growth factor 10 (FGF10) signaling. With the help of the infected and uninfected bronchial organoid models generated from primary human bronchial epithelial cells (hBEpCs), Fang et al. [Fang 2021] investigated targeting colony-stimulating factor 3 (CSF3), which was identified as an upregulated gene, as a potential therapeutic approach.
3.1.1.2. Alveolar organoids
Alveolar organoids aim to model the alveoli, the small sacs that terminate the branching of the bronchioles. They have also been applied to elucidate the pathogenesis of human lung failure caused by the SARS-CoV-2 virus. Alveolar organoids are dramatically different in cellular composition and function when compared to airway organoids. In alveoli, cuboidal AT2 cells account for about half of the alveolar epithelial cells and function in secreting pulmonary surfactant, while flat delicate AT1 cells cover most of the alveolar surface, mediating gas exchange to adjacent capillary vessels, and serve as an important component of the blood-air barrier [Yamamoto 2020]. Alveolar organoids are composed of AT1 and AT2 cells and may also contain mesenchymal cells. Pei et al. [Pei 2021] derived human alveolar organoids that contained AT1 and AT2 cells from hESCs, and found that SARS-CoV-2 infected the AT2 cells due to their highly expressed ACE2 receptor and TMPRSS2 serine protease. Utilizing hPSCs to create an alveolar organoid model, Han et al. [Han 2020] found that the inflammatory changes seen in human COVID-19 infection were mimicked by the upregulation of cytokine/chemokine signaling, and the treatment of identified drugs significantly inhibited the SARS-CoV-2 infection. Similarly, Tiwari et al. [Tiwari 2021] generated human induced pluripotent stem cell (iPSC)-derived lung organoids (Figure 3 (c-d)), and the results showed that they were permissive to SARS-CoV-2 infection due to the highly expressed TMPRSS2 (Figure 3(e)) and ACE2 (Figure 3 (f)). These studies have verified the fidelity of the alveolar organoid disease models to investigate the human lung pathophysiology of SARS-CoV-2 infection as well as screen new drugs.
3.1.1.3. Bronchioalveolar organoids
Although bronchioalveolar organoids are less common, they are significant in providing models for cellular tropism analysis and treatment testing. The bronchioalveolar organoid models the alveoli and the respiratory bronchioles that are conjoined at the bronchioalveolar duct junction. Lamers et al. [Lamers 2021] developed a bronchioalveolar-like model from human small airway stem cell-derived lung bud tip progenitor organoids, and the bronchioalveolar organoids were composed of both alveolar-like and bronchiolar-like cells, merging the alveolar organoid and bronchiolar organoid models to resemble the bronchoalveolar duct junction. The cells present in this region included AT1 and AT2 cells, club cells, ciliated cells, basal cells, and neuroendocrine cells, and the results showed that SARS-CoV-2 accurately replicated within the organoids, proving the model to be an ideal model for virus infection and drug screening.
3.1.2. Typical cell sources and extracellular matrix
Various cells and ECMs have been utilized for the development of lung organoid cultures, and they are summarized in Table 1 and further discussed as follows.
Table 1.
Typical cell sources and ECMs used for establishing human lung organoid cultures.
| Human lung organoid |
Cell source | ECM | Reference |
|---|---|---|---|
| Airway and bronchial organoid | Solid human lung tissue | Matrigel | [Zhou 2018, Sachs 2019] |
| Human lung fibroblasts (HLF) | Matrigel | [Tan 2017] | |
| Human lung microvascular lung endothelial cells (HMVEC-L) | |||
| Human bronchial epithelial cells (NHBEs) | |||
| NHBEs | Matrigel | [Sano 2022] | |
| Human bronchial epithelial cells (hBEpCs) | N.A. | [Fang 2021] | |
| Alveolar organoid | Human pluripotent stem cells (hPSCs) | Matrigel | [Han 2021, Han 2022] |
| Induced pluripotent stem cells (iPSCs) | Matrigel | [Tiwari 2021] | |
| Immortalized human alveolar epithelial cells | Matrigel | [Tran 2022b] | |
| Bronchioalveolar organoid | Human small airway stem cells | Matrigel, collagen I | [Lamers 2021] |
3.1.2.1. Cell Sources
It is evident that lung organoids yield very useful models for COVID-19 research, providing a greater understanding of pathogenesis and effective therapies. The replication of both the histology and function of the lungs allows for an in-depth examination of COVID-19 kinetics, tropism, and the host responses [Wang 2022]. Current lung organoid cultures are usually derived from hPSCs or ASCs with inductive factors and a supportive 3D environment. The most common starting cell type to encourage proliferation and differentiation into a lung organoid are hPSCs, including induced pluripotent stem cells (iPSCs), and embryonic stem cells (ESCs) [Kong 2021]. hPSCs have been designated as the most promising due to the limited availability of primary human lung samples and because they possess cell types that are found in the organ environment [Tian 2021]. To regenerate lung models, multiple types of cells must be differentiated to replicate the cellular architecture and function. Chen et al. [Chen 2017] generated lung bud organoids using hPSCs over a 20-day period that developed into branching airways and early alveolar structures. Since the emergence of the pandemic, iPSCs and hESCs have been used to derive human lung organoids for COVID-19 infection and drug testing studies [Pei 2021, Tiwari 2021].
Cells from adult tissues can also be used to prepare organoids. For example, Tan et al. [Tan 2017] incorporated normal human bronchial epithelial cells (NHBEs), lung fibroblasts, and lung microvascular endothelial cells into a 3D multicellular environment to generate airway organoids, and found that the randomly mixed cell populations self-organized into discrete epithelial and endothelial structures. Sachs et al. [Sachs 2019] reported a versatile approach to establishing adult human airway epithelial organoids, containing basal cells, functional multi-ciliated cells, mucus-producing secretory cells, and CC10-secreting club cells from patient materials (such as lavages, biopsies, and resections). Human lung epithelial cells such as NHBEs [Sano 2022] and primary human bronchial epithelial cells (hBEpCs) [Fang 2021] were successfully used for COVID-19 studies.
