Abstract
Bone related diseases such as osteoporosis, osteoarthritis, metastatic bone cancer, osteogenesis imperfecta, and Paget’s disease, are primarily treated with pharmacologic therapies that often exhibit limited efficacy and substantial side effects. Bone injuries or fractures are primarily repaired with biocompatible materials that produce mixed results in sufficiently regenerating healthy and homogenous bone tissue. Each of these bone conditions, both localized and systemic, use different strategies with the same goal of achieving a healthy and homeostatic bone environment. In this study, we developed a new type of bone-based nanoparticle (BPs) using the entire organic extracellular matrix (ECM) of decellularized porcine bone, additionally encapsulating indocyanine green dye (ICG) for an in vivo monitoring capability. Utilizing the regenerative capability of bone ECM and the functionality of nanoparticles, the ICG encapsulated BPs (ICG/BPs) have been demonstrated to be utilized as a therapeutic option for localized and systemic orthopedic conditions. Additionally, ICG enables an in situ monitoring capability in the Short-Wave Infrared (SWIR) spectrum, capturing the degradation or the biodistribution of the ICG/BPs after both local implantation and intravenous administration, respectively. The efficacy and safety of the ICG/BPs shown within this study lay the foundation for future investigations, which will delve into optimization for clinical translation.
1. Introduction
Bone diseases and physiological conditions, such as osteoporosis, Paget’s disease, osteosarcomas, osteoarthritis, metastatic bone cancer, hormonal status and chronic diseases, and traumatic bone injury, affect millions of people worldwide and impose an increasingly heavy burden on an aging society.1–5 Although bone is a living tissue that is capable of continuous regeneration, disturbances in bone homeostasis can lead to impaired bone healing.6,7 Pharmacologic therapy is the primary clinical treatment for such bone diseases;8 however, unlike soft organs such as the liver or kidney, bone is highly mineralized and less vascularized, which ultimately decreases the efficiency of drug delivery to bone.9,10 Another limitation of current pharmacologic therapies is that many drugs have a short circulation half-life, resulting in poor assimilation and a low bioavailability in the diseased bone.11,12 Thus, drugs are normally administered at high dosage levels at an increased frequency. This often leads to side effects resulting from systemic toxicity due to the large amount of drug accumulation in other unintended organs.13,14
Bone is a heterogeneous composite tissue, composed of a dynamic extracellular matrix (ECM) network that consists of 50–70% inorganic minerals, 20–40% organic matrix, and 5–10% water.6,7 The inorganic minerals, primarily consisting of calcium phosphate (CaP), specifically hydroxyapatite (HA), are the source for hardness and strength of the bone, while the organic matrix, including collagens and non-collagenous proteins, provide the tissue with flexibility and a template to regulate new bone formation.15,16 Collagens, especially collagen type I, are the most abundant proteins found in bone and play a critical role in their structure, tissue organization, and mechanical support.17,18 Non-collagenous proteins, such as proteoglycans, glycoproteins, γ Carboxy glutamic acid-containing proteins, and other serum-derived proteins, are associated with regulating fundamental cellular processes (such as attachment, proliferation, differentiation, and migration), tissue mineralization, and driving functions related to tissue regeneration.19,20
Decellularized bone matrix (DBM) is a widely used bone grafting material in clinical orthopedic treatment, as DBM is naturally derived from bone tissues that have undergone a meticulous decellularization process that removes the cellular and antigen components while preserving the ECM components (collagens, proteoglycans, glycoproteins, γ Carboxy glutamic acid-containing proteins, serum-derived proteins, etc.) and mechanical and structural properties.21–24 There is a wide diversity of DBMs that have been approved by the U.S. Food and Drug Administration (FDA) for clinical use.23,25–28 DBM offers numerous advantages over other bone grafting materials like bone cements, bone glasses, hydroxyapatite (HA), and synthetic polymers, due to its preservation of natural signaling cues necessary for enhancing cell activity, osteoinduction, osteoconduction, and osteogenesis.29–32 Our previous work had established a decellularization protocol for porcine bone and demonstrated a near-to-complete cellular and porcine antigen removal of the obtained DBM. The DBM was further digested with pepsin and desalted with acetone precipitation to obtain a gellable DBM. We demonstrated excellent biocompatibility and osteogenesis capability of the gellable DBM.33 The flexibility of the gellable DBM also allows it to be further processed into various forms, such as nanomaterials, that may best meet clinical needs. These promising results have motivated the development of a new nanostructured biomaterial based on the bone ECM with the aim of providing new strategies for treatments and therapies designed for treating bone injury and disease.
Nanomedicine is focused on developing nanoscale materials that has valuable applications in the diagnosis, prevention, and treatment of a number of diseases. As an important tool of nanomedicine, nanoparticles (NPs) have been extensively studied in such areas as drug and gene delivery, vaccine adjuvants, disease diagnosis and therapy, and biomedical imaging, due to their extraordinary advantages such as a low toxicity, enlarged drug-loading ability, tailorable characteristics, controllable physical stability, enhanced intracellular uptake, and targeted and on-demand drug release.34–37 Numerous types of inorganic (hydroxyapatite, silica, metals, calcium phosphate, etc.) and organic materials (synthetic polymers, chitosan, liposomes, proteins, etc.) have been formulated into NPs.38–41 Unfortunately, no NP has yet been approved by the FDA for clinical orthopedic treatment as current strategies have many limitations, including elevated risks of low biocompatibility and delivery efficiency, potential accumulation in healthy organs, and induced inflammation and tissue damage.42–47 More efforts are needed to develop new NPs for improving safety and therapeutic efficiency for hard-to-treat bone diseases.48,49
A key technology for translating nanomedicine-based approaches is theranostics, or combined imaging and therapeutic NP approaches, which can efficiently track NP pharmacokinetics as well as pharmacodynamics. Image-guided interventions are playing an increasing role in diagnosis and treatment for bone diseases, as these strategies can provide a less invasive and more accurate way not only for detecting and monitoring of disease but also for improving treatment outcomes. Radiographic imaging, such as plain X-ray, Computed Tomography (CT), and Dual Energy X-ray Absorptiometry (DEXA), are the most common methods for clinical bone diagnosis, but many studies find that radiography alone offers an incomplete assessment of bone healing and is unreliable in determining the healing stages of bone.50–52 Other drawbacks of radiographic imaging include frequent radiation exposure and high cost, which can be especially detrimental for children, pregnant women, and immune-compromised or ill patients. Recent efforts have focused on developing image-guided therapies that can monitor the host-material interaction of implants, quantitatively assess bone healing, and evaluate the performance of drugs or grafts in a real-time, non-invasive, highly sensitive, and cost-effective way. One such method, Near-Infrared Spectroscopy imaging (NIR), has been used to study the in vitro and in vivo degradation of biomaterials that have been doped with fluorescent markers. Indocyanine Green (ICG) is an FDA approved image contrast dye that is often viewed in the near-IR (NIR) or NIR-I window (700–900 nm) for a wide variety of preclinical and clinical applications. It has been found that extending fluorescence imaging into shortwave IR, also known as SWIR or NIR-II (950–1700 nm) enhances the advantages of NIR imaging due to lower tissue autofluorescence and increased sensitivity, leading to increased tissue penetration depth and decreased scattering.53–57
In this study, we developed a new type of bone-based nanoparticle (BP) using the entire ECM of decellularized porcine bone. To enable the BPs to be monitored in vivo, ICG was additionally encapsulated within the BPs (ICG/BPs). We sought to characterize the properties of the ICG/BPs including their morphology and size, surface properties, protein content, and their effect on stem cell differentiation. Capitalizing on the enhanced functionality of the ICG/BPs, we explored their in situ monitoring capability for both localized and systemic applications using fluorescence imaging in both the NIR and SWIR windows. To our knowledge, this is the first instance of developing protein-based NPs using the entirety of the decellularized ECM of bone tissue.
2. Results
2.1. Morphological characterization of ICG/BPs
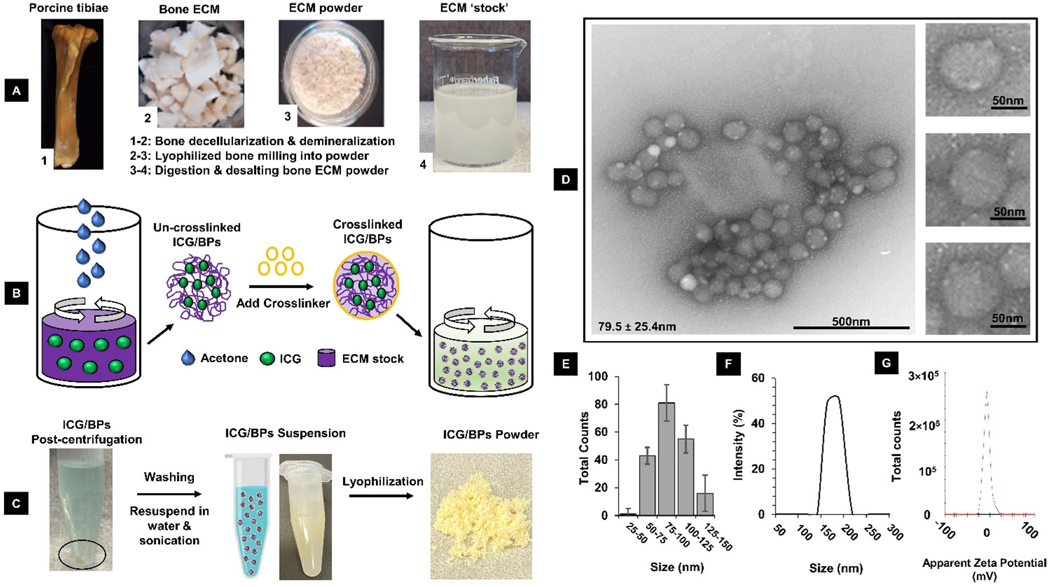
TEM images of the ICG/BPs showed a small, spherical morphology with an average size of 79.5 ± 25.4 nm (Fig. 1D). It was not uncommon to see the particles agglomerate into small clusters. The distribution of particle diameters based on TEM images showed a large percentage within 75–100 nm, but there were small counts of larger or smaller particles also found (Fig. 1E). After re-suspension of the lyophilized ICG/BPs into distilled water and sonication, DLS measurements found the average size of ICG/BPs to be 171 ± 11.41 nm (Fig. 1F). The discrepancy in particle size differences between TEM and DLS measurements may be attributed to observations of the particles in a dry or wet state, or the size ranging from a single NP up to particle assemblies.58,59 The surface charge, also measured as the zeta potential (), falls in the range of −6.3 ± 1.9 mV when resuspended in water (Fig. 1G). NPs with a zeta potential between −10 and +10 mV are considered neutral, and a zeta potential between −30 mV to +30 mV is considered to have sufficient repulsive forces in order to attain better physical colloidal stability.60 These parameters are critical in understanding the in vitro and in vivo behavior of the ICG/BPs with cells and in the bloodstream, respectively. Protein characterization of ICG/BPs by mass spectrometry (ESI. 3†) showed that several major ECM proteins were preserved and this result was consistent with our previous findings.33 Thermal characterization of the ICG/BPs using thermogravimetric analysis (TGA) showed the loss of mass with increasing temperature was consistent with protein-only degradation (ESI. 4†).
