Adult stem cells seem to have the capacity to “transdifferentiate” into cells of many different tissue types; now further work is needed to establish their role in treating degenerative diseases
The discovery of adult tissue specific stem cells, such as haematopoietic stem cells, which have the ability to “transdifferentiate” into other tissues, has generated much excitement among cell biologists and transplant clinicians. It opens new avenues for basic biological research by using stem cells from adults as an alternative to stem cells from embryos. It also carries important implications for the treatment of many liver, heart, and neurodegenerative diseases. Despite strong evidence that multipotent stem cells (stem cells with the potential to differentiate into several cell types) reside in many adult organs and can be manipulated in ways that may confer a therapeutic advantage, several questions remain to be addressed before the development of clinical applications. We review recent findings on the “plasticity” of adult stem cells to adopt a fate different from their originally intended one, and we discuss the potential clinical importance of this finding. We also point out some directions for research in the future.
Summary points
Pluripotent embryonic stem cells divide indefinitely and ostensibly generate all cell types
Stem cells from adults regenerate their resident tissue but may have broader potential for differentiation (multipotential)
Further work must establish the broad differentiation and functional potential of stem cells from adults
Clinical utility of all stem cells awaits further validation and development
Methods
We searched Medline with both general keywords such as “stem cells,” “stem cell plasticity,” “adult stem cells,” and “embryonal stem cells” and with more specific terms such as “haematopoietic, hepatic or muscle stem cells,” “Parkinson's disease,” and “osteogenesis imperfecta.” We also cite published and unpublished data generated in our laboratory.
Adult stem cells reconsidered
After development of the embryo most tissues are fully differentiated, and further growth or repair of damaged sites is undertaken by stem cells residing in particular tissues. Adult stem cell populations have been most thoroughly characterised in mouse and human bone marrow, where they continuously replenish the differentiated cells of the peripheral blood lost through attrition. From studies of the haematopoietic system it has been possible to define a stem cell as a cell with the capacity to self renew and to generate cells of multiple diverse lineage within the tissue in which the stem cell resides. The ability of the haematopoietic stem cells within bone marrow to give rise to all blood elements has been extensively exploited in the clinic for transplantation of bone marrow and stem cells. By contrast, despite the well documented presence of stem cells in several mature tissues, including muscle, brain, skin, liver, and mammary gland, they remain poorly defined and underexploited in the clinic.1 Recent reports suggest that some of these stem cells can differentiate outside of their tissue of origin (box and fig 1).
Recent reports of potential plasticity of stem cells
The following reported experiments involved transplantation of marked donor cells into whole animals or directly into injured tissue and examination of the recipient tissue for incorporation of the marked donor derived cells
Whole bone marrow reportedly generated:Skeletal muscle2Cardiac muscle3Liver4–6Endothelial cells7Brain8–10
Muscle reportedly generated:Bone marrow11,12,13
Neural stem cells reportedly generated:Blood14Skeletal muscle15Multiple embryonic tissues16
Enriched or purified haematopoietic stem cells reportedly generated:Skeletal muscle11Cardiac muscle17,18,19Endothelial cells17Liver hepatocytes and bile duct20Multiple epithelial tissues21
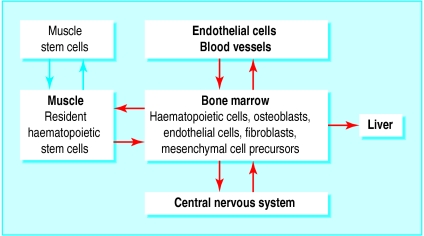
Figure 1.
Possible pathways for differentiation in adult stem cells. Cells within bone marrow may give rise to tissues such as endothelial cells, liver, muscle, and neurones (red arrows). Alternatively haematopoietic stem cells in muscle or muscle stem cells in bone marrow give rise to respective progeny (blue arrows)
Adult stem cells versus embryonic stem cells
Since the isolation of stem cells from human embryos two years ago, the prospect of using such cells to generate cells and tissues for cell therapy has stimulated much scientific and public interest. The stem cells are obtained from the inner cell mass of the blastocyst (fig 2). As numerous studies with their mouse counterparts have shown, the stem cells from human embryos are inherently primitive, can proliferate indefinitely, and have the capacity to generate all cell types of the adult.22 Yet because the embryo is destroyed to isolate the cells, a host of ethical issues has deeply divided researchers and politicians alike.