3.1.2.2. Extra cellular matrix
The ECM is a 3D network that provides a cellular scaffold that helps cells to attach and supports signaling among different cell types. Due to the complex function of native ECM, a model capable of fostering long-term expansion of basal cells while maintaining their phenotypic stability has not yet been developed. The composition of ECM changes throughout the lung development phase, ranging from fetal to neonatal, and finally adult tissue, which makes the replication of ECM extremely difficult [Busch 2021]. Currently, natural matrices, such as Matrigel, fibrin, and collagen, in which cells can be successfully embedded, have been explored as the culturing environment and altered to mimic characteristics of native ECM. Of different reagents, thus far, Matrigel is the most widely used ECM material for organoid cultures [Chen 2017, de Carvalho 2019]. However, more studies are still needed to form a more accurate understanding of how a specific ECM affects and facilitates cellular differentiation.
3.2. Engineered lung organoids
3.2.1. Transwell ALI models
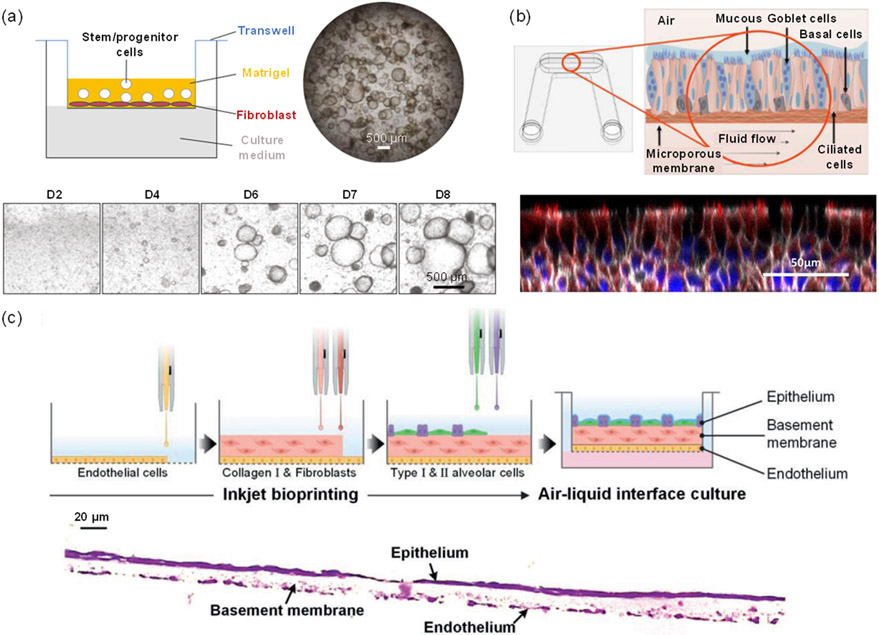
As COVID-19 emerged, self-assembled organoids were widely grown in a submerged Transwell ALI setting, which encourages the proliferation and differentiation of stem cells, followed by the formation of a biomimetic ALI structure [Lu 2020, Lukassen 2020, Sungnak 2020, Zhu 2020a, Zhu 2020b]. In particular, 3D ALI models allow for cellular interactions on a semi-dry cell surface, consisting of an apical surface exposed to air and a basal surface submerged in a liquid culture medium (Figure 4 (a)) [Li 2020]. As opposed to traditional fully submerged cell cultures, ALI cultures directly mimic the in vivo structure of the respiratory tract [Zscheppang 2018] [Gard 2021]. The ALI culture is composed of cells that are more irregular in shape with rougher surfaces and an abundance of secretions, which are superior to the smooth and less matured cells in the submerged culture [Wu 2017]. In addition, multicellular ALI models that are composed of more than one cell type can better resemble the cellular arrangement in vivo than monoculture, and they were used to study disorders of the respiratory tract in response to viral infection [Baldassi 2021].
Figure 4.
Approaches for lung organoid engineering. (a) (Upper) Schematic of an ALI organoid culture loaded into a Transwell filter insert and (lower) images of the organoids over an 8-day period after seeding [Li 2020]. (b) (Upper) Schematic of an ALI microfluidic platform including differentiated cell populations and basolateral fluid flow and (lower) a confocal image of the pseudostratified epithelium established on an ALI [Gard 2021]. (c) (Upper) Schematic of inkjet-printing process for fabrication of a 3D alveolar barrier model and (lower) a H&E-stained cross-sectional image of alveolar barrier model showing the epithelium, basement membrane, and endothelium layers [Kang 2021].
Overall, ALI models, either as 2D or 3D constructs, can serve to study the cell biology of the respiratory system, model respiratory diseases and infections, and be used for drug discovery. They have also been used for COVID-19 studies. Sano et al. [Sano 2022] compared the SARS-CoV-2 infection efficiency between the spherical bronchial organoid from NHBEs and the suspended organoid in ALI culture and found that the ALI model was much more efficiently infected due to viral infection from the luminal side. Sasaki et al. [Sasaki 2021] reported that A549 alveolar epithelial cells in ALI culture, as opposed to those in conventional submerged culture, intriguingly became susceptible to SARS-CoV-2 infection due to the increased expression of ACE2 and TMPRSS2.
3.2.2. Lung-on-a-chip models
The development of microfluidic or organ-on-a-chip devices can better provide organ-level functionalities required to serve as a disease model and maintain differentiation and tissue-specific functions. Organ-on-a-chip devices are usually microfluidic-based, allowing continuous perfusion of medium and/or air to recirculate nutrients, remove waste products, and oxygenate the media. Lung-on-a-chip integrates multiple physiological components of in vitro system, such as the ALI structure and flow condition, making their replication of microenvironment physiologically relevant. Huh et al. [Huh 2010] developed a multifunctional microdevice that reproduces key structural, functional, and biomechanical properties of the human lung alveolar-capillary interface, which is the fundamental functional unit of a living lung. Their micro-engineered lung-on-a-chip containing channels that were lined by mammalian lung epithelial and endothelial cells exposed to air, fluid flow, and cyclic mechanical strain, mimicking normal breathing motions. The microdevice uniquely recreated the physiological breathing movements seen in the human lung while providing oxygenated air to the cells as well as causing a physical stretch of the membrane. Results demonstrated that cyclic mechanical strain accentuated toxic and inflammatory responses of the lung to silica nanoparticles as well as epithelial and endothelial uptake of nanoparticulates, and it reproduced complex integrated organ-level responses to bacteria and inflammatory cytokines introduced into the alveolar space, which led to the discovery of new therapeutic agents.