Fig. 1.
Summary of bone decellularization, digestion, and ICG/BP synthesis process. (A) Porcine tibiae were harvested, decellularized, demineralized, and digested to prepare the ECM stock solution. (B) ICG/BPs were formulated by mixing free ICG dye with the ECM stock, using desolvation via acetone precipitation, and chemical crosslinking. (C) The ICG/BPs could be made into an aqueous solution or lyophilized into a powder. (D) TEM imagery of the ICG/BPs, with multiple particles displaying a small, spherical morphology (79.5 ± 25.4 nm). (E) Size distribution of the ICG/BPs based on TEM imaging. (F) Measured size destitution of the ICG/BPs using DLS (171 ± 11.4 nm). (G) Measured Zeta potential of the ICG/BPs (−6.3 ± 1.9 mV).
2.2. Loading efficiency and optical characterization of ICG/BPs
Loading efficiency.
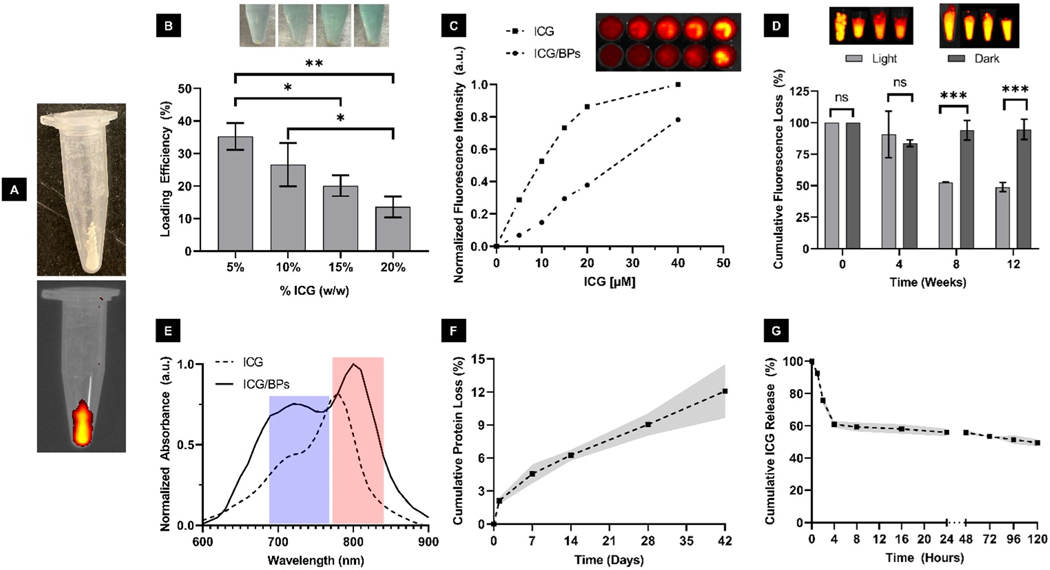
After synthesis, the ICG/BPs appeared whitish in color with a slight green appearance, indicating entrapped ICG, which was confirmed using fluorescence imaging in the NIR window (Fig. 2A). We determined the loading efficiency of ICG within the ICG/BPs to be ∼34% in ICG/BPs with a loading concentration of ICG at 5% w/w to stock solution (mg), finding that the loading efficiency decreased with larger amounts of ICG pre-mixed with the stock prior to encapsulation (Fig. 2B). We examined if the decreased loading efficiency had any impact on the resulting fluorescent intensity. When starting with a loading concentration of 5% w/w and increasing to 10% w/w, there is nearly double the resulting fluorescent output, however any increase in ICG after 10% w/w (up to 20% w/w) shows a potential quenching effect (ESI. 5†). Due to this, all of the ICG/BPs used in this study are fabricated with a loading concentration of 10% ICG w/w.
Fig. 2.
Optical characterization and stability of ICG/BPs. (A) ICG/BPs post-synthesis and the observed fluorescence in the NIR window (840 nm). (B) Loading efficiency (LE) of the ICG/BPs using different initial concentrations of ICG during synthesis, with a decreased loading efficiency consistent with a greener supernatant. (C) Absorption measurements of ICG (5 ) and the ICG/BPs (10 encapsulated ICG), showing two characteristic peaks in the NIR region represented by the blue bar (∼720 nm) and red bar (∼780 nm), with the ICG/BPs displaying a slight red shift of ∼10–20 nm. (D) Fluorescent intensity of ICG and ICG/BPs using equivalent concentrations of ICG for each respective solution, measured in the NIR window (840 nm). (E) Long-term photostability of the ICG/BPs when either constantly exposed to or sheltered from light, measured in the SWIR window (950 nm). (F) Degradation of the ICG/BPs as measured by cumulative protein loss (mg) over 42 days. (G) ICG released from the ICG/BPs at different timepoints over the course of 5 days. (One way ANOVA followed by group post hoc comparison was performed on select data, statistical significance is quantified as: ****; ***; **; *, ns = no significance).
Optical characterization.
The optical characterization of free ICG and the ICG/BPs was done using absorption and emission spectroscopy. We observed a significant enhancement in both the absorption spectrum and the emission curve of ICG/BPs. In comparison to free ICG in water, a noticeable bathochromic shift of approximately 10–20 nm in the absorption spectrum was identified. This shift slightly altered the position of the second peak, moving it approximately 15–30 nm from around 780 to 810 nm (Fig. 2C). The red shift towards longer wavelengths is indicative of changes in the physiochemical environment, likely attributed to polaric-* transitions resulting from electrostatic interactions between ICG molecules and the nanoparticles.61 The florescent intensity of the ICG/BPs based on total concentration was determined using the NIR window (840 nm). Using a starting sample of 5 mg of ICG/BPs resuspended into Milli-Q water, 100uL aliquots that would equate to 40, 20, 15, 10, and 5 ICG (assuming a 100% loading efficiency) were measured out. We found that each aliquot of ICG/BPs was equivalent to ∼ 12.5, 4.85, 3.52, 2.75, and 1.52 of encapsulated ICG, respectively (Fig. 2D). The average percent of expected signal from each concentration is consistent with the average loading efficiency we had previously determined (∼30%). Our characterization demonstrated a linear relationship in the intensity relative to increasing ICG/BP concentration. However, the resulting intensity of the ICG/BPs when compared to their ICG counterpart () is not equivalent, more than likely due to their average loading efficiency as previously mentioned. To summarize, this would mean that when measuring out ICG/BPs that would equate to 40 of ICG, the resulting fluorescent output more closely resembles that of ∼15 ICG.
Photostability.
We examined the long-term photostability of the ICG/BPs in both light and dark conditions (Fig. 2E), using the SWIR window (950 nm). The ICG/BPs kept in dark conditions retained up to 80% of their original fluorescent intensity after 12 weeks, whereas the ICG/BPs exposed to constant light retained ∼47% of their original fluorescent intensity. There were observed instances of samples retaining more than 50% of the original fluorescence up to 1 year after synthesis, lyophilization, and being stored under dark conditions (ESI. 6†). This increased retention of fluorescent performance allows for the extended storage and use of the ICG/BPs, providing ease-of-use advantages for clinicians.
2.3. Material stability of ICG/BPs
Material degradation rate.
To assess the in vitro degradation rate of the ICG/BPs, we quantified the cumulative protein loss by mass (mg). Over a 42 days period, we found a gradual degradation of the ICG/BPs, exhibiting an approximately 12% total mass loss over 7 weeks without external perturbation (Fig. 2F).
ICG release rate.
To measure the release profile of the ICG/BPs, pre-weighed amounts were studied at different timepoints over the course of 5 days after incubation with rat plasma at a temperature of 37 °C to best simulate physiological conditions (Fig. 2G). We found that the amount of ICG released from the ICG/BPs was initially (0.5–4 hours) very high, and then slowly declined to stabilize over 5 days. There was an initial large decrease over the first 4 hours, indicating ∼40% of ICG by mass had been released. This initial loss was followed by a slow linear decay over the next 5 days, during which an additional 11% of ICG was released. Cumulatively, ∼51% of the ICG had been released over 5 days of incubation.
2.4. Intracellular uptake, cytotoxicity, and osteogenic differentiation of BMSCs after ICG/BP co-culture
Intracellular uptake.
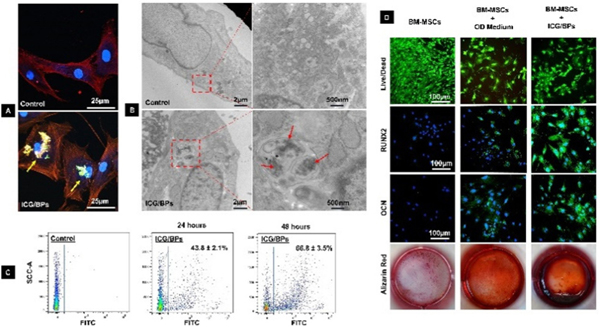
After 24 hours of co-culturing BMSCs with ICG/BPs, the cellular uptake of the particles was analyzed using a confocal laser scanning microscope (Fig. 3A) and TEM imagery (Fig. 3B). Both results demonstrated the intracellular localization of particles within the cellular environment. The percentage of cellular uptake was determined by flow cytometry after co-culturing BMSCs with ICG/BPs for 24 and 48 hours (Fig. 3C), which showed that the cellular uptake rate was about 44.1 ± 4.6% after 24 hours and 64.5 ± 4.3% after 48 hours of co-culturing.
Fig. 3.
Cellular uptake and compatibility of ICG/BPs. After 24 hours of co-culturing BMSCs with ICG/BPs, the cellular uptake of the particles was analysed using (A) confocal laser scanning microscopy and (B) TEM imaging. (C) The percentage of cellular uptake was determined by flow cytometry after co-culturing BMSCs with ICG/BPs for 24 and 48 hours. (D) After 4 weeks of cell culture, the cytotoxicity of the ICG/BPs was determined by Live/Dead staining and osteogenic differentiation was evaluated with RUNX2 and OCN staining, and calcium deposition measured with alizarin red S staining.
Cytotoxicity and osteogenic differentiation.