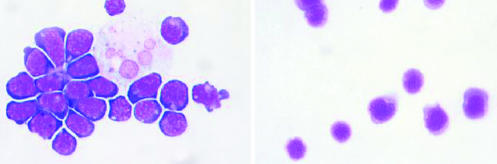
Figure 2.
(Left) Embryonic stem cells from mouse (Wright Giemsa). Large pale staining cell (upper right) is either a stromal cell, with which these cells were grown, or a differentiated daughter of an embryonic stem cell. (Right) Haematopoietic stem cells from mouse
Some researchers argue that stem cells derived from adults have sufficient developmental potential that they can be substituted for stem cells from the embryo in therapeutic situations, without any appreciable loss of regenerative efficacy. Others contend that the extensive capacity of adult stem cells to differentiate has not been proved and that their capacity proliferation is more limited than that of embryonic stem cells, so they may not provide a long term source of progenitor cells to replace tissue lost to chronic disease. Clearly there is a need to rigorously evaluate claims of differentiation “plasticity” of adult stem cells and their long term therapeutic potential.
Evidence for stem cell plasticity
The first suggestion that stem cells from one tissue of an adult could generate cells of an unrelated tissue came from studies of whole bone marrow transplantation in humans and animal models, in which the transplanted cells could be distinguished from the recipient's tissues by using conventional histological techniques. Examination of several different organs of the recipient showed the presence of cells derived from the donor in tissues other than the blood, including endothelial, skeletal, and cardiac muscle cells, liver oval cells, hepatocytes, and cells of the central nervous system.2–9,22 All of these studies were technically difficult because the donor cells formed most of the haematopoietic system after bone marrow transplantation, and haematopoietic cells infiltrated all tissues. The investigators therefore had to distinguish between donor blood cells and donor cells that had become something else, such as hepatocytes. The most striking suggestion of stem cell plasticity was published in 1998 by an Italian group, which found that genetically marked bone marrow cells from a mouse can migrate to sites of muscle injury and participate in muscle regeneration, albeit at low efficiency.2 The implication of this and similar studies performed with whole bone marrow—that the diverse donor cell types observed after transplantation could all be derived from the haematopoietic system—captured the imagination of researchers in tissue specific stem cells. It seemed that given the appropriate environmental signals, adult cells derived from bone marrow have the capacity to transdifferentiate into cells of many different organs, including liver hepatocytes, endothelial cells, and even neurones.
Two studies found that transplanted bone marrow cells from adults could migrate into the brain and differentiate into cells expressing proteins specific for neuronal cells.8,9 These findings suggest that cells contained within the bone marrow of the adult transdifferentiated and assumed a neuronal fate. The flaw in this interpretation is that besides haematopoietic stem cells, whole bone marrow contains a spectrum of diverse cell types (for example, differentiated haematopoietic cells, osteoblasts, endothelial cells, fibroblasts, mesenchymal precursors that can differentiate into cartilage, bone, and fat23,24), which are transferred to the recipient and which could participate in the apparent differentiation events of non-haematopoietic origin.
Turning blood into muscle, liver, and brain
To garner support for the hypothesis that stem cells from the haematopoietic system are capable of participating in the differentiation into diverse tissue types, several groups have transplanted purified cells in a variety of settings. Gussoni and others transplanted several thousand haematopoietic stem cells from male mice into female mdx mice, a model of Duchenne muscular dystrophy.11 They were able to track the fate of the transplanted cells by detecting the Y chromosome with fluorescent in situ hybridisation. The donor cells efficiently replenished the bone marrow of the recipients as expected, and cells from the males expressing dystrophin were found at low levels in host muscle fibres, indicating differentiation of the transplanted cells into muscle. As in previous studies,evidence of the ostensibly multipotent capacity of haematopoietic stem cells was based on the examination of tissues with markers specific for that tissue, such as dystrophin in skeletal muscle, and markers that distinguish resident cells from those of the donor. Because of the low level of engraftment and the lack of clinically evident weakness in the mdx mice, it was not possible to assess the contribution of the engrafted cells to cardiac function or muscle strength.