Lung-on-a-chip models have had extraordinary implications in COVID-19 research regarding pathogenesis and the development of effective therapies [Bates 2021, Wang 2022]. Generally, the microfluidic aspect fosters a multicellular culture in an environment more accurately resembling in vivo functions and interactions with surrounding systems. These advantages create the opportunity to analyze SARS-CoV-2 viruses and pathological interactions with existing chronic respiratory diseases in a host. Si et al. [Si 2021] developed an airway chip using primary human lung bronchial-airway basal stem cells cultured on one side of the membrane (namely ‘airway channel’) and a primary human lung endothelium cultured on the other side (namely ‘vascular channel’), which was exposed to a continuous medium flow. They found that the antiviral performance of drugs tested on the chips infected with pseudo-typed SARS-CoV-2 virus appeared more clinically relevant than when cells were cultured under static conditions. Gard et al. [Gard 2021] fabricated a microfluidic ALI culture platform by culturing NHBEs (Figure 4(b)) to evaluate viral infection kinetics and antiviral agent dosing and were able to demonstrate the infection of SARS-CoV-2 into the airway epithelial cells of the model via the ACE2 receptor and TMPRSS2.
3.2.3. 3D-printed models
In recent years, 3D bioprinting has been deemed one of the most promising technologies for tissue engineering [Huang 2015, Huang 2018]. In 3D bioprinting, biological materials and living cells can be printed layer-by-layer to create biomedical models. Cells are usually suspended in a hydrogel solution with a required number and density to form bioinks for 3D printing, and certain rheology properties of bioinks are required to enable the printing and gelation [Zhang 2018, Song 2021, Ren 2022, Wu 2022]. This type of mechanical assembly creates a precise and consistent spatial pattern within a 3D culture environment. Thus, bioprinting organoids significantly eliminates variation across culture batches, which is a factor present when constructing organoid cultures using conventional production methods [Parihar 2022]. Furthermore, bioprinting bridges the gap between the importance of cellular self-organization within cultures and the challenge of a culture self-assembling into a large and controlled tissue unit. Allowing the cells to naturally self-organize and facilitating the process through bioprinting techniques encourages faster and more complex organoid formation [Ren 2021].
Brassard et al. [Brassard 2021] used organoids as building blocks to 3D print certain patterns within a highly permissive ECM, demonstrating the successful integration of bioprinting and organoid technology. The pattern geometry and cellular composition could be easily controlled by the extrusion printing technique. After cultivation, the printed organoids showed self-organized features such as lumens, branched vasculature, and tubular intestinal epithelia, indicating that the printed patterns could facilitate effective multicellular self-organization and tissue morphogenesis. Kang et al. [Kang 2021] used a high-resolution inkjet printing technique to fabricate a three-layered 3D alveolar barrier model by the sequential deposition of four types of alveolar cells: AT1 and AT2 epithelial cells, fibroblasts, and lung microvascular endothelial cells (Figure 4(c)). Functional and structural characteristics of the 3D model were evaluated, and the results indicated that due to the multi-layered structure, this 3D-printed alveolar barrier model showed better lung tissue morphologies and functions than conventional 2D culture models or 3D non-structured models. The utilization of 3D bioprinting to produce lung organoid cultures provides an alternative tool to COVID-19 infection studies and drug discovery applications [Kabir 2021].
4. Lung organoid cultures for COVID-19 applications
4.1. Viral infection applications
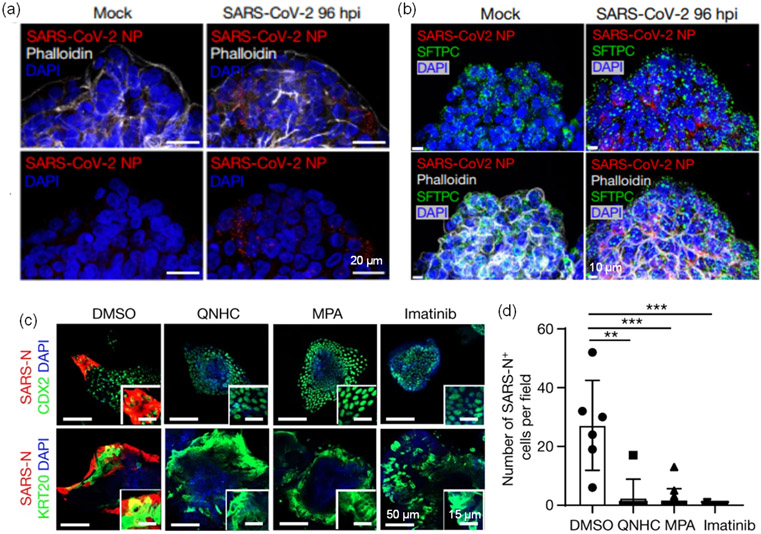
Early in the pandemic, researchers successfully isolated the SARS-CoV-2 virus and investigated its transmission, morphogenesis, and pathogenesis using standard immortalized cells, such as Vero E6 and Huh7 cells [Lu 2020, Zhu 2020b]. However, these cells do not express the main receptors of the virus, ACE2 and TMPRSS2. Alternatively, Zhu et al. [Zhu 2020a] used human airway epithelial cells (HAECs) to develop an in vitro infection model. Do et al. [Do 2021] grew human tracheal airway epithelial cells (HtAEC) and human small airway epithelial cells (HsAEC) in an ALI modeled system where the apical side was infected with SARS-CoV-2. Human lung organoids, as well as other organoid types with highly expressed ACE2 and TMPRSS2 proteins, have been used for SARS-CoV-2 infection studies, and the infection efficiencies have been characterized [Elbadawi 2020, Zhao 2020, Tiwari 2021, Zhao 2021, Han 2022, Tran 2022a]. Expression of SARS-CoV-2 nucleocapsid protein (NP) was found in the infected basal organoid (Figure 5a) and AT2 organoid (Figure 5b) after 96 h [Salahudeen 2020]. It is noted that even though these epithelial cells are permissive to a variety of respiratory viral infections, the variety of cells for the models is still limited and assembling all relevant cells of the respiratory tract (such as ciliated, goblet, and basal cells) into a model is still challenging.
Figure 5.
Lung organoids that used for infection and drug discovery applications. (a-b) Lung organoids with SARS-CoV-2 infection and (c-d) drug treatment. (a) SARS-CoV-2 nucleocapsid protein (NP) appeared in basal organoids and (b) apical-out AT2 organoids at 96 h post infection [Salahudeen 2020]. (c-d) QNHC, MPA, and imatinib can inhibit the SARS-COV-2 infection [Han 2021].