The cytotoxicity of the ICG/BPs was determined by Live/Dead staining. After 4 weeks of cell culture, most of the cells in different groups retained green staining (Fig. 3D, row 1), which demonstrated the co-culturing of ICG/BPs with the BMSCs did not affect the viability and survival of cells. The osteogenic differentiation of BMSCs in different groups after 4 weeks cell culture was evaluated with RUNX-related transcription factor 2 (RUNX2) and osteocalcin (OCN) staining, and calcium deposition was verified by alizarin red staining. Enhanced RUNX2 and OCN fluorescence (Fig. 3D, rows 2 & 3, respectively) and mineralized nodules (Fig. 3D, row 4) were found in the BMSCs that were co-cultured with the ICG/BPs, suggesting that the ICG/BPs could additionally enhance the osteogenic differentiation of stem cells.
2.5. Bone regeneration and in situ monitoring of ICG/BPs in non-healing tibial defects
In vivo bone regeneration.
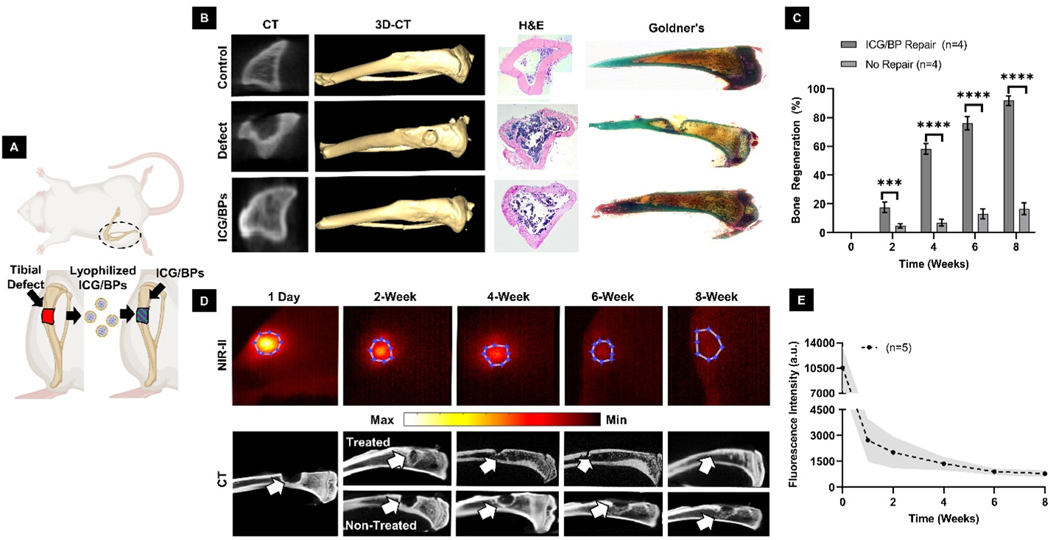
For our in vivo evaluation of any bone-regenerating stimulation effects, ICG/BPs were implanted into a critical sized, non-healing tibial defect (Fig. 4A). New bone formation was assessed using CT after 8 weeks of implantation. Comparing each group, CT analysis and 3D-CT reconstruction indicated that the ICG/BPs stimulate bone growth (Fig. 4B, columns 1 & 2). The calculated bone regeneration using CT imaging indicated the ICG/BPs with the highest bone regeneration (89 ± 5.1%) after 8 weeks, compared to the incomplete bone healing (17 ± 3.8%) in that of the defect group (Fig. 4C). Histological analysis with H&E showed a large amount of fibrous tissue invasion in the defect group, whereas there is significantly less present in the ICG/BP group (Fig. 4B, column 3). Goldner’s Trichrome stain on un-decalcified samples indicated that the defect area that is treated with the ICG/BPs showed comparable morphology to the tibia in the control group (Fig. 4B, column 4).
Fig. 4.
Bone regeneration and in situ SWIR monitoring. (A) A surgically created tibial defect was filled with ICG/BP powder. (B) CT of the axial plane (column 1), 3D-CT of the longitudinal plane (column 2), H&E (column 3), and Masson-Goldner’s Trichrome Stain (column 4) were used to compare the morphological and histological differences among the control group (row 1), defect group (row 2), and ICG/BP group (row 3). (C) Total percent of bone regeneration and comparison between the defect group (No Repair) and ICG/BP group (ICG/BP Repair). (D) SWIR imaging of a tibial defect with implanted ICG/BPs post-surgery (top) over the course of 8 weeks, and the corresponding CT of the bone (bottom). (E) Mean collected data of SWIR fluorescence intensity over 8 weeks. (One way ANOVA followed by group post hoc comparison was performed on select data, statistical significance is quantified as: ****; ***; **; *, ns = no significance).
In situ monitoring of ICG/BP degradation.
The SWIR window was used to monitor and quantify the degradation of the ICG/BPs after their implantation into the non-healing tibia defect (Fig. 4D, row 1). By distinguishing the Region-of-Interest (ROI) using MATLAB®, we were able to quantify the SWIR signal using the mean intensity value of the measured area. The shape of the ROI was created by using known anatomical distances measured on the leg and re-creating the measured defect size at the onset of the experiment (4 × 4 mm). Immediately following implantation, the ICG/BPs exhibited an intense fluorescent signal within the defect area. The fluorescence signal emanating from the implanted ICG/BPs exhibited an abrupt decline during the initial week post-implantation. Following the first week, a discernible, consistent reduction in fluorescence signal was observed, and after 8 weeks of ICG/BPs implantation, only a limited fluorescent signal remained. Quantitative analysis using the mean values calculated from the ROI revealed a continual decrease in fluorescence signal over time, indicating the degradation of the particle graft with approximately ∼5–10% of the signal remaining after 8 weeks (Fig. 4E). Additionally, we measured bone samples ex vivo via CT to verify the bone growth corresponding to fluorescence decay (Fig. 4D, rows 2 & 3). At the end of 8 weeks, there is roughly 10 ± 3.8% fluorescence remaining, which is consistent with the near complete bone regeneration shown in Fig. 5D.
Fig. 5.
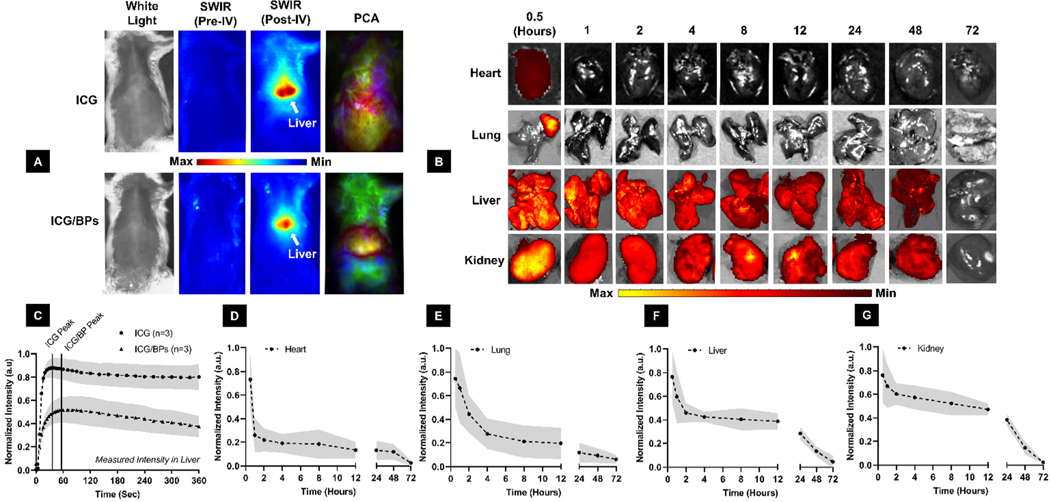
Dynamic in situ monitoring of ICG/BPs and biodistribution. (A) White light images (column 1) were used to subtract background fluorescence to obtain the pure SWIR spectrum image both pre- (column 2) and post-IV administration (column 3). PCA (column 4) was used to quantify segment anatomical regions of interest, in this case the liver, over the course of the experiment. (B) Ex vivo fluorescence imaging of the heart, lungs, liver, and kidneys to determine the biodistribution of the ICG/BPs up to 72 hours post-administration, measured in the NIR window (840 nm) (C) Normalized fluorescence intensity of both free ICG and the ICG/BPs collected from PCA analysis, with a noticeably later peak of intensity in the liver for the ICG/BPs than the free ICG dye. Mean fluorescence intensity in the heart (D), lungs (E), liver (F), and kidney (G) collected from (B).
2.6. In vivo evaluation of ICG/BPs for systemic administration
In situ monitoring using dynamic SWIR imaging.
SWIR dynamic imaging demonstrated the in situ monitoring capability of the ICG/BPs after IV injection (Fig. 5A, columns 1–3). Using the previously established methods for PCA decomposition,62 we segmented the liver and quantified the biodistribution of the ICG/BPs and free ICG, demonstrating the difference in fluorescent intensity and the pharmacokinetics of each substance over time (Fig. 5A, column 4). The data extracted from each ROI were used partially to determine that intensity was true ICG/BP signal and not just free ICG dye, and we determined that the lower intensity and difference in kinetics through the liver verifies the ICG is still bound within the BPs (Fig. 5C).
Biodistribution and systemic clearance of ICG/BPs.
Our visual comparisons of free ICG and ICG/BP clearance over 6 minutes showed an initial pass through the plural cavity and eventual passage into the liver and intestines (ESI. V1 & V2,† respectively). PCA analysis further showed an initial increase in fluorescence as the ICG/BPs were filtered into the liver and the gradual decrease after roughly 4 minutes post-administration. To determine the full clearance method of the ICG/BPs, the biodistribution was further analyzed ex vivo using fluorescent analysis of each organ in the NIR window (Fig. 5B). We found there was minimal ICG/BP presence in the heart after 1 hour (Fig. 5D), and a strong decrease in the lung during the first 4 hours (Fig. 5E). The large majority of signal was present in the liver (Fig. 5F) and the kidney (Fig. 5G). While the liver contains the highest signal for that of any tissue, the kidney is the organ that retains most of its original signal over the first 12 hours after injection. Excluding the heart, each organ showed a gradual decrease in fluorescence, with negligible signal in any organ after 72 hours. The only exception to this trend was the spleen; the ICG/BPs were cleared from the spleen after 72 hours, but varying amounts of the ICG/ BPs were found at each time point (ESI. 7†).
Systemic biocompatibility of ICG/BPs.
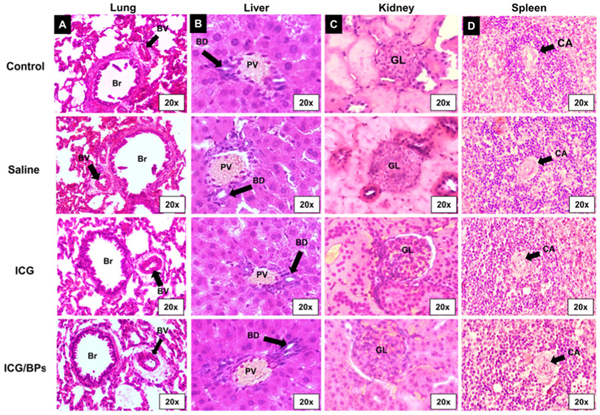
Our first observations came from the animals themselves immediately after IV administration; upon returning from the state of anesthesia, animals were alert and mobile with no typical indication of stress (rough hair, hunched posture, lethargy), indicating the initial biocompatibility of the ICG/BPs. We also performed histological analysis using H&E staining on major organs including lung, liver, kidney, and spleen (Fig. 6). After 15 days, we used anatomical markers relevant for each organ to evaluate potential damage, including necrosis, scarring, or any other abnormalities, and we determined there was no noticeable damage.