Analogous studies in our laboratory have shown that the progeny of transplanted haematopoietic stem cells can also contribute to the repair of capillaries (fig 3) and cardiomyocytes in a mouse model of coronary artery infarction.17 Orlic and others also observed that when a population enriched in haematopoietic stem cells was injected directly into injured hearts it could participate in the regeneration of cardiac muscle, leading to an apparent improvement of cardiac function.18 They also recently showed that treatment of mice with granulocyte colony stimulating factor, which is known to mobilise stem cells from bone marrow into blood, protected mice from some of the damage of induced myocardial infarction.19 This suggested that the increased number of circulating stem cells was protective, perhaps through engraftment of cells into the heart, although this was not shown.
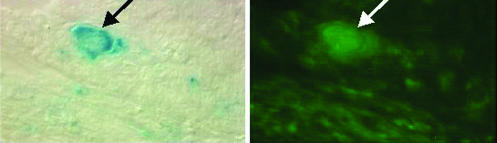
Figure 3.
Enriched haematopoietic stem cells engrafted in endothelial layer of blood vessels. (Left) lacZ-positive cells derived from stem cells (arrow). (Right) Endothelial cells (arrow). Haematopoietic stem cells were purified from Rosa26 transgenic mice, which express the lacZ transgene (β galactosidase) widely, and transplanted into the bone marrow of irradiated recipients. Several months after transplantation the tibialis anterior was injured by injection of cardiotoxin. Two weeks later, cryosections were made and stained with X-gal, to detect β galactosidase, and anti-PE-CAM1 antibody, to detect endothelial cells
Lagasse and others were the first to provide strong support for the concept of transdifferentiation at a functional level by using small numbers of purified stem cells. They showed that as few as 30 highly purified haematopoietic stem cells injected into mice with an inducible lethal hereditary liver disease, tyrosinaemia type 1, could repopulate the haematopoietic system as well as differentiate into hepatocytes and rescue the animals from hepatic failure and death.20
Although these studies support the idea of the differentiation “plasticity” of adult stem cells, definitive proof is still lacking. In all of these experiments multiple cells were transplanted, from between 30 and several thousand. Even with as few as 30 highly purified cells, multiple stem cell types could be present—for example, a progenitor of both haematopoietic and non-haematopoietic origin—accounting for differentiation into multiple tissue types. Definitive proof requires experiments to show that a single stem cell can differentiate into more than one tissue type.
What does the future hold for adult stem cells of non-haematopoietic origin?
Haematopoietic stem cells can be isolated prospectively by using combinations of surface markers, but such strategies have not yet been exploited for stem cells specific for other organs. These are usually identified by their anatomical location or by markers associated with their tissue progenitor cells. Surface markers that may be specific for adult stem cells are beginning to emerge and may soon allow a prospective evaluation of the transdifferentiative capacity of adult stem cells of non-haematopoietic origin.
Muscle stem cells
Skeletal muscle contains a potent myogenic stem cell population with the ability to regenerate muscle in vivo. Our group transplanted mice with a crude mixture of muscle derived mononuclear cells that displayed the hallmarks of primitive haematopoietic stem cells. The transplanted cells gave rise to all haematopoietic cells and possessed the capacity for self renewal, as shown by their ability to contribute to blood production after serial transplantation into secondary recipients.12 This suggested that the haematopoietic activity of muscle originated from myogenic stem cells that had switched fate on introduction to the regenerating bone marrow, which had been highly stimulated after lethal irradiation. Equally tenable interpretations were that a progenitor common to both the haematopoietic and the myogenic lineage was present in the muscle specimens or, more likely, that true haematopoietic stem cells were present in muscle, for unknown reasons. Recent data from Ogawa's and our own group suggest that bone marrow derived haematopoietic stem cells contained within the muscle are responsible for the haematopoietic potential initially thought to be derived from muscle cells.25,26 This finding emphasises the need for caution when interpreting the results of studies in transdifferentiation.