4.2. Drug discovery and screening applications
Lung organoid cultures infected with SARS-CoV-2 can help identify potential therapeutic drugs against COVID-19 [Han 2020, Duarte 2021]. After the lung organoids are infected with SARS-CoV-2 virus or pseudovirus, drug candidates can be applied to the infected organoids to evaluate the drug efficacy. One strategy to inhibit viral entry into host cells is to interfere with the binding of virus spike protein and host cell receptors. Tiwari et al. [Tiwari 2021] reported that AEC2 and TMPRSS2 inhibitors could block viral entry into lung organoids. Spitalieri et al. [Spitalieri 2022] proposed a neutralizing antibody and a synthetic peptide that can bind directly to the spike protein as two potential approaches, and the results showed that the infection efficacy in the treated cells was highly decreased. Han et al. [Han 2021] used a lung organoid culture derived from human pluripotent stem cells as a high-throughput screen platform to identify entry inhibitors of SARS-COV-2, and treatment with identified drugs including quinacrine dihydrochloride (QNHC), mycophenolic (MPA), and imatinib could significantly inhibit the SARS-COV-2 infection (Figure 5(c-d)). Using the same lung organoid culture [Han 2021], Duarte et al. [Duarte 2021] identified atorvastatin from thousands of FDA-approved drugs that blocked viral entry into the lung organoid. In search of therapeutic options, researchers have proposed antiviral resveratrol [Ramdani 2020, Ter Ellen 2021, van Brummelen 2022] as an advantageous antiviral therapy for SARS-CoV-2 infection. Ellen et al. [Ter Ellen 2021] provided evidence that resveratrol and pterostilbene exhibited a strong antiviral effect for ALI cultured human primary bronchial epithelial cells up to 48 hours post-infection.
5. Challenges and future perspectives
5.1. Physiologically relevant complexity and functionality
While mimicking the whole human lung and communications between different sections in an organoid is challenging, the improvement of the physiological relevance in regard to phenotypes and functionalities of organoid cultures still benefits the model validity. Engineering a specialized lung hydrogel scaffold to guide the cells into appropriate complex branching structures merits further exploration [Vazquez-Armendariz 2021]. The lack of physiologically relevant representation of typical lung microenvironment poses a technical drawback that may alter the host response observed in vivo after COVID-19 infection. Appropriate modeling of the roles that the immune system holds in the lungs can certainly enhance the understanding of important events taking place after viral infection, and this may be achieved by co-culturing lung organoid cultures with immune cells and stromal cells, which reside in the peribronchovascular interstitium and interact with alveoli in vivo. Vascularization is another crucial physiological process in vivo that has been largely overlooked in organoid developments thus far [Teuwen 2020, Lampejo 2022, Ren 2023]. Establishing a vascularized lung organoid culture to facilitate ALI morphogenesis and cellular interactions would be promising. Moreover, inadequate standardization of protocols, which may result in abnormal self-assembled architectures with poor reproducibility, is one of the key challenges.
5.2. Advanced extracellular matrix
Optimized ECMs allowing dynamic changes in biophysical, chemical, and mechanical properties are also needed to recapitulate the in vivo environment. Matrigel, the most commonly utilized ECM to develop organoids, is isotropic and chemically undefined [Li 2019]. It might not be able to adequately simulate dynamic environmental changes with respect to the time and stage of cellular development. In addition, xenobiotic factors within Matrigel might have unanticipated effects on the cellular responses and chemical screening results, which could pose a concern when translating the basic research findings to clinical applications. As such, effective substitutes for Matrigel are needed.
5.3. Organoids-on-a-chip
Despite the current progress, advanced microfluidic organoid cultures for enhanced organoid maturation and branching morphogenesis need to be further established. Integrating a system where the fluidic networks replicate the natural flow conditions of blood in the surrounding capillaries as well as including a pliable membrane to imitate the fluctuations of alveoli would ensure that mechanical forces in vitro are appropriately generated. Additionally, incorporating more flow channels to resemble different components of the lung may be beneficial to mimic the in vivo structure. Furthermore, investigation of the integration of microfluidics with self-assembled organoids to enable the fabrication of organoids-on-a-chip appears highly promising [Homan 2019].
5.4. Viral infection effectiveness
Although organoid cultures serve as promising models to mimic lung functions and investigate the mechanism of infection, there are many challenges in perfecting them. Due to the variance among labs and protocols, the infection results and efficiency may be inconsistent. Improvements in infection efficiency in organoid models should be made for further use and discoveries. Another major challenge in the production of an effective platform is that it should not only recapitulate the pathogenesis, but also allow the study of various aspects of the toxicology, immunology, and prophylactic and therapeutic approaches. A single platform might not meet all these requirements because of the complicated crosstalk between the host and the virus [Ramezankhani 2022], so a systematic approach is needed for organoid culture development. Additionally, since organoids can be generated from ethnically/racially diverse patients with different health backgrounds, an understanding of disparities in COVID-19 infection is expected.
5.5. High-throughput drug screening
Drug screening on a large scale remains a challenge for organoid technology. While organoids have been successfully used for drug discovery [Han 2021, Hashimoto 2023], the anti-viral response and cell tropism identified among studies using organoids were unfortunately inconsistent [Kim 2022]. The limited batch-to-batch reproducibility of organoids hampers their application as a high-throughput drug screening approach due to varying self-assembled cellular constitutions and architectures and intricate in vivo microenvironments. In addition, the absence of vasculature and immune cells in current organoid cultures limits the understanding of viral infection processes and cellular responses to drugs.
6. Summary
The outbreak of SARS-CoV-2 has prompted the scientific community to explore novel technologies to fully understand the host response and potential therapies. Conventional 2D cultures and animal models have failed to adequately recapitulate the morphological and functional characteristics of in vivo human respiratory systems. Fortunately, organoids have been regarded as the most promising in vitro platform for COVID-19 and similar applications as they have provided ample directions specifically towards acquiring enhanced understanding of viral pathogenesis and identifying therapeutic agent candidates. However, current organoid cultures still have some limitations such as the absence of stromal tissue that includes vasculature and immune and various support cells. There is a need for more advanced models with enhanced physiological relevance, improved maturity, and better scalability. In addition, the variability of organoid cultures in size, shape, and/or cellular composition may lead to unsatisfactory reproducibility, which is the main challenge in comparing experimental results across different application scenarios. Despite these limitations, the use of organoid cultures to study COVID-19 and other emerging pandemic diseases seems promising and highly feasible with the advance in stem cell techniques, materials science engineering, and engineering tools.