Fig. 6.
H&E staining of organs 15 days after systemic administration. Images of the (A) lung, (B) liver, (C) kidney, and (D) spleen in different experimental groups, with no noticeable changes between groups. (Br = Bronchiole, BV = Blood Vessel, PV = Portal Vein, BD = Bile Duct, GL = Glomerulus, BC = Bowman’s Capsule, DCT = Distal Convoluted Tubule, PCT = Proximal Convoluted Tubule, CA = Central Arteriole).
3. Discussion
Bone ECM-based scaffolds derived from decellularized bone have been widely utilized as a regenerative therapy for bone repair because of their close-to-native microstructures, mechanical properties, and osteoinductivity/osteoconductivity features.23,25–28 To better meet clinical needs, ECM-based biomaterials can be made or incorporated into a variety of forms that can include patches, scaffolds, powders, bio-inks, hydrogels, and nanomaterials. To our knowledge, this study is the first instance of transforming the entire ECM of bone tissue into NPs using our combinative fabrication methodology.
Protein-based NPs can be fabricated with many methods, including desolvation, coacervation, self-assembly, thermal gelation, spray-drying, emulsification, and chemical crosslinking.63–66 During particle formation, proteins undergo conformational changes depending on the composition and concentration of protein. Other environmental factors also play an important role in NP formation, such as solution pH, temperature, stirring rate, ionic strength, ratios of chemical agents, and crosslinking method, all of which influences the size, electrostatic interactions, surface charge, and stability of the final particles.67 During design, NPs can be generated to encapsulate various types of molecules, drugs, or dyes as an in vivo guidance and nano-delivery system, capable of targeting and binding to specific moieties and then releasing cargo with controlled and sustained release mechanisms. In this study, we encapsulated free ICG dye within these novel BPs, designed for fluorescent-guided imaging practices in bone treatments and therapy, while further demonstrating their potential use in localized or systemic applications by using different rat models.
The size, shape, and surface properties of NPs are critical parameters on their performance in vivo. These parameters will ultimately influence their therapeutic and diagnostic applications, as they impact many different physiological functions including cellular uptake, blood circulation half-life, organ accumulation, and subsequent clearance.68–72 In this study, we report a large discrepancy in the size of the ICG/BPs using TEM (79.5 ± 25.4 nm) and DLS (171 ± 11.41 nm) measurements. Classifying the “true” size of NPs is difficult, as there is no metrological technique that can accurately characterize the morphological features of a NP. Electron microscopy techniques, including TEM and SEM, are still considered the gold standard as their high spatial resolution (<5 nm) allows for visualizing the morphology of particles with excellent quality.73–77 Overall, both TEM and DLS measurements and Zeta Potential measurements provide us with a good estimate of the “true” size of the ICG/BPs and their surface charge, respectively. In general, NPs with an overall size range of 10 nm to 200 nm are considered optimal for intravenous injection without embolization concerns. NPs that are smaller than 10 nm will be rapidly cleared by the kidney, whereas NPs that are larger than 200 nm will be mostly cleared by the mononuclear phagocytic system.71,72,78–80
Recent attempts, especially in cancer related research,81–83 have been made to encapsulate ICG in nanocarriers to overcome limitations of ICG that include a poor photostability, concentration-dependent aggregation, non-specific binding, and a short half-life (1.5–3 minutes in rats62,84 and 2–4 minutes in humans85–87). We demonstrate in this study the successful encapsulation of ICG within the BPs and the effect this encapsulation has on its newly enabled optical capabilities based on these experimental findings.
First, we investigated the impact of various starting concentrations of ICG on the loading efficiency of BPs. Our objective was to determine an optimal ICG-to-ECM ratio that maximizes loading efficiency without wasting excess ICG and without compromising the final fluorescent output. During encapsulation, we observed that an increase in the initial amount of ICG had an adverse effect on loading efficiency, establishing an inverse relationship between the initial ICG quantity and subsequent loading efficiency. However, we found fluorescence saturation and potential quenching with values above 10%, leading us to conclude that 10% is optimal for our experiments.
Second, we examined the in vitro degradation of the ICG/BPs, finding a slower degradation rate with ∼12% total protein loss over the course of 7 weeks without perturbation. The degradation rate of a biomaterial is an important characteristic for bonerelated applications. An ideal biomaterial should have a degradation rate that is commensurate with the new bone growth-ratio to better maintain the growth of new bone. Moreover, the degradation rate of protein-based NPs is also closely related to the release rate of any encapsulated cargos.88–93
Third, we determined the release rate of ICG from the ICG/BPs during in vitro incubation with blood plasma. We found that the amount of ICG released from the ICG/BPs was initially very high, suggesting that the interaction of the ICG/BPs in the bloodstream disrupts the bonds of proteins that are relatively weak, resulting a quick release of ICG that is most likely near the surface of the particles. While our study provides valuable insights, it’s important to acknowledge that we did not extensively investigate the impact of synthesis variables on the characteristics of the ICG/BPs, which include such things as the pH and temperature of the solution, or the desolvating and crosslinking agent concentrations. These variables, in turn, would likely influence parameters such as particle size, zeta potential, degradation, and the loading efficiency and release rate of ICG. These aspects remained relatively underexplored in our current study but will be a central focus of future research aimed at comprehensively characterizing the ICG/BPs.
After particle characterization, we explored basic optical properties of the ICG/BPs using absorbance and fluorescence spectra. We found there is a broad enhancement of the absorption spectrum and the ICG emission curve. The much broader peak in absorbance within the ICG/BPs is most likely due to the complex diffraction patterns of the many proteins within the ICG/BP solution itself, causing uneven aggregation. It has been previously reported that encapsulation of ICG may have each spectrum due to stokes’ shifting and changes in the chemical structure of ICG.94,95
While traditional NIR windows serve well in various clinical applications, the use of shortwave IR (SWIR) extends the observation depth to over 1 cm within living animals.96 This feature proved invaluable for our study on the in situ degradation of the ICG/BPs in a tibial defect model, as SWIR enables robust fluorescence monitoring through skin, periosteum, muscle, and bone tissue. With the promising results in the NIR window during our in vitro studies, we chose to evaluate their performance in the SWIR window (950 nm) in vivo. In this window, the ICG/BPs exhibited a high fluorescent profile when compared to increasing concentrations of free ICG in water. The encapsulation of ICG within the BPs altered the fluorescent profile of free ICG, a change attributed to the exposure to proteins during the synthesis process. During this step, free ICG binds to various proteins in the stock solution, though the specific nature of this binding and the proteins involved remain unexplored. Importantly, encapsulation within the BPs resulted in an increased photostability of the free ICG dye, likely stemming from the interaction between ICG and ECM proteins during synthesis and subsequently modifying the initial fluorescence.
The cellular fate of NPs is intricately governed by their physiochemical attributes, encompassing composition, size, shape, surface charge, and hydrophobicity/hydrophilicity.97,98 To test the cellular uptake and cellular compatibility, ICG/BPs were co-cultured with BMSCs and the success of entry into cells and intracellular localization of the ICG/BPs were confirmed by confocal microscopy and TEM imaging after 24 hours of culture time, with no apparent toxic effects on the BMSCs. ICG/BPs not only demonstrated biocompatibility but also augmented osteogenic differentiation of stem cells, evidenced by upregulated expression of OCN and RUNX2 transcription factors, along with increased matrix mineralization as observed through alizarin red staining. These findings underscore the potential of the ICG/BPs to not only be efficiently taken up intracellularly but also to induce osteogenic differentiation in stem cells, indicating promising applications in bone regeneration.
Building upon the encouraging outcomes of our in vitro studies, we investigated the translational potential of ICG/BPs for localized and systemic applications in orthopedic treatments. Initial experiments focused on using the ICG/BPs as a bone grafting substance for repairing critical sized, nonhealing tibial defects in rats. Eight weeks after locally implanting the ICG/BPs in the surgically created tibial defect, both CT and histological evaluations demonstrated that the ICG/BPs could efficiently stimulate and promote new bone regeneration and formation compared to a defect without any bone grafting. Furthermore, the locally implanted ICG/BPs were capable of in situ monitoring in the SWIR window over the course of 8 weeks. The observed phenomena of rapid degradation at the onset of the experiment may be due to the clearance of loosely associated material by the lymphatic system, resulting in the confinement of the signal to within the defect site. Additionally, we suggest that a plausible contributor to the initial steep decline in vivo is the prompt release of ICG due to the disruption of weaker protein bonds upon contact with blood, post-surgical fluids from swelling over the defect area, and alterations in temperature and pH, leading to the disruption of weaker protein bonds and accelerating the degradation process.
After incubation with blood plasma in vitro, Fig. 2G illustrates this initial rapid degradation of the ICG/BPs and the release pattern of the encapsulated ICG. During this initial steep decline, roughly ∼40% of the initial amount of free ICG dye bye mass had been released from the ICG/BPs. However, only another ∼11% of ICG was lost over the next 4 days, resulting in roughly ∼51% of ICG remaining. Our findings demonstrate that this rapid onset of ICG loss was consistent with the interaction of the ICG/BPs and blood plasma both in vitro and in vivo; however, this initial rapid degradation was not as apparent when the ICG/BPs were incubated with PBS.
During our fabrication process, the ICG/BPs are thoroughly washed, removing any excess ICG dye that wasn’t encapsulated during fabrication, thus ensuring any fluorescence is coming from encapsulated ICG. The in vitro degradation of the ICG/BPs when switching between different conditions, such as PBS and blood plasma (Fig. 2F and G, respectively) is vastly different, indicating a much more rapid onset of degradation when conditions are changed from a more neutral state (PBS) to a more dynamic state (plasma). Future studies will more closely examine this degradation phenomena and how the interaction with the aforementioned physiological conditions influence the characteristics and performance of the ICG/BPs.
We found that the ICG/BPs aid in the reconstruction and regeneration of local bone defects while simultaneously offering continuous qualitative assessment of bone healing and regeneration using SWIR fluorescence imaging. The outcomes align with prior studies utilizing NIR optical imaging techniques for monitoring the degradation of bone scaffolds.99,100
There are inherent differences between the BPs and traditional DBM products. First, many DBM products are not solely comprised of proteins as they still retain the mineral components naturally found in bone tissue, such as Hydroxyapatite or Calcium Phosphates. Second, the interactions each of these grafting materials would have with the surrounding environment may differ due to their structure, as the BPs are capable of being taken up by the cells compared to the DBM which may induce the cells to behave differently. As a nanoparticle-based graft, our results suggest they have osteoconductive and osteoinductive properties and provide a naturally rich environment for cellular migration, differentiation, and growth. Future studies will look closer into the interactions of the BPs with cells and how they might behave both in and around cells.