Neuronal stem cells
Over the past two decades the transplantation of cells or stem cells has emerged as an attractive approach to the restoration of function in neurodegenerative diseases such as Parkinson's and Alzheimer's. Implantation of dopaminergic neuroblasts, from embryos, which are already committed by fate into the brains of patients with Parkinson's disease has yielded promising results, including clinical improvement in some patients.27,28 The problems with this strategy—need for embryonic tissue, localised delivery of dopaminergic cells, and limited graft survival—have impeded its broader use. Neuronal stem cells have also been isolated from the brains of embryos and adults.29,30 Adult neural stem cells have the potential to differentiate into multiple cell types of the brain, mainly oligodendrocytes, astrocytes, and neurones, giving them a major therapeutic advantage over committed progenitor cells such as those used in transplants for Parkinson's disease. Moreover, these neural stem cells may be multipotent: when injected into blastocysts of mice they contributed to multiple types of tissues in the embryos.16 One study reported the generation of blood from neuronal stem cells when transplanted into lethally irradiated recipients, but this has not yet been reproduced.14 These neuronal stem cells have also been observed to generate skeletal muscle when cultured with a cell line capable of differentiating into muscle or when injected into regenerating muscle.15 Further experiments are needed to determine whether the transdifferentiative potential of neuronal stem cells resides in specific clones or defines this cell type in general and whether these properties will be generalisable to neural stem cells in humans.
Recent clinical observations
Although most published information on adult stem cells draws heavily from studies with animal models, there is increasing clinical evidence to support the concept of stem cell transdifferentiation. Theise and others examined the livers of patients who received either bone marrow from someone of the opposite sex or liver transplants. In both cases the authors found hepatocytes and cholangiocytes from the opposite sex, suggesting that a circulating bone marrow cell could home to and transdifferentiate in the liver.6 Horwitz and others showed engraftment of donor osteoblasts and enhanced bone formation after bone marrow transplantation in children with the most severe form of osteogenesis imperfecta, a genetic disorder of collagen production.31 The patients also showed major clinical benefits, including fewer fractures and increased growth velocity. The authors concluded that functional mesenchymal progenitor cells residing in the bone marrow compartment were responsible for the changes, although part of the therapeutic effect may have derived from haematopoietic stem cells with the capacity for transdifferentiation.
Conclusions
Stem cells derived from bone marrow, whether multipotent haematopoietic stem cells or other tissue specific stem cells resident in the bone marrow, have a major advantage over stem cells from other organs: they are well defined, easy to isolate, and can be injected systemically, reaching other tissues through the bloodstream. Moreover, transplantation of bone marrow or haematopoietic stem cells leads to induction of donor tolerance, permitting transdifferentiation or transplantation of other tissue specific stem cells from the same donor without the need for prolonged immunosuppression of the recipient. None the less, the transdifferentiation events described in most of these studies were rare, even under extreme selective pressure. Do such events occur simply by chance or do they reflect a genetic programme that can be activated by specific signals?
Key progress needed in research into plasticity of stem cells from adults
Proof that a single stem cell from an adult can differentiate into more than one cell or tissue type
Proof of functionality: that progeny of “transdifferentiated” adult stem cells functionally integrate in the new tissue
Proof of functional benefit in non-autochtonous tissue
Improvements in efficiency of engraftment and transdifferentiation
Establishment of practical utility: histocompatibility issues? Expansion and differentiation of stem cells? Clinical delivery issues?
As studies are published, some of the earlier results are being questioned. Do some of the reported observations of transdifferentiation represent events of cell fusion, as recently suggested for bone marrow and neural progenitor cells?32,33 Are there other potential explanations for this apparently unorthodox behaviour of stem cells? Before adult stem cells are used therapeutically in patients with degenerative disorders of the liver, heart, or brain, the properties of such cells must be characterised, the factors responsible for their regulation defined, and functionality proved.