Acknowledgments
This research was partially supported by the US National Science Foundation (1762941) and the US National Institutes of Health (HL162405, U54CA233396, U54CA233444, & U54CA233465). The U54 grants support the Florida-California Cancer Research, Education and Engagement (CaRE2) Health Equity Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The final peer-reviewed manuscript is subject to the National Institutes of Health Public Access Policy.
Footnotes
Conflicts of Interests
The authors have no conflicts to disclose.
Ethical approval
This study does not contain any studies with human or animal subjects performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no new data was created or analyzed in this study.
References
- Amore BM, Gibbs JP and Emery MG, "Application of in vivo animal models to characterize the pharmacokinetic and pharmacodynamic properties of drug candidates in discovery settings." Combinatorial chemistry & high throughput screening, 13(2): 207–218, 2010. [DOI] [PubMed] [Google Scholar]
- Baldassi D, Gabold B and Merkel O, "Air-liquid interface cultures of the healthy and diseased human respiratory tract: promises, challenges and future directions." Adv Nanobiomed Res, 1(6), 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Deng W, Huang B, Gao H, Liu J, Ren L, Wei Q, Yu P, Xu Y, Qi F, et al. , "The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice." Nature, 583(7818): 830–833, 2020. [DOI] [PubMed] [Google Scholar]
- Bates M., "Fighting COVID-19 With Lung-Chips." IEEE pulse, 12(3): 6–10, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brassard JA, Nikolaev M, Hubscher T, Hofer M and Lutolf MP, "Recapitulating macro-scale tissue self-organization through organoid bioprinting." Nat. Mater, 20(1): 22–29, 2021. [DOI] [PubMed] [Google Scholar]
- Busch SM, Lorenzana Z and Ryan AL, "Implications for Extracellular Matrix Interactions With Human Lung Basal Stem Cells in Lung Development, Disease, and Airway Modeling." Front Pharmacol, 12: 645858, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway E., "Monkeys and mice enlisted to fight coronavirus." Nature, 579(183): 10–1038, 2020. [Google Scholar]
- Cespedes MDS and Souza J, "Coronavirus: a clinical update of Covid-19." Rev Assoc Med Bras (1992), 66(2): 116–123, 2020. [DOI] [PubMed] [Google Scholar]
- Chen YW, Huang SX, de Carvalho A, Ho SH, Islam MN, Volpi S, Notarangelo LD, Ciancanelli M, Casanova JL, Bhattacharya J, et al. , "A three-dimensional model of human lung development and disease from pluripotent stem cells." Nat Cell Biol, 19(5): 542–549, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary SJ, Pitchford SC, Amison RT, Carrington R, Robaina Cabrera CL, Magnen M, Looney MR, Gray E and Page CP, "Animal models of mechanisms of SARS-CoV-2 infection and COVID-19 pathology." British journal of pharmacology, 177(21): 4851–4865, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva da Costa FA, Soares MR, Malagutti-Ferreira MJ, da Silva GR, Livero F and Ribeiro-Paes JT, "Three-Dimensional Cell Cultures as a Research Platform in Lung Diseases and COVID-19." Tissue Eng Regen Med, 18(5): 735–745, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho A, Strikoudis A, Liu HY, Chen YW, Dantas TJ, Vallee RB, Correia-Pinto J and Snoeck HW, "Glycogen synthase kinase 3 induces multilineage maturation of human pluripotent stem cell-derived lung progenitors in 3D culture." Development, 146(2), 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi S, Serrano-Aroca A, Tambuwala MM, Uhal BD, Brufsky AM and Takayama K, "SARS-CoV-2 research using human pluripotent stem cells and organoids." Stem Cells Transl Med, 10(11): 1491–1499, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do TND, Donckers K, Vangeel L, Chatterjee AK, Gallay PA, Bobardt MD, Bilello JP, Cihlar T, De Jonghe S, Neyts J, et al. , "A robust SARS-CoV-2 replication model in primary human epithelial cells at the air liquid interface to assess antiviral agents." Antiviral Res, 192: 105122, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte RRR, Copertino DC Jr., Iniguez LP, Marston JL, Bram Y, Han Y, Schwartz RE, Chen S, Nixon DF and Powell TR, "Identifying FDA-approved drugs with multimodal properties against COVID-19 using a data-driven approach and a lung organoid model of SARS-CoV-2 entry." Mol Med, 27(1): 105, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbadawi M and Efferth T, "Organoids of human airways to study infectivity and cytopathy of SARS-CoV-2." The Lancet Respiratory Medicine, 8(7): e55–e56, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang C, Mei J, Tian H, Liou YL, Rong D, Zhang W, Liao Q and Wu N, "CSF3 Is a Potential Drug Target for the Treatment of COVID-19." Front Physiol, 11: 605792, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard AL, Luu RJ, Miller CR, Maloney R, Cain BP, Marr EE, Burns DM, Gaibler R, Mulhern TJ, Wong CA, et al. , "High-throughput human primary cell-based airway model for evaluating influenza, coronavirus, or other respiratory viruses in vitro." Sci Rep, 11(1): 14961, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith LG and Swartz MA, "Capturing complex 3D tissue physiology in vitro." Nat Rev Mol Cell Biol, 7(3): 211–224, 2006. [DOI] [PubMed] [Google Scholar]
- Haefeli-Bleuer B and Weibel ER, "Morphometry of the human pulmonary acinus. .” The Anatomical Record, 220(4): 401–414, 1988. [DOI] [PubMed] [Google Scholar]
- Han Y, Yang L, Duan X, Duan F, Nilsson-Payant BE, Yaron TM, Wang P, Tang X, Zhang T, Zhao Z, et al. , "Identification of Candidate COVID-19 Therapeutics using hPSC-derived Lung Organoids." bioRxiv, 2020. [Google Scholar]
- Han Y, Duan X, Yang L, Nilsson-Payant BE, Wang P, Duan F, Tang X, Yaron TM, Zhang T, Uhl S, et al. , "Identification of SARS-CoV-2 inhibitors using lung and colonic organoids." Nature, 589(7841): 270–275, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Yang L, Lacko LA and Chen S, "Human organoid models to study SARS-CoV-2 infection." Nat Methods, 19(4): 418–428, 2022. [DOI] [PubMed] [Google Scholar]
- Hashimoto R, Takahashi J, Shirakura K, Funatsu R, Kosugi K, Deguchi S, Yamamoto M, Tsunoda Y, Morita M, Muraoka K, et al. , "SARS-CoV-2 disrupts respiratory vascular barriers by suppressing Claudin-5 expression." Sci Adv, 8(38): p.eabo678, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Tamura T, Watanabe Y, Sakamoto A, Yasuhara N, Ito H, Nakano M, Fuse H, Ohta A, Noda T, et al. , "Evaluation of Broad Anti-Coronavirus Activity of Autophagy-Related Compounds Using Human Airway Organoids." Mol Pharm, 20(4): 2276–2287, 2023. [DOI] [PubMed] [Google Scholar]
- Health, N. I. o., “Animal Models and Resources for Coronavirus Research." from https://orip.nih.gov/animal-models-and-resources-coronavirus-research, 2021.