We have demonstrated that the novel BPs are capable of stimulating bone growth as a stand-alone grafting substance. However, a limitation of this study was exploring the optimization of the fabrication process for potentially enhancing the therapeutic efficacy of the BPs. Our fabrication process shows an easy and straightforward way by which to enhance their characteristics, such as by attaching or encapsulating other biological agents like the growth factor or bone morphogenetic proteins (BMPs). Our future studies will look towards optimizing the BPs for different therapeutic applications and fully utilizing both their innate regenerative capability and their nanoparticle functionality.
Moving to the systemic level, our second objective involved in situ monitoring of the ICG/BPs after intravenous administration and comparing the ICG/BPs to free ICG dye. The use of SWIR dynamic imaging enabled quantitative assessment of the in vivo biodistribution, organ accumulation, and systemic clearance of each injected substance. Following intravenous administration of the ICG/BPs, all animals exhibited normal behavior without acute or chronic reactions. No observable signs of physical abnormalities or weight loss were noted. The in vivo biodistribution of ICG/BPs was systematically tracked using SWIR imaging over 7 minutes, and Principal Component Analysis (PCA) decomposition of epifluorescence images enabled anatomical segmentation of the liver, revealing a delayed arrival of the ICG/BPs, indicative of an extended circulation time in the bloodstream compared to the free ICG dye. Our results underscore the in situ monitoring capabilities, in vivo stability, and initial biocompatibility of ICG/BPs. Ex vivo analysis confirmed preferential accumulation in the liver and kidneys, suggesting efficient clearance through the hepatic and renal pathways to mitigate potential toxicity. Histological examinations of major organs of animals that received ICG/BPs revealed no notable changes, which was determined by identifying and observing anatomical landmarks, affirming the biocompatibility and safety of ICG/BPs. However, despite these promising findings, rapid clearance from the ICG/BPs from the bloodstream was observed due to a lack of bone-specific targeting and binding. To enhance circulation times within the bloodstream, our future research will focus on modifying the surface properties of the ICG/BPs by coating the surface with targeting peptides or ligands and adjusting properties such as the size and surface charge of the particles, all of which can influence interactions with the protein corona in the bloodstream. These modifications will be explored in subsequent studies to improve the functionality of the ICG/BPs as a potential delivery system and extend their circulation time within the bloodstream.
4. Materials and methods
4.1. Bone decellularization and preparation of bone ECM ‘stock’ solution
Fresh porcine tibias were collected from approved Medical College of Wisconsin (MCW) vendors and decellularized with the same protocol as previously described [68], in which the tibias were sectioned into small pieces and fully demineralized with 0.5 M hydrochloric acid (HCL, Sigma-Aldrich, St Louis, MO) for ∼3 days and decellularized with 0.5% sodium dodecyl sulfate (SDS) and 1% Triton X-100 mixed solution (Sigma-Aldrich, St Louis, MO) for another ∼5 days under agitation at room temperature until all the bone pieces became pale white and porous appearance (Fig. 1A). After entirely washing with distilled water, the bone ECMs were freeze-dried at −80 °C (Cole-Parmer, Vernon Hills, IL) and milled into a fine powder using a Thomas Wiley® Mini Cutting Mill (Thomas Scientific, Swedesboro, NJ).
The ECM powders were pre-treated with HCL solution (1 gram of ECM powder per 100 ml of 1 M HCL) under constant stirring for 48 hours at 4 °C. After the 48 hours, the pH of the solution was adjusted to 2.5 by adding 50% sodium hydroxide (NaOH, Sigma-Aldrich, St Louis, MO) solution. After stirring for another 15 minutes at 4 °C, the whole solution was moved to a hot stir plate with the temperature set to 45 °C. Pepsin (Sigma-Aldrich, St Louis, MO) was then added into the whole solution at 15% w/w (g) and left to stir for 48 hours at 45 °C. A final adjustment to the pH was made using more NaOH solution, bringing the final pH to ∼7. The digestion is then removed from heat while still under constant stirring, and after coming to room temperature, the solution is vacuum filtered using a 140 nylon net filter. After filtration, the final bone ECM digestion was additionally desalted by adding acetone dropwise to the solution at a 1 : 1 v/v (mL) ratio following by centrifugation at 8000 RPM (6000g) for 20 minutes at 4 °C. The resulting pellet was collected and resuspended in distilled water by vertexing and immersion blending, hereby making the ECM ‘stock’ solution (Fig. 1A). The protein concentration of the ‘stock’ solution was measured using the Pierce™ bicinchoninic acid assay (BCA assay, ThermoFisher Scientific, Waltham, MA) and further adjusted to a final concentration of 330 ml−1. The final stock solution could be stored at −20 °C until needed.
4.2. ICG/BP fabrication
As indicated in Fig. 1B, ICG dye (Sigma-Aldrich, St Louis, MO) was dissolved in the ECM ‘stock’ solution at 10% w/w based on the total protein amount (mg) of the ECM ‘stock’ solution. Acetone was then added dropwise into the ICG and ECM mixed solution (the volume ratio of acetone to the ICG/ECM solution was 3 : 1) at a rate of 1 mL min−1 with a syringe pump under constant stirring at 500 RPM at room temperature. After desolvation, 50% glutaraldehyde solution was then added into the above solution for chemical crosslinking, with a ratio of 33 of Glutaraldehyde per 1 mL of the initial ‘stock’ solution volume (pre-desolvation) under constant stirring at 500 RPM for 30 minutes at room temperature. The final ICG/BPs were washed with distilled water three times by centrifugation, and the resulting pellet was redispersed into distilled water by sonication using a Branson® Sonifier 150 (Brookfield, CT) for 2 minutes at 9 W while on ice.
We also prepared non-ICG-encapsulated BPs (BPs) for cell culture and immunofluorescence staining. The BPs were prepared with the same protocol as making the ICG/BPs excluding the ICG loading step. The obtained ICG/BPs or BPs were freeze-dried at −80 °C for experimental characterization or long-term storage (Fig. 1C).
4.3. Morphological characterization of ICG/BPs
For transmission electron microscopy (TEM), the lyophilized ICG/BPs were dissolved in 0.1 M sodium phosphate buffer (PBS, pH = 7.4) by sonification and fixed in 1% Osmium tetroxide (Sigma-Aldrich, St Louis, MO) for 2 hours at room temperature. After fixation, the ICG/BPs were washed with PBS buffer following by another wash with distilled water. The final particle samples were deposited onto a 400 mesh formvar-carbon-coated copper grid, air-dried at room temperature, and imaged with a JEOL 2100 LaB6 microscope (200 kV).
A Malvern Zetasizer 3000 dynamic light scattering (DLS) system was used to measure the hydrodynamic size distribution of the fabricated ICG/BPs. For these measurements, pellets of ICG/BPs were resuspended in 1 mL of Milli-Q water and sonicated for 2 minutes on ice at 9 W. DLS measurements were taken at 25 °C. The approximate net charge on the surface of the ICG/BPs was calculated as the Zeta (ζ) potential. Zeta potential was calculated using the Smoluchowski approximation, and parameters optimized for proteins in solution (water) were chosen for particle characterization. Raw data was exported and analyzed using Microsoft Excel. For reproducibility, five measurements () of each sample were collected.
4.4. Determination of loading efficiency
For Loading efficiency (LE) measurements of ICG within the ICG/BPs, the absorbance of the samples at wavelength ∼780 nm was measured using a Gen5® plate reader. The absorbance of each sample was compared with the standard calibration curve (ESI. 1†) of ICG to determine the approximate ICG concentration within each sample. ICG was dissolved in the ECM ‘stock’ solution at different concentrations (5, 10, 15, 20% w/w) based on the total protein amount (mg) of the ECM ‘stock’ solution, and ICG/BPs were synthesized as described as section 2.2. LE was used to estimate the loaded amount of ICG within the BPs, using the following eqn (1):
| (1) |
4.5. In vitro ICG/BP degradation and ICG release rate
In vitro particle degradation.
To investigate the in vitro degradation rate of the ICG/BPs, lyophilized ICG/BP samples were pre-measured, fully immersed into 2 mL of PBS solution, and incubated at 37 °C for 6 weeks. Each week during ICG/BP incubation ( at each time point), 100 of the top solution was collected and the protein concentration in the collected solution was measured by a BCA assay according to the manufacturer protocol. In vitro degradation rate of the ICG/BPs was calculated using the following eqn (2):
| (2) |
In vitro release of ICG.
To investigate the in vitro release of ICG from the ICG/BPs during degradation, 10 mg of lyophilized ICG/BPs was dissolved in a 1 mL Eppendorf tube containing plasma isolated from rat blood and incubated at 37 °C for 5 days. At pre-determined time intervals (0.5, 1, 2, 3, 4, 8, and 16 hours and 1, 2, 3, 4, and 5 days), the tubes were centrifuged, and the supernatant was removed and replaced with the same amount of fresh plasma. The ICG/BPs in each tube were re-suspended and placed back into incubation at 37 °C. The absorbance of the supernatant at each time point was measured at 780 nm, the peak absorbance value for ICG, using a Gen5™ Microplate Reader and Imager Software package (BioTek, Winooski, VT). The release rate of ICG from the ICG/BPs at each time point was calculated using the eqn (3):
| (3) |
where was the absorbance of the amount of ICG measured in the supernatant at each time point and being the absorbance of the amount of ICG in the ICG/ECM mixed solution prior to ICG/BP formation. The release study was performed in triplicate for each time point (), with all samples kept in black test tubes in an effort to prevent samples from any unnecessary light exposure during sample handling.
4.6. Fluorescence measurements and photostability
Imaging methods.
The SWIR imaging system used for static and dynamic fluorescence imaging was comprised of an 808 nm diode laser (Diomed, D15 Plus) used for illumination, an InGaAs focal plane array (Princeton Instruments, NIRvana 640ST) with a 25 mm lens (Navitar, SWIR-25), in conjunction with 808 nm notch (Semrock, StopLine®, NF03–808E-50,) and 950 nm long pass (Semrock, EdgeBasic™, BLP01–980R-50) filters. For static fluorescence imaging of bone regeneration, an 808 nm power density of approximately 5 mW cm−2 was used. Dynamic contrast enhanced imaging was performed with approximately 5–25 mW cm−2 for free ICG and the ICG/BPs respectively, with a temporal resolution of 100 ms.