Additional educational resources
Useful publications
Lagasse E, Shizuru JA, Uchida N, Tsukamoto A, Weissman IL. Toward regenerative medicine. Immunity 2001;14:425-36
Very good overview of the properties and potential of haematopoietic stem cells
Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell 2001;105:829-41
Provides historical account of plasticity of stem cells from adults
Wulf GG, Jackson KA, Goodell MA. Somatic stem cell plasticity: current evidence and emerging concepts. Exp Hematol 2001;29:1361-70
Comprehensive review of the specialty of stem cells from adults, with summary of published data
Lachmann P. Stem cell research—why is it regarded as a threat? EMBO Rep 2001;2:165-8
An investigation of the economic and ethical arguments made against research with stem cells from human embryos
Useful websites
National Institute of Health Stem Cell Primer (www.nih.gov/news/stemcell/primer.htm)
Defines pluripotent stem cells, how they are derived, and why they are important to science and for advances in health care. Also defines stem cells from adults
Report on stem cell research from the American Association for the Advancement of Science (www.aaas.org/spp/dspp/sfrl/projects/stem/main.htm and www.aaas.org/spp/cstc/issues/stemcells.htm)
Discussion of scientific and ethical issues and policies surrounding stem cell research
St Jude's Children's Hospital (www.stjude.org/)
Recent trials of bone marrow transplantation and mesenchymal progenitor cell therapy in osteogenesis imperfecta
National Institutes of Health (www.ninds.nih.gov/parkinsonsweb/nigral.htm)
Recent trials of fetal tissue transplantation in Parkinson's disease
Acknowledgments
MAG is a scholar of the Leukemia and Lymphoma Society of America. We thank Maryellen R Goodell for critical reading of the manuscript and Kathyjo A Jackson for producing figure 3.
Footnotes
Competing interests: None declared.
References
- 1.Lagasse E, Shizuru JA, Uchida N, Tsukamoto A, Weissman IL. Toward regenerative medicine. Immunity. 2001;14:425–436. doi: 10.1016/s1074-7613(01)00123-6. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- 3.Bittner RE, Schofer C, Weipoltshammer K, Ivanova S, Streubel B, Hauser E, et al. Recruitment of bone-marrow-derived cells by skeletal and cardiac muscle in adult dystrophic mdx mice. Anat Embryol (Berl) 1999;199:391–396. doi: 10.1007/s004290050237. [DOI] [PubMed] [Google Scholar]
- 4.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase M, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 5.Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, et al. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235–240. doi: 10.1002/hep.510310135. [DOI] [PubMed] [Google Scholar]
- 6.Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, et al. Liver from bone marrow in humans. Hepatology. 2000;32:11–16. doi: 10.1053/jhep.2000.9124. [DOI] [PubMed] [Google Scholar]
- 7.Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- 8.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 9.Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 10.Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc Natl Acad Sci USA. 1997;94:4080–4085. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gussoni E, Soneoka Y, Strickland CD, Buzney EA, Khan MK, Flint AF, et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 12.Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci USA. 1999;96:14482–14486. doi: 10.1073/pnas.96.25.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pang W. Role of muscle-derived cells in hematopoietic reconstitution of irradiated mice. Blood. 200;95:1106–1108. [PubMed] [Google Scholar]
- 14.Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- 15.Galli R, Borello U, Gritti A, Minasi MG, Bjornson C, Coletta M, et al. Skeletal myogenic potential of human and mouse neural stem cells. Nat Neurosci. 2000;3:986–991. doi: 10.1038/79924. [DOI] [PubMed] [Google Scholar]
- 16.Clarke DL, Johansson CB, Wilbertz J, Veress B, Nilsson E, Karlstrom H, et al. Generalized potential of adult neural stem cells. Science. 2000;288:1660–1663. doi: 10.1126/science.288.5471.1660. [DOI] [PubMed] [Google Scholar]
- 17.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107:1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 20.Lagasse E, Connors H, Al Dhalimy M, Reitsma M, Dohse M, Osborne L, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 21.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 22.Odorico JS, Kaufman DS, Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19:193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- 23.Pereira RF, Halford KW, O'Hara MD, Leeper DB, Sokolov BP, Pollard MD, et al. Cultured adherent cells from marrow can serve as long-lasting precursors for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci USA. 1995;92:4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 25.Kawada H, Ogawa M. Bone marrow origin of hematopoietic progenitors and stem cells in murine muscle. Blood. 2001;98:2008–2013. doi: 10.1182/blood.v98.7.2008. [DOI] [PubMed] [Google Scholar]
- 26.McKinney-Freeman SL, Jackson KA, Camargo FD, Ferrari G, Mavilio F, Goodell MA. Muscle-derived hematopoietic stem cells are hematopoietic in origin. Proc Natl Acad Sci USA. 2002;99:1341–1346. doi: 10.1073/pnas.032438799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Lozano JJ, Mata M, Bravo G. Neural transplants in Parkinson disease: clinical results of 10 years of experience. Group of Neural Transplants of the CPH. Rev Neurol. 2000;30:1077–1083. [PubMed] [Google Scholar]
- 28.Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 30.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 31.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WWK, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 32.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 33.Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]