- Hofer M and Lutolf MP, "Engineering organoids." Nat Rev Mater, 6(5): 402–420, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, et al. , "Flow-enhanced vascularization and maturation of kidney organoids in vitro." Nat. Methods, 16(3): 255–262, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Guo H, Zhou P and Shi ZL, "Characteristics of SARS-CoV-2 and COVID-19." Nat Rev Microbiol, 19(3): 141–154, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Liu T, Liao J, Maharjan S, Xie X, Pérez M, Anaya I, Wang S, Tirado Mayer A, Kang Z, et al. , "Reversed-engineered human alveolar lung-on-a-chip model." Proc. Natl. Acad. Sci. U.S.A, 118(19): e2016146118, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Leu MC, Mazumder J and Donmez A, "Additive Manufacturing: Current State, Future Potential, Gaps and Needs, and Recommendations." J. MANUF. SCI. E, 137(1): 014001, 2015. [Google Scholar]
- Huang Y and Schmid SR, "Additive Manufacturing for Health: State of the Art, Gaps and Needs, and Recommendations." J. MANUF. SCI. E, 140(9), 2018. [Google Scholar]
- Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY and Ingber DE, "Reconstituting Organ-Level Lung Functions on a Chip." Science, 328(5986): 1662–1668, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE, "Human organs-on-chips for disease modelling, drug development and personalized medicine." Nat Rev Genet, 23(8): 467–491, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir A, Datta P, Oh J, Williams A, Ozbolat V, Unutmaz D and T. O. I, "3D Bioprinting for fabrication of tissue models of COVID-19 infection." Essays Biochem, 65(3): 503–518, 2021. [DOI] [PubMed] [Google Scholar]
- Kang D, Park JA, Kim W, Kim S, Lee HR, Kim WJ, Yoo JY and Jung S, "All-Inkjet-Printed 3D Alveolar Barrier Model with Physiologically Relevant Microarchitecture." Adv Sci (Weinh), 8(10): 2004990, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapalczynska M, Kolenda T, Przybyla W, Zajaczkowska M, Teresiak A, Filas V, Ibbs M, Blizniak R, Luczewski L and Lamperska K, "2D and 3D cell cultures - a comparison of different types of cancer cell cultures." Arch. Med. Sci, 14(4): 910–919, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid N, Arif S, Kobayashi I and Nakajima M, "Lab-on-a-chip techniques for high-throughput proteomics and drug discovery." In Microfluidics for Pharmaceutical Applications (pp. ). William Andrew Publishing.: 371–422, 2019. [Google Scholar]
- Kim J, Koo BK and Knoblich JA, "Human organoids: model systems for human biology and medicine." Nat Rev Mol Cell Biol, 21(10): 571–584, 2020a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Koo BK and Clevers H, "Organoid Studies in COVID-19 Research." Int J Stem Cells, 15(1): 3–13, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YI, Kim SG, Kim SM, Kim EH, Park SJ, Yu KM, Chang JH, Kim EJ, Lee S, Casel MAB, et al. , "Infection and Rapid Transmission of SARS-CoV-2 in Ferrets." Cell Host Microbe, 27(5): 704–709 e702, 2020b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konar D, Devarasetty M, Yildiz DV, Atala A and Murphy SV, "Lung-On-A-Chip Technologies for Disease Modeling and Drug Development." Biomed Eng Comput Biol, 7(Suppl 1): 17–27, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Wen S, Cao W, Yue P, Xu X, Zhang Y, Luo L, Chen T, Li L, Wang F, et al. , "Lung organoids, useful tools for investigating epithelial repair after lung injury." Stem Cell Res Ther, 12(1): 95, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers MM, van der Vaart J, Knoops K, Riesebosch S, Breugem TI, Mykytyn AZ, Beumer J, Schipper D, Bezstarosti K, Koopman CD, et al. , "An organoid-derived bronchioalveolar model for SARS-CoV-2 infection of human alveolar type II-like cells." EMBO J, 40(5): e105912, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampejo AO, Hu NW, Lucas D, Lomel BM, Nguyen CM, Dominguez CC, Ren B, Huang Y and Murfee WL, "A Challenge for Engineering Biomimetic Microvascular Models: How do we Incorporate the Physiology?" Front Bioeng Biotechnol, 10: 912073, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhans SA, "Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning." Front Pharmacol, 9: 6, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M and Izpisua Belmonte JC, "Organoids - Preclinical Models of Human Disease." N Engl J Med, 380(6): 569–579, 2019. [DOI] [PubMed] [Google Scholar]
- Li Y, Wu Q, Sun X, Shen J and Chen H, "Organoids as a Powerful Model for Respiratory Diseases." Stem Cells Int, 2020: 5847876, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, et al. , "Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding." The Lancet, 395(10224): 565–574, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukassen S, Chua RL, Trefzer T, Kahn NC, Schneider MA, Muley T, Winter H, Meister M, Veith C, Boots AW, et al. , "SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells." EMBO J, 39(10): e105114, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Fontela C, Dowling WE, Funnell SGP, Gsell PS, Riveros-Balta AX, Albrecht RA, Andersen H, Baric RS, Carroll MW, Cavaleri M, et al. , "Animal models for COVID-19." Nature, 586(7830): 509–515, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni K, Che B, Yang C, Qin Y, Gu R, Wang C, Luo M and Deng L, "Emerging toolset of three-dimensional pulmonary cell culture models for simulating lung pathophysiology towards mechanistic elucidation and therapeutic treatment of SARS-COV-2 infection." Front Pharmacol, 13: 1033043, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs M, Nyengaard JR, Jung A, Knudsen L, Voigt M, Wahlers T, Richter J and Gundersen HJ, "The number of alveoli in the human lung." Am J Respir Crit Care Med, 169(1): 120–124, 2004. [DOI] [PubMed] [Google Scholar]
- Parihar A, Pandita V and Khan R, "3D printed human organoids: High throughput system for drug screening and testing in current COVID-19 pandemic." Biotechnol Bioeng, 119(10): 2669–2688, 2022. [DOI] [PubMed] [Google Scholar]