An IVIS Spectrum CT fluorescent animal imager (PerkinElmer, MA, USA) was additionally used for fluorescence analysis in the NIR window only. Samples were placed into the imaging chamber and presets were chosen using the LivingImage® software package specific to ICG (excitation/emission = 745/840 nm). Images were analyzed using the LivingImage® software package, and the quantified fluorescent intensity was represented by the average radiant efficiency [(p s−1 cm−2 sr−1)/( cm−2)] after background subtraction.
Fluorescence characterization.
Free ICG dye was mixed into Milli-Q water at a starting concentration of 1000 and diluted into various concentrations to create a standard reference curve (ESI. 2†). To better simulate physiological conditions of ICG in blood plasma, a standard reference curve was also created using free ICG dye diluted in 10% Bovine Serum Albumin (BSA) solution. All references were aliquoted into black-bottomed well plates to prevent fluorescent bleed-through during imaging.
A theoretical loading efficiency of ICG at 100% was used to create the scale for a concentration curve of the ICG/BPs. The fluorescence intensity of aliquots of ICG/BPs (100 ) that are equivalent to 40, 20, 15, 10, and 5 of ICG were measured and compared to the standard reference curves of the free ICG dye.
Long-term photostability.
To investigate the long-term fluorescence stability of the ICG/BPs, 10 mg samples were placed in Eppendorf tubes and kept either exposed to light constantly or stored in the dark at room temperature. The fluorescent intensity was measured weekly for up to 12 weeks.
4.7. Cellular uptake of ICG/BPs and cellular compatibility
ICG/BPs Co-cultured with BMSCs.
Human bone marrow-derived mesenchymal stem cells (BMSCs) were purchased from Lonza Inc. (Morristown, NJ). The cells were resuspended and seeded in 75 mm flasks at a density of 103 cells per cm2 with Mesenchymal Stem Cell Growth Medium (Lonza Inc.) at 37 °C and 5% CO2 atmosphere. The third passage of the BMSCs were seeded at a density of 103 cells per cm2 in each well of a 24-well cell culture plate. After the cells reach 80% confluence, they were randomly divided into the following groups: (1) Co-culture group (BMSCs co-cultured with ICG/BPs): lyophilized ICG/BPs were dispersed in the osteogenic differentiation medium [DMEM, 10% FBS, 1% penicillin, streptomycin and dexamethasone (0.1 ), β-glycerophosphate (10 mM), and ascorbic acid (50 ), Lonza Inc.] at a concentration of 50 ml−1 by sonification, and 0.5 ml of the ICG/BP-infused medium was added into each well of the cell culture plate. To better characterize the cell viability and osteogenic differentiation of the BMSCs, BPs (non-ICG encapsulated) were co-cultured with BMSCs using the same method as the ICG/BPs. After 24 hours of co-culturing, the medium was changed to the osteogenic differentiation medium (no ICG/BPs or BPs in the medium). (2) Differentiation control group: BMSCs were cultured with 0.5 ml of osteogenic differentiation medium in a cell culture plate (no particle co-culturing). (3) Control group: BMSCs were cultured with 0.5 ml of Mesenchymal Stem Cell Growth Medium in a cell culture plate (no particle co-culturing). All the cells from the above groups were cultured at 37 °C in a 5% CO2 atmosphere for 4 weeks with the medium changed twice per week.
Intracellular uptake.
After BMSCs were co-cultured with the ICG/BPs for 24 hours, the cells were washed three times with PBS and incubated with Rhodamine Phalloidin (abcam, Waltham, MA) for 20 minutes at room temperature for actin filament staining. The cell nucleus was double stained with 4′,6-diamidino-2-phenylindole (DAPI, abcam, Waltham, MA) at 1 mL−1. The cells were observed using a Nikon A1 Laser Scanning Confocal Microscope. Cells with no ICG/BPs co-culturing were used as controls.
TEM imaging was used to identify the intracellular location of the ICG/BPs after co-culturing. After co-culturing the BMSCs with ICG/BPs for 24 hours, the cells were fixed in 2.5% glutaraldehyde at 4 °C overnight, washed with PBS, and fixated with 1% osmium tetroxide at 4 °C for 4 hours. After fixation, the cells were stained with 2% uranyl acetate, dehydrated in a graded ethanol-acetone series, and then embedded in pure Epon resin. After slicing with a ultramicrotome (Leica, Wetzlar, Germany), the final cell sections were mounted on 200 mesh copper grids and examined with a JEOL 2100 LaB6 microscope operated at an accelerating voltage of 100 kV. Cells that were not co-cultured with ICG/BPs were used as controls.
Flow cytometry was used to quantify the intracellular uptake of the ICG/BPs. After co-culturing the BMSCs with ICG/ BPs for 24 hours and 48 hours, respectively, the cells were washed three times with PBS solution, detached with TrypLE Reagent (Fisher scientific, Hampton, NH), and collected by centrifugation at 2000 rpm, followed with two washing cycles using PBS. The cell suspension was filtered through 400-mesh sieves and subjected to a BD LSRFortessa™ X-20 Cell Analyzer (BD Biosciences, Franklin Lakes, NJ). Cells that were not co-cultured with ICG/BPs were used as controls.
Cell viability.
After 4 weeks of co-culture, cells from all the groups were stained with the Live/Dead Cell Viability Assay (ThermoFisher Scientific Inc., Waltham, MA) to determine cell viability, and the cell nuclei were double stained with 1 ml−1 DAPI.
Osteogenic differentiation.
To determine the osteogenic differentiation after 4 weeks of co-culture, cells from all the groups were fixed with 4% paraformaldehyde for 30 minutes and blocked with 5% normal serum/0.3% Triton X-100 solution for 60 minutes. The cells were then incubated with primary antibodies [Monoclonal Anti-osteocalcin antibody (1 : 100 dilution, abcam, Cambridge, MA) and the Anti-RUNX2 antibody (1 : 100 dilution, abcam, Cambridge, MA)] overnight at 4 °C. After that, cells were washed three times with PBS and stained with the secondary antibody, Goat Anti-Rabbit IgG H&L (Alexa Fluor® 488) (1 : 200 dilution, abcam, Cambridge, MA), for 1 hour at room temperature. Negative control samples were not subjected to primary antibody incubation. After washing three times with PBS, the nuclei were double stained with 1 mL−1 DAPI. The cells were observed using a Nikon A1 Laser Scanning Confocal Microscope. To measure calcium deposition, cells from different groups were fixed with 10% formalin for 30 minutes followed with three times of washing with PBS and three times of washing with distilled water. The cells were then stained with 1× alizarin red S solution (Millipore, Billerica, MA) for 30 minutes, and washed four times with distilled water. Cell images were then captured with a light microscope.
4.8. In vivo evaluation of ICG/BPs for tibial defect repair
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Medical College of Wisconsin (MCW) and approved by the Institutional Animal Care and Use Committees (IACUC) at MCW.
Animals.
Sprague Dawley (SD) rats (Charles Rivers, Inc., 8–10-week-old, males and females) were used and split into 3 groups: (1) Tibial receiving no defect (control group, ), (2) tibial defect without particle implantation (defect group, ), and (3) tibia defect implanted with ICG/BPs (ICG/BP group, ). The rats in the control group didn’t undergo any surgical procedures. All the rats in the defect group and the ICG/BP group were anesthetized using isoflurane via inhalation. The fur on the right leg was shaved and skin was sterilized using iodine solution. A small incision was made to expose the tibia in right leg. A cylindrical bone defect, large enough to classify as non-healing (4 mm diameter × 2 mm thickness) was created in the proximal portion of the tibia using a surgical drill while irrigating with cold 0.9% sterile saline solution. The defect was either not treated (defect group) or fully filled with lyophilized ICG/BPs (ICG/BP group, Fig. 4A). Muscle over the defect was sutured with 6–0 sutures, and skin was closed over the wound with 4–0 sutures. Animals were monitored over the course of 8 weeks for any leg abnormalities and possible compound fractures, in which case animals were euthanized.
Evaluation of bone growth using computed tomography (CT).
An IVIS Spectrum CT was used to analyze the bone healing of the tibia defects using the following imaging parameters: 90 kV, 8 mA, and 0.3 mm voxel sizes. Eight weeks post-surgery, animals were euthanized and the right tibias from all animals in each group were harvested. Samples were washed with PBS and fixed with 10% neutral buffered formalin for 24 hours prior to CT imaging. The total remaining area of the defect detected from CT images was measured using ImageJ® software. 3D renderings of the tibia and defect area were created using the Living Image® software package included with the IVIS Spectrum CT. Total percentage of the bone healing was calculated using following eqn (4):
| (4) |
In situ monitoring of ICG/BP degradation with SWIR after implantation.
Fluorescent intensity was used to evaluate the in vivo degradation of ICG/BPs after implantation into tibia defects using the SWIR imaging system and methodology previously described in section 2.6. After implanting the ICG/BPs into the defect and suturing the muscle and skin over the defect, the tibia was imaged immediately after surgery and 1 and 3 days and every week post-surgery up to 8 weeks. SWIR imaging data was analyzed, converted, and filtered into a raw image using algorithms developed for MATLAB®. A region-of-interest (ROI) was drawn around the defect area, and signal intensity values were calculated using mean filtering.
Histology.
The right tibias in all groups were isolated and fixed with 10% neutral buffered formalin for 24 hours followed by washing three times with PBS. For tibias used for the Goldner’s Masson trichrome staining, samples were dehydrated with increasing concentrations of ethanol and embedded in methacrylate. Longitudinal sections of the tibia (200–300 ) were prepared with a diamond-precision parallel saw (EXAKT300CP, Norderstedt Germany). The defect region was then reduced to a thickness of 10–15 by micro grinding and polishing. The slides were stained with the Goldner’s Masson trichrome staining kit (Sigma, St Louis, Missouri) following manufacturer’s instructions. For Hematoxylin and Eosin (H&E) staining, tibias were decalcified using OSTEOMALL® solution (SigmaAldrich, St Louis, Missouri) following manufacturer’s instructions. After decalcification, samples were washed thoroughly with PBS, dehydrated with increasing concentrations of ethanol, washed with xylene solution, and placed into paraffin for embedding. Cross sections measuring 8 thickness were used, placed on glass slides for H&E staining (Sigma, St Louis, Missouri), and observed using a brightfield microscope.
4.9. In vivo evaluation of ICG/BPs for systemic administration
Animals.
SD rats (8–10-week-old, males and females) were used for systematic administration of ICG/BPs and compared with control groups including (1) no injection, (2) saline injection, and (3) free ICG dye injection. For the free ICG group, ICG dye was dissolved in Milli-Q water and concentrated to 400 . For the ICG/BP group, lyophilized ICG/BPs were dissolved in PBS solution at 5 mg ml−1 based on the dry weight of ICG/BPs (∼440 ICG) and sonicated. Rats under isoflurane anesthesia were placed on a heating pad underneath the SWIR imaging system and 0.5 ml of free ICG dye, the ICG/BPs, or saline solution was intravenously (IV) injected via tail vein at a rate of 0.1 ml s−1 using a syringe pump.
In situ monitoring of ICG/BPs using dynamic SWIR imaging.
Immediately after IV injection, each rat was in situ, real-time imaged to capture the in vivo delivery and biodistribution of free ICG or the ICG/BPs for up to 7 minutes post-administration. Dynamic imaging data was collected using the SWIR imaging system and methodology previously described in section 2.6. The free ICG dose for dynamic contrast enhanced imaging was approximately 0.25 mg kg−1, while the ICG/BPs dosage was 4 mg kg−1. Both free ICG and the ICG/BPs were administered IV via tail vein with a 24-gauge catheter and syringe pump set to 0.2 mL s−1. Dynamic imaging was performed for 6 minutes to assess biodistribution. Data was analyzed, filtered, and converted into video using algorithms developed for MATLAB®. Principal component analysis (PCA) was performed in accordance with previously published methods.62 At the completion of the experiment, rats were kept on a heating pad and monitored for any signs of complications.
Biodistribution and systemic clearance.
To investigate the subsequent accumulation and clearance of ICG/BPs in major organs after systematic administration, rats were euthanized at different timepoints (0.5, 1, 2, 4, 8, 12, 24, 48, and 72 hours, at each timepoint) after IV injection of the ICG/BPs and the heart, lungs, liver, and kidney were harvested for ex vivo analysis. The fluorescent intensity of each organ was measured by the IVIS spectrum CT, with preset settings for ICG in the NIR window (excitation = 745 nm/emission = 840 nm).
Histology.
To evaluate any potential damage to major organs after ICG/BP administration, all rats from different groups were euthanized 15 days post-administration and major organs including the lung, liver, kidney, and spleen were harvested. Samples were washed with PBS solution, fixed with 10% formalin solution for 24 hours, dehydrated with increasing concentrations of ethanol, washed with xylene solution, and placed into paraffin for embedding. Ground sections measuring 8 were used placed on glass slides for H&E staining (Sigma, St Louis, Missouri) and observed using a brightfield microscope.
4.10. Statistical analysis
Data was analyzed using Excel or GraphPad Prism® and expressed as mean ± standard error. Comparisons were determined by ANOVA (SAS 9.0). The Holm-Sidak Test, which can be used for pairwise comparisons and comparisons versus a control group, was used for post-hoc comparisons. Data correlations were determined from mean values using a Pearson-product moment correlation (parametric) or Spearman Rank Order Correlation (nonparametric). Results are considered significantly different at .
5. Conclusions
This study introduced a new type of NPs, denoted as ICG/BPs, formed by encapsulating ICG dye within a bone-based NP crafted from the complete decellularized bone ECM. The ICG/BPs demonstrated a nano-sized structure, incorporating organic matrix proteins reminiscent of those naturally occurring in native bone tissue. This composition not only promotes biocompatibility but also augments osteoblast differentiation during in vitro co-culturing with BMSCs. In vivo assessments demonstrated the capacity of the ICG/BPs to promote bone regeneration when utilized for locally repairing large-size, nonhealing tibial defects in animals. Additionally, the degradation of the ICG/BPs is able to be monitored using SWIR imaging in vivo and aligns temporally with bone regeneration and healing.
To explore the systemic potential of the ICG/BPs, we conducted in vivo evaluations on rats, focusing on biodistribution, systemic clearance, and the overall general safety following their intravenous administration. The use of SWIR imaging revealed the biodistribution pattern, highlighting significant accumulation primarily in the liver and kidneys. This indicated effective elimination through hepatic and renal pathways, mitigating the risk of accumulation-related toxicity. The observed well-being and normal behavior of treated animals, coupled with the absence of acute and chronic toxicities in vital organs during histological examination, affirmed the favorable safety profile of the ICG/BPs. Notably, the real-time, in situ monitoring capabilities of the ICG/BPs in both the NIR and SWIR windows gave insights into their in vivo dynamics. This real-time monitoring additionally provided valuable insight for enhancing the assessment of their safety, determining their potential applicability, and identifying any potential downstream effects.
The demonstrated efficacy and safety of the ICG/BPs in this study serve as a foundational basis for their prospective applications in orthopedic treatments. Subsequent investigations will focus on further optimization, clinical translation, and a comprehensive exploration of synthesis variables, addressing specific targeting challenges to enhance therapeutic outcomes.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the startup fund from The Marquette University and Medical College of Wisconsin Joint Department of Biomedical Engineering to Dr Bo Wang and the NIH 2R01CA193343 and NIH S10OD032237 awarded to Dr Amit Joshi.
Footnotes
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4bm00391h
Conflicts of interest
The authors declare that they have no conflict of interest.
References
- 1.Laurencin CT, Ambrosio AM, Borden MD and Cooper JA Jr., Annu. Rev. Biomed. Eng, 1999, 1, 19–46. [DOI] [PubMed] [Google Scholar]
- 2.Bhatt RA and Rozental TD, Handb. Clin, 2012, 28, 457–468. [DOI] [PubMed] [Google Scholar]
- 3.Bullock G, Atkinson J, Gentile P, Hatton P and Miller C, J. Funct. Biomater, 2021, 12, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lobb DC, DeGeorge BR Jr. and Chhabra AB, J. Hand Surg., Am., 2019, 44, 497–505.e2. [DOI] [PubMed] [Google Scholar]
- 5.Rupp M, Klute L, Baertl S, Walter N, Mannala GK, Frank L, Pfeifer C, Alt V and Kerschbaum M, J. Biomed. Mater. Res., Part B, 2022, 110, 350–357. [DOI] [PubMed] [Google Scholar]
- 6.Einhorn TA, Clin. Orthop. Relat. Res, 1998, S7–21, DOI: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 7.Oryan A, Monazzah S and Bigham-Sadegh A, Biomed. Environ. Sci, 2015, 28, 57–71. [DOI] [PubMed] [Google Scholar]
- 8.Sun S, Expert Opin. Ther. Targets, 2008, 12, 239–251. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Newman MR and Benoit DSW, Eur. J. Pharm. Biopharm, 2018, 127, 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porter JR, Ruckh TT and Popat KC, Biotechnol. Prog, 2009, 25, 1539–1560. [DOI] [PubMed] [Google Scholar]
- 11.Lin JH, Bone, 1996, 18, 75–85. [DOI] [PubMed] [Google Scholar]
- 12.Aoki K, Alles N, Soysa N and Ohya K, Adv. Drug Delivery Rev., 2012, 64, 1220–1238. [DOI] [PubMed] [Google Scholar]
- 13.Carbone EJ, Rajpura K, Allen BN, Cheng E, Ulery BD and Lo KW, Nanomedicine, 2017, 13, 37–47. [DOI] [PubMed] [Google Scholar]
- 14.Gu W, Wu C, Chen J and Xiao Y, Int. J. Nanomed, 2013, 8, 2305–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alford AI, Kozloff KM and Hankenson KD, Int. J. Biochem. Cell Biol., 2015, 65, 20–31. [DOI] [PubMed] [Google Scholar]
- 16.Madhurakkat Perikamana SK, Lee J, Lee YB, Shin YM, Lee EJ, Mikos AG and Shin H, Biomacromolecules, 2015, 16, 2541–2555. [DOI] [PubMed] [Google Scholar]
- 17.Palmer LC, Newcomb CJ, Kaltz SR, Spoerke ED and Stupp SI, Chem. Rev, 2008, 108, 4754–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beniash E, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol, 2011, 3, 47–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorski JP, Front. Biosci.-Landmark, 2011, 16, 2598–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garnero P, BoneKEy Rep., 2012, 1, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolf MT, Daly KA, Reing JE and Badylak SF, Biomaterials, 2012, 33, 2916–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sicari BM, Dearth CL and Badylak SF, Anat. Rec, 2014, 297, 51–64. [DOI] [PubMed] [Google Scholar]
- 23.Hinderer S, Layland SL and Schenke-Layland K, Adv. Drug Delivery Rev., 2016, 97, 260–269. [DOI] [PubMed] [Google Scholar]
- 24.Duisit J, Amiel H, Wuthrich T, Taddeo A, Dedriche A, Destoop V, Pardoen T, Bouzin C, Joris V, Magee D, Vogelin E, Harriman D, Dessy C, Orlando G, Behets C, Rieben R, Gianello P and Lengele B, Acta Biomater., 2018, 73, 339–354. [DOI] [PubMed] [Google Scholar]
- 25.Chen FM and Liu X, Prog. Polym. Sci, 2016, 53, 86–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Browning KN, Physiol J., 2018, 596, 3831–3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hussey GS, Keane TJ and Badylak SF, Nat. Rev. Gastroenterol. Hepatol, 2017, 14, 540–552. [DOI] [PubMed] [Google Scholar]
- 28.Liao J, Xu B, Zhang R, Fan Y, Xie H and Li X, J. Mater. Chem. B, 2020, 8, 10023–10049. [DOI] [PubMed] [Google Scholar]
- 29.Sawkins MJ, Bowen W, Dhadda P, Markides H, Sidney LE, Taylor AJ, Rose FR, Badylak SF, Shakesheff KM and White LJ, Acta Biomater., 2013, 9, 7865–7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Q, Chen C and Lyu GB, Zhonghua Yixue Zazhi, 2023, 103, 787–792. [DOI] [PubMed] [Google Scholar]
- 31.Kim D, Lee H, Lee GH, Hoang TH, Kim HR and Kim GH, Bioeng. Transl. Med, 2022, 7, e10317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amirazad H, Dadashpour M and Zarghami N, J. Biol. Eng, 2022, 16, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li W, Wang F, Barnett C and Wang B, J. Biomed. Mater. Res., Part B, 2021, 109, 2131–2141. [DOI] [PubMed] [Google Scholar]