- Pei R, Feng J, Zhang Y, Sun H, Li L, Yang X, He J, Xiao S, Xiong J, Lin Y, et al. , "Host metabolism dysregulation and cell tropism identification in human airway and alveolar organoids upon SARS-CoV-2 infection." Protein Cell, 12(9): 717–733, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plebani R, Bai H, Si L, Li J, Zhang C and Romano M, "3D Lung Tissue Models for Studies on SARS-CoV-2 Pathophysiology and Therapeutics." Int J Mol Sci, 23(17), 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdani LH and Bachari K, "Potential therapeutic effects of Resveratrol against SARS-CoV-2." Acta Virol, 64(3): 276–280, 2020. [DOI] [PubMed] [Google Scholar]
- Ramezankhani R, Solhi R, Chai YC, Vosough M and Verfaillie C, "Organoid and microfluidics-based platforms for drug screening in COVID-19." Drug Discov Today, 27(4): 1062–1076, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez RD, Sheridan S, Girard L, Sato M, Kim Y, Pollack J, Peyton M, Zou Y, Kurie JM, DiMaio JM, et al. , "Immortalization of human bronchial epithelial cells in the absence of viral oncoproteins." Cancer Res, 64(24): 9027–9034, 2004. [DOI] [PubMed] [Google Scholar]
- Rauth S, Karmakar S, Batra SK and Ponnusamy MP, "Recent advances in organoid development and applications in disease modeling." Biochim Biophys Acta Rev Cancer, 1875(2): 188527, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddel RR, Ke Y, Gerwin BI, McMenamin MG, Lechner JF, Su RT, Brash DE, Park JB, Rhim JS and Harris CC, "Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes." Cancer Res, 48(7): 1904–1909, 1988. [PubMed] [Google Scholar]
- Ren B, Song K, Sanikommu AR, Chai Y, Longmire MA, Chai W, Murfee WL and Huang Y, "Study of sacrificial ink-assisted embedded printing for 3D perfusable channel creation for biomedical applications." Applied Physics Reviews, 9(1): 011408, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren B, Jiang Z, Murfee WL, Katz AJ, Siemann D and Huang Y, "Realizations of vascularized tissues: from in vitro platforms to in vivo grafts." Biophysics Reviews, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y, Yang X, Ma Z, Sun X, Zhang Y, Li W, Yang H, Qiang L, Yang Z, Liu Y, et al. , "Developments and Opportunities for 3D Bioprinted Organoids." Int J Bioprint, 7(3): 364, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs N, Papaspyropoulos A, Zomer-van Ommen DD, Heo I, Bottinger L, Klay D, Weeber F, Huelsz-Prince G, Iakobachvili N, Amatngalim GD, et al. , "Long-term expanding human airway organoids for disease modeling." EMBO J, 38(4), 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahudeen AA, Choi SS, Rustagi A, Zhu J, van Unen V, de la OS, Flynn RA, Margalef-Catala M, Santos AJM, Ju J, et al. , "Progenitor identification and SARS-CoV-2 infection in human distal lung organoids." Nature, 588(7839): 670–675, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano E, Suzuki T, Hashimoto R, Itoh Y, Sakamoto A, Sakai Y, Saito A, Okuzaki D, Motooka D, Muramoto Y, et al. , "Cell response analysis in SARS-CoV-2 infected bronchial organoids." Commun Biol, 5(1): 516, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Kishimoto M, Itakura Y, Tabata K, Intaruck K, Uemura K, Toba S, Sanaki T, Sato A, Hall WW, et al. , "Air-liquid interphase culture confers SARS-CoV-2 susceptibility to A549 alveolar epithelial cells." Biochem Biophys Res Commun, 577: 146–151, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. , "Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche." Nature, 459(7244): 262–265, 2009. [DOI] [PubMed] [Google Scholar]
- Shan C, Yao YF, Yang XL, Zhou YW, Gao G, Peng Y, Yang L, Hu X, Xiong J, Jiang RD, et al. , "Infection with novel coronavirus (SARS-CoV-2) causes pneumonia in Rhesus macaques." Cell Res, 30(8): 670–677, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran A, Prasad K, Chaudhari S, Brand A and Satyamoorthy K, "Advances in development and application of human organoids." 3 Biotech, 11(6): 257, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen MA, Khan S, Kazmi A, Bashir N and Siddique R, "COVID-19 infection: Origin, transmission, and characteristics of human coronaviruses." J Adv Res, 24: 91–98, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou S, Liu M, Yang Y, Kang N, Song Y, Tan D, Liu N, Wang F, Liu J and Xie Y, "Animal Models for COVID-19: Hamsters, Mouse, Ferret, Mink, Tree Shrew, and Non-human Primates." Front Microbiol, 12: 626553, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha J, Ghadiri M, Shanmugavel M, Razavi Bazaz S, Vasilescu S, Ding L and Ebrahimi Warkiani M, "A rapidly prototyped lung-on-a-chip model using 3D-printed molds." Organs-on-a-Chip, 1: 100001, 2019. [Google Scholar]
- Si L, Bai H, Rodas M, Cao W, Oh CY, Jiang A, Moller R, Hoagland D, Oishi K, Horiuchi S, et al. , "A human-airway-on-a-chip for the rapid identification of candidate antiviral therapeutics and prophylactics." Nat Biomed Eng, 5(8): 815–829, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Ren B, Zhai Y, Chai W and Huang Y, "Effects of transglutaminase cross-linking process on printability of gelatin microgel-gelatin solution composite bioink." Biofabrication, 14(1), 2021. [DOI] [PubMed] [Google Scholar]
- Spitalieri P, Centofanti F, Murdocca M, Scioli MG, Latini A, Di Cesare S, Citro G, Rossi A, Orlandi A, Miersch S, et al. , "Two Different Therapeutic Approaches for SARS-CoV-2 in hiPSCs-Derived Lung Organoids." Cells, 11(7), 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucki AO, Stucki JD, Hall SR, Felder M, Mermoud Y, Schmid RA, Geiser T and Guenat OT, "A lung-on-a-chip array with an integrated bio-inspired respiration mechanism." Lab Chip, 15(5): 1302–1310, 2015. [DOI] [PubMed] [Google Scholar]
- Sungnak W, Huang N, Becavin C, Berg M, Queen R, Litvinukova M, Talavera-Lopez C, Maatz H, Reichart D, Sampaziotis F, et al. , "SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes." Nat Med, 26(5): 681–687, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Q, Choi KM, Sicard D and Tschumperlin DJ, "Human airway organoid engineering as a step toward lung regeneration and disease modeling." Biomaterials, 113: 118–132, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Ellen BM, Dinesh Kumar N, Bouma EM, Troost B, van de Pol DPI, van der Ende-Metselaar HH, Apperloo L, van Gosliga D, van den Berge M, Nawijn MC, et al. , "Resveratrol and Pterostilbene Inhibit SARS-CoV-2 Replication in Air-Liquid Interface Cultured Human Primary Bronchial Epithelial Cells." Viruses, 13(7), 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuwen LA, Geldhof V, Pasut A and Carmeliet P, "COVID-19: the vasculature unleashed." Nat Rev Immunol, 20(7): 389–391, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Gao J, Garcia IM, Chen HJ, Castaldi A and Chen YW, "Human pluripotent stem cell-derived lung organoids: Potential applications in development and disease modeling." Wiley Interdiscip Rev Dev Biol, 10(6): e399, 2021. [DOI] [PubMed] [Google Scholar]
- Tiwari SK, Wang S, Smith D, Carlin AF and Rana TM, "Revealing Tissue-Specific SARS-CoV-2 Infection and Host Responses using Human Stem Cell-Derived Lung and Cerebral Organoids." Stem Cell Reports, 16(3): 437–445, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran BM, Deliyannis G, Hachani A, Earnest L, Torresi J and Vincan E, "Organoid Models of SARS-CoV-2 Infection: What Have We Learned about COVID-19?" Organoids, 1(1): 2–27, 2022a. [Google Scholar]
- Tran E, Shi T, Li X, Chowdhury AY, Jiang D, Liu Y, Wang H, Yan C, Wallace WD, Lu R, et al. , "Development of human alveolar epithelial cell models to study distal lung biology and disease." iScience, 25(2): 103780, 2022b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Brummelen R and van Brummelen AC, "The potential role of resveratrol as supportive antiviral in treating conditions such as COVID-19 - A formulator's perspective." Biomed Pharmacother, 148: 112767, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vaart J and Clevers H, "Airway organoids as models of human disease." J Intern Med, 289(5): 604–613, 2021a. [DOI] [PubMed] [Google Scholar]
- van der Vaart J, Lamers MM, Haagmans BL and Clevers H, "Advancing lung organoids for COVID-19 research." Dis Model Mech, 14(6), 2021b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Armendariz AI and Herold S, "From Clones to Buds and Branches: The Use of Lung Organoids to Model Branching Morphogenesis Ex Vivo." Front. Cell. Dev. Biol, 9: 631579, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang P and Qin J, "Human Organoids and Organs-on-Chips for Addressing COVID-19 Challenges." Adv Sci (Weinh), 9(10): e2105187, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodall MNJ, Masonou T, Case KM and Smith CM, "Human models for COVID-19 research." J Physiol, 599(18): 4255–4267, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wang Y, Liu G, Jia Y, Yang J, Shi J, Dong J, Wei J and Liu X, "Characterization of air-liquid interface culture of A549 alveolar epithelial cells." Braz J Med Biol Res, 51(2): e6950, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Song K, Zhang D, Ren B, Sole-Gras M, Huang Y and Yin J, "Embedded extrusion printing in yield-stress-fluid baths." Matter, 5(11): 3775–3806, 2022. [Google Scholar]
- Xu H, Lyu X, Yi M, Zhao W, Song Y and Wu K, "Organoid technology and applications in cancer research." J Hematol Oncol, 11(1): 116, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, Zhong W and Hao P, "Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission." Sci China Life Sci, 63(3): 457–460, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Korogi Y, Hirai T and Gotoh S, "A method of generating alveolar organoids using human pluripotent stem cells." Methods Cell Biol, 159: 115–141, 2020. [DOI] [PubMed] [Google Scholar]
- Zhang M, Wang P, Luo R, Wang Y, Li Z, Guo Y, Yao Y, Li M, Tao T, Chen W, et al. , "Biomimetic Human Disease Model of SARS-CoV-2 Induced Lung Injury and Immune Responses on Organ Chip System." Adv. Sci: 2002928, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Jin Y, Yin J, Xu C, Xiong R, Christensen K, Ringeisen BR, Chrisey DB and Huang Y, "Evaluation of bioink printability for bioprinting applications." Applied Physics Reviews, 5(4): 041304, 2018. [Google Scholar]
- Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, Lv T, Liang J, Zhang Q, Xu W, et al. , "Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids." Protein Cell, 11(10): 771–775, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Li C, Liu X, Chiu MC, Wang D, Wei Y, Chu H, Cai JP, Hau-Yee Chan I, Kak-Yuen Wong K, et al. , "Human Intestinal Organoids Recapitulate Enteric Infections of Enterovirus and Coronavirus." Stem Cell Reports, 16(3): 493–504, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Li C, Sachs N, Chiu MC, Wong BH, Chu H, Poon VK, Wang D, Zhao X, Wen L, et al. , "Differentiated human airway organoids to assess infectivity of emerging influenza virus." Proc Natl Acad Sci U S A, 115(26): 6822–6827, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N, Wang W, Liu Z, Liang C, Wang W, Ye F, Huang B, Zhao L, Wang H, Zhou W, et al. , "Morphogenesis and cytopathic effect of SARS-CoV-2 infection in human airway epithelial cells." Nat Commun, 11(1): 3910, 2020a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. , "A Novel Coronavirus from Patients with Pneumonia in China, 2019." N Engl J Med, 382(8): 727–733, 2020b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zscheppang K, Berg J, Hedtrich S, Verheyen L, Wagner DE, Suttorp N, Hippenstiel S and Hocke AC, "Human Pulmonary 3D Models For Translational Research." Biotechnol J, 13(1), 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data was created or analyzed in this study.