- 34.Aoki K and Saito N, Pharmaceutics, 2020, 12, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dorati R, DeTrizio A, Modena T, Conti B, Benazzo F, Gastaldi G and Genta I, Pharmaceuticals, 2017, 10, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogay V, Mun EA, Kudaibergen G, Baidarbekov M, Kassymbek K, Zharkinbekov Z and Saparov A, Polymers, 2020, 12, 2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xinluan W, Yuxiao L, Helena NH, Zhijun Y and Ling Q, Curr. Pharm. Des, 2015, 21, 1575–1583. [DOI] [PubMed] [Google Scholar]
- 38.Feng L, Zhu C, Yuan H, Liu L, Lv F and Wang S, Chem. Soc. Rev, 2013, 42, 6620–6633. [DOI] [PubMed] [Google Scholar]
- 39.Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK and Hua S, Front. Pharmacol, 2015, 6, 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luther DC, Huang R, Jeon T, Zhang X, Lee YW, Nagaraj H and Rotello VM, Adv. Drug Delivery Rev., 2020, 156, 188–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maiti D, Tong X, Mou X and Yang K, Front. Pharmacol, 2018, 9, 1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Roemeling C, Jiang W, Chan CK, Weissman IL and Kim BYS, Trends Biotechnol., 2017, 35, 159–171. [DOI] [PubMed] [Google Scholar]
- 43.Nizzero S, Ziemys A and Ferrari M, Trends Cancer, 2018, 4, 277–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar S, Anselmo AC, Banerjee A, Zakrewsky M and Mitragotri S, J. Controlled Release, 2015, 220, 141–148. [DOI] [PubMed] [Google Scholar]
- 45.Xie X, Liao J, Shao X, Li Q and Lin Y, Sci. Rep, 2017, 7, 3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garapaty A and Champion JA, PLoS One, 2019, 14, e0217022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganson NJ, Povsic TJ, Sullenger BA, Alexander JH, Zelenkofske SL, Sailstad JM, Rusconi CP and Hershfield MS, J. Allergy Clin. Immunol, 2016, 137, 1610–1613.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao X, Li L, Cai X, Huang Q, Xiao J and Cheng Y, Biomaterials, 2021, 265, 120404. [DOI] [PubMed] [Google Scholar]
- 49.Cheng H, Chawla A, Yang Y, Li Y, Zhang J, Jang HL and Khademhosseini A, Drug Discovery Today, 2017, 22, 1336–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mangus CW, Klein BL, Miller M, Stewart D and Ryan LM, J. Invest. Med, 2019, 67, 59–62. [DOI] [PubMed] [Google Scholar]
- 51.Kaneko A, Matsushita I, Kanbe K, Arai K, Kuga Y, Abe A, Matsumoto T, Nakagawa N and Nishida K, Mod. Rheumatol, 2013, 23, 1053–1062. [DOI] [PubMed] [Google Scholar]
- 52.Krishnan A, Shirkhoda A, Tehranzadeh J, Armin AR, Irwin R and Les K, Radiographics, 2003, 23, 1371–1383discussion 1384–1377. [DOI] [PubMed] [Google Scholar]
- 53.Hong G, Diao S, Chang J, Antaris AL, Chen C, Zhang B, Zhao S, Atochin DN, Huang PL, Andreasson KI, Kuo CJ and Dai H, Nat. Photonics, 2014, 8, 723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simpson CR, Kohl M, Essenpreis M and Cope M, Phys. Med. Biol, 1998, 43, 2465–2478. [DOI] [PubMed] [Google Scholar]
- 55.Sordillo LA, Pu Y, Pratavieira S, Budansky Y and Alfano RR, J. Biomed. Opt, 2014, 19, 056004. [DOI] [PubMed] [Google Scholar]
- 56.Zhang H, Salo D, Kim DM, Komarov S, Tai YC and Berezin MY, J. Biomed. Opt, 2016, 21, 126006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carr JA, Valdez TA, Bruns OT and Bawendi MG, Proc. Natl. Acad. Sci. U. S. A, 2016, 113, 9989–9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shvedchenko DO and Suvorova EI, Microsc. Res. Tech, 2017, 80, 1113–1122. [DOI] [PubMed] [Google Scholar]
- 59.Teulon JM, Godon C, Chantalat L, Moriscot C, Cambedouzou J, Odorico M, Ravaux J, Podor R, Gerdil A, Habert A, Herlin-Boime N, Chen SW and Pellequer JL, Nanomaterials (Basel), 2018, 9, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mondejar-Lopez M, Lopez-Jimenez AJ, Abad-Jorda M, Rubio-Moraga A, Ahrazem O, Gomez-Gomez L and Niza E, Molecules, 2021, 26, 4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Niu C, Fan S, Li Y, Li X, Dai Y, Shi J and Wang X, Int. J. Mol. Sci, 2020, 21, 4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jagtap J, Sharma G, Parchur AK, Gogineni V, Bergom C, White S, Flister MJ and Joshi A, Biomed. Opt. Express, 2018, 9, 543–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Habibi N, Mauser A, Raymond JE and Lahann J, Beilstein J. Nanotechnol, 2022, 13, 274–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Girott i A, Gonzalez-Valdivieso J, Alonso-Sampedro I, Escalera-Anzola S, Ramos-Diez S and Arias FJ, Methods Mol. Biol, 2022, 2465, 41–72. [DOI] [PubMed] [Google Scholar]
- 65.Upreti T, Wolfe KM, Van Bavel N, Anikovskiy M and Labouta HI, Phys. Chem. Chem. Phys, 2022, 24, 5610–5617. [DOI] [PubMed] [Google Scholar]
- 66.Habibi N, Mauser A, Ko Y and Lahann J, Adv. Sci, 2022, 9, e2104012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang G and Uludag H, Expert Opin. Drug Delivery, 2008, 5, 499–515. [DOI] [PubMed] [Google Scholar]
- 68.de Barros AB, Tsourkas A, Saboury B, Cardoso VN and Alavi A, EJNMMI Res., 2012, 2, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elias DR, Poloukhtine A, Popik V and Tsourkas A, Nanomedicine, 2013, 9, 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li F, Chen Y, Liu S, Pan X, Liu Y, Zhao H, Yin X, Yu C, Kong W and Zhang Y, Int. J. Nanomed, 2019, 14, 9917–9928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kang JH, Cho J and Ko YT, J. Drug Targeting, 2019, 27, 103–110. [DOI] [PubMed] [Google Scholar]
- 72.Agarwal R, Jurney P, Raythatha M, Singh V, Sreenivasan SV, Shi L and Roy K, Adv. Healthc. Mater, 2015, 4, 2269–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sikes JC, Wonner K, Nicholson A, Cignoni P, Fritsch I and Tschulik K, ACS Phys. Chem. Au, 2022, 2, 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee B, Yoon S, Lee JW, Kim Y, Chang J, Yun J, Ro JC, Lee JS and Lee JH, ACS Nano, 2020, 14, 17125–17133. [DOI] [PubMed] [Google Scholar]
- 75.Hayashida M, Paraguay-Delgado F, Ornelas C, Herzing A, Blackburn AM, Haydon B, Yaguchi T, Wakui A, Igarashi K, Suzuki Y, Motoki S, Aoyama Y, Konyuba Y and Malac M, Micron, 2021, 140, 102956. [DOI] [PubMed] [Google Scholar]
- 76.Maugi R, Hauer P, Bowen J, Ashman E, Hunsicker E and Platt M, Nanoscale, 2020, 12, 262–270. [DOI] [PubMed] [Google Scholar]
- 77.Jackson P, Periasamy S, Bansal V and Geso M, Australas. Phys. Eng. Sci. Med, 2011, 34, 243–249. [DOI] [PubMed] [Google Scholar]
- 78.Brown TD, Habibi N, Wu D, Lahann J and Mitragotri S, ACS Biomater. Sci. Eng, 2020, 6, 4916–4928. [DOI] [PubMed] [Google Scholar]
- 79.Guo H, Stan G and Liu Y, Soft Matter, 2018, 14, 1311–1318. [DOI] [PubMed] [Google Scholar]
- 80.Horechyy A, Paturej J, Nandan B, Jehnichen D, Gobel M, Reuter U, Sommer JU and Stamm M, J. Colloid Interface Sci., 2020, 578, 441–451. [DOI] [PubMed] [Google Scholar]
- 81.Wu MR, Huang YY and Hsiao JK, Molecules, 2019, 24, 2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun Z, Wang X, Liu J, Wang Z, Wang W, Kong D and Leng X, Mol. Pharm, 2021, 18, 928–939. [DOI] [PubMed] [Google Scholar]
- 83.Zhang X, Li Y, Wei M, Liu C, Yu T and Yang J, Drug Delivery, 2019, 26, 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moulder JE, Fish BL, Holcenberg JS and Sun GX, Int. J. Radiat. Oncol., Biol., Phys., 1990, 19, 1389–1396. [DOI] [PubMed] [Google Scholar]
- 85.Aguree S and Gernand AD, MethodsX, 2019, 6, 1072–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bell OH, Carreno E, Williams EL, Wu J, Copland DA, Bora M, Kobayter L, Fruttiger M, Sim DA, Lee RWJ, Dick AD and Chu CJ, PLoS One, 2020, 15, e0226311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nystrom NN, Yip LCM, Carson JJL, Scholl TJ and Ronald JA, Radiol. Imaging Cancer, 2019, 1, e190035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leo E, Scatturin A, Vighi E and Dalpiaz A, J. Nanosci. Nanotechnol, 2006, 6, 3070–3079. [DOI] [PubMed] [Google Scholar]
- 89.Uhrich KE, Cannizzaro SM, Langer RS and Shakesheff KM, Chem. Rev, 1999, 99, 3181–3198. [DOI] [PubMed] [Google Scholar]
- 90.Yang F, Zhang X, Song L, Cui H, Myers JN, Bai T, Zhou Y, Chen Z and Gu N, ACS Appl. Mater. Interfaces, 2015, 7, 9410–9419. [DOI] [PubMed] [Google Scholar]
- 91.Yuan Q, Shah J, Hein S and Misra RD, Acta Biomater., 2010, 6, 1140–1148. [DOI] [PubMed] [Google Scholar]
- 92.Huang G, Gao J, Hu Z, St John JV, Ponder BC and Moro D, J. Controlled Release, 2004, 94, 303–311. [DOI] [PubMed] [Google Scholar]
- 93.Trewyn BG, Giri S, I Slowing I and Lin VS, Chem. Commun, 2007, 3236–3245, DOI: 10.1039/b701744h. [DOI] [PubMed] [Google Scholar]
- 94.Kneipp J, Kneipp H, Rice WL and Kneipp K, Anal. Chem, 2005, 77, 2381–2385. [DOI] [PubMed] [Google Scholar]
- 95.Wang X, Ku G., Wegiel MA, Bornhop DJ, Stoica G and Wang LV, Opt. Lett, 2004, 29, 730–732. [DOI] [PubMed] [Google Scholar]
- 96.Carr JA, Franke D, Caram JR, Perkinson CF, Saif M, Askoxylakis V, Datta M, Fukumura D, Jain RK, Bawendi MG and Bruns OT, Proc. Natl. Acad. Sci. U. S. A, 2018, 115, 4465–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V and Thompson M, Nat. Mater, 2009, 8, 543–557. [DOI] [PubMed] [Google Scholar]
- 98.Qiu Y, Liu Y, Wang L, Xu L, Bai R, Ji Y, Wu X, Zhao Y, Li Y and Chen C, Biomaterials, 2010, 31, 7606–7619. [DOI] [PubMed] [Google Scholar]
- 99.Kim SH, Lee JH, Hyun H, Ashitate Y, Park G, Robichaud K, Lunsford E, Lee SJ, Khang G and Choi HS, Sci. Rep, 2013, 3, 1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim SH, Park JH, Kwon JS, Cho JG, Park KG, Park CH, Yoo JJ, Atala A, Choi HS, Kim MS and Lee SJ, Biomaterials, 2020, 258, 120267